Abstract
OBJECTIVE
To assess the efficacy of the insulin pen cap Insulclock on improving glycemic control, treatment adherence, and user satisfaction in people with type 1 diabetes.
RESEARCH DESIGN AND METHODS
This multicenter, open-label, randomized controlled trial comprised a 4-week run-in phase and a 6-week double-arm phase in which participants were randomly assigned into an active or masked mode.
RESULTS
Fifty-five participants were evaluable (active group, n = 26, masked group, n = 29). The increase in time in range was higher in the active versus masked group (5.2% vs. −0.8%; P = 0.016). The active group showed a higher reduction in mean glucose, glucose management indicator, time above range, and high blood glucose index. On-time insulin doses increased in the active group and decreased in the masked group.
CONCLUSIONS
Insulclock system use was associated with improved glycemic control, glycemic variability, hyperglycemia risk, and treatment adherence in people with uncontrolled type 1 diabetes.
Introduction
Insulclock is a small insulin pen cap that records the dose, time, duration, and temperature of insulin injections (1). The Insulclock system obtains continuous glucose monitoring (CGM) and glucometer data and integrates this information with insulin injection information in its application (app). The aim of this study was to assess the efficacy of this digital system on improving glycemic control, treatment adherence, and quality of life in patients with type 1 diabetes.
Research Design and Methods
Design
This multicenter, open-label, randomized controlled trial was conducted at four Spanish centers. The study was approved by the ethics review committees of the recruiting hospitals. The study followed the ethical principles of the Declaration of Helsinki. Each participant provided written informed consent before inclusion in the study.
The study comprised a 4-week run-in phase and a 6-week double-arm phase scheduled over seven visits. In the run-in phase, participants were trained on using the device and app in a masked mode. In the double-arm phase, participants in the active group had access to all the Insulclock system functionalities, such as insulin reminders and CGM, with insulin dose information integrated in the app. All patients were using the FreeStyle Libre 2 CGM (FGM) as usual diabetes care and continued using it during the study with all its functionalities, including alarms.
Population
We included participants aged 14–80 years with uncontrolled type 1 diabetes (HbA1c ≥6.5% and/or HbA1c variations ≥1% within the previous 2 years) and regularly attending (≥2 per year) follow-up visits at the endocrinology department. Exclusion criteria were pregnancy or lactation, history or current alcohol or drug abuse, dementia diagnosis, acute infection, inability to give informed consent or to use study devices, or any medical condition that could interfere with the study’s procedures.
Outcomes
The study compared differences between the active and masked groups during the double-arm phase in glycemic control and variability by assessing the following variables: time in range (TIR) 70–180 mg/dL (3.9–10.0 mmol/L) (2) (primary objective), glucose management indicator (GMI), time above range (TAR), time below range, mean amplitude of glycemic excursions, SD, coefficient of variation, high blood glucose index (HGBI), and low blood glucose index.
The glucose rate increase detector algorithm was used to automatically detect meal glucose excursions through the rate of change of glucose from FGM data (3). An on-time insulin injection was considered when a bolus insulin injection was detected within 45 min before the glycemic meal excursion. Meal-time insulin doses not detected by the glucose rate of change methodology were categorized as on time. A bolus was deemed mistimed when the insulin injection occurred within 60 min after the meal glycemic excursion start. A bolus was considered missed when the meal excursion was not associated with an insulin injection 45 min before to 60 min after the FGM rise.
Statistical Analyses
Statistical analyses were performed using SPSS version 17.0 software (IBM Corporation, Chicago, IL). The magnitude of group differences was calculated with Cohen d, with an effect size >0.2 considered small, ≥0.50 to <0.80 moderate, and ≥0.80 large. Based on our pilot study (4) and assuming a 30% dropout rate, 75 individuals were required to obtain data from 51 participants (26 per arm) with a 5% type I (α) error.
Results
Seventy-five participants were randomly assigned, and data from 55 were evaluable (active group, n = 26; masked group, n = 29; mean age 40.9 years) (Supplementary Table 1). None of the variables assessed showed differences between groups at baseline or randomization. However, a different dropout rate in both groups during the double-arm phase affected the glycemic profile of the final evaluable participants. Patients who completed the study in the active group (evaluable participants) had worse glycemic control at randomization but a more significant improvement during the double-arm phase than those in the masked group. Reasons for discontinuation in these 20 participants are described in Supplementary Fig. 1.
Glycemic Control
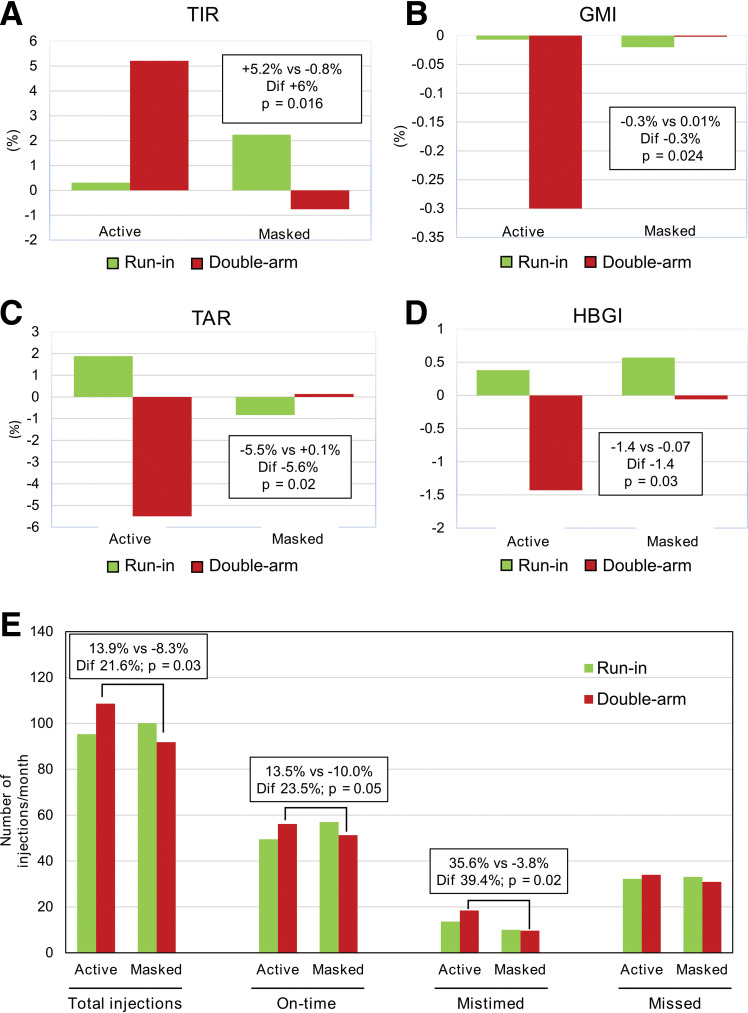
The TIR increased by 5.2% in the active group and decreased by 0.8% in the masked group (6%, P = 0.016, Cohen d = 0.67) (Fig. 1). Mean glucose levels decreased in the active group and remained stable in the masked group (−8.7 mg/dL, P = 0.024, Cohen d = −0.63). Differences between groups in GMI were significant (−0.31%, P = 0.024, Cohen d = −0.63). The TAR decreased by 5.5% in the active group and increased by 0.13% in the masked group (−5.6%, P = 0.018, Cohen d = −0.66). A significantly higher decrease in HBGI was observed in the active group compared with the masked group (−1.4, P = 0.029, Cohen d = −0.61) (Fig. 1 and Supplementary Figs. 2–3). Participants in both groups showed comparable data describing FGM use (active time and FGM readings) both in the run-in and double-arm phases.
Figure 1.
Effect of Insulclock on glycemic control and treatment adherence. A–D: Graphs show the mean change from baseline to randomization (green) and from randomization to final double-arm phase (red) in the final evaluable participants. E: Bar graphs show the number of insulin injections taken per month, including total injections, on-time injections, mistimed injections, and missed injections. Dif, differences between the active and masked groups in change during the double-arm phase.
Adherence
The total number of insulin injections and insulin doses taken on time per month increased in the active group (13.9% and 13.5%, respectively) and decreased in the masked group (−8.3% [P = 0.029, Cohen d = 0.66] and −10.0% [P = 0.046, Cohen d = 0.60], respectively). An increase in mis-timed insulin doses per month was observed in the active group versus a similar number in the masked group (P = 0.017, Cohen d = 0.73) (Fig. 1).
Quality of Life
Participants in the active group stated that the Insulclock system reduced their treatment burden. Seven Insulin Treatment Satisfaction Questionnaire items worsened in the masked group and two in the active group (Supplementary Fig. 4).
Conclusions
The 6% increase in TIR found in our study is similar to the 8% shown in our pilot study (4). An observational study with the connected insulin pen NovoPen 6 showed an 8% improvement in TIR (5). Interestingly, the 6% TIR increase associated with a 0.3% GMI decrease found in our study is considered clinically relevant (6).
Active Insulclock was associated with improvements in the glycemic variability glucose metrics TIR, TAR, and HBGI and numerical reductions in SD and mean amplitude of glycemic excursions. These findings may indicate that Insulclock mainly contributes to avoiding hyperglycemic excursions because it improves insulin injection adherence, which directly reflects on postprandial control.
Using the functionalities of Insulclock resulted in a 13.9% increase in the total number of insulin injections in the active group mainly due to an improvement in the doses taken on time (13.5%). The increased number of mistimed doses in this group probably reflects a rescue of otherwise lost injections.
Insulin Treatment Satisfaction Questionnaire items related to hypoglycemia control were the most considerably improved, as observed in the pilot study (4). Notably, the improvement in treatment burden perception indicates that although using the device is an additional task for participants, they felt it eased their daily life.
There was a considerable dropout rate. A high study dropout rate is frequent in people with uncontrolled type 1 diabetes and in studies assessing technology research. Despite that variables showed similar values between groups before randomization, the final evaluable participants had worse glycemic control in those completing the double-arm phase in the active group. They probably appreciated more the balance task/benefits of using the device. Therefore, the study’s main limitations are the reduced sample size and follow-up period and the considerable dropout rate.
In conclusion, this randomized controlled trial is the first multicenter study to report a significant improvement in glycemic control and adherence in people with type 1 diabetes using an insulin pen cap and app. These encouraging results should be confirmed by further research.
Article Information
Acknowledgments. The authors acknowledge the editorial assistance of Carla Granados, Trialance SCCL.
Funding. This study was funded by the European Commission (grant 739148) and Insulcloud, S.L., which provided the Insulclock devices and telemedicine system tested. Medical writing and statistical assistance were funded by the European Union Horizon 2020 (project identifier 674505).
Duality of Interest. F.G.-P. has taken part in advisory panels for Insulcloud S.L., Sanofi, and Novo Nordisk; has received research support from Sanofi, Novo Nordisk, Boehringer Ingelheim, and EliLilly; and has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, and Eli Lilly. C.A. has received research support from Sanofi, Novo Nordisk, Boehringer Ingelheim, and Eli Lilly and has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim, AstraZeneca, and Bristol-Myers Squibb. E.F.-R. has acted as a speaker for Novo Nordisk, Eli Lilly, and AstraZeneca. L.C. has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim, AstraZeneca, and Eli Lilly. P.P. has received research support from Novo Nordisk and has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim, AstraZeneca, and Eli Lilly. D.B. has received research support from and acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim, and Eli Lilly. E.M.T. has received research support from Sanofi and Novo Nordisk and has acted as a speaker for Sanofi, Novo Nordisk, and Eli Lilly. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. F.G.-P. designed and supervised the study, researched data, and wrote the manuscript. C.A., E.F.-R., L.C., P.P., S.G., D.B., and E.M.T. researched data and reviewed the manuscript. S.R.-V., H.B., X.V., J.P.-G., and L.R.-V. analyzed data and reviewed the manuscript. F.G.-P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 15th International Conference on Advanced Technologies & Treatments for Diabetes, Barcelona, Spain, 27–30 April 2022.
Footnotes
Clinical trial reg. no. NCT04847778; clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.21397506.
References
- 1. Gomez-Peralta F, Abreu C, Gomez-Rodriguez S, Ruiz L. Insulclock: a novel insulin delivery optimization and tracking system. Diabetes Technol Ther 2019;21:209–214 [DOI] [PubMed] [Google Scholar]
- 2. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harvey RA, Dassau E, Zisser H, Seborg DE, Doyle FJ 3rd. Design of the glucose rate increase detector: a meal detection module for the health monitoring system. J Diabetes Sci Technol 2014;8:307–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gomez-Peralta F, Abreu C, Gomez-Rodriguez S, et al. Efficacy of Insulclock in patients with poorly controlled type 1 diabetes mellitus: a pilot, randomized clinical trial. Diabetes Technol Ther 2020;22:686–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adolfsson P, Hartvig NV, Kaas A, Møller JB, Hellman J. Increased time in range and fewer missed bolus injections after introduction of a smart connected insulin pen. Diabetes Technol Ther 2020;22:709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol 2019;13:614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]