SUMMARY
The breast implant capsule is a dynamic structure that forms following the implantation of a device. Although normally benign, increased awareness of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) highlights that disease may arise from the capsule. BIA-ALCL presents as a late seroma or mass but explains few of the late seromas found in breast implant patients. To date, many of these seromas lack a clear etiology and are often described as “idiopathic.” Several benign and malignant breast implant capsular pathologies can cause a late seroma or mass. Similar to early reports of BIA-ALCL, these conditions are rare and largely limited to case reports or series.
The purpose of this special topic is to present a narrative review highlighting capsular pathologies that contribute to the formation of late seroma or mass in an attempt to broaden the differential diagnosis and help plastic surgeons identify the etiology. Specifically, we review the presentation and management of BIA-ALCL, synovial metaplasia, capsular epithelialization, late hematoma, double capsule, breast cancer, squamous cell carcinoma, mesenchymal tumor, and B-cell lymphoma. Although rare, plastic surgeons should consider these capsular conditions as causes of late seromas and masses. Usually, these conditions may be diagnosed by following the National Comprehensive Cancer Network (NCCN) screening guidelines for BIA-ALCL. Thorough evaluation and workup of late seromas and masses may lead to improved characterization of these rare breast implant capsular conditions and improve our understanding of their pathophysiology and management.
INTRODUCTION
Breast implant capsular pathology has gained increased attention among plastic surgeons because of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). While BIA-ALCL dominates the discussion on pathologies associated with the breast implant capsule, there are other benign and malignant capsular conditions involving the breast implant capsule that warrant consideration by plastic surgeons treating patients with a late seroma or capsular mass.
The breast implant capsule forms via a foreign body reaction to the implant. Immediately after implant placement, capsule formation begins with coagulation and accumulation of cellular debris and progresses through protein adsorption, acute inflammation, chronic inflammation, and fibrous encapsulation.1 The breast implant capsule contains three layers: 1) an inner layer of fibroblasts and macrophages, 2) an intermediate layer of loose connective tissue fibrils rich in vasculature, and 3) a dense, vascular, outer collagen layer.2 Capsular evolution occurs in response to several factors, such as implant texture, infection or biofilm, and radiation.3 While most capsules remain benign, some undergo varying degrees of fibrosis and/or develop a seroma or mass.4
A seroma is defined as “late” if it occurs more than 1 year after implantation.5 One to two percent of breast implant placements result in late seroma; most are associated with textured implants.6–8 While plastic surgeons routinely screen for BIA-ALCL when encountering late periprosthetic fluid collection, most late seromas occur due to conditions and capsular pathologies other than BIA-ALCL. DiNapoli reported that only 9% of late seromas in breast implant patients were positive for BIA-ALCL.4 A more recent study on magnetic resonance imaging (MRI) to screen for silent rupture found that 1.7% of patients had periprosthetic fluid collection; 14 out of 15 were benign and only one was positive for BIA-ALCL.9
The rapid accumulation of data on BIA-ALCL has, quite appropriately, led plastic surgeons to assess for BIA-ALCL in patients presenting with a late seroma or mass. However, BIA-ALCL only contributes to a small proportion of these cases, which when combined with the absence of trauma, infection, or other explainable cause for late seroma underscores the need to expand the differential diagnosis to recognize other etiologies to explain what is otherwise referred to as “idiopathic.” In this narrative review, we present a broad overview of breast implant capsular pathologies associated with late presentation of intracapsular fluid accumulation or mass formation.
BREAST IMPLANT-ASSOCIATED ANAPLASTIC LARGE CELL LYMPHOMA (BIA-ALCL)
BIA-ALCL is a T-cell lymphoma of the breast implant capsule that typically presents as sudden-onset unilateral breast swelling 7–10 years after implantation.10, 11 While BIA-ALCL also occurs in native breast tissue, the incidence is greater in the presence of breast implants.12 Further examination most commonly reveals a seroma or, less commonly, a mass.13 Cordeiro et al and Nelson et al recently demonstrated that the incidence of BIA-ALCL in textured implant breast reconstruction patients may be as high as 1:355 to 559.14, 15 Among patients with known implant type, all patients with BIA-ALCL were exposed to macrotextured implants, including 11% of patients who already had undergone exchange to a smooth device.16 However, the pathophysiology linking macrotextured implants and BIA-ALCL remains unknown. Leading theories include genetic predisposition and chronic inflammation—potentially induced by implant-surface biofilm or shedding of silicone particles.17 The U.S. Food and Drug Administration recently reported one case of BIA-ALCL in a patient exposed only to a smooth implant,18 underscoring the inadequacy of our current understanding of the BIA-ALCL disease process.
The National Comprehensive Cancer Network (NCCN) has recommended guidelines for BIA-ALCL diagnosis and management.19 First, suspected cases should undergo ultrasound or MRI with needle aspiration of any effusion or fine-needle aspiration of a capsular mass. The pathology is positive for BIA-ALCL if flow cytometry and immunohistochemistry demonstrate clonal CD30+ALK-T-cells. If the pathology is indeterminate, the specimens should be sent to a specialized center for further analysis. If the disease is confined to the capsule, the patient should have a complete capsulectomy. If there is an extracapsular mass, the patient should undergo complete capsulectomy with excision of the extracapsular mass but should also be referred to hematology for potential adjuvant chemotherapy or radiotherapy.
CAPSULAR PATHOLOGY PRESENTING WITH INTRACAPSULAR FLUID ACCUMULATION
Synovial Metaplasia
Synovial metaplasia often presents as a seroma or unilateral breast swelling and is usually found incidentally on pathology.20, 21 Case reports have described synovial metaplasia as the etiology of an intracapsular fluid collection that was thought in one instance to be BIA-ALCL and in another to be a ruptured saline implant.20, 22 Synovial metaplasia is a benign condition occurring in 40–77% of breast implant capsules independent of implant texture and fill.23, 24 In response to microtrauma from the implant moving in the capsule pocket, the capsule attempts to repair itself by developing a synovial lining analogous to an articular joint.21, 23–25 Synovial metaplasia is associated with time elapsed since implantation, with increasing prevalence during the first 5 years, and decreasing prevalence thereafter.24, 26
Clinically, entry of a pocket that has undergone synovial metaplasia will typically reveal a viscous, cellular fluid; diagnosis is confirmed through assessment of the capsule by pathology.20, 21 Capsules that have undergone synovial metaplasia display a layer of cells with secretory properties on the inner lining.21, 23, 25 While definitive diagnostic imaging modalities are lacking, synovial metaplasia occasionally shows capsular thickening in conjunction with peri-implant fluid.22 This condition is managed by capsulectomy with or without implant exchange.
Capsular Epithelialization
Capsular epithelialization is a rarely reported, with only four reports published in the literature to date.27–30 The reported presentations include non-specific unilateral breast enlargement, breast implant extrusion, and contour irregularity with non-specific fluid on MRI.27–30 The etiology of capsular epithelialization is unknown; leading hypotheses include ingrowth of epithelial cells from the wound margin, metaplasia of breast duct cells, or surgical seeding of keratinocytes at the time of breast implant placement.27, 31
Clinically, the few reports of capsular epithelialization describe scattered islands of epithelialization and cystic structures in the capsule, along with fluid whose consistency ranges from serosanguinous to milky and keratinaceous. Capsular assessment by pathology will typically reveal epithelial cells or keratinaceous debris, differentiating capsular epithelialization from other capsular pathology.27–30 Capsulectomy to ensure removal of any residual intracapsular epithelial tissue appropriately treated this condition in case reports.
Late Hematoma
Late hematomas in breast implant patients usually present as unilateral swelling of the breast that is more commonly chronic and progressive but can on occasion present acutely.29, 32–35 The exact incidence is unknown, and descriptions of late hematomas are limited to several case reports and small case series.33, 36, 37 Many of the published cases have been deemed idiopathic, but some patients report an inciting traumatic event.29, 32–35 Several causes have been attributed to their onset, including capsular tear, erosion of a capsular artery with prevention of retraction by the fibrotic capsule, chronic inflammation, and chronic microtrauma from motion of the implant in a contracted capsule.29, 32, 33, 35, 36, 38, 39 After these inciting events, progression of late hematomas has been compared to that of chronic subdural hematomas, with small recurrent bleeds forming clots that create osmotic environments that slowly pull fluid into the capsule over time.34
To diagnose a late hematoma, computed tomography (CT), MRI, or ultrasound are used to identify fluid with or without an organized mass (clot). An aspirate of the fluid is sent for pathology and/or cytology for definitive diagnosis. The management of late hematoma involves evacuation of the hematoma, clot, and fluid from the breast pocket, capsulectomy, and coagulation of any bleeding blood vessels. Upon entering the pocket of a breast affected by late hematoma, the surgeon typically notices the presence of serosanguinous fluid, blood or a clot with varying degrees of organization, and possibly inflamed or granulation tissue.29, 33, 37, 40 Pathology reports for the cases in the literature have described engorged blood vessels, which could represent the etiology of the sentinel bleed and reinforce the need for meticulous hemostasis.29, 33
Double Capsule
A double capsule is another cause of unilateral breast swelling. A double capsule is associated with a more chronic or indolent onset of unilateral breast effusion and more commonly occurs in patients with macrotextured implants.10, 41, 42 The exact etiology of the double capsule is unclear but is thought to be either mechanical, infectious, or both. The mechanical hypothesis describes the integration of the textured implant into the capsule; then, as force is transmitted through the chest, the portion of the capsule adherent to the textured breast implant delaminates from the outer portion of the capsule. This results in the creation of a potential space and through micromotion of the two delaminated layers of capsule, inflammation results and fluid accumulates.41–43 The presence of biofilm at the implant-capsule interface has also been linked to delamination of the inner layer of capsule, which again results in the creation of a potential space for fluid to accumulate.44
A double capsule is usually diagnosed at the time of revision surgery. Although often an incidental finding, the presence of a double capsule may explain why the patient developed a recurrent seroma. The surgeon observes a capsule adherent to the implant and a residual capsule in the breast pocket. A double capsule is best managed with capsulectomy and implant exchange.
CAPSULAR PATHOLOGY PRESENTING WITH DEVELOPMENT OF A MASS
B-Cell Malignancy
B-cell lymphoma of the breast represents <0.5% of all breast malignancy.45 Only eight case reports describing B-cell lymphoma in patients with breast implants have been published.46–53 It is questionable whether implants play a causal role in B-cell lymphoma given that four of the nine reported patients also had systemic lymphoma, and only about half of these cases involved the luminal aspect of the implant capsule.47, 50, 52–54 Of the different types of B-cell malignancy, large B-cell lymphoma is thought to be the most likely to be linked to implants because it can be activated by chronic inflammation in a manner similar to BIA-ALCL.54
In terms of clinical presentation, about half of the reported cases had breast pain and swelling, while others had axillary lymphadenopathy, recurrent fevers, and systemic symptoms.47–53 Patients with any of these symptoms, especially in the context of a history of systemic lymphoma, should prompt consultation with a hematologist and surgical oncologist. Following the NCCN guidelines for BIA-ALCL workup will facilitate diagnosis and should include aspiration and cytology of any intracapsular fluid with CT or MRI as needed to better characterize a mass. A PET scan is also valuable for the evaluation of extra-mammary disease. Definitive management should be directed by a hematologist specializing in B-cell lymphoma, with the plastic surgeon and surgical oncologist offering surgical support for complete capsulectomy and excision of the mass if indicated.
Squamous Cell Carcinoma
Seven cases of squamous cell carcinoma in the breast implant capsule have been published,28, 55–59 and our group recently treated another case. Squamous cell carcinoma arising from the capsule typically presents as unilateral breast pain and enlargement with possible axillary lymphadenopathy.56, 58 Most of the reported cases have occurred in patients who have had their implants for more than 15 years and have incomplete knowledge or documentation of their history of breast surgeries (capsulectomies, exchanges, implant types and textures).56, 58, 59 The etiology is thought to be similar to a Marjolin’s ulcer, with chronic inflammation or irritation of a capsule that has undergone squamous metaplasia. Similar to capsular epithelialization, the source of the epithelial cells is debatable and could be seeding from surgical incisions, metaplasia of the breast ductal cells, or transformation of the capsular cells. Notably, free silicone injection into the breast has also been associated with breast squamous cell carcinoma.60 Squamous cell carcinoma of the breast also occurs in the absence of breast implants, albeit rarely, representing only 0.1% of all invasive breast cancers.61
Patients presenting with unilateral breast swelling, pain, and periprosthetic fluid collection or capsular mass should be managed using the NCCN BIA-ALCL guidelines to arrive at a diagnosis. The aspirated fluid is typically keratin-rich, and masses usually display dysplastic keratinized epithelium of capsule histologically.55–59 While this is a rare disease, the devastating outcomes underscore the importance of rapid diagnosis and urgent management to maximize the survival of patients affected by breast implant capsular squamous cell carcinoma. Of the seven patients in the published case reports, four died from the disease. Once the tumor extends beyond the capsule, the risk of locoregional metastasis is high. Therefore, these patients should also undergo positron emission tomography (PET) to assess for spread of the disease and be promptly referred to surgical oncology. Surgical management should be deferred to the surgical oncologist but should at least include explant and capsulectomy. The reported cases have at minimum involved mastectomy and occasionally chest wall resection and lymphadenectomy. As with patients without implants who develop breast squamous cell carcinoma,62 patients with capsular squamous cell carcinoma should also receive adjuvant chemotherapy and radiation therapy under the direction of a multidisciplinary tumor board. We recently treated a patient with capsular squamous cell carcinoma according to these management strategies, using neoadjuvant chemotherapy, mastectomy, and chest wall resection, resulting in disease-free survival over 2 years after reconstruction (Figures 1 and 2).
Figure 1.
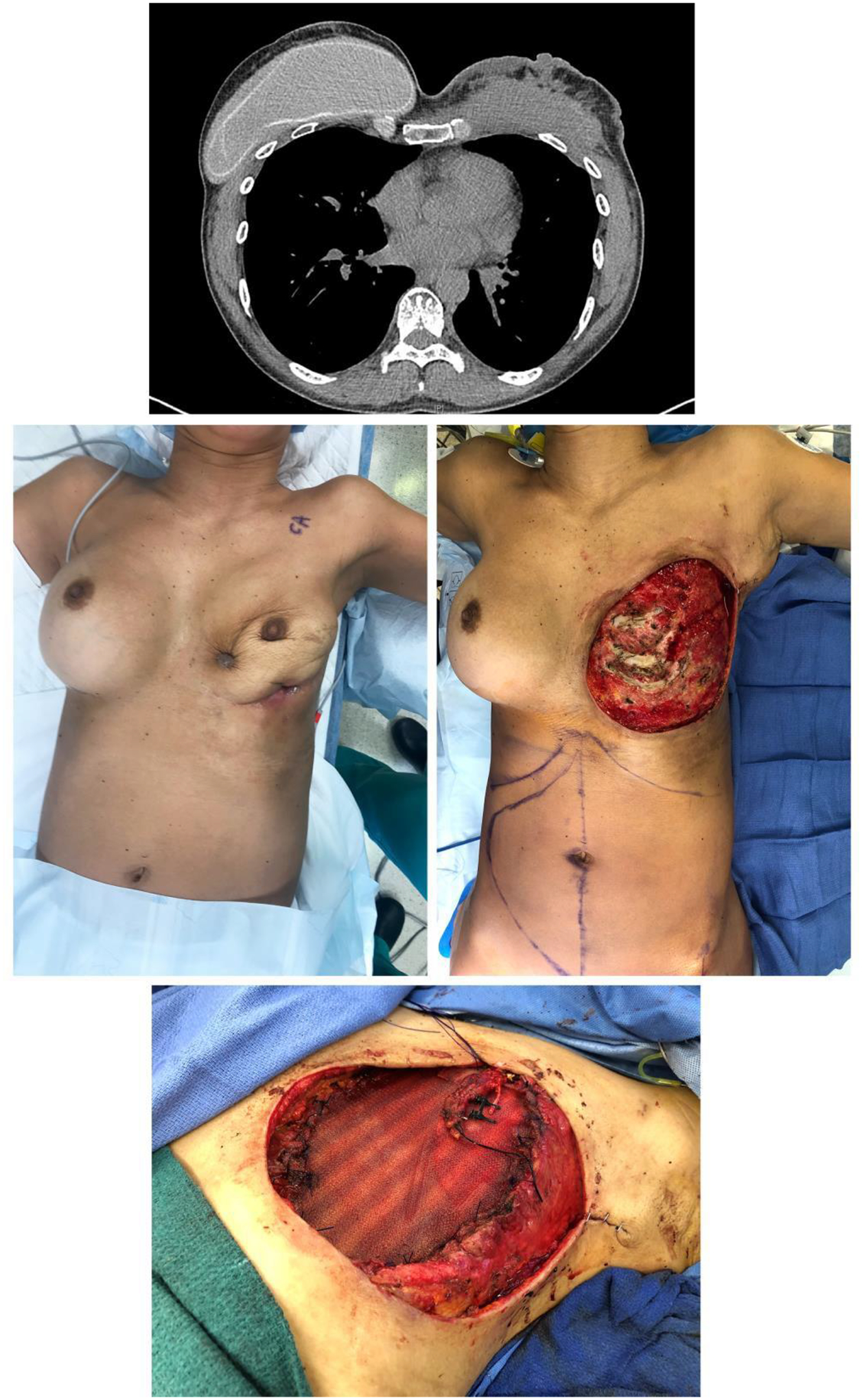
Preoperative CT scan of the chest (A) and photo (B) of a forty-eight-year-old female patient with a history of bilateral breast augmentation show development of squamous cell carcinoma in the right breast implant capsule. Following diagnosis, the patient underwent chemotherapy followed by radical mastectomy and full thickness chest wall resection (C) and reconstruction with Phasix mesh (Bard Davol Inc., Warwick, RI) (D) and anterolateral thigh free tissue transfer.
Figure 2.
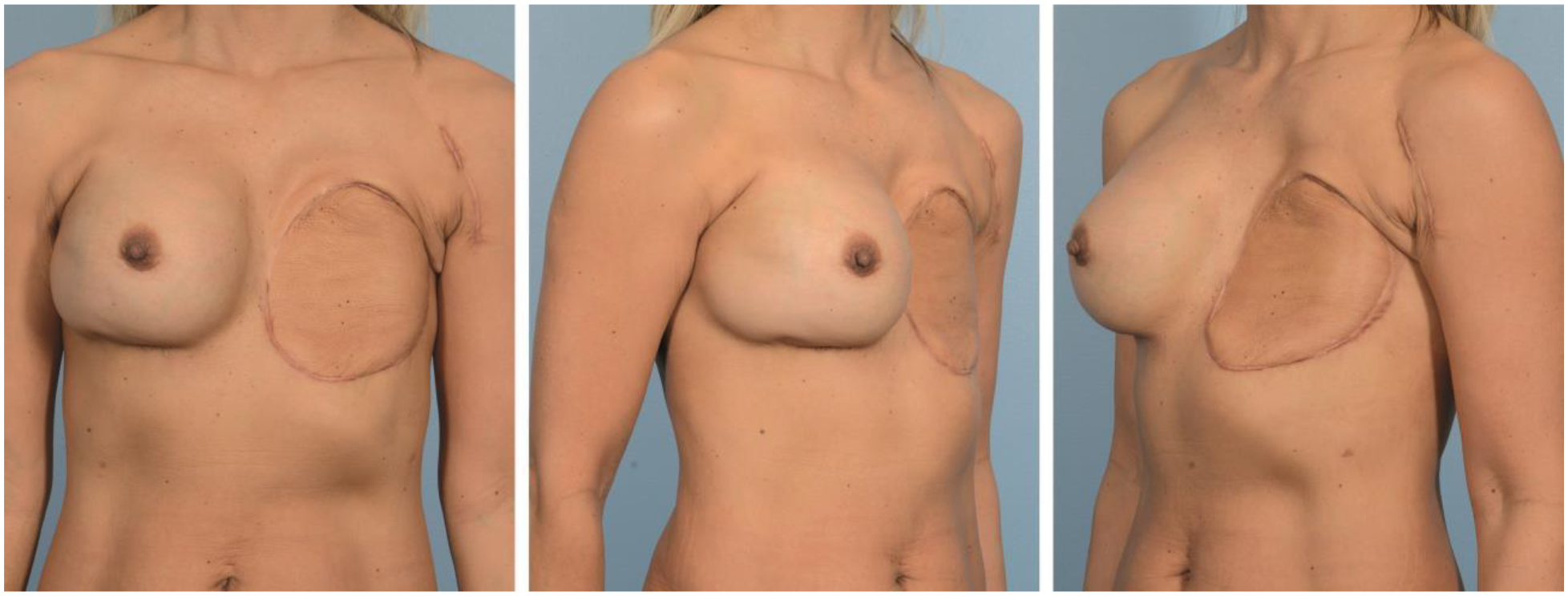
Postoperative photos of patient at 3 years after reconstruction. She has remained disease-free.
Breast Cancer
Only one case series describing breast cancer within the implant capsule has been published.63 The authors of this paper and the case series acknowledge that this is probably due to underreporting. In the published series of three patients, patients either had multiple nodules along the implant capsule or a tumor invading the capsule. Interestingly, the tumor was found in the area of the capsule against breast parenchyma and was absent in the capsule against muscle in the case of subpectoral implants. All three patients presented with masses that had evolved, with satellite lesions and/or spread along the capsule.
Primary breast carcinoma occurs as frequently in patients with cosmetic implants as in patients without cosmetic implants.64 The breast cancer pathology in this case series was not discussed; however, there was concern for tumor cells that invaded or spread to the outer vascular layer of the implant capsule from a mass that developed in the breast parenchyma. Given the vascularity of the breast implant capsule, it is hypothesized that this represents a vascular plane where the primary breast cancer could infiltrate and spread.
The management of a palpable mass in a patient with breast implants should follow the same guidelines as for a patient without breast implants. Providers should immediately schedule an ultrasound or MRI and promptly refer patients to a surgical oncologist. For breast implant patients, MRI may be preferred since it provides better resolution of the tumor and capsule than ultrasound. Ultimately, the surgical management of the tumor should be guided by the surgical oncologist, but if capsular involvement is suspected, a complete capsulectomy should be performed due to the potential for hematologic spread of tumor cells through the vascular plane of the capsule.
Mesenchymal Tumors
Mesenchymal tumors represent 0.2–0.3% of all breast tumors, and 10% of mesenchymal tumors occur in patients with breast implants.65, 66 Mesenchymal tumors arising from the breast implant capsule are even rarer, with only a few reported cases in the literature.67–69 Mesenchymal tumors, in general, arise from the benign proliferation of fibroblasts and myofibroblasts and are associated with trauma, such as surgical trauma from implant placement.70 In the breast, mesenchymal tumors can develop from smooth muscle cells in the breast ducts or chest wall muscles.65, 71 Mesenchymal breast tumors are typically solitary and benign but locally invasive.65 The association between breast implants and mesenchymal tumor development is debatable since the frequency of these tumors in patients with and without implants is similar.66, 72
As with any patient presenting with a breast mass, a thorough physical examination is important, including an assessment for lymphadenopathy, since mesenchymal breast tumors often present as a palpable mass that has grown over time. Detecting the tumor is easier on examination when it is superficial, but it can be easily missed if it is deep, such as on the deep surface of the pectoralis muscle. Additionally, these tumors usually are not accompanied by any lymphadenopathy.65 In terms of diagnostic imaging, MRI is the best modality, followed by ultrasound; on the other hand, mammograms rarely detect mesenchymal tumors.65, 73, 74 Ultimately, these patients should be referred to a surgical oncologist for further characterization of the mass, which is diagnosed by core biopsy showing spindle cells.75, 76 Surgical oncologists manage the mesenchymal tumor by attempting a complete excision. Where it involves the breast implant capsule, a capsulectomy should be performed to reduce the risk of local recurrence.
MANAGEMENT RECOMMENDATIONS FOR A PERIPROSTHETIC EFFUSION OR MASS
The conditions described in this overview have been rarely described. However, plastic surgeons had a similar experience with BIA-ALCL, whereby the incidence was extremely rare. With increased recognition and screening, seromas and masses that were previously thought to be idiopathic have now been diagnosed as BIA-ALCL. We suggest that by following the NCCN guidelines for BIA-ALCL screening for more routine late seromas and masses, we might be able to assign a diagnostic reason for the approximately 90% of late seromas that presently arise from an unknown etiology.
Upon presentation of an implant patient with breast pain or swelling, providers should perform a physical examination, palpating for any fluid collections, masses, or lymphadenopathy. Providers should also assess for any concerning systemic symptoms. Then, providers should adhere to the NCCN BIA-ALCL screening guidelines for patients presenting with a late seroma or mass, which will diagnose many of these rare capsular pathologies. Ultrasound or MRI can be used to identify the presence of a fluid collection or mass and should be followed by ultrasound-guided aspiration or biopsy and pathologic workup. For the most part, these pathologies, like BIA-ALCL, are also managed by capsulectomy, implant exchange, and consultation with other oncologists when extracapsular disease is present.
Plastic surgeons have given significant attention to the indications for different types of capsulectomy in light of concerns about BIA-ALCL and breast implant illness. The FDA does not recommend capsulectomy for asymptomatic patients concerned about disease. However, upon finding irregularities in the capsule, recent expert opinions suggest to surgically excise suspicious capsule.77 Depending on the distribution of disease, this could result in a partial or total capsulectomy.78 If there is extra-capsular involvement, the surgeon should consult surgical oncology and/or tumor board for additional guidance on excision. It is important, however, to appropriately counsel patients about the risks of capsulectomy and highlight that to date there is no data to describe the rate of complications.78, 79
Limited data exists regarding reconstruction or implant replacement after capsulectomy for capsular disease, in part due to the rare diagnosis. The best recommendations in this regard were published by Lamaris et al concerning reconstruction following treatment for BIA-ALCL.80 They recommend immediate reconstruction if the disease is limited to the capsule, but to wait 6 to 12 months if an extra-capsular component exists. However, such management following BIA-ALCL is debated, and the most conservative approach is likely no immediate reconstruction even if the process is limited to the capsule.
Table I provides a summary of diagnoses and treatments for non-BIA-ALCL capsular conditions.
Table I.
Non-ALCL Breast Implant Capsular Pathologies
| Capsule Pathology | Incidence | Presentation | Diagnosis | Pathology | Management |
|---|---|---|---|---|---|
| Fluid accumulation | |||||
| Synovial metaplasia | 40–77% of breast implant capsules22, 23 |
|
|
|
|
| Capsular epithelialization | 4 case reports/series26–29 |
|
|
|
|
| Late hematoma | Unknown |
|
|
|
|
| Double capsule | Unknown |
|
|
|
|
| Mass | |||||
| B-cell malignancy | 9 reported cases46–53 |
|
|
|
|
| Squamous cell carcinoma | 7 reported cases28, 55–59 |
|
|
|
|
| Breast cancer | 3 reported cases63 |
|
|
|
|
| Mesenchymal tumor | Rare |
|
|
|
|
CONCLUSIONS
Breast implant capsules are dynamic tissues that change over time. However, as BIA-ALCL has taught us, the capsule can evolve to have significant clinical implications. In addition to BIA-ALCL, plastic surgeons should be familiar with the broad differential diagnosis for capsular pathology that could explain “idiopathic” late seromas or masses. Our knowledge about BIA-ALCL has grown tremendously since it was first described in 1997. Not only have we diagnosed and treated a rising number of BIA-ALCL cases over time, but we have also discovered that BIA-ALCL occurs far more frequently than ever imagined. Similarly, these rare capsular conditions may not be as rare as we think. By continuing to follow the NCCN BIA-ALCL guidelines for the management of late seromas and masses, we may soon understand with greater accuracy the true incidence, etiology, and clinical implications of these conditions arising from the breast implant capsule.
Funding:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Financial disclosures: None
REFERENCES
- 1.Klopfleisch R, Jung F. The pathology of the foreign body reaction against biomaterials. J Biomed Mater Res A. Mar 2017;105(3):927–940. doi: 10.1002/jbm.a.35958 [DOI] [PubMed] [Google Scholar]
- 2.de Bakker E, van den Broek LJ, Ritt M, Gibbs S, Niessen FB. The Histological Composition of Capsular Contracture Focussed on the Inner Layer of the Capsule: An Intra-Donor Baker-I Versus Baker-IV Comparison. Aesthetic Plast Surg. Dec 2018;42(6):1485–1491. doi: 10.1007/s00266-018-1211-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachour Y, Bargon CA, de Blok CJM, Ket JCF, Ritt M, Niessen FB. Risk factors for developing capsular contracture in women after breast implant surgery: A systematic review of the literature. J Plast Reconstr Aesthet Surg. Sep 2018;71(9):e29–e48. doi: 10.1016/j.bjps.2018.05.022 [DOI] [PubMed] [Google Scholar]
- 4.Di Napoli A Achieving Reliable Diagnosis in Late Breast Implant Seromas: From Reactive to Anaplastic Large Cell Lymphoma. Plast Reconstr Surg. Mar 2019;143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):15S–22S. doi: 10.1097/PRS.0000000000005565 [DOI] [PubMed] [Google Scholar]
- 5.Bengtson B, Brody GS, Brown MH, et al. Managing late periprosthetic fluid collections (seroma) in patients with breast implants: a consensus panel recommendation and review of the literature. Plast Reconstr Surg. Jul 2011;128(1):1–7. doi: 10.1097/PRS.0b013e318217fdb0 [DOI] [PubMed] [Google Scholar]
- 6.Mazzocchi M, Dessy LA, Carlesimo B, Marchetti F, Scuderi N. Late seroma formation after breast surgery with textured silicone implants: a problem worth bearing in mind. Plast Reconstr Surg. Apr 2010;125(4):176e–177e. doi: 10.1097/PRS.0b013e3181cb664d [DOI] [PubMed] [Google Scholar]
- 7.Pinchuk V, Tymofii O. Seroma as a late complication after breast augmentation. Aesthetic Plast Surg. Jun 2011;35(3):303–14. doi: 10.1007/s00266-010-9607-6 [DOI] [PubMed] [Google Scholar]
- 8.Spear SL, Rottman SJ, Glicksman C, Brown M, Al-Attar A. Late seromas after breast implants: theory and practice. Plast Reconstr Surg. Aug 2012;130(2):423–35. doi: 10.1097/PRS.0b013e3182589ea9 [DOI] [PubMed] [Google Scholar]
- 9.Sutton EJ, Dashevsky BZ, Watson EJ, et al. Incidence of benign and malignant peri-implant fluid collections and masses on magnetic resonance imaging in women with silicone implants. Cancer Med. May 2020;9(10):3261–3267. doi: 10.1002/cam4.2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemens MW, Nava MB, Rocco N, Miranda RN. Understanding rare adverse sequelae of breast implants: anaplastic large-cell lymphoma, late seromas, and double capsules. Gland Surg. Apr 2017;6(2):169–184. doi: 10.21037/gs.2016.11.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemens MW, Miranda RN. Coming of Age: Breast Implant-Associated Anaplastic Large Cell Lymphoma After 18 Years of Investigation. Clin Plast Surg. Oct 2015;42(4):605–13. doi: 10.1016/j.cps.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 12.de Boer M, van Leeuwen FE, Hauptmann M, et al. Breast Implants and the Risk of Anaplastic Large-Cell Lymphoma in the Breast. JAMA Oncol. Mar 1 2018;4(3):335–341. doi: 10.1001/jamaoncol.2017.4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brody GS, Deapen D, Taylor CR, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg. Mar 2015;135(3):695–705. doi: 10.1097/PRS.0000000000001033 [DOI] [PubMed] [Google Scholar]
- 14.Cordeiro PG, Ghione P, Ni A, et al. Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured breast implants. J Plast Reconstr Aesthet Surg. Jan 20 2020; doi: 10.1016/j.bjps.2019.11.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson JA, Dabic S, Mehrara BJ, et al. Breast Implant-associated Anaplastic Large Cell Lymphoma Incidence: Determining an Accurate Risk. Ann Surg. Sep 1 2020;272(3):403–409. doi: 10.1097/SLA.0000000000004179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts JM, Carr LW, Jones A, Schilling A, Mackay DR, Potochny JD. A Prospective Approach to Inform and Treat 1340 Patients at Risk for BIA-ALCL. Plast Reconstr Surg. Jul 2019;144(1):46–54. doi: 10.1097/PRS.0000000000005703 [DOI] [PubMed] [Google Scholar]
- 17.Marra A, Viale G, Pileri SA, et al. Breast implant-associated anaplastic large cell lymphoma: A comprehensive review. Cancer Treat Rev. Mar 2020;84:101963. doi: 10.1016/j.ctrv.2020.101963 [DOI] [PubMed] [Google Scholar]
- 18.U.S. Food & Drug Administration. Medical Device Reports of Breast Implant Associated Anaplastic Large Cell Lymphoma. Updated 08/20/2020. Accessed 03/10/2021, https://www.fda.gov/medical-devices/breast-implants/medical-device-reports-breast-implant-associated-anaplastic-large-cell-lymphoma
- 19.Clemens MW, Jacobsen ED, Horwitz SM. 2019 NCCN Consensus Guidelines on the Diagnosis and Treatment of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthet Surg J. Jan 31 2019;39(Supplement_1):S3–S13. doi: 10.1093/asj/sjy331 [DOI] [PubMed] [Google Scholar]
- 20.Krishnanandan S, Abbassian A, Sharma AK, Cunnick G. Capsular synovial metaplasia mimicking silicone leak of a breast prosthesis: a case report. J Med Case Rep. Aug 15 2008;2:277. doi: 10.1186/1752-1947-2-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone JL, Boost T. Cytological features of breast peri-implant synovial metaplasia. Acta Cytol. 2014;58(5):511–3. doi: 10.1159/000369054 [DOI] [PubMed] [Google Scholar]
- 22.Linden O, Kelil T, Greenwood H, Lauw M, Strachowski L. Capsular synovial metaplasia mimicking radiographic features of implant-associated anaplastic lymphoma. Clin Imaging. Feb 2020;59(2):144–147. doi: 10.1016/j.clinimag.2019.10.019 [DOI] [PubMed] [Google Scholar]
- 23.Copeland M, Choi M, Bleiweiss IJ. Silicone breakdown and capsular synovial metaplasia in textured-wall saline breast prostheses. Plast Reconstr Surg. Oct 1994;94(5):628–33; discussion 634–6. [PubMed] [Google Scholar]
- 24.Ko CY, Ahn CY, Ko J, Chopra W, Shaw WW. Capsular synovial metaplasia as a common response to both textured and smooth implants. Plast Reconstr Surg. Jun 1996;97(7):1427–33; discussion 1434–5. doi: 10.1097/00006534-199606000-00017 [DOI] [PubMed] [Google Scholar]
- 25.Jimenez-Heffernan JA, Barcena C, Munoz-Hernandez P. Cytological features of breast peri-implant papillary synovial metaplasia. Diagn Cytopathol. Sep 2018;46(9):769–771. doi: 10.1002/dc.23947 [DOI] [PubMed] [Google Scholar]
- 26.Kamel M, Fornasier VL, Peters W. Cartilaginous metaplasia in the capsule of a Dacron-backed silicone gel breast prosthesis. Ann Plast Surg. Feb 1999;42(2):202–6. [PubMed] [Google Scholar]
- 27.Alikhan MB, Nassar A, Mansoor I. Squamous metaplasia on the breast implant capsule. Int J Surg Pathol. Dec 2010;18(6):570–4. doi: 10.1177/1066896908329587 [DOI] [PubMed] [Google Scholar]
- 28.Kitchen SB, Paletta CE, Shehadi SI, Bauer WC. Epithelialization of the lining of a breast implant capsule. Possible origins of squamous cell carcinoma associated with a breast implant capsule. Cancer. Mar 1 1994;73(5):1449–52. doi: [DOI] [PubMed] [Google Scholar]
- 29.Peters W, Fornasier V. Late unilateral breast enlargement after insertion of silicone gel implants: A histopathological study. Can J Plast Surg. Spring 2007;15(1):19–28. doi: 10.1177/229255030701500107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh K, DiazGomez B, Lomme M. Squamous epithelialization of bilateral breast implant capsules complicated by implant extrusion. Breast J. Jul 2018;24(4):654–655. doi: 10.1111/tbj.12988 [DOI] [PubMed] [Google Scholar]
- 31.van Diest PJ, Beekman WH, Hage JJ. Pathology of silicone leakage from breast implants. J Clin Pathol. Jul 1998;51(7):493–7. doi: 10.1136/jcp.51.7.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McArdle B, Layt C. A case of late unilateral hematoma and subsequent late seroma of the breast after bilateral breast augmentation. Aesthetic Plast Surg. Jul 2009;33(4):669–70. doi: 10.1007/s00266-009-9325-0 [DOI] [PubMed] [Google Scholar]
- 33.Peters W, Fornasier V, Howarth D. Late unilateral hematoma after breast augmentation. Plast Surg (Oakv). Spring 2014;22(1):18–21. [PMC free article] [PubMed] [Google Scholar]
- 34.Roman S, Perkins D. Progressive spontaneous unilateral enlargement of the breast twenty-two years after prosthetic breast augmentation. Br J Plast Surg. Jan 2005;58(1):88–91. doi: 10.1016/j.bjps.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 35.Seth AK, Kim JY. Acute symptomatic hematoma with defined etiology seven years after breast reconstruction: A case report and literature review. Can J Plast Surg. Summer 2010;18(2):e27–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Grippaudo FR, Renzi L, Costantino B, Longo B, Santanelli F. Late unilateral hematoma after breast reconstruction with implants: case report and literature review. Aesthet Surg J. Aug 1 2013;33(6):830–4. doi: 10.1177/1090820X13496249 [DOI] [PubMed] [Google Scholar]
- 37.Kim L, Castel N, Parsa FD. Case of late hematoma after breast augmentation. Arch Plast Surg. Mar 2018;45(2):177–179. doi: 10.5999/aps.2016.01718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brickman M, Parsa NN, Parsa FD. Late hematoma after breast implantation. Aesthetic Plast Surg. Mar-Apr 2004;28(2):80–2. doi: 10.1007/s00266-004-3120-8 [DOI] [PubMed] [Google Scholar]
- 39.Cheng NX, Chen B, Li Q, et al. Late haematoma and seroma in patients with silicone mammary prosthesis: our reports and literature review. J Plast Reconstr Aesthet Surg. Jul 2011;64(7):e185–6. doi: 10.1016/j.bjps.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 40.Park SH, Park ES, Kang SG. Late Capsular Hematoma after Prosthesis Removal Following Aesthetic Breast Augmentation: A Case Report. Arch Aesthetic Plast Surg. 6 2017;23(2):96–100. doi: 10.14730/aaps.2017.23.2.96 [DOI] [Google Scholar]
- 41.Hall-Findlay EJ. Breast implant complication review: double capsules and late seromas. Plast Reconstr Surg. Jan 2011;127(1):56–66. doi: 10.1097/PRS.0b013e3181fad34d [DOI] [PubMed] [Google Scholar]
- 42.Pandya AN, Dickson MG. Capsule within a capsule: an unusual entity. Br J Plast Surg. Jul 2002;55(5):455–6. doi: 10.1054/bjps.2002.3864 [DOI] [PubMed] [Google Scholar]
- 43.Giot JP, Paek LS, Nizard N, et al. The double capsules in macro-textured breast implants. Biomaterials. Oct 2015;67:65–72. doi: 10.1016/j.biomaterials.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 44.Danino MA, Nizard N, Paek LS, Govshievich A, Giot JP. Do Bacteria and Biofilm Play a Role in Double-Capsule Formation around Macrotextured Implants? Plast Reconstr Surg. Nov 2017;140(5):878–883. doi: 10.1097/PRS.0000000000003767 [DOI] [PubMed] [Google Scholar]
- 45.Surov A, Holzhausen HJ, Wienke A, et al. Primary and secondary breast lymphoma: prevalence, clinical signs and radiological features. Br J Radiol. Jun 2012;85(1014):e195–205. doi: 10.1259/bjr/78413721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beydoun AS, Ovalle F, Brannock K, Gobble RM. A Case Report of a Breast Implant-Associated Plasmacytoma and Literature Review of Non-ALCL Breast Implant-Associated Neoplasms. Aesthet Surg J. Jun 21 2019;39(7):NP234–NP239. doi: 10.1093/asj/sjy315 [DOI] [PubMed] [Google Scholar]
- 47.Chen VW, Hoang D, Clancy S. Breast Implant-Associated Bilateral B-Cell Lymphoma. Aesthet Surg J. Jan 29 2020;40(2):NP52–NP58. doi: 10.1093/asj/sjy093 [DOI] [PubMed] [Google Scholar]
- 48.Cook PD, Osborne BM, Connor RL, Strauss JF. Follicular lymphoma adjacent to foreign body granulomatous inflammation and fibrosis surrounding silicone breast prosthesis. Am J Surg Pathol. Jun 1995;19(6):712–7. doi: 10.1097/00000478-199506000-00012 [DOI] [PubMed] [Google Scholar]
- 49.Kraemer DM, Tony HP, Gattenlohner S, Muller JG. Lymphoplasmacytic lymphoma in a patient with leaking silicone implant. Haematologica. Apr 2004;89(4):ELT01. [PubMed] [Google Scholar]
- 50.Moling O, Piccin A, Tauber M, et al. Intravascular large B-cell lymphoma associated with silicone breast implant, HLA-DRB1*11:01, and HLA-DQB1*03:01 manifesting as macrophage activation syndrome and with severe neurological symptoms: a case report. J Med Case Rep. Sep 15 2016;10(1):254. doi: 10.1186/s13256-016-0993-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nichter LS, Mueller MA, Burns RG, Stallman JM. First report of nodal marginal zone B-cell lymphoma associated with breast implants. Plast Reconstr Surg. Mar 2012;129(3):576e–578e. doi: 10.1097/PRS.0b013e3182419caa [DOI] [PubMed] [Google Scholar]
- 52.Said JW, Tasaka T, Takeuchi S, et al. Primary effusion lymphoma in women: report of two cases of Kaposi’s sarcoma herpes virus-associated effusion-based lymphoma in human immunodeficiency virus-negative women. Blood. Oct 15 1996;88(8):3124–8. [PubMed] [Google Scholar]
- 53.Smith BK, Gray SS. Large B-cell lymphoma occurring in a breast implant capsule. Plast Reconstr Surg. Oct 2014;134(4):670e–1e. doi: 10.1097/PRS.0000000000000535 [DOI] [PubMed] [Google Scholar]
- 54.Bacon CM, O’Donoghue JM. Commentary on: Breast Implant-Associated Bilateral B-Cell Lymphoma. Aesthet Surg J. Jan 29 2020;40(2):NP59–NP62. doi: 10.1093/asj/sjz304 [DOI] [PubMed] [Google Scholar]
- 55.Buchanan PJ, Chopra VK, Walker KL, Rudolph R, Greco RJ. Primary Squamous Cell Carcinoma Arising From a Breast Implant Capsule: A Case Report and Review of the Literature. Aesthet Surg J. Jun 13 2018;38(7)doi: 10.1093/asj/sjy092 [DOI] [PubMed] [Google Scholar]
- 56.Olsen DL, Keeney GL, Chen B, Visscher DW, Carter JM. Breast implant capsule-associated squamous cell carcinoma: a report of 2 cases. Hum Pathol. Sep 2017;67:94–100. doi: 10.1016/j.humpath.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 57.Paletta C, Paletta FX Jr., Paletta FX Sr. Squamous cell carcinoma following breast augmentation. Ann Plast Surg. Nov 1992;29(5):425–9; discussion 429–32. doi: 10.1097/00000637-199211000-00009 [DOI] [PubMed] [Google Scholar]
- 58.Zhou YM, Chaudhry HE, Shah A, Andrews J. Breast Squamous Cell Carcinoma Following Breast Augmentation. Cureus. Oct 3 2018;10(10):e3405. doi: 10.7759/cureus.3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zomerlei TA, Samarghandi A, Terando AM. Primary Squamous Cell Carcinoma Arising from a Breast Implant Capsule. Plast Reconstr Surg Glob Open. Dec 2015;3(12):e586. doi: 10.1097/GOX.0000000000000567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Talmor M, Rothaus KO, Shannahan E, Cortese AF, Hoffman LA. Squamous cell carcinoma of the breast after augmentation with liquid silicone injection. Ann Plast Surg. Jun 1995;34(6):619–23. doi: 10.1097/00000637-199506000-00009 [DOI] [PubMed] [Google Scholar]
- 61.Yadav S, Yadav D, Zakalik D. Squamous cell carcinoma of the breast in the United States: incidence, demographics, tumor characteristics, and survival. Breast Cancer Res Treat. Jul 2017;164(1):201–208. doi: 10.1007/s10549-017-4251-3 [DOI] [PubMed] [Google Scholar]
- 62.Ogita M, Shiraishi K, Karasawa K, et al. Clinical outcome of adjuvant radiotherapy for squamous cell carcinoma of the breast; a multicenter retrospective cohort study. Breast. Aug 2020;52:88–94. doi: 10.1016/j.breast.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tokin CA, Wallace AM. Breast cancer presenting within or adjacent to the breast implant capsule: a case series and clinical recommendations. Clin Breast Cancer. Aug 2012;12(4):296–9. doi: 10.1016/j.clbc.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 64.Brinton LA, Lubin JH, Burich MC, Colton T, Brown SL, Hoover RN. Breast cancer following augmentation mammoplasty (United States). Cancer Causes Control. Oct 2000;11(9):819–27. doi: 10.1023/a:1008941110816 [DOI] [PubMed] [Google Scholar]
- 65.Neuman HB, Brogi E, Ebrahim A, Brennan MF, Van Zee KJ. Desmoid tumors (fibromatoses) of the breast: a 25-year experience. Ann Surg Oncol. Jan 2008;15(1):274–80. doi: 10.1245/s10434-007-9580-8 [DOI] [PubMed] [Google Scholar]
- 66.Tzur R, Silberstein E, Krieger Y, Shoham Y, Rafaeli Y, Bogdanov-Berezovsky A. Desmoid Tumor and Silicone Breast Implant Surgery: Is There Really a Connection? A Literature Review. Aesthetic Plast Surg. Feb 2018;42(1):59–63. doi: 10.1007/s00266-017-0948-2 [DOI] [PubMed] [Google Scholar]
- 67.Hammoudeh ZS, Darian VB. Desmoid tumor (fibromatosis) of the breast after augmentation with saline implants. Plast Reconstr Surg. Apr 2012;129(4):753e–4e. doi: 10.1097/PRS.0b013e318245e918 [DOI] [PubMed] [Google Scholar]
- 68.Jewett ST Jr., Mead JH. Extra-abdominal desmoid arising from a capsule around a silicone breast implant. Plast Reconstr Surg. Apr 1979;63(4):577–9. doi: 10.1097/00006534-197904000-00031 [DOI] [PubMed] [Google Scholar]
- 69.Vandeweyer E, Deraemaecker R. Desmoid tumor of the breast after reconstruction with implant. Plast Reconstr Surg. Jun 2000;105(7):2627–8. doi: 10.1097/00006534-200006000-00065 [DOI] [PubMed] [Google Scholar]
- 70.Wongmaneerung P, Somwangprasert A, Watcharachan K, Ditsatham C. Bilateral desmoid tumor of the breast: case seriesand literature review. Int Med Case Rep J. 2016;9:247–51. doi: 10.2147/IMCRJ.S106325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taylor TV, Sosa J. Bilateral breast fibromatosis: case report and review of the literature. J Surg Educ. Jul-Aug 2011;68(4):320–5. doi: 10.1016/j.jsurg.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 72.Balzer BL, Weiss SW. Do biomaterials cause implant-associated mesenchymal tumors of the breast? Analysis of 8 new cases and review of the literature. Hum Pathol. Nov 2009;40(11):1564–70. doi: 10.1016/j.humpath.2009.03.020 [DOI] [PubMed] [Google Scholar]
- 73.Godwin Y, McCulloch TA, Sully L. Extra-abdominal desmoid tumour of the breast: review of the primary management and the implications for breast reconstruction. Br J Plast Surg. Apr 2001;54(3):268–71. doi: 10.1054/bjps.2001.3548 [DOI] [PubMed] [Google Scholar]
- 74.Leibman AJ, Kossoff MB. Sonographic features of fibromatosis of the breast. J Ultrasound Med. Jan 1991;10(1):43–5. doi: 10.7863/jum.1991.10.1.43 [DOI] [PubMed] [Google Scholar]
- 75.Lopez-Ferrer P, Jimenez-Heffernan JA, Vicandi B, Ortega L, Viguer JM. Fine-needle aspiration cytology of mammary fibromatosis: report of two cases. Diagn Cytopathol. Nov 1997;17(5):363–8. doi: [DOI] [PubMed] [Google Scholar]
- 76.Pettinato G, Manivel JC, Petrella G, Jassim AD. Fine needle aspiration cytology, immunocytochemistry and electron microscopy of fibromatosis of the breast. Report of two cases. Acta Cytol. Jul-Aug 1991;35(4):403–8. [PubMed] [Google Scholar]
- 77.Asaad M, Offodile AC, Santanelli Di Pompeo F, et al. Management of Symptomatic Patients with Textured Implants. Plast Reconstr Surg. May 1 2021;147(5S):58S–68S. doi: 10.1097/PRS.0000000000008047 [DOI] [PubMed] [Google Scholar]
- 78.Calobrace MB, Mays C. An Algorithm for the Management of Explantation Surgery. Clin Plast Surg. Jan 2021;48(1):1–16. doi: 10.1016/j.cps.2020.09.005 [DOI] [PubMed] [Google Scholar]
- 79.Abi-Rafeh J, Safran T, Winocour S, Dionisopoulos T, Davison P, Vorstenbosch J. Lack of Evidence on Complication Profile of Breast Implant Capsulectomy: A Call to Action for Plastic Surgeons. Plast Reconstr Surg. Jul 1 2021;148(1):157e–158e. doi: 10.1097/PRS.0000000000008010 [DOI] [PubMed] [Google Scholar]
- 80.Lamaris GA, Butler CE, Deva AK, et al. Breast Reconstruction Following Breast Implant-Associated Anaplastic Large Cell Lymphoma. Plast Reconstr Surg. Mar 2019;143(3S A Review of Breast Implant-Associated Anaplastic Large Cell Lymphoma):51S–58S. doi: 10.1097/PRS.0000000000005569 [DOI] [PubMed] [Google Scholar]


