Abstract
BACKGROUND
The ISCHEMIA trial compared an initial invasive versus an initial conservative management strategy for patients with chronic coronary disease and moderate or severe ischemia, with no major difference in most outcomes over a median of 3.2 years. Extended follow-up for mortality is ongoing.
METHODS
ISCHEMIA participants were randomized to an initial invasive strategy (INV) added to guideline-directed medical therapy or a conservative strategy (CON). Patients with moderate or severe ischemia, ejection fraction ≥35%, and no recent acute coronary syndromes were included. Those with an unacceptable level of angina were excluded. Extended follow-up for vital status is being conducted by sites or through central death index search. Data obtained through December 2021 are included in this interim report. We analyzed all-cause, cardiovascular, and non-cardiovascular mortality by randomized strategy, using nonparametric cumulative incidence estimators, Cox regression models and Bayesian methods. Undetermined deaths were classified as cardiovascular as pre-specified in the trial protocol.
RESULTS
Baseline characteristics for 5179 original ISCHEMIA trial participants included median age 65 years, 23% women, 16% Hispanic, 4% Black, 42% diabetes, and median EF 0.60. A total of 557 deaths accrued over a median follow-up of 5.7 years, with 268 of these added in the extended follow-up phase. This included a total of 343 cardiovascular deaths, 192 non-cardiovascular deaths and 22 unclassified deaths. All-cause mortality was not different between randomized treatment groups (7-year rate 12.7% in INV, 13.4% in CON; adjusted hazard ratio (HR)=1.00, 95% CI: 0.85–1.18). There was a lower 7-year rate cardiovascular mortality (6.4% vs. 8.6%, adjusted HR=0.78, 95% CI: 0.63–0.96) with an initial invasive strategy but a higher 7-year rate of non-cardiovascular mortality (5.6% vs. 4.4%, adjusted HR=1.44, 95% CI: 1.08–1.91) compared with the conservative strategy. No heterogeneity of treatment effect was evident in prespecified subgroups, including multivessel coronary disease.
CONCLUSIONS
There was no difference in all-cause mortality with an initial invasive strategy compared with an initial conservative strategy, but there was lower risk of cardiovascular mortality and higher risk of non-cardiovascular mortality with an initial invasive strategy over a median follow-up of 5.7 years.
Keywords: catheterization, medical therapy, revascularization, stable coronary disease, Myocardial Infarction, Chronic Coronary Disease, Stable Ischemic Heart Disease
BACKGROUND
The ISCHEMIA trial compared initial invasive vs. conservative management strategies for patients with chronic coronary disease and moderate or severe ischemia on stress testing.1 After a median follow-up of 3.2 years, there was no net benefit for the initial invasive strategy on the primary or major secondary clinical outcomes. While there was no significant difference in the rate of total myocardial infarction (MI), the invasive strategy led to more peri-procedural MIs, all centrally adjudicated, but fewer spontaneous MIs. MI events were associated with a higher risk of subsequent mortality,2 with a stronger association for spontaneous MI than for peri-procedural MI. There appeared to be a late divergence of the cardiovascular mortality curves in favor of the invasive strategy over the conservative strategy with 4-year rates of 4.1 vs. 5.0%; HR 0.87 [95% CI: 0.66, 1.15]. In contrast, the 4-year rates of non-cardiovascular mortality were higher in the invasive strategy (2.5% vs 1.4%; HR 1.63 [95% CI: 1.06–2.52])3 and all-cause mortality was not different (6.5% vs. 6.4%; HR 1.05 [95% CI: 0.83, 1.32]).1 The severity of coronary artery disease on coronary computed tomography angiography (CCTA) was strongly associated with primary and secondary outcome events.4 Herein we report the interim 7-year all-cause, cardiovascular and non-cardiovascular mortality rates for the ongoing NHLBI-funded ISCHEMIA Extended Follow-up (ISCHEMIA-EXTEND), including findings across subgroups.
METHODS
STUDY DESIGN
ISCHEMIA and ISCHEMIA-EXTEND were sponsored by the National Institutes of Health/National Heart, Lung, and Blood Institute, and the trial data sets will be made available through the National Institutes of Health BioData Catalyst (BDC) website (https://biodatacatalyst.nhlbi.nih.gov/). ISCHEMIA trial and ISCHEMIA-EXTEND designs have been reported.5, 6 In brief, ISCHEMIA randomized patients with chronic coronary disease and moderate or severe ischemia to an initial invasive strategy of cardiac catheterization and revascularization, when feasible, added to guideline-directed medical therapy; or an initial conservative strategy of guideline-directed medical therapy alone, with catheterization and revascularization reserved for failure of medical therapy. Major exclusion criteria for the trial included left main stenosis ≥50%, ejection fraction <35%, acute coronary syndrome within 2 months, and angina that could not be managed with medical therapy alone. The trial protocol included long-term assessment with up to 20-year follow-up in the consent form.
All 5179 randomized trial participants’ baseline and survival data are included in this report. ISCHEMIA-EXTEND is continuing to follow participants that survived the initial trial phase and had not withdrawn consent (referred to as EXTEND eligible) for collection of vital status and cause of death data. Thirty-six of the original 37 countries obtained vital status information: 33 contacted participants or their designated surrogate one to two times a year. One country is pending regulatory approval. Three of the 36 countries had central data available. One of these was not able to provide cause of death, and these deaths were “unclassified”. Consistent with the ISCHEMIA trial phase in which deaths of undetermined cause after CEC adjudication were included in the protocol definition of cardiovascular death,5 we grouped undetermined deaths during the extended follow-up period as cardiovascular deaths. Death dates including year, month, and day for the extended follow-up period were available for all but one participant whose date included only the year. For this participant, we substituted the midpoint of the indicated year.
Information on whether death was COVID-related was collected, when available, as of July 2020. Cause of death was not centrally adjudicated during extended follow-up. All sites had local ethics committee or institutional review board approval and participants gave informed consent.
The findings from subgroups1,5 of interest that were prespecified at trial inception, including those previously found to be independently associated with higher risk of mortality, are reported. For the subset of participants that had core-lab interpreted CCTA, severity of coronary artery disease (CAD) was categorized as single or multivessel disease, by both ≥50% and ≥70% stenosis criteria, when possible.4 Multi-vessel disease was assessed when either all key segments required to determine the number of diseased vessels were evaluable, or when two of three major vessels were evaluable as diseased (MVD present) or not diseased (MVD absent).
STATISTICAL ANALYSIS
All analyses are performed according to intention-to-treat based on initial randomized trial strategy assignment. We compared baseline characteristics of participants included in the ISCHEMIA-EXTEND eligible study population versus the original trial population and ineligible participants who withdrew from the trial with no database search allowable or who declined extended follow-up.
Intention-to-treat analysis was used to estimate the effect of an assigned management strategy on risk of all-cause, cardiovascular, and non-cardiovascular mortality from the time of randomization. Using the Kaplan-Meier method, we estimated the cumulative event rate of mortality by assigned management strategy and used the log rank test to assess differences in the survival distributions. We estimated yearly mortality differences through 7 years follow-up. For the competing events of cardiovascular and non-cardiovascular mortality, we used a non-parametric cumulative incidence function estimator and the Fine-Gray method to test for differences in the cumulative incidence functions by strategy.
Using separate Cox proportional hazards regression models for all-cause, cardiovascular, and non-cardiovascular mortality, we estimated the adjusted hazard ratio for the invasive versus conservative strategy, after controlling for prespecified baseline participant characteristics as done in the initial ISCHEMIA trial phase,1 namely, sex, age, diabetes status, eGFR, and ejection fraction. In randomized clinical trials, adjustment for a prespecified, parsimonious set of covariates is recommended to improve precision of the estimated treatment effect and safeguard against potential covariate imbalances between treatment groups.7–11 For cardiovascular and non-cardiovascular mortality, we estimated cause-specific Cox models to obtain cause-specific hazard ratios. We assessed the proportional hazards assumption with the score test of the null hypothesis of no association between the scaled Schoenfeld residuals for management strategy and log time. The null hypothesis was not rejected at the 5% significance level (all-cause mortality, p=0.27; cardiovascular mortality, p=0.06; non-cardiovascular mortality, p=0.26).
To further characterize the effect of assigned management strategy, we used Bayesian piecewise exponential survival modeling12. For all-cause mortality, we estimated the posterior mean adjusted absolute percent difference for the invasive versus conservative strategy in the cumulative event rate at 7 years (controlling for the aforementioned baseline characteristics). We quantified the posterior probability that the difference was below or above varying thresholds. For cardiovascular and non-cardiovascular mortality, we modified the piecewise survival model to account for competing events in the spirit of the model for competing risk failure times in Andrinopoulou et al (2014).13 We extended the piecewise exponential survival model to jointly model the hazard of each event of interest (cardiovascular and non-cardiovascular mortality). We used this model to estimate the posterior mean adjusted absolute percent difference for the invasive versus conservative strategy in the 7-year cumulative incidence of cardiovascular mortality and non-cardiovascular mortality (accounting for the respective competing risk). Details about model specification, assignment of prior distributions, and model fitting, convergence, and diagnostics are available in the Supplement.
To assess heterogeneity of treatment effect in prespecified subgroups of interest, we estimated the adjusted hazard ratio for the invasive versus conservative strategy in each pre-specified subgroup. We tested the null hypothesis that the treatment effect did not differ by subgroup using the Wald test for interaction.
We conducted sensitivity analyses to examine whether the estimated effect of treatment strategy at 7 years of follow-up was robust to the classification of new undetermined deaths during the extended follow-up phase as cardiovascular deaths, and the proportional hazards assumption. To conduct sensitivity analysis about our assumption that new undetermined deaths during the extended follow-up phase have cardiovascular-related causes (as per the ISCHEMIA trial protocol definition),5 we assumed that these new undetermined deaths were instead non-cardiovascular deaths. For one country in which cause of death during the extended follow-up was unavailable, we ran models based on either censoring new deaths from the country at the end of the trial phase or treating these new deaths as undetermined deaths – as in countries collecting cause of death data. To evaluate the proportional hazards assumption, we extended the Bayesian piecewise exponential model to allow time-varying treatment effects (see Supplement).
All analyses were conducted using R statistical software,14 with Bayesian modeling conducted using JAGS.15
RESULTS
BASELINE CHARACTERISTICS
Figure 1 presents the participant flow for long-term follow-up. In baseline data and survival analyses, all 5,179 trial participants are included, with because participants who withdrew or declined extended follow-up are censored at their last known alive date. Among 5179 participants initially randomized, 289 (5.6%) had died by the end of the original trial follow-up in June 2019, 29 (0.6%) withdrew with no database search allowable, and 36 (0.7%) participants declined extended follow-up. Thus, 4,825 participants were eligible for additional follow-up for mortality in ISCHEMIA-EXTEND, including 2,407 in the invasive strategy and 2,418 participants in the conservative strategy. Median follow-up among the 5,179 participants was 5.7 years.
Figure 1. Participant Flow (CONSORT).
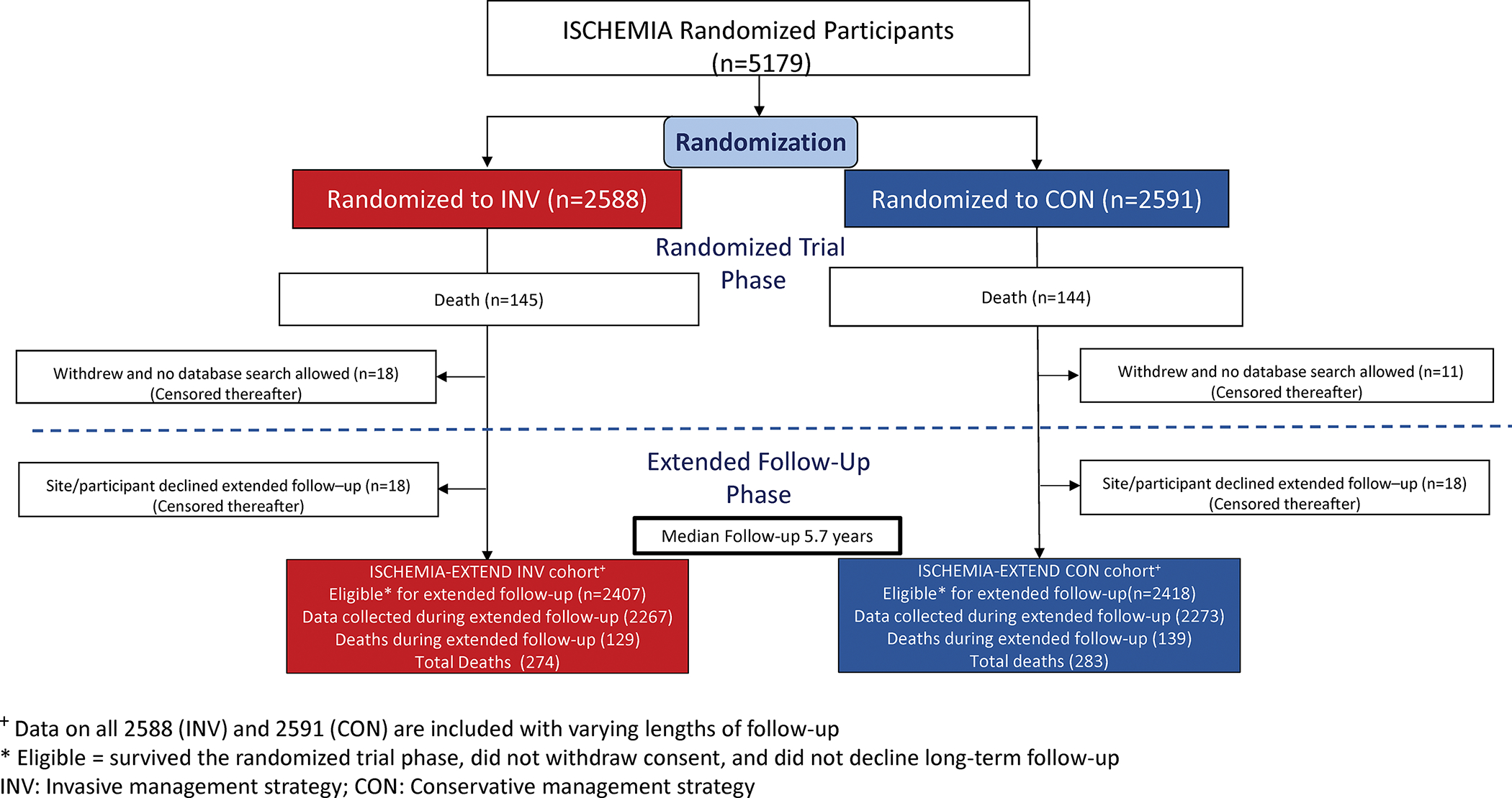
The top portion, labeled randomized trial phase, shows 5179 randomized to either invasive (red) or conservative (blue) strategy between 2011 and 2018. The original reported trial findings included follow-up through June 2019. Twenty-nine participants withdrew from follow-up during the trial phase with no database search allowable, and 36 participants declined extended follow-up. The median follow-up was 5.7 years. In survival analyses, all 5,179 trial participants are included with participants who withdrew or declined extended follow-up censored at their last known alive date. Numbers at risk at each time point are shown below the horizontal axis on survival plots. Hazard ratios and 95% confidence intervals are derived from adjusted analyses.
Baseline characteristics for the 5179 ISCHEMIA trial participants overall and by eligibility status are shown in Table S1. Among the 5,179 trial participants, the median age was 65 years, 23% women, 16% Hispanic, 4% Black, 42% had diabetes, and median EF was 60%. There were no major differences in baseline characteristics between those who were eligible for extended follow-up and the original randomized cohort (Table S1). Compared with participants eligible for extended follow up, participants who withdrew with no database search allowable during the initial trial phase or who declined extended follow-up were older and more likely to be of Asian race.
CLINICAL OUTCOMES
There were 268 additional deaths during the extended follow-up period, leading to a total of 557 deaths at a median of 5.7 years. This total included 343 cardiovascular deaths, 192 non-cardiovascular deaths, and 22 deaths with cause not classified (from a country without cause of death data available at the time of this report). The cumulative all-cause mortality rate was not different between assigned management strategies (Figure 2A, log rank p=0.74). A small early excess risk of mortality at 1 year in the invasive versus conservative strategy resolved by 2 years (Figure 2A and Table 1). The Cox adjusted all-cause mortality hazard ratio for invasive versus conservative management was 1.00 (95% CI: 0.85, 1.18). In contrast, the cumulative incidence of cardiovascular mortality by management strategies diverged at approximately 2.5 years in favor of the invasive strategy (Figure 2B, Fine and Gray p=0.008), with an estimated 7-year difference in the cumulative incidence for invasive versus conservative management of −2.19% (95% CI: −3.85%, −0.53%) and adjusted hazard ratio of 0.78 (95% CI: 0.63, 0.96) (Table 1). Non-cardiovascular mortality cumulative incidence curves by assigned strategy diverged at approximately 2.5–3 years in favor of the conservative strategy (Figure 2C, Fine and Gray p=0.015). Between 4 and 6 years, the cumulative incidence of non-cardiovascular mortality was significantly higher in the invasive versus conservative strategy (Table 1). At 7 years, the estimated difference in the cumulative incidence of non-cardiovascular mortality for invasive versus conservative management was 1.20% with a 95% confidence interval just covering the null (95% CI: −0.32%, 2.72%) (Table 1). Adjusting for baseline characteristics, the hazard ratio for non-cardiovascular mortality was 1.44 for the invasive strategy compared with the conservative strategy (95% CI: 1.08, 1.91) (Table 1). Twenty-one deaths were noted to have a proximate COVID diagnosis.
Figure 2. Cumulative incidence of mortality for invasive versus conservative-strategy.
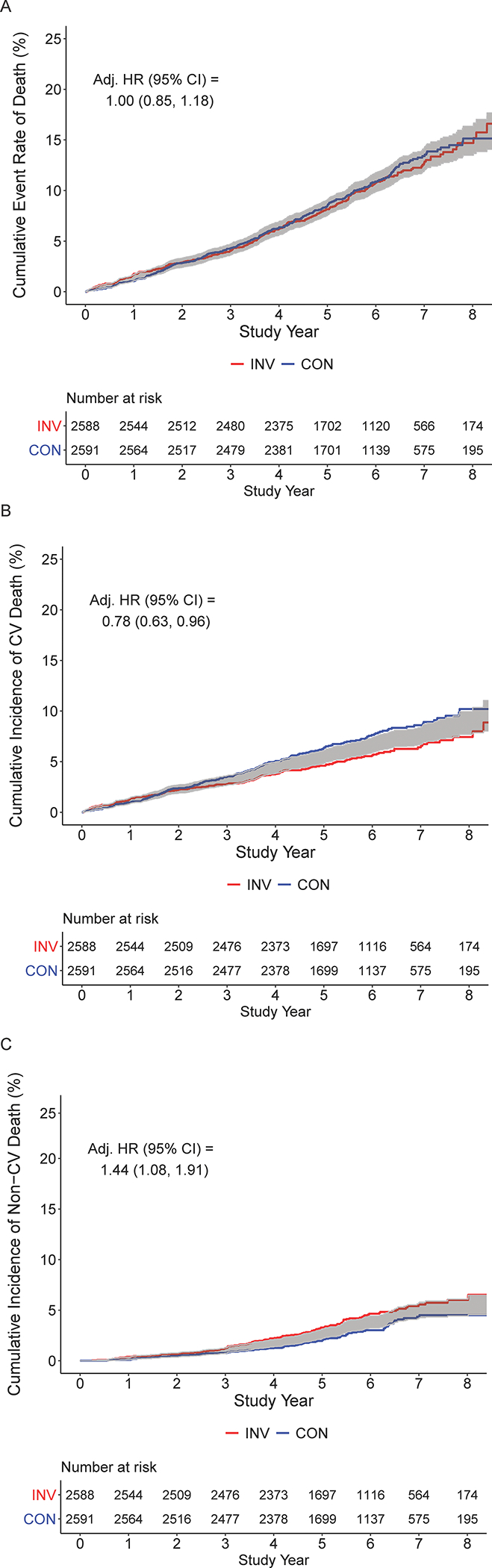
A. All-cause mortality cumulative event rate by initial randomized assignment to invasive (INV, red) vs conservative (CON, blue) management strategy. The adjusted HR is 1.00 (95% CI: 0.85, 1.18). Numbers at risk for each group are below the x-axis. Shading indicates the half width of the 95% confidence interval for the difference. Lack of overlap between the lines and shading indicates that the 95% CI for the difference excludes zero. B. Cumulative incidence function for cardiovascular mortality by initial randomized assignment to invasive (red) vs conservative (blue) management strategy (accounting for competing risks.) The adjusted HR is 0.78 (95% CI: 0.63, 0.96). For countries collecting cause of death data, cases with undetermined cause of death are included as cardiovascular death, as was prespecified in the trial cardiovascular death definition. In one country, where cause of death data are not available after the end of the trial phase on June 30, 2019, twenty-two deaths post-June 30, 2019 were censored as of June 30, 2019. Numbers at risk for each group are below the horizontal axis. Shading indicates the half width of the 95% confidence interval for the difference. Lack of overlap between the lines and shading indicates that the 95% CI for the difference excludes zero. C. Cumulative incidence function for non-cardiovascular mortality by initial randomized assignment to invasive (red) vs conservative (blue) management strategy, accounting for competing risks. The adjusted HR is 1.44 (95% CI: 1.08, 1.91). For countries collecting cause of death data, unknown cause of death are included as cardiovascular death as was prespecified in the trial definition. In one country where cause of death data are not available after the end of the trial phase on June 30, 2019, twenty-two deaths post-June 30, 2019 were censored as of June 30, 2019. Numbers at risk for each group are below the horizontal axis. Shading indicates the half width of the 95% confidence interval for the difference. Lack of overlap between the lines and shading indicates that the 95% CI for the difference excludes zero.
Table 1.
Effect of an invasive versus conservative-strategy on all-cause mortality, cardiovascular mortality, and non-cardiovascular mortality.
| INV | CON | Estimated % Difference for INV versus CON* (95% CI) | Adjusted Hazard Ratio† (95% CI) | |
|---|---|---|---|---|
| All-cause mortality | 1.00 (0.85, 1.18) | |||
| Number of participants with event | 274 | 283 | ||
| 1-year cumulative event rate | 1.70% | 1.04% | 0.66% (0.02%, 1.29%) | |
| 2-year cumulative event rate | 2.90% | 2.86% | 0.04% (-0.87%, 0.95%) | |
| 3-year cumulative event rate | 4.06% | 4.28% | −0.23% (−1.31%, 0.86%) | |
| 4-year cumulative event rate | 6.19% | 6.29% | −0.11% (−1.42%, 1.21%) | |
| 5-year cumulative event rate | 8.15% | 8.48% | −0.33% (−1.87%, 1.21%) | |
| 6-year cumulative event rate | 10.78% | 10.88% | −0.10% (−1.96%, 1.76%) | |
| 7-year cumulative event rate | 12.70% | 13.40% | −0.70% (−2.95%, 1.56%) | |
| Cardiovascular mortality | 0.78 (0.63, 0.96) | |||
| Number of participants with event | 147 | 196 | ||
| 1-year cumulative incidence | 1.31% | 0.96% | 0.35% (−0.23%, 0.93%) | |
| 2-year cumulative incidence | 2.24% | 2.39% | −0.15% (−0.97%, 0.67%) | |
| 3-year cumulative incidence | 2.78% | 3.51% | −0.73% (−1.68%, 0.22%) | |
| 4-year cumulative incidence | 3.75% | 5.02% | −1.27% (−2.38%, −0.15%) | |
| 5-year cumulative incidence | 4.60% | 6.35% | −1.74% (−3.01%, −0.48%) | |
| 6-year cumulative incidence | 5.62% | 7.64% | −2.02% (−3.48%, −0.56%) | |
| 7-year cumulative incidence | 6.41% | 8.60% | −2.19% (−3.85%, −0.53%) | |
| Non-cardiovascular mortality | 1.44 (1.08, 1.91) | |||
| Number of participants with event | 112 | 80 | ||
| 1-year cumulative incidence | 0.39% | 0.08% | 0.31% (0.05%, 0.57%) | |
| 2-year cumulative incidence | 0.66% | 0.46% | 0.19% (−0.21%, 0.60%) | |
| 3-year cumulative incidence | 1.24% | 0.77% | 0.47% (−0.08%, 1.01%) | |
| 4-year cumulative incidence | 2.21% | 1.24% | 0.97% (0.26%, 1.68%) | |
| 5-year cumulative incidence | 3.29% | 1.96% | 1.32% (0.42%, 2.23%) | |
| 6-year cumulative incidence | 4.65% | 3.02% | 1.63% (0.46%, 2.81%) | |
| 7-year cumulative incidence | 5.56% | 4.36% | 1.20% (−0.32%, 2.72%) |
CON = conservative strategy; CI = confidence interval; cardiovascular = cardiovascular; INV = invasive strategy
For mortality, we estimated the K-M based event rate at each time point. For the competing events of cardiovascular and non-cardiovascular mortality, we used a non-parametric cumulative incidence function to estimate the cumulative incidence at each time point.
Adjusted hazard ratios were estimated from separate multivariable Cox proportional hazards regression models for each outcome. Models were adjusted for prespecified participant baseline characteristics, namely, sex, age, diabetes, eGFR, and ejection fraction. For competing events cardiovascular and non-cardiovascular mortality, we estimated cause-specific hazard ratios.
The Bayesian posterior distributions of the adjusted absolute percent difference between the invasive versus conservative strategy in the cumulative all-cause mortality rate at 7 years, and the cumulative incidence of cardiovascular and non-cardiovascular mortality at 7 years are shown in Figure 3, with detailed posterior summaries presented in Table S2. The posterior mean adjusted absolute percent difference in the 7-year all-cause mortality rate was near null (absolute difference = 0.09% (95% credible interval (CrI): −1.85%, 1.99%). The cumulative incidence of cardiovascular mortality at 7 years was 1.70% lower in the invasive versus conservative strategy (95% credible interval (CrI): −3.14%, −0.26%). The posterior probability that the 7-year difference in the incidence of cardiovascular mortality favored the invasive strategy by at least 1% compared with the conservative strategy was 82%. In contrast, the cumulative incidence of non-cardiovascular mortality at 7 years was an estimated 1.65% higher in the invasive versus conservative strategy (95% CrI: 0.37%, 2.82%). There was an 85% posterior probability that the 7-year difference in the incidence of non-cardiovascular mortality favored the conservative strategy by at least 1%.
Figure 3. Probability that one strategy is better than another for 7-year all-cause mortality.
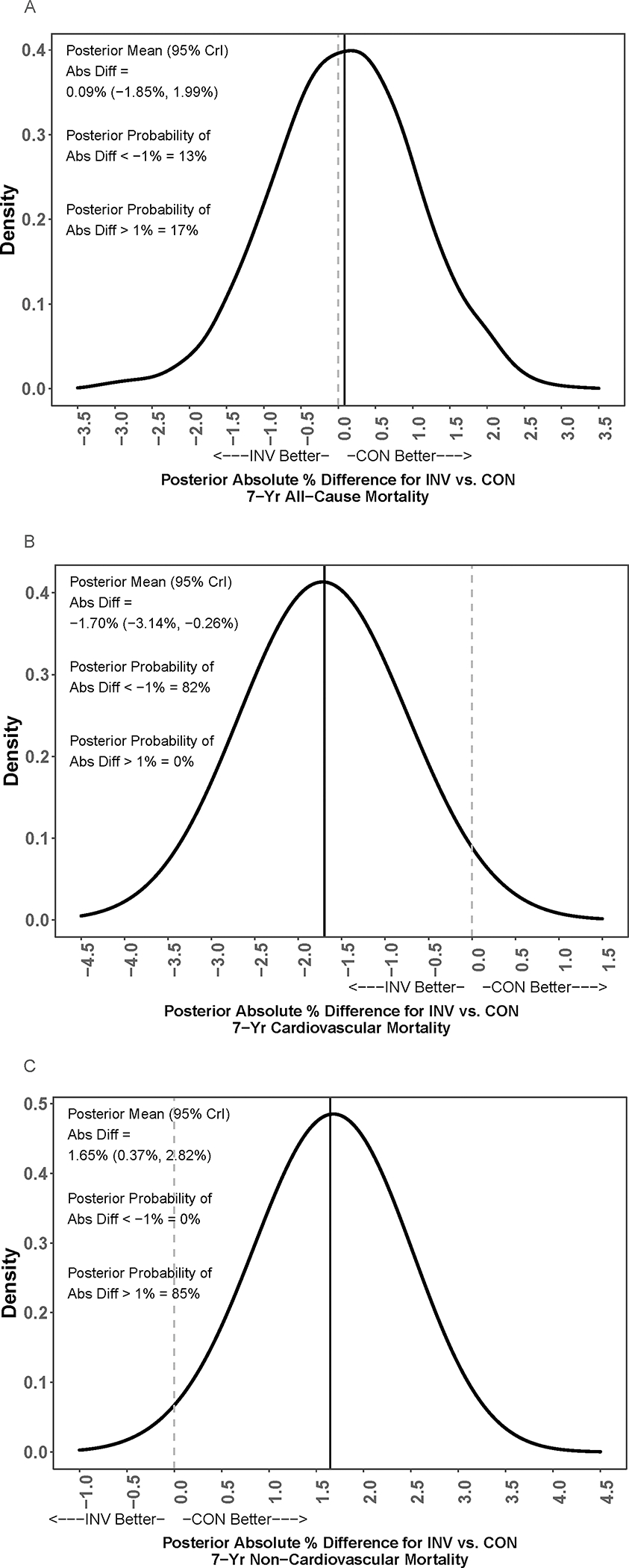
Posterior distribution of the adjusted absolute % difference (Abs Diff) in risk of mortality at 7 years for an invasive (INV) vs. conservative (CON) strategy. The gray dashed vertical bar is the null value indicating no difference. The solid black vertical bar is the posterior mean value of the difference. Positive values represent lower mortality for a conservative strategy, and negative values represent lower mortality for an invasive strategy. Panel A shows the posterior distribution of the adjusted absolute % difference (Abs Diff) in risk of all-cause mortality at 7 years for an invasive (INV) vs. conservative (CON) strategy. The solid line is close to the grey-dashed null value line indicating no difference between the groups. Panel B shows the posterior distribution of the adjusted absolute % difference (Abs Diff) in risk of cardiovascular mortality at 7 years for an invasive (INV) vs. conservative (CON) strategy. The concentration of values around −2 indicates a benefit to an invasive over conservative strategy by approximately 2 percentage points. In contrast, in Panel C for non-cardiovascular mortality, the posterior distribution of the adjusted absolute % difference (Abs Diff) in risk of non-cardiovascular mortality at 7 years for an invasive (INV) vs. conservative (CON) strategy shows a concentration of values around +2 indicates a benefit to a conservative over invasive strategy by approximately 2 percentage points.
SUBGROUP ANALYSES
Subgroup analyses for all-cause mortality, cardiovascular mortality and non-cardiovascular mortality are presented in Figures 4A, 4B, and 4C, respectively. After adjusting for baseline characteristics, there were no significant differences between management strategies. Baseline CCTA was performed in 3913 (76%) of the 5,179 randomized trial participants and was analyzable for multivessel disease (using the ≥70% stenosis definition) in 3047 (78%) (Table S3). Figure 5 shows the cumulative event rate for all-cause mortality and the cumulative incidence of cardiovascular and non-cardiovascular mortality for the invasive versus conservative strategy, stratified by whether or not multivessel disease was present using the ≥70% stenosis definition (Figure 5).
Figure 4. Forest plot for heterogeneity of treatment effect. A. All-cause mortality. B. Cardiovascular mortality. C. Non-cardiovascular mortality.
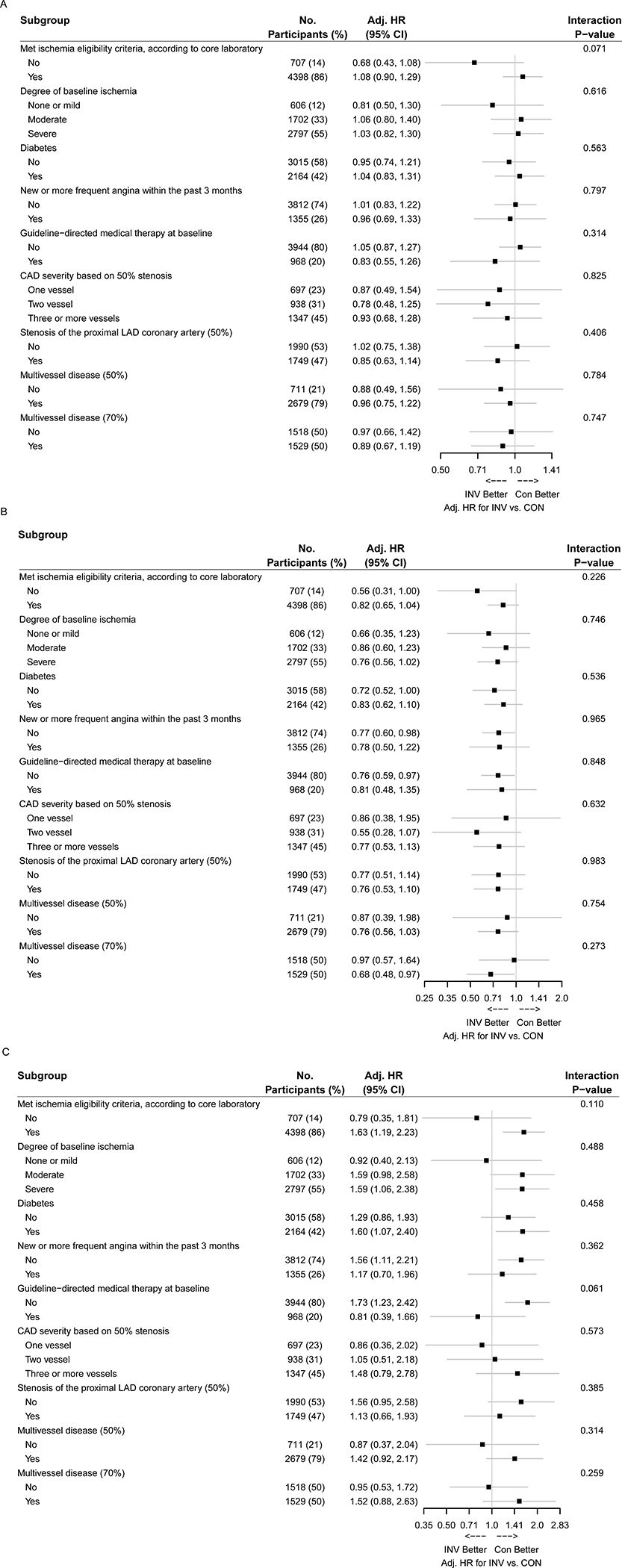
Adjusted hazard ratios and associated 95% confidence intervals for an invasive (INV) versus conservative (CON) strategy in prespecified subgroups are shown. The subgroup-specific treatment effects are adjusted for sex, age, diabetes status, eGFR, and ejection fraction. Denominators in a given subgroup may vary by data availability. MVD- multivessel disease; MVD (50, 70) indicates the stenosis threshold for determination of a diseased vessel was ≥50% or ≥70%, respectively. For CAD severity based on ≥50% stenosis, 4 participants with 0 vessel disease were excluded from the analysis.
Figure 5. All-cause, cardiovascular, and non-cardiovascular mortality among participants by presence of multivessel disease (N=3,047).
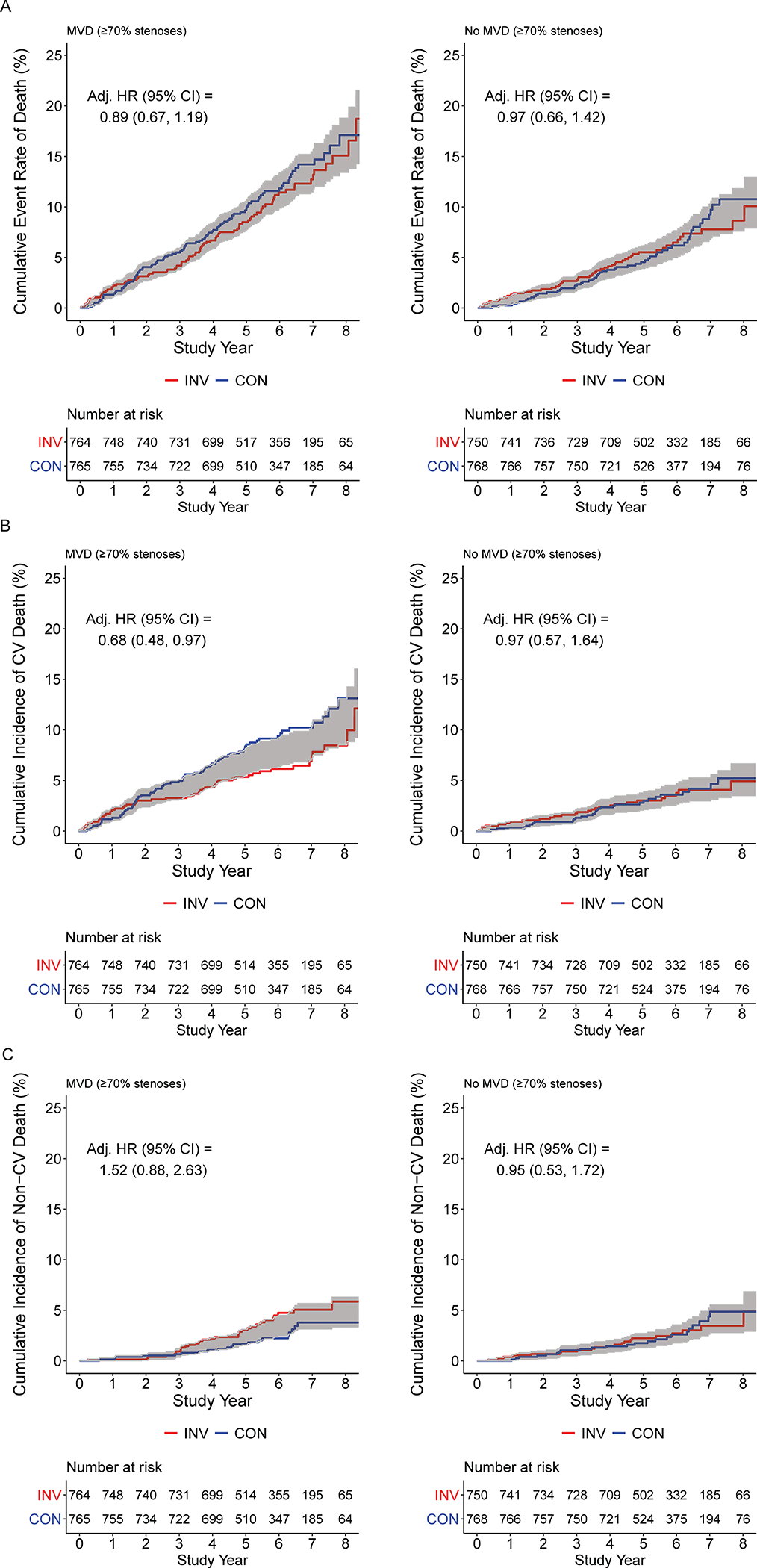
For each of all-cause, cardiovascular, and non-cardiovascular, the p values for interaction between the presence or absence of multivessel disease and treatment assignment were >0.05 (see Figure 4). A. Cumulative all-cause mortality rate for participants with CCTA data evaluable for multivessel disease (MVD) (≥70% stenosis) by initial randomized assignment to invasive (INV, red) vs conservative (CON, blue) management strategy, stratified by participants with MVD on CCTA (Panel A) and those without MVD on CCTA (Panel B). B. Cumulative incidence of cardiovascular mortality for participants with CCTA data evaluable for multivessel disease (MVD) (≥70% stenosis) by initial randomized assignment to invasive (red) vs conservative (blue) management strategy, stratified by participants with MVD on CCTA (Panel A) and those without MVD on CCTA (Panel B). C. Cumulative incidence of non-cardiovascular mortality for participants with CCTA data evaluable for multivessel disease (MVD) (≥70% stenosis) by initial randomized assignment to invasive (red) vs conservative (blue) management strategy, stratified by participants with MVD on CCTA (Panel A) and those without MVD on CCTA (Panel B).
SENSITIVITY ANALYSES
Over the trial phase and extended follow-up, there are 267 CV deaths; 192 non-CV deaths; and 98 undetermined deaths. This totals 557 deaths. Of the 98 undetermined deaths, 60 occurred during the extended follow-up period. Of the 60 undetermined deaths occurring during the extended follow-up period, 22 were from the country that could not provide cause of death. We conducted sensitivity analyses with respect to the classification on new undetermined deaths from extended follow-up as cardiovascular deaths (Table S4, cardiovascular mortality, Table S5, non-cardiovascular mortality). In the analyses of cardiovascular and non-cardiovascular mortality, the estimated hazard ratio for an invasive versus conservative strategy was robust to grouping new undetermined deaths as non-cardiovascular deaths, including grouping deaths from the one country without cause of death data available during extended follow-up.
For all-cause, cardiovascular, and non-cardiovascular mortality, Tables S6–S8 (respectively) compare the estimated effect of treatment strategy based on the proportional hazards assumption with non-proportional hazards specifications where the treatment effect is allowed to vary according to time intervals over the follow-up period. Estimated treatment effects do not appear sensitive to the proportional hazards assumption.
DISCUSSION
In this interim report of the extended follow-up of the ISCHEMIA trial, there was no difference in all-cause mortality out to 7 years, but there was a lower risk of 7-year cardiovascular mortality and a higher risk of non-cardiovascular mortality with the initial invasive strategy as compared with the initial conservative strategy. Because these two mortality patterns were of approximately equal magnitude, all-cause mortality rates showed no net treatment difference. This interim report of extended follow-up of participants adds almost twice as many deaths compared with that reported in the trial phase. Although not well powered for all-cause mortality, these additional deaths afford greater precision around the estimated adjusted hazard ratio for invasive vs conservative management strategies (hazard ratio of 1.00 with 95% confidence intervals from 0.85 to 1.18). Using Bayesian analysis there was a 82% probability that an invasive strategy is superior to a conservative strategy by at least 1 absolute percentage point for cardiovascular mortality, and a 85% probability that a conservative strategy is superior by at least 1 absolute percentage point for non-cardiovascular mortality. The probability of near 50% for either a survival benefit with an invasive strategy or a conservative strategy suggests that there is no clinically meaningful difference in 7-year all-cause mortality between groups.
The incremental value of an initial invasive strategy was tested in the context of the population randomized, the procedures performed and the use of guideline-directed medical therapy.16 The strategy did not test routine revascularization for those with angiographic findings suitable for revascularization; rather, we tested routine cardiac catheterization compared with selective use of catheterization and revascularization based on clinical need, e.g., acute coronary syndrome or refractory angina. During the trial phase there was greater use of revascularization in the invasive strategy group (mean 0.9 procedures per invasive strategy participant and 0.3 per conservative strategy participant),1 consistent with the trial randomization. We did not collect information on revascularization during the extended follow-up phase. The 4-year cumulative rate of revascularization in the conservative group was 23%.1 Dual antiplatelet therapy use was higher in the invasive strategy group throughout the trial phase.
Our results are consistent with prior randomized trials of revascularization versus medical therapy alone, which have reported similar rates of all-cause mortality between groups.17–22 A meta-analysis of such randomized trials, including the initial trial phase of ISCHEMIA, has also reported similar all-cause mortality between groups (odds ratio 0.99; 95% CI 0.90–1.09).23 When considering cardiovascular mortality, it has previously been suggested that longer term follow-up24 will demonstrate a benefit of revascularization on all-cause mortality. Although not powered for all-cause mortality, the current analysis shows an effect size similar to the above meta-analysis with a HR of 1.00 and 95% confidence intervals from 0.85 to 1.18. We estimated a probability of 13% that there was at least a 1% absolute percentage-point difference in all-cause mortality at 7 years in favor of an invasive strategy, and a 17% probability of at least a 1% advantage in all-cause mortality in favor of a conservative strategy.
Accrual of additional deaths during extended follow-up allowed us to detect a lower rate of cardiovascular death with the invasive strategy. This is consistent with a prior meta-analysis reporting a 21% reduction in the odds of cardiovascular mortality associated with an invasive strategy whether ISCHEMIA was included (OR=0.79; 95% CI 0.67–0.93) or not.24 The trial phase demonstrated an excess of peri-procedural MI events and a reduction in spontaneous MI events with the invasive strategy.1, 2 Because spontaneous MI in this trial and other studies has been associated with greater risk of subsequent death compared with peri-procedural MI, we postulated that these differences in the rates and impact of MI during the trial would translate to reduction in long-term all-cause and cardiovascular mortality.2 We observed lower cardiovascular mortality with the invasive strategy, but that benefit was offset by higher non-cardiovascular mortality of approximately equal magnitude in with the invasive strategy resulting in no difference in all-cause mortality.
The higher rate of non-cardiovascular death in the invasive group was unexpected, and remains unexplained. The low rate of all-cause death makes it unlikely that the observed excess risk of non-CV death among INV participants is explained by the phenomenon of competing risks; the rate of CV death would have to be substantially higher to explain the apparent observed difference in non-CV death between the two treatment groups based on competing risks alone. Common causes of non-CV death in ISCHEMIA and other studies of CCD are typically cancer and infection. 3, 25 We previously reported that non-CV death was higher in INV and that there were more deaths from malignancy in the INV group despite equal baseline prevalence of cancer in the two groups. There was a significant association between the number of procedures with radiation exposure (i.e., stress nuclear test, CCTA, cardiac catheterization and PCI) and death from malignancy. The higher use of DAPT in the invasive arm of ISCHEMIA was not associated with a higher rate of incident malignancy during the trial.3 As noted previously, the timing of the association between radiation exposure, new malignancy, and malignancy-related death does not seem biologically plausible as the cause of an increase in non-cardiovascular death because the latency period between radiation damage to a clinically diagnosable cancer and death is expected to be much longer than our trial follow-up period.
Cause of death is not being centrally adjudicated during extended follow-up. However, during the trial phase when all deaths were centrally adjudicated for cause, the sensitivity of site-determined trial-defined cardiovascular death was 91% and when the site reported death as cardiovascular, it was confirmed as cardiovascular by the events committee in 96%.6 Determination of cause of death is inherently limited based on variation in the amount of information available from case to case, as well as intrinsic uncertainties about causal mechanisms of death in relation to chronic coronary disease and comorbidity.
The key finding remains that with 557 deaths, all-cause mortality was not different between groups. ISCHEMIA-EXTEND will continue to follow surviving participants into 2025 for a projected median of approximately 10 years to increase the precision around these mortality estimates. We note the absence of significant interaction on outcomes between the presence or absence of multivessel coronary artery disease based on CCTA and the randomized initial strategy. This subgroup was selected for analysis based on our prior publication demonstrating that coronary artery disease severity was strongly associated with mortality. A more detailed subgroup analysis related to ischemia severity was not performed because of its previously demonstrated lack of increased risk after adjustment for CAD severity.4
Studies of patient preferences demonstrate that quality-of-life, functional status and survival rank highly.26 We have previously shown that quality-of-life was improved with an initial invasive strategy and the extent of benefit was related to the degree of angina on a medically tolerated regimen.27 Those without angina did not experience quality-of-life benefits. We believe the data from this interim follow-up report demonstrating no difference in survival between groups at 7 years will add to the evidence base for shared decision-making between patients and their physicians.
LIMITATIONS
ISCHEMIA trial tested two commonly used clinical management strategies – invasive vs conservative – and did not test revascularization vs no revascularization. ISCHEMIA-EXTEND was designed as a pragmatic long-term follow-up study of mortality, with limited data collection. Therefore, no data were collected on non-fatal events, use of medications or revascularization procedures, angina burden, or quality of life after the initial median 3.2-year follow-up. The cause of death (cardiovascular vs. non-cardiovascular) was adjudicated during the trial phase but not during the extended phase.
CONCLUSIONS
An initial invasive strategy of cardiac catheterization and revascularization, when feasible, added to guideline-directed medical therapy resulted in no difference in all-cause mortality, but a lower risk of cardiovascular mortality and a higher risk of non-cardiovascular mortality as compared with an initial conservative strategy with catheterization and revascularization reserved for failure of medical therapy in patients with moderate or severe ischemia during a median follow-up of 5.7 years.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
An initial invasive versus an initial conservative management strategy for patients with chronic coronary disease and moderate or severe ischemia resulted in lower cardiovascular mortality at median 5.7 years.
The previously observed excess of non-cardiovascular mortality with initial invasive strategy persisted.
In this interim report of extended follow-up of ISCHEMIA, with a total of 557 deaths (nearly twice the number of deaths in the initial phase), the probability of a survival benefit at 7 years with either initial management strategy was not different.
What are the clinical implications?
These findings provide important evidence for patients with chronic coronary disease and their physicians as they decide whether to add invasive management to guideline-directed medical therapy.
Acknowlegements
The authors are indebted to the site investigators and to the participants who made this study possible. We thank Anna Naumova for her expert editorial assistance in the preparation of this report. Disclaimer: The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the United States Department of Health and Human Services.
Disclosures
Judith S. Hochman is PI for the ISCHEMIA trial for which, in addition to support by National Heart, Lung, and Blood Institute grant, devices and medications were provided by Abbott Vascular; Medtronic, Inc.; St. Jude Medical, Inc.; Volcano Corporation; Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Merck Sharp & Dohme Corp.; Omron Healthcare, Inc.; and financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP. She is PI for ISCHEMIA-EXTEND.
Rebecca Anthopolos: none.
Harmony R. Reynolds reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study, she receives support from Abbott Vascular (donation of optical coherence tomography catheters for an unrelated research study) and Biotelemetry Inc (donation of telemetry monitors for an unrelated research study)
Sripal Bangalore reports receiving research grant from NHLBI and Abbott Vascular and advisory board from Abbott Vascular, Pfizer, Amgen, Biotronik, Meril and Reata.
Yifan Xu reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Sean O’Brien reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Stavroula Mavromichalis: none.
Michelle Chang: none.
Aira Contreras: none.
Yves Rosenberg: none.
Ruth Kirby: none.
Balram Bhargava reports grants from National Heart, Lung and Blood Institute, during the conduct of the study.
Roxy Senior reports grants from National Heart, Lung and Blood Institute, during the conduct of the study.
Ann Banfield: none.
Shaun G. Goodman reports receiving research grant and salary support, and speaker/consulting honoraria from: Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Daiichi-Sankyo, Eli Lilly, Fenix Group International, Ferring Pharmaceuticals, GlaxoSmithKline, Janssen/Johnson & Johnson, Luitpold Pharmaceuticals, Matrizyme, Merck, Novartis, Pfizer, Regeneron, Sanofi, Servier, Tenax Therapeutics; Heart and Stroke Foundation of Ontario/University of Toronto, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Duke Clinical Research Institute, PERFUS.
Renato D. Lopes reports grants from National Heart, Lung and Blood Institute, during the conduct of the study; other from Bayer, other from Boehringer Ingleheim, grants and other from Bristol-Myers Squibb, other from Daiichi Sankyo, grants and other from Glaxo Smith Kline, grants and other from Medtronic, other from Merck, grants and other from Pfizer, other from Portola, grants and other from Sanofi, outside the submitted work.
Radoslaw Pracon reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
José López-Sendón reports grants from National Heart, Lung and Blood Institute, during the conduct of the study; other from Bayer, other from Boehringer Ingleheim, grants and other from Bristol-Myers Squibb, other from Daiichi Sankyo, grants and other from Glaxo Smith Kline, grants and other from Medtronic, other from Merck, grants and other from Pfizer, other from Portola, grants and other from Sanofi, outside the submitted work.
Aldo Pietro Maggioni reports grants from the National Heart, Lung, and Blood Institute during the conduct of the study; and personal fees from Bayer, Fresenius, and Novartis, outside the submitted work.
Jonathan D. Newman reports receiving funding from the National Heart, Lung, and Blood Institute.
Jeffery S. Berger reports grants from National Heart, Lung and Blood Institute during the conduct of the study.
Mandeep S. Sidhu reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; personal fees from Astra Zeneca, personal fees from Sanofi-Regeneron, outside the submitted work.
Harvey D. White reports grants from National Heart, Lung and Blood Institute during the conduct of the study; reports receiving grant support paid to the institution and fees for serving on a steering committee for the ODYSSEY OUTCOMES trial (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) from Sanofi-Aventis and Regeneron Pharmaceuticals, for the ACCELERATE study (A Study of Evacetrapib in High-Risk Vascular Disease) from Eli Lilly, for the STRENGTH trial (Outcomes Study to Assess Statin Residual Risk Reduction With EpaNova in High CV Risk Patients With Hypertriglyceridemia) from Omthera Pharmaceuticals, for the HEART-FID study (Randomized Placebo-Controlled Trial of FCM as Treatment for Heart Failure With Iron Deficiency) from American Regent; for the CAMELLIA-TIMI study (A Study to Evaluate the Effect of Long-term Treatment With BELVIQ [Lorcaserin HC] on the Incidence of Major Adverse Cardiovascular Events and Conversion to Type 2 Diabetes Mellitus in Obese and Overweight Subjects With Cardiovascular Disease or Multiple Cardiovascular Risk Factors) from Eisai Inc, for the dal-GenE study (Effect of Dalcetrapib vs Placebo on CV Risk in a Genetically Defined Population With a Recent ACS) from DalCor Pharma UK Inc, for the AEGIS-II study from CSL Behring, for the SCORED trial (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk) and the SOLOIST-WHF trial (Effect of Sotagliflozin on Cardiovascular Events in Patients With Type2 Diabetes Post Worsening Heart Failure) from Sanofi-Aventis Australia Pty Ltd, and for the CLEAR Outcomes Study (Evaluation of Major Cardiovascular Events in Patients With, or at High Risk for, Cardiovascular Disease Who Are Statin Intolerant Treated With Bempedoic Acid. [ETC-1002] or Placebo) from Esperion Therapeutics Inc. He was on the Advisory Board for Genentech, Inc. and received lecture fees from AstraZeneca.
Andrea B. Troxel: none.
Robert A. Harrington: none.
William E. Boden reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants from Abbvie, grants from Amarin, grants from Amgen, personal fees from Amgen, personal fees from Cleveland Clinic Clinical Coordinating Center, personal fees from Janssen, outside the submitted work.
Gregg W. Stone has received speaker honoraria from Medtronic, Pulnovo, Infraredx; has served as a consultant to Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Abiomed, Ancora, Elucid Bio, Occlutech, CorFlow, Apollo Therapeutics, Impulse Dynamics, Cardiomech, Gore, Amgen, Adona Medical, Millennia Biopharma; and has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, Xenter. Dr. Stone’s daughter is an employee at Medtronic. Institutional disclosure: Dr. Stone’s employer, Mount Sinai Hospital, receives research support from Abbott, Abiomed, Bioventrix, Cardiovascular Systems Inc, Phillips, Biosense-Webster, Shockwave, Vascular Dynamics, Pulnovo and V-wave.
Daniel B. Mark: none.
John A. Spertus reports grants from NHLBI, Abbott Vascular, Janssen, Bristol Meyers Squibb, American College of Cardiology Foundation, Professional Consulting fees from Bayer, Merck, Janssen, Bristol Meyers Squibb, AstraZeneca, Terumo, Ionis Pharmaceuticals and United Healthcare, Board of Directors for Blue Cross Blue Shield of Kansas City Intellectual Property with Licenses Paid by Seattle Angina Questionnaire, Kansas City Cardiomyopathy Questionnaire and Peripheral Artery Questionnaire.
David J. Maron reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study.
Sources of Funding:
NIH grant R01HL149888. This project was supported in part by Clinical Translational Science Award No. 11UL1 TR001445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences, the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Department of Health and Human Services.
Nonstandard Abbreviations and Acronyms:
- BP
Blood Pressure
- CAD
Coronary artery disease
- CCTA
Coronary computed tomography angiography
- CON
Conservative management strategy
- eGFR
Estimated glomerular filtration rate
- INV
Invasive management strategy
- LDL-C
Low-density lipoprotein cholesterol
- MI
Myocardial infarction
- MVD
Multivessel disease
APPENDIX
ISCHEMIA-EXTEND Research Group
Clinical Coordinating Center Faculty and Staff - NYU Grossman School of Medicine:
Judith S. Hochman, MD, David J. Maron, MD, Harmony R. Reynolds, MD, Sripal Bangalore, MD, MHA, Stavroula Mavromichalis, MS, Michelle Chang, MPH, Aira Contreras, MA, Shari Esquenazi-Karonika, PhD(c), MPH, MS, Margaret Gilsenan, Ewelina Gwiszcz, Patenne Mathews, MPH, Samaa Mohamed, Anna Naumova, MA, Arline Roberts, RN, Kerrie VanLoo
Statistical and Data Coordinating Center (SDCC):
NYU Grossman School of Medicine:
Rebecca Anthopolos, DrPH, Yifan Xu, MPH, Andrea B. Troxel, ScD, Ying Lu, MS
Duke Clinical Research Institute (DCRI):
Zhen Huang, MS, Samuel Broderick, MS
Academic Research Organizations (AROs)/Country Leaders/Country Coordinators:
Argentina: Luis Guzmán, MD, PhD
Australia: Joseph Selvanayagam, MBBS, DPhil
Brazil (BCRI): Renato D. Lopes, MD, PhD
Canada: Shaun G. Goodman, MD, MSc
France: Gabriel Steg, MD, Jean-Michel Juliard, MD
Germany: Rolf Doerr, MD
Hungary: Matyas Keltai, MD, PhD, MSc
India: Balram Bhargava, MD, MSc, (Independent Consultant: Boban Thomas, MD)
Israel: Tali Sharir, MD, Eugenia Nikolsky, MD, PhD
Italy (ANMCO): Aldo P. Maggioni, MD
Japan: Shun Kohsaka, MD
Mexico: Jorge Escobedo, MD, MSc
New Zealand: Harvey D. White, DSc
Poland: Radosław Pracoń, MD, PhD
Russia: Olga Bockeria, MD, PhD
Spain (FIBHULP): José López-Sendón, MD
Sweden: Claes Held, MD, PhD
United Kingdom: Roxy Senior, MD, DM, Ann Banfield, RGN, BSc
Core Laboratories Directors and Readers:
Leslee J. Shaw, PhD, Lawrence Phillips, MD, Daniel Berman, MD, Raymond Y. Kwong, MD, MPH, Michael H. Picard, MD, Bernard R. Chaitman, MD, Ziad Ali, MD, DPhil, James Min, MD, G B John Mancini, MD, Jonathon Leipsic, MD
Site principal investigators conducting extended follow-up and/or site investigators who made a substantial contribution to the initial trial phase:
Argentina
Instituto Medico DAMIC: Luis Guzmán, MD, PhD
Australia
Royal Perth Hospital: Graham Hillis, MBChB
John Hunter Hospital: Suku Thambar, MD
Flinders Medical Centre: Majo Joseph, MD
Flinders Medical Centre: Joseph Selvanayagam, MBBS, DPhil
Queen Elizabeth Hospital: John Beltrame, BMBS, PhD
Austria
University of Vienna Allgemeines Krankenhaus: Irene Lang, MD, PhD
LKH Graz West Austria: Herwig Schuchlenz, MD
Wilhelminen Hospital Vienna: Kurt Huber, MD
Belgium
University Hospital Gasthuisberg: Kaatje Goetschalckx, MD
Brazil
Heart Instituto do Coração - University of São Paulo: Whady Hueb, MD, PhD
Hospital São Lucas da Pontificia Universidade Católica do Rio Grande do Sol: Paulo Ricardo Caramori, MD
Instituto de Cardiologia de Porto Alegre: Alexandre de Quadros, MD
Instituto Dante Pazzanese de Cardiologia: Paola Smanio, MD
Hospital Pró Cardiaco: Claudio Mesquita, MD
University Federal Hospital of São Paulo: Renato D. Lopes, MD, PhD
Quanta Diagnóstico e Terapia: João Vitola, MD, PhD
Hospital das Clinicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo: José Marin-Neto, MD, PhD
Hospital Samaritano Paulista: Expedito Ribeiro da Silva, MD, PhD
Hospital São Vicente de Paulo: Rogério Tumelero, MD
Hospital Da Bahia: Marianna Andrade, PhD
Fundação Bahiana de Cardiologia: Alvaro Rabelo Alves Junior, MD
Hospital Maternidade e Pronto Socorro Santa Lúcia: Frederico Dall’Orto, PhD
Hospital de Clinicas de Porto Alegre: Carisi Polanczyk, PhD
Hospital Vera Cruz: Estevão Figueiredo, MD
Canada
Foothills Medical Centre: Andrew Howarth, MD
Montreal Heart Institute: Gilbert Gosselin, MD
Saint Michael’s Hospital: Asim Cheema, MD, PhD
University of Alberta Hospital: Kevin Bainey, MD, MSc
CSSS du Sud de Lanaudière: Denis Phaneuf, MD
Centre Hospitalier Régional de Trois-Rivières: Ariel Diaz, MSc, MD
University Hospital: Pallav Garg, MBBS, MSc
Hamilton General Hospital: Shamir Mehta, MD, MSc
Vancouver General Hospital: Graham Wong, MD MPH
West Lincoln Memorial Hospital: Andy Lam, MD
James Cha, MD
Corcare Cardiovascular Research: Paul Galiwango, MD
University Health Network: Amar Uxa, MD
University of Ottawa Heart Institute: Benjamin (Ben) Chow, MD
Saint Catharines General Hospital: Adnan Hameed, MD
Women’s College Hospital: Jacob Udell, MD, MPH
Dixie Medical Group: Asim Cheema, MD, PhD
Egypt
Cairo University Faculty of Medicine: Magdy Hamid, MD
France
Ambroise Paré Hospital: Marie Hauguel-Moreau, MD
Centre Hospitalier Universitaire d’Angeres: Alain Furber, MD
Centre Hospitalier Sud Francilien: Pascal Goube, MD
Bichat Hospital: Philippe-Gabriel Steg, MD
Grenoble University Hospital: Gilles Barone-Rochette, MD, PhD
C.H. Louis Pasteur: Christophe Thuaire, MD
Bichat Hospital: Michel Slama, MD
Germany
Praxisklinik Herz Und Gefaesse: Rolf Doerr, MD
Universitätsklinikum Bonn: Georg Nickenig, MD
Robert Bosch Hospital: Raffi Bekeredjian, MD
University Hospital Jena: P. Christian Schulze, MD, PhD
Hungary
Semmelweis University: Bela Merkely, MD, PhD, MSc, DSc
George Gottsegen National Institute of Cardiology: Geza Fontos, MD
Szent Istvan Hospital: András Vértes, PhD
University of Szeged: Albert Varga, MD, DSc
India
All India Institute of Medical Sciences: Balram Bhargava, MD, DM
Sree Chitra Tirunal Institute for Medical Sciences and Technology: Ajit Kumar, PhD
Government Medical College: Rajesh G Nair, MsC
Ruby Hall Clinic: Purvez Grant, MD
Sri Jayadeva Institute of Cardiovascular Sciences and Research, Bangalore-Karnataka: Cholenahally Manjunath, MD, Nagaraja Moorthy, MD
Jawaharlal Institute of Postgraduate Medical Education and Research: Santhosh Satheesh, MD, DM
Ram Manohar Lohia Hospital: Ranjit Kumar Nath, MD, DM
Hero DMC Heart Institute Unit Dayanand Medical: Gurpreet Wander, DM
Gurunanak CARE Hospital: Johann Christopher, MD, PhD
King George’s Medical University: Sudhanshu Dwivedi, MD, DM
Apollo Hospitals: Abraham Oomman, DM, MD
Fortis Escorts Heart Institute: Atul Mathur, PhD
KEM Hospital: Milind Gadkari, MD, MRCP
Apollo Health City Campus: Sudhir Naik, MD
MOSC Medical College Hospital, Kolenchery: Eapen Punnoose, MD, DM
Fortis Healthcare Fl.t Lt. Rajan Dhall Hospital: Ranjan Kachru, MD
CARE Hospital, CARE Nampally: Johann Christopher, MBBS, MD, DNB
Batra Hospital and Medical Research Center: Upendra Kaul, MD, DM
Israel
Assuta Medical Centers: Tali Sharir, MD
Rambam Medical Center: Arthur Kerner, MD
Italy
University of Padua: Giuseppe Tarantini, PhD
Ospedali Riuniti of Ancona: Gian Piero Perna, MD
Azienda Ospedaliera Santa Croce e Carle: Emanuela Racca, PhD
Policlinico di Monza: Andrea Mortara, MD
Istituto Clinico Humanitas: Lorenzo Monti, MSc
Clinica Mediterranea: Carlo Briguori, MD, PhD
Osepdale Regionale Umberto Parini: Gianpiero Leone, MD
UO Cardiologia Ospedale SS Cosma e Damiano: Roberto Amati, MD
Ospedale Casa Sollievo della Sofferenza: Mauro Salvatori, MD
Ospedale Civile S. Antonio Abate: Antonio Di Chiara, MD
Second University of Naples - Monaldi Hospital: Paolo Calabro, MD, PhD
Ospedale GB Morgagni L. Pierantoni di Forli: Marcello Galvani, MD
Ospedale di Circolo e Fondazione Macchi: Stefano Provasoli, MD
Japan
Keio University Hospital: Keiichi Fukuda, MD, PhD, Shun Kohsaka, MD
Saitama Medical University: Shintaro Nakano, MS
Lithuania
Vilnius University Santaros Klinikos: Aleksandras Laucevicius, MD
Macedonia
University Clinic of Cardiology: Sasko Kedev, MD, PhD
Malaysia
Institut Jantung Negara: Ahmad Khairuddin, MD
Mexico
Instituto Mexicano del Seguro Social: Jorge Escobedo, MD
Netherlands
Cardio Research Hartcentrum OLVG: Robert Riezebos, MD, PhD
Isala Klinieken Weezenlanden: Jorik Timmer, MD, PhD
New Zealand
Waikato Hospital: Spencer Heald, MD
Auckland City Hospital: Ralph Stewart, MD
Peru
Instituto Neuro Cardiovascular de las Americas: Walter Mogrovejo Ramos, MD
Poland
Coronary and Structural Heart Diseases Department, Institute of Cardiology, Warsaw: Marcin Demkow, MD
Medical University of Warsaw: Tomasz Mazurek, MD, PhD
Cardiology Clinic, Medical University of Lodz: Jarozlaw Drozdz, MD
Institute of Cardiology, Warsaw: Hanna Szwed, MD, PhD
Department of Interventional Cardiology & Angiology, Institute of Cardiology: Adam Witkowski, MD, PhD
Portugal
Centro Hospitalar de Vila Nova de Gaia/Espinho, EPE: Nuno Ferreira, PhD
CHLN - Hospital Santa Maria: Fausto Pinto, MD, PhD
Hospital de Santa Marta: Ruben Ramos, MD
Romania
Emergency Institute of Cardiovascular Diseases: Bogdan Popescu, PhD
County Emergency Hospital Baia Mare: Calin Pop, MD, PhD
Russia
Bakulev Scientific Center for Cardiovascular Surgery: Leo Bockeria, MD, Olga Bockeria, MD, PhD
Almazov National Medical Research Centre: Elena Demchenko, MD, PhD
E. Meshalkin National Medical Research Center of the Ministry of Health of the Russian Federation: Alexander Romanov, MD
North Western State Medical University: Leonid Bershtein, MD, PhD
Saudi Arabia
King Abdullah International Medical Research Center (KAIMRC): Ahmed Jizeeri, MD
Serbia
Clinical Center of Serbia: Goran Stankovic, MD, PhD
Clinic for cardiovascular diseases, Clinical Center Nis: Svetlana Apostolovic, MD, PhD
Institute of Cardiovascular Diseases, Vojvodina - Sremska Kamenica: Nada Cemerlic Adjic, MD
University Hospital Center Bezanijska Kosa: Marija Zdravkovic, MD
Cardiology Clinic at Clinical Center of Serbia: Branko Beleslin, MD, PhD
University Clinical Hospital Zvezdara: Milica Dekleva, MD, PhD
Clinical Center Kragujevac: Goran Davidovic, MD
Singapore
National Heart Centre Singapore: Terrance Chua, MD
Tan Tock Seng Hospital: David Foo, MD
National University Heart Centre: Kian Keong Poh, MD
South Africa
Groote Schuur Hospital: Mpiko Ntsekhe, MD
Spain
Hospital de Sant Pau Barcelona: Alessandro Sionis, MD
Hospital Virgen de la Arrixaca: Francisco Marin, MSc
Hospital Universitario y Politecnico La Fe: Vicente Miró, MD
Hospital Universitario La Paz: José López-Sendón, MD
Hospital de Bellvitge: Montserrat Gracida Blancas, MD
Hospital Clinico Universitario de Santiago: José González-Juanatey, MD
Hospital General Universitario Gregorio Maranon: Francisco Fernández-Avilés, MD, PhD
Complejo Hospitalario Universitario A Coruña: Jesús Peteiro, MD
Hospital Universitario Miguel Servet: Jose Enrique Castillo Luena, MD
Sweden
Uppsala University: Claes Held, MD, PhD
Danderyds Hospital: Johannes Aspberg, MD
Switzerland
Cardiocentro Ticino: Mariagrazia Rossi, PhD
Thailand
Chiang Mai University Hospital: Srun Kuanprasert, MD
Ramathibodi Hospital: Sukit Yamwong, MD
United Kingdom
The Belfast Health and Social Care Trust: Nicola Johnston, BA, MS
Ulster Hospital: Patrick Donnelly, PhD
Craigavon Area Hospital: Andrew Moriarty, PhD
Northwick Park Hospital-Royal Brompton Hospital, London: Roxy Senior, MD, DM, Ahmed Elghamaz, MB, BCh, Sothinathan Gurunathan, MBChB. Nikolaos Karogiannis, MBBS. Benoy N Shah, MD, MBBS, BSc (Hons), Richard HJ Trimlett, MBBS, CCST, Michael B Rubens, LRCP, MRCS, MBBS, DMRD, Edward D Nicol, MD, BMedSci, MBBS, DTM&H, Tarun K Mittal, MD, Reinette Hampson, BSc (Hons), BA (Hons)
Broomfield Hospital NHS Trust: Reto Gamma, MBBS
The James Cook University Hospital: Mark De Belder, MD
Southend University Hospital NHS Trust: Thuraia Nageh, MD, MRCP
Bradford Royal Infirmary: Steven Lindsay, MD
United States
Atlanta V.A. Medical Center: Kreton Mavromatis, MD
Mayo Clinic: Todd Miller, MD
V.A. North Texas Health Care System: Subhash Banerjee, MD, FACC, FCSCAI
NYU Medical Center/Bellevue Hospital: Harmony Reynolds, MD
Henry Ford Health System: Khaled Nour, MD
Brigham and Women’s Hospital: Peter Stone, MD
Footnotes
Supplemental Material
ISCHEMIA-EXTEND Study Organization
Bayesian Modeling of the Effect of Management Strategy on All-Cause, Cardiovascular, and Non-Cardiovascular Mortality
Site Investigators
References
- 1.Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, Chaitman BR, Senior R, López-Sendón J, Alexander KP, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. New England Journal of Medicine. 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaitman BR, Alexander KP, Cyr DD, Berger JS, Reynolds HR, Bangalore S, Boden WE, Lopes RD, Demkow M, Piero Perna G, et al. Myocardial Infarction in the ISCHEMIA Trial: Impact of Different Definitions on Incidence, Prognosis, and Treatment Comparisons. Circulation. 2021;143:790–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sidhu MS, Alexander KP, Huang Z, O’Brien SM, Chaitman BR, Stone GW, Newman JD, Boden WE, Maggioni AP, Steg PG, et al. Causes of cardiovascular and noncardiovascular death in the ISCHEMIA trial. American Heart Journal. 2022;248:72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds HR, Shaw LJ, Min JK, Page CB, Berman DS, Chaitman BR, Picard MH, Kwong RY, O’Brien SM, Huang Z, et al. Outcomes in the ISCHEMIA Trial Based on Coronary Artery Disease and Ischemia Severity. Circulation. 2021;144:1024–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maron DJ, Hochman JS, O’Brien SM, Reynolds HR, Boden WE, Stone GW, Bangalore S, Spertus JA, Mark DB, Alexander KP, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. American Heart Journal. 2018;201:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anthopolos R, Maron DJ, Bangalore S, Reynolds HR, Xu Y, O’Brien SM, Troxel AB, Mavromichalis S, Chang M, Contreras A, et al. ISCHEMIA-EXTEND studies: Rationale and design. American Heart Journal. 2022;254:228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Medicines Agency. Guideline on adjustment for baseline covariates in clinical trials. EMA/CHMP/295050/2013. February 26, 2015. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-adjustment-baseline-covariates-clinical-trials_en.pdf.
- 8.Chow S-C and Liu J-P. Design and Analysis of Clinical Trials : Concepts and Methodologies, Chapter 12 Issues in Efficacy Evaluation. 3rd ed. Hoboken, N.J. John Wiley & Sons; 2013. [Google Scholar]
- 9.U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. COVID-19: Developing drugs and biological products for treatment or prevention. Guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/covid-19-developing-drugs-and-biological-products-treatment-or-prevention. 2020. Date Accessed 18 Oct. 2022. [Google Scholar]
- 10.U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. (E9 statistical principles for clinical trials. Guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e9-statistical-principles-clinical-trials. 1998. Date Accessed 18 Oct. 2022. [Google Scholar]
- 11.Benkeser D, Diaz I, Luedtke A, Segal J, Scharfstein D and Rosenblum M. Improving precision and power in randomized trials for COVID-19 treatments using covariate adjustment, for binary, ordinal, and time-to-event outcomes. Biometrics. 2021;77:1467–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim JG, Chen M-H and Sinha D. Bayesian survival analysis. New York: Springer; 2001. [Google Scholar]
- 13.Andrinopoulou ER, Rizopoulos D, Takkenberg JJ and Lesaffre E. Joint modeling of two longitudinal outcomes and competing risk data. Statistics in Medicine. 2014;33:3167–78. [DOI] [PubMed] [Google Scholar]
- 14.R Core Team. A language and environment for statistical computing. 2022. [Google Scholar]
- 15.Plummer M JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. Proceedings of the 3rd International Workshop on Distributed Statistical Computing (DSC 2003), Vienna, 20–22 March 2003, 1–10. [Google Scholar]
- 16.Newman JD, Alexander KP, Gu X, O’Brien SM, Boden WE, Govindan SC, Senior R, Moorthy N, Rezende PC, Demkow M, et al. Baseline Predictors of Low-Density Lipoprotein Cholesterol and Systolic Blood Pressure Goal Attainment After 1 Year in the ISCHEMIA Trial. Circulation: Cardiovascular Quality and Outcomes. 2019;12:e006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, et al. Optimal medical therapy with or without PCI for stable coronary disease. New England Journal of Medicine. 2007;356:1503–16. [DOI] [PubMed] [Google Scholar]
- 18.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Mobius-Winkler S, Rioufol G, Witt N, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. New England Journal of Medicine. 2012;367:991–1001. [DOI] [PubMed] [Google Scholar]
- 19.BARI 2D Study Group, Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. New England Journal of Medicine. 2009;360:2503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hueb W, Lopes N, Gersh BJ, Soares PR, Ribeiro EE, Pereira AC, Favarato D, Rocha AS, Hueb AC and Ramires JA. Ten-year follow-up survival of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation. 2010;122:949–57. [DOI] [PubMed] [Google Scholar]
- 21.Sedlis SP, Hartigan PM, Teo KK, Maron DJ, Spertus JA, Mancini GB, Kostuk W, Chaitman BR, Berman D, Lorin JD, et al. Effect of PCI on Long-Term Survival in Patients with Stable Ischemic Heart Disease. New England Journal of Medicine. 2015;373:1937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, Engstrom T, Kaab S, Dambrink JH, Rioufol G, et al. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. New England Journal of Medicine. 2018;379:250–259. [DOI] [PubMed] [Google Scholar]
- 23.Bangalore S, Maron DJ, Stone GW and Hochman JS. Routine Revascularization Versus Initial Medical Therapy for Stable Ischemic Heart Disease: A Systematic Review and Meta-Analysis of Randomized Trials. Circulation. 2020;142:841–857. [DOI] [PubMed] [Google Scholar]
- 24.Navarese EP, Lansky AJ, Kereiakes DJ, Kubica J, Gurbel PA, Gorog DA, Valgimigli M, Curzen N, Kandzari DE, Bonaca MP, et al. Cardiac mortality in patients randomised to elective coronary revascularisation plus medical therapy or medical therapy alone: a systematic review and meta-analysis. European Heart Journal. 2021;42:4638–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang EY, Dixson J, Schiller NB and Whooley MA. Causes and Predictors of Death in Patients With Coronary Heart Disease (from the Heart and Soul Study). American Journal of Cardiology. 2017;119:27–34. [DOI] [PubMed] [Google Scholar]
- 26.Stevenson LW, Hellkamp AS, Leier CV, Sopko G, Koelling T, Warnica JW, Abraham WT, Kasper EK, Rogers JG, Califf RM, et al. Changing preferences for survival after hospitalization with advanced heart failure. Journal of the American College of Cardiology. 2008;52:1702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spertus JA, Jones PG, Maron DJ, O’Brien SM, Reynolds HR, Rosenberg Y, Stone GW, Harrell FE Jr., Boden WE, Weintraub WS, et al. Health-Status Outcomes with Invasive or Conservative Care in Coronary Disease. New England Journal of Medicine. 2020;382:1408–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


