INTRODUCTION
ROS1 (ROS1 proto-oncogene receptor tyrosine kinase) encodes a tyrosine kinase of the insulin receptor family that plays a role in regulating cellular growth and differentiation. ROS1 is activated by chromosomal rearrangement involving its kinase domain (exons 36 to 42) in a variety of human cancers and is most commonly associated with non–small-cell lung cancer (NSCLC).1 Several techniques are used to detect these ROS1 fusions, including DNA sequencing, RNA sequencing, reverse transcription polymerase chain reaction (RT-PCR), and fluorescence in situ hybridization (FISH). Immunohistochemistry (IHC) of the ROS1 protein can also be considered as a prescreening test because high-expression staining is suggestive of possible activating fusions present in the sample.
ROS1 aberrations are present in 1% to 2% of patients with NSCLC, and ROS1 inhibitor therapy (crizotinib,2 ceritinib,3 lorlatinib4) is active in these tumors. In the expansion cohort of the phase I study of crizotinib in this indication, the objective response rate was 72%, and the median progression-free survival was 19.2 months. ROS1 inhibitors have also shown evidence of activity in other tumor types, including pediatric inflammatory myofibroblastic tumors (crizotinib),5-7 melanoma (entrectinib),8 pediatric high-grade glioma (entrectinib),6 acral lentiginous melanoma (entrectinib),9 and pancreatic cancer (entrectinib).10 Here we report the successful treatment of an intrahepatic cholangiocarcinoma (ICC) harboring a ROS1 fusion with crizotinib.
CASE REPORT
A 56-year-old non-Hispanic white woman with a past medical history of nontoxic goiter and hypothyroidism presented to her primary care provider with right-sided abdominal pain and lower extremity swelling of 2 months duration. Abdominal ultrasound demonstrated a large liver mass that was later visualized on a computed tomography (CT) scan. The mass was approximately 7 cm in its largest dimension in the right lobe of the liver. Several prominent periportal/portocaval lymph nodes were also noted. Biopsy of the mass showed moderately differentiated adenocarcinoma consistent with intrahepatic biliary tract cancer (BTC) or cholangiocarcinoma (immunostaining was positive for CK7, CK19, and MUC-1 and negative for CK20, CDX-2, GATA-3, and thyroid transcription factor); S100 showed weak nonspecific staining). Laboratory evaluation showed mildly elevated alkaline phosphatase and carbohydrate antigen 19-9 (CA19-9) of 278 U/mL. Her case was presented at a multidisciplinary tumor board and she was determined to have unresectable disease because of the presence of involved nonregional lymph nodes.
Systemic therapy with gemcitabine and cisplatin was initiated. She tolerated the chemotherapy well, but repeat CT imaging approximately 10 weeks after the start of chemotherapy showed progression with new lesions in segments 2 and 8 of the liver in addition to the continued presence of lesions in segments 6 and 4 and the caudate. Disease progression was also noted in the periportal/portocaval lymph nodes. CA19-9 had increased to 482 U/mL.
Molecular profiling of the tumor (FoundationOne CDx, Foundation Medicine) revealed an RDX-ROS1 fusion as well as a TERT promotor 124 C>T mutation. Variants of undetermined significance were noted in BCORL1, BRCA2, LTK, and PDK1 genes. Second-line treatment was initiated with the ROS1 inhibitor crizotinib at the standard oral dose of 250 mg twice per day. She tolerated crizotinib well; on review, her only complaints were intermittent constipation along with mild abdominal pain and indigestion. A few weeks after initiating systemic therapy with crizotinib, her CA19-9 declined from 482 to 178 U/mL, and approximately 4 months after the start of crizotinib it declined to 33 U/mL (Fig 1). CT evaluation at month 3 showed a significant tumor response. Her dominant lesion decreased from 7.3 cm to 4.1 cm (–43.8%). The segment 2 lesion and enlarged periportal/portocaval lymph nodes comparably decreased in size, and the lesions in segments 4, 8, and the caudate lobe were no longer identified (Fig 1). The patient’s response to crizotinib is ongoing at the time of this report approximately 6 months after initiating therapy. The patient provided consent to publish this case report.
FIG 1.
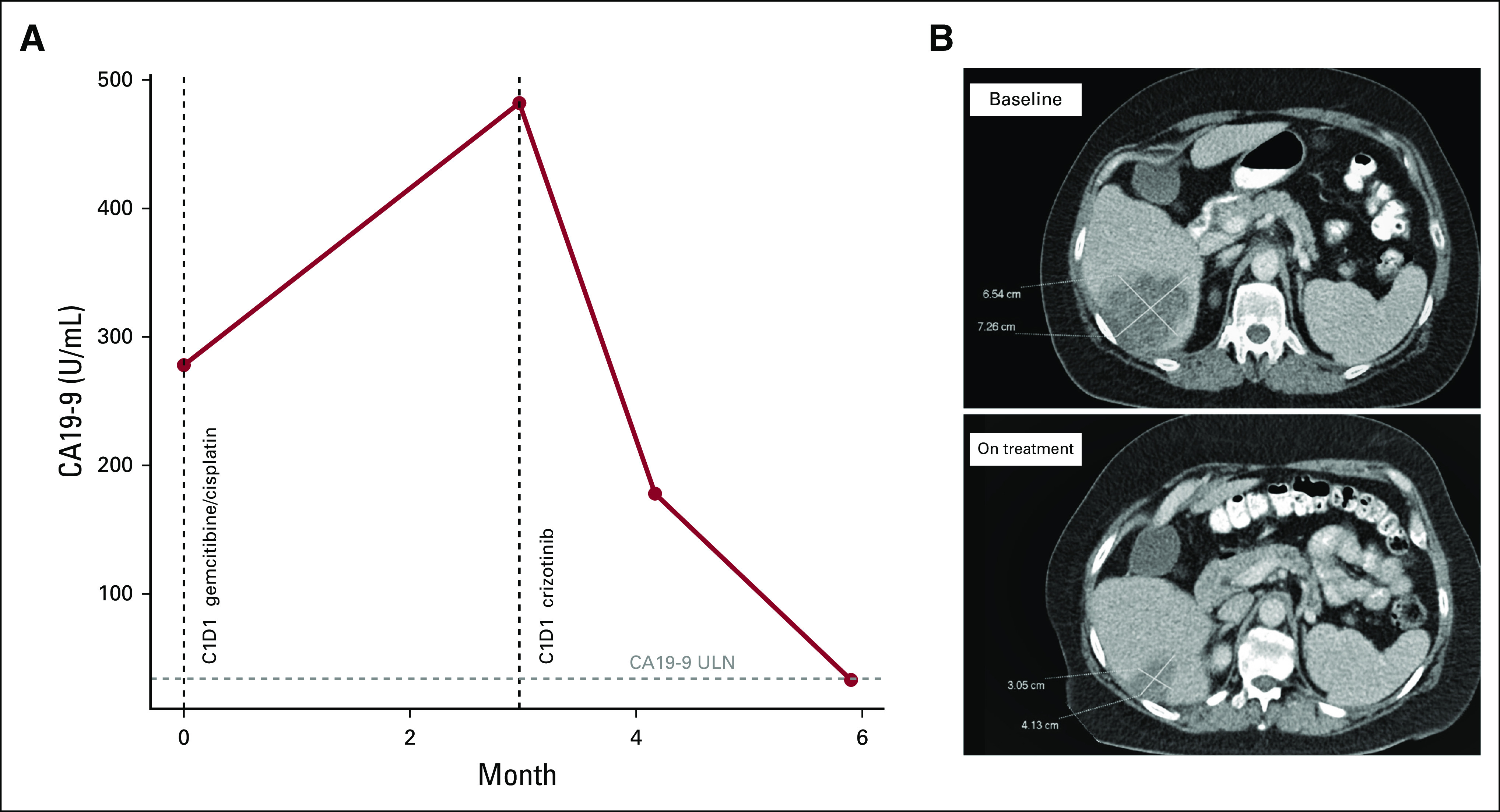
(A) Carbohydrate antigen 19-9 (CA19-9) at baseline, in response to chemotherapy, and then in response to crizotinib. (B) Target lesion measurements before and after crizotinib treatment. ULN, upper limit of normal (36.0 U/mL).
DISCUSSION
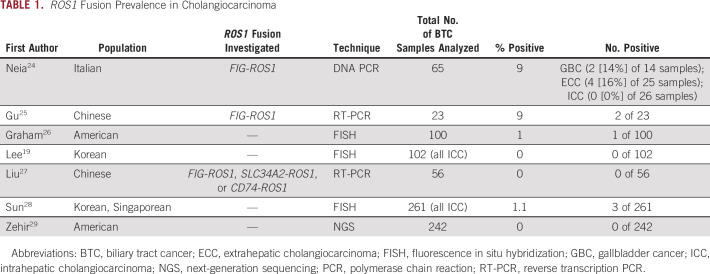
Cholangiocarcinomas arise from epithelial cells lining the biliary tree. Standard treatment options are limited, and the median survival for patients with advanced cholangiocarcinoma is approximately 1 year. Recently, actionable mutations in cholangiocarcinoma (particularly ICC) have been described, including FGFR fusion rearrangements and IDH1 mutations.11,12 Several reports have described apparent ROS1 translocations in cholangiocarcinoma. The Catalogue of Somatic Mutations in Cancer (COSMIC) database reports ROS1 fusions in less than 1% of BTCs and 1% of gallbladder adenocarcinomas (GBCs). ROS1 fusions have been reported in ICC, extrahepatic cholangiocarcinoma, and GBC with varying frequencies (Table 1).
TABLE 1.
ROS1 Fusion Prevalence in Cholangiocarcinoma
The specific fusion in this patient detected by FoundationOne CDx was an RDX-ROS1 fusion. The RDX gene (NM_002906.3) encodes the protein radixin, a cytoskeleton protein involved in linking actin to the plasma membrane.13 This is the first time this fusion partner has been seen in cholangiocarcinoma and potentially across solid tumors either partnered with ROS1 or another oncogene on review of the COSMIC database.1,14
Additional studies highlight the additional molecular and clinical importance of ROS1 in BTC. A preclinical study described the FIG-ROS1 fusion that induces ICC oncogenic development in an orthotopic allograft mouse model in cooperation with Kras and p53. Other conditional expression experiments using the models suggested oncogenic addiction to ROS1 signaling despite additional mutations, including Kras, which predicted the clinical efficacy of crizotinib described in this case report.15 In another report, expression levels of ROS1 measured by IHC were correlated with response to gemcitabine and oxaliplatin combined with cetuximab in advanced BTC.16 ROS1-expressing BTC tumors from patients with advanced disease were grouped with ALK- and c-MET–expressing BTC tumors because the 3 genes have been previously implicated in anti-EGFR resistance in NSCLC.17,18 ROS1, ALK, or c-MET (RAM) high-expressing tumors were all ICC tumors and had worse outcomes compared with RAM low expressers. RAM low expressers were also found to benefit from the addition of cetuximab to gemcitabine and oxaliplatin chemotherapy. The authors concluded that RAM expression may be site-specific (more common in ICC), and RAM expression should potentially be used in treatment decisions, specifically anti-EGFR therapy. In contradiction to this study, ROS1 IHC expression levels were associated with well-differentiated histology and better survival in a series of ICC patients who underwent curative resection.19
The current evidence suggests that ROS1 fusions are rare events in BTC, but the low incidence may partly be a result of the challenge in detecting them. FISH is a gold standard for ROS1 detection because it has the ability to detect translocations of ROS1 with any fusion partner; however, there can be multiple FISH patterns in addition to the classic split pattern.20,21 FISH and RT-PCR can also be technically challenging, time-consuming, costly, and limited by tissue quality and availably. Performing IHC studies as a prescreen is a consideration, and in lung cancer, ROS1 IHC has good concordance with FISH and RT-PCR methods and is used as a screening biomarker.22 This same concordance was not seen in ICC in 1 study because the ROS1 gene rearrangement by break-apart FISH was not positive in the 72 tumors reported to have moderate to strong IHC staining for ROS1.19 Discordance may be suggestive of ROS1 activation through alterative means such as epigenetic mechanisms.23 Molecular techniques need to be refined because identifying these rare drivers can be key to successful and sustained treatment options.
Although ROS1 fusions have been described in cholangiocarcinoma, to our knowledge this is the first definitive report of a patient with ROS1 fusion–positive cholangiocarcinoma treated with a ROS1 inhibitor. Additional information about crizotinib activity in cholangiocarcinoma is anticipated from the French National AcSé Program crizotinib basket study (ClinicalTrials.gov identifier: NCT02034981), which is enrolling a cohort of patients across multiple tumor types who harbor alterations in ALK, MET, or ROS1. An initial report from this study indicated that crizotinib has clinical activity in biomarker-selected patients across various tumor types, but it did not explicitly report the response rate of crizotinib in cholangiocarcinoma.5 Other basket trials are enrolling patients with solid tumors, including cholangiocarcinoma with ROS1 gene fusions (ClinicalTrials.gov identifier: NCT02568267). Our report highlights the potential value of molecular profiling in cholangiocarcinoma and the significance of ROS1 in this disease. It also adds to the growing list of tyrosine kinase inhibitor responses to ROS1 fusions across solid tumors, and these reports collectively provide support for a tumor agnostic approach to targeting ROS1 fusions outside NSCLC.
Supplementary Material
SUPPORT
Supported by the National Institutes of Health (NIH) under Award No. T32 CA009071, Molecular Targets for Cancer Detection and Treatment (C.D.J.). Also supported by the NIH under the National Cancer Institute Specialized Program of Research Excellence (SPORE) in Gastrointestinal Cancers (P50 CA062924), and NIH Center Core Grant No. P30 CA006973.
AUTHOR CONTRIBUTIONS
Conception and design: Christopher D. Jakubowski, Ihab R. Kamel, Mark Yarchoan
Collection and assembly of data: Christopher D. Jakubowski, Ihab R. Kamel, Mark Yarchoan
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Christopher Jakubowski
Honoraria: Oncology Business Review
Mark Yarchoan
Consulting or Advisory Role: Eisai, Exelixis, AstraZeneca, Geneos
Research Funding: Bristol-Myers Squibb (Inst), Merck & Co (Inst), Exelixis (Inst), Incyte (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Uguen A, De Braekeleer M: ROS1 fusions in cancer: A review. Future Oncol 12:1911-1928, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Shaw AT, Ou S-HI, Bang Y-J, et al. : Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 371:1963-1971, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subbiah V, Hong DS, Meric-Bernstam F: Clinical activity of ceritinib in ROS1-rearranged non-small cell lung cancer: Bench to bedside report. Proc Natl Acad Sci U S A 113:E1419-E1420, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw AT, Felip E, Bauer TM, et al. : Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 18:1590-1599, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vassal G, Cozic N, Ferretti G, et al. : Biomarker-driven access to crizotinib in ALK, MET, or ROS1 positive (+) malignancies in adults and children: The French National AcSé Program. J Clin Oncol 36, 2018. (suppl; abstr 2504) [Google Scholar]
- 6.Robinson GW, Gajjar AJ, Gauvain KM, et al. : Phase 1/1B trial to assess the activity of entrectinib in children and adolescents with recurrent or refractory solid tumors including central nervous system (CNS) tumors. J Clin Oncol 37, 2019. (suppl; abstr 10009) [Google Scholar]
- 7.Lovly CM, Gupta A, Lipson D, et al. : Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 4:889-895, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drilon A, Siena S, Ou S-HI, et al. : Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: Combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov 7:400-409, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couts KL, et al. : Acral Lentiginous Melanoma Harboring a ROS1 Gene Fusion With Clinical Response to Entrectinib. JCO Precis Oncol 2017. 10.1200/PO.16.00013 [DOI] [PubMed] [Google Scholar]
- 10.Pishvaian MJ, Rolfo CD, Liu SV, et al. : Clinical benefit of entrectinib for patients with metastatic pancreatic cancer who harbor NTRK and ROS1 fusions. J Clin Oncol 36, 2018. (suppl; abstr 521) [Google Scholar]
- 11.Abou-Alfa GK, Sahai V, Hollebecque A, et al. : Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol 21:671-684, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 21:796-807, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. NCBI Nucleotide Database: Homo sapiens radixin (RDX), transcript variant 3, mRNA. (2019). http://www.ncbi.nlm.nih.gov/nuccore/NM_002906.3.
- 14.Roskoski R, Jr: ROS1 protein-tyrosine kinase inhibitors in the treatment of ROS1 fusion protein-driven non-small cell lung cancers. Pharmacol Res 121:202-212, 2017. [DOI] [PubMed] [Google Scholar]
- 15.Saborowski A, Saborowski M, Davare MA, et al. : Mouse model of intrahepatic cholangiocarcinoma validates FIG-ROS as a potent fusion oncogene and therapeutic target. Proc Natl Acad Sci U S A 110:19513-19518, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiang N-J, Hsu C, Chen J-S, et al. : Expression levels of ROS1/ALK/c-MET and therapeutic efficacy of cetuximab plus chemotherapy in advanced biliary tract cancer. Sci Rep 6:25369, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergethon K, Shaw AT, Ou S-HI, et al. : ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 30:863-870, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwak EL, Bang Y-J, Camidge R, et al. : Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363:1693-1703, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee K-H, Lee K-B, Kim T-Y, et al. : Clinical and pathological significance of ROS1 expression in intrahepatic cholangiocarcinoma. BMC Cancer 15:721, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mescam-Mancini L, Lantuéjoul S, Moro-Sibilot D, et al. : On the relevance of a testing algorithm for the detection of ROS1-rearranged lung adenocarcinomas. Lung Cancer 83:168-173, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida A, Kohno T, Tsuta K, et al. : ROS1-rearranged lung cancer: A clinicopathologic and molecular study of 15 surgical cases. Am J Surg Pathol 37:554-562, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Bubendorf L, Büttner R, Al-Dayel F, et al. : Testing for ROS1 in non-small cell lung cancer: a review with recommendations. Virchows Arch 469:489-503, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HJ, Seol HS, Kim JY, et al. : ROS1 receptor tyrosine kinase, a druggable target, is frequently overexpressed in non-small cell lung carcinomas via genetic and epigenetic mechanisms. Ann Surg Oncol 20:200-208, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Peraldo Neia C, Cavalloni G, Balsamo A, et al. : Screening for the FIG-ROS1 fusion in biliary tract carcinomas by nested PCR. Genes Chromosomes Cancer 53:1033-1040, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Gu T-L, Deng X, Huang F, et al. : Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PLoS One 6:e15640, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham RP, Barr Fritcher EG, Pestova E, et al. : Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol 45:1630-1638, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Liu P, Wu Y, Sun L, et al. : ROS kinase fusions are not common in Chinese patients with cholangiocarcinoma. Nan Fang Yi Ke Da Xue Xue Bao 33:474-478, 2013 [PubMed] [Google Scholar]
- 28.Lim SM, Yoo JE, Lim KH, et al. : Rare incidence of ROS1 rearrangement in cholangiocarcinoma. Cancer Res Treat 49:185-192, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zehir A, Benayed R, Shah RH, et al. : Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703-713, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]