Abstract
Anaplasma marginale is transmitted biologically by infected ticks or mechanically by biting flies and contaminated fomites. In tick-free areas, such as southern Uruguay, horseflies could be the principal vectors of this pathogen for bovines, causing anaplasmosis. The objective of this work was to detect the presence of A. marginale by MSP-5 PCR and Sanger sequencing in the most prevalent species of horseflies obtained using different collection methods in Colonia, Tacuarembó and Paysandú, Uruguay. Eight horsefly species were tested (Dasybasis missionum, Poeciloderas lindneri, Tabanus campestris, T. claripennis, T. fuscofasciatus, T. platensis, T. tacuaremboensis and T. triangulum); four species were found to be positive for A. marginale, with D. missionum and P. lindneri having the most frequent infections, while only one individual each of T. fuscofasciatus and T. tacuaremboensis was positive. Both D. missionum and P. lindneri were positive for A. marginale in tick-free areas, and the implications are discussed in this report.
Subject terms: Molecular biology, Parasitology, Pathogens
Introduction
Anaplasma marginale Theiler, 1910 (Rickettsiales: Anaplasmataceae) is an intracellular pathogen endemic to tropical and subtropical areas worldwide. Infection of cattle with A. marginale causes bovine anaplasmosis, a mild to severe haemolytic disease that results in considerable economic losses to both the dairy and beef industries. Transmission of A. marginale to cattle occurs biologically by ticks and mechanically by biting flies and by blood-contaminated fomites1. In recent studies, biological transmission by ticks was reported to be more efficient than mechanical transmission by Stomoxys calcitrans Linnaeus, 1758 (Diptera: Muscidae), the stable fly2, and the horsefly Tabanus fuscicostatus Hine, 1906 (Diptera: Tabanidae) in the southeastern United States3. Despite this, mechanical transmission by horseflies is considered important to anaplasmosis epidemiology4–6. In tick-free areas where anaplasmosis outbreaks are often detected, transmission by flies and fomites deserves further investigation3,5,7.
Uruguay is located between latitudes 30° and 35°S and longitudes 53° and 58°W, has a temperate climate and is considered marginal for the development of cattle ticks8. The country has a bovine population that exceeded 11.8 million animals in 20219 in two areas with the occurrence of Rhipicephalus (Boophilus) microplus (Canestrini, 1888) (Ixodida, Ixodidae), a biological vector of A. marginale. The northern area of the Rio Negro is considered tick infested, and the southern area has variable infestations with a tick-free area10. This generates enzootic instability in a herd. Due to this instability, tick-borne diseases are widely distributed in the Uruguayan territory and cause substantial economic losses in the country due to the cost of control measures and animal losses11–13.
There is no information on the potential of horseflies to carry A. marginale in tick-free areas or in infested areas. The Tabanidae diversity in Uruguay comprises 46 species in 14 genera14,15 and allows us to infer that some species may be positive for this pathogenic agent and that, therefore, they have great epidemiological importance in anaplasmosis as well as in bovine parasitic sadness. The importance of horseflies can be even greater in locations where there is no occurrence of the biological vector, where there was no introduction of animals from areas with high infestation of ticks or where there was no possibility of fomites contacting animals on a property. Therefore, the objective of this work was to detect the presence of A. marginale DNA by PCR and DNA sequencing in the most prevalent species of horseflies using manual (feeding on cattle) or NZI trap collection methods in tick-free areas and infested areas in Uruguay.
Results
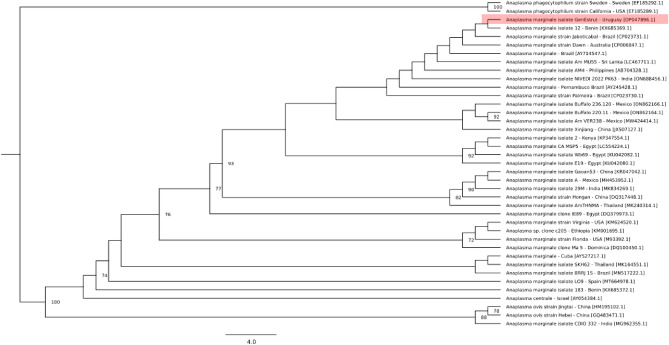
The GenBank search along with our sequences resulted in a final dataset of 39 sequences from 19 countries. The msp5 sequences obtained from GenBank varied in length (351–1146 nt), and after alignment and removal of ambiguous regions with Gblocks, resulted in an alignment of 341 nt, of which 122 nt were parsimony-informative and 193 nt were constant sites. The best-fitting evolutionary model indicated by ModelFinder (according to the Bayesian information criterion (BIC)) was K2P + G4. The GenEstrut isolate was grouped with strains and isolates of A. marginale from Brazil, Benin, Australia, Sri Lanka, the Philippines, India, Mexico, China, Kenya, Egypt and Thailand with 93% bootstrap support (Fig. 1).
Figure 1.
Characterization of the A. marginale partial msp5 sequence. The tree was constructed using the maximum likelihood method with the evolutionary model K2P + G4. The numbers in the tree indicate bootstrap values for the branch nodes. Sequences of A. phagocytophilum were used as outgroups.
Frequency
According to visualization of the electrophoresis gel, the molecular weight of the gene amplified by PCR was approximately 354 bp. The gene sequence is available from GenBank under accession OP047896. PCR for the MSP-5 gene indicated the presence of A. marginale in 26 (26.8%) of 98 specimens of tabanids. A higher prevalence was observed in P. lindneri, with 85% PCR positivity. The second highest prevalence was detected in D. missionum, at 26%, followed by T. fuscofasciatus and T. tacuaremboensis, with just one positive case each (Table 1). T. campestris, T. claripennis, T. aff. platensis and T. triangulum were not PCR positive for A. marginale (Table 1).
Table 1.
Species and total specimens of Tabanidae collected using different methods to detect A. marginale by PCR and Sanger sequencing in the departments of Tacuarembó, Paysandú and Colonia, Uruguay. M, manually; NZi, NZi trap. Number of individuals (positive individuals).
| Species | Tacuarembó | Paysandú | Colonia | Total | ||
|---|---|---|---|---|---|---|
| Methods | ||||||
| M | M | NZi | M | NZi | ||
| Number of individuals (Positive individuals) | ||||||
| D. missionum (Macquart), 1838 | 7 (5) | 0 | 0 | 0 | 15 (1) | 22 (6) |
| P. lindneri (Kröber), 1929 | 0 | 3 (2) | 11 (11) | 4 (3) | 2 (2) | 20 (18) |
| T. aff. platensis Brèthes, 1910 | 0 | 0 | 2 | 0 | 1 | 3 |
| T. campestris Brèthes, 1910 | 0 | 0 | 1 | 0 | 0 | 1 |
| T. claripennis (Bigot), 1892 | 0 | 0 | 0 | 0 | 2 | 2 |
| T. fuscofasciatus Macquart, 1838 | 0 | 14 (1) | 0 | 4 | 0 | 18 (1) |
| T. triangulum Wiedemann, 1828 | 0 | 0 | 0 | 0 | 21 | 21 |
| T. tacuaremboensis Krolow, Lucas & Henriques, 2022 | 0 | 1 (1) | 0 | 0 | 8 | 9 (1) |
| Total | 7 (5) | 18 (4) | 14 (11) | 8 (3) | 49 (3) | 96 (26) |
More MSP-5 PCR-positive tabanids were detected with manual sampling from animals being fed upon (68.8%) than with NZI traps (18.3%), independent of the location (Table 1).
The A. marginale-positive specimens were more proportionally abundant at Taquarembó, with 71% of D. missionum individuals positive, followed by Paysandu, with 47%, and Colonia, with 10% of individuals positive for A. marginale. At the three locations with manual collections, 75% positive individuals were obtained in Tacuarembó, 37% were positive for A. marginale in Colonia, and 22% were positive in Paysandú. Regarding the collections with NZI traps, Paysandú included 78% of the individuals positive for A. marginale, while in Colonia, the percentage of positive individuals was 6.1%.
Discussion
This is the first study on the molecular detection of A. marginale in horseflies in South America and the first record of this pathogen in the species D. missionum, P. lindneri and T. fuscofasciatus. These three species join a list of more than 30 species in which A. marginale has already been detected. This represents the first occurrence of this parasite in the Poeciloderas genus and the second occurrence in a species of Dasybasis. In the genus Tabanus, A. marginale has already been detected in approximately 20 species4–6.
Another important result is the occurrence of horsefly specimens positive for A. marginale in areas free of R. microplus ticks, the main vector of this pathogen in Uruguay. On farms in the department of Colonia, tests were carried out on seven species of horsefly with high abundance that were collected in these areas, and positive specimens were found among P. lindneri, the species most frequently collected in the area15, and D. missionum.
Even though horseflies do not have the same vectorial capacity as ticks in transmitting A. marginale, it is worth noting that transmission factors may also depend on the abundance of these flies in the environment, as well as on tick biology, particularly the capacity to move from one host to another. This capacity is considered normal for 3-host ticks, such as the rocky mountain tick Dermacentor andersoni Stiles, 19083, but it may be limited or very restricted in one-host ticks such as R. microplus6. In such cases, the objective of the tick is to multiply and reinject parasites on the same animal. This amplifies the parasite burden, leading to immune failure and the appearance of clinical signs. In contrast, biting flies can transmit a small quantity of blood, acting as mechanical vectors of Anaplasma, which is responsible for epizootics, especially in areas without efficient biological vectors6, such as tick-free areas.
Until now, the role of blood-sucking flies in the transmission of A. marginale has been poorly understood, despite outbreaks of anaplasmosis occurring in tick-free areas. In the absence of substantiating evidence regarding the transmission of Anaplasma in tick-free areas, the relationship between anaplasmosis outbreaks and a high abundance of horseflies has indicated that these factors are related7,16, but this has not been effectively proven17. However, in tick-free areas of Argentina, anaplasmosis outbreaks occur for unknown reasons at 4- to 7-year intervals18. In tick-free areas, the occurrence of anaplasmosis outbreaks on farms where there were no previously infected animals or contaminated fomites draws attention to haematophagous dipterans, such as horseflies and stable flies, as potential mechanical vectors7,19. From this perspective, our results point to P. lindneri and D. missionum as potential vectors of this pathogen.
Our point of view on the importance of horseflies in the epidemiology of anaplasmosis in Uruguay and especially in tick-free areas has arisen because samples collected from organisms on animals showed a higher prevalence of Anaplasma than those collected with NZI traps. The contact between horseflies and animals can increase the probability of occurrence of Anaplasma in vectors, as observed in this work. Thus, mechanical transmission is likely the major route of dissemination for A. marginale in certain areas of the USA1, Central and South America and Africa where tick vectors are absent4,20. In addition, special attention should be given to potential reservoirs of A. marginale, which could serve as a source of infective blood for mechanical spread by various routes and biological transmission by ticks1. These are perhaps the first animals to suffer haematophagy by horseflies and can mechanically contaminate the flies (see Figure 3 in De La Fuente et al.16).
The species D. missionum and P. lindneri can be important vectors of Anaplasma in areas of enzootic instability, especially in dairy farms, feedlots and other intensive production systems, where the animals are very close to each other. The high density of animals favours the transmission of pathogens by horseflies due to their sensitive behaviour regarding the host's reaction and aggressive behaviour due to the need to ingest large amounts of blood for maturation of their oocytes21,22. This finding may also be important for addressing the geographic distribution of the species D. missionum and P. lindneri that coincide with areas of high production of beef and dairy cattle in South America. The species P. lindneri occurs in Argentina (Formosa, Chaco, Santa Fé, and Entre Ríos), Paraguay, Uruguay (Montevideo, Colonia, Paysandu and Tacuarembó)15,23 and Brazil (Mato Grosso do Sul and Rio Grande do Sul)24. D. missionum is one of the four most frequent species in Tacuarembó, Uruguay15, and is considered rare in collections from the coastal plain of Rio Grande do Sul, Pampa biome25. It was originally described from specimens collected during the Jesuit missions in Rio Grande do Sul, and its distribution extends to Argentina (Misiones, Buenos Aires, Santa Fé)23.
A. marginale was detected in only one individual each of T. fuscofasciatus and T. tacuaremboensis. T. fuscofasciatus has a wide distribution, including the Cerrado biome in the state of Goiás to Rio Grande do Sul in Brazil23,25, Uruguay (Tacuarembó and Paysandú)15, Bolivia, Argentina (Salta, Santa Fe, Formosa, Chaco, Entre Ríos, and Misiones) and Paraguay23. This species is less abundant than others15,25. T. tacuaremboensis was recently described26, and for now, this species is restricted to the Uruguayan Pampa.
The absence of positive detection in T. campestris, T. claripennis and T. aff. platensis may have been due to the low number of specimens used for each of these species to detect A. marginale, with the exception of T. triangulum. This species accounted for 22% of the specimens tested, showing no detection of the pathogen, despite being one of the most frequent species in NZI traps in the department of Colonia15. Recently, studies detected the presence of Trypanosoma kaiowa27 in 33% of specimens of T. triangulum in the coastal plain of Rio Grande do Sul and did not find other parasites of veterinary importance, despite the high abundance of this horsefly species in southern Rio Grande do Sul, Brazil25,28.
Although further studies are needed to determine the role of horseflies in the transmission of A. marginale3, especially in tick-free areas, these findings have significant implications for understanding the epidemiology of this important disease in South America.
Methods
Data collection
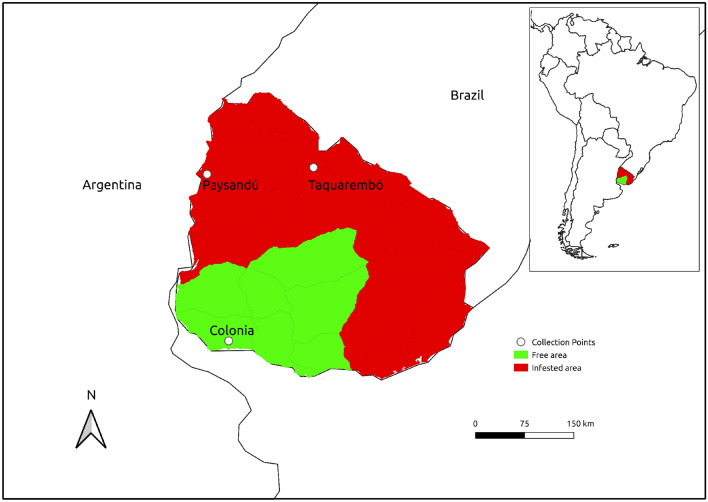
The collections were performed on farms located in the departments of Tacuarembó (three farms), Paysandú (one farm) and Colonia (two farms) and occurred between December 2017 and March 2019. These samplings were performed manually and/or with NZI traps. Using the manual sampling protocol, the horse flies were caught while feeding on animals. A defined collection time pattern was not established for the manual (feeding on cattle) or NZI trap15 method (Table 2, Fig. 2). The most abundant species were Dasybasis missionum (Macquart, 1838), Poeciloderas lindneri (Kröber, 1929), Tabanus campestris Brèthes, 1910, Tabanus claripennis Bigot, 1892, Tabanus fuscofasciatus Macquart, 1838, Tabanus platensis Brèthes, 1910, Tabanus tacuaremboensis Krolow, Lucas e Henriques, 2022 and Tabanus triangulum Wiedemann, 182815.
Table 2.
Location and capture method used for the nonsystematic horsefly collections in Uruguay.
| Identifier | Location | Department | Capture method | Total N |
|---|---|---|---|---|
| M | 31°21′37.8″S, 56°05′14.6″W | Tacuarembó | Manual (feeding on cattle) | 8 |
| M | 31°28′29.4″S, 57°53′44.4″W | Paysandú | Manual (feeding on cattle) | 4 |
| NZI | 31°28′29.4″S, 57°53′44.4″W | Paysandú | NZI trap | 33 |
| NZI | 34°17′30.2″S, 57°37′41.4″W | Colonia | NZI trap | 49 |
| M | 34°18′14.1″S, 57°31′42.7″W | Colonia | Manual (feeding on cattle) | 4 |
Figure 2.
Locations of horsefly collections in tick-free and tick-infested areas of Uruguay. The map was created using QGIS 3.22.11 (http://qgis.org).
DNA extraction, PCR and sequencing
Total DNA extraction from whole Tabanidae individuals was performed using the PureLink® Genomic DNA Mini Kit (Thermo Fisher Scientific Inc., USA). DNA concentration and quality were verified by spectrophotometry using a NanoVue™ Plus (GE Healthcare Life Sciences, USA), and only samples with absorption ratios in the range of 1.8–2.0 were subjected to PCR, following the same methodology used by Rodrigues et al.28.
The molecular detection of A. marginale was carried out using Major Surface Protein (MSP-5). This fragment is highly conserved for the genus and in all isolates of A. marginale and is widely used for the detection and confirmation of this species29. The primers used were forward (5′-GCATAGCCTCCGCGTCTTTC-3′) and reverse (5′-TCCTCGCCTTGGCCCTCAGA-3′), and the expected amplicon was 458 bp in length. The A. marginale DNA positive control was kindly supplied by F. Riet-Correa. The optimal PCR parameters were initial denaturation for 4 min at 94 °C, followed by 35 cycles of 94 °C for 1 min, 60 °C for 30 s, and 72 °C for 45 s, with a final extension of 4 min at 72 °C30.
The 25 µl PCR mixture consisted of 2 µl of DNA template (100 ng total input), 12.5 µl of GoTaq® Green Master Mix 2 ×, 1 µl each of forward and reverse primer (10 µM each primer) and enough nuclease-free water to reach the total volume. Electrophoresis of PCR products was carried out at a constant voltage of 10 V/cm by using a Bio-Rad electrophoresis assembly with a 1.2% agarose gel in 0.5 × Tris Borate EDTA (TBE) buffer28. Products were purified by excluding unwanted components from the PCR using a PureLink® PCR Purification Kit (Life Technologies, USA) following the manufacturer's instructions28.
Purified PCR products were quantified by using a UV spectrophotometer and inserted into the pCR™2.1-TOPO® vector using the TOPO® TA Cloning® Kit (Life Technologies, USA). The cloning reaction with a 6 µl volume containing 4 µl of purified PCR products, 1 µl of salt solution and 1 µl of pCR™2.1-TOPO® vector was incubated for 5 min at room temperature28. Each cloning reaction was used to transform electrocompetent Escherichia coli DH5α cells. The transformed cells were poured into Luria–Bertani agar (LB agar) plates with 100 mg/ml ampicillin and incubated overnight at 37 °C. The automatic sequencing of the positive clones was performed on a Biosystems 3500 Genetic Analyser® (Life Technologies, USA) by using a Big Dye® v3.1 Terminator Kit (Applied Biosystems, USA) (Life Technologies, USA)28.
Phylogenetic analysis
A set of reference sequences was obtained by a BLAST search of the msp5 sequence from A. marginale isolate GenEstrut against the GenBank database. Sequences with query coverage = 100%, a length > 350 bp, and available country information were selected. Additionally, msp5 sequences from Anaplasma ovis (HM195102.1 and GQ483471.1) and Anaplasma phagocytophilum (EF185292.1 and EF185289.1) were retrieved from GenBank. The nucleotide sequences were aligned using the webPRANK webserver (https://www.ebi.ac.uk/goldman-srv/webprank/)31, followed by removal of ambiguous regions with Gblocks (http://phylogeny.lirmm.fr/)32. The phylogenetic analysis was performed on the IQ-TREE webserver (http://iqtree.cibiv.univie.ac.at/)33 using the ModelFinder application to select the best evolutionary model. Branch support was assessed using ultrafast bootstrap approximation (UFBoot) with 1000 replicates34, an approximate likelihood-ratio test based on a Shimodaira-Hasegawa-like procedure (SH-aLRT) with 1000 replicates35 and an approximate Bayes test.
Acknowledgements
Tiago Kütter Krolow received a research grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-310214/2021-1). Gratchela Dutra Rodrigues received a research grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-128699/2019-1).
Author contributions
M.L. and A.S. collected the data. F.R.C., V.F.C, R.F.K. planned and supervised the study, analysed the data and wrote the manuscript. T.K.K. performed the taxonomic identification and wrote the manuscript. A.M., G.D.R., P.P., and R.F.K. analysed the data and wrote the manuscript. H.G.O., L.S.G, W.B.D., E.B. and G.D.R. performed molecular detection and wrote the manuscript. L.S.N. Phylogenetic Analysis and wrote the manuscript.
Data availability
The sequences generated and/or analysed during the current study are available in the GenBank repository: https://www.ncbi.nlm.nih.gov/nuccore/OP047896.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kocan KM, et al. The natural history of Anaplasma marginale. Vet. Parasitol. 2010;167(2–4):95–107. doi: 10.1016/j.vetpar.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Scoles GA, Broce AB, Lysyk TJ, Palmer GH. Relative efficiency of biological transmission of Anaplasma marginale (Rickettsiales: Anaplasmataceae) by Dermacentor andersoni (Acari: Ixodidae) compared with mechanical transmission by Stomoxys calcitrans (Diptera: Muscidae) J. Med. Entomol. 2005;42:668–675. doi: 10.1093/jmedent/42.4.668. [DOI] [PubMed] [Google Scholar]
- 3.Scoles GA, Miller JA, Foil LD. Comparison of the efficiency of biological transmission of Anaplasma marginale (Rickettsial: Anaplasmataceae) by Dermacentor andersoni Stiles (Acari: Ixodidae) with mechanical transmission by the horse fly, Tabanus fuscicostatus Hine (Diptera: Muscidae) J. Méd. Éntomol. 2008;45:109–114. doi: 10.1093/jmedent/45.1.109.0000-0003-3128-685X. [DOI] [PubMed] [Google Scholar]
- 4.Krinsky WL. Animal-disease agents transmitted by horse flies and deer flies (Diptera, Tabanidae) J. Med. Éntomol. 1976;13:225–275. doi: 10.1093/jmedent/13.3.225. [DOI] [PubMed] [Google Scholar]
- 5.Foil LD. Tabanids as vectors of disease agents. Parasitol. Today. 1989;5:88–96. doi: 10.1016/0169-4758(89)90009-4. [DOI] [PubMed] [Google Scholar]
- 6.Baldacchino F, et al. Tabanids: Neglected subjects of research, but vectors of disease agents Important! Infect. Genet. Evol. 2014;28:596–615. doi: 10.1016/j.meegid.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 7.Hornok S, et al. Molecular identification of Anaplasma marginale and rickettsial endosymbionts in blood-sucking flies (Diptera: Tabanidae, Muscidae) and hard ticks (Acari: Ixodidae) Vet. Parasitol. 2008;154:354–359. doi: 10.1016/j.vetpar.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Nari A. Strategies for the control of one-host ticks and relationship with tick-borne diseases in South America. Vet. Parasitol. 1995;57:153–165. doi: 10.1016/0304-4017(94)03117-F. [DOI] [PubMed] [Google Scholar]
- 9.MGAP. Anuario estadístico Agropecuario. https://descargas.mgap.gub.uy/DIEA/Anuarios/Anuario2021/LIBRO%20ANUARIO%202021%20Web.pdf (2021).
- 10.Miraballes AC, Riet-Correa F. A review of the history of research and control of Rhipicephalus (Boophilus) microplus, babesiosis and anaplasmosis in Uruguay. Exp. Appl. Acarol. 2018;75(4):383–398. doi: 10.1007/s10493-018-0278-3. [DOI] [PubMed] [Google Scholar]
- 11.De Vos AJ, Jorgensen WK. Tick Vector Biology. Springer; 1992. Protection of cattle against babesiosis in tropical and subtropical countries with a live, frozen vaccine; pp. 159–174. [Google Scholar]
- 12.Bock R, Jackson L, de Vos A, Jorgensen W. Babesiosis of cattle. Parasitology. 2004;129:247–269. doi: 10.1017/S0031182004005190. [DOI] [PubMed] [Google Scholar]
- 13.Miraballes C, Aráoz V, Riet-Correa F. Rhipicephalus microplus, babesiosis and anaplasmosis in Uruguay: Current situation and control or elimination programs on farms. Exp. Appl. Acarol. 2019;78(4):579–593. doi: 10.1007/s10493-019-00405-0. [DOI] [PubMed] [Google Scholar]
- 14.Coscarón S, Martinez M. Checklist of tabanidae (Insecta: Diptera) from Uruguay. J. Éntomol. Soc. Argent. 2019;78:40–46. doi: 10.25085/rsea.780105. [DOI] [Google Scholar]
- 15.Lucas M, et al. Diversity and seasonality of horse flies (Diptera: Tabanidae) in Uruguay. bioRxiv. 2020 doi: 10.1101/794479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De La Fuente J, et al. Potential vertebrate reservoir hosts and invertebrate vectors of Anaplasma marginale and A. phagocytophilum in Central Spain. Vector Borne Zoonot. Dis. 2005;5:390–401. doi: 10.1089/vbz.2005.5.390. [DOI] [PubMed] [Google Scholar]
- 17.Guglielmone AA, et al. Different seasonal occurrence of anaplasmosis outbreaks in beef and dairy cattle in an area of Argentina free of Boophilus microplus ticks. Vet. Q. 1997;19(1):32–33. doi: 10.1080/01652176.1997.9694735. [DOI] [PubMed] [Google Scholar]
- 18.Guglielmone AA. Epidemiology of babesiosis and anaplasmosis in South and Central America. Vet. Parasitol. 1995;57(1–3):109–119. doi: 10.1016/0304-4017(94)03115-D. [DOI] [PubMed] [Google Scholar]
- 19.Bautista-Garfias, C. et al. Molecular detection of Anaplasma marginale in stable flies Stomoxys calcitrans (Diptera: Muscidae) feeding on a tick-free bovine herd. Veterinaria México OA5(1) (2018).
- 20.Ewing SA. Transmission of Anaplasma marginale by arthropods. In: Hidalgo RJ, Jones EW, editors. Proc. 7th Nat. Anaplasmosis Conf. Mississippi State University; 1981. pp. 395–423. [Google Scholar]
- 21.Foil LD, Hogsette JA. Biology and control of tabanids, stable flies and horn flies. Rev. Sci. Tech. 1994;13:1125–1158. doi: 10.20506/rst.13.4.821. [DOI] [PubMed] [Google Scholar]
- 22.Barros ATM, Foil LD. The influence of distance on movement of tabanids (Diptera: Tabanidae) between horses. Vet. Parasitol. 2007;144:380–384. doi: 10.1016/j.vetpar.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 23.Coscarón S, Papavero N. Catalogue of neotropical Diptera. Tabanidae. Neotrop. Diptera. 2009;16:1–199. [Google Scholar]
- 24.Krolow, T. K. & Henriques, A. L. Checklist das espécies de mutucas (Diptera, Tabanidae) do estado do Mato Grosso do Sul, Brasil. Iheringia. Série Zool.107, suppl. 10.1590/1678-4766e2017131 (2017).
- 25.Krüger RF, Krolow TK. Seasonal patterns of horse fly richness and abundance in the Pampa biome of southern Brazil. J. Ecol. Vector. 2015;40:364–372. doi: 10.1111/jvec.12175. [DOI] [PubMed] [Google Scholar]
- 26.Krolow TK, Lucas M, Henriques AL. Revisiting the tabanid fauna (Diptera: Tabanidae) of Uruguay: Notes on the species of the genus Tabanus Linnaeus, with the description of a new species. Neotrop. Entomol. 2022;1:1. doi: 10.1007/s13744-022-00958-7. [DOI] [PubMed] [Google Scholar]
- 27.Fermino BR, et al. Shared species of crocodilian trypanosomes carried by tabanid flies in Africa and South America, including the description of a new species from caimans, Trypanosoma kaiowa n. sp. Parasites Vectors. 2019;12:1–17. doi: 10.1186/s13071-019-3463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodrigues GD, et al. Molecular Detection of Trypanosoma kaiowa in Tabanus triangulum (Diptera: Tabanidae) from the Coastal Plain of Rio Grande do Sul, Southern Brazil. Acta Parasitol. 2021;67:518–522. doi: 10.1007/s11686-021-00440-1. [DOI] [PubMed] [Google Scholar]
- 29.Torioni de Echaide S, et al. Detection of cattle naturally infected with Anaplasma marginale in a region of endemicity by nested PCR and a competitive enzyme-linked immunosorbent assay using recombinant major surface protein 5. J. Clin. Microbiol. 1998;36(3):777–782. doi: 10.1128/JCM.36.3.777-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parodi P, et al. Validation of a multiplex PCR assay to detect Babesia spp. and Anaplasma marginale in cattle in Uruguay in the absence of a gold standard test. J. Vet. Diagn. Investig. 2021;33(1):73–79. doi: 10.1177/1040638720975742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Löytynoja A, Goldman N. webPRANK: A phylogeny-aware multiple sequence aligner with interactive alignment browser. BMC Bioinform. 2010;11(1):1–7. doi: 10.1186/1471-2105-11-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dereeper A, et al. Phylogeny. fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36(suppl 2):465–469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen L-T, et al. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32(1):268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minh B, et al. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013;30(5):1188–1195. doi: 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst. Biol. 2006;55(4):539–552. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequences generated and/or analysed during the current study are available in the GenBank repository: https://www.ncbi.nlm.nih.gov/nuccore/OP047896.