Abstract
Periodontal disease is a chronic multifactorial disease triggered by a complex of bacterial species. These interact with host tissues to cause the release of a broad array of pro-inflammatory cytokines, chemokines, and tissue remodelers, such as matrix metalloproteinases (MMPs), which lead to the destruction of periodontal tissues. Patients with severe forms of periodontitis are left with a persistent pro-inflammatory transcriptional profile throughout the periodontium, even after clinical intervention, leading to the destruction of teeth-supporting tissues. The oral spirochete, Treponema denticola , is consistently found at significantly elevated levels at sites with advanced periodontal disease. Of all T. denticola virulence factors that have been described, its chymotrypsin-like protease complex, also called dentilisin, has demonstrated a multitude of cytopathic effects consistent with periodontal disease pathogenesis, including alterations in cellular adhesion activity, degradation of various endogenous extracellular matrix–substrates, degradation of host chemokines and cytokines, and ectopic activation of host MMPs. Thus, the following model of T. denticola –human periodontal ligament cell interactions may provide new knowledge about the mechanisms that drive the chronicity of periodontal disease at the protein, transcriptional, and epigenetic levels, which could afford new putative therapeutic targets.
This protocol was validated in: PLOS Pathog (2021), DOI: 10.1371/journal.ppat.1009311
Keywords: Periodontal disease , Host–microbe interactions , Periodontal ligament cells , Treponema denticola , Dentilisin
Background
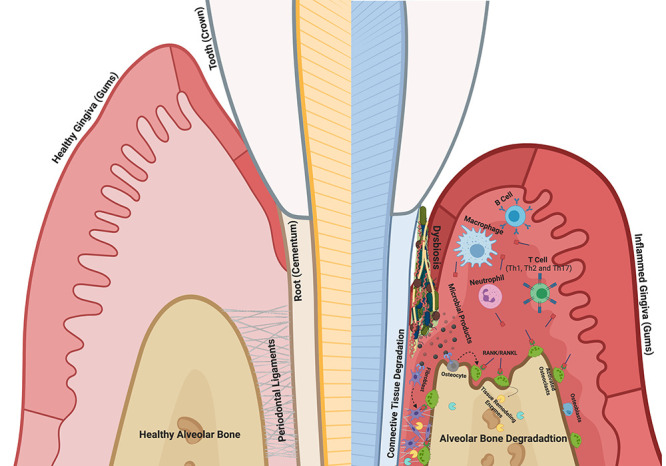
The human periodontal ligament serves as a connector between the tooth and the surrounding alveolar bone proper. Its main role is to convert mechanical forces into chemical signals, which largely mediate tissue turnover through the expression of various proteases, primarily matrix metalloproteinases (MMPs) ( Ho et al., 2007 ; Sokos et al., 2015 ; Takimoto et al., 2015 ; Jiang et al., 2016 ; Lin et al., 2017 ; Kim et al., 2020 ). Periodontal diseases are caused by bacterially derived factors, leading to a chronic infectious inflammatory disease that affects the periodontium and gradually destroys the tooth-supporting alveolar bone ( Sela et al., 1997 ; Asai et al., 2003 ; Hayashi et al., 2010 ; Trindade et al., 2014 ; Cecil et al., 2017 ; Deng et al., 2017 ; Gao et al., 2020 ) ( Figure 1 ). As periodontal disease progresses, periodontopathogenic bacteria invade deeper into the subgingival space, compromising the periodontal ligament (PDL) function and contributing to tooth loss.
Figure 1. Pathophysiology of periodontal disease .
Among over 500 species of bacterium, the oral spirochete, Treponema denticola , is consistently found at significantly elevated levels in advanced lesions (Ateia et al., 2018; Solbiati and Frias- Lopez, 2018 ). Additionally, elevated T. denticola biofilm levels, combined with elevated MMP levels in host tissue, display robust combinatorial characteristics in predicting advanced periodontal disease severity. Thus, clinical data regarding the increased presence of T. denticola in periodontal lesions, together with basic research results involving the role of T. denticola products, suggest that it plays a pivotal role in driving periodontal disease progression. Therefore, delineation of causative mechanisms that T. denticola uses to drive host modulation may help to identify novel targets for better or alternative treatments for this chronic disease.
Conserved lipid moieties of the protease complex recognized by host receptor complexes may contribute to the activation of innate immune responses ( Schenk et al., 2009 ). Because predominant host responses to lipoproteins are believed to be to their lipid moieties, most studies have focused on diacylated lipopeptide, Pam2CSK4, and triacylated lipopeptide, Pam3CSK4, which mimic bacterial lipoproteins for their potent immunostimulatory and osteoclastogenic activities (Schenk et al., 2009; Kim et al., 2013; Wilson and Bernstein, 2016 ). Recent studies have demonstrated that synthetic di- and tri-acylated lipopeptides, which preferentially activate TLR2/6 and TLR2/1-dependent pathways respectively, are sufficient to induce alveolar bone loss in mice ( Kim et al., 2013 ; Souza et al., 2020 ), broadening the avenues of investigation into the role of lipoproteins underpinning the pathogenesis of periodontal disease. However, studies that utilize endogenously expressed bacterial lipopeptides are lacking.
Several proteinases and peptidases secreted by T. denticola have been identified as causative factors that likely contribute to periodontal disease pathogenesis, due to their roles in processing host tissue proteins and peptides to fulfill the nutritional requirements of these highly motile and invasive organisms (Veith et al., 2009; Ellis and Kuehn, 2010 ; Visser et al., 2011; Asai et al., 2003; Cecil et al., 2016). Of all T. denticola surface-expressed proteins that have been described, a chymotrypsin-like protease complex, also called dentilisin, has demonstrated a multitude of cytopathic effects consistent with periodontal disease pathogenesis, including changes in cellular adhesion activity ( Bamford et al., 2007 ; Sano et al., 2014 ), degradation of various endogenous extracellular matrix–substrates ( Bamford et al., 2007 ; Miao et al., 2011 ; Inagaki et al., 2016 ), and degradation of host chemokines and cytokines ( Miyamoto et al., 2006 ; McDowell et al., 2012 ). Despite the many studies demonstrating its role at the protein level, few direct links have been reported between the activity of T. denticola ’s protease and the cellular and tissue processes driving periodontal tissue destruction. The following protocol delineates the experimental setup to study the interactions between T. denticola’s dentilisin protease and human periodontal ligament cells.
Materials and Reagents
6-well clear multi-well plate (Fisher Scientific, catalog number: 25373-187)
10 cm Falcon tissue culture plate (The Lab Depot, catalog number: 25373-10)
Minimal essential medium-α (MEM-α) (ThermoFisher Scientific, catalog number: 12571063)
Phosphate buffered saline (PBS) (Thermo Fisher Scientific, catalog number: 14190-094)
Penicillin/streptomycin (P/S) (Thermo Fisher Scientific, Gibco, catalog number: 15140122)
Amphotericin B (Thermo Fisher Scientific, Gibco, catalog number: 15290018)
0.25% trypsin with phenol red (Thermo Fisher, catalog number: 25200-05
Heat-inactivated fetal bovine serum (FBS) (Gibco, catalog number: 10-438-026)
BCA protein assay kit (Millipore Sigma, catalog number: 71285-3)
Equipment
96-well SpectraMax Plus microplate reader (VWR, catalog number: 89212-396)
Labomed Lx400 phase contrast HD digital microscope (Microscope Central, catalog number: 9126017T-HDS)
SteriCycle 370 CO 2 incubator (Thermo Fisher Scientific, catalog number: TH-370N)
NanoDrop One Microvolume UV-Vis spectrophotometer (Fisher Scientific, catalog number: 13-400-519)
Bright line hemacytometer chamber (Carolina, catalog number: 700722)
Thermo Sorvall ST16R refrigerated centrifuge (Thermo Fisher Scientific, catalog number: 75-004-240)
Procedure
Human periodontal ligament cell cultures (hPDL)
-
As described previously ( Scanlon et al., 2011 ), prepare the primary culture of human periodontal ligament cells via the direct cell outgrowth method, by isolating cells from the periodontal ligament tissue around the middle third of healthy human teeth extracted. Maintain cells in approximately 10 mL of MEM-α augmented with 10% FBS, 1% P/S, and 1% amphotericin B in 10 cm Falcon tissue culture plates in a humid atmosphere with 95% air and 5% CO 2 at 37 °C.
Passage cell outgrowths when they reach approximately 90% confluency using 0.25% trypsin and a Thermo Sorvall ST16R refrigerated centrifuge. Although variation will be observed from patient to patient, cells usually take approximately three to four days to become confluent after seeding cells at approximately 70%.
Use cells passaged three to six times for experimentation.
-
Validate human periodontal ligament cell parameters by 1) measuring mRNA expression of a specific isoform of the POSTN gene, which is exclusively expressed by periodontal ligament cells, 2) evaluating cell morphology, and 3) examining expression of other confident cell biomarkers, such as vimentin (general fibroblast marker) and CD45 , which ensure cultures are free of macrophages ( Marchesan et al., 2011 ).
If cultures are contaminated with other cell types, hPDL cells can be further purified via FACS sorting using the biomarkers reported above ( Basu et al., 2010 ).
-
Seed approximately 9 × 10 5 cells into 6-well clear multi-well plates and allow them to adhere in the presence of MEM-α with 10% FBS, 1% amphotericin B, and 1% P/S for approximately 24 h before stimulating or challenging the cells.
Stimulation of human periodontal ligament cells using purified dentilisin
Use a BCA protein assay kit according to the manufacturer’s recommendations to determine purified dentilisin (UniProt Accession #: P96091) sample concentrations
Scan the plates using a 96-well SpectraMax Plus Microplate Reader.
Determine enzymatic activity using gelatin zymography as described previously ( Cathcart, 2016 ).
Wash cells with PBS 2–3 times.
Add the purified dentilisin/PBS solution to MEM-α media with no phenol red, no serum, and no antibiotics to a final concentration of 1 μg/mL.
-
Add this dentilisin solution to healthy human periodontal ligament cell cultures and incubate in a humid atmosphere containing 95% air and 5% CO 2 at 37 °C for 2 h.
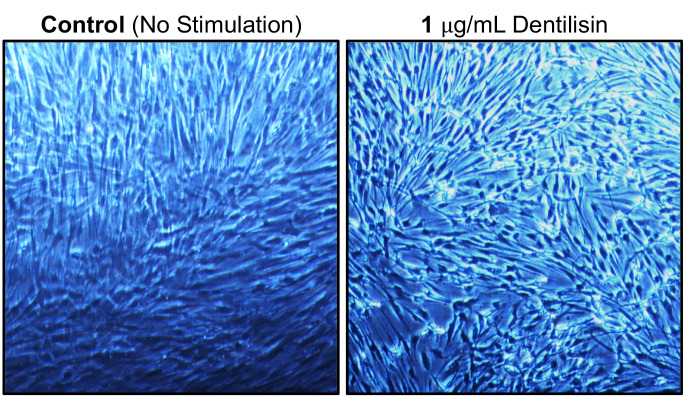
Stimulation with purified dentilisin will cause significant changes to the cells’ morphology ( Figure 2 ).
Gently wash cells with PBS twice and incubate in a humid atmosphere containing 95% air and 5% CO 2 at 37 °C for an additional 22 h in MEM-α with no FBS, P/S, or amphotericin B.
Figure 2. Morphological Change of hPDL Cells Following Purified Dentilisin Stimulation.
Healthy human periodontal ligament fibroblasts were challenged with purified dentilisin at a final concentration of 1 μg/mL for 2 h in MEM-α without serum or antibiotics, followed by a 22 h incubation in a humid atmosphere containing 95% air and 5% CO 2 at 37 °C in MEM-α media with no supplementation.
Data analysis
Following this experimental setup, harvest cells for analysis of various parameters. In our studies, we harvested cells or conditioned media to evaluate MMP enzymatic activity (gelatin zymography) and protein and RNA expression (Western Blot and qRT-PCR), and for cell imaging (immunofluorescence) ( Ganther et al., 2021 ).
Notes
If collecting conditioned media samples, it is highly recommended to use media without phenol red, serum, or antibiotics as dentilisin’s protease activity can cleave numerous substates and may interfere with protein concentration measurements.
Acknowledgments
We thank Dr. Christopher Fenno for his donation of purified dentilisin samples and isogenic mutants. These studies were supported by funding from the NIH (R01 DE025225) to YLK ( https://www.nih.gov/ ) and to SG by a Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant (T32DE007306) ( https://www.nih.gov/ ). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of this manuscript.
Competing interests
The authors declare no conflict of interest.
Ethics
Approval to conduct human subjects’ research was obtained from the University of California San Francisco Institutional Review Board (# 16-20204; reference #227030).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
Q&A
Post your question about this protocol in Q&A and get help from the authors of the protocol and some of its users.
References
- 1. Asai Y. , Jinno T. and Ogawa T. ( 2003 . ). Oral Treponemes and Their Outer Membrane Extracts Activate Human Gingival Epithelial Cells through Toll-Like Receptor 2. Infect Immun 71 ( 2 ): 717 - 725 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ateia I. M. , Sutthiboonyapan P. , Kamarajan P. , Jin T. , Godovikova V. , Kapila Y. L. and Fenno J. C. ( 2018 . ). Treponema denticola increases MMP-2 expression and activation in the periodontium via reversible DNA and histone modifications . Cell Microbiol 20 ( 4 ): 10.1111/cmi.12815 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bamford C. V. , Fenno J. C. , Jenkinson H. F. and Dymock D. ( 2007 . ). The chymotrypsin-like protease complex of Treponema denticola ATCC 35405 mediates fibrinogen adherence and degradation . Infect Immun 75 ( 9 ): 4364 - 4372 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basu S. , Campbell H. M. , Dittel B. N. and Ray A. ( 2010 . ). Purification of specific cell population by fluorescence activated cell sorting(FACS) . J Vis Exp ( 41 ): 1546 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cathcart J. ( 2016 . ). Assessment of Matrix Metalloproteinases by Gelatin Zymography . Methods Mol Biol 1406 : 151 - 159 . [DOI] [PubMed] [Google Scholar]
- 6. Cecil J. D. , O'Brien-Simpson N. M. , Lenzo J. C. , Holden J. A. , Singleton W. , Perez-Gonzalez A. , Mansell A. and Reynolds E. C. ( 2017 . ). Outer Membrane Vesicles Prime and Activate Macrophage Inflammasomes and Cytokine Secretion In Vitro and In Vivo . Front Immunol 8 : 1017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cecil J. D. , O'Brien-Simpson N. M. , Lenzo J. C. , Holden J. A. , Chen Y. Y. , Singleton W. , Gause K. T. , Yan Y. , Caruso F. and Reynolds E. C. ( 2016 . ). Differential Responses of Pattern Recognition Receptors to Outer Membrane Vesicles of Three Periodontal Pathogens . PLoS One 11 ( 4 ): e0151967 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deng Z. L. , Szafranski S. P. , Jarek M. , Bhuju S. and Wagner-Dobler I. ( 2017 . ). Dysbiosis in chronic periodontitis: Key microbial players and interactions with the human host . Sci Rep 7 ( 1 ): 3703 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellis T. N. and Kuehn M. J. ( 2010 . ). Virulence and immunomodulatory roles of bacterial outer membrane vesicles . Microbiol Mol Biol Rev 74 ( 1 ): 81 - 94 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ganther S. , Radaic A. , Malone E. , Kamarajan P. , Chang N. N. , Tafolla C. , Zhan L. , Fenno J. C. and Kapila Y. L. ( 2021 . ). Treponema denticola dentilisin triggered TLR2/MyD88 activation upregulates a tissue destructive program involving MMPs via Sp1 in human oral cells . PLoS Pathog 17 ( 7 ): e1009311 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao L. , Kang M. , Zhang M. J. , Reza Sailani M. , Kuraji R. , Martinez A. , Ye C. , Kamarajan P. , Le C. , Zhan L. , et al. .( 2020 . ). Polymicrobial periodontal disease triggers a wide radius of effect and unique virome . NPJ Biofilms Microbiomes 6 ( 1 ): 10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayashi C. , Gudino C. V. , Gibson F. C. 3rd Genco C. A. ( 2010 . ). Review: Pathogen-induced inflammation at sites distant from oral infection: bacterial persistence and induction of cell-specific innate immune inflammatory pathways . Mol Oral Microbiol 25 ( 5 ): 305 - 16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ho S. P. , Marshall S. J. , Ryder M. I. and Marshall G. W. ( 2007 . ). The tooth attachment mechanism defined by structure, chemical composition and mechanical properties of collagen fibers in the periodontium . Biomaterials 28 ( 35 ): 5238 - 5245 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inagaki S. , Kimizuka R. , Kokubu E. , Saito A. and Ishihara K. ( 2016 . ). Treponema denticola invasion into human gingival epithelial cells . Microb Pathog 94 : 104 - 111 . [DOI] [PubMed] [Google Scholar]
- 15. Jiang N. , Guo W. , Chen M. , Zheng Y. , Zhou J. , Kim S. G. , Embree M. C. , Songhee Song K. , Marao H. F. and Mao J. J. ( 2016 . ). Periodontal Ligament and Alveolar Bone in Health and Adaptation: Tooth Movement . Front Oral Biol 18 : 1 - 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim J. , Yang J. , Park O. J. , Kang S. S. , Kim W. S. , Kurokawa K. , Yun C. H. , Kim H. H. , Lee B. L. and Han S. H. ( 2013 . ). Lipoproteins are an important bacterial component responsible for bone destruction through the induction of osteoclast differentiation and activation . J Bone Miner Res 28 ( 11 ): 2381 - 2391 . [DOI] [PubMed] [Google Scholar]
- 17. Kim K. , Kang H. E. , Yook J. I. , Yu H. S. , Kim E. , Cha J. Y. and Choi Y. J. ( 2020 . ). Transcriptional Expression in Human Periodontal Ligament Cells Subjected to Orthodontic Force: An RNA-Sequencing Study . J Clin Med 9 ( 2 ): 358 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin J. D. , Jang A. T. , Kurylo M. P. , Hurng J. , Yang F. , Yang L. , Pal A. , Chen L. and Ho S. P. ( 2017 . ). Periodontal ligament entheses and their adaptive role in the context of dentoalveolar joint function . Dent Mater 33 ( 6 ): 650 - 666 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marchesan J. T. , Scanlon C. S. , Soehren S. , Matsuo M. and Kapila Y. L. ( 2011 . ). Implications of cultured periodontal ligament cells for the clinical and experimental setting: a review . Arch Oral Biol 56 ( 10 ): 933 - 943 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDowell J. V. , Miller D. P. , Mallory K. L. and Marconi R. T. ( 2012 . ). The Pathogenic Spirochetes: strategies for evasion of host immunity and persistence . Embers, M. E.(Ed.): 43-62. Springer ; . [Google Scholar]
- 21. Miao D. , Fenno J. C. , Timm J. C. , Joo N. E. and Kapila Y. L. ( 2011 . ). The Treponema denticola chymotrypsin-like protease dentilisin induces matrix metalloproteinase-2-dependent fibronectin fragmentation in periodontal ligament cells . Infect Immun 79 ( 2 ): 806 - 811 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyamoto M. , Ishihara K. and Okuda K. ( 2006 . ). The Treponema denticola surface protease dentilisin degrades interleukin-1 beta(IL-1 beta), IL-6, and tumor necrosis factor alpha . Infect Immun 74 ( 4 ): 2462 - 2467 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sano Y. , Okamoto-Shibayama K. , Tanaka K. , Ito R. , Shintani S. , Yakushiji M. and Ishihara K. ( 2014 . ). Dentilisin involvement in coaggregation between Treponema denticola and Tannerella forsythia . Anaerobe 30 : 45 - 50 . [DOI] [PubMed] [Google Scholar]
- 24. Scanlon C. , Marchesan J. , Soehren S. , Matsuo M. and Kapila Y. ( 2011 . ). Capturing the regenerative potential of periodontal ligament fibroblasts . J Stem Cells Regen Med 7 ( 1 ): 54 - 56 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schenk M. , Belisle J. T. and Modlin R. L. ( 2009 . ). TLR2 Looks at Lipoproteins . Immunity 31 : 847 - 849 . [DOI] [PubMed] [Google Scholar]
- 26. Sela M. N. , Bolotin A. , Naor R. , Weinberg A. and Rosen G. ( 1997 . ). Lipoproteins of Treponema denticola : their effect on human polymorphonuclear neutrophils . J Periodontal Res 32 ( 5 ): 455 - 466 . [DOI] [PubMed] [Google Scholar]
- 27. Sokos D. , Everts V. and de Vries T. J. ( 2015 . ). Role of periodontal ligament fibroblasts in osteoclastogenesis: a review . J Periodontal Res 50 ( 2 ): 152 - 159 . [DOI] [PubMed] [Google Scholar]
- 28. Solbiati J. and Frias-Lopez J. ( 2018 . ). Metatranscriptome of the Oral Microbiome in Health and Disease . J Dent Res 97 ( 5 ): 492 - 500 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Souza J. A. C. , Magalhaes F. A. C. , Oliveira G. , RS D. E. M. , Zuanon J. A. and Souza P. P. C. ( 2020 . ). Pam2CSK4(TLR2 agonist) induces periodontal destruction in mice . Braz Oral Res 34 : e012 . [DOI] [PubMed] [Google Scholar]
- 30. Takimoto A. , Kawatsu M. , Yoshimoto Y. , Kawamoto T. , Seiryu M. , Takano-Yamamoto T. , Hiraki Y. and Shukunami C. ( 2015 . ). Scleraxis and osterix antagonistically regulate tensile force-responsive remodeling of the periodontal ligament and alveolar bone . Development 142 ( 4 ): 787 - 796 . [DOI] [PubMed] [Google Scholar]
- 31. Trindade F. , Oppenheim F. G. , Helmerhorst E. J. , Amado F. , Gomes P. S. and Vitorino R. ( 2014 . ). Uncovering the molecular networks in periodontitis . Proteomics Clin Appl 8 ( 9-10 ): 748 - 761 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Veith P. D. , Dashper S. G. , O'Brien-Simpson N. M. , Paolini R. A. , Orth R. , Walsh K. A. and Reynolds E. C. ( 2009 . ). Major proteins and antigens of Treponema denticola . Biochim Biophys Acta 1794 ( 10 ): 1421 - 1432 . [DOI] [PubMed] [Google Scholar]
- 33. Visser M. B. , Koh A. , Glogauer M. and Ellen R. P. ( 2011 . ). Treponema denticola major outer sheath protein induces actin assembly at free barbed ends by a PIP2-dependent uncapping mechanism in fibroblasts . PLoS One 6 ( 8 ): e23736 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilson M. M. and Bernstein H. D. ( 2016 . ). Surface-Exposed Lipoproteins: An Emerging Secretion Phenomenon in Gram-Negative Bacteria . Trends Microbiol 24 ( 3 ): 198 - 208 . [DOI] [PMC free article] [PubMed] [Google Scholar]