Abstract
PURPOSE:
We sought to examine tumor subtype, stage at diagnosis, time to surgery (TTS), and overall survival (OS) among Hispanic patients of different races and among Hispanic and non-Hispanic (NH) women of the same race.
METHODS:
Women 18 years of age or older who had been diagnosed with stage 0-IV breast cancer and who had undergone lumpectomy or mastectomy were identified in the National Cancer Database (2004-2014). Tumor subtype and stage at diagnosis were compared by race/ethnicity. Multivariable linear regression and Cox proportional hazards modeling were used to estimate associations between race/ethnicity and adjusted TTS and OS, respectively.
RESULTS:
A total of 44,374 Hispanic (American Indian [AI]: 79 [0.2%]; Black: 1,011 [2.3%]; White: 41,126 [92.7%]; Other: 2,158 [4.9%]) and 858,634 NH women (AI: 2,319 [0.3%]; Black: 97,206 [11.3%]; White: 727,270 [84.7%]; Other: 31,839 [3.7%]) were included. Hispanic Black women had lower rates of triple-negative disease (16.2%) than did NH Black women (23.5%) but higher rates than did Hispanic White women (13.9%; P < .001). Hispanic White women had higher rates of node-positive disease (23.2%) versus NH White women (14.4%) but slightly lower rates than Hispanic (24.6%) and NH Black women (24.5%; P < .001). Hispanic White women had longer TTS versus NH White women regardless of treatment sequence (adjusted means: adjuvant chemotherapy, 42.71 v 38.60 days; neoadjuvant chemotherapy, 208.55 v 201.14 days; both P < .001), but there were no significant racial differences in TTS among Hispanic patients. After adjustment, Hispanic White women (hazard ratio, 0.77 [95% CI, 0.74 to 0.81]) and Black women (hazard ratio, 0.75 [95% CI, 0.58 to 0.96]) had improved OS versus NH White women (reference) and Black women (hazard ratio, 1.15 [95% CI, 1.12 to 1.18]; all P < .05).
CONCLUSION:
Hispanic women had improved OS versus NH women, but racial differences in tumor subtype and nodal stage among Hispanic women highlight the importance of disaggregating racial/ethnic data in breast cancer research.
INTRODUCTION
An estimated 57 million people in the United States, constituting 17% of the population, identify as Hispanic or Latinx.1,2 By 2060, this figure is projected to double, making Hispanics the largest, fastest-growing racial or ethnic minority group in the country.1,2 Breast cancer is the most commonly diagnosed malignancy and leading cause of cancer death in Hispanic women in the United States.3 Several epidemiologic studies have shown that Hispanic women are more likely to be diagnosed at a younger age, with triple-negative or human epidermal growth factor receptor 2 (HER2)–enriched (HER2+) disease, and with advanced-stage disease when compared with non-Hispanic (NH) White women.4-7
In these studies and others, researchers have overwhelmingly treated Hispanic patients as a single ethnic group and have compared them with NH White and Black patients. In reality, the Hispanic population in the United States is genetically, culturally, and geographically heterogeneous; individuals can have varying degrees of African, European, Indigenous, and (to a lesser extent) Asian ancestry; and Hispanic individuals may self-identify as any race or as multiple races.8
In breast cancer, where research has shown that race/ethnicity can be associated with disease predisposition, access to care, and outcomes after diagnosis,4,9-12 studies that combine Hispanic women of all races into a single group may overlook opportunities to identify important characteristics within this heterogeneous group that could more accurately predict outcomes and reveal opportunities to improve care. Accordingly, we sought to compare tumor subtype, stage at diagnosis, time to surgery (TTS), and overall survival (OS) among Hispanic patients of different races and among Hispanic and NH women of the same race.
METHODS
Patient Cohort
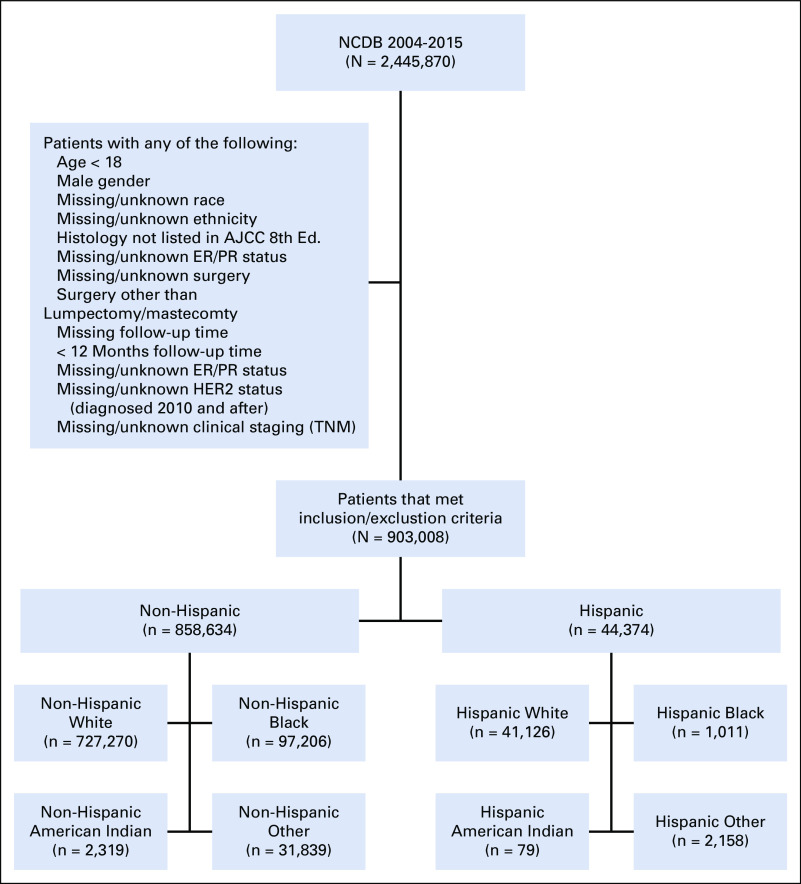
Women 18 years of age or older who had been diagnosed with stage 0-IV breast cancer between 2004 and 2015 and who had undergone lumpectomy or mastectomy were identified in the National Cancer Database (NCDB). Patients with unknown surgery or unknown duration of follow-up and/or those with a follow-up of < 12 months (including all patients diagnosed in 2015) were excluded. Patients with missing or unknown clinical stage, estrogen receptor (ER) status, or progesterone receptor (PR) status were also excluded, as were patients with missing HER2 status who were diagnosed in or after 2010, when HER2 coding became standardized in the NCDB.
The Facility Oncology Registry Data Standards (FORDS) coding manual guides data entry for the hospital-based cancer registries from which the NCDB is sourced.13 According to the FORDS manual, each patient may have up to five race entries (Race1-Race5), with Race1 defined as representing the patient’s primary racial affiliation. It is not indicated whether these categorizations are self-reported. If a multiracial individual has “White” listed as one of her racial groups, White race is always coded as Race2 or higher, with Race1 defaulting to one of her other non-White racial identities. Ethnicity is defined per the FORDS manual as being either of Spanish/Hispanic Origin or not. For the purpose of ascribing race and ethnicity to each patient, ethnicity is always paired and analyzed in conjunction with Race1. For this study, we used NCDB terminology to define eight patient race/ethnicity categorizations: Hispanic and NH White; Hispanic and NH Black; Hispanic and NH American Indian (AI); and Hispanic and NH Other, which includes patients who reported Asian or Pacific Islander (PI) race, because the number of Hispanic Asian and PI women was small. In addition, we had concern that some patients (in particular, those who identified as Filipino and had Spanish surnames) might have been miscoded through inappropriate use of the FORDS manual’s guidelines for designating patients as Hispanic.13
Hormone receptor positive (HR+) was defined as ER positive and/or PR positive, whereas hormone receptor negative (HR−) was defined as ER negative and PR negative. Patients diagnosed in or after 2010 were divided into three tumor subtypes on the basis of combinations of hormone receptor and HER2 status: (1) HR+/HER2−, (2) HER2+ (including both HR+ and HR−), and (3) HR−/HER2− (ie, triple negative).
Statistical Analysis
Patient characteristics were summarized as No. (%) for categorical variables and as median (interquartile range [IQR]) for continuous variables. Analysis of variance and χ2 tests were used to assess intergroup differences for continuous and categorical variables, respectively. Median follow-up time was estimated using the reverse Kaplan-Meier method.
Linear regression models were used to estimate the association of race/ethnicity with time to definitive surgery (lumpectomy or mastectomy), stratified by timing and receipt of chemotherapy (no chemotherapy, neoadjuvant chemotherapy [NACT], or adjuvant chemotherapy [ACT]) and after adjustment for variables captured within the NCDB that could have influenced patterns of care (itemized under Fig 1). Adjusted mean TTS in days and 95% CIs were estimated from these adjusted models, and pairwise comparisons were conducted with application of a Tukey adjustment, as appropriate. These models were built in the generalized estimating equations framework and included a compound symmetry covariance structure to account for the correlation of patients treated at the same facility.
FIG 1.
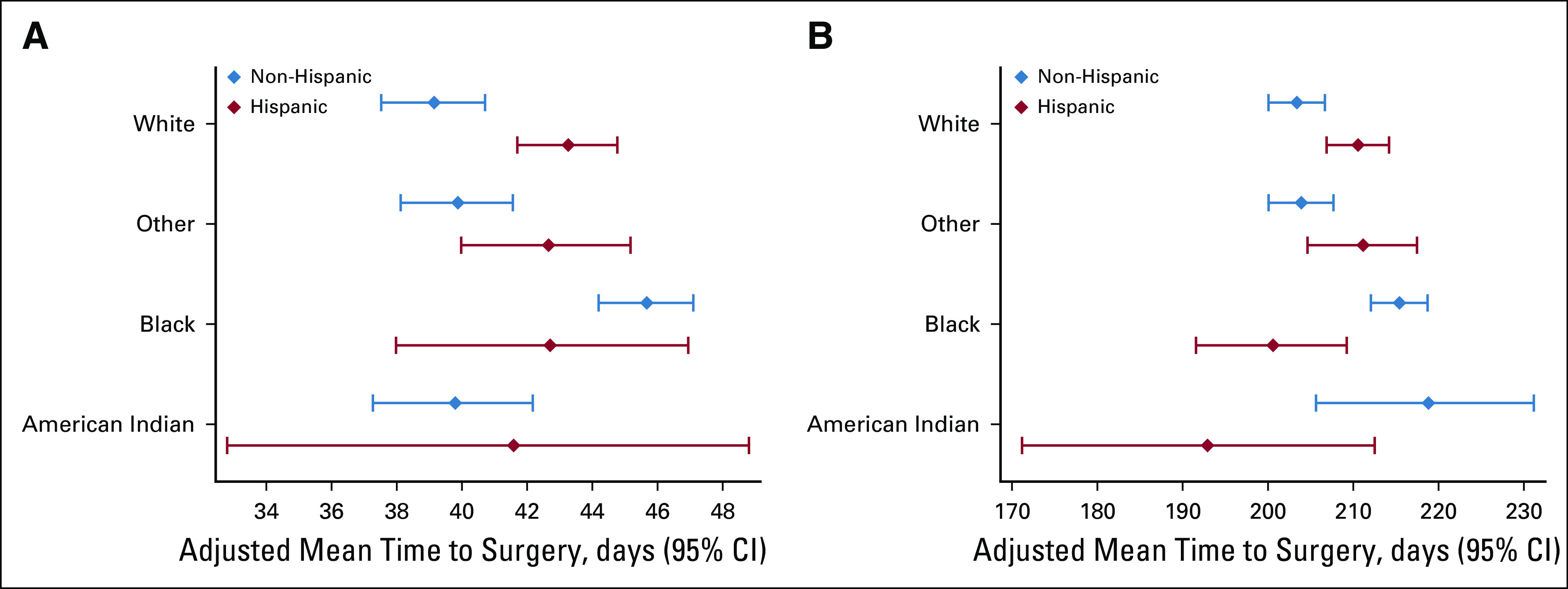
Time to surgery by race and treatment sequence among Hispanic and non-Hispanic women with breast cancer, National Cancer Database, 2004-2014. (A) Adjuvant chemotherapy only. (B) Neoadjuvant chemotherapy. Models were adjusted for year of diagnosis, distance traveled for treatment, Charlson-Deyo comorbidity score, education, income, insurance status, facility type, facility location, clinical TNM classifications, grade, histology, estrogen receptor/progesterone receptor status, and surgery type, all of which were significant.
OS was defined as the time from diagnosis to death or last follow-up. Kaplan-Meier curves were used to visualize unadjusted OS, and we report 5-year and 10-year survival rates and 95% CIs. Cox proportional hazards models were used to estimate the effect of race/ethnicity on OS after adjustment for the same covariables included in the TTS models plus receipt of chemotherapy, endocrine therapy, and radiation (itemized under Fig 2). A sensitivity survival analysis was conducted to also include TTS. Because the results of this analysis did not significantly differ from the primary model, only the results from the model without TTS are presented. We report hazard ratios and 95% CIs. Robust sandwich covariance estimators were included in all adjusted survival models to account for the correlation of patients treated at the same facility.
FIG 2.
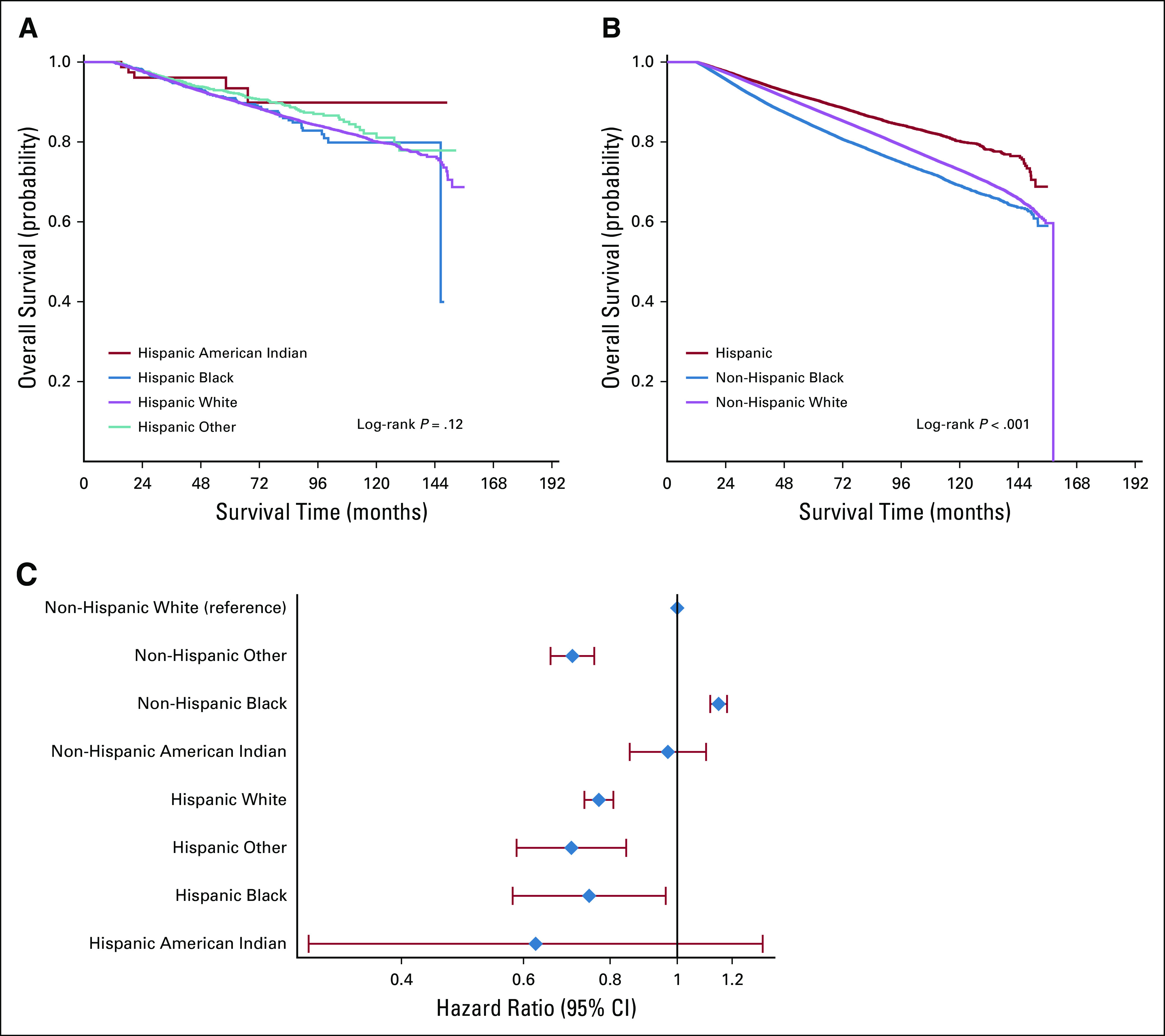
Unadjusted and adjusted overall survival (OS) among Hispanic and non-Hispanic women, National Cancer Database, 2004-2014. (A) Unadjusted OS: Hispanic women, all races (n = 44,374). (B) Unadjusted OS: Hispanic, non-Hispanic Black, and non-Hispanic White women (n = 903,008). (C) Adjusted OS: Hispanic and non-Hispanic women, all races (n = 770,691). Model was adjusted for year of diagnosis, distance traveled for treatment, Charlson-Deyo comorbidity score, education, income, insurance status, facility type, facility location, clinical TNM classifications, grade, histology, estrogen receptor/progesterone receptor status, surgery type, and receipt of chemotherapy, endocrine therapy, and radiation, all of which were significant.
On the basis of the low rate (approximately 5%) of missing values among included covariables, it was assumed that overall missing data in our cohort were not informative. Accordingly, only patients with available data for all covariables were included in each regression model (complete case analysis), and effective sample sizes are reported for each table/figure. No adjustments were made for multiple comparisons. Instead, we present the effect size and measure of variability for all estimated statistics. P values < .05 were considered statistically significant, but given the large sample sizes for some comparisons, results should be interpreted with plausible clinical relevance in mind. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient, Tumor, and Treatment Characteristics
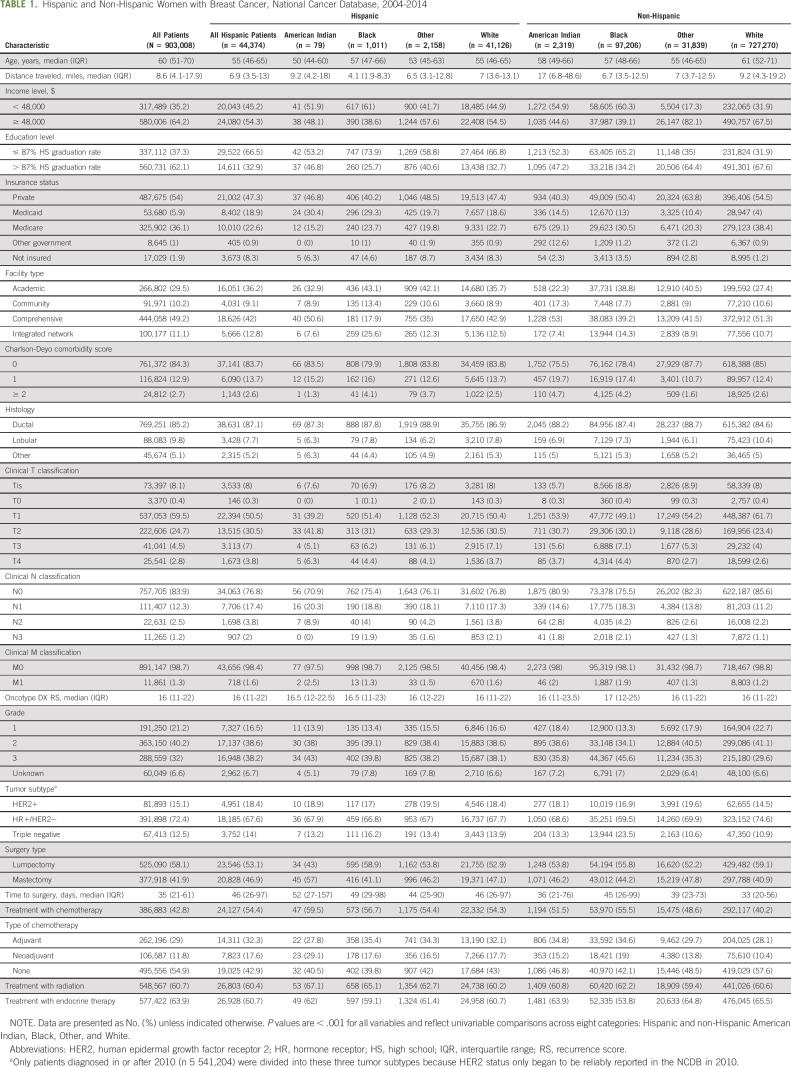
We identified 44,374 Hispanic and 858,634 NH women (Table 1; Appendix Fig A1, online only). Among Hispanic women, 41,126 (92.7%) were White, 1,011 (2.3%) were Black, 79 (0.2%) were AI, and 2,158 (4.9%) were of another race. Among NH women, 727,270 (84.7%) were White, 97,206 (11.3%) were Black, 2,319 (0.3%) were AI, and 31,839 (3.7%) were of another race. Among Hispanic patients for whom a country of origin was reported (n = 14,983), 6,767 (45.2%) were from Mexico. Median follow-up time was 60.8 months (95% CI, 60.7 to 60.9 months).
TABLE 1.
Hispanic and Non-Hispanic Women with Breast Cancer, National Cancer Database, 2004-2014
Proportions of triple-negative breast cancer (TNBC) were highest in NH Black women (23.5%) and lowest in NH women of White or Other races (approximately 10% to 11%). Among Hispanic women, rates of TNBC ranged from 16.2% in Hispanic Black women to approximately 13% among Hispanic women reported as AI or of Other race. In addition, Hispanic women of all races had higher rates of HER2+ disease than did NH White women (eg, Hispanic White, 18.4% v NH White, 14.5%; P < .001). Compared with NH White women, Hispanic White women had a higher proportion of tumors that were ≥ cT2 at diagnosis (41.3% v 29.9%) but similar rates to those of Hispanic and NH Black women (41.5% and 41.7%). Hispanic White women had more node-positive (cN1-3) disease (23.2%) compared with NH White women (14.4%) but only a slightly lower proportion compared with Hispanic Black (24.6%) and NH Black (24.5%) women. Hispanic AI women had the highest proportions of node-positive disease (29.1%).
Hispanic AI women had the highest rates of mastectomy (57%), whereas among other groups, mastectomy rates ranged from 40% to 48%. Hispanic White women had higher rates of treatment with chemotherapy than did NH White women (54.3% v 40.2%) whereas Hispanic and NH Black women received chemotherapy at similar rates (56.7% and 55.5%). Among Hispanic women, AI women had the highest rates of chemotherapy receipt (59.5%).
Time to Definitive Surgery
Median unadjusted TTS ranged from 33 days (IQR, 20-56 days) in NH White women to 52 days (IQR, 27-152 days) in Hispanic AI women (Table 1). After adjustment, Hispanic White women had longer TTS compared with NH White women, regardless of treatment sequence. Among women who received ACT, Hispanic White women had longer TTS compared with NH White women (adjusted mean, 42.71 v 38.60 days; P < .001; Fig 1A). Among women who received NACT, Hispanic White women experienced longer TTS compared with NH White women (adjusted mean, 208.55 v 201.14 days; P < .001), whereas Hispanic Black women experienced shorter TTS compared with NH Black women (adjusted mean, 198.33 v 213.70 days; P = .009, Fig 1B). However, there were no significant racial differences in TTS among Hispanic patients.
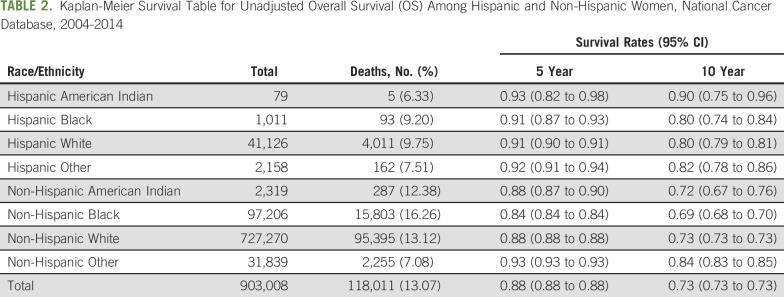
OS
There was no difference in unadjusted OS among Hispanic women of different races (Fig 2A; Table 2), but Hispanic women as a whole had better unadjusted OS than did NH women (Fig 2B; Table 2). Compared with NH White women, Hispanic Black (hazard ratio, 0.75 [95% CI, 0.58 to 0.96]) and Hispanic White women (hazard ratio, 0.77 [95% CI, 0.74 to 0.81]) had improved OS, whereas NH Black women had worse OS after adjusting for covariables (hazard ratio, 1.15 [95% CI, 1.12 to 1.18]; all P < .05; Fig 2C). Although TTS was statistically significantly associated with OS, the association was not clinically meaningful, and adjustment for TTS did not significantly alter the association of race/ethnicity with OS.
TABLE 2.
Kaplan-Meier Survival Table for Unadjusted Overall Survival (OS) Among Hispanic and Non-Hispanic Women, National Cancer Database, 2004-2014
DISCUSSION
In our examination of breast cancer among women of Hispanic and NH ethnicity, there was no difference in OS among Hispanic women of different races, and Hispanic ethnicity was independently associated with improved OS. Our findings are consistent with the findings of some reports showing lower breast cancer mortality among Hispanic women compared with NH White women1 but are contrary to the findings of others that have shown Hispanic women as having comparable4 or worse survival.14,15 However, rates of TNBC differed between women of different races among both Hispanic and NH women. Furthermore, although Hispanic Black women seemed to share some prognostic features with NH Black women, including similar rates of large tumors and node-positive disease, they had markedly better OS.
Our findings support the merit of disaggregating race and Hispanic ethnicity in certain aspects of breast cancer research. However, it is also important to recognize that race is a social construct, despite its often being used as an approximate and imperfect proxy for genetic ancestry.16,17 The incidence of breast cancer varies significantly among countries in Latin America. There is, for example, a much higher incidence of breast cancer in Argentina, where rates are comparable to those among White women in the United States and whose population includes many individuals of predominantly European ancestry, as compared with Ecuador, where a higher proportion of people have a mixture of indigenous, European, and/or African ancestry.18,19 However, it is unclear how individuals from these countries would identify with regard to race in the United States. Thus, when racial trends are observed in epidemiologic studies such as ours, one must consider the extent to which genetic ancestry, versus other cultural and social factors that also contribute to racial self-identification, is driving the findings one observes.
For example, Hispanic Black women in our study had higher proportions of TNBC than did Hispanic White women but lower proportions than did NH Black women, and Hispanic White women had a higher proportion of TNBC than did NH White women. These findings are interesting in light of our increased understanding of the role that African ancestry may play in the development of TNBC.9,20 In one admixture-based genome-wide study of African-American women, ancestry was found to be associated with ER/PR status and disease stage,21 whereas another study identified single-nucleotide polymorphisms as being associated with both African ancestry and risk of TNBC, demonstrating that the risk of TNBC increased with higher proportions of global African ancestry in a given individual.22 Because Hispanic women have varying proportions of African ancestry, the association between African ancestry and risk of TNBC may be relevant not only to Hispanic women who identify as Black but also to those who identify as White.
As with TNBC, some studies have found levels of HER2 expression to be associated with having higher proportions of African and indigenous ancestry.23,24 Our study found more HER2+ disease among Hispanic women of all races compared with NH White women, including among Hispanic women who identified as White, again reflecting the significant racial admixture that exists among Hispanic people. Thus, in aggregate, our findings support our understanding of race as a social construct and our recommendation not only to disaggregate race and Hispanic ethnicity but also to prioritize investigation of genetic ancestry rather than ethnic or racial categorization to further elucidate the pathogenesis and incidence of triple-negative and HER2+ breast cancer.
Our finding of more node-positive disease among Hispanic women of all races versus NH White women is consistent with previous work and suggests a need for improved early detection.6 Diagnosis before nodal involvement would potentially help women avoid extensive axillary treatment and associated lifelong treatment sequelae such as lymphedema. Many studies have looked at mammography among Hispanic women and the factors that affect screening rates in this population.25-29 For the most part, screening rates across ethnic and racial groups in the United States (with the notable exception of Native Americans) are high and largely similar.30-34 Furthermore, mammography does not typically detect pathologic axillary lymphadenopathy. Screening alone may not be sufficient to address this disparity in presentation, and it may be that better connectivity to the health care system overall, including having a primary care provider, is a better strategy for promoting early detection.
We also examined TTS, which is a marker of quality in breast cancer care35 and is associated with overall prognosis.36 There were no significant differences in TTS among Hispanic women of different races, suggesting that race is much less associated with differential access to care and quality of care coordination among Hispanic women than among NH women. Regardless of treatment sequence, however, Hispanic White women had longer TTS than did NH White women, suggesting yet another opportunity for improving breast cancer care for Hispanic women, because delaying care can be associated with worse outcomes.36
Notably, despite presenting at higher stages and experiencing longer TTS, Hispanic women as a whole had improved OS compared with NH White women. The Hispanic Paradox has been described as an epidemiologic phenomenon wherein Hispanic individuals in the United States have lower mortality compared with NH White individuals, despite having factors generally associated with a worse prognosis, including a lower socioeconomic status or a greater burden of comorbidities.37-40 Several factors have been proposed to explain this phenomenon. One is “the healthy migrant effect,” (ie, comparatively better health among newly arrived immigrants to the United States compared with subsequent American-born generations in part because of the adverse effect of cumulative systemic bias as well as American lifestyle characteristics on the latter). Another is the presence of strong social support structures and cultural capital ascribed to close-knit immigrant communities even in the presence of other socioeconomic aggravators.37,41 Our findings suggest evidence of a Hispanic Paradox among women with breast cancer, and notably, this phenomenon was observed among Hispanic women regardless of race.
Banegas and Li14 previously compared Hispanic Black, Hispanic White, NH Black, and NH White women in the SEER database. They found that Hispanic Black, Hispanic White, and NH Black women all had a greater risk of being diagnosed with more advanced-stage breast cancer compared with NH White women, a finding we also observed. However, they also found that Hispanic Black and Hispanic White women, as well as NH Black women, had a greater risk of dying as a result of breast cancer than did NH White women, whereas we found that Hispanic women of all races did better than NH women with regard to OS. The NCDB captures only OS; thus, we were unable to discern from the data available to us whether cause-specific mortality in our cohort might have run counter to OS and been in keeping with the previous SEER analysis. In addition, the study by Banegas and Li14 included only patients diagnosed up to 2008 and did not include information about HER2 status, a critical criterion for contemporary personalized breast cancer treatment.
We used the NCDB because we were interested in TTS, a quality metric for which disparities have been seen. To calculate and interpret adjusted TTS appropriately, it must be stratified by treatment sequence; otherwise, groups with higher rates of NACT receipt (eg, Black women with TNBC) will have skewed results, and SEER does not provide sufficiently granular systemic therapy or surgical treatment data to address this issue.42 Accordingly, the divergence of our findings from the SEER analysis could be explained by a number of factors related to the differential characteristics of the NCDB and SEER and also by the time periods over which our respective analyses were conducted.
Limitations in our study include those inherent to the use of a large retrospective data set, such as selection bias, and those specific to the NCDB, as described earlier in the text. Another issue is the accuracy and completeness of race/ethnicity reporting in the NCDB. Data are supplied to the NCDB by reporting institutions, so although we presume that race and ethnicity were self-reported on intake forms by most individuals, this is not definitively known. The NCDB, for the purposes of coding, requires each patient to have one primary race, despite the fact that a growing number of residents in the United States identify as multiracial.43 Notably, the vast majority of Hispanic patients in our cohort identified as White, despite the fact that most would have had some degree of African and/or indigenous ancestry.44 Multiple social and cultural factors contribute to racial self-identification among Hispanics in the United States (and, indeed, in Latin America), including the extent to which race was associated with class in an individual’s country of origin and the ability (and desirability) of “passing” as White.44
Missing race/ethnicity information was among the criteria that excluded the most patients from our study. As an example, only 79 women in our final cohort identified as Hispanic AI, which limits our ability to draw significant conclusions about this group. NH White patients constituted 6% more of the final analytic cohort than the full initial patient cohort, suggesting that patients of color may be relatively over-represented among those with missing race/ethnicity data. Thus, although proportions were similar between the full initial cohort and the final analytic cohort for all racial and ethnic groups other than NH Whites, it is possible that our exclusion criteria differentially affected Hispanic and non-White patients, thereby contributing to bias in our cohort. People of color and undocumented individuals may also be underrepresented in the NCDB, which is sourced from hospitals accredited by the Commission on Cancer (CoC), and these facilities may be somewhat less diverse than non-CoC–accredited hospitals. Nevertheless, as the largest cancer registry in the United States, the NCDB still offers our best opportunity for examining representative national trends and rates of the kind we report here. However, the completeness and quality of race/ethnicity data in large data sets, especially for people of color, must be improved.
In our review, Hispanic women had better OS after a diagnosis of breast cancer than did both NH Black and NH White women but higher rates of lymph node involvement at presentation and longer TTS compared with NH White women. Among Hispanic women, there were no racial differences in TTS or OS, but proportions of TNBC differed by race among both Hispanic and NH women. To our knowledge, our study of racial and ethnic trends and outcomes in a contemporary cohort of Hispanic and NH women with breast cancer is the largest ever conducted, and it adds significantly to the literature by examining not only racial and ethnic differences in survival but also rates of HER2+ and TNBC, as well as TTS stratified by receipt and timing of chemotherapy. Hispanic women in the United States represent a diverse and heterogeneous group. We hope that our findings will highlight the importance of disaggregating race/ethnicity data in future research on breast cancer in this population.
APPENDIX
FIG A1.
Hispanic and non-Hispanic women with breast cancer, National Cancer Database (NCDB), 2004-2014. AJCC, American Joint Committee on Cancer; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Samantha M. Thomas
Consulting or Advisory Role: AbbVie
Jennifer K. Plichta
Research Funding: Color Genomics
Edgardo Parrilla Castellar
Employment: Q2 Solutions - Genomics Laboratory, Genomic Services
Rachel A. Greenup
Honoraria: Novartis, Genentech
Terry Hyslop
Consulting or Advisory Role: AbbVie
Travel, Accommodations, Expenses: AbbVie
No other potential conflicts of interest were reported.
DISCLAIMER
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC's NCDB and its participating hospitals are the source of the de-identified data used herein; the CoC has not verified these data and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
PRIOR PRESENTATION
Presented in part at the 2019 Duke Cancer Institute Scientific Retreat, October 28, 2019, Durham, NC.
SUPPORT
Supported by Grant No. 1K08CA241390 from the National Institutes of Health (NIH; O.M.F.), by Grant No. K12HD043446 from the NIH Building Interdisciplinary Research Careers in Women’s Health (R.A.G.), and by the Duke Cancer Institute through Grant No. P30CA014236 from the NIH.
Conflicts of Interest Statement: The authors have declared that no competing interests exist.
AUTHOR CONTRIBUTIONS
Conception and design: Cosette D. Champion, Oluwadamilola M. Fayanju
Financial support: Oluwadamilola M. Fayanju, Rachel A. Greenup
Administrative support: Oluwadamilola M. Fayanju
Collection and assembly of data: Samantha M. Thomas
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disparities at the Intersection of Race and Ethnicity: Examining Trends and Outcomes in Hispanic Women With Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Samantha M. Thomas
Consulting or Advisory Role: AbbVie
Jennifer K. Plichta
Research Funding: Color Genomics
Edgardo Parrilla Castellar
Employment: Q2 Solutions - Genomics Laboratory, Genomic Services
Rachel A. Greenup
Honoraria: Novartis, Genentech
Terry Hyslop
Consulting or Advisory Role: AbbVie
Travel, Accommodations, Expenses: AbbVie
No other potential conflicts of interest were reported.
REFERENCES
- 1.Miller KD, Goding Sauer A, Ortiz AP, et al. Cancer statistics for Hispanics/Latinos, 2018 CA Cancer J Clin 68425–4452018 [DOI] [PubMed] [Google Scholar]
- 2.Vespa J, Armstrong DM, Medina L. Demographic Turning Points for the United States: Population Projections for 2020 to 2060. Washington, DC: US Census Bureau; 2018. [Google Scholar]
- 3.Lynce F, Graves KD, Jandorf L, et al. Genomic disparities in breast cancer among Latinas Cancer Contr 23359–3722016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iqbal J, Ginsburg O, Rochon PA, et al. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States JAMA 313165–1732015 [DOI] [PubMed] [Google Scholar]
- 5. doi: 10.1089/jwh.2010.2558. Hines LM, Risendal B, Byers T, et al: Ethnic disparities in breast tumor phenotypic subtypes in Hispanic and non-Hispanic white women. J Women's Health (Larchmt) 20:1543-1550, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nahleh Z, Otoukesh S, Mirshahidi HR, et al. Disparities in breast cancer: A multi-institutional comparative analysis focusing on American Hispanics Cancer Med 72710–27172018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boone SD, Baumgartner KB, Joste NE, et al. The joint contribution of tumor phenotype and education to breast cancer survival disparity between Hispanic and non-Hispanic white women Cancer Causes Control 25273–2822014 [DOI] [PubMed] [Google Scholar]
- 8.González Burchard E, Borrell LN, Choudhry S, et al. Latino populations: A unique opportunity for the study of race, genetics, and social environment in epidemiological research Am J Public Health 952161–21682005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study JAMA 2952492–25022006 [DOI] [PubMed] [Google Scholar]
- 10.Menashe I, Anderson WF, Jatoi I, et al. Underlying causes of the black-white racial disparity in breast cancer mortality: A population-based analysis J Natl Cancer Inst 101993–10002009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warner ET, Tamimi RM, Hughes ME, et al. Racial and ethnic differences in breast cancer survival: Mediating effect of tumor characteristics and sociodemographic and treatment factors J Clin Oncol 332254–22612015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman LA, Kaljee LM.Health disparities and triple-negative breast cancer in African American women: A review JAMA Surg 152485–4932017 [DOI] [PubMed] [Google Scholar]
- 13. Commission on Cancer: FORDS Facility Oncology Registry Data Standards. Revised 2016. Chicago, IL, American College of Surgeons, 2002. [Google Scholar]
- 14.Banegas MP, Li CI.Breast cancer characteristics and outcomes among Hispanic Black and Hispanic White women Breast Cancer Res Treat 1341297–13042012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ooi SL, Martinez ME, Li CI.Disparities in breast cancer characteristics and outcomes by race/ethnicity Breast Cancer Res Treat 127729–7382011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonham VL, Green ED, Pérez-Stable EJ.Examining how race, ethnicity, and ancestry data are used in biomedical research JAMA 3201533–15342018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster MW, Sharp RR.Race, ethnicity, and genomics: Social classifications as proxies of biological heterogeneity Genome Res 12844–8502002 [DOI] [PubMed] [Google Scholar]
- 18. Forman D, Bray F, Brewster DH, et al (eds): Cancer Incidence in Five Continents (vol X). Lyon, France, International Agency for Research on Cancer, 2014. [Google Scholar]
- 19. Homburger JR, Moreno-Estrada A, Gignoux CR, et al. Genomic insights into the ancestry and demographic history of South America. PLoS Genet. 2015;11:e1005602. doi: 10.1371/journal.pgen.1005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman LA.Breast cancer disparities: High-risk breast cancer and African ancestry Surg Oncol Clin N Am 23579–5922014 [DOI] [PubMed] [Google Scholar]
- 21.Fejerman L, Haiman CA, Reich D, et al. An admixture scan in 1,484 African American women with breast cancer Cancer Epidemiol Biomarkers Prev 183110–31172009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer JR, Ruiz-Narvaez EA, Rotimi CN, et al. Genetic susceptibility loci for subtypes of breast cancer in an African American population Cancer Epidemiol Biomarkers Prev 22127–1342013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marker KM, Zavala VA, Vidaurre T, et al. Human epidermal growth factor receptor 2-positive breast cancer is associated with indigenous American ancestry in Latin American women Cancer Res 801893–19012020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serrano-Gomez SJ, Sanabria-Salas MC, Hernández-Suarez G, et al. High prevalence of luminal B breast cancer intrinsic subtype in Colombian women Carcinogenesis 37669–6762016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jerome-D’Emilia B.A systematic review of barriers and facilitators to mammography in Hispanic women J Transcult Nurs 2673–822015 [DOI] [PubMed] [Google Scholar]
- 26. Kadivar H, Kenzik KM, Dewalt DA, et al. The association of English functional health literacy and the receipt of mammography among Hispanic women compared to non-Hispanic U.S.-born White women. PLoS One. 2016;11:e0164307. doi: 10.1371/journal.pone.0164307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelman KK, Cizik AM, Ellerbeck EF, et al. Perceptions of the screening mammography experience by Hispanic and non-Hispanic White women Womens Health Issues 22e395–e4012012 [DOI] [PubMed] [Google Scholar]
- 28. doi: 10.1089/jwh.2008.1009. Borrayo EA, Hines L, Byers T, et al: Characteristics associated with mammography screening among both Hispanic and non-Hispanic white women. J Womens Health (Larchmt) 18:1585-1894, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. doi: 10.1089/jwh.2008.0793. Mack KP, Pavao J, Tabnak F, et al: Adherence to recent screening mammography among Latinas: Findings from the California Women's Health Survey. J Womens Health (Larchmt) 18:347-354, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Peek ME, Han JH.Disparities in screening mammography. Current status, interventions and implications J Gen Intern Med 19184–1942004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roubidoux MA.Breast cancer and screening in American Indian and Alaska Native women J Cancer Educ 27S66–S722012 [DOI] [PubMed] [Google Scholar]
- 32.Tangka FK, Subramanian S, Mobley LR, et al. Racial and ethnic disparities among state Medicaid programs for breast cancer screening Prev Med 10259–642017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013 CA Cancer J Clin 6452–622014 [DOI] [PubMed] [Google Scholar]
- 34.DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019 CA Cancer J Clin 69438–4512019 [DOI] [PubMed] [Google Scholar]
- 35.Polverini AC, Nelson RA, Marcinkowski E, et al. Time to treatment: Measuring quality breast cancer care Ann Surg Oncol 233392–34022016 [DOI] [PubMed] [Google Scholar]
- 36.Eriksson L, Bergh J, Humphreys K, et al. Time from breast cancer diagnosis to therapeutic surgery and breast cancer prognosis: A population-based cohort study Int J Cancer 1431093–11042018 [DOI] [PubMed] [Google Scholar]
- 37.Franzini L, Ribble JC, Keddie AM.Understanding the Hispanic paradox Ethn Dis 11496–5182001 [PubMed] [Google Scholar]
- 38.Ruiz JM, Steffen P, Smith TB.Hispanic mortality paradox: A systematic review and meta-analysis of the longitudinal literature Am J Public Health 103e52–e602013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vega WA, Rodriguez MA, Gruskin E.Health disparities in the Latino population Epidemiol Rev 3199–1122009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markides KS, Coreil J.The health of Hispanics in the southwestern United States: An epidemiologic paradox Public Health Rep 101253–2651986 [PMC free article] [PubMed] [Google Scholar]
- 41.Thomson EF, Nuru-Jeter A, Richardson D, et al. The Hispanic paradox and older adults’ disabilities: Is there a healthy migrant effect? Int J Environ Res Public Health 101786–18142013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohanty S, Bilimoria KY.Comparing national cancer registries: The National Cancer Data Base (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) program J Surg Oncol 109629–6302014 [DOI] [PubMed] [Google Scholar]
- 43.Jones NA, Bullock J. The Two or More Races Population: 2010. Washington, DC: US Census Bureau, US Department of Commerce; 2012. [Google Scholar]
- 44.Amaro H, Zambrana RE.Criollo, mestizo, mulato, LatiNegro, indígena, white, or black? The US Hispanic/Latino population and multiple responses in the 2000 census Am J Public Health 901724–17272000 [DOI] [PMC free article] [PubMed] [Google Scholar]