Abstract
Background:
Self-glucose monitoring is critical for management of diabetes in pregnancy; yet validated reports of adherence to testing recommendations and associated perinatal outcomes are limited.
Objective:
Using cloud-based, self-glucose monitoring technology, we sought to answer the following questions:
Are there differences in rates of testing adherence based on type of diabetes in pregnancy?
Is adherence to glucose monitoring recommendations associated with perinatal outcomes in pregnancies complicated by diabetes?
We hypothesized that adherence to glucose testing recommendations varies by type of diabetes and that increased adherence to testing recommendations would be associated with improved perinatal outcomes.
Study Design:
This single-center, prospective cohort study included women with type 2 diabetes (T2DM) and gestational diabetes (GDM) enrolled in a perinatal diabetes program before 29 weeks gestation between December 2015 and June 2018. All women received a cellular-enabled glucometer that uploaded glucose values to a cloud-based, HIPAA compliant platform in real time, ensuring transmission of accurate glucose values. The primary outcome was adherence to self-glucose monitoring recommendations. Four glucose checks were advised daily and percent adherence was calculated. Secondary outcomes were preeclampsia, cesarean delivery, large for gestational age neonates and neonatal hypoglycemia. The study was powered to detect a 10% difference in the primary outcome of adherence to advised self-glucose monitoring by diabetes type. Adjusted risk ratios and 95% confidence intervals were generated using logistic regression.
Results:
This study included 103 eligible women. Baseline characteristics differed between groups with women with T2DM having higher initial HgbA1c and BMI when compared to women with GDM. No differences were noted in age or parity. Adherence was calculated over 20±6 weeks for women with T2DM compared to 9±4 weeks for women with GDM. Overall adherence to glucose monitoring was significantly less for women with T2DM compared to those with GDM. Mean testing adherence rates were 51%, 66% and 70% for T2DM, GDM,A1 and GDM,A2 respectively (p=0.016).
We found that for every 10% increase in adherence to testing recommendations, the odds of Cesarean delivery, neonatal hypoglycemia, and large for gestational age fetuses decreases by 15–20%. There was no association between adherence and rates of preeclampsia.
Conclusion:
This study shows that overall adherence to testing recommendations differs by diabetes type and is associated with neonatal outcomes. Improved outcomes with higher adherence may reflect more timely medication adjustments in response to real-time glucose values. Programs aimed at improving adherence could prove beneficial.
Keywords: Pregnancy, Type 2 diabetes, Gestational Diabetes, Adherence, Self-glucose monitoring, cellular enabled glucometer
Condensation:
Adherence to self-glucose monitoring recommendations in pregnancy varies by type of diabetes and decreased adherence is associated with adverse perinatal outcomes.
Introduction:
Self-monitoring of blood glucose provides the foundation for clinical management of diabetes in pregnancy. Women with type 2 diabetes (T2DM) and those diagnosed with gestational diabetes (GDM) in pregnancy are advised to test glucose levels 4 times daily: fasting and one or two hours post-prandially1–3. Most patients record these values on a supplied log and review them with providers at regular intervals. In some practices, glucometer logs are also reviewed at the time of clinic visits. Prior work has shown that depending on type of diabetes, up to 35% of patient supplied blood glucose values are modified or omitted in pregnancy4. In a general population, only 71% of reported values were considered reliable due to under reporting, over reporting and lack of concordance5.
There has been increasing interest in the impact of adherence to testing recommendations with perinatal outcomes6–8. In one recent report, only 61% of women with GDM completed greater than 80% of recommended tests over the first 13 days of testing and lower adherence was associated with increased risk of preeclampsia and higher HgbA1c at delivery7. Absolute rates of adherence were not reported in this study, and it did not assess the impact of adherence on neonatal outcomes. Given difficulties of accessing reliable self-glucose monitoring values in pregnancy, other groups have attempted to correlate surrogate markers of adherence to care including attendance at prenatal visits with perinatal outcomes6,8. Adherence rates are often dichotomized into high or low, though it remains unclear at which threshold this adherence distinction should be made, which limits practical counseling to patients.
Our diabetes program has recently adopted use of a cellular-enabled glucometer platform for management of all pregnancies complicated by diabetes, which eliminates under-, over-, and discordant self-reporting. Additionally, values are tagged to meal time and do not require any manual entry of glucometer values. The purpose of this study was to apply this novel technology to ask two questions fundamental to care of women with diabetes: 1) Does adherence to glucose testing recommendations vary by type of diabetes? 2) Is adherence to self-glucose monitoring recommendations associated with perinatal outcomes? We hypothesized that adherence to glucose testing recommendations varied by type of diabetes and that lower rates of adherence to glucose testing would be associated with adverse perinatal outcomes.
Methods:
We performed a prospective cohort study of pregnant women with T2DM or GDM enrolled in a perinatal diabetes program at the University of Iowa between December 2015 and June of 2018. All women were managed by the same care team. Type 2 diabetes was defined by HgbA1c >6.5% in first trimester or established pre-existing diagnosis3. Our institution practices a two-step approach to diagnosis of GDM. GDM was defined by elevation of a 3 hour glucose tolerance test following an elevated 1 hour glucose tolerance test in accordance with ACOG guidelines.2 Pregnant women with GDM managed with diet and exercise were classified as GDM, A1 and those requiring medication were classified as GDM, A2 in accordance with ACOG guidelines. All women had similar glucose goals in pregnancy: less than 95 mg/dL for fasting values and less than 140 mg/dL for post prandial values1–3. In labor, all women had a target glucose of 70–110 mg/dL and insulin drips were used per our hospital’s labor glucose management protocol.
Women were included if they were enrolled in our perinatal diabetes program and delivered an infant at our institution. All patients were provided and used a cellular-enabled glucometer to ensure reliability of testing. This cellular-enabled glucometer (Telcare) allows for real time transmission of self-monitored blood glucoses to a HIPAA compliant, cloud-based platform9. This transmission is independent of patient cellular data plans or access to wireless internet. As a quality improvement initiative, this project was reviewed by the University of Iowa Institutional Review Board and was deemed not human subjects research (IRB No 201509749).
Women were excluded if they delivered at an outside facility (Figure 1). Additionally, our study excluded women with type 1 diabetes due to differences in frequency of self-glucose monitoring regimens (7 times daily) and complexity of this population’s use of continuous glucose monitoring to supplement self-monitoring of blood glucose, adding further challenges to assessment of adherence to testing recommendations.
Figure 1:
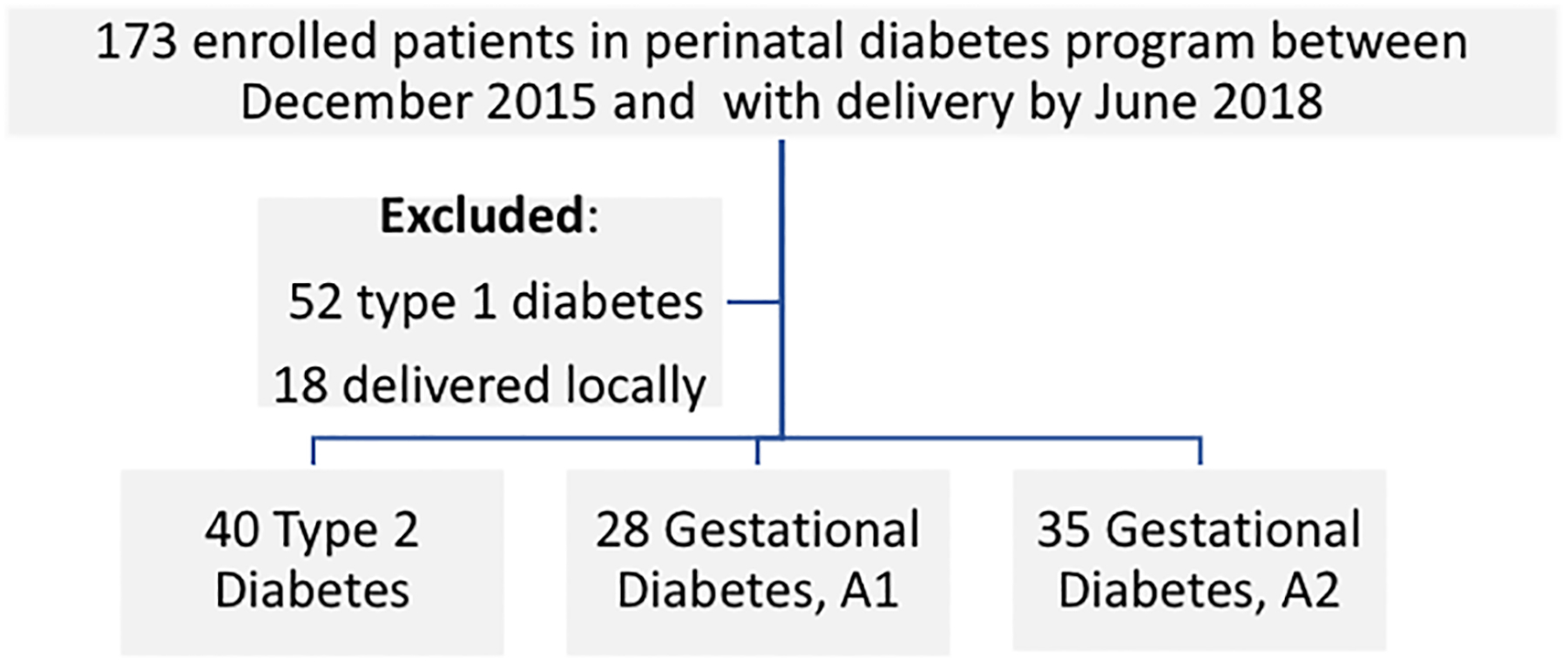
Study sample of women enrolled in perinatal diabetes program
Current guidelines by both the ADA and ACOG recommend that women with T2DM and GDM monitor glucose four times daily—fasting and post-prandial1,2. In our perinatal diabetes program, we advise women to test glucose 1 hour post-prandially. Adherence was calculated as the number of tests completed daily at appropriate times divided by the four tests recommended daily. The primary outcome was average adherence to self-glucose monitoring recommendations. Average daily adherence was calculated at two time points: over the first week of recommended testing in pregnancy compared and over the entire course of monitoring in pregnancy. Secondary outcomes assessed were preeclampsia, cesarean delivery, neonatal hypoglycemia and large for gestational age. Hypoglycemia was defined as a neonate with any blood glucose less than 47 mg/dL per hospital protocol. Large for gestational age was determined by birthweight greater than the 95th percentile for expected gestational age.10 Preeclampsia was defined by 2 blood pressures greater than 140 mmHg systolic and/or 90 mmHg diastolic and either 24 hour urine protein > 300 mg, a urine protein to creatinine ratio >0.3, or evidence of end organ damage11.
Based on adherence over the course of pregnancy for 56 pregnant women with T2DM enrolled in our program but ineligible for this study, we assumed a 54% adherence in T2DM with a standard deviation of 24%. Our preliminary analysis showed the ratio of women with T2DM, GDM,A2, and GDM,A1 diabetes enrolled in our program to be 8:7:5. Using this information, our power analysis showed that we would need a sample of 93 patients to detect a 10% difference in overall adherence between groups with a type I error rate of 5% and power of 80%. Therefore, it was determined that the sample of 103 women enrolled in the program between December 2015 and June of 2018 would be a sufficient sample for the study. Additionally, a sample of 103 women was determined sufficient to detect a 23% absolute difference in large for gestational age neonates assuming a rate of 37.5% in patients with poor control historically seen at our institution. The study was not powered to detect a difference in pre-eclampsia, Cesarean delivery, or neonatal hypoglycemia. All results were analyzed by Chi squared, Fisher’s exact or t-test as indicated. Logistic regression was used to control for type of diabetes. To calculate odds for adverse outcomes for every 10% increase in adherence, regression was performed with modified adherence values. Adherence values were modified by dividing calculated adherence by 10 so every point increase in the modified variable indicated a 10% increase in adherence.
Results:
This study included 103 eligible women. Baseline characteristics differed between groups with women with T2DM having higher HgbA1c and BMI when compared to women with GDM (Table 1). No differences were noted in age or parity. Adherence was calculated over 20±6 weeks for women with T2DM compared to 9±4 weeks for women with GDM. Overall adherence to self-glucose monitoring was significantly lower for women with T2DM compared to those with GDM (Table 2). Testing adherence rates were 51%, 66% and 70% for T2DM, GDM,A1 and GDM,A2 respectively (p=0.016). Adherence to the fasting test was higher than post prandial tests in all groups (Table 2). However, testing adherence to individual tests throughout the day was similar to calculated overall adherence.
Table 1:
Baseline Characteristics
| Type 2 | A1 Gestational | A2 Gestational | P | |
|---|---|---|---|---|
| Number | 39 | 28 | 35 | |
| Age | 33 ± 0.87 | 31 ± 0.96 | 31 ± 6.04 | 0.290 |
| BMI at NOB visit | 39 ± 1.31 | 32 ± 1.28 | 38 ± 9.32 | 0.002 |
| HgbA1c at NOB | 7.6 ± 0.27 | 5.3 ± 0.091 | 5.3 ± 0.342 | <0.001 |
| Nulliparous (Y/N) | 8 (21%) | 7 (25%) | 13 (37%) | 0.262 |
| Medicaid (Y/N) | 31 (80%) | 11 (39%) | 17 (49%) | 0.002 |
| Gestational age at first contact (weeks) | 17 ± 0.97 | 29 ± 3.90 | 27 ± 4.70 | <0.001 |
| Weeks of glucose testing | 20.22 ± 1.11 | 9.20 ± 4.31 | 10.31 ± 5.27 | <0.001 |
| Medication:Insulin | 33 (85%) | N/A | 22 (63%) | <0.001 |
| Medication:Metformin | 1 (3%) | N/A | 2 (6%) | * |
| Medication:Glyburide | 4 (10%) | N/A | 11 (31%) | * |
| GA at delivery (wks) | 36.87 ± 0.58 | 37.96 ± 2.10 | 37.51 ± 2.02 | 0.270 |
| HgbA1c % at delivery | 6.3 ± 0.18 | 5.1 ± 0.103 | 5.4 ± 0.074 | <0.001 |
| Birthweight (gms) | 3235 ± 162 | 3069 ± 137 | 3239 ± 110 | 0.657 |
| Preeclampsia | 7 (18%) | 3 (11%) | 8 (23%) | * |
| Cesarean delivery | 21 (54%) | 13 (46%) | 18 (51%) | 0.834 |
| Primary Cesarean delivery | 7 (33%) | 6 (46%) | 8 (44%) | * |
| Hypoglycemia | 22 (61%) | 12 (43%) | 20 (57%) | 0.322 |
| Large for Gestational Age | 11 (28%) | 2 (7%) | 5 (14%) | * |
Data are expressed as mean ± SE or n (%). P values are for One-way ANOVA or Chi-Square as appropriate.
Missing data for 1 participant (n=27)
Missing data for 3 participants (n=32)
Missing data for 10 participants (n=18)
Missing data for 13 participants (n=22)
Data violate Chi-square minimum expected counts assumption.
Table 2:
Comparison of overall adherence to self-glucose monitoring by type of diabetes
| Type 2 | A1 Gestational | A2 Gestational | P | |
|---|---|---|---|---|
| Overall Adherence to glucose monitoring % | 51.17 ± 4.76 | 66.05 ± 5.6 | 70.26 ± 4.83 | 0.016 |
| Fasting Adherence % | 54.15 ± 5.28 | 72.68 ± 5.69 | 81.22 ± 4.27 | 0.001 |
| Post BF Adherence % | 47.36 ± 5.02 | 62.56 ± 6.09 | 66.94 ± 5.26 | 0.023 |
| Post Lunch Adherence % | 46.81 ± 4.84 | 65.37 ± 5.62 | 64.92 ± 5.51 | 0.018 |
| Post Dinner Adherence % | 52.14 ± 5.09 | 64.08 ± 6.08 | 68.25 ± 5.36 | 0.083 |
Data are expressed as mean ± SE. P values are for One-way ANOVA
We sought to compare rates of adherence to self-glucose monitoring over the first week of recommended testing in pregnancy compared to adherence over the entire course of testing in pregnancy. We found that rates of adherence to self-glucose monitoring for women with T2DM was similar over the first week of testing compared to the entire testing range in pregnancy (55.3% +/− 5.2% compared to 51.17 +/− 4.76%, p=0.251). In contrast, women with GDM demonstrated decreasing adherence to self-glucose monitoring recommendations over the course of pregnancy. For women with GDM,A1 and GDM,A2, adherence to testing recommendations decreased by 7–10% over the course of pregnancy (Table 2).
We hypothesized that increased rates of adherence to self-glucose monitoring would be associated with improved perinatal outcomes. Overall, we found no difference in absolute rates of these outcomes in our populations (Table 1). However, we compared odds of adverse maternal and neonatal outcomes with adherence to self-glucose monitoring. We found that for every 10% increase in adherence to testing recommendations, the odds of Cesarean delivery, neonatal hypoglycemia and large for gestational age neonates decreases by 15–20% (Table 3). The decrease in Cesarean delivery rate and neonatal hypoglycemia persists after adjusting for type of diabetes (Table 3).
Table 3:
Comparison of adherence to self-glucose monitoring during first week of recommended testing and entire pregnancy
| 1 week adherence% | Overall adherence% | Difference %a | P | |
|---|---|---|---|---|
| Type 2 (n=39) | 55.30 ± 5.19 | 51.17 ± 4.76 | 4.14 (3.04 – 11.32) | 0.251 |
| A1 Gestational (n=28) | 76.54 ± 5.88 | 66.05 ± 5.63 | 10.48 (4.73 – 16.24) | 0.001 |
| A2 Gestational (n=35) | 77.66 ± 4.46 | 70.26 ± 4.83 | 7.41 (3.01–11.80) | 0.002 |
Data are expressed as mean ± SE. P values are for paired t-tests.
Data expressed as mean (95% CI)
Discussion:
In this study, we show that rates of adherence to self-glucose monitoring recommendations in pregnancy differ by type of diabetes and tend to decrease over time. Further, we show that increased adherence to testing recommendations is associated with a decreased risk of adverse neonatal outcomes including hypoglycemia and large for gestational age. This data corroborates clinical intuition that adherence to self-glucose monitoring is critical to neonatal outcomes. The association of improved neonatal outcomes with higher adherence to self-glucose monitoring may reflect more timely treatment adjustments in response to transmission of accurate and timely glucose values to care teams.
The rates of testing remain stable over the course of pregnancy for women with T2DM, suggesting that previously established testing habits persist throughout pregnancy. In contrast, women with GDM,A1 and GDM,A2 showed decreased adherence to testing recommendations over the course of pregnancy. For women with GDM,A1 this may be due to the fact that as no medication is needed, patients perceive testing feedback is less necessary.
A major strength of this study is use of cellular-enabled glucometers that allow real time transmission of glucose values to a secure web-based portal in pregnancy. Data transmission and text messaging through this meter occurs automatically and does not require patient internet access or cellular data plans. This ensures the transmission of timely and accurate glucose values. Paper glucose logs show high rates of modified values4,12. This study is also strengthened by assessment of all self-monitored glucose values in pregnancy as opposed to the 1–2 week time frame that has previously been reported. Studies that estimate adherence to testing recommendations over a brief period of logging may overestimate adherence to testing recommendations.
The major limitations of this study are its relatively small sample size from a single Academic Perinatal Diabetes Program and its limited power to detect differences in neonatal outcomes. To reduce bias we only included women cared for by our perinatal diabetes program with a consistent diabetes care nurse and maternal-fetal medicine specialists. All patients received weekly communication with our care team. While we show that increases in adherence to testing recommendations are associated with improved neonatal outcomes, we cannot delineate if this is exclusively due to self-glucose monitoring adherence. For instance, women who are more adherent to testing recommendations may be more likely to take medication as prescribed or follow dietary and exercise recommendations that can contribute to improved outcomes. Additionally, adherence is likely linked to provider medication adjustments since it is challenging to increase insulin without glucose values. Further studies will be needed to see if interventions to improve testing adherence specifically can improve maternal and neonatal outcomes.
Additionally, we cannot eliminate the possibility that decreased adherence is associated with socioeconomic stress, which has been described as a barrier to diabetes management in both pregnant and non-pregnant populations13–15. For instance, food insecurity may limit testing according to recommendations because regular meals are not available. We have not adjusted our adherence calculations for this reality, noting that we recommend similar testing regimens and meal plans to all patients. Further, women with less flexible work environments may have outside influences limiting adherence to testing recommendations. We noted that twice as many women with T2DM have Medicaid coverage compared to those with GDM, suggesting the potential impact of socioeconomic state on pregnancy outcomes. However, in an effort to reduce socioeconomic barriers to reporting, our program provides the glucometer and strips to women without respect to insurance coverage. Evaluating adherence to self-glucose monitoring may provide insights into other social determinants of health that could contribute to improved outcomes.
While we see an association between increased adherence to testing recommendations and improvements in neonatal outcomes even after controlling for diabetes type, we acknowledge that we cannot completely control for differences in underlying disease pathology that may contribute to worse perinatal outcomes and the fact that women with type 2 diabetes in our study tend to be less adherent to glucose testing. Women with type 2 diabetes may have a higher level of comfort with diabetes management than those with gestational diabetes and perceive less need to obtain regular glucose values. We also note that until August 2017 when ACOG recommended insulin as first line medication for treatment of GDM,A22, glyburide was regularly used in our practice and potentially contributed to elevated rates of neonatal hypoglycemia noted in our GDM,A2 population. However, given the higher rates of adherence to testing recommendations in this population, this may mask some of the impact adherence on neonatal hypoglycemia. Finally, while all women were managed by the same care team and had similar glucose goals in pregnancy, we have not controlled for impact of individual glucose values on outcomes. Future studies will be needed to address these limitations.
Overall, this study uses a novel technology to assess adherence throughout pregnancy and we show that adherence to testing recommendations differ for women with type 2 and gestational diabetes in our population. We provide evidence that increased adherence to self-glucose monitoring recommendations is associated with decreased risk of adverse pregnancy outcomes even after controlling for type of diabetes, though acknowledge limitations to this conclusion. Overall, we feel that these data support everyday clinical practices to encourage patients that even small increases in adherence can potentially improve maternal and neonatal outcomes. Additionally, it lays the foundation for funding program initiatives aimed at improving adherence to self-glucose monitoring to potentially improve outcomes. Accurate assessment of adherence over the course of pregnancy will be critical for the measurement of success in any intervention.
Table 4:
Association of overall adherence to self-glucose monitoring to maternal and neonatal outcomes
| Uncontrolled OR (95% CI) | P | Adjusted OR (95% CI) controlling for diabetes type | P | |
|---|---|---|---|---|
| Maternal Outcomes | ||||
| Preeclampsia | 0.959 (0.812 – 1.133) | 0.622 | 0.950 (0.798 – 1.132) | 0.567 |
| Cesarean delivery | 0.793 (0.687 – 0.916) | 0.002 | 0.782 (0.672 – 0.911) | 0.002 |
| Neonatal Outcomes | ||||
| Hypoglycemia* | 0.851 (0.739 −0.980) | 0.025 | 0.851 (0.735 – 0.986) | 0.032 |
| Large for Gestational Age | 0.818 (0.689 – 0.970) | 0.021 | 0.839 (0.701 – 1.005) | 0.057 |
Odds ratios calculated for every 10 % points increase in adherence.
3 cases missing data
AJOG at a Glance:
Why was the study conducted?
Validated reports of testing adherence to self-glucose monitoring have previously been limited by unreliability of patient reported glucose logs, surrogate markers and brief periods of assessment.
What are the key findings?
Adherence to glucose monitoring recommendations varies by diabetes type.
Women with gestational diabetes are more adherent to monitoring recommendations than those with type 2 diabetes.
Increased adherence to self-glucose monitoring decreases risk of adverse perinatal outcomes
What does the study add to what is already know?
Using novel technology, this study reports absolute adherence to glucose testing throughout pregnancy
Clinical practice that encourages adherence to testing recommendations contributes to improved clinical outcomes.
Funding source:
Sarah A Wernimont is funded by T32DK112751-01.
Footnotes
presented as an original abstract at the 2019 Society for Maternal Fetal Medicine Annual Meeting, Las Vegas NV
Disclosures:
The authors report no conflict of interests
REFERENCES
- 1.American Diabetes A 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2019. Diabetes care. 2019;42(Suppl 1):S165–S172. [DOI] [PubMed] [Google Scholar]
- 2.Gestational Diabetes Mellitus. ACOG Practice Bulletin No 190. The American College of Obstetricains and Gynecologists. 2018. [Google Scholar]
- 3.Pregestational Diabetes Mellitus. ACOG Practice Bulletin No 201. The American College of Obstetricains and Gynecologists. 2018. [Google Scholar]
- 4.Kendrick JM, Wilson C, Elder RF, Smith CS. Reliability of reporting of self-monitoring of blood glucose in pregnant women. J Obstet Gynecol Neonatal Nurs. 2005;34(3):329–334. [DOI] [PubMed] [Google Scholar]
- 5.Given JE, O’Kane MJ, Coates VE, Moore A, Bunting BP. Comparing patient generated blood glucose diary records with meter memory in type 2 diabetes. 2014;104(3):358–362. [DOI] [PubMed] [Google Scholar]
- 6.Carter EB, Tuuli MG, Odibo AO, Macones GA, Cahill AG. Prenatal visit utilization and outcomes in pregnant women with type II and gestational diabetes. J Perinatol. 2017;37(2):122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosson E, Baz B, Gary F, et al. Poor Reliability and Poor Adherence to Self-Monitoring of Blood Glucose Are Common in Women With Gestational Diabetes Mellitus and May Be Associated With Poor Pregnancy Outcomes. Diabetes care. 2017;40(9):1181–1186. [DOI] [PubMed] [Google Scholar]
- 8.Sperling JD, Maggio L, Has P, Daley J, Khander A, Coustan DR. Prenatal Care Adherence and Neonatal Intensive Care Unit Admission or Stillbirth among Women with Gestational and Preexisting Diabetes Mellitus. Am J Perinatol. 2018;35(2):103–109. [DOI] [PubMed] [Google Scholar]
- 9.Wernimont SA, Sheng JS, Fleener D, Summers KM, Syrop C, Andrews JI. Cellular-Enabled Glucometers and Maternal Glucose Control: A Quality Improvement Initiative. Journal of diabetes science and technology. 2019:1932296819856360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer MS PR, Wen SW, Joseph KS, Allen A, Abrahamowicz M, Blondel B, Bréart G; Fetal/Infant Health Study Group of the Canadian Perinatal Surveillance System. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108(2):E35. [DOI] [PubMed] [Google Scholar]
- 11.Gestational Hypertension and Preeclampsia ACOG Practice Bulletin No 202. The American College of Obstetricains and Gynecologists. 2018. [Google Scholar]
- 12.Given JE, O’Kane MJ, Bunting BP, Coates VE. Comparing patient-generated blood glucose diary records with meter memory in diabetes: a systematic review. Diabetic Medicine. 2013;30(8):901–913. [DOI] [PubMed] [Google Scholar]
- 13.Laraia BA, Siega-Riz AM, Gundersen C. Household food insecurity is associated with self-reported pregravid weight status, gestational weight gain, and pregnancy complications. J Am Diet Assoc. 2010;110(5):692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandit AU, Bailey SC, Curtis LM, et al. Disease-related distress, self-care and clinical outcomes among low-income patients with diabetes. J Epidemiol Community Health. 2014;68(6):557–564. [DOI] [PubMed] [Google Scholar]
- 15.Vijayaraghavan M, Jacobs EA, Seligman H, Fernandez A. The association between housing instability, food insecurity, and diabetes self-efficacy in low-income adults. J Health Care Poor Underserved. 2011;22(4):1279–1291. [DOI] [PubMed] [Google Scholar]


