Abstract
Objective:
Selinexor is a first-in-class, oral selective inhibitor of nuclear export (SINE) compound which blocks Exportin-1 (XPO1). Our objective was to determine maximum tolerated dose (MTD) and recommended phase II dose (RP2D) of selinexor and weekly paclitaxel.
Methods:
This was an open label, single-center, multi-arm phase 1b study utilizing a “3 + 3” design and a “basket-type” expansion in recurrent solid tumors. Selinexor (60mg or 80 mg twice weekly orally) and weekly paclitaxel (80mg IV 2 week on, 1 week off) were one of 13 parallel arms. Efficacy was evaluated using RECIST version 1.1.
Results:
All 35 patients treated were evaluable for toxicity and 31 (88%) were evaluable for response. Patient diagnoses included platinum-resistant/refractory ovarian (n=28), breast (n=4), prostate (n=2), and cervical (n=1) cancer. Patients had a median of four prior therapies (range 1-10), and 47% had a prior taxane in the recurrent setting. There were no DLTs and 60mg was chosen as the RP2D due to long-term tolerability. Ninety-seven percent of patients had at least one treatment-emergent adverse event (TEAE), and the most common grade ≥ 3 TEAE were neutropenia (46%), anemia (31%), and nausea (21%). Among 24 evaluable patients with ovarian cancer, response rate was 17%, CBR was 58%, and median PFS was 6.8 months (95% CI 3.7, not reached (NR)).
Conclusions:
Oral selinexor in combination with weekly paclitaxel demonstrated promising clinical activity with manageable toxicity. This combination should be considered for further exploration in a randomized study, especially in ovarian malignancies.
Keywords: Selinexor, Paclitaxel, Metastatic solid tumors, Ovarian cancer, Selective inhibitor of nuclear export (SINE)
Introduction
The process of nuclear-cytoplasmic transport to maintain the intracellular localization of proteins is a critical mechanism to preserve cellular homeostasis. Exportins are nuclear export proteins that regulate transfer of molecules through the nuclear pore1,2. Of the seven known exportins, exportin-1 (XPO-1) or chromosomal region maintenance 1 (CRM1), is the most well described and is involved in translocation of at more than 200 unique proteins3–7. Amongst these, the transport of tumor suppressor proteins and growth-regulating oncoprotein mRNAs from the nucleus to the cytoplasm of the cell is a key component of normal cell function and development. Over-expression of XPO1 can lead to tumorigenesis secondary to neutralization of tumor suppressor protein function through increased transfer into the cytoplasm8. As expected, the over-expression of XPO1 has been associated with poor prognosis across solid and liquid malignancies, including ovarian cancer1,9–12. Collectively, these findings support exploration of agents to target exportins, and specifically, XPO1.
Selective inhibitors of nuclear export (SINE) compounds block XPO1-mediated transport, forcing the intranuclear accumulation of oncoprotein mRNAs along with “re-activation” of tumor suppressor proteins. Forced nuclear retention of mRNAs prevents their translation leading to reduced oncoprotein levels. Along with tumor suppressor protein re-activation, cell cycle checkpoint activity is restored, and tumor cell growth is hindered and tumor cell apoptosis follows. Selinexor (KPT-330) is a first in class, novel, potent, oral SINE which blocks XPO1 through formation of a reversible covalent bond with cysteine 528 of the XPO1 cargo-binding pocket13–16. In preclinical models, selinexor yielded tumor growth inhibition through blockage of XPO1-mediated nuclear transport and inhibition of attenuation of DNA damage repair. Phase I and II trials of selinexor as a single agent have demonstrated modest activity, with objective responses in patients with gynecologic malignancies13–16.
Ovarian cancer is the deadliest gynecologic malignancy with 5-year overall survival estimated at approximately 30%. Patients with platinum-resistant or platinum-refractory disease have limited options and decreased survival. As in other solid tumors, elevated levels of XPO1 are associated with worse outcomes in ovarian cancer17. Selinexor has demonstrated preclinical activity in ovarian cancer cell lines and patient-derived platinum-resistant models18. Further, the combination of selinexor with chemotherapy was found to exert antitumor activity across a number of solid tumor in vivo models15. The greatest activity appeared to be in combination wiath paclitaxel15, supporting use in this study. Single agent activity was explored in a phase II trial across all gynecologic malignancies, where modest 8% objective response and approximately 30% disease control rates were observed in a heavily pre-treated ovarian cancer population19. Given the role of weekly paclitaxel in the treatment of ovarian cancer \and the observed preclinical synergy, we sought to perform a phase Ib study of the combination of selinexor and weekly paclitaxel in advanced solid tumors with a planned expansion in recurrent ovarian cancer.
Patients and Methods
Study Design
This was an open-label, single-center, multi-arm, investigator-initiated, phase Ib of selinexor in combination with standard treatments in patients with advanced or metastatic solid tumors (Supplemental Figure 1; ClinicalTrial.gov identifier: NCT02419495). The study was conducted in multi-arms utilizing a standard 3 plus 3 design and a “basket type” expansion. Selinexor in combination with weekly paclitaxel was employed as one of the 13 parallel arms. The study protocol and all modifications were approved by the Institutional Review Board at our institution and was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice, and all local and federal regulatory guidelines.
The primary objective of this study was to determine the maximum tolerated dose (MTD), and recommended phase 2 dose (RP2D) of the combinations and further explore safety and tolerability of the RP2D of oral selinexor given with weekly intravenous paclitaxel. Secondary objectives included estimation of preliminary antitumor activity with objective response rate (ORR), duration of response (DOR), clinical benefit rate (CBR) per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.120) and progression free survival (PFS).
Patient Population
Eligible patients had histologically confirmed advanced or metastatic solid tumors which were unresponsive or had relapse following prior systemic therapy. There was no limit to number of prior lines of therapy, prior taxanes were allowed in upfront and/or recurrent setting. All patients had at least one measurable target lesion by RECIST v1.120. For the dose expansion, all patients were required to have a platinum resistant or refractory disease ovarian, fallopian tube, or peritoneal cancer (defined as progression during or within 6 months of a platinum-based therapy). For dose escalation, all patients were required to have adequate bone marrow (absolute neutrophil count (ANC) > 1,500/mcL, hemoglobin > 10.0 g/dL, platelets > 125,000/mcL), liver (total bilirubin within institutional limits and AST/ALT < 2 x institutional upper limit of normal) (ULN)), and renal function (creatinine within institutional normal limits or creatinine clearance >/= 30 mL/min/1.72 m2). In the expansion cohorts, acceptable levels were as follows: ANC > 1,000/mcL, hemoglobin > 9.0 g/dL, platelets > 100,000/mcL, AST/ALT </=2x ULN (patients with known liver involvement could have AST/ALT </= 5x ULN). Key exclusion criteria included patients with primary central nervous system tumor or active central nervous system involvement, residual toxicity not resolved to </= grade 1 (excluding alopecia), evidence of bowel obstruction or need for total parenteral nutrition, prior treatment with an agent targeting the exportin, and unstable cardiovascular function. All subjects provided written informed consent.
Treatment Plan
Cycle length was 21 days. Selinexor was dosed orally twice a week for the first two weeks of each cycle. Selinexor doses were taken 48 hours apart, typically on Monday/Wednesday or Tuesday/Thursday. Selinexor dose was 60mg for dose level 1 and 80mg for dose level 2. Paclitaxel was given at a fixed dose of 80mg/m2 intravenously on day 1 and day 8 of each cycle.
Assessments
Toxicities were monitored through the duration of the study and 30 days after cessation of study treatment. Common Terminology Criteria for Adverse Events version 4.03 (CTCAE v4.03) was utilized to grade treatment-emergent adverse events (TEAEs) and treatment-related adverse events (TRAEs). DLT was defined as any investigational treatment-related grade 4 hematologic adverse event, grade ≥ 3 thrombocytopenia associated with clinically significant bleeding, febrile neutropenia, or non-hematologic adverse event grade ≥ 3 in severity per CTCAE v4.03 despite optimal supportive medications, excluding electrolyte abnormalities that are reversible, asymptomatic or hair loss which is not dose-limiting. The MTD was defined as the highest dose level at which ≤ 33% of patients experience DLTs during cycle 1. After the MTD was defined, the study was expanded to include additional evaluable patients at the MTD. A safety monitoring committee comprised of investigators and the study sponsor reviewed all safety information and made consensus decisions about dose escalation. All patients underwent disease radiographic assessment with CT or MRI based on RECIST v1.120 every 3 cycles and responses were confirmed.
Statistical Methods
Patient characteristics, TEAEs, TRAEs, and antitumor activity were summarized using descriptive statistics. Clinical benefit rate (CBR) was defined as proportion of patients with complete response, partial response, or stable disease longer than 4 months from treatment start by RECIST v1.120. PFS was defined as the time from treatment to the time of progression disease or death, whichever occurred first, or off study for those who had not experienced progression. Duration of clinical benefit was defined as from the time of treatment start to the time of progression disease or death, whichever occurred first, or off study among the patients with clinical benefit. The distribution of PFS, duration of response, and duration of clinical benefit were summarized by Kaplan-Meier method.
A Simon minimax two-stage design for 10% vs. 30% targeting alpha = beta = 10% was utilized in the 25-patient tumor-specific cohorts. This design planned for 16 patients in the first stage and concludes if there are fewer than two successes. If there are at least 2 successes in the first 16 patients, 9 more patients will be enrolled for a total of 25 patients. This design has an obtained alpha 9.5%, power of 90%, and has an early stopping probability if the true success rate is 10% of 0.51. The probability of stopping early if the true success rate is 5% is 0.81. All analyses were performed in SAS 9.4 (Cary, NC).
Results
Patient characteristics
Between 7/2015 and 9/2020, 35 patients were enrolled on the selinexor/weekly paclitaxel arm of the basket study. All patients were evaluable for toxicity and 31 (88%) were evaluable for response. Patient characteristics are described in Table 1. Median age was 64 years (range, 38 - 77) with 94% female. The median number of prior systemic therapies received was 4 (range, 1 - 10) and 47% had previously received a taxane in the recurrent setting. The most common diagnosis was ovarian cancer (n=28, 80%) followed by breast (n=4, 11%), prostate (n=2, 6%), and cervical (n=1, 3%) cancer. Among the patients with ovarian cancer, high grade serous (82%) was the most common histology, all had received a taxane in the primary setting, and 71% had received bevacizumab in the recurrent setting.
Table 1.
Patient demographics and clinical characteristics (n=35)
| Characteristic | |
|---|---|
| Race, n (%) | |
| Caucasian | 25 (71) |
| Black | 5 (14) |
| Hispanic | 3 (9) |
| Asian | 1 (3) |
| Other | 1 (3) |
| Primary Site, n (%) | |
| Ovarian/Peritoneal/Fallopian tube | 28 (80) |
| Breast | 4 (11) |
| Prostate | 2 (6) |
| Cervical | 1 (3) |
| Ovarian Histology, n (%) | |
| High grade serous | 23 (82) |
| Low grade serous | 2 (7) |
| Clear cell | 2 (7) |
| Adenosquamous | 1 (4) |
| Prior Taxane in Recurrent Setting, n (%) | |
| No | 18 (53) |
| Yes | 16 (47) |
| Age, median years (range) | 64 |
| (38-77) | |
| Prior lines, median number (range) | 4 |
| (1-10) |
During the dose escalation phase, six patients were treated on dose level 1 and six patients were treated on dose level 2. Twenty-three patients were treated on the expansion phase of this arm.
Safety and Tolerability
Ninety-seven percent of patients had at least one treatment-emergent adverse event (TEAE) and the most common TEAEs were anemia (83%), nausea (63%), leukopenia (60%), fatigue (57%), neutropenia (51%), thrombocytopenia (49%), hyponatremia (54%), and vomiting (40%) (Table 2). The most prevalent grade ≥ 3 TEAE were anemia (83%), nausea (63%), leukopenia (60%), fatigue (57%), neutropenia (51%), and thrombocytopenia (49%). In regard to most common adverse events related to study treatment, the most common TRAEs were anemia (74%), nausea (57%), fatigue (51%), leukopenia (51%), neutropenia (49%), thrombocytopenia (46%), hyponatremia (34%), and vomiting (31%) (Table 2). Elevations in aspartate aminotransferase (23%), alanine aminotransferase (20%), and alkaline phosphatase (17%) were also observed without more serious liver toxicity (e.g., bilirubin elevation).
Table 2-.
Treatment-related and treatment-emergent adverse events experienced by ≥ 10% patients on selinexor and weekly paclitaxel
| Adverse Event | Treatment-related adverse events n, (%) | Treatment-emergent adverse events n, (%) | ||
|---|---|---|---|---|
| All grades | G3/G4 | All grades | G3/G4 | |
| Anemia | 26 (74) | 11 (31) | 29 (83) | 11 (31) |
| Nausea | 20 (57) | 1 (3) | 22 (63) | 1 (3) |
| Leukopenia | 18 (51) | 6 (17) | 21 (60) | 6 (17) |
| Fatigue | 18 (51) | 3 (9) | 20 (57) | 4 (11) |
| Neutropenia | 17 (49) | 16 (46) | 18 (51) | 16 (46) |
| Thrombocytopenia | 16 (46) | 2 (6) | 17 (49) | 3 (9) |
| Hyponatremia | 12 (34) | 3 (9) | 19 (54) | 6 (17) |
| Vomiting | 11 (31) | 1 (3) | 14 (40) | 2 (6) |
| Aspartate aminotransferase increased | 8 (23) | 1 (3) | 11 (31) | 1 (3) |
| Alanine aminotransferase increased | 7 (20) | 1 (3) | 10 (29) | 1(3) |
| Alkaline phosphatase increased | 6 (17) | 0 (0) | 7 (20) | 0 (0) |
| Peripheral sensory neuropathy | 6 (17) | 2 (6) | 7 (20) | 2 (6) |
| Mucositis - oral | 5 (14) | 0 (0) | 6 (17) | 0 (0) |
| Anorexia | 4 (11) | 0 (0) | 6 (17) | 0 (0) |
| Blurred vision | 4 (11) | 0 (0) | 4 (11) | 0 (0) |
| Diarrhea | 4 (11) | 1 (3) | 12 (34) | 1 (3) |
| Lymphopenia | 4 (11) | 2 (6) | 6 (17) | 3 (9) |
| Dyspnea | 2 (6) | 0 (0) | 12 (34) | 1 (3) |
| Abdominal Pain | 1 (3) | 0 (0) | 10 (29) | 3 (9) |
| Edema – limbs | 2 (6) | 0 (0) | 9 (26) | 1 (3) |
| Constipation | 2 (6) | 0 (0) | 8 (23) | 0 (0) |
| Hypokalemia | 1 (3) | 0 (0) | 8 (23) | 0 (0) |
| Hypoalbuminemia | 2 (6) | 0 (0) | 7 (20) | 0 (0) |
| Cough | 0 (0) | 0 (0) | 6 (17) | 0 (0) |
| Creatinine increased | 1 (3) | 1 (3) | 5 (14) | 1 (3) |
| Hypophosphatemia | 2 (6) | 0 (0) | 5 (14) | 1 (3) |
| Abdominal distention | 1 (3) | 0 (0) | 5 (14) | 0 (0) |
| Dizziness | 3 (9) | 0 (0) | 5 (14) | 0 (0) |
| Headache | 0 (0) | 0 (0) | 5 (14) | 0 (0) |
| Thromboembolic event | 1 (3) | 1 (3) | 4 (11) | 2 (6) |
| Hyperuricemia | 1 (3) | 1 (3) | 4 (11) | 1 (3) |
| Fever | 0 (0) | 0 (0) | 4 (11) | 0 (0) |
| Hyperkalemia | 2 (6) | 0 (0) | 4 (11) | 0 (0) |
| Syncope | 2 (6) | 2 (6) | 2 (6) | 2 (6) |
| CPK increased | 1 (3) | 1 (3) | 2 (6) | 1 (3) |
| Sepsis | 1 (3) | 1 (3) | 1 (3) | 1 (3) |
| Stroke | 1 (3) | 1 (3) | 1 (3) | 1 (3) |
| Colonic Fistula | 0 (0) | 0 (0) | 1 (3) | 1 (3) |
| Hypertension | 0 (0) | 0 (0) | 1 (3) | 1 (3) |
| Non-cardiac chest pain | 0 (0) | 0 (0) | 1 (3) | 1 (3) |
| Pain | 0 (0) | 0 (0) | 1 (3) | 1 (3) |
| Small intestinal obstruction | 0 (0) | 0 (0) | 1 (3) | 1 (3) |
| Thrombotic thrombocytopenia purpura | 0 (0) | 0 (0) | 1 (3) | 1 (3) |
There were no DLTs observed on either dose level. In dose level 2, despite no DLTs, 2 of 3 patients experienced grade 3 or 4 toxicity requiring dose interruption beyond cycle 1. Significant toxicities included fatigue, anemia, neutropenia, and syncope. The patients ultimately discontinued therapy for progressive disease prior to any dose reduction. Thus, DL1 was chosen as the recommended phase II due to better long term tolerability. Ten (29%) patients reported having 15 serious adverse events related to study intervention including G3 anemia (13%), G2 acute kidney injury (13%), G4 creatinine phosphokinase elevation (7%), G3 creatinine increased (7%), G2 dysarthria (7%), G2 fatigue (13%), G3 fatigue (7%), G4 hyperuricemia (7%), G3 nausea (8%), G4 sepsis (7%), G3 stroke (7%), G3 syncope (7%), and G3 nausea (7%). One patient died on study secondary to an unrelated adverse event of colonic perforation due to disease burden.
Antitumor Activity
Median cycle number was 4 (range, 1-10). Best overall tumor response among 31 evaluable patients is depicted in Figure 1. Four patients achieved a partial response (13% ORR) and an additional 10 patients (32%) had stable disease for greater than 4 months for CBR of 45%. All patients who achieved clinical benefit had a diagnosis of ovarian cancer. Among the entire cohort, 16 patients (47%) had prior exposure to a taxane, including one patient who achieved a partial response.
Figure 1: Antitumor activity of the combination of selinexor and weekly paclitaxel.
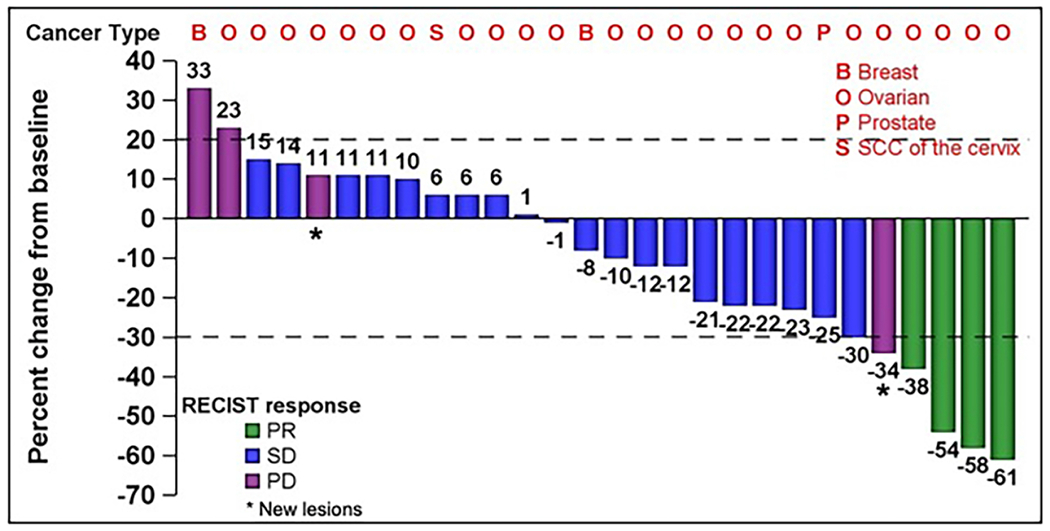
Abbreviations: B = Breast cancer; O = Ovarian Cancer; P = Prostate Cancer; S = Squamous Cell Carcinoma of the Cervix; PR = Partial Response; SD = Stable Disease; PD = Progressive Disease
Among the 24 evaluable patients with ovarian cancer, response rate was 17% and clinical benefit rate was 58%. 6 (25%) received a taxane in the recurrent setting. Duration of clinical benefit among patients with ovarian cancer was 7.6 months (95% CI: 4.4 – not reached, Figure 2). Among the four responders, 3 had high grade serous (HGS) histology and 1 had low grade serous (LGS) histology. The three responders with HGS had known pathogenic p53 mutations, the patient with LGS did not have a known molecular profile.
Figure 2:
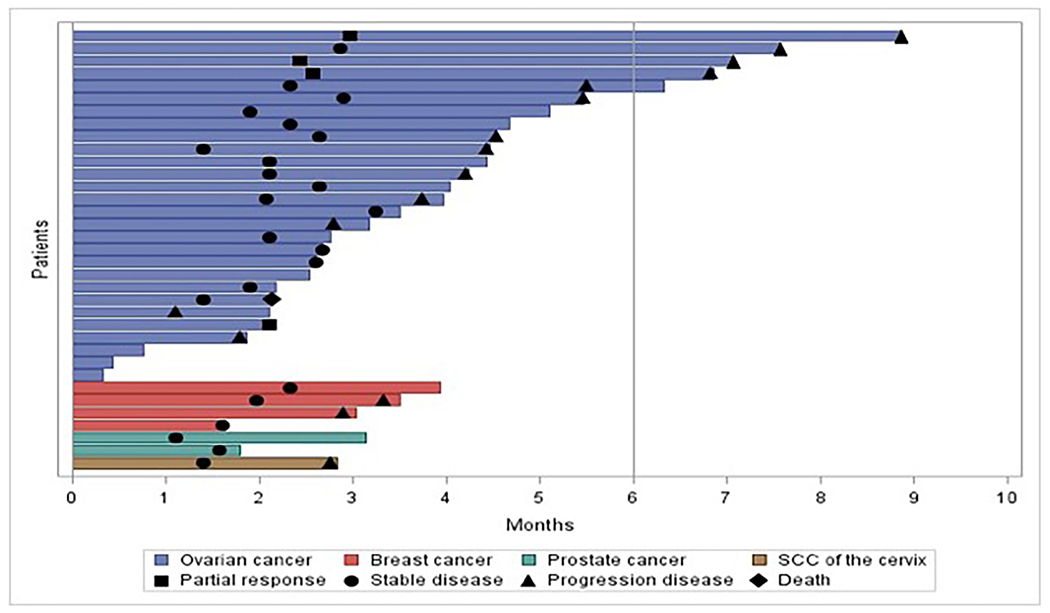
Time on treatment on selinexor and weekly paclitaxel
The median PFS for all patients and patients with ovarian cancer were 6.8 months (95% CI 2.9, not reached) and 6.8 months (95% CI 3.7, not reached) (Figure 3A and 3B), respectively.
Figure 3:
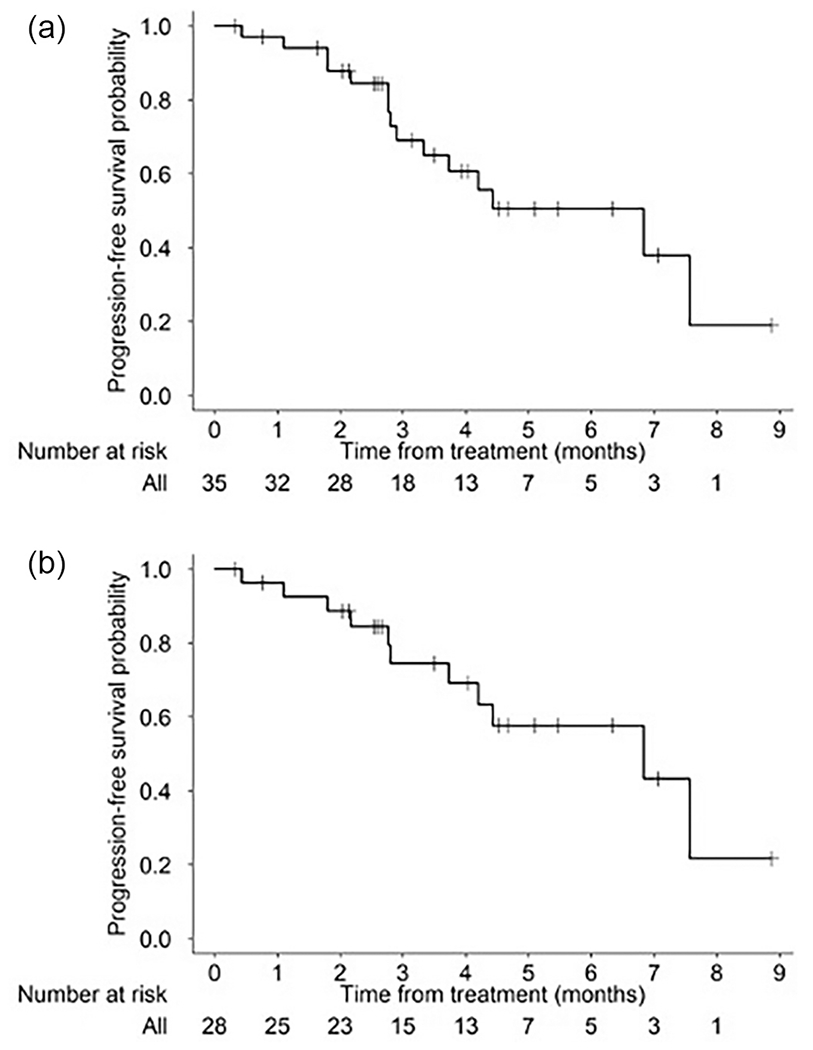
Progression free survival in A) entire cohort and B) ovarian cancer cohort
Discussion
This is the first study to report on the combination of selinexor with weekly paclitaxel, one of the most commonly used regimens for recurrent ovarian cancer, as well as other solid tumors. This basket phase 1b trial demonstrated the combination of selinexor and weekly paclitaxel was tolerable at up to 80mg selinexor twice weekly; the RP2D of selinexor 60mg orally twice a week and paclitaxel 80mg/m2 intravenously weekly on a two week on, one week off schedule was chosen due to good long-term tolerability. Further, intriguing clinical activity was demonstrated, regardless of prior taxane use.
The majority of patients treated with the combination experienced at least one treatment-emergent adverse event. Similar to other studies of selinexor, the most common adverse events were hematologic, gastrointestinal, and constitutional. Overall, these toxicities were low-grade and easily managed with supportive care. Serious adverse events were experienced in 29% of patients and were generally reversible. The increase in grade 3/4 bone marrow toxicity as compared to studies of selinexor19 alone, including neutropenia, anemia, and leukopenia, was likely due to overlapping toxicity between the agents. This is similar to what has been observed in other trials combining selinexor and chemotherapy21–23. In fact, the rates of grade 3/4 leukopenia, neutropenia, and leukopenia were much lower in the current study as compared to both phase 1b trials combining selinexor with paclitaxel and carboplatin.
There was encouraging activity of the combination, especially among women with recurrent, platinum-resistant ovarian cancer where a 17% RR and 58% CBR was achieved. Further, progression free survival was 6.8 months in this platinum-resistant/refractory population, which is more than twice what is expected with chemotherapy alone, especially in a heavily pretreated population24. Initial studies of selinexor as a single agent demonstrated modest response rates, with the first in human trial yielding 4% objective response, including a patient with ovarian cancer25. A subsequent phase II trial in recurrent gynecologic malignancies demonstrated an 8% response rate and 30% disease control rate (CR, PR, SD ≥ 12 weeks) in patients with progressive ovarian cancer after a median of five prior regimens19.
There is a lack of understanding regarding which molecular aberrations may be associated with benefit from selinexor. In a study of selinexor as a maintenance strategy in advanced and recurrent endometrial cancer, patients with wildtype p53 appeared to garner the greatest benefit. Our study is lacking the depth of molecular data to assess any associations, although three patients with objective response to the combination did have a pathogenic p53 mutation.
In ovarian cancer, response rates for weekly paclitaxel have been reported as anywhere from 20-60%26, with higher response rates achieved in taxane-naïve patients and in patients generally less heavily pretreated (1-2 prior therapies) than those who entered the current study. However, duration of response and progression free survival are limited with this regimen. The combination of chemotherapy (weekly paclitaxel, topotecan or pegylated doxorubicin) with bevacizumab is FDA-approved in the platinum-resistant setting due to demonstration of improved PFS with the combination of chemotherapy alone in the AURELIA trial (HR 0.48 (95% CI, 0.38 to 0.60; P < .001). Of note, the AURELIA trial was specific to patients with only 1-2 prior regimens and no prior bevacizumab in the recurrent setting. Thus, rates of objective response, clinical benefit, and median progression free survival in the current trial are noteworthy given the heavily pre-treated nature of our population (median 4 cycles) and the high proportion of patients with prior bevacizumab (71%).
The combination of selinexor and weekly paclitaxel appears to have the potential for synergitic activity over either single agent. However, these results should be interpreted with caution in this small patient population, given results of other randomized trials that did not show benefit with addition of other targeted agents, such as pazopanib or Reolysin, to weekly paclitaxel27,28.
In summary, the SINE inhibitor, selinexor, in combination with paclitaxel demonstrated promising clinical activity with manageable toxicity, and further evaluation is warranted. This combination should be considered for further exploration in a randomized study, especially in ovarian malignancies.
Supplementary Material
Supplemental Figure 1: Selinexor basket trial schema
Highlights.
The combination of the selective inhibitor of nuclear export (SINE), selinexor, and weekly paclitaxel is tolerable.
Most common toxicity from the combination of selinexor and paclitaxel included bone marrow suppression, nausea, and fatigue.
Selinexor and weekly paclitaxel yielded clinical benefit in heavily pre-treated patients with platinum-resistant ovarian cancer.
Funding:
Karyopharm, NIH SPORE in Ovarian Cancer (NCT P50 CA217685), NIH Cancer Center Support Grant (P30 CA016672), NIH R35 Grant (R35 CA209904), GOG Foundation Scholar Investigator Award, American Cancer Society
Conflict of Interest Statement:
Shannon N. Westin receives research support to institution from AstraZeneca, Bayer, Clovis Oncology, Cotinga Pharmaceuticals, GSK, Mereo, Novartis, OncXerna, Roche/Genentech, Zentalis and consulting fees from Agenus, AstraZeneca, Caris, Clovis Oncology, Eisai, EQRX, GSK, ImmunoGen, Lilly, Merck, Mereo, Mersana, Roche/Genentech, Vincerx, and Zentalis; Siqing Fu receives research support to the institution from Abbisko, BeiGene, BioAtla, LLC. Boehringer Ingelheim, Eli Lilly & Co., Exelisis, Green2Bio, Inc., Hookipa Biotech, IMV, Inc., Innovent Biologics, Co., Ltd., K-Group Beta, Lyvgen Biopharm, MacroGenics, Millennium Pharmaceuticals, Nerviano Medical Sciences, NeuPharma, NextCure, Ningbo NewBay Technology Development, Novartis, NovoCure, Parexel International, LLC, PureTech Health, LLC, Sellas Life Sciences Group, Soricimed Biopharma, Inc., SQZ Biotechnologies, Sumitomo Dainippon, Taiho Oncology and NCCN, Treadwell Therapeutics, Turnstone Biologics, Tyligand Bioscience, Ltd., and Vaccibody AS. Apostolia Tsimberidou receives research support to the institution from BI Pharma, IMMATICS, Parker Institute for Cancer Immunotherapy, Agenus, Tempus, Tvardi, Boston Biomedical, Karus Therapeutics, Novacure, Ltd., and consulting fees from Vincerx and Diaccurate; Sarina Piha-Paul receives research support to the institution from AbbVie, Inc., ABM Therapeutics, Inc., Acepodia, Inc, Alkermes, Aminex Therapeutics, Amphivena Therapeutics, Inc., BioMarin Pharmaceutical, Inc, Boehringer Ingelheim, Bristol Myers Squib, Cerulean Pharma, Inc., Chugai Pharmaceutical Co., Ltd, Curis, Inc., Cyclacel Pharmaceuticals, Daiichi Sankyo, Eli Lilly, ENB Therapeutics, Five Prime Therapeutics, F-Star Beta Limited, F-Star Therapeutics, Gene Quantum, Genmab A/S, Gilead Sciences, Inc., GlaxoSmithKline, Helix BioPharma Corp., HiberCell, Inc., Immunomedics, Inc., Incyte Corp., Jacobio Pharmaceuticals Co., Ltd., Lytix Biopharma AS, Medimmune, LLC., Medivation, Inc., Merck Sharp and Dohme Corp., Novartis Pharmaceuticals, Pieris Pharmaceuticals, Inc., Pfizer, Phanes Therapeutics, Principia Biopharma, Inc., Puma Biotechnology, Inc., Purinomia Biotech, Inc., Rapt Therapeutics, Inc., Seattle Genetics, Silverback Therapeutics, Synlogic Therapeutics, Taiho Oncology, Tesaro, Inc., TransThera Bio, ZielBio, Inc., and consulting fees from CRC Oncology; David S. Hong receives research support to the institution from AbbVie, Adaptimmune, Adlai-Nortye, Amgen, Astra-Zeneca, Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Deciphera, Eisai, Endeavor, Erasca, F. Hoffmann-La Roche, Fate Therapeutics, Genentech, Genmab, Ignyta, Infinity, Kite, Kyowa Kirin, Lilly, LOXO, Merck, Medimmune, Mirati, Mologen, Navier, NCI-CTEP, Novartis, Numab, Pfizer, Pyramid Bio, SeaGen, Takeda,TCR2, Teckro, Turning Point Therapeutics, Verstatem, VM Oncology, travel accommodations/expenses from Bayer, Genmab, AACR, ASCO, SITC, Telperian, consulting fees from Adaptimmune, Alpha Insights, Acuta, Alkermes, Amgen, Aumbiosciences, Axiom, Baxter, Bayer, Boxer Capital, BridgeBio, COR2ed, COG, Ecor1, Genentech, Gilead, GLG, Group H, Guidepoint, HCW Precision, Immunogen, Infinity, Janssen, Liberium, Medscape, Numab, Oncologia Brasil, Pfizer, Pharma Intelligence, POET Congress, Prime Oncology, Seattle Genetics, ST Cube, Takeda, Tavistock, Trieza Therapeutics, Turning Point, WebMD, Ziopharm and other ownership interests with Molecular Match (Advisor), OncoResponse (Founder, Advisor), Telperian (Founder,Advisor); Shubham Pant receives consulting fees from Merck, GSK, Kiyatec, and has stocks in Bio-Path; Amir Jazaeri receives research support to the institution from Iovance, BMS, Eli Lilly, Pfizer, Ziopharm, AstraZeneca, Aravivie, Merck, Immatics USA, consulting fees from Aravive, Agenus, Macrogenics, BMS, Alkermes, Instill Bio, Immune-Onc, GSK, Obsedian, AvengeBio, NuProbe, EMD-Serono, Guidepoint, Gherson Lehrman Group, participation in a DSMB for Roche, and stock/stock options from AvengeBio. David Gershenson receives research support to the institution from NRG Oncology, Novartis, royalties/licenses from Elsevier, UpToDate, consulting fees from Genentech, payment for lectures from CFS PER Conference, participation in DSMB/advisory board for Onconova, Springworks, leadership role in International Consortium for Low-Grade Serous Cancer, and stock or stock options from Johnson & Johnson, Bristol Myers Squibb, and Procter and Gamble; Anil K. Sood receives research support to institution from M-Trap, royalties/licensure from BioPath, consulting fees from AstraZeneca, Merck, GSK, Kiyatec, and; Robert L. Coleman receives research support to institution from AstraZeneca, Clovis, Genelux, Genmab, Merck, Immunogen Janssen, Roche/Genentech, consulting fees from Agenus, Alkermes, AstraZeneca, Clovis, Deciphera, Genelux, Genmab, GSK, Immunogen Janssen, OncoQuest, Onxeo, Onxerna, Regeneron, Roche/Genentech, and participates in DSMB for VBL Therapeutics; Jatin Shah is employed and receives stock/stock options from Karyopharm; Funda Meric-Bernstam receives research support to the institution from Aileron Therapeutics, Inc. AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences Inc., Curis Inc., CytomX Therapeutics Inc., Daiichi Sankyo Co. Ltd., Debiopharm International, eFFECTOR Therapeutics, Genentech Inc., Guardant Health Inc., Klus Pharma, Takeda Pharmaceutical, Novartis, Puma Biotechnology Inc., Taiho Pharmaceutical Co., consulting fees from AbbVie, Aduro BioTech Inc., Alkermes, AstraZeneca, DebioPharm, eFFECTOR Therapeutics, F. Hoffman-La Roche Ltd., Genentech Inc., IBM Watson, Infinity Pharmaceuticals, Jackson Laboratory, Kolon Life Science, Lengo Therapeutics, OrigiMed, PACT Pharma, Parexel International, Pfizer Inc., Samsung Bioepis, Seattle Genetics Inc., Tallac Therapeutics, Tyra Biosciences, Xencor, Zymeworks, payment for lecture from Chugai Biopharmaceuticals, and participation on DSMB/Advisory Board with Black Diamond, Biovica, Eisai, Immunomedics, Inflection Biosciences, Karyopharm Therapeutics, Loxo Oncology, Mersana Therapeutics, OnCusp Therapeutics, Puma Biotechnology Inc., Seattle Genetics, Silverback Therapeutics, Spectrum Pharmaceuticals, and Zentalis; Aung Naing receives research support to institution from NCI, EMD Serono, MedImmune, Healios Onc. Nutrition, Atterocor/Millendo, Amplimmune, ARMO BioSciences, Karyopharm Therapeutics, Incyte, Novartis, Regeneron, Merck, Bristol- Myers Squibb, Pfizer, CytomX Therapeutics, Neon Therapeutics, Calithera Biosciences, TopAlliance Biosciences, Eli Lilly, Kymab, PsiOxus, Arcus Biosciences, NeoImmuneTech, ImmuneOncia, Surface Oncology, Monopteros Therapeutics, BioNTech SE, Seven & Eight Biopharma, SOTIO Biotech AG, Immune Deficiency Foundation, Jeffery Modell Foundation and Chao physician-scientist, Baxalta, consulting fees from ARMO BioSciences, CytomX Therapeutics, Novartis, Genome & Company, OncoSec KEYNOTE-695, Kymab, STCube Pharmaceuticals, Deka Biosciences, Takeda, CSL, Behring, Horizon, and Pharming.
All other authors note no relevant conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT Author Statement: Shannon N. Westin: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing - original draft; Writing - review & editing; Siqing Fu: Resources, Writing - review & editing; Apostolia Tsimberidou: Resources, Writing - review & editing; Sarina Piha-Paul: Resources, Writing - review & editing; Fechukwu Akhmedzhanov: Data Curation, Investigation, Resources, Writing- review and editing, Project Administration; Bulent Yilmaz: Data Curation, Investigation, Resources, Writing- review and editing, Project Administration; Lacey McQuinn: Data Curation, Investigation, Resources, Writing- review and editing, Project Administration; Amanda L. Brink: Data Curation, Investigation, Resources, Writing- review and editing, Project Administration; Jing Gong: Data Curation, Investigation, Resources, Writing- review and editing, Project Administration; Cheuk Hong Leung: Investigation, Resources, Writing- review and editing; Heather Lin: Investigation, Resources, Writing- review and editing; David S. Hong: Resources, Writing - review & editing; Shubham Pant: Resources, Writing - review & editing; Bret Carter: Investigation, Resources, Writing - review & editing; Amir Jazaeri: Resources, Writing - review & editing; David Gershenson: Resources, Writing - review & editing; Anil K. Sood: Resources, Writing - review & editing; Robert L. Coleman: Resources, Writing - review & editing; Jatin Shah: Conceptualization, Funding Acquisition, Resources, Writing - review & editing; Funda Meric-Bernstam: Resources, Writing - review & editing; Aung Naing: Conceptualization, Data curation, Formal analysis, Funding Acquisition, Investigation, Methodology, Project Administration, Resources, Writing - original draft; Writing - review & editing;
References
- 1.Turner JG, Dawson J, Sullivan DM: Nuclear export of proteins and drug resistance in cancer. Biochem Pharmacol 83:1021–32, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukuda M, Asano S, Nakamura T, et al. : CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390:308–11, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Tan DS, Bedard PL, Kuruvilla J, et al. : Promising SINEs for embargoing nuclear-cytoplasmic export as an anticancer strategy. Cancer Discov 4:527–37, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Gravina GL, Senapedis W, McCauley D, et al. : Nucleo-cytoplasmic transport as a therapeutic target of cancer. J Hematol Oncol 7:85, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh K, Cang S, Sekhri A, et al. : Selective inhibitors of nuclear export (SINE)--a novel class of anti-cancer agents. J Hematol Oncol 7:78, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu D, Grishin NV, Chook YM: NESdb: a database of NES-containing CRM1 cargoes. Mol Biol Cell 23:3673–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen KT, Holloway MP, Altura RA: The CRM1 nuclear export protein in normal development and disease. Int J Biochem Mol Biol 3:137–51, 2012 [PMC free article] [PubMed] [Google Scholar]
- 8.Azizian NG, Li Y: XPO1-dependent nuclear export as a target for cancer therapy. J Hematol Oncol 13:61, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyake T, Pradeep S, Wu SY, et al. : XPO1/CRM1 Inhibition Causes Antitumor Effects by Mitochondrial Accumulation of eIF5A. Clin Cancer Res 21:3286–97, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertucci F, Finetti P, Birnbaum D: XPO1, therapeutic … and prognostic target in sarcomas. Oncoscience 3:143–4, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birnbaum DJ, Finetti P, Birnbaum D, et al. : XPO1 Expression Is a Poor-Prognosis Marker in Pancreatic Adenocarcinoma. J Clin Med 8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Chong Y, Tu Y, et al. : CRM1/XPO1 is associated with clinical outcome in glioma and represents a therapeutic target by perturbing multiple core pathways. J Hematol Oncol 9:108, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senapedis WT, Baloglu E, Landesman Y: Clinical translation of nuclear export inhibitors in cancer. Semin Cancer Biol 27:74–86, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Kashyap T, Argueta C, Unger T, et al. : Selinexor reduces the expression of DNA damage repair proteins and sensitizes cancer cells to DNA damaging agents. Oncotarget 9:30773–30786, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arango NP, Yuca E, Zhao M, et al. : Selinexor (KPT-330) demonstrates anti-tumor efficacy in preclinical models of triple-negative breast cancer. Breast Cancer Res 19:93, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung HY, Chook YM: Atomic basis of CRM1-cargo recognition, release and inhibition. Semin Cancer Biol 27:52–61, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noske A, Weichert W, Niesporek S, et al. : Expression of the nuclear export protein chromosomal region maintenance/exportin 1/Xpo1 is a prognostic factor in human ovarian cancer. Cancer 112:1733–43, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Camacho SC, Silvers TR, et al. : Inhibition of the Nuclear Export Receptor XPO1 as a Therapeutic Target for Platinum-Resistant Ovarian Cancer. Clin Cancer Res 23:1552–1563, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Vergote IB, Lund B, Peen U, et al. : Phase 2 study of the Exportin 1 inhibitor selinexor in patients with recurrent gynecological malignancies. Gynecol Oncol 156:308–314, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–47, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Thein KZ, Piha-Paul SA, Tsimberidou A, et al. : Selinexor in combination with standard chemotherapy in patients with advanced or metastatic solid tumors. Exp Hematol Oncol 10:59, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thein KZ, Karp DD, Tsimberidou A, et al. : Selinexor in combination with carboplatin and paclitaxel in patients with advanced solid tumors: Results of a single-center, multi-arm phase Ib study. Invest New Drugs, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubinstein MM, Grisham RN, Cadoo K, et al. : A phase I open-label study of selinexor with paclitaxel and carboplatin in patients with advanced ovarian or endometrial cancers. Gynecol Oncol 160:71–76, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayson GC, Kohn EC, Kitchener HC, et al. : Ovarian cancer. Lancet 384:1376–88, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Abdul Razak AR, Mau-Soerensen M, Gabrail NY, et al. : First-in-Class, First-in-Human Phase I Study of Selinexor, a Selective Inhibitor of Nuclear Export, in Patients With Advanced Solid Tumors. J Clin Oncol 34:4142–4150, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baird RD, Tan DS, Kaye SB: Weekly paclitaxel in the treatment of recurrent ovarian cancer. Nat Rev Clin Oncol 7:575–82, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Cohn DE, Sill MW, Walker JL, et al. : Randomized phase IIB evaluation of weekly paclitaxel versus weekly paclitaxel with oncolytic reovirus (Reolysin(R)) in recurrent ovarian, tubal, or peritoneal cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 146:477–483, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson DL, Sill MW, Coleman RL, et al. : Paclitaxel With and Without Pazopanib for Persistent or Recurrent Ovarian Cancer: A Randomized Clinical Trial. JAMA Oncol 4:196–202, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Selinexor basket trial schema


