Abstract
Zonulin is a physiologic epithelial and endothelial permeability modulator. Zonulin increases antigen trafficking from the gut lumen into the bloodstream and in between body compartments, mechanisms linked to many chronic inflammatory diseases. Upon its initial discovery, it was noted that zonulin was not a single protein, rather a family of structurally and functionally related proteins referred to as the zonulin family proteins (ZFPs). ZFPs are members of the mannose associated serine proteases (MASP) family and are the result of high mutation rates leading to many zonulin polymorphisms. Pre-haptoglobin 2, the precursor of haptoglobin 2, was identified as the first eukaryotic member of the ZFPs, and properdin, a key positive regulator of the alternative pathway, as a second member. In this study, we report two additional proteins that are likely ZFPs. Human coagulation factor X (FX) and CD5 antigen-like (CD5L). Both FX and CD5L recombinant proteins were detected by anti-zonulin antibody in Western immunoblot analysis, and both proteins decreased epithelial barrier competency of Caco-2 cell monolayers as established by the Trans Epithelial Electrical Resistance (TEER) assay. These results indicate that FX and CD5L have structural and functional similarities with previously identified ZFPs and, therefore, can be considered new members of this family of proteins.
Keywords: Zonulin, Pre-haptoglobin 2 (pre-HP2), Properdin, Zonulin family proteins (ZFPs), Coagulation factor X (FX), CD5 antigen-like (CD5L)
1. Introduction
Zonulin is a tight junction (TJ)-regulating protein that induces TJ disassembly and a subsequent increase in intestinal permeability [1]. TJ, the most apical junctional complex, are epithelial cell-cell junctions that regulate the flow of solutes through the paracellular pathway and maintains cell polarity [2]. They have an important function in regulating paracellular antigen (Ag) trafficking. Dysregulation of TJs causes mammalian epithelial sheet dysfunction and leads to various human diseases of the gastrointestinal tract, liver, vascular system and respiratory tract as well as facilitate viral infections [3, 4]. Zonulin is the only reversible intestinal permeability modulator identified so far [1, 5, 6]. Zonulin expression increases gut permeability and Ag trafficking in intestinal epithelium, which has been linked to many chronic inflammatory diseases (CIDs), metabolic disorders and cancer [6, 7].
Since its discovery, it was reported that zonulin was not a single protein, rather a family of structurally and functionally related proteins, now referred to as the zonulin family proteins (ZFPs) [5]. These proteins are derived from the complement-associated protein, mannose-binding lectin-associated serine protease (MASP), which have no protease function due to mutations in the catalytic domain leading to the acquisition of new functions such as the capability of modulating intercellular TJs [6]. The development of the ZFP is likely secondary to high mutation rates during evolution that led to frequent zonulin polymorphisms. [8]. The first discovered eukaryotic member of the ZFP was pre-haptoglobin 2 (pre-HP2), the precursor of haptoglobin 2 for which previously no function had been described [9]. Properdin, a key positive regulator of the alternative pathway, was reported as a second member of the ZFPs [10]. Multiple additional ZFPs are likely to exist. In the present study, we aimed at exploring new ZFPs, and we identified human coagulation factor X (FX) and CD5 antigen-like protein (CD5L) as two new potential members of this family based on their structural (spectrometry analysis, Western immunoblot analysis) and functional (by using transepithelial electrical resistance (TEER) assay) similarities to ZFPs.
2. Material and methods
2.1. Human Serum samples and Reagents
Human serum samples were collected and processed for phenotyping as previously described [11, 12]. HP consists of a ~ 45 kDa β-chain that is expressed in all HP phenotypes and two potential a-chains, a ~ 8.9 kDa α-1 chain and a ~ 16 kDa α-2 chain [13]. The combination of the expression of these chains makes three human HP phenotypes, which are called HP1-1, 2-1 and 2-2, and only patients with the HP2-1 or HP2-2 express zonulin as pre-HP2 [13]. HP phenotypes were determined by western blot analysis using anti-HP antibody (#GW20080F, Sigma-Aldrich, Burlington, MA) as previously described [12]. All human serum samples were IgG and albumin depleted using the Pierce™ Albumin/IgG Removal Kit (#89875, Thermo Fisher Scientific, Waltham, MA) and then concentrated with the Amicon® Ultra 0.5 mL Centrifugal Filters (#UFC505024, Merck Millipore, Burlington, MA). Briefly, 30 uL of each human serum sample was loaded to this albumin and IgG removal kit and 150 uL of albumin and IgG depleted serum samples were obtained. Then, each 150 uL of albumin and IgG depleted serum sample was loaded to unto the protein concentrator and approximately 20 uL of protein concentrated sample was obtained. Recombinant human coagulation factor Xa protein (FXa) (#1063-SE-010), recombinant human CD5L protein (CD5L) (#2797-CL-050) and recombinant human properdin protein (#8216-PR-050) were purchased from Bio-Techne Corporation (Minneapolis, MN). Recombinant human FX was not commercially available and therefore recombinant human Factor Xa was used as a proxy. Recombinant human zonulin protein (NP_005134 C-His tag) was synthesized by GenScript (Piscataway, NJ). The prototype of zonulin member is pre-HP2 [9], and this recombinant zonulin refers to recombinant pre-HP2.
2.2. Western immunoblot analysis
Human serum samples phenotyped as HP1-1 and 2-2, and recombinant proteins (zonulin, properdin, FXa and CD5L) were analyzed by Western blot. 10 μL aliquots of albumin/IgG depleted and concentrated human serum samples and 0.25μg, 0.5μg, 1μg or 2μg aliquots of the recombinant proteins were mixed with a sample buffer containing beta-mercaptoethanol and sodium dodecyl sulfate (SDS). SDS was diluted from 4X to 2X wiht distilled water (dH2O). Samples were then denatured at 99 °C for 5 min, loaded into 4 -20 % tris-glycine gels with LI-COR Chameleon Duo ladder and run at 80 volts for 90 minutes. Gels were then transferred onto PVDF membranes and rinsed with dH2O. The PVDF membranes were blocked with 5% non-fat milk in Tris buffered saline (TBS), and incubated overnight at 4 C with primary antibody diluted in TBS-Tween 0.1% (TBST). The membranes were then washed with TBST three times and incubated for 1h at room temperature with a secondary antibody (1:20000) that was diluted in TBST + 0.02% SDS. Western blot signal was visualized in the 680 and 800 channels by using the Bio-Rad ChemiDoc MP Imaging system (Bio-Rad, Hercules, CA). In this western blot analysis, we used the polyclonal anti-coagulation factor X antibody from rabbit (1:1000) (MBS821249, MyBioSource, San Diego, CA), polyclonal anti-CD5L antibody from rabbit (1:1000) (MBS1496603, MyBioSource, San Diego, CA) and polyclonal anti-zonulin antibody from goat (1:1000) (KBMO Diagnostics, Hopedale, MA) as primary antibodies. The prototype of zonulin member is pre-HP2 [9], and this anti-zonulin antibody is the same as anti-pre-HP2 antibody. For secondary antibodies, we used IRDye 800CW Donkey anti-Rabbit IgG Secondary Antibody (LICOR Biosciences, Lincoln, NE) and IRDye 680RD Donkey anti-Goat IgG Secondary Antibody (LICOR Biosciences, Lincoln, NE).
2.3. Mass Spectrometry
For mass spectrometry analysis of human serum samples Western immunoblot procedure was performed as previously described except for possible modification as noted below. Following gel electrophoresis, the gel was stained with Coomassie Blue (#24594, Thermo Fisher Scientific, Waltham, MA). Gel bands with a molecular weight of approximately 50 kDa identified by anti-HP antibody (#GW20080F, Sigma-Aldrich, Burlington, MA), excised and sent to Mass Spectrometry Core Facility (Taplin Mass Spectrometry Facility at Harvard Medical School). We reviewed peptide and protein data detected from each band focusing on proteins with similar molecular weight as previously identified ZFPs (~50 kDa), overlapping motifs and possible members of the MASP family. We used UniProt (https://www.uniprot.org/) and MOTIF search (https://www.genome.jp/tools/motif/) for structural characterization.
2.4. Cell line culture
Human Caco-2 intestinal epithelial cells (HTB-37™, American Type Culture Collection (ATCC), Manassas, VA) were maintained in Dulbecco's Modified Eagle Medium (DMEM, high glucose, pyruvate, Gibco™, Thermo Fisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich, St. Louis, MO). The medium contained 100U/ml of penicillin (Gibco™, Thermo Fisher Scientific, Waltham, MA). Cells were plated on 150cm2 cell culture flask (Corning, Lowell, MA) and incubated in a humidified 5% CO2 incubator at 37 °C. Monolayers were grown on 1.12 cm2 permeable polycarbonate membrane with 0.4 um pore size (Corning, Lowell, MA) until they reached a confluent state.
2.5. Measurement of TEER
Transepithelial electric cell resistance (TEER) was measured to monitor and analyze the epithelial barrier function of Caco-2 epithelial monolayers by using the Epithelial Volt/Ohm Meter EVOM2 and Chopstick Electrode for EVOM2 (World Precision Instruments, Sarasota, FL). Caco-2 monolayers with TEER values of approximately 1000 Ω.cm2 were considered to have an appropriate barrier function and were treated with 5 μg/mL of recombinant zonulin (rZonulin), recombinant properdin (rProperdin), recombinant FXa (rFXa) or recombinant CD5L (rCD5L).
2.6. ELISA measurements
Zonulin ELISA was done by using the zonulin ELISA kit from Immundiagnostik, Inc. (#KR5601, Manchester, NH) and per manufacturer’s protocol. 10 μg/mL of recombinant zonulin (rZonulin), recombinant properdin (rProperdin), rFXa, and rCD5L were used for the assay. Each recombinant protein was pre-incubated at different temperatures (4°C, 37°C, 65°C) and then tested with the ELISA assay.
2.7. Data analysis
Each set of results shown is representative of at least three separate experiments. Results are given as means ± SEM. Statistical analysis was tested by one-way analysis of variance (ANOVA) with the Tukey-Kramer method. Statistical significance was set at *p < 0.05.
3. Results
3.1. Identification of FX and CD5L as potential zonulin family proteins by mass spectrometry analysis
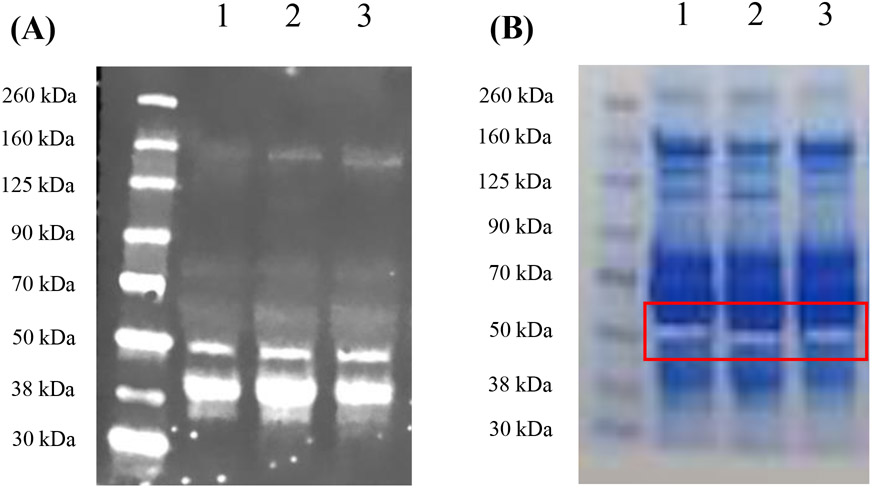
Mass spectrometry analysis on human sera was performed to identify potential new members of ZFPs. HP1-1 (lane 1), HP2-1 (lane 2) and HP2-2 (lane 3) human serum samples were loaded unto the gel for comparison (Figure 1A). The 50 kDa gel band of each human serum sample identified by the anti-HP antibody was excised and sent for mass spectrometry analysis (Figure 1B). A total of 1,485 peptides and 112 proteins, 1,404 peptides and 130 proteins, and 1,339 peptides and 118 were detected from the HP1-1, HP2-1, and HP2-2 human serum samples, respectively (Table 1). We selected for proteins with similar molecular weight, motifs in common with zonulin, and an evolutionary relationship to MASPs. Based on these selection criteria, we identified FX and CD5L in all three phenotype (HP1-1, HP2-1 and HP2-2) serum samples as ZFP candidates.
Figure 1. Gel bands for mass spectrometry analysis.
(A) The western blot image using anti-HP antibody for mass spectrometry. The gel was run to focus on bands with a molecular weight (MW) greater than 30 kDa since the target protein, zonulin as pre-HP2, has a MW ~50 kDa. Lane 1: HP1-1 human serum, Lane 2: HP2-1 human serum, Lane 3: HP2-2 human serum. (B) Image of the gel sent for mass spectrometry analysis with the excised bands. HP:haptoglobin
Table 1.
The number of peptides and proteins detected by mass spectrometry analysis from each band.
| Band 1 | Band 2 | Band 3 | |
|---|---|---|---|
| Total peptides | 1485 | 1404 | 1339 |
| Total proteins | 112 | 130 | 118 |
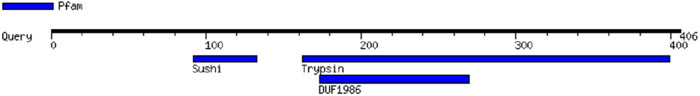
FX, which has a MW of 54.7 kDa, has a trypsin motif in common with haptoglobin (Table 2), an epidermal growth factor (EGF) motif in common with pre-HP-2 [6], and as a complement-activating protein, it has an evolutionary relationship with MASPs [14]. Similarly, zonulin has been reported to activate the complement system via lectin and the classical pathways [15]. The sequence of CD5L, which belongs to the scavenger receptor cysteine-rich (SRCR) family of proteins, contains SRCR domains (Table 2), which consists of Factor I, a protein with similar functions to MASPs [16, 17]. CD5L also has serine-type endopeptidase activity [18].
Table 2.
Protein motifs of Haptoglobin, Properdin, FX and CD5L.
| Haptoglobin |
|
| Properdin |

|
| FX (Human coagulation factor X) |
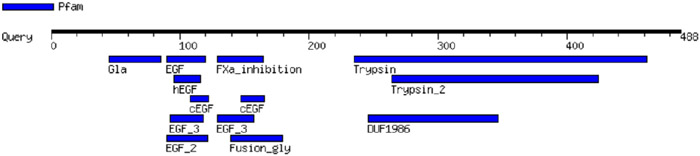
|
| CD5L (CD5 antigen-like) |
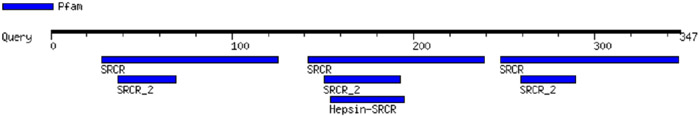
|
Motif images were created by MOTIF Search, https://www.genome.jp/tools/motif/
3.2. FXa and CD5L were recognized by anti-zonulin antibody
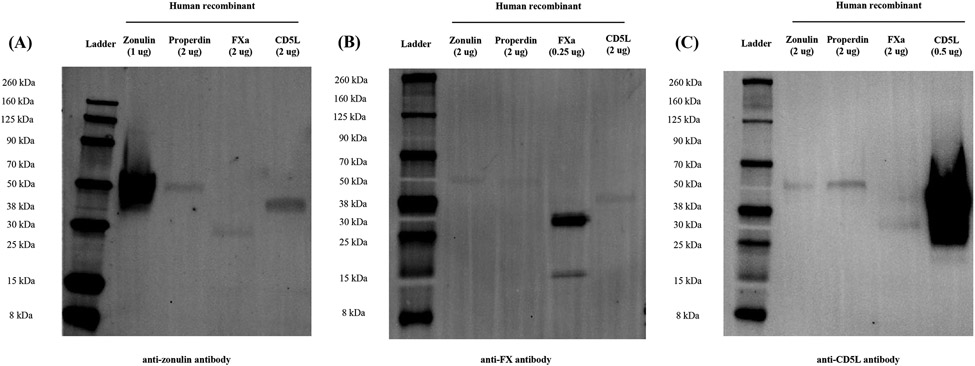
To investigate whether FX and CD5L had overlapping peptide motifs with zonulin, we performed Western immunoblot analysis. rFXa and rCD5L were used as target proteins and rZonulin and rProperdin, previously identified members of the ZFPs [10], as controls. The anti-zonulin antibodies recognized all recombinant proteins (Figure 2A). The signal of rZonulin and rProperdin with anti-zonulin antibody was ~ 50 kDa (Figure 2A). The signal of rFXa with anti-zonulin antibody was ~30 kDa, and that of rCD5L was ~38 kDa (Figure 2A). We also examined whether FX and CD5L were identified in human samples using anti-FX antibody and anti-CD5L antibody and whether there was cross-reactivity between anti-FX, anti-CD5L and anti-zonulin antibodies. The signal for the anti-FX antibody and zonulin cross-reactivity in both HP1-1 and HP2-2 phenotype human sera was at ~38 kDa (Supplemental figure 1). The signal for the anti-CD5L antibody and zonulin cross-reactivity in both HP1-1 and HP2-2 phenotype human sera was between 30 and 38 kDa (Supplemental figure 2).
Figure 2. Western blot images of human zonulin, properdin, FXa and CD5L recombinant proteins.
(A) Fluorescence western blot image of human recombinant proteins with goat polyclonal anti-zonulin antibody. Inverted image of 680 channel. Bands for all recombinant proteins were identified with the anti-zonulin antibody. (B) Fluorescence western blot image of human recombinant proteins with the rabbit polyclonal anti-FX antibody. Inverted image of 800 channel. Bands for all recombinant proteins were identified with the anti-FX antibody. (C) Fluorescence western blot image of human recombinant proteins with rabbit polyclonal anti-CD5L antibody. Inverted image of 800 channel. Bands for all recombinant proteins were identified with the anti-CD5L antibody. FX: Factor X; FXa: Factor Xa; CD5L:CD5-like
3.3. Zonulin and Properdin were recognized by anti-FX or anti-CD5L antibodies
We also examined whether zonulin and properdin were recognized by anti-FX and anti-CD5L antibodies by Western immunoblot analysis. The signal of rZonulin and rProperdin with anti-FX antibody was ~50 kDa, and the signals of rFXa were at ~30 kDa and ~15 kDa (Figure 2B). The signal of rCD5L by anti-FX antibody was also confirmed at ~38 kDa (Figure 2B). The signal of rZonulin and rProperdin with anti-CD5L antibody was ~50 kDa, and the signal of rCD5L was at ~38 kDa (Figure 2C). The signal of rFXa by anti-CD5L antibody was also confirmed at ~30 kDa (Figure 2B).
3.4. Zonulin, Properdin, FXa and CD5L were under-estimated by commercial zonulin ELISA kit
We performed zonulin ELISA by using a commercially available kit (Immunodiagnostik, Germany) to check the detection of these two new putative members of the ZFPs. Of note, neither pre-HP2 nor properdin are detected by this ELISA, even though the kits antibody can identify both by Western immunoblot [10]. Similarly, this ELISA under-estimated the loaded concentration of rFXa and rCD5L (10 μg/mL). To establish whether the tertiary conformation of these two proteins could affect their detection by the ELISA assay, each recombinant protein was pre-incubated at different temperatures (4°C, 37°C, 65°C) and then tested with the ELISA assay. Despite these treatments, the concentrations of all recombinant proteins continued to be under-estimated by the ELISA, and there was no statistical difference between different temperatures in all recombinant proteins (Supplemental figure 3).
3.5. rFXa and rCD5L decreased epithelial barrier competency
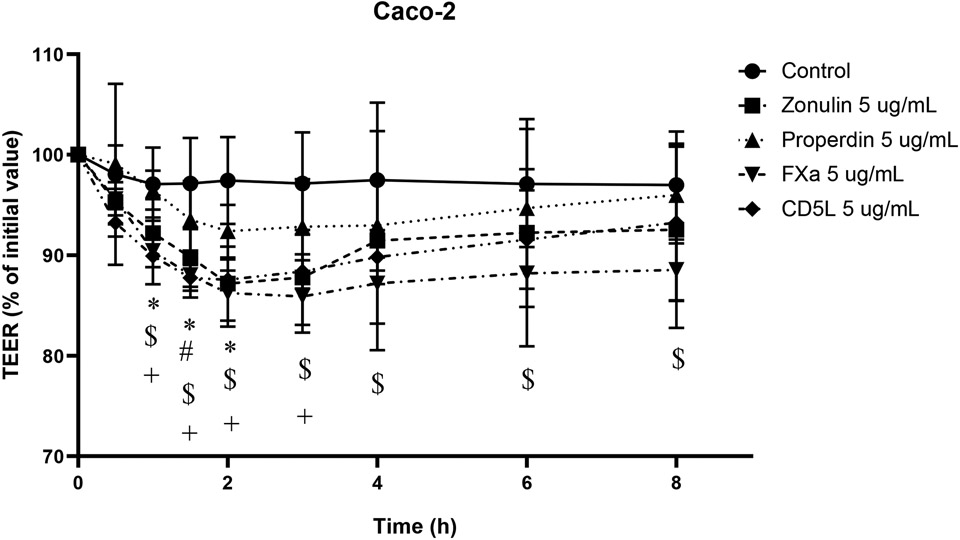
To investigate whether FXa and CD5L have a biological effect on epithelial barrier function, we performed the TEER measurement assay using Caco-2 cell culture monolayers. The Caco-2 monolayers were either untreated (negative control) or apically treated with positive controls (rZonulin or rProperdin), rFXa, or rCD5L, and the TEER was recorded up to 8h after the start of treatment as denoted in Figure 3. TEER decreased by 15% with 5 μg/mL of rZonulin from baseline to 2 hours after treatment as compared to control, and then began to recover (Figure 3). TEER was decreased within 10% with 5 μg/mL of rProperdin from initial treatment to 2 hours after treatment as compared to control, and then began to recover, (Figure 3). Compared to the positive controls, rFXa and rCD5L showed comparable TEER drops and it was also similar to the previously reported for pre-HP2 [6, 9]. Specifically, TEER decreased by 15% with 5 μg/mL of rFXa from baseline to 3 hours after treatment as compared to control, and then began to recover (Figure 3). TEER was decreased by 15% with 5 μg/mL of rCD5L from initial treatment to 2 hours after treatment as compared to control, and then began to recover (Figure 3).
Figure 3. The TEER (% of initial value) of Caco-2 monolayer with human recombinants.
Transepithelial electrical resistance (TEER) in Caco-2 control cells or treated with 5 μg/mL of recombinant zonulin, properdin, FXa or CD5L for 8 h. TEER values were reported in terms of percentage with respect to the initial value. There was a decrease in the TEER % initial value of the monolayer treated with each recombinant compared to control. * Control vs. 5 μg/mL of zonulin-treated cells. # Control vs. 5 μg/mL of properdin-treated cells. $ Control vs. 5 μg/mL of FXa-treated cells. + Control vs. 5 μg/mL of CD5L-treated cells (*: p < 0.05, #: p < 0.05, $: p < 0.05, +: p < 0.05). FX: Factor X; FXa: Factor Xa; CD5L:CD5-like antigen
4. Discussion
Zonulin is a reversible regulator of intercellular TJs in human health and disease [19]. TJ are the most apical junctional complexes of the paracellular pathway thereby separating the apical and basolateral cellular compartments by forming the epithelial barrier [2]. They regulate paracellular Ag trafficking and are dynamic structures that operate in several important functions of the human epithelium under both physiological and pathological circumstances including in the human intestine [3, 4]. Chronic inflammatory diseases including autoimmune [7, 20], metabolic [21], gastrointestinal [22, 23] and neurologic disorders [24] have been associated with epithelial barrier disruption and Ag trafficking secondary to zonulin. Zonulin is a family of related proteins [6, 7, 19] that evolved from complement-associated proteins (MASP). These proteins lost their protease function due to mutations in the catalytic domain which leads to the acquisition of new functions, such as regulating intercellular TJs [6]. It is considered that the frequent zonulin polymorphisms secondary to high mutation rates during evolution led to the development of the ZFP [7]. The first identified member of the ZFP was pre-HP2, the precursor protein to HP, which is a complement-associated protein [9]. The second member was properdin, a regulator of the alternative pathway by significantly increasing the half-life of the complement component 3 (C3) and 5 (C5) convertases and also a member of MASP family [10]. Other members of the MASP family include a series of plasminogen-related growth factors, such as EGF, and hepatocyte growth factor (HGF), involved in cell growth, proliferation, differentiation and migration, and disruption of intercellular junctions [15]. In this context, it is likely that other MASP members are also ZFP members with additional functions that affect the epithelial barrier. Therefore, this study aimed at identifying additional ZFP members using mass spectrometry, and western blot and TEER assays to confirm overlapping structural components and function. As a result, we identified FX and CD5L as new ZFP candidates.
FX is a vitamin-K dependent serine protease zymogen that is activated in the first common step of the intrinsic and extrinsic pathways of blood coagulation. FXa, the activated form of FX, is an active serine protease and has high homology to the trypsin family of serine proteases [25]. Fxa is involved in propagation of the coagulant response and cell signaling pathways linked with the inflammatory response [26]. This signaling function of FXa is mediated through protease activated receptor (PAR) cleavage and PAR2 activation occurs in extravascular environments specifically by macrophage synthesized FX [27]. Zonulin regulates intestinal permeability through direct activation of EGF receptor (EGFR) or PAR2 mediated EGFR transactivation [6]. Using MOTIF search, (https://www.genome.jp/tools/motif/), HP and FX have the protein motifs of trypsin and domains of unknown function (DUF) 1986 in common (Table 2). According to the UniProt (https://www.uniprot.org/) [28], which is a freely accessible database of protein sequences annotated with functional information, DUF1986 is one of the DUF members, and it was found in serine proteases and is predicted to contain disulphide bonds. From the above, it is conceivable that FX has some connection with zonulin/pre-HP2 in terms of cell signaling and molecular function. In our experiments, we confirmed the structural similarity of FXa with zonulin by Western immunoblot. We also confirmed functional similarity as FXa decreased epithelial barrier function of Caco-2 epithelial cell cultures.
CD5L is a macrophage-secreted protein, originally named Sp alpha [29]. It belongs to the SRCR family of proteins, and has SRCR domains 1, 2 and 3 [17, 30]. The SRCR domain consists of 90 – 110 residues containing 6 – 8 cysteines with a well-conserved disulfide bond pattern [30, 31], and SRCR domains are present in over 30 different secreted and/or membrane anchored proteins. Although the genes that encode SRCR domain-containing proteins are easy to predict from genome sequences, these proteins are difficult to classify in a particular family of proteins [32]. Factor I is a major regulator of complement activation, and FI can degrade activated C3b and C4b in the presence of its cofactors and dismantle C3b convertase of all pathways. FI has five domains one of which is SRCR [16], and FI has also been reported to have similar function as an enzyme with MASP-3. This is pertinent as homology in the evolution of immune reactions between SRCR and MASP have been reported and they are considered to have some overlapping functions [16, 33]. Like CD5L’s function within the complement system, properdin causes production of chemotactic anaphylatoxin C3a and C5a with subsequent formation of immune complexes that leads to the increase of endothelial permeability [34], and pre-HP2 also causes the increase of vascular permeability by generation of C3a and C5a in several tissues, including lung [15]. Moreover, according to the GO (Gene Ontology) Annotations, which are statements about the function of a particular gene and created by associating a gene or gene product, of UniProt (https://www.uniprot.org/) [18, 28], it is considered that CD5L has serine-type endopeptidase activity. Hence, CD5L was considered a potential ZFP member. In this study, we confirmed the structural similarity of CD5L with zonulin by western blot. We also confirmed CD5L decreased epithelial barrier function of Caco-2 epithelial, and this indicated that CD5L has functional similarity with zonulin.
These findings and the similarities in structure and function suggest that FX and CD5L are new ZFP members. However, recombinant FX and CD5L were not recognized by commercial zonulin ELISA, as is the case with pre-HP2 and properdin. One conceivable explanation of these results is that zonulin, properdin, FX and CD5L are not the main targets detected by this ELISA. Considering that the signal could be detected by this ELISA kit in human serum sample [10] and that the anti-zonulin Ab of this kit recognize pre-HP2 and properdin, it is also possible that tertiary and quaternary (multimers) structure arrangements present in sera samples but not in recombinant proteins are necessary to properly detect any ZFP member by this ELISA.
In conclusion, our research contributes to the growing family of zonulin proteins. Additional proteins are likely to be identified, we thereby propose a new nomenclature for ZFP members as follows, pre-HP2: Zonulin-A, Properdin: Zonulin-B, FX: Zonulin-C, and CD5L: Zonulin-D. The role of these new ZFPs in human disease related to gastrointestinal epithelial barrier dysfunction must be further examined.
Supplementary Material
Supplemental figure 1. Fluorescence western blot image of human serum samples (HP1-1 and HP2-2 phenotype) with anti-FX and anti-zonulin antibodies. Colored images of rabbit anti-FX antibody (800 green channel) and goat anti-zonulin antibody (680 red channel), and merged image of 800 green and 680 red channels. Predicted molecular mass weight of FX is 12 kDa and 30 kDa. There was overlap in the bands around 38 kDa and 15 kDa by anti-FX and anti-zonulin antibodies in patient serum samples. HP: haptoglobin]
Supplemental figure 2. Fluorescence western blot image of human serum samples (HP1-1 and HP2-2 phenotype) with anti-CD5L and anti-zonulin antibody. Colored images of rabbit anti-CD5L antibody (800 green channel) and goat anti-zonulin antibody (680 red channel), and merged image of 800 green and 680 red channels. Predicted molecular mass weight of CD5L is 38 kDa. There was overlap in the bands around 38 kDa by anti-CD5L and anti-zonulin antibodies in patient serum samples. HP: haptoglobin; r-CD5L: recombinant human CD5L, r-zonulin: recombinant human zonulin.
Supplemental figure 3. Zonulin ELISA results of human recombinant zonulin, properdin, FXa and CD5L in different incubation temperatures. All recombinant proteins were loaded at 10 μg/mL. The concentrations of all recombinant proteins were lower than predicted concentration (10 μg/mL), and at a similar level between all recombinant proteins. There was also no statistical difference between different temperatures.
Highlights.
- Zonulin is not a single protein, rather a family of structurally and functionally related proteins, now referred to as the zonulin family proteins (ZFPs).
- Human coagulation factor X (FX) and CD 5 antigen-like (CD5L) were identified as new members of ZFPs based on evolutionary links, similar protein structure by Mass spectrometry and Western Blotting analysis and function by Trans Epithelial Electrical Resistance (TEER) changes, so structurally and functionally resembling pre-HP2 and properdin, the two other ZFPs identified so far.
- Additional ZFPs are likely to be identified, therefore we propose the following new nomenclature for ZFPs: pre-HP2: Zonulin-A, Properdin: Zonulin-B, FX: Zonulin-C, and CD5L: Zonulin-D.
Funding
This study was supported by a grant from the National Institutes of Health [NIH 1K23DK128634-01 (EEM)] and by a grant from the European Commission by means of the Horizon 2020 program [H2020-SC1-BHC-03-2018, the project ID 825033 (AF)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics statement
Human serum samples were obtained as part of a protocol examining proteins associated with gastrointestinal function. This protocol for human study was reviewed and approved by the ethics committee of the Boston Children’s Hospital (BCH) Institutional Review Board (IRB). All study procedures were performed in accordance with BCH’s research guidelines and Massachusetts General Hospital’s (MGH) guidelines. All enrolled patient’s parents/guardians provided informed consent, and patients provided consent or assent when applicable.
Declaration of competing interests
TK was financially supported by The Uehara Memorial Foundation Overseas Postdoctoral Fellowships and The Ito Foundation for the Promotion of Medical Science Travel Grants for Overseas Exchange. Other authors have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum SE. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000. Apr 29;355(9214):1518–9. doi: 10.1016/S0140-6736(00)02169-3. [DOI] [PubMed] [Google Scholar]
- [2].Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009. Aug;1(2):a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005. Sep;2(9):416–22. doi: 10.1038/ncpgasthep0259. [DOI] [PubMed] [Google Scholar]
- [4].Sawada N Tight junction-related human diseases. Pathol Int. 2013. Jan;63(1):1–12. doi: 10.1111/pin.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000. Dec;113 Pt 24:4435–40. doi: 10.1242/jcs.113.24.4435. [DOI] [PubMed] [Google Scholar]
- [6].Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011. Jan;91(1):151–75. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- [7].Fasano A All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020. Jan 31;9:F1000 Faculty Rev–69. doi: 10.12688/f1000research.20510.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fasano A Zonulin measurement conundrum: add confusion to confusion does not lead to clarity. Gut. 2021. Oct;70(10):2007–2008. doi: 10.1136/gutjnl-2020-323367. [DOI] [PubMed] [Google Scholar]
- [9].Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, Zhao A, Yang S, Arrietta MC, Meddings JB, Fasano A. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci U S A. 2009. Sep 29;106(39):16799–804. doi: 10.1073/pnas.0906773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Scheffler L, Crane A, Heyne H, Tönjes A, Schleinitz D, Ihling CH, Stumvoll M, Freire R, Fiorentino M, Fasano A, Kovacs P, Heiker JT. Widely Used Commercial ELISA Does Not Detect Precursor of Haptoglobin2, but Recognizes Properdin as a Potential Second Member of the Zonulin Family. Front Endocrinol (Lausanne). 2018. Feb 5;9:22. doi: 10.3389/fendo.2018.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Martinez EE, Zurakowski D, Pereira L, Freire R, Emans JB, Nurko S, Duggan CP, Fasano A, Mehta NM. Interleukin-10 and Zonulin Are Associated With Postoperative Delayed Gastric Emptying in Critically Ill Surgical Pediatric Patients: A Prospective Pilot Study. JPEN J Parenter Enteral Nutr. 2020. Nov;44(8):1407–1416. doi: 10.1002/jpen.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Martinez EE, Lan J, Konno T, Miranda-Ribera A, Fiorentino M, Mehta NM, Fasano A. Novel role of zonulin in the pathophysiology of gastro-duodenal transit: a clinical and translational study. Sci Rep. 2021. Nov 17;11(1):22462. doi: 10.1038/s41598-021-01879-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, Kalet-Litman S, Anbinder Y, Lache O, Nakhoul FM, Asaf R, Farbstein D, Pollak M, Soloveichik YZ, Strauss M, Alshiek J, Livshits A, Schwartz A, Awad H, Jad K, Goldenstein H. Haptoglobin: basic and clinical aspects. Physiol Rev. Antioxid Redox Signal 2010. Feb;12(2):293–304. doi 10.1089/ars.2009.2793. [DOI] [PubMed] [Google Scholar]
- [14].Kurosky A, Barnett DR, Lee TH, Touchstone B, Hay RE, Arnott MS, Bowman BH, Fitch WM. Covalent structure of human haptoglobin: a serine protease homolog. Proc Natl Acad Sci U S A. 1980. Jun;77(6): 3388–3392. doi: 10.1073/pnas.77.6.3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang MS, Grailer JJ, Zetoune FS, Andjelkovic AV, Fasano A, Ward PA. Zonulin as prehaptoglobin2 regulates lung permeability and activates the complement system. Am J Physiol Lung Cell Mol Physiol. 2013. Jun 15; 304(12): L863–L872. doi: 10.1152/ajplung.00196.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dobó J, Kocsis A, Dani R, Gál P. Proprotein Convertases and the Complement System. Front Immunol. 2022. Jul 6;13:958121. doi: 10.3389/fimmu.2022.958121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sanchez-Moral L, Ràfols N, Martori C, Paul T, Téllez É, Sarrias MR. Multifaceted Roles of CD5L in Infectious and Sterile Inflammation. Int J Mol Sci. 2021. Apr 15;22(8):4076. doi: 10.3390/ijms22084076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gaudet P, Livstone MS, Lewis SE, Thomas PD Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief Bioinform. 2011. Sep;12(5):449–62. doi: 10.1093/bib/bbr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016. Oct 21;4(4):e1251384. doi: 10.1080/21688370.2016.1251384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wood Heickman LK, DeBoer MD, Fasano A. Zonulin as a potential putative biomarker of risk for shared type 1 diabetes and celiac disease autoimmunity. Diabetes Metab Res Rev. 2020. Jul;36(5):e3309. doi: 10.1002/dmrr.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernandez-Real JM. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS One. 2012;7(5):e37160. doi: 10.1371/journal.pone.0037160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Caviglia GP, Dughera F, Ribaldone DG, Rosso C, Abate ML, Pellicano R, Bresso F, Smedile A, Saracco GM, Astegiano M. Serum zonulin in patients with inflammatory bowel disease: a pilot study. Minerva Med. 2019. Apr;110(2):95–100. doi: 10.23736/S0026-4806.18.05787-7. [DOI] [PubMed] [Google Scholar]
- [23].Tarko A, Suchojad A, Michalec M, Majcherczyk M, Brzozowska A, Maruniak-Chudek I. Zonulin: A Potential Marker of Intestine Injury in Newborns. Dis Markers. 2017;2017:2413437. doi: 10.1155/2017/2413437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Díaz-Coránguez M, Segovia J, López-Ornelas A, Puerta-Guardo H, Ludert J, Chávez B, Meraz-Cruz N, González-Mariscal L. Transmigration of neural stem cells across the blood brain barrier induced by glioma cells. PLoS One. 2013. Apr 5;8(4):e60655. doi: 10.1371/journal.pone.0060655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hertzberg M Biochemistry of factor X. Blood Rev. 1994. Mar;8(1):56–62. doi: 10.1016/0268-960x(94)90007-8. [DOI] [PubMed] [Google Scholar]
- [26].Neuenschwander PF. COAGULATION CASCADE ∣ Factor X. Encyclopedia of Respiratory Medicine. 2006, Pages 499–503. doi.org/ 10.1016/B0-12-370879-6/00085-5 [DOI] [Google Scholar]
- [27].Ruf W Roles of factor Xa beyond coagulation. J Thromb Thrombolysis. 2021. Aug;52(2):391–396. doi: 10.1007/s11239-021-02458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].UniProt Consortium. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021. Jan 8;49(D1):D480–D489. doi: 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gebe JA, Kiener PA, Ring HZ, Li X, Francke U, Aruffo A. Molecular cloning, mapping to human chromosome 1 q21-q23, and cell binding characteristics of Spalpha, a new member of the scavenger receptor cysteine-rich (SRCR) family of proteins. J Biol Chem. 1997. Mar 7;272(10):6151–8. doi: 10.1074/jbc.272.10.6151. [DOI] [PubMed] [Google Scholar]
- [30].Sarrias MR, Padilla O, Monreal Y, Carrascal M, Abian J, Vives J, Yélamos J, Lozano F. Biochemical characterization of recombinant and circulating human Spalpha. Tissue Antigens. 2004. Apr;63(4):335–44. doi: 10.1111/j.0001-2815.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- [31].Sarrias MR, Grønlund J, Padilla O, Madsen J, Holmskov U, Lozano F. The Scavenger Receptor Cysteine-Rich (SRCR) domain: an ancient and highly conserved protein module of the innate immune system. Crit Rev Immunol. 2004;24(1):1–37. doi: 10.1615/critrevimmunol.v24.i1.10. [DOI] [PubMed] [Google Scholar]
- [32].Sanjurjo L, Aran G, Roher N, Valledor AF, Sarrias MR. AIM/CD5L: a key protein in the control of immune homeostasis and inflammatory disease. J Leukoc Biol. 2015. Aug;98(2):173–84. doi: 10.1189/jlb.3RU0215-074R. [DOI] [PubMed] [Google Scholar]
- [33].Dzik JM. The ancestry and cumulative evolution of immune reactions. Acta Biochim Pol. 2010;57(4):443–66. [PubMed] [Google Scholar]
- [34].Kouser L, Abdul-Aziz M, Nayak A, Stover CM, Sim RB, Kishore U. Properdin and factor h: opposing players on the alternative complement pathway “see-saw”. Front Immunol. 2013. Apr 23;4:93. doi: 10.3389/fimmu.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. Fluorescence western blot image of human serum samples (HP1-1 and HP2-2 phenotype) with anti-FX and anti-zonulin antibodies. Colored images of rabbit anti-FX antibody (800 green channel) and goat anti-zonulin antibody (680 red channel), and merged image of 800 green and 680 red channels. Predicted molecular mass weight of FX is 12 kDa and 30 kDa. There was overlap in the bands around 38 kDa and 15 kDa by anti-FX and anti-zonulin antibodies in patient serum samples. HP: haptoglobin]
Supplemental figure 2. Fluorescence western blot image of human serum samples (HP1-1 and HP2-2 phenotype) with anti-CD5L and anti-zonulin antibody. Colored images of rabbit anti-CD5L antibody (800 green channel) and goat anti-zonulin antibody (680 red channel), and merged image of 800 green and 680 red channels. Predicted molecular mass weight of CD5L is 38 kDa. There was overlap in the bands around 38 kDa by anti-CD5L and anti-zonulin antibodies in patient serum samples. HP: haptoglobin; r-CD5L: recombinant human CD5L, r-zonulin: recombinant human zonulin.
Supplemental figure 3. Zonulin ELISA results of human recombinant zonulin, properdin, FXa and CD5L in different incubation temperatures. All recombinant proteins were loaded at 10 μg/mL. The concentrations of all recombinant proteins were lower than predicted concentration (10 μg/mL), and at a similar level between all recombinant proteins. There was also no statistical difference between different temperatures.