Abstract
With the outbreak of the new coronavirus disease 2019 (COVID-19), the rapid spread of the virus has brought huge economic losses and life threats to the world. So far, we have entered the third year of the epidemic and there is an urgent need to provide more anti-viral treatment along with vaccination. Recent studies have confirmed that Cepharanthine (CEP) has strong antiviral efficacy, which is a potential drug against COVID-19. As a natural active alkaloid, the development of CEP-incorporated products is dependent on the extraction, purification and identification of CEP. This review gives a brief introduction of CEP, including its origin and classification, and its conventional and novel extraction techniques. In addition, the purification and identification techniques are summarized. In the last, the future research directions are proposed. It can be found from this review that the extraction from plants is still the main way to obtain CEP, and it is necessary to use innovative techniques and their hybrid extractions to extract CEP. More efficient extraction and purification techniques should be used to extract CEP in the future. This review provides a basis for the development of novel extraction and purification techniques and industrial utilization of CEP.
Keywords: Cepharanthine, Extraction, Purification, Identification, COVID-19
1. Introduction
The ongoing global epidemic coronavirus disease, namely COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first identified as the causative agent of a new respiratory syndrome in January 2020 [1], [2]. The common symptoms of COVID-19 include fever, sore throat, fatigue, cough and shortness of breath, which are similar to the clinical symptoms of respiratory infections, making it often difficult to diagnose accurately. Symptoms can appear from 2 days to 2 weeks after exposure to the virus. Some COVID-19 patients are asymptomatic, while others develop pneumonia, acute respiratory distress syndrome (ARDS), stroke, and multiple organ failure [1], [3], [4]. The rapid spread of COVID-19 is causing social and economic shocks around the world [5], [6]. WHO reported that as of July 7, 2022, there have been 550, 218, 992 confirmed cases of COVID-19 worldwide, including nearly 6, 343, 783 deaths [7]. Despite the successful developments and relatively widespread applications of COVID-19 vaccines (Fig. 1 ), the emergence of new variants of SARS-CoV-2, which escape the immunity that comes from vaccinations, poses severe challenges for COVID-19 surveillance and control [7], [8], [9]. Moreover, people in some areas of the world have not been vaccinated due to issues related to hesitancy, unavailability or compromised immune systems, which could undermine efforts to end the pandemic through vaccines [8]. Therefore, there is an urgent need for additional anti-viral treatment options which can be widely produced and distributed [10].
Fig. 1.
Update of COVID-19 pandemic worldwide. As of July 7, 2022, the cumulative number of reported cases worldwide has exceeded 550 million, while the cumulative death toll has exceeded 6.3 million. Till to July 3, 2022, 12.1 billion doses of vaccine have been administered globally [7].
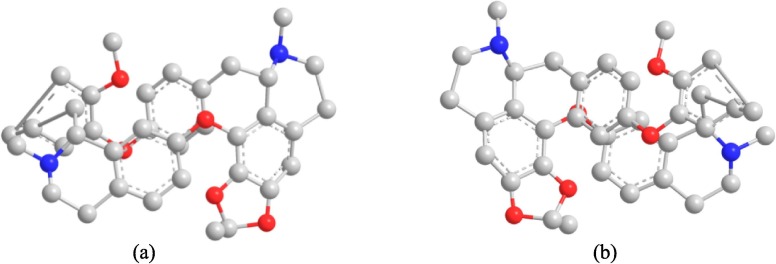
Herbal medicines and natural antiviral drugs have been used to prevent, diagnose and treat diseases for thousands of years in China, and have attracted extensive attention in fighting SARS-CoV-2 due to their safety and effectiveness. Cepharanthine (CEP), which is extracted from a famous Chinese herb Stephania cepharantha Hayata, is a natural bisbenzylisoquinoline alkaloid (Fig. 2 ) with large elliptical ring structure [11]. As a natural alkaloid, CEP has been shown to possess anti-inflammatory, antiparasitic, immunomodulatory and antiviral effects, indicating its possible application against viral diseases [9], [11], [12]. More specifically, the inhibitory effect of CEP on SARS-CoV, HCoV-OC43 and other coronaviruses has been confirmed in preclinical studies [13]. The clinical application showed that CEP was safe and no obvious side effects were found. In addition, the virus infection experiments also showed that the traditional Chinese medicine made from CEP also had good antiviral effect [14], [15]. With the outbreak of COVID-19, accumulating evidence suggests that CEP may be a potential natural antiviral agent against SARS-CoV-2 infection [16], [17]. In recent research conducted by Chinese scientists, CEP was identified as the most effective drug against SARS-CoV-2 associated pangolin coronavirus xCoV, a new coronavirus isolated from pangolin that is 92.5 % homologous to SARS-CoV-2 spike (S) protein, in a large drug screening of 2,406 clinically approved drugs [6], [18]. The results have been granted a national invention patent. The patent specification showed that 10 μM CEP inhibited the replication of pangolin coronavirus xCoV by 15,393 times and it had EC50 values of 0.9851 μM 20. Besides, American scientists also confirmed that CEP had excellent therapeutic effects on COVID-19 through a study of 26 drugs and its EC50 values against SARS-CoV-2 is 0.13 μM in a paper published in Science [2]. By the results of molecular docking simulations of CEP into target proteins, angiotensin-converting enzyme 2 (ACE2), it was revealed that CEP most strongly binds to ACE2 with binding affinity 9.8 kcal/mol [12], thus interfere with the ACE2-spike (S) protein interaction. In another in silico docking simulation study, there is a prospect that CEP molecules can bind the SARS-CoV-2 spike (S) protein, and interfere with S engagement to its receptor (ACE2) [19], [20], [21]. According to these docking models, CEP is thought to inhibit the entry phase in viral infection [19], [22]. In the light of these findings, CEP has become a promising drug for treating COVID-19.
Fig. 2.
Chemical structure of Cepharanthine: (a) front view; (b) back view (The red icon is an oxygen atom and the blue icon is a nitrogen atom). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
As a natural active alkaloid, CEP extraction and purification are the premise of its clinical application. The quality of extraction operation directly affects the yield of active ingredients in medicinal plants and the difficulty of subsequent processing. Moreover, clinical trials and subsequent industrial production of CEP drugs all depend on the extraction and purification processes. Hence, in order to design an efficient extraction and purification process, it is necessary to comprehensively consider current different extraction and purification processes and their advantages and limitations. However, current studies mainly focus on the efficacy and mechanism of CEP, and there is no literature that comprehensively describes the extraction and purification methods of CEP to the best of our knowledge. This review gives a brief introduction to CEP and mainly highlights the advantages and limitations of extraction and purification techniques. Moreover, the identification methods of CEP were summarized. Overall, this review provides a basis for the development of novel extraction and purification techniques for this potential antiviral drug, and a reference for the industrial production and application of CEP.
2. A brief overview of cepharanthine
CEP molecule (C37H38N2O6) has 83 atoms, with a molecular weight of 607. Since CEP was discovered and purified from S. cepharantha in 1934 by Japanese pharmacist Heisaburo Kondo [23], it has been reported in Japan for more than 70 years to treat a number of acute and chronic diseases, including malaria, alopecia and leukopenia [23]. From a chemical point of view, CEP is classified as a biscoclaurine alkaloid, which belongs to a member of the large family of structurally close bisbenzylisoquinoline cyclic alkaloids [9], [24]. It is worth noting that there have been several unsuccessful attempts to synthesize CEP in a cost-effective manner [9]. Therefore, the separation and purification of alkaloids is the key to the development of CEP drugs. The traditional separation and purification technology are based on this fact, that is, different alkaloid monomers have different polarity and solubility in some solvents, to separate alkaloids, or separated alkaloids from the mixture by distillation according to the solubility of different alkaloids or alkaloid salts [25].
CEP is extracted from the genus Stephania which is belonging to the Menispermaceae family. Stephania. japonica (Thunb) Miers, Stephania. epigaea Lo and Stephania cepharantha Hayata are three common plants used to extract CEP [26], as shown in Fig. 3 .
Fig. 3.
Three common plants from which CEP is extracted. (a) Stephania japonica (Thunb) Miers; (b) Stephania epigaea Lo; (c) Stephania cepharantha Hayata.
Stephania japonica (Thunb.) Miers (Fig. 3 (a)) has glabrous whole plant, strip-shaped root, brownish yellow, slender twigs, and straight lines. Leaves are papery or hard papery, usually triangular nearly round or triangular broad ovate, umbrella shaped cymes axillary, nearly pedicel. Fruit is obovate to nearly round, red when mature. In China, it is found in southern Henan, Sichuan, Chongqing, Hubei, Hunan, Jiangsu, Zhejiang, Anhui, Jiangxi, Fujian, and grows in village or wilderness shrubs. The roots of Stephania japonica (Thunb) Miers contain a variety of alkaloids, which are commonly used in Chinese folk herbs. They are bitter in taste and cold in nature, and have the effects of dispelling wind and activating collaterals, promoting diuresis and detumescence [27].
Stephania epigaea Lo (Fig. 3 (b)) is herbaceous, deciduous liana, glabrous; roots large, generally oblate globose, usually exposed to the ground. Lower part of stem slightly lignified, tender branch slightly fleshy, often purple red, dry lines. Leaf blade oblate or nearly round, base slightly round, upper dark green, slightly glossy, below powdery. Male plants are single umbrella-shaped cyme, female plants are single umbrella-shaped cyme. Stephania epigaea Lo is born in mountain bushes, forest margins or rock crevices. It is distributed in southern Sichuan, Yunnan and Guizhou. The root tuber of this plant is a famous Chinese herbal medicine for clearing heat, detoxification, sedation, regulating qi and relieving pain [27].
Stephania cepharantha Hayata (Fig. 3 (c)) is herbaceous, deciduous, hairless vine, usually 1–2 m high or over, its roots are lumpy or nearly conical, and branches are purple red and slender. Leaves are papery, triangular oblate to suborbicular, usually 2–6 cm long, 2.5–6.5 cm wide, base round or nearly flat, margin entire or slightly undulate, palmate veins 7–9, downward very slender, petiole 1.5–7 cm long, slender. It is more adaptive, both in the village edge, wilderness, forest edge and other places deep and fertile soil (root tuber often deep soil), and in limestone areas in the stone or gravel (root tuber floating ground). Stephania cephalantha Hayata is widely distributed in China, from northwest to the Hanzhong area of Shanxi, east to Zhejiang, Jiangsu and Taiwan, southwest to eastern and southeastern Sichuan, eastern and southern Guizhou, and south to Guangxi and Guangdong. Tuberous root contains a variety of alkaloids, which is a commonly used folk medicine. It has bitter in taste and cold in nature, and has the functions of clearing heat and detoxifying, detumescence and relieving pain [27]. Moreover, affected by geographical environment, climatic conditions and other factors, the content of CEP in the extract of the same medicinal material varies from region to region and CEP is generally more abundant in the tubers than the roots [28], [29].
3. Extraction techniques of CEP
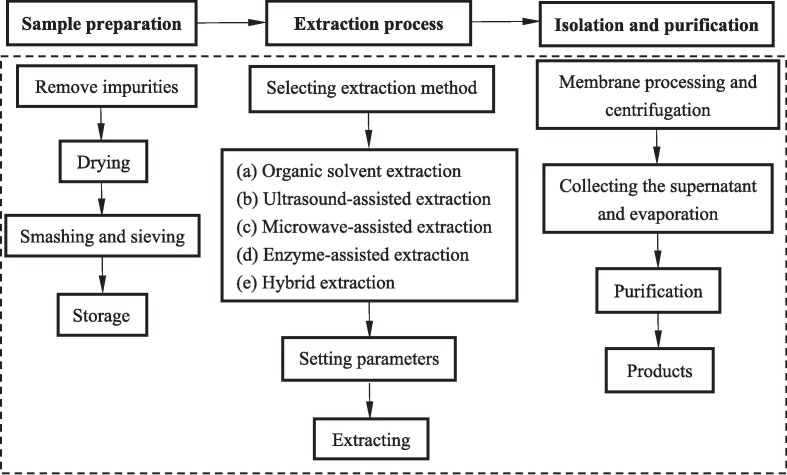
Extraction is one of the most important steps in the recovery of valuable biological components [30]. Theoretically, the ideal extraction method should be able to extract as many target components as possible in a shorter time and remove the unwanted compounds [31]. In the past few years, several methods have been developed for the extraction of CEP and the extraction process is shown in Fig. 4 [28], [29], [32], [33]. These methods can be broadly divided into conventional and innovative techniques [34].
Fig. 4.
Schematic procedure of different extraction methods.
The most commonly used traditional method is organic solvent extraction, which is a solid–liquid extraction containing numerous organic solvents or mixtures of aqueous-organic solvents. Commonly used organic solvents include methanol, ethanol, methylene chloride, etc. [35]. Due to its simple operation and simple instrument, this method has been widely used in industry in recent decades [36]. However, this method not only needs to consume large amounts of organic solvents, but also may produce solvent residues, affecting the final quality of the product, so its application is limited. In order to realize economical, green and rapid extraction of bioactive compounds, several innovative technologies, including ultrasound, microwave and enzyme-assisted extraction, have been widely used (Fig. 5 ) [28], [29] and the advantages/disadvantages of extraction techniques of CEP be summarized in Table.1 . This section focuses on recent advances in traditional and innovative technologies for extracting CEP.
Fig. 5.
Laboratory scale experimental set-up for conventional extraction of CEP from plant matrices. (a) Organic solvent extraction; (b) Ultrasound-assisted extraction; (c) Microwave-assisted extraction; (d) Enzyme-assisted extraction; (e) Hybrid extraction.
Table 1.
Comparison of extraction methods of CEP.
| Method | Advantage | Disadvantage |
|---|---|---|
| Organic solvent extraction | Simple equipment, easy to implement, the most widely used. | Time-consuming, laborious and harmless. |
| Ultrasound-assisted extraction | Simple and time-saving, low energy consumption, wide application. | It is easy to affect the consistency of CEP yield in different batches. |
| Microwave-assisted extraction | The most time-saving, solvent saving. | It can induce undesirable structural modifications and is not conductive to large-scale production. |
| Enzyme-assisted extraction | The conditions are mild, low temperature, high efficiency and reduction of equipment corrosion. | High cost, large amount of enzyme, and is not suitable for industrial scales |
| Ultrasound-assisted extraction and aqueous two-phase | Advance extraction yields, environmentally-friendly, energy-saving. | Long phase separation time at aqueous two-phase step. |
3.1. Organic solvent extraction
The principle of organic solvent extraction of target compounds is the similar dissolve mutually theory [37], [38], so the choice of solvent type is very important, which depends on not only the solubility of the target component in the solvent, but also the avoidance of extraction of other unwanted compounds [39]. Based on a previous literature, it was found that dichloromethane could be used to extract CEP from different parts (tubers, roots, stems and leaves) of Stephania rotunda, as shown in Fig. 5(a), In this process, a certain amount of plant dry powder was first dipped in acidic-water (1:99, v/v) for four hours, then extracted with dichloromethane and the highest CEP content in tubers was 1.90 % [40]. In another study, the dry powder of Stephania cepharantha Hayata was soaked in 80 % aqueous ethanol (v/v) for 1.5 h at a solid–liquid ratio of 1:12 g/mL, and then extracted by hot reflux for 1 h, repeated three times. Finally, the content of CEP accounted for 0.58 % of the crude extract [33]. To sum up, organic solvent extraction of CEP has certain advantages, such as simple equipment and easy to implement, but it also has significant disadvantages, such as time-consuming, laborious, and so on. In addition, some organic solvents, such as dichloromethane, will harm human health, pollute the environment, and increase the cost of waste treatment, which is not in line with the concept of green environmental protection development.
3.2. Ultrasound-assisted extraction (UAE)
In order to overcome the shortcomings of traditional extraction, UAE has been proposed to extract bioactive components from plants (Fig. 5(b)) [41]. When ultrasound is applied to the solvent, it can generate and transfer strong energy that leads to the production of bubbles [42], and the subsequent collapse of these bubbles at the surface of the plant matrix will produce intense shear forces, turbulences, micro-mixing and acoustic streaming in a very short time and a very small space [43], [44], which will destroy the structure of cell wall and favor the mass transfer. At the same time, the rupture of the cell walls also facilitates solvent penetration into raw material matrix to accelerate the dissolution of bioactive components and increase the extraction rate [37]. Based on this theory, UAE has been widely used in the extraction of various bioactive compounds [45], [46]. Using aqueous ethanol (50:50, v/v) as solvent, under the optimal extraction conditions of solid–liquid ratio of 1:30 g/mL and ultrasonic power of 120 W for 10 min, Desgrouas et al. extracted CEP from the tubers of Stephania rotunda by ultrasonic combined with organic solvent [29]. Compared with the traditional extraction method with dichloromethane as solvent, the CEP yield obtained by UAE was 2.5 times higher than that by traditional method. By measuring the CEP-containing residues after rotary evaporation drying, the yield of UAE was 11.5 times higher than that of traditional extraction. At the same time, ultrasonic and dichloromethane extraction of CEP comparative experiments showed that in addition to the influence of ultrasonic, the selection of solvent type also had a significant impact on the final extraction yield. Under optimal conditions, the CEP yield with dichloromethane as solvent by UAE is 0.72 %, and the yield with ethanol–water (50: 50, v/v) as solvent is 17.35 % [29]. UAE is a non-thermal process, which avoids the problem of thermal degradation during the extraction of bioactive components [47]. However, the uniformity of UAE is poor, because the intensity of ultrasonic decreases with the increase of distance of the emitter [48] . Besides, the rupture of cavitation bubbles produces hydroxyl radicals [42], which will affect the consistency of CEP yield in different batches.
3.3. Microwave-assisted extraction (MAE)
MAE is another innovative and green method that uses microwave energy (electromagnetic waves with a frequency of 300 MHz to 300 GHz) to extract bioactive compounds. The principle of MAE is based on non-ionizing radiation to the solvent, which passes through the cell wall and reaches the interior of the plant matrix [43], [47], [49]. The radiation treatment can cause the molecular motion, ionic conduction and dipole rotation. It induces an increase of temperature and pressure in the cell, leading to the destruction of the cell wall and the increase of porosity [50], [51], which is conducive to the penetration of solvent into the sample matrix and promote the mass transfer process [52]. MAE process is assumed to consist of three successive steps: Purification of the sample matrix, penetration of the solvent into the matrix, and diffusion of the solute into the solvent [42]. Compared with conventional extraction, its main advantage is to reduce the consumption of organic solvents and greatly shorten the extraction time [53]. Due to the feasibility and the advantage of this technique, MAE has gained wide attention in green extraction of the valuable compounds from natural sources and industrial by-products [54].
As can be seen from Fig. 5 (c), under the optimal extraction conditions of solid–liquid ratio of 1:30 g/mL for 15 min, the extraction yield of CEP obtained by MAE was 12.4 times higher than that by dichloromethane extraction [29]. In addition, under the condition of microwave power of 100 W, solid–liquid ratio of 1:10 g/mL, microwave irradiation for 15 min, the moistening step of extracted samples in different solvents (dichloromethane, ethanol, methanol, hydrochloric acid solution) was tested in this study, and the statistical test showed that the effect of moistening step on the CEP yield was not significant (p = 0.235) [29]. In another study of extracting four active alkaloids from Stephania sinica, MAE had the highest extraction efficiency, the shortest extraction time (90 s) and the least consumption of ethanol solvent (12 mL) compared with Soxhlet and UAE [55]. Notably, MAE is a new auxiliary technology with great development potential to enhance solid–liquid extraction process [43]. However, this technique is relatively expensive and its low absorption efficiency limits its application in industry [56], [57].
3.4. Enzyme-assisted extraction (EAE)
The plant cell walls consist of complex structural polysaccharides (pectin, cellulose, hemicellulose, etc.) and protein, conferring the stability of the plant cell walls and resistance of intracellular component extraction [45], [58]. Thus, enzymes with specific catalytic hydrolysis properties can deconstruct the plant cell walls, improving the yield of the target compounds [59].
EAE is considered as a promising alternative technique to the conventional solvent methods because of its mild reaction conditions, high efficiency and low processing temperature [60] and has been applied to the extraction of CEP (Fig. 5 (d)). In a previous patent report, it was found that the optimal extraction condition of EAE was to add 2–3 L of water and 0.5–5 mL of mixed biological enzyme solution (one or several components of hemicellulose, pectin lyase, medium temperature liquefying enzyme, low temperature liquefying enzyme, snailase, and so on) to the crushed material, soak for 1 h and then add 9–11 L of 1–2 % hydrochloric acid solution. After soaking again for 3 h, the liquid was separated, and then 9–11 L of 1–2 % hydrochloric acid solution was added again into the matrix. After extraction for 3 h, the crude extract containing CEP was obtained by filtration [28].
EAE is has attracted more and more attention due to its simplicity, high efficiency, elimination of harsh extraction conditions and reduction of equipment corrosion. Generally, lab-scale enzymatic extraction of CEP requires relatively low concentration of matrix, however, the low concentrations of matrix make scale-up of the extraction process is difficult [61]. Therefore, EAE is not suitable for industrial scales due to its high cost in industrial applications [62], [63].
3.5. Hybrid extraction.
Although innovative technologies have many advantages over traditional solvent extraction, as mentioned above, these green technologies also show some shortcomings. Some studies have found that the combination of some green techniques can overcome these shortcomings and increase the extraction yield of target active components [51]. In a previous study, Sun et al. integrated two techniques, aqueous two-phase (ATP) and UAE, to extract CEP from Stephania Epigaea (Fig. 4 (e)). It was found when ATP, whose optimal composition were 4 mL ethanol, 5 mL water and 1.2 g ammonium sulfate combined with UAE under the condition of solid–liquid ratio 1:90 g/mL, extraction time 10 min, the maximum yield of adenosine triphosphate could reach 0.071 mg/g [32]. Due to the disruptive ability of ultrasound, the combination of ATP and UAE recovered more CEP than UAE alone. Since the ATP has many advantages, such as good biocompatibility, low interfacial tension, short extraction time, high yield, easy to scale up, easy to combine with other extraction techniques, and so on, it has been recognized as a superior downstream processing technique for biological molecules [64], [65]. Therefore, the combination of ATP and other innovation extraction techniques should be further studied in the future.
4. Purification techniques of CEP
After successful extraction by various methods, purification of the crude extract is an important step to obtain high quality and strong physiological activity of CEP. At present, there are many purification methods for CEP, mainly including macroporous resins, high-speed counter-current chromatography (HSCCC) and aqueous two-phase extraction (ATPE).
4.1. Macroporous resins
Macroporous resins are porous cross-linked polymer beads that can interact with absorbents through electrostatic interaction, hydrogen bonds, and molecular sieve action [66], [67]. As useful adsorbents, macroporous resins have been widely applied to the purification and enrichment of natural compounds of herbal plants including CEP, attributing to their distinguishing advantages such as large specific surface area, simple operation process, low cost, low contamination and easy regeneration [66], [67], [68]. In a previous study, the adsorption and purification capabilities of 3 different resins (HPD100, AB-8, and D101) on crude CEP extracts were compared [33]. The D101macroporous resin showed the highest purification efficiency, whose specific surface area was more than 400 m2/g, and particle diameter was 0.3–1.25 mm with pore sizes of 10.0–11.0 nm. Under optimal conditions, the maximum extraction yield of total alkaloids and CEP was 3.4 % and 2.9 %, respectively. It is expected that more microporous resins can be synthesized in the future for the purification of CEP.
4.2. High-speed counter-current chromatography
HSCCC is a two-phase chromatographic technique using liquid phase and stationary phase. It has been successfully applied to the preparation and separation of various natural products. The principle of HSCCC is the precise separation of the target extract based on the partition coefficient between the two solvents, which is determined by hydrophobicity [69]. At the same time, because the liquid is used as the stationary phase, the solute can fully contact with the liquid [70], so as to avoid the loss of the adsorbed sample and the chemical degradation of the target component [69].
HSCCC combined with dynamic pH junction has been successfully applied to the separation and purification of CEP from Stephania cepharantha. In this study, Hydrochloric acid (HCL) of 5.0 mmol/L and 60 mL the upper phase of hexane–ethyl acetate–methanol-water (1:1:1:1, v/v/v/v) were introduced into the sample solution with 10 % triethylamine at 50 °C and 400 W irradiation power for 10 min, and then the solvent system were directly separated and purified by high-speed counter-current chromatography. Meanwhile, the addition of inorganic acid (HCL) and organic base (triethylamine) in mobile phase and sample solution contributed to the formation of dynamic pH junction, leading to the concentration of alkaloids [70]. Finally, CEP with a purity of 99.3 % was obtained from 6 g sample.
Because crude plants extracts contain complex mixtures, it is unlikely to obtain different types of compounds from the crude extract of HSCCC using a single solvent system. Therefore, before the HSCCC method is carried out, it is usually necessary to fractionate the crude extract into discrete fractions containing a group of compounds with similar polarity or molecular size.
4.3. Aqueous two-phase extraction (ATPE)
Aqueous biphasic system is usually made from a mixture of hydrophilic organic solvents (such as ethanol, methanol, acetone, etc.), and inorganic salts (phosphates, sulfates, citrates) [32]. At a certain critical concentration, two hydrophilic phases in equilibrium with each other will be formed. For many biological substrates, aqueous two-phase extraction (ATPE) is a mild one-step process that can improve the purity and yield of the extract [71]. In the case conducted by Sun et al. [32], CEP was extracted by UAE from a traditional herbal plants stephania epigaea and purified by ATPE based on aqueous ethanol and ammonium sulfate, the highest yield was obtained with a recovery of 102.4 % compared with the other solvents.
In conclusion, all purification techniques have their advantages and limitations. The macroporous resin is a simple and efficient purification method for many biological substrates, but this method has lower selectivity and is difficult to be used in industrial applications. The HSCCC method is a unique liquid–liquid separation chromatography and has been successfully used for the extraction of CEP, however, its high cost and complex operation may limit its industrial application. ATPE technology has the advantages of low equipment, low cost and 70–90 % reduction in water consumption, which is a reasonable method for large-scale purification of bioactive compounds [72].
5. Identification techniques of CEP
Due to the confusion of CEP raw materials in the market, its clinical safety and quality performance are affected [73]. Therefore, it is necessary to consider the identification method of CEP to ensure its antiviral effect and clinical safety. The identification techniques of CEP, such as HPLC (high performance liquid chromatography) [40], [74], RP-HPLC (reversed phase high performance liquid chromatography) [75] and UV–vis (ultraviolet-visable) [76], [77], are presented in Table.2 . The results of HPLC identification showed that the content of CEP in different parts of the same plant was different, and CEP was mainly distributed in the stem of Stephania japonica (Thunb.) Miers originated from Hunan province [74]. In another experiment, the research results showed that the content of alkaloids in tubers was higher than that in roots, stems and leaves [40]. It was found that this method was simple, accurate, effective and reproducible in the determination of CEP in Japanese Stephania root, Epigeal Stephania root tuber and Stephanotis [40], [74], [78]. The RP-HPLC method is simple, accurate and reliable [78]. When detecting the content of CEP in Stephania epigaea Lo, the average content of CEP was 3.57 mg/g [75]. The content of CEP in its tablets was determined by UV spectrophotometry. The results showed that UV spectrophotometry was not only rapid, simple and accurate, but also had good precision [76]. The solid sample was ground and mixed with the adsorbent by MSPD (matrix solid-phase dispersion) method, so that the components to be tested were fully adsorbed on the carrier. The MSPD-HPLC method not only shortened the required time, but also quickly and accurately determined the CEP content in Stephania cepharantha Hayata [79]. In addition, 1H NMR and 13C NMR spectra have been used to identify CEP [12].
Table 2.
A description of the different identification methods for CEP.
| Methods | Operating conditions | Results | Reference |
|---|---|---|---|
| HPLC | Angela C18 column (250 mm × 4.6 mm, 5 µm); The mobile phase (v/v): acetonitrile–water (52:48,0.5 % triethylamine and 0.01 % phosphoric acid); The column temperature (℃):30; The injection volume (µL): 20 | A good linearity (μg/mL): 9–216 (r = 0.9997), RSD value at 2.0 % | 71 |
| HPLC | Symmetry C8 column (250 mm × 4.6 mm, 5 µm); The mobile phase (v/v): 25 mM potassium phosphate buffer (pH 3.5, adjusted with orthophosphoric acid) - acetonitrile (8:2); The mobile phase flow rate (mL/min): 1.0; The injection volume (µL): 20; The column temperature (℃): 25; The UV spectra detection wavelength(nm): 282 | A good linear behavior was observed with values of r2 > 0.9964 | 37 |
| RP-HPLC | AlltechRP C18 column (250 mm × 4.6 mm, 5 µm); The mobile phase (v/v): methanol-0.05 % triethanolamine buffered (75:25); The mobile phase flow rate (mL/min): 0.8; The injection volume (µL): 10; The column temperature (℃): 30; The UV spectra detection wavelength (nm): 283 | A good linearity (μg/mL): 0.10032–1.0032 (r = 0.9999), RSD value at 1.84 % | 74 |
| RP-HPLC | Hypersil C18 column (250 mm × 4.6 mm, 5 µm); The mobile phase (v/v/v): methanol–water- triethanolamine (75:23:0.20); The mobile phase flow rate (mL/min): 1.0; The injection volume (µL):20; The column temperature (℃): 30; The UV spectra detection wavelength (nm): 282 | A good linearity (μg/mL): 0.12–0.13 (r = 0.994) | 72 |
| MSPD-HPLC | Phenomenex Gemini C18 column (250 mm × 4.6 mm, 5 µm); The mobile phase (v/v): acetonitrile-triethanolamine (70:30); The mobile phase flow rate (mL/min): 1.0; The injection volume (µL):20; The column temperature (℃): 40; The UV spectra detection wavelength (nm): 282 | A good linearity (μg/mL): 14.88–238 (r = 0.9994) | 75 |
| UPLC-QTOF-MS | UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 µm); The mobile phase (v/v): acetonitrile and aqueous formic acid (0.1 %); The mobile phase flow rate (mL/min): 0.4; The injection volume (µL): 5; The column temperature (℃): 40;MS: Scan monitoring (m/z): 100–1200; Capillary voltage (kV): 0.5; Sample cone voltage (V): 40; Extraction cone voltage (V): 4; Source temperature (℃): 100; Desolvation temperature (℃): 300; Desolvation gas flow (L/h): 800; |
MS/ MS Fragmentation: 564.2397[M + H –NH2CH3]+ Molecular Formula: C37H38N2O6 |
70 |
| UV–vis | Solvent: anhydrous ethanol solution; Solid-liquid ratio (µg /ml): 40:1; The UV spectra detection wavelength (nm): 282; | A good linearity (μg/mL): 10–70 (r = 0.9995) | 73 |
Abbreviations: RSD: relative standard deviation; HPLC: high performance liquid chromatography; RP-HPLC: reversed phase high performance liquid chromatography; MSPD: matrix solid-phase dispersion; UPLC: ultra-high performance liquid chromatography; QTOF: quadrupole time-of fligh; MS: mass spectrometry; UV–vis: ultraviolet-visable.
6. Conclusions and perspectives
The biological and antiviral effects of CEP have been widely studied and confirmed, and it has become a promising drug for the treatment of COVID-19. It is very crucial to study and optimize the extraction and purification process of active ingredients in medicinal plants. Conventionally, CEP can be directly extracted by organic solvents, but some solvents are toxic to humans and pose a threat to the environment. UAE, MAE and EAE are considered as novel and green alternatives for the extraction of CEP from plant sources. These techniques can destroy the cell walls of matrix and accelerate the mass transfer of bioactive substances. The combination of ATP with UAE is effective for the extraction of CEP and can promote the yield. HSCCC and resins can separate CEP with high purity. Various identification techniques, such as HPLC, RP-HPLC and UV–vis have been employed for the identification of CEP.
All of these techniques have their advantages. However, there is a long way to go before an efficient extraction and purification process can be designed. The future research prospects of extraction and purification of CEP and the current research gaps in this field are given as follows:
(1) In addition to the extraction techniques mentioned in this paper, modern extraction techniques include pulsed electric field, high voltage discharge, supercritical fluid extraction, high pressure processing and mechanochemical assisted extraction [80], [81]. These novel techniques can shorten the extraction time and simplify the purification [82], and should be researched to extract CEP in the future.
(2) From an economic point of view, in recent years, membrane processing (MP) technologies,such as ultra-(UF) and nano-(NF) filtration, has been used to extract polyphenols [83], capsaicin [80] and anthocyanins [84], etc. Compared with the traditional extraction technology, MP is a highly selective target solute treatment technology, which has the advantages of simple operation, large-scale feasibility, less operation steps and energy saving. Therefore, MP can be used as an alternative method to extract CEP in aqueous solution, depending on the membrane properties (e.g., surface topography, hydrophilicity/hydrophobicity, pore size, charge, morphology, etc.) [80], [85]. It should be noted that there is no report on the treatment of CEP extract by MP.
(3) With more and more attention being paid to hybrid extraction, in addition to the combination of ATP and UAE, the combination of other new technologies is worthy of further discussion and research in the future. Further, these technologies are still lacking in industrial scale applications, so it is necessary to develop simple and efficient extraction processes to improve industrial applicability. Besides, in order to improve the separation efficiency, more macroporous resins with different physical properties need to be synthesized.
(4) Due to the fact that the unique chemical structure of CEP plays an important role in enhancing its antiviral efficacy, it is necessary to investigate the effects of different extraction methods and operating conditions on the chemical structure of CEP in order to better understand and fully utilize CEP compounds in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We apologize to those authors whose research could not be cited due to space limits.
Data availability
Data will be made available on request.
References
- 1.Yin J., et al. Advances in the development of therapeutic strategies against COVID-19 and perspectives in the drug design for emerging SARS-CoV-2 variants. Comput. Struct. Biotechnol. J. 2022;20:824–837. doi: 10.1016/j.csbj.2022.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drayman1 N., et al. Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2. Science. 2021;373:931–936. doi: 10.1126/science.abg5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srivastava A., Gupta R.C., Doss R.B., Lall R. Trace minerals, vitamins and nutraceuticals in prevention and treatment of COVID-19. J. Dietary Supplements. 2022;19:395–429. doi: 10.1080/19390211.2021.1890662. [DOI] [PubMed] [Google Scholar]
- 4.A.G. Ayele, E.F. Enyew, Z.D. Kifle, Roles of existing drug and drug targets for COVID-19 management. Metabolism Open 11, 100103-100103, 10.1016/j.metop.2021.100103 (2021). [DOI] [PMC free article] [PubMed]
- 5.Li J., et al. Bioavailability enhancement of cepharanthine via pulmonary administration in rats and its therapeutic potential for pulmonary fibrosis associated with COVID-19 infection. Molecules. 2022;27 doi: 10.3390/molecules27092745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S., et al. Transcriptome analysis of cepharanthine against a SARS-CoV-2-related coronavirus. Brief. Bioinform. 2021;22:1378–1386. doi: 10.1093/bib/bbaa387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO, WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed July 7, 2022, 2022.
- 8.Chitsike L., Krstenansky J., Duerksen-Hughes P.J. ACE2: S1 RBD Interaction-targeted peptides and small molecules as potential COVID-19 therapeutics. Adv. Pharmacol. Sci. 2021;2021 doi: 10.1155/2021/1828792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogosnitzky M., Okediji P., Koman I. Cepharanthine: a review of the antiviral potential of a Japanese-approved alopecia drug in COVID-19. Pharmacol. Rep. 2020;72:1509–1516. doi: 10.1007/s43440-020-00132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar P., et al. Identification of potential COVID-19 treatment compounds which inhibit SARS Cov2 prototypic. Delta and Omicron variant infection. Virology. 2022;572:64–71. doi: 10.1016/j.virol.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodoira R., et al. An overview on extraction, composition, bioactivity and food applications of peanut phenolics. Food Chem. 2022;381 doi: 10.1016/j.foodchem.2022.132250. [DOI] [PubMed] [Google Scholar]
- 12.Celik S., Akyuz S., Ozel A.E. Vibrational spectroscopic characterization and structural investigations of Cepharanthine, a natural alkaloid. J. Mol. Struct. 2022;1258 doi: 10.1016/j.molstruc.2022.132693. [DOI] [Google Scholar]
- 13.Kim D.E., et al. Natural bis-benzylisoquinoline alkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus oc43 infection of mrc-5 human lung cells. Biomolecules. 2019;9 doi: 10.3390/biom9110696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z.R., et al. Berbamine hydrochloride potently inhibits SARS-CoV-2 infection by blocking S protein-mediated membrane fusion. PLoS Negl. Trop. Dis. 2022;16:e0010363. doi: 10.1371/journal.pntd.0010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seubwai W., et al. Cepharanthine exerts antitumor activity on cholangiocarcinoma by inhibiting NF-kappa B. Cancer Sci. 2010;101:1590–1595. doi: 10.1111/j.1349-7006.2010.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang, et al. Comparison of viral rna-host protein interactomes across pathogenic rna viruses informs rapid antiviral drug discovery for SARS-CoV-2. Cell Res. 2022;32:9–23. doi: 10.1038/s41422-021-00581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, et al. Identification of bis-benzylisoquinoline alkaloids as sars-cov-2 entry inhibitors from a library of natural products. Signal Transduct. Target. Ther. 2021;6:131. doi: 10.1038/s41392-021-00531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Y. Tong, et al., Pangolin coronavirus xCoV and its application and application of drugs against coronavirus infection (Patent No. CN 113046327-A), 2021.
- 19.Ohashi H., et al. Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. iScience. 2021;24 doi: 10.1016/j.isci.2021.102367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A.C. Walls, et al., Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein, Cell 181, 281-292 e286, 10.1016/j.cell.2020.02.058 (2020). [DOI] [PMC free article] [PubMed]
- 21.Abdullah, Weiss J., Zhang H. Recent advances in the composition, extraction and food applications of plant-derived oleosomes. Trends Food Sci. Tech. 2020;106:322–332. doi: 10.1016/j.tifs.2020.10.029. [DOI] [Google Scholar]
- 22.Hijikata A., et al. Evaluating cepharanthine analogues as natural drugs against SARS-CoV-2. FEBS Open Bio. 2022;12:285–294. doi: 10.1002/2211-5463.13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailly C. Cepharanthine: An update of its mode of action, pharmacological properties and medical applications. Phytomedicine. 2019;62 doi: 10.1016/j.phymed.2019.152956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogosnitzky M., Danks R. Therapeutic potential of the biscoclaurine alkaloid, cepharanthine, for a range of clinical conditions. Pharmacol. Rep. 2011;63:337–347. doi: 10.1016/S1734-1140(11)70500-X. [DOI] [PubMed] [Google Scholar]
- 25.Adejokea., et al. A review on classes, extraction, purification and pharmaceutical importance of plants alkaloid article info abstract. J. Med. Chem. Sci. 2019;2:130–139. doi: 10.13140/RG.2.2.12867.96809. [DOI] [Google Scholar]
- 26.Semwal D.K., et al. The genus Stephania (Menispermaceae): chemical and pharmacological perspectives. J. Ethnopharmacol. 2010;132:369–383. doi: 10.1016/j.jep.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 27.Chinese Flora Editing Committee & Sciences, C. A. o. Flora of China, Science Press, 1993.
- 28.Cai, Q. Separation and extraction of cepharanthine by adding water and mixed biological enzyme into crushed root, adding hydrochloric acid water solution, warming, soaking, separating liquid, and adding hydrochloric acid water solution to slag (Patent No. CN 102146083-A). 102146083-A (2011).
- 29.Desgrouas C., et al. Rapid and green extraction, assisted by microwave and ultrasound of cepharanthine from Stephania rotunda Lour. Sep. Purif. Technol. 2014;123:9–14. doi: 10.1016/j.seppur.2013.12.016. [DOI] [Google Scholar]
- 30.Barba F.J., Zhu Z., Koubaa M., Sant'Ana A.S., Orlien V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Tech. 2016;49:96–109. doi: 10.1016/j.tifs.2016.01.006. [DOI] [Google Scholar]
- 31.Lord H., Pawliszyn J. Evolution of solid-phase microextraction technology. J. Chromatogr. A. 2000;885:153–193. doi: 10.1016/s0021-9673(00)00535-5. [DOI] [PubMed] [Google Scholar]
- 32.S. Sun, et al., Simultaneous Electrochemiluminescence determination of sinomenine, cepharanthine and tetrahydropalmatine in Stephania epigaea by capillary electrophoresis coupled with ultrasonic-assisted aqueous two-phase extraction, Int. J. Electrochem. Sci 15, 5002-5017, 10.20964/2020.06.33 (2020).
- 33.Xiao J., et al. High performance liquid chromatography determination and optimization of the extraction process for the total alkaloids from traditional herb Stephania cepharantha Hayata. Molecules. 2019;24 doi: 10.3390/molecules24030388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sagar N.A., Pareek S., Sharma S., Yahia E.M., Lobo M.G. Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018;17:512–531. doi: 10.1111/1541-4337.12330. [DOI] [PubMed] [Google Scholar]
- 35.Farias C.A.A., et al. Microwave hydrodiffusion and gravity model with a unique hydration strategy for exhaustive extraction of anthocyanins from strawberries and raspberries. Food Chem. 2022;383 doi: 10.1016/j.foodchem.2022.132446. [DOI] [PubMed] [Google Scholar]
- 36.Li Z., Fan Y., Xi J. Recent advances in high voltage electric discharge extraction of bioactive ingredients from plant materials. Food Chem. 2019;277:246–260. doi: 10.1016/j.foodchem.2018.10.119. [DOI] [PubMed] [Google Scholar]
- 37.Miranda P.H.S., et al. A scientific approach to extraction methods and stability of pigments from Amazonian fruits. Trends Food Sci. Tech. 2021;113:335–345. doi: 10.1016/j.tifs.2021.04.047. [DOI] [Google Scholar]
- 38.Johnson J., Collins T., Walsh K., Naiker M. Solvent extractions and spectrophotometric protocols for measuring the total anthocyanin, phenols and antioxidant content in plums. Chem. Pap. 2020;74:4481–4492. doi: 10.1007/s11696-020-01261-8. [DOI] [Google Scholar]
- 39.Chan K.N., et al. Effect of extraction solvent on the bioactivity of an herbal formulation. Ind. Eng. Chem. Res. 2009;48:4852–4857. doi: 10.1021/ie8012538. [DOI] [Google Scholar]
- 40.Sothavireak B., et al. Simultaneous HPLC determination of three bioactive alkaloids in the asian medicinal plant Stephania rotunda. Nat. Prod. Commun. 2010;5:877–882. doi: 10.1177/1934578x1000500611. [DOI] [PubMed] [Google Scholar]
- 41.Gulzar S., et al. Oil and pigments from shrimp processing by-products: Extraction, composition, bioactivities and its application - A review. Trends Food Sci. Tech. 2020;100:307–319. doi: 10.1016/j.tifs.2020.04.005. [DOI] [Google Scholar]
- 42.Kumar M., et al. Recent trends in extraction of plant bioactives using green technologies: A review. Food Chem. 2021;353 doi: 10.1016/j.foodchem.2021.129431. [DOI] [PubMed] [Google Scholar]
- 43.Huang G., Chen F., Yang W., Huang H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci. Tech. 2021;109:564–568. doi: 10.1016/j.tifs.2021.01.038. [DOI] [Google Scholar]
- 44.Zhang R., Li S., Zhu Z., He J. Recent advances in valorization of Chaenomeles fruit: A review of botanical profile, phytochemistry, advanced extraction technologies and bioactivities. Trends Food Sci. Tech. 2019;91:467–482. doi: 10.1016/j.tifs.2019.07.012. [DOI] [Google Scholar]
- 45.Jiang T., Ghosh R., Charcosset C. Extraction, purification and applications of curcumin from plant materials - A comprehensive review. Trends Food Sci. Tech. 2021;112:419–430. doi: 10.1016/j.tifs.2021.04.015. [DOI] [Google Scholar]
- 46.Vinatoru M., Mason T.J., Calinescu I. Ultrasonically assisted extraction and microwave assisted extraction of functional compounds from plant materials. TrAC -Trend Anal Chem. 2017;97:159–178. doi: 10.1016/j.trac.2017.09.002. [DOI] [Google Scholar]
- 47.Cui J., et al. Pectins from fruits: Relationships between extraction methods, structural characteristics, and functional properties. Trends Food Sci. Tech. 2021;110:39–54. doi: 10.1016/j.tifs.2021.01.077. [DOI] [Google Scholar]
- 48.Wang L., Weller C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Tech. 2006;17:300–312. doi: 10.1016/j.tifs.2005.12.004. [DOI] [Google Scholar]
- 49.Echegaray N., et al. Recent advances in food products fortification with anthocyanins. Crit. Rev. Food Sci. Nutr. 2022;62:1553–1567. doi: 10.1080/10408398.2020.1844141. [DOI] [PubMed] [Google Scholar]
- 50.Wang H., Ding J., Ren N. Recent advances in microwave-assisted extraction of trace organic pollutants from food and environmental samples. TrAC - Trend Anal Chem. 2016;75:197–208. doi: 10.1016/j.trac.2015.05.005. [DOI] [Google Scholar]
- 51.Bruno S.F., Ekorong F.J.A.A., Karkal S.S., Cathrine M.S.B., Kudre T.G. Green and innovative techniques for recovery of valuable compounds from seafood by-products and discards: A review. Trends Food Sci. Tech. 2019;85:10–22. doi: 10.1016/j.tifs.2018.12.004. [DOI] [Google Scholar]
- 52.Angiolillo L., Del Nobile M.A., Conte A. The extraction of bioactive compounds from food residues using microwaves. Curr. Opin. Food Sci. 2015;5:93–98. doi: 10.1016/j.cofs.2015.10.001. [DOI] [Google Scholar]
- 53.Wang J., Zhang M., Fang Z. Recent development in efficient processing technology for edible algae: A review. Trends Food Sci. Tech. 2019;88:251–259. doi: 10.1016/j.tifs.2019.03.032. [DOI] [Google Scholar]
- 54.Ameer K., Shahbaz H.M., Kwon J.H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2017;16:295–315. doi: 10.1111/1541-4337.12253. [DOI] [PubMed] [Google Scholar]
- 55.Xie D.T., et al. Microwave-assisted extraction of bioactive alkaloids from Stephania sinica. Sep. Purif. Technol. 2014;130:173–181. doi: 10.1016/j.seppur.2014.04.026. [DOI] [Google Scholar]
- 56.Ghareaghajlou N., Hallaj-Nezhadi S., Ghasempour Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021;365 doi: 10.1016/j.foodchem.2021.130482. [DOI] [PubMed] [Google Scholar]
- 57.Xiang B., Zhou X., Qin D., Li C., Xi J. Infrared assisted extraction of bioactive compounds from plant materials: Current research and future prospect. Food Chem. 2022;371 doi: 10.1016/j.foodchem.2021.131192. [DOI] [PubMed] [Google Scholar]
- 58.Panouille M., Thibault J.F., Bonnin E. Cellulase and protease preparations can extract pectins from various plant byproducts. J. Agr. Food Chem. 2006;54:8926–8935. doi: 10.1021/jf0617824. [DOI] [PubMed] [Google Scholar]
- 59.Puri M., Sharma D., Barrow C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012;30:37–44. doi: 10.1016/j.tibtech.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 60.Garcia-Vaquero M., Rajauria G., O'Doherty J.V., Sweeney T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017;99:1011–1020. doi: 10.1016/j.foodres.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 61.Khodaei N., Karboune S., Orsat V. Microwave-assisted alkaline extraction of galactan-rich rhamnogalacturonan I from potato cell wall by-product. Food Chem. 2016;190:495–505. doi: 10.1016/j.foodchem.2015.05.082. [DOI] [PubMed] [Google Scholar]
- 62.Michalak I., Chojnacka K. Algal extracts: Technology and advances. Eng. Life Sci. 2014;14:581–591. doi: 10.1002/elsc.201400139. [DOI] [Google Scholar]
- 63.Hahn T., Lang S., Ulber R., Muffler K. Novel procedures for the extraction of fucoidan from brown algae. Process Biochem. 2012;47:1691–1698. doi: 10.1016/j.procbio.2012.06.016. [DOI] [Google Scholar]
- 64.Chethana S., Nayak C.A., Raghavarao K.S.M.S. Aqueous two phase extraction for purification and concentration of betalains. J. Food Eng. 2007;81:679–687. doi: 10.1016/j.jfoodeng.2006.12.021. [DOI] [Google Scholar]
- 65.Zhu L., Lu Y., Sun Z., Han J., Tan Z. The application of an aqueous two-phase system combined with ultrasonic cell disruption extraction and HPLC in the simultaneous separation and analysis of solanine and Solanum nigrum polysaccharide from Solanum nigrum unripe fruit. Food Chem. 2020;304 doi: 10.1016/j.foodchem.2019.125383. [DOI] [PubMed] [Google Scholar]
- 66.Liu B., Liu J., Huang D., Wei J., Di D. Boric acid modified macroporous adsorption resin and its adsorption properties for catechol compounds. Colloid. Surface. A. 2020;595 doi: 10.1016/j.colsurfa.2020.124674. [DOI] [Google Scholar]
- 67.Bao J., et al. Effects of macroporous adsorption resin on antibiotic resistance genes and the bacterial community during composting. Bioresour. Technol. 2020;295 doi: 10.1016/j.biortech.2019.121997. [DOI] [PubMed] [Google Scholar]
- 68.Du Z., Wang K., Tao Y., Chen L., Qiu F. Purification of baicalin and wogonoside from Scutellaria baicalensis extracts by macroporous resin adsorption chromatography. J. Chromatogr. B. 2012;908:143–149. doi: 10.1016/j.jchromb.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 69.Yanagida A., et al. Analytical separation of tea catechins and food-related polyphenols by high-speed counter-current chromatography. J. Chromatogr. A. 2006;1112:195–201. doi: 10.1016/j.chroma.2005.09.086. [DOI] [PubMed] [Google Scholar]
- 70.Yuan Z., Xiao X., Li G. Dynamic pH junction high-speed counter-current chromatography coupled with microwave-assisted extraction for online separation and purification of alkaloids from Stephania cepharantha. J. Chromatogr. A. 2013;1317:203–210. doi: 10.1016/j.chroma.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 71.Varadavenkatesan T., Pai S., Vinayagam R., Pugazhendhi A., Selvaraj R. Recovery of value-added products from wastewater using aqueous two-phase systems-a review. Sci. Total Environ. 2021;778 doi: 10.1016/j.scitotenv.2021.146293. [DOI] [PubMed] [Google Scholar]
- 72.Lu M., Ho C.T., Huang Q. Extraction, bioavailability, and bioefficacy of capsaicinoids. J. Food Drug Anal. 2017;25:27–36. doi: 10.1016/j.jfda.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiao J., et al. Rapid characterization of TCM Qianjinteng by UPLC-QTOF-MS and its application in the evaluation of three species of Stephania. J. Pharm. Biomed. Anal. 2018;156:284–296. doi: 10.1016/j.jpba.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 74.Xiao J., et al. Determination of cepharanthine in plants of Stephania Lour. from various habitats by HPLC. Drugs & Clinic. 2016;31:591–594. doi: 10.7501/j.issn.1674-5515.2016.05.006. [DOI] [Google Scholar]
- 75.Li X., Zeng Y., Zhang H., Wang J. Determination of the cepharanthine and cyleanine in Stephania epigaea Lo by RP-HPLC. Res. Practice on Chin. Med. 2010;24:61–63. doi: 10.13728/j.1673-6427.2010.02.020. [DOI] [Google Scholar]
- 76.Ran Y., Huang M., Xu W., Ye S. Determination of cephalotene and its tablets by Ultraviolet spectrophotometry. China J. Chin. Materia Medica. 1993;18:481–483. [PubMed] [Google Scholar]
- 77.Fei W., et al. Optimization of cepharanthine hydroxypropyl-β-cyclodextrin microspheres preparation process by response surface methodology. Contemp. Chem. Ind. 2022;51:1845–1849. doi: 10.13840/j.cnki.cn21-1457/tq.2022.08.047. [DOI] [Google Scholar]
- 78.Wang Y., Mu X. Determination of cephalotene in Stephania cepharantha Hayata by RP-HPLC. J. Med. Pharmacy Chin. Minorities. 2010;16:44–45. doi: 10.3969/j.issn.1006-6810.2010.04.028. [DOI] [Google Scholar]
- 79.Y. Wang, et al., Determination of cepharanthine in Stephania cepharantha Hayata by MSPD-HPLC, Chin. Traditional Patent Med. 36, 2557-2560, 10. 3969/j.issn.1001-1528. 2014.12.025 (2014).
- 80.Castro-Muñoz R., et al. Up-to-date strategies and future trends towards the extraction and purification of capsaicin: A comprehensive review. Trends Food Sci. Tech. 2022;123:161–171. doi: 10.1016/j.tifs.2022.03.014. [DOI] [Google Scholar]
- 81.Castro-Muñoz R., et al. Current role of membrane technology: From the treatment of agro-industrial by-products up to the valorization of valuable compounds. Waste Biomass Valoriz. 2017;9:513–529. doi: 10.1007/s12649-017-0003-1. [DOI] [Google Scholar]
- 82.Wang S., et al. Mechanochemical-assisted extraction of active alkaloids from plant with solid acids. ACS Sustain. Chem. Eng. 2018;7:197–207. doi: 10.1021/acssuschemeng.8b02902. [DOI] [Google Scholar]
- 83.Castro-Munoz R., et al. Phenolic compounds recovered from agro-food by-products using membrane technologies: An overview. Food Chem. 2016;213:753–762. doi: 10.1016/j.foodchem.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 84.Castro-Muñoz R., et al. Membrane-based technologies as an emerging tool for separating high-added-value compounds from natural products. Trends Food Sci. Tech. 2018;82:8–20. doi: 10.1016/j.tifs.2018.09.017. [DOI] [Google Scholar]
- 85.Castro-Muñoz R., et al. Membrane technologies assisting plant-based and agro-food by-products processing: A comprehensive review. Trends Food Sci. Tech. 2020;95:219–232. doi: 10.1016/j.tifs.2019.12.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.