Abstract
Human metapneumovirus (HMPV) infection is one of the leading causes of hospitalization in young children with acute respiratory illness. In this study, we prospectively collected respiratory tract samples from children who were hospitalized with acute lower respiratory tract infection in six hospitals in China from 2017 to 2019. HMPV was detected in 145 out of 2733 samples (5.3%) from the hospitalized children. The majority of HMPV-positive children were under the age of two (67.6%), with a median age of one year. HMPV can independently cause acute lower respiratory tract infection in young children, while all patients showed mild clinical symptoms. Of all the co-infected patients, HMPV was most commonly detected with enterovirus (EV) or rhinovirus (RhV) (38.0%, followed by respiratory syncytial virus (RSV) (32.0%). The highest detection rate occurred from March to May in both northern and southern China. Out of 145 HMPV positive samples, 48 were successfully typed, of which 36 strains were subgrouped into subtypes A2c (75%), eight strains were included in subtype B1 (16.7%), and four strains were included in subtype B2 (8.3%). Moreover, 16 A2c strains contained 111-nucleotide duplications in the G gene. Twenty-seven complete HMPV genomes were successfully obtained, and 25 (92.6%) strains belonged to subtype A2c, whereas one strain was included in subgroup B1 and another was included in subgroup B2. A total of 277 mutations were observed in the complete genomes of 25 A2c strains. All results presented here improve our understanding of clinical characteristics and molecular epidemiology of HMPV infection in children.
Keywords: Human metapneumovirus (HMPV), Acute lower respiratory tract infection, Clinical characteristics, Molecular epidemiology, Multicentre prospective study
Highlights
-
•
This is the first research which focus on HMPV multicentre data in China.
-
•
5.3% of HMPV-infected children were detected from 2733 enrolled patients with fever, coughing, sputum, and runny noses.
-
•
Most of HMPV obtained in this study belong to the new Cluster A2c, which might be recombined from A2b and A2a strains.
1. Introduction
Human metapneumovirus (HMPV), discovered in 2001, has been frequently found to be associated with respiratory tract illnesses among infants and children worldwide and usually causes mild and self-limiting infections. However, HMPV also results in severe pneumonia among children, older adults, and persons with underlying chronic conditions, including asthma, cancer, and chronic obstructive pulmonary disease (Edwards et al., 2013). Prevalent worldwide, HMPV is an important respiratory pathogen that manifests in children less than 5 years of age (Gray et al., 2006; Sloots et al., 2006; Legrand et al., 2011; Li et al., 2012; Edwards et al., 2013). The rates of HMPV infection range from 5.5% to 25% among children hospitalized with respiratory illnesses (Gray et al., 2006; Legrand et al., 2011; Edwards et al., 2013). Our previous study from 2015 to 2017 revealed that HMPV was the third most common virus associated with coinfection and accounted for 7.8% (213/2721) or sixth most commonly detected virus in children hospitalized with lower respiratory tract infections (Zhu et al., 2021). However, in this research, only viral detection rates, demographic and clinical features of all common 18 respiratory viruses were described. A multicentre study focused on the clinical and molecular epidemiological characteristics of HMPV in hospitalized children with acute lower respiratory tract infections in China is currently needed.
HMPV is a nonsegmented, negative-stranded RNA virus that belongs to the family Pneumoviridae with a genome of approximately 13 kb in length and contains eight genes (N, P, M, F, M2, SH, G, and L) (Nao et al., 2020). These genes encode a total of nine proteins, including three surface glycoproteins: F (fusion glycoprotein), SH (small hydrophobic protein), and G (attachment glycoprotein). HMPV has one serotype with two genotypes, A and B, that are further divided into six lineages, including A1, A2a, A2b, A2c, B1, and B2, based mainly on variations in the G gene (Tulloch et al., 2021). Until now, there is limited multicentre research on the clinical characteristics and molecular epidemiology of HMPV in children with acute lower respiratory tract infections in China.
In this study, we conducted a multicentre observational study aimed to determine the overall burden of hospitalizations for HMPV among young children in different areas of China and to determine whether there was variation in the epidemic pattern of HMPV. Additionally, phylogenetic, amino acid mutation and recombination analyses were performed on the HMPV sequences to explore the molecular epidemiological characteristics of the virus.
2. Materials and methods
2.1. Patients and clinical samples
We performed a multicentre prospective study with children under the age of 18 years who were admitted to the hospital for acute lower respiratory tract infections from November 2017 to November 2019. Patients were enrolled from six cities, representing six different areas of China. The hospitals included Beijing Children's Hospital in Beijing (central China), Yinchuan Maternal and Child Health Hospital in Yinchuan (north-western China), Shengjing Hospital of China Medical University in Shenyang (north-eastern China), The First Affiliated Hospital of Guangzhou Medical University in Guangzhou (southern China), Yuying Children's Hospital of Wenzhou Medical University in Wenzhou (south-eastern China), and Guiyang Women and Children Healthcare Hospital in Guiyang (south-western China). Patients with nosocomial respiratory infections, or with inhaled airway foreign bodies, and acute upper respiratory infection were excluded. Enrolled children and/or their caregivers were interviewed using a standardized questionnaire and medical charts were abstracted after discharge. Demographic, epidemiologic, and clinical data were systematically collected. Patient clinical records and information were anonymized and deidentified prior to analysis. A standard sampling method for nasopharyngeal aspirates (NPAs) specimen collection was used for all six hospitals as follows: NPAs specimens were collected using sterile flocked Dacron swabs with flexible shafts. Swabs were passed through one nostril to the nasopharynx and rotated to collect epithelial tissue and absorb secretions. Swabs were combined in 3 mL sterile universal transport medium. All specimens were obtained in the first 24 h of hospitalization and stored frozen (−80 °C) until analysis. All patients were enrolled in a network where viral pathogens were monitored in children with acute lower respiratory tract infections, and all the patients' information was submitted to the Infectious Disease Surveillance System of China.
2.2. Molecular detection
Viral nucleic acids in the clinical samples were extracted using the NucliSens easyMAG system (bioMérieux, Marcy-l’Etoile, France) according to the manufacturer's instructions. The Luminex xTAG respiratory viral panel (RVP) assay and Luminex 200 instrument (Luminex, Austin, TX) were used to detect 18 common respiratory viral pathogens and subtypes in the nucleic acid samples, including HMPV, influenza A, influenza A subtype H1, influenza A subtype H3, 2009 H1N1, influenza B, human adenoviruses (HAdVs), human parainfluenza virus (HPIV) 1–4, respiratory syncytial virus (RSV) A and B, enteroviruses and rhinoviruses (EVs/RhVs), human coronavirus (HCoV) HKU1, 229E, NL63 and OC43, and human bocavirus (HBoV). An internal positive control (MS2) was added into each specimen before the nucleic acid extraction, and a positive PCR control (Lambda DNA) was added in every PCR batch run, following the manufacturer's manual instruction. Bacterial infection tests were not performed in our study.
2.3. PCR amplification of the HMPV G gene
For specimens tested HMPV positive by using Luminex xTAG RVP assay, the viral RNA was reverse transcribed into cDNA using a SuperScript™ III First-Strand Synthesis System (Invitrogen, USA). Then, the complete coding sequence of G gene was amplified, using traditional nested PCR with the following primers: hMPV-G-F: 5′-GAGAACATTCGRRCRATWGAYATG -3′, hMPV-G-R1: 5′-AGATARACATTRACAGTRGAYTCA-3′, and hMPV-G-R2: 5′-C AAYAACAGGGTTTTCYAYAGCA-3′ (Yi et al., 2019). The PCR products were purified and sequenced using an ABI3730xl DNA Analyser at Sino Geno Max (Beijing, China).
2.4. HMPV full-length genome sequencing
Total RNA was extracted from the NPAs using a RNeasy Mini Kit (Qiagen, Hilden, Germany). The extracted viral RNA was reverse-transcribed and tagged with index adaptors using the NEB Next Ultra II RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA, USA). The resulting cDNA libraries were verified using the MultiNA System (Shimadzu, Kyoto, Japan) and quantified using a Quantus Fluorometer (Promega, Madison, WI, USA). The indexed libraries were then pooled and sequenced (300-bp paired-end reads) using the MiSeq instrument (Illumina Inc., San Diego, CA, USA). After sequencing, the reads were subjected to de novo assembly using Shovill software (version 1.0.4) with the default settings. Reads with alignments that exhibited both high percent identity and high query coverage were retained, with the exception of reads that aligned with any mitochondrial or plasmid reference sequences. All random distributions of reads covered 100% of the full length HMPV genome.
2.5. Phylogenetic and amino acid substitution analysis
Multiple-sequence alignments were constructed with MAFT (https://www.ebi.ac.uk/Tools/msa/mafft/) software (version 7.407) using the accuracy-oriented method (L-INS-i). Phylogenetic trees were generated using the neighbour-joining method, and bootstrap values of MEGA software version 7 with 1000 replicates were calculated to evaluate confidence estimates. Regions of the F gene were compared to reference strains from the GenBank database to identify amino acid substitutions.
2.6. Recombination analysis
Recombination analysis of the whole HMPV genomic sequences was carried out using Simplot v3.5.1 software with the selected parameters, including a window size of 1000, a step size of 200 and Kimura (two parameter) distance correction.
3. Results
3.1. Enrolled patients and epidemiological analyses
Of the 3114 children enrolled in the network for viral pathogen monitoring, 2733 (87.8%) patients were finally included in this study. Children whose specimens were unavailable for HMPV testing or children whose guardians did not provide consent for long-term specimen storage were excluded. A flowchart of our enrolled patients is shown in Fig. 1. A Luminex xTAG respiratory viral panel was used for pathogen detection, resulting in 1553 (56.8%) virus-positive samples, among which 145 were HMPV-positive (5.3% in total).
Fig. 1.
Flowchart of enrolled patients and viral pathogen-positive patients.
The highest prevalence was shown in Wenzhou City, with a positive rate up to 13.6%. The detection rate in Guiyang City (4.7%) had the second highest prevalence, followed by Cities of Yinchuan (4.1%), Guangzhou (3.8%), Shenyang (3.3%) and Beijing (2.9%) (Table 1). The incidence of HMPV infection seems to be higher in southern China, as deduced from our results. The positivity rate and monthly distribution of HMPV infections in China from 2017 to 2019 are shown in Fig. 2. In northern China, positive rates for HMPV infections varied from 0% to 17.0% across the study period, while in southern China, positive rates varied between 0 and 23.9%. From March to May, significant increases in detection rates occurred both in northern and southern China.
Table 1.
Positive rate of HMPV detected in different cities of China in 2017–2019.
| City | HMPV-positive rates of the three years |
Total (%) | ||
|---|---|---|---|---|
| 2017 (%) | 2018 (%) | 2019 (%) | ||
| Wenzhou | 0 | 41 (13.1) | 20 (16.4) | 61 (13.6) |
| Guangzhou | 3 (1.4) | 16 (8.0) | 4 (2) | 23 (3.8) |
| Guiyang | 1 (2.3) | 8 (4.0) | 9 (7.4) | 18 (4.7) |
| Shenyang | 0 | 6 (4.1) | 4 (3.4) | 10 (3.3) |
| Beijing | 2 (1.2) | 11 (4.2) | 4 (2.4) | 17 (2.9) |
| Yinchuan | 3 (1.6) | 11 (7.0) | 2 (4.7) | 16 (4.1) |
| Total | 9 (1.9) | 93 (6.2) | 43 (5.9) | 145 (5.3) |
Fig. 2.
Monthly distribution of HMPV positive rates in 2017–2019 in north and south China.
3.2. Clinical characteristics of the study cohort
The median age of all enrolled children was 12 months with interquartile age ranged from 6.5 to 34.5 months (Table 2). The age group distribution showed that 67.6% of HMPV-positive children were younger than 23 months of age; moreover, in children under one year of age, the positive rates were 46.2%, which accounted for nearly half of all HMPV-positive children. The majority of HMPV-positive children were male (60.0%). Fever, cough and sputum are the most common symptoms in all age groups, however, younger children showed more respiratory tract symptoms than the elder, and runny nose only seen in children under three years (Table 3). Most of the patients showed signs of pneumonia in chest radiographs, featured as light transmittance changes and cloudy flocculent shadows in lungs. The most common diagnosis by the time of discharge was pneumonia (75.2%). The median duration of hospitalization was six days. All of our patients showed mild clinical symptoms, and none of them were admitted to the paediatric intensive care unit (PICU) during their stay in the hospital. No family clustering and reinfection of HMPV were found in this study.
Table 2.
Clinical and demographic Characteristics of children with HMPV infections.
| HMPV-Positive (%, n = 145) | |
|---|---|
| Age of month | |
| Median | 12 |
| Interquartile range | 6.5–34.5 |
| Age group-no. (%) | |
| <6 m | 28 (19.3) |
| 6–11 m | 39 (26.9) |
| 12–23 m | 31 (21.4) |
| 24–35 m | 10 (6.9) |
| 36–59 m | 25 (17.2) |
| ≥60 m | 12 (8.3) |
| Gender- no. (%) | |
| Female | 58 (40.0) |
| Male | 87 (60.0) |
| Hospitalization characteristics-no. | |
| Length of stay-median days | 6 |
| Interquartile range for length of stay | 4–7 |
| ICU admission | 0 |
| Death | 0 |
| Co-morbidity diseases | |
| Congenital heart disease | 4 (2.8) |
| Bronchial asthma | 2 (1.4) |
| After surgery | 1 (0.7) |
| Familial genetic disease | 3 (2.1) |
| Final diagnosis-no.(%) | |
| Pneumonia | 109 (75.2) |
| Bronchiolitis | 22 (15.2) |
| Bronchitis | 1 (0.7) |
Table 3.
Clinical symptoms of children in different age groups with HMPV infections.
| Age of month | No. of fever (%) | No. of cough (%) | No. of sputum (%) | No. of runny nose (%) |
|---|---|---|---|---|
| <6 m | 10 (35.7) | 28 (100) | 9 (32.1) | 5 (17.9) |
| 6–11 m | 15 (38.5) | 31 (79.5) | 7 (17.9) | 4 (10.2) |
| 12–23 m | 15 (48.4) | 25 (80.7) | 5 (16.1) | 5 (16.1) |
| 24–35 m | 6 (60.0) | 9 (90.0) | 1 (10.0) | 1 (10.0) |
| 36–59 m | 8 (32.0) | 15 (60.0) | 5 (20.0) | 0 (0) |
| ≥ 60 m | 6 (50.0) | 7 (58.3) | 3 (25.0) | 0 (0) |
| Total | 60 (41.4%) | 115 (79.3%) | 30 (20.7%) | 15 (10.3%) |
3.3. Coinfection with other respiratory viruses
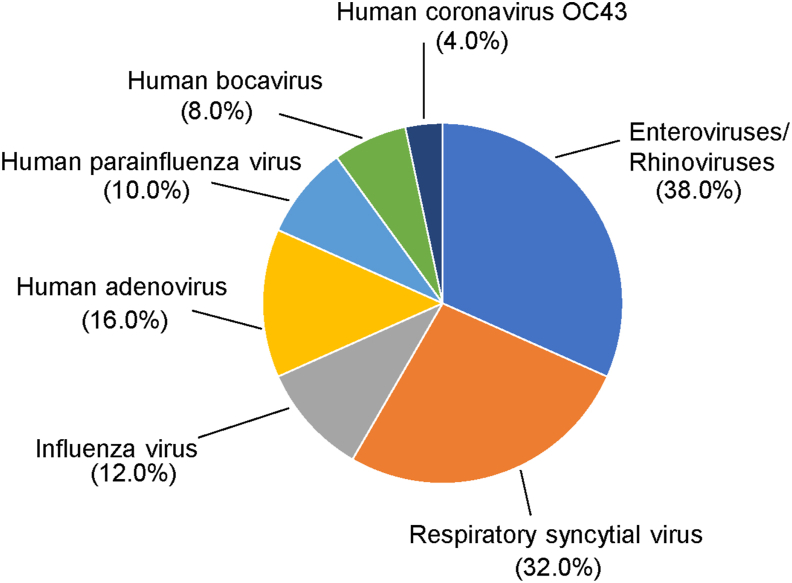
Among the 145 HMPV-infected patients, 50 (34.5%) were coinfected with other respiratory viruses (Table 4). Forty-one of the patients were coinfected with only one virus, and eight patients were coinfected with three viruses. Only one patient was coinfected with four viruses. However, all these multipathogen infections showed no special clinical characteristics compared with single pathogen infections. Coinfection of HMPV with EV or RhV was most common (38.0%, but EV and RhV were not further differentiated), followed by RSV (16/50, 32.0%), HAdV (8/50, 16%), IFV (6/50, 12.0%), PIV (5/50, 10.0%), HBoV (4/50, 8.0%), OC43 (2/50, 4.0%), and Mycoplasma pneumoniae (1.8%, detected based on a clinical test in the routine care and not obtained as part of this study, Fig. 3).
Table 4.
Coinfection of HMPV and other respiratory viruses detected in the study.
| Viruses detected in coinfection | No. (%, n = 145) | |
|---|---|---|
| Single infection | 95 (65.5) | |
| Coinfection | ||
| 1 virus | 41 (28.3) | |
| HMPV + EV/RhV | 14 (9.7) | |
| HMPV + RSV | 12 (8.3) | |
| HMPV + IFV | 4 (2.8) | |
| HMPV + HAdV | 4 (2.8) | |
| HMPV + HBoV | 4 (2.8) | |
| HMPV + PIV | 2 (1.4) | |
| HMPV + OC43 | 1 (1.4) | |
| 2 viruses (n = 8) | ||
| HMPV + EV/RhV + RSV | 1 (1.4) | |
| HMPV + EV/RhV + HAdV | 1 (1.4) | |
| HMPV + EV/RhV + IFV | 1 (1.4) | |
| HMPV + EV/RhV + PIV | 1 (1.4) | |
| HMPV + RSV + HAdV | 1 (1.4) | |
| HMPV + RSV + PIV | 1 (1.4) | |
| HMPV + HAdV + IFV | 1 (1.4) | |
| HMPV + HAdV + PIV | 1 (1.4) | |
| 3 viruses (n = 1) | 1 (1.4) | |
| HMPV + RSV + OC43+ EV/RhV | 1 (1.4) | |
| Total | 145 | |
Fig. 3.
Percentage of coinfection of HMPV and other respiratory viruses detected in the study.
3.4. Sequence alignment and phylogenetic analysis
The G gene of the HMPV-positive samples was amplified, and the PCR products were sequenced and subjected to BLAST analysis. Out of 145 positive samples, 48 were successfully typed (Table 5). The results revealed that 36 out of the 48 strains belonged to the A2c subtype (75.0%), 8 were typed as B1 (16.7%), and 4 were typed as B2 (8.3%).
Table 5.
Time and spatial distributions of 48 HMPV G gene sequences amplified from 145 positive samples.
| Wenzhou (WZ) | Guangzhou (GZFE) | Beijing (BCH) | Yinchuan (YC) | Guiyang (GY) | Total | |
|---|---|---|---|---|---|---|
| 2017 | 1 | 8 | – | – | – | 9 |
| 2018 | 11 | – | 4 | 1 | 4 | 20 |
| 2019 | 14 | 2 | 2 | 1 | – | 19 |
| Total | 26 | 10 | 6 | 2 | 4 | 48 |
Twenty-seven complete HMPV genomes were successfully obtained by high-throughput metagenomic whole genome sequencing method. The whole-genome sequences of all 27 strains have been deposited in GenBank under accession numbers OM262393 to OM262418 and OK644703 (Supplementary Table S1). Time and spatial distributions of the 27 strains were shown in Table 6. Phylogenetic analysis was conducted with the 27 complete genomes (Fig. 4) and their F/G/SH genes respectively (Figs. S1A–C). Reference genomes were downloaded from the GenBank database.
Table 6.
Time and spatial distributions of 27 HMPV strains with full-length genome sequences.
| Wenzhou (WZ) | Guangzhou (GZFE) | Beijing (BCH) | Yinchuan (YC) | Guiyang (GY) | Total | |
|---|---|---|---|---|---|---|
| 2017 | – | 5 | – | – | 1 | 6 |
| 2018 | 5 | – | 3 | 1 | 2 | 11 |
| 2019 | 7 | 2 | 1 | – | – | 10 |
| Total | 12 | 7 | 4 | 1 | 3 | 27 |
Fig. 4.
Phylogenetic tree constructed based on the complete genome. The phylogenetic tree was generated using the neighbour-joining method based on the Kimura two-parameter model with 1000 replicates. The strains isolated in different years are marked by • with different colours: strains from 2017 are marked with blue, strains from 2018 are marked with green, and strains from 2019 are marked with purple. Sequences were named with abbreviations of cities they were collected. BCH, WZ, GZFE, GY and YC were abbreviation of samples from Beijing Children's Hospital (Beijing), the 2nd Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University (Wenzhou), Guangzhou Women and Children's Medical Center (Guangzhou), Guiyang Women and Children Healthcare Hospital (Guiyang) and Yinchuan Maternal and Child Health Hospital (Yinchuan) respectively.
The complete genome sequence alignment showed that 25 out of the 27 (92.6%) strains belonged to subtype A2c, whereas only two strains belonged to genotype B, with one in subgroup B1 and the other in subgroup B2. Both genotype B strains were obtained from Guangzhou in 2019. Phylogenetic trees constructed by the F gene, G gene and SH gene were consistent with the results obtained based on the whole genome sequence, while there was closer genetic distance between groups in the F gene tree than in the G gene tree.
3.5. Nucleotide duplication of the G gene
180- or 111-nucleotide duplications (180 nt-dup or 111 nt-dup) in the G gene have been reported as unique variants of HMPV A2c strains in recent years (Saikusa et al., 2019) and were mainly detected in Spain, Japan, Croatia, and Guangzhou City of China. In our study, we identified 16 strains containing 111 nt-dup variants, and none of the viruses contained 180 nt-dup variants in the G gene. The 16 strains shared the same duplications in the same region (duplicated in 475–585 bp of the G gene, Fig. 5). These 16 A2c strains with 111 nt-dup were separated from each other in the phylogenetic tree. Of all the 111 nt-dup strains, only 1 was derived from 2017 (1/9, 11.1% of all the year), 5 from 2018 (5/93, 5.4% of the year) and 10 from 2019 (10/43, 23.3% of the year). Patients infected with 111 nt-dup strains did not show any particular symptoms compared with patients infected with nonduplication strains.
Fig. 5.
Sequence alignment of 111 nt-dup in the G gene of the 16 HMPV strains of A2c subgroup isolated in this study. Reference strains were chosen according to published literatures. Reference strains for A1 subtype (GenBank accession number NC_039199.1) were isolated in Netherlands in 2001; reference strains for A2a and A2b subtypes were isolated from USA (2003), China (2010) respectively; B1 and B2 strains were both isolated from Netherlands, 2008. A2c subtype does not have a recognized reference sequence until now.
3.6. Amino acid variation analysis
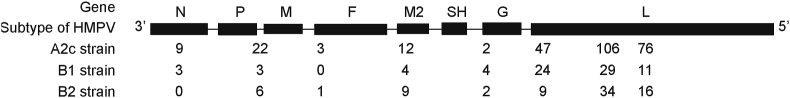
As shown in Fig. 6, we analyzed the amino acid variations in all 27 genome-wide sequences. Twenty-five A2c sequences aligned with the HMPV reference sequence (A1 subtype, GenBank accession number NC_039199.1) and 277 amino acid variations were found in the coding region, including 106 mutations in the G gene, 76 in the L gene, and 47 in the SH gene. The B1 and B2 strains were compared with the reference sequence of the subtype (B1 subtype reference sequence GenBank accession number: AY525843; B2 subtype reference sequence GenBank accession number: FJ168778), and 78 and 77 mutations were found, respectively. The mutations were concentrated in the G gene, L gene and SH gene (Fig. 6).
Fig. 6.
Amino acid variations in all 27 genome-wide sequences of HMPV strains isolated in this study. Twenty-five A2c sequences aligned with the HMPV reference sequence (A1 subtype, GenBank accession number NC_039199.1). The B1 and B2 strains were compared with the reference sequence of the subtype (B1 subtype reference sequence GenBank accession number: AY525843; B2 subtype reference sequence GenBank accession number: FJ168778).
The F gene is relatively conserved as the antigen neutralization site. We further compared the F genes of our strains with the HMPV prototype strain (GenBank accession number NC_039199.1), and the 25 A2c strains in this study had 11 amino acid substitutions in the F protein coding region (Table 7). Seven mutations were found in most A strains submitted worldwide (Ala185Asp, Phe258Ile, Gly294Glu, Ser466Asn, Thr504Ser, Val510Ile, and Asn539Ser). The frequency of the Lys518Arg mutation in our sequences was 88.0%, parallel to only 20.7% in all HMPV A strains.
Table 7.
Amino acid variations in the F gene region of 25 strains from HMPV A2c subgroup isolated in this study.
| Amino acid site | Amino acid substitutions | Frequency (%, n = 25) | Other F gene from Group A in the GenBank database (%, n = 184) |
|---|---|---|---|
| 171 | Lys171Arg | 2 (8.0) | 4 (2.2) |
| 185 | Ala185Asp | 25 (100.0) | 167 (90.8) |
| 258 | Phe258Ile | 25 (100.0) | 184 (100.0) |
| 294 | Gly294Glu | 25 (100.0) | 180 (97.8) |
| 466 | Ser466Asn | 25 (100.0) | 164 (89.1) |
| 495 | Ile495Thr | 1 (4.0) | 1 (0.5) |
| 504 | Thr504Ser | 25 (100.0) | 168 (91.3) |
| 510 | Val510Ile | 25 (100.0) | 128 (69.6) |
| 511 | Phe511Leu | 1 (4.0) | 0 |
| 518 | Lys518Arg | 22 (88.0) | 38 (20.7) |
| 539 | Asn539Ser | 25 (100.0) | 156 (84.8) |
3.7. Recombination analysis of the A2c subgroup
A2c subgroup (also named as A2b2 subgroup) was described as a novel HMPV clade in some articles (Nidaira et al., 2012), which was considered to be a subdivision of A2b subtype (Nao et al., 2020). However, there was no recombination analysis conducted for the new clade yet. We performed recombination analysis on 25 complete genome sequences belonging to the A2c subgroup together with reference strains from every subgroup (no reference sequence for A2c subgroup in GenBank database, Fig. 7). The results revealed that recombination events occurred between the genome sequences of the representative strains of each subgroup. As indicated in the results from SimPlot scans, the sequences isolated in this study showed the highest similarity and the closest phylogenetic relationship with the A2b subtype; however, in the P, M, G and L gene segments, the strains showed lower similarity with A2b representative strains. The four segments may be recombined from the A2a subtype. The bootscanning results further confirmed the phylogenetic relationships.
Fig. 7.
Recombination analysis of 25 A2c subgroup sequences of HMPV strains from China, 2017–2019. (A) Simplot and (B) Bootscan analysis of the whole genomes of the A2c strain compared with all the prototype sequences from every subtype. Recombination analysis was performed by using Simplot with the following inputs: the window size (1000 nucleotides (nt)), step size (200 nt), distance model (Kimura), and tree model (neighbour-joining). The GenBank accession numbers of the prototype strains of each HMPV are as follows: A1 strain, AF371337; A2a strain, AY297749; A2b strain, DQ843659; B1 strain, AY525843; and B2 strain, FJ168778.
4. Discussion
HMPV has been identified as a cause of acute lower respiratory tract infections since it was discovered in 2001; however, according to a serological study, HMPV infections have existed for at least 70 years (Van Den Hoogen et al., 2001). Almost all children eventually become infected with HMPV, and adults can be reinfected by HMPV throughout their lives due to incomplete immunity (Haas et al., 2013; Schuster and Williams, 2014). The prevalence of HMPV among patients hospitalized with acute respiratory infection (ARI) is approximately 6% worldwide according to previous studies based on meta-analyses (Lefebvre et al., 2016; Divarathna et al., 2020). A multicentre study in China from 2009 to 2021 showed HMPV was responsible for 2.54% of all 11,660 febrile respiratory syndrome cases (Cui et al., 2021). In 1460 ARI patients in Guangzhou from January 2013 to December 2017, HMPV was detected in 5.2% samples (Yi et al., 2019). 4.1% of ARI patients were positive for HMPV in Beijing from April 2017 to March 2018 (Wang et al., 2021). During October 2017 to June 2019, HMPV was identified in 7.1% of severe ARI patients in Luohe, China (Xie et al., 2021a, Xie et al., 2021b). In our multicentre prospective study of 2733 hospitalized paediatric patients with ARI, HMPV was detected in 5.3% of the respiratory specimens from 2017 to 2019. This prevalence was consistent with previous studies (Sloots et al., 2006; Legrand et al., 2011; Li et al., 2012; Arnott et al., 2013; Edwards et al., 2013; Lefebvre et al., 2016). The differences in positive rates may be related to the enrolled population, detection methods, regional economic level, study time, sample size or other factors. A significant difference in the detection rates among age groups was observed, with patients aged ≤ 2 years (67.6%) having a higher rate of detection, especially in children under one year of age (46.2%). The positive rate decreased as patients became older, which might be because HMPV infections in older children usually lead to mild symptoms and hospitalization is unnecessary.
The clinical features of HMPV infections in our study were consistent with previous reports (Edwards et al., 2013). HMPV-infected patients showed mild respiratory symptoms, the majority of HMPV-positive children presented pneumonia and stayed in the hospital for 4–7 days, the median duration of hospitalization was 6 days, and none of the children were admitted to the ICU. According to some studies, HMPV infections seemed to be associated with morbidity and mortality in high-risk groups, whether children or adults (Choi et al., 2019). Here, almost all the children enrolled with normal immune system function, 10 patients were immunocompromised for different reasons, however, all of whom showed mild and self-limiting symptoms and recovered completely.
Vincente et al. found that the clinical severity of HMPV A infections was higher than that of HMPV B infections (Vicente et al., 2006). Matsuzaki et al. indicated that wheezing was more prevalent with both HMPV B1 and B2 infections, whereas laryngitis was associated more with HMPV B1 infection (Tsukagoshi et al., 2013). There were also studies that showed no direct association between the genotypes and the clinical courses (Papenburg et al., 2013). However, in Asia, patients with severe HMPV infections have rarely been reported, which may be determined by the pathogenicity of dominant epidemic strains (Xie et al., 2021a, Xie et al., 2021b).
The positive rate observed in Wenzhou was much higher than that in other areas, which indicated that it may be a local small-scale epidemic of HMPV infections. We compared the monthly distribution of HMPV positive rates in southern and northern China, and similar seasonal patterns were observed in different climatic zones. Both in northern and southern China, HMPV was detected mainly from March through May, which was consistent with results of other HMPV studies in China (Kong et al., 2016, Xie et al., 2021a, Xie et al., 2021b). However, some areas did not have samples in some months, which made the positive rate decrease sharply (for example, January 2019, northern China).
Patients with HMPV who are coinfected with other respiratory viruses have been reported to be more likely to have a fever, leading to severe pneumonia. We reported that the coinfection proportion was 34.5%, with EV or RhV being the most frequently coinfecting viruses. However, because of few specimens obtained, we could not further classify EV or RhV infections separately. Eight children were coinfected with three viruses, and their coinfection pathogens were different from each other and showed no specific infection patterns or clinical characteristics. There are also studies reported that the viral load of asymptomatic children infected with HMPV is significantly lower than that of symptomatic children, which needs to be further verified (Bosis et al., 2008). One study demonstrated that children with HMPV had higher risks of bacteraemia (Choe et al., 2020); however, we did not collect clinical examination results about patients’ bacterial pathogens in this study.
The G gene sequences of 48 strains of HMPV obtained in this study showed the co-prevalence of three genotypes, among which the A2c genotype dominated, which was consistent with the results of HMPV studies in China in recent years (Yi et al., 2019; Wang et al., 2021, Xie et al., 2021a, Xie et al., 2021b). This subtype was described as a novel HMPV clade in recent years and was previously described as the A2b-2 subtype in some studies (Nao et al., 2019). Naganori Nao et al. constructed Markov chain Monte Carlo (MCMC) trees with HMPV F and G genes, indicating that the most recent common ancestor (tMRCA) of HMPV subtype A2b-2 was more recent, with estimates ranging from 2002 to 2005, much later than those of subtype A2a (1989–1994) and subtype A2b (1980–1996) (Nao et al., 2020). According to a systematic review, the presence of many HMPV genotypes (A1, A2, B1, B2) and sub-genotypes (A2a, A2b, A2c, B2a, B2b) suggests a rapid evolution of the virus with limited influence by time and geography from 9/54 African, 11/35 American, 20/50 Asian, 2/14 Australian/Oceanian and 20/51 European countries from 1976 to 2012 (Divarathna et al., 2020). In Europe, a study in Croatia from 2011 to 2014 showed the emergence of the A2c genotype and with no detection of A1. The epidemiological study of HMPV in Asia began in Israel from 2002 to 2004 (Regev et al., 2006). After 2005, clusters of HMPV mainly composed by strains in A2b, and A2c lineages became dominant in Asian countries. A study in Wuhan, China, from 2008 to 2013 showed the detection rate of A1 genotype gradually decreased, and finally vanished, while the detection rate of A2c gradually increased (Kong et al., 2016). More recently, A2c was the predominant lineage identified in HMPV epidemics in Guangzhou, China, 2013–2015 (Yi et al., 2019).
180-nt and 111-nt duplicate variants in the G gene were first reported in Japan (Saikusa et al., 2017, 2019) and then detected in different regions of the world, including Spain, Japan, Croatia, and Guangzhou, China (Pinana et al., 2017; Saikusa et al., 2017; Yi et al., 2019). A recent study also found that HMPV subtype A2c variants 111 nt and 180 nt duplications predominated in the central region of China from 2017 to 2019 (Xie et al., 2021b). We also found 16 sequences with 111-nt dup variants in our study, which demonstrated their continuing geographic spread in China. However, these 111-nt dup variants dispersed with each other in the phylogenetic tree, which indicates that the duplication variants evolved by independent patterns in different evolutionary lineages. Although no difference in clinical symptoms was observed between the patients infected with HMPV duplication variants and those with nonduplication-variant viruses in this study, the increased transmission frequency of the duplication variants suggests a role of duplication in the G gene in potentially expanding its transmission. Similar nucleotide duplicate variants had been reported in the G gene of RSV, an important member of Pneumovirus family, and the two variants spread quickly and finally dominated the season (Song et al., 2017a, 2017b). Therefore, further study is needed to clarify whether and how duplications result in an evolutionary advantage for HMPV.
The recombination of subtype A2c had not been studied since it was separated from the A2b branch, so we conducted a recombination analysis with 25 A2c strains isolated in our study. The results demonstrated that the A2c strain was based on the structure of A2b, with partial regions of the P gene, M gene and L gene recombined from the A2a strain.
The F protein is an essential protein for viral adsorption and fusion and is a major target antigen for neutralizing antibodies (Burstein et al., 2019). We compared all of our genome sequences with reference sequences from every clade. Most mutations were found in the G gene, followed by the SH gene and L gene, revealing that the F gene was relatively conserved and can be a target for vaccine production. However, the meaning of these mutations is still not clear. Further research based on infectious clone, single and multiple site mutations, and cross-neutralization experiments are needed to detect whether the pathogenicity and antigenicity of the virus have changed.
There were also some limitations of this study. We enrolled patients from six areas in China, but these regions could still not completely reflect the national epidemic pattern; in particular, some regions had no samples collected in some months, which may influence the results of our study. For the coinfection analysis, we detected viral pathogens only, lacking information about bacterial coinfections. Only 48 out of the 145 positive samples were successfully typed. Due to the low volume of samples, we could not amplify the G gene of all the positive samples to conduct further analysis.
5. Conclusions
This is the first research which focuses on HMPV multicentre data in China. In this study, we detected 5.3% of HMPV-infected children from a total of 2733 enrolled patients with clinical symptoms of fever, coughing, sputum, and runny noses. The virus can cause acute lower respiratory tract infections independently, and coinfection with EV or RhV was most common. The highest detection rate occurred from March to May in both northern and southern China. The molecular epidemiology analysis demonstrated that most of our sequences belong to the new Cluster A2c, which might be recombined from the A2b and A2a strains.
Data availability
The datasets supporting the conclusions of this article are included within the article.
Ethics statement
This study was performed in strict accordance with the human subject protection guidance and was approved by the Ethical Review Committees of Beijing Children's Hospital, Guangzhou Women and Children's Medical Center, Yinchuan Maternal and Child Health Hospital, Yuying Children's Hospital of Wenzhou Medical University, Guiyang Women and Children Healthcare Hospital, and Shengjing Hospital of China Medical University. Written informed consent was obtained from the participants (≥ 8 years) or their parents/guardian (< 8 years).
Author contributions
Hongwei Zhao: investigation, visualization, writing – original draft. Qianyu Feng: data curation, formal analysis, writing – original draft. Ziheng Feng: data curation, formal analysis. Yun Zhu: data curation, formal analysis. Junhong Ai: data curation, formal analysis. Baoping Xu: resources, data curation. Li Deng: resources, data curation. Yun Sun: resources, data curation. Changchong Li: resources, data curation. Rong Jin: resources, data curation. Yunxiao Shang: resources, data curation. Xiangpeng Chen: Validation. Lili Xu: conceptualization, supervision, writing – original draft, review & editing. Zhengde Xie: funding acquisition, project administration.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (82172275) and the CAMS Innovation Fund for Medical Sciences, China (CIFMS, 2019-I2M-5-026).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virs.2022.08.007.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Arnott A., Vong S., Sek M., Naughtin M., Beaute J., Rith S., Guillard B., Deubel V., Buchy P. Genetic variability of human metapneumovirus amongst an all ages population in Cambodia between 2007 and 2009. Infect. Genet. Evol. 2013;15:43–52. doi: 10.1016/j.meegid.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosis S., Esposito S., Osterhaus A.D., Tremolati E., Begliatti E., Tagliabue C., Corti F., Principi N., Niesters H.G. Association between high nasopharyngeal viral load and disease severity in children with human metapneumovirus infection. J. Clin. Virol. 2008;42:286–290. doi: 10.1016/j.jcv.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R., Henry N.J., Collison M.L., Marczak L.B., Sligar A., Watson S., Marquez N., Abbasalizad-Farhangi M., Abbasi M., Abd-Allah F., et al. Mapping 123 million neonatal, infant and child deaths between 2000 and 2017. Nature. 2019;574:353–358. doi: 10.1038/s41586-019-1545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe Y.J., Park S., Michelow I.C. Co-seasonality and co-detection of respiratory viruses and bacteraemia in children: a retrospective analysis. Clin. Microbiol. Infect. 2020;26 doi: 10.1016/j.cmi.2020.09.006. 1690 e1695-1690 e1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.H., Hong S.B., Huh J.W., Jung J., Kim M.J., Chong Y.P., Kim S.H., Sung H., Koo H.J., Do K.H., Lee S.O., Lim C.M., Kim Y.S., Woo J.H., Koh Y. Outcomes of severe human metapneumovirus-associated community-acquired pneumonia in adults. J. Clin. Virol. 2019;117:1–4. doi: 10.1016/j.jcv.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui A., Xie Z., Yu P., Zhu R., Ma Y., Xiang X., Zhang L., Zhu Y., Wu J., Gao Z., Zhang R., Han G., Xu W., Zhang Y. Viral infection and epidemiological characteristics of human metapneumovirus in febrile respiratory syndrome cases in nine provinces in China from 2009 to 2021. Chin. J. Appl. Clin. Pediatr. 2021;36:1861–1865. (in Chinese) [Google Scholar]
- Divarathna M.V.M., Rafeek R.a.M., Noordeen F. A review on epidemiology and impact of human metapneumovirus infections in children using TIAB search strategy on PubMed and PubMed Central articles. Rev. Med. Virol. 2020;30:e2090. doi: 10.1002/rmv.2090. [DOI] [PubMed] [Google Scholar]
- Edwards K.M., Zhu Y., Griffin M.R., Weinberg G.A., Hall C.B., Szilagyi P.G., Staat M.A., Iwane M., Prill M.M., Williams J.V., New Vaccine Surveillance, N. Burden of human metapneumovirus infection in young children. N. Engl. J. Med. 2013;368:633–643. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G.C., Capuano A.W., Setterquist S.F., Erdman D.D., Nobbs N.D., Abed Y., Doern G.V., Starks S.E., Boivin G. Multi-year study of human metapneumovirus infection at a large US Midwestern Medical Referral Center. J. Clin. Virol. 2006;37:269–276. doi: 10.1016/j.jcv.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas L.E., Thijsen S.F., Van Elden L., Heemstra K.A. Human metapneumovirus in adults. Viruses. 2013;5:87–110. doi: 10.3390/v5010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W., Wang Y., Zhu H., Lin X., Yu B., Hu Q., Yang X., Guo D., Peng J., Zhou D. Circulation of human metapneumovirus among children with influenza-like illness in Wuhan, China. J. Med. Virol. 2016;88:774–781. doi: 10.1002/jmv.24411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre A., Manoha C., Bour J.B., Abbas R., Fournel I., Tiv M., Pothier P., Astruc K., Aho-Glele L.S. Human metapneumovirus in patients hospitalized with acute respiratory infections: a meta-analysis. J. Clin. Virol. 2016;81:68–77. doi: 10.1016/j.jcv.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand L., Vabret A., Dina J., Petitjean-Lecherbonnier J., Stephanie G., Cuvillon D., Tripey V., Brouard J., Freymuth F. Epidemiological and phylogenic study of human metapneumovirus infections during three consecutive outbreaks in Normandy, France. J. Med. Virol. 2011;83:517–524. doi: 10.1002/jmv.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang Z., Gonzalez R., Xiao Y., Zhou H., Zhang J., Paranhos-Baccala G., Vernet G., Jin Q., Wang J., Hung T. Prevalence of human metapneumovirus in adults with acute respiratory tract infection in Beijing, China. J. Infect. 2012;64:96–103. doi: 10.1016/j.jinf.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nao N., Saikusa M., Sato K., Sekizuka T., Usuku S., Tanaka N., Nishimura H., Takeda M. Recent molecular evolution of human metapneumovirus (HMPV): subdivision of HMPV A2b strains. Microorganisms. 2020;8 doi: 10.3390/microorganisms8091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nao N., Sato K., Yamagishi J., Tahara M., Nakatsu Y., Seki F., Katoh H., Ohnuma A., Shirogane Y., Hayashi M., Suzuki T., Kikuta H., Nishimura H., Takeda M. Consensus and variations in cell line specificity among human metapneumovirus strains. PLoS One. 2019;14:e0215822. doi: 10.1371/journal.pone.0215822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nidaira M., Taira K., Hamabata H., Kawaki T., Gushi K., Mahoe Y., Maeshiro N., Azama Y., Okano S., Kyan H., Kudaka J., Tsukagoshi H., Noda M., Kimura H. Molecular epidemiology of human metapneumovirus from 2009 to 2011 in Okinawa, Japan. Jpn. J. Infect. Dis. 2012;65:337–340. doi: 10.7883/yoken.65.337. [DOI] [PubMed] [Google Scholar]
- Papenburg J., Carbonneau J., Isabel S., Bergeron M.G., Williams J.V., De Serres G., Hamelin M.E., Boivin G. Genetic diversity and molecular evolution of the major human metapneumovirus surface glycoproteins over a decade. J. Clin. Virol. 2013;58:541–547. doi: 10.1016/j.jcv.2013.08.029. [DOI] [PubMed] [Google Scholar]
- Pinana M., Vila J., Gimferrer L., Valls M., Andres C., Codina M.G., Ramon J., Martin M.C., Fuentes F., Saiz R., Alcubilla P., Rodrigo C., Pumarola T., Anton A. Novel human metapneumovirus with a 180-nucleotide duplication in the G gene. Future Microbiol. 2017;12:565–571. doi: 10.2217/fmb-2016-0211. [DOI] [PubMed] [Google Scholar]
- Regev L., Hindiyeh M., Shulman L.M., Barak A., Levy V., Azar R., Shalev Y., Grossman Z., Mendelson E. Characterization of human metapneumovirus infections in Israel. J. Clin. Microbiol. 2006;44:1484–1489. doi: 10.1128/JCM.44.4.1484-1489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikusa M., Kawakami C., Nao N., Takeda M., Usuku S., Sasao T., Nishimoto K., Toyozawa T. 180-Nucleotide duplication in the G gene of human metapneumovirus A2b subgroup strains circulating in Yokohama city, Japan, since 2014. Front. Microbiol. 2017;8:402. doi: 10.3389/fmicb.2017.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikusa M., Nao N., Kawakami C., Usuku S., Tanaka N., Tahara M., Takeda M., Okubo I. Predominant detection of the subgroup A2b human metapneumovirus strain with a 111-nucleotide duplication in the G gene in Yokohama city, Japan in 2018. Jpn. J. Infect. Dis. 2019;72:350–352. doi: 10.7883/yoken.JJID.2019.124. [DOI] [PubMed] [Google Scholar]
- Schuster J.E., Williams J.V. Human Metapneumovirus. Microbiol. Spectr. 2014;2 doi: 10.1128/microbiolspec.AID-0020-2014. [DOI] [PubMed] [Google Scholar]
- Sloots T.P., Mackay I.M., Bialasiewicz S., Jacob K.C., Mcqueen E., Harnett G.B., Siebert D.J., Masters I.B., Young P.R., Nissen M.D. Human metapneumovirus, Australia, 2001–2004. Emerg. Infect. Dis. 2006;12:1263–1266. doi: 10.3201/eid1208.051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Wang H., Shi J., Cui A., Huang Y., Sun L., Xiang X., Ma C., Yu P., Yang Z., Li Q., Ng T.I., Zhang Y., Zhang R., Xu W. Emergence of BA9 genotype of human respiratory syncytial virus subgroup B in China from 2006 to 2014. Sci. Rep. 2017;7:16765. doi: 10.1038/s41598-017-17055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Zhang Y., Wang H., Shi J., Sun L., Zhang X., Yang Z., Guan W., Zhang H., Yu P., Xie Z., Cui A., Ng T.I., Xu W. Emergence of ON1 genotype of human respiratory syncytial virus subgroup A in China between 2011 and 2015. Sci. Rep. 2017;7:5501. doi: 10.1038/s41598-017-04824-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi H., Ishioka T., Noda M., Kozawa K., Kimura H. Molecular epidemiology of respiratory viruses in virus-induced asthma. Front. Microbiol. 2013;4:278. doi: 10.3389/fmicb.2013.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch R.L., Kok J., Carter I., Dwyer D.E., Eden J.S. An amplicon-based approach for the whole-genome sequencing of human metapneumovirus. Viruses. 2021;13 doi: 10.3390/v13030499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Hoogen B.G., De Jong J.C., Groen J., Kuiken T., De Groot R., Fouchier R.A., Osterhaus A.D. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente D., Montes M., Cilla G., Perez-Yarza E.G., Perez-Trallero E. Differences in clinical severity between genotype A and genotype B human metapneumovirus infection in children. Clin. Infect. Dis. 2006;42:e111–113. doi: 10.1086/504378. [DOI] [PubMed] [Google Scholar]
- Wang C., Wei T., Ma F., Wang H., Guo J., Chen A., Huang Y., Xie Z., Zheng L. Epidemiology and genotypic diversity of human metapneumovirus in paediatric patients with acute respiratory infection in Beijing, China. Virol. J. 2021;18:40. doi: 10.1186/s12985-021-01508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Xu J., Ren Y., Cui A., Wang H., Song J., Zhang Q., Hu M., Xu W., Zhang Y. Emerging human metapneumovirus gene duplication variants in patients with severe acute respiratory infection, China, 2017-2019. Emerg. Infect. Dis. 2021;27:275–277. doi: 10.3201/eid2701.201043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z.B., Xu J., Ren Y.H., Cui A.L., Wang H.L., Song J.H., Zhang Q., Hu M.L., Xu W.B., Zhang Y. Emerging human metapneumovirus gene duplication variants in patients with severe acute respiratory infection, China, 2017-2019. Emerg. Infect. Dis. 2021;27:275–277. doi: 10.3201/eid2701.201043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L., Zou L., Peng J., Yu J., Song Y., Liang L., Guo Q., Kang M., Ke C., Song T., Lu J., Wu J. Epidemiology, evolution and transmission of human metapneumovirus in Guangzhou China, 2013-2017. Sci. Rep. 2019;9:14022. doi: 10.1038/s41598-019-50340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Xu B., Li C., Chen Z., Cao L., Fu Z., Shang Y., Chen A., Deng L., Bao Y., Sun Y., Ning L., Yu S., Gu F., Liu C., Yin J., Shen A., Xie Z., Shen K. A multicenter study of viral aetiology of community-acquired pneumonia in hospitalized children in Chinese mainland. Virol. Sin. 2021;36:1543–1553. doi: 10.1007/s12250-021-00437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.