Abstract
Heparan sulfate proteoglycans (HSPGs) are part of proteoglycan family. They are composed of heparan sulfate (HS)-type glycosaminoglycan (GAG) chains covalently linked to a core protein. By interacting with growth factors and/or receptors, they regulate numerous pathways including Wnt, hedgehog (Hh), bone morphogenic protein (BMP) and fibroblast growth factor (FGF) pathways. They act as inhibitor or activator of these pathways to modulate embryonic and adult stem cell fate during organ morphogenesis, regeneration and homeostasis. This review summarizes the knowledge on HSPG structure and classification and explores several signaling pathways regulated by HSPGs in stem cell fate. A specific focus on hair follicle stem cell fate and the possibility to target HSPGs in order to tackle hair loss are discussed in more dermatological and cosmeceutical perspectives.
Subject terms: Stem cells, Growth factor signalling
Introduction
Hair follicles (HFs) are mini-organs under the skin allowing to hair shaft growth1. A HF can be divided into three parts. The infundibulum is the upper portion between the skin surface and the sebaceous duct outlet. This duct connects the infundibulum of the hair follicle to the sebaceous gland, allowing the excretion of sebum along the hair shaft to hydrate the scalp. The middle part of the HF is the isthmus which extends from the sebaceous duct to the bulb. It is formed by different concentric layers forming the canal where the hair shaft grows1, from the outermost to the innermost layer: the connective tissue sheath, the outer root sheath (ORS), and the inner root sheath (IRS). This layer is in contact with the cuticle of the hair shaft until the level of the sebaceous canal where it disappears. The bulb, the deepest portion of the HF, is composed the hair matrix which surrounds the dermal papilla2.
Over the course of its life, hair follicle undergoes cyclic changes3. Forty to one hundred hairs are lost per day. Their renewal occurs during the hair growth cycle which is characterized by three main phases: anagen, catagen and telogen. An additional phase, called exogen phase, is controlled separately and leads to hair shaft loss4,5. The anagen phase allows the generation and growth of new hair shafts6. It lasts between three to 6 years on the average, divided into six stages. This phase is characterized by a remodeling of the HF morphology due to the activation (at the end of the telogen phase) and the intense proliferation of different cell types7,8. During the next catagen phase, the hair shaft stops growing and the transient segment of HF regresses8. It is divided into eight stages8 and lasts between 15 and 20 days. During this phase, apoptosis of keratinocytes is observed in a localized area, particularly at the junction of the secondary hair germ (SHG) and the dermal papilla (DP)8. The telogen phase is characterized by a dormant state of the DP and hair follicle stem cells making it a resting phase8. During this phase, the hair shaft remains anchored in the hair follicle6. After several months, the HF will return to the anagen phase thanks to stimuli.
The hair growth cycle is centered on the activation of HF pluripotent cells to differentiate and to provide the different cell lineages of the HF. Indeed, the hair shaft formation and the HF remodeling involve hair stem cells, located at the bulge, which contribute to generate the different cell lineages of the sebaceous gland, epidermis, and HF9. The process of the hair stem cell differentiation is complex and its study has revealed different populations derived from the hair stem cells. Five major distinct cell populations are defined in the literature based on their location, provenance, cell fate during the cycle phases, and their cellular markers: bulge stem cells, ORS progenitor cells, SHG progenitor cells, matrix transit-amplifying (TA) cells, and terminally differentiating cells (of the hair shaft, IRS and ORS)10–13. Moreover, the process of the hair stem cell differentiation involves many other cell types and specific niches: keratinocytes, fibroblasts of the DP, endothelial cells, fat cells, and immune system cells. The set of possible interactions between these different cell types complicates the study of the regulation of hair stem cell differentiation during the hair growth cycle.
All these cellular interactions are still poorly characterized, but it is known that growth factors (GFs) regulate the passage between the different phases of the hair cycle14. In particular, for the telogen to anagen transition and the hair shaft growth, several GFs are involved (such as Wnts, bone morphogenic proteins (BMPs), hedgehogs (Hhs) and fibroblast growth factors (FGFs)). A fine regulation of the GFs involved in the hair shaft growth is essential for the process of the hair cycle. The mechanisms involved in the regulation of these growth factors are still poorly understood, but several studies suggest that heparan sulfate proteoglycans (HSPG) are involved. These studies have shown an evolution of HSPG expression and distribution on the HF according to the phases of the hair cycle15–18. It has been demonstrated that the morphogenesis of a correct hair shaft requires a complex control of HSPGs production and sulfation19. Moreover, HSPGs are known to regulate many GFs involved in tissue or organ development and regeneration, such as those described for regulating the hair cycle20–25.
The purpose of this review is to make an update of the pivotal role of HSPGs in stem cell fate. Moreover, a specific focus on hair follicle stem cell differentiation during hair shaft growth is reported. Further, applications of these findings in the context of alopecia are also discussed.
HSPG structure, synthesis, trafficking and location
Structure of glycosaminoglycans and proteoglycans
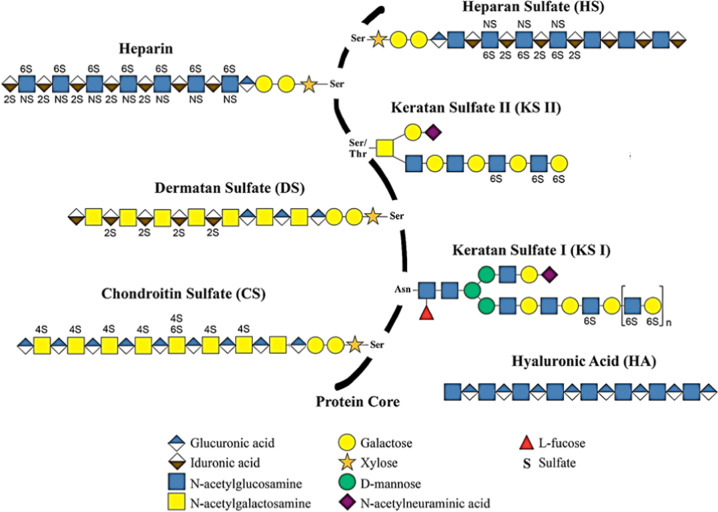
In this section, the structure, the synthesis, the trafficking and the location are briefly described and summarized in Table 1. Proteoglycans (PGs), components of the extracellular matrix (ECM) and cell membranes, are produced by many cell types26. They are composed of a core protein to which one or more linear polysaccharide chains of glycosaminoglycans (GAGs) are covalently attached27. GAG chains are composed of a repeat of disaccharide units formed from a hexosamine (N-acetyl-glucosamine or N-acetyl-galactosamine) and an uronic acid (D-glucuronic acid or L-iduronic acid) or a single ose27. The detailed structure of the six different types of GAG has been already described27–30 and is illustrated in Fig. 1.
Table 1.
Type, specific structural characteristics, and main roles of HSPG.
| Location | Classification | Name | Specific structural characteristics | Roles | References |
|---|---|---|---|---|---|
| Cytoplasm | Secretory granules | Serglycin | Small core protein Serine-glycine repeats | Hematopoietic cell protease activity Inflammatory response | Avraham et al.44 Iozzo et Schaefer 45, Korpetinou et al.46, Kjellén et al.47, Schick et Senkowski-Richardson48, Kolset et al.49, Kolset et Tveit 50, Schick et al.51, Schick et al.52, Yurt et al.53, Pejler et al.54, Manou et al. 2020a, Bouris et al.56, Manou et al. 2020b |
| Membrane | Transmembrane | Betaglycan | Presents two forms: with or without GAG chains | Growth factor co-receptors | Couchman58, Karamanos et al.59. |
| Neuropilin-1 | |||||
| CD44 | |||||
| Syndecans | Specific motif (cleavage zone) Variable region and two highly conserved domains | Cell processes (adhesion, cytoskeletal remodeling, migration) Growth factor co-receptors | Bernfield et al.60, Gondelaud et Ricard-Blum 2019, Kimet al. 1994, Choi et al.63, Chung et al.64, Echtermeyer et al.65, Leadbeater et al.66, Noguer et al.67 | ||
| GPI-anchored | Glypicans | Unique motif of 14 cysteine residues Highly conserved serine/glycine repeats | Regulation of cell signaling during tissue or organ development and regeneration | Filmus et al.23, Karamanos et al.59, Fransson 68, Veugelers et al.69, Hancock71, Mertens et al.72, Traister et al.73 Kawahara et al.74, Hereld et al.75, McGough et al.76 | |
| Pericellular | Basement membrane | Perlecan | Laminin-like domain | Integrity and function of basement membranes | Iozzo et Schaefer 45, Costell et al.77, Iozzo78, Amenta et al.79, McCarthy80, French et al.81, Mazzon et al.82, Mazzon et al.83. |
| Agrin | |||||
| Type XVIII collagen | Thrombospondin-like domain | ||||
| Type XV collagen | |||||
| Extracellular | SPOCK | Testicans | Composed of five domains | Regulation of the CNS development Adipose tissue maturation | Iozzo et Schaefer 45, Vannahme et al.84, Kohfeldt et al.85, Bonnet et al.86, Hartmann et al.87, Charbonnier et al.88, Schnepp et al.89, Yamamoto et al.90, Alshargabi et al.91. |
CNS central nervous system; GAG glycosaminoglycan.
Fig. 1. Representation of the structure of glycosaminoglycans, their sulfation sites and their covalent attachments to the core protein.
The composition of the disaccharide unit repeats is schematically illustrated for heparan sulfate (HS), heparin, dermatan sulfate (DS), keratan sulfates I and II (KS I and II), chondroitin sulfate (CS) and hyaluronic acid (HA). Hyaluronic acid is the only GAG in free and unsulfated form. All the other GAGs are attached to a protein and present O-sulfation at the 2 (2 S) or 4 (4 S) and/or 6 (6 S) carbons, and/or N-sulfation (NS). (Figure adapted from Merida-de-Barros et al. 201829; Copyright Elsevier).
Due to their structure and composition, GAGs are capable of binding to many components31–37.
The nature of the HS GAG chains, in addition to the amino-acid sequence of the core protein, defines the characteristics and properties of the HSPGs as well as their trafficking.
Biosynthesis and trafficking of HSPGs
HS biosynthesis takes place in the Golgi apparatus38. The HS GAG chain is formed in a stepwise manner by glycosyltransferases and sulfotransferases39. They use as substrate uridine diphosphate (UDP)-sugar and 3'-phosphoadenosine-5'-phosphosulfate (PAPS), respectively.
The first step of GAG chain initiation on core protein is the formation of the “linker”38. This step consists of the binding of a xylose to a serine of the core protein by xylose transferase. Then the addition of the other three monosaccharides forming the tetrasaccharide unit is catalyzed by galactosyl transferase I and glucuronic acid transferase I39. The next step consists of the elongation of the HS GAG chain by the addition of repeated disaccharide units38,39. Studies have shown that several factors influence this step and predetermine the type of GAG produced. For example, the amino-acid sequence surrounding the serine residue where the GAG chain covalent binding occurs;40,41 the production and transport of UDP-sugars in the Golgi apparatus;39 or the “linker” phosphorylation, epimerization or sulfation42,43.
Once the HSPGs have been synthesized, they are transported to the membrane or secreted to the ECM (except serglycin which is cytoplasmic). These two processes are influenced by different conditions (pH, specific amino-acid sequence, nature of sugars, glycosylphosphatidylinositol (GPI) anchor…). Studies, conducted on polarized cells, show that HSPGs are sorted to be secreted basolaterally to bind to the plasma membrane or apically to join the ECM39.
Classification of HSPGs according to their location
There are three main families of proteoglycans differing in their location. HSPGs are either cytoplasmic, either bound into the cell membrane, or composing the basement membrane or secreted in the ECM.
Cytoplasmic HSPGs
Only one PG belongs to the cytoplasmic PG family: serglycin. It is composed of a small core protein, about 16 kDa44, characterized by serine-glycine repeats45 and different GAG chains depending on the cell type46. Most commonly, serglycin has GAG chains of HEP and/or CS47 but can also rarely exhibit GAG chains of HS and CS48.
Serglycin is found in different cell types of hematopoietic origin such as cytotoxic T lymphocytes, neutrophils, and eosinophilic polymorphs49. It is also detected in macrophages50, endothelial cells51 and in embryonic stem cells52. When associated with HEP or HS chains, this PG is predominantly present in mast cells rich in secretory granules50,53.
Serglycin plays an important role in hematopoietic cell protease activity54. Indeed, it regulates the storage of proteases in the secretory granules as well as their protease activity during their intracellular release. It also plays a role in the immune response, in particular in the inflammatory response, by interacting with many components of the immune system54. Moreover several studies have shown the pro-tumorigenic functions of the serglycin55 and its ability to induce epithelial to mesenchymal transition56,57.
Membranes HSPGs
Membrane HSPGs are composed of several members grouped into two major families: transmembrane and GPI-anchored HSPGs45.
The transmembrane HSPG family is largely represented by syndecans (SDCs), and three other members: betaglycan, neuropilin-1 and CD4458,59 (Fig. 2). The three latter’s can occur in two forms: with or without GAG chains that may be HS and/or CS or DS58. They regulate many cell signaling pathways as GF co-receptors58,59.
Fig. 2. Schematic representation of transmembrane proteoglycans.
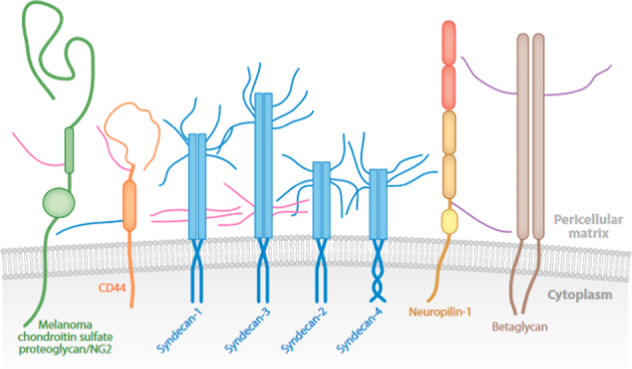
The different proteoglycans of this family are represented with their GAG chains (CS in pink, HS in blue, HS or DS or CS in purple). (Figure from Couchman, 201058; Copyright Anal Review license).
The SDC group is composed of four members: SDC1 to 4, in order of discovery. They differ from each other largely by their extracellular domain, which is the most variable area of the core protein, with only 10-20% similarity60. Within the highly conserved amino-acid sequence of SDC family, there is a specific motif, close to the transmembrane domain, that is recognized by proteases responsible for cleaving the extracellular domain of SDCs and sequences involved in the attachment of GAG chains61. All four syndecans have HS chains at their N-terminus. In addition, SDC1 and 3 also carry CS chains (Fig. 2). The transmembrane and cytoplasmic regions of the syndecans are highly homologous, with 60 to 70% sequence homology60. The cytoplasmic domain is characterized by a variable region V surrounded by two highly conserved domains C1 and C2. These two domains are involved in functions common to all the four SDCs, whereas the V domain has a role in the specific functions of each syndecan.
SDCs are present on the surface of most of the cells and their expression is finely regulated. Depending on the stage of cell, tissue or organ differentiation, the four syndecans are expressed differently and selectively, reflecting their distinct function62. SDCs regulate many cell processes such as adhesion, cytoskeletal remodeling, and migration63. They act as co-receptors for several cell signaling pathways such as FGF2, vascular endothelial growth factor (VEGF), granulocyte-macrophage colony-stimulating factor (GM-CSF) or hepatocyte growth factor (HGF)63. They are also known for their implication in tissue repair and regeneration such as wound healing, vascular or neuronal repair64–67.
Glypicans (GPCs) form the GPI-anchored proteoglycan family68. It is characterized by a unique motif of 14 cysteine residues, which is conserved in all GPCs, including those of Drosophila, Dally and Dally-like-protein (Dlp)23. In human, six GPCs have been identified, GPC1 to 6 (Fig. 3). They are divided into two subfamilies: GPC1, 2, 4 and 6 (related to Dlp) and GPC3 and 5 (related to Dally). These two subgroups present 25 % of similarity including the sequences of the insertion site of HS type GAG chains23. These sites are composed of highly conserved serine/glycine repeats located near the C-terminus23,69. The GAGs of the glypicans are exclusively HS type except for GPC5 which can also have CS-type GAG chains. All GPCs have a core protein of ~60 kDa. They are expressed by many tissues and cells. Usually in polarized cells, GPI-anchored proteins are found in lipid rafts located at the apical pole70, involved in various cell signaling71. In these cells, GPI-anchored GPCs can also be present in large quantities at the basolateral region72. Interestingly, it has been shown that unglycosylated GPCs are localized at the apical pole, demonstrating that HS chains play a role in the trafficking of GPCs72. GPCs can be cleaved by different molecules such as Notum73, disintegrin and metalloproteinase 17 (ADAM17)74 or phospholipase C (PLC)75. GPCs play a role in modulating cell signaling during tissue or organ development and regeneration by regulating many cell signaling pathways such as Wnt, Hh, BMP, and FGF23,59,76.
Fig. 3. Schematic representation of the six glypicans.
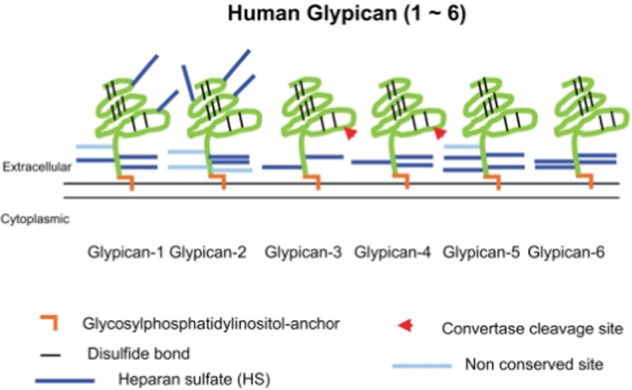
The core protein (green) is connected to the membrane by a GPI-anchor (brown) and the GAG chains (light and dark blue) on the core proteins are represented. Arrowheads represent the cleavage site of the core protein by convertase. (Figure from Yoneda et al., 2012252; Copyright Journal of Histochemistry and Cytochemistry license).
Pericellular HSPGs
There are four pericellular PGs or basal membrane PGs: perlecan, agrin, type XVIII and type XV collagens. These PGs exhibit HS chains but, the type XV collagen can also have CS chains. They are associated with laminin or type IV collagen allowing the integrity of basement membranes77,78. They can also be associated with the cell surface via integrins45 ensuring signal transmission79, an essential function of the basement membrane. Moreover they are essential for embryogenesis and tissue maturation80. For example, perlecan promotes the chondrocyte differentiation81 and agrin plays a crucial role in the hematopoietic niches82 and monocyte maturation83.
ECM HSPGs
The ECM HSPGs are represented by the testican family. They are also named secreted protein acidic and cysteine rich (SPARC)/Osteonectin and Kazal-like domain proteoglycans (SPOCKs). This family is composed of three members: testican 1, 2 and 3. Their other name, SPOCK, is due to their characteristic protein domains. Their core protein is composed of five domains84. The N-terminal domain I is specific to the testican family, hence the name testican-specific domain, and corresponds to a signal peptide84. The domain II is called follistatin domain and is a module rich in cysteine. The domain III is characterized by binding sites of low affinity to calcium85, earning it the name of calcium binding domain. The domain IV, or thyroglobulin domain, contains a tetrapeptide sequence CWCV stabilized by three disulfide bridges corresponding to a thyroglobulin-like domain45. The domain V, also specific to this family, contains the two potential binding sites for GAG chains.
The GAG chains of SPOCKs are predominantly of the HS type45. They are expressed almost exclusively in the brain84,86,87 and studies suggest that they play a regulatory role during central nervous system development88–90. A recent study has shown that SPOCK1 induces adipocyte differentiation and adipose tissue maturation through the up-regulation of adipogenesis-related genes91.
Role of HSPGs in the regulation of stem cell differentiation during morphogenesis and regeneration of organs
In this section, the role of HSPGs in the regulation of major signaling pathways known to be involved in embryonic and adult stem cell differentiation are presented (Table 2). The stem cell differentiation needs to be finely regulated during the development or the regeneration of organs. Several studies have shown the role of HSPGs in this orchestrated process. The mechanism of action of HSPGs is well described in numerous studies conducted on Drosophila. Indeed, it is a powerful model to understand the complex processes involved in human stem cell fate because they present very high homology with human HSPGs (Fig. 4).
Table 2.
Role of HSPGs in the regulation of major signaling pathways involved in embryonic and adult stem cell fate.
| Cells | HSPGs | Growth factors/Signaling pathways | Promoting effect of HSPGs on stem cell fate and organ formation/regeneration/homeostasis | References |
|---|---|---|---|---|
| Drosophila embryonic stem cells | Dlp | Wg | Establishment of the dorso-ventral axis | Kreuger et al.24 |
| Dally | Shh | Establishment of the anterior-posterior axis | Ayers et al.20 | |
| Dpp | Development of wing | Akiyama et al.149, Fujise et al.151 | ||
| Trol | Wg | Formation of pre- and postsynaptic structures | Kamimura et al.106 | |
| Wg and Dpp | Formation of second instar brain | Lindner et al.107 | ||
| Hh | Differentiation of neural stem cell | |||
| Hh and FGF | Activation of neural stem cell division | Park et al.134 | ||
| Vertebrate embryonic stem cells | HS | Maintenance of embryonic stem cell pluripotency (attachment and growth) | Stelling et al.188 | |
| BMP and FGF | Differentiation of embryonic stem cell during mesoderm formation | Kraushaar et al.152 | ||
| HS sulfation | Wnt, BMP and FGF | Maintenance and differentiation of mouse embryonic stem cells | Sasaki et al.110 | |
| FGF | Differentiation of embryonic stem cells into neural progenitor cells | Johnson et al.168 | ||
| Differentiation of embryonic stem cells | Hirano et al.189, Hirano et al.190 | |||
| Glypican-4 | Wnt | Inhibition of embryonic stem cell differentiation | Fico et al.108 | |
| Wtn and FGF | Migration of lateral line collective cell during zebrafish embryogenesis | Venero Galanternik et al.109 | ||
| Agrin | FGF | Formation of zebrafish retina | Liu et al.169 | |
| Neuroepithelial cells | HSPG | Essential before and during neurogenesis | Yamaguchi et al. 2001 | |
| FGF | Differentiation of murine neuroepithelial tissue | Nurcombe et al.173, Brickman et al.170 | ||
| HS | FGF | Proliferation, survival and differentiation of neuroepithelial cells | Murphy et al.172, Guilemot and Zimmer 2011 | |
| HS sulfation | FGF | Proliferation and differentiation of neural stem cell | Yamada et al.174 | |
| Syndecan-1 | Wnt | Maintenance and proliferation of neural progenitor cells during cortical neurogenesis | Wang et al.111 | |
| Syndecan-3 | HB-GAM | Facilitation of neuroblast migration during brain development | Raulo et al.191 | |
| GDNF | Bespalov et al.192 | |||
| Syndecan-4 | Regulation of neural stem cell proliferation during zebrafish neurogenesis | Luo et al. 2016 | ||
| Glypican-1 | FGF | Control of the brain size during neurogenesis | Jen et al.175 | |
| Glypican-4 | FGF | Maintenance of murine neuroepithelial cells | Hagihara et al.176 | |
| FGF | Regulation of forebrain patterning of Xenopus | Galli 2003 | ||
| Glypican-6 | FGF | Development of mouse cerebral cortex | Salehi178 | |
| Perlecan | FGF | Proliferation and differentiation of neural stem cells during neural tube formation | Joseph et al.180, Haubst et al.179, Giros et al. 2007 | |
| Hh | Regulation of neurogenesis | Giros et al. 2007, Palma et al.136 | ||
| Agrin | Wnt | Differentiation of neuroepithelial cell during the formation of neuromuscular junctions | Henríquez and Salinas 2011 | |
| Hh and FGF | Development of GABAergic and glutamatergic neuron in zebrafish brain | Zhang et al.137 | ||
| Drosophila adult stem cells | HS sulfation | Hh, EGFR and Jak/Stat | Division and differentiation of intestinal stem cell during regeneration | Takemura and Nakato138 |
| Dlp and Dally | Wg, Hh and Jak/Stat | Maintenance of ovarian adult stem cells | Su et al.113 | |
| Dally | Dpp | Regulation of stem cell number in germline stem cell niche | Hayashi et al.153, Dejima et al150. | |
| Hematopoietic progenitor cells | HSPG/HS | Adhesion of stem and progenitor cells to stromal cells | Siczkowski et al.193, Zweegman et al.194 | |
| HSPG | SDF-1 | Migration, homing and retention of progenitor cells | Netelenbos et al.195 | |
| HS | SDF-1 | Migration of progenitor cells | Netelenbos et al.198 | |
| GM-CSF | Promotion of haematopoiesis | Gordon et al.196 | ||
| Il-3 | Regulation of hematopoietic lineage formation | Roberts et al.197 | ||
| HS/heparin | Il-6, PF4 and TGFβ | Differentiation of megakaryocyte progenitors | Han et al.199 | |
| Osteogenic and Chondrogenic progenitors | HS | BMP | Potentiation of bone repair | Bramono et al.154 |
| TGFβ | Promotion of chondrogenic differentiation | Chen et al202. | ||
| HS/Heparin | Promotion of chondrogenesis and cartilage nodule formation | San Antonio et al.200 | ||
| Heparin | Wnt | Differentiation of osteogenic progenitor cell | Ling et al.114 | |
| Syndecan-3 | Hh | Proliferation and maturation of chondrocyte | Shimo et al.142 | |
| BMP | Inhibition of chondrogenesis during cartilage differentiation | Fisher et al.155 | ||
| FGF | Proliferation of chondrogenic progenitors | Kirsch et al.181, Shimazu et al.182 | ||
| Glypicans-1 and -3 | BMP | Inhibition of osteogenesis during bone regeneration | Dwivedi et al.222 | |
| Glypican-3 sulfation | Regulation of osteogenic lineage formation | Haupt et al. 2009, Zhao et al. 2015 | ||
| Glypican-6 | Hh | Growth of developing long bones | Capurro et al.21 | |
| Perlecan | Promotion of chondrogenesis | Gomes et al.201 | ||
| BMP | Improvement of osteogenesis | Decarlo et al.157 | ||
| Stimulation of chondrogenic differentiation | Jha et al.156 | |||
| Agrin | BMP | Differentiation of osteoblast | Souza et al.158 | |
| Wnt | Differentiation of chondrogenic stem cell during osteochondral regeneration | Eldridge et al.115 | ||
| Intestinal progenitor cells | HS | Wnt | Differentiation of intestinal progenitor cells during the regeneration of intestinal crypt | Yamamoto et al.25 |
| HS sulfation | Regulation of colonic epithelial cell differentiation | Jao et al.203 | ||
| Muscle satellite cells and myoblasts | HS | FGF | Regulation of muscle satellite cells and differentiation of myoblasts | Rapraeger et al.184, Olwin and Rapraeger183 |
| Syndecan-3 | FGF | Inhibition of myogenic differentiation | Fuentealba et al.185 | |
| Notch | Regulation of adult myogenesis | Pisconti et al.204 | ||
| Syndecan-4 | Expression by self-renewing muscle stem cell | Tanaka et al. 2009 | ||
| Glypican-1 | FGF | Formation of muscle | Gutierrez and Brandan 2010 | |
| Skin progenitor cells | HSPG | Evolution of its expression within the epidermis | Caughmman et al. 1987, Horiguchi et al.206 | |
| Syndecan | TRPC | Regulation of adhesion, adherens junction composition, and early differentiation | Gopal et al.208 | |
| Syndecan-1 | Evolution of its expression within the epidermis | Sanderson et al.207 | ||
| Glypican-1 | Evolution of its expression within the epidermis | Perrot et al.187 | ||
| FGF | Proliferation of keratinocyte progenitors | |||
| Hair follicle stem cells and progenitors | HSPG | Expression structure dependent in the hair follicle | Bernard218, Botchkarev and Kishimoto219, Couchman220, Westgate et al.221 | |
| HSPG sulfation | Necessity for correct morphogenesis of hair shaft | Coulson-Thomas et al.19 | ||
| Syndecan-1 | Diminution of its expression in ORS during telogen phase | Bayer-Garner et al.15 | ||
| Glypican-1 | Maintenance of its expression in the hair matrix and hair shaft along the hair cycle | Colin-Pierre et al.215 | ||
| Glypican-1 sulfation | Variation of the type and/or the degree of sulfation during hair cycle | |||
| Perlecan | Diminution of its expression in dermal papilla in late catagen phase | Malgouries et al.17 | ||
| Versican* | Wnt | Induction of anagen phase and hair inductivity | Yang et al.224 | |
| Decorin* | TGFβ | Induction of anagen phase | Inui and Itami225 |
HSPG heparan sulfate proteoglycan; Dlp Dally-like-protein; Wg Wingless; Hh Hedgehog; Dpp BMP2 and 4 Drosophila homologous; FGF fibroblast growth factor; HS heparan sulfate; BMP bone morphogenic protein; HB-GAM pleiotrophin; GDNF glial cell line-derived neurotrophic factor; EGFR epithelial growth factor receptor; SDF-1 stromal cell-derived factor-1; GM-CSF granulocyte-macrophage colony-stimulating factor; IL interleukin; PF4 platelet factor 4; TGF transforming growth factor beta; TRPC transient receptor potential canonical channel; * other types of proteoglycan.
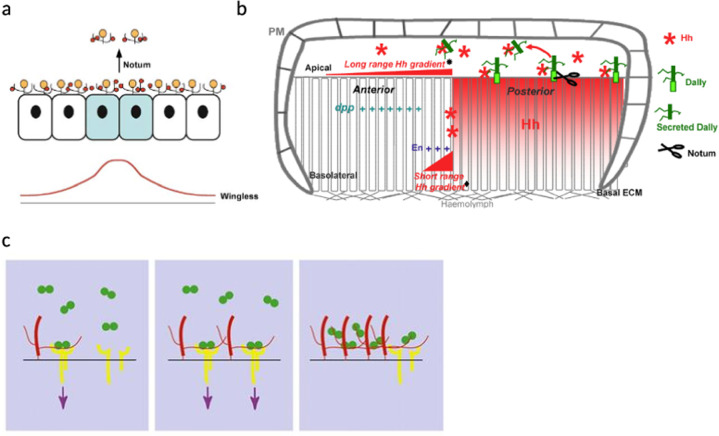
Fig. 4. Drosophila glypicans regulate Wnt, Hh and BMP signaling pathways during Drosophila embryonic development.
a Dlp regulates the activation of the Wnt pathway and the establishment of the dorsoventral (DV) axis during Drosophila embryonic development. Wg growth factor (red) and Notum enzyme are produced by the cells at the DV boundary (marked in blue). Dlp (yellow) sequestrates Wg and Notum cleaves the GPI anchor of Dlp. These processes lead to the formation of the Wg gradient (red line). (Figure from Kreuger et al., 200424; Copyright Elsevier license). b Dally (green) regulates Hh (red star) activity during the establishment of the anterior-posterior axis during Drosophila embryonic development. Hh is secreted by future posterior cells and a short-range gradient (♦) is formed by diffusion of Hh to the future anterior cells (basolateral level). A long-range Hh gradient (∗) is enabled by Hh-related Dally cleavage by Notum at the apical pole of the cells. ECM extracellular matrix; PM plasma membrane. (Figure from Ayers et al., 201020; Copyright Elsevier license). c Regulation of the activation of Dpp signaling pathways by Dally. Left: Dally (red) favors the interaction between Dpp (green) and its receptor (yellow) allowing the activation of the signaling pathway (purple arrows). Middle: This activation can be enhanced by an augmentation of Dally levels. Right: An excess of Dally levels leads to an inhibition of the signaling pathway activation via the sequestration of Dpp. (Figure reproduced with permission of the journal Development from Fujise et al., 2003151).
Implication of HSPGs in Wnt signaling pathways
The human Wnt family is composed of 19 members that bind to Frizzled transmembrane receptors (7 members) involving a coreceptor recruitment92,93. The Wnt signaling pathways were demonstrated to regulate the stem cell differentiation process occurring during embryogenesis and development/regeneration of numerous organs94–105. Wnts are able to induce different signaling pathways93 and to exhibit different effects on the stem cell fate, depending on the coreceptor with which Frizzled receptors are associated.
The regulation of Wnt signaling pathways is crucial for a correct differentiation of stem cell and thus for a correct morphogenesis or regeneration of organs. Some studies have shown the regulation of Wnt signaling pathways by HSPGs during the stem cell differentiation.
Regulation of Wnt signaling by HSPGs during Drosophila embryogenesis
The two first examples highlight the role of HSPGs during Drosophila embryogenesis. Kreuger and collaborators have studied the regulation of dorso-ventral axis establishment during Drosophila embryogenesis24. They were able to show that the glypican Dlp is involved in the formation of the Wingless (Wg) gradient necessary for the establishment of the dorso-ventral axis (Fig. 4a). Drosophila perlecan homologue Trol was shown to have an important role during Drosophila nervous system development106,107. Indeed, by regulating Wg signaling, it was demonstrated to regulate the formation of pre- and postsynaptic structures106 and a second brain instar107.
Regulation of Wnt signaling by HSPGs modulates vertebrate embryonic stem cell fate
In the case of vertebrate development, several studies have demonstrated the roles of HSPGs in the regulation of embryonic stem cells and neuroepithelial cells by modulating Wnt signaling. In particular, GPC4 regulates Wnt/β-catenin signaling inhibiting the differentiation of mouse embryonic stem cells and promoting their self-renewal108. GPC4 was also shown to play a role during zebrafish development109. Indeed, it regulates Wnt/β-catenin signaling to promote the essential migration of lateral line collective cells during embryogenesis. Moreover, Sasaki and collaborators have demonstrated the crucial role of HS sulfation for the regulation of self-renewal and differentiation of mouse embryonic stem cells110. A study conducted in vivo on mouse cortical neurogenesis has demonstrated that SDC1 promotes the activation of Wnt signaling pathways during neural progenitor cell differentiation111. This activation permits to maintain their phenotype and their proliferation potent. As last example, it has been shown that a collaboration between Wnts and agrin promotes cell differentiation for the formation of vertebrate post-synaptic neuromuscular junctions112.
Regulation of Wnt signaling by HSPGs modulates adult stem cell fate
Some studies have been conducted on adult stem cell differentiation to understand how HSPGs regulate the Wnt signaling pathways during regeneration of organs. In the case of Drosophila adult stem cells, Dally and Dlp are expressed in the niche of ovarian adult stem cells and play a role in the self-renewal of these cells by regulating different pathways including Wg113. As first example in the case of vertebrate adult stem cells, it has been shown that HSPGs promote the activation of Wnt signaling pathways by modulating the binding of Wnts on mouse intestinal progenitor cells25. The activation of the leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) progenitor cells induces their differentiation and leads to the regeneration of intestinal crypt. Similarly, in the context of osteoblast differentiation, the association of heparin with Wnt3a leads to murine osteogenic progenitor cell differentiation114. As third example, a study conducted on osteochondral regeneration has demonstrated the important role of agrin in the mouse chondrogenic stem cells by downregulation of Wnt/β-catenin signaling pathway115.
Implication of HSPGs in Hh signaling pathways
The human Hh family is composed of 3 members: Desert hedgehog (Dhh), Indian hedgehog (Ihh) and Sonic hedgehog (Shh). They are secreted and bind to Patched membrane receptor116. Several studies have demonstrated the involvement of Hhs in the regulation of vertebrate embryogenesis and vertebrate organs development/regeneration117–121. These studies have shown the role of Hhs in the regulation of stem cell differentiation. Moreover, it is known that Hh proteins can act at short or long-range, especially during embryogenesis, to regulate organ development (imaginal discs of Drosophila or vertebrate neural tubes for example)122–126.
Thus, the Hh short or long-range gradients need a fine regulation to be set up and allow a correct stem cell differentiation. Several studies have demonstrated the capability of HSPG to regulate the gradient and the effect of Hhs during morphogenesis or regeneration of organs.
Regulation of Hh signaling by HSPGs during Drosophila and vertebrate embryogenesis
In the case of embryogenesis, the establishment of Drosophila127,128, mouse129,130 or zebrafish131 mutant embryos deficient for UDP-glucose (required for GAG synthesis) or loss-of-function mutation for an HSPG provides evidence on the essential role of HSPGs in the regulation of Hh signalization during organ development. Moreover, some studies have demonstrated that the establishment of Hh gradients requires HSPGs132,133. For example, Ayers and collaborators have been able to demonstrate the involvement of the glypican Dally in the establishment of the Drosophila anterior-posterior axis thanks to the regulation of the Hh gradient (Fig. 4b). Future cells of the posterior zone secrete Hhs, two gradients are then formed: a short-range gradient at the basolateral level of the cells by limited diffusion and a long-range gradient at the apical pole due to the sequestration of Hhs by Dally and its release by Notum, allowing to transport Hhs to act over a long distance20. Drosophila perlecan homologue Trol was shown to induce the activation of neural stem cell134 and their differentiation into a specific neuroblast population by regulating Hh pathway107.
Some studies have demonstrated the implication of Hh signaling modulation by HSPGs during vertebrate development in particular in the case of brain formation. For example, perlecan has been shown to regulate mouse neurogenesis by mediation of Shh concentration gradient135,136 and agrin has been demonstrated to control neuron development in zebrafish brain by regulating Shh137.
Regulation of Hh signaling by HSPGs modulates adult stem cell fate
Several studies have revealed the importance of HSPGs on adult stem cell differentiation regulated by Hh signaling. In the case of Drosophila adult stem cells, Dally and Dlp regulate ovarian stem cell maintenance by regulating Hh signaling113 and the HS sulfation is essential for intestinal stem cell division and differentiation during regeneration138. Other studies conducted on the differentiation of osteogenic and chondrogenic progenitors in vertebrate have shown that the HS GAG chains are involved in the Hh long-range gradient formation139–141 essential for the regulation of chondrocytes differentiation. More precisely, Capurro and collaborators have demonstrated in vitro the capability of GPC6 to form a ternary complex with Hh and its receptor21. Indeed, GPC6 binds the Hh ligand with its core protein and the Hh receptor with its HS chains to promote their interaction and the long bone growth. SDC3 was also shown to be involved in chick chondrocyte proliferation and maturation by regulating Ihh signaling142.
Implication of HSPGs in BMP signaling pathways
The 20 secreted BMPs composed the human BMP family. They bind to transmembrane BMP receptors (BMPR)143,144. The signaling pathways of BMPs are involved in the regulation of vertebrate embryogenesis and vertebrate organs development/regeneration145,146 and they are particularly known to regulate bone formation and osteoblast differentiation147,148.
The regulation of the activation of BMP signaling pathways is crucial for a correct differentiation of stem cell and thus for a correct morphogenesis or regeneration of organs.
Regulation of BMP signaling by HSPGs during Drosophila and vertebrate embryogenesis
Several studies conducted on Drosophila embryos have shown the regulation of these pathways through the interaction between HSPGs and BMPs149–151. More precisely, as shown in Fig. 4c, Dally could regulate Dpp (BMP2 and 4 Drosophila homologous) through the stabilization of the interaction between Dpp and its receptor or the sequestration of Dpp151. Moreover, Dally could participate to the internalization and the degradation of the Dpp-receptor complex149 during wind development. Trol is known to play a role during Drosophila second brain instar formation by regulating Dpp in a Trol dependent manner107.
In the case of vertebrate embryogenesis, few evidence show the implication of HSPGs in the regulation of BMP signaling. In particular, the HS chain and its sulfation are crucial for a correct regulation of mouse embryonic stem cell differentiation110 and for mesoderm formation from mouse embryonic stem cells152 induced by BMP.
Regulation of BMP signaling by HSPGs modulates adult stem cell fate
Some studies have been conducted on adult stem cell differentiation to understand how HSPGs regulate the BMP signaling pathways in the context of organ homeostasis or regeneration. As first example, two studies conducted on Drosophila adult stem cells have demonstrated that Dally is the co-receptor of Dpp in the germline stem cell niche and it regulates the number of these stem cells150,153.
In the case of vertebrate adult stem cells, several studies performed on osteogenic and chondrogenic progenitors have revealed the role of HSPGs in the regulation of BMP signaling involved in cell maintenance or differentiation. HS chains have been shown in vitro to potentiate the BMP2-induced bone repair by prolonging BMP-2 half-life, reducing interactions between BMP-2 with its antagonist noggin, and modulating BMP2 distribution on the cell surface154. SDC3 has been demonstrated in vitro to impair the interaction between BMP2 and its receptors leading to an inhibition of chondrogenesis during cartilage differentiation155. Similarly, GPC1 and 3 were able to inhibit BMP signaling and the osteogenesis mediated by BMP2 in human primary cranial suture mesenchymal cells22. BMP2 is also known to be regulated by perlecan. In contrast, perlecan has been shown in vitro to stimulate chondrogenic differentiation by modulating BMP2156 and to improve osteogenesis by increasing BMP2 signaling157. Finally, agrin has been demonstrated to play a role in osteoblast differentiation by regulating BMP signaling pathways158.
Implication of HSPGs in FGF signaling pathways
The human FGF family is composed of eighteen secreted proteins that can induce various different actions by binding one of the four FGF receptors (FGFR)159. The FGF signaling is known to regulate stem cell pluripotency and differentiation160,161. Numerous studies have demonstrated the implication of FGF in embryogenesis105,162 and in development/regeneration of various organs161 such as bones163,164, spinal cord165 or lung166,167.
The FGF signaling pathways need a fine regulation for a correct morphogenesis or regeneration of organs and numerous studies have proven the role of HSPGs in these processes.
Regulation of FGF signaling by HSPGs modulates embryonic stem cell fate
Studies performed on mouse embryonic stem cells have highlighted the importance of the HS chain sulfation during the formation of the mesoderm152. Indeed, the HS chain sulfation has been demonstrated to regulate FGF signaling involved in the mouse embryonic stem cell differentiation110. Johnson and collaborators have shown in culture of mouse embryonic stem cells that HS sulfation increases the FGF2 cell surface binding, inducing a cell differentiation into neural progenitor cells168. In the case of zebrafish development, GPC4 was demonstrated to induce the migration of lateral line collective cells during embryogenesis by the regulation of FGF signaling109 and agrin is necessary for the retina formation of zebrafish probably by the regulation of FGF8 signaling169.
Other studies, carried out on neuroepithelial cells have proven evidence of the role of different HSPGs on FGF signaling regulation during embryogenesis. In the case of neural stem cell proliferation, survival and differentiation, it has been shown that the HS chains of HSPGs and their sulfation are essential for the regulation of FGF distribution and binding and thus for the correct neuroepithelial tissue development170–174. The regulation of FGF signaling by GPCs seem to be highly implicated in the brain development processes. For example, GPC1 was shown to regulate the interaction between FGF17 and its receptor during early neurogenesis controlling the size of the mouse brain175. In mouse neuroepithelial cells, the HS chains of GPC4 are able to sequestrate FGF2 to prevent the binding with its receptor leading to the maintenance of their proliferative stem cell phenotype176. In contrast, in the case of Xenopus neurulation, GPC4 was demonstrated to bind FGF2 to facilitate the binding with its receptor regulating the dorsoventral forebrain patterning177. As last example for GPCs, a study conducted on mouse cerebral cortical development suggests a role of GPC6 in the regulation of FGF2 signaling during this process178. Perlecan was also shown to be important in the regulation of the brain development modulated by FGF signaling pathways. In the case of Drosophila, Trol is able to mediate FGF signaling to activate neural stem cell division134. In the case of vertebrate, FGF2 signaling is modulated by perlecan to regulate proliferation and differentiation of neural stem cells135,179,180. Agrin was demonstrated to regulate GABAergic and glutamatergic neuron development in zebrafish forebrain by the modulation of FGF signaling137.
Regulation of FGF signaling by HSPGs modulates vertebrate adult stem cell fate
Numerous studies have provided evidence on the role of HSPGs in vertebrate adult stem cell differentiation modulated by FGF signaling pathways. SDC3 was shown to induce the proliferation of chick chondrogenic progenitors by modulation of FGF2 signaling181,182. The regulation of FGF2 signaling by the HS chains of HSPGs was demonstrated to be essential for the regulation of mouse muscle satellite cells and myoblasts differentiation183,184. SDC3 is able to facilitate the interaction between FGF2 and its receptor leading to the repression of myogenic differentiation of murine skeletal myoblasts185. In contrast, GPC1 was reported to sequester FGF2, preventing its binding to its receptor, to promote mouse muscle differentiation186. The last following example highlights the role of HSPGs on skin progenitor cell fate modulated by FGF signaling. GPC1 is expressed by the epidermis keratinocytes mainly in the basal layer where the progenitors are present. In parallel, GPC1 cleavage is able to decrease human keratinocyte proliferation induced by FGF2187. These results tend to indicate a role of GPC1 in skin precursor cell proliferation during skin regeneration.
Implication of HSPGs in modulation of other signaling pathways
Additional roles of HSPGs in the regulation of vertebrate embryonic stem cell fate are described (Table 2) in particular during the central nervous system development188–192. There is also evidence that HSPGs play a critical role in the differentiation of hematopoietic progenitors and stem cells193–199.
In case of adult stem cell fate, the role of HSPGs on chondrogenesis and cartilage formation was confirmed by other studies conducted on HS chains200 and perlecan201. Moreover, the HS chains have been shown to potentiate the TGFβ3 signaling promoting chondrogenic differentiation of human mesenchymal stem cells202.
The sulfation of HS chains is crucial for a correct mouse colonic epithelial cell differentiation203. SDC3 interacts with Notch and regulates mouse adult myogenesis204.
As last examples, the role of HSPGs on the regulation of skin progenitor cell differentiation are presented. The first evidence was proven by the HSPG distribution within the epidermis which depends on the state of keratinocyte differentiation205,206. For example, SDC1 is weakly detected in the basal layer of epidermis and highly expressed in the suprabasal layers207. On the contrary, GPC1 is distributed throughout the epidermis but preferentially in the basal layer187. Few studies have been conducted on the pathways regulated by HSPGs involved in epidermis progenitor cell differentiation (see FGF subsection). Another example is the regulation of adhesion and early differentiation of keratinocytes progenitor cells by the formation of a complex between transient receptor potential canonical channel 4 (TRPC4) and SDC, demonstrating in Caenorhabditis elegans208.
All these examples demonstrate the role of HSPGs on the stem cell fate via the regulation of growth factor distribution, sequestration and downstream signaling pathway. Moreover, they highlight the opposing effects that HSPGs can exhibit on stem cell behaviors, either by favoring the maintenance of pluripotency or by promoting differentiation. Finally, these examples also show the different mechanisms of action of HSPGs. Indeed, they can sequester growth factors to prevent or facilitate the interaction with the receptors thanks to their HS chains or they can promote the interaction by their core protein.
Importance of HSPG in hair follicle stem cells differentiation
Major signaling pathways involving in hair follicle stem cell differentiation
In the case of hair follicles, the major signaling pathways of growth factors (Wnt, Shh, BMP and FGF) are particularly important to regulate stem cell maintenance or differentiation during hair cycle and hair shaft growth (Fig. 5).
Fig. 5. Regulation of stem cell differentiation during the telogen to anagen transition.
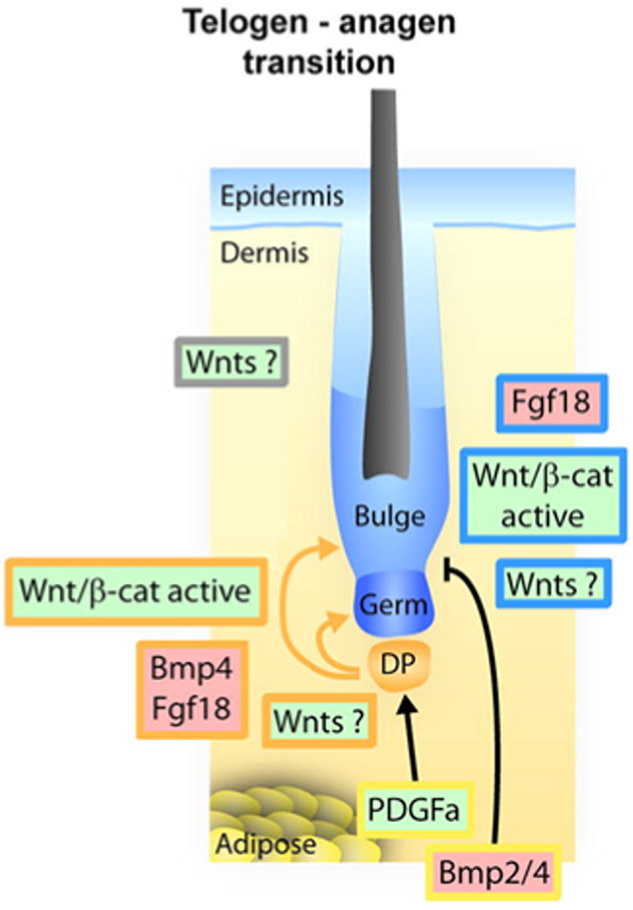
The dermal papilla secretes Wnts which activate the stem cells of the bulge and the progenitor cells of the secondary germ. In contrast, the dermal papilla also produced BMPs promoting quiescence of these cells and prevent their activation. (This Figure was published in Sennett and Rendl, 2012211; Copyright Elsevier).
The platelet-derived growth factor (PDGF) secreted by fat cells activates the Wnt pathway within the DP209. In parallel, an inhibition of the BMP pathway is observed in the adipose macro-environment and in the dermal papilla210,211. The BMP pathway is active throughout the telogen phase and allows the maintenance of quiescence of SHG progenitor cells.
At the end of the telogen phase, various BMP inhibitors are secreted from the dermal papilla, particularly Noggin211 (Fig. 5). With the BMP pathway inhibition, secretion of Wnts from the dermal papilla has an activating effect on SHG progenitor cells and bulge stem cells at early anagen phase. Then, the Wnt/β-cat pathway will be activated in SHG progenitor cells and in bulge stem cells211.
The activation of these cell types leads to the secretion of different growth factors regulating the hair stem cell differentiation and the formation of a new hair shaft. For example, the production of insulin-like growth factor 1 (IGF1) by the DP controls the proliferation and the differentiation of SHG progenitor cells to regenerate the hair matrix212. The keratinocyte growth factor (KGF or FGF7), produced by the DP, stimulates the hair matrix cells, which then produce keratinocytes to form the new hair shaft212. The secretion of HGF by the DP promotes the elongation of the hair shaft213. Hedgehog (Hh), secreted by the hair matrix cells, stimulates the bulge stem cells to provide a new pool of SHG progenitor cells necessary for the next growth cycle214. In parallel, angiogenic pathways are also involved. The fibroblasts of the DP as well as the keratinocytes of the matrix and the ORS secrete VEGF which induces the formation of new blood vessels which provide the nutrient supply necessary for the formation of the hair shaft212,215,216.
Other growth factors are very important for the anagen-catagen transition. For example, epithelial growth factor (EGF) and FGF5 are necessary for this transition217. TGFβ and brain-derived neurotrophic factor (BDNF) inhibit HGF expression and VEGF secretion, respectively14. Moreover, the inhibition of Noggin and Wnt pathway signaling, as well as FGF18 and BMP2, 4 secretion by the dermal papilla, promote the quiescence of bulge stem cell14,211.
This brief summary provides an overview of the intense complexity of the crosstalk required for the maintenance and regulation of the hair cycle. This level of regulation is permitted by multiple growth factors, several signaling pathways and includes numerous different cell types. These regulations are still poorly understood but some studies provide evidence of the implication of HSPGs in these regulatory processes.
Involvement of HSPGs in hair follicle stem cell differentiation
In this section, the roles of HSPGs in hair follicle stem cell fate are presented (Table 2).
Studies have been conducted on the distribution of HSPGs in the different hair structures during hair growth cycle. During the anagen phase, HS chains are detected in the basement membrane, connective tissue sheath and the dermal papilla of hair follicle17,218–221. Experiments conducted on specific HSPGs have demonstrated that perlecan is expressed in basement membrane and the dermal papilla;17 SDC1 is expressed in the ORS, in the hair shaft and lower in the IRS and dermal papilla;15,17 GPC1 is expressed in the hair matrix (more strongly in the differentiation zone) and less in the hair shaft16.
Moreover, it has been shown that HSPG distribution in hair follicle evolves during hair growth cycle. For example, it has been demonstrated that perlecan is still expressed in basement membrane and connective tissue sheath during catagen phase but in dermal papilla a decrease of its expression is observed in late catagen17. Bayer-Garner and collaborators have shown that SDC1 expression in ORS decreases in telogen phase15. In contrast, the distribution of GPC1 in the hair matrix and hair shaft seems to be the same all along the hair cycle16. Moreover, it has been demonstrated that a fine regulation of HSPG sulfation is necessary for a correct formation of hair shaft19. It is interesting because the type and/or the degree of sulfation vary during hair cycle16. These studies suggest a role of HSPGs on hair follicle stem cell fate. Indeed, the tissular or cellular distribution of growth factors is associated with the expression of HSPGs during Drosophila embryogenesis20,24,151, bone formation222 or skin regeneration187,208 where HSPGs are demonstrated to regulate the pathways involved during these processes.
One another evidence is the fact that other proteoglycans, such as CSPG or DSPG regulate the signaling pathways involved in hair follicle stem cell fate. In particular, versican expressed in the dermal papilla is well described. Several studies showed the ability of versican to induce anagen phase and hair inductivity219,223 via the Wnt/β-cat pathway224. In addition, decorin was shown to be an anagen inducer probably by downregulating TGFβ signaling225. The similarity between the mechanisms of action of HSPGs and other sulfated proteoglycans emphasizes the role of HSPGs in the regulation of signaling pathways involved in hair follicle stem cell differentiation.
To conclude this section, the key role of HSPGs in the regulation of hair growth cycle and hair shaft formation is well established. Unfortunately, despite several studies carried out on the distribution of HSPGs on hair follicles, only few studies have investigated the mechanism by which HSPGs regulate these processes and which growth factor and signaling pathways are involved. Further works are necessary to better understand HSPG mechanisms of action and to develop HS proteoglycan-based therapies for hair disorders.
HSPGs as therapeutic targets for androgenetic alopecia
Androgenetic alopecia accounts for 90% of alopecia cases and affects 50% of women and 80% of men in their lifetime226. In men, it manifests as hair loss in localized areas227 and diffused loss in women228. According to Grand View Research, Inc., the global alopecia market size was valued in 2020 at USD 7.6 billion. From 2021 to 2028, it is expected to expand at a Compound Annual Growth Rate of 8.1% and to reach USD 14.2 billion. Androgenetic alopecia is due to an excessive supply of androgen to the dermal papilla causing physiological disruptions in hair follicles229. As described in the previous chapter, the Wnt signaling is crucial for hair stem cell differentiation and for the growth of the new hair shaft. In vitro, androgens were shown to inhibit the production of Wnt by dermal papilla cells230. This type of inhibition could explain the deregulation in vivo of the various growth factors secreted during the anagen phase. These deregulations during the anagen phase promote hair follicle miniaturization231. For example, the inhibition of IGF1 and KGF promotes hair thinning231. Shh inhibition disrupts the SHG formation and VEGF inhibition disrupts perifollicular revascularization. In addition, it has been shown that androgens stimulate the secretion of TGFβ231 and IL-6232 that induces premature transition to the catagen phase. In other context, androgens modulate the HSPG expression. For instance, the SDC1 expression decreased in the mouse mammary tumor cells after incubation with testosterone233. In addition, the steroid hormone estradiol has been demonstrated to modulate SDC3 expression and distribution playing a role in rat uterine growth234.
Currently, two major drug treatments exist, Minoxidil (lotion) and Finasteride (oral tablet) as well as surgical and low-level laser treatments227,235. Several cosmetic active ingredients, such as Stemoxydine, have also been developed and packaged in the form of shampoo, hair lotion, etc. These active ingredients reduce inflammation and/or improve micro-vascularization to promote the growth phase of the hair shaft and stop hair loss (Table 3). These drug treatments act to restore cellular signaling pathways dysregulated in androgenetic alopecia. For example, Minoxidil was demonstrated to activate the Wnt pathway in the mouse dermal papilla in vivo and in human dermal papilla cells in vitro236. This activation may be related to a stimulation of adipocyte precursors to secrete PDGF that activates the dermal papilla237. The stimulation of Wnt signaling in dermal papilla is able to restore the secretion of IGF1, HGF, and VEGF238. Furthermore, during the catagen phase, Minoxidil was shown to inhibit TGFβ-induced apoptosis in matrix TA cells and to increase the Bcl-2/Bax ratio protecting cells from apoptosis239.
Table 3.
Mode of administration, mode of action and side effects of alopecia treatments and active ingredients.
| Treatments/ Cosmetic active ingredients | Application/Administration | Mode of action | Side effects | References |
|---|---|---|---|---|
| Minoxidil | Topical application | Increases hair growth by prolonging anagen duration. Other possible mechanisms of action: stimulation of angiogenesis throught VEGF | Inflammatory skin reaction, eczema and allergies | York et al. 2020 |
| Finasteride | Oral administration | Prevents androgen dependent miniaturization of hair follicles by competitively inhibiting 5-alpha-reductase | Sexual disorders | York et al. 2020 |
| Stemoxydine | Topical application | Mimics the effects of the hypoxic environment essential for hair stem cells | No observable side effects | https://www.anabolichealth.com/stemoxydine-review/ |
| Nourkrin | Oral administration | Intake of proteoglycan to the hair follicle leading to re-establishment of the hair follicle metabolism | No observable side effects | Thom243 |
Some studies have highlighted the link between hair growth disorder and the alteration of the expression and/or the distribution of proteoglycans. In particular, it has been shown that the dermal papilla of hair follicle isolated from a bald area presents a lower gene and protein expression of versican compared to those isolated from a hairy area240. The alteration of the expression and/or the distribution of HS proteoglycans in case of hair loss could be explained by the fact that androgens are able to modify the HSPG expression. Finally, evidence demonstrates the role of HSPGs in hair follicle stem cell fate during hair growth cycle. Altogether, these data highlight the role of HSPGs is the hair follicle physiopathology and make HSPGs as interesting targets for treatment of androgenetic alopecia. Despite this fact, few studies have been conducted to develop proteoglycan-based treatments for hair loss (and no one on HSPG-based treatments). After many years of proteoglycans related studies on their potential therapeutic development, Wadstein and Thom were the first to demonstrate the effect of proteoglycan concentration or synthesis in hair follicle growth18. Based on a clinical study, a cocktail of proteoglycans (oral administration) was shown to induce changes in hair follicle structure241. A specific cocktail of proteoglycans (Nourkrin), rich in lectican and decorin, was formulated. Clinical studies, conducted on patient with hair loss, have proven its efficacy on hair growth in monotherapy242,243 or in add-on treatment244 by increasing the hair count and reducing hair loss. Nourkrin is developed as oral treatment. It seems that the oral ingestion of proteoglycans leads to an increase of proteoglycan concentration in the hair follicles by direct deposition and/or synthesis of proteoglycans without secondary effect18. Nourkrin is a drug-free bioactive proteoglycan formula, based on natural ingredients. Studies are required to develop topical HS proteoglycan formula. However, some studies highlight possible vehicle or formula to induce the HS proteoglycan skin penetration such as polymersomes, vesicular nanocarriers, liposomes, nanoparticles, topical solutions and gels245. For example, in the case of wound healing, proteoglycans were topically applied using 30% glycerol formula246. Minoxidil was shown to present long term benefits although it has been demonstrated to induce a transient post-treatment hair shedding247. Interestingly, Nourkrin treatment was shown to do not induce post-treatment hair shedding with an uncharacterized molecular mechanism18. Nevertheless, it has been demonstrated that proteoglycans purified from salmon cartilage promote the wound healing of dermal fibroblasts248 and induce their proliferation, by the MAPK/ERK signaling pathway activation249. Similarly, a versican treatment on fibroblast stimulates their proliferation250. Interestingly, the application of proteoglycans purified from salmon cartilage on hematopoietic progenitor cells induce their differentiation into progenitor cells for granulocyte-macrophages, erythrocytes and/or megakaryocytes251. All these studies provide evidence that application or oral administration of proteoglycans can modulate the cell fate by the regulation of signaling pathways. Moreover, the first studies conducted on proteoglycan-based therapy for hair loss are promising. The mechanism of action of HSPGs on signaling pathway involved in hair follicle stem cell fate remains to be elucidated in order to develop HSPG-based treatment for hair loss.
Conclusion
It is clear that our knowledge on the role of HSPGs on stem cell fate becomes greater by the day. Several publications and reviews have reported the capacity of HSPGs to modulate signaling pathways in stem cells in various different ways. Indeed, they can interact directly with the growth factors and/or their associated receptors by their core protein or their HS GAG chains. This interaction can facilitate the binding between receptor and growth factor or can inhibit the pathway by the sequestration of the growth factor. Moreover, membrane HSPGs can be cleaved to favor long-range activity of a growth factor. The understanding of the molecular mechanisms of action of HSPGs on stem cell fate highlights their key role on the regulation of organ morphogenesis, homeostasis and regeneration. In the case of hair follicle stem cells, there is still a long way to characterize the role of HSPGs. Indeed, no studies have reported a link between HSPGs and growth factors/signaling pathways involved in hair follicle stem cell fate. Moreover, few studies have shown a regulation of hair follicle stem cells by other proteoglycans (versican, decorin). Evidence on the role of HSPGs on the regulation of signaling pathways involved in hair follicles stem cell differentiation are proven by several studies that have demonstrated changes in the distribution of HSPGs during hair growth cycle and by the fact that HSPG sulfation is necessary for hair shaft growth. Currently, the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have only approved the topical Minoxidil and the oral Finasteride as treatment of androgenetic alopecia. These two drugs have demonstrated their ability to induce hair regrowth. However, both present side effects limiting their efficacy in a still unclear mechanism. In particular, the Finasteride is known to induce sexual disorder when the oral use is prolonged245. The Minoxidil topical application required specific formulation known to induce inflammatory skin reaction, such as eczema and allergy in case of repetitive application247. The fact that the treatment of alopecia requires long periods of application or oral administration represents the greatest limitation of these treatments. The Nourkrin oral tablet administration has shown good results on hair growth. Drug-free, it does not induce any side effect even during long period of application243. The intake of proteoglycan to the hair follicle leads to re-establishment of the hair follicle metabolism. The studies of the role HS proteoglycans in the regulation of the hair follicle metabolism will contribute to mastering the mode of action of the treatment based on HSPG delivery or to develop therapies targeting HSPGs. Proteoglycan-based treatment by oral delivery of proteoglycans appears to be a promising method to tackle androgenetic alopecia. Indeed, it has been shown to increase the hair counts on scalp. This kind of treatment can be applied with HSPGs oral delivery or topical application on scalp. Another way of investigation would be a treatment able to regulate the cellular expression of HSPGs to modulate their local distribution in hair follicles.
Acknowledgements
This study was made in collaboration with BASF Beauty Care Solutions. Ms Charlie Colin-Pierre was a BASF /CNRS funded PhD fellow.
Author contributions
C.C.-P. prepared the overall structure of the manuscript, performed the literature search and analysis, and contributed to drafting all manuscript sections. All authors critically revised the work. All authors have read and agreed with the submission of the manuscript and have agreed to be personally accountable for the author’s own contribution.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Laurent Ramont, Stéphane Brézillon.
References
- 1.Bernard BA. La vie révélée du follicule de cheveu humain. Méd. Sci. 2006;22:138–143. doi: 10.1051/medsci/2006222138. [DOI] [PubMed] [Google Scholar]
- 2.Geras, A. J. Dermatology: A Medical Artist’s Interpretation (Sandoz Medical Publications, Sandoz Pharma Limited, 1990).
- 3.Sada A, Tumbar T. New insights into mechanisms of stem cell daughter fate determination in regenerative tissues. Int. Rev. Cell Mol. Biol. 2013;300:1–50. doi: 10.1016/B978-0-12-405210-9.00001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenn K. Exogen is an active, separately controlled phase of the hair growth cycle. J. Am. Acad. Dermatol. 2005;52:374–375. doi: 10.1016/j.jaad.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 5.Milner Y, et al. Exogen, shedding phase of the hair growth cycle: characterization of a mouse model. J. Invest. Dermatol. 2002;119:639–644. doi: 10.1046/j.1523-1747.2002.01842.x. [DOI] [PubMed] [Google Scholar]
- 6.Bernard BA, Commo S, Gerst C, Mahé FY, Pruche F. Données récentes sur la biologie du cheveu. Bull. Esthet. Dermatol Cosmetol. 1996;4:55–64. [Google Scholar]
- 7.Chase HB, Rauch R, Smith VW. Critical stages of hair development and pigmentation in the mouse. Physiol. Zool. 1951;24:1–8. doi: 10.1086/physzool.24.1.30152098. [DOI] [PubMed] [Google Scholar]
- 8.Müller-Röver S, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 9.Alonso L, Fuchs E. Stem cells of the skin epithelium. Proc. Natl Acad. Sci. USA. 2003;100:11830–11835. doi: 10.1073/pnas.1734203100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue K, et al. Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. Lab. Investig. J. Tech. Methods Pathol. 2009;89:844–856. doi: 10.1038/labinvest.2009.48. [DOI] [PubMed] [Google Scholar]
- 11.Kloepper JE, et al. Immunophenotyping of the human bulge region: the quest to define useful in situ markers for human epithelial hair follicle stem cells and their niche. Exp. Dermatol. 2008;17:592–609. doi: 10.1111/j.1600-0625.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- 12.Panteleyev AA. Functional anatomy of the hair follicle: the secondary hair germ. Exp. Dermatol. 2018;27:701–720. doi: 10.1111/exd.13666. [DOI] [PubMed] [Google Scholar]
- 13.Purba TS, et al. Human epithelial hair follicle stem cells and their progeny: current state of knowledge, the widening gap in translational research and future challenges. BioEssays N. Rev. Mol. Cell. Dev. Biol. 2014;36:513–525. doi: 10.1002/bies.201300166. [DOI] [PubMed] [Google Scholar]
- 14.Botchkarev VA, Paus R. Molecular biology of hair morphogenesis: development and cycling. J. Exp. Zool. B Mol. Dev. Evol. 2003;298:164–180. doi: 10.1002/jez.b.33. [DOI] [PubMed] [Google Scholar]
- 15.Bayer-Garner IB, Sanderson RD, Smoller BR. Syndecan-1 is strongly expressed in the anagen hair follicle outer root sheath and in the dermal papilla but expression diminishes with involution of the hair follicle. Am. J. Dermatopathol. 2002;24:484–489. doi: 10.1097/00000372-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Colin-Pierre, C. et al. Hair histology and glycosaminoglycans distribution probed by infrared spectral imaging: focus on Heparan sulfate proteoglycan and glypican-1 during hair growth cycle. Biomolecules11, 192 (2021). [DOI] [PMC free article] [PubMed]
- 17.Malgouries S, Thibaut S, Bernard BA. Proteoglycan expression patterns in human hair follicle. Br. J. Dermatol. 2008;158:234–242. doi: 10.1111/j.1365-2133.2007.08339.x. [DOI] [PubMed] [Google Scholar]
- 18.Wadstein, J., Thom, E. & Gadzhigoroeva, A. Integral roles of specific proteoglycans in hair growth and hair loss: mechanisms behind the bioactivity of proteoglycan replacement therapy with Nourkrin® with Marilex® in pattern hair loss and telogen Effluvium. Dermatol. Res. Pract. 2020, 8125081 (2020). [DOI] [PMC free article] [PubMed]
- 19.Coulson-Thomas VJ, Gesteira TF, Esko J, Kao W. Heparan sulfate regulates hair follicle and sebaceous gland morphogenesis and homeostasis. J. Biol. Chem. 2014;289:25211–25226. doi: 10.1074/jbc.M114.572511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayers KL, Gallet A, Staccini-Lavenant L, Thérond PP. The long-range activity of Hedgehog is regulated in the apical extracellular space by the glypican Dally and the hydrolase Notum. Dev. Cell. 2010;18:605–620. doi: 10.1016/j.devcel.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Capurro M, et al. Glypican-6 promotes the growth of developing long bones by stimulating Hedgehog signaling. J. Cell Biol. 2017;216:2911–2926. doi: 10.1083/jcb.201605119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dwivedi PP, et al. Regulation of bone morphogenetic protein signalling and cranial osteogenesis by Gpc1 and Gpc3. Bone. 2013;55:367–376. doi: 10.1016/j.bone.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Filmus J, Capurro M, Rast J. Glypicans. Genome Biol. 2008;9:224. doi: 10.1186/gb-2008-9-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreuger J, Perez L, Giraldez AJ, Cohen SM. Opposing activities of Dally-like glypican at high and low levels of Wingless morphogen activity. Dev. Cell. 2004;7:503–512. doi: 10.1016/j.devcel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto S, et al. Heparan sulfate on intestinal epithelial cells plays a critical role in intestinal crypt homeostasis via Wnt/β-catenin signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305:G241–G249. doi: 10.1152/ajpgi.00480.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallagher JT. The extended family of proteoglycans: social residents of the pericellular zone. Curr. Opin. Cell Biol. 1989;1:1201–1218. doi: 10.1016/S0955-0674(89)80072-9. [DOI] [PubMed] [Google Scholar]
- 27.Yung S, Chan TM. Glycosaminoglycans and proteoglycans: overlooked entities? Perit. Dial. Int. 2007;27:S104–S109. doi: 10.1177/089686080702702s18. [DOI] [PubMed] [Google Scholar]
- 28.Sasarman F, et al. Biosynthesis of glycosaminoglycans: associated disorders and biochemical tests. J. Inherit. Metab. Dis. 2016;39:173–188. doi: 10.1007/s10545-015-9903-z. [DOI] [PubMed] [Google Scholar]
- 29.Merida-de-Barros DA, Chaves SP, Belmiro CLR, Wanderley JLM. Leishmaniasis and glycosaminoglycans: a future therapeutic strategy? Parasit. Vectors. 2018;11:536. doi: 10.1186/s13071-018-2953-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razi N, Lindahl U. Biosynthesis of heparin/heparan sulfate. The D-glucosaminyl 3-O-sulfotransferase reaction: target and inhibitor saccharides. J. Biol. Chem. 1995;270:11267–11275. doi: 10.1074/jbc.270.19.11267. [DOI] [PubMed] [Google Scholar]
- 31.Ricard-Blum S. Protein–glycosaminoglycan interaction networks: focus on heparan sulfate. Perspect. Sci. 2017;11:62–69. doi: 10.1016/j.pisc.2016.10.004. [DOI] [Google Scholar]
- 32.Oh J-H, et al. Intrinsic aging- and photoaging-dependent level changes of glycosaminoglycans and their correlation with water content in human skin. J. Dermatol. Sci. 2011;62:192–201. doi: 10.1016/j.jdermsci.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host‐associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 34.Takemae H, et al. Toxoplasma gondii RON4 binds to heparan sulfate on the host cell surface. Parasitol. Int. 2018;67:123–130. doi: 10.1016/j.parint.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J. Clin. Invest. 2001;108:349–355. doi: 10.1172/JCI200113738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uijtdewilligen PJE, et al. Dynamic expression of genes involved in proteoglycan/glycosaminoglycan metabolism during skin development. BioMed. Res. Int. 2018;2018:9873471. doi: 10.1155/2018/9873471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shworak NW, et al. Pathway-specific regulation of the synthesis of anticoagulantly active heparan sulfate. J. Biol. Chem. 1994;269:24941–24952. doi: 10.1016/S0021-9258(17)31481-3. [DOI] [PubMed] [Google Scholar]
- 38.Annaval T, et al. Heparan sulfate proteoglycans biosynthesis and post synthesis mechanisms combine few enzymes and few core proteins to generate extensive structural and functional diversity. Mol. Basel Switz. 2020;25:E4215. doi: 10.3390/molecules25184215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prydz K, Dalen KT. Synthesis and sorting of proteoglycans. J. Cell Sci. 2000;113:193–205. doi: 10.1242/jcs.113.2.193. [DOI] [PubMed] [Google Scholar]
- 40.Dolan M, Horchar T, Rigatti B, Hassell JR. Identification of sites in domain I of perlecan that regulate heparan sulfate synthesis. J. Biol. Chem. 1997;272:4316–4322. doi: 10.1074/jbc.272.7.4316. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Esko JD. Amino acid determinants that drive heparan sulfate assembly in a proteoglycan. J. Biol. Chem. 1994;269:19295–19299. doi: 10.1016/S0021-9258(17)32166-X. [DOI] [PubMed] [Google Scholar]
- 42.Aikawa J, Esko JD. Molecular cloning and expression of a third member of the heparan sulfate/heparin GlcNAc N-deacetylase/ N-sulfotransferase family. J. Biol. Chem. 1999;274:2690–2695. doi: 10.1074/jbc.274.5.2690. [DOI] [PubMed] [Google Scholar]
- 43.Fransson LA, Silverberg I, Carlstedt I. Structure of the heparan sulfate-protein linkage region. Demonstration of the sequence galactosyl-galactosyl-xylose-2-phosphate. J. Biol. Chem. 1985;260:14722–14726. doi: 10.1016/S0021-9258(17)38632-5. [DOI] [PubMed] [Google Scholar]
- 44.Avraham S, et al. Molecular cloning of a cDNA that encodes the peptide core of a mouse mast cell secretory granule proteoglycan and comparison with the analogous rat and human cDNA. Proc. Natl Acad. Sci. USA. 1989;86:3763–3767. doi: 10.1073/pnas.86.10.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. J. Int. Soc. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korpetinou A, et al. Serglycin: At the crossroad of inflammation and malignancy. Front. Oncol. 2014;3:327. doi: 10.3389/fonc.2013.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kjellén L, et al. Primary structure of a mouse mastocytoma proteoglycan core protein. Biochem. J. 1989;263:105–113. doi: 10.1042/bj2630105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schick BP, Senkowski-Richardson S. Proteoglycan synthesis in human erythroleukaemia (HEL) cells. Biochem. J. 1992;282:651–658. doi: 10.1042/bj2820651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolset SO, Prydz K, Pejler G. Intracellular proteoglycans. Biochem. J. 2004;379:217–227. doi: 10.1042/bj20031230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolset SO, Tveit H. Serglycin—Structure and biology. Cell. Mol. Life Sci. 2008;65:1073–1085. doi: 10.1007/s00018-007-7455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schick BP, Gradowski JF, Antonio JDS. Synthesis, secretion, and subcellular localization of serglycin proteoglycan in human endothelial cells. Blood. 2001;97:449–458. doi: 10.1182/blood.V97.2.449. [DOI] [PubMed] [Google Scholar]
- 52.Schick BP, Ho H-CK, Brodbeck KC, Wrigley CW, Klimas J. Serglycin proteoglycan expression and synthesis in embryonic stem cells. Biochim. Biophys. Acta BBA Mol. Cell Res. 2003;1593:259–267. doi: 10.1016/S0167-4889(02)00396-8. [DOI] [PubMed] [Google Scholar]
- 53.Yurt RW, Leid RW, Austen KF. Native heparin from rat peritoneal mast cells. J. Biol. Chem. 1977;252:518–521. doi: 10.1016/S0021-9258(17)32747-3. [DOI] [PubMed] [Google Scholar]
- 54.Pejler G, Åbrink M, Wernersson S. Serglycin proteoglycan: regulating the storage and activities of hematopoietic proteases. BioFactors. 2009;35:61–68. doi: 10.1002/biof.11. [DOI] [PubMed] [Google Scholar]
- 55.Manou D, et al. Serglycin activates pro-tumorigenic signaling and controls glioblastoma cell stemness, differentiation and invasive potential. Matrix Biol. 2020;6–7:100033. doi: 10.1016/j.mbplus.2020.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bouris P, et al. Serglycin promotes breast cancer cell aggressiveness: induction of epithelial to mesenchymal transition, proteolytic activity and IL-8 signaling. Matrix Biol. J. Int. Soc. Matrix Biol. 2018;74:35–51. doi: 10.1016/j.matbio.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 57.Manou D, Karamanos NK, Theocharis AD. Tumorigenic functions of serglycin: regulatory roles in epithelial to mesenchymal transition and oncogenic signaling. Semin. Cancer Biol. 2020;62:108–115. doi: 10.1016/j.semcancer.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Couchman JR. Transmembrane signaling proteoglycans. Annu. Rev. Cell Dev. Biol. 2010;26:89–114. doi: 10.1146/annurev-cellbio-100109-104126. [DOI] [PubMed] [Google Scholar]
- 59.Karamanos NK, et al. Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics. Chem. Rev. 2018;118:9152–9232. doi: 10.1021/acs.chemrev.8b00354. [DOI] [PubMed] [Google Scholar]
- 60.Bernfield M, et al. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu. Rev. Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 61.Gondelaud F, Ricard-Blum S. Structures and interactions of syndecans. FEBS J. 2019;286:2994–3007. doi: 10.1111/febs.14828. [DOI] [PubMed] [Google Scholar]
- 62.Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol. Biol. Cell. 1994;5:797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi Y, Chung H, Jung H, Couchman JR, Oh E-S. Syndecans as cell surface receptors: unique structure equates with functional diversity. Matrix Biol. J. Int. Soc. Matrix Biol. 2011;30:93–99. doi: 10.1016/j.matbio.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 64.Chung H, Multhaupt HAB, Oh E-S, Couchman JR. Minireview: Syndecans and their crucial roles during tissue regeneration. FEBS Lett. 2016;590:2408–2417. doi: 10.1002/1873-3468.12280. [DOI] [PubMed] [Google Scholar]
- 65.Echtermeyer F, et al. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J. Clin. Invest. 2001;107:R9–R14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leadbeater WE, et al. Intracellular trafficking in neurones and glia of fibroblast growth factor-2, fibroblast growth factor receptor 1 and heparan sulphate proteoglycans in the injured adult rat cerebral cortex. J. Neurochem. 2006;96:1189–1200. doi: 10.1111/j.1471-4159.2005.03632.x. [DOI] [PubMed] [Google Scholar]
- 67.Noguer O, Villena J, Lorita J, Vilaró S, Reina M. Syndecan-2 downregulation impairs angiogenesis in human microvascular endothelial cells. Exp. Cell Res. 2009;315:795–808. doi: 10.1016/j.yexcr.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 68.Fransson L-A. Glypicans. Int. J. Biochem. Cell Biol. 2003;35:125–129. doi: 10.1016/S1357-2725(02)00095-X. [DOI] [PubMed] [Google Scholar]
- 69.Veugelers M, et al. Glypican-6, a new member of the Glypican family of cell surface Heparan sulfate proteoglycans. J. Biol. Chem. 1999;274:26968–26977. doi: 10.1074/jbc.274.38.26968. [DOI] [PubMed] [Google Scholar]
- 70.Mayor S, Riezman H. Sorting GPI-anchored proteins. Nat. Rev. Mol. Cell Biol. 2004;5:110–120. doi: 10.1038/nrm1309. [DOI] [PubMed] [Google Scholar]
- 71.Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat. Rev. Mol. Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mertens G, Van der Schueren B, van den Berghe H, David G. Heparan sulfate expression in polarized epithelial cells: the apical sorting of glypican (GPI-anchored proteoglycan) is inversely related to its heparan sulfate content. J. Cell Biol. 1996;132:487–497. doi: 10.1083/jcb.132.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Traister A, Shi W, Filmus J. Mammalian Notum induces the release of glypicans and other GPI-anchored proteins from the cell surface. Biochem. J. 2008;410:503–511. doi: 10.1042/BJ20070511. [DOI] [PubMed] [Google Scholar]
- 74.Kawahara R, et al. Mass spectrometry-based proteomics revealed Glypican-1 as a novel ADAM17 substrate. J. Proteom. 2017;151:53–65. doi: 10.1016/j.jprot.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 75.Hereld D, Krakow JL, Bangs JD, Hart GW, Englund PT. A phospholipase C from Trypanosoma brucei which selectively cleaves the glycolipid on the variant surface glycoprotein. J. Biol. Chem. 1986;261:13813–13819. doi: 10.1016/S0021-9258(18)67092-9. [DOI] [PubMed] [Google Scholar]
- 76.McGough IJ, et al. Glypicans shield the Wnt lipid moiety to enable signalling at a distance. Nature. 2020;585:85–90. doi: 10.1038/s41586-020-2498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Costell M, et al. Perlecan maintains the integrity of Cartilage and some basement membranes. J. Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iozzo RV. MATRIX PROTEOGLYCANS: From molecular design to cellular function. Annu. Rev. Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 79.Amenta PS, et al. Proteoglycan-collagen XV in human tissues is seen linking banded collagen fibers subjacent to the basement membrane. J. Histochem. Cytochem. J. Histochem. Soc. 2005;53:165–176. doi: 10.1369/jhc.4A6376.2005. [DOI] [PubMed] [Google Scholar]
- 80.McCarthy KJ. The basement membrane proteoglycans perlecan and agrin: something old, something new. Curr. Top. Membr. 2015;76:255–303. doi: 10.1016/bs.ctm.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 81.French MM, et al. Expression of the heparan sulfate proteoglycan, perlecan, during mouse embryogenesis and perlecan chondrogenic activity in vitro. J. Cell Biol. 1999;145:1103–1115. doi: 10.1083/jcb.145.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mazzon C, et al. The critical role of agrin in the hematopoietic stem cell niche. Blood. 2011;118:2733–2742. doi: 10.1182/blood-2011-01-331272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mazzon C, et al. Agrin is required for survival and function of monocytic cells. Blood. 2012;119:5502–5511. doi: 10.1182/blood-2011-09-382812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vannahme C, et al. Molecular cloning of testican-2: defining a novel calcium-binding proteoglycan family expressed in brain. J. Neurochem. 1999;73:12–20. doi: 10.1046/j.1471-4159.1999.0730012.x. [DOI] [PubMed] [Google Scholar]
- 85.Kohfeldt E, Maurer P, Vannahme C, Timpl R. Properties of the extracellular calcium binding module of the proteoglycan testican. FEBS Lett. 1997;414:557–561. doi: 10.1016/S0014-5793(97)01070-3. [DOI] [PubMed] [Google Scholar]
- 86.Bonnet F, et al. Structure and cellular distribution of mouse brain testican: association with the postsynaptic area of hippocampus pyramidal cells (∗) J. Biol. Chem. 1996;271:4373–4380. doi: 10.1074/jbc.271.8.4373. [DOI] [PubMed] [Google Scholar]
- 87.Hartmann U, et al. Testican-3: a brain-specific proteoglycan member of the BM-40/SPARC/osteonectin family. J. Neurochem. 2013;125:399–409. doi: 10.1111/jnc.12212. [DOI] [PubMed] [Google Scholar]
- 88.Charbonnier F, Périn JP, Roussel G, Nussbaum JL, Alliel PM. [Cloning of testican/SPOCK in man and mouse. Neuromuscular expression perspectives in pathology] C. R. Seances Soc. Biol. Fil. 1997;191:127–133. [PubMed] [Google Scholar]
- 89.Schnepp A, et al. Mouse testican-2: expression, glycosylation, and effects on neurite outgrowth*. J. Biol. Chem. 2005;280:11274–11280. doi: 10.1074/jbc.M414276200. [DOI] [PubMed] [Google Scholar]
- 90.Yamamoto A, et al. Structural abnormalities of corpus callosum and cortical axonal tracts accompanied by decreased anxiety-like behavior and lowered sociability in Spock3-mutant mice. Dev. Neurosci. 2014;36:381–395. doi: 10.1159/000363101. [DOI] [PubMed] [Google Scholar]
- 91.Alshargabi R, et al. SPOCK1 induces adipose tissue maturation: new insights into the function of SPOCK1 in metabolism. Biochem. Biophys. Res. Commun. 2020;533:1076–1082. doi: 10.1016/j.bbrc.2020.09.129. [DOI] [PubMed] [Google Scholar]
- 92.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 93.Grumolato L, et al. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Amerongen R, Bowman AN, Nusse R. Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 2012;11:387–400. doi: 10.1016/j.stem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 95.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 96.Barker N, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 97.ten Berge D, et al. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat. Cell Biol. 2011;13:1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bowman AN, van Amerongen R, Palmer TD, Nusse R. Lineage tracing with Axin2 reveals distinct developmental and adult populations of Wnt/β-catenin-responsive neural stem cells. Proc. Natl Acad. Sci. USA. 2013;110:7324–7329. doi: 10.1073/pnas.1305411110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheung P, et al. Regenerative reprogramming of the intestinal stem cell state via Hippo signaling suppresses metastatic colorectal cancer. Cell Stem Cell. 2020;27:590–604.e9. doi: 10.1016/j.stem.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Flesken-Nikitin A, et al. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495:241–245. doi: 10.1038/nature11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Q, et al. Lats1/2 sustain intestinal stem cells and Wnt activation through TEAD-dependent and independent transcription. Cell Stem Cell. 2020;26:675–692.e8. doi: 10.1016/j.stem.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rinkevich Y, et al. In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep. 2014;7:1270–1283. doi: 10.1016/j.celrep.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tan SH, et al. Wnts produced by Osterix-expressing osteolineage cells regulate their proliferation and differentiation. Proc. Natl Acad. Sci. USA. 2014;111:E5262–E5271. doi: 10.1073/pnas.1420463111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang B, Zhao L, Fish M, Logan CY, Nusse R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature. 2015;524:180–185. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu L, et al. Derivation of intermediate pluripotent stem cells Amenable to primordial germ cell specification. Cell Stem Cell. 2021;28:550–567.e12. doi: 10.1016/j.stem.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 106.Kamimura K, et al. Perlecan regulates bidirectional Wnt signaling at the Drosophila neuromuscular junction. J. Cell Biol. 2013;200:219–233. doi: 10.1083/jcb.201207036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lindner JR, et al. The Drosophila Perlecan gene trol regulates multiple signaling pathways in different developmental contexts. BMC Dev. Biol. 2007;7:121. doi: 10.1186/1471-213X-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fico A, et al. Modulating Glypican4 suppresses tumorigenicity of embryonic stem cells while preserving self-renewal and pluripotency. Stem Cells Dayt. Ohio. 2012;30:1863–1874. doi: 10.1002/stem.1165. [DOI] [PubMed] [Google Scholar]
- 109.Venero Galanternik M, Lush ME, Piotrowski T. Glypican4 modulates lateral line collective cell migration non cell-autonomously. Dev. Biol. 2016;419:321–335. doi: 10.1016/j.ydbio.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 110.Sasaki N, et al. The 3’-phosphoadenosine 5’-phosphosulfate transporters, PAPST1 and 2, contribute to the maintenance and differentiation of mouse embryonic stem cells. PloS One. 2009;4:e8262. doi: 10.1371/journal.pone.0008262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Q, Yang L, Alexander C, Temple S. The niche factor syndecan-1 regulates the maintenance and proliferation of neural progenitor cells during mammalian cortical development. PloS One. 2012;7:e42883. doi: 10.1371/journal.pone.0042883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Henríquez JP, Salinas PC. Dual roles for Wnt signalling during the formation of the vertebrate neuromuscular junction. Acta Physiol. Oxf. Engl. 2012;204:128–136. doi: 10.1111/j.1748-1716.2011.02295.x. [DOI] [PubMed] [Google Scholar]
- 113.Su T-Y, Nakato E, Choi PY, Nakato H. Drosophila Glypicans regulate follicle stem cell maintenance and Niche competition. Genetics. 2018;209:537–549. doi: 10.1534/genetics.118.300839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ling L, et al. Synergism between Wnt3a and heparin enhances osteogenesis via a phosphoinositide 3-kinase/Akt/RUNX2 pathway. J. Biol. Chem. 2010;285:26233–26244. doi: 10.1074/jbc.M110.122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eldridge SE, et al. Agrin induces long-term osteochondral regeneration by supporting repair morphogenesis. Sci. Transl. Med. 2020;12:eaax9086. doi: 10.1126/scitranslmed.aax9086. [DOI] [PubMed] [Google Scholar]
- 116.Walterhouse DO, Yoon JW, Iannaccone PM. Developmental pathways: sonic hedgehog-patched-GLI. Environ. Health Perspect. 1999;107:167–171. doi: 10.1289/ehp.99107167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hebrok M, Kim SK, St Jacques B, McMahon AP, Melton DA. Regulation of pancreas development by hedgehog signaling. Dev. Camb. Engl. 2000;127:4905–4913. doi: 10.1242/dev.127.22.4905. [DOI] [PubMed] [Google Scholar]
- 118.Karp SJ, et al. Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Dev. Camb. Engl. 2000;127:543–548. doi: 10.1242/dev.127.3.543. [DOI] [PubMed] [Google Scholar]
- 119.Kenney AM, Rowitch DH. Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol. Cell. Biol. 2000;20:9055–9067. doi: 10.1128/MCB.20.23.9055-9067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Krüger M, et al. Sonic hedgehog is a survival factor for hypaxial muscles during mouse development. Dev. Camb. Engl. 2001;128:743–752. doi: 10.1242/dev.128.5.743. [DOI] [PubMed] [Google Scholar]
- 121.Miura H, et al. Shh and Ptc are associated with taste bud maintenance in the adult mouse. Mech. Dev. 2001;106:143–145. doi: 10.1016/S0925-4773(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 122.Alexandre C, Lecourtois M, Vincent J. Wingless and Hedgehog pattern Drosophila denticle belts by regulating the production of short-range signals. Dev. Camb. Engl. 1999;126:5689–5698. doi: 10.1242/dev.126.24.5689. [DOI] [PubMed] [Google Scholar]
- 123.Briscoe J, Chen Y, Jessell TM, Struhl G. A hedgehog-insensitive form of patched provides evidence for direct long-range morphogen activity of sonic hedgehog in the neural tube. Mol. Cell. 2001;7:1279–1291. doi: 10.1016/S1097-2765(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 124.Hynes M, et al. The seven-transmembrane receptor smoothened cell-autonomously induces multiple ventral cell types. Nat. Neurosci. 2000;3:41–46. doi: 10.1038/71114. [DOI] [PubMed] [Google Scholar]
- 125.Ingham PW, Fietz MJ. Quantitative effects of hedgehog and decapentaplegic activity on the patterning of the Drosophila wing. Curr. Biol. CB. 1995;5:432–440. doi: 10.1016/S0960-9822(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 126.Teleman AA, Cohen SM. Dpp gradient formation in the Drosophila wing imaginal disc. Cell. 2000;103:971–980. doi: 10.1016/S0092-8674(00)00199-9. [DOI] [PubMed] [Google Scholar]
- 127.Lin X, Perrimon N. Role of heparan sulfate proteoglycans in cell-cell signaling in Drosophila. Matrix Biol. J. Int. Soc. Matrix Biol. 2000;19:303–307. doi: 10.1016/S0945-053X(00)00073-1. [DOI] [PubMed] [Google Scholar]
- 128.Williams EH, et al. Dally-like core protein and its mammalian homologues mediate stimulatory and inhibitory effects on Hedgehog signal response. Proc. Natl Acad. Sci. USA. 2010;107:5869–5874. doi: 10.1073/pnas.1001777107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Capurro MI, et al. Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Dev. Cell. 2008;14:700–711. doi: 10.1016/j.devcel.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 130.Capurro MI, Li F, Filmus J. Overgrowth of a mouse model of Simpson-Golabi-Behmel syndrome is partly mediated by Indian hedgehog. EMBO Rep. 2009;10:901–907. doi: 10.1038/embor.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Superina S, Borovina A, Ciruna B. Analysis of maternal-zygotic ugdh mutants reveals divergent roles for HSPGs in vertebrate embryogenesis and provides new insight into the initiation of left-right asymmetry. Dev. Biol. 2014;387:154–166. doi: 10.1016/j.ydbio.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 132.Gallet A, Rodriguez R, Ruel L, Therond PP. Cholesterol modification of hedgehog is required for trafficking and movement, revealing an asymmetric cellular response to hedgehog. Dev. Cell. 2003;4:191–204. doi: 10.1016/S1534-5807(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 133.Han C, Belenkaya TY, Wang B, Lin X. Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process. Dev. Camb. Engl. 2004;131:601–611. doi: 10.1242/dev.00958. [DOI] [PubMed] [Google Scholar]
- 134.Park Y, Ng C, Datta S. Induction of string rescues the neuroblast proliferation defect in trol mutant animals. Genes. N. Y. N. 2000. 2003;36:187–195. doi: 10.1002/gene.10216. [DOI] [PubMed] [Google Scholar]
- 135.Girós A, Morante J, Gil-Sanz C, Fairén A, Costell M. Perlecan controls neurogenesis in the developing telencephalon. BMC Dev. Biol. 2007;7:29. doi: 10.1186/1471-213X-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Palma V, et al. SHh activity and localization is regulated by perlecan. Biol. Res. 2011;44:63–67. doi: 10.4067/S0716-97602011000100008. [DOI] [PubMed] [Google Scholar]
- 137.Zhang C, Ojiaku P, Cole GJ. Forebrain and hindbrain development in zebrafish is sensitive to ethanol exposure involving agrin, Fgf, and sonic hedgehog function. Birt. Defects Res. A. Clin. Mol. Teratol. 2013;97:8–27. doi: 10.1002/bdra.23099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Takemura M, Nakato H. Drosophila Sulf1 is required for the termination of intestinal stem cell division during regeneration. J. Cell Sci. 2017;130:332–343. doi: 10.1242/jcs.195305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gritli-Linde A, Lewis P, McMahon AP, Linde A. The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev. Biol. 2001;236:364–386. doi: 10.1006/dbio.2001.0336. [DOI] [PubMed] [Google Scholar]
- 140.Hilton MJ, Gutiérrez L, Martinez DA, Wells DE. EXT1 regulates chondrocyte proliferation and differentiation during endochondral bone development. Bone. 2005;36:379–386. doi: 10.1016/j.bone.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 141.Koziel L, Kunath M, Kelly OG, Vortkamp A. Ext1-dependent heparan sulfate regulates the range of Ihh signaling during endochondral ossification. Dev. Cell. 2004;6:801–813. doi: 10.1016/j.devcel.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 142.Shimo T, et al. Indian hedgehog and syndecans-3 coregulate chondrocyte proliferation and function during chick limb skeletogenesis. Dev. Dyn. Publ. Am. Assoc. Anat. 2004;229:607–617. doi: 10.1002/dvdy.20009. [DOI] [PubMed] [Google Scholar]
- 143.Liu F, Ventura F, Doody J, Massagué J. Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol. Cell. Biol. 1995;15:3479–3486. doi: 10.1128/MCB.15.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yamashita H, Ten Dijke P, Heldin CH, Miyazono K. Bone morphogenetic protein receptors. Bone. 1996;19:569–574. doi: 10.1016/S8756-3282(96)00259-1. [DOI] [PubMed] [Google Scholar]
- 145.Kattman SJ, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 146.McCarthy N, et al. Distinct mesenchymal cell populations generate the essential intestinal BMP signaling gradient. Cell Stem Cell. 2020;26:391–402.e5. doi: 10.1016/j.stem.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen G, Deng C, Li Y-P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 2011;13:27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 149.Akiyama T, et al. Dally regulates Dpp morphogen gradient formation by stabilizing Dpp on the cell surface. Dev. Biol. 2008;313:408–419. doi: 10.1016/j.ydbio.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dejima K, Kanai MI, Akiyama T, Levings DC, Nakato H. Novel contact-dependent bone morphogenetic protein (BMP) signaling mediated by heparan sulfate proteoglycans. J. Biol. Chem. 2011;286:17103–17111. doi: 10.1074/jbc.M110.208082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fujise M, et al. Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Dev. Camb. Engl. 2003;130:1515–1522. doi: 10.1242/dev.00379. [DOI] [PubMed] [Google Scholar]
- 152.Kraushaar DC, et al. Heparan sulfate facilitates FGF and BMP signaling to drive mesoderm differentiation of mouse embryonic stem cells. J. Biol. Chem. 2012;287:22691–22700. doi: 10.1074/jbc.M112.368241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hayashi Y, Kobayashi S, Nakato H. Drosophila glypicans regulate the germline stem cell niche. J. Cell Biol. 2009;187:473–480. doi: 10.1083/jcb.200904118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bramono DS, et al. Bone marrow-derived heparan sulfate potentiates the osteogenic activity of bone morphogenetic protein-2 (BMP-2) Bone. 2012;50:954–964. doi: 10.1016/j.bone.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fisher MC, Li Y, Seghatoleslami MR, Dealy CN, Kosher RA. Heparan sulfate proteoglycans including syndecan-3 modulate BMP activity during limb cartilage differentiation. Matrix Biol. J. Int. Soc. Matrix Biol. 2006;25:27–39. doi: 10.1016/j.matbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 156.Jha AK, Yang W, Kirn-Safran CB, Farach-Carson MC, Jia X. Perlecan domain I-conjugated, hyaluronic acid-based hydrogel particles for enhanced chondrogenic differentiation via BMP-2 release. Biomaterials. 2009;30:6964–6975. doi: 10.1016/j.biomaterials.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Decarlo AA, et al. Perlecan domain 1 recombinant proteoglycan augments BMP-2 activity and osteogenesis. BMC Biotechnol. 2012;12:60. doi: 10.1186/1472-6750-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Souza ATP, et al. The extracellular matrix protein Agrin is expressed by osteoblasts and contributes to their differentiation. Cell Tissue Res. 2021;386:335–347. doi: 10.1007/s00441-021-03494-9. [DOI] [PubMed] [Google Scholar]
- 159.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mossahebi-Mohammadi M, Quan M, Zhang J-S, Li X. FGF signaling pathway: a key regulator of stem cell pluripotency. Front. Cell Dev. Biol. 2020;8:79. doi: 10.3389/fcell.2020.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xie Y, et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020;5:181. doi: 10.1038/s41392-020-00222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thisse B, Thisse C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev. Biol. 2005;287:390–402. doi: 10.1016/j.ydbio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 163.Marie PJ, Miraoui H, Sévère N. FGF/FGFR signaling in bone formation: progress and perspectives. Growth Factors Chur Switz. 2012;30:117–123. doi: 10.3109/08977194.2012.656761. [DOI] [PubMed] [Google Scholar]
- 164.Teven CM, Farina EM, Rivas J, Reid RR. Fibroblast growth factor (FGF) signaling in development and skeletal diseases. Genes Dis. 2014;1:199–213. doi: 10.1016/j.gendis.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Diez Del Corral R, Morales AV. The multiple roles of FGF signaling in the developing spinal cord. Front. Cell Dev. Biol. 2017;5:58. doi: 10.3389/fcell.2017.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jacob A, et al. Differentiation of human Pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell. 2017;21:472–488.e10. doi: 10.1016/j.stem.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang L, et al. FGF/FGFR signaling: from lung development to respiratory diseases. Cytokine Growth Factor Rev. 2021;62:94–104. doi: 10.1016/j.cytogfr.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 168.Johnson CE, et al. Essential alterations of heparan sulfate during the differentiation of embryonic stem cells to Sox1-enhanced green fluorescent protein-expressing neural progenitor cells. Stem Cells Dayt. Ohio. 2007;25:1913–1923. doi: 10.1634/stemcells.2006-0445. [DOI] [PubMed] [Google Scholar]
- 169.Liu I-H, Zhang C, Kim MJ, Cole GJ. Retina development in zebrafish requires the heparan sulfate proteoglycan agrin. Dev. Neurobiol. 2008;68:877–898. doi: 10.1002/dneu.20625. [DOI] [PubMed] [Google Scholar]
- 170.Brickman YG, et al. Structural modification of fibroblast growth factor-binding heparan sulfate at a determinative stage of neural development. J. Biol. Chem. 1998;273:4350–4359. doi: 10.1074/jbc.273.8.4350. [DOI] [PubMed] [Google Scholar]
- 171.Guillemot F, Zimmer C. From cradle to grave: the multiple roles of fibroblast growth factors in neural development. Neuron. 2011;71:574–588. doi: 10.1016/j.neuron.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 172.Murphy M, Drago J, Bartlett PF. Fibroblast growth factor stimulates the proliferation and differentiation of neural precursor cells in vitro. J. Neurosci. Res. 1990;25:463–475. doi: 10.1002/jnr.490250404. [DOI] [PubMed] [Google Scholar]
- 173.Nurcombe V, Ford MD, Wildschut JA, Bartlett PF. Developmental regulation of neural response to FGF-1 and FGF-2 by heparan sulfate proteoglycan. Science. 1993;260:103–106. doi: 10.1126/science.7682010. [DOI] [PubMed] [Google Scholar]
- 174.Yamada T, et al. Heparan sulfate alterations in extracellular matrix structures and fibroblast growth factor-2 signaling impairment in the aged neurogenic niche. J. Neurochem. 2017;142:534–544. doi: 10.1111/jnc.14081. [DOI] [PubMed] [Google Scholar]
- 175.Jen Y-HL, Musacchio M, Lander AD. Glypican-1 controls brain size through regulation of fibroblast growth factor signaling in early neurogenesis. Neural Dev. 2009;4:33. doi: 10.1186/1749-8104-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hagihara K, Watanabe K, Chun J, Yamaguchi Y. Glypican-4 is an FGF2-binding heparan sulfate proteoglycan expressed in neural precursor cells. Dev. Dyn. Publ. Am. Assoc. Anat. 2000;219:353–367. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1059>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 177.Galli A, Roure A, Zeller R, Dono R. Glypican 4 modulates FGF signalling and regulates dorsoventral forebrain patterning in Xenopus embryos. Dev. Camb. Engl. 2003;130:4919–4929. doi: 10.1242/dev.00706. [DOI] [PubMed] [Google Scholar]
- 178.Salehi Z. In vivo injection of fibroblast growth factor-2 into the cisterna magna induces glypican-6 expression in mouse brain tissue. J. Clin. Neurosci. J. Neurosurg. Soc. Australas. 2009;16:689–692. doi: 10.1016/j.jocn.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 179.Haubst N, Georges-Labouesse E, De Arcangelis A, Mayer U, Götz M. Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Dev. Camb. Engl. 2006;133:3245–3254. doi: 10.1242/dev.02486. [DOI] [PubMed] [Google Scholar]
- 180.Joseph SJ, et al. A proteoglycan that activates fibroblast growth factors during early neuronal development is a perlecan variant. Dev. Camb. Engl. 1996;122:3443–3452. doi: 10.1242/dev.122.11.3443. [DOI] [PubMed] [Google Scholar]
- 181.Kirsch T, Koyama E, Liu M, Golub EE, Pacifici M. Syndecan-3 is a selective regulator of chondrocyte proliferation. J. Biol. Chem. 2002;277:42171–42177. doi: 10.1074/jbc.M207209200. [DOI] [PubMed] [Google Scholar]
- 182.Shimazu A, et al. Syndecan-3 and the control of chondrocyte proliferation during endochondral ossification. Exp. Cell Res. 1996;229:126–136. doi: 10.1006/excr.1996.0350. [DOI] [PubMed] [Google Scholar]
- 183.Olwin BB, Rapraeger A. Repression of myogenic differentiation by aFGF, bFGF, and K-FGF is dependent on cellular heparan sulfate. J. Cell Biol. 1992;118:631–639. doi: 10.1083/jcb.118.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252:1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- 185.Fuentealba L, Carey DJ, Brandan E. Antisense inhibition of syndecan-3 expression during skeletal muscle differentiation accelerates myogenesis through a basic fibroblast growth factor-dependent mechanism. J. Biol. Chem. 1999;274:37876–37884. doi: 10.1074/jbc.274.53.37876. [DOI] [PubMed] [Google Scholar]
- 186.Gutiérrez J, Brandan E. A novel mechanism of sequestering fibroblast growth factor 2 by glypican in lipid rafts, allowing skeletal muscle differentiation. Mol. Cell. Biol. 2010;30:1634–1649. doi: 10.1128/MCB.01164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Perrot G, et al. Decreased expression of GPC1 in human skin keratinocytes and epidermis during ageing. Exp. Gerontol. 2019;126:110693. doi: 10.1016/j.exger.2019.110693. [DOI] [PubMed] [Google Scholar]
- 188.Stelling MP, Lages YMV, Tovar AMF, Mourão PAS, Rehen SK. Matrix-bound heparan sulfate is essential for the growth and pluripotency of human embryonic stem cells. Glycobiology. 2013;23:337–345. doi: 10.1093/glycob/cws133. [DOI] [PubMed] [Google Scholar]
- 189.Hirano K, et al. 3-O-sulfated heparan sulfate recognized by the antibody HS4C3 contributes [corrected] to the differentiation of mouse embryonic stem cells via fas signaling. PloS One. 2012;7:e43440. doi: 10.1371/journal.pone.0043440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hirano K, Van Kuppevelt TH, Nishihara S. The transition of mouse pluripotent stem cells from the naïve to the primed state requires Fas signaling through 3-O sulfated heparan sulfate structures recognized by the HS4C3 antibody. Biochem. Biophys. Res. Commun. 2013;430:1175–1181. doi: 10.1016/j.bbrc.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 191.Raulo E, Chernousov MA, Carey DJ, Nolo R, Rauvala H. Isolation of a neuronal cell surface receptor of heparin binding growth-associated molecule (HB-GAM). Identification as N-syndecan (syndecan-3) J. Biol. Chem. 1994;269:12999–13004. doi: 10.1016/S0021-9258(18)99975-8. [DOI] [PubMed] [Google Scholar]
- 192.Bespalov MM, et al. Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J. Cell Biol. 2011;192:153–169. doi: 10.1083/jcb.201009136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Siczkowski M, Clarke D, Gordon MY. Binding of primitive hematopoietic progenitor cells to marrow stromal cells involves heparan sulfate. Blood. 1992;80:912–919. doi: 10.1182/blood.V80.4.912.912. [DOI] [PubMed] [Google Scholar]
- 194.Zweegman S, et al. Bone marrow stromal proteoglycans regulate megakaryocytic differentiation of human progenitor cells. Exp. Cell Res. 2004;299:383–392. doi: 10.1016/j.yexcr.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 195.Netelenbos T, et al. Proteoglycans guide SDF-1-induced migration of hematopoietic progenitor cells. J. Leukoc. Biol. 2002;72:353–362. doi: 10.1189/jlb.72.2.353. [DOI] [PubMed] [Google Scholar]
- 196.Gordon MY, Riley GP, Watt SM, Greaves MF. Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. Nature. 1987;326:403–405. doi: 10.1038/326403a0. [DOI] [PubMed] [Google Scholar]
- 197.Roberts R, et al. Heparan sulphate bound growth factors: a mechanism for stromal cell mediated haemopoiesis. Nature. 1988;332:376–378. doi: 10.1038/332376a0. [DOI] [PubMed] [Google Scholar]
- 198.Netelenbos T, et al. Proteoglycans on bone marrow endothelial cells bind and present SDF-1 towards hematopoietic progenitor cells. Leukemia. 2003;17:175–184. doi: 10.1038/sj.leu.2402738. [DOI] [PubMed] [Google Scholar]
- 199.Han ZC, et al. Glycosaminoglycans enhance megakaryocytopoiesis by modifying the activities of hematopoietic growth regulators. J. Cell. Physiol. 1996;168:97–104. doi: 10.1002/(SICI)1097-4652(199607)168:1<97::AID-JCP12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 200.San Antonio JD, Winston BM, Tuan RS. Regulation of chondrogenesis by heparan sulfate and structurally related glycosaminoglycans. Dev. Biol. 1987;123:17–24. doi: 10.1016/0012-1606(87)90422-2. [DOI] [PubMed] [Google Scholar]
- 201.Gomes RR, Farach-Carson MC, Carson DD. Perlecan functions in chondrogenesis: insights from in vitro and in vivo models. Cells Tissues Organs. 2004;176:79–86. doi: 10.1159/000075029. [DOI] [PubMed] [Google Scholar]
- 202.Chen J, et al. Exogenous Heparan sulfate enhances the TGF-β3-induced chondrogenesis in human mesenchymal stem cells by activating TGF-β/Smad signaling. Stem Cells Int. 2016;2016:1520136. doi: 10.1155/2016/1520136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jao T-M, et al. Alteration of colonic epithelial cell differentiation in mice deficient for glucosaminyl N-deacetylase/N-sulfotransferase 4. Oncotarget. 2016;7:84938–84950. doi: 10.18632/oncotarget.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pisconti A, Cornelison DDW, Olguín HC, Antwine TL, Olwin BB. Syndecan-3 and Notch cooperate in regulating adult myogenesis. J. Cell Biol. 2010;190:427–441. doi: 10.1083/jcb.201003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Caughman SW, Krieg T, Timpl R, Hintner H, Katz SI. Nidogen and heparan sulfate proteoglycan: detection of newly isolated basement membrane components in normal and epidermolysis bullosa skin. J. Invest. Dermatol. 1987;89:547–550. doi: 10.1111/1523-1747.ep12461192. [DOI] [PubMed] [Google Scholar]
- 206.Horiguchi Y, Couchman JR, Ljubimov AV, Yamasaki H, Fine JD. Distribution, ultrastructural localization, and ontogeny of the core protein of a heparan sulfate proteoglycan in human skin and other basement membranes. J. Histochem. Cytochem. J. Histochem. Soc. 1989;37:961–970. doi: 10.1177/37.7.2659664. [DOI] [PubMed] [Google Scholar]
- 207.Sanderson RD, Hinkes MT, Bernfield M. Syndecan-1, a cell-surface proteoglycan, changes in size and abundance when keratinocytes stratify. J. Invest. Dermatol. 1992;99:390–396. doi: 10.1111/1523-1747.ep12616103. [DOI] [PubMed] [Google Scholar]
- 208.Gopal S, et al. Transmembrane proteoglycans control stretch-activated channels to set cytosolic calcium levels. J. Cell Biol. 2015;210:1199–1211. doi: 10.1083/jcb.201501060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Festa E, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Plikus MV, et al. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science. 2011;332:586–589. doi: 10.1126/science.1201647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sennett R, Rendl M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin. Cell Dev. Biol. 2012;23:917–927. doi: 10.1016/j.semcdb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Idali, T. A. Hypertrichose Congénitale chez l’enfant. http://hdl.handle.net/123456789/15057 (2016).
- 213.Shimaoka S, et al. Hepatocyte growth factor/scatter factor expressed in follicular papilla cells stimulates human hair growth in vitro. J. Cell. Physiol. 1995;165:333–338. doi: 10.1002/jcp.1041650214. [DOI] [PubMed] [Google Scholar]
- 214.Hsu Y-C, Li L, Fuchs E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell. 2014;157:935–949. doi: 10.1016/j.cell.2014.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Colin-Pierre C, et al. The Glypican-1/HGF/C-Met and Glypican-1/VEGF/VEGFR2 ternary complexes regulate hair follicle angiogenesis. Front. Cell Dev. Biol. 2021;9:781172. doi: 10.3389/fcell.2021.781172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Invest. 2001;107:409–417. doi: 10.1172/JCI11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Suzuki S, Ota Y, Ozawa K, Imamura T. Dual-mode regulation of hair growth cycle by two Fgf-5 gene products. J. Invest. Dermatol. 2000;114:456–463. doi: 10.1046/j.1523-1747.2000.00912.x. [DOI] [PubMed] [Google Scholar]
- 218.Bernard BA. Advances in understanding hair growth. F1000Res. 2016;5:F1000 Faculty Rev-147. doi: 10.12688/f1000research.7520.1. [DOI] [Google Scholar]
- 219.Botchkarev VA, Kishimoto J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J. Investig. Dermatol. Symp. Proc. 2003;8:46–55. doi: 10.1046/j.1523-1747.2003.12171.x. [DOI] [PubMed] [Google Scholar]
- 220.Couchman JR. Hair follicle proteoglycans. J. Invest. Dermatol. 1993;101:60S–64S. doi: 10.1016/0022-202X(93)90502-9. [DOI] [PubMed] [Google Scholar]
- 221.Westgate GE, Messenger AG, Watson LP, Gibson WT. Distribution of proteoglycans during the hair growth cycle in human skin. J. Invest. Dermatol. 1991;96:191–195. doi: 10.1111/1523-1747.ep12461019. [DOI] [PubMed] [Google Scholar]
- 222.Dwivedi PP, Lam N, Powell BC. Boning up on glypicans-opportunities for new insights into bone biology. Cell Biochem. Funct. 2013;31:91–114. doi: 10.1002/cbf.2939. [DOI] [PubMed] [Google Scholar]
- 223.Kishimoto J, et al. Selective activation of the versican promoter by epithelial- mesenchymal interactions during hair follicle development. Proc. Natl Acad. Sci. USA. 1999;96:7336–7341. doi: 10.1073/pnas.96.13.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yang Y, et al. Versican gene: regulation by the β-catenin signaling pathway plays a significant role in dermal papilla cell aggregative growth. J. Dermatol. Sci. 2012;68:157–163. doi: 10.1016/j.jdermsci.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 225.Inui S, Itami S. A newly discovered linkage between proteoglycans and hair biology: decorin acts as an anagen inducer. Exp. Dermatol. 2014;23:547–548. doi: 10.1111/exd.12471. [DOI] [PubMed] [Google Scholar]
- 226.Piraccini BM, Alessandrini A. Androgenetic alopecia. G. Ital. Dermatol. E Venereol. Organo Uff. Soc. Ital. Dermatol. E Sifilogr. 2014;149:15–24. [PubMed] [Google Scholar]
- 227.Dardour J-C, Hennebert H. [Baldness surgery] Ann. Chir. Plast. Esthet. 2003;48:364–370. doi: 10.1016/j.anplas.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 228.Ioannides D, Lazaridou E. Female pattern hair loss. Curr. Probl. Dermatol. 2015;47:45–54. doi: 10.1159/000369404. [DOI] [PubMed] [Google Scholar]
- 229.Pillon F, Allaert F-A. Rôle de la complémentation orale pour lutter contre la chute de cheveux. Actual. Pharm. 2011;50:39–40. [Google Scholar]
- 230.Leirós GJ, Attorresi AI, Balañá ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br. J. Dermatol. 2012;166:1035–1042. doi: 10.1111/j.1365-2133.2012.10856.x. [DOI] [PubMed] [Google Scholar]
- 231.Ceruti JM, Leirós GJ, Balañá ME. Androgens and androgen receptor action in skin and hair follicles. Mol. Cell. Endocrinol. 2018;465:122–133. doi: 10.1016/j.mce.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 232.Kwack MH, Ahn JS, Kim MK, Kim JC, Sung YK. Dihydrotestosterone-inducible IL-6 inhibits elongation of human hair shafts by suppressing matrix cell proliferation and promotes regression of hair follicles in mice. J. Invest. Dermatol. 2012;132:43–49. doi: 10.1038/jid.2011.274. [DOI] [PubMed] [Google Scholar]
- 233.Ruohola JK, Valve EM, Vainikka S, Alitalo K, Härkönen PL. Androgen and fibroblast growth factor (FGF) regulation of FGF receptors in S115 mouse mammary tumor cells. Endocrinology. 1995;136:2179–2188. doi: 10.1210/endo.136.5.7536664. [DOI] [PubMed] [Google Scholar]
- 234.Russo LA, Calabro SP, Filler TA, Carey DJ, Gardner RM. In vivo regulation of syndecan-3 expression in the rat uterus by 17 beta-estradiol. J. Biol. Chem. 2001;276:686–692. doi: 10.1074/jbc.M004106200. [DOI] [PubMed] [Google Scholar]
- 235.Darwin E, Heyes A, Hirt PA, Wikramanayake TC, Jimenez JJ. Low-level laser therapy for the treatment of androgenic alopecia: a review. Lasers Med. Sci. 2018;33:425–434. doi: 10.1007/s10103-017-2385-5. [DOI] [PubMed] [Google Scholar]
- 236.Kwack MH, Kang BM, Kim MK, Kim JC, Sung YK. Minoxidil activates β-catenin pathway in human dermal papilla cells: a possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011;62:154–159. doi: 10.1016/j.jdermsci.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 237.Choi, N., Shin, S., Song, S. U. & Sung, J.-H. Minoxidil promotes hair growth through stimulation of growth factor release from adipose-derived stem cells. Int. J. Mol. Sci. 19, 691 (2018). [DOI] [PMC free article] [PubMed]
- 238.Otomo, S. [Hair Growth Effect of Minoxidil]. Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica Vol. 119 https://pubmed.ncbi.nlm.nih.gov/11915519/ (2002). [DOI] [PubMed]
- 239.Han JH, et al. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J. Dermatol. Sci. 2004;34:91–98. doi: 10.1016/j.jdermsci.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 240.Soma T, Tajima M, Kishimoto J. Hair cycle-specific expression of versican in human hair follicles. J. Dermatol. Sci. 2005;39:147–154. doi: 10.1016/j.jdermsci.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 241.Lassus A, Jeskanen L, Happonen HP, Santalahti J. Imedeen for the treatment of degenerated skin in females. J. Int. Med. Res. 1991;19:147–152. doi: 10.1177/030006059101900208. [DOI] [PubMed] [Google Scholar]
- 242.Thom E. Efficacy and tolerability of Hairgain in individuals with hair loss: a placebo-controlled, double-blind study. J. Int. Med. Res. 2001;29:2–6. doi: 10.1177/147323000102900101. [DOI] [PubMed] [Google Scholar]
- 243.Thom E. Nourkrin: objective and subjective effects and tolerability in persons with hair loss. J. Int. Med. Res. 2006;34:514–519. doi: 10.1177/147323000603400508. [DOI] [PubMed] [Google Scholar]
- 244.Kingsley DH, Thom E. Cosmetic hair treatments improve quality of life in women with female pattern hair loss. J. Appl Cosmetol. 2012;30:49–59. [Google Scholar]
- 245.Khan MZU, et al. Finasteride topical delivery systems for androgenetic alopecia. Curr. Drug Deliv. 2018;15:1100–1111. doi: 10.2174/1567201815666180124112905. [DOI] [PubMed] [Google Scholar]
- 246.Liu X-J, et al. Lumican accelerates wound healing by enhancing α2β1 integrin-mediated fibroblast contractility. PLoS One. 2013;8:e67124. doi: 10.1371/journal.pone.0067124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rossi A, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat. Inflamm. Allergy Drug Disco. 2012;6:130–136. doi: 10.2174/187221312800166859. [DOI] [PubMed] [Google Scholar]
- 248.Ito G, Kobayashi T, Takeda Y, Sokabe M. Proteoglycan from salmon nasal cartridge promotes in vitro wound healing of fibroblast monolayers via the CD44 receptor. Biochem. Biophys. Res. Commun. 2015;456:792–798. doi: 10.1016/j.bbrc.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 249.Sano M, Shang Y, Nakane A, Saito T. Salmon nasal cartilage proteoglycan enhances growth of normal human dermal fibroblast through Erk1/2 phosphorylation. Biosci. Biotechnol. Biochem. 2017;81:1379–1385. doi: 10.1080/09168451.2017.1318695. [DOI] [PubMed] [Google Scholar]
- 250.Zhang Y, Cao L, Kiani CG, Yang BL, Yang BB. The G3 domain of versican inhibits mesenchymal chondrogenesis via the epidermal growth factor-like motifs. J. Biol. Chem. 1998;273:33054–33063. doi: 10.1074/jbc.273.49.33054. [DOI] [PubMed] [Google Scholar]
- 251.Kashiwakura I, Takahashi K, Takagaki K. Application of proteoglycan extracted from the nasal cartilage of salmon heads for ex vivo expansion of hematopoietic progenitor cells derived from human umbilical cord blood. Glycoconj. J. 2007;24:251–258. doi: 10.1007/s10719-007-9033-4. [DOI] [PubMed] [Google Scholar]
- 252.Yoneda A, Lendorf ME, Couchman JR, Multhaupt HAB. Breast and ovarian cancers. J. Histochem. Cytochem. 2012;60:9–21. doi: 10.1369/0022155411428469. [DOI] [PMC free article] [PubMed] [Google Scholar]