Abstract
Objective:
Our objectives were to: identify trajectories of palpitations over the menopause transition; characterize them; and examine associations with subclinical cardiovascular disease (CVD).
Methods:
We analyzed the following data from the multi-site, multi-ethnic Study of Women Across the Nation (SWAN): reported palpitations occurrence over time, baseline sociodemographic, reproductive, medication, and health-related factors, and follow-up visit subclinical CVD (carotid atherosclerosis, vascular stiffness). Trajectories of palpitations (n=3276), their characteristics, and their associations with subclinical CVD (n=1559) were identified using group-based trajectory modeling and linear and logistic regression models.
Results:
Three trajectories emerged: high probability of palpitations in perimenopause to early postmenopause diminishing in late postmenopause (15.9% of women), moderate probability of palpitations in perimenopause- to early postmenopause diminishing in late postmenopause (34.3%), and sustained low probability of palpitations (49.8%). In the fully adjusted multivariable model, the high probability group had a more adverse reproductive and health-related profile at baseline (higher gravidity, early perimenopause, vasomotor symptoms, poorer overall health, higher depressive symptoms, higher perceived stress, greater sleep problems, higher blood pressure). In fully adjusted multivariable models, palpitations trajectories were not related to atherosclerosis or arterial stiffness.
Conclusions:
Distinct patterns of palpitations emerged, with a substantial portion of women having palpitations during the perimenopause and early postmenopause. Palpitations were not associated with subclinical CVD. Findings can help identify women at risk for palpitations during the menopause transition who may need symptom relief.
Keywords: Palpitations, menopause, cardiovascular disease
INTRODUCTION
Palpitations (a feeling of rapid, irregular, or exaggerated heart beats)1, 2 are common in women during the menopause transition, yet have received relatively little research attention. A limited number of cross-sectional studies assessed palpitations over the past 2 weeks to year. In these studies, palpitations affected up to 42% of perimenopausal3 and 54% of postmenopausal women3 and were associated with worse sleep, depression, stress, and menopause quality of life (QOL).4
The trajectories of palpitations over the menopause transition have not been well-described or characterized. In a study of 200 Swedish women, changes in palpitations over five years from premenopause to postmenopause were only broadly described (e.g., decreasing [15%], no change [44%], increasing [41%]).5 In addition, the women most likely to experience palpitations over time have not been well-characterized as the only two longitudinal studies included only women with breast cancer. It is unknown whether sociodemographic, reproductive, and health-related factors shown to increase the likelihood of reporting palpitations in cross-sectional studies4, 6, 7 are also related to the trajectories of palpitations over time.
Palpitations may have implications for women’s cardiovascular health. Although relationships have been studied in cross-sectional studies, they have not been examined over time. Two cross-sectional studies reported no significant relationships between palpitations and atherosclerosis,8 arrhythmias,8 carotid intima-media thickness (CIMT), coronary artery calcium,9 cardio-ankle vascular index,8 ankle brachial pulse index,8 heart rate,8 or blood pressure.8 It is unkown whether trajectories of palpitations over time are related to a more adverse subclinical CVD profile (i.e., greater CIMT, carotid plaque, and arterial stiffness).
The Study of Women’s Health Across the Nation (SWAN) is one of the largest, most comprehensive, multi-ethnic, longitudinal study of the menopause transition. SWAN provides a unique opportunity to evaluate trajectories of palpitations during the menopause transition, characterize factors that may be associated with those distinct trajectories, and elucidate the relationship of those trajectories to subclinical CVD later in the transition. The current study aimed to: (1) describe trajectories of palpitations over the menopause transition; (2) characterize them; and (3) evaluate their relationship to subclinical CVD. Hypotheses included: (1) distinct trajectories of palpitations exist; (2) more symptomatic trajectories will be associated with a more adverse socioeconomic, reproductive, and health-related profile; and (3) more symptomatic trajectories will be associated with a more adverse subclinical CVD profile.10 Our goal was to generate foundational, descriptive information necessary for understanding palpitations during the menopause transition and their clinical importance.
METHODS
Design
SWAN is a multi-site, prospective, longitudinal, observational, cohort study of biological and psychosocial changes over the menopause transition in a racially and ethnically diverse, community-based population in the United States. Participants were initially recruited from 1995 to 1997 and have been followed for up to 17 clinical visits over 22 years.11 Clinical visits included physical measures, interviews and questionnaires, phlebotomy, and at select visits at select study sites, vascular ultrasound indices of subclinical CVD. The data used in the analyses were as follows. Trajectories of palpitations were mapped based on the final menstrual period (FMP). Observations ranged from 6 years before the FMP to 10 years after the FMP. Participant characteristics at baseline and subclinical measures of CVD assessed at visit 12 or 13 were then related to palpitations trajectories.
Setting and Sample
Recruitment at each clinical site included women of non-Hispanic white race/ethnicity and women of one other predetermined racel/ethnicity. The latter included Black women in Pittsburgh, Pennsylvania, Boston, Massachusetts, Chicago, Illinois, and Ypsilanti, Michigan; Japanese women in Los Angeles, California; Hispanic women in Newark, New Jersey; and Chinese women in Oakland, California. Protocols were approved by institutional review boards at each site. All participants provided signed, written informed consent.
Inclusion and exclusion criteria have been previously described.11 Briefly, women were aged 42-52 years old, had an intact uterus and at least one ovary, were not using hormone therapy or other medications known to affect ovarian function, were not pregnant or lactating, and had at least one menses in the 3 months prior to study enrollment. Thus, all women were premenopausal or early perimenopausal at baseline.
The sample used for this analysis included all participants who completed the palpitation symptom item at baseline and at least two other time points. We excluded women (1) with baseline arrhythmias or baseline anti-arrhythmic medications (n=1) or those (2) taking anticoagulants (n=25). For analyses linking palpitation trajectories to subclinical CVD measures, we also excluded women who did not have a carotid ultrasound or arterial stiffness measurement (n=1717). Six of the seven sites assessed subclinical CVD at visit 12 or 13. The smaller sample size for the CVD measures is due to the number of participating sites and attrition of study participants over time.
Main Outcomes and Measures
Palpitations
Palpitations were assessed at each study visit. Women self-reported the number of days in the past two weeks that they experienced “heart pounding/racing” (none, 1-5 days, 6–8 days, 9–13 days, every day). For analysis, women were categorized at each visit as having palpitations if they endorsed any number of days of “heart pounding/racing” and as not having palpitations if they endorsed “none”.
Baseline sociodemographic, reproductive, and health-related factors
Baseline sociodemographics included self-reported race, educational attainment, and financial strain (difficulty paying for basic household expenses; not very hard, somewhat hard, or very hard).
Reproductive factors included self-reported gravidity, parity, menopausal status, and vasomotor symptoms as well as laboratory values of estradiol (E2) and follicle-stimulating hormone (FSH). Menopausal status was defined using the Stages of Reproductive Aging Workshop +10 system.12 Premenopausal status was defined as menses in the 3 months prior to the baseline interview with no change in menstrual cycle variability over the prior year. Early perimenopause was defined as menses in the prior 3 months with increased menstrual cycle variability over the prior year. Late perimenopause was defined as the 1-3 years prior to the final menstrual period when intervals of amenorrhea are 60 days are longer.12 Early postmenopause was defined as the first 2-6 years of amenorrhea with late perimenopause defined as the remaining years of amenorrhea.12 Vasomotor symptoms were considered present if women endorsed any hot flashes or night sweats in the prior two weeks. Serum E2 assays were done in duplicate with an automated CIBA Corning Diagnostics ACS:180 analyzer (Bayer Diagnostics Corporation, Norwood, MA),13 with a lower limit of detection of 1 to 7 pg/mL. E2 inter-assay and intra-assay coefficients of variation were 10.6% and 6.4%, respectively. Serum FSH assays were done in singlicate with a two-site chemiluminometric immunoassay, with a lower limit of detection of 1.9 to 3.2 nM. FSH interassay and intra-assay coefficients of variation were 9.9% and 6.1%, respectively.
Medication use was assessed using self-reports and confirmed by study staff through visual inspection of pill bottles. Medications were then coded by therapeutic class and subclass using the Iowa Drug Information system.14 For this analysis, we considered any medications used for arrhythmias or affecting heart rate including: antiarrhythmics (mexiletine, dofetilide, sotalol, flecainide, propafenone), beta-blockers (metoprolol, propanolol, atenolol, carvedilol, bisopropolol, nadolol, acebutolol, timolol, labetalol, nebivolol), nondihydropyridine calcium channel blockers (verapamil, diltiazem), dihydropyridine calcium channel blockers (nifedipine, amlodipine), beta-agonists (albuterol, formoterol, terbutaline, metaproterenol, levalbuterol, pirbuterol), vagolytics (neostigmine, physostigmine), and ivrabadine, clonidine, amiodarone, and theophylline.15
Baseline health-related and CVD risk factors included self-rated general health, comorbidities (i.e., anemia, thyroid disease), depressive symptoms, perceived stress, sleep problems, smoking status, alcohol use, caffeine intake, physical activity, body mass index (BMI), blood pressure, and fasting levels of high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), total cholesterol, glucose, and homeostatic model assessment insulin resistance (HOMA-IR). General health was assessed as a single item with options of excellent, very good, good, fair, and poor. Depressive symptoms were assessed using total scores on the well-validated 20-item Centers for Epidemiological Studies Depression Scale.16 Perceived stress was assessed using total scores on the well-validated 10-item Perceived Stress Scale.17 Sleep problems over the past two weeks were assessed as trouble falling asleep, early morning awakenings, and sleep interruptions. We dichotomized each as present versus absent and summed categories to determine the number of sleep problems reported (0 to 3). Smoking status was based on American Thoracic Society questions18 and analyzed as current vs. past/never smoker. Alcohol consumption reported over the past year was categorized as any versus none. Daily caffeine intake (mg/day) was based on responses to a modified 1995 Block Food Frequency Questionnaire.19–21 Physical activity levels were a continuous score based on usual participation in activities using items from the Kaiser Physical Activity Survey, which is a modification of the Baecke questionnaire.22, 23 BMI (weight [kg]/height [m2]) was assessed using stadiometers and calibrated scales. Blood pressure was the average of two seated measurements.
Fasting levels of CVD risk biomarkers were assessed as follows. EDTA-treated plasma was separated, frozen at −20°C, and sent on dry ice to the Medical Research Laboratories (Highland Heights, KY). Twelve-hour fasting plasma lipid levels were analyzed by standard enzymatic methods on a Hitachi 747 analyzer (Boehringer Mannheim Diagnostics, Baden-Wurttemberg, Germany) and high-density lipoprotein cholesterol (HDL-c) was isolated using heparin-2M manganese chloride.24 HDL-c coefficient of variation was approximately 4%. Low-density lipoprotein cholesterol (LDL-c) was calculated using the Friedewald equation (total cholesterol - (triglycerides / 5) - HDL) in women with fasting triglyceride level below 400 mg/dl.25 Coefficients of variation for total cholesterol and triglycerides were 0.8% and 1.2%, respectively. Glucose was measured in serum by automated enzymatic assay on a Hitachi 747-200 chemistry analyzer using the hexokinase reaction and Roche Diagnostic reagents with coefficient of variation of 1.6%. HOMA-IR was calculated as: [glucose (mmol/ L) x insulin (mIU/mL)] / 22.5.26 Insulin inter-assay coefficients of variation were: 4.2% at 12 ng/mL, 3.5% at 78, and 4.7% at 167 mU/L. Insulin intra-assay coefficients of variation were: 2.1% at 12 ng/mL, 1.5% at 78 and 2.7% at 167 mU/L.
Subclinical CVD (subgroup)
Bilateral ultrasound carotid images were obtained using a Terason t3000 Ultrasound System (Teratech Corp, Burlington, MA) equipped with a variable frequency 5-12 Mhz linear array transducer and were read centrally at the SWAN Ultrasound Reading Center (University of Pittsburgh Ultrasound Research Lab [URL], Pittsburgh, PA). Two digitized images were obtained of the left and right distal common carotid artery. From each of the 4 images, using the Artery Measurement System (AMS) semi-automated edge detection software,27 intima-media thickness measures were obtained by electronically tracing and measuring the distance between the lumen-intima and the media-adventitia interfaces across a 1-cm segment proximal to the carotid bulb. One measurement was generated for each pixel over the area, for a total of approximately 140 measures for each segment. The mean of the average readings of all four images were used in analyses.
The presence and extent of plaque were evaluated in each of the following five segments of the left and right carotid artery: the proximal and distal common carotid, carotid bulb, and proximal internal and external carotid arterial segments. Plaque was defined as a distinct area protruding into the vessel lumen that was at least 50% thicker than the adjacent intimal media thickness (IMT) and summarized as the presence or absence of any plaque. Additionally, for each of the bilateral carotid segments, the degree of plaque was graded between 0 (no observable plaque) to 3 (plaque covering 50% or more of the vessel diameter). The grades from all segments of the combined left and right carotid artery were summed to create the plaque index (possible range: 0-30) and categorized as 0 or ≥ 1. The intra-class correlation was ≥ 0.77 for site sonographers; for readers at the URL, the reading center for SWAN, the intra-class correlation was > 0.90 for IMT intra- and inter-reads and 0.93 for plaque index assessment. These scanning and reading protocols have been used in prior studies.28–30
Mean brachial-ankle pulse wave velocity (baPWV) was measured using the VP2000 system (Omron Health Care Co., Kyoto, Japan), a non-invasive automated waveform analyzer. Cuffs placed around both upper arms and ankles were connected to a plethysmograph sensor that determines volume pulse waveforms. This device provides measures of baPWV, a mixed measure of central and peripheral arterial stiffness, on both right and left sides. Our analyses used the average of the two sides. The baPWV is the distance in centimeters between the brachial and ankle arterial recording sites divided by the time delay in seconds between the foot of the respective waveforms obtained at each recording site. The distance or path length for brachial/ankle arterial sites was calculated using a height-based algorithm.31 The reproducibility was excellent, with intra and inter-technician correlation coefficients of > 0.93 for all sites.
Data analysis
Group-based trajectory modeling was conducted using the SAS procedure Proc Traj,32 to identify homogeneous subgroups of distinct palpitations trajectories over time. Age at FMP was used to quantify time. For women without an observed FMP (n=1510), imputed FMP was used per processes previously described.33 Observations ranged from 6 years before the FMP to 10 years after the FMP (e.g., visits 1 to 17). Visits in which women reported hormone therapy use were dropped. Each trajectory was examined for linear, quadratic, or cubic patterns and the best function was determined via significance and fit statistics (Bayesian information Criterion). We estimated the probability of belonging to each palpitation trajectory group and the subgroup with the highest estimated probability was assigned to the woman.
We estimated unadjusted associations of baseline characteristics with the trajectory groups using chi-squared tests for categorical variables and analysis of variance for continuous variables. Multivariable associations were estimated with multinomial logistic regression. Baseline age and all covariates associated with the palpitation trajectories at p < 0.10 were included in the model: demographics (race/ethnicity, education, financial strain), reproductive factors (gravidity, menopause status, VMS), and health-related factors (overall health, depression, stress, sleep, smoking, BMI, and blood pressure). Due to multicollinearity and model fit, we did not include the following variables in models: anxiolytic medication, anemia, parity, thyroid medication, and blood pressure-lowering medication. We considered two-tailed p-values < 0.05 as statistically significant for individual predictors.
Next, the palpitation trajectories were related to measures of subclinical CVD in separate linear (carotid IMT, baPWV) and logistic (plaque) regression models adjusted for age, race/ethnicity, education, study site, and covariates associated with palpitation trajectories and subclinical CVD outcome measures at p < 0.05. We used covariates from the SWAN visit corresponding to the carotid ultrasound and arterial stiffness assessment (visit 12 or 13). We performed sensitivity analyses excluding women with an imputed FMP (n=1510). Residual analysis and diagnostic plots were used to verify model assumptions. Variables with a variance inflation factor ≥ 10 that were highly correlated (≥ 0.7) with other variables, were removed from models. We report the most parsimonious model with the highest Akaike Information Criterion. Analyses were performed with SAS v9.2 (SAS, Cary, NC).
RESULTS
Trajectories (n=3,276)
A total of 3,276 women were included in the trajectories analysis. At study baseline, participants were a mean age of 46.3 (SD=2.7) and premenopausal (54.2%) or perimenopausal (45.8%). Almost half of women (47%) were non-Hispanic white, 28% were Black, 7.7% Hispanic/Latino, 8.5% Chinese, and 8.6% Japanese.
As shown in Figure 1, three distinct trajectories were identified: high probability of palpitations in the perimenopause to early postmenopause diminishing in late postmenopause (15.9% of the sample), moderate probability of palpitations in the perimenopause to early postmenopause diminishing in late postmenopause (34.3%), and sustained low probability of palpitations across the menopause transition (49.8%). Trajectory grouping and probability did not change significantly (< 10%) when excluding women with imputed FMP.
Figure 1.
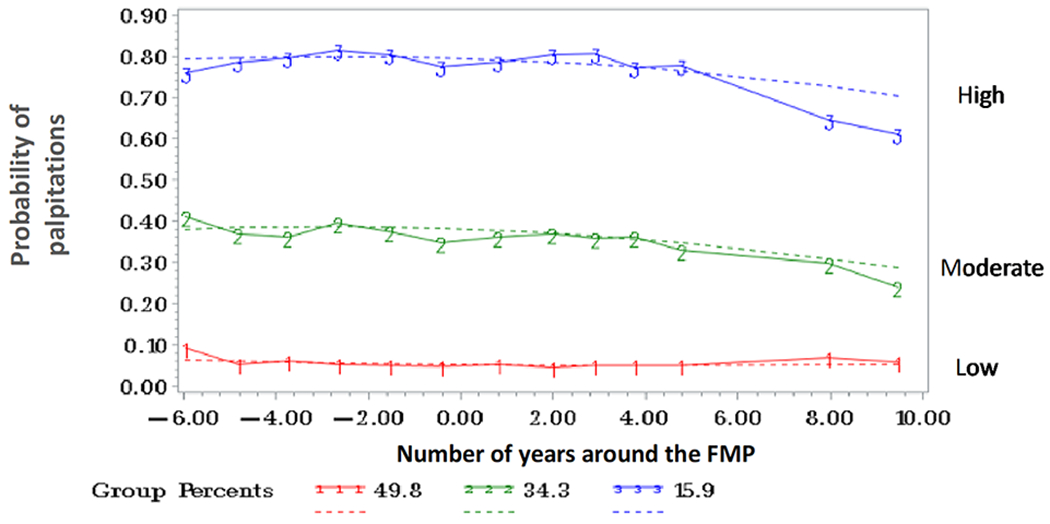
Trajectories of Palpitations/Heart Pounding
Factors Related to Trajectories (n=3,276)
In bivariate analyses (Table 1), trajectories varied by sociodemographics (race/ethnicity, educational level, financial strain), reproductive factors (gravidity, menopausal status, vasomotor symptoms), medication use (beta-blockers, calcium channel blockers, anxiolytics, and thyroid medications), and health-related and CVD risk factors (overall health ratings, anemia, depressive symptoms, perceived stress, sleep problems, being a current smoker, physical activity, BMI, and systolic and diastolic blood pressure). As hypothesized, bivariate analyses showed women in the high probability palpitations trajectory group were more likely to be Black, had higher gravidity, were earlier in the transition at baseline, and showed an overall more adverse socioeconomic, reproductive, and health-related profile (e.g., less educated, more financial strain, more VMS, using medications, reporting poorer overall health, reporting history of anemia, higher depressive symptoms, higher perceived stress, greater sleep problems, more often a smoker, lower physical activity, higher BMI, and higher blood pressure.
Table 1.
Baseline profile of palpitations trajectory subgroups (n=3,276)
| Low (n=1750) | Moderate (n=1062) | High (n=464) | P-value | |
|---|---|---|---|---|
|
| ||||
| Socio-demographic characteristics | ||||
|
| ||||
| Age, mean ± SD | 46.3 ±2.7 | 46.3 ±2.6 | 46.5 ±2.7 | 0.63 |
|
| ||||
| Race/Ethnicity, n(%) | <0.0001 | |||
| White | 866 (49.5) | 486 (45.7) | 189 (40.7) | |
| Black/African-American | 464 (26.5) | 288 (27.2) | 170 (36.6) | |
| Hispanic | 135 (7.7) | 78 (7.3) | 37 (8.0) | |
| Chinese | 162 (9.3) | 90 (8.5) | 27 (5.8) | |
| Japanese | 123 (7.0) | 120 (11.3) | 41 (8.8) | |
|
| ||||
| Education, n(%) | <0.0001 | |||
| ≤High school | 399 (22.8) | 291 (27.4) | 158 (34.1) | |
| >High school | 1351 (77.2) | 771 (72.6) | 306 (66.0) | |
|
| ||||
| Difficulty paying for basics | <0.0001 | |||
| Not hard at all | 1360 (77.8) | 717 (67.5) | 241 (52.0) | |
| Somewhat/very hard | 388 (22.2) | 345 (32.5) | 223 (48.0) | |
|
| ||||
| Reproductive factors | ||||
|
| ||||
| Gravidity, mean ± SD | 2.8 ±1.8 | 3.0 ±2.0 | 3.4 ±2.1 | <0.0001 |
|
| ||||
| Parity, mean ± SD | 2.3 ±1.3 | 2.3 ±1.3 | 2.3 ±1.7 | 0.46 |
|
| ||||
| E2 (log pg/ml), mean ± SD | 4.0 ±0.8 | 4.0 ±0.8 | 4.0 ±0.7 | 0.58 |
|
| ||||
| FSH (log pg/ml), mean ± SD | 2.9 ±0.8 | 2.9 ±0.7 | 2.9 ±0.7 | 0.99 |
|
| ||||
| Menopausal status | <0.0001 | |||
| Premenopause | 1018 (58.4) | 534 (50.4) | 256 (46.0) | |
| Early perimenopause | 721 (41.4) | 524 (49.4) | 218 (54.0) | |
| Unknown | 4 (0.2) | 2 (0.2) | 0 | |
|
| ||||
| Any vasomotor symptoms | 308 (17.6) | 350 (32.9) | 220 (46.5) | <0.0001 |
|
| ||||
| Medications | ||||
|
| ||||
| Blood pressure medication | 212 (12.1) | 142 (13.4) | 106 (22.8) | <0.0001 |
| Beta-blocker | 40 (2.3) | 33 (3.1) | 33 (7.1) | <0.0001 |
| Calcium channel blockera | 66 (3.8) | 40 (3.8) | 36 (7.8) | 0.0005 |
|
| ||||
| Steroid inhaler | 96 (5.5) | 59 (5.6) | 9 (4.9) | 0.73 |
|
| ||||
| Anxiolytic medication | 126 (7.2) | 121 (11.4) | 75 (16.2) | <0.0001 |
|
| ||||
| Thyroid medication | 114 (6.5) | 91 (8.6) | 44 (9.5) | 0.03 |
|
| ||||
| Health-related | ||||
|
| ||||
| Overall health (n=3273) | <0.0001 | |||
| Excellent/Very good | 362 (20.9) | 249 (23.6) | 86 (18.7) | |
| Good | 1188 (68.6) | 605 (57.4) | 246 (53.4) | |
| Fair/Poor | 183 (10.6) | 201 (19.1) | 129 (28.0) | |
|
| ||||
| Ever told you have anemia | 556 (32.6) | 381 (36.0) | 198 (43.0) | 0.0001 |
|
| ||||
| CES-D score, mean ± SD | 8.4 ±8.3 | 12.5 ±10.1 | 15.5 ±11.2 | <0.0001 |
|
| ||||
| Perceived stress scale | 8.0 ±2.8 | 9.0 ±2.9 | 9.7 ±3.0 | <0.0001 |
|
| ||||
| Number of sleep problems | <0.0001 | |||
| None | 921 (52.6) | 466 (43.8) | 142 (30.6) | |
| One | 402 (23.0) | 212 (19.9) | 106 (22.8) | |
| Two | 271 (15.5) | 206 (19.4) | 103 (22.2) | |
| Three | 156 (8.9) | 179 (16.8) | 113 (24.4) | |
|
| ||||
| Current smoker (ever) | 258 (14.8) | 207 (19.5) | 104 (22.1) | 0.0001 |
|
| ||||
| Alcohol use (any) | 1200 (68.6) | 699 (65.8) | 291 (62.9) | 0.33 |
|
| ||||
| Caffeine (mg/day), mean ± SD | 211.4 ± 179.7 | 213.5 ± 177.3 | 217.8 ± 179.9 | 0.79 |
|
| ||||
| Physical activity score | 7.8 ± 1.8 | 7.6 ± 1.8 | 7.4 ± 1.7 | <0.0001 |
|
| ||||
| Body mass index, mean ± SD | 27.8±7.0 | 28.2±7.0 | 29.8±8.1 | <0.0001 |
|
| ||||
| Systolic BP, mean ± SD | 116.5 ±16.7 | 118.9 ±16.8 | 120.3 ±18.0 | <0.0001 |
|
| ||||
| Diastolic BP, mean ± SD | 75.0 ±10.3 | 76.1 ±10.5 | 75.9 ±11.1 | 0.02 |
Includes nondihydropyridine (diltiazem and verapamil) and dihydropyridine (amlodipine, nifedipine) calcium channel blockers.
Sample is 3,276 unless otherwise indicated. All data shown as n (%) except where indicated. . BP, blood pressure. CESD, Center for Epidemiological Studies Depression scale. E2, estradiol. FSH, follicle stimulating hormone. SD, standard deviation.
As hypothesized, multivariable analysis (see Table 2) showed that (1) women in the moderate probability of reporting palpitations trajectory group were more likely to report higher gravidity, be earlier in the menopause transition at baseline, report more VMS, and report more depressive symptoms, stress, and sleep problems and (2) women in the high probability of reporting palpitations trajectory group were more likely to report higher gravidity, report more VMS, and report poorer overall health, more depressive symptoms, stress, and sleep problems.
Table 2.
Adjusted odds of belonging to moderate or high palpitation trajectory group relative to the low palpitation trajectory group (n=3,276)
| AOR (95% CI) | ||
|---|---|---|
|
| ||
| Moderate | High | |
|
| ||
| Socio-demographic characteristics | ||
|
| ||
| Age | 1.00 (0.96 to 1.03) | 1.03 (0.98 to 1.08) |
|
| ||
| Race/Ethnicity | ||
| White | Reference | Reference |
| Black/African-American | 0.91 (0.71 to 1.18) | 1.13 (0.81 to 1.57) |
| Hispanic | 0.58 (0.23 to 1.51) | 0.81 (0.20 to 3.30) |
| Chinese | 1.30 (0.81 to 2.09) | 1.48 (0.74 to 2.95) |
| Japanese | 0.91 (0.60 to 1.41) | 0.96 (0.46 to 1.99) |
|
| ||
| Education, n(%) | ||
| ≤High school | Reference | Reference |
| >High school | 0.94 (0.74 to 1.19) | 0.84 (0.61 to 1.14) |
|
| ||
| Financial strain | ||
| Not hard at all | Reference | Reference |
| Somewhat/very hard | 1.10 (0.89 to 1.35) | 1.09 (0.82 to 1.44) |
|
| ||
| Reproductive factors | ||
|
| ||
| Gravidity | 1.06 (1.00 to 1.11) | 1.09 (1.02 to 1.17) |
|
| ||
| Menopausal status | ||
| Premenopause | Reference | Reference |
| Early perimenopause | 1.22 (1.01 to 1.47) | 1.15 (0.88 to 1.50) |
|
| ||
| Vasomotor symptoms | 1.70 (1.36 to 2.13) | 2.66 (2.00 to 3.54) |
|
| ||
| Health-related and CVD risk | ||
|
| ||
| Overall health | ||
| Excellent/Very good | Reference | Reference |
| Good | 0.80 (0.64 to 1.00) | 1.08 (0.78 to 1.51) |
| Fair/Poor | 1.18 (0.86 to 1.63) | 2.14 (1.42 to 3.25) |
|
| ||
| CESD score (per 5-unit) | 1.18 (1.10 to 1.25) | 1.18 (1.09 to 1.28) |
|
| ||
| PSS score (per 5-unit) | 1.28 (1.06 to 1.54) | 1.76 (1.38 to 2.26) |
|
| ||
| Number of sleep problems | ||
| None | Reference | Reference |
| One | 0.93 (0.74 to 1.19) | 1.46 (1.05 to 2.04) |
| Two | 1.14 (0.88 to 1.47) | 1.50 (1.04 to 2.15) |
| Three | 1.41 (1.03 to 1.94) | 2.14 (1.43 to 3.19) |
|
| ||
| Current smoker | 1.13 (0.87 to 1.47) | 1.12 (0.80 to 1.57) |
|
| ||
| Body mass index | 0.99 (0.98 to 1.01) | 1.01 (0.99 to 1.03) |
|
| ||
| Systolic BP (per 10-unit) | 1.03 (0.95 to 1.12) | 1.04 (0.94 to 1.08) |
|
| ||
| Diastolic BP(per 5-unit) | 1.01 (0.94 to 1.08) | 0.98 (0.90 to 1.08) |
AOR, adjusted odds ratio. BP, blood pressure. CESD, Center for Epidemiological Studies Depression scale. CVD, cardiovascular disease. PSS, Perceived Stress Scale.
Model adjusted for the study site (not listed). Due to multicollinearity, the following variables were not included: physical activity, anxiolytic medication, anemia, parity, thyroid medication, blood pressure-lowering medication. No significant interactions with race/ethnicity.
Trajectories and Subclinical Cardiovascular Disease (n=1,559)
For the subclinical CVD analysis, 1559 of the 3,276 had a carotid ultrasound or arterial stiffness measurement. In this subpopulation, CVD risk factors varied by trajectory subgroups (Table 3). Although the multivariable age-adjusted model showed that women in the high palpitation trajectory group had a higher IMT and baPWV (Table 4), these associations did not hold in fully adjusted models.
Table 3.
Cardiovascular disease risk profile of palpitation trajectory subgroups at time of carotid assessmenta (n=1559)
| Low (n=828) |
Moderate (n=497) |
High (n=234) |
P-value | |
|---|---|---|---|---|
|
| ||||
| Age, mean ± SD | 61.9 ±2.7 | 61.7 ±2.6 | 62.2 ±2.7 | 0.04 |
|
| ||||
| Overall health (n=1481) | <0.0001 | |||
| Excellent/Very good | 460 (58.2) | 236 (50.0) | 60 (27.6) | |
| Good | 238 (30.1) | 186 (39.5) | 85 (38.9) | |
| Fair/Poor | 93 (11.8) | 50 (10.5) | 73 (33.5) | |
|
| ||||
| Current smoker | 51 (6.2) | 36 (7.2) | 32 (13.7) | 0.0001 |
|
| ||||
| Alcohol use | 530 (64.0) | 295 (59.4) | 133 (56.9) | 0.04 |
|
| ||||
| Physical activity | 7.6 ± 1.8 | 7.4 ± 1.8 | 7.5 ± 2.0 | 0.36 |
|
| ||||
| CES-D score, mean ± SD | 5.0 ±6.2 | 7.9 ±7.9 | 11.9 ±10.2 | <0.0001 |
|
| ||||
| Perceived stress scale | 6.2 ±2.5 | 7.1 ±3.0 | 8.2 ±3.0 | <0.0001 |
|
| ||||
| Number of sleeping complaints | <0.0001 | |||
| None | 338 (40.8) | 155 (31.2) | 54 (23.1) | |
| One | 230 (27.8) | 112 (22.5) | 52 (13.2) | |
| Two | 157 (19.0) | 125 (25.2) | 61 (17.8) | |
| Three | 103 (12.4) | 105 (21.1) | 67 (28.6) | |
|
| ||||
| Body mass index | 29.4±7.1 | 30.1±7.2 | 30.8±7.8 | 0.03 |
|
| ||||
| Systolic blood pressure | 122.4 ±16.6 | 124.2 ±16.8 | 126.4 ±16.2 | 0.006 |
|
| ||||
| Diastolic blood pressure | 74.1 ±9.6 | 74.5 ±9.3 | 74.4 ±9.6 | 0.75 |
|
| ||||
| Glucose (mg/dL) | 96.4 ±27.2 | 99.0 ±27.0 | 106.1 ±38.2 | 0.0001 |
|
| ||||
| LDL-c (mg/dL) | 110.3 ±30.2 | 107.0 ±29.8 | 108.2 ±32.0 | 0.21 |
|
| ||||
| HDL-c (mg/dL) | 66.5 ±16.5 | 65.8 ±16.8 | 64.4 ±16.2 | 0.27 |
|
| ||||
| Blood pressure medication | 295 (35.6) | 219 (44.0) | 123 (52.6) | <0.0001 |
|
| ||||
| Anxiolytic medication | 144 (18.2) | 126 (26.6) | 54 (24.1) | 0.001 |
|
| ||||
| Thyroid medication | 121 (15.3) | 71 (15.0) | 43 (19.2) | 0.31 |
|
| ||||
| Common carotid IMT (mm) | 0.79 ± 0.11 | 0.80 ± 0.12 | 0.82 ± 0.13 | 0.002 |
|
| ||||
| baPWV (cm/s) (n=1341) | 1218.7 ± 214.1 | 1240.8 ± 219.3 | 1267.1 ± 227.2 | 0.16 |
|
| ||||
| Carotid plaque | 0.24 | |||
| 0 | 480 (58.0) | 270 (54.4) | 125 (53.6) | |
| ≥ 1 | 348 (42.0) | 227 (45.6) | 109 (46.4 | |
All covariates included are from the time of carotid assessment (SWAN Visit 12 and 13). SD, standard deviation.
baPWV, brachial-a pulse wave velocity. CES-D, Center for Epidemiological Studies Depression. cm/sec, centimeters per second. scaleHDL-c, high density lipoprotein cholesterol. IMT, intima media thickness… LDL-c, low density lipoprotein cholesterol. mm, millimeters. SD, standard deviation.
Table 4.
Associations between palpitation trajectory group and measures of subclinical cardiovascular disease (n=1,559)
| CCA IMT (mm) | baPWV (m/s) | Plaque Index | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Age-adjusted ß (SE) |
Fully-adjusted ß (SE) |
Age-adjusted ß (SE) |
Fully-adjusted ß (SE) |
Age-adjusted OR (95% CI) |
Fully-adjusted OR (95% CI) |
|
|
| ||||||
| Total Sample (n=1559) | ||||||
|
| ||||||
| Low | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate | 0.012 (0.007) | 0.001 (0.008) | 23.7 (13.2) | 18.1 (13.4) | 0.87 (0.70 - 1.08) | 0.84 (0.63 - 1.12) |
| High | 0.029 (0.009)** | 0.012 (0.01) | 48.6 (17.5)** | −5.1 (17.3) | 0.90 (0.68 - 1.19) | 1.11 (0.77 - 1.60) |
|
| ||||||
| Non-Hispanic White (n=787) | ||||||
|
| ||||||
| Low | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate | 0.009 (0.009) | 0.009 (0.01) | 7.6 (16.9) | 15.4 (17.7) | 0.81 (0.60 - 1.11) | 0.87 (0.58 - 1.32) |
| High | 0.026 (0.012)* | 0.019 (0.015) | 21.1 (24.8) | −5.6 (25.1) | 0.81 (0.53 - 1.25) | 1.14 (0.63 - 2.06) |
|
| ||||||
| Non-Hispanic Black (n=482) | ||||||
|
| ||||||
| Low | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate | 0.017 (0.014) | 0.007 (0.017) | 19.8 (23.9) | 19.9 (25.1) | 0.81 (0.54 - 1.21) | 0.67 (0.41 - 1.12) |
| High | 0.030 (0.016) | 0.017 (0.02) | 44.6 (28.3)* | 11.0 (30.0) | 0.84 (0.53 - 1.35) | 1.15 (0.61 - 2.19) |
|
| ||||||
| Hispanic/Latina (n=95) | ||||||
|
| ||||||
| Low | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate | −0.010 (0.026) | −0.045 (0.03) | 17.0 (35.2) | 22.4 (60.3) | 0.94 (0.34 - 2.58) | 1.00 (0.21 - 4.91) |
| High | −0.001 (0.035) | 0.002 (0.041) | 0.5 (49.1) | −35.6 (81.7) | 1.29 (0.32 - 5.13) | 1.57 (0.21 - 11.94) |
|
| ||||||
| Chinese (n=195) | ||||||
|
| ||||||
| Low | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate | 0.009 (0.019) | −0.029 (0.025) | −0.9 (15.1) | −12.4 (42.9) | 0.90 (0.49 - 1.67) | 1.91 (0.72 - 5.04) |
| High | −0.009 (0.023) | −0.031 (0.025) | −3.7 (22.6) | −47.8 (51.6) | 1.00 (0.47 - 2.13) | 1.21 (0.49 - 3.00) |
Sample is 1,559 unless otherwise indicated. Fully-adjusted = age, site, race/ethnicity (in the total sample), education, financial strain, smoking status, alcohol use, sleep, BMI, systolic blood pressure, and glucose at Visit 12/13.
p<0.05,
p<0.01.
ß, beta. baPWV, brachial-a pulse wave velocity. CCA IMT, common carotid intima media thickness. CI, confidence interval. SE, standard error.
DISCUSSION
This analysis of data from the large, multi-site, multi-ethnic SWAN study aimed to describe trajectories of palpitations over the menopause transition, characterize them, and examine their association with subclinical CVD. Consistent with the first hypothesis three distinct trajectories emerged: (1) high probability of reporting palpitations occurrence during perimenopause to early postmenopause and diminishing in late postmenopause, (2) moderate probability of reporting palpitations during perimenopause to early postmenopause and diminishing in late postmenopause, and (3) sustained low probability of reporting palpitations over the menopause transition. Half the sample was in the sustained low probability group, suggesting that about half of women are unlikely to report palpitations during perimenopause through late postmenopause. The other half were in the high to moderate probability groups, suggesting that about half of women are moderately to highly likely to report palpitations during perimenopause through early postmenopause. These findings suggesting palpitations are common during the menopause transition are consistent with a prior review showing that up to 42% of perimenopausal and up to 54% of postmenopausal women endorsed having had palpitations over the prior 2 weeks, month, or year.3 However, the findings are only partially consistent with a longitudinal study of 200 Norwegian women who provided data annually over 5 years from premenopause to postmenopause showing palpitations (measured as frequency) decreased in 15% of women, increased in 41%, and did not change in 44%.5 In our high and moderate probability trajectories, we did not see decreasing palpitations until late postmenopause and it was only in the low probability trajectory that we saw no change over time. We did not see increasing palpitations, possibly due to our focus on symptom occurrence rather than other symptom dimensions. Relying on a measure of palpitations occurrence (e.g., yes vs. no) in this analysis may have masked changes in frequency, severity, or other symptom characteristics (e.g., yes but more frequent or more severe). Additional analyses are needed to understand how other palpitations symptom dimensions may change over time.
Our second hypothesis that trajectories would be associated with baseline sociodemographics, reproductive factors, medications known to affect heart rhythm or rate, and/or health-related factors was also supported. The sustained high probability trajectory group had an overall pattern of greater reproductive and health-related stressors at baseline (e.g., higher gravidity, early perimenopause status, having vasomotor symptoms, poorer overall health, higher depressive symptoms, higher perceived stress, and greater sleep problems). Importantly, our longitudinal SWAN findings differ from a SWAN cross-sectional analysis of screening data (n=16,065) showing increased odds of reporting baseline palpitations occurrence with Hispanic race/ethnicity, lower education, greater financial strain, greater parity, menopausal status (perimenopausal or postmenopausal), past smoking, and lower physical activity.6 Our null findings are consistent with a limited number of cross-sectional studies in perimenopausal and/or postmenopausal women showing no relationship between palpitations and parity,34 estradiol levels,9 alcohol use,4, 8 and caffeine intake.8 The present study is strengthened by its longitudinal design that leveraged over 16 years of follow-up data.
Due to the limited scope of prior research, our findings can only be compared to the limited number of cross-sectional studies characterizing women reporting palpitations. Findings from these studies are mixed. For gravidity, prior research is limited. Our findings differ from the single available study showing Jordanian women reporting 1-2 pregnancies were more likely to report palpitations than women with zero or ≥ 3 pregnancies.35 For menopause status, prior research is equivocal. Two studies showed palpitations were more common in perimenopausal versus postmenopausal women36, 37 and two other studies showed the opposite.38, 39 Our findings suggest palpitations are evidenced in perimenopause and continue unchanged into early postmenopause. For vasomotor symptoms, prior research is also equivocal. Our findings are similar to two studies showing vasomotor symptoms were associated with higher prevalence and severity of palpitations40, 41 but differ from another study showing vasomotor symptom frequency, severity, and bother were not related to palpitations distress.4 For overall health, our findings can only be compared to studies on QOL. Palpitations were associated with poorer physical and general QOL in some articles 42–44 but not to mental42 or general45 QOL in other articles. For depression, prior research is equivocal with our findings matching one study46 but not another.47 For stress, our findings are similar to two studies showing higher stress was related to greater palpitations occurrence48 and distress.4 For sleep, our findings are similar to three studies showing poor sleep being related to higher palpitations occurrence,43, 49 severity,50 and distress.4
Our third hypothesis that more symptomatic trajectories would be associated with a more adverse subclinical CVD profile was not supported in age-adjusted models. We identified only one study evaluating palpitations in menopausal women in relation to subclinical CVD. An analysis of screening data from 868 predominantly White women enrolled in the Kronos early estrogen prevention study (KEEPS) found no significant associations between palpitations (measured as severity) and carotid intima-media thickness (p=.817) or coronary artery calcium scores (p=.091).9 In the general population, palpitations are the second leading reason for cardiologist visits,51 account for 16% of primary care visits,51 and are more likely to be reported by women than men during outpatient or emergency department visits52 and during an acute cardiac event.53, 54
Limitations
Findings should be interpreted in consideration of some limitations. The measure of palpitations was limited in breadth and recall period and under-reporting of symptoms may have occurred. Women were asked about “heart pounding/racing” in the past two weeks and not about other similar sensations over a longer period of time. In a review of how palpitations have been measured during the menopause transition, Sheng et al. described finding a great deal of variability in what sensation, over what time period, and what dimension (e.g., frequency, severity, bother, etc.) women were asked to report.55 The item used in SWAN may have missed women with palpitations who may have felt sensations other than “heart pounding/racing” and may have endorsed items such as “heart discomfort”, “heart skipping”, “tightness in chest”, or “butterflies”.55 Similarly, the item used in SWAN focused on frequency in the past two weeks and may have missed women having palpitations in the past month or more, but not in the past two weeks.55 The exclusion criterion of arrythmias was ascertained by self-report. We did not report anxiety in the models because of high multicollinearity with depression, sleep, and overall health (p ≥0.70) and better goodness of fit with the variables we reported. Historically, palpitations have been mostly attributed to psychosomatic (e.g., anxiety, stress) rather than cardiac causes.56 In perimenopausal and postmenopausal women, there are two available studies with limited anxiety assessments, with both showing greater anxiety was associated with palpitations.8, 57 More detailed study of the relationship between palpitations and anxiety is needed. Sleep problems and not sleep deprivation was measured. In addition, because palpitations were assessed via a self-reported measure, we do not know if the palpitations women reported corresponded to any electrocardiographic abnormalities. Because palpitations can be associated with serious, life-threatening arrhythmias,58, 59 it will be important for future research to determine whether and what arrhythmias are associated with the palpitations that women report across the menopause transition.
CONCLUSIONS
Findings show distinct temporal patterns in the way women endorse having had palpitations over the past two weeks over the menopause transition. The trajectories we identified suggest palpitations are common from early perimenopause through early postmenopause and for some women diminish but remain common into the late postmenopause. Characterizing the trajectories suggests there are reproductive and health-related factors that can be used to help identify women with a moderate and high probability of reporting palpitations across the menopause transition. The clinical significance of these palpitations in relation to subclinical CVD was not demonstrated here but additional research over a longer period of time may be needed.
Acknowledgment:
Clinical Centers: University of Michigan, Ann Arbor - Carrie Karvonen-Gutierrez, PI 2021- present, Siobán Harlow, PI 2011-2021, Mary Fran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA - Sherri-Ann Burnett-Bowie, PI 2020 – present, Joel Finkelstein, PI 1999-present, Robert Neer, PI 1994-1999; Rush University, Rush University Medical Center, Chicago, IL - Imke Janssen, PI 2020 – present, Howard Kravitz, PI 2009-2020, Lynda Powell, PI 1994-2009; University of California, Davis/Kaiser - Elaine Waetjen and Monique Hedderson, PIs 2020 – Present; Ellen Gold, PI 1994 - 2020; University of California, Los Angeles - Arun Karlamangla, PI 2020 - present; Gail Greendale, PI 1994 - 2020; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 - present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry, New Jersey Medical School, Newark - Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Rebecca Thurston, PI 2020 – present; Karen Matthews, PI 1994 - 2020. NIH Program Office: National Institute on Aging, Bethesda, MD - Rosaly Correa-de-Araujo 2020 - present; Chhanda Dutta 2016- present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers. Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services). SWAN Repository: University of Michigan, Ann Arbor – Siobán Harlow 2013 - 2018; Dan McConnell 2011 - 2013; MaryFran Sowers 2000 – 2011. Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001. Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair. The authors thank the study teams at each site and the women who participated in SWAN.
Sources of funding:
The Study of Women Across the Nation (SWAN) is funded through the National Institutes of Health (NIH); DHHS, through the National Institute on Aging (NIA); the National Institute of Nursing Research (NINR); and the NIH Office of Research on Women’s Health (ORWH) (grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). Other support from the Indiana University Ethel Clarke Fellowship and a Collaboration in Translational Research Pilot Grant (Carpenter/Tisdale MPI) from the Indiana Clinical and Translational Sciences Institute funded, in part by Grant Number UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical, and Translational Sciences Award. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NIA, NINR, or ORWH.
Financial Disclosures/Conflicts of Interest:
Dr. Carpenter reports personal fees from Mapi/ICON and the University of Wisconsin Milwaukee. Dr. Tisdale reports institutional fees from the National Institute of Health, the American Heart Association, and Agency for Healthcare Quality and research; funding from Pharmacotherapy Publications Inc; an annual stipend for serving as Scientific Editor Nova Southeastern University; honorarium for giving a presentation at a symposium American Society of Health; book royalties from Systems Pharmacists. Dr. Jackson has research support from NIH and Amgen, is a consultant for UpToDate and McKesson, sreves on the Editorial Board for AHA, is a consultant to ACC, and is an expert witness for DeBlase Brown Everly LLP. Dr. Thurston is a consultant/advisory board member for Astellas, Bayer, Vira Health, and Happify Health, and was a past consultant for Pfizer and Virtue Health. The other authors have nothing to disclose.
REFERENCES
- 1.Cho L Ever since I started menopause, my heart flutters from time to time. It usually lasts several seconds. Is this normal? Heart Advis. 2006;9(10):8. [PubMed] [Google Scholar]
- 2.Sievert LL, Obermeyer CM. Symptom clusters at midlife: a four-country comparison of checklist and qualitative responses. Menopause. 2012;19(2):133–44. doi: 10.1097/gme.0b013e3182292af3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpenter JS, Sheng Y, Elomba C, et al. A systematic review of palpitations prevalence by menopausal status. Curr Obstet Gynecol Rep. 2021;10:7–13. doi: 10.1007/s13669-020-00302-z [DOI] [Google Scholar]
- 4.Carpenter JS, Tisdale JE, Chen CX, et al. A Menopause Strategies-Finding Lasting Answers for Symptoms and Health (MSFLASH) investigation of self-reported menopausal palpitation distress. J Womens Health (Larchmt). 2021;30(4):533–8. doi: 10.1089/jwh.2020.8586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holte A Influences of natural menopause on health complaints: a prospective study of healthy Norwegian women. Maturitas. 1992;14(2):127–41. doi: 10.1016/0378-5122(92)90005-o [DOI] [PubMed] [Google Scholar]
- 6.Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol. 2000;152(5):463–73. doi: 10.1093/aje/152.5.463 [DOI] [PubMed] [Google Scholar]
- 7.Carpenter JS, Sheng Y, Pike C, et al. Correlates of palpitations during menopause: A scoping review. Womens Health. 2022;18:1–14. doi: 10.1177/17455057221112267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enomoto H, Terauchi M, Odai T, et al. Independent association of palpitation with vasomotor symptoms and anxiety in middle-aged women. Menopause. 2021;28(7):741–7. doi: 10.1097/gme.0000000000001776 [DOI] [PubMed] [Google Scholar]
- 9.Wolff EF, He Y, Black DM, et al. Self-reported menopausal symptoms, coronary artery calcification, and carotid intima-media thickness in recently menopausal women screened for the Kronos early estrogen prevention study (KEEPS). Fertil Steril. 2013;99(5):1385–91. doi: 10.1016/j.fertnstert.2012.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tepper PG, Brooks MM, Randolph JF Jr., et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause. 2016;23(10):1067–74. doi: 10.1097/GME.0000000000000676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Khoudary SR, Greendale G, Crawford SL, et al. The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health Across the Nation (SWAN). Menopause. 2019;26(10):1213–27. doi: 10.1097/GME.0000000000001424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Fertil Steril. 2012;97(4):843–51. doi: 10.1016/j.fertnstert.2012.01.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.England BG, Parsons GH, Possley RM, McConnell DS, Midgley AR. Ultrasensitive semiautomated chemiluminescent immunoassay for estradiol. Clin Chem. 2002;48(9):1584–6. doi: [PubMed] [Google Scholar]
- 14.Iowa Drug Information Service (IDIS). IDIS Drug Vocabulary and Thesaurus Description. Coralville, IA: University of Iowa College of Pharmacy Division of Drug Information Service; 2012. doi:10.1.1.674.2511 [Google Scholar]
- 15.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138(13):e272–e391. doi: 10.1161/CIR.0000000000000549 [DOI] [PubMed] [Google Scholar]
- 16.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1(3):385–401. doi: [Google Scholar]
- 17.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. doi: [PubMed] [Google Scholar]
- 18.Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis. 1978;118(6 Pt 2):1–120 [PubMed] [Google Scholar]
- 19.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453–69. doi: 10.1093/oxfordjournals.aje.a114416 [DOI] [PubMed] [Google Scholar]
- 20.Gold EB, Bair Y, Block G, et al. Diet and lifestyle factors associated with premenstrual symptoms in a racially diverse community sample: Study of Women’s Health Across the Nation (SWAN). J Womens Health (Larchmt). 2007;16(5):641–56. doi: 10.1089/jwh.2006.0202 [DOI] [PubMed] [Google Scholar]
- 21.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–99. doi: 10.1093/aje/154.12.1089 [DOI] [PubMed] [Google Scholar]
- 22.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28(3):313–23. doi: 10.1006/pmed.1998.0470 [DOI] [PubMed] [Google Scholar]
- 23.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–42. doi: 10.1093/ajcn/36.5.936 [DOI] [PubMed] [Google Scholar]
- 24.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19(1):65–76. doi: [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. doi: [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 27.Wendelhag I, Gustavsson T, Suurkula M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles and description of a computerized analysing system. Clin Physiol. 1991;11(6):565–77. doi: 10.1111/j.1475-097x.1991.tb00676.x [DOI] [PubMed] [Google Scholar]
- 28.Njoroge JN, El Khoudary SR, Fried LF, Barinas-Mitchell E, Sutton-Tyrrell K. High urinary sodium is associated with increased carotid intima-media thickness in normotensive overweight and obese adults. Am J Hypertens 2011;24(1):70–6. doi: 10.1038/ajh.2010.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutton-Tyrrell K, Kuller LH, Matthews KA, et al. Subclinical atherosclerosis in multiple vascular beds: an index of atherosclerotic burden evaluated in postmenopausal women. Atherosclerosis. 2002;160(2):407–16. doi: 10.1016/s0021-9150(01)00591-3 [DOI] [PubMed] [Google Scholar]
- 30.Sutton-Tyrrell K, Wolfson SK Jr., Thompson T, Kelsey SF. Measurement variability in duplex scan assessment of carotid atherosclerosis. Stroke. 1992;23(2):215–20. doi: 10.1161/01.str.23.2.215 [DOI] [PubMed] [Google Scholar]
- 31.Sugawara J, Hayashi K, Tanaka H. Arterial path length estimation on brachial-ankle pulse wave velocity: validity of height-based formulas. J Hypertens. 2014;32(4):881–9. doi: 10.1097/HJH.0000000000000114 [DOI] [PubMed] [Google Scholar]
- 32.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29(3):374–93. doi: 10.1177/0049124101029003005 [DOI] [Google Scholar]
- 33.Samargandy S, Matthews KA, Brooks MM, et al. Arterial stiffness accelerates within 1 Year of the final menstrual period: the SWAN heart study. Arterioscler Thromb Vasc Biol. 2020;40(4):1001–8. doi: 10.1161/ATVBAHA.119.313622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdullah B, Moize B, Ismail BA, Zamri M, Mohd Nasir NF. Prevalence of menopausal symptoms, its effect to quality of life among Malaysian women and their treatment seeking behaviour. Med J Malays. 2017;72(2):94–9 [PubMed] [Google Scholar]
- 35.Jaber RM, Khalifeh SF, Bunni F, Diriye MA. Patterns and severity of menopausal symptoms among Jordanian women. J Women Aging. 2017;29(5):428–36. doi: 10.1080/08952841.2016.1213110 [DOI] [PubMed] [Google Scholar]
- 36.Rahman SA, Zainudin SR, Mun VL. Assessment of menopausal symptoms using modified Menopause Rating Scale (MRS) among middle age women in Kuching, Sarawak, Malaysia. Asia Pac Fam Med. 2010;9(1):5. doi: 10.1186/1447-056x-9-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman S, Salehin F, Iqbal A. Menopausal symptoms assessment among middle age women in Kushtia, Bangladesh. BMC Res Notes. 2011;4:188. doi: 10.1186/1756-0500-4-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruan X, Cui Y, Du J, Jin F, Mueck AO. Prevalence of climacteric symptoms comparing perimenopausal and postmenopausal Chinese women. J Psychosom Obstet Gynaecol. 2017;38(3):161–9. doi: 10.1080/0167482X.2016.1244181 [DOI] [PubMed] [Google Scholar]
- 39.Blümel JE, Chedraui P, Baron G, et al. Menopausal symptoms appear before the menopause and persist 5 years beyond: a detailed analysis of a multinational study. Climacteric. 2012;15(6):542–51. doi: 10.3109/13697137.2012.658462 [DOI] [PubMed] [Google Scholar]
- 40.Blümel JE, Chedraui P, Baron G, et al. A large multinational study of vasomotor symptom prevalence, duration, and impact on quality of life in middle-aged women. Menopause 2011;18(7):778–85. doi: 10.1097/gme.0b013e318207851d [DOI] [PubMed] [Google Scholar]
- 41.Chen R, Chang T, Chow S. Perceptions of and attitudes toward estrogen therapy among surgically menopausal women in Taiwan. Menopause. 2008;15(3):517–23. doi:doi: 10.1097/gme.0b013e3181591dc9. [DOI] [PubMed] [Google Scholar]
- 42.Conde DM, Pinto-Neto AM, Santos-Sa D, Costa-Paiva L, Martinez EZ. Factors associated with quality of life in a cohort of postmenopausal women. Gynecol Endocrinol. 2006;22(8):441–6. doi: 10.1080/09513590600890306 [DOI] [PubMed] [Google Scholar]
- 43.Dotlic J, Kurtagic I, Nurkovic S, et al. Factors associated with general and health-related quality of life in menopausal transition among women from Serbia. Women Health. 2018;58(3):278–96. doi: 10.1080/03630242.2017.1306604 [DOI] [PubMed] [Google Scholar]
- 44.Gazibara T, Nurkovic S, Kovacevic N, et al. Factors associated with sexual quality of life among midlife women in Serbia. Qual Life Res. 2017;26(10):2793–804. doi: 10.1007/s11136-017-1608-3 [DOI] [PubMed] [Google Scholar]
- 45.Chou MF, Wun YT, Pang SM. Menopausal symptoms and the menopausal rating scale among midlife chinese women in Macau, China. Women Health. 2014;54(2):115–26. doi: 10.1080/03630242.2013.871767 [DOI] [PubMed] [Google Scholar]
- 46.Fu JX, Luo Y, Chen MZ, et al. Associations among menopausal status, menopausal symptoms, and depressive symptoms in midlife women in Hunan Province, China. Climacteric. 2020;23(3):259–66. doi: 10.1080/13697137.2019.1703936 [DOI] [PubMed] [Google Scholar]
- 47.Bashar M, Ahmed K, Uddin MS, Ahmed F, Emran AA, Chakraborty A. Depression and quality of life among postmenopausal women in Bangladesh: a cross-sectional study. J Menopausal Med. 2017;23(3):172–81. doi: 10.6118/jmm.2017.23.3.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu P, Yuan Y, Liu M, et al. Factors associated with menopausal symptoms among middle-aged registered nurses in Beijing. Gynecol Endocrinol. 2015;31(2):119–24. doi: 10.3109/09513590.2014.971237 [DOI] [PubMed] [Google Scholar]
- 49.Asplund R, Aberg HE. Nightmares, cardiac symptoms and the menopause. Climacteric. 2003;6(4):314–20. doi: 10.1080/713605432 [DOI] [PubMed] [Google Scholar]
- 50.Tao MF, Sun DM, Shao HF, Li CB, Teng YC. Poor sleep in middle-aged women is not associated with menopause per se. Braz J Med Biol Res. 2016;49(1):e4718. doi: 10.1590/1414-431x20154718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raviele A, Giada F, Bergfeldt L, et al. Management of patients with palpitations: A position paper from the European Heart Rhythm Association. Europace. 2011;13(7):920–34. doi: 10.1093/europace/eur130 [DOI] [PubMed] [Google Scholar]
- 52.Misiri J, Candler S, Kusumoto FM. Evaluation of syncope and palpitations in women. J Womens Health (Larchmnt). 2011;20(10):1505–15. doi: 10.1089/jwh.2010.2636 [DOI] [PubMed] [Google Scholar]
- 53.Mujtaba SF, Rizvi SN, Talpur A, Younis F, Minhas K, Farooqui Z. Gender based differences in symptoms of acute coronary syndrome. Journal of the College of Physicians and Surgeons--Pakistan : JCPSP. 2012;22(5):285–8. doi:05.2012/JCPSP.285288 [PubMed] [Google Scholar]
- 54.O’Donnell S, McKee G, O’Brien F, Mooney M, Moser DK. Gendered symptom presentation in acute coronary syndrome: a cross sectional analysis. Int J Nurs Stud. 2012;49(11):1325–32. doi: 10.1016/j.ijnurstu.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 55.Sheng Y, Carpenter JS, Elomba CD, et al. Review of menopausal palpitations measures. Womens Midlife Health. 2021;7(1):5. doi: 10.1186/s40695-021-00063-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muskin PR. Panics, prolapse, and PVCs. Gen Hosp Psychiatry. 1985;7(3):219–23. doi: 10.1016/0163-8343(85)90072-6 [DOI] [PubMed] [Google Scholar]
- 57.Ishizuka B, Kudo Y, Tango T. Cross-sectional community survey of menopause symptoms among Japanese women. Maturitas. 2008;61(3):260–7. doi: 10.1016/j.maturitas.2008.07.006 [DOI] [PubMed] [Google Scholar]
- 58.Thavendiranathan P, Bagai A, Khoo C, Dorian P, Choudhry NK. Does this patient with palpitations have a cardiac arrhythmia? JAMA. 2009;302(19):2135–43. doi: 10.1001/jama.2009.1673 [DOI] [PubMed] [Google Scholar]
- 59.Clementy N, Fourquet A, Andre C, et al. Benefits of an early management of palpitations. Medicine. 2018;97(28):e11466. doi: 10.1097/MD.0000000000011466 [DOI] [PMC free article] [PubMed] [Google Scholar]


