Abstract
We recently described a new afimbrial adhesin, AfaE-VIII, produced by animal strains associated with diarrhea and septicemia and by human isolates associated with extraintestinal infections. Here, we report that the afa-8 operon, encoding AfaE-VIII adhesin, from the human blood isolate Escherichia coli AL862 is carried by a 61-kb genomic region with characteristics typical of a pathogenicity island (PAI), including a size larger than 10 kb, the presence of an integrase-encoding gene, the insertion into a tRNA locus (pheR), and the presence of a small direct repeat at each extremity. Moreover, the G+C content of the afa-8 operon (46.4%) is lower than that of the E. coli K-12/MG1655 chromosome (50.8%). Within this PAI, designated PAI IAL862, we identified open reading frames able to code for products similar to proteins involved in sugar utilization. Four probes spanning these sequences hybridized with 74.3% of pathogenic afa-8-positive E. coli strains isolated from humans and animals, 25% of human pathogenic afa-8-negative E. coli strains, and only 8% of fecal strains (P = 0.05), indicating that these sequences are strongly associated with the afa-8 operon and that this genetic association may define a PAI widely distributed among human and animal afa-8-positive strains. One of the distinctive features of this study is that E. coli AL862 also carries another afa-8-containing PAI (PAI IIAL862), which appeared to be similar in size and genetic organization to PAI IAL862 and was inserted into the pheV gene. We investigated the insertion sites of afa-8-containing PAI in human and bovine pathogenic E. coli strains and found that this PAI preferentially inserted into the pheV gene.
Pathogenic Escherichia coli strains have the potential to cause a wide variety of infectious diseases, including septicemia, newborn meningitis, and intestinal and urinary tract infections (UTIs). These strains carry virulence-associated genes, which may encode toxins, capsules, invasins, adhesins, and other virulence factors that enable them to overcome host defenses, to proliferate, and to cause tissue damage and disease. These determinants are usually clustered on the chromosome in pathogenicity islands (PAIs) (24). The PAIs of uropathogenic strains were the first to be described in E. coli species. At least four PAIs are present in the genome of uropathogenic E. coli (UPEC) strain 536. PAI I536 and PAI II536 encode the hemolysin and the P-related fimbrial adhesin, while PAI III536 encodes the S fimbrial adhesin (9, 24, 25, 33) PAI IV536 carries the fyuA (ferrin yersiniabactin uptake) and irp1 through irp5 (iron-repressible protein) genes originally found in the PAI (HPI) of various Yersinia species (24). Two PAIs were described in UPEC strain J96 and reported to encode the hemolysin and the P or P-related fimbrial adhesins. PAI IIJ96 also encodes the cytotoxic necrotizing factor 1 (CNF1) (8, 55). One PAI (PAI ICFT073) has been identified in UPEC strain CFT073 and was reported to encode the hemolysin and the P fimbrial adhesin (23, 32). In diarrheagenic E. coli strains, several pathotypes have been reported to carry PAIs. Enteropathogenic E. coli (EPEC) strains carry the locus of enterocyte effacement (LEE) PAI (39, 40). Like EPEC, enterohemorrhagic E. coli (EHEC) strains are generally considered to contain the LEE (39, 44). Most of the enteroaggregative E. coli (EAEC) strains harbor the HPI of Yersinia enterocolitica (51). A potential PAI has been described in a prototypical enterotoxigenic E. coli (ETEC) strain, H10407, which contains a tia locus that mediates in vitro invasion into cultured intestinal epithelial cells (J. M. Fleckenstein, N. J. Snellings, E. A. Elsinghorst, and L. E. Lindler, Abstr. Meet. Microb. Pathogenesis Host Response, p. 37, 1997.)
Among the adhesins produced by pathogenic E. coli strains, afimbrial adhesins encoded by the afa family of gene clusters have been extensively studied (18, 19, 30, 34, 37, 42). We recently described a new afa operon (afa-8) encoding an afimbrial adhesin widespread among bovine E. coli isolates associated with diarrhea and/or septicemia (35) and human E. coli isolates associated with extraintestinal infections (20, 35; C. Le Bouguénec, L. Lalioui, L. du Merle, M. Jouve, P. Courcoux, S. Bouzari, R. Selvarangan, B. J. Nowicki, Y. Germani, A. Andremont, P. Gounon, and M. I. Garcia, submitted for publication). This gene cluster is chromosome or plasmid borne (20, 35), suggesting that it may be carried by a mobile element, facilitating its dissemination among pathogenic E. coli strains. Moreover, Garcia et al. (19) showed that the afa-3 gene cluster, carried by human pathogenic E. coli strains, is flanked by insertion sequence elements and is able to translocate from a plasmid to the chromosome by an IS1-mediated recombination mechanism.
The aim of this study was to investigate the possible association of the afa-8 operon with a PAI. Sequence analysis of the chromosomal regions downstream from the afa-8 operon identified a potential P4 integrase gene and a phenylalanine-specific tRNA gene, consistent with the definition of PAIs. Partial characterization of the afa-8-containing PAI from E. coli AL862 indicated that this PAI is a 61-kb chromosomal region that carries the afa-8 operon as the only known virulence determinant. Moreover, this afa-8-containing PAI has a distinctive feature: the ability to insert into the two tRNAPhe loci present on the chromosome of the same strain.
MATERIALS AND METHODS
Bacterial strains, cosmids, and culture conditions.
Four partially characterized collections of human E. coli strains were used in this study. The first consisted of 44 isolates from urine specimens, 18 of which carried the afa-8 operon (Le Bouguénec et al, submitted; C. Le Bouguénec, personal communication). The second collection consisted of 40 blood isolates from cancer patients (26), 14 of which carried the afa-8 operon (Le Bougénec et al., submitted). The third collection consisted of 16 strains isolated from stool specimens from children with diarrhea (21). The fourth collection consisted of 35 E. coli strains isolated from the feces of healthy volunteers (26).
We also studied 39 strains isolated from calves (36 strains) and piglets (3 strains) with intestinal and extraintestinal disorders. All 39 strains have been reported to carry the afa-8 operon (35; J. P. Girardeau, personal communication).
E. coli HB101 (10) was used as a host for maintaining cosmid clones. E. coli K-12/MG1655 (7) was used as a control for PCR assays.
The cosmid vector pHC79 (16) was used in cloning experiments.
E. coli strains were grown in Luria broth without glucose (10 g of tryptone, 5 g of yeast extract, and 5 g of NaCl per liter [pH 7.0]) or on Luria agar plates (containing 1.5% agar) at 37°C. E. coli-harboring cosmid clones were grown with 100 μg of carbenicillin per ml.
DNA analysis and genetic techniques.
Total plasmid DNA was extracted by the Kado method (31). Recombinant cosmids were routinely isolated by alkaline lysis (38), and whole-cell DNA was prepared by cesium chloride gradient (34). Standard procedures were used for restriction endonuclease digestions and other common DNA manipulations (38). Pulsed-field gel electrophoresis of genomic DNA from E. coli AL862, using restriction enzyme NotI, was performed as previously described (11). Primers, sequences, and the predicted sizes of the PCR products are given in Table 1. The cycling conditions were initial denaturation at 95°C for 5 min followed by 30 cycles at 95°C for 30 s, 65°C for 30 s, and 72°C for 1 min. For amplification of the iuC gene from the aerobactin operon, annealing was performed at 55°C. For amplification of afaE-8-pheR, afaE-8-yjdC, afaE-8-pheV, and afaE-8-yqgA regions, each cycle consisted of 1 min at 94°C, 1 min at 65°C, and 2 min at 72°C.
TABLE 1.
PCR primers used in this study
| Specificity | Nucleotide sequences of primers | Size of PCR product (bp) | Representative strain | Reference |
|---|---|---|---|---|
| afaE-8 | 5′-CTAACTTGCCATGCTGTGACAGTA-3′ | 302 | 239KH89 | 35 |
| 5′-TTATCCCCTGCGTAGTTGTGAATC-3′ | ||||
| iuC | 5′-AAACCTGGCTTACGCAACTGT-3′ | 269 | J96 | 6 |
| 5′-ACCCGTCTGCAAATCATGGAT-3′ | ||||
| sfaD/sfaEa,b | 5′-CGGAGGAGTAATTACAAACCTGGCA-3′ | 410 | J96 | 36 |
| 5′-CTCCGGAGAACTGGGTGCATCTTAC-3′ | ||||
| cadC | 5′-CCATTTTCAATCCAGTAAAGGG-3′ | 692 | MG1655 | This study |
| 5′-ATCAGCGCCAATACCGTGCTC-3′ | ||||
| cadC/pheRa | 5′-AGCCGCGCTTTGGTACAGTAGC-3′ | 823 | MG1655 | This study |
| 5′-CCGAACTCAACCAGATTCTCCCC-3′ | ||||
| cadC/yjdCa | 5′-AGCCGCGCTTTGGTACAGTAGC-3′ | 1,168 | MG1655 | This study |
| 5′-CAGATGGAACTGGTGCTGGAAGG-3′ | ||||
| afaE-8/inta | 5′-GATTCACAACTACGCAGGGG-3′ | 1,102 | 239KH89 | This study |
| 5′-CTGTCGCGTATTGACGGTTATAAAG-3′ | ||||
| afaE-8/pheRa | 5′-GATTCACAACTACGCAGGGG-3′ | 1,850 | 239KH89 | This study |
| 5′-CCGAACTCAACCAGATTCTCCCC-3′ | ||||
| afaE-8/yjdCa | 5′-GATTCACAACTACGCAGGGG-3′ | 2,255 | AL862 | This study |
| 5′-CAGATGGAACTGGTGCTGGAAGG-3′ | ||||
| int/pheRa | 5′-CTGAAGATGCCAGACTGTACGGC-3′ | 657 | 239KH89 | This study |
| 5′-CCGAACTCAACCAGATTCTCCCC-3′ | ||||
| int/yjdCa | 5′-CTGAAGATGCCAGACTGTACGGC-3′ | 2,065 | AL862 | This study |
| 5′-CAGATGGAACTGGTGCTGGAAGG-3′ | ||||
| pheR/yjdCa | 5′-GGGGAGAATCTGGTTGAGTTCGG-3′ | 495 | AL862 | This study |
| 5′-CAGATGGAACTGGTGCTGGAAGG-3′ | ||||
| afaE-8/yqgAa | 5′-GATTCACAACTACGCAGGGG-3′ | 2,200c | AL862 | This study |
| 5′-CCGGATTACGCATCTGTGGCATT-3′ | ||||
| afaE-8/pheVa | 5′GATTCACAACTACGCAGGGG-3′ | 1,800c | AL862 | This study |
| 5′-ATTTGATTGACGAGACGAGGCGAA-3′ | ||||
| Probe A | 5′-ATCAGATGCCTAAAGAAGGAGAAAC-3′ | 831 | AL862 | This study |
| 5′-CAATACTCGGATAAGATGATTGC-3′ | ||||
| Probe B | 5′-TTTGATGAGCGATGTACTTTCCGAA-3′ | 991 | AL862 | This study |
| 5′-GCAGATACAACGTGAACATACCGA-3′ | ||||
| Probe C | 5′-GGACGATAATGTGATCGTCTATAAG-3′ | 821 | AL862 | This study |
| 5′-GTGGAAGATACTCATCTGCTACACG-3′ | ||||
| Probe D | 5′-CTGCTCGGCAATGTCTTTGGTGC-3′ | 1,119 | AL862 | This study |
| 5′-CTGTGTACCAGATGCAAGGGCG-3′ | ||||
| Probe Ed | 5′-CCAATCAGACAGTCTTATCCCATC-3′ | 474 | AL862 | This study |
| 5′-GGGCGCAGGAAAGTCACCATCC-3′ |
Primer specific for a given region.
Detect sfa and foc sequences.
Sized approximately on agarose gel.
Deduced from sequencing of 576 bp at the right end of the insert of cosmid 3 (Fig. 4). This sequence displays no significant similarities to any known sequence in the database.
Cosmid library.
Genomic DNA was extracted from E. coli AL862 isolated from the blood of a cancer patient and partially digested with restriction endonuclease Sau3A. Restriction fragments (35 to 50 kb) were sized on a sucrose gradient (10 to 40%) and ligated to the BamHI-digested and alkaline phosphatase-treated cosmid vector pHC79 DNA as previously described (38). Cosmids were packaged in vitro into phage lambda particles by using the λ DNA in vitro packaging module (Stratagene, Austin, Tex.) and used to infect E. coli HB101. Carbenicillin-resistant HB101 transductants were screened by colony hybridization.
The absence of rearrangements of the inserts from the recombinant cosmids during the molecular cloning processes was confirmed by Southern blot hybridization experiments.
Hybridization.
Bacteria grown for 3 h on nitrocellulose filters were used for colony hybridization as described by Grunstein and Hogness (22). For Southern blot hybridization, total plasmid DNA and DNA restriction fragments were submitted to electrophoresis and transferred to nitrocellulose sheets (0.45-mm-diameter pore size; Schleicher and Schuell, Inc.) by the Southern blotting technique (53). Hybridization was performed under stringent conditions (65°C), with PCR products (Table 1) labeled with 32P by using the Megaprime DNA labeling system (Amersham International) as probes and signals detected by autoradiography with Amersham Hyperfilm-MP.
DNA sequencing.
Double-stranded DNA was sequenced by Big Dye Terminator chemistry (Perkin-Elmer Applied Biosystems, Foster, Calif.). For each cycle, the sequencing reaction mixture contained 16 μl of Big Dye Terminator mix, 13 pmol of primer, and 0.4 to 0.8 μg of DNA in a total volume of 40 μl. The cycling conditions were initial denaturation at 95°C for 5 min followed by 75 cycles at 95°C for 30 s, 55°C for 30 s, and 60°C for 4 min. Excess dye terminators were removed with a spin column (Millipore S.A., Molsheim, France), and reaction mixtures were dried in a vacuum system. Each sample was resuspended in 15 μl of template suppression reagent (TSR) and denatured by heating at 95°C for 2 min, and the entire volume was loaded on an ABI 310 automated DNA sequencing instrument (Perkin-Elmer). Sequence data were analyzed by ABI version 3.0.1b3 software. Sequences were screened for similarity to previously published sequences by using the computer programs BLASTN and BLASTX at the National Center for Biotechnology Information. Multiple alignments were performed with the CLUSTAL W program. We analyzed the partial sequence of the island for the presence of open reading frames (ORFs) of at least 45 codons.
Statistical analysis.
Proportions were compared by using the chi-square test.
Nucleotide sequence accession number.
The GenBank accession numbers for the sequences reported herein are AF072900, AF286670, and AF286671.
RESULTS
Analysis of the sequence of the afa-8 operon.
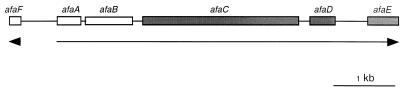
The recombinant cosmid pILL1211 was previously described as a cosmid carrying the afa-8 gene cluster cloned from the chromosome of the bovine pathogenic E. coli strain 239KH89 (35). A 4.2-kb sequence from this cosmid was published (accession no. AF072900) and reported to carry the afaC, afaD, and afaE genes encoding the outer membrane protein anchor (AfaC), the invasin (AfaD), and the afimbrial adhesin (AfaE), respectively (Fig. 1) (35). In this study, we completed the genetic characterization of the afa-8 operon by sequencing 2 kb upstream from the afaC gene. Computer analysis revealed three ORFs, ORF1, ORF2, and ORF3, which mapped to the same loci as and had similar sequences to the afaF, afaA, and afaB genes from the afa-3 operon (19), respectively (Fig. 1). These results confirmed that the genetic organization of the afa-8 operon was similar to that of the afa-3 operon.
FIG. 1.
Genetic organization of the afa-8 operon. White boxes indicate ORFs sequenced in this study. Gray boxes indicate ORFs previously sequenced (35). Arrows show the direction of gene transcription.
The first ORF, afaF, was transcribed in the opposite orientation to the other ORFs. It encoded a peptide of 62 amino acids (aa) with a molecular mass of 7.22 kDa, similar to those of the E. coli regulatory proteins DaaF (5) (67% identity and 74% similarity) and AfaF encoded by the afa-3 operon (19) (66% identity and 72% similarity). Five hundred ninety-nine base pairs downstream from the afaF gene was the afaA ORF, encoding a peptide of 127 aa with a calculated molecular mass of 14.4 kDa. This peptide exhibited homologies with E. coli regulators such as DaaA (5) (58% identity and 65% similarity), AfaA encoded by the afa-3 operon (19) (54% identity and 61% similarity), and PapB (3) (36% identity and 47% similarity). The afaA gene is followed by an 80-bp noncoding region and the afaB ORF, encoding a 255-aa protein similar to the periplasmic chaperone proteins involved in the biogenesis of bacterial adhesive structures. The predicted sequence of the AfaB product was similar to those of the AfaB protein involved in the production of the afimbrial adhesin AfaE-III (19) (66% identity and 71% similarity), the NfaE protein chaperone involved in the production of the nonfimbrial adhesin NFA-I (1) (63% identity and 69% similarity), and the AggD chaperone of EAEC (49) (59% identity and 66% similarity). A gram-negative pilus assembly chaperone motif (FPEDRESLQWLCVKGIPP) was found in the AfaB protein encoded by the afa-8 operon (28, 29, 58). A putative signal sequence was identified in the AfaB protein, resulting in a predicted mature peptide of 232 aa with a deduced molecular mass of 24.9 kDa and a pI of 8.71.
Determination and analysis of the complete sequence of the afa-8 operon (6,246 bp) showed that the G+C content of this operon (46.4%) was slightly different from that of the genome of E. coli MG1655 (50.8%) (7).
Analysis of sequence of the region located downstream from the afa-8 gene cluster.
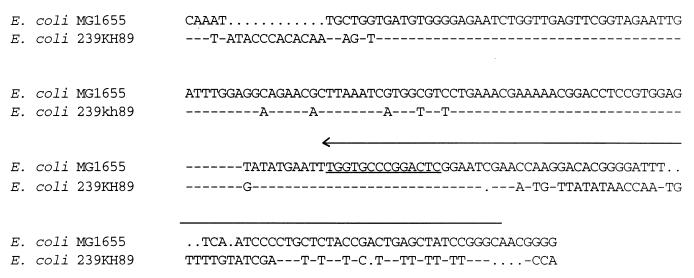
To determine whether the afa-8 operon was associated with a PAI, we sequenced 2 kb downstream from this operon in pILL1211. The afaE-8 gene was followed by a 300-bp noncoding region and a 1,263-bp ORF transcribed in the opposite orientation and encoding a putative protein of 421 aa. The deduced amino acid sequence of this putative protein displayed the highest percentage of identity and similarity with the E. coli MG1655 prophage P4 integrase (13) (67% identity and 74% similarity) and the bacteriophage P4 integrase (45) (54% identity and 66% similarity) (Fig. 2). This integrase-encoding gene (int gene) is intact and has two possible AUG start codons, but only the first is adjacent to a sequence resembling a ribosome-binding sequence (52; data not shown). Phage integration results from homologous recombination between the attachment site attB (20 bp) on the bacterial chromosome and an identical site (attP) on the phage chromosome (15). The site of bacteriophage P4 integration into the E. coli chromosome has been previously identified and was shown to reside within the leuX tRNA gene (45). Although not entirely identical to the attP gene, we identified, 197 bp downstream from the int gene, an attB-like site that displayed 14 identical nucleotides over a 20-bp sequence (Fig. 3). The int gene is followed by a noncoding region (219 bp) carrying a 136-bp sequence that is 95% identical to a region carrying the phenylalanine-specific tRNA-encoding gene (pheR) in E. coli MG1655 (Fig. 3). Only the 22 bp at the 3′ end of the 76 bp encoding the tRNAPhe were conserved (pheR′), with a single internal base pair deletion, and these residues carried the attB-like site (Fig. 3).
FIG. 2.
Comparison of predicted protein sequences for integrases of prophage P4 of E. coli MG1655, E. coli 239KH89, and bacteriophage P4. Shaded residues are identical to those from the integrase of prophage P4 of E. coli. Gaps have been inserted to optimize the alignment.
FIG. 3.
Sequence alignment of the 219-bp region downstream from the int gene in E. coli 239KH89 with the region carrying the phenylalanine-specific tRNA gene (pheR) in E. coli MG1655. pheR is indicated by a horizontal arrow above the sequence in the direction of transcription. Underlined nucleotides represent the attB-like site. Dashes represent identical nucleotides. Gaps have been inserted to optimize the alignment.
The G+C content of the afa-8 operon and the association of this operon with a P4 integrase-encoding gene and the pheR′ gene suggest that this gene cluster is carried by a PAI designated PAI I239KH89.
In E. coli K-12, the pheR gene is preceded by the lysine decarboxylase regulatory gene cadC and is immediately followed by the hypothetical yjdC gene and maps to position 94 min in the chromosome (2, 7, 13). Surprisingly, sequence analysis of the region downstream from the pheR′ gene in E. coli 239KH89 showed a noncoding region (900 bp) that was not similar to sequences in the database. Moreover, amplification and sequence analysis of the cad-yjdC region (Table 1) in strain 239KH89 showed the presence of an intact pheR gene, not disturbed by insertion (data not shown), suggesting that the chromosome of this strain carries at least two copies of the pheR gene, a complete copy between the cad and yjdC genes, and a truncated copy (pheR′) containing PAI I239KH89.
To characterize the afa-8-containing PAI, we selected E. coli AL862, a human blood isolate, carrying the afa-8 operon inserted into the pheR loci, near the yjdC gene, according to the PCR results (Table 1). This PAI was designated PAI IAL862.
Determination of the right junction of PAI IAL862.
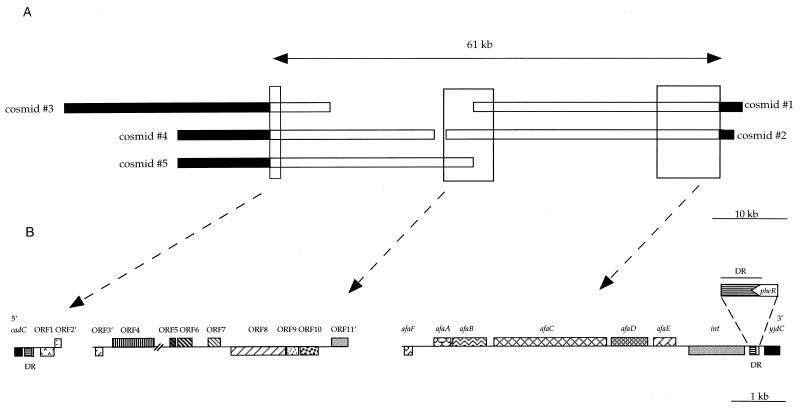
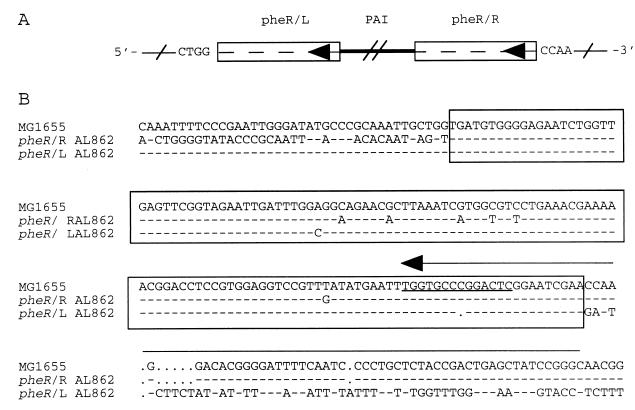
Three hundred recombinant clones from the cosmid library of E. coli AL862 were screened by colony hybridization with the afaE-8 gene as a probe (Table 1) to identify the cosmids carrying the right junction of PAI IAL862 with E. coli K-12-type sequences. Two positive cosmids (cosmids 1 and 2) (Fig. 4A) were selected for further studies. Analysis of the sequences downstream from the afa-8 operon on cosmid 1 revealed the presence of a 300-bp noncoding region followed by an integrase-encoding gene identical (100% identity) to those described in E. coli 239KH89. Downstream from the int gene, we identified the 76-bp sequence encoding the tRNAPhe and carrying the attB-like site at its 3′ end (Fig. 5B), which was followed by the hypothetical yjdC gene.
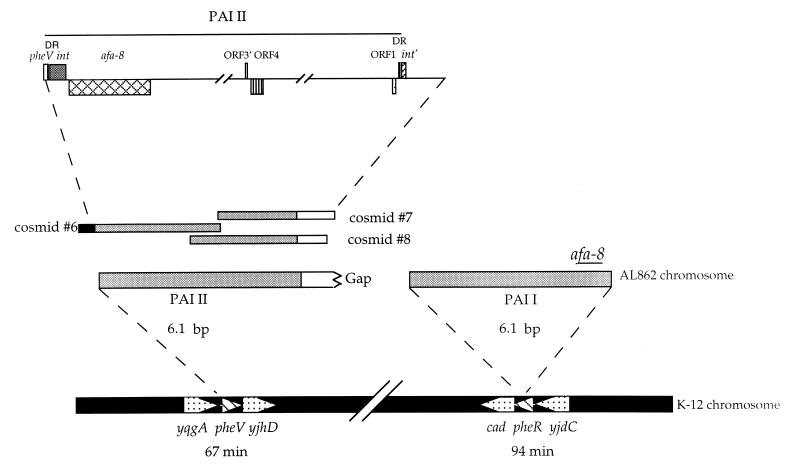
FIG. 4.
Partial structure of PAI IAL862. (A) Schematic diagram of the inserts of overlapping cosmids derived from PAI IAL862. The solid bars represent the E. coli K-12 chromosome. PAI IAL862-specific DNA is represented by white bars. The estimated size of PAI IAL862 is shown above. (B) ORFs deduced from the sequences at the left (5′) and right (3′) ends as well as the central region of PAI IAL862 are represented by boxes. Direction of transcription (left or right) is indicated by boxes (below and above the line, respectively). DR, direct repeat. Cosmids 1, 2, 3, 4, and 5 correspond to pILL1259, pILL1255, pILL1267, pILL1269, and pILL1270, respectively.
FIG. 5.
Schematic diagram and nucleotide sequences of the junction sites of PAI IAL862. (A) The boxes represent the 136-bp direct repeat with the left and right junctions indicated (see below); the 5′- and 3′-end nucleotides are shown for reference. The single diagonal lines represent the chromosomal DNA of E. coli K-12. The arrows represente pheR sequences. The double diagonal lines represent intervening PAI-specific DNA. (B) pheR regions were derived from E. coli MG1655. pheR/L and pheR/R, represent the left and right junction sequences of PAI IAL862, respectively. pheR is indicated by a horizontal arrow above the sequence in the direction of transcription. Underlined nucleotides indicate the attB-like site. The 136-bp direct repeat sequence is shown in boxes. Dashes represent nucleotides identical to the sequence of E. coli MG1655. Gaps have been inserted to optimize the alignment.
Determination of the left junction of PAI IAL862.
To identify the left junction of PAI IAL862, an internal fragment of the cadC gene was amplified (Table 1) and used as a probe to screen, by DNA hybridization, 200 recombinant cosmids from the cosmid library of E. coli AL862. Three positive cosmids (cosmids 3, 4, and 5) (Fig. 4A) were selected for further studies. An oligonucleotide primer, internal to the cadC gene, was used to sequence the PAI IAL862-specific DNA sequences from the left junction. The cadC gene was found to be followed by a 136-bp nearly perfect duplication of the right junction (Fig. 4B and 5). A segment consisting of 22 bp of the 3′ end of the pheR gene with a single internal base pair deletion, carrying the attB-like site, was found at the left junction (Fig. 5B). A 204-bp noncoding region, immediately adjacent to the duplicated region within the PAI, showed no similarity to sequences in the database. This noncoding region is followed by two ORFs (ORF1 completely sequenced and ORF2 partially sequenced) (Fig. 4B). The sequence of the N terminus (48 aa over 88 aa) of the putative protein encoded by ORF1 was very similar (91% identity and 97% similarity) to the 61-aa product of ORF L12 previously described in a putative P4 family prophage of the LEE of the EHEC strain EDL933 (44) (Table 2). The product of ORF2′, which was partially sequenced (141 bp), was truncated by a stop codon and exhibited 80% identity (88% similarity) to the C terminus of a putative product of ORF L11 in the same prophage (Table 2).
TABLE 2.
ORFs identified by partial sequencing of PAI IAL862
| ORF | Predicted size (aa) | Characteristic of homologous protein
|
||||
|---|---|---|---|---|---|---|
| Function | Species (strain) | Length (aa) | %Identity/ % similarity | Accession no. (reference) | ||
| ORF1 | 88 | L12 putative protein | Escherichia coli (EDL933) | 61 | 91/97 | AAC31491 (44) |
| ORF2′ | >47 | L11 putative protein | Escherichia coli (EDL933) | 76 | 80/88 | AAC31490 (44) |
| ORF3′ | >57 | DeoR transcriptional repressor of deoxyribose operon | Escherichia coli (MG1655) | 252 | 58/71 | P06215 (41, 56, 57) |
| DeoR transcriptional activator | S. enterica serovar Typhimurium | 252 | 53/69 | AAB80741 | ||
| ORF4 | 314 | RbsK putative ribokinase | Schizosaccharomyces pombe | 318 | 36/46 | O60116 |
| RbsK ribokinase | Lactobacillus sakei | 302 | 34/42 | AAD34338 (54) | ||
| RbsK putative ribokinase | Haemophilus influenzae | 306 | 33/43 | P44331 (17) | ||
| RbsK ribokinase | Escherichia coli (MG1655) | 309 | 32/41 | P05054 (27) | ||
| ORF5 | 48 | No significant similarities | ||||
| ORF6 | 111 | No significant similarities | ||||
| ORF7 | 94 | L13 IS2-like protein | Escherichia coli (EDL933) | 133 | 37/48 | AAC31492 (44) |
| Transposase | Burkholderia glumae | 401 | 27/39 | BAA24920 | ||
| ORF8 | 418 | Putative integral membrane protein | Streptomyces coelicolor | 516 | 49/61 | CAB52363 (47) |
| SgaT putative transport protein | Escherichia coli (MG1655) | 484 | 30/40 | P39301 (13, 48) | ||
| ORF9 | 95 | Hypothetical protein SCJ21.24c | Streptomyces coelicolor | >150 | 35/46 | CAB52364 (47) |
| B component (PtxB) of a putative phosphotransferase enzyme II | Escherichia coli (MG1655) | 101 | 25/36 | P39302 (13, 48) | ||
| ORF10 | 147 | A component (PtyA) of a putative phosphotransferase enzyme II | Escherichia coli (MG1655) | 147 | 40/52 | P32058 (13, 48) |
| A component (PtxA) of a putative phosphotransferase enzyme II | Escherichia coli (MG1655) | 154 | 36/49 | P39303 (13, 48) | ||
| A component (SgcA) of a putative phosphotransferase enzyme II | Escherichia coli (MG1655) | 143 | 36/47 | P39363 (13, 48) | ||
| ORF11′ | >124 | Regulator of gluconate operon | Escherichia coli (MG1655) | 313 | 38/47 | AAC6463 (7) |
| Gluconate repressor | Pseudomonas aeruginosa | 315 | 36/47 | AAD01801 | ||
Determination of the size of PAI IAL862.
A walking method, involving the hybridization of probes derived from the sequences of the ends of cosmids carrying the right and left junctions to filters containing cosmids from the library, was used to order and to identify overlapping clones harboring PAI sequences in the library. Positive hybridization was confirmed by PCR. One miniset of five overlapping cosmid clones covers the afa-8-containing PAI, including the right and left junctions and giving a total size of approximately 61 kb (Fig. 4A).
Virulence factors carried by PAI IAL862.
In addition to the afa-8 operon, E. coli AL862 carries sfa/foc sequences encoding the fimbrial adhesin of the S family and the iuC gene from the aerobactin-encoding operon (Table 1). Hybridization and PCR assays showed that none of these determinants is carried by PAI IAL862, suggesting that the afimbrial AfaE-VIII adhesin is the only known virulence factor encoded by this island.
Partial characterization of PAI IAL862 was initiated by sequencing the 3′ ends of the inserts of cosmids 1 and 2 and the right end of the insert of cosmid 5 (Fig. 4A). Analysis of the sequence of these regions led to the identification of seven complete ORFs (ORF4 to -10) and two partial ORFs (ORF3′ and -11′) (Fig. 4B and Table 2). Comparison of the products of these ORFs to the sequences from the database showed that PAI IAL862 contains a high density of genes that may be involved in sugar utilization: the ORF3′ to ORF4 products showed homologies with proteins involved in ribose metabolism, while ORF8 to ORF11′ products showed homologies with proteins of phosphotransferase systems. The ORF7 product was similar to the L13 IS2-like protein previously described in a putative P4 family prophage of the LEE PAI of the EHEC EDL933. The ORF5 and ORF6 products showed no similarity to any known protein in the database.
Although the putative proteins encoded by the central region of PAI IAL862 were similar to proteins mainly described in E. coli MG1655, the nucleotide sequences of this region showed no similarity to the sequence genome of this strain. Comparison of these sequences with unfinished genome sequences from the database revealed that the region carrying ORF3′ to ORF6 is highly similar (81 to 95% identity) to a chromosomal DNA sequence of Salmonella enterica serovar Typhimurium. Despite this high level of similarity, the G+C content of this region (39.7%) differs from that of the Salmonella genome (52%) (43). The region comprising ORF8 to ORF11′ was very similar (81% identity) to a chromosomal DNA sequence of Yersinia pestis and had a similar G+C content (46.6%) (4).
PAI IAL862 is duplicated on the chromosome of E. coli AL862.
Hybridization assays with total DNA from E. coli AL862 digested with NotI, with the afaE gene from the afa-8 operon used as a probe, revealed two hybridizing fragments greater than 300 kb in size, suggesting that there are two copies of this operon on the chromosome of E. coli AL862 (data not shown). The absence of hybridization on total plasmid DNA of E. coli AL862 with the same probe confirmed the chromosomal location of the two copies of the afa-8 operon. Moreover, analysis of E. coli AL862 cosmid library also showed other cosmids that hybridized with the regions carrying ORF4 to ORF11 from PAI IAL862 (Fig. 4B) and carried the afa-8 operon, but tested negative for the yjdC gene. Sequence analysis of one of these cosmids (cosmid no. 6) (Fig. 6) confirmed the presence of an int gene, identical (100% identity over 440 bp) to that found in the right boundary of PAI I, followed by the phenylalanine-specific tRNA pheV gene and the hypothetical yqgA gene. In E. coli K-12, the pheV gene maps to position 67 min on the chromosome (7), between the yqgA and yghD genes (7, 46). We investigated whether the two copies of the afa-8 operon were present on the chromosome of the same clone of E. coli AL862, by using PCR to test 12 single colonies for genetic associations of the afaE-8 gene with yjdC and of the afaE-8 gene with yqgA (Table 1). Both PCRs were positive for all 12 colonies, indicating that the chromosome of E. coli AL862 carries two afa-8-carrying PAIs: one inserted into the pheR gene (PAI IAL862) and a second inserted into the pheV gene, designated PAI IIAL862.
FIG. 6.
Schematic diagram showing the positions of PAI I and PAI II from E. coli AL862. The gray bars represent sequences of PAI I and PAI II. The black bars represent the E. coli K-12 chromosome. The stippled arrows within the black bars indicate the tRNAPhe genes, and the boxes indicate the yqgA, yjhD, cadC, and yjdC genes, showing their orientation and their position on the E. coli K-12 chromosome. The PAIs and their estimated sizes are shown above the E. coli K-12 chromosome map. DR, direct repeat. Cosmids 7, 8, and 9 correspond to pILL1254, pILL1266, and pILL1271, respectively.
The right junction of PAI IIAL862 was determined by analysis of E. coli AL862 cosmid library. This revealed cosmids 7 and 8 (Fig. 6), which carried ORF1, ORF3′, and ORF4 previously described in the PAI IAL862, but not the cadC gene. These data confirmed that the insertion sites of PAI I and PAI II are different and suggested that these two PAIs are similar. Moreover, reciprocal hybridizations between restriction fragments from the cosmids carrying PAI I (Fig. 4A) and the cosmids carrying PAI II (Fig. 6) indicated that the two sets of cosmids shared a region spanning PAI I. An oligonucleotide primer designated in ORF1 was used to identify the right boundary of PAI II. Sequence analysis of cosmid 7 (Fig. 6) revealed 448 bp, carrying the 3′ end of ORF1 followed by a 204-bp noncoding region identical (100% identity) to those found in the left junction of PAI I. Immediately downstream from the noncoding region, we identified a segment consisting of 22 bp of the 3′ end of the tRNAPhe-encoding gene, which carries the attB-like site. Forty-three base pairs downstream from this short repeat, we found instead of the yghD gene another ORF, partially sequenced (138 bp), able to encode a putative protein similar to the N termini of integrases previously described in the LEE PAI of EHEC strain EDL933 (54% identity and 69% similarity) (44) and the HPI of Yersinia pseudotuberculosis (63% identity and 76% similarity) (11). It appears that the left junction of PAI IIAL862 is not adjacent to the sequences described in E. coli K-12. Moreover, the sequence of the 3′ ends of cosmids 7 and 8 showed no similarity to sequences in the database.
Analysis of the distribution of afa-8-carrying PAI.
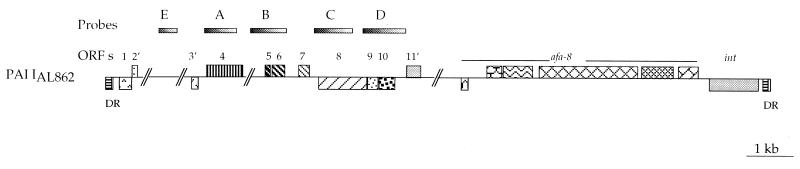
To determine whether sequences within the PAI IAL862 were specific for pathogenic E. coli strains carrying the afa-8 operon, we investigated the frequency of occurrence of the A, B, C, and D regions (Fig. 7 and Table 3). These regions were amplified (Table 1) and used as probes to screen by colony hybridization three collections of clinical isolates. These collections comprised 70 afa-8-positive strains isolated from animals (calves and piglets) with diarrhea or septicemia and from humans with UTI or septicemia; 68 afa-8-negative clinical isolates from humans with septicemia, UTI, or diarrhea; and 35 afa-8-negative strains isolated from healthy individuals. The distribution of the A, B, C, and D regions in strains from various origins and the correlation of these regions with the presence of the afa-8 operon are shown in Fig. 7 and Table 3. The four probes were detected more frequently in pathogenic strains, regardless of whether or not these strains carried the afa-8 operon, than in strains isolated from healthy individuals. The proportion of pathogenic strains testing positive with all of the probes was significantly higher for afa-8-positive strains (74.3%) than for afa-8-negative strains (25%) (P = 0.05). This difference was confirmed by hybridization experiments of ABCD-positive strains with probe E (Fig. 7), which reacted with 100% of the afa-8-positive strains and with only 65% of afa-8-negative strains. In contrast, the proportion of strains testing negative with all of the A, B, C, and D probes was significantly higher in nonpathogenic strains and pathogenic afa-8-negative strains (71.5% and 63.2%, respectively) than in pathogenic afa-8-positive strains (17.1%). Interestingly, hybridization experiments with plasmid DNA isolated from the latter strains with the afaE probe indicated in all of these strains that the afa-8 operon is plasmid borne. All of these data strongly suggested the presence of a genetic element similar to PAI IAL862 in most of the afa-8-positive pathogenic strains.
FIG. 7.
Distribution of sequences from PAI IAL862 among pathogenic and nonpathogenic E. coli strains. Regions from PAI IAL862 used as probes are shown. Results of hybridization experiments are shown in Table 3.
TABLE 3.
Results of hybridization studies
| Strain type (n) | No. (%) of isolates
that hybridized with probe(s)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ABCD | BCD | ABD | ABC | CD | AB | A | B | C | D | None | |
| Pathogenic | |||||||||||
| afa-8 positive (70) | 52 (74.3)ab | 2 (2.9) | 1 (1.4) | 0 (0) | 1 (1.4) | 0 (0) | 1 (1.4) | 0 (0) | 1 (1.4) | 0 (0) | 12 (17.1)c |
| afa-8 negative (68) | 17 (25)b | 0 (0) | 0 (0) | 2 (2.9) | 0 (0) | 3 (4.4) | 1 (1.5) | 1 (1.5) | 0 (0) | 1 (1.5) | 43 (63.2) |
| Nonpathogenic (35) | 3 (8.5)b | 1 (3) | 0 (0) | 0 (0) | 2 (5.5) | 0 (0) | 4 (11.5) | 0 (0) | 0 (0) | 0 (0) | 25 (71.5) |
Including strain 239KH89.
Among the ABCD-positive strains, 100% of the afa-8-positive, 65% of the afa-8-negative, and 33% of the nonpathogenic strains also hybridized with the E probe.
Strains carrying the afa-8 gene cluster on a plasmid (this study; J. Gérardin, personal communication).
Variability in the chromosomal location of afa-8-carrying PAI.
Sixteen strains (7 bovine diarrheagenic strains and 9 human blood isolates) that reacted with the A, B, C, and D probes and tested positive for the afaE-8–int genetic association were used to investigate the chromosomal location of the afa-8-containing PAI. Genetic association of the afa-8 operon with either pheR or pheV or yjdC or yqgA was investigated with several set of primers, as indicated in Table 1. In one human strain, PCR results suggested that the afa-8 operon inserted into the pheR gene next to the yjdC gene, indicating that, like PAI IAL862, the afa-8-containing PAI mapped to position 94 min on the chromosome. In 13 strains (7 human and 6 bovine), PCR results suggested that the afa-8 operon inserted into the pheV gene next to the yqgA gene, indicating that, like PAI IIAL862, the afa-8-containing PAIs mapped to position 67 min on the chromosome. In the two remaining strains (one human and one bovine), although the afa-8 operon inserted into the pheR gene, it was not associated with the yjdC gene. These results suggested that as in E. coli 239KH89, afa-8-containing PAI was located at an unknown position on the chromosome. The chromosomal location of the afa-8-carrying PAI in strain 239KH89 was further investigated by sequencing of 4 kb downstream from the pheR gene, on cosmid pILL1211. This revealed a 947-bp noncoding region followed by nine ORFs. The products of ORF1, ORF2, ORF3, ORF4, ORF5, ORF6, and ORF7 displayed significant similarities to the peptides encoded by the L12 (78% identity), L11 (73% identity), L10 (73% identity), L9 (88% identity), L8 (90% identity), and L7 (95% identity) ORFs, respectively, previously described in the putative P4 family prophage carried by the LEE PAI of EHEC strain EDL933 (44). L7 and L8 were related to genes found only in the P4-like family of cryptic prophages from E. coli K-12, whereas L9 to L12 were completely unknown (44). The products of ORF2 and ORF4 were truncated by a stop codon and a frameshift, respectively. The products of ORF6 and ORF7 also matched two hypothetical proteins of E. coli MG1655, YeeW (63% identity) and YeeV (88% identity), respectively. The products of ORF8, ORF9, and ORF10 were similar to YeeU (89% identity), YeeT (94% identity), and YeeS (98% identity) of E. coli MG1655, respectively (7). The genes encoding the putative proteins YeeW to YeeS are contiguous on the chromosome of E. coli MG1655 and map to position 45 min.
All of these data taken together indicate the variability of the chromosomal location of afa-8 containing PAIs and suggest that these PAIs are preferentially inserted into the pheV gene.
DISCUSSION
Genes encoding important virulence factors are often located on mobile genetic elements such as phages, plasmids, transposons, and PAIs. They may therefore be transferred from one cell to another, and this horizontal transfer represents a key genetic mechanism in the evolution of pathogens. E. coli represents an example of a pathogen that has developed by lateral gene transfer. Several PAIs carrying virulence genes introduced into the genome via lateral transfer have been described in pathogenic E. coli strains associated with intestinal or extraintestinal infections in humans. In previous studies, we have described a new afa-8 operon, encoding an afimbrial adhesin (AfaE-VIII), carried by bovine E. coli strains associated with diarrhea or septicemia (35) and human E. coli isolates associated with extraintestinal infections (20, 35). This operon may be borne on a plasmid or on the chromosome (20, 35), suggesting that it is associated with mobility genes. In this report, we used E. coli AL862, a human blood isolate, as a prototype afa-8-carrying E. coli strain. We studied a putative PAI carrying this operon to identify and to improve our understanding of the mechanisms involved in dissemination of the afa-8 operon among pathogenic isolates.
The results reported here indicate that the afa-8 operon is carried by a genomic region that fits within the category of PAIs as defined by Hacker et al. (25). In strain AL862, the afa-8 operon is (i) located within a 61-kb chromosomal region, (ii) in the vicinity of a mobility gene (int gene), or (iii) associated with the phenylalanine-specific tRNA gene (pheR). Moreover, the G+C content of the afa-8 operon, which has been completely sequenced in the bovine pathogenic E. coli strain 239KH89, is slightly lower (46.4%) than that of the chromosome of E. coli MG1655 (50.8%) (7). This new PAI was designated PAI IAL862. The presence of a putative integrase gene, highly similar to that of bacteriophage P4, and a pheR gene at the right extremity of this PAI, as well as a 14-bp sequence resembling the attP site of bacteriophage P4 at both the right and left extremities, strongly argues in favor of the hypothesis that PAI IAL862 was acquired via horizontal transfer from a bacteriophage.
E. coli AL862 carries the sfa/foc sequences, encoding a fimbrial adhesin, and the iuC gene from the aerobactin-encoding operon, but none of these determinants is carried by PAI IAL862, indicating that AfaE-VIII adhesin is the only known virulence factor encoded by this new island. However, the partial nucleotide sequence of PAI IAL862 revealed new ORFs with sequences similar to those of determinants encoding proteins involved in the utilization of various sugars. These regions showed heterogeneous G+C contents and were similar to sequences from different bacteria (S. enterica serovar Typhimurium and Y. pestis), suggesting a stepwise acquisition of these DNA fragments from heterogeneous sources, leading to the mosaic-like structure of this island. These sequences, as assayed by colony hybridization, are highly frequent in afa-8-positive strains, (81.5%), less frequent in human pathogenic afa-8-negative strains (25%), and generally absent from strains isolated from healthy individuals and considered to be nonpathogenic. We therefore suggest that these newly described genes are particularly found in pathogenic isolates and are preferentially associated with the afa-8 operon. They probably define PAIs similar to PAI IAL862. These new sequences may contribute to the survival of the strains in certain ecological niches and do not directly contribute to host damage and infection.
One of the interesting features revealed by analysis of the E. coli AL862 cosmid library is that the chromosome of this strain carries two afa-8-containing PAIs: PAI I, described above and located in the vicinity of the pheR gene; and PAI II, located in the vicinity of the pheV gene. Similarly, the PAI I and PAI II of UPEC strain J96 were inserted into the pheV and pheR genes, respectively. These two PAIs differ in size and in the virulence factors they encode (8, 9, 55). In contrast, the PAI I and PAI II of E. coli AL862 are similar in size and genetic organization. In addition, they have identical sequences at their extremities, suggesting at a first approximation that the afa-8-containing PAI is present in two copies on the chromosome of E. coli AL862. To our knowledge, this is the first report of such a phenomenon. Buchrieser et al. (11) previously reported insertion of the HPI into any of the three asn tRNA genes present on the bacterial chromosome of Y. pseudotuberculosis, but insertion occurred in different variants of the same serotype and not in the same variant. Further studies are necessary to confirm that PAI I and PAI II from E. coli AL862 are similar along their entire length. Unlike PAI IAL862, both extremities of which were adjacent to the E. coli K-12 chromosome, the right junction of PAI IIAL862 was adjacent to a putative integrase-encoding gene and to other unknown sequences. It is likely that PAI IIAL862 is adjacent to an extrachromosomal segment that may define a putative PAI, designated PAI IIIAL862. Further studies are required to determine whether this putative PAI carries the aer operon and the sfa/ foc sequences. E. coli AL862 also carries the fyuA, irp1, and irp2 genes (Girardeau, personal communication) found in the HPIs of various Yersinia species (11, 12, 14) and various pathotypes of E. coli (50, 51), as well as a disturbed asnT locus, suggesting that HPI has been acquired by this strain. All of these data suggest that the chromosome of E. coli AL862 carries at least four PAIs (PAIs I, II, and III and an HPI) and confirms the capacity of E. coli species to evolve by horizontal gene transfer.
Analysis of the chromosomal location of afa-8-containing PAI in pathogenic E. coli strains indicated that this PAI inserted preferentially into the pheV gene, rather than the pheR gene. It is well known that the pheR gene is followed by the cadC gene on the chromosome of the prototype E. coli strain MG1655 (7). However, analysis of the sequence of the insertion site of the afa-8-containing PAI in the bovine E. coli strain 239KH89 indicated that this PAI is adjacent to a truncated copy of the pheR gene (pheR′) and mapped to position 45 min on the chromosome. Sequence analysis suggested that this truncated copy of the pheR gene corresponded to the left junction of a remnant PAI carrying ORFs similar to those previously described in the putative prophage of the LEE PAI of E. coli EDL933, rather than to the right junction of the afa-8-containing PAI. The significance of the integration of this PAI into this truncated pheR gene is currently unclear, but suggests that, as for the PAI II of E. coli AL862, the adjacent regions of this PAI may have undergone considerable recombination over time. Whether such events occurred before or after the acquisition of afa-8-containing PAI is unclear. Differences in the chromosomal location of PAIs carrying the afa-8 operon indicate diversity in PAI evolution. These PAIs probably have a common ancestor that inserted into the pheR and pheV loci of E. coli. The possibility that these PAIs also inserted into other tRNA-encoding genes should not be eliminated. The identification of an intact int gene, which presumably encodes a functional integrase protein, suggests that this integrase is involved in the mobility of these PAIs. In addition, direct repeat elements flanking PAIs may act as targets for specific recombinases, thereby playing an important role in the integration and/or excision of PAIs. Interestingly, PAI IAL862 is flanked by a 136-bp imperfect direct repeat carrying an attB-like site. We therefore suggest that PAI IAL862 has probably retained the capacity to excise from the chromosome.
In summary, we found that the afa-8 operon of the human blood isolate AL862 is carried by a 61-kb PAI (PAI IAL862) integrated into the pheR gene and possesses several characteristics suggestive of potential mobility. We also demonstrated that E. coli AL862 contains another afa-8-containing PAI, probably similar to PAI I, integrated into the pheV gene. Finally, we report that the afa-8-containing PAIs from human and bovine isolates are preferentially inserted into the pheV gene. Determination of the other genes carried by the afa-8-containing PAIs will be an interesting field for future research.
ACKNOWLEDGMENTS
We are grateful to A. Labigne, in whose unit this work was carried out, for continuing interest and helpful discussions. We also thank Y. Germani (Pasteur Institute, Bangui, Republic of Central Africa) for the gift of E. coli diarrheal isolates, J. P. Girardeau (INRA, Clermont Ferrand-Theix, France) and J. Mainil (Bacteriology, Faculty of Veterinary Medicine, University of Liege, Liege, Belgium) for the gift of animal E. coli isolates, and A. Andremont (Bichat Claude-Bernard Hospital, Paris, France) for the gift of human blood E. coli isolates. We thank J. Hacker for critical reading of the manuscript and E. Carniel, in whose laboratory the pulsed-field gel electrophoresis assays were performed, for helpful discussions. We also thank L. du Merle for technical assistance.
This work was supported by grant 1335 from the European Community program FAIR and a grant from the Programme de Recherche Fondamentale en Microbiologie et Maladies Infectieuses et Parasitaires (PRFMMIP-MENRT). L. Lalioui received a fellowship from the Marcel Mérieux Fondation and the Fondation pour La Recherche Médicale.
REFERENCES
- 1.Ahrens R, Ott M, Ritter A, Hoschützky H, Bühler T, Lottspeich F, Boulnois G J, Jann K, Hacker J. Genetic analysis of the gene cluster encoding nonfimbrial adhesin I from an Escherichia coliuropathogen. Infect Immun. 1993;61:2505–2512. doi: 10.1128/iai.61.6.2505-2512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann B J. Linkage map of Escherichia coliK-12, edition 8. Microbiol Rev. 1990;54:130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baga M, Goransson M, Normark S, Uhlin B E. Transcriptional activation of a pap pilus virulence operon from uropathogenic Escherichia coli. EMBO J. 1985;4:3887–3893. doi: 10.1002/j.1460-2075.1985.tb04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bercovier H, Mollaret H H. Yersinia. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams & Wilkins; 1984. pp. 498–506. [Google Scholar]
- 5.Bilge S S, Apostol J M, Jr, Fullner K J, Moseley S L. Transcriptional organization of the F1845 fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1993;7:993–1006. doi: 10.1111/j.1365-2958.1993.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 6.Bingen E, Picard B, Brahimi N, Mathy S, Desjardins P, Elion J, Denamur E. Phylogenetic analysis of Escherichia colistrains causing neonatal meningitis suggests horizontal gene transfer from a predominant pool of highly virulent B2 group strains. J Infect Dis. 1998;177:642–650. doi: 10.1086/514217. [DOI] [PubMed] [Google Scholar]
- 7.Blattner F R, Plunkett G R, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coliK-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 8.Blum G, Falbo V, Caprioli A, Hacker J. Gene clusters encoding the cytotoxic necrotizing factor type 1, Prs-fimbriae and alpha-hemolysin form the pathogenicity island II of the uropathogenic Escherichia colistrain J96. FEMS Microbiol Lett. 1995;126:189–195. doi: 10.1111/j.1574-6968.1995.tb07415.x. [DOI] [PubMed] [Google Scholar]
- 9.Blum G, Ott M, Lischewski A, Ritter A, Imrich H, Tschäpe H, Hacker J. Excision of large DNA regions termed pathogenicity islands from tRNA-specific loci in the chromosome of an Escherichia coliwild-type pathogen. Infect Immun. 1994;62:606–614. doi: 10.1128/iai.62.2.606-614.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Microbiol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 11.Buchrieser C, Brosch R, Bach S, Guiyoule A, Carniel E. The high-pathogenicity island of Yersinia pseudotuberculosis can be inserted into any of the three chromosomal asntRNA genes. Mol Microbiol. 1998;30:965–978. doi: 10.1046/j.1365-2958.1998.01124.x. [DOI] [PubMed] [Google Scholar]
- 12.Buchrieser C, Rusniok C, Frangeul L, Couve E, Billault A, Kunst F, Carniel E, Glaser P. The 102-kilobase pgm locus of Yersinia pestis: sequence analysis and comparison of selected regions among different Yersinia pestis and Yersinia pseudotuberculosisstrains. Infect Immun. 1998;67:4851–4861. doi: 10.1128/iai.67.9.4851-4861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burland V, Plunkett G R, Sofia H J, Daniels D L, Blattner F R. Analysis of the Escherichia coligenome VI: DNA sequence of the region from 92.8 through 100 minutes. Nucleic Acids Res. 1995;23:2105–2119. doi: 10.1093/nar/23.12.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carniel E, Guilvout I, Prentice M. Characterization of a large chromosomal “high-pathogenicity island” in biotype 1B Yersinia enterocolitica. J Bacteriol. 1996;178:6743–6751. doi: 10.1128/jb.178.23.6743-6751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheetham B F, Katz M E. A role for bacteriophages in the evolution and transfer of bacterial virulence determinants. Mol Microbiol. 1995;18:201–208. doi: 10.1111/j.1365-2958.1995.mmi_18020201.x. [DOI] [PubMed] [Google Scholar]
- 16.Collins J. Escherichia coli plasmids packageable in vitroin lambda bacteriophage particles. Methods Enzymol. 1979;68:309–326. doi: 10.1016/0076-6879(79)68022-9. [DOI] [PubMed] [Google Scholar]
- 17.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 18.Garcia M I, Gounon P, Courcoux P, Labigne A, Le Bouguénec C. The afimbrial adhesive sheath encoded by the afa-3 gene cluster of pathogenic Escherichia coliis composed of two adhesins. Mol Microbiol. 1996;19:683–693. doi: 10.1046/j.1365-2958.1996.394935.x. [DOI] [PubMed] [Google Scholar]
- 19.Garcia M-I, Labigne A, Le Bouguenec C. Nucleotide sequence of the afimbrial-adhesin-encoding afa-3 gene cluster and its translocation via flanking IS1insertion sequences. J Bacteriol. 1994;176:7601–7613. doi: 10.1128/jb.176.24.7601-7613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gérardin J, Lalioui L, Jacquemin E, Le Bouguénec C, Mainil J G. The afa-related gene cluster in necrotoxigenic and other Escherichia coli from animals belongs to the afa-8variant. Vet Microbiol. 2000;76:155–184. doi: 10.1016/s0378-1135(00)00234-0. [DOI] [PubMed] [Google Scholar]
- 21.Germani Y, Morillon M, Begaud E, Dubourdieu H, Costa R, Thevenon J. Two-year study of endemic enteric pathogens associated with acute diarrhea in New Caledonia. J Clin Microbiol. 1994;32:1532–1536. doi: 10.1128/jcm.32.6.1532-1536.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunstein M, Hogness D S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci USA. 1975;72:3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyer D M, Kao J-S, Mobley H L T. Genomic analysis of a pathogenicity island in uropathogenic Escherichia coliCFT073: distribution of homologous sequences among isolates from patients with pyelonephritis, cystitis, and catheter-associated bacteriuria and from fecal samples. Infect Immun. 1998;66:4411–4415. doi: 10.1128/iai.66.9.4411-4417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hacker J, Blum-Oehler G, Janke B, Nagy G, Goebel W. Pathogenicity islands of extraintestinal Escherichia coli. In: Kaper J, Hacker J, editors. Pathogenicity islands and other mobile virulence elements. Washington, D.C.: ASM Press; 1999. pp. 59–76. [Google Scholar]
- 25.Hacker J, Blum-Oehler G, Muhldorfer I, Tschape H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 26.Hilali F, Ruimy R, Saulnier P, Barnabé B, Le Bouguénec C, Tibayrenc M, Andremont A. Prevalence of virulence genes and clonality in Escherichia colistrains that cause bacteremia in cancer patients. Infect Immun. 2000;68:3983–3989. doi: 10.1128/iai.68.7.3983-3989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hope J N, Bell A W, Hermodson M A, Groarke J M. Ribokinase from Escherichia coli K12. Nucleotide sequence and overexpression of the rbsKgene and purification of ribokinase. J Biol Chem. 1986;15:7663–7668. [PubMed] [Google Scholar]
- 28.Hung D L, Knight S D, Woods R M, Pinkner J S, Hultgren S J. Molecular basis of two subfamilies of immunoglobulin-like chaperones. EMBO J. 1996;15:3792–3805. [PMC free article] [PubMed] [Google Scholar]
- 29.Jacob-Dubuisson F, Pinkner J, Xu Z, Striker R, Padmanhaban A, Hultgren S J. PapD chaperone function in pilus biogenesis depends on oxidant and chaperone-like activities of DsbA. Proc Natl Acad Sci USA. 1994;91:11552–11556. doi: 10.1073/pnas.91.24.11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jouve M, Garcia M-I, Courcoux P, Labigne A, Gounon P, Le Bouguénec C. Adhesion to and invasion of HeLa cells by pathogenic Escherichia coli carrying the afa-3 gene cluster are mediated by the AfaE and AfaD proteins, respectively. Infect Immun. 1997;65:4082–4089. doi: 10.1128/iai.65.10.4082-4089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kado C I, Liu S-T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kao J-S, Stucker D M, Warren J W, Mobley H L T. Pathogenicity island sequences of pyelonephritogenic Escherichia coliCFT073 are associated with virulent uropathogenic strains. Infect Immun. 1997;65:2812–2820. doi: 10.1128/iai.65.7.2812-2820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knapp S, Hacker J, Jarchau T, Goebel W. Large unstable inserts in the chromosome affect virulence properties of uropathogenic Escherichia coliO6 strain 536. J Bacteriol. 1986;168:22–30. doi: 10.1128/jb.168.1.22-30.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labigne-Roussel A F, Lark D, Schoolnik G, Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia colistrain. Infect Immun. 1984;46:251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lalioui L, Jouve M, Gounon P, Le Bouguénec C. Molecular cloning and characterization of the afa-7 and afa-8 gene clusters encoding afimbrial adhesins in Escherichia colistrains associated with diarrhea or septicemia in calves. Infect Immun. 1999;67:5048–5059. doi: 10.1128/iai.67.10.5048-5059.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Bouguénec C, Archambaud M, Labigne A. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia colistrains by polymerase chain reaction. J Clin Microbiol. 1992;30:1189–1193. doi: 10.1128/jcm.30.5.1189-1193.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Bouguénec C, Garcia M I, Ouin V, Desperrier J-M, Gounon P, Labigne A. Characterization of plasmid-borne afa-3 gene clusters encoding afimbrial adhesins expressed by Escherichia colistrains associated with intestinal or urinary tract infections. Infect Immun. 1993;61:5106–5114. doi: 10.1128/iai.61.12.5106-5114.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 39.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDaniel T K, Kaper J B. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coliK-12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 41.Mortensen L, Dandanell G, Hammer K. Purification and characterization of the deoR repressor of Escherichia coli. EMBO J. 1989;8:325–331. doi: 10.1002/j.1460-2075.1989.tb03380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nowicki B, Labigne A, Moseley S, Hull R, Hull S, Moulds J. The Dr hemagglutinin, afimbrial adhesins AFA-I and AFA-III, and F1845 fimbriae of uropathogenic and diarrhea-associated Escherichia colibelong to a family of hemagglutinins with Dr receptor recognition. Infect Immun. 1990;58:279–281. doi: 10.1128/iai.58.1.279-281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ochman H, Lawrence J G. Phylogenetics and the amelioration of bacterial genomes. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C.: ASM Press; 1996. pp. 2627–2637. [Google Scholar]
- 44.Perna N T, Mayhew G F, Pósfai G, Elliott S, Donnenberg M S, Kaper J B, Blattner F R. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli. O157:H7. Infect Immun. 1998;66:3810–3815. doi: 10.1128/iai.66.8.3810-3817.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierson L S D, Kahn M L. Integration of satellite bacteriophage P4 in Escherichia coli. DNA sequences of the phage and host regions involved in site-specific recombination. J Mol Biol. 1987;196:487–496. doi: 10.1016/0022-2836(87)90026-x. [DOI] [PubMed] [Google Scholar]
- 46.Pittard J, Praszkier J, Certoma A, Eggertsson G, Gowrishankar J, Narasaiah G, Whipp M J. Evidence that there are only two tRNAPhe genes in Escherichia coli. J Bacteriol. 1990;152:6077–6083. doi: 10.1128/jb.172.10.6077-6083.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redenbach M, Kieser H M, Denapaite D, Eichner A, Cullum J, Kinashi H, Hopwood D A. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolorA3(2) chromosome. Mol Microbiol. 1996;21:77–96. doi: 10.1046/j.1365-2958.1996.6191336.x. [DOI] [PubMed] [Google Scholar]
- 48.Reizer J, Charbit A, Reizer A, Saier M H J. Novel phosphotransferase system genes revealed by bacterial genome analysis: operons encoding homologues of sugar-specific permease domains of the phosphotransferase system and pentose catabolic enzymes. Genome Sci Technol. 1996;1:53–75. [Google Scholar]
- 49.Savarino S J, Fox P, Yikang D, Nataro J P. Identification and characterization of a gene cluster mediating enteroaggregative Escherichia coliaggregative adherence fimbria I biogenesis. J Bacteriol. 1994;156:4949–4957. doi: 10.1128/jb.176.16.4949-4957.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schubert S, Rakin A, Fischer D, Sorsa J, Heesemann J. Characterization of the integration site of Yersinia high-pathogenicity island in Escherichia coli. FEMS Microbiol Lett. 1999;159:409–414. doi: 10.1111/j.1574-6968.1999.tb08756.x. [DOI] [PubMed] [Google Scholar]
- 51.Schubert S, Rakin A, Karch H, Carniel E, Heesemann J. Prevalence of the “high-pathogenicity island” of Yersinia species among Escherichia colistrains that are pathogenic to humans. Infect Immun. 1998;66:480–485. doi: 10.1128/iai.66.2.480-485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shine J, Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975;254:34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- 53.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–515. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 54.Stentz R, Zagorec M. Ribose utilization in Lactobacillus sakei: analysis of the regulation of the rbsoperon and putative involvement of a new transporter. J Mol Microbiol Biotechnol. 1999;1:165–173. [PubMed] [Google Scholar]
- 55.Swenson D L, Bukanov N O, Berg D E, Welch R A. Two pathogenicity islands in uropathogenic Escherichia coliJ96: cosmid cloning and sample sequencing. Infect Immun. 1996;64:3736–3743. doi: 10.1128/iai.64.9.3736-3743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valentin-Hansen P, Hojrup P, Short S. The primary structure of the DeoR repressor from Escherichia coliK-12. Nucleic Acids Res. 1985;13:5927–5936. doi: 10.1093/nar/13.16.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valentin-Hansen P, Svenningsen B A, Munch-Petersen A, Hammer-Jespersen K. Regulation of the deo operon in Escherichia coli: the double negative control of the deo operon by the cytR and deoR repressors in a DNA directed in vitrosystem. Mol Gen Genet. 1978;159:191–202. doi: 10.1007/BF00270893. [DOI] [PubMed] [Google Scholar]
- 58.Van Rosmalen M, Saier M H., Jr Structural and evolutionary relationships between two families of bacterial extracytoplasmic chaperone proteins which function cooperatively in fimbrial assembly. Res Microbiol. 1993;144:507–527. doi: 10.1016/0923-2508(93)90001-i. [DOI] [PubMed] [Google Scholar]