Abstract
OBJECTIVE
To examine the association of race/ethnicity and socioeconomic deprivation with initiation of guideline-recommended diabetes medications with cardiovascular benefit (glucagon-like peptide 1 receptor agonists [GLP1-RA] and sodium–glucose cotransporter 2 inhibitors [SGLT2i]) among older adults with type 2 diabetes (T2D) and either incident atherosclerotic cardiovascular disease (ASCVD) or congestive heart failure (CHF).
RESEARCH DESIGN AND METHODS
Using Medicare data (2016–2019), we identified 4,057,725 individuals age >65 years with T2D and either incident ASCVD or CHF. We estimated incidence rates and hazard ratios (HR) of GLP1-RA or SGLT2i initiation within 180 days by race/ethnicity and zip code–level Social Deprivation Index (SDI) using adjusted Cox proportional hazards models.
RESULTS
Incidence rates of GLP1-RA or SGLT2i initiation increased over time but remained low (<0.6 initiations per 100 person-months) in all years studied. Medication initiation was less common among those of Black or other race/ethnicity (HR 0.81 [95% CI 0.79–0.84] and HR 0.84 [95% CI 0.75–0.95], respectively) and decreased with increasing SDI (HR 0.96 [95% CI 0.96–0.97]). Initiation was higher in ASCVD than CHF (0.35 vs. 0.135 initiations per 100 person-months). Moderate (e.g., nephropathy, nonalcoholic fatty liver disease) but not severe (e.g., advanced chronic kidney disease, cirrhosis) comorbidities were associated with higher probability of medication initiation.
CONCLUSIONS
Among older adults with T2D and either ASCVD or CHF, initiation of GLP1-RA or SGLT2i was low, suggesting a substantial deficit in delivery of guideline-recommended care or treatment barriers. Individuals of Black and other race/ethnicity and those with higher area-level socioeconomic deprivation were less likely to initiate these medications.
Introduction
Beginning in 2015, data began to rapidly accumulate reporting cardiovascular benefits of glucagon-like peptide 1 receptor agonists (GLP1-RA) and sodium–glucose cotransporter 2 inhibitors (SGLT2i) in patients with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD), with SGLT2i also showing benefit among patients with T2D and congestive heart failure (CHF) (1–8). These findings resulted in a paradigm shift in the treatment of T2D, prompting American Diabetes Association and European Association for the Study of Diabetes consensus report updates in 2018 and 2019 (9,10). These updates first recommended use of GLP1-RA and SGLT2i as preferred second-line agents after metformin among patients with ASCVD or CHF whose hemoglobin A1c (HbA1c) was above goal and later recommended use of these medications among those with either comorbidity, regardless of baseline glycemic control.
Despite the manifold benefits of these medications, uptake has been slow, both among the total population with diabetes (11–15) and among those with both T2D and ASCVD (15–18). Further, as seen with other novel and/or costly diabetes therapies (19–22), racial/ethnic and socioeconomic differences in GLP1-RA (12,14,15,18) and SGLT2i (11,15,16,18) use have already begun to emerge. To our knowledge, uptake has not been explored within certain key populations, including U.S. older adults or publicly insured individuals, and the degree to which race/ethnicity and socioeconomic status contribute independently to these disparities remains unclear.
In this study, we examine rates of initiation of GLP1-RA or SGLT2i among older adults with T2D and either ASCVD or CHF, a population for whom recent guidelines would recommend universal treatment with these medications (9,10). We also examine racial/ethnic and socioeconomic disparities in medication initiation, including the independent contribution of these factors and trends in these disparities over time. Finally, we report the associations of age, sex, region, medical comorbidities, medications, and health care use with initiation of these medications in order to define patient populations most likely to receive GLP1-RA and SGLT2i based on current practice patterns.
Research Design and Methods
Data Source and Study Cohort
We leveraged Medicare fee-for-service claims data from Parts A (inpatient coverage), B (outpatient coverage), and D (prescription benefits) from July 2016 through December 2019, which were the latest data available. July 2016 was chosen as the study start date as it followed the publications of at least one study supporting use of a GLP1-RA [Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER), June 2016 (2)] and an SGLT2i [BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial, September 2015 (6)] for prevention of macrovascular complications in patients with ASCVD or CHF.
In this data set, we examined a cohort of individuals aged ≥65 years with at least 365 days of continuous enrollment and diagnoses of T2D and either incident ASCVD or incident CHF, as defined by validated ICD codes (Supplementary Table 1). The date of diagnosis of either ASCVD or CHF, or the first clinical documentation (e.g., diagnostic code entry) of these indications following July 2016, was considered the cohort entry date. We excluded individuals who had preexisting ASCVD or CHF or previous exposure to either GLP-1-RA or SGLT2i prior to cohort entry date, who had any diagnosis codes for type 1 diabetes, or who would not be eligible for either medication based on diagnosis of either chronic kidney disease stage V or end-stage renal disease. Lastly, we excluded individuals who were missing key variables (age, sex, race/ethnicity, or zip code from which to assign area-level Social Deprivation Index [SDI]) or who were admitted to a nursing home (where diagnoses and medications would not be individually charged to insurance) during the covariate assessment period. The study was approved by the institutional review board of the Brigham and Women’s Hospital, and a data use agreement was in place.
Outcome and Covariate Definitions
The primary outcome was filling of either a GLP1-RA (including liraglutide, dulaglutide, semaglutide, exenatide, albiglutide, or lixisenatide) or an SGLT2i (including empagliflozin, canagliflozin, dapagliflozin, or ertugliflozin) within 180 days of cohort entry. Race and ethnicity were defined as documented in the Medicare data set (enrollment database variable). SDI, a measure incorporating area-level demographics, educational attainment, and other socioeconomic measures, was assigned at the zip code level (23).
Baseline characteristics that might associate with filling of GLP1-RA or SGLT2i were assessed during the 365-day covariate assessment period prior to the cohort entry date. Demographic covariates included age, sex, region, and calendar year of cohort entry. Comorbidities, including key metabolic and cardiovascular comorbidities and relative contraindications for either GLP1-RA or SGLT2i use, were defined based on the presence of at least one ICD code for that diagnosis (Supplementary Table 1). Frailty and combined comorbidity were assessed with use of validated, claims-based indices (24,25). Medication use was defined based on the presence of at least one filling of that medication (Supplementary Table 2). Measures of health care use were also captured, including number of individual prescriptions, diabetes prescriptions, primary care visits, endocrinologist visits, cardiologist visits, emergency room visits, and hospitalizations.
Follow-up began on the cohort entry date and continued until the first of the following events occurred: 180 days of follow-up, disenrollment from Medicare, nursing home admission, death, or end of the study period (31 December 2019). Reasons for censoring are reported in Supplementary Table 3.
Statistical Analysis
Baseline characteristics among those who did and did not fill GLP1-RA or SGLT2i and stratified by race/ethnicity are presented with mean and SD for continuous variables and number and percent for categorical variables. Standardized differences were calculated to compare those who did and did not initiate GLP1-RA or SGLT2i, and incidence rates of medication initiation were calculated as total initiations divided by total person-months of follow-up. In the primary analysis, Cox proportional hazards models were used to assess differences in time to occurrence of the primary outcome (filling of either GLP1-RA or SGLT2i), with results reported as hazard ratios (HR) and 95% CIs for each exposure and covariate. We used a sequential modeling approach was used to understand the association of race/ethnicity and SDI with prescribing, independent of one other and related factors like health care access and use. The base model (model 1) included only demographic characteristics, comorbidities, and medication use. Model 2 was additionally adjusted for race/ethnicity. Model 3 was additionally adjusted for SDI, and the final model (model 4) also included health care use measures. SDI and number of primary care visits were analyzed per SD for interpretability. Finally, we included additional models with examination of multiplicative interactions between 1) year and race/ethnicity, 2) year and SDI, and 3) sex and race/ethnicity to understand whether any observed disparities were evolving over time and whether associations between sex and medication initiation were similar across race/ethnicity groups.
We performed multiple sensitivity analyses to confirm the stability of results under different assumptions, including 1) multivariable logistic regression models of the primary outcome, 2) multivariable linear regression models with examination of proportion of days covered with a GLP1-RA or SGLT2i during the 180-day follow-up period among those who filled a GLP1-RA or SGLT2i at least once, and time-to-event analyses 3) allowing for up to 365 days of follow-up, 4) only including medications with strong evidence for cardiovascular benefit (liraglutide, semaglutide, dulaglutide, empagliflozin, canagliflozin, dapagliflozin) as the outcome, and 5) restricting to individuals with cohort entry dates in 2018 or 2019 when updated guidelines were publicized recommending use of GLP1-RA or SGLT2i therapy in these patients regardless of glycemic control. We additionally performed subgroup analyses restricting the cohort to only metformin users, as a sample taking at least one diabetes medication and as a means of ruling out advanced renal disease, which had not been coded), and restricting the cohort to only those with at least 180 days of follow-up (a.k.a., not including those who disenrolled, were admitted to a nursing home, or died prior to 180 days) in order to account for differential censoring, which might reflect clinical differences in disease severity or life expectancy, which are not well captured by codes. Lastly, we performed time-to-event analyses stratifying the cohort by 1) indication for GLP1-RA or SGLT2i (nonmutually exclusive ASCVD or CHF cohorts), 2) year of cohort entry, 3) age at cohort entry (65–74 years vs. ≥75 years), and 4) race/ethnicity. In the analysis stratified by indication, the primary outcome was filling of either GLP1-RA or SGLT2i among patients in the ASCVD cohort, with additional analyses examining initiation of each medication class individually; among patients in the CHF cohort, the primary outcome was filling of an SGLT2i. All analyses were performed with SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
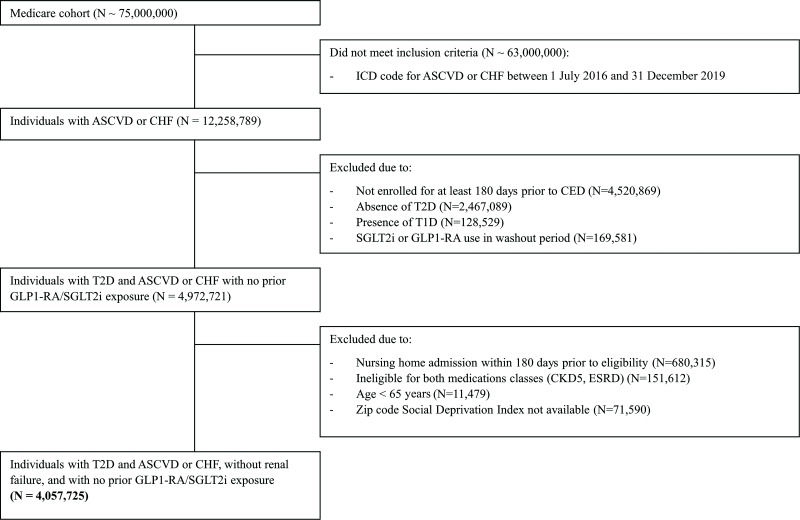
A total of 4,057,725 individuals were included in the analysis (Fig. 1). Of those potentially eligible, only 169,581 (3.3%) were excluded due to GLP1-RA or SGLT2i use prior to ASCVD or CHF diagnosis (Fig. 1). Within the included sample, mean age was 75.6 years, 50.8% of individuals were women, and 80.0% were non-Hispanic White (demographic characteristics presented in Table 1, full characteristics presented in Supplementary Table 4, full characteristics stratified by race/ethnicity presented in Supplementary Table 5). Given the strong recommendations for treatment of this group, and thus the aspiration that nearly 100% of patients with ASCVD or CHF should receive these medications, incidence rates of initiation of GLP1-RA or SGLT2i were low (0.34 initiations per 100 person-months) but increased over time from 0.23 initiations per 100 person-months in 2016 to 0.58 initiations per 100 person-months in 2019.
Figure 1.
Flow diagram. CED, cohort entry date; CKD5, chronic kidney disease stage 5; ESRD, end-stage renal disease.
Table 1.
Baseline demographic characteristics of patients who did and did not fill prescriptions for a GLP1-RA or an SGLT2i
| Total | GLP1-RA or SGLT2i initiators | GLP1-RA or SGLT2i noninitiators | Standardized difference | |
|---|---|---|---|---|
| n | 4,057,725 | 68,893 | 3,988,832 | |
| Age, mean (SD) | 75.6 (7.50) | 71.6 (5.60) | 75.6 (7.49) | −0.61038 |
| Male, n (%) | 1,996,028 (49.19) | 37,168 (53.95) | 1,958,860 (49.11) | 0.09698 |
| Year of cohort entry, n (%) | ||||
| 2016 | 1,706,022 (42.04) | 21,050 (30.55) | 1,684,972 (42.24) | −0.24471 |
| 2017 | 1,043,432 (25.71) | 18,437 (26.76) | 1,024,995 (25.70) | 0.02421 |
| 2018 | 700,058 (17.25) | 15,716 (22.81) | 684,342 (17.16) | 0.1418 |
| 2019 | 608,213 (14.99) | 13,690 (19.87) | 594,523 (14.90) | 0.13131 |
| Race/ethnicity, n (%) | ||||
| Non-Hispanic White | 3,245,234 (79.98) | 55,225 (80.16) | 3,190,009 (79.97) | 0.00468 |
| Non-Hispanic Black | 417,317 (10.28) | 5,557 (8.07) | 411,760 (10.32) | −0.07817 |
| Hispanic | 111,070 (2.74) | 2,290 (3.32) | 108,780 (2.73) | 0.03486 |
| Asian | 124,869 (3.08) | 2,385 (3.46) | 122,484 (3.07) | 0.022 |
| Other | 21,392 (0.53) | 357 (0.52) | 21,035 (0.53) | −0.00125 |
| Missing | 137,843 (3.40) | 3,079 (4.47) | 134,764 (3.38) | 0.05616 |
| SDI, mean (SD) | 48.919 (28.00) | 48.569 (27.94) | 48.925 (28.00) | −0.01273 |
| Region, n (%) | ||||
| South | 1,611,393 (39.71) | 28,936 (42.00) | 1,582,457 (39.67) | 0.0474 |
| Northeast | 873,219 (21.52) | 13,618 (19.77) | 859,601 (21.55) | −0.04405 |
| Midwest | 897,362 (22.11) | 13,627 (19.78) | 883,735 (22.16) | −0.05837 |
| West | 675,751 (16.65) | 12,712 (18.45) | 663,039 (16.62) | 0.04814 |
Associations With GLP1-RA and SGLT2i Initiation
In fully adjusted models, older age (HR 0.94 per year of age, 95% CI 0.94–0.94) was associated with lower probability and male sex (HR 1.08, 95% CI 1.06–1.10) with higher probability of GLP1-RA or SGLT2i initiation (Table 2). Non-Hispanic Black and other race/ethnicity and greater socioeconomic deprivation (as measured by higher SDI) were also associated with decreased likelihood of initiation of GLP1-RA or SGLT2i (respectively, HR 0.81, 95% CI 0.79–0.83; HR 0.87, 95% CI 0.79–0.97; and HR 0.96 per 1 SD increase, 95% CI 0.96–0.97). Adjusting for SDI (model 3) did not significantly attenuate the association between race/ethnicity and medication initiation observed in model 2 (Supplementary Table 6). Patients in the Western U.S. were most likely to fill prescriptions for GLP1-RA or SGLT2i, followed by those in the South, Northeast, and Midwest.
Table 2.
Cox proportional hazards models for filling a new prescription for a GLP1-RA or an SGLT2i
| HR (95% CI) | P | |
|---|---|---|
| Age | 0.94 (0.94–0.94) | <0.0001 |
| Male | 1.078 (1.06–1.1) | <0.0001 |
| Year of cohort entry | ||
| 2016 | Ref | |
| 2017 | 1.455 (1.43–1.48) | <0.0001 |
| 2018 | 1.832 (1.79–1.87) | <0.0001 |
| 2019 | 2.275 (2.23–2.33) | <0.0001 |
| Race/ethnicity | ||
| Non-Hispanic White | Ref | |
| Non-Hispanic Black | 0.810 (0.79–0.83) | <0.0001 |
| Hispanic | 0.989 (0.95–1.03) | 0.6104 |
| Asian | 1.034 (0.99–1.08) | 0.1263 |
| Other | 0.874 (0.79–0.97) | 0.0112 |
| Missing | 1.032 (0.99–1.07) | 0.093 |
| SDI, per SD28 | 0.964 (0.96–0.97) | <0.0001 |
| Region | ||
| South | Ref | |
| Northeast | 0.935 (0.92–0.96) | <0.0001 |
| Midwest | 0.815 (0.80–0.83) | <0.0001 |
| West | 1.064 (1.04–1.09) | <0.0001 |
| Comorbidities | ||
| Hypertension | 1.053 (1.02–1.08) | 0.0004 |
| Hyperlipidemia | 1.105 (1.08–1.13) | <0.0001 |
| Atrial fibrillation/flutter | 0.945 (0.92–0.97) | <0.0001 |
| Obesity | 1.377 (1.35–1.40) | <0.0001 |
| Cirrhosis | 0.852 (0.80–0.91) | <0.0001 |
| NAFLD, hepatosteatosis | 1.236 (1.19–1.28) | <0.0001 |
| Tobacco use | 0.869 (0.83–0.91) | <0.0001 |
| Alcohol use | 0.845 (0.78–0.91) | <0.0001 |
| Diabetes complications | ||
| Neuropathy | 1.225 (1.20–1.25) | <0.0001 |
| Retinopathy | 1.179 (1.15–1.20) | <0.0001 |
| Nephropathy | 1.151 (1.12–1.19) | <0.0001 |
| Comorbidity indices | ||
| Gagne index | 0.980 (0.98–0.98) | <0.0001 |
| Frailty index | 0.802 (0.61–1.05) | 0.1129 |
| SGLT2i contraindications | ||
| Diabetic ketoacidosis | 1.173 (1.00–1.37) | 0.0487 |
| Urinary tract infection | 0.956 (0.93–0.98) | 0.0002 |
| Cellulitis of the groin or Fournier gangrene | 1.384 (1.31–1.46) | <0.0001 |
| Nontraumatic amputation | 0.961 (0.87–1.06) | 0.4309 |
| Hypotension | 0.924 (0.88–0.96) | 0.0004 |
| Falls | 0.992 (0.96–1.03) | 0.6466 |
| Fracture | 1.002 (0.98–1.03) | 0.8928 |
| GLP1-RA contraindications | ||
| Pancreatitis | 0.896 (0.82–0.98) | 0.0185 |
| Cholelithiasis, cholecystitis | 0.844 (0.80–0.89) | <0.0001 |
| Medullary thyroid cancer | 1.374 (0.34–5.49) | 0.6532 |
| SGLT2i and GLP1-RA relative contraindication | ||
| Chronic kidney disease, stage 4 | 0.998 (0.98–1.02) | 0.8524 |
| Diabetes medications | ||
| Metformin | 1.421 (1.38–1.46) | <0.0001 |
| Sulfonylureas | 1.593 (1.54–1.64) | <0.0001 |
| Meglitinides | 1.509 (1.42–1.60) | <0.0001 |
| DPP-4 inhibitors | 1.867 (1.82–1.92) | <0.0001 |
| Thiazolidinediones | 1.432 (1.38–1.49) | <0.0001 |
| α-Glucosidase inhibitors | 1.397 (1.27–1.54) | <0.0001 |
| Amylin analogs | 1.590 (1.04–2.44) | 0.034 |
| Basal insulins | 1.707 (1.65–1.77) | <0.0001 |
| Bolus insulins | 1.163 (1.12–1.21) | <0.0001 |
| Mixed insulins | 1.378 (1.31–1.45) | <0.0001 |
| Cardiovascular medications | ||
| β-Blockers | 1.028 (1.01–1.04) | 0.0009 |
| ACE inhibitors, ARBs | 1.038 (1.02–1.06) | <0.0001 |
| Mineralocorticoid receptor antagonists | 1.037 (1.01–1.07) | 0.0105 |
| Sacubitril | 1.373 (1.25–1.51) | <0.0001 |
| Thiazide diuretics | 0.931 (0.91–0.95) | <0.0001 |
| Loop diuretics | 0.986 (0.97–1.01) | 0.1662 |
| Calcium channel blockers | 0.893 (0.88–0.91) | <0.0001 |
| Statins | 1.010 (0.99–1.03) | 0.2839 |
| Ezetimibe | 1.177 (1.14–1.22) | <0.0001 |
| PCSK9 inhibitors | 1.873 (1.68–2.09) | <0.0001 |
| Fibrates | 1.079 (1.05–1.11) | <0.0001 |
| Niacin | 0.908 (0.83–1.00) | 0.0469 |
| n-3 formulations | 1.420 (1.36–1.48) | <0.0001 |
| Bile acid sequestrants | 1.158 (1.09–1.23) | <0.0001 |
| Use measures | ||
| Primary care visits, per SD (5 visits) | 0.984 (0.98–0.99) | 0.0001 |
| Endocrine visits | 1.062 (1.06–1.07) | <0.0001 |
| Cardiology visits | 1.024 (1.02–1.03) | <0.0001 |
| ER visits | 0.976 (0.97–0.99) | <0.0001 |
| Hospitalizations | 0.867 (0.85–0.88) | <0.0001 |
| Total prescriptions | 1.063 (1.05–1.07) | <0.0001 |
| Total diabetes prescriptions | 1.100 (1.07–1.13) | <0.0001 |
Full model (model 4) is adjusted for demographic characteristics, comorbidities, medication use, race/ethnicity, SDI, and health care use measures. ARB, angiotensin II receptor blocker; DPP-4, dipeptidyl peptidase 4; ER, emergency room; NAFLD, nonalcoholic fatty liver disease; PCSK9, proprotein convertase subtilisin/kexin type 9; Ref, reference.
Multiple medical factors were associated with increased or decreased probability of GLP1-RA or SGLT2i initiation (Table 2). Cardiovascular risk factors (e.g., hypertension, hyperlipidemia, obesity, nonalcoholic fatty liver disease) were associated with increased probability, whereas markers of end-stage or more severe disease (e.g., cirrhosis, combined comorbidity index score [25]) were associated with decreased probability of initiation. The association of medication side effects and relative contraindications to medication use with medication initiation was variable (e.g., urinary tract infection associated with lower rates and diabetic ketoacidosis with higher rates of medication initiation). Prior filling of medications used for treatment of ASCVD and heart failure (e.g., β-blockers, ACE inhibitors, sacubitril) was associated with higher rates of GLP1-RA or SGLT2i initiation, whereas filling of other first-line antihypertensive agents more commonly used for uncomplicated hypertension (e.g., thiazide diuretics and calcium channel blockers) was associated with lower rates of GLP1-RA or SGLT2i initiation. Presence of any microvascular complication and use of any other diabetes medication were associated with higher rates of GLP1-RA or SGLT2i initiation. Number of outpatient encounters with a specialist (endocrinologist or cardiologist) positively associated with GLP1-RA or SGLT2i initiation, while encounters for more severe or uncontrolled disease (e.g., emergency department visits or hospitalizations) associated with lower rates of medication initiation.
Sensitivity and Stratified Analyses
Racial/ethnic and socioeconomic disparities persisted in all sensitivity analyses, including in those with examination of binary receipt of GLP1-RA or SGLT2i (Supplementary Table 7) and percent days covered with a GLP1-RA or an SGLT2i in the first 180 days after diagnosis of ASCVD or CHF, among those who filled the medication at least once (Supplementary Table 8). Disparities also persisted in analyses restricting to patients with at least 180 days of follow-up or allowing for up to 365 days of follow-up (Supplementary Table 9), examining initiation of only medications with clear cardiovascular benefit (Supplementary Table 10), restricting to metformin users (Supplementary Table 11), and restricting to those with a qualifying event in 2018 or 2019 (Supplementary Table 12).
In analyses stratified by indication (ASCVD vs. CHF, nonmutually exclusive) (Table 3), the incidence rate of initiating a GLP1-RA or an SGLT2i was higher among patients with ASCVD than among those with CHF (0.35 vs. 0.13 initiations per 100 person-months). Racial/ethnic and socioeconomic disparities in GLP1-RA or SGLT2i initiation persisted in those with ASCVD, while only Black race/ethnicity was associated with lower medication initiation in those with CHF (Table 3 and Fig. 2). Among those with ASCVD, analyses examining either GLP1-RA or SGLT2i initiation in isolation (rather than as a combined outcome) showed lower rates of initiation of either medication among those reporting non-Hispanic Black race/ethnicity, with variable effects among those of other race/ethnicity groups based on the medication studied (Supplementary Table 13).
Table 3.
Cox proportional hazards models for filling a new prescription for a GLP1-RA or an SGLT2i among patients with ASCVD and for an SGLT2i among patients with CHF
| ASCVD cohort | CHF cohort | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 0.939 (0.94–0.94) | <0.0001 | 0.949 (0.95–0.95) | <0.0001 |
| Male | 1.081 (1.06–1.1) | <0.0001 | 1.199 (1.13–1.27) | <0.0001 |
| Year of cohort entry | ||||
| 2016 | Ref | Ref | ||
| 2017 | 1.447 (1.42–1.48) | <0.0001 | 1.395 (1.30–1.50) | <0.0001 |
| 2018 | 1.828 (1.79–1.87) | <0.0001 | 1.697 (1.57–1.83) | <0.0001 |
| 2019 | 2.241 (2.19–2.29) | <0.0001 | 2.803 (2.61–3.01) | <0.0001 |
| Race/ethnicity | ||||
| Non-Hispanic White | Ref | Ref | ||
| Non-Hispanic Black | 0.811 (0.79–0.84) | <0.0001 | 0.818 (0.74–0.90) | <0.0001 |
| Hispanic | 0.995 (0.95–1.04) | 0.8294 | 1.182 (1.03–1.36) | 0.0183 |
| Asian | 1.034 (0.99–1.08) | 0.1423 | 1.294 (1.13–1.48) | 0.0002 |
| Other | 0.842 (0.75–0.95) | 0.0038 | 0.777 (0.54–1.11) | 0.1709 |
| Missing | 1.025 (0.99–1.07) | 0.2191 | 1.220 (1.08–1.38) | 0.0014 |
| SDI, per SD28 | 0.964 (0.96–0.97) | <0.0001 | 0.991 (0.96–1.02) | 0.529 |
| Region | ||||
| South | Ref | Ref | ||
| Northeast | 0.929 (0.91–0.95) | <0.0001 | 0.964 (0.90–1.04) | 0.3286 |
| Midwest | 0.813 (0.80–0.83) | <0.0001 | 0.793 (0.74–0.85) | <0.0001 |
| West | 1.060 (1.04–1.09) | <0.0001 | 1.142 (1.06–1.23) | 0.0003 |
| Comorbidities | ||||
| Hypertension | 1.070 (1.04–1.10) | <0.0001 | 1.016 (0.93–1.12) | 0.7367 |
| Hyperlipidemia | 1.101 (1.07–1.13) | <0.0001 | 1.123 (1.04–1.21) | 0.0019 |
| Atrial fibrillation/flutter | 0.947 (0.92–0.97) | <0.0001 | 0.943 (0.89–1.00) | 0.0571 |
| Obesity | 1.375 (1.35–1.40) | <0.0001 | 1.128 (1.07–1.19) | <0.0001 |
| Cirrhosis | 0.842 (0.79–0.90) | <0.0001 | 0.924 (0.77–1.11) | 0.4017 |
| NAFLD, hepatosteatosis | 1.231 (1.18–1.28) | <0.0001 | 1.265 (1.11–1.45) | 0.0006 |
| Tobacco use | 0.855 (0.82–0.89) | <0.0001 | 1.034 (0.91–1.18) | 0.6166 |
| Alcohol use | 0.841 (0.77–0.92) | <0.0001 | 0.977 (0.78–1.22) | 0.8398 |
| Diabetes complications | ||||
| Neuropathy | 1.221 (1.19–1.25) | <0.0001 | 1.202 (1.12–1.29) | <0.0001 |
| Retinopathy | 1.179 (1.15–1.21) | <0.0001 | 1.093 (1.01–1.18) | 0.0255 |
| Nephropathy | 1.16 (1.12–1.20) | <0.0001 | 0.956 (0.85–1.07) | 0.4349 |
| Comorbidity indices | ||||
| Gagne index | 0.982 (0.98–0.99) | <0.0001 | 0.958 (0.94–0.97) | <0.0001 |
| Frailty index | 0.773 (0.58–1.04) | 0.087 | 0.331 (0.13–0.85) | 0.0223 |
| SGLT2i contraindications | ||||
| Diabetic ketoacidosis | 1.107 (0.93–1.32) | 0.2576 | 1.722 (1.13–2.62) | 0.0113 |
| Urinary tract infection | 0.948 (0.92–0.97) | <0.0001 | 0.872 (0.80–0.95) | 0.0009 |
| Cellulitis of the groin or Fournier gangrene | 1.389 (1.31–1.47) | <0.0001 | 1.447 (1.19–1.76) | 0.0002 |
| Nontraumatic amputation | 0.935 (0.84–1.04) | 0.2096 | 1.013 (0.66–1.56) | 0.9548 |
| Hypotension | 0.904 (0.86–0.95) | <0.0001 | 1.045 (0.93–1.18) | 0.4698 |
| Falls | 0.986 (0.95–1.02) | 0.4711 | 0.927 (0.82–1.04) | 0.2066 |
| Fracture | 1.000 (0.97–1.03) | 0.9874 | 1.022 (0.94–1.12) | 0.6323 |
| GLP1-RA contraindications | ||||
| Pancreatitis | 0.88 (0.80–0.97) | 0.0118 | 1.219 (0.93–1.60) | 0.1495 |
| Cholelithiasis, cholecystitis | 0.846 (0.8–0.89) | <0.0001 | 0.771 (0.65–0.91) | 0.0028 |
| Medullary thyroid cancer | 1.710 (0.43–6.84) | 0.4482 | 0 (0–1.11 × 10193) | 0.9696 |
| SGLT2i and GLP1-RA relative contraindication | ||||
| Chronic kidney disease, stage 4 | 0.992 (0.97–1.02) | 0.5363 | 0.752 (0.70–0.81) | <0.0001 |
| Diabetes medications | ||||
| Metformin | 1.412 (1.37–1.46) | <0.0001 | 1.936 (1.75–2.14) | <0.0001 |
| Sulfonylureas | 1.607 (1.55–1.66) | <0.0001 | 1.769 (1.59–1.97) | <0.0001 |
| Meglitinides | 1.532 (1.44–1.63) | <0.0001 | 1.457 (1.17–1.82) | 0.0009 |
| DPP-4 inhibitors | 1.871 (1.82–1.93) | <0.0001 | 2.485 (2.26–2.74) | <0.0001 |
| Thiazolidinediones | 1.405 (1.35–1.46) | <0.0001 | 1.873 (1.65–2.13) | <0.0001 |
| α-Glucosidase inhibitors | 1.431 (1.29–1.59) | <0.0001 | 1.201 (0.84–1.72) | 0.3197 |
| Amylin analogs | 1.583 (1.00–2.51) | 0.0521 | 0 (0–8.29 × 1092) | 0.9365 |
| Basal insulins | 1.706 (1.65–1.77) | <0.0001 | 1.284 (1.14–1.44) | <0.0001 |
| Bolus insulins | 1.166 (1.12–1.21) | <0.0001 | 1.273 (1.11–1.46) | 0.0005 |
| Mixed insulins | 1.389 (1.32–1.46) | <0.0001 | 1.181 (0.99–1.42) | 0.071 |
| Cardiovascular medications | ||||
| β-Blockers | 1.026 (1.01–1.04) | 0.0036 | 1.003 (0.95–1.06) | 0.9129 |
| ACE inhibitors, ARBs | 1.033 (1.01–1.05) | 0.0008 | 1.005 (0.95–1.07) | 0.8804 |
| Mineralocorticoid receptor antagonists | 1.013 (0.98–1.05) | 0.435 | 1.068 (0.99–1.15) | 0.0796 |
| Sacubitril | 1.372 (1.20–1.57) | <0.0001 | 1.424 (1.20–1.69) | <0.0001 |
| Thiazide diuretics | 0.917 (0.90–0.94) | <0.0001 | 1.016 (0.94–1.10) | 0.6783 |
| Loop diuretics | 0.985 (0.96–1.01) | 0.1825 | 0.880 (0.83–0.93) | <0.0001 |
| Calcium channel blockers | 0.892 (0.88–0.91) | <0.0001 | 0.853 (0.80–0.90) | <0.0001 |
| Statins | 1.006 (0.99–1.03) | 0.5306 | 0.986 (0.93–1.05) | 0.6553 |
| Ezetimibe | 1.175 (1.13–1.22) | <0.0001 | 1.166 (1.02–1.33) | 0.0252 |
| PCSK9 inhibitors | 1.877 (1.67–2.10) | <0.0001 | 1.337 (0.79–2.26) | 0.2797 |
| Fibrates | 1.083 (1.05–1.11) | <0.0001 | 1.043 (0.94–1.16) | 0.4277 |
| Niacin | 0.900 (0.81–1.00) | 0.0397 | 0.872 (0.60–1.27) | 0.4708 |
| n-3 formulations | 1.421 (1.36–1.48) | <0.0001 | 1.442 (1.24–1.68) | <0.0001 |
| Bile acid sequestrants | 1.161 (1.08–1.24) | <0.0001 | 1.219 (0.97–1.53) | 0.0877 |
| Use measures | ||||
| Primary care visits, per SD (5 visits) | 0.987 (0.98–1.00) | 0.0034 | 0.981 (0.96–1.01) | 0.1749 |
| Endocrine visits | 1.065 (1.06–1.07) | <0.0001 | 1.050 (1.04–1.06) | <0.0001 |
| Cardiology visits | 1.026 (1.02–1.03) | <0.0001 | 1.020 (1.00–1.04) | 0.0524 |
| ER visits | 0.978 (0.97–0.99) | <0.0001 | 0.991 (0.96–1.02) | 0.5301 |
| Hospitalizations | 0.872 (0.85–0.89) | <0.0001 | 0.936 (0.89–0.99) | 0.0128 |
| Total prescriptions | 1.067 (1.06–1.08) | <0.0001 | 1.000 (0.97–1.04) | 0.9841 |
| Total diabetes prescriptions | 1.094 (1.06–1.12) | <0.0001 | 1.032 (0.94–1.13) | 0.4892 |
Full model (model 4) is adjusted for demographic characteristics, comorbidities, medication use, race/ethnicity, SDI, and health care use measures. ARB, angiotensin II receptor blocker; DPP-4, dipeptidyl peptidase 4; ER, emergency room; NAFLD, nonalcoholic fatty liver disease; PCSK9, proprotein convertase subtilisin/kexin type 9; Ref, reference.
In analyses stratified by year (Supplementary Table 14), racial/ethnic and socioeconomic disparities persisted for all years studied, with the degree of disparity decreasing over time among non-Hispanic Black and other race individuals (with a significant race-by-year multiplicative interaction when added to model 4; P = 0.01 in 2019 vs. 2,016 for non-Hispanic Black and P < 0.0001 in 2019 vs. 2016 for other). No change in socioeconomic disparities was seen during the study period (P for interaction >0.05 for all years vs. 2016). In analyses stratified by age at cohort entry, non-Hispanic Black race/ethnicity remained associated with lower rates of medication initiation for all age-groups, with other race/ethnicity and greater socioeconomic deprivation associated with lower rates of initiation in older (age ≥75 years) and younger (age 65–74 years) groups, respectively (Supplementary Table 15). Finally, in analyses stratified by race/ethnicity (Supplementary Table 16), negative associations between social deprivation and GLP1-RA or SGLT2i filling existed for individuals of non-Hispanic White and non-Hispanic Black race/ethnicity (the two largest groups) but not for other reported race/ethnicities. In individuals of Hispanic, other, and missing race/ethnicity, there was no significant association between SDI and filling, while in non-Hispanic Asian individuals, higher social deprivation was counterintuitively associated with higher rates of GLP1-RA or SGLT2i filling (HR 1.05 [95% CI 1.01–1.09] per 1 SD increase in SDI). Additionally, a significant interaction existed between sex and race/ethnicity, with non-Hispanic Black men (P < 0.0001) and Hispanic men (P = 0.02) less likely to initiate GLP1-RA or SGLT2i therapy.
Conclusions
We found that among individuals for whom GLP1-RA or SGLT2i therapy is recommended (those with T2D and incident ASCVD or CHF and without advanced renal failure), rates of GLP1-RA or SGLT2i filling were very low (<0.6 initiations per 100 person-months) in all populations and at all time points. Further, men, non-Hispanic Black individuals and those of other race/ethnicity, and those with higher social deprivation had lower probability of initiating a GLP1-RA or an SGLT2i, with race/ethnicity and social deprivation contributing independently to these disparities. Findings were robust to a variety of sensitivity analyses, and disparities largely persisted over time but seemed to be narrowing slightly for non-Hispanic Black and other race individuals by 2019. GLP1-RA or SGLT2i filling seemed to be more common among individuals with moderately complex disease (e.g., requiring multiple diabetes medications and therapies for ASCVD and CHF, requiring cardiology or endocrinology care) and less common among those with advanced or poorly controlled disease (e.g., as measured according to high frailty [24] or combined comorbidity [25] indices, recent emergency department visits or hospitalizations).
Among the most concerning findings of this study are the very low rates of GLP1-RA or SGLT2i initiation in this population, a group who universally carried indications for therapy. Significant cardiovascular, renal, and even all-cause mortality benefits of GLP1-RA and SGLT2i have repeatedly been shown in studies of patients with known ASCVD or CHF (1–8), and current guidelines recommend these therapies for patients at risk (9,10). Despite this, as has been seen in other medical conditions and especially following the release of novel medications (26,27), rates of guideline-recommend GLP1-RA and SGLT2i use in individuals within this high-risk population remain very low (15–18), often even lower than in populations with T2D and without these indications (11,12,15). The rates of medication initiation reported in our study are even lower than those reported in previous studies, possibly related to the older age of participants in our study (as lower rates of medication filling have been noted previously in Medicare Advantage vs. other commercially insured individuals [15]), the use of public rather than private insurance, and the evaluation of medication initiation after incident diagnosis of ASCVD or CHF, which excluded individuals using these therapies prior to diagnosis of ASCVD or CHF (although only 3.3% were excluded for prior GLP1-RA or SGLT2i use) (Fig. 1). Efforts are urgently needed to improve guideline implementation and to prevent the exacerbation of preexisting health disparities, including efforts to improve access to and filling of these medications in high-risk groups.
Significant racial/ethnic and socioeconomic disparities existed in GLP1-RA or SGLT2i filling. Although the effect size of SDI was small, this was measured per SD change in SDI, such that those at more extreme levels of social deprivation may have much larger reductions in medication initiation. Previous analyses examining a general T2D population (with and without cardiovascular or renal indications for these therapies) in commercially insured data sets and among individuals with T2D and cardiovascular disease in commercially insured, electronic medical record, and nationally representative data sets (14,16,17) have shown similar trends, as well as lower rates of medication use among those with cardiovascular or renal indications compared with those without these indications (11,12). Many factors, including decreased access to health care overall or subspecialty care, receipt of care in different health care settings (18), limited health literacy and ability to advocate for oneself, financial barriers, and structural racism, may be contributing to these differences. However, the fact that race/ethnicity and SDI were independently associated with lower rates of medication filling, including in stratified models (Supplementary Table 16), suggests that the association between race/ethnicity and medication filling is not completely mediated by social deprivation. Further, socioeconomic disparities have also been described in Denmark, with persistently lower use of GLP1-RA or SGLT2i in those with lower socioeconomic position despite access to universal health care (28), suggesting that the association between SDI and lower filling may not be mediated exclusively by limited access to care. Our results similarly showed that adjusting for health care use measures did not explain the effect of either race/ethnicity or social deprivation on GLP1-RA or SGLT2i filling. A recent analysis identified racial/ethnic differences in primary nonadherence (defined by failing to fill a prescription within 30 days) of GLP1-RA and SGLT2i (29), so even if prescribing were equal across race/ethnicities, further research is needed to understand barriers to first medication filling. Our analysis builds on and strengthens these findings in examining a population for whom these medications are strongly indicated, studying older adults with public rather than private insurance, and showing narrowing of disparities over time in non-Hispanic Black individuals.
In addition to racial/ethnic disparities, we also identified lower rates of medication initiation in women than in men. Previous studies have described this phenomenon for SGLT2i use in other populations (11,14–16,18), although several studies show the opposite trend for GLP1-RA use (12,18). Research shows that women are less likely than men to receive guideline-directed care in several areas (30–33), including diabetes (34), and it is believed that this may result from implicit sex bias (35). Although studies show that heart disease is less likely to be recognized in women than in men (36), this is an unlikely contributor to our findings, as all individuals included in our study had received a diagnosis of ASCVD or CHF. Of note, the association between sex and medication initiation was reversed among non-Hispanic Black individuals in our study, with a significant sex-by-race/ethnicity interaction. The reasons for this warrant further study, although Black men’s health outcomes may be disproportionately affected by experiences of racism (37), and Black men face a host of unique barriers to optimal health care (38,39).
In examining the association of medication initiation and the spectrum of comorbidities, health care use measures, and burden of disease, a pattern emerged suggesting a narrow index of illness at which GLP1-RA or SGLT2i are most likely to be initiated. Medication initiation was more common among those with diagnoses of disease precursors but not end-stage disease (e.g., nonalcoholic fatty liver disease but not cirrhosis, diabetic neuropathy without amputations, diabetic nephropathy without advanced chronic kidney disease). Older age, frailty index, and combined comorbidity index were all associated with lower rates of medication filling. Similarly, subspecialty care by an endocrinologist or cardiologist was associated with higher rates of medication filling, while emergency or inpatient care was associated with lower rates. This may represent hesitation on the part of clinicians or patients to initiate new and relatively lesser studied medications among individuals with advanced comorbidities, polypharmacy, or limited life expectancy for whom adverse medication effects may be more severe than in less ill patients (40–44). However, the association of SGLT2i with lower rates of hospitalization for heart failure in many trials suggests that patients with frequent hospitalizations, at least for this indication, may be among those who receive the greatest absolute benefit from these therapies. Our findings are consistent with those of a previous study in a privately insured population with T2D in whom rates of SGLT2i use were found to be paradoxically lower among those with indications (ASCVD or chronic kidney disease) (11). In parallel, relative contraindications to GLP1-RA or SGLT2i did not necessarily correlate with lower rates of medication initiation. For example, history of diabetic ketoacidosis and cellulitis of the groin, known complications of SGLT2i, were associated with higher rates of GLP1-RA or SGLT2i use, including in the analysis limited to individuals with CHF and for which only SGLT2i initiation was considered an outcome.
This study has multiple strengths, including use of a large sample representing a diverse population of older adults across the U.S., the restriction to only individuals who have a strong indication for GLP1-RA or SGLT2i initiation and who are eligible for both medication classes studied, the longitudinal outcome assessment, and the multiple sensitivity analyses, which confirmed the robustness of our findings. However, this analysis must be interpreted in light of its limitations. Medicare data include only billing information, and thus important clinical factors, such as hemoglobin A1c or blood pressure and lipid levels from which longitudinal risk of ASCVD could be calculated, could not be considered in the analysis. Similarly, billing codes are underused for certain comorbidities (e.g., early-stage chronic kidney disease [45]) leading to underestimation of the prevalence of these conditions. The outcome of medication filling captures elements of both provider and patient behavior and cannot speak directly to prescribing patterns or patient adherence to therapy, although the proportion of days covered sensitivity analysis (Supplementary Table 8) examining number of medication fillings has been used as a proxy for medication adherence in other studies. Race and ethnicity variables in claims databases are not based on self-report and may be more accurate for White and Black individuals than for other race/ethnicities (46). Our analysis considered only individuals with fee-for-service Medicare insurance and may not be representative of all elderly adults in the U.S. (e.g., those with Medicare Advantage plans). Medication filling using secondary insurance coverage may not be captured in this data set, so individuals with secondary insurance may be undercounted in calculating rates of GLP1-RA or SGLT2i initiation. Finally, although our analysis was able to identify disparities in medication filling, this observational data set cannot confirm the reasons underlying these disparities.
In conclusion, using a data set of >4.5 million older adults in the U.S. for whom guidelines clearly recommend GLP1-RA or SGLT2i therapy, we found that overall initiation of these medications was exceedingly low, that individuals of non-Hispanic Black or other race/ethnicity and with higher social deprivation experienced even lower likelihood of starting treatment with these agents, and that rates of medication use seemed to be highest among individuals with moderate but not severe disease processes. Further studies are needed to examine the reasons underlying these findings and their consequences in population health and health disparities. Additionally, interventions are warranted to improve uptake of these novel therapies among patients for whom they are indicated.
Article Information
Funding. This study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA. S.J.C. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (F32DK127545), and by National Institutes of Health grant T32DK007028. J.C.L. was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (K01HL141538). E.P. was supported by National Institute on Aging career development grant K08AG055670 and research grants from the Patient-Centered Outcomes Research Institute (PCORI) (DB-2020C2-20326) and the U.S. Food and Drug Administration (5U01FD007213).
The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health or PCORI or its Board of Governors or Methodology Committee.
Duality of Interest. S.J.C. reports employment of a close family member by a Johnson & Johnson company. E.P. is co-investigator of a research grant to the Brigham and Women’s Hospital from Boehringer Ingelheim, outside the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.J.C., J.C.L., and E.P. contributed to the design and analysis plan of this manuscript. R.L. performed the analysis. S.J.C. drafted the manuscript with critical feedback from all authors. S.J.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.21313254.
References
- 1. Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 2. Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019. 29;381:841–851 [DOI] [PubMed] [Google Scholar]
- 4. Gerstein HC, Colhoun HM, Dagenais GR, et al.; REWIND Investigators . Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–130 [DOI] [PubMed] [Google Scholar]
- 5. Holman RR, Bethel MA, Mentz RJ, et al.; EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 7. Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 8. Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 9. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43:487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eberly LA, Yang L, Eneanya ND, et al. Association of race/ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open 2021;4:e216139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eberly LA, Yang L, Essien UR, et al. Racial, ethnic, and socioeconomic inequities in glucagon-like peptide-1 receptor agonist use among patients with diabetes in the US. JAMA Health Forum 2021;2:e214182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nargesi AA, Jeyashanmugaraja GP, Desai N, Lipska K, Krumholz H, Khera R. Contemporary national patterns of eligibility and use of novel cardioprotective antihyperglycemic agents in type 2 diabetes mellitus. J Am Heart Assoc 2021;10:e021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Limonte CP, Hall YN, Trikudanathan S, et al. Prevalence of SGLT2i and GLP1RA use among US adults with type 2 diabetes. J Diabetes Complications 2022;36:108204. [DOI] [PubMed] [Google Scholar]
- 15. Nargesi AA, Clark C, Liu M, et al. U.S. patterns of drug utilization and prescription fills for proven cardioprotective anti-hyperglycemic agents. J Am Coll Cardiol 2022;79(Suppl.):1446 [Google Scholar]
- 16. Chahine N, Al-Kindi S. Under-prescription of SGLT2 inhibitors in patients with diabetes and cardiovascular disease in the United States. J Am Coll Cardiol 2020;75(Suppl. 1):1915 [Google Scholar]
- 17. Nelson AJ, Ardissino M, Haynes K, et al. Gaps in evidence-based therapy use in insured patients in the United States with type 2 diabetes mellitus and atherosclerotic cardiovascular disease. J Am Heart Assoc 2021;10:e016835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mahtta D, Ramsey DJ, Lee MT, et al. Utilization rates of SGLT2 inhibitors and GLP-1 receptor agonists and their facility-level variation among patients with atherosclerotic cardiovascular disease and type 2 diabetes: insights from the Department of Veterans Affairs. Diabetes Care 2022;45:372–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cromer SJ, Wexler DJ, Kazemian P. Correlates of analog vs human basal insulin use among individuals with type 2 diabetes: a cross-sectional study. Diabetes Res Clin Pract 2021;175:108825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Agarwal S, Schechter C, Gonzalez J, Long JA. Racial-ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther 2021;23:306–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Briesacher B, Limcangco R, Gaskin D. Racial and ethnic disparities in prescription coverage and medication use. Health Care Financ Rev 2003;25:63–76 [PMC free article] [PubMed] [Google Scholar]
- 22. Whyte MB, Hinton W, McGovern A, et al. Disparities in glycaemic control, monitoring, and treatment of type 2 diabetes in England: a retrospective cohort analysis. PLoS Med 2019;16:e1002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Butler DC, Petterson S, Phillips RL, Bazemore AW. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Serv Res 2013;48:539–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim DH, Glynn RJ, Avorn J, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci 2019;74:1271–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 2011;64:749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med 2003;348:2635–2645 [DOI] [PubMed] [Google Scholar]
- 27. Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med 2011;104:510–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Falkentoft AC, Andersen J, Malik ME, et al. Impact of socioeconomic position on initiation of SGLT-2 inhibitors or GLP-1 receptor agonists in patients with type 2 diabetes - a Danish nationwide observational study. Lancet Reg Health Eur 2022;14:100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luo J, Feldman R, Rothenberger S, Korytkowski M, Fischer MA, Gellad WF. Incidence and predictors of primary nonadherence to sodium glucose co-transporter 2 inhibitors and glucagon-like peptide 1 agonists in a large integrated healthcare system. J Gen Intern Med 2022;37:3562–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee KK, Ferry AV, Anand A, et al.; High-STEACS Investigators . Sex-specific thresholds of high-sensitivity troponin in patients with suspected acute coronary syndrome. J Am Coll Cardiol 2019;74:2032–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao M, Woodward M, Vaartjes I, et al. Sex differences in cardiovascular medication prescription in primary care: a systematic review and meta-analysis. J Am Heart Assoc 2020;9:e014742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen EH, Shofer FS, Dean AJ, et al. Gender disparity in analgesic treatment of emergency department patients with acute abdominal pain. Acad Emerg Med 2008;15:414–418 [DOI] [PubMed] [Google Scholar]
- 33. Yancy CW, Fonarow GC, Albert NM, et al. Influence of patient age and sex on delivery of guideline-recommended heart failure care in the outpatient cardiology practice setting: findings from IMPROVE HF. Am Heart J 2009;157:754–62.e2 [DOI] [PubMed] [Google Scholar]
- 34. Yu MK, Lyles CR, Bent-Shaw LA, Young BA. Sex disparities in diabetes process of care measures and self-care in high-risk patients. J Diabetes Res 2013;2013:575814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paulson E. Recognizing, addressing unintended gender bias in patient care, 2020. Accessed 8 June 2022. Available from https://physicians.dukehealth.org/articles/recognizing-addressing-unintended-gender-bias-patient-care
- 36. Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med 2000;342:1163–1170 [DOI] [PubMed] [Google Scholar]
- 37. Assari S, Lee DB, Nicklett EJ, Moghani Lankarani M, Piette JD, Aikens JE. Racial discrimination in health care is associated with worse glycemic control among Black men but not Black women with type 2 diabetes. Front Public Health 2017;5:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheatham CT, Barksdale DJ, Rodgers SG. Barriers to health care and health-seeking behaviors faced by Black men. J Am Acad Nurse Pract 2008;20:555–562 [DOI] [PubMed] [Google Scholar]
- 39. Blackwell D, Villarroel M. Tables of Summary Health Statistics for U.S. Adults: 2017 National Health Interview Survey. Hyattsville, MD, National Center for Health Statistics, 2018 [Google Scholar]
- 40. Mata-Cases M, Benito-Badorrey B, Roura-Olmeda P, et al.; GEDAPS (Primary Care Group for the study of Diabetes) of the Catalonian Society of Family and Community Medicine . Clinical inertia in the treatment of hyperglycemia in type 2 diabetes patients in primary care. Curr Med Res Opin 2013;29:1495–1502 [DOI] [PubMed] [Google Scholar]
- 41. Mata-Cases M, Franch-Nadal J, Real J, et al. Therapeutic inertia in patients treated with two or more antidiabetics in primary care: Factors predicting intensification of treatment. Diabetes Obes Metab 2018;20:103–112 [DOI] [PubMed] [Google Scholar]
- 42. Ali DH, Kiliç B, Hart HE, et al. Therapeutic inertia in the management of hypertension in primary care. J Hypertens 2021;39:1238–1245 [DOI] [PubMed] [Google Scholar]
- 43. Mitchell A, Snowball J, Welsh TJ, Watson MC, McGrogan A. Prescribing of direct oral anticoagulants and warfarin to older people with atrial fibrillation in UK general practice: a cohort study. BMC Med 2021;19:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lublóy Á. Factors affecting the uptake of new medicines: a systematic literature review. BMC Health Serv Res 2014;14:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maciejewski M, Onstad K, Tamayo L. Chronic kidney disease often undiagnosed in Medicare beneficiaries. Baltimore, MD, Centers for Medicare & Medicaid Services Office of Minority Health, 2020. Accessed 10 January 2022. Available from https://www.cms.gov/files/document/ckd-data-highlight102020-2.pdf
- 46. Jarrín OF, Nyandege AN, Grafova IB, Dong X, Lin H. Validity of race and ethnicity codes in Medicare administrative data compared with gold-standard self-reported race collected during routine home health care visits. Med Care 2020;58:e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]