Abstract
Objective:
To assess whether vascular FDG-PET activity is associated with angiographic change in large-vessel vasculitis (LVV).
Methods:
Patients with LVV were recruited into a prospective cohort. All patients underwent magnetic resonance (MR) or computed tomography (CT) angiography, FDG-PET, and follow-up studies ≥6 months later per a standardized imaging protocol. Arterial damage, defined as stenosis, occlusion, or aneurysm, and corresponding FDG uptake was evaluated in 17 arterial territories. On follow-up, development of new lesions was recorded, and existing lesions were characterized as improved, worsened, or unchanged.
Results:
1,091 arterial territories were evaluated from 70 patients (TAK=38; GCA=32). Over 1.6 years of median follow-up, new lesions developed only in 8 arterial territories, exclusively in 5 patients with TAK. Arterial lesions improved in 16 territories and worsened in 6 territories. Most arterial territories without FDG-PET activity at baseline [787 out of 793 (99%)] remained unchanged over time by angiography. Few territories with PET activity changed over time [24 of 298 (8%)], but of the territories with angiographic change, the majority had baseline PET activity (80%). Within a patient, an arterial territory with baseline PET activity had 20 times increased odds for angiographic change compared to a paired arterial territory without PET activity (p<0.01). Concomitant edema and wall thickness further increased risk for angiographic change.
Conclusion:
Development of angiographic change was infrequent in this cohort of patients with LVV. Lack of PET activity was strongly associated with stable angiographic disease. In cases of angiographic progression, change was preceded by the presence of FDG-PET activity.
INTRODUCTION
Disease activity assessment in large-vessel vasculitis (LVV) can be challenging as patients may not have overt clinical symptoms or elevated acute phase reactants during periods of active disease. Non-invasive angiography with use of magnetic resonance angiography (MRA) or computed tomography angiography (CTA) has become essential to detect and monitor vascular disease in patients with LVV(1–3). The development of new areas of arterial damage on angiography during periods of apparent clinical remission has been reported in LVV, but it remains unknown whether this is a common or rare phenomenon(4, 5). Previous studies have shown a range of frequency of angiographic progression in LVV, and some studies have demonstrated that either progressive arterial damage or improvement can occur over time(6–8). Current guidelines differ on the role and frequency of periodic angiography to monitor arterial damage in patients with LVV, in part due to limited prospective data characterizing angiographic progression of disease over time and a lack of standardized imaging protocols when assessing change(3, 9).
Multimodal imaging assessment, incorporating the use of 18F-flurodeoxyglucose (FDG) positron emission tomography (PET) with non-invasive angiography, has become increasingly used in LVV(1, 10, 11). Abnormal metabolic activity on FDG-PET within the walls of large arteries due to activated immune cells can be used as a surrogate for vascular inflammation in LVV but the degree to which vascular FDG-PET abnormalities are specific for inflammation is difficult to define (1, 12, 13). Limited longitudinal data on use of FDG-PET in LVV has shown patients may have vascular PET activity during periods of clinical remission(12, 14, 15). Whether FDG-PET activity predicts long-term outcomes, including angiographic progression of disease, in patients with LVV remains unknown.
The objectives of this study were to: 1) Characterize progression of disease over time by non-invasive angiography using a standardized imaging protocol in a prospective, longitudinal cohort of patients with Takayasu’s arteritis (TAK) and giant cell arteritis (GCA) and 2) Evaluate whether the presence of vascular FDG-PET activity predicts angiographic progression of disease in LVV.
PATIENTS AND METHODS
Study population:
Patients with LVV were recruited into a prospective, observational cohort at the National Institutes of Health (NIH) in Bethesda, MD, USA. Patients fulfilled the 1990 American College of Rheumatology (ACR) Classification Criteria for TAK(16) or modified 1990 ACR Criteria for GCA(17, 18). Patients could be enrolled at various stages during the disease course.
Clinical Assessment:
All patients underwent baseline clinical evaluation, laboratory testing, magnetic resonance angiography (MRA) or computed tomography angiography (CTA), and FDG-PET imaging at the NIH Clinical Center. The investigative study team performed all clinical assessments within 24 hours prior to imaging assessment. Clinical disease was recorded as active or remission, prior to conducting imaging studies. Active disease was defined as presence at the time of assessment of any clinical disease feature directly attributed to vasculitis (e.g. carotidynia, headache). Fatigue or elevated acute phase reactant levels alone were not considered sufficient evidence of active disease. Remission was defined as the absence of any clinical symptoms directly attributable to vasculitis, regardless of acute phase reactants. Patients underwent follow-up clinical assessments at least 6 months later. Changes in clinical disease activity, C-reactive protein (CRP) levels, and treatment over the follow-up interval were assessed. Increased treatment was defined as the addition of a new disease-modifying antirheumatic drug (DMARD), new biologic therapy, or increase in glucocorticoid dose by ≥50% over the follow-up period.
Non-Invasive Angiography Protocol and Assessment:
All patients underwent a baseline MRA or CTA of the aorta and primary branches and a follow-up study on the same image modality at least six months after baseline per a standardized imaging protocol, as previously described(10). In patients who had more than two study visits, the baseline and most recent follow-up images were compared to assess for change over the longest available time interval (see online Supplementary Methods for additional details and imaging protocol).
Vascular damage, defined as stenosis, occlusion, or aneurysm, was evaluated by visual inspection in 4 segments of the aorta (ascending, arch, descending thoracic, and abdominal) and 13 branch arteries (innominate, carotids, vertebrals, subclavians, axillaries, common iliacs, femorals) by a single reader (MA) blinded to clinical status. Only luminal changes were considered, and wall thickness, edema, and contrast enhancement were not included in the definition of vascular damage. On follow-up angiography, the development of new angiographic damage in these same arterial territories was recorded, and existing arterial damage was characterized as improved, worsened, or unchanged by visual inspection, with confirmation by an independent reader (JM). Angiographic change over time was evaluated at the patient level and within each individual arterial territory.
Wall morphologic changes were defined by expert review (MA, >10 yrs experience in multimodality vascular imaging followed by expert consensus opinion for challenging cases) (see online Supplementary Methods for additional details).
FDG-PET Imaging Protocol and Assessment:
All patients underwent whole-body FDG-PET studies on the same dates as the baseline and follow-up angiograms, per a standardized imaging protocol. A single reader (MA) reviewed all FDG-PET scans, blinded to clinical status and angiogram assessment. Excellent intra-rater agreement (kappa=0.76) and inter-rater agreement was previously reported (kappa=0.84) for this cohort(12, 19). Qualitative assessment of FDG uptake was assessed in each of the 17 corresponding arterial territories evaluated by angiography. The degree of arterial uptake was visually assessed relative to liver uptake as 0=no uptake; 1=less than liver uptake; 2=same as liver uptake; 3=greater than liver uptake. Active vasculitis in an arterial territory was defined as greater FDG uptake in the arterial wall compared to liver. Semi-quantitative assessment of arterial FDG uptake was performed to obtain corresponding maximum standardized uptake values (SUVmax) (see online Supplementary Methods for additional details and imaging protocol).
Statistical Analysis:
Clinical characteristics among patients with improved arterial damage, new/worsened arterial damage, and unchanged arterial damage were compared using Chi-square or Kruskal-Wallis test, as appropriate. FDG-PET and angiographic findings were compared within specific arterial territories. Baseline FDG-PET activity was studied in association with angiographic change (new, worsening, or improved) versus no change to determine if FDG-PET activity predicted angiographic progression of disease. Logistic regression was used to identify clinical features associated with angiographic change using the following predictor variables: type of LVV (TAK vs GCA), baseline clinical disease activity (active/remission), disease duration, baseline CRP levels, baseline global FDG-PET interpretation (active/inactive), and baseline treatment (yes/no). Only variables with p <0.10 in univariable analyses were included in the multivariable model. Analyses were conducted using JMP version 14.0.
Within-person, arterial territory-matched analysis:
To evaluate the association between baseline FDG-PET activity and risk for subsequent angiographic change in the same specific arterial territories on the follow up study, we used a within-person, arterial territory-matched approach with conditional logistic regression adjusting for correlated measures. For this approach, we identified participants with discordant angiographic change in paired arteries (e.g., a patient who developed worsening angiographic damage in the left carotid artery over study follow-up, while there were no changes in the right carotid artery). In this way, each person serves as their own control, allowing comparison of baseline FDG-PET activity in the angiographic territory that changed versus the contralateral territory that did not change over time within the same patient. As such, the effects of all person-level confounders are eliminated in this type of analysis (e.g., differences in disease duration at baseline imaging study, differences in length of follow-up time, differences in treatment, etc.) (20). Fourteen arterial territories were assessed and grouped into 7 symmetric paired sets: 1) bilateral carotids, 2) bilateral vertebrals, 3) bilateral subclavians, 4) bilateral axillaries, 5) bilateral common iliacs, 6) bilateral femorals, and 7) descending thoracic and abdominal aorta. The remaining three arterial territories assessed by angiography (ascending aorta, aortic arch, innominate artery) were not included in the paired analyses. Conditional logistic regression analysis was conducted using R version 4.0.2.
Ethics and Informed Consent:
All patients provided written informed consent. An institutional review board and radiation safety committee at the NIH approved the research (National Institutes of Arthritis and Musculoskeletal and Skin Diseases IRB Protocol: 14-AR-0200).
RESULTS
Study Population:
A total of 70 patients with LVV were recruited into the study. There were 38 patients with TAK and 32 patients with GCA. Baseline demographics of the study population are shown in Table 1. Median disease duration at time of study enrollment was 2.2 years in TAK and 0.7 years in GCA. Many patients (37 out of 70, 53%) had ongoing active clinical symptoms at the baseline visit. Comparing TAK and GCA, there were no significant differences in the proportion of patients with active disease at the baseline study visit (TAK=45%, GCA=63%, p=0.14), nor were there differences in the proportion of patients who achieved clinical remission over follow-up (TAK=79% vs GCA=88%, p=0.53).
TABLE 1:
Study Population Characteristics at the Baseline Visit
| TAK n = 38 |
GCA n = 32 |
p-value | |
|---|---|---|---|
| Age (years, IQR) | 29.5 (18.4–39.5) | 70.5 (61.1–75.9) | <0.01 |
| Gender (n, % female) | 30 (79%) | 23 (72%) | 0.49 |
| Disease duration (years, IQR) | 2.2 (0.6–5.5) | 0.7 (0.1–2.6) | <0.01 |
| Clinical disease activity (n, % active) | 17 (45%) | 20 (63%) | 0.14 |
| Temporal artery biopsy positivea
LV-GCA (angiographic involvementb) Both |
N/A | 14 (44%) 11 (34%) 7 (22%) |
N/A |
| Acute phase reactants ESR (mm/h, IQR) CRP (mg/L, IQR) |
18.5 (11–34) 3.8 (0.9–14) |
19 (9–26) 6.3 (1–11) |
0.81 0.58 |
| Type of angiography MRA CTA |
35 (92%) 3 (8%) |
29 (91%) 3 (9%) |
1.0 |
| Treatment Prednisone (mg/day, IQR) Other immune therapy Conventional DMARD Biologic DMARD |
5 (0–10) 26 (70%) 22 (85%) 11 (42%) |
7.5 (0–34) 16 (50%) 11 (69%) 3 (19%) |
0.21 0.08 0.27 0.17 |
26 of 32 patients (81%) with GCA underwent temporal artery biopsy
All patients underwent MRA or CTA at time of baseline FDG-PET scan. LV-GCA was defined as stenosis, occlusion, or aneurysm of large arteries on MRA or CTA
TAK=Takayasu’s arteritis; GCA=giant cell arteritis; IQR=interquartile range; LV=large-vessel; ESR=erythrocyte sedimentation rate; CRP=C-reactive protein; MRA=magnetic resonance angiography; CTA=computed tomography angiography; DMARD=disease-modifying antirheumatic drug
Angiographic Change Over Study Follow-Up:
Arterial territory-level analysis:
A total of 1,190 arterial territories were evaluated from 70 patients with LVV. Ninety-nine territories with unacceptable image quality due to technical factors (e.g. patient positioning, movement, image acquisition error) were excluded. The remaining 1,091 arterial territories were assessed (TAK=577 arterial territories, GCA=514 arterial territories) over a median follow up of 1.6 years [Interquartile range (IQR) 1.0–2.7 years]. Patients with TAK were followed for similar lengths of time compared to patients with GCA [TAK = 1.5 years (1.0–2.6) vs GCA = 1.8 years (0.7–2.9); p=0.96]. There was no change in angiographic findings (i.e. new, worsening, or improved stenosis, occlusion, or aneurysm) from baseline to the most recent follow-up visit in 1,061 out of 1,091 territories (97.3%). New, worsening, or improved areas of arterial damage were observed in 30 of 1,091 (2.7%) of all territories, which occurred in 16 participants overall. Out of 843 arterial territories with no arterial damage at the baseline visit, new damage occurred in 8 territories (1%) in 5 participants. A total of 248 arterial territories had existing arterial luminal damage at the baseline visit, with aneurysmal disease observed in 52 (21%) of these territories (TAK=35 and GCA=17). Most of these arterial territories remained unchanged over follow-up [226 of 248 territories (91%)]. Change in luminal damage was bidirectional, with improvement in 16 territories (7%) and worsening in 6 territories (2%) (FIGURE 1 and SUPPLEMENTARY FIGURE 1). Change was only observed in stenosing lesions, and angiographic change only occurred in 9 of the 17 specific arterial territories assessed (SUPPLEMENTARY FIGURE 2).
FIGURE 1: Angiographic Progression in Arterial Territories.
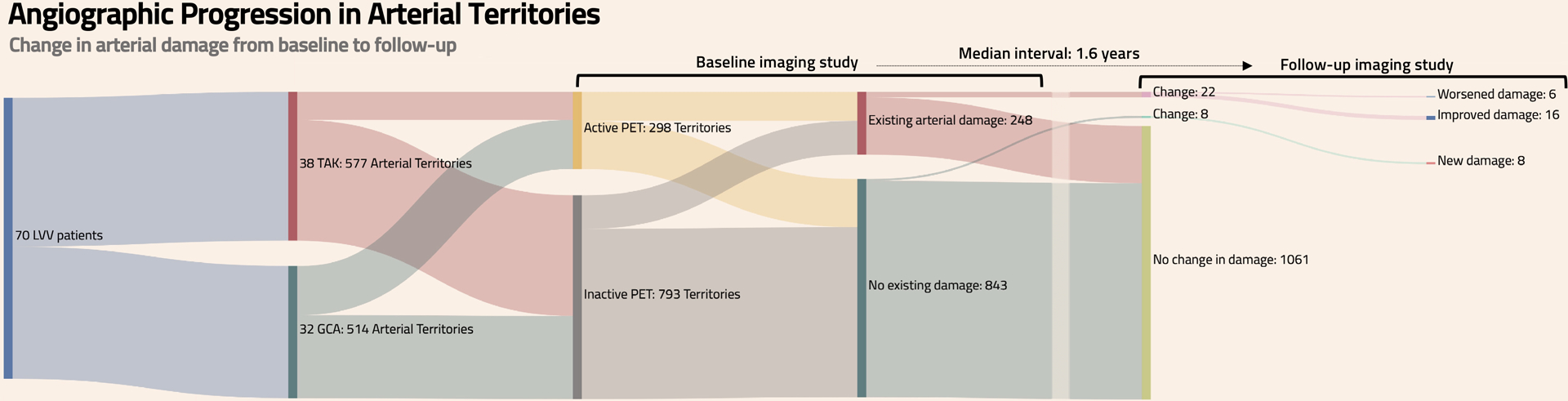
Change in arterial damage from baseline to follow-up is depicted in 1,091 arterial territories from 70 patients with large-vessel vasculitis (577 arterial territories from patients with TAK and 514 arterial territories from patients with GCA). The presence of FDG-PET activity and existing arterial damage in each arterial territory was assessed at time of baseline imaging studies. Over a median 1.6-year follow-up period, the number of arterial territories with improved damage, worsened damage, new damage, or no change was evaluated. TAK=Takayasu’s arteritis; GCA=giant cell arteritis
Patient-level analysis:
Of the 70 patients with LVV, 9 patients (6 TAK, 3 GCA) had improved damage in at least one arterial territory (without any territories with new or worsening damage), 7 patients (7 TAK, 0 GCA) had at least one arterial territory with new or worsening damage (without any territories with improvement), and 54 patients (25 TAK, 29 GCA) had no change on follow-up angiogram (TABLE 2).
Table 2:
Clinical Characteristics of Patients with Improved Damage, New or Worsened Damage, or No Angiographic Change
| Improved n = 9 |
New/worsened n = 7 |
No change N = 54 |
p-value | |
|---|---|---|---|---|
| Disease duration at baseline (years, IQR) | 0.4 (0.1–2.8) | 1.5 (0.6–3.6) | 1.5 (0.4–5.1) | 0.19 |
| Follow-up duration (years, IQR) | 1.5 (1.0–2.7) | 1.9 (1.5–4.3) | 1.6 (1.0–2.7) | 0.52 |
| LVV type | GCA=3 TAK=6 |
GCA=0 TAK=7 |
GCA=29 TAK=25 |
0.02 |
| Clinical activity Active at any point during the study Persistent remission |
9 (100%) 0 (0%) |
7 (100%) 0 (0%) |
25 (46%) 29 (54%) |
<0.01 |
| CRP Baseline (mg/L, IQR) Follow-up (mg/L, IQR) |
1.8 (0.7–28) 0.4 (0.2–0.9) |
13.4 (5.1–49) 6.1 (4–20) |
4.2 (1–11) 0.6 (0.2–2) |
0.20 0.02 |
| CRP Elevated (>10mg/L) during the study Persistently normal |
3 (33%) 6 (67%) |
5 (71%) 2 (29%) |
17 (31%) 37 (69%) |
0.11 |
| Baseline PET global interpretation (active, %) | 7 (78%) | 5 (71%) | 40 (77%) | 0.96 |
| Baseline PETVAS (median, IQR) | 17 (11–26) | 17 (10–21) | 18 (14–24) | 0.95 |
| Follow-up PET global interpretation (active, %) | 5 (56%) | 3 (43%) | 28 (52%) | 0.87 |
| PET global interpretation Active at any point during the study Persistently inactive |
7 (78%) 2 (22%) |
6 (86%) 1 (14%) |
44 (81%) 10 (19%) |
0.92 |
| Treatment | ||||
| Median prednisone Baseline (mg/day, IQR) Follow-up (mg/day, IQR) |
15 (0–50) 0 (0–6) |
2 (0–30) 0 (0–20) |
5 (0–16) 0 (0–5) |
0.55 0.35 |
| Conventional DMARDa Baseline Follow-up |
4 (44%) 4 (44%) |
3 (43%) 5 (71%) |
23 (43%) 23 (43%) |
0.99 0.35 |
| Biologic DMARDb Baseline Follow-up |
2 (22%) 8 (89%) |
4 (57%) 4 (57%) |
10 (19%) 27 (50%) |
0.07 0.09 |
LVV=large-vessel vasculitis; CRP=C-reactive protein; PETVAS=PET Vascular Activity Score(12)
At baseline, the most commonly used conventional DMARDs were methotrexate (TAK=12, GCA=10) and mycophenolate mofetil (TAK=6, GCA=0). At follow up, the most commonly used conventional DMARDs were methotrexate (TAK=14, GCA=3) and mycophenolate mofetil (TAK=6, GCA=1).
At baseline, and the most commonly used biologic DMARDs were tumor necrosis factor inhibitors (TAK=6, GCA=0) or tocilizumab (TAK=2, GCA=1). At follow up, the most commonly used biologic DMARDs were tumor necrosis factor inhibitors (TAK=10, GCA=0) or tocilizumab (TAK=9, GCA=20).
In univariable logistic regression, angiographic change was not significantly associated with baseline CRP levels, baseline FDG-PET interpretation (active versus inactive vasculitis), disease duration, length of follow-up time, or baseline treatment status. A diagnosis of TAK compared to GCA [Odds ratio = 3.10 (95% CI 1.54–7.17); p<0.01] and active clinical disease at the baseline visit [Odds ratio = 3.94 (95% CI 1.86–10.80); p<0.01] were independent predictors of angiographic change in multivariable regression.
New damage occurred in 5 of 38 (13%) patients with TAK, and worsened damage occurred in 3 of 38 (8%) patients with TAK (1 patient had a territory with new damage and another territory with worsened damage). None of the 32 patients with GCA developed new or worsened areas of arterial damage over follow-up. Seventy-two percent of patients with new or worsening damage had clinically active disease at the baseline visit, and the remaining patients developed a clinical flare over the follow-up interval, with no patient developing “silent” angiographic progression. Compared to patients with improved damage, patients with new or worsening arterial damage were often initially evaluated later in the disease course [median disease duration 1.5 years (IQR 0.6–3.6 years)], had the highest CRP levels at baseline visit [median 13.4 mg/L (IQR 5.1–49 mg/L)], and the majority (57%) were receiving biologic therapies at the baseline visit but were on low doses of glucocorticoids [median prednisone dose 2 mg/day (IQR 0–30 mg/day)] (TABLE 2). Two patients were non-adherent to treatment over the follow-up interval and one patient developed a severe disease relapse with delayed initiation of treatment.
Improved damage occurred in 6 of 38 (16%) patients with TAK and 3 of 32 (9%) patients with GCA. All patients who had improved damage had clinical disease activity at the baseline visit. Compared to patients with new/worsening arterial damage, patients with improved damage were frequently early in the disease course [median disease duration 0.4 years (IQR 0.1–2.8 years)], were on higher doses of glucocorticoids at the baseline visit [median prednisone dose 15 mg/day (IQR 0–50 mg/day)], and had the greatest increase in treatment over the follow-up interval, with 89% patients on a biologic therapy at time of follow-up assessment (TABLE 2).
No angiographic change occurred in 25 (66%) patients with TAK and 29 (91%) patients with GCA. The majority of patients with no change were in persistent clinical remission [29 of 54 (54%)] (SUPPLEMENTARY FIGURE 3). Of these 29 patients in persistent clinical remission, most had normal acute phase reactants at baseline [24 of 29 (83%)], but many [20 of 29 (69%)] had baseline subclinical FDG-PET activity. Treatment was increased in eight patients in clinical remission throughout the study who had an active FDG-PET scan at baseline, and there was no angiographic progression of disease over follow-up in any of these patients. Four of these patients were being treated with glucocorticoid monotherapy at baseline, and steroid-sparing therapy was added. The other 4 patients had severe elevations in CRP or severe FDG-PET activity for whom biologic therapy was added (SUPPLEMENTARY FIGURE 4). There were 12 patients in clinical remission throughout the study who had active FDG-PET scans at baseline (all with normal CRP) and did not receive increased treatment. No angiographic progression of disease was observed over follow-up in these patients.
FDG-PET Activity and Angiographic Change:
Assessment of angiographic change in all territories with and without FDG-PET activity:
Baseline FDG-PET activity was evaluated in the 1,091 corresponding arterial territories evaluated by angiography. There was no change in angiographic findings over follow-up for 787 out of 793 (99%) arterial territories with no FDG-PET activity at baseline. Most arteries territories with FDG-PET activity at baseline also did not develop angiographic change over the follow-up interval, with only 24 of 298 arterial territories (8%) developing angiographic change. However, of the 30 arterial territories that developed angiographic change, the majority had FDG-PET activity at baseline [24 of 30 (80%) territories] (FIGURE 2). Of the 6 territories (4 patients) with angiographic change in absence of baseline PET activity, 3 patients had clinical relapse with PET activity in the corresponding territory on follow-up. The other patient had associated arterial thrombosis with progressive damage in absence of PET activity. Overall, FDG-PET activity in an arterial territory at baseline was associated with change in that arterial territory on follow-up angiography with a positive predictive value of 8% and negative predictive value of 99%.
FIGURE 2: Assessment of Angiographic Progression of Disease in Arterial Territories With and Without FDG-PET Activity.
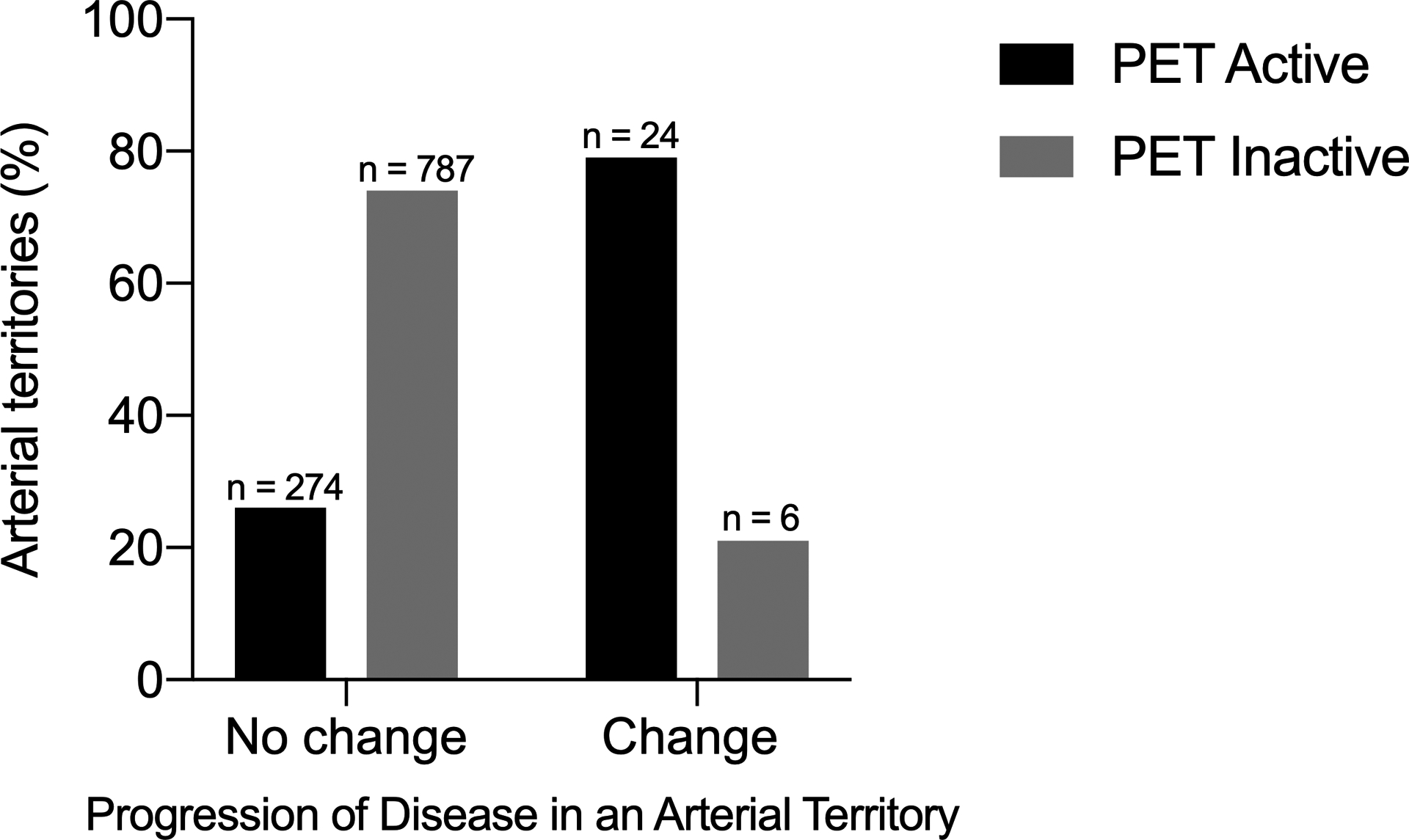
Arterial territories were divided into those that did versus did not change on follow-up angiogram. The presence (black bars) or absence (gray bars) of FDG-PET activity was evaluated in each arterial territory.
Association of FDG-PET activity and angiographic change in the within-person arterial territory-matched approach using conditional logistic regression:
Of the 30 arterial territories that developed angiographic change over follow-up, 26 of the 30 territories were asymmetric in paired arteries (e.g. left carotid artery had angiographic progression, right carotid artery had no change). Specifically, we identified 16 participants who had 26 asymmetric angiographic changes in paired arterial territories that were eligible for this analysis (10 had more than one discordant pair). An arterial territory with baseline FDG-PET activity had significantly higher odds for angiographic progression of disease over follow-up compared to the matched arterial territory without FDG-PET activity (Odds ratio = 19.49, 95% CI 2.44–156.02; p <0.01). A representative image of asymmetric angiographic change in paired arteries is shown in FIGURE 3.
FIGURE 3: Angiographic Progression of Disease in a Patient with Takayasu’s Arteritis.
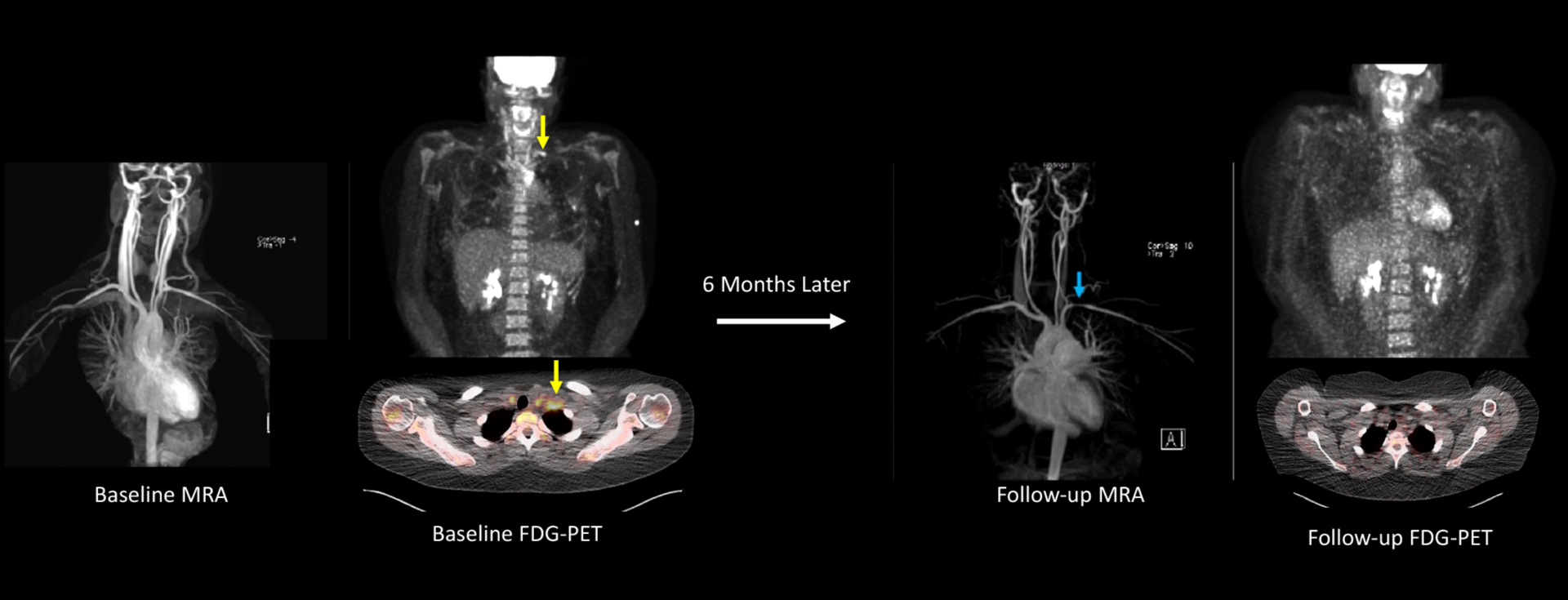
This 22-year-old female with Takayasu’s arteritis self-discontinued methotrexate/infliximab after approximately 2 years of treatment. Six months later she developed constitutional symptoms, frontal headaches, carotidynia, and left arm claudication. She had significant elevations in acute phase reactants (ESR 104, CRP 85 mg/L). An FDG-PET scan showed severe vascular FDG uptake throughout the aorta and bilateral common carotid arteries with a prominent area of focal inflammation in the left subclavian artery (yellow arrows) on whole-body and axial views. MRA obtained the same date did not show vascular damage. Treatment was re-initiated with excellent clinical response. Six months later, the patient had a repeat MRA showing a new left subclavian artery stenosis (blue arrow) with resolution of vascular PET activity.
Additional imaging characteristics associated with angiographic change:
Because most instances of angiographic change were asymmetric in paired arterial territories and were associated with corresponding baseline FDG-PET activity, we next examined all instances of asymmetric FDG-PET activity in paired arteries across the cohort at the baseline study visit. Of a total of 490 paired arterial territories, FDG-PET activity was asymmetric in 42 paired territories (e.g. left carotid artery had baseline FDG-PET activity, right carotid artery had no FDG-PET activity). When examining the 42 arterial territories with baseline FDG-PET activity, corresponding angiographic change occurred in 13 (31%) territories and there was no change in the other 29 (69%) territories. We then studied if additional factors present on the baseline imaging studies besides presence of FDG-PET activity were associated with angiographic change, including the degree of FDG-PET activity (SUVmax values) and the presence of wall morphologic changes (increased wall thickening and edema). There were no differences in baseline median SUVmax values among territories with asymmetric FDG-PET activity that did [SUVmax 3.6, IQR (2.2–4.5) versus did not [SUVmax 4.2, IQR (3.1–5.3)] develop angiographic progression; p=0.35. (FIGURE 4A). All 13 (100%) cases of asymmetric FDG-PET activity in which angiographic change occurred had concomitant wall morphologic changes (increased wall thickening and edema), compared with 11 of 29 (38%) cases where there was no angiographic change; p<0.01 (FIGURE 4B).
FIGURE 4: Imaging Characteristics Associated With Angiographic Change.
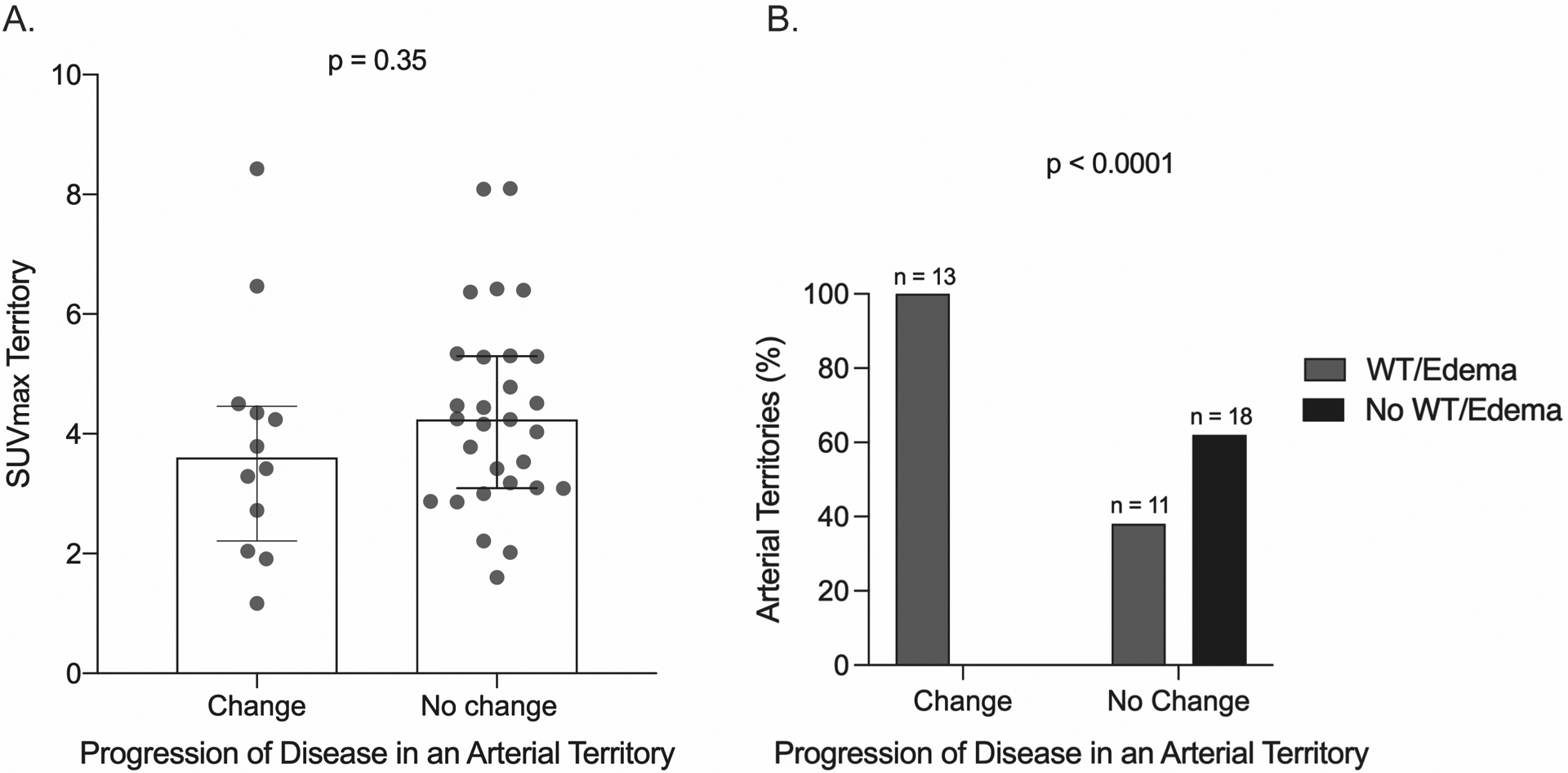
A. SUVmax values on baseline FDG-PET scan were assessed among territories with asymmetric FDG-PET activity that did versus did not develop angiographic progression of disease over follow-up. B. Corresponding wall morphologic changes (i.e. wall thickening and edema) on baseline angiography were assessed among territories with asymmetric FDG-PET activity that did versus did not develop angiographic progression of disease over the follow-up interval. SUVmax=maximum standardized uptake values; WT=wall thickening.
DISCUSSION
This study provides some of the first and only available data about the longitudinal relationship between FDG-PET activity and angiographic change in LVV. Complex associations between FDG-PET activity and angiographic change were found in this cohort where patients underwent standardized clinical and imaging assessments. Overall, little angiographic change occurred, and a lack of FDG-PET activity was strongly associated with stable angiographic disease. The majority of arterial territories with FDG-PET activity did not develop angiographic change; however, in cases where angiographic change did occur, change was frequently preceded by the presence of FDG-PET activity in the arterial territory at baseline.
The development of angiographic change was uncommon over a median 1.6-year follow-up period in this cohort of patients with LVV. In cases where angiographic change occurred, it was more frequently observed in TAK compared to GCA. A wide range of frequency of angiographic progression in LVV has been reported in previous studies, with several studies also showing angiographic change is an uncommon occurrence(8, 21), while other studies have reported more frequent angiographic change(4, 6). Studies where angiographic change has been reported more frequently have been retrospective, not employed standardized imaging protocols, and not used centralized review of images, all of which could have led to over-estimation of the amount of angiographic change. In this study, patients were frequently enrolled in the later phases of disease, which may have contributed to the infrequent change observed; however, despite being later in the disease course, many patients had clinical disease activity at the baseline study visit or developed disease flares over the follow-up interval. More patients in this cohort were also being treated with biologic therapy, compared to other cohorts(6), which could have impacted the amount of angiographic change observed. Additionally, as LVV is a slowly progressive disease, the duration of follow-up may have contributed to the low frequency of angiographic change observed. However, some patients did develop new or worsening arterial damage later in the disease course, highlighting the importance of continued monitoring of patients with LVV, even in later phases of disease.
In cases of existing arterial luminal damage, this study demonstrated that both progressive arterial damage or improvement could occur over time, similar to findings from prior studies(7, 21). Whether improvement or worsening occurs in a territory with existing arterial damage may depend on where a patient is in the course of their disease, disease severity, and treatment received. Patients in this study who developed new or worsening arterial damage were often non-adherent to treatment or had delayed initiation of treatment outside the window of possible prevention of damage. These data provide insight into the natural history of vascular lesions in LVV, as a window of intervention may exist to prevent new or worsening damage with treatment. Additionally, new or worsening arterial damage was only observed in TAK rather than GCA, despite comparable proportions of patients who achieved clinical remission throughout the study. Progressive arterial damage despite effective therapeutic intervention has been attributed to “healing fibrosis” in TAK. Thus, the window for effective intervention may be narrower in TAK compared to GCA due to differential rates of time required to develop angiographic damage in these diseases.
This study is some of the only available evidence to evaluate whether FDG-PET activity is associated with angiographic change. This study does not address the specificity of FDG-PET signal and whether FDG-PET activity represents subclinical vascular inflammation, vascular remodeling, or a combination of factors. In the absence of a corresponding histologic gold standard, some level of uncertainty about whether FDG-PET activity truly represents active vasculitis will always remain, but this data reframes the focus to whether FDG-PET activity carries prognostic information for patients and therefore should guide management decisions.
The majority of arterial territories with vascular FDG-PET activity did not develop angiographic change over the follow-up period. Normalization of FDG-PET activity should not be the goal for all patients with LVV, particularly for GCA, where metabolic activity in the arterial wall without subsequent angiographic change was a frequent occurrence. While overall most arterial territories with FDG-PET activity were not associated with angiographic change, there are still many potential scenarios for which serial FDG-PET monitoring may be useful including: clarification of symptoms that could be secondary to ongoing vascular inflammation or reflect prior vascular damage, evaluation of unexplained laboratory abnormalities later in the disease course, or assessment of treatment response at the vascular level. On the other hand, absence of vascular FDG-PET activity in arterial territories was associated with stable angiographic findings on follow-up imaging. FDG-PET data could potentially be incorporated into more stringent definitions of disease remission. In cases of angiographic change, FDG-PET activity preceded angiographic change in most cases, suggesting FDG-PET is tracking an aspect of disease activity. Future research is needed to better define what additional imaging risk factors contribute to change over time. Our data provide preliminary insight into potential risk factors. Focal, asymmetric areas of arterial FDG-PET activity with concomitant wall edema and wall thickness on angiography were most likely to develop angiographic change (SUPPLEMENTARY FIGURE 5).
This study also shows that treatment decisions should not be based solely on FDG-PET data. Overall, the majority of patients in clinical remission with abnormal FDG-PET activity did not receive increased treatment, and none of these patients had angiographic change over follow-up. On the other hand, some patients with subclinical FDG-PET activity (with or without elevations in acute phase reactants) did receive increased treatment. There may be subsets of patients at greatest risk for angiographic progression who may benefit from increased treatment based on imaging findings alone, such as those with focal areas of FDG-PET activity and concomitant wall morphologic changes in critical vascular regions. However, this study was done in the context of an observational cohort making it difficult to know what would have happened in these cases if treatment remained unchanged.
These data should inform future guideline recommendations for imaging monitoring in LVV. Current guidelines recommend regular noninvasive imaging for long-term monitoring of angiographic progression, but do not specify an optimal imaging modality or interval(3, 9). These data demonstrate the value and support the use of multi-modal imaging assessment in LVV with concomitant FDG-PET and non-invasive angiography. Additionally, more frequent imaging monitoring may be needed in patients with TAK compared to patients with GCA. This study also suggests that for patients in stable, persistent clinical remission, the value of serial imaging is likely less than for patients with active clinical symptoms, as patients in this cohort who developed new or worsening angiographic damage had corresponding clinical symptoms suggestive of a disease flare. No patient had silent angiographic progression which may be a less frequent phenomenon than previously described(4). However, the fact that no patient had silent angiographic progression of disease in this cohort needs to be interpreted with caution as some patients in persistent clinical remission had treatment increased based on elevated acute phase reactants and/or FDG-PET activity. Additionally, all patients underwent rigorous, standardized clinical assessments in a research setting, which may have led to detection of clinical disease activity that may have otherwise been missed and contributed to the lack of “silent” angiographic progression observed in this study.
There are several strengths of this study. For each patient, clinical data, laboratory data, angiography, and FDG-PET scans were obtained at baseline and patients were prospectively followed with the same clinical and imaging data obtained at the follow-up study visit. Each patient underwent MRA/CTA and FDG-PET scans per a standardized imaging protocol, enabling direct comparison of baseline and follow-up images without a need to account for potential technical differences in image acquisition. Additionally, baseline and follow-up MRA/CTA and FDG-PET scan images were directly reviewed by a central reader.
Some additional limitations of this study should be noted. The NIH is a referral center with possible selection bias, but there were no geographic or socioeconomic restrictions to enrollment. When assessing angiographic change, territories with worsening or improvement were studied in composite. There were too few angiographic events to evaluate angiographic worsening or improvement separately. However, the degree of baseline PET activity was not associated with angiographic improvement or worsening in the patient-level analyses. Whether there is angiographic improvement or worsening over time is more likely related to effectiveness of therapeutic intervention rather than differences in the amount of PET activity at the baseline visit. This study was done in the context of an observational cohort where patients received various treatments and were followed for variable time intervals. To address these differences, a within-person arterial territory-matched approach was used to limit confounding between patients, such as differences in treatment. However, this approach can only be applied to persons with discordant angiographic change in paired arteries; thus, those with symmetric angiographic change (while infrequent) cannot be evaluated by this approach.
In conclusion, this study underscores the complex longitudinal associations between FDG-PET activity and angiographic change in LVV. Clinicians should be aware of the potential advantages and challenges of incorporating multimodal imaging into clinical practice to assess disease activity and vascular damage over time in patients with LVV.
Supplementary Material
Financial Disclosures:
This study was supported by the Intramural Research Program at the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. LaValley is supported by NIH/NIAMS CCCR P30 AR072571.
Footnotes
Statements: The authors declare no conflicts of interest related to this work.
REFERENCES
- 1.Slart R, Writing g, Reviewer g, Members of EC, Members of EI, Inflammation, et al. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging. 2018;45(7):1250–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamada I, Nakagawa T, Himeno Y, Kobayashi Y, Numano F, Shibuya H. Takayasu arteritis: diagnosis with breath-hold contrast-enhanced three-dimensional MR angiography. J Magn Reson Imaging. 2000;11(5):481–7. [DOI] [PubMed] [Google Scholar]
- 3.Maz M, Chung SA, Abril A, Langford CA, Gorelik M, Guyatt G, et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Giant Cell Arteritis and Takayasu Arteritis. Arthritis Rheumatol. 2021;73(8):1349–65. [DOI] [PubMed] [Google Scholar]
- 4.Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, et al. Takayasu arteritis. Ann Intern Med. 1994;120(11):919–29. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Martinez A, Arguis P, Prieto-Gonzalez S, Espigol-Frigole G, Alba MA, Butjosa M, et al. Prospective long term follow-up of a cohort of patients with giant cell arteritis screened for aortic structural damage (aneurysm or dilatation). Ann Rheum Dis. 2014;73(10):1826–32. [DOI] [PubMed] [Google Scholar]
- 6.Kermani TA, Diab S, Sreih AG, Cuthbertson D, Borchin R, Carette S, et al. Arterial lesions in giant cell arteritis: A longitudinal study. Semin Arthritis Rheum. 2019;48(4):707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tombetti E, Godi C, Ambrosi A, Doyle F, Jacobs A, Kiprianos AP, et al. Novel Angiographic Scores for evaluation of Large Vessel Vasculitis. Sci Rep. 2018;8(1):15979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besutti G, Muratore F, Mancuso P, Ferrari M, Galli E, Spaggiari L, et al. Vessel inflammation and morphological changes in patients with large vessel vasculitis: a retrospective study. RMD Open. 2022;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77(5):636–43. [DOI] [PubMed] [Google Scholar]
- 10.Quinn KA, Ahlman MA, Malayeri AA, Marko J, Civelek AC, Rosenblum JS, et al. Comparison of magnetic resonance angiography and (18)F-fluorodeoxyglucose positron emission tomography in large-vessel vasculitis. Ann Rheum Dis. 2018;77(8):1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lariviere D, Benali K, Coustet B, Pasi N, Hyafil F, Klein I, et al. Positron emission tomography and computed tomography angiography for the diagnosis of giant cell arteritis: A real-life prospective study. Medicine (Baltimore). 2016;95(30):e4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grayson PC, Alehashemi S, Bagheri AA, Civelek AC, Cupps TR, Kaplan MJ, et al. (18) F-Fluorodeoxyglucose-Positron Emission Tomography As an Imaging Biomarker in a Prospective, Longitudinal Cohort of Patients With Large Vessel Vasculitis. Arthritis Rheumatol. 2018;70(3):439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Incerti E, Tombetti E, Fallanca F, Baldissera EM, Alongi P, Tombolini E, et al. (18)F-FDG PET reveals unique features of large vessel inflammation in patients with Takayasu’s arteritis. Eur J Nucl Med Mol Imaging. 2017;44(7):1109–18. [DOI] [PubMed] [Google Scholar]
- 14.Blockmans D, de Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L, Bobbaers H. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum. 2006;55(1):131–7. [DOI] [PubMed] [Google Scholar]
- 15.Arnaud L, Haroche J, Malek Z, Archambaud F, Gambotti L, Grimon G, et al. Is (18)F-fluorodeoxyglucose positron emission tomography scanning a reliable way to assess disease activity in Takayasu arteritis? Arthritis Rheum. 2009;60(4):1193–200. [DOI] [PubMed] [Google Scholar]
- 16.Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129–34. [DOI] [PubMed] [Google Scholar]
- 17.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33(8):1122–8. [DOI] [PubMed] [Google Scholar]
- 18.Langford CA, Cuthbertson D, Ytterberg SR, Khalidi N, Monach PA, Carette S, et al. A Randomized, Double-Blind Trial of Abatacept (CTLA-4Ig) for the Treatment of Takayasu Arteritis. Arthritis Rheumatol. 2017;69(4):846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn KA, Rosenblum JS, Rimland CA, Gribbons KB, Ahlman MA, Grayson PC. Imaging acquisition technique influences interpretation of positron emission tomography vascular activity in large-vessel vasculitis. Semin Arthritis Rheum. 2020;50(1):71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neogi T, Felson D, Niu J, Nevitt M, Lewis CE, Aliabadi P, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prieto-Gonzalez S, Garcia-Martinez A, Tavera-Bahillo I, Hernandez-Rodriguez J, Gutierrez-Chacoff J, Alba MA, et al. Effect of glucocorticoid treatment on computed tomography angiography detected large-vessel inflammation in giant-cell arteritis. A prospective, longitudinal study. Medicine (Baltimore). 2015;94(5):e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


