Abstract
Discovered in 1819 in the tropical waters off Singapore, the magnificent Neptune’s cup sponge Cliona patera (Hardwicke, 1820) was harvested for museums and collectors until it was presumed extinct worldwide for over a century since 1907. Recently in 2011, seven living individuals were rediscovered in Singapore with six relocated to a marine protected area in an effort to better monitor and protect the population, as well as to enhance external fertilisation success. To determine genetic diversity within the population, we sequenced the complete mitochondrial genomes and nuclear ribosomal DNA of these six individuals and found extremely limited variability in their genes. The low genetic diversity of this rediscovered population is confirmed by comparisons with close relatives of C. patera and could compromise the population’s ability to recover from environmental and anthropogenic pressures associated with the highly urbanised coastlines of Singapore. This lack of resilience is compounded by severe predation which has been shrinking sponge sizes by up to 5.6% every month. Recovery of this highly endangered population may require ex situ approaches and crossbreeding with other populations, which are also rare.
Subject terms: Biodiversity, Conservation biology
Introduction
The marine fauna of the world is facing an extinction crisis1–3, with various anthropogenic pressures such as climate change and overharvesting driving losses in animal species living in the sea4–6. Compounding the problem, modern threats such as coastal urbanisation are wiping out entire populations of marine animals7,8. On the conservation front, well-studied fauna such as corals receive much of the limelight, typically with ominous predictions9,10. Nevertheless, a comprehensive review on sponges found no pressing need for special provisions to be made for most species globally, albeit acknowledging the lack of information on anthropogenic effects could be to their detriment11. Furthermore, recent studies predict sponges will outlast the onslaught of warming oceans wrought by climate change, even with impacts such as anoxic conditions caused by eutrophication4,12,13. Conversely, opinions on the vulnerability of populations in the near future have been gaining traction14–20.
With over 9000 species found throughout the world’s marine ecosystems21, sponges form integral components of benthic environments. They not only stabilise coral reefs via bioerosion and substrate consolidation, but are also heavily involved in nutrient cycling22,23. Recent reviews on the ecology of sponges emphasised their critical contributions to carbon, nitrogen and phosphorous biogeochemical cycling24,25. Furthermore, sponges are a rich source of secondary metabolites, with potential for novel compounds as drug candidates26–29. Despite their ecological and economic importance, many populations along coastlines are facing declines, having to endure a barrage of problems such as urbanisation, pollution, and overfishing20,30–32. Exemplifying the negative effects of anthropogenic-related pressures on natural populations in Southeast Asia, the magnificent Neptune’s cup sponge Cliona patera (Hardwicke, 1820) was harvested for museums and collectors until it became presumably extinct with the last living specimen recorded in 1907 off the coast of present day Banten, Indonesia33. Notably, in the nineteenth century, C. patera was recorded to be present in large numbers in Singapore, whose native people used to collect the sponges in droves for Europeans demanding specimens due to the species’ unique morphology34 (Fig. 1).
Figure 1.

A live specimen of Cliona patera in Singapore, photographed in November 2018.
Since the 1990s, specimens of C. patera from Australia and Southeast Asia have emerged from trawling and biodiversity surveys, suggesting that at least some remnant populations persisted26,34–37. Indeed, C. patera was rediscovered in 2011 from its type locality, Singapore, with just seven living individuals that are currently known to exist. Coastal populations of marine fauna are more adversely impacted by human activities31,32, which could reduce the rate of population recovery of C. patera in Singapore. In a bid to conserve this species, six of the seven sponges have been relocated to the Sisters’ Islands Marine Park (SIMP), a marine protected area managed by Singapore’s National Parks Board. Within that area, strict protection laws are enforced, and the site facilitates regular monitoring efforts. There is an added benefit of the aggregation which enhances the probability of external fertilisation in benthic organisms38–41. However, aggregating the sponges in a protected area might result in increased predation, particularly from large predators such as turtles, thus adversely hampering sponge recovery. Furthermore, small populations generally suffer from low genetic diversity42, limiting the long-term viability of this sponge population. The efficacy of this measure in recovering the local population of this critically endangered species has hitherto not been assessed43.
This study aims to determine the genetic diversity of the local C. patera population across multiple loci, thereby estimating their resilience. We further characterised its ecology at the relocated site to assess risks and threats to the threatened population. With some individuals facing severe predatory stress on relocation, exacerbated by negligible population genetic variability, we propose urgent recovery and conservation measures for this enigmatic species to prevent it from becoming extinct, again.
Methods
Sampling and DNA sequencing
Tissue samples of 2 cm3 were taken from six relocated C. patera individuals and fixed in 100% molecular-grade ethanol. Genomic DNA and library preparation were conducted following Quek et al.44. Briefly, genomic DNA (gDNA) was extracted using EZNA Mollusc DNA Kit with a modified elution protocol to ensure high quality gDNA was obtained. Following which, purification of gDNA was conducted using Zymo DNA Clean-up and Concentrator Kit. Bioruptor Pico (Diagenode) and KAPA dual-indexed adapters were ligated to sheared fragments using KAPA HyperPrep kit. Final libraries were size-selected following manufacturers’ protocol using Agencourt AMPure XP beads (Beckman Coulter). The samples were then pooled in equimolar concentrations with other libraries not associated with this study and sequenced on a single Illumina HiSeq 4000 (150 × 150 bp) lane to recover the complete mitochondrial genome and nuclear rRNA sequences.
Separately, amplification of the internal transcribed spacer (ITS) for sponges was conducted using ITSRA2 (5′-GTC CCT GCC CTT TGT ACA CA-3′) and ITS2.2 (5′-CCT GGT TAG TTT CTT TTC CTC CGC-3′)45. Amplified products were purified using AMPure XP (Beckman Coulter) and cycle sequenced in both directions separately using the BigDye™ terminator method and sequenced using the ABI 3130 XL Genetic Analyzer (Applied Biosystems). Chromatograms produced were then edited on Geneious Prime (Biomatters) and assembled de novo.
Genome skimming and loci identification
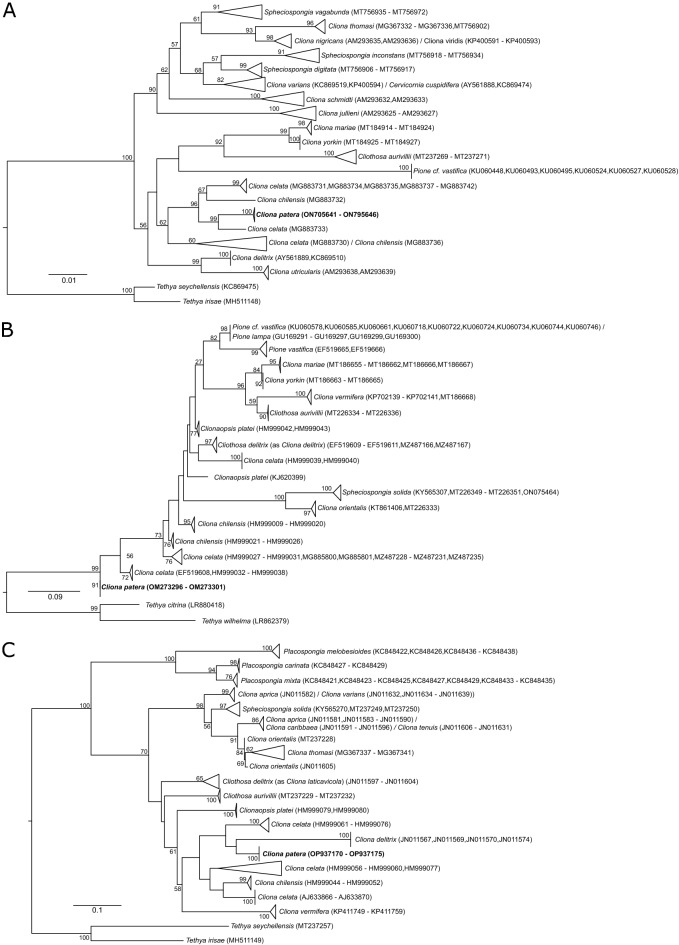
Raw reads were demultiplexed and both low quality bases and adapters were trimmed and assembled using fastp v0.20.146 and SPAdes v3.12.047 respectively, under default settings. Mitochondrial genomes were identified by BLASTn (e-value 10−6), searching against other clionaid cytochrome c oxidase subunit I (COI) sequences downloaded from GenBank (Fig. 2), with the longest contig selected as the mitochondrial genome, and circularisation of the genome was executed by circules.py48. Annotation of mitochondrial genomes was conducted using MITOS249.
Figure 2.
Maximum likelihood phylogeny of Clionaidae, with Tethya spp. as outgroup, based on three genes: (A) nuclear 28S rRNA; (B) mitochondrial cytochrome c oxidase subunit I (COI); (C) nuclear internal transcribed spacers (ITS). Numbers on nodes represent bootstrap support (≥ 50 only). Samples in bold represent those sequenced in this study.
Sequences of nuclear ribosomal gene 28S were identified in a similar fashion to that of COI, by searching against poriferan 28S sequences downloaded from GenBank using BLASTn (e-value 10−6) (Fig. 2). Only contigs with ≥ 200 bp overlap of ≥ 90% similarity to the references and minimum kmer coverage of 100 (determined by SPAdes v3.12.0) were extracted and separately assembled in Geneious Prime (Biomatters). Finally, to ensure overlapping regions between the sequences downloaded from GenBank with the assembled ribosomal sequences, we repeated the BLASTn search and extracted the overlapping region with the highest bitscore from the contig.
Pairwise sequence comparisons
To compute pairwise distances among members of Clionaidae according to the World Porifera Database21, sequences of the following genes—COI, ITS, and 28S rRNA—were downloaded from GenBank (Fig. 2). To account for inconsistent identification of sponges that could inflate intraspecific distances, we first reconstructed a maximum likelihood (ML) phylogeny for each gene separately with Tethya spp. as outgroup. Sequences downloaded were aligned by MAFFT v7.271 under –auto settings50. Alignments were manually inspected to check for overlap between individuals of each nominal taxon. The best model of DNA evolution was identified using ModelTest-NG51 and specified in ML phylogeny reconstruction using RAxML-NG v0.8.1 with 10 random and 10 parsimonious trees, and 200 bootstrap pseudoreplicates52. Each gene tree was inspected, and we only kept sequences from named taxa that were recovered as a monophyly with a minimum bootstrap support value of 50 for downstream pairwise comparisons. Retained sequences were realigned by MAFFT as above and the alignments were imported into MEGA X53 to compute intraspecific pairwise distances.
Ecological data
Growth
Monthly photographs of relocated sponges were tracked each month from June 2020 to December 2020 to estimate growth rates via SCUBA dive surveys. Photos were taken with a scale from a fixed distance and angle using an Olympus TG-5. The total height of each sponge was measured using ImageJ54. For months where visibility in the photo was low, the data were not used. Growth rate (cm/month) was computed based on the height data obtained monthly.
Regeneration capability
To determine the regeneration ability of C. patera, we cored the cup section of all six relocated individuals using a 2 cm diameter stainless steel apple corer and estimated the rate of tissue recovery in relocated C. patera individuals. Weekly surveys were performed until the hole had fully recovered. During each survey, the diameter of the cored hole was measured, and any other visual observations noted.
The surface area of the remaining hole was calculated based on the measured diameter. Recovery rate was measured by taking the difference in surface area every week, normalised by the number of days between two surveys. Average recovery rate of each sponge was calculated until the holes had completely sealed during the survey.
Results
Mitochondrial genome assembly and genetic diversity
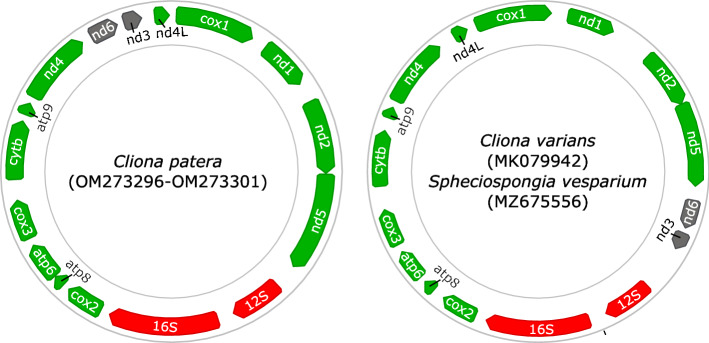
The mitochondrial genomes of all six samples sequenced were identical, with a length of 19,133 bp. Comparison of gene order of ribosomal and protein-coding genes (PCGs) between C. patera and congener C. varians found slight differences, with the block of genes comprising nad6 and nad3 adjacent to the 12S rRNA gene in C. varians, whereas it is between nad4 and nad4L in C. patera (Fig. 3).
Figure 3.
Circular maps depicting the mitochondrial gene arrangement in Clionaidae, partially reconstructed using Geneious Prime (Biomatters). Rearrangements between Cliona patera and confamilials are highlighted in grey. Annotation of C. varians and Spheciospongia vesparium was conducted using MITOS249 as GenBank records were not formally annotated.
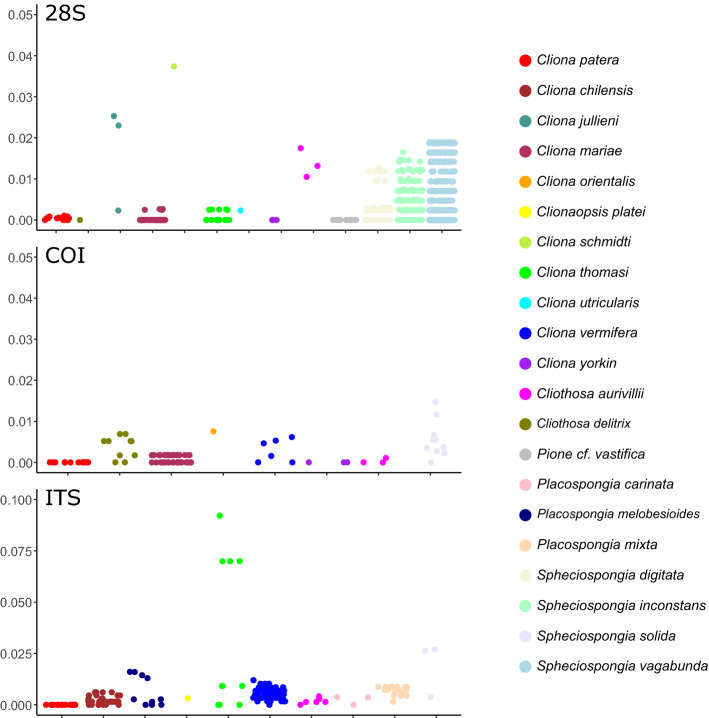
The phylogeny reconstructed showed a number of inconsistencies in taxon identification based on GenBank data (Fig. 3), corroborating studies such as those identifying cryptic species complexes in C. celata55,56. After extracting and analysing sequences only from taxa that form monophyletic groups, we found higher intraspecific pairwise differences in other clionaids, compared to genetic distances from nuclear and mitochondrial loci of C. patera that were nearly zero (Fig. 4, Tables S1–S3). Specifically, mean intraspecific pairwise distances for 28S rRNA locus in C. patera stood at 0.0423% (± SD 0.0324%), and no variability was detected for ITS and COI sequences.
Figure 4.
Intraspecific pairwise distances of clionaid species for three genes: nuclear 28S rRNA (top); mitochondrial cytochrome c oxidase subunit I (COI; middle), and nuclear internal transcribed spacers (ITS; bottom).
Growth and recovery
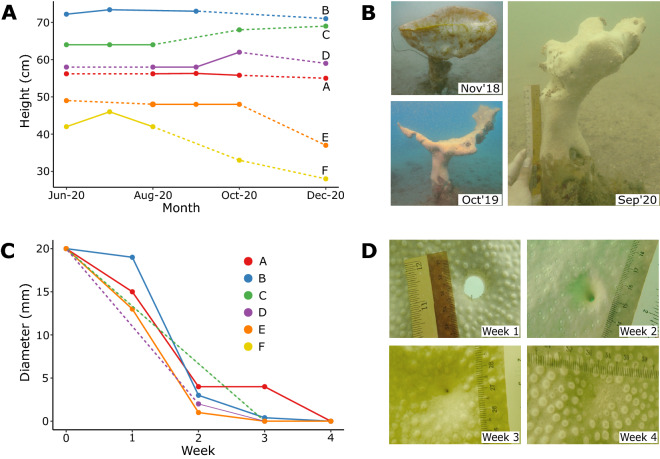
Of the six sponges observed over a period of six months, three of them (A, B, D) maintained relatively constant heights (Fig. 5A) and one individual (C) grew appreciably by 5 cm (8%). For the remaining two individuals (E and F), we recorded a loss of between 12 and 14 cm (24–33%), likely due to severe predation. Their cups were almost entirely consumed and visible bite marks were recorded on their stems (Fig. 5A,B). Nevertheless, C. patera generally exhibited remarkable rates of recovery after boring, sealing the 20 mm diameter core hole within three weeks (Fig. 5C,D). However, one of the individuals (F) did not show any sign of recovery after two weeks, and by the third week of observation, the tissue at its core had been consumed by predators.
Figure 5.
(A) Changes in height of Cliona patera sponges A–F over six months; (B) images showing changes to sponge F; (C) changes in diameter of 20 mm bored hole in sponges A–E over four weeks; sponge F was omitted due to consumption of bored area after just two weeks; (D) sponge B demonstrating full recovery by the fourth week. Solid and dotted lines connect between points with and without monthly data respectively.
Discussion
This study has provided clear evidence for two main threats against the long-term viability of the C. patera population in Singapore: low genetic variability and heavy predation pressure. Predation was so severe in some individuals that the entire cup was consumed, and would likely take a long time to recover, if at all possible. Indeed, sponge E is no longer alive (died sometime in February 2022) and only a small area of living tissue remains for sponge F. The known population in Singapore now stands at just six individuals.
The mitochondrial genome of C. patera presented a novel gene rearrangement when compared to confamilials C. varians and Spheciospongia vesparium, with the block nad6-nad3 being translocated to between nad4 and nad4l in the former, and being between nad5 and 12S rRNA in C. varians. Examination of mitochondrial gene arrangements in Demospongiae has revealed multiple rearrangements, and is particularly rife in Heteroscleromorpha that contains Clionaida57. Rapid and unique mitochondrial evolution has also been observed in other sponges, such as in Calcaronea with linear chromosomes58,59, and in Hexactinellida with a frameshifting translation strategy to cope with their deep-water environments60. Furthermore, phylogeny reconstructions of Clionaidae have found C. varians to be more closely related to Spheciospongia than C. patera (Fig. 2), though all are nested within Clionaidae. These patterns support the identical gene arrangement between C. varians and S. vesparium, but also suggest that the rearrangement occurred recently. Nevertheless, this is only the second complete mitochondrial genome sequenced for Cliona and third for Clionaidae to date. More representative data for Clionaida are needed to determine if there is indeed rapid mitochondrial gene evolution within the order (Fig. 3).
The lack of variability for the COI gene among sponge species concurs with numerous past studies, as sponges have relatively slow rates of mitochondrial evolution (Fig. 4)61–63, which could be caused by their comparatively long generation times and low metabolic rates64. Nevertheless, in contrast to other members of Clionaidae, the C. patera population in Singapore had virtually no variation for the three gene loci analysed (Fig. 4, Tables S1–S3). For example, León-Pech et al.65 found a minimum of 0.2% divergence within C. vermifera, and we detected at least some sequence variability in all species compared except C. yorkin. Alternative markers commonly used in phylogenetic and taxonomic studies of sponges include nuclear markers such as ITS and 28S loci of the ribosomal RNA (rRNA) region66, with the former being common in phylogeographic studies due to their higher degree of intraspecific variability65,67 (but see Wörheide et al.45).
The lack of variability across the whole mitochondrial genome and ribosomal genes examined here could be caused by common maternal inheritance within C. patera. Considering that this species was once thought to be extinct, the population bottleneck in the early twentieth century and the resultant small remnant population that arose from a few ancestral individuals would have diminished the gene pool markedly. Low genetic diversity increases the risk of extinction by reducing the capacity of populations to adapt to environmental changes due to the lack of variation. Furthermore, high levels of urbanisation (e.g., land reclamation) and associated anthropogenic pressures (e.g., pollution, high sedimentation rates, ship groundings) along the coastlines of the city state exert further duress on the C. patera population in Singapore. These threats are known to be detrimental for marine invertebrates68,69, even leading to the local extirpation of several species8,70. The relocation of the sponges in Singapore to a marine park would partially ameliorate some of the threats faced by the C. patera population, but this measure (i.e., the no-fishing zone) may introduce other stressors such as increased abundance of large spongivores.
With advanced sequencing technologies, it is now possible to trace population genetic patterns from museum samples collected centuries ago, based on remnant ancient DNA (aDNA) using highly rigorous techniques71. For sponges, mini-barcodes have been developed for sponge identification of museum specimens72,73 that can be extended in future studies to multi-marker assays for reconstructing population histories. Considering the large collections of C. patera available in museums around the world due to the wanton harvesting in the nineteenth century, population genetic studies of C. patera specimens around the world can be performed to detect bottlenecks in the past74. This would shed more light on the detrimental effects that anthropogenic activities have on sponge populations75,76 and potentially drive conservation efforts to limit overharvesting globally.
Predation by spongivores, especially larger taxa, are able to shape sponge communities77–79. A recent review showed that fishes are known to influence sponge distributions in the Atlantic, with some 50 sponge genera serving as a prey80. Furthermore, large predators such as the hawksbill turtle appear to have a preference for certain sponge prey, such as those with a lower spicule content81. More specifically, predators of clionaids include both vertebrates (e.g., parrotfish, pufferfish, damselfish and turtles) and invertebrates (e.g., gastropods, decapods, isopods and echinoids)82–86. For example, Mortimer et al.87 investigated sponge consumption based on 18S metabarcoding and found members of Clionaida within the gut content of a number of different spongivorous fishes in Wakatobi Marine National Park, Indonesia. Clionaids appear to be palatable to spongivores as numerous bite marks on C. patera have been recorded (Table S4).
Rapid regeneration and anti-predatory mechanisms are critical for the survival of sponges, particularly in large reef sponges such as Neofibularia nolitangere, Ircinia strobilina and Agelas clathrodes in the Caribbean88. In C. celata, rapid regeneration enabled papillae consumed to regenerate as quickly as within 12 days89. Similarly, we found that C. patera generally had remarkably high rates of recovery, taking only about three weeks to seal a 20 mm diameter core hole (Fig. 5C,D). In C. celata, regeneration rates were generally correlated with high current flow (present in SIMP), possibly aiding regeneration by reducing the amount of energy expended for feeding or waste removal89. Cliona patera could employ a similar strategy to their Caribbean counterparts, coupling mechanical defences (spicules) with rapid regeneration to maximise their survival chances under predatory stress.
Upon relocation, three of the six sponges were able to either maintain constant height, and one even grew by 8% (Fig. 5A). Nevertheless, two individuals fared poorly, with their cups almost entirely consumed (e.g. Fig. 5B). A recent paper by Wulff79 debunked the popular binary of sponges being either “palatable” or “deterrent” based on frequency of consumption of sponge pellets, proposing that sponge defenses are predator- and habitat-specific. By transplanting the sponges to SIMP, the sponges might have inadvertently been exposed to increased opportunistic predation, particularly by aggregating them together. In addition, wounding of the sponge during regeneration experiments could have altered their gene expression, requiring allocation of metabolic resources toward regeneration and possibly triggering the release of other spongivore-attracting metabolites90,91. Future conservation efforts on this sponge need to carefully consider the diversity of potential spongivores in the target site prior to any relocation of individuals. We note that only two out of the six individuals at SIMP were preyed on severely, so more data on the effects of sponge aggregation on predation are needed to further assess this strategy. Finally, considering the trade-off in resource allocation between growth and gametogenesis in clionaids92, it is highly likely that individuals E and F (Fig. 5A,B) would prioritise recovery and growth and not be sexually active for some time.
The reproduction biology of Cliona has been studied extensively for several species, including Cliothosa delitrix93, C. tenuis92, C. vermifera94, C. celata and C. viridis95. In these studies, C. vermifera and C. tenuis were determined to be gonochoric, C. celata and C. viridis hermaphoroditic, and Cliothosa delitrix mostly gonochoric with some hermaphrodites observed. With the exception of C. vermifera, all other clionaids were oviparous. In addition, gamete release generally occurred under warmer temperatures, with up to 90% of sexual reproduction occurring annually. Interestingly, despite the high percentage of Cliothosa delitrix individuals found to contain reproductive structures93, a population study across the Greater Caribbean found > 12% of the samples were clones (n = 495)96, contrary to an earlier study which found no clones (n = 47)97. Based on available literature, we propose that C. patera is unlikely to reproduce by fragmentation, similar to other stalked sponges due to its inability to regenerate the attachment stalk and poor attachment98,99. Therefore, it is critical for cross-fertilization to be the main driver for increasing not only the threatened species’ genetic diversity but also population abundance to ensure its long-term viability.
The low abundance and genetic diversity of this sponge population are pressing concerns for the species. Despite stringent legislative protection from associated anthropogenic impacts (e.g., seabed dredging, anchoring, coastal reclamation and harvesting) on the transplanted individuals, inbreeding between the small pool of individuals could continue to erode their genetic fitness, resulting in increased risk of extinction. In the future, by enacting partnerships between countries harbouring C. patera populations, with in situ individuals currently found only in Cambodia, Singapore and Thailand, the global genetic diversity of this species can be estimated across populations. Using high-throughput sequencing techniques such as those applied in this study, the global population genomics of C. patera can be examined to guide conservation action plans and enhance the species’ genetic diversity. Critically, its exceptional rarity throughout the region may require ex situ conservation efforts, including transplanting and propagating genetically distinct individuals in aquarium settings. Public aquaria harbour a rich diversity of marine organisms, often including threatened species, and many have been involved in conservation and reintroduction programmes100. However, while some clionaids have been kept in laboratory aquaria successfully for experiments, these are mostly performed for encrusting forms101–103. Clearly, the reproductive biology of C. patera and its viability in the aquarium must be carefully assessed to mitigate the risk of losing the entire population. If successful, individuals reared in captivity may be relocated back to the wild, within suitably identified sites after careful assessment as outlined here.
Supplementary Information
Acknowledgements
This research is supported by the National Research Foundation, Prime Minister’s Office, Singapore under its Marine Science R&D Programme (MSRDP-P03).
Author contributions
Z.B.R.Q. designed the research and performed the genetic analyses, J.Y.N., K.T. and D.H. designed the research and performed the field work, Z.B.R.Q., S.S.J., J.X.S.L., S.C.L. contributed to data collection. Z.B.R.Q. wrote the first draft of the manuscript, and all authors contributed to the final version.
Data availability
The datasets generated and/or analysed during the current study are available in SRA/GenBank under BioProject PRJNA796519 (raw reads), accession numbers OM273296–OM273301 (mitochondrial genomes), ON705641–ON705646 (28S rRNA) and OP937170–OP937175 (ITS sequences).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-26970-w.
References
- 1.McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Warner RR. Marine defaunation: Animal loss in the global ocean. Science. 2015;347(6219):1255641. doi: 10.1126/science.1255641. [DOI] [PubMed] [Google Scholar]
- 2.Webb TJ, Mindel BL. Global patterns of extinction risk in marine and non-marine systems. Curr. Biol. 2015;25(4):506–511. doi: 10.1016/j.cub.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 3.Pinsky ML, Fredston A. A stark future for ocean life. Science. 2022;376(6592):452–453. doi: 10.1126/science.abo4259. [DOI] [PubMed] [Google Scholar]
- 4.Bell JJ, Bennett HM, Rovellini A, Webster NS. Sponges to be winners under near-future climate scenarios. Bioscience. 2018;68(12):955–968. doi: 10.1093/biosci/biy142. [DOI] [Google Scholar]
- 5.Dulvy NK, et al. Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 2021;31(21):4773–4787.e8. doi: 10.1016/j.cub.2021.08.062. [DOI] [PubMed] [Google Scholar]
- 6.Penn JL, Deutsch C. Avoiding ocean mass extinction from climate warming. Science. 2022;376(6592):524–526. doi: 10.1126/science.abe9039. [DOI] [PubMed] [Google Scholar]
- 7.Hubbard DM, Dugan JE, Schooler NK, Viola SM. Local extirpations and regional declines of endemic upper beach invertebrates in southern California. Estuar. Coast. Shelf Sci. 2014;150(Part A):67–75. doi: 10.1016/j.ecss.2013.06.017. [DOI] [Google Scholar]
- 8.Poquita-Du RC, et al. Last species standing: loss of Pocilloporidae corals associated with coastal urbanization in a tropical city state. Mar. Biodivers. 2019;49:1727–1741. doi: 10.1007/s12526-019-00939-x. [DOI] [Google Scholar]
- 9.Hughes TP, et al. Coral reefs in the Anthropocene. Nature. 2017;546:82–90. doi: 10.1038/nature22901. [DOI] [PubMed] [Google Scholar]
- 10.Bellwood DR, et al. Coral reef conservation in the Anthropocene: Confronting spatial mismatches and prioritizing functions. Biol. Conserv. 2019;236:604–615. doi: 10.1016/j.biocon.2019.05.056. [DOI] [Google Scholar]
- 11.Bell, et al. Global conservation status of sponges. Conserv. Biol. 2015;29(1):42–53. doi: 10.1111/cobi.12447. [DOI] [PubMed] [Google Scholar]
- 12.Kelmo F, Bell JJ, Attrill MJ. Tolerance of sponge assemblages to temperature anomalies: Resilience and proliferation of sponges following the 1997–8 El-Niño southern oscillation. PLoS ONE. 2013;8(10):e76441. doi: 10.1371/journal.pone.0076441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Micaroni V, et al. Adaptive strategies of sponges to deoxygenated oceans. Glob. Change Biol. 2022;28(6):1972–1989. doi: 10.1111/gcb.16013. [DOI] [PubMed] [Google Scholar]
- 14.Di Camillo CG, Bartolucci I, Cerrano C, Bavestrello G. Sponge disease in the Adriatic Sea. Mar. Ecol. 2013;34(1):62–71. doi: 10.1111/j.1439-0485.2012.00525.x. [DOI] [Google Scholar]
- 15.Pérez T, Vacelet J. Effect of climatic and anthropogenic disturbances on sponge fisheries. In: Goffredo S, Dubinsky Z, editors. The Mediterranean Sea. Springer; 2014. pp. 577–587. [Google Scholar]
- 16.Ereskovsky A, Ozerov DA, Pantyulin AN, Tzetlin AB. Mass mortality event of White Sea sponges as the result of high temperature in summer 2018. Polar Biol. 2019;42:2313–2318. doi: 10.1007/s00300-019-02606-0. [DOI] [Google Scholar]
- 17.Lesser MP, Slattery M. Will coral reef sponges be winners in the Anthropocene? Glob. Change Biol. 2020;26(6):3202–3211. doi: 10.1111/gcb.15039. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson A, et al. Warming and acidification threaten glass sponge Aphrocallistes vastus pumping and reef formation. Sci. Rep. 2020;10:8176. doi: 10.1038/s41598-020-65220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beepat SS, Davy SK, Woods L, Bell JJ. Short-term responses of tropical lagoon sponges to elevated temperature and nitrate. Mar. Environ. Res. 2020;157:104922. doi: 10.1016/j.marenvres.2020.104922. [DOI] [PubMed] [Google Scholar]
- 20.Shore A, et al. On a reef far, far away: Anthropogenic impacts following extreme storms affect sponge health and bacterial communities. Front. Mar. Sci. 2021;8:608036. doi: 10.3389/fmars.2021.608036. [DOI] [Google Scholar]
- 21.de Voogd et al. World Porifera Databasehttps://www.marinespecies.org/porifera/ (2022).
- 22.Wulff JL. Assessing and monitoring coral reef sponges: Why and how? Bull. Mar. Sci. 2001;69(2):831–846. [Google Scholar]
- 23.Bell JJ. The functional roles of marine sponges. Estuar. Coast. Shelf Sci. 2008;79(3):341–353. doi: 10.1016/j.ecss.2008.05.002. [DOI] [Google Scholar]
- 24.Folkers M, Rombouts T. Sponges revealed: a synthesis of their overlooked ecological functions within aquatic ecosystems. In: Jungblut S, Liebich V, Bode M, editors. YOUMARES 9—The Oceans: Our Research, Our Future. Springer; 2019. pp. 181–194. [Google Scholar]
- 25.Pawlik JR, McMurray SE. The emerging ecological and biogeochemical importance of sponges on coral reefs. Ann. Rev. Mar. Sci. 2020;12:315–337. doi: 10.1146/annurev-marine-010419-010807. [DOI] [PubMed] [Google Scholar]
- 26.Sawangwong P, et al. Secondary metabolites from a marine sponge Cliona patera. Biochem. Syst. Ecol. 2008;36(5):493–496. doi: 10.1016/j.bse.2008.01.002. [DOI] [Google Scholar]
- 27.Zhang H, et al. Bioactive secondary metabolites from the marine sponge genus Agelas. Mar. Drugs. 2017;15(11):351. doi: 10.3390/md15110351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He Q, Miao S, Ni N, Man Y, Gong K. A review of the secondary metabolites from the marine sponges of the genus Aaptos. Nat. Prod. Commun. 2020;15(9):1–12. [Google Scholar]
- 29.Ho, et al. Assessing the diversity and biomedical potential of microbes associated with the Neptune’s Cup sponge, Cliona patera. Front. Microbiol. 2021;12:631445. doi: 10.3389/fmicb.2021.631445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pronzato R. Mediterranean sponge fauna: A biological, historical and cultural heritage. Biogeographia. 2003;24(1):91–99. [Google Scholar]
- 31.DiBattista JD, et al. Environmental DNA can act as a biodiversity barometer of anthropogenic pressures in coastal ecosystems. Sci. Rep. 2020;10:8365. doi: 10.1038/s41598-020-64858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halpern BS, et al. A global map of human impact on marine ecosystems. Science. 2008;319(5865):948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 33.Vosmaer GCJ. Poterion a boring sponge. K. Ned. Akad. Wet. Proc. 1908;11:37–41. [Google Scholar]
- 34.Lim, S. C., Tun, K. & Goh, E. Rediscovery of the Neptune’s Cup sponge in Singapore: Cliona or Poterion? Contributions to Marine Science 2012, 49–56 (2012).
- 35.Low MEY. The date of publication of Cliona patera (Hardwicke), the ‘sponge plant from the shores of Singapore’ (Porifera: Hadromerida: Clionaidae) Nat. Singap. 2012;5:223–227. [Google Scholar]
- 36.Knight, K. Super-rare giant sponge discovered in seahorse hotspot. Fauna & Floral Internationalhttps://www.fauna-flora.org/news/super-rare-sponge-discovered-seahorse-hotspot/ (2018).
- 37.The State of Queensland (Queensland Museum). Cliona patera. Queensland Museum Networkhttps://collections.qm.qld.gov.au/objects/73638/cliona-patera (2012–2022).
- 38.Heath DJ. Simultaneous hermaphroditism; Cost and benefit. J. Theor. Biol. 1977;64:363–373. doi: 10.1016/0022-5193(77)90363-0. [DOI] [PubMed] [Google Scholar]
- 39.André C, Lindegarth M. Fertilization efficiency and gamete viability of a sessile, free-spawning bivalve, Cerastoderma edule. Ophelia. 1995;43(3):215–227. doi: 10.1080/00785326.1995.10429833. [DOI] [Google Scholar]
- 40.Bayer SR, et al. Fertilization success in scallop aggregations: Reconciling model predictions and field measurements of density effects. Ecosphere. 2018;9(8):e02359. doi: 10.1002/ecs2.2359. [DOI] [Google Scholar]
- 41.Yund PO. How severe is sperm limitation in natural populations of marine free-spawners? Trends Ecol. Evol. 2000;15(1):10–13. doi: 10.1016/S0169-5347(99)01744-9. [DOI] [PubMed] [Google Scholar]
- 42.Frankham R. Relationship of genetic variation to population size in wildlife. Conserv. Biol. 1996;10(6):1500–1508. doi: 10.1046/j.1523-1739.1996.10061500.x. [DOI] [Google Scholar]
- 43.Lim, S. C. Porifera. Singapore Red Data Book.https://www.nparks.gov.sg/biodiversity/wildlife-in-singapore/species-list/sponge (2022).
- 44.Quek ZBR, Chang JJM, Ip YCA, Chan YKS, Huang D. Mitogenomes reveal alternative initiation codons and lineage-specific gene order conservation in echinoderms. Mol. Biol. Evol. 2021;38(3):981–985. doi: 10.1093/molbev/msaa262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wörheide G, Nichols SA, Goldberg J. Intragenomic variation of the rDNA internal transcribed spacers in sponges (Phylum Porifera): Implications for phylogenetic studies. Mol. Phylogenet. Evol. 2004;33(3):816–830. doi: 10.1016/j.ympev.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Chen S, Zhou Y, Chen Y, Gu J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bankevich A, et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19(5):455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hahn C, Bachmann L, Chevreux B. Reconstructing mitochondrial genomes directly from genomic next-generation sequencing reads: A baiting and iterative mapping approach. Nucleic Acids Res. 2013;41(13):e129. doi: 10.1093/nar/gkt371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donath A, et al. Improved annotation of protein-coding genes boundaries in metazoan mitochondrial genomes. Nucleic Acids Res. 2019;47(20):10543–10552. doi: 10.1093/nar/gkz833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Darriba D, et al. ModelTest-NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol. 2020;37(1):291–294. doi: 10.1093/molbev/msz189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics. 2019;35(21):4453–4455. doi: 10.1093/bioinformatics/btz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xavier JR, et al. Molecular evidence of cryptic speciation in the “cosmopolitan” excavating sponge Cliona celata (Porifera, Clionaidae) Mol. Phylogenet. Evol. 2010;56(1):13–20. doi: 10.1016/j.ympev.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 56.de Paula TS, Zilberberg C, Hajdu E, Lôbo-Hajdua G. Morphology and molecules on opposite sides of the diversity gradient: Four cryptic species of the Cliona celata (Porifera, Demospongiae) complex in South America revealed by mitochondrial and nuclear markers. Mol. Phylogenet. Evol. 2012;62(1):529–541. doi: 10.1016/j.ympev.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Plese B, et al. Mitochondrial evolution in the Demospongiae (Porifera): Phylogeny, divergence time, and genome biology. Mol Phylogenet Evol. 2021;155:107011. doi: 10.1016/j.ympev.2020.107011. [DOI] [PubMed] [Google Scholar]
- 58.Lavrov DV, Adamski M, Chevaldonné P, Adamska M. Extensive mitochondrial mRNA editing and unusual mitochondrial genome organization in calcaronean sponges. Curr. Biol. 2016;26(1):86–92. doi: 10.1016/j.cub.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 59.Lavrov DV, Pett W. Animal mitochondrial DNA as we do not know it: mt-genome organization and evolution in nonbilaterian lineages. Genome Biol. Evol. 2016;8(9):2896–2913. doi: 10.1093/gbe/evw195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haen KM, Pett W, Lavrov DV. Eight new mtDNA sequences of glass sponges reveal an extensive usage of + 1 frameshifting in mitochondrial translation. Gene. 2014;535(2):336–344. doi: 10.1016/j.gene.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 61.Shearer TL, van Oppen MJH, Romano SL, Wörheide G. Slow mitochondrial DNA sequence evolution in the Anthozoa (Cnidaria) Mol. Ecol. 2002;11(12):2475–2487. doi: 10.1046/j.1365-294X.2002.01652.x. [DOI] [PubMed] [Google Scholar]
- 62.Lavrov DV, Forget L, Kelly M, Lang BF. Mitochondrial genomes of two demosponges provide insights into an early stage of animal evolution. Mol. Biol. Evol. 2005;22(5):1231–1239. doi: 10.1093/molbev/msi108. [DOI] [PubMed] [Google Scholar]
- 63.Huang D, Meier R, Todd PA, Chou LM. Slow mitochondrial COI sequence evolution at the base of the metazoan tree and its implications for DNA barcoding. J. Mol. Evol. 2008;66(2):167–174. doi: 10.1007/s00239-008-9069-5. [DOI] [PubMed] [Google Scholar]
- 64.Wörheide G. Low variation in partial cytochrome oxidase subunit I (COI) mitochondrial sequences in the coralline demosponge Astrosclera willeyana across the Indo-Pacific. Mar. Biol. 2006;148:907–912. doi: 10.1007/s00227-005-0134-y. [DOI] [Google Scholar]
- 65.León-Pech MG, Cruz-Barraza JA, Carballo JL, Calderon-Aguilera LE, Rocha-Olivares A. Pervasive genetic structure at different geographic scales in the coral-excavating sponge Cliona vermifera (Hancock, 1867) in the Mexican Pacific. Coral Reefs. 2015;34:887–897. doi: 10.1007/s00338-015-1316-9. [DOI] [Google Scholar]
- 66.Yang Q, Franco CMM, Sorokin SJ, Zhang W. Development of a multilocus-based approach for sponge (phylum Porifera) identification: Refinement and limitations. Sci. Rep. 2017;7:41422. doi: 10.1038/srep41422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wörheide G, Epp LS, Macis L. Deep genetic divergences among Indo-Pacific populations of the coral reef sponge Leucetta chagosensis (Leucettidae): Founder effects, vicariance, or both? BMC Evol. Biol. 2008;8:24. doi: 10.1186/1471-2148-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lai S, Loke LHL, Hilton MJ, Bouma TJ, Todd PA. The effects of urbanisation on coastal habitats and the potential for ecological engineering: A Singapore case study. Ocean Coast. Manag. 2015;103:78–85. doi: 10.1016/j.ocecoaman.2014.11.006. [DOI] [Google Scholar]
- 69.Kuempel CD, et al. Identifying management opportunities to combat climate, land, and marine threats across less climate exposed coral reefs. Conserv. Biol. 2022;36(3):e13856. doi: 10.1111/cobi.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neo ML, et al. et al. Giant clams (Bivalvia: Cardiidae: Tridacninae): A comprehensive update of species and their distribution, current threats and conservation status. In: Hawkins SJ, et al.et al., editors. Oceanography and Marine Biology: An Annual Review. CRC Press; 2017. pp. 87–388. [Google Scholar]
- 71.Orlando L, et al. Ancient DNA analysis. Nat. Rev. Methods Prim. 2021;1:14. doi: 10.1038/s43586-020-00011-0. [DOI] [Google Scholar]
- 72.Cárdenas P, Moore JA. First records of Geodia demosponges from the New England seamounts, an opportunity to test the use of DNA mini-barcodes on museum specimens. Mar. Biodiv. 2019;49:163–174. doi: 10.1007/s12526-017-0775-3. [DOI] [Google Scholar]
- 73.Erpenbeck D, et al. Minimalist barcodes for sponges: A case study classifying African freshwater Spongillida. Genome. 2019;62(1):1–10. doi: 10.1139/gen-2018-0098. [DOI] [PubMed] [Google Scholar]
- 74.Chang D, Shapiro B. Using ancient DNA and coalescent-based methods to infer extinction. Biol. Lett. 2016;12(2):20150822. doi: 10.1098/rsbl.2015.0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pacioni C, et al. Genetic diversity loss in a biodiversity hotspot: Ancient DNA quantifies genetic decline and former connectivity in a critically endangered marsupial. Mol. Ecol. 2015;24(23):5813–5828. doi: 10.1111/mec.13430. [DOI] [PubMed] [Google Scholar]
- 76.Lombal AJ, et al. Using ancient DNA to quantify losses of genetic and species diversity in seabirds: A case study of Pterodroma petrels from a Pacific island. Biodivers. Conserv. 2020;29:2361–2375. doi: 10.1007/s10531-020-01978-8. [DOI] [Google Scholar]
- 77.Ruzicka R, Gleason DF. Sponge community structure and anti-predator defenses on temperate reefs of the South Atlantic Bight. J. Exp. Mar. Biol. Ecol. 2009;380(1–2):36–46. doi: 10.1016/j.jembe.2009.08.011. [DOI] [Google Scholar]
- 78.Loh TL, Pawlik JR. Chemical defenses and resource trade-offs structure sponge communities on Caribbean coral reefs. Proc. Natl. Acad. Sci. U.S.A. 2014;111(11):4151–4156. doi: 10.1073/pnas.1321626111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wulff JL. Targeted predator defenses of sponges shape community organization and tropical marine ecosystem function. Ecol. Monogr. 2021;91(2):e01438. doi: 10.1002/ecm.1438. [DOI] [Google Scholar]
- 80.Coppock AG, Kingsford MJ, Battershill CN, Jones GP. Significance of fish–sponge interactions in coral reef ecosystems. Coral Reefs. 2022;41:1285–1308. doi: 10.1007/s00338-022-02253-8. [DOI] [Google Scholar]
- 81.Baumbach DS, Zhang R, Hayes CT, Wright MK, Dunbar SG. Strategic foraging: Understanding hawksbill (Eretmochelys imbricata) prey item energy values and distribution within a marine protected area. Mar. Ecol. 2022;00:e12703. [Google Scholar]
- 82.Guida VG. Sponge predation in the oyster reef community as demonstrated with Cliona celata Grant. J. Exp. Mar. Biol. Ecol. 1976;25(2):109–122. doi: 10.1016/0022-0981(76)90012-5. [DOI] [Google Scholar]
- 83.Verdín PCJ, Carballo JL, Camacho ML. A qualitative assessment of sponge-feeding organisms from the Mexican Pacific coast. Open Mar. Biol. J. 2010;4:39–46. doi: 10.2174/1874450801004010039. [DOI] [Google Scholar]
- 84.Márquez JC, Zea S. Parrotfish mediation in coral mortality and bioerosion by the encrusting, excavating sponge Cliona tenuis. Mar. Ecol. 2012;33(4):417–426. doi: 10.1111/j.1439-0485.2011.00506.x. [DOI] [Google Scholar]
- 85.González-Rivero M, Ferrari R, Schönberg CHL, Mumby PJ. Impacts of macroalgal competition and parrotfish predation on the growth of a common bioeroding sponge. Mar. Ecol. Prog. Ser. 2012;444:133–142. doi: 10.3354/meps09424. [DOI] [Google Scholar]
- 86.von Brandis RG, Mortimer JA, Reilly BK, van Soest RWM, Branch GM. Diet composition of hawksbill turtles (Eretmochelys imbricata) in the Republic of Seychelles. Western Indian Ocean J. Mar. Sci. 2014;13(1):81–91. [Google Scholar]
- 87.Mortimer C, Dunn M, Haris A, Jompa J, Bell J. Estimates of sponge consumption rates on an Indo-Pacific reef. Mar. Ecol. Prog. Ser. 2021;672:123–140. doi: 10.3354/meps13786. [DOI] [Google Scholar]
- 88.Hoppe WF. Growth, regeneration and predation in three species of large coral reef sponges. Mar. Ecol. Prog. Ser. 1988;50(12):117–125. doi: 10.3354/meps050117. [DOI] [Google Scholar]
- 89.Bell JJ. Regeneration rates of a sublittoral demosponge. J. Mar. Biol. Assoc. U.K. 2002;82(1):169–170. doi: 10.1017/S0025315402005295. [DOI] [Google Scholar]
- 90.Wu Y-C, et al. Opisthobranch grazing results in mobilisation of spherulous cells and re-allocation of secondary metabolites in the sponge Aplysina aerophoba. Sci. Rep. 2020;10:21934. doi: 10.1038/s41598-020-78667-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu Y-C, Franzenburg S, Ribes M, Pita L. Wounding response in Porifera (sponges) activates ancestral signaling cascades involved in animal healing, regeneration, and cancer. Sci. Rep. 2022;12:1307. doi: 10.1038/s41598-022-05230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.González-Rivero M, et al. Life-history traits of a common Caribbean coral-excavating sponge, Cliona tenuis (Porifera: Hadromerida) J. Nat. Hist. 2013;47(45–46):1–20. [Google Scholar]
- 93.Chaves-Fonnegra A, Maldonado M, Blackwelder P, Lopez JV. Asynchronous reproduction and multi-spawning in the coral-excavating sponge Cliona delitrix. J. Mar. Biol. Assoc. U.K. 2015;96(2):515–528. doi: 10.1017/S0025315415000636. [DOI] [Google Scholar]
- 94.Bautista-Guerrero E, Carballo JL, Maldonado M. Abundance and reproductive patterns of the excavating sponge Cliona vermifera: A threat to Pacific coral reefs? Coral Reefs. 2014;33:259–266. doi: 10.1007/s00338-013-1094-1. [DOI] [Google Scholar]
- 95.Piscitelli M, Corriero G, Gaino E, Uriz M-J. Reproductive cycles of the sympatric excavating sponges Cliona celata and Cliona viridis in the Mediterranean Sea. Invertebr. Biol. 2011;130(1):1–10. doi: 10.1111/j.1744-7410.2010.00216.x. [DOI] [Google Scholar]
- 96.Chaves-Fonnegra A, Feldheim KA, Secord J, Lopez JV. Population structure and dispersal of the coral-excavating sponge Cliona delitrix. Mol. Ecol. 2015;24(7):1447–1466. doi: 10.1111/mec.13134. [DOI] [PubMed] [Google Scholar]
- 97.Zilberberg C, Maldonado M, Solé-Cava A. Assessment of the relative contribution of asexual propagation in a population of the coral-excavating sponge Cliona delitrix from the Bahamas. Coral Reefs. 2006;25:297–301. doi: 10.1007/s00338-006-0094-9. [DOI] [Google Scholar]
- 98.Wulff JL. Effects of a hurricane on survival and orientation of large erect coral reef sponges. Coral Reefs. 1995;14:55–61. doi: 10.1007/BF00304073. [DOI] [Google Scholar]
- 99.Wilkinson CR, Thompson JE. Experimental sponge transplantation provides information on reproduction by fragmentation. Proc. 8th Int. Coral Reef Symp. 1997;2:1417–1420. [Google Scholar]
- 100.da Silva R, et al. Assessing the conservation potential of fish and corals in aquariums globally. J. Nat. Conserv. 2019;48:1–11. doi: 10.1016/j.jnc.2018.12.001. [DOI] [Google Scholar]
- 101.Neumann AC. Observations on coastal erosion in Bermuda and measurements of the boring rate of the sponge, Cliona lampa. Limnol. Oceanogr. 1966;11(1):92–108. doi: 10.4319/lo.1966.11.1.0092. [DOI] [Google Scholar]
- 102.Rosell D, Uriz MJ. Do associated zooxanthellae and the nature of the substratum affect survival, attachment and growth of Cliona viridis (Porifera: Hadromerida)? An experimental approach. Mar. Biol. 1992;114:503–507. doi: 10.1007/BF00350042. [DOI] [Google Scholar]
- 103.Ramsby BD, Hoogenboom MO, Smith HA, Whalan S, Webster NS. The bioeroding sponge Cliona orientalis will not tolerate future projected ocean warming. Sci. Rep. 2018;8:8302. doi: 10.1038/s41598-018-26535-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available in SRA/GenBank under BioProject PRJNA796519 (raw reads), accession numbers OM273296–OM273301 (mitochondrial genomes), ON705641–ON705646 (28S rRNA) and OP937170–OP937175 (ITS sequences).