Highlights:
-
•
Around half of patients with recurrent/metastatic cervical cancer treated at first-line received second-line treatment.
-
•
The proportion of patients receiving second-line treatment varied by geographic region of the United States.
-
•
The proportion of patients receiving second-line treatment also varied by therapy used in first-line.
-
•
Further research is needed to clarify barriers to accessing second-line treatment in this patient population.
Keywords: Barriers to care, Cervical cancer, Epidemiology, Geographic variation, Second-line treatment, United States
Abstract
Purpose
Contemporary, real-world data on eligible patients receiving treatment following progression on first-line (1L) recurrent or metastatic cervical cancer (r/mCC) therapy are needed to inform treatment algorithms and identify potential gaps in the r/mCC care continuum.
Methods
This study estimated the prevalence and predictors of second-line (2L) r/mCC therapy among 1L-treated patients using the 2015–2020 IBM MarketScan® commercial claims database. Women ≥ 18 years diagnosed with cervical cancer and treated with first-line systemic therapies were identified and followed for 12 months from their 1L therapy end date. Women with claims for a new therapy after 60 days but no later than 365 days from the end of 1L treatment were identified as those who progressed and received 2L therapy for r/mCC. Descriptive statistics examined baseline cohort characteristics and multivariable logistic regression model examined the factors associated with receiving 2L treatment.
Results
We identified 384 1L-treated patients with r/mCC with ≥ 12 months of follow-up post-1L treatment. During follow-up, over half (51.0 %) of the 1L-treated r/mCC patients received 2L treatment. Patients from the South and Midwest had a lower likelihood of receiving 2L treatment compared with those living in the Northeast (adjusted odds ratio [aOR] = 0.43; 0.23–0.84) and (aOR = 0.52; 0.28–0.95, respectively). Patients not treated with bevacizumab in 1L were also less likely to receive 2L therapy (aOR = 0.65; 0.43–0.99).
Conclusion
Additional research and targeted outreach efforts are needed to understand geography-, population-, or practice-specific barriers impacting access to 2L therapy among patients with r/mCC.
1. Introduction
An estimated 14,100 women will be diagnosed with invasive cervical cancer in 2022 in the United States (US), with approximately 16 % metastatic at diagnosis, and up to 61 % of patients with earllier stage diagnosis will develop metastatic cervical cancer within the first 2 years of completing treatment (National Cancer Institute: Surveillance, Epidemiology, and End Results Program, 2022a, National Cancer Institute: Surveillance, Epidemiology, and End Results (SEER) Program, 2022b, McLachlan et al., 2017, Pfaendler and Tewari, 2016). Although the recurrent or metastatic cervical cancer (r/mCC) setting has been characterized by poor prognosis with limited treatment options, recent approvals offer new treatment options to address the unmet needs for first-line (1L) or second-line or later (2L+) r/mCC patients (National Cancer Institute: Surveillance, Epidemiology, and End Results Program, 2022a, Marabelle et al., 2020, Colombo et al., 2021, U.S. Food Drug Administration, 2021).
In 2018, the U.S. Food and Drug Administration granted accelerated approval to pembrolizumab as monotherapy for previously treated patients with r/mCC whose tumors express PD-L1 (Marabelle et al., 2020). In 2021, pembrolizumab received full approval for use in combination with chemotherapy +/- bevacizumab for patients with PD-L1 expression in the 1L r/mCC setting (Colombo et al., 2021). Also in 2021; tisotumab vedotin-tftv, an antibody-drug conjugate targeting tissue factor, was granted accelerated approval for treatment of patients with r/mCC with disease progression on or after chemotherapy (U.S. Food Drug Administration, 2021).
As the r/mCC treatment landscape continues to evolve, quantifying the proportion of patients needing 2L therapy and the predictors of 2L therapy uptake will help inform treatment algorithms, identify potential gaps in the care continuum, and provide insights into underlying drivers of r/mCC treatment continuity for future research. Data on these aspects of r/mCC treatment are so far limited. Therefore, the objective of this study was to determine the prevalence and predictors of 2L therapy among 1L treated patients with r/mCC.
2. Methods
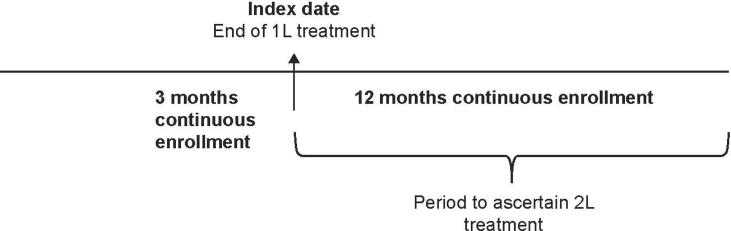
We analyzed the 2015–2020 IBM MarketScan® commercial claims database. The database comprises member enrollment information consisting of demographic variables such as age, sex, geographic location (identified as four census regions, Northeast, Midwest, South, or West), and health plan enrollment/disenrollment dates, as well as medical and prescription drug claims. We utilized a previously-validated claims-based algorithm to identify patients with r/mCC (Musa et al., 2022). Briefly, a cohort design was used; we identified women ≥ 18 years with one or more inpatient claim or two outpatient claims with a diagnosis for malignant neoplasm of the cervix (identified by the International Classification of Diseases 9th and 10th Revisions, Clinical Modification Codes, 180.XX and C53.XX), followed by utilization of one or more systemic therapy indicative of 1L r/mCC treatment. Therapies that included concomitant radiation therapy or surgery within 60 days were excluded. The last recorded date of 1L treatment was assigned as the index date for each patient. Continuous enrollment criteria of a minimum 3-month pre-index and 12-month post-index were applied (Fig. 1). Women with claims for a new therapy after 60 days but no later than 365 days from the end of 1L treatment were identified as those who received subsequent r/mCC therapy.
Fig. 1.
Study design.
1L, first-line; 2L, second-line.
We used descriptive statistics to examine the baseline characteristics of the final analytical cohort. A multivariable logistic regression model examined the factors associated with receiving 2L treatment. All analyses were performed using SAS®, Cary, NC. P-value was tested at 0.05.
3. Results
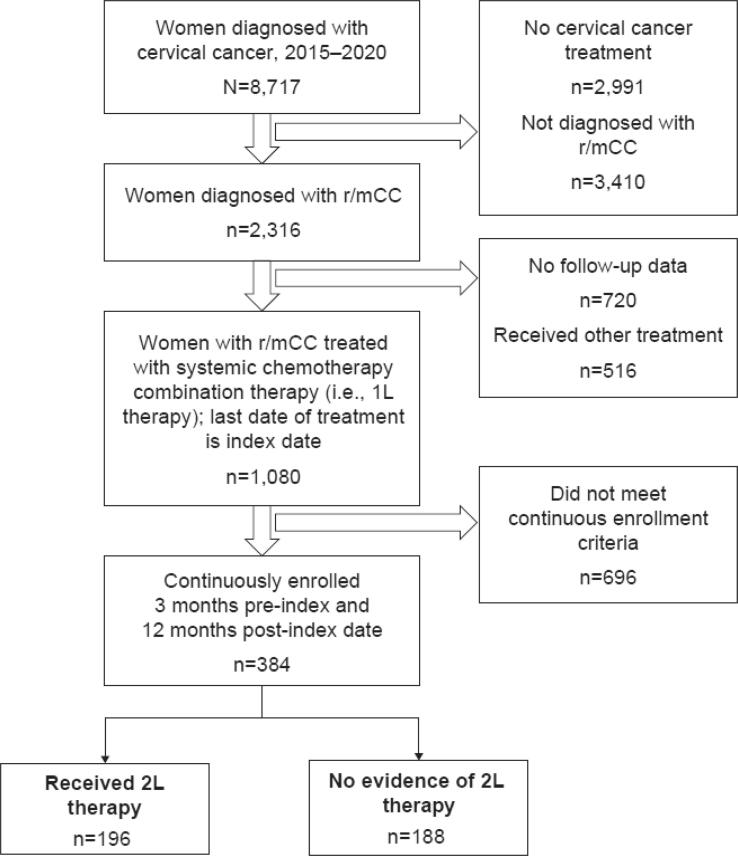
A total of 1080 patients with 1L-treated r/mCC were identified, of whom 384 met the study criteria (Fig. 2). The cohort comprised women with a mean age of 54.5 years, largely enrolled in a non-health maintenance organization health plan (88.8 %), and most women had no comorbid conditions (55.0 %). Approximately 40 % of these women were previously treated with bevacizumab (Table 1).
Fig. 2.
Study sample flow.
1L, first-line; 2L, second-line; r/mCC, recurrent or metastatic cervical cancer.
Table 1.
Demographic characteristics of 1L-treated patients with r/mCC.
| Characteristics | N | Percent |
|---|---|---|
| Total | 384 | 100.0 |
| Age at Index, years, mean (SD) | 54.52 | 11.38 |
| Index year | ||
| 2015 | 100 | 26.0 |
| 2016 | 78 | 20.3 |
| 2017 | 82 | 21.4 |
| 2018 | 68 | 17.7 |
| 2019 | 56 | 14.6 |
| Region | ||
| Northeast | 62 | 16.2 |
| Midwest | 93 | 24.2 |
| South | 183 | 47.7 |
| West | 46 | 12.0 |
| 1L contains bevacizumab | 155 | 40.4 |
| Charlson Comorbidity Score, mean (SD) | 0.82 | 1.23 |
| CCI categories | ||
| 0 | 211 | 55.0 |
| 1 | 97 | 25.3 |
| 2 | 40 | 10.4 |
| 3+ | 36 | 9.4 |
| Baseline comorbidities | ||
| Myocardial infarction | 8 | 2.1 |
| Congestive heart failure | 8 | 2.1 |
| Peripheral vascular disease | 23 | 6.0 |
| Dementia | 1 | 0.3 |
| Chronic pulmonary disease | 45 | 11.7 |
| Rheumatic disease | 5 | 1.3 |
| Peptic ulcer disease | 3 | 0.8 |
| Liver disease | 50 | 13.0 |
| Diabetes without complications | 58 | 15.1 |
| Diabetes with complications | 16 | 4.2 |
| Paralysis | 2 | 0.5 |
| Renal disease | 29 | 7.6 |
| AIDS | 1 | 0.3 |
1L, first-line; AIDS, acquired immunodeficiency syndrome; CCI, Charlson Comorbidity Index; HMO, health maintenance organization; r/mCC, recurrent or metastatic cervical cancer.
Post-1L treatment, 196 (51.0 %) patients initiated a subsequent therapy within a median duration of 122 days from the end date of 1L therapy. The baseline characteristics of patients who received 2L therapy were generally similar to those who did not receive 2L therapy (Table 2). The geographic location of the patient and prior exposure to bevacizumab were significant predictors of receiving 2L therapy (Table 3). Specifically, patients from the South (adjusted odds ratio [aOR] = 0.43 [0.23–0.84]) and Midwest (aOR = 0.52 [0.28–0.95]) regions had a lower likelihood of receiving 2L treatment after 1L therapy compared with those living in the Northeast. Women without prior bevacizumab treatment were also less likely to receive subsequent therapy (aOR = 0.65 [0.43–0.99]). Age, type of health plan, and comorbidity score were not associated with the likelihood of receipt of 2L therapy.
Table 2.
Baseline characteristics of 1L treated patients with r/mCC with and without 2L therapy during follow-up.
| Characteristics | Total | Received 2L |
Did Not Receive 2L |
P-value | ||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | |||
| Total | 384 | 196 | 100.0 | 188 | 100.0 | |
| Age at index, years | 0.85 | |||||
| Mean (SD) | 54.52 | 54.63 | 10.65 | 54.41 | 12.13 | |
| Index year | 0.32 | |||||
| 2015 | 100 | 57 | 29.1 | 43 | 22.9 | |
| 2016 | 78 | 40 | 20.4 | 38 | 20.2 | |
| 2017 | 82 | 45 | 23.0 | 37 | 19.7 | |
| 2018 | 68 | 30 | 15.3 | 38 | 20.2 | |
| 2019 | 56 | 24 | 12.2 | 32 | 17.0 | |
| Region | 0.08 | |||||
| Northeast | 62 | 40 | 20.4 | 22 | 11.7 | |
| Midwest | 93 | 41 | 20.9 | 52 | 27.7 | |
| South | 183 | 90 | 45.9 | 93 | 49.5 | |
| West | 46 | 25 | 12.8 | 21 | 11.2 | |
| Index line contains bevacizumab | 0.08 | |||||
| No | 229 | 108 | 55.1 | 121 | 64.4 | |
| Yes | 155 | 88 | 44.9 | 67 | 35.6 | |
| Providers seen in 60 days prior to index | ||||||
| Oncologist | 180 | 90 | 45.9 | 90 | 47.9 | 0.76 |
| Gynecologist | 122 | 64 | 32.7 | 58 | 30.9 | 0.74 |
| Others | 193 | 97 | 49.5 | 96 | 51.1 | 0.76 |
| Charlson Comorbidity Score | 0.66 | |||||
| Mean (SD) | 0.82 | 0.79 | 1.22 | 0.85 | 1.24 | |
| CCI categories | 0.74 | |||||
| 0 | 211 | 112 | 57.1 | 99 | 52.7 | |
| 1 | 97 | 47 | 24.0 | 50 | 26.6 | |
| 2 | 40 | 18 | 9.2 | 22 | 11.7 | |
| 3+ | 36 | 19 | 9.7 | 17 | 9.0 | |
| Baseline comorbidities | ||||||
| Myocardial infarction | 8 | 5 | 2.6 | 3 | 1.6 | 0.72 |
| Congestive heart failure | 8 | 4 | 2.0 | 4 | 2.1 | 1.00 |
| Peripheral vascular disease | 23 | 10 | 5.1 | 13 | 6.9 | 0.52 |
| Cerebrovascular diseases | 13 | 5 | 2.6 | 8 | 4.3 | 0.41 |
| Dementia | 1 | 0 | 0.0 | 1 | 0.5 | 0.49 |
| Chronic pulmonary disease | 45 | 24 | 12.2 | 21 | 11.2 | 0.75 |
| Rheumatic disease | 5 | 4 | 2.0 | 1 | 0.5 | 0.37 |
| Peptic ulcer disease | 3 | 1 | 0.5 | 2 | 1.1 | 0.62 |
| Mild liver disease | 50 | 28 | 14.3 | 22 | 11.7 | 0.54 |
| Diabetes without complications | 58 | 28 | 14.3 | 30 | 16.0 | 0.67 |
| Diabetes with complications | 16 | 7 | 3.6 | 9 | 4.8 | 0.62 |
| Paralysis | 2 | 1 | 0.5 | 1 | 0.5 | 1.00 |
| Renal disease | 29 | 12 | 6.1 | 17 | 9.0 | 0.34 |
| AIDS | 1 | 1 | 0.5 | 0 | 0.0 | 1.00 |
1L, first-line; 2L, second-line; AIDS, acquired immunodeficiency syndrome; CCI, Charlson Comorbidity Index; HMO, health maintenance organization; r/mCC, recurrent or metastatic cervical cancer.
Table 3.
Likelihood of receipt of 2L treatment among 1L treated patients with r/mCC.
| Effect | Odds Ratio* | 95 % Confidence Limits | P-value | |
|---|---|---|---|---|
| Age | 1.01 | 0.99 | 1.04 | 0.33 |
| Region | ||||
| South versus Northeast | 0.43 | 0.23 | 0.84 | 0.03 |
| Midwest versus Northeast | 0.52 | 0.28 | 0.95 | 0.01 |
| West versus Northeast | 0.64 | 0.29 | 1.40 | 0.26 |
| Plan type (Non-HMO vs HMO) | 1.23 | 0.64 | 2.38 | 0.53 |
| Group type (Medicare vs. commercial) | 0.79 | 0.35 | 1.75 | 0.55 |
| Bevacizumab history (No vs Yes) | 0.65 | 0.43 | 0.99 | 0.04 |
| Charlson Comorbidity Index | 0.96 | 0.81 | 1.14 | 0.65 |
1L, first-line; 2L, second-line; HMO, health maintenance organization; r/mCC, recurrent or metastatic cervical cancer.
*Odds ratio and 95% confidence limits for multivariable logistic regression model simultaneously adjusted for age, region, plan type, group type, bevacizumab treatment history, and comorbidity index.
4. Discussion
Our finding that nearly half of 1L-treated patients with r/mCC received 2L therapy is consistent with a recent study that followed patients with r/mCC from 2014 to 2020 in the US Oncology Network, reporting that 48 % of 1L-treated patients received 2L therapy (Alholm et al., 2022). To our knowledge, these are the only two studies so far that estimated real-world receipt of 2L therapy and its predictors among contemporary patients with r/mCC. Collectively, these data provide highlight potential gaps in the r/mCC care continuum.
We found that geography is an important predictor in the receipt of 2L treatment. Due to data limitations, however, it was not possible to capture geographic-level factors contributing to a lower likelihood of receiving 2L r/mCC treatment for patients living in the South and the Midwest, compared with those in the Northeast. Previous studies have pointed to a high correlation between treatment discontinuation and/or interruption due to longer travel times, lack of gynecologic oncology workforce, and suboptimal treatment with patients’ area of residence and distance from the care facility (Barrington et al., 2016, Temkin et al., 2015, Ricci et al., 2017, Hung et al., 2020). Southern and Midwestern states were reported to have fewer gynecological oncologists and fewer National Cancer Institute (NCI)-designated cancer centers compared with the Northeast (Alimena et al., 2021). Spees et al. also reported that travel time of ≥15 miles from residence is associated with a nearly 30 % higher risk of lack of timely cervical cancer treatment (Spees et al., 2019). It is possible that patients with r/mCC in our study who live in the South or the Midwest experienced these barriers, which decreased their likelihood of receiving 2L treatment. More granular patient- and geography-level indicators of healthcare access are needed to better understand drivers of geographic disparities in r/mCC treatment.
Patients without prior exposure to bevacizumab were also less likely to receive 2L treatment for r/mCC. Previous reports have suggested that factors associated with the likelihood of receiving 2L r/mCC treatment were similar to those predicting survival, including prior bevacizumab exposure (other factors cited included disease stage, histology, metastases, tumor size, and tumor burden) (Tewari et al., 2014, Kim et al., 2012, Chen et al., 2021, Kato et al., 2021, Zhang et al., 2018, Endo et al., 2015, Rose et al., 2015), although we were unable to directly assess such an association due to data limitations. Future studies should seek to understand patient characteristics or other factors influencing bevacizumab inclusion in a patient’s treatment.
Our study findings should be interpreted within the context of study limitations. Claims databases do not contain information on pathology, biomarkers, and qualitative indicators pertaining to treatment; therefore, our model does not account for these factors. Patients who may have initiated a subsequent line of therapy beyond the 12-month follow-up duration study were not captured in our analysis. Finally, our cohort was derived from a nationwide sample of women enrolled in a commercial health plan, which precludes generalizability to uninsured patients and patients enrolled under public health insurance plans.
Despite limitations, our study points to clear contributors of eligible patients not receiving 2L r/mCC therapy. Taken together, results suggest that the difference across the US in proportion of patients receiving subsequent therapy are likely influenced by both local- and patient-level factors. Further investigation into these relationships will help in understanding more clearly drivers of treatment and health disparities in r/mCC.
Funding
This study was funded by Seagen Inc.
CRediT authorship contribution statement
Kalyani Sonawane: Conceptualization, Methodology, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. Tara Castellano: Conceptualization, Methodology, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. Christina Washington: Methodology, Validation, Writing – review & editing. Jie Ting: Conceptualization, Methodology, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. Andy Surinach: Investigation, Data curation, Data analysis, Writing – review & editing. Carol Kirshner: Investigation, Data curation, Data analysis, Writing – review & editing. Jagpreet Chhatwal: Conceptualization, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. Turgay Ayer: Conceptualization, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. Kathleen Moore: Conceptualization, Methodology, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
Kalyani Sonawane has received consulting fees from, and has held a leadership role with Value Analytics Labs; Jie Ting is an employee of, and holds stock in Seagen Inc.; Andy Surinach is an employee of Genesis Research, which received consulting fees from Seagen Inc. in connection with this study; Jagpreet Chhatwal received funding from Seagen Inc. in connection with this study, and has received consulting fees and honoria from Novo Nordisk, and Bayer; Turgay Ayer received funding from Seagen Inc. in connection with this study, and holds a leadership role with Value Analytics Labs; Kathleen Moore has received consulting fees from Green Fire Bio, and payment or honoria from, and participated in data monitoring or advisory boards for AstraZeneca, Aravive, Alkemeres, Addi, Blueprint pharma, Clovis, Elevar, Eisai, EMD Serono, GSK/Tesaro, Genentech/Roche, Hengrui, Immunogen, INxmed, IMab, Mersana, Merck, Myriad, Mereo, Novartis, OncXerna, Onconova, SQZ, Tarveda, VBL Therapeutics and Verastem, received support for attending meetings from AstraZeneca and GSK/Tesaro and holds a leadership role with GOG partners; Tara Castellano, Christina Washington and Carol Kirshner have no competing interests to disclose.
Acknowledgements
Editorial support was provided by Philip Ruane of Curo Consulting, a division of Envision Pharma Group, and funded by Seagen Inc.
Contributor Information
Kalyani Sonawane, Email: sonawane@musc.edu.
Tara Castellano, Email: tcaste@lsuhsc.edu.
Christina Washington, Email: christina-washington@ouhsc.edu.
Jie Ting, Email: jting@seagen.com.
Carol Kirshner, Email: ckirshner@valueanalyticslabs.com.
Jagpreet Chhatwal, Email: JagChhatwal@mgh.harvard.edu.
Turgay Ayer, Email: ayer@isye.gatech.edu.
Kathleen Moore, Email: kathleen-moore@ouhsc.edu.
References
- Alholm Z., He D., Ting J., Zhang Y.J., Sudharshan L., Leong T., Coleman R.L., Monk B.J. Real-world treatment drop-off among recurrent or metastatic cervical cancer patients: a US community oncology-based analysis. Gynecol. Oncol. 2022;166(3):567–575. doi: 10.1016/j.ygyno.2022.07.026. [DOI] [PubMed] [Google Scholar]
- Alimena S., Davis M., Pelletier A., Terry K., King M., Feldman S. Regional variation in access to oncologic care and racial disparities among cervical cancer patients. Gynecol. Oncol. 2021;162:S261. doi: 10.1097/COC.0000000000000944. [DOI] [PubMed] [Google Scholar]
- Barrington D.A., Dilley S.E., Landers E.E., Thomas E.D., Boone J.D., Straughn J.M., et al. Distance from a Comprehensive Cancer Center: a proxy for poor cervical cancer outcomes? Gynecol. Oncol. 2016;143:617–621. doi: 10.1016/j.ygyno.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute: Surveillance, Epidemiology, and End Results Program (SEER*Explorer), Cervix Uteri Cancer Stage Distribution of SEER Incidence Cases, 2008-2017, https://seer.cancer.gov/statistics-network/explorer/application.html (Accessed January, 2022).
- National Cancer Institute: Surveillance, Epidemiology, and End Results (SEER) Program, Cancer Stat Facts: Cervical Cancer, https://seer.cancer.gov/statfacts/html/cervix.html (Accessed August 11, 2022).
- Chen H.-H., Meng W.-Y., Li R.-Z., Wang Q.-Y., Wang Y.-W., Pan H.-D., et al. Potential prognostic factors in progression-free survival for patients with cervical cancer. BMC Cancer. 2021;21:531. doi: 10.1186/s12885-021-08243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo N., Dubot C., Lorusso D., Caceres M.V., Hasegawa K., Shapira-Frommer R., Tewari K.S., Salman P., Hoyos Usta E., Yañez E., Gümüş M., Olivera Hurtado de Mendoza M., Samouëlian V., Castonguay V., Arkhipov A., Toker S., Li K., Keefe S.M., Monk B.J. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N. Engl. J. Med. 2021;385(20):1856–1867. doi: 10.1056/NEJMoa2112435. [DOI] [PubMed] [Google Scholar]
- Endo D., Todo Y., Okamoto K., Minobe S., Kato H., Nishiyama N. Prognostic factors for patients with cervical cancer treated with concurrent chemoradiotherapy: a retrospective analysis in a Japanese cohort. J. Gynecol. Oncol. 2015;26:12–18. doi: 10.3802/jgo.2015.26.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung P., Deng S., Zahnd W.E., Adams S.A., Olatosi B., Crouch E.L., et al. Geographic disparities in residential proximity to colorectal and cervical cancer care providers. Cancer. 2020;126:1068–1076. doi: 10.1002/cncr.32594. [DOI] [PubMed] [Google Scholar]
- Kato M.K., Tanase Y., Uno M., Ishikawa M., Kato T. Brain metastases from uterine cervical and endometrial cancer. Cancers (Basel) 2021;13(3):519. doi: 10.3390/cancers13030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.-E., Park B.-J., Kwack H.-S., Kwon J.-Y., Kim J.-H., Yoon S.-C. Outcomes and prognostic factors of cervical cancer after concurrent chemoradiation. J. Obstet. Gynaecol. Res. 2012;38:1315–1320. doi: 10.1111/j.1447-0756.2012.01871.x. [DOI] [PubMed] [Google Scholar]
- Marabelle A., Le D.T., Ascierto P.A., Di Giacomo A.M., De Jesus-Acosta A., Delord J.P., et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan J., Boussios S., Okines A., Glaessgen D., Bodlar S., Kalaitzaki R., Taylor A., Lalondrelle S., Gore M., Kaye S., Banerjee S. The impact of systemic therapy beyond first-line treatment for advanced cervical cancer. Clin. Oncol. (R. Coll Radiol.) 2017;29(3):153–160. doi: 10.1016/j.clon.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Musa F.B., Brouwer E., Ting J., Schwartz N.R.M., Surinach A., Bloudek L., Ramsey S.D. Trends in treatment patterns and costs of care among patients with advanced stage cervical cancer. Gynecol. Oncol. 2022;164(3):645–650. doi: 10.1016/j.ygyno.2021.12.028. [DOI] [PubMed] [Google Scholar]
- Pfaendler K.S., Tewari K.S. Changing paradigms in the systemic treatment of advanced cervical cancer. Am. J. Obstet. Gynecol. 2016;214(1):22–30. doi: 10.1016/j.ajog.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci S., Tergas A.I., Long Roche K., Fairbairn M.G., Levinson K.L., Dowdy S.C., Bristow R.E., Lopez M., Slaughter K., Moore K., Fader A.N. Geographic disparities in the distribution of the U.S. gynecologic oncology workforce: a Society of Gynecologic Oncology study. Gynecol. Oncol. Rep. 2017;22:100–104. doi: 10.1016/j.gore.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose P.G., Java J., Whitney C.W., Stehman F.B., Lanciano R., Thomas G.M., DiSilvestro P.A. Nomograms Predicting Progression-Free Survival, Overall Survival, and Pelvic Recurrence in Locally Advanced Cervical Cancer Developed From an Analysis of Identifiable Prognostic Factors in Patients From NRG Oncology/Gynecologic Oncology Group Randomized Trials of Chemoradiotherapy. J. Clin. Oncol. 2015;33(19):2136–2142. doi: 10.1200/JCO.2014.57.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spees L.P., Brewster W.R., Varia M.A., Weinberger M., Baggett C., Zhou X., et al. Examining urban and rural differences in how distance to care influences the initiation and completion of treatment among insured cervical cancer patients. Cancer Epidemiol. Biomarkers & Prev. 2019;28(5):882–889. doi: 10.1158/1055-9965.EPI-18-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin S.M., Fleming S.A., Amrane S., Schluterman N., Terplan M. Geographic disparities amongst patients with gynecologic malignancies at an urban NCI-designated cancer center. Gynecol. Oncol. 2015;137(3):497–502. doi: 10.1016/j.ygyno.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Tewari K.S., Sill M.W., Long H.J., Penson R.T., Huang H., Ramondetta L.M., Landrum L.M., Oaknin A., Reid T.J., Leitao M.M., Michael H.E., Monk B.J. Improved survival with bevacizumab in advanced cervical cancer. N. Engl. J. Med. 2014;370(8):734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food & Drug Administration, 2021. FDA grants accelerated approval to tisotumab vedotin-tftv for recurrent or metastatic cervical cancer, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-tisotumab-vedotin-tftv-recurrent-or-metastatic-cervical-cancer (Accessed August 11, 2022).
- Zhang Y., Guo X., Wang G., Ma W., Liu R., Han X., Li L., Baklaushev V.P., Bryukhovetskiy A.S., Wang W., Wang X., Zhang C. Real-world study of the incidence, risk factors, and prognostic factors associated with bone metastases in women with uterine cervical cancer using surveillance, epidemiology, and end results (SEER) data analysis. Med. Sci. Monit. 2018;24:6387–6397. doi: 10.12659/MSM.912071. [DOI] [PMC free article] [PubMed] [Google Scholar]