Abstract
Purpose
There are no nationally representative data on oncologists’ use of next-generation sequencing (NGS) testing in practice. The purpose of this study was to investigate how oncologists in the United States use NGS tests to evaluate patients with cancer and to inform treatment recommendations.
Methods
The study used data from the National Survey of Precision Medicine in Cancer Treatment, which was mailed to a nationally representative sample of oncologists in 2017 (N = 1,281; cooperation rate = 38%). Weighted percentages were calculated to describe NGS test use. Multivariable modeling was conducted to assess the association of test use with oncologist practice characteristics.
Results
Overall, 75.6% of oncologists reported using NGS tests to guide treatment decisions. Of these oncologists, 34.0% used them often to guide treatment decisions for patients with advanced refractory disease, 29.1% to determine eligibility for clinical trials, and 17.5% to decide on off-label use of Food and Drug Administration–approved drugs. NGS test results informed treatment recommendations often for 26.8%, sometimes for 52.4%, and never or rarely for 20.8% of oncologists. Oncologists younger than 50 years of age, holding a faculty appointment, having genomics training, seeing more than 50 unique patients per month, and having access to a molecular tumor board were more likely to use NGS tests.
Conclusion
In 2017, most oncologists in the United States were using NGS tests to guide treatment decisions for their patients. More research is needed to establish the clinical usefulness of these tests, to develop evidence-based clinical guidelines for their use in practice, and to ensure that patients who can benefit from these new technologies receive appropriate testing and treatment.
INTRODUCTION
The increased affordability, accessibility, and reliability of DNA and RNA high-throughput sequencing platforms and bioinformatics capabilities enable oncologists to provide more personalized care, often referred to as precision oncology.1 However, the accelerated development and diffusion of new commercial and noncommercial tumor gene sequencing panels have made this a challenging time for oncologists to effectively incorporate these new tests into routine patient care, particularly with the paucity of data on their clinical usefulness and the limited availability of evidence-based clinical guidelines.
Cancer treatment decisions are increasingly made on the basis of genomic information, and there are currently large numbers of genomic tests available to oncologists.2 Genomic tests designed to facilitate decisions about treatment management include those that identify alterations in single genes and multimarker tumor panels . Multimarker panels include targeted gene-expression profiling tests that are used to estimate prognosis and/or the likelihood of recurrence. Multimarker panels also include DNA and RNA analysis through next-generation sequencing (NGS) technologies, including custom panels that profile multiple actionable driver genes and tumor characteristics that may guide the selection of targeted therapies.
Despite advances in precision oncology, comparatively little empirical research is available that assesses the expectations and experiences of oncologists in the United States who may use these genomic tools to care for patients with cancer. To date, most studies have evaluated physicians’ experiences with specific genomic tests for individual gene mutations.3-5 Studies that have attempted to assess providers’ experiences with custom NGS tumor panels6-9 have focused on intended rather than actual use. Furthermore, previous studies of physicians’ use of NGS tests were on the basis of small samples and were conducted in tertiary referral centers; as a result, their findings may not represent how oncology is practiced in the United States. Currently, there are no nationally representative data describing oncologists’ awareness, knowledge, and use of NGS testing to inform patient care, especially in community practice settings.
With the increasing application of precision oncology, understanding how oncologists use genomic tests in their practice and the factors that affect their use is essential to ensure that patients who can benefit receive appropriate testing and follow-up. The purpose of this study was to investigate how oncologists in the United States use NGS tests to evaluate patients with cancer and inform treatment recommendations.
METHODS
Data Source
This study used data from the National Survey of Precision Medicine in Cancer Treatment, a nationally representative survey of hematologists, hematologists/oncologists, and oncologists sponsored by the National Cancer Institute (NCI), the National Human Genomic Research Institute, and the American Cancer Society. Practicing oncologists were identified from the American Medical Association Physician Masterfile, a database of all licensed physicians in the United States. Oncologists who were retired, in training (ie, residents), not in clinical practice, older than 75 years of age, or deceased were excluded. Oncologists were selected using probability sampling, stratified by specialty, census region, size of the physician’s metropolitan statistical area, and a combined sex by age variable. Eligibility and contact information for sampled oncologists were verified by telephone calls to the physicians’ offices. Throughout data collection, 87 oncologists were identified as ineligible. Of the remaining 3,378 oncologists with verified contact information, 1,281 completed the survey (cooperation rate = 38%10).
A survey packet containing a personalized invitation letter from the NCI, an endorsement letter from ASCO, a pen with the study’s name printed on it, a self-administered paper questionnaire, and a business reply envelope was mailed to eligible oncologists. Up to two e-mail reminders and two follow-up mailings with a replacement paper questionnaire were sent to nonresponders. A telephone reminder call was placed to physicians who had not responded to previous contact attempts. Participants received a $50 honorarium for completing the survey.
The questionnaire (Data Supplement) took 20 minutes to complete on average, and data were collected from February through May of 2017. Information was ascertained about oncologists’ demographics, training, academic affiliation, specialty, and patient volume, and their practice characteristics, including practice setting, structure, and resources to support genomic testing. Information about oncologists’ use of commercially available multimarker tumor panels and noncommercial tumor panels performed at academic medical centers was collected, as were data on the clinical scenarios in which NGS test were used. Because of differences in how survey questions were worded (eg, some explicitly stated the exclusion of Oncotype DX), sensitivity analyses were conducted to examine whether oncologists who reported using other gene expression tests, including the Breast Cancer Index, Mammaprint, Prosigna, and/or myPlan Lung Cancer, differed in their use of NGS tests from those who did not use these gene expression tests. Detailed information about the survey’s data collection methods has been published elsewhere,,11 and details describing the collected data are included in the Appendix.
Statistical Analyses
Survey weights were calculated using the age, sex, and geographic location information available on the sample frame. The weights also adjust for the complex survey design by accounting for the probability of selection as well as the probability of noncontact and the probability of noncooperation. The survey weights were applied in all analyses of the data and provide statistical adjustment so that respondents are representative of the population of practicing oncologists in the United States.
Each oncologist was classified as a user of NGS tests if he or she reported using at least one specific multimarker panel with NGS technologies profiling multiple actionable driver genes for guiding treatment decisions in the past 12 months. The weighted percentage of NGS testing among oncologists was calculated overall and was stratified by physician demographic and practice characteristics. Multivariable models were estimated to examine the independent association between each physician demographic and practice characteristic and the likelihood of NGS testing, when adjusting for the other characteristics in the model. Results are presented as adjusted odds ratios and adjusted weighted percentages (ie, predicted probabilities)12 with corresponding 95% CIs. All analyses were conducted using SUDAAN release 11.0.1 (RTI International, Research Triangle Park, NC).
RESULTS
Characteristics of Survey Respondents
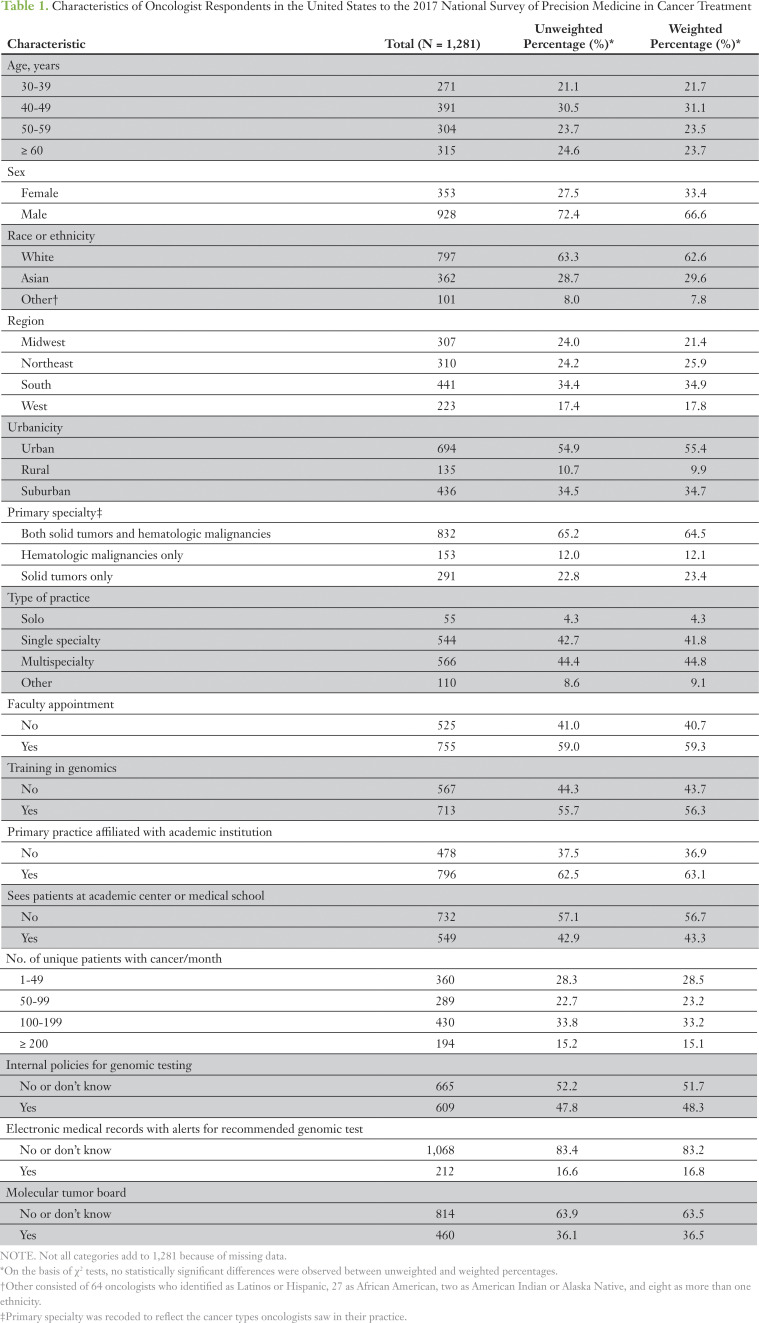
Characteristics of the 1,281 oncologists who completed the survey are listed in Table 1. Most respondents were male (66.4%) and white (62.6%). Nearly one third (31.1%) of respondents were in the 40- to 49-year age group. Approximately 10% reported practicing in a rural setting. Most respondents (64.5%) reported treating patients with solid tumors as well as hematologic malignancies. Most respondents reported that their practices were affiliated with an academic institution: 43.3% saw patients at an academic center and 59.3% held a faculty appointment. More than one half of respondents (56.3%) reported having some training in genomic testing. Respondents also, on average, saw 101 unique patients with cancer per month. More than one half of respondents (56.9%) spent at least 90% of their time in patient care and almost one half (49.3%) spent less than 10% of their time teaching (data not shown).
Table 1.
Characteristics of Oncologist Respondents in the United States to the 2017 National Survey of Precision Medicine in Cancer Treatment
Prevalence of NGS Test Use and Predictors of Test Use to Guide Treatment Decisions
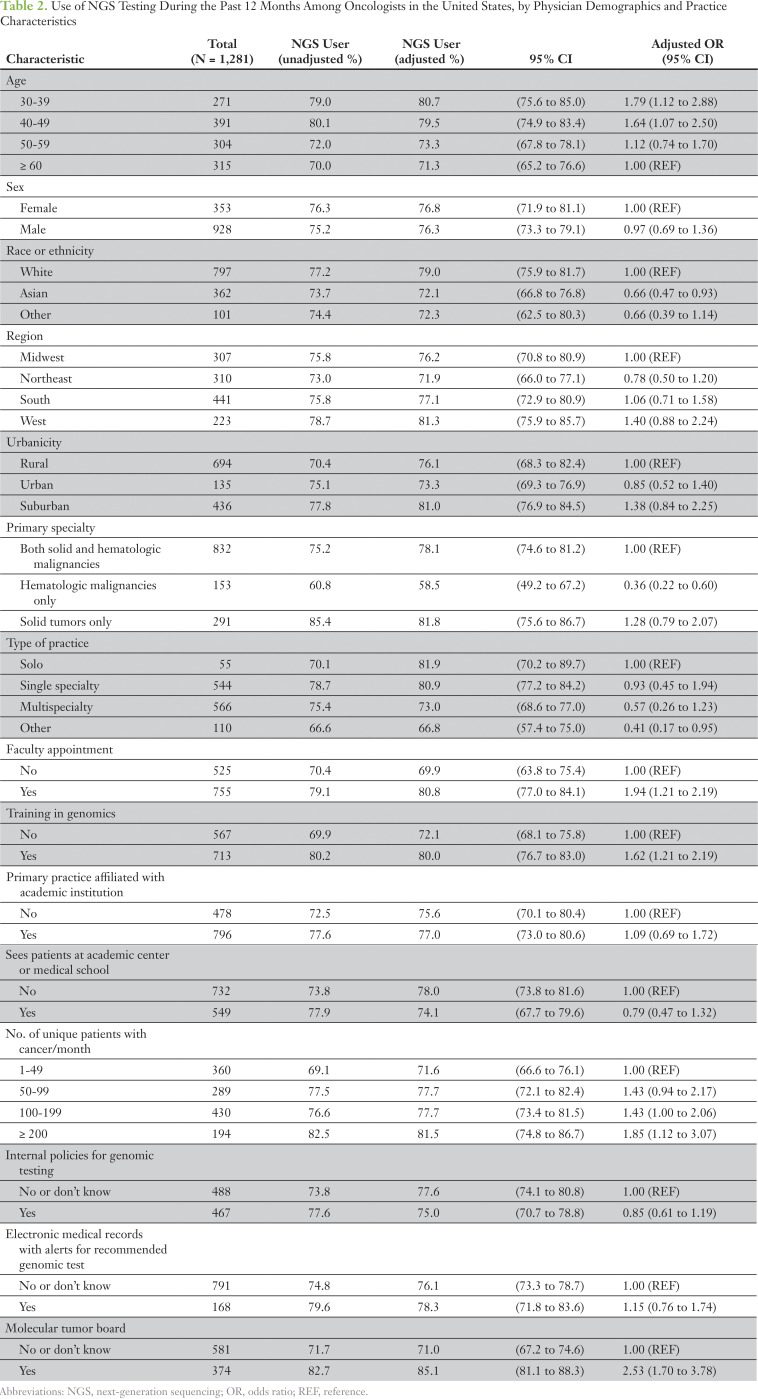
Overall, 75.6% of oncologists reported using NGS tests in the past 12 months to guide treatment decisions; use differed according to the physician’s demographic and practice characteristics (Table 2). NGS test use for treatment decisions was higher among younger oncologists (age < 50 years) than among those who were 60 years of age or older. Oncologists who treated only patients with hematologic malignancies were less likely to use NGS tests than were oncologists who treated both solid and hematologic malignancies and those who only treated patients with solid tumors. Oncologists who held a faculty appointment, had some training in genomics, or were part of a practice that had a molecular tumor board were more likely to use NGS tests than were those who did not have these characteristics. In addition, those who treated fewer than 50 patients with cancer per month were more likely to use NGS tests than were oncologists who treated more than 50 patients with cancer per month. In multivariable analyses, results were somewhat attenuated but largely unchanged. The likelihood of NGS use was not statistically significantly associated with other provider and practice characteristics assessed.
Table 2.
Use of NGS Testing Over the Past 12 Months Among Oncologists in the United States, by Physician Demographics and Practice Characteristics
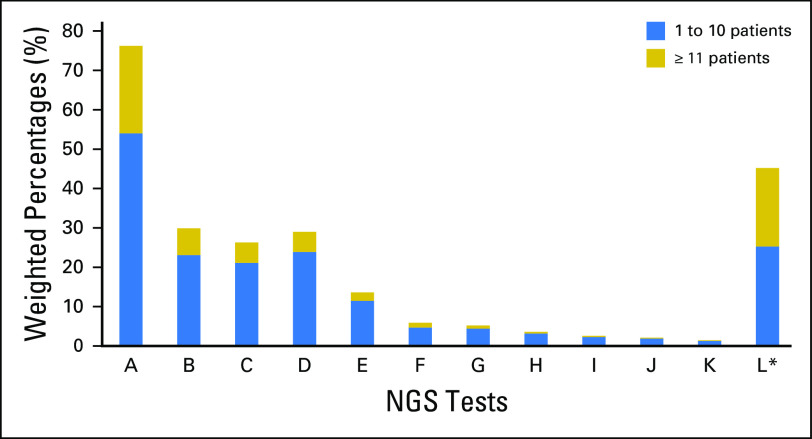
In this survey, oncologists in the United States were asked how often they used 11 commercially available NGS tests or a noncommercial NGS test over the past 12 months (Fig 1). Less than 7% of oncologists reported using 10 of the commercially available NGS tests in more than 10 patients (range, 0.2% to 6.8%); for the other two NGS tests surveyed, approximately 20% of oncologists reported using them in more than 10 patients. Moreover, among oncologists who ordered any of these NGS tests (n = 959), 28.2% ordered one test, 31.7% ordered two tests, 23.3% ordered three tests, and 16.7% ordered four or more tests in the past 12 months.
Fig 1.
Use of specific commercially available next-generation sequencing (NGS) and noncommercial NGS tests during the past 12 months by oncologists in the United States. (*) Noncommercial NGS tests.
NGS Test Use Practice Patterns
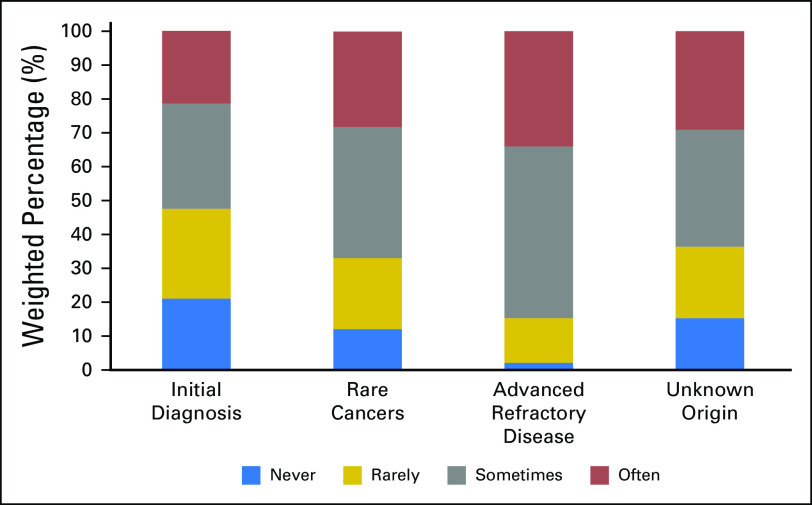
Oncologists’ use of NGS test results varied by clinical presentation (Fig 2). For 34.0% of oncologists (95% CI, 31.0% to 37.2%), NGS test results were used often over the past 12 months to guide treatment decisions for patients with advanced refractory disease. Fewer oncologists reported using NGS tests results often when treating patients with rare cancers (28.2% [95% CI, 25.2% to 31.5%]), patients with cancers of unknown origin (29.1% [95% CI, 25.9% to 32.4%]), or patients with an initial diagnosis of cancer (21.4% [95% CI, 18.4% to 24.2%]).
Fig 2.
Use of next-generation sequencing tests by clinical presentation over the past 12 months among oncologists in the United States.
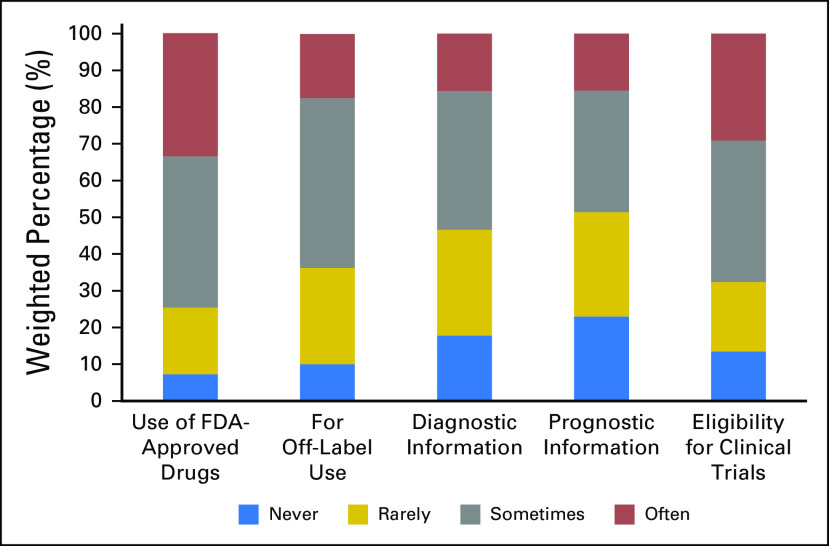
The frequency of NGS testing varied by clinical purpose. Approximately one third of oncologists reported ordering NGS tests often to guide the use of a Food and Drug Administration (FDA)–approved therapy (33.5% [95% CI, 30.5% to 36.6%]) and to determine eligibility for clinical trials (29.1% [95% CI, 26.2% to 32.1%]). Fewer oncologists reported using NGS tests results often in decisions about off-label use of FDA-approved drugs (17.5% [95% CI, 15.2% to 20.1%]) or to provide diagnostic (15.6% [95% CI, 13.3% to 18.2%]) or prognostic (15.5% [95% CI, 13.3% to 18.1%]) information (Fig 3).
Fig 3.
Use of next-generation sequencing tests by clinical purpose over the past 12 months among oncologists in the United States.
Respondents were explicitly directed to exclude Oncotype DX when answering questions about NGS testing for different clinical purposes. We conducted sensitivity analyses to examine whether oncologists who used other gene expression tests (ie, Breast Cancer Index, Mammaprint, Prosigna, and/or myPlan Lung Cancer) that were not explicitly included in those survey questions, answered these questions differently from oncologists who did not. We found that oncologists who used the Breast Cancer Index, Mammaprint, Prosigna, and/or myPlan Lung Cancer did not differ in the way they answered these questions in nine out of 10 comparisons. However, oncologists who used the Breast Cancer Index, Mammaprint, Prosigna, and/or myPlan Lung Cancer reported using multimarker tumor panels less often to determine eligibility for clinical trials (23.1% [95% CI, 18.9% to 27.8%]) than did those who did not (32.9% [95% CI, 29.1% to 37.0%]). When analyses were restricted to oncologists who treated a high volume of patients with breast cancer, there were no differences in test use between oncologists who used the Breast Cancer Index, Mammaprint, and Prosigna and those who did not (data not shown).
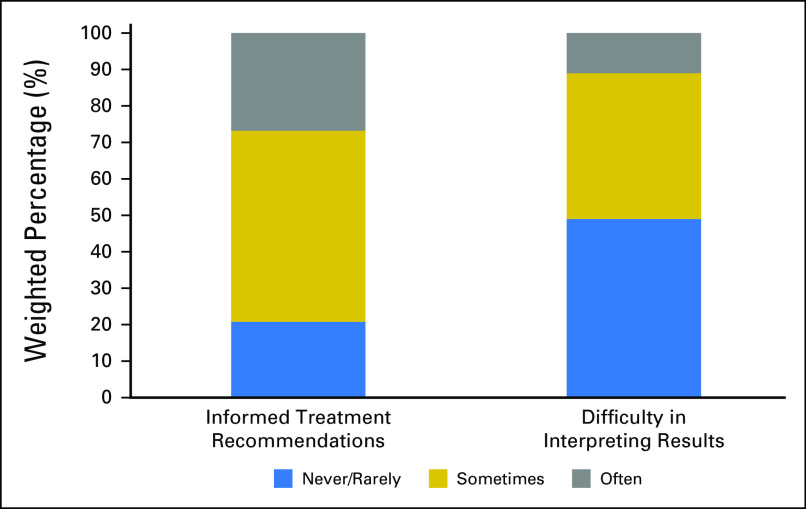
There was variability in how frequently oncologists used NGS testing to guide clinical care. For 26.8% of oncologists (95% CI, 24.0% to 29.8%), NGS tests results informed treatment recommendations often, whereas 52.4% (95% CI, 49.2% to 55.7%) reported sometimes, and 20.8% (95% CI, 18.3% to 23.6%) indicated that results never or rarely informed their treatment recommendations (Fig 4). There was also variability in the difficulty oncologists reported having in interpreting the test results. For 11.0% of oncologists (95% CI, 9.1% to 13.2%), NGS test results were often difficult to interpret, whereas 40.0% (95% CI, 36.8% to 43.2%) reported this was the case sometimes (data not shown). For 49% of oncologists (95% CI, 45.8% to 52.3%), the results were never or rarely difficult to interpret.
Fig 4.
Usefulness of NGS testing over the past 12 months among oncologists in the United States.
Oncologists also reported whether they referred their patients with cancer to another practice or provider for NGS testing. Twenty-five percent (n = 319) indicated that they referred their patients to another location or provider for NGS testing; among these oncologists, 84.4% (n = 257) referred to an academic medical center, 82.1% (n = 240) to a clinical trial, and 18.2% (n = 51) to an oncologist outside of their practice (data not shown).
DISCUSSION
In this nationally representative study of 1,281 oncologists, we examined how NGS tests are currently used in clinical practice and whether use is influenced by physician and practice characteristics. Approximately 75% of those oncologists reported using NGS test results to guide patient care, and the use of NGS tests differed by oncologists’ demographic and practice characteristics. For example, younger oncologists were more likely to be NGS test users, suggesting that they may have had more recent training in genomic testing or that they are more receptive to the incorporation of NGS testing in their practice. Having a faculty appointment and access to a molecular tumor board were also associated with NGS test use, perhaps reflecting greater access to in-house NGS tests in academic settings or more involvement in clinical research. Oncologists whose practices had higher patient volumes were also more likely to use NGS tests. Not surprisingly, oncologists who treated only patients with hematologic malignancies were less likely to use NGS tests than were oncologists who treated both solid and hematologic malignancies or those who treated only patients with solid tumors, suggesting that NGS tests may be ordered reflexively by pathologists rather than by oncologists. Moreover, it may be common practice to order other non-NGS tests such as fluorescent in situ hybridization and molecular testing for patients with hematologic malignancies.
Oncologists reported using NGS most often for patients with advanced refractory disease, but also used these tests often for patients diagnosed with a rare cancer or cancers of unknown origin and/or for patients with an initial diagnosis of cancer. These results may reflect oncologist’s use of NGS testing to inform treatment strategies when established therapies have failed or when there is uncertainty about the usefulness of existing treatment guidelines for less common clinical situations. With the recent approval of multiple therapeutic agents targeting specific driver mutations, oncologists may send a patient’s tumors for sequencing with the hope of identifying treatments that are potentially efficacious for their patient’s particular molecular tumor subtype. In our survey, more than one third of oncologists reported using NGS test results often to guide the use of an FDA-approved therapy.
Advances in precision oncology pose challenges for oncologists. The first challenge is posed by the lack of published, evidence-based guidelines informing the use of currently available NGS tests. At the time of the survey in May 2017, non–small-cell lung cancer was the only cancer type for which there was a consensus recommendation for NGS use.13 Furthermore, no professional group other than the National Comprehensive Cancer Network has yet issued evidence-based guidelines recommending the use of NGS testing. However, as the number of single-gene or focused-indication (eg, microsatellite instability) tests that are components of the standardized evaluation of common malignancies has grown, oncologists are increasingly faced with decisions about whether to order several targeted tests or a single NGS panel.
There has also been an effort by pharmaceutical companies to develop tissue-agnostic cancer drugs that target a specific genetic marker independent of tumor type.14 Given the prospect of tissue-agnostic labeling, the use of NGS will only grow, and in the absence of clinical guidelines informing NGS use, it is likely that many providers will experience uncertainty about how to clearly integrate this information into clinical care decisions. In our survey, 75% of oncologists report using NGS tests to guide treatment recommendations, suggesting that oncologists are already confronted with a large volume of genomic information that they must interpret.
That NGS test results are frequently ambiguous presents another challenge for oncologists. More than 50% of oncologists in our study reported that NGS test results were difficult to interpret either often or sometimes. In addition, 25% indicated that they referred patients to other providers for NGS testing, possibly suggesting a lack of expertise or resources for ordering and interpreting NGS tests. Several academic and commercial groups have responded to this need, developing online decision support resources for genomic medicine, such as Personalized Cancer Therapy, My Cancer Genome, and OncoKB, with the goal of providing an accessible platform to oncologists.15 However, more work is needed to ensure that patients who can benefit from these new technologies receive appropriate testing and follow-up.
A third challenge for oncologists is the lack of clinical usefulness data for many of the available NGS tests. Two major, ongoing clinical trials, NCI-MATCH (National Cancer Institute–Molecular Analysis for Therapy Choice),16 and Pediatric MATCH (National Cancer Institute–Children’s Oncology Group Pediatric MATCH Trial), will generate evidence to establish clinical usefulness to fill some of these gaps. Our study suggests that NGS tests play an important role in identifying patients for clinical trials. Furthermore, ASCO’s Targeted Agent and Profiling Utilization Registry (TAPUR) Study17 and CureOne (formerly called MED-C) are building registries of NGS testing data to enhance understanding of how testing is being used and its impact on patient outcomes.18
Insurers are also recognizing the cost efficiencies of NGS panel testing as opposed to multiple single-gene testing for specific cancer-site indications but are grappling with a lack of clinical usefulness data for testing cancers broadly. Effective March 16, 2018, the Centers for Medicare & Medicaid Services has allowed non–FDA-approved assays run in Clinical Laboratory Improvement Amendments–certified laboratories to be reimbursed, dependent on decisions made by local Medicare administrative contractors.19 It is unclear whether and under what circumstances commercial private payers will decide to reimburse for NGS testing broadly for cancer. Our findings offer an important baseline that could be used to evaluate the impact of these coverage decisions on NGS test use because our data were collected before implementation of these key changes in coverage.
Our study has several limitations. First, the cooperation rate was lower than that of previous surveys of physicians on the topic of genomic and genetic test use,7,20 and responders may have differed from nonresponders in terms of their genomic testing practices and other characteristics such as academic affiliation. However, respondents are representative of the population of practicing oncologists in the United States in terms of age, sex, and geographic location on the basis of statistical adjustment for nonresponse using data available on the survey's sample frame.
Another limitation is that we were unable to assess how oncologists use the tests in specific clinical situations and for which cancer types they are ordering these tests. In addition, precision oncology is a rapidly evolving field, and our findings reflect NGS test use in 2017. However, these limitations are offset by several strengths of the study, including the nationally representative sample of practicing oncologists in a wide variety of practice settings and the large sample size, which allowed us to analyze multiple factors associated with NGS use and to examine a variety of practice patterns.
The majority of oncologists in the United States use NGS tests to guide patient care, even in the absence of evidence-based practice guidelines. The rapid commercialization and adoption of genomic tests in practice highlights the importance of generating evidence for the clinical usefulness of NGS panels. There is also a need to monitor the use of these new technologies in practice to ensure that patients with cancer have access to appropriate testing and effective therapies.
Supplementary Material
Appendix
Physician characteristics included age, sex, race or ethnicity, faculty appointment, training in genomic testing (eg, instruction throughout residency and/or fellowship, professional lectures or seminars, symposiums, conferences, or continuing medical education). Practice characteristics, such as region, urbanicity, type of practice (solo, single specialty group, multispecialty group, other), academic affiliation, and number of unique patients with cancer seen per month were also collected. In addition, questions were asked about whether the oncologist’s practice setting has a genomic or molecular tumor board, policies for genomic testing use, and/or an electronic medical record that provides alerts when a genomic test is recommended. An oncologist’s specialty was defined using information on type(s) of patients with cancer seen: only patients with solid tumors, only those with hematologic malignancies, or both. Age and demographic characteristics other than race or ethnicity were obtained from the American Medical Association Masterfile.
An oncologists was classified as a user of next-generation sequencing (NGS) tests if he or she reported use, in the past 12 months, of specific multimarker panels with NGS technologies profiling multiple actionable driver genes used to guide treatment decisions. The following tests were included in this definition: CancerSELECT or Cancer Complete, Caris Molecular Intelligence or Target Now, CGI Complete, FoundationOne, FoundationOneHeme, FoundationACT, GPS Cancer, Guardant360, Omniseq Comprehensive, OnkoSight Tumor Panels, Solid Tumor Mutation Panel (ARUP Laboratories), and noncommercial tumor panels. Multimarker tests that use gene-expression profiling (eg, Oncotype DX for breast cancer), primarily used to predict prognosis and/or the likelihood of recurrence, were not classified as NGS tests. This list reflects the multimarker tumor panel tests that were commonly available at the time the survey was developed. To capture less commonly used tests or newly developed panels, there was an option to write in any additional multimarker tumor panel tests used in the past 12 months that were not listed on the questionnaire.
Footnotes
Supported by the National Institutes of Health and American Cancer Society (contract Nos. HHSN261201400011I to Scientific Consulting Group [N.I.S.] and HHSN261201000086I to RTI International [K.W.]).
AUTHOR CONTRIBUTIONS
Conception and design: Andrew N. Freedman, Carrie N. Klabunde, Kristine Wiant, Lindsey Enewold, Stacy W. Gray, Kelly K. Filipski, Nancy L. Keating, Debra G.B. Leonard, Lori Minasian, Arnold L. Potosky, Donna R. Rivera, Richard L. Schilsky, Deborah Schrag, Helmneh M. Sineshaw, Jeffery P. Struewing, Gordon Willis, Janet S. de Moor
Financial support: Andrew N. Freedman, Helmneh M. Sineshaw, Jeffery P. Struewing
Administrative support: Andrew N. Freedman, Carrie N. Klabunde
Provision of study material or patients: Andrew N. Freedman
Collection and assembly of data: Carrie N. Klabunde, Kristine Wiant, Kelly K. Filipski, Timothy S. McNeel, Helmneh M. Sineshaw
Data analysis and interpretation: Andrew N. Freedman, Carrie N. Klabunde, Lindsey Enewold, Stacy W. Gray, Nancy L. Keating, Debra G.B. Leonard, Tracy Lively, Timothy S. McNeel, Arnold L. Potosky, Donna R. Rivera, Deborah Schrag, Naoko I. Simonds, Helmneh M. Sineshaw, Jeffery P. Struewing, Gordon Willis, Janet S. de Moor
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Andrew N. Freedman
No relationship to disclose
Carrie N. Klabunde
No relationship to disclose
Kristine Wiant
No relationship to disclose
Lindsey Enewold
No relationship to disclose
Stacy W. Gray
No relationship to disclose
Kelly K. Filipski
No relationship to disclose
Nancy L. Keating
No relationship to disclose
Debra G.B. Leonard
No relationship to disclose
Tracy Lively
No relationship to disclose
Timothy S. McNeel
No relationship to disclose
Lori Minasian
No relationship to disclose
Arnold L. Potosky
No relationship to disclose
Donna R. Rivera
No relationship to disclose
Richard L. Schilsky
Research Funding: AstraZeneca (Inst), Bayer AG (Inst), Bristol-Myers Squibb (Inst), Genentech/Roche (Inst), Eli Lilly (Inst), Merck (Inst), Pfizer (Inst)
Deborah Schrag
Consulting or Advisory Role: Journal of the American Medical Association
Research Funding: Pfizer
Other Relationship: Journal of the American Medical Association
Naoko I. Simonds
Stock and Other Ownership Interests: CVS Health (I), Walgreens Boots Alliance, Anthem Foundation, Anthem Foundation (I), Celgene (I), CVS Health, Johnson & Johnson, Johnson & Johnson (I), Walgreens Boots Alliance (I), LabCorp, LabCorp (I), Valeant Pharmaceuticals International, Valeant Pharmaceuticals International (I), Endo Pharmaceuticals (I), Aetna (I)
Helmneh M. Sineshaw
No relationship to disclose
Jeffery P. Struewing
No relationship to disclose
Gordon Willis
No relationship to disclose
Janet S. de Moor
Employment: Biogen (I)
Travel, Accommodations, Expenses: Biogen (I)
REFERENCES
- 1.Collins FS, Varmus H: A new initiative on precision medicine. N Engl J Med 372:793-795, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NCBI : GTR: Genetic testing registry. https://www.ncbi.nlm.nih.gov/gtr/
- 3.Wideroff L, Freedman AN, Olson L, et al. : Physician use of genetic testing for cancer susceptibility: Results of a national survey. Cancer Epidemiol Biomarkers Prev 12:295-303, 2003 [PubMed] [Google Scholar]
- 4.Miller FA, Krueger P, Christensen RJ, et al. : Postal survey of physicians and laboratories: Practices and perceptions of molecular oncology testing. BMC Health Serv Res 9:131, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schink JC, Trosman JR, Weldon CB, et al. : Biomarker testing for breast, lung, and gastroesophageal cancers at NCI designated cancer centers. J Natl Cancer Inst 106:pii:dju256, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller FA, Hayeems RZ, Bytautas JP, et al. : Testing personalized medicine: Patient and physician expectations of next-generation genomic sequencing in late-stage cancer care. Eur J Hum Genet 22:391-395, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray SW, Hicks-Courant K, Cronin A, et al. : Physicians’ attitudes about multiplex tumor genomic testing. J Clin Oncol 32:1317-1323, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray SW, Park ER, Najita J, et al. : Oncologists’ and cancer patients’ views on whole-exome sequencing and incidental findings: Results from the CanSeq study. Genet Med 18:1011-1019, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson LM, Valdez JM, Quinn EA, et al. : Integrating next-generation sequencing into pediatric oncology practice: An assessment of physician confidence and understanding of clinical genomics. Cancer 123:2352-2359, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Association of Public Opinion Research : https://www.aapor.org/Standards-Ethics/Standard-Definitions-(1).aspx
- 11.Wiant K, Geisen E, Creel DV, et al. : Risks and rewards of using pre-paid vs. post-paid incentive checks on a survey of physicians. BMC Med Res Methodol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graubard BI, Korn EL: Predictive margins with survey data. Biometrics 55:652-659, 1999 [DOI] [PubMed] [Google Scholar]
- 13.NCCN : 2016. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer Version 3.2016. http://www.hts.org/gr/assets/files/omades_ergasias/cancer/NCCN%20guidelines%20NCLC%202016.pdf
- 14.Garber K: In a major shift, cancer drugs go ‘tissue-agnostic’. Science 356:1111-1112, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Kurnit KC, Bailey AM, Zeng J, et al. : Personalized cancer therapy: A publicly available precision oncology resource. Cancer Res 77:e123-e126, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute : 2017. https://www.cancer.gov/about-cancer/treatment/clinical -trials/nci-supported/nci-match
- 17.ASCO : TAPUR. http://www.tapur.org
- 18.genomeweb : Varmus urges payors to use coverage with evidence development to advance cancer genomics. https://www.genomeweb.com/reimbursement/varmus-urges-payors-use-coverage-evidence-development-advance-cancer-genomics#.WvW6nZcpDD4
- 19.Centers for Medicare & Medicaid Services : Decision memo for next generation sequencing (NGS) for Medicare beneficiaries with advanced cancer (CAG-00450N). https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=290
- 20.Wideroff L, Vadaparampil ST, Greene MH, et al. : Hereditary breast/ovarian and colorectal cancer genetics knowledge in a national sample of US physicians. J Med Genet 42:749-755, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]