Abstract
Objective:
Medication nonadherence is common among patients with systemic lupus erythematosus (SLE), and adherence often fluctuates with time. Underrepresented racial minorities have disproportionately lower rates of medication adherence and more severe SLE manifestations. We aimed to identify modifiable factors associated with persistent medication nonadherence.
Methods:
Patients taking ≥1 SLE medication were enrolled. Adherence data was obtained at baseline and at follow-up roughly one year later using both self-reported adherence and pharmacy refill data. Covariates included patient-provider interaction, patient self-efficacy, and clinical factors. We compared characteristics of patients in three groups using the Kruskal-Wallis H test: Persistent Nonadherence (low adherence by self-report and refill rates at both time points), Persistent Adherence (high adherence by self-report and refill rates at both time points), and Inconsistent Adherence (the remainder).
Results:
Among 77 patients (median age 44, 53% Black, 96% female), 48% had Persistent Nonadherence. Compared with other adherence groups, patients with Persistent Nonadherence were younger and more likely to be Black, have lower income, take ≥2 SLE medications, have higher SLE-related damage at baseline, and have higher physician global assessment at follow-up. Persistently non-adherent patients also rated more hurried communication with providers – particularly fast speech and difficult word choice – and had lower self-efficacy in managing medications.
Conclusions:
Potential avenues to improve medication adherence include optimizing patient-provider communication, specifically avoiding difficult vocabulary and fast speech, and enhancing patient self-efficacy, particularly among younger Black patients with lower income who are at higher risk for nonadherence.
Medication nonadherence is common among patients with systemic lupus erythematosus (SLE), with reported rates between 43% and 75%1. Reasons for medication nonadherence are complex and can be explained by the interaction of factors from four domains: the illness severity, perceived susceptibility to the effects of that illness, perceived benefits of taking a medication, and barriers to taking that medication2. Poor adherence to medications is a major cause of morbidity and mortality in lupus and is associated with worse disease activity, higher frequency of end-stage renal disease, and greater utilization of the Emergency Department3–6. Patients who are underrepresented racial minorities have disproportionately higher rates of medication nonadherence compared to non-Hispanic White patients, and this has been hypothesized to partially account for known racial outcome disparities in SLE7.
As medication nonadherence is ubiquitous in SLE and clearly associated with worse clinical outcomes, modifiable factors that contribute to low adherence are important to ascertain. One sub-focus of adherence research examines the physician-patient relationship and quality of physician-patient communication. Prior studies have shown that effective communication contributes to improved patient understanding of illness and of the risks and benefits associated with treatment; such communication is strongly correlated with adherence8,9. More patient-centered communication is also associated with better patient self-efficacy, and this association is strongest for patients with high chronic disease burden and concomitant mental illness, two features frequently noted in patients with SLE10. Results from our previous cross-sectional study indicated differences in patient-provider communication and patient self-efficacy between adherent and nonadherent patients, suggesting that these may be modifiable factors in a subset of nonadherent patients11. We aimed to explore this relationship further in this longitudinal analysis.
Methods:
Study setting and population:
Patients included in this analysis were recruited from the Duke Lupus Registry (DLR), a prospective cohort comprised of patients with SLE followed at the Duke Lupus Clinic. The Duke Lupus Clinic is staffed by six attending rheumatologists (four Caucasian, two Asian) who share the clinical care for all patients. Inclusion criteria for the DLR are age ≥18 years, fluency in English, having no cognitive or other physical barriers to provide informed consent, and meeting American College of Rheumatology 1997 or SLE International Collaborating Clinics 2012 SLE classification criteria12,13. All enrolled subjects in the DLR provided signed informed consent to participate in research and are followed regularly as clinically indicated.
To be included in the current analysis, subjects must be prescribed one or more SLE medication and have completed assessments as described below. Adherence was assessed at two time points (baseline and follow-up, both face-to-face office visits) roughly a year apart. Patients were excluded if they were a new patient to the clinic, allowing us to focus on patients who have an ongoing relationship with their providers. Exclusion criteria also included being pregnant or nursing, which may temporarily alter medication-taking behavior, and having significant cognitive or language barriers that prevented questionnaire completion. Both the DLR and the current study were approved by the institutional review board at Duke University (IRB study #Pro00008875 and #Pro00100861, respectively).
Data collection:
Data were obtained through electronic medical record review and questionnaires at baseline (7/2018–1/2019) and follow-up (9/2019–1/2020). At baseline, the following were collected from medical record review: age, gender, insurance status, disease duration, hospitalizations/emergency room visits in the past 12 months, and number of SLE medications. Patient-reported race, marital status, annual household income, education level, and disability status were also assessed at baseline. In addition, the following measures were obtained during the study.
Adherence measures (baseline and follow-up):
For the purpose of adherence research, we used both self-reported adherence and pharmacy refill data to measure adherence to SLE medications. Self-reported adherence to SLE medications was assessed using the Medication Adherence Self-Report Inventory (MASRI), a questionnaire validated in SLE. The MASRI asks patients to provide a numerical estimate of their adherence from 0%−100% over the preceding month. In accordance with published cutoffs14, we defined High Self-reported Adherence as MASRI ≥ 90%. Additionally, we obtained pharmacy refill information for all SLE medications prescribed in the prior 3 months through chart review and/or phone calls to each patient’s pharmacy. High Refill was defined as having a medication possession ratio (MPR) of ≥80%. High Composite Adherence was defined as having both High Self-reported Adherence and High Refills7. Those with High Composite Adherence at both baseline and follow-up were considered to have Persistent Adherence, those with Low Composite Adherence at both time points were considered to have Persistent Nonadherence, and those with Low Composite Adherence at either but not both time points were considered to have Inconsistent Adherence.
Patient-provider interaction (baseline)
The Interpersonal Processes of Care Survey (IPC-29) was used to assess seven domains of patient-provider interaction via a total of 29 items each on a five-point Likert scale. The seven domains included were: “Hurried communication,” “Elicit concerns, responded,” “Explained results, medications,” “Patient-centered decision making,” “Compassionate, respectful,” “Discrimination,” and “Disrespectful office staff.” Scores for each domain ranged from 1–5, with higher scores indicating greater perception of that domain. Thus, a score of 1 is optimal for “Hurried communication,” “Discrimination,” and “Disrespectful office staff,” while a score of 5 is optimal for the other domains.
Patient self-efficacy (baseline)
We used the Patient Reported Outcomes Measurement Information System (PROMIS) short forms to measure both general self-efficacy as well as self-efficacy in managing medications and treatments. Raw scores were obtained and uploaded to the scoring service, where T-scores were obtained15. A T-score of 50 corresponds to the reference population mean, with a five-point difference (one-half standard deviation) considered a clinically significant difference16,17.
SLE-related damage (baseline) and disease activity (follow-up)
The Systemic Lupus International Collaborating Clinics (SLICC) Damage Index18 at time of baseline visit was obtained via chart review by an attending rheumatologist. The SLE Disease Activity Index (SLEDAI) assessment19 and Physician Global Assessment (PGA) were completed at the follow-up visit by the attending rheumatologist.
Statistics:
We described categorical variables using percentages and continuous variables using medians due to skewed data. Characteristics of adherence groups were compared using the Kruskal-Wallis H test. We used the Chi squared or the Fisher’s exact test to compare the proportion of patients reporting less than optimal scores on IPC-29 domains among adherence groups. All statistical analysis was performed using STATA (version 14.2 College Station, TX).
Results:
Study Population:
Among the 77 patients included in this analysis, median age was 44 (IQR 34–51), 96% were female, 53% were Black, 36% were on disability, and 51% had private insurance (Table 1). On average, patients had been diagnosed with SLE for 15 years and were prescribed two SLE medications, with 88% prescribed Hydroxychloroquine and 65% a disease modifying anti-rheumatic drug (DMARD) (Table 1).
Table 1.
Study population characteristics at baseline.
| Patient Characteristics | |
|---|---|
| Age, years, median [IQR] | 44 [34–51] |
| Race, n (%) | |
| Caucasian | 33 (43%) |
| African American | 41 (53%) |
| Other | 3 (4%) |
| Female gender, n (%) | 74 (96%) |
| ≥ College education, n (%) | 46 (60%) |
| Married or cohabiting, n (%) | 34 (45%) |
| Annual Income >$100,000, n (%) | 12 (16%) |
| Disability, n (%) | 28 (36%) |
| Insurance, n (%) | |
| Medicaid | 12 (16%) |
| Medicare | 23 (30%) |
| Private | 39 (51%) |
| Other | 3 (4%) |
| SLE duration, years, median [IQR] | 15 [8–22] |
| No. prescribed SLE medications, median [IQR] | 2 [1–3] |
| SLE Medications, n (%) | |
| Hydroxychloroquine | 68 (88%) |
| DMARDs | 50 (65%) |
| Mycophenolate mofetil | 28 (36%) |
| Azathioprine | 11 (14%) |
| Methotrexate | 12 (16%) |
| Leflunomide | 3 (4%) |
| Belimumab | 4 (5%) |
IQR: Interquartile range; SLE: Systemic Lupus Erythematosus; DMARD: Disease Modifying Anti-Rheumatic Drug
Medication Adherence:
At baseline, 70% had High Self-Reported Adherence (MASRI ≥90%), 49% High Refills (MPR ≥80%), and 40% High Composite Adherence (MASRI ≥90% and MPR ≥80%) for all SLE medications prescribed. Longitudinally, 30% had Persistent Adherence (High Composite Adherence at both baseline and follow-up), while 48% had Persistent Nonadherence (Low Composite Adherence at both baseline and follow-up), and 22% had Inconsistent Adherence (Low Composite Adherence at either baseline or follow-up). Persistent Adherence was 49% for Hydroxychloroquine and 36% for DMARDs.
Comparing across adherence groups, those with Persistent Nonadherence, and to a lesser degree those with Inconsistent Adherence, were more likely to be younger, Black, and have an annual household income of ≤$100,000. There were no statistically significant differences between adherence groups in terms of having a college education, being on disability, or insurance type (Table 2).
Table 2.
Patient characteristics among adherence groups.
| Patient characteristics | Persistent Nonadherence (n=37) | Inconsistent Adherence (n=17) | Persistent Adherence (n=23) | P-value |
|---|---|---|---|---|
| Age, years, median [IQR] | 37[32–47] | 38[31–50] | 49[43–57] | 0.007 |
| Race, n (%) | ||||
| White | 10 (27%) | 8 (47%) | 15 (65%) | 0.01 |
| Black | 26 (70%) | 7 (41%) | 8 (35%) | |
| Other | 1 (3%) | 2 (12%) | 0 (0%) | |
| Female gender, n (%) | 37 (100%) | 16 (94%) | 21 (91%) | 0.1 |
| ≥ College education, n (%) | 20 (54%) | 12 (71%) | 14 (61%) | 0.6 |
| Annual Income ≤$100,000, n (%) | 35 (94%) | 15 (88%) | 15 (60%) | 0.008 |
| Married or cohabiting, n (%) | 13 (36%) | 8 (47%) | 13 (57%) | 0.3 |
| Disability, n (%) | 17 (46%) | 6 (35%) | 5 (22%) | 0.2 |
| Insurance, n (%) | 0.3 | |||
| Medicaid | 9 (24%) | 2 (12%) | 1 (4%) | |
| Medicare | 12 (32%) | 4 (24%) | 7 (30%) | |
| Private | 15 (41%) | 10 (59%) | 14 (61%) | |
| Other | 1 (3%) | 1 (6%) | 1 (4%) | |
| ≥2 SLE medications, n (%) | 34 (92%) | 13 (76%) | 10 (43%) | <0.001 |
| SLICC damage score, median [IQR] | 3 [1–4] | 1 [0–2] | 1 [0–2] | 0.0004 |
| SLEDAI, median [IQR] | 2 [0–5] | 2 [0–3] | 0 [0–4] | 0.2 |
| PGA, median [IQR] | 0.5 [0.1–0.8] | 0 [0–0.5] | 0 [0–0.5] | 0.03 |
| 1+ ER visits or hospitalizations since prior visit, n (%) | 3 (12.5%) | 1 (8.3%) | 6 (29%) | 0.3 |
IQR: Interquartile range; SLE: Systemic Lupus Erythematosus; SLICC: Systemic Lupus International Collaborating Clinics; SLEDAI: Systemic Lupus Erythematosus Disease Activity Index; PGA: Physician Global Assessment
Clinically, disease duration was not significantly different by adherence group. Patients with Persistent Nonadherence were more likely to be prescribed two or more SLE-specific medications and had higher SLICC damage scores at baseline. At follow-up, patients with Persistent Nonadherence had higher PGA scores but similar SLEDAI scores compared to other adherence groups (Table 2).
Patient-Provider Communication and Self-Efficacy:
Those with Persistent Nonadherence rated overall more “Hurried communication” with their providers (median 1.4) compared to patients in the other adherence groups (median 1.0, p=0.01) (Table 3). When examining the proportion of patients reporting less than optimal scores on the IPC-29 domains, 70% of patients with Persistent Nonadherence report “Hurried communication” compared to 35% and 48% of those with Inconsistent Adherence and Persistent Adherence respectively (p=0.04) (Figure 1). In particular, patients with Persistent Nonadherence noted that their providers spoke more quickly (“How often did doctors speak too fast?”) and used more difficult vocabulary (“How often did doctors use words that were hard to understand?”). There was no significant difference among the adherence groups in the rest of the IPC-29 domains (Table 3).
Table 3.
Patient-provider communication and self-efficacy among adherence groups.
| Patient Reported Outcome | Persistent Nonadherence (n=37) | Inconsistent Adherence (n=17) | Persistent Adherence (n=23) | P-value |
|---|---|---|---|---|
| Hurried communication*, median [IQR] | 1.4[1.0–2.2] | 1.0[1.0–1.2] | 1.0[1.0–1.6] | 0.01 |
| Speak fast§, median [IQR] | 2.0[1.0–3.0] | 1.0[1.0–1.5] | 1.0[1.0–2.0] | 0.01 |
| Difficult words§, median [IQR] | 2.0[1.0–3.0] | 1.0[1.0–2.0] | 1.0[1.0–2.0] | 0.01 |
| Discrimination, median [IQR] | 1.0[1.0–1.0] | 1.0[1.0–1.0] | 1.0[1.0–1.0] | 0.85 |
| Disrespectful office staff, median [IQR] | 1.0[1.0–1.0] | 1.0[1.0–1.0] | 1.0[1.0–1.0] | 0.67 |
| Elicit concerns, median [IQR] | 4.7[4.0–5.0] | 5.0[4.7–5.0] | 4.7[3.7–5.0] | 0.46 |
| Explain results, medications, median [IQR] | 4.5[3.5–5.0] | 5.0[4.3–5.0] | 4.5[3.5–5.0] | 0.40 |
| Patient-centered decision making, median [IQR] | 4.0[3.8–5.0] | 4.8[4.5–5.0] | 4.3[3.0–5.0] | 0.17 |
| Compassionate respectful, median [IQR] | 4.8[4.0–5.0] | 5.0[5.0–5.0] | 5.0[3.6–5.0] | 0.12 |
| Self-efficacy^, general, median [IQR] | 46.9[41.8–55.3] | 52.1[49.4–64.7] | 52.3[44.3–64.7] | 0.18 |
| Self-efficacy^, managing treatments & medications, median [IQR] | 42.9[40.4–52.8] | 52.2[46.9–60.6] | 54.3[47.7–60.6] | 0.04 |
Interpersonal Processes of Care survey, score ranges 1–5, with higher score indicating more of the domains. 1 is best score for Hurried communication and its sub-domains. Scores of other domains of this survey (Elicited concerns, Explained results, Patient-centered decision making, Discrimination, and Disrespectful office staff) were not significantly different between adherence groups.
Subdomains of Hurried Communication, as reflected by answers to the questions “how often did doctors speak too fast?” and “how often did doctors use words that were hard to understand?”
Patient Reported Outcomes Measurement Information System (PROMIS®) measures, general population mean score is 50, clinically significant difference is ≥ 5. IQR: Interquartile range.
Figure 1.
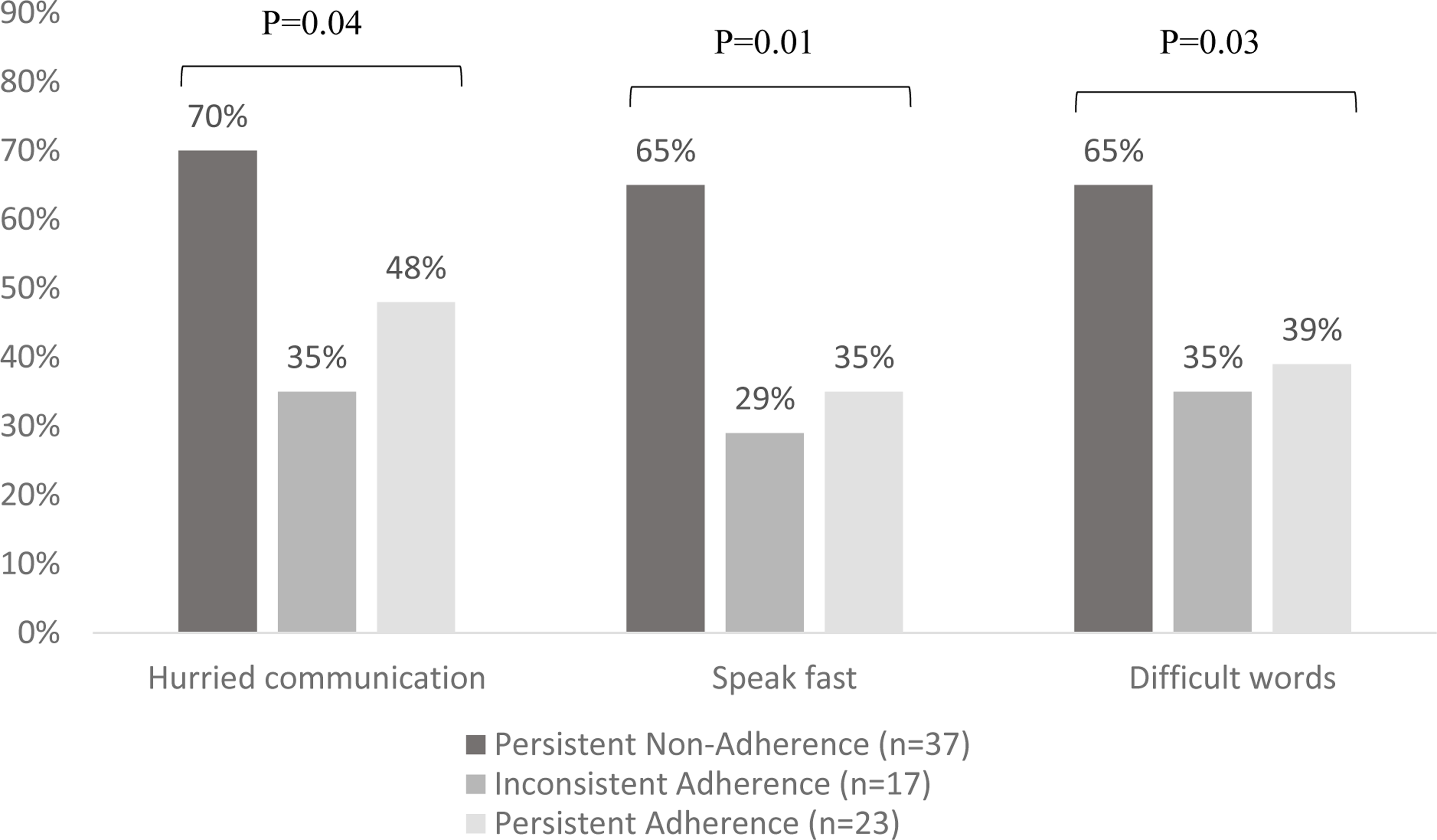
Comparing the proportion of patients reporting “Hurried communication,” specifically doctors speaking fast and using difficult words, among adherence groups.
Patients with Persistent Nonadherence also rated themselves as having lower self-efficacy in terms of managing medications and treatments (median 42.9) compared to those with Inconsistent Adherence (median 52.2) and Persistent Adherence (median 54.3, p=0.04). There was no statistically significant difference between adherence groups in terms of general self-efficacy (Table 3).
Discussion:
We identified that persistent medication nonadherence was associated with two modifiable factors for potential intervention: patient perceived hurried communication with their providers, particularly with respect to fast speech and difficult word choice, and lower patient self-efficacy in treatment management. This is significant because of the prevalence of nonadherence: we found less than one-third of patients both refilled and reported taking their lupus medications consistently across a one-year period, and nearly half of patients were persistently nonadherent. Further, Persistent Nonadherence was associated with poorer outcomes, both higher lupus-related damage at baseline and higher physician-rated disease activity at follow-up, underscoring the clinical impact of medication nonadherence.
The relationship between patient-provider communication, patient self-efficacy, and medication adherence has not been extensively studied among patients with SLE. We previously found that worse communication and lower patient self-efficacy were associated with having more SLE-related damage among Black patients11. Drenkard, et al. found a similar association between worse communication and higher SLE disease activity in Black patients20. Interpreted through the lens of the current analysis, we hypothesize that persistent nonadherence is a mediator for damage and current lupus disease activity, and this relationship should be investigated further in a larger longitudinal study.
Findings from our current study add to evidence that exists in other chronic diseases, where more collaborative patient-provider communication has been linked to improved medication adherence21–23. It has been described that Black patients are more likely than their White counterparts to rate their doctor visits as being shorter, less participatory, and less satisfactory, especially when they are in race-discordant relationships with their providers24,25. This may help explain our finding that Persistent Nonadherence was more common among Black patients seen in the Duke Lupus Clinic, where six attending rheumatologists (four Caucasian, two Asian) share the clinical care for all lupus patients, over half of whom are Black. This fact, combined with the extremely high rate of patient-provider racial discordance in the care of patients with SLE in the United States26 and racial disparities in SLE outcomes27,28, further underscores the significance of our results.
Additionally, our study highlights the importance of patient self-efficacy in maintaining medication adherence. Efforts are made to develop programs that enhance self-efficacy of patients with SLE, such as patient education, patient navigators, and peer mentoring programs31,32,33,34. Beyond these efforts, further provider training in communication may be similarly important to optimizing care for patients with SLE, particularly those from underrepresented racial and ethnic backgrounds, because patient-provider communication can also impact a patient’s health-related self-efficacy10,29–31. Turning our attention to the patient encounter and critically examining how care can be delivered in ways that promote good communication practices and foster patient self-efficacy could help mitigate racial disparities in SLE medication adherence and outcomes. In particular, speaking more slowly and avoiding words that may be difficult to understand, such as medical jargon, are potential avenues for improving patient perceived communication with their providers.
We also found that patients who were persistently nonadherent were more likely to be prescribed a larger number of lupus medications. Polypharmacy has been reported to be associated with nonadherence35, and our previous qualitative work also point to pill burden as a barrier to adherence36. While it may not always be possible to reduce the number of medications a patient is prescribed due to disease activity and comorbidities, it is worthwhile considering the relative importance of each specific medication for patients who are receiving polypharmacy.
Major strengths of our study include use of both subjective and objective measures of adherence, limiting social desirability biases from using solely self-reported adherence measures. We also assessed adherence at two separate time points over the course of about a year. This allowed us to identify patients with Persistent Nonadherence over time, a group at particularly high-risk for poor outcomes. Lastly, many of the instruments used (e.g., MASRI, SLEDAI, PGA) have been validated in SLE populations.
There are also several limitations to this study. First, our small sample size limited statistical power to control for potential confounders or stratify our analysis by race or medication type. The limited sample size could explain the lack of association between adherence groups with other factors historically correlated with adherence, such as education and marital status1,7. Future studies should include a larger sample to allow for multivariable analysis. Second, patients were recruited from one tertiary care lupus clinic in the Southeastern United States. Therefore, our sample may not be representative of patients with SLE from other clinical settings. For example, our clinic has very few patients of Hispanic ethnicity, a population who may experience more communication challenges due to cultural and language barriers. Third, the median duration of SLE disease in our sample was 15 years, so findings may not extrapolate to patients who are earlier in their disease course. Fourth, we only collected data on SLE-specific medications while most patients with SLE also take other medications, and the impact of polypharmacy accounting for all prescribed medications warrants further study. Lastly, we classified patients based on adherence assessment at two time points roughly one year apart. As adherence can be dynamic, the two time points may not be representative of the period in between. Therefore, some patients classified to have Persistent Adherence and Persistent Nonadherence may actually have Inconsistent Adherence, making the groups more similar to each other. The fact that we identified significant differences among the three groups despite this limitation further underscores the potential implications of our results.
In conclusion, by assessing medication adherence longitudinally, we identified a group of patients with potential modifiable risk factors for Persistent Nonadherence. Future studies should explore ways to optimize patient-provider communication, specifically avoiding difficult vocabulary and fast speech, and enhance patient self-efficacy as potential avenues for improving adherence, particularly for young Black patients with SLE who are at highest risk of nonadherence and poor outcomes.
Significance and Innovations:
We assessed medication adherence longitudinally and identified that nearly half of patients were persistently non-adherent over a one-year period.
Persistent Nonadherence was more common among patients who are younger, Black, and have a lower annual household income.
Persistent Nonadherence was associated with patient perception of hurried communication with providers and lower patient self-efficacy in managing medications, two modifiable factors that may help improve adherence and mitigate racial disparities in SLE outcomes.
Grant support:
Research reported in this publication was supported by the Duke Center for REsearch to AdvanCe Healthcare Equity (REACH Equity) Career Development Award (NIH 5U54MD012530-02) and National Center for Advancing Translational Sciences of the National Institutes of Health (NIH 1KL2TR002554). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr Eudy receives research funding from NIH NCATS Award Number 1KL2TR002554, Pfizer, and Exagen, and consults for Amgen. Dr. Rogers receives research funding from GSK, Pfizer, and Exagen, and consults for Eli Lilly, Immunovant, Exagen, Janssen, and Northwestern. Dr. Clowse receives research funding from Exagen, and Pfizer, and consults for GSK and UCB. Dr. Sadun receives research funding from Rheumatology Research Foundation.
References:
- 1.Mehat P, Atiquzzaman M, Esdaile JM, AviÑa-Zubieta A, De Vera MA. Medication Nonadherence in Systemic Lupus Erythematosus: A Systematic Review. Arthritis Care Res (Hoboken). 2017;69(11):1706–1713. doi: 10.1002/ACR.23191 [DOI] [PubMed] [Google Scholar]
- 2.Johnson MJ. The Medication Adherence Model: a guide for assessing medication taking. Res Theory Nurs Pract. 2002;16(3):179–192. doi: 10.1891/RTNP.16.3.179.53008 [DOI] [PubMed] [Google Scholar]
- 3.Feldman CH, Yazdany J, Guan H, Solomon DH, Costenbader KH. Medication Nonadherence Is Associated with Increased Subsequent Acute Care Utilization among Medicaid Beneficiaries with Systemic Lupus Erythematosus. Arthritis Care Res. 2015;67(12):1712–1721. doi: 10.1002/acr.22636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petri M, Perez-Gutthann S, Longenecker JC, Hochberg M. Morbidity of systemic lupus erythematosus: role of race and socioeconomic status. Am J Med. 1991;91(4):345–353. doi: 10.1016/0002-9343(91)90151-M [DOI] [PubMed] [Google Scholar]
- 5.Adler M, Chambers S, Edwards C, Neild G, Isenberg D. An assessment of renal failure in an SLE cohort with special reference to ethnicity, over a 25-year period. Rheumatology (Oxford). 2006;45(9):1144–1147. doi: 10.1093/RHEUMATOLOGY/KEL039 [DOI] [PubMed] [Google Scholar]
- 6.A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med. 1991;324(3):150–154. doi: 10.1056/NEJM199101173240303 [DOI] [PubMed] [Google Scholar]
- 7.Sun K, Eudy AM, Criscione‐Schreiber LG, et al. Racial Disparities in Medication Adherence between African American and Caucasian Patients With Systemic Lupus Erythematosus and Their Associated Factors. ACR open Rheumatol. 2020;2(7):430–437. doi: 10.1002/ACR2.11160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haskard Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47(8):826–834. doi: 10.1097/MLR.0B013E31819A5ACC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koneru S, Kocharla L, Higgins GC, et al. Adherence to medications in systemic lupus erythematosus. J Clin Rheumatol. 2008;14(4):195–201. doi: 10.1097/RHU.0b013e31817a242a [DOI] [PubMed] [Google Scholar]
- 10.Finney Rutten LJ, Hesse BW, St. Sauver JL, et al. Health Self-Efficacy Among Populations with Multiple Chronic Conditions: the Value of Patient-Centered Communication. Adv Ther. 2016;33(8):1440–1451. doi: 10.1007/S12325-016-0369-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun K, Eudy AM, Criscione-Schreiber LG, et al. Racial Differences in Patient-provider Communication, Patient Self-efficacy, and Their Associations With Systemic Lupus Erythematosus-related Damage: A Cross-sectional Survey. J Rheumatol. 2021;48(7):1022–1028. doi: 10.3899/JRHEUM.200682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petri M, Orbai AM, Alarcõn GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi: 10.1002/ART.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/ART.1780400928 [DOI] [PubMed] [Google Scholar]
- 14.Koneru S, Shishov M, Ware A, et al. Effectively measuring adherence to medications for systemic lupus erythematosus in a clinical setting. Arthritis Rheum. 2007;57(6):1000–1006. doi: 10.1002/ART.22898 [DOI] [PubMed] [Google Scholar]
- 15.HealthMeasures Scoring Service. Accessed March 27, 2022. https://www.healthmeasures.net//score-and-interpret/interpret-scores/promis
- 16.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C [DOI] [PubMed] [Google Scholar]
- 17.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64(5):507–516. doi: 10.1016/J.JCLINEPI.2010.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–369. doi: 10.1002/ART.1780390303 [DOI] [PubMed] [Google Scholar]
- 19.Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–640. doi: 10.1002/ART.1780350606 [DOI] [PubMed] [Google Scholar]
- 20.Drenkard C, Bao G, Lewis TT, Pobiner B, Priest J, Lim SS. Physician-patient interactions in African American patients with systemic lupus erythematosus: Demographic characteristics and relationship with disease activity and depression. Semin Arthritis Rheum. 2019;48(4):669–677. doi: 10.1016/j.semarthrit.2018.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoenthaler A, Chaplin WF, Allegrante JP, et al. Provider communication effects medication adherence in hypertensive African Americans. Patient Educ Couns. 2009;75(2):185–191. doi: 10.1016/J.PEC.2008.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenthaler A, Allegrante JP, Chaplin W, Ogedegbe G. The effect of patient-provider communication on medication adherence in hypertensive black patients: does race concordance matter? Ann Behav Med. 2012;43(3):372–382. doi: 10.1007/S12160-011-9342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Náfrádi L, Nakamoto K, Schulz PJ. Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PLoS One. 2017;12(10). doi: 10.1371/JOURNAL.PONE.0186458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA. 1999;282(6):583–589. doi: 10.1001/JAMA.282.6.583 [DOI] [PubMed] [Google Scholar]
- 25.Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139(11). doi: 10.7326/0003-4819-139-11-200312020-00009 [DOI] [PubMed] [Google Scholar]
- 26.American College of Rheumatology Workforce Study Taskforce. The 2015 Workforce Study of Rheumatology Specialists in the United States: Survey Results.; 2016. https://www.rheumatology.org/portals/0/files/ACR-Workforce-Study-2015.pdf
- 27.Costenbader KH, Desai A, Alarcón GS, et al. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum. 2011;63(6):1681–1688. doi: 10.1002/ART.30293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim SS, Helmick CG, Bao G, et al. Racial Disparities in Mortality Associated with Systemic Lupus Erythematosus - Fulton and DeKalb Counties, Georgia, 2002–2016. MMWR Morb Mortal Wkly Rep. 2019;68(18):419–422. doi: 10.15585/MMWR.MM6818A4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marks R, Allegrante JP, Lorig K. A review and synthesis of research evidence for self-efficacy-enhancing interventions for reducing chronic disability: implications for health education practice (part II). Health Promot Pract. 2005;6(2):148–156. doi: 10.1177/1524839904266792 [DOI] [PubMed] [Google Scholar]
- 30.Marks R, Allegrante JP, Lorig K. A review and synthesis of research evidence for self-efficacy-enhancing interventions for reducing chronic disability: implications for health education practice (part I). Health Promot Pract. 2005;6(1):37–43. doi: 10.1177/1524839904266790 [DOI] [PubMed] [Google Scholar]
- 31.Karlson EW, Liang MH, Eaton H, et al. A randomized clinical trial of a psychoeducational intervention to improve outcomes in systemic lupus erythematosus. Arthritis Rheum. 2004;50(6):1832–1841. doi: 10.1002/ART.20279 [DOI] [PubMed] [Google Scholar]
- 32.Falasinnu T, Bao G, Brady TJ, Lim SS, Drenkard C. Factors Associated with the Initiation and Retention of Patients with Lupus in the Chronic Disease Self-Management Program. Arthritis Care Res (Hoboken). Published online November 4, 2021. doi: 10.1002/ACR.24811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White AA, Ba A, Faith TD, et al. The Care-coordination Approach to Learning Lupus Self-Management: a patient navigator intervention for systemic lupus inpatients. Lupus Sci Med. 2021;8(1). doi: 10.1136/lupus-2021-000482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams EM, Dismuke CL, Faith TD, et al. Cost-effectiveness of a peer mentoring intervention to improve disease self-management practices and self-efficacy among African American women with systemic lupus erythematosus: analysis of the Peer Approaches to Lupus Self-management (PALS) pilot study. Lupus. 2019;28(8):937–944. doi: 10.1177/0961203319851559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marengo MF, Waimann CA, de Achaval S, et al. Measuring therapeutic adherence in systemic lupus erythematosus with electronic monitoring. Lupus. 2012;21(11):1158–1165. doi: 10.1177/0961203312447868 [DOI] [PubMed] [Google Scholar]
- 36.Sun K, Corneli AL, Dombeck C, et al. Barriers to taking medications for systemic lupus erythematosus: a qualitative study of racial minority patients, lupus providers, and clinic staff. Arthritis Care Res (Hoboken). Published online March 4, 2021. doi: 10.1002/ACR.24591 [DOI] [PMC free article] [PubMed] [Google Scholar]


