Abstract
The ability of heparan sulfate, heparin, and other glycosaminoglycans to inhibit the infectivity of Chlamydia trachomatis serovars E and LGV was examined using a simple competitive inhibition assay with three cell types from the human female reproductive tract, including primary human endosalpingeal cells. With the majority of the glycosaminoglycans tested, LGV was more significantly inhibited than serovar E. We have compared chlamydial infectivity between a wild-type Chinese hamster ovary cell line and two glycosaminoglycan-deficient cell lines. LGV was shown to be unable to infect heparan sulfate-deficient and GAG-deficient Chinese hamster ovary cell lines, whereas the E serovar infected these cells as efficiently as the control (nondeficient) cells. These two sets of experiments confirmed that serovar LGV is more dependent on a heparan sulfate-related mechanism of infectivity than is serovar E. This is further supported by the fact that attempts to purify a heparan sulfate-like molecule from either serovar cultured in glycosaminoglycan-deficient cell lines were nonproductive. Previous reports have suggested that chlamydia are able to produce a heparan sulfate-like molecule that is important for attachment and infectivity. We have attempted to detect possible binding of a specific heparan sulfate antibody to C. trachomatis by flow cytometry. Results showed no binding of the heparan sulfate antibody to C. trachomatis serovar LGV or E. Our results strongly indicate that chlamydiae do not produce a heparan sulfate-like molecule but rather use host cell heparan sulfate in order to infect cells.
Chlamydia trachomatis serovars D to K are a common cause of sexually transmitted infections such as urethritis, cervicitis, salpingitis, and pelvic inflammatory disease in females and urethritis in males (30, 37). Because of these infections, the organism has been associated with female infertility caused by ascending chlamydial infections to the uterine tubes and as a possible contributor to male infertility (3, 30). In the developing world, C. trachomatis serovars A to C cause ocular infections, particularly trachoma, and the serovar lymphogranuloma venereum (LGV) (L1, L2, L2a, L3) is responsible for a condition involving generalized lymphoadenopathy (37). C. trachomatis infections can be treated by antibiotics once diagnosed, but the problem in control lies within the large number of asymptomatic patients. In spite of considerable research there is no commercially available vaccine (36).
C. trachomatis is an obligate intracellular gram-negative bacterium that during its developmental cycle alternates between two forms: the infectious elementary body (EB) and the noninfectious replicating form, the reticulate body. The attachment process of the EB to a host cell is the event most crucial to a successful infection, since C. trachomatis is an obligate intracellular pathogen. EBs attach to columnar epithelial cells followed by endocytosis and inhibition of lysosomal fusion (29). A number of chlamydial ligands have been identified and characterized. These include the major outer membrane protein (MOMP) (41, 42, 44, 45) as well as the cysteine-rich OmcB (Omp2) protein, hsp70, the polymorphic outer membrane proteins, and the thermolabile 34-kDa membrane protein (18). In addition to these proposed ligands, there is a considerable amount of experimental evidence to suggest that the glycosaminoglycan (GAG), heparan sulfate (HS) is involved in the chlamydial attachment-infectivity process (5, 6, 7, 12, 25, 32, 38, 39, 43, 50, 51). What remains currently controversial is whether HS is present on chlamydiae or the host cell.
HS belongs to the family of GAGs that are linear, negatively charged polymers consisting of repeating disaccharide repeats of an amino sugar and uronic acid. GAG residues are covalently linked to core proteins to form proteoglycans and are found on the surface of most nucleated cell types. The other three members of the GAG family include hyaluronic acid, chondroitin sulfate (CS), and keratan sulfate (16, 17). HS has the most complex molecular structure and is made up of a backbone of N-acetylated or N-sulfated amino sugars of d-glucosamine or galactosamine linked to glucuronic or iduronic acid and complex patterns of O-sulfate substitutions. HS has a wide range of functions, including the binding to extracellular matrix components such as fibronectin, collagen, laminin, and other HS-containing proteoglycans (1, 10). In the last few years there has been a considerable increase in the number of microorganisms that have been shown to use HS as a host cell receptor. Examples include Neisseria gonorrhoeae, Bordetella pertussis, Listeria monocytogenes, and herpes simplex virus (33, 48).
In 1992, Zhang and Stephens (51) first presented data to support the hypothesis that C. trachomatis produces a unique HS-like molecule that binds to a complementary mammalian host cell receptor. This model proposes a trimolecular mechanism of infection where the chlamydial derived HS acts as a bridge between a chlamydial ligand and the host cell receptor (32, 38, 51). Evidence to support this model included the inhibitory effect of heparin (HP) and HS on attachment and infectivity, the decreased ability of heparitinase-treated chlamydia (LGV) to attach to host cells, and the ability of C. trachomatis (LGV) to infect an HS-deficient cell line. The successful purification of an HS-like chlamydial derived molecule, said to be derived by culturing the organism in HS-deficient cells, provided stronger evidence to support this model (51). The role of HS was given further support by a series of experiments showing that polystyrene microspheres coated with HP or HS could be endocytosed by HeLa cells and that this process could be competitively inhibited by live EBs (39). More recently, Rasmussen-Lathrop et al., (32) reported that chlamydial infectivity was neutralized by an HS-specific antibody that bound to EBs of C. trachomatis as well as Chlamydia pneumoniae.
In contrast to the model described by Zhang and Stephens (51), it has been suggested that C. trachomatis does not produce its own HS-like molecule but instead binds to HS found on host cells. Su and coworkers performed a series of experiments using recombinant Escherichia coli expressing MOMP as a fusion protein with E. coli maltose binding protein (43). HS was found to inhibit the attachment of this recombinant E. coli to HeLa cells similar to the evidence of Zhang and Stephens (51). The same recombinant E. coli was not able to attach to HS-deficient cells, suggesting that MOMP binds to HS found on host cells in order to attach to epithelial cells. The fact that heparitinase treatment of host cells decreased attachment of C. trachomatis was contradictory to the Stephens model (43, 51).
Other reports have suggested the inhibitory effect that HP and HS exhibit is due to the high negative charge that these molecules possess. The fact that dextran sulfate, another highly negatively charged polysaccharide, is able to inhibit chlamydial attachment has supported the idea that chlamydial adherence is mediated by a nonspecific charge interaction (50). Surprisingly, it has also been reported that HP is not able to inhibit chlamydial attachment in vivo in spite of its high inhibitory effect in vitro (40).
Due to the existing controversies over the involvement of HS in chlamydial attachment we have carried out a series of experiments to further investigate this topic. We performed infectivity inhibition experiments using a range of GAGs, with both commercially available cell lines of the epithelium from the human genital tract lines including primary endosalpingeal cell cultures. Two proteoglycan-deficient cell lines were used in order to compare C. trachomatis infectivity. We attempted to purify any chlamydial derived HS by culturing the organism in proteoglycan-deficient cells, in addition to using flow cytometry to investigate if an HS-specific antibody (11) could bind EBs.
In our experiments we have used both chlamydial serovars E and LGV since previous work has revealed interesting differences between C. trachomatis serovars. The trachoma serovar (B) was found to be less sensitive to HS inhibition of attachment than the LGV serovar (5). Moreover, experiments using a wider range of C. trachomatis serovars confirmed that the LGV serovar appeared to predominantly use a GAG-dependent mechanism of attachment, whereas the other serovars used a GAG-independent mechanism of attachment as well as a GAG-dependent mechanism (6, 12).
MATERIALS AND METHODS
C. trachomatis serovars.
C. trachomatis serovar E was isolated from a clinical sample obtained from the Department of Genitourinary Medicine, Royal Hallamshire Hospital, Sheffield, United Kingdom, and serovar LGV (L1) was kindly provided by M. Ward (University of Southampton, Southampton, United Kingdom). Confirmation of the genotype was conducted by restriction analysis of a nested PCR product of each serovar according to the method of Lan et al. (26).
Cell lines.
McCoy cells (mouse fibroblast cell line), HeLa 229 cells (cervical carcinoma), Hec-1B cells (endometrial carcinoma), CHO-K1, pgsD-677 (CRL-2244) (HS deficient CHO-K1 cells) and pgsA-745 (CRL-2242) (HS- and CS-deficient CHO-K1 cells) were all obtained from the American Type Culture Collection (ATCC) (Manassas, Va.) and maintained according to the supplier's instructions. Human endosalpingeal cells were obtained from four women who donated their fallopian tubes for research purposes at the time of their hysterectomy. Details of the surgical procedure and methods used to provide primary human endosalpingeal cells for culture and experimentation are described by Pacey et al. (31). All women were previously fertile and had normal endocrinology. Two women were in the follicular phase of their cycles at the time of surgery and the other two were in the secretory phase. All primary cultures of human endosalpinx were checked for C. trachomatis infection prior to analysis. Briefly, DNA was extracted from cells of the original biopsy sample and subjected to a β-globin PCR according to Saiki et al. (34) to confirm the presence of DNA in the sample. Furthermore, a single C. trachomatis plasmid PCR was carried out according to the method of Claas et al. (8) to determine any possible C. trachomatis infection.
Propagation of C. trachomatis.
Chlamydiae were grown in semiconfluent McCoy cells for 48 to 72 h in maintenance medium (RPMI supplemented with cycloheximide [2 μg ml−1]). In order to harvest EBs, cell monolayers were disrupted by sterile glass beads and sonicated twice for 10 s at a 15-μm amplitude. Cell debris were removed by centrifugation at 500 × g for 15 min, and the remaining suspension of chlamydiae was further purified by centrifugation at 30,000 × g for 1 h at 4°C. The resulting pellet was resuspended in 8 ml of phosphate-buffered saline (PBS), sonicated as described above, layered over 30% urografin (Schering, West Sussex, United Kingdom) and centrifuged at 30,000 × g. The pellet formed was resuspended in 15 ml of PBS, sonicated, and centrifuged as described above, and the final pellet was resuspended in SPG (sucrose phosphate buffer is 5 mM glutamine, 0.2 M sucrose. 0.2 M phosphate buffer) and stored at −70°C for further use.
Quantification of C. trachomatis and direct immunofluorescence.
McCoy cell monolayers were grown in 24-well tissue culture plates on sterile coverslips and subsequently infected with a dilution series of purified EBs (10−2 to 10−6); plates were centrifuged for 1 h at 2,000×g. Following incubation for 48 h (37°C, 5% CO2), cells were fixed and stained with an antichlamydial monoclonal antibody (Syva MicroTrak, San Jose, Calif.), and the number of inclusions were counted by fluorescence microscopy at a × 400 magnification. The number of inclusion bodies per coverslip was calculated for each dilution and was used to determine the number of inclusion forming units (IFU) per milliliter.
Reagents.
The GAGs—HP (H3149), bovine intestinal derived HS (H9902), CS A (C8529), and CS B (dermatan sulfate) (C2413)— and the chemically modified HPs—N-acetyl HP (A8036), de-N-sulfated HP (D4776), and de-N-sulfated acetylated HP (D9808)— were all purchased from Sigma (Poole, United Kingdom). The HP fractionation product Clexane was purchased from Rhone Poulenc Rorer (Paris, France). The GAG lyases, heparitinase (heparinase III) and chondroitinase ABC, were obtained from Seikagaku (Tokyo, Japan). Pronase was purchased from Boehringer (Mannheim, Germany). The radiolabel Na235SO4 was obtained from ICN International (Costa Mesa, Calif.). DEAE-Sepharose and PD-10 Sephadex G-25 desalting columns were obtained from Amersham Pharmacia (Uppsala, Sweden). Polyclonal fluorescein isothiocyanate (FITC)-conjugated antichlamydial antibody was purchased from Biogenesis (Poole, United Kingdom). The HS antibody F58-10E4 (mouse immunoglobulin M [IgM]) (11) was purchased from Seikagaku, and the secondary anti-mouse IgM phycorethrin (PE)-conjugated antibody was purchased from Serotec (Oxford, United Kingdom).
Infectivity inhibition assays.
Confluent HeLa, Hec-1B, and human endosalpingeal cell monolayers were prepared and subsequently infected with an inoculum that was adjusted so that not more than ∼50 inclusions were present in each field at a ×400 magnification (approximately 5 × 105 IFU) of the required serovar in 100 μl of PBS. Inocula were supplemented with HP, HS, CS A, CS B, N-acetyl HP, de-N-sulfated HP, de-N-sulfated N-acetylated HP, and Clexane (0.5 mg ml−1) as appropriate. Cells were incubated for 1 h at 37°C and then washed with PBS before adding 1 ml of tissue culture fluid supplemented with cycloheximide (2 μg ml−1) to each well. After incubation at 37°C in 5% CO2 for 48 h, infected monolayers were fixed, stained, and counted using direct immunofluorescence as described above. The average number of inclusion bodies for 25 fields (×400 magnification) was determined. For statistical analysis a total of six experiments were performed for each cell type.
Comparison of CHO-K1, pgsA-745, and pgsD-677 cells.
Monolayers of CHO-K1, pgsA-745, and pgsD-677 were grown to confluency in 24-well tissue culture plates. Cells were washed using PBS and subsequently inoculated with a suitable inoculum of EBs (adjusted so that not more than ∼50 inclusions were present at a ×400 magnification). Cells were then incubated with bacteria for 1 h at 37°C (5% CO2) in air and washed three times with PBS. Tissue culture fluid containing cycloheximide (2 μg ml−1) was then added to each well before further incubation at 37°C (5% CO2) for 48 h. Cells were fixed, stained, and counted as previously described.
Radiolabeling of HS from chlamydia-infected host cell cultures.
Semiconfluent 25-cm2 tissue culture flasks of cells (CHO-K1, pgsA-745, and pgsD-677) were prepared. Growth media were replaced with maintenance media, and cells were infected with C. trachomatis (an appropriate inoculum was used so that approximately 70 to 80% infection was achieved after 3 days of incubation). In the case of the E serovar, chlamydiae were centrifuged onto cells at 2,000 × g for 1h. At 12 h postinfection 35SO4 (25 μCi ml−1) was added, and cells were further incubated for 48 h. Cells were then washed three times using ice-cold PBS to remove any free sulfate and were solubilized using 0.1 M NaOH (15 min at room temperature). Cell lysates were treated overnight with nonspecific pronase, 0.2 mg ml−1 in 0.32 M NaCl, 0.14 M Na acetate (pH 7.4) at 42°C. Anion-exchange chromatography was carried out using 1.5-ml DEAE-Sepharose columns. Columns were preequilibrated using 15 ml of PBS, 15 ml of 0.25 M NaCl, and 15 ml of PBS. The radiolabeled GAGs (each cell lysate was made up to a 20-ml volume using PBS) were loaded onto the column and washed with 0.25 M NaCl in order to remove any weakly bound material. The radiolabeled GAGs were finally eluted using 1.0 M NaCl (15 0.5-ml fractions), and a further 15 fractions were eluted using 1.5 M NaCl. The radioactivity of the flowthrough, the 0.25 M NaCl wash, and each fraction was determined by liquid scintillation counting (1% volume of each fraction) using a 1211 Rackbeta liquid scintillation counter (LKB, Turku, Finland).
Enzymatic treatment of purified GAGs.
Fractions that contained the radiolabeled GAGs were pooled together and desalted using PD-10 Sephadex G-25 desalting columns. The desalted GAGs were then treated with 2.5 mU of heparitinase (heparinase III) per reaction for 16 h at 37°C (buffer: 100 mM Na acetate, pH 7.0, with 0.1 mM Ca acetate) followed by 25 mU of chondroitinase ABC per reaction for 4 h at 37°C (buffer: 20 mM Tris-acetate, pH 8.0). Nontreated, heparitinase- and chondroitinase ABC-only treated controls were also included. The products were analyzed using anion-exchange chromatography as described above. Columns were eluted using 1 M NaCl, and 20 0.5-ml fractions were collected.
Binding of HS-specific antibody to C. trachomatis EBs.
The method followed for staining and detecting antibody binding to bacteria has been previously described by Jack et al. (23). In brief, an aliquot of EBs (approximately 2.5 × 106 IFU per sample) was spun at 9,780 × g for 5 min. The supernatant was removed, and EBs were resuspended in 50 μl of PBS containing 1:25 antichlamydial FITC-conjugated antibody. Following a 15-min incubation at 37°C, the EB suspensions were washed twice (5 min at 9,780 × g). EBs were subsequently resuspended in 50 μl of PBS containing 1:50 anti-HS antibody, incubated at 37°C for 15 min, and washed twice, and finally EBs were resuspended in 50 μl of PBS containing 1:50 antimouse IgM (PE conjugated). Bacteria were incubated at 37°C for 15 min and washed twice with PBS, and the final pellet was resuspended in 100 μl of 2% paraformaldehyde in PBS. EB suspensions were then transferred to sterile fluorescence-activated cell sorter tubes containing 200 μl of PBS. Flow cytometry was performed on a FACS Calibur (Becton Dickinson, Oxford, United Kingdom) at low flow rate using CellQuest software. Chlamydial EBs were selected on the basis of size, granularity, and positive FITC staining. In each experiment nonstained controls and single- and double-stained samples were included. Furthermore, an anti-HS control isotype antibody was also used in every experiment.
Statistical analysis.
Statistical analysis of the results was carried out using Instat (Graph Pad Software Inc., San Diego, Calif.).
RESULTS
Infectivity inhibition experiments.
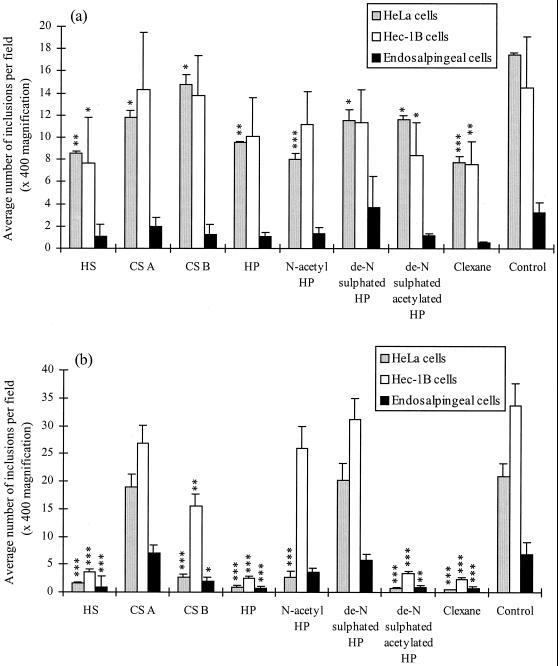
Figure 1 summarizes the results of infectivity experiments in which serovars E and LGV were incubated with Hec-1B, HeLa, and human primary endosalpingeal cells in the presence of a variety of GAGs. In these experiments, HS was found to have a strong inhibitory effect on LGV infectivity (P < 0.001 for all cell types), but with serovar E was found to inhibit infectivity of HeLa (P < 0.01) and Hec-1B cells (P < 0.05) only. CS A had no significant effect on the infectivity of LGV but did inhibit the effect of serovar E on HeLa cells (P < 0.05). In contrast, CS B was found to inhibit the infectivity by serovar LGV of all three cell types (Hec1-B and HeLa cells, P < 0.01; primary cells, P < 0.05) but to only significantly inhibit the effect of serovar E on HeLa cells (P < 0.05).
FIG. 1.
Infectivity inhibition results with serovar E (a) and serovar LGV (b) at 37°C Comparison of C. trachomatis infectivity levels incubated on HeLa, Hec-1B, and endosalpingeal cell monolayers with addition of different GAGs: HP and chemically modified HPs (all used at 0.5 mg ml−1). This figure represents the results of six different experiments. Error bars: standard error. Results were statistically analyzed using a one-way-paired analysis of variance test, and a Tukey-Kramer test was performed if there was a significant difference between the columns. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
The GAGs HP, de-N-sulfated acetylated HP, and Clexane were found to have a very high inhibitory effect (∼90% inhibition) on LGV infectivity with all three cell types (P < 0.001 for all cell types). However, in the case of serovar E these molecules were not as strong inhibitors of infectivity. HP only significantly decreased serovar E infectivity of HeLa cells (P < 0.01), and de-N-sulfated acetylated HP inhibited infectivity by approximately 50% on Hec-1B and HeLa cells (P < 0.05 in both cases). Clexane also inhibited serovar E infectivity of Hec-1B (P < 0.01) and HeLa cells (P < 0.001), but not to the same level as observed with the LGV serovar.
Of the remaining GAGs tested, it was surprising that de-N-sulfated HP was only found to decrease E serovar infectivity of HeLa cells (P < 0.05) and that no inhibitory effect was recorded with the LGV serovar for any cell type. Moreover, for N-acetyl HP, the infectivity of HeLa cells by both serovars E and LGV was equally significant (P < 0.001) with no reduction of infectivity of any other cell type. Interestingly, in the case of the primary human endosalpingeal cells, none of the inhibitors used significantly decreased serovar E infectivity, whereas with serovar LGV the reduction of infectivity was very similar to that in the other two cell lines used. Overall, these data indicate that selective modifications of HP alter its ability to interfere with infectivity, suggesting that subtle but specific structural determinants are required for its mechanism of action.
Although the above experiments were performed at a standard dose of 0.5 mg ml−1 for all GAGs tested, dose-response experiments were carried out with HP over a twofold-dilution series from 1.0 to 0.031 mg ml−1. These experiments were performed with both chlamydial serovars and with HeLa and Hec-1B cells (data not shown). Primary endosalpingeal cells were unavailable at the time that dose-response experiments were performed. In summary, LGV infectivity was dose dependent in both cell lines with up to 90% inhibition of infectivity at doses as low as 0.031 mg ml−1. In contrast, serovar E infectivity was inhibited by only 50% at doses as high as 1 mg ml−1. This confirms the GAG-dependent infectivity of LGV and indicates that it can be significantly inhibited at relatively low doses of HP.
CHO-K1-deficient cell lines.
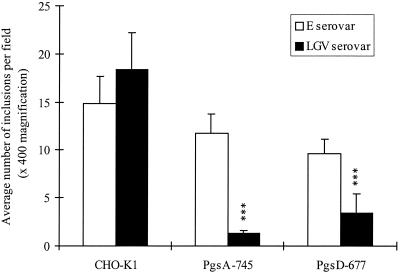
Figure 2 summarizes the results of infectivity experiments in which the abilities of serovars E and LGV to infect GAG-deficient CHO-K1 cell lines were examined. The cell lines chosen were pgsA-745 (deficient for all GAGs) and pgsD-677 (only deficient for HS but able to produce CS) and each were compared to CHO-K1 (wild-type) cells. The ability of serovar LGV to infect pgsA-745 and pgsD-677 cells was significantly lower than that of the CHO-K1 wild-type cells (P < 0.001 for both cell types). Moreover, there was no significant difference between the ability of serovar E to infect the control CHO-K1 and the GAG-deficient cell lines. These results suggest that serovar LGV uses host-cell HS in order to infect cells, whereas serovar E appears to be less dependent or does not use host cell HS for infectivity.
FIG. 2.
Comparison of serovar E and LGV infectivity of CHO-K1, pgsA-745 and pgsD-677 cells at 37°C. pgsA-745 and pgsD-677 cells are defective CHO-K1 cell lines. pgsA-745 cells do not produce any GAGs, and pgsD-677 are not able to produce any HS (they do produce CS). This figure represents the results of six different experiments. Error bars: standard error. Results were statistically analyzed using a one-way-paired analysis of variance test, and a Tukey-Kramer test was performed if there was a significant difference between the columns. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P <.001.
Radiolabeling of HS from chlamydia-infected host cell cultures.
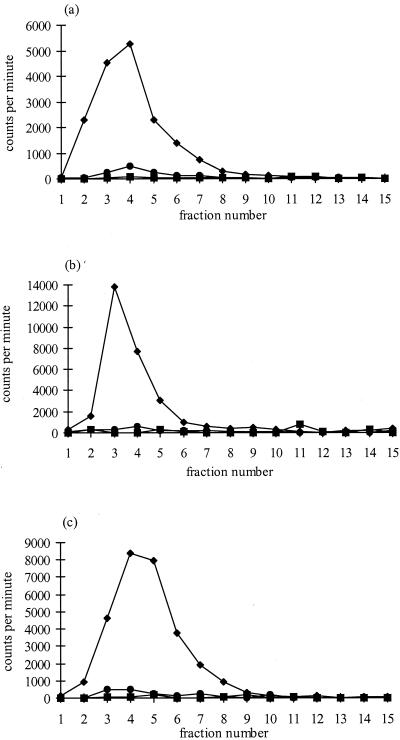
In an attempt to purify any GAGs derived from chlamydia, serovars E and LGV were grown in CHO-K1 (wild-type), pgsA-745 (HS- and CS-deficient), and pgsD-677 (HS-deficient) cells in the presence of 35SO4, in a similar manner to that described by Zhang and Stephens (51). Figure 3 illustrates the subsequent elution profiles through a DEAE-Sepharose column of radiolabeled GAGs in the cell lysate of uninfected controls and serovar E or LGV infected cells. In Fig. 3a, the non infected CHO-K1 cells produced the typical GAG elution profile, with the peak of radioactivity found in the first 10 fractions eluted with 1 M NaCl. No radiolabeled material was present in noninfected pgsA-745 cells since they are defective in GAG synthesis and the small peak of radiolabeled material which was produced by noninfected pgsD-677 cells is probably due to the production of CS only (Fig. 3a). The elution profiles of E or LGV infected cells were very similar to the noninfected controls (Fig. 3b and c), and no radiolabeled material was present in pgsA-745 and pgsD-677 cells infected with either serovar following elution by 1.0 M NaCl.
FIG. 3.
Elution profiles of radiolabeled GAGs prepared from control non infected cells (a), LGV-infected cells (b), and E serovar infected cells (c). ⧫, CHO-K1; ■, pgsA-745; ●, pgsD-677. Fractions 1 to 15 were eluted using 1.0 M NaCl, and a further 15 fractions (not shown) were eluted using 1.5 M NaCl (no radiolabeled material was eluted in these further 15 fractions). The radioactivity in each fraction was determined by liquid scintillation counting (1% volume of each 0.5-ml fraction collected).
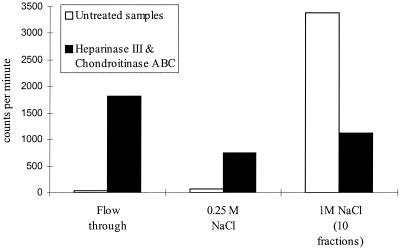
In order to confirm the presence of GAGs in these experiments, the first 10 fractions of noninfected CHO cells (Fig. 3a) were pooled together and treated with specific GAG lyases, heparitinase, and chondroitinase ABC. The results of the incubation with heparitinase and chondroitinase ABC are shown in Figure 4. Most of the radiolabeled 35SO4 was recovered in the flowthrough and 0.25 M NaCl wash, indicating HS and CS were digested in the samples. This result confirmed the presence of HS and CS in the pooled fractions from Fig. 4a.
FIG. 4.
Scintillation counting of heparitinase- and chondroitinase ABC-digested samples compared with that of untreated controls. GAGs from noninfected CHO-K1 cells (fractions 2 to 9) were pooled (Fig. 3a), desalted, and then treated with 2.5 mU of heparitinase and 25 mU of chondroitinase ABC. Total counts per minute of the column flowthrough, 0.25 M NaCl, and first 10 fractions of 1 M NaCl are illustrated in this figure. In the untreated control GAGs bound to the DEAE-Sepharose column and were eluted in the 1 M NaCl fractions. In the digested samples most of the counts were collected in the flowthrough and 0.25 M NaCl, since degraded GAGs did not bind to the column. Material remaining bound after the enzyme treatments probably represents sulfated HS oligosaccharides which are resistant to heparitinase degradation. The radioactivity in a 1% volume of each sample was recorded by scintillation counting.
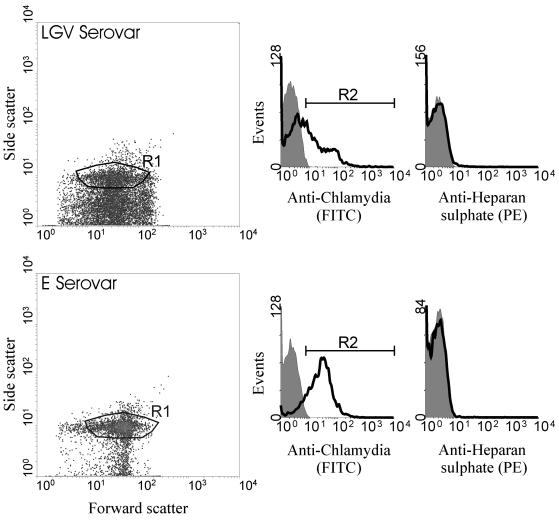
Binding of HS antibody to C. trachomatis EBs.
Using flow cytometry of serovar LGV and E it was possible to identify a cell population based on forward and side scatter. To confirm the identity of these events, we used a FITC-conjugated antibody specific for chlamydial EBs (Fig. 5). Using a combination of forward- and side-scatter characteristics and positive staining for FITC antichlamydia antibody it was possible to identify EBs (region R1 as shown in Fig. 5). We then examined the binding of anti-HS using a PE-conjugated antibody. The binding of anti-HS was the same as the background fluorescence for both serovars used, indicating the absence of HS (as shown in righthand panel histograms [Fig. 5]). To ensure that the antichlamydia antibody was not interfering with anti-HS binding, in certain experiments the antichlamydia antibody was omitted and the cell population was determined by size and granularity alone. The absence of antichlamydia antibody did not alter the lack of anti-HS binding (data not shown). Experiments using an isotype-matched control antibody gave similar results to the anti-HS antibody (data not shown).
FIG. 5.
Representative flow cytometric analysis of serovar LGV (top panel) and serovar E (bottom panel) EBs stained with a chlamydial antibody (FITC conjugated) and/or an (HS-specific antibody followed by a secondary antibody. The left-hand panels illustrate typical density blots of forward versus side scatter, with region 1 (R1) representing the population of particles analyzed in the corresponding histograms. The middle panels illustrate chlamydial staining with an antichlamydia FITC-conjugated antibody (solid line) and unstained controls (shaded area). Only particles included in region R2 (positively stained with antichlamydia antibody) were analyzed for positive staining with an anti-HS antibody. The righthand panels illustrate anti-HS (PE) staining (solid line) and unstained controls (shaded area).
To confirm the sensitivity and specificity of our experimental method, we used the same indirect immunofluorescence conditions to detect anti-HS binding to HS-positive (CHO-K1) and -negative (pgsA-745) cells. We were able to detect clear binding of anti-HS to CHO-K1 cells but not to pgsA-745 cells (data not shown). These findings confirmed the specificity of our antibody, the efficacy of the experimental methods, and also the phenotype of the cell types used in these experiments. As such, we can conclude that EBs (serovar LGV or E) do not contain HS, further supporting the hypothesis that HS-related chlamydial infectivity is mediated through HS on the host cell rather than on the chlamydial organism.
DISCUSSION
GAGs are thought to play a crucial role in many aspects of microbiological pathogenicity (33, 48). In the infections caused by N. gonorrhoeae, L. monocytogenes, Helicobacter pylori, and herpes simplex virus, for example, it is a GAG that provides one of the receptors on the eukaryotic host cell to which the microorganism attaches. In the case of C. trachomatis, however, it is controversial whether the EBs bind to a GAG HS on the host cell (43), or whether the EBs produce their own HS-like molecule (32, 38, 39, 51).
To study the role of GAGs in chlamydial infection of host cells, we chose four experimental approaches. The first utilized a simple in vitro infection inhibition assay, similar to that previously used by other authors (4, 5, 6, 12, 21, 51). However, unlike previous authors, we used three different cell types to represent the variety of epithelium found in the human female reproductive tract, which is known to differ markedly between the cervix, the uterus, and the fallopian tubes. The cell lines HeLa and Hec-1B are derived from the human cervix and endometrium respectively and as passaged cell lines are significantly different from the polarized nature of their in vivo counterparts. Therefore, we also used primary cultures of endosalpingeal cells (the epithelium of the fallopian tube). Although these cells were not grown on an extracellular matrix to maintain their polarity, actively beating cilia on their apical surface suggest that in their unpassaged state they still have retained some polarity. Moreover, they are considered to be a good model of the endosalpinx in other experimental systems (20, 28, 31). The decision to use both serovars E and LGV in this experiment was made on the basis of known differences between them, in terms of their response to GAGs (5, 6, 12) and the differences in the types of clinical presentations that result from their infection (12, 29). Finally, the selection of the GAG or GAG-like molecules to use in the infectivity inhibition experiments was made on the basis of previous reports (e.g., reference (33) and on details of their structure. For example, HS and HP are complex, highly negatively charged molecules comprised of a backbone of disaccharide repeats of hexuronic acid (d-glucuronic or l-iduronic acids) alternating with d-glucosamine residues. The variation of the N-acetyl, N-sulfate, and O-sulfate groups on these disaccharide repeats makes the number of different oligosaccharide sequences virtually unlimited (16, 17, 46). To see the effect these acetyl and sulfate groups have on chlamydial infectivity, we also chose to include a number of chemically modified HPs. De-N-sulfated N-acetylated HP (derived from porcine intestinal mucosa) has some similarities to HS because of its high N-acetyl content but lacks N-sulfate groups. N-acetyl HP and de-N-sulfated HP (completely de-N-sulfated and approximately 20% N-acetylated) also differ in terms of the number and position of sulfate and acetyl group. CS A and CS B (dermatan sulfate) are other members of the GAG family that are less sulfated (16, 17), with CS A being the less sulfated of the two. Finally, Clexane is the commercial name for the fractionation product of HP called enoxaparin, clinically used as an anticoagulant (2, 9, 35). The molecular weight of this molecule is 4.3 kDa, compared to the average molecular mass of HP, at 12 kDa (14). All of the GAGs and GAG-related molecules were used at a concentration of 0.5 mg ml−1, as this concentration has been used by others looking at their effect on attachment and infectivity (50, 51). However, dose-response experiments indicate that the infectivity of serovar LGV can be significantly inhibited at concentrations as low as 0.031 mg ml−1, whereas serovar E is largely unaffected, even at high doses (e.g., 1 mg ml− 1).
The results of our experiments indicate that LGV infectivity is more markedly inhibited by GAG or GAG-like molecules than is serovar E (Fig. 1). This confirms previous reports (5, 6, 12) that LGV infection is dependent on an HS-related mechanism. Interestingly, most of the GAG inhibition of serovar E infectivity was observed in HeLa cells. Moreover, none of the GAGs and GAG-related molecules used inhibited serovar E infectivity of primary human endosalpingeal cells. This further confirms the non-HS mechanism of infectivity of serovar E. However, it also raises the possibility of infectivity of different cell types being dependent on different mechanisms. Human primary cultures morphologically differ from HeLa and Hec-1B cells, and it is possible that other aspects of their function vary also. In a study similar to ours by Davis and Wyrick (12), serovar E and LGV attachment and infectivity were compared between nonpolarized as well as polarized cultures of Hec-1B cells, and it was concluded that serovar LGV was markedly affected by HS and HP whereas serovar E was not. Su and Caldwell (40) showed that sulfated polysaccharides were able to inhibit infectivity of cells in vitro but they were not effective in inhibiting the infectivity of C. trachomatis in a murine infection model of the female genital tract. This raises the possibility that the results obtained during in vitro experimentation with cell lines could be somewhat different from those that might be obtained in native cells. This concern is equally applicable to the results obtained with serovar LGV as much as they are with serovar E. In considering the role of host cell HS, however, it is important to consider its localization on host cells. Data have been published that show the presence of HS in the female genital tract, with HS staining being more intense in the basolateral surface of the epithelial cells than in the apical surface (19, 22). It has been postulated that this may explain the more invasive nature of an LGV infection (12) but does not undermine the hypothesis that the lesser amount of HS on the apical surface is sufficient to allow the attachment and infectivity of EBs across the apical surface. Indeed, it is thought that HS proteoglycans may play a role in tethering embryos to the apical surface of uterine epithelia during the early stages of implantation (24), suggesting that there might be sufficient GAGs expressed apically to also allow chlamydial attachment to and infection of the reproductive epithelium.
HS, HP, de-N-sulfated acetylated HP, and Clexane all have a related structure (16, 17, 33, 46), and it is interesting that they all inhibited LGV infection in all cell types to a similar level. However, not all of the GAGs tested gave the expected results. CS B, for example, was able to inhibit LGV infection of all three cell types, but this is inconsistent with an HS-related mechanism. Again, some of the inhibitors caused an inhibition in some cell types but not others, suggesting that there may be differences between the cells in terms of mechanisms of infectivity or mode of action of exogenously applied GAGs. The fact that neither N-acetyl HP nor de-N-sulfated HP inhibited serovar LGV infection of either Hec-1B or endosalpingeal cells suggested the importance of the N-sulfate groups for LGV infectivity in these cell types. This is consistent with the report by Chen et al. (7) who used different modified HPs in similar experiments and concluded that moderately sulfated GAGs were essential for attachment to host cells.
None of the inhibitors had a marked effect on the infectivity of both serovars, although Clexane was the most effective inhibitor, blocking approximately 90% of infectivity of serovar LGV for all cell types and reducing the infectivity of serovar E for HeLa and Hec-1B cells by approximately 50% (it has no effect on the infectivity of primary endosalpingeal cells). Clexane is an interesting molecule because of its current clinical applications (9, 35). We hypothesize that its strong inhibitory effect could be accounted for by the fact that it is a smaller molecule than HP. There is little experimental evidence about the effect of low-molecular-weight HPs on bacterial adherence. Vela et al. (47) attempted to evaluate the protective effect of HP and low-molecular-weight HP on the development of intra-abdominal adhesions and abscess formation after rats were subjected to a peritoneal challenge. Their data showed a significant decrease in adhesion and abscess formation between HP and low-molecular-weight HP treatment compared to the untreated control but no significant difference between HP and the low-molecular-weight HP. Our data have shown that Clexane has a strong inhibitory effect on infectivity and could suggest the possible development of a vaginal formulation based on a low-molecular-weight HP to block chlamydial infection.
In order to explore whether or not infectivity was dependent upon GAGs present on the host cells or on the chlamydial organism, we performed a second series of infectivity experiments using CHO cells that are known to be defective in key GAG-related genes (13, 27). This approach had been used previously by Zhang and Stephens (51) and Stephens et al. (39), who proposed that it was HS on chlamydia that is important in attachment and infectivity. However, the HS-deficient cell line that these authors used in both series of experiments to purify chlamydial HS (CHO-761) is known to produce 5% wild-type HS (13, 33), and therefore it is quite possible that these results occurred because of the small amount of HS being produced by these cells. For our experiments, we carefully chose cell lines with clearly defined properties: pgsA-745 cells are defective in xylosyltransferase (the first sugar transfer in GAG chain synthesis) and therefore do not produce any GAGs (13). The cell line pgsD-677 lacks both N-acetylglucosaminyltransferase and glucuronyltransferase activities required for synthesis of HS, but they do produce CS (27). Infectivity experiments with these cells clearly showed (Fig. 3) that while serovar E was able to infect each cell line to the same extent, LGV failed to infect the GAG-deficient cells. This confirms that LGV infection is dependent upon GAGs (and particularly HS) and provides strong evidence that these GAGs are on host cells. This is in agreement with the study by Su et al. (43), who showed a marked reduction of binding of a recombinant MOMP to pgsA-745 and pgsD-677 cells. The results of this experiment also confirm that serovar E must infect host cells by a predominantly GAG-independent mechanism.
To investigate the possible role of chlamydial HS in infection, we attempted to isolate any HS-like molecules from both the E and LGV serovars cultured in the GAG-deficient and HS-deficient cells. The advantage of using GAG-deficient cells was that any chlamydial HS-like molecule produced could be detected without being masked by the host cell GAGs. The results of this experiment (Fig. 4) showed that neither E or LGV was able to produce any HS-like molecule, or if they did, the amounts produced were below the level of detection by the sensitive 35SO4 radiolabeling procedure followed. This view is further supported by the recent completion of the C. trachomatis genome project that has indicated there are no GAG biosynthesis-related genes in the genome (18). It remains possible that chlamydia could exploit the cellular machinery of host cells in order to produce GAGs, although under the experimental conditions described here, this would not have been the case for the pgsA-745 and pgsD-677 cells. Moreover, it is possible that the EBs used to infect the various cells used in these experiments did have GAG-like molecules associated with them, as they had been initially grown in McCoy cells that had intact GAG biosynthesis machinery. Although the cycloheximide added to the McCoy cell cultures to inhibit eukaryotic protein synthesis is also known to inhibit GAG biosynthesis (13), it does so at higher concentrations than we employed. Therefore, although we did not measure the GAG biosynthesis of McCoy cells, we assumed that all EBs from McCoy cells could have HS-like molecules associated with them if chlamydiae are able to produce any HS-like molecules. What is perhaps a more interesting question is whether the EBs of serovar LGV produced in the pgsA-745 and pgsD-677 cells are as infective as those grown in wild-type CHO cells. This was impossible to test, due to the relatively low infectivity of GAG-deficient cells by serovar LGV. In their experiments, Zhang and Stephens (51) suggested that EBs derived from chlamydial infection in their deficient cells were equally infective as those of the control cells. However, as we have already discussed, their GAG-deficient cells were still able to produce some HS that could have effectively contaminated the experiment.
Lastly, we used flow cytometry to assay anti-HS antibody binding to EBs. We were able to identify EBs using a combination of physical characteristics and positive labeling of antibody to chlamydia. We were unable to detect anti-HS binding above background fluorescence, and therefore we conclude that EBs do not express HS (Fig. 5). This provides further proof, by another experimental approach, that the GAG-dependent mechanism of chlamydial infection must occur by the presence of GAGs on host cells rather than on chlamydiae. In addition to flow cytometry experiments, the same HS-specific antibody (11) was used to stain serovar LGV-infected monolayers of pgsA-745 using a similar method to that used by Rasmussen-Lathrop et al. (32), although in our study cells were examined by confocal microscopy. CHO-K1 noninfected and infected monolayers were also included in these experiments to ensure the detection of HS in CHO-K1 cells. No binding of the antibody was observed in pgsA-745 infected cells, suggesting that EBs do not produce any HS-like molecule that is recognized by the HS-specific antibody (data not shown).
Our data as described above are in contrast with the hypothesis proposed by Stephens et al. over the past few years (32, 39, 51). Possible reasons for this include the fact that we have used LGV strain L1 rather than L2 as used in the work of Stephens and coworkers (32, 39, 51). Also, we have chosen to maintain our chlamydial cultures in McCoy cells rather than L cells. However, we cannot envisage how such small differences might lead to the widely conflicting results described in the present paper. Other possibilities include subtle differences in the method to purify EBs or in the host cells used for experimentation. We note that in their recent report (32) the Stephens group have used the same GAG-deficient cell lines (pgsA-745 and pgsD-677) that we have also used in our work. However, they have frequently used CHO-761, which, as we have described, is known to produce some wild-type HS (13, 33). Even in their most recent paper (32), the anti-HS antibody (F58-10E4) was used to immunoprecipitate a putative chlamydial HS-like ligand from infected CHO-761 and pgsA-745 cells. Importantly, evidence for heparitinase degradation of the immunoprecipitated material (diagnostic for the presence of HS) was presented. However, the host cell source (CHO-761 or pgsA-745) for this crucial data was not made clear. In contrast to their data, work recently presented by Wuppermann et al. (49) showed that C. pneumoniae infectivity was inhibited highly by HP, to a lesser extent by HS, and by heparitinase treatment of monolayers. Furthermore, it was shown that C. pneumoniae failed to infect HS- and CS-deficient CHO-K1 cells. Thus, according to these results, C. pneumoniae uses host cell HS as a possible receptor, similar to what we propose with C. trachomatis.
In conclusion, our set of data strongly suggest that C. trachomatis does not produce HS, and we propose that HS acts as a host cell receptor in a mechanism similar to that suggested by Su et al. (43) who showed binding of MOMP to host cell HS. The list of microorganisms proven to use HS as a host cell receptor is growing; thus, it would not be surprising if chlamydiae have also evolved a mechanism to exploit HS. N. gonorrhoeae, another important genital tract pathogen, has been suggested to use HS as a host cell receptor, and recent experiments carried out by Freissler et al. (15) have shown that overexpression of syndecans in HeLa cells results in increased adhesion and invasion of gonococci, strengthening the concept that gonococci bind to host HS. Similar studies with chlamydiae could provide more information as to whether they bind to HS or not. Further work is required to identify this molecule more precisely and also to establish the mechanism of interaction employed by serovar E that is clearly GAG independent.
ACKNOWLEDGMENTS
We thank Dominic L. Jack for his help with the flow cytometry experiments.
This work was supported by a bursary from the University of Sheffield and the Infertility Research Trust (registered charity 512973), United Kingdom.
REFERENCES
- 1.Bernfield M, Gotte M, Park P P, Reizes O, Fitzgerald M L, Lincenum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 2.Brieger D, Dawes J. Production method affects the pharmacokinetic and ex vivo biological properties of low molecular weight heparins. Thromb Haemost. 1997;77:317–322. [PubMed] [Google Scholar]
- 3.Cates W, Wasserheit J N. Genital chlamydial infections: epidemiology and reproductive sequelae. Am J Obstet Gynecol. 1991;164:1771–1781. doi: 10.1016/0002-9378(91)90559-a. [DOI] [PubMed] [Google Scholar]
- 4.Cesar B, Gutierrez-Martin C B, Ojcius D M, Hsia R, Hellio R, Bavoil P M, Dautry-Varsat A. Heparin-mediated inhibition of Chlamydia psittaci to HeLa cells. Microb Pathog. 1997;22:47–57. doi: 10.1006/mpat.1996.0090. [DOI] [PubMed] [Google Scholar]
- 5.Chen J C R, Stephens R S. Trachoma and LGV biovars of Chlamydia trachomatis share the same glycosaminoglycan-dependent mechanism for infection of eukaryotic cells. Mol Microbiol. 1994;11:501–507. doi: 10.1111/j.1365-2958.1994.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen J C R, Stephens R S. Chlamydia trachomatis glycosaminoglycan-dependent and independent attachment to eukaryotic cells. Microb Pathog. 1997;22:23–30. doi: 10.1006/mpat.1996.0087. [DOI] [PubMed] [Google Scholar]
- 7.Chen J C R, Zhang J P, Stephens R S. Structural requirements of heparin binding to Chlamydia trachomatis. J Biol Chem. 1996;10:11134–11140. [PubMed] [Google Scholar]
- 8.Claas H C, Wagenvoort J H, Niesters H G, Tio T T, Van Rijsoort-Vos J H, Quint W G. Diagnostic value of the polymerase chain reaction for Chlamydia detection as determined in a follow-up study. J Clin Microbiol. 1991;29:42–45. doi: 10.1128/jcm.29.1.42-45.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creekmore F. Low molecular weight heparins in acute coronary syndrome. S Dak J Med. 2000;53:21–23. [PubMed] [Google Scholar]
- 10.David G. Biology and pathology of the pericellular heparan sulfate proteoglycans. Biochem Soc Trans. 1991;19:816–820. doi: 10.1042/bst0190816. [DOI] [PubMed] [Google Scholar]
- 11.David G, Bai X M, Van der Schueren B, Cassiman J J, Van den Berghe H. Developmental changes in heparan sulfate expression: in situ detection with mAbs. J Cell Biol. 1992;119:961–975. doi: 10.1083/jcb.119.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis C H, Wyrick P B. Differences in the association of Chlamydia trachomatis serovar E and serovar L2 with epithelial cells in vitro may reflect biological differences in vivo. Infect Immun. 1997;65:2914–2924. doi: 10.1128/iai.65.7.2914-2924.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esko J D, Steward T E, Taylor W H. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci USA. 1985;82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fareed J, Jeske W, Hoppensteadt D, Clarizio R, Walenga J M. Low-molecular weight heparins: pharamacologic profile and product differentiation. Am J Cardiol. 1998;82:3L–10L. doi: 10.1016/s0002-9149(98)00105-2. [DOI] [PubMed] [Google Scholar]
- 15.Freissler E, Heyde A M A D, David G, Meyer T F, Dehio C. Syndecan-1 and syndecan-4 can mediate the invasion of OpaHSPG-expressing Neisseria gonorrhoeae into epithelial cells. Cell Microbiol. 2000;2:69–82. doi: 10.1046/j.1462-5822.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher J T, Lyon N, Steward W P. Structure and function of heparan sulfate proteoglycans. Biochem J. 1986;236:313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher J T, Turnbull J E, Lyon M. Heparan sulphate proteoglycans. Biochem Soc Trans. 1990;18:207–209. doi: 10.1042/bst0180207. [DOI] [PubMed] [Google Scholar]
- 18.Hackstadt T. Cell biology. In: Stephens R S, editor. Chlamydia: intracellular biology, pathogenesis, and immunity. Washington, D.C.: ASM Press; 1999. pp. 101–138. [Google Scholar]
- 19.Hayashi K, Hayashi M, Boutin E, Cunha G R, Bernfield M, Trelstad R T. Hormonal modification of epithelial differentiation and expression of cell surface heparan sulfate proteoglycan in the mouse vaginal epithelium. Lab Investig. 1988;58:68–76. [PubMed] [Google Scholar]
- 20.Henriksen T, Tanbo T, Åbyholm T, Oppedal B R, Claussen O P, Hovig T. Epithelial cells from human fallopian tube in culture. Hum Reprod. 1990;5:25–31. doi: 10.1093/oxfordjournals.humrep.a137034. [DOI] [PubMed] [Google Scholar]
- 21.Herold B C, Siston A, Bremer J, Kirkpatrick R, Wilbanks G, Fugedi P, Peto C, Cooper M. Sulphated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Infect Immun. 1997;41:2776–2780. doi: 10.1128/aac.41.12.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inki P. Expression of syndecan-1 in female reproductive tract tissues and cultured keratinocytes. Mol Hum Reprod. 1997;3:299–305. doi: 10.1093/molehr/3.4.299. [DOI] [PubMed] [Google Scholar]
- 23.Jack D L, Dodds A W, Anwar N, Ison C A, Law A, Frosch M, Turner M W, Klein N J. Activation of complement by mannose-binding lectin in isogenic mutants of Neisseria meningitidis serogroup B1. J Immunol. 1998;160:1346–1353. [PubMed] [Google Scholar]
- 24.Kirn-Safran C B, Carson D D. Dynamics of uterine glycoconjugate expression and function. Semin Reprod Endocrinol. 1999;17:217–227. doi: 10.1055/s-2007-1016229. [DOI] [PubMed] [Google Scholar]
- 25.Kuo C C, Grayston T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976;13:1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lan J, Ossewaarde J M, Walboomers J M, Meijer C J, van den Brule A J. Improved PCR sensitivity for direct genotyping of Chlamydia trachomatis serovars by using a nested PCR. J Clin Microbiol. 1994;32:528–530. doi: 10.1128/jcm.32.2.528-530.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lidholt K, Weinke J L, Kiser C S, Lugemwa F N, Bame K J, Cheifetz S, Massague J, Lindahl U, Esko J D. A single mutation affects both N-acetylglucosaminyltransferase and glucuronosyltransferase activities in a Chinese hamster ovary cell mutant defective in heparan sulfate biosynthesis. Proc Natl Acad Sci USA. 1992;89:2267–2271. doi: 10.1073/pnas.89.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morales P, Palmas V, Salgado A M, Villalón M. Sperm interaction with human oviductal cells in vitro. Hum Reprod. 1996;11:1504–1509. doi: 10.1093/oxfordjournals.humrep.a019426. [DOI] [PubMed] [Google Scholar]
- 29.Moulder J. Interaction of chlamydiae and the host cells in vitro. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paavonen J, Eggert- Kruse W. Chlamydia trachomatis: impact on human reproduction. Hum Reprod. 1999;5:433–447. doi: 10.1093/humupd/5.5.433. [DOI] [PubMed] [Google Scholar]
- 31.Pacey A A, Hill C J, Scudamore I W, Warren M A, Barratt C L, Cooke I D. The interaction in vitro of human spermatozoa with epithelial cells from the human uterine (fallopian) tube. Hum Reprod. 1995;10:360–366. doi: 10.1093/oxfordjournals.humrep.a135943. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen-Lathrop S J, Koshiyama K, Phillips N, Stephens R S. Chlamydia-dependent biosynthesis of a heparan sulfate-like compound in eukaryotic cells. Cell Microbiol. 2000;2:137–144. doi: 10.1046/j.1462-5822.2000.00039.x. [DOI] [PubMed] [Google Scholar]
- 33.Rostand K S, Esko J D. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saiki R K, Scharf S, Faloona F, Mullis K B, Horn G T, Erlich H A, Arnheim N. Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 35.Saltissi D, Morgan C, Westhuyzen J, Healy H. Comparison of low molecular weight heparin (enoxaparin sodium) and standard unfractionated heparin for haemodialysis anticoagulation. Nephr Dial Transplant. 1999;14:2698–2703. doi: 10.1093/ndt/14.11.2698. [DOI] [PubMed] [Google Scholar]
- 36.Stagg A J. Vaccines against Chlamydia: approaches and progress. Mol Med Today. 1998;4:166–173. doi: 10.1016/s1357-4310(98)01232-5. [DOI] [PubMed] [Google Scholar]
- 37.Stamm W E, Holmes K K. Chlamydia trachomatis infections of the adult. In: Holmes K K, Mardh P A, Sparling P F, Weisner P J, Cates W, Lemon S M, Stamm W E, editors. Sexually transmitted diseases. New York, N.Y: McGraw-Hill; 1990. pp. 181–193. [Google Scholar]
- 38.Stephens R S. Molecular mimicry and Chlamydia trachomatis infection of eukaryotic cells. Trends Microbiol. 1994;2:99–101. doi: 10.1016/0966-842x(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 39.Stephens R S, Fawaz F S, Kennedy K A, Koshiyama K, Nichols B, vanOoij C, Engel J N. Eukaryotic cell uptake of heparin-coated microspheres: a model of host cell invasion by Chlamydia trachomatis. Infect Immun. 2000;68:1080–1085. doi: 10.1128/iai.68.3.1080-1085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su H, Caldwell H D. Sulfated polysaccharides and a synthetic sulfated polymer are potent inhibitors of Chlamydia trachomatis infectivity in vitro but lack protective effect in an in vivo model of chlamydial genital tract infection. Infect Immun. 1998;66:1258–1260. doi: 10.1128/iai.66.3.1258-1260.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su H, Zhang Y X, Barrera O, Watkins N G, Caldwell H D. Differential effect of trypsin on infectivity of Chlamydia trachomatis: loss of infectivity requires cleavage of major outer membrane protein variable domains II and IV. Infect Immun. 1988;56:2094–2100. doi: 10.1128/iai.56.8.2094-2100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su H, Watkins N G, Zhang Y X, Caldwell H D. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect Immun. 1990;58:1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su H, Raymond L, Rockey D D, Fischer E, Hackstadt T, Caldwell H D. A recombinant Chlamydia trachomatis major outer membrane protein binds to heparan sulfate receptors on epithelial cells. Proc Natl Acad Sci USA. 1996;93:11143–11148. doi: 10.1073/pnas.93.20.11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swanson A F, Kuo C C. Evidence that the major outer membrane protein of Chlamydia trachomatis is glycosylated. Infect Immun. 1991;59:2120–2125. doi: 10.1128/iai.59.6.2120-2125.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swanson A F, Kuo C C. Binding of the glycan of the major outer membrane protein of Chlamydia trachomatis to HeLa cells. Infect Immun. 1994;62:24–28. doi: 10.1128/iai.62.1.24-28.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turnbull J E, Gallagher J T. Molecular organisation of heparan sulfate from human skin fibroblasts. Biochem J. 1990;265:715–724. doi: 10.1042/bj2650715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vela A R, Littleton J C, O'Leary J P. The effects of minidose heparin and low molecular weight heparin on peritonitis in the rat. Am Surg. 1999;65:473–477. [PubMed] [Google Scholar]
- 48.Wadstrom T, Ljungh A. Glycosaminoglycan-binding microbial proteins in tissue adhesion and invasion: key events in microbial pathogenicity. J Med Microbiol. 1999;48:223–233. doi: 10.1099/00222615-48-3-223. [DOI] [PubMed] [Google Scholar]
- 49.Wuppermann F N, Hegemann J H, Jantos C A. Proceedings of the 4th Meeting of the European Society for Chlamydia Research. Helsinki, Finland: Universitat Helsingiensis; 2000. Heparan sulfate is a cellular receptor for Chlamydia pneumoniae; p. 31. [Google Scholar]
- 50.Zaretzky F R, Pearce-Pratt R, Phillips D M. Sulfated polyanions block Chlamydia trachomatis infection of cervix-derived human epithelia. Infect Immun. 1995;63:3520–3526. doi: 10.1128/iai.63.9.3520-3526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J P, Stephens R S. Mechanism of Chlamydia trachomatis attachment to eukaryotic host cell. Cell. 1992;69:861–869. doi: 10.1016/0092-8674(92)90296-o. [DOI] [PubMed] [Google Scholar]