Abstract
Legionella pneumophila is a facultative intracellular gram-negative rod that causes pneumonia in humans. Free-living amoebas are thought to serve as a reservoir for Legionella infections. Signature-tagged mutagenesis was employed to identify Legionella pneumophila genes necessary for survival in the amoeba Acanthamoeba castellanii. Six mutant strains were defective in assays of invasion and intracellular growth. Four mutants also exhibited invasion and replication defects in Hartmannella vermiformis, an amoeba linked to hospital outbreaks of Legionella pneumonia. The six mutants also were tested in macrophages derived from peripheral blood mononuclear cells. Two mutants had intracellular replication defects, and two different strains entered cells less efficiently. Two transposon insertions were in known L. pneumophila genes, lspK and aroB. The other four were in novel genes. One gene has similarity to a cytochrome c-type biogenesis protein of Pseudomonas fluorescens. Another has similarity to a transcriptional activator regulating flagellar biosynthesis in Vibrio cholera. The third is similar to traA of Rhizobium sp. strain NGR234, which is involved in conjugal transfer of DNA. The fourth has no homology. By using survival in amoeba as a selection, we have isolated mutant strains with a range of phenotypes; and we have potentially identified new L. pneumophila virulence genes.
Legionella pneumophila is a facultative intracellular gram-negative rod that causes epidemic, sporadic, and nosocomial pneumonia (33, 43, 54, 55). Epidemiological evidence suggests that the environmental source of these infections is freshwater, including potable water supplies and cooling towers (54, 58). In water sources linked to outbreaks of Legionnaires' disease, Legionella is found replicating within protozoa such as free-living amoebas, implicating amoebas as important in the transmission of Legionnaires' disease (7, 32). Inside free-living amoebas, L. pneumophila resides within a phagosome (4). To evade host cell defenses, virulent L. pneumophila prevents phagosome-lysosome fusion. Instead, rough endoplasmic reticulum is recruited to the phagosome, and a replicative vacuole is formed (1, 10). L. pneumophila has a similar lifestyle in human macrophages (45, 46, 80), a host cell type that is thought to be a niche for Legionella replication in human infection (42, 85).
Many genes that are important for the initial steps of L. pneumophila invasion of macrophages have been identified and studied (67, 84); fewer genes that are specifically important in amoebas have been studied (12, 30, 38, 39). Although there is overlap between gene products necessary for infection of both cell types (35, 76), different gene products are likely to be necessary for survival in free-living amoebas and not macrophages. For example, L. pneumophila cells grown within amoebas are more infectious than broth-grown L. pneumophila for macrophage-like cell lines (20), suggesting that L. pneumophila genes important for human infection are activated within amoebas. Identifying genes important for survival in amoebas versus macrophages may give us insight into what makes L. pneumophila infectious for humans.
Recently, a negative-selection strategy, signature-tagged transposon mutagenesis, has been successfully employed to identify genes important for in vivo virulence in a variety of gram-negative and gram-positive bacteria (15, 18, 21, 27, 41, 56, 62). In brief, this technique involves creating a library of bacterial transposon mutants, with each transposon tagged with a unique DNA sequence. Pools of mutants, rather than individual strains with transposon insertions, are then screened in host cells or animals. The output pool—containing strains capable of entry into cells, intracellular replication, and cell-to-cell spread—is compared to the input pool by hybridizing the unique tags present in the output pool to a filter containing input DNA. Those clones missing from the output pool are potentially avirulent.
In this study, a library of L. pneumophila mutants containing signature-tagged transposon insertions was screened in free-living Acanthamoeba castellanii amoebas to identify genes necessary for infection (27, 41). Because L. pneumophila can undergo a 1,000-fold amplification within these cells, it is possible to detect the loss of clones not competent for intracellular replication. From the first 700 transposon mutants screened, we isolated six clones that were attenuated in virulence in A. castellanii. To determine if the mutants had different phenotypes in different host cells, we tested these six mutants in three additional cell types: (i) Hartmannella vermiformis, a free-living amoeba that has been linked to nosocomial Legionnaires' disease (11, 32); (ii) phorbol myristate acetate (PMA)-treated U-937 cells, a macrophage-like cell line used to study L. pneumophila (61); and (iii) peripheral blood mononuclear cell (PBMC)-derived human macrophages. For the six mutants, the DNA transposon junction was cloned and sequenced. Four of the genes flanking the transposon insertions encode putative proteins with amino acid similarity to known virulence factors from Legionella or other bacteria. These data, together with the sequence similarities, are presented.
MATERIALS AND METHODS
Bacterial strains and cultures.
A streptomycin-resistant mutant of L. pneumophila serogroup 1 (130b) (20, 28), designated LpAA100jm (27), was cultured in (buffered yeast extract α-ketoglutarate–morpholinepropanesulfonic acid (BYEα-MOPS) or BYEα–N-(2-acetamido)-2-aminoethanesulfonic acid (BYEα-ACES) at 37°C in a roller or on buffered charcoal yeast extract agar α-ketoglutarate–MOPS (BCYEα-MOPS) at 37° in a 5% CO2 incubator (25, 26, 29, 60). BYEα-MOPS (pH 6.9) contains (per liter) 2 g of MOPS, 2 g of α-ketoglutaric acid, 15 g of yeast extract, 2 g of KOH, 0.4 g of l-cysteine, and 0.025 g of ferric pyrophosphate. BCYEα-MOPS agar contains (per liter) 2 g of activated charcoal and 15 g of Bacto Agar in addition to the ingredients in BYEα-MOPS. BYEα-ACES (pH 6.9) contains (per liter) 10 g of ACES, 0.77 g of α-ketoglutaric acid, 10 g of yeast extract, 2.6 g of KOH, 0.4 g of l-cysteine, and 0.025 g of ferric pyrophosphate. Bacto Agar and yeast extract were obtained from Difco, and all other components were from Sigma.
A salt-resistant mutant, AP1, was isolated by 10 serial passages of wild-type L. pneumophila on BCYEα-MOPS agar containing 125 mM NaCl. The salt-resistant mutant does not replicate in A. castellanii, H. vermiformis, U-937 cells, or PBMC-derived macrophages (this study). A signature-tagged derivative of the salt-resistant strain AP2 was derived as described below. Antibiotic concentrations in L. pneumophila media were 200 μg ml−1 for streptomycin and 25 μg ml−1 for kanamycin.
Growth curves for wild-type and mutant L. pneumophila were performed by recording the optical densities at 600 nm in triplicate as a function of time, and the doubling times during exponential growth were determined.
DNA manipulations.
Plasmid DNA was made by the alkaline lysis method (70) or with a QIAGEN plasmid midi kit or a QIAprep spin miniprep kit (QIAGEN) according to the manufacturer's instructions. L. pneumophila chromosomal DNA was purified by the cetyltrimethylammonium bromide method (69). Restriction enzymes and T4 DNA ligase were obtained from New England Biolabs or GibcoBRL. Transformations were performed in Escherichia coli DH5α (GibcoBRL).
PCR for signature-tagged mutagenesis and inverse PCR was carried out using Taq DNA polymerase or eLONGase enzyme mix (GibcoBRL), respectively, in a programmable thermal controller (MJ Research Inc.).
Signature-tagged mutagenesis.
The isolation of the signature-tagged library in L. pneumophila has been described (27). The library was enlarged by mating plasmid pC6HT (27) from SM10λpir (24, 77) into LpAA100jm and collecting more transconjugants. Two hundred fifty mutants were screened from this library, and 450 were screened from the already published library. As a control for the screening, pC6HT was mated into the salt-resistant strain AP1 and a salt-resistant, signature-tagged clone, AP2, was added to one 96-well plate containing the library. To screen the mutants, 96 strains were grown on BCYEα-MOPS agar for 72 h and pooled. On average, 5 × 106 CFU of input pool (multiplicity of infection [MOI] of 10:1) were used to infect a monolayer of amoebas as described below. The output pool was isolated from amoebas as described below at 72 h. Typically 106 to 108 CFU were isolated. Under these screening conditions, the hybridization signal from AP2 was reproducibly absent. All screens were performed twice.
Filters containing DNA from each of the input pools were made by transferring the strains from a 96-well plate onto a Hybond N+ filter (Amersham) on a BCYEα-MOPS agar plate containing kanamycin and streptomycin. Colonies formed after 3 days. DNA was isolated on the filter according to the manufacturer's instructions. Portions of the input pool and the output pool were used to make [α-32P]dCTP-radiolabeled tags by PCR with primers P2 (5′-TACCTACAACCTCAAGCT-3′) and P4 (5′-TACCCATTCTAACCAAGC-3′) (41) in a two-step process. In the first step chromosomal DNA was amplified in a 50-μl PCR mixture (a 1 μM concentration of primers, 0.2 μg of DNA, a 40 μM concentration of each deoxynucleoside triphosphate, 1.2 mM MgCl2) with the following PCR program: 93°C for 3 min; 30 cycles of 93°C for 1 min, 48°C for 1.5 min, and 72°C for 1 min; and an extension at 72°C for 4 min. The 80-bp PCR product was then purified using a 1.6% SeaKem GTG (BioWhittaker Molecular Applications) agarose gel. The agarose piece was melted in 1 ml of Tris-EDTA at 65°C, and 2 μl was reamplified under the same conditions except that 0.7 μM [α-32P]dCTP was used instead of dCTP. The probes were hybridized to filters made from the 96-well input pool microtiter dish. Mutant strains that hybridized to the input pool probe, but not to the output pool probes, in two experiments were chosen for further study.
Data analysis.
Each experiment described below was performed a minimum of two times. Each data point in each experiment was determined in triplicate. The figures in this work are representative single experiments performed with a single batch of cells, each with a wild-type control. Two-tailed P values were calculated using Student's t test.
Amoeba cultures.
Axenic A. castellanii, ATCC 30234 (American Type Culture Collection), was propagated at room temperature in T-75 flasks (Falcon) in 15 ml of PYG broth (57). Amoeba monolayers were formed by adding 1 ml of PYG broth containing 105 to 106 amoeba to 24-well tissue culture plates (Costar) and incubating at room temperature overnight.
Axenic H. vermiformis, ATCC 50237 (31), cultures were grown in ATCC medium 1034 (49) at 30°C in T-75 flasks. Amoeba monolayers were formed by adding 0.5 ml of medium 1034 and 0.5 ml of 1034 medium containing 5 × 104 to 105 amoebas to 24-well tissue culture plates and incubating at 37°C overnight.
L. pneumophila invasion of and replication in A. castellanii.
Wild-type or mutant strains of L. pneumophila were grown to post-exponential phase in a roller at 37°C in BYEα-MOPS. Prior to use in invasion or attachment assays, the amoeba were washed twice with A. castellanii salts (AC salts) (57) and incubated at 37°C for 30 min to induce starvation. Bacteria were added at an MOI of 10:1 to amoeba monolayers for 2 h. After the monolayers were washed two times with AC salts, gentamicin 200 μg ml−1 was added for 2 h to kill extracellular bacteria. Each well was then washed three times with AC salts, and then either 1 ml of sterile distilled water or 1 ml of PYG buffer with streptomycin (40 μg ml−1) was added. For time points after 4 h, assays were performed in PYG buffer because amoebas died when incubated in AC salts for longer than 24 h (data not shown). Monolayers were lysed with four passes through a 27-gauge needle, and L. pneumophila cells were counted by plating on BCYEα-MOPS agar. For time points after 4 h, the entire sample was transferred to a microcentrifuge tube and spun for 5 min at 15,000 × g. The pellet was resuspended in 1 ml of sterile distilled water, and the amoebas were lysed as described above.
L. pneumophila attachment to A. castellanii.
Attachment assays were performed in triplicate in the same manner as invasion assays except that all steps were carried out in the presence of cycloheximide (100 μg ml−1, Sigma). Cyclohexiamide has been shown to prevent L. pneumophila uptake by amoebas (2). Previous experiments measuring wild-type L. pneumophila attachment to A. castellanii as a function of time showed that after 30 min the percent attachment did not change (data not shown). Therefore, attachment was allowed to occur for 30 min and the monolayers were washed five times in AC salts prior to disrupting the amoeba-L. pneumophila interaction in 1 ml of sterile distilled water with a 27-gauge needle.
A. castellanii cytotoxicity assays.
Invasion assays were performed as described above using bacteria grown in BYEα-ACES at an MOI of 100:1. Prior to processing the sample, gentamicin was added at 200 μg ml−1 for 2 h to kill L. pneumophila from lysed amoeba in the extracellular media, since L. pneumophila in high concentrations can interfere with the assay (data not shown). For each sample, the monolayer and supernatant were collected and spun at 5,000 × g for 5 min. The supernatant was removed, and the cells were resuspended in 1 ml of AC salts. The 5-min spin was repeated, the supernatant was again removed, and the sample was resuspended in 100 μl of AC salts and transferred to a 96-well plate. For quantitation, an uninfected amoeba culture was added to 10 wells in duplicate in a range of concentrations. To calculate the number of amoebas per control well, an amoeba control culture cell count was determined on a hemocytometer in the presence of 1% Eosin Y (Sigma) in phosphate-buffered saline. A Celltiter 96 AQueous kit (Promega), based on a modification of the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] technology (22), was then used to develop the samples. In viable cells, MTT is reduced to insoluble blue formazan. The Celltiter 96 AQueous kit uses a modification of this compound that is soluble when reduced and can be monitored colorimetrically. The samples were read on a Microplate autoreader (Bio-tek Instruments) at 490 nm. The controls were plotted and fitted to a line. The mutants were tested by the same method except that only a 96-h time point sample was taken.
L. pneumophila invasion of and replication in H. vermiformis.
Wild-type or mutant strains of L. pneumophila were grown to post-exponential phase in a roller at 37°C in BYEα-ACES. To induce starvation, 1 ml of Pucks Saline F was added and the amoeba were incubated at 37°C for 30 min (49). Bacteria were added at an MOI of 10:1 to amoeba monolayers. Invasion was allowed to occur over a 2-h period. Gentamicin at 200 μg ml−1 was added for 2 h to kill extracellular bacteria. Each well was then washed three times with medium 1034, and then either 1 ml of sterile distilled water or 1 ml of medium 1034 was added. Monolayers were processed as described above.
L. pneumophila invasion of and replication in U-937 cells.
Tissue culture assays were performed at 37°C in a 5% CO2 incubator using RPMI 1640 with glutamine and 10% fetal bovine serum (FBS) (GibcoBRL) unless otherwise stated. U-937 cells (ATCC CRL-1593.2) (79) were differentiated with 12-myristate 13-acetate (PMA; Sigma) for 48 h (8, 61). Wild-type and mutant L. pneumophila strains were grown to post-exponential phase in BYEα-MOPS and added to differentiated U-937 monolayers in 24-well tissue culture dishes for 1 h at an MOI of 5:1. After the monolayers were washed three times with RPMI 1640, 10% FBS, gentamicin at 200 μg ml−1 was added for 2 h to kill extracellular bacteria. Each well was then washed three times with RPMI 1640–10% FBS and then either 1 ml of sterile distilled water or 1 ml of RPMI 1640–10% FBS, with streptomycin (40 μg ml−1) being added for later time points. Monolayers were processed as described above.
L. pneumophila invasion of and replication in macrophages.
PBMCs were isolated as previously described using a Ficoll gradient (Sigma) (47, 80). PBMCs were allowed to adhere to 24-well, tissue culture dishes for 24 h at 37°C in RPMI 1640 and 15% human serum (Sigma) and then were washed three times with the same medium. The monolayers were allowed to differentiate for an additional 6 days in the same medium. After the monolayers were washed once, the invasion and intracellular replication assays were carried out in RPMI 1640–10% FBS at 37°C. Wild-type and mutant L. pneumophila strains were grown to post-exponential phase in BYEα-ACES broth and added to monolayers in 24-well, tissue culture dishes for 2 h at an MOI of 1:1. Gentamicin at 200 μg ml−1 was added for 2 h to kill extracellular bacteria. Each well was then washed three times with RPMI 1640–10% FBS and then either 1 ml of sterile distilled water or 1 ml of RPMI 1640–10% FBS, with streptomycin (40 μg ml−1) added for later time points. Monolayers were processed as described above.
Sodium sensitivity.
The sodium sensitivity of wild-type L. pneumophila and mutant strains was determined by growing each of the strains to post-exponential phase and plating serial dilutions in sterile distilled water on BCYEα-MOPS and BCYEα-MOPS containing 125 mM NaCl.
Inverse PCR to obtain sequence flanking transposon insertions and further sequencing.
Inverse PCR was used to clone the 5′ end of the transposon and its junction with the L. pneumophila chromosomal sequences. Chromosomal DNA from each of the mutants was purified, and 1.5 μg was partially digested with Sau3A I or fully digested with HhaI prior to self-ligation overnight at 14°C in a total volume of 300 μl as described (59). The primers used for the inverse PCR were ISau5x (5′-AAGATCTAGAAATAGCGGCAAAAATAATACC-3′), starting at nucleotide 107 within the transposon, and IPCR3 (5′-GCAGCGTTGGGTCCTGGAGATCCTCTA-3′), starting at nucleotide 53 within the transposon (23). DNA was amplified in a 50-μl reaction mixture (a 300 nM concentration of each primer, 0.75 μg of ligated DNA, 2.2 mM MgCl2, and a 200 μM concentration of each deoxynucleoside triphosphate) using the following PCR program: 94°C for 3 min; five cycles of 94°C for 30 s, 55°C for 30 s, and 68°C for 4 min; 25 cycles of 94°C for 30 s and 68°C for 4.5 min; and finishing with an extension at 68°C for 20 min. PCR products were gel purified with a SPIN-X kit (Costar) and ligated to the TA vector pCR 2.1 (Invitrogen) or digested with XbaI prior to ligating into either pUC18 (86) or pAP128 (A. Polesky, unpublished data). Sequence was obtained using M13 universal primers and a primer within the transposon, Kan′ (5′-GCCTCTTCCGACCATCAAGC-3′), at either Protein Design Labs or the Howard Hughes PAN Facility at Stanford University. Sequences were compared to those of known genes within the NCBI combined databases by using the BLAST search program (3).
For a subset of the strains, further DNA sequence flanking the transposon insertion was obtained by subcloning from a cosmid library. The cosmid library was a kind gift from N. Cary Engleberg and is similar to the one published by J. Arroyo et al. (6). Probes were made from the library by PCR using the IPCR3 and Kan′ primers described above. PCR products were labeled with [α-32P]dCTP (Amersham) using Ready-To-Go DNA labeling beads (Amersham) according to the manufacturer's instructions. For signature-tagged mutant 2 (STM-2) and STM-6, an SstI and EcoRI cosmid fragment, respectively, was identified by Southern blot analysis (78) and subcloned into pUC18 and sequenced. Additional sequence flanking the STM-1 insertion was obtained by directly sequencing from the cosmid.
Nucleotide sequence accession numbers.
The sequences flanking the transposon insertions in STM-1, -2, -4, and -6 have been deposited in the GenBank database under the following accession numbers, respectively: AF317390, AF315463, AF315577, and AF315650.
RESULTS
Negative selection of L. pneumophila transposon mutants defective for intracellular replication.
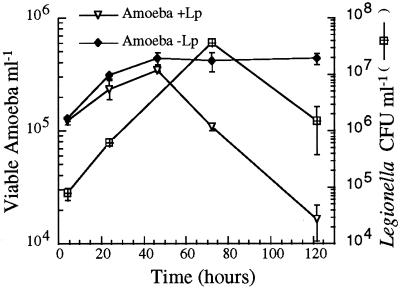
Virulent L. pneumophila is able to replicate and/or persist in free-living amoebas (4, 44, 66, 83). To determine the most ideal conditions for the identification of genes important for the ability of L. pneumophila to replicate in amoebas, we first explored the relationship between intracellular bacterial growth and host cell survival. L. pneumophila cells were allowed to invade amoebas for 2 h, and then gentamicin was added to kill extracellular bacteria. At various time points, amoeba viability was determined and intracellular bacterial CFU counts were determined. At 37°C, L. pneumophila grew well within amoebas (undergoing a 478-fold increase in number). Amoeba viability declined between 48 and 120 h postinfection (Fig. 1). Therefore, the negative selection procedure was performed at 37°C, and the output pool for the signature-tagged mutagenesis was isolated 72 h after infection, at the end of bacterial replication but before amoeba viability significantly declined.
FIG. 1.
Survival of A. castellanii and growth of intracellular L. pneumophila at 37°C. The y axis on the left represents the number of viable amoeba as a function of time in PYG broth determined by the Celltiter 96 AQueous assay. The y axis on the right represents the number of L. pneumophila CFU ml−1 as a function of time. +Lp, with L. pneumophila; −Lp, without L. pneumophila. Error bars, standard deviations.
A single input pool (one microtiter dish containing 96 unique mutant strains) was grown on BCYEα-MOPS agar for 72 h and combined. Infections of amoeba monolayers (approximately 5 × 105 cells per well) were performed at an MOI of 10:1. Average L. pneumophila invasion under these conditions was 0.3 to 1%. This MOI made it likely that each amoeba was infected with a single mutant strain and that each mutant strain was represented about 100 times. Output pools, consisting of between 106 and 108 CFU, were collected at 72 h and plated. Each mutant pool was independently screened twice to minimize the number of false-positive strains identified. [α-32P]dCTP radiolabeled tags were prepared by PCR from the input pool and both output pools for each experiment and hybridized to replica dot blots containing DNA from the input pool. From the first 700 clones screened, 12 hybridization signals were absent from both output pools, and these isolates were evaluated further.
Ability of wild-type and mutant L. pneumophila strains to invade and replicate within A. castellanii.
Each of the 12 mutants missing from the output pools was tested individually for its ability to invade and replicate in A. castellanii. Wild-type L. pneumophila and a salt-resistant mutant, selected by serial passage on salt-containing media, were used as positive and negative controls, respectively. The salt-resistant strain is avirulent in all cell types tested (see below).
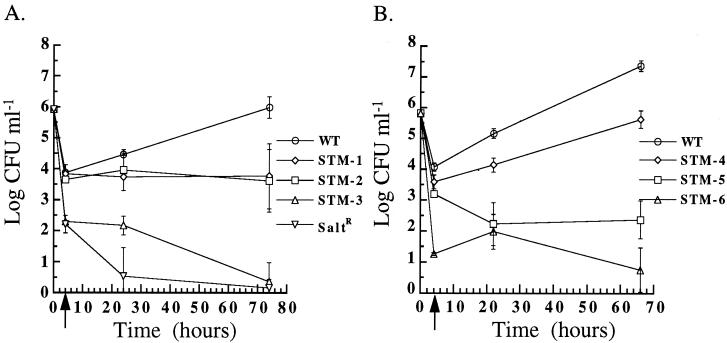
Six mutants differed significantly from the wild type in their ability to invade and grow within A. castellanii (Fig. 2). None of the six mutants had a growth defect in broth (data not shown). STM-1 and STM-2 invaded A. castellanii as efficiently as the wild type and either persisted and did not replicate or were killed at the same rate that they replicated. STM-4 entered A. castellanii at a slightly lower rate than the wild type and replicated intracellularly, but not as efficiently as the wild type, as indicated by the slope of its intracellular growth curve.
FIG. 2.
Invasion and intracellular growth of L. pneumophila strains in A. castellanii at 37°C. After growth to post-exponential phase in BYEα-MOPS, wild-type L. pneumophila (WT), the salt-resistant mutant (SaltR), and STM-1, -2, and -3 (A) and STM-4, -5, and -6 (B) were incubated for 2 h with A. castellanii. The initial time point (T = 0 h) is the log CFU milliliter−1 of input bacteria. The arrow at T = 4 h, after 2 h of gentamicin killing of extracellular bacteria, represents invasion. Subsequent time points represent intracellular replication. Error bars, standard deviations.
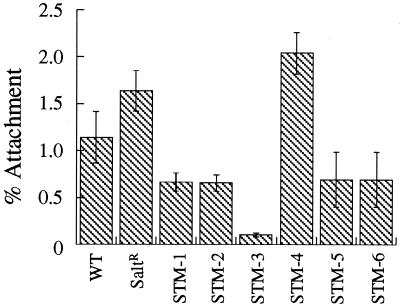
STM-3, -5, and -6 were 39.8-, 7.6-, and 778.3-fold less invasive, respectively, than the wild type (P < 0.05), as indicated by the decrease in CFU recovered at 4 h after invasion. To determine whether the defects in invasion were related to defects in adherence to A. castellanii, the mutants were tested for their ability to attach to amoebas pretreated with cycloheximide. Cycloheximide previously has been shown to prevent uptake by amoebas and subsequent intracellular replication of L. pneumophila (2). As shown in Fig. 3, STM-3 was the only mutant significantly different from the wild type, with an 8.1-fold decrease in attachment efficiency (P < 0.05). However, the 8.1-fold adherence defect observed with STM-3 probably does not fully account for its 39.8-fold decrease in invasion, suggesting an independent invasion defect. From these experiments, we cannot determine whether STM-3 and STM-6 strains were unable to enter cells or were being killed immediately after entry; experiments are under way to assess these possibilities. The number of colonies isolated for STM-3, -5, and -6 decreased as a function of time, suggesting that these strains could not survive intracellularly.
FIG. 3.
Attachment of wild-type (WT) and mutant L. pneumophila to A. castellanii in the presence of cycloheximide (100 μg ml−1). SaltR, salt resistant. Error bars, standard deviations.
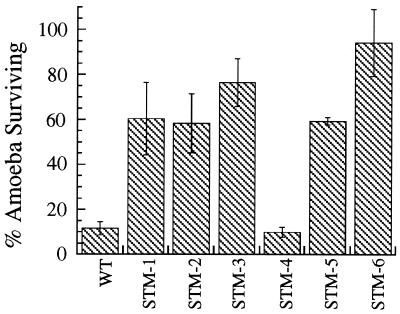
Since wild-type L. pneumophila is cytotoxic to A. castellanii, we next tested the cytotoxicity of mutant strains to amoeba cultures (Fig. 4). Ninety-six hours after infection with wild-type L. pneumophila only 11.7% of the amoebas survived, whereas 96 h after infection with STM-6 94.3% of the amoebas survived. Infection with STM-1, -2, -3, and -5 also resulted in a decreased level of cytotoxicity compared to the wild type, with 58.4 to 76.5% of the host cells surviving. STM-1, -2, -3, -5, and -6 do not replicate within A. castellanii (Fig. 2) and are cytotoxic to a lesser degree than the wild type (P < 0.05). In contrast, STM-4, which did replicate intracellularly, although at a lower rate than the wild type, killed amoebas as efficiently as the wild type did.
FIG. 4.
Cytotoxicity of L. pneumophila strains to A. castellanii. A. castellanii survival after infection with wild-type (WT) and mutant L. pneumophila strains compared to an uninfected control after 96 h. Error bars, standard deviations.
Six of the mutants tested individually in A. castellanii were not significantly different from the wild type in invasion and intracellular replication assays (data not shown). There are several possible explanations for the identification of six clones with intracellular growth characteristics similar to those of wild type. First, the strains were selected in a competition but subsequently were tested individually rather than in a mixed infection with the wild type and a single mutant (21). Second, the growth conditions prior to invasion (see below) and the growth phase of the mutants are known to affect invasion frequency (14; A. Polesky, unpublished data); for technical reasons, the initial screening was performed using plate-grown bacteria, while the testing of individual mutants was performed with bacteria grown to post-exponential phase in aerated liquid culture. Finally, these six mutants may be false positives. Given the ease of testing individual mutants within amoebas (as opposed to within animals), optimization of the pool complexity and further repetitions of the screens were not performed (19, 21, 27).
Ability of wild-type and mutant L. pneumophila strains to replicate within H. vermiformis and effect of growth media on invasion frequency.
To test whether the disrupted genes are also important in another free-living amoeba, all six mutants were next tested in H. vermiformis. Although H. vermiformis and A. castellanii are both found in potable water supplies, H. vermiformis is isolated more often at higher temperatures and has been found in water sources linked to hospital outbreaks of Legionella pneumonia (11, 32). The correlation between H. vermiformis isolation from water supplies and nosocomial Legionnaires' disease suggests that H. vermiformis may be important for Legionella spread to humans.
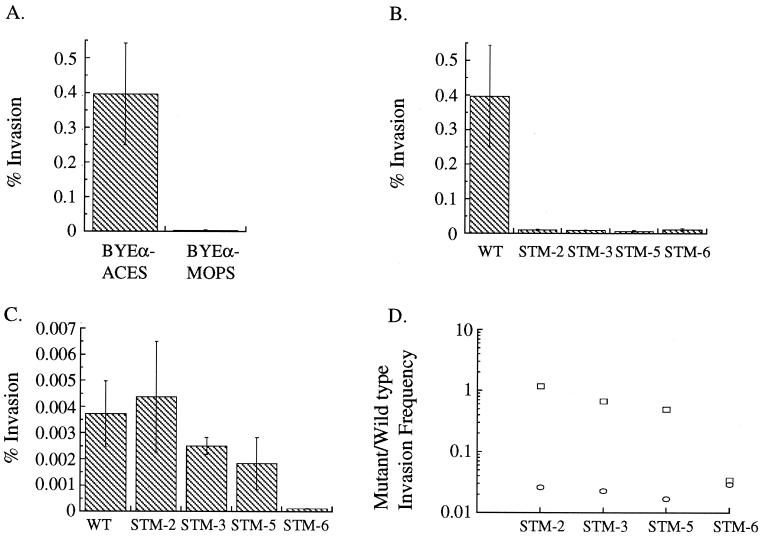
Preliminary experiments to establish conditions for assaying L. pneumophila invasion and replication in H. vermiformis showed that the invasion frequency of wild-type L. pneumophila grown in BYEα-MOPS (the medium used for previous experiments) was less than 0.1%. However, L. pneumophila grown to post-exponential phase in BYEα-ACES invaded H. vermiformis 100-fold more efficiently than L. pneumophila grown in BYEα-MOPS (Fig. 5A). Subsequent experiments have shown that growth of wild-type L. pneumophila in BYEα-ACES improved invasion frequency of all of the cell types used in this work from 10- to 100-fold (data not shown). These two media differ primarily in the buffer used and the amount of yeast extract and α-ketoglutarate present (Materials and Methods).
FIG. 5.
Wild-type and mutant L. pneumophila invasion of H. vermiformis after growth to post-exponential phase in BYEα-ACES or BYEα-MOPS broth. (A). Comparison of wild-type L. pneumophila invasion of H. vermiformis after growth in BYEα-ACES or BYEα-MOPS broth. (B) Wild-type (WT) and STM-2, -3, -5, and -6 invasion after growth in BYEα-ACES broth. (C) Wild-type (WT) and STM-2, -3, -5, and -6 invasion after growth in BYEα-MOPS broth. (D) Ratio of mutant to wild-type invasion frequencies in H. vermiformis, after growth in BYEα-ACES broth (circle) and in BYEα-MOPS broth (square). Error bars, standard deviations.
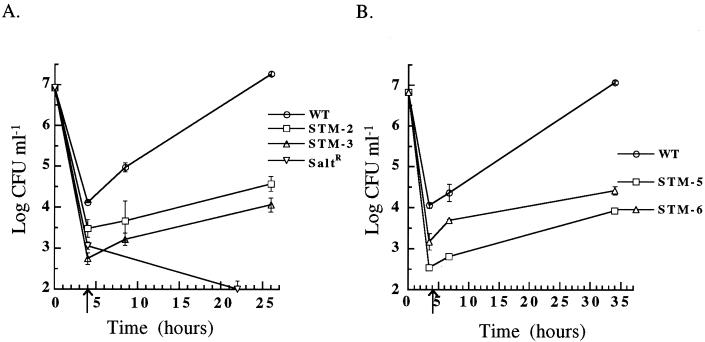
Because wild-type L. pneumophila invades H. vermiformis more efficiently after growth in BYEα-ACES, we chose to grow the STMs in BYEα-ACES to evaluate H. vermiformis invasion. The results of the experiments using H. vermiformis are shown in Fig. 5B. STM-2, -3, -5, and -6 invade H. vermiformis between 33.9- and 58.9-fold less efficiently than does the wild type. Two of the mutants, STM-2 and STM-5, have invasion deficits of greater magnitude in H. vermiformis than in A. castellanii (Fig. 2), and the invasion frequencies of STM-2, -3, -5, and -6 are significantly different from the invasion frequency of the wild type (P < 0.05).
We suspected that these additional invasion defects were a result of changing the growth medium from BYEα-MOPS to BYEα-ACES. To address this question, the H. vermiformis invasion frequencies for STM-2, -3, -5, and -6 grown to post-exponential phase in BYEα-MOPS were measured. After growth in BYEα-MOPS, the differences in invasion frequency between STM-2, -3, and -5 and the wild type diminished significantly and the invasion frequencies of STM-2, -3, and -5 were no longer significantly different from that of the wild type (Fig. 5C and d). Although the invasion frequencies of STM-2, -3, and -5 were affected by the media they were grown in, the intracellular growth rates of the mutants once internalized were unaffected (data not shown), suggesting that the small number of organisms measured are intracellular (L. pneumophila does not grow in 1034 media). When STM-2, -3, and -5 were grown in BCYEα-ACES rather than BCYEα-MOPS, a BYEα-ACES-dependent invasion deficit was also present in A. castellanii and PBMC-derived macrophages (data not shown). These data suggest that BYEα-ACES broth activates a pathway in L. pneumophila which significantly increases the ability of the wild type to invade multiple cell types and that the mutations in strains STM-2, -3, and -5 interfere with this pathway. On the other hand, STM-6 invaded H. vermiformis poorly in both types of media and has a broth-independent invasion phenotype.
Intracellular replication of the mutants was also assessed during these experiments. STM-2, -3, -5, and -6 all have a replication defect in H. vermiformis (Fig. 6). STM-1 and -4 have a <10-fold defect in H. vermiformis invasion, and the slopes of the intracellular growth curves are not significantly different from the slope of the wild-type growth curve (data not shown). These results suggest that STM-1 and STM-4 may contain insertions in genes more important for intracellular replication within A. castellanii.
FIG. 6.
Invasion and intracellular growth of L. pneumophila strains in H. vermiformis at 37°C. After growth in BYEα-ACES, wild-type L. pneumophila (WT), the salt-resistant mutant, (SaltR), and STM-2 and -3 (A) or STM-5 and -6 (B) were incubated with H. vermiformis for 2 h. The initial time point (T = 0 h) is the log CFU milliliter−1 of input bacteria. The arrow at T = 4 h, after 2 h of gentamicin killing of extracellular bacteria, represents invasion. Subsequent time points represent intracellular replication. Error bars, standard deviations.
Ability of wild-type and mutant L. pneumophila to invade and replicate within macrophage-like cell lines and PBMC-derived macrophages.
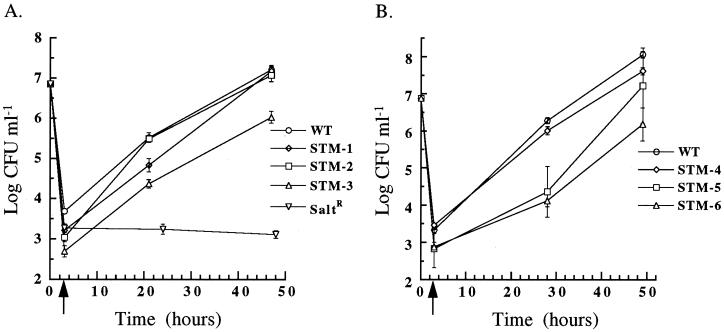
Although our main goal was to identify L. pneumophila genes important for infection of amoebas we also wanted to know if any of the mutants isolated had phenotypes in macrophages. First, the mutants were tested for their ability to invade and replicate within PMA-differentiated U-937 cells (Fig. 7), a macrophage-like cell line used previously in other laboratories to isolate L. pneumophila mutants with virulence defects (35, 36). All of the mutants replicated within U-937 cells. Those mutants unable to replicate in amoebas had only subtle defects in U-937 cells compared to the wild type. As expected, the salt-resistant control was unable to grow intracellularly.
FIG. 7.
Invasion and intracellular growth of L. pneumophila strains in PMA-differentiated U-937 cells at 37°C. After growth to post-exponential phase in BYEα-MOPS, wild-type L. pneumophila (WT) the salt-resistant mutant (saltR), and STM-1, -2, and -3 (A) or STM-4, -5 and -6 (B) were incubated with PMA-differentiated U-937 cells for 1 h. The initial time point (T = 0 h) is the log CFU milliliter−1 of input bacteria. The arrow at T = 3 h, after 2 h of gentamicin killing of extracellular bacteria, represents invasion. Subsequent time points represent intracellular replication. Error bars, standard deviations.
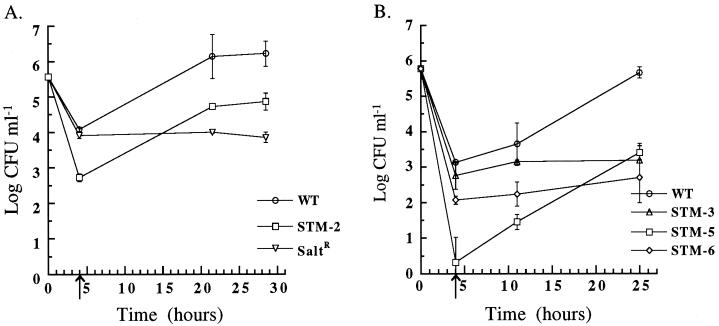
We postulated that the U-937 tissue culture cell line may be different in its response to L. pneumophila infection compared to primary macrophages. The mutants were therefore tested for their ability to invade and replicate within human macrophages derived from PBMCs (Fig. 8). In human macrophages, STM-1 and STM-4 entered and replicated as well as the wild type (data not shown), but the other four mutants did not. STM-2 and STM-5 entered between 23- and 648-fold less efficiently than the wild type (after growth in BYEα-ACES) (P < 0.05), but replicated at the same rate as wild type. STM-3 and STM-6 did not replicate effectively within macrophages. Our results indicate that PMA-differentiated U-937 cells may not be as efficient as human macrophages in killing these strains, and additional experiments in U-937 cells were not pursued.
FIG. 8.
Invasion and intracellular growth of L. pneumophila strains in PBMC-derived macrophages cells at 37°C. After growth to post-exponential phase in BYEα-ACES, wild-type L. pneumophila (WT), the salt-resistant mutant (SaltR), and STM-2 (A) or STM-3, -5, and -6 (B) were incubated with PBMC-derived macrophages for 2 h. The initial time point (T = 0 h) is the log CFU milliliter−1 of input bacteria. The arrow at T = 4 h, after 2 h of gentamicin killing of extracellular bacteria, represents invasion. Subsequent time points represent intracellular replication. Error bars, standard deviations.
Salt resistance of wild-type and mutant L. pneumophila strains.
Because some avirulent L. pneumophila mutants are salt resistant, the growth of the STMs on sodium chloride-containing media was tested (16, 68, 82). The connection between salt resistance and avirulence is important because wild-type L. pneumophila become salt sensitive during post-exponential phase, a period of growth when other L. pneumophila potential virulence characteristics are expressed (cytotoxicity and flagella) (14). To determine if any STMs were salt resistant, the percent salt-resistant bacteria was determined by plating post-exponential cultures on BCYEα-MOPS with and without 125 mM NaCl. Only 0.01% of the wild-type input was able to grow on salt-containing media, compared to 110% of the salt-resistant control. The results, presented in Table 1, indicate that only STM-6 was completely salt resistant. Interestingly, STM-4 was partially salt resistant, even though it was only mildly defective in intracellular replication assays.
TABLE 1.
Salt resistance of wild-type and mutant L. pneumophila strains
| Strain | Mean % NaCl-resistant CFUa ± SD |
|---|---|
| Wild type | 0.01 ± 0.003 |
| Salt-resistant | 110.0b ± 14 |
| STM-1 | 0.06 ± 0.01 |
| STM-2 | 0.005 ± 0.002 |
| STM-3 | 0.002 ± 0.0006 |
| STM-4 | 0.8b ± 0.1 |
| STM-5 | 0.05 ± 0.03 |
| STM-6 | 74.0b ± 17 |
Percent NaCl-resistant CFU was determined for strains grown to post-exponential phase with the following formula: (CFU on BCYEα-MOPS plus 125 mM NaCl/CFU on BCYEα-MOPS) ×100.
Statistically different from wild type (P < 0.05).
Cloning of DNA flanking the transposon insertions.
Each of the mutants was found to contain a single transposon insertion at a unique location within the L. pneumophila chromosome by Southern blot analysis (data not shown). The DNA junctions between the transposon insertions and flanking L. pneumophila DNA were cloned using inverse PCR, and 30 to 300 nucleotides of sequence was obtained for each of the six clones. Five clones (Table 2) had amino acid sequences with statistically significant similarities to sequences from other known proteins, and the L. pneumophila DNA sequence from these five PCR fragments was used to probe a cosmid library to obtain more complete sequence. STM-3 and STM-5 contain insertions within known L. pneumophila genes. The STM-3 insertion is in the lspK gene, encoding part of a type II secretion system (38). The gene disrupted in STM-5 is aroB (27). STM-1 has an insertion within a gene with amino acid similarity to the cytochrome c-type biogenesis protein, CycK (ccmF gene), of Pseudomonas fluorescens (accession no. P52225). STM-2 contains a disruption in a gene encoding a protein similar in amino acid sequence to a transcriptional activator in Vibrio cholera and Pseudomonas aeruginosa that regulates flagellar biosynthesis (51, 64). Finally, STM-6 contains an insertion in a gene showing similarity to the traA gene of Rhizobium sp. Strain NGR234 that is involved in conjugation of plasmid DNA (34).
TABLE 2.
Sequence similarities of L. pneumophila genes identified by signature-tagged mutagenesisa
| Mutant strain | Homologous gene | Organism | Putative function | % Identity | % Positive | Length (amino acids) | Score | E value |
|---|---|---|---|---|---|---|---|---|
| STM-1 | ccmF | Pseudomonas fluorescens | Cytochrome c biogenesis | 46 | 56 | 213 | 159 | 4e−38 |
| STM-2 | flrC | Vibrio cholera | Transcriptional activator of a two-component regulatory system that regulates flagella biosynthesis | 60 | 74 | 258 | 280 | 1e−74 |
| STM-3 | lspK | Legionella pneumophila | Type II secretion system structural protein | |||||
| STM-4 | Unknown (207 nucleotides) | |||||||
| STM-5 | aroB | Legionella pneumophila | Aromatic amino acid biosynthesis | |||||
| STM-6 | traA | Rhizobium sp. NGR234 | Protein involved in conjugal transfer | 36 | 56 | 271 | 166 | 1e−39 |
The values are taken from a Basic Local Alignment Search Tool for amino acid comparison (blastx program) (3). The amino acid length refers to a contiguous length of sequence similarity.
DISCUSSION
Screening of STMs in A. castellanii is a successful strategy for isolating L. pneumophila mutants attenuated in multiple cell types. The isolated mutants had a range of phenotypes affecting both invasion and intracellular replication, and a subgroup of the mutants was selectively defective within amoebas and not within PBMC-derived macrophages. During these experiments, we showed that the phenotype of mutants within a particular macrophage-like cell line may not be the same as in PBMC-derived macrophages and that different growth media significantly affect L. pneumophila invasion.
The invasion and replication phenotypes of the six mutants are summarized in Table 3. In A. castellanii, STM-3 and STM-6 were 39.8- and 778-fold less invasive than the wild type, respectively, independent of the growth medium used. All six mutants studied also had a defect in intracellular replication. A subset of the mutants tested, STM-2, -3, -5, and -6, did not replicate at the same rate (slope of the intracellular growth curve after invasion) as the wild type in the amoeba H. vermiformis. STM-1 and STM-4 grew almost as well as the wild type in H. vermiformis, suggesting that the affected genes may be more important for replication within A. castellanii. H. vermiformis appears to be less effective than A. castellanii in controlling intracellular infection with L. pneumophila because the doubling time of L. pneumophila is faster in this host and those mutants which are killed in A. castellanii (STM-3 and STM-6) can replicate at least to some extent in H. vermiformis.
TABLE 3.
Summary of the invasion and replication phenotypes of the STMs in amoeba and PBMC-derived macrophages
| Mutant strain | Invasion defecta
|
Replication defect
|
|||
|---|---|---|---|---|---|
| BYEα-MOPS (amoeba) | BYEα-ACES (multiple cell types)c | A. castellanii | H. vermiformis | PBMC-derived macrophages | |
| STM-1 | + | ||||
| STM-2 | + | + | + | ||
| STM-3 | +b | + | + | + | + |
| STM-4 | + | ||||
| STM-5 | + | + | + | ||
| STM-6 | + | + | + | + | |
Tenfold or greater difference from wild type.
Acanthamoeba castellanii only.
The cell types tested were A. castellanii, H. vermiformis, and PBMC-derived macrophages.
The phenotype of the mutants in PMA-differentiated U-937 cells was different from that in amoebas in that all of the mutants were able to replicate intracellularly. Furthermore, STM-3 and STM-6 were able to grow within U-937 cells but did not replicate well in primary macrophages, similar to the results seen in H. vermiformis. In contrast to H. vermiformis, STM-2 and STM-5, once within PBMC-derived macrophages, grow at the same rate as the wild type. Macrophage-like cell lines, compared to one another and compared to primary macrophages, control the replication of intracellular pathogens to different extents (13, 71); consequently, use of transformed cell lines may be misleading for strains with partial defects in virulence. Because PMA-differentiated U-937 cells are inefficient at killing all but the most severely affected L. pneumophila mutants, it is hard to interpret the results of experiments performed with this cell line.
The invasion frequency, relative to that of the wild type, of three mutants, STM-2, -3, and -5, was dependent upon the broth in which they were grown. This was true in all of the cell types tested. We do not yet understand which component or combination of components in the two media gives rise to this difference. Hammer et al. have shown that presence of ppGpp (a signal during amino acid starvation) is important for the expression of virulence characteristics (40). However, simply lowering the amount of yeast extract in BYEα-MOPS does not improve invasion frequency (A. Polesky and J. Ross, unpublished data), suggesting that the signal in BYEα-ACES is more complex than merely the amino acid pool of the broth. As a tool, however, this observation has allowed us to identify genes that are in some way important for the expression of these virulence traits. We are currently investigating the basis for this result, as it is likely to increase our understanding of conditions that promote L. pneumophila virulence.
In summary, six L. pneumophila mutants identified by screening a signature-tagged transposon library in A. castellanii were studied in detail in four cell types (Table 3). Two of these mutants, STM-1 and STM-4, were most defective in the host cell in which they were selected. Four of the mutants, STM-2, -3, -5, and -6, did not replicate as efficiently in the two types of amoeba tested. STM-2 and STM-5 exhibited a defect in invasion of human macrophages but, once internalized, replicated well. Thus, STM-2 and STM-5 appear to have an amoeba-specific replication defect. Two of the mutants, STM-3 and STM-6, do not replicate efficiently in amoebas or macrophages.
For each of the mutants described, we have been able to clone the region of L. pneumophila DNA containing the transposon insertion. Five sequences have amino acid similarities to other sequenced genes; the sixth sequence, flanking the STM-4 transposon insertion, has no known sequence homology. The location of four of the transposon insertions are intriguing, since they occur in genes known to be involved in virulence in L. pneumophila or in other bacterial species. Final proof of the role of each individual gene will require either complementation of the disrupted gene or replication of the phenotype in a strain with an in-frame deletion within the putative virulence gene, work that is currently in progress. Nevertheless, the gene sequence and homology information revealed varied classes of genes as important for L. pneumophila virulence.
The location of the STM-1 insertion is in a gene with similarity to the cytochrome c-type biogenesis gene, ccmF (encoding the CycK protein), of P. fluorescens (accession no. P52225). Cytochrome c-type proteins play a role in anaerobic respiration and nitrogen symbiosis (48, 53). CycK has been proposed to play a role in the covalent linkage of the heme moiety to apocytochrome c (48, 65). The function of cytochrome c-type biogenesis proteins in legionellae is unknown. Potentially, they could allow the bacteria to adapt to a more anaerobic environmental niche. It is interesting that STM-1 has a growth defect only in A. castellanii, suggesting that the intracellular milieu of A. castellanii may be different than that of H. vermiformis.
The STM-2 insertion is within a sequence with homology to transcriptional activators within two-component regulatory systems. The sequences to which the gene is most closely related are the transcriptional activators flrC, within the two-component regulatory system flrB and flrC of V. cholera, and fleS, within the two-component regulatory system fleR and fleS of P. aeruginosa. Both these systems regulate flagellar biosynthesis. In addition, fleR and fleS regulate P. aeruginosa adhesion to mucin. Both two-component systems are themselves regulated via an alternative sigma factor, ς54 (51, 64). STM-2 does not replicate well inside of amoebas and has a BYEα-ACES-dependent invasion deficit, raising the possibility that a pathway important for invasion when bacteria are grown in BYEα-ACES media may be interrupted in this mutant.
The STM-3 insertion is in the lspK gene from L. pneumophila. This gene is part of the lspFGHIJK operon, which encodes a type II secretory system (38). A deletion in the lspGH genes impaired L. pneumophila replication in A. castellanii, but not within the macrophage-like cell line HL-60 (38). In agreement with this result, STM-3 has a more subtle phenotype in U-937 cells than in amoebas. We propose that in PBMC-derived macrophages, the lspK gene product may be more important for intracellular replication than in macrophage-like cell lines.
STM-5 has an insertion within the aroB gene of L. pneumophila. The aroB gene encodes a metabolic enzyme involved in biosynthesis of aromatic amino acids. This mutation was also isolated in a guinea pig screen of L. pneumophila signature-tagged mutants using an overlapping library and is the only gene present both in the six mutants presented in this paper and the 16 identified by Edelstein et al. in their guinea pig screen of L. pneumophila STMs (27). In Salmonella enterica serovar Typhimurium, a mutation within the aroB gene leads to a strain with an attenuated phenotype in mice (37). It is thought that mutations within the aromatic amino acid synthesis pathway can lead to decreased virulence because the bacteria cannot obtain aromatic amino acids or appropriate precursors within the experimental environment (17); this is one possible explanation why STM-5 cannot replicate within amoebas but can replicate within human cells. The availability of different metabolites within amoebas and the media as well as the actual metabolic pathways within amoebas may not be the same as that in mammalian cells. Whether the BYEα-ACES-dependent invasion deficit in STM-5 is due to the mutation in aroB, a polar effect, or an impaired regulatory pathway has not been determined yet.
The STM-6 transposon insertion is located in a gene with similarity to a conjugal transfer protein gene, traA, of Rhizobium sp. strain NGR234 (34). This gene is not within the previously isolated dot-icm or lvh loci. These loci encode putative proteins with similarity to the Tra and Trb proteins of the plasmid collb-P9 and the vir secretion system of Agrobacterium tumifaciens (lvh locus), type IV secretion systems involved in DNA transfer (52, 73). In L. pneumophila, the dot-icm-encoded putative secretory system appears to be necessary for pore formation, as well as the proper trafficking of virulent L. pneumophila (50, 75). Mutations in some of the dot-icm genes also abolish conjugation of plasmids (72, 81). Like many of the strains with mutations in the dot and icm loci, STM-6 cannot replicate in amoebas and PBMC-derived macrophages. STM-6 is unable to kill A. castellanii and is salt resistant, as are many of the dot and icm mutant strains (5, 9, 63, 68, 72, 74, 82).
In summary, transposon mutagenesis of L. pneumophila and screening of signature-tagged strains in the amoeba A. castellanii have yielded mutants with an array of phenotypes in invasion and intracellular replication. Mutations within two genes appear to be A. castellanii specific; two additional mutations lead to intracellular replication defects that are amoeba-specific. It will be interesting to determine why these genes are required for intracellular replication in amoebas but not macrophages. Finally, two mutants were unable to replicate in amoebas and human macrophages and are likely to have a more global role in virulence. Of the six genes sequenced to date, one of them was contained in the 16 genes isolated by an overlapping library in guinea pigs (27). Interestingly, no dot or icm genes were identified during this pilot screening. This is likely a result of the small number of clones screened. The use of free-living amoebas to perform the selection has led to the isolation of strains with decreased virulence in amoeba as well as in vitro in PBMC-derived macrophages. The locations of the transposon insertions suggest that different classes of L. pneumophila genes are involved in virulence.
ACKNOWLEDGMENTS
This work was supported by the Parker B. Francis Fellowship to A.H.P., the Cell and Molecular Biology Training Grant to J.T.D.R., and NIH grant AI38459 to L.S.T and S.F.
We thank Paul Edelstein for the kind gift of a plasmid, pC6HT, and a portion of the STM library used in these experiments as well as advice and support. We thank N. Cary Engelberg for the cosmid library. We are grateful to Daniel Martin, Erin Gaynor, and Corrella Detweiler for advice, support, helpful discussions, and critical reading of the manuscript. We thank Sara Fisher and Edward Leonard II for their editorial assistance and John Cha for technical support.
REFERENCES
- 1.Abu Kwaik Y. The phagosome containing Legionella pneumophila within the protozoan Hartmannella vermiformis is surrounded by the rough endoplasmic reticulum. Appl Environ Microbiol. 1996;62:2022–2028. doi: 10.1128/aem.62.6.2022-2028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Kwaik Y, Fields B S, Engleberg N C. Protein expression by the protozoan Hartmannella vermiformis upon contact with its bacterial parasite Legionella pneumophila. Infect Immun. 1994;62:1860–1866. doi: 10.1128/iai.62.5.1860-1866.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 4.Anand C M, Skinner A R, Malic A, Kurtz J B. Interaction of L. pneumophila and a free living amoeba (Acanthamoeba palestinensis) J Hyg (London) 1983;91:167–178. doi: 10.1017/s0022172400060174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews H L, Vogel J P, Isberg R R. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect Immun. 1998;66:950–958. doi: 10.1128/iai.66.3.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arroyo J, Hurley M C, Wolf M, McClain M S, Eisenstein B I, Engleberg N C. Shuttle mutagenesis of Legionella pneumophila: identification of a gene associated with host cell cytopathicity. Infect Immun. 1994;62:4075–4080. doi: 10.1128/iai.62.9.4075-4080.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbaree J M, Fields B S, Feeley J C, Gorman G W, Martin W T. Isolation of protozoa from water associated with a legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. Appl Environ Microbiol. 1986;51:422–424. doi: 10.1128/aem.51.2.422-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger K H, Isberg R R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 9.Berger K H, Merriam J J, Isberg R R. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol Microbiol. 1994;14:809–822. doi: 10.1111/j.1365-2958.1994.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 10.Bozue J A, Johnson W. Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect Immun. 1996;64:668–673. doi: 10.1128/iai.64.2.668-673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breiman R F, Fields B S, Sanden G N, Volmer L, Meier A, Spika J S. Association of shower use with Legionnaires' disease. Possible role of amoeba. JAMA. 1990;263:2924–2926. [PubMed] [Google Scholar]
- 12.Brieland J, McClain M, LeGendre M, Engleberg C. Intrapulmonary Hartmannella vermiformis: a potential niche for Legionella pneumophila replication in a murine model of legionellosis. Infect Immun. 1997;65:4892–4896. doi: 10.1128/iai.65.11.4892-4896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchmeier N A, Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989;57:1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrne B, Swanson M S. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camacho L R, Ensergueix D, Perez E, Gicquel B, Guilhot C. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol Microbiol. 1999;34:257–267. doi: 10.1046/j.1365-2958.1999.01593.x. [DOI] [PubMed] [Google Scholar]
- 16.Catrenich C E, Johnson W. Virulence conversion of Legionella pneumophila: a one-way phenomenon. Infect Immun. 1988;56:3121–3125. doi: 10.1128/iai.56.12.3121-3125.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cersini A, Salvia A M, Bernardini M L. Intracellular multiplication and virulence of Shigella flexneri auxotrophic mutants. Infect Immun. 1998;66:549–557. doi: 10.1128/iai.66.2.549-557.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang S L, Mekalanos J J. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol Microbiol. 1998;27:797–805. doi: 10.1046/j.1365-2958.1998.00726.x. [DOI] [PubMed] [Google Scholar]
- 19.Chiang S L, Mekalanos J J, Holden D W. In vivo genetic analysis of bacterial virulence. Annu Rev Microbiol. 1999;53:129–154. doi: 10.1146/annurev.micro.53.1.129. [DOI] [PubMed] [Google Scholar]
- 20.Cirillo J D, Falkow S, Tompkins L S. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect Immun. 1994;62:3254–3261. doi: 10.1128/iai.62.8.3254-3261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darwin A J, Miller V L. Identification of Yersinia enterocolitica genes affecting survival in an animal host using signature-tagged transposon mutagenesis. Mol Microbiol. 1999;32:51–62. doi: 10.1046/j.1365-2958.1999.01324.x. [DOI] [PubMed] [Google Scholar]
- 22.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 23.Derbyshire K M. An IS903-based vector for transposon mutagenesis and the isolation of gene fusions. Gene. 1995;165:143–144. doi: 10.1016/0378-1119(95)00512-5. [DOI] [PubMed] [Google Scholar]
- 24.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edelstein P H. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J Clin Microbiol. 1981;14:298–303. doi: 10.1128/jcm.14.3.298-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edelstein P H, Edelstein M A. Comparison of three buffers used in the formulation of buffered charcoal yeast extract medium. J Clin Microbiol. 1993;31:3329–3330. doi: 10.1128/jcm.31.12.3329-3330.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edelstein P H, Edelstein M A, Higa F, Falkow S. Discovery of virulence genes of Legionella pneumophila by using signature tagged mutagenesis in a guinea pig pneumonia model. Proc Natl Acad Sci USA. 1999;96:8190–8195. doi: 10.1073/pnas.96.14.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engleberg N C, Drutz D J, Eisenstein B I. Cloning and expression of Legionella pneumophila antigens in Escherichia coli. Infect Immun. 1984;44:222–227. doi: 10.1128/iai.44.2.222-227.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feeley J C, Gibson R J, Gorman G W, Langford N C, Rasheed J K, Mackel D C, Baine W B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979;10:437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fettes P S, Susa M, Hacker J, Marre R. Characterization of the Legionella pneumophila gene ligA. Int J Med Microbiol. 2000;290:239–250. doi: 10.1016/S1438-4221(00)80121-6. [DOI] [PubMed] [Google Scholar]
- 31.Fields B S, Nerad T A, Sawyer T K, King C H, Barbaree J M, Martin W T, Morrill W E, Sanden G N. Characterization of an axenic strain of Hartmannella vermiformis obtained from an investigation of nosocomial legionellosis. J Protozool. 1990;37:581–583. doi: 10.1111/j.1550-7408.1990.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 32.Fields B S, Sanden G N, Barbaree J M, Morrill W E, Wadowsky R M, White E H, Feeley J C. Intracellular multiplication of Legionella pneumophila in amoebae isolated from hospital hot water tanks. Curr Microbiol. 1989;18:131–137. [Google Scholar]
- 33.Fraser D W, Tsai T R, Orenstein W, Parkin W E, Beecham H J, Sharrar R G, Harris J, Mallison G F, Martin S M, McDade J E, Shepard C C, Brachman P S. Legionnaires' disease: description of an epidemic of pneumonia. N Engl J Med. 1977;297:1189–1197. doi: 10.1056/NEJM197712012972201. [DOI] [PubMed] [Google Scholar]
- 34.Freiberg C, Fellay R, Bairoch A, Broughton W J, Rosenthal A, Perret X. Molecular basis of symbiosis between Rhizobium and legumes. Nature. 1997;387:394–401. doi: 10.1038/387394a0. [DOI] [PubMed] [Google Scholar]
- 35.Gao L Y, Harb O S, Abu Kwaik Y. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao L Y, Harb O S, Abu Kwaik Y. Identification of macrophage-specific infectivity loci (mil) of Legionella pneumophila that are not required for infectivity of protozoa. Infect Immun. 1998;66:883–892. doi: 10.1128/iai.66.3.883-892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunel-Ozcan A, Brown K A, Allen A G, Maskell D J. Salmonella typhimurium aroB mutants are attenuated in BALB/c mice. Microb Pathog. 1997;23:311–316. doi: 10.1006/mpat.1997.0157. [DOI] [PubMed] [Google Scholar]
- 38.Hales L M, Shuman H A. Legionella pneumophila contains a type II general secretion pathway required for growth in amoebae as well as for secretion of the Msp protease. Infect Immun. 1999;67:3662–3666. doi: 10.1128/iai.67.7.3662-3666.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hales L M, Shuman H A. The Legionella pneumophila rpoS gene is required for growth within Acanthamoeba castellanii. J Bacteriol. 1999;181:4879–4889. doi: 10.1128/jb.181.16.4879-4889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammer B K, Swanson M S. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol Microbiol. 1999;33:721–731. doi: 10.1046/j.1365-2958.1999.01519.x. [DOI] [PubMed] [Google Scholar]
- 41.Hensel M, Shea J E, Gleeson C, Jones M D, Dalton E, Holden D W. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 42.Hernandez F J, Kirby B D, Stanley T M, Edelstein P H. Legionnaires' disease. Postmortem pathologic findings of 20 cases. Am J Clin Pathol. 1980;73:488–495. doi: 10.1093/ajcp/73.4.488. [DOI] [PubMed] [Google Scholar]
- 43.Hoge C W, Brieman R F. Advances in the epidemiology and control of legionella infections. Epidemiol Rev. 1991;13:329–340. doi: 10.1093/oxfordjournals.epirev.a036076. [DOI] [PubMed] [Google Scholar]
- 44.Holden E P, Winkler H H, Wood D O, Leinbach E D. Intracellular growth of Legionella pneumophila within Acanthamoeba castellanii Neff. Infect Immun. 1984;45:18–24. doi: 10.1128/iai.45.1.18-24.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horwitz M A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horwitz M A, Silverstein S C. Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J Clin Investig. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson W D, Jr, Mei B, Cohn Z A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977;146:1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kereszt A, Slaska-Kiss K, Putnoky P, Banfalvi Z, Kondorosi A. The cycHJKL genes of Rhizobium meliloti involved in cytochrome c biogenesis are required for “respiratory” nitrate reduction ex planta and for nitrogen fixation during symbiosis. Mol Gen Genet. 1995;247:39–47. doi: 10.1007/BF00425819. [DOI] [PubMed] [Google Scholar]
- 49.King C H, Fields B S, Shotts E B, Jr, White E H. Effects of cytochalasin D and methylamine on intracellular growth of Legionella pneumophila in amoebae and human monocyte-like cells. Infect Immun. 1991;59:758–763. doi: 10.1128/iai.59.3.758-763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirby J E, Isberg R R. Legionnaires' disease: the pore macrophage and the legion of terror within. Trends Microbiol. 1998;6:256–258. doi: 10.1016/s0966-842x(98)01310-9. [DOI] [PubMed] [Google Scholar]
- 51.Klose K E, Mekalanos J J. Distinct roles of an alternative sigma factor during both free-swimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol Microbiol. 1998;28:501–520. doi: 10.1046/j.1365-2958.1998.00809.x. [DOI] [PubMed] [Google Scholar]
- 52.Komano T, Yoshida T, Narahara K, Furuya N. The transfer region of IncI1 plasmid R64: similarities between R64 tra and Legionella icm/dot genes. Mol Microbiol. 2000;35:1348–1359. doi: 10.1046/j.1365-2958.2000.01769.x. [DOI] [PubMed] [Google Scholar]
- 53.Kranz R, Lill R, Goldman B, Bonnard G, Merchant S. Molecular mechanisms of cytochrome c biogenesis: three distinct systems. Mol Microbiol. 1998;29:383–396. doi: 10.1046/j.1365-2958.1998.00869.x. [DOI] [PubMed] [Google Scholar]
- 54.Lowry P W, Tompkins L S. Nosocomial legionellosis: a review of pulmonary and extrapulmonary syndromes. Am J Infect Control. 1993;21:21–27. doi: 10.1016/0196-6553(93)90203-g. [DOI] [PubMed] [Google Scholar]
- 55.McDade J E, Shepard C C, Fraser D W, Tsai T R, Redus M A, Dowdle W R. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977;297:1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- 56.Mei J M, Nourbakhsh F, Ford C W, Holden D W. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol Microbiol. 1997;26:399–407. doi: 10.1046/j.1365-2958.1997.5911966.x. [DOI] [PubMed] [Google Scholar]
- 57.Moffat J F, Tompkins L S. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect Immun. 1992;60:296–301. doi: 10.1128/iai.60.1.296-301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen M H, Stout J E, Yu V L. Legionellosis. Infect Dis Clin N Am. 1991;5:561–584. [PubMed] [Google Scholar]
- 59.Pang K M, Knecht D A. Partial inverse PCR: a technique for cloning flanking sequences. BioTechniques. 1997;22:1046–1048. doi: 10.2144/97226bm07. [DOI] [PubMed] [Google Scholar]
- 60.Pasculle A W, Feeley J C, Gibson R J, Cordes L G, Myerowitz R L, Patton C M, Gorman G W, Carmack C L, Ezzell J W, Dowling J N. Pittsburgh pneumonia agent: direct isolation from human lung tissue. J Infect Dis. 1980;141:727–732. doi: 10.1093/infdis/141.6.727. [DOI] [PubMed] [Google Scholar]
- 61.Pearlman E, Jiwa A H, Engleberg N C, Eisenstein B I. Growth of Legionella pneumophila in a human macrophage-like (U937) cell line. Microb Pathog. 1988;5:87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- 62.Polissi A, Pontiggia A, Feger G, Altieri M, Mottl H, Ferrari L, Simon D. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect Immun. 1998;66:5620–5629. doi: 10.1128/iai.66.12.5620-5629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Purcell M, Shuman H A. The Legionella pneumophila icmGCDJBF genes are required for killing of human macrophages. Infect Immun. 1998;66:2245–2255. doi: 10.1128/iai.66.5.2245-2255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ritchings B W, Almira E C, Lory S, Ramphal R. Cloning and phenotypic characterization of fleS and fleR, new response regulators of Pseudomonas aeruginosa which regulate motility and adhesion to mucin. Infect Immun. 1995;63:4868–4876. doi: 10.1128/iai.63.12.4868-4876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ritz D, Thony-Meyer L, Hennecke H. The cycHJKL gene cluster plays an essential role in the biogenesis of c-type cytochromes in Bradyrhizobium japonicum. Mol Gen Genet. 1995;247:27–38. doi: 10.1007/BF00425818. [DOI] [PubMed] [Google Scholar]
- 66.Rowbotham T J. Current views on the relationships between amoebae, legionellae and man. Isr J Med Sci. 1986;22:678–689. [PubMed] [Google Scholar]
- 67.Roy C R, Berger K H, Isberg R R. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- 68.Sadosky A B, Wiater L A, Shuman H A. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun. 1993;61:5361–5373. doi: 10.1128/iai.61.12.5361-5373.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sal G D, Manfioletti G, Schneider C. The CTAB-DNA precipitation method: a common mini-scale preparation of template DNA from phagemids, phages or plasmids suitable for sequencing. BioTechniques. 1989;7:514–519. [PubMed] [Google Scholar]
- 70.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 71.Schwan W R, Huang X Z, Hu L, Kopecko D J. Differential bacterial survival, replication, and apoptosis-inducing ability of Salmonella serovars within human and murine macrophages. Infect Immun. 2000;68:1005–1013. doi: 10.1128/iai.68.3.1005-1013.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Segal G, Purcell M, Shuman H A. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc Natl Acad Sci USA. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Segal G, Russo J J, Shuman H A. Relationships between a new type IV secretion system and the icm/dot virulence system of Legionella pneumophila. Mol Microbiol. 1999;34:799–809. doi: 10.1046/j.1365-2958.1999.01642.x. [DOI] [PubMed] [Google Scholar]
- 74.Segal G, Shuman H A. Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect Immun. 1997;65:5057–5066. doi: 10.1128/iai.65.12.5057-5066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Segal G, Shuman H A. How is the intracellular fate of the Legionella pneumophila phagosome determined? Trends Microbiol. 1998;6:253–235. doi: 10.1016/s0966-842x(98)01308-0. [DOI] [PubMed] [Google Scholar]
- 76.Segal G, Shuman H A. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect Immun. 1999;67:2117–2124. doi: 10.1128/iai.67.5.2117-2124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 78.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 79.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 80.Swanson M S, Isberg R R. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vogel J P, Andrews H L, Wong S K, Isberg R R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 82.Vogel J P, Roy C, Isberg R R. Use of salt to isolate Legionella pneumophila mutants unable to replicate in macrophages. Ann NY Acad Sci. 1996;797:271–272. doi: 10.1111/j.1749-6632.1996.tb52975.x. [DOI] [PubMed] [Google Scholar]
- 83.Wadowsky R M, Butler L J, Cook M K, Verma S M, Paul M A, Fields B S, Keleti G, Sykora J L, Yee R B. Growth-supporting activity for Legionella pneumophila in tap water cultures and implication of hartmannellid amoebae as growth factors. Appl Environ Microbiol. 1988;54:2677–2682. doi: 10.1128/aem.54.11.2677-2682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wiater L A, Dunn K, Maxfield F R, Shuman H A. Early events in phagosome establishment are required for intracellular survival of Legionella pneumophila. Infect Immun. 1998;66:4450–4460. doi: 10.1128/iai.66.9.4450-4460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Winn W C, Jr, Myerowitz R L. The pathology of the Legionella pneumonias. A review of 74 cases and the literature. Hum Pathol. 1981;12:401–422. doi: 10.1016/s0046-8177(81)80021-4. [DOI] [PubMed] [Google Scholar]
- 86.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]