Abstract
Purpose
Anaplastic thyroid cancer (ATC) is a deadly form of thyroid cancer. BRAF V600E is the only actionable mutation for which there is a Food and Drug Administration–approved drug combination. Rapid detection of BRAF V600E and initiation of therapy is critical. We explored the ability of droplet digital polymerase chain reaction (ddPCR) to identify this mutation in circulating cell-free DNA (cfDNA) in plasma.
Materials and Methods
The ddPCR assay was evaluated for its sensitivity, specificity for detection of BRAF V600E cfDNA, and concordance with tumor tissue. The assay also was used to evaluate its potential role as a biomarker of response.
Results
Forty-four patients with ATC who were tested for the BRAF mutation by tumor tissue DNA sequencing or immunohistochemistry were included. Sixteen BRAF V600E–positive patients had treatment samples to evaluate cfDNA levels as a biomarker of response in correlation with restaging scans. Concordance of ddPCR with tumor tissue was 93%, with a sensitivity of 85% and specificity of 100%. Area under the curve by Wilcoxon rank sum test was 0.9 (95% CI, 0.80 to 0.99; P < .001). As a biomarker of response to treatment, 94% of ddPCR samples were concordant with tumor shrinkage in restaging scans, and 47% were concordant with tumor growth (Fisher’s exact test P = .0061). In addition, cfDNA levels by ddPCR were predictive of treatment response in 71% of samples.
Conclusion
cfDNA detection by ddPCR is highly sensitive, specific, and concordant with mutation status on ATC tumors. ddPCR also can be used for monitoring cfDNA levels in conjunction with imaging scans in patients with ATC.
INTRODUCTION
Anaplastic thyroid carcinoma (ATC) is responsible for one third of thyroid cancer deaths and has a high mortality rate.1,2 BRAF V600E is the most common actionable driver mutation in ATC, which is seen in approximately 45% of these cancers.3,4 Indeed, targeted treatment strategies are extending patient survival,5-8 and the BRAF plus MEK inhibitor combination dabrafenib plus trametinib was recently approved by the Food and Drug Administration for BRAF V600E–mutated ATC.9,10
Diagnosis of ATC usually is made by biopsy. Unlike differentiated tumors, the lack of sufficient viable tissue hampers molecular diagnostic testing.2,11,12 Given the rapid progression of ATC, rapid testing is needed for timely therapeutic intervention. Liquid biopsy (mutation testing performed on blood samples) provides a potential alternative to tissue biopsy in the management of certain malignancies.13-15 The detection of mutations in circulating cell-free DNA (cfDNA), such as BRAF V600E, can serve as a surrogate for mutation testing on tissue biopsy, and its overall levels provide a novel marker in predicting thyroid cancer burden.16,17
The need for molecular testing on tissue before treatment with selective BRAF inhibitors may substantially delay the time to initiation of a beneficial treatment in patients with BRAF-mutated ATC.8,18-22 We have demonstrated previously that BRAF V600E cfDNA can be detected in the blood of patients with ATC by a next-generation sequencing (NGS) platform with a faster turnaround time, which therefore serves as a surrogate for tissue biopsy.23
A study at our center has demonstrated that droplet digital polymerase chain reaction (ddPCR) can be used as a targeted analysis of cfDNA in the circulation of patients with medullary thyroid cancer and that changes in circulating tumor DNA levels correspond to treatment responses.24 Therefore, we hypothesized that the detection of BRAF-mutated cfDNA by ddPCR will correlate with tissue-based molecular testing results and that changes in the level of the cfDNA will correlate with response to treatment in patients with ATC.
MATERIALS AND METHODS
Study Population for Testing for Concordance Between Circulating BRAF V600E cfDNA and Tumor Tissue and cfDNA by NGS
Patients with suspected ATC were prospectively enrolled between March 2016 and July 2017. Informed consent was obtained from the patients before collecting blood under an institutional review board protocol. All patients’ tumor tissues were read by an independent head and neck pathologist at our center to confirm their diagnosis. Individuals who had unconfirmed ATC or no evidence of disease or who were being managed by observation for low disease burden were excluded. Our standard of care is to obtain mutation tests on tumor tissue by NGS or immunohistochemistry (IHC) or cfDNA by NGS before initiation of treatment. In the absence of readily available tumor mutation profiling, we obtain both BRAF V600E by IHC on tissue biopsy and cfDNA on plasma by NGS simultaneously before initiation of treatment. Whenever feasible, these results are confirmed through testing on tumor tissue by NGS to validate treatment choice. The IHC analysis antibody is anti-BRAF p.V600E (clone VE1, dilution 1:50; Spring Bioscience, Pleasanton, CA) and is performed on a Ventana BenchMark ULTRA autostainer (Ventana Medical Systems, Tucson, AZ).
Patients who had a cfDNA analysis by NGS more than 2 weeks before the research ddPCR sample draw were excluded from concordance testing. However, if patients tested positive for the BRAF V600E mutation on their tumor tissue and were receiving treatment, serial plasma samples were collected for ddPCR assay during their treatment to examine the correlation of cfDNA levels with response to treatment.
Study Population for Correlation of Changes in cfDNA Levels With Response to Treatment
Patients with tissue-positive BRAF V600E mutation were followed prospectively during their respective treatments to evaluate whether levels of circulating BRAF V600E cfDNA by ddPCR could serve as a biomarker for monitoring response to therapy. Blood samples for cfDNA analysis were drawn at routinely scheduled laboratory appointments.
BRAF V600E Mutation Analysis
Tumor tissue was tested for BRAF mutation status by either IHC or NGS at our center (50-gene somatic mutation analysis panel,25 Solid Tumor Genomics Assay version 1 that examines 134 genes,26 or FoundationOne genomic test27), depending on the quantity of viable tumor tissue available for testing. Some patients had mutation testing by PCR before referral to our center. Blood samples were drawn for ddPCR testing as described in the Appendix and sent for cfDNA analysis by NGS using the Guardant360 sequencing test28 (Guardant Health, Redwood City, CA).
Evaluation of Response to Treatment
A single independent radiologist reviewed the images at baseline and at the time of restaging while patients were receiving treatment. We determined clinical responses by calculating the percent change (from baseline and after/during treatment) in the size of target lesions identified using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1,29 but we modified slightly the definitions of response. We calculated the change in BRAF-mutated cfDNA level and correlated that with the change in the sum of the size of target lesions. For the purpose of our study, an increase in tumor size was defined as an increase in the sum of target lesions greater than or equal to 10 mm. RECIST progression in nontarget lesions was assigned an additional 20 mm to the sum of target lesions. A decrease in tumor size was defined as a decrease in sum of target lesions greater than or equal to 10 mm. Stable tumor size was defined as a change in tumor burden between 9 and −9 mm.
Data Analysis
Testing for concordance between circulating BRAF V600E cfDNA by ddPCR and tumor tissue and cfDNA by NGS and tumor tissue.
We defined a true-positive sample by the presence of BRAF V600E mutation in cfDNA (by either ddPCR or NGS) and on tissue testing. Similarly, true-negative samples had the absence of BRAF V600E mutation detection in both cfDNA and tissue testing. A false positive was defined by the presence of a mutation in cfDNA when tumor tissue tested negative, and a false negative was indicated when the mutation was not detected in cfDNA but tumor tissue tested positive for BRAF V600E mutation.
Concordance between BRAF V600E mutation in cfDNA by ddPCR with BRAF mutation status on tumor tissue by NGS, IHC, and PCR was calculated as number of concordant samples / (number of concordant samples + number of discordant samples). Concordance also was calculated for the detection of BRAF mutation in cfDNA samples by NGS and tumor tissue.
Testing for correlation of change in circulating BRAF V600E ddPCR levels with changes in tumor size.
In patients who were followed prospectively, concordance was calculated between the change in the level of BRAF-mutated cfDNA by ddPCR and change in tumor size on restaging scans during treatment. Any increase in the level of cfDNA was called concordant if that correlated with an increase in tumor size of greater than or equal to 10 mm. Similarly, any decrease in the level of cfDNA was called concordant if it correlated with a decrease in tumor size of greater than or equal to 10 mm. If there was no change in the level of cfDNA correlating with 9 to −9 mm, the sample was called concordant. If the changes in the levels of cfDNA did not meet these three criteria, they were called discordant. Samples drawn in between restaging scans were studied in correlation with the change in tumor size on follow-up imaging to evaluate whether the test was predictive of response to therapy.
Statistical Tests
Sensitivity and specificity of detection of BRAF-mutated cfDNA by ddPCR and by NGS were calculated and reported with exact 95% CIs. Changes between levels of circulating cfDNA and change in the sum of target lesions at the time of each restaging were studied using Spearman rank correlation coefficient. Fisher's exact test was conducted to calculate concordance between cfDNA levels and the corresponding radiologic events, specifically, increase in tumor size, tumor shrinkage, and stable tumor size.
RESULTS
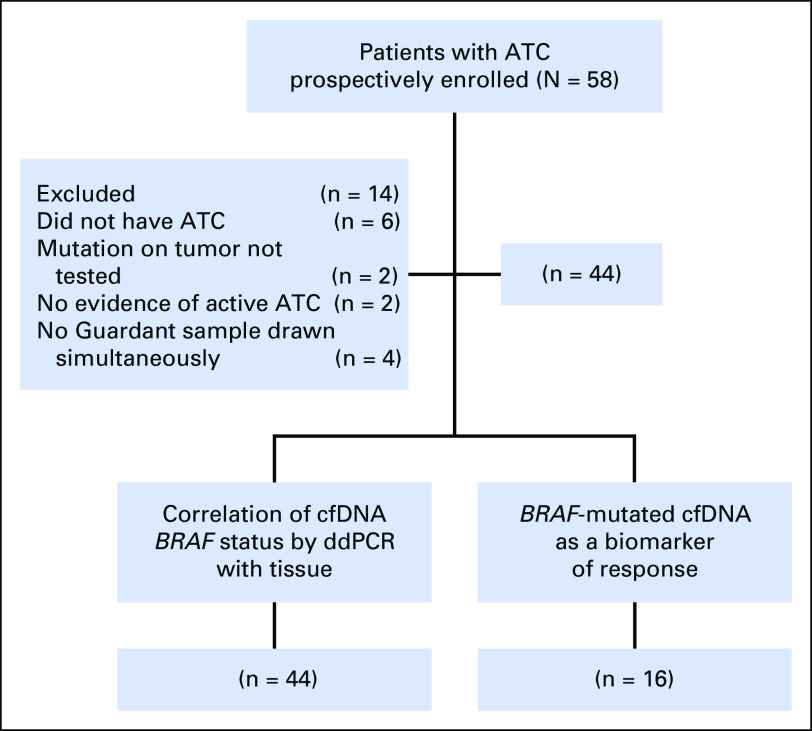
Fifty-eight patients with a suspected ATC diagnosis were prospectively enrolled in the study. Fourteen were excluded (Fig 1). Of the remaining 44 patients, 16 who had a BRAF V600E–positive tumor were followed for correlation changes in cfDNA with response to treatment.
Fig 1.
Patient selection and distribution. The study population was selected between March 2016 and July 2017; informed consent was obtained at the time of enrollment. ATC, anaplastic thyroid carcinoma; cfDNA, cell-free DNA, ddPCR, droplet digital polymerase chain reaction.
Patient Characteristics
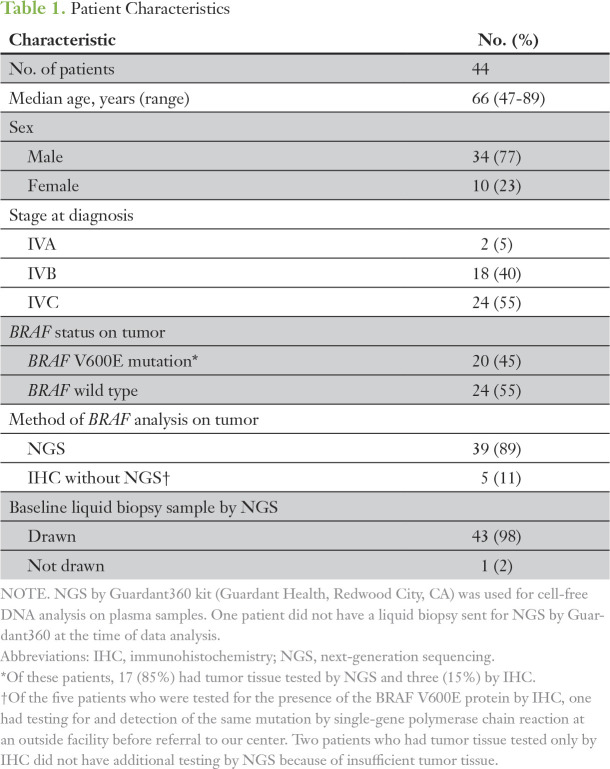
Patient characteristics are listed in Table 1. Median age was 66 years (range, 47 to 89 years). The majority were men (77%). At the time of diagnosis, 24 patients (55%) had stage IVC disease. Tumor somatic mutation testing was performed by NGS in 39 patients (89%), with the remaining patients evaluated by BRAF mutation–specific IHC. Twenty patients (45%) were positive for BRAF V600E mutation. Forty-three (98%) also had blood samples sent for cfDNA by NGS.
Table 1.
Patient Characteristics
Detection of BRAF V600E cfDNA on Liquid Biopsy
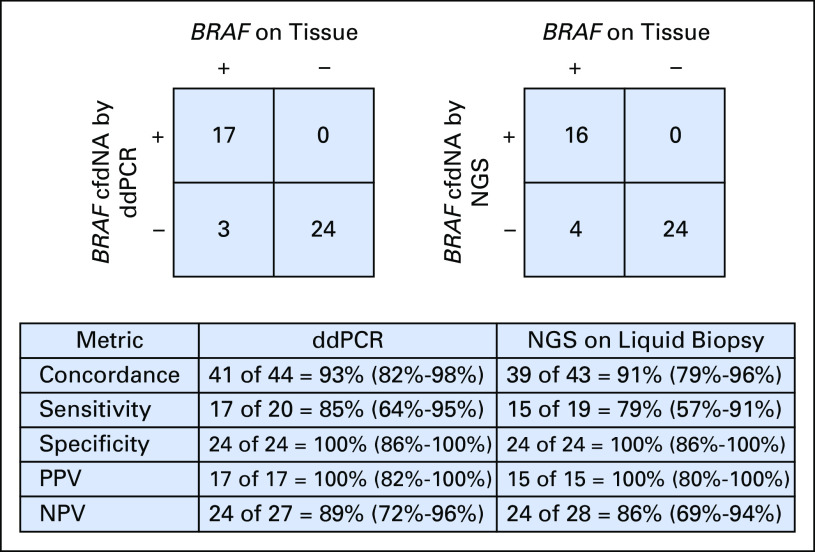
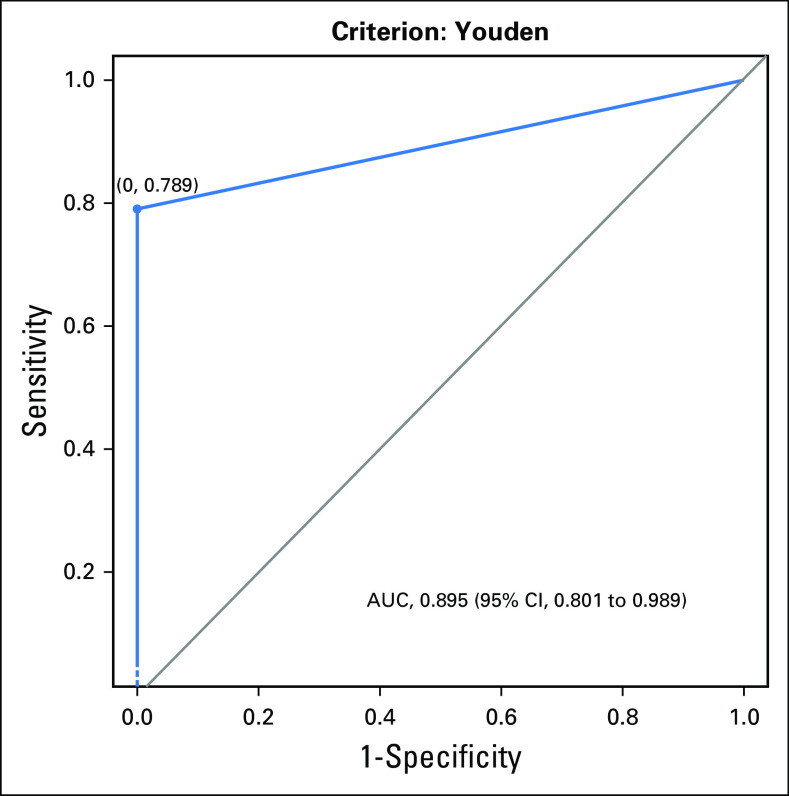
The lowest allelic fraction (AF) for BRAF V600E detected by ddPCR was 0.07% and for cfDNA by NGS, 0.1%. The median AF was 0.42% (range, 0.07% to 20.9%) and 1.1% (range, 0.1% to 26.4%) by ddPCR and NGS assays, respectively. Of the 20 patients who tested positive for BRAF mutation on tissue, 17 (85%) were positive for BRAF V600E cfDNA by ddPCR and 16 (80%) were positive for cfDNA by NGS (Fig 2). One patient was true positive for cfDNA by NGS but false negative by ddPCR. Two patients with BRAF-mutated tumor tissue tested negative for cfDNA by NGS but tested positive by ddPCR. An additional two patients with BRAF V600E–positive tumor tissue were negative by both liquid biopsy approaches. Overall, three patients tested as false negative by ddPCR cfDNA analysis. All 24 patients whose tumor tissue tested negative for BRAF V600E were also negative for cfDNA by NGS and ddPCR liquid biopsy. The sensitivity of BRAF V600E cfDNA detection was 85% for ddPCR and 79% for NGS. Thus, a 6% difference was found in the sensitivity of the two tests (95% CI, −18% to 29%). The specificity of both liquid biopsy tests for the detection of BRAF V600E cfDNA was 100%. Figure 3 shows the receiver operating characteristic curve for this data set. The two data sets produced identical results, so only a single curve is shown (area under the curve, 0.90; 95% CI, 0.80 to 0.99; Wilcoxon rank sum test P < .001). Detection of BRAF-mutated cfDNA by ddPCR was 93% concordant with tumor testing, whereas detection of cfDNA by NGS was 91% (95% CI, −8% to 14%). Both liquid biopsy assays were found to be similar in accuracy and concordance.
Fig 2.
Concordance between detection of circulating BRAF V600E cell-free DNA (cfDNA) by droplet digital polymerase chain reaction (ddPCR) and next-generation sequencing (NGS) on liquid biopsy with mutation status on tumor. Differences in the ability to detect and concordance with BRAF positivity on tumor tissue are shown. One patient had cfDNA tested by ddPCR, but no sample was drawn simultaneously for NGS on liquid biopsy. Confidence intervals are shown in parentheses. NPV, negative predictive value; PPV, positive predictive value.
Fig 3.
Receiver operator characteristic curve. One patient with BRAF positivity on tumor did not get testing done by next-generation sequencing (NGS) on liquid biopsy; therefore, the analysis was done on 43 patient samples. Because all 24 of the patients without BRAF mutation tested negative for cell-free DNA by droplet digital polymerase chain reaction (ddPCR) and NGS, the receiver operating characteristic curve is degenerate. On cell-free DNA testing by NGS, three of 20 patients with BRAF positivity on tumor tissue tested negative. On ddPCR, four of 19 patients with BRAF positivity on tumor tissue tested negative. The plots and areas under the curve (AUCs) are identical for ddPCR and NGS on liquid biopsy. Wilcoxon rank sum test P < .001.
Correlation Between Changes in BRAF V600E cfDNA by ddPCR and Changes in Tumor Size
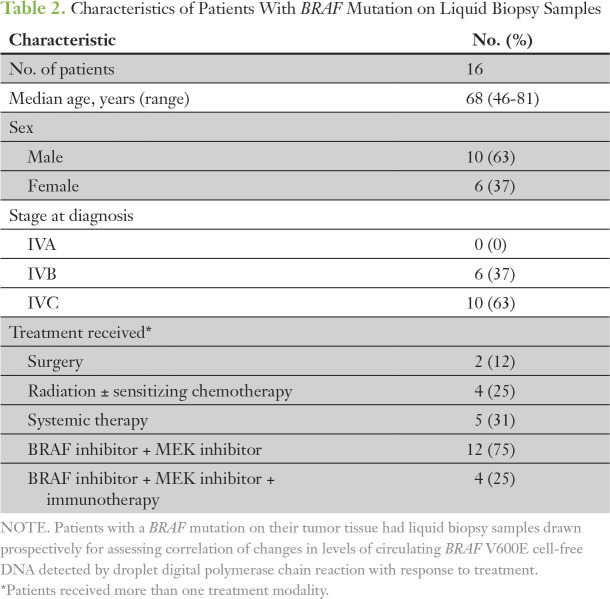
Sixteen patients whose tumors tested positive for BRAF mutation were prospectively followed and had plasma samples drawn during scheduled laboratory appointments before the clinic visit. The details of these patients are listed in Table 2. Median time between the samples was 3.83 weeks (range, 1.3 to 18 weeks). Median staging interval for scans was 6 weeks (range, 1.6 to 20.4 weeks). Study patients underwent one to eight post-treatment scans (median, 3.5 scans) and were exposed to one to three treatments. Thirty-six radiologic assessments were available for direct comparison with plasma samples. At the time of baseline cfDNA sampling, the median tumor burden by imaging was 76.8 mm (range, 8 to 146.9 mm). BRAF V600E cfDNA was detected in 13 of 16 patients. In the three patients who tested false negative by ddPCR, tumor burden at baseline ranged from 31 to 33.3 mm. These patients had had their primary tumor treated with surgery (thyroidectomy in two patients and hemithyroidectomy in one patient). The BRAF V600E cfDNA was detectable in tumors larger than 52.5 mm, with the exception of a single patient who had an 8-mm lung nodule that presented while he was receiving adjuvant radiation to the thyroid bed with chemotherapy.
Table 2.
Characteristics of Patients With BRAF Mutation on Liquid Biopsy Samples
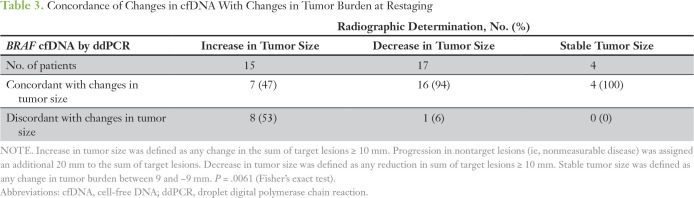
Of 36 time points, 27 (75%) provided concordant findings between changes in cfDNA levels and response to therapy (Table 3). Seventeen scans had findings consistent with decreases in tumor size, of which 16 (94%) were concordant with reductions in BRAF V600E cfDNA. We included the three patients who tested false negative by ddPCR in this group because the BRAF V600E cfDNA levels continued to remain undetectable, which correlated with these patients’ continued response to treatment with BRAF inhibitor. Increased tumor size was observed in 15 scans, of which seven (47%) were concordant with increased cfDNA levels. Stable tumor size was observed for four restaging scans, which correlated with no change in cfDNA levels. Overall comparison of response determined by imaging versus levels of cfDNA by ddPCR was statistically significant when analyzed using Fisher's exact test (P = .0061). On analyzing the change in levels of cfDNA with changes in tumor size, the Spearman rank correlation coefficient was 0.37 (95% CI, 0.05 to 0.62; P = .029).
Table 3.
Concordance of Changes in cfDNA With Changes in Tumor Burden at Restaging
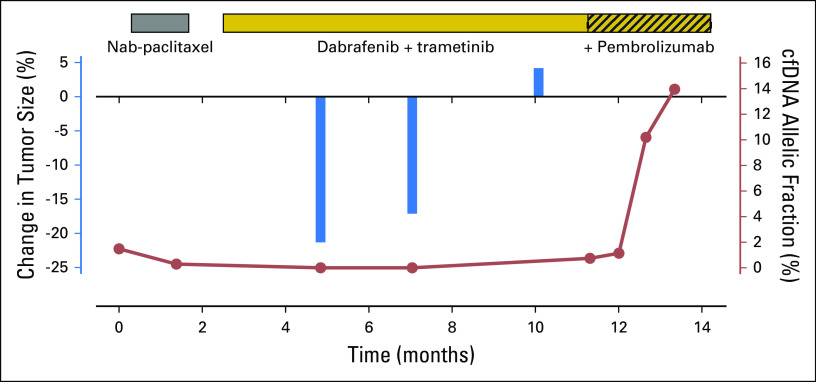
Eleven patients (65%) had a total of 17 samples drawn between restaging scans. Of these, 12 (71%) revealed a change in cfDNA level predictive of the image-based change in tumor size. The median time between treatment start and the next sample draw was 2.9 weeks (range, 0.14 to 5.9 weeks). The earliest predictor of response was seen at 1 day after surgery and 2 weeks after starting BRAF inhibitor therapy. One patient showed an increase in cfDNA levels 2 weeks after starting BRAF inhibitor therapy, which was consistent with the increased tumor size observed on his first restaging scans 2 months after starting this treatment. The median time between a sample and follow-up imaging was 3.7 weeks (range, 1.14 to 3.9 weeks). Figure A1 shows an example of the trends in cfDNA levels that correlated with changes in tumor size on imaging in a patient who was initially treated with nab-paclitaxel followed by BRAF and MEK inhibitor therapy and then with the addition of immunotherapy (pembrolizumab). The levels of BRAF cfDNA trended upward despite the addition of pembrolizumab because the patient was not able to swallow the BRAF inhibitor, which led to nonadherence and eventual progression.
DISCUSSION
The BRAF V600E mutation is the most common actionable mutation in ATC tumors. Recent studies have shown promising results with dabrafenib plus trametinib (BRAF and MEK inhibitors) in terms of clinical response and survival,9,18,30 and this combination is now Food and Drug Administration approved for BRAF-mutated ATC. Hence, identification of this mutation for timely initiation of treatment is important.
In addition to the ability to identify an actionable mutation for initiation of therapy, development of biomarkers is needed to predict and assess treatment response in ATC. In differentiated thyroid cancer (DTC), serum thyroglobulin (Tg) levels are a biomarker of response to therapy. In ATC, Tg measurements play a small role because the dedifferentiation associated with these tumors generally results in loss of Tg expression. Given the successes observed for other cancers where tumor-specific biomarkers do not exist, we believe that changes in BRAF V600E cfDNA could provide a specific biomarker for monitoring response to treatment in patients with ATC.19 Although response can be assessed by imaging, a marker to predict response allows sufficient time to change the treatment plan before the patient becomes too ill.
In this study, we show that cfDNA by ddPCR is a sensitive and highly specific technique for determining BRAF status in patients with ATC. This test was found to have a concordance similar to cfDNA by NGS in relation to the BRAF status on the tumor. BRAF cfDNA and RNA in liquid biopsy have been shown to be useful biomarkers in patients with DTC as well as in a murine model of ATC.31,32 The current study is the first in our knowledge to evaluate ddPCR to detect BRAF-mutated cfDNA in the circulation of patients with ATC. In a phase II clinical trial in radioiodine-refractory DTC, BRAF V600E cfDNA was detectable by ddPCR in only 35% of patients,33 whereas in our study, it was detected in 85% of patients with ATC. The release of tumor DNA into the circulation is believed to be a direct measure of tumor cell death. Unlike DTC tumors, greater cellular turnover occurs in ATC tumors, which leads to greater shedding of genetic material into the circulation. However, even with high tumor cell turnover, tumor burden may not be sufficient to permit the detection of circulating tumor DNA. In the current study cohort, ddPCR failed to detect mutation in three patients with BRAF V600E–mutated tumors. The inability to detect mutation could be explained by the low overall disease burden on imaging compared with positive patients. Therefore, we suggest that in patients with low measurable disease, reliance solely on cfDNA for identifying the BRAF mutation may not be advisable, and molecular testing on tumor tissue should be awaited.
A murine model of BRAF-mutated ATC circulating RNA levels has been shown to be a useful biomarker of response to therapy.32 In the current study, we show that ddPCR is a useful technique for serial monitoring of cfDNA levels as a predictive biomarker in patients with ATC on treatment. Changes in levels of cfDNA were seen as early as 1 day after surgery and 2 weeks after starting BRAF inhibitor therapy, consistent with tumor shrinkage. The changes in cfDNA levels were concordant with changes in tumor size on imaging. These findings were similar to that observed in patients with melanoma.34,35 In patients whose BRAF V600E cfDNA levels rise after initiating BRAF and MEK inhibitor therapy, resistance to treatment should be considered, prompting early restaging scans and consideration of alternative treatment options upon confirmation. Our findings also highlight the limitation of using a single-gene cfDNA as a biomarker in a heterogeneous tumor such as ATC. In patients who experience an increase in tumor size, fewer than one half showed the expected concomitant increases in BRAF V600E cfDNA. One possible explanation is that targeted treatment indeed led to a reduction in the BRAF V600E–harboring tumor cells but led to clonal expansion of tumor cells that harbored other driver mutations as a mechanism of resistance. Such a possibility could be addressed through tumor biopsy or cfDNA by NGS to look for the emergence of new mutations.
Our results strongly support the use of ddPCR for identifying the BRAF V600E mutation in patients with ATC. However, the small sample size limited us from evaluating the association of the AF of the BRAF V600E cfDNA with prognosis and overall survival.
In conclusion, ddPCR is a novel, noninvasive technique for detecting BRAF V600E cfDNA in patients with ATC and should be considered in routine standard-of-care testing. The results show that cfDNA potentially could serve as a biomarker to predict response in patients with ATC before restaging scans, which is important in the management of an aggressive cancer like ATC where timely treatment is crucial for prolonging survival.
Supplementary Material
ACKNOWLEDGMENT
We thank the patients with ATC who agreed to provide samples, Karen Bohannon-Johnson for phlebotomy support, and clinical colleagues who provided access to their patients for this study.
Appendix
Isolation of Cell-Free DNA for Droplet Digital Polymerase Chain Reaction
After obtaining patient consent, approximately 8 to 10 mL of blood were drawn into each of two EDTA tubes. Plasma was separated from the cell components after centrifuging twice at 2,000 rpm for 10 minutes at 4°C and then stored at −80°C to allow for batch isolation of cell-free DNA (cfDNA). Before cfDNA isolation, plasma was thawed on ice and then centrifuged again to remove particulate. QIAmp Circulating Nucleic Acid Kit (QIAGEN, Venlo, the Netherlands) was used to isolate cfDNA from plasma. A standardized plasma volume of 3 mL was adapted for each sample, with a 50 μL elution volume. The amount of DNA was quantified using a Qubit fluorometer (Thermo Fisher Scientific, Waltham, MA).
Droplet Digital Polymerase Chain Reaction
The detection of the BRAF V600E mutation was performed in a research laboratory setting using the QX200 Droplet Digital PCR system (Bio-Rad, Hercules, CA) and the validated PrimePCR ddPCR Mutation Assay (Bio-Rad), which was multiplexed with an assay for detection of BRAF unmutated codon (wild type). Each reaction contained 11 μL of 2× Supermix, 1.1 μL of 20× Assay 1 HEX (wild-type allele), and 1.1 μL of 20× Assay 2 FAM (mutant allele) per reaction for a total of 13.2 μL per reaction. The remaining 8.8 μL contained cfDNA sample. The cfDNA yields using this protocol ranged from 2 to 132 ng/mL when measured by Qubit fluorometer, which allowed a median estimated input of 5 ng per assay. A nontemplate control of water was run for each assay as well as a BRAF V600E positive control (KTC1 cell line DNA). Droplets were counted with the QX200 Droplet Reader, and data were analyzed using QuantaSoft software (Bio-Rad), with thresholds for FAM and HEX detection manually set on the basis of results from the no-template control. We required a minimum of 8,000 counted droplets. The detection of BRAF V600E was considered positive if two or more positive droplets were detected in a single assay. The allelic fraction was calculated, with the ratio of channel 1 counts (BRAF V600E cfDNA) over channels 1 and 2 (BRAF V600E and BRAF WT) and the value standardized as a percentage.
Fig A1.
Trends in cell-free DNA (cfDNA) levels that correlate with changes in tumor size on imaging. cfDNA declined at the time of response to therapy correlated with tumor shrinkage from baseline, and an increase in cfDNA levels at the time of progression correlated with a radiologic increase in tumor burden.
Supported by an Endocrine Fellows Foundation grant (P.C.I.) and National Cancer Institute Grant No. P50 CA168505 (G.J.C.). The Tissue Biospecimen and Pathology Resource and Sequencing and Microarray Facility are institutional resources funded by National Cancer Institute Grant No. P30 CA016672.
Presented at the 87th Annual Meeting of the American Thyroid Association, Victoria, British Columbia, Canada, October 18-22, 2017.
P.C.I and G.J.C. share first authorship.
AUTHOR CONTRIBUTIONS
Conception and design: Priyanka C. Iyer, Gilbert J. Cote, Ramona Dadu, Mark Zafereo, Naifa L. Busaidy, Brandon G. Gunn, Maria E. Cabanillas
Financial support: Gilbert J. Cote
Provision of study material or patients: Tao Hai, Naifa L. Busaidy, Vivek Subbiah, Neil Gross, Maria E. Cabanillas
Collection and assembly of data: Priyanka C. Iyer, Gilbert J. Cote, Tao Hai, Maria Gule-Monroe, Jacquelin Bui-Griffith, Renata Ferrarotto, Vivek Subbiah, Maria E. Cabanillas
Data analysis and interpretation: Priyanka C. Iyer, Gilbert J. Cote, Maria Gule-Monroe, Michelle D. Williams, Kenneth Hess, Marie-Claude Hofmann, Ramona Dadu, Mark Zafereo, Naifa L. Busaidy, Renata Ferrarotto, Vivek Subbiah, Neil Gross, Heath D. Skinner, Adam S. Garden, Maria E. Cabanillas
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Priyanka C. Iyer
No relationship to disclose
Gilbert J. Cote
No relationship to disclose
Tao Hai
No relationship to disclose
Maria Gule-Monroe
No relationship to disclose
Jacquelin Bui-Griffith
No relationship to disclose
Michelle D. Williams
No relationship to disclose
Kenneth Hess
No relationship to disclose
Marie-Claude Hofmann
No relationship to disclose
Ramona Dadu
Research Funding: AstraZeneca, Merck, Eisai, Genentech, Bristol-Myers Squibb
Mark Zafereo
No relationship to disclose
Naifa L. Busaidy
Consulting or Advisory Role: Loxo Oncology, Eisai
Research Funding: GlaxoSmithKline, Novartis
Renata Ferrarotto
Consulting or Advisory Role: AYALA
Research Funding: OncoMed Pharmaceuticals (Inst), G1 Therapeutics (Inst), AstraZeneca (Inst), MedImmune (Inst), EMD Serono (Inst), Genentech (Inst), Roche (Inst), Merck Serono (Inst)
Vivek Subbiah
Consulting or Advisory Role: MedImmune
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), NanoCarrier (Inst), Northwest Biotherapeutics (Inst), Genentech (Inst), Roche (Inst), BERG (Inst), Bayer AG (Inst), Incyte (Inst), Fujifilm (Inst), PharmaMar (Inst), D3 Oncology Solutions (Inst), Pfizer (Inst), Amgen (Inst), AbbVie (Inst), MultiVir (Inst), Blueprint Medicines (Inst), Loxo Oncology (Inst), Vegenics (Inst), Takeda Pharmaceuticals (Inst), Alfasigma (Inst), Agensys (Inst), Idera (Inst), Boston Biomedical (Inst), Inhibrx (Inst), Exelixis (Inst)
Travel, Accommodations, Expenses: PharmaMar, Bayer AG
Neil Gross
Honoraria: Intuitive Surgical
Consulting or Advisory Role: PDS Biotechnology, Verb Surgical (Inst)
Research Funding: Regeneron Pharmaceuticals, MedImmune (Inst), Genentech (Inst)
Brandon G. Gunn
No relationship to disclose
Heath D. Skinner
No relationship to disclose
Adam S. Garden
Consulting or Advisory Role: Galera Therapeutics
Maria E. Cabanillas
Consulting or Advisory Role: Loxo Oncology, Blueprint Medicines
Research Funding: Kura Oncology, Eisai, Roche, Genentech, Exelixis
REFERENCES
- 1.Landa I, Ibrahimpasic T, Boucai L, et al. : Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 126:1052-1066, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smallridge RC, Copland JA: Anaplastic thyroid carcinoma: Pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol) 22:486-497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao SN, Busaidy NL, Dadu R, et al. : Patterns of failure in anaplastic thyroid cancer. 98th Annual Meeting of the Endocrine Society, Boston, MA, April 1-4, 2016 [Google Scholar]
- 4.Pozdeyev N, Gay LM, Sokol ES, et al. : Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res 24:3059-3068, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi S, Kiyota N, Yamazaki T, et al. : Phase II study of lenvatinib in patients with differentiated, medullary, and anaplastic thyroid cancer: Final analysis results. J Clin Oncol 34, 2016. (suppl; abstr 6088) [Google Scholar]
- 6.Brose MS, Cabanillas ME, Cohen EEW, et al. : Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: A non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol 17:1272-1282, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dadu R, Shah K, Busaidy NL, et al. : Efficacy and tolerability of vemurafenib in patients with BRAF(V600E) -positive papillary thyroid cancer: MD Anderson Cancer Center off label experience. J Clin Endocrinol Metab 100:E77-E81, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyer P, Dadu R, Busaidy NL, et al. : Harvesting high-hanging Fruit: Targeted therapy for BRAF mutant (BRAFm) and BRAF wild-type (BRAFwt) anaplastic thyroid cancer (ATC). 86th Annual Meeting of the American Thyroid Association, Denver, CO, September 21-25, 2016 [Google Scholar]
- 9.Subbiah V, Kreitman RJ, Wainberg ZA, et al. : Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol 36:7-13, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration : FDA approves dabrafenib plus trametinib for anaplastic thyroid cancer with BRAF V600E mutation. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm606708.htm
- 11.Cabanillas ME, Zafereo M, Gunn GB, et al. : Anaplastic thyroid carcinoma: Treatment in the age of molecular targeted therapy. J Oncol Pract 12:511-518, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Cabanillas ME, Dadu R, Hu MI, et al. : Thyroid gland malignancies. Hematol Oncol Clin North Am 29:1123-1143, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Stroun M, Anker P, Lyautey J, et al. : Isolation and characterization of DNA from the plasma of cancer patients. Eur J Cancer Clin Oncol 23:707-712, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Schwarzenbach H, Hoon DSB, Pantel K: Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 11:426-437, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Scherer F, Kurtz DM, Newman AM, et al. : Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA. Sci Transl Med 8:364ra155, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cradic KW, Milosevic D, Rosenberg AM, et al. : Mutant BRAF(T1799A) can be detected in the blood of papillary thyroid carcinoma patients and correlates with disease status. J Clin Endocrinol Metab 94:5001-5009, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Chuang TCY, Chuang AYC, Poeta L, et al. : Detectable BRAF mutation in serum DNA samples from patients with papillary thyroid carcinomas. Head Neck 32:229-234, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Hyman DM, Puzanov I, Subbiah V, et al. : Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 373:726-736, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabanillas ME, Busaidy NL, Khan SA, et al. : Molecular diagnostics and anaplastic thyroid carcinoma: The time has come to harvest the high hanging fruit. Int J Endocr Oncol 3:221-233, 2016 [Google Scholar]
- 20.Agarwal R, Wang J, Wilson K, et al. : Response to targeted therapy in BRAF mutant anaplastic thyroid cancer. J Natl Compr Canc Netw 14:1203-1207, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Prager GW, Koperek O, Mayerhoefer ME, et al. : Sustained response to vemurafenib in a BRAFV600E-mutated anaplastic thyroid carcinoma patient. Thyroid 26:1515-1516, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Rosove MH, Peddi PF, Glaspy JA: BRAF V600E inhibition in anaplastic thyroid cancer. N Engl J Med 368:684-685, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Sandulache VC, Williams MD, Lai SY, et al. : Real-time genomic characterization utilizing circulating cell-free DNA in patients with anaplastic thyroid carcinoma. Thyroid 27:81-87, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cote GJ, Evers C, Hu MI, et al. : Prognostic significance of circulating RET M918T mutated tumor DNA in patients with advanced medullary thyroid carcinoma. J Clin Endocrinol Metab 102:3591-3599, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luthra R, Patel KP, Routbort MJ, et al. : A targeted high-throughput next-generation sequencing panel for clinical screening of mutations, gene amplifications, and fusions in solid tumors. J Mol Diagn 19:255-264, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Singh RR, Patel KP, Routbort MJ, et al. : Clinical massively parallel next-generation sequencing analysis of 409 cancer-related genes for mutations and copy number variations in solid tumours. Br J Cancer 111:2014-2023, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foundation Medicine : Genomic testing. https://www.foundationmedicine.com/genomic-testing
- 28.Lanman RB, Mortimer SA, Zill OA, et al. : Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PLoS One 10:e0140712, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Iyer P, Dadu R, Ferrarotto R, et al. : Real-world experience with targeted therapy for the treatment of anaplastic thyroid carcinoma. Thyroid 28:79-87, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pupilli C, Pinzani P, Salvianti F, et al. : Circulating BRAFV600E in the diagnosis and follow-up of differentiated papillary thyroid carcinoma. J Clin Endocrinol Metab 98:3359-3365, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Lubitz CC, Zhan T, Gunda V, et al. : Circulating BRAFV600E levels correlate with treatment in patients with thyroid carcinoma. Thyroid 28:328-339, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konda B SM, Wei L, Espinosa AV, et al. : Evaluation of BRAFV600E levels in cell free DNA (cfDNA) as a biomarker of response in BRAF V600E mutated radioiodine refractory differentiated thyroid cancer (DTC) treated with dabrafenib alone or in combination with trametinib. 87th Annual Meeting of the American Thyroid Association, Victoria, BC, Canada, October 18-22, 2017 [Google Scholar]
- 34.Schreuer M, Meersseman G, Van Den Herrewegen S, et al. : Quantitative assessment of BRAF V600 mutant circulating cell-free tumor DNA as a tool for therapeutic monitoring in metastatic melanoma patients treated with BRAF/MEK inhibitors. J Transl Med 14:95, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen G, McQuade JL, Panka DJ, et al. : Clinical, molecular, and immune analysis of dabrafenib-trametinib combination treatment for BRAF inhibitor-refractory metastatic melanoma: A phase 2 clinical trial. JAMA Oncol 2:1056-1064, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]