Abstract
Clostridium difficile is a major cause of nosocomial diarrhea in industrialized countries. Although most illnesses respond to available therapy, infection can increase morbidity, prolong hospitalization, and produce life-threatening colitis. Vaccines are being explored as an alternative means for protecting high-risk individuals. We assessed the safety, immunogenicity, and dose response of a parenteral vaccine containing C. difficile toxoids A and B. Thirty healthy adults were assigned to receive four spaced inoculations on days 1, 8, 30, and 60 with one of three doses of vaccine (6.25, 25, or 100 μg). At each dose level, subjects were randomized, in a double-blind fashion, to receive either the soluble toxoids (n = 5) or toxoids adsorbed to alum (n = 5). Subjects were monitored for clinical and immunologic responses to vaccination. Vaccination was generally well tolerated, with occasional, usually mild, systemic reactions (abdominal pain, arthralgia, and diarrhea). The most common local reaction, mild arm pain, was reported by all recipients of the toxoid-alum formulation. Nearly all subjects (≥90%) developed vigorous serum antibody responses to both toxins, as measured by immunoglobulin G (IgG) enzyme-linked immunosorbent assay and neutralization of cytotoxicity, whereas fecal IgA increases occurred in approximately 50%. Statistically significant effects of dose and formulation on immunogenicity were not seen, although antibody levels tended to be higher with the alum-adjuvanted formulations and with increasing doses of soluble toxoid. Serum antibody responses among the toxoid-alum group appeared to plateau at 25 μg. We concluded that the C. difficile toxoid vaccine is safe and immunogenic in healthy volunteers. Further development as a prophylactic vaccine or for producing C. difficile hyperimmune globulin is justified.
Clostridium difficile is the major infectious cause of nosocomial diarrhea in industrialized countries (1, 24) and a well-recognized source of recalcitrant diarrheal outbreaks, particularly affecting units for the elderly (26) and immunocompromised (4). In a recent study, it was found that 17% of patients who are hospitalized for 2 or more days and receive antibiotics will develop C. difficile-associated diarrhea (CDAD) (19). Reports from several countries suggest that the incidence of CDAD has been rising in recent years (10, 38). Although most illnesses resolve with discontinuation of antibiotic therapy and provision of specific antibiotic treatment, these infections may prolong hospitalization (14), inflate medical costs (28, 31), increase morbidity (21), and produce severe, life-threatening colitis (11). Furthermore, there are drawbacks to antimicrobial treatment for CDAD, including secondary infection with multiresistant organisms (29) and clinical relapse, which occurs in 5 to 30% of patients after antibiotic therapy is discontinued (9, 25). Innovative approaches for prevention and treatment are thus needed, particularly those that reduce the need for antibiotic therapy (9).
The pathogenesis of CDAD is mediated by the actions of two protein exotoxins, toxin A and toxin B, which induce mucosal injury and inflammation of the colon (17, 27). Strategies that provide antibody-mediated antitoxic immunity are being explored. An effective vaccine could be used to elicit protective immunity in individuals at high risk for CDAD or to produce hyperimmune globulin preparations as a means for passive immunoprophylaxis or treatment. Herein we report the results of a phase 1 study to assess the safety, immunogenicity, and dose response, in healthy adults, of a parenteral vaccine containing C. difficile toxoids A and B with and without alum adjuvant.
MATERIALS AND METHODS
Vaccine manufacture and administration.
The formalin-inactivated toxoid A and B vaccine was produced using good manufacturing practices at the Centre for Applied Microbiological Research, Porton Down, United Kingdom. Anaerobic cultures of C. difficile (strain ATCC 43255) were grown in dialysis sacs suspended in growth medium. Viable C. difficile and spores were removed from pooled sacs by centrifugation followed by submicron filtration, the filtrate was concentrated and diafiltered, and then the toxin was precipitated at 4°C with 60% ammonium sulfate and the pellets were frozen. At Forest Glen (building 501), Walter Reed Army Institute of Research, the pellets were dissolved in phosphate buffer and applied to an S300 Sephacryl size exclusion column. The peak containing both toxin A and toxin B was collected, concentrated, and then inactivated for 18 days with formaldehyde at 4.25 mg/ml at 4 to 6°C in a solution containing lysine at 4.25 mg/ml. The inactivation period exceeds three times the interval needed for complete elimination of in vivo biological activity (lethality in mice). After formalin treatment, activity in the IMR90 cytopathology cell culture assay was at the threshold of detectability (cyotoxicity reduced by 8 to 9 log10). The formaldehyde concentration was then reduced by diafiltration to 0.016%, thus retaining a low concentration to prevent toxoid reversion.
The final product (lot no. 98F03) was used to fill glass vials at a volume of 0.6 ml and stored at 2 to 8°C. The total protein concentration was 0.52 mg/ml, of which toxins A and B comprised about 44% (by gel densitometry) at a 1.5:1 toxin A to toxin B ratio (as measured by capture enzyme-linked immunosorbent assay [ELISA]). The final product passed safety tests developed at OraVax (Cambridge, Mass.) for mouse toxicity (0.5-mg intraperitoneal dose) and cytotoxicity (up to 0.25 mg/ml) in IMR90 cells. In addition, no toxicity occurred when hamsters were injected with four intramuscular (i.m.) doses of 500 μg/kg (with and without alum) or when cynomolgus monkeys were given either a single i.m. dose of 20 μg/kg (with and without alum) or a single i.m. dose of 2,700 μg/kg without alum. The potency of the final product was tested by inoculating, via intraperitoneal injection on days 1 and 7, groups of eight 10- to 12-week-old mice, using graded doses of toxoid adsorbed to alum. Mice were bled on day 14 to determine the 50% effective dose, which was defined as the toxoid dose that elicited a toxin A and B neutralizing antibody titer of ≥1:25 (a titer that protects mice against intraperitoneal challenge with 10 50% lethal doses of toxins A and B). The vaccine lot had a 50% effective dose comparable to that of a reference control (a preclinical lot of vaccine manufactured by the same process as the clinical lot). Using this reference control lot, the stability of the toxoid state at 37°C was verified by the IMR90 cytotoxicity assay and mouse toxicity for 3 months and, in a second series of studies, by the IMR90 cytotoxicity assay for 300 days at 4, 28, and 37°C. The clinical protocol was conducted under an approved investigational new drug application (BB-IND 7932) filed with the U.S. Food and Drug Administration.
On the morning of vaccination, the investigator performed serial dilutions using phosphate-buffered saline (PBS) with 0.016% formaldehyde (pH 7.4) to achieve the desired dose in 0.5 ml. The final dose (6.25, 25, or 100 μg) represented the total protein content, of which toxoids A and B represented 44%, at a ratio of 1.5 to 1. Rehydragel aluminum hydroxide adjuvant (Reheis; 723 to 757 μg) was added to half of the vaccine doses as specified by the randomization scheme described below. Vaccine was administered by an unblinded nurse who was not involved in subsequent clinical assessment of volunteers.
Recruitment, study design, and clinical evaluation. (i) Recruitment and medical screening.
Healthy adults 18 to 50 years of age were recruited from the Baltimore-Washington community and the University of Maryland College Park campus. After medical history determination, physical examination, and a battery of laboratory tests, individuals meeting the following criteria were excluded: significant medical or psychiatric abnormalities, a history of gastrointestinal disease including previous antibiotic-associated diarrhea, a positive fecal C. difficile stool cytotoxin assay, antibiotic therapy during the previous month, and failure to achieve a passing grade (70%) on a written examination which tested the volunteer's comprehension of the trial. Women were required to have a negative pregnancy test within 7 days of each vaccination. Volunteers who satisfied all eligibility requirements and provided informed, written consent in accordance with the guidelines of the Institutional Review Boards at the University of Maryland in Baltimore and in College Park were enrolled in the study.
(ii) Allocation and vaccination procedures.
Volunteers were sequentially assigned, in groups of 10, to receive one of three increasing doses of vaccine (6.25, 25, or 100 μg) as shown in Table 1. Five subjects at each dose level were randomized, in a double-blind fashion, to receive soluble toxoid vaccine, and five subjects were randomized to receive an equivalent dose of toxoid adsorbed to alum. The regimen consisted of four inoculations administered by i.m. injection into the deltoid muscle (alternating arms) on study days 1, 8, 30, and 60.
TABLE 1.
Study designa
| Cohort no. | Dose of toxoid (μg) | Randomized assignment | No. of subjects |
|---|---|---|---|
| 1 | 6.25 | Toxoid | 5 |
| Toxoid-alum formulationb | 5 | ||
| 2 | 25 | Toxoid | 5 |
| Toxoid-alum formulationb | 5 | ||
| 3 | 100 | Toxoid | 5 |
| Toxoid-alum formulationb | 5 |
Vaccine was administered as an i.m. injection into the deltoid muscles of alternating arms on days 1, 8, 30, and 60.
723 to 757 μg of alum.
To increase the margin of safety, initial exposure to each dose of vaccine was limited by vaccinating two subjects in each cohort 1 week before the remaining eight subjects. While maintaining the double-blind condition; one subject received the soluble toxoid and the other received toxoid with alum. These two subjects were observed for 1 week for clinical tolerance before the remaining eight subjects were inoculated at that dose level.
(iii) Clinical monitoring.
Subjects were monitored at the study site for 30 min after receiving vaccine to detect immediate reactions. For 7 days after each inoculation, they kept a diary in which they recorded their evening oral temperature; the time, date, and consistency (loose or formed) of all stools passed; and the following signs and symptoms: anorexia, malaise, joint swelling, numbness or tingling in fingers or toes, rash, abdominal pain, joint pain, injection site pain, and other, unsolicited complaints. Subjects graded their pain as mild (aware of symptom but symptom easily tolerated), moderate (symptom interferes with usual activity), or severe (symptom prevents work or ability to perform usual activity). In addition, a medical history was obtained at each visit to the study site and by telephone interview 3 days after each inoculation. A panel of clinical laboratory tests (complete blood count with differential and platelet counts, creatinine, alanine aminotransferase, and urinalysis) was performed 1 week after each inoculation.
Study personnel inspected the injection site 1 and 7 days after each inoculation for the presence of local reactions (swelling, erythema, or heat). Swelling and erythema were graded (maximum diameter) as mild (≤4.0 cm), moderate (5.0 to 6.0 cm), or severe (≥7.0 cm).
Laboratory methods. (i) ASC responses.
Peripheral blood mononuclear cells (PBMC) were collected before and 7 days after administration of each dose of vaccine to measure circulating immunoglobulin A (IgA) and IgG antibody-secreting cells (ASC) recognizing toxin A and toxin B, by adapting a previously described ELISPOT assay (33). Briefly, microdilution plates were coated with either toxin A (5 μg/ml) or B (1 μg/ml) antigen (lots VC011199 and 06986006, respectively, obtained from culture filtrates, highly purified by anion-exchange chromatography, and supplied by OraVax). Samples from each subject at each time point were run at initial concentrations of 2.5 × 105 PBMC/well and at dilutions of 1:10 and 1:100 for each immunoglobulin isotype and antigen, in quadruplicate. Negative controls (uncoated plates) were run for each subject and assay, and the values were subtracted from the observed response. Positive controls (antitoxin A IgG2a-producing PCG-4 mouse hybridoma, lot 8/26/98, obtained from OraVax) were run on each day of testing. A positive ELISPOT response was defined as a geometric mean postvaccination count per 106 PBMC ≥3 standard deviations above the mean prevaccination count, in the log metric. Actual cutoff values designating a positive response for antitoxin A were 3.7 (IgA) and 1.2 (IgG) ASC per 106 PBMC, and for antitoxin B they were 0.9 (IgA) and 0 (IgG) ASC per 106 PBMC.
(ii) Serum antibody responses.
Sera were collected prevaccination and 8, 15, 30, 37, 60, 67, and 90 days after immunization and tested for antibodies to toxins A and B by ELISA and by neutralization assay. To detect neutralizing antibody, IMR90 cells were seeded 1 day prior to use into microtiter plates at 1.8 × 104 cells/well in Eagle minimum essential medium (EMEM) supplemented with 10% heat-inactivated fetal calf serum and 1% l-glutamine (EMEM-10). The toxins were diluted to eight times their 50% cytotoxicity titer for use in the assay. An equal volume of serum, diluted 1:5 in triplicate using EMEM-10 and titrated by serial twofold dilutions, was added to the diluted toxin and placed at 37°C for 1 h. Medium was then removed from plates and replaced with 100 ml of the serum-toxin mixture. The cell plates containing the serum-toxin mixtures, along with positive and negative controls, were incubated for 16 to 20 h in a 37°C, 5% CO2 incubator. The percentage of cells rounded was scored as 0 to 4 (0 = 0 to 10% of cells rounded, 1 = 11 to 39% rounded, 2 = 40 to 60% rounded, 3 = 61 to 89% rounded, and 4 = 90 to 100% rounded). The final data were reported as the 50% neutralizing titer (highest serum dilution at which the rounding score was ≥2). Seroconversion was defined as a fourfold or greater rise in titer following vaccination.
To detect specific IgA and IgG by ELISA, plates were coated with toxin antigens as described above for the ELISPOT assay, stored overnight at 4°C, washed with PBS, and then blocked using PBS with 5% fetal bovine serum (FBS). Eight serial twofold dilutions of the following were run, in duplicate, in PBS-Tween with 1% FBS: each serum specimen starting at 1:100, control wells with no antigen, and the IgG and IgA reference standards starting at 1:200 for the IgG reference (IVIG [Baxter, Deerfield, Ill.], containing anti-toxin A and B antibody at 50,000 ELISA units [EU]/ml) and the IgA reference (IgAbulin [OraVax], containing anti-toxin A and B antibody at 100,000 EU/ml). After incubation and washing, alkaline phosphatase-labeled goat anti-human IgG or IgA antibody (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) diluted in PBS with 1% FBS was added. The plates were incubated and washed, substrate was added, and the standard optical density at 500 EU/ml was monitored. The reaction was stopped when the IgG or IgA reference reached maximum antibody binding as indicated by predetermined maximal absorbance values of the 1:100 and 1:200 dilutions of the IgG and IgA standards. Serum samples were read at a dilution corresponding to the linear portion of the run's reference curve after it was ascertained that the run's reference curve and the standard reference curve absorbance values had a correlation, r, of ≥0.9. Limits of detection of anti-toxin A and anti-toxin B IgG were 31.25 and 62.5 EU/ml, respectively; limits of detection of anti-toxin A and anti-toxin B IgA were 7.81 and 15.62 EU/ml. Seroconversion was defined as a fourfold or greater rise in ELISA units following vaccination.
(iii) Fecal antibody responses.
Specific fecal IgA antibody responses were measured before and 7 days after each dose of vaccine by ELISA using previously described assays adapted for toxin A and B antigens (22). Briefly, volunteers submitted a fresh (<24 h old) stool sample, which they maintained in an insulated bag containing a cold pack. A 10% stool supernatant containing protease inhibitors was assayed for total IgA using a reference standard of purified IgA from human colostrum and then standardized to 2 mg% total IgA to ensure that specific antibody would be detected at levels of ≥0.25% of the total IgA. The anti-toxin A- and B-specific IgA ELISAs were performed as described above for serum IgA, except that the reference standard used was the colostrum IgA reference curve and results were reported as nanograms of specific antibody per milliliter. This method of quantifying antibodies is based on the equivalence of absorbances between a reference ELISA and anti-toxin ELISA performed in parallel under identical laboratory conditions using the same buffers, temperatures, incubations, enzyme conjugates, and reagent dilutions (39). A response was defined as a fourfold increase in titer postvaccination.
Statistical methods.
One-tailed Fisher's exact tests were performed to determine whether alum increased the proportion of subjects (in each randomized group of five) that experienced systemic or local reactions; linear regression analyses were used to assess whether alum amplified the severity of these reactions. Analyses were performed independently for each formulation to evaluate whether reactions were more frequent (Cochran-Armitage tests for trend) or more severe (regression analyses) at higher doses. To assess whether either alum or dose level enhanced the immune response, separate logistic regression analyses were performed for each immunologic assay, with response as the dichotomous dependent variable; analysis of variance (ANOVA) was used to determine whether alum or a dose increase increased the magnitude of these responses. For both logistic and ANOVA models, the independent variables also included the interaction between alum and dose level. Relationships among preimmunization and peak postimmunization titers and among peak postvaccination titers for the various immunologic assays were examined using Spearman's correlations. Statistical significance was assessed at the 5% level.
RESULTS
Description of study subjects.
The median age of the 30 subjects was 23 years; 23 (77%) were men and seven (23%) were women. The distribution by race and ethnicity was as follows: Caucasian, 47%; African American, 33%; Hispanic, 3%; and Asian or Pacific Islander, 17%. All 30 subjects enrolled received four doses of vaccine and were included in the analysis.
Clinical tolerance. (i) Systemic reactions.
There were no serious adverse events during the study, and the vaccine was generally well tolerated (Table 2). No subject experienced fever. The most common complaint was rash (eight subjects), which uniformly was localized, did not involve urticaria or petechiae, and usually could be attributed to a cause other than vaccination, such as contact dermatitis. Six subjects reported eight episodes of abdominal pain; most episodes were rated mild (n = 5) or moderate (n = 2), lasting 3 or fewer days, although one subject experienced fleeting (5-min duration) abdominal pain on 2 nonconsecutive days that he rated severe. Two subjects noted arthralgia for 1 to 3 days; one attributed the pain to muscle soreness after vigorous exercise, and the other attributed it (elbow pain) to the contiguous tender injection site. Two subjects experienced five episodes of nonbloody diarrhea (defined as three or more loose stools within 24 h), with passage of three to eight loose stools. There were no statistically significant differences in the frequency, duration, or severity of systemic reactions between dosage groups or between formulations (alum versus soluble toxoid). Clinically significant laboratory abnormalities that appeared to be vaccine related were not seen.
TABLE 2.
Systemic complaints reported in a standardized diary within 7 days following each inoculation with C. difficile toxoid vaccine, by dose and formulation
| Complaint | No. of subjectsa who received:
|
|||||
|---|---|---|---|---|---|---|
| 6.25 μg of toxoid
|
25 μg of toxoid
|
100 μg of toxoid
|
||||
| Toxoid | Toxoid-alum | Toxoid | Toxoid-alum | Toxoid | Toxoid-alum | |
| Anorexia | 1 | 0 | 0 | 0 | 1 | 1 |
| Malaise | 2 | 0 | 0 | 2 | 1 | 0 |
| Joint swelling | 0 | 0 | 0 | 0 | 0 | 1 |
| Numbness | 1 | 0 | 0 | 0 | 1 | 1 |
| Rash | 1 | 2 | 0 | 2 | 2b | 1b |
| Abdominal pain | 1c | 1 | 1 | 0 | 2 | 1 |
| Joint pain | 0 | 1 | 0 | 1 | 0 | 0 |
| Fever | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 1c | 0 | 0 | 0 | 1b | 0 |
| Any of the above | 3 | 2 | 1 | 3 | 2 | 2 |
There were five subjects in each treatment group.
It should be noted that one subject in each group experienced two episodes of the specified complaint (after different inoculations).
This subject experienced three episodes of the specified complaint (after different inoculations).
(ii) Local reactions.
Pain at the site of injection was the most frequent local reaction, occurring in 100% of subjects who received toxoid adsorbed to alum, compared with zero, two, and one of the five subjects who received soluble toxoid, at each increasing dose level (Table 3). Most episodes of pain (55%) lasted for a single day, and all resolved within 4 days. Whereas no subject experienced local heat, self-limited swelling or erythema was observed at the two higher doses (Table 3). All subjects graded their local reactions as mild, except for one recipient of 25 μg of toxoid with alum who experienced mild-to-moderate arm pain after the third vaccination and moderate-to-severe pain after the fourth dose. Recipients of alum were more likely to experience arm pain (P = 0.004, 0.08, and 0.02 for the 6.25-, 25-, and 100-μg dose groups, respectively). The frequency of local swelling (P < 0.001 in the alum formulation) and erythema (P = 0.04 and 0.02 in the formulations with and without alum, respectively) increased with increasing doses of vaccine.
TABLE 3.
Local reactions occurring within 7 days following administration of each dose of C. difficile toxoid vaccine, by dose and formulationa
| Reaction | No. of subjectsb who received:
|
|||||
|---|---|---|---|---|---|---|
| 6.25 μg of toxoid
|
25 μg of toxoid
|
100 μg of toxoid
|
||||
| Toxoid | Toxoid-alum | Toxoid | Toxoid-alum | Toxoid | Toxoid-alum | |
| Pain | 0 | 5 | 2 | 5 | 1 | 5 |
| Swelling | 0 | 0 | 0 | 1 | 1 | 4 |
| Erythema | 0 | 0 | 0 | 1 | 3 | 3 |
| Heat | 0 | 0 | 0 | 0 | 0 | 0 |
| Any local reaction | 0 | 5 | 2 | 5 | 3 | 5 |
The reactions were solicited in a systematic fashion. In addition, six of the subjects spontaneously reported pruritus at the injection site after receiving either 6.25 μg of toxoid (two subjects), 25 μg of toxoid-alum (two subjects), 100 μg of toxoid (one subject), or 100 μg of toxoid-alum (one subject).
There were five subjects per treatment group.
It is notable that six subjects reported pruritus (without urticaria) at the site of injection, even though this symptom was not specifically solicited. These responses were seen with equal frequency at all dose levels (2 of 10 subjects per dose group) and among subjects who received the alum-adjuvanted formulation and those who did not (3 of 15 subjects per formulation). The onset was 1 to 6 days after injection in five subjects and 30 min after inoculation in the sixth, with a duration of 1 to 4 days. Pruritus was experienced by three subjects after the second injection only, by two after the third injection only, and by one after the second and third injections.
Immune responses. (i) Serum neutralizing antibody responses.
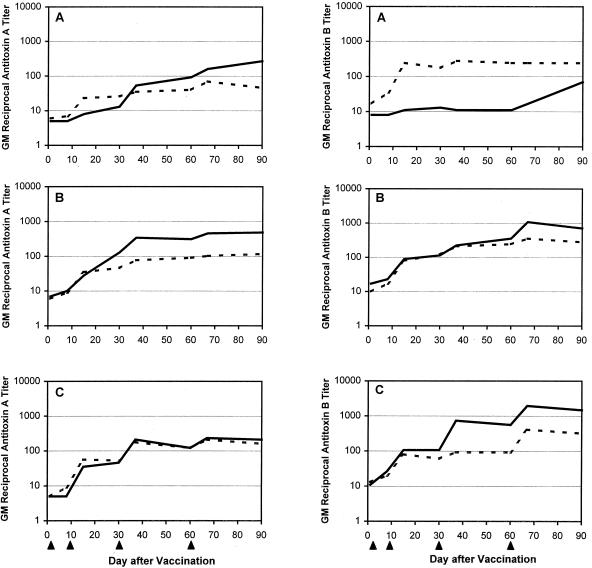
The vaccine elicited fourfold rises in neutralizing antibody to toxins A and B in all but two subjects in the 6.25-μg group (one received alum, and one did not). For toxin A, the highest geometric mean titers followed the 25-μg dose of toxoid-alum formulation and the 100-μg dose of toxoid alone; for toxin B, the titers consistently increased with increasing doses (Table 4 and Fig. 1). In most instances, the responses following toxoid-alum administration exceeded those following soluble-toxoid administration. Nonetheless, statistically significant effects of dose and formulation on the magnitude of the responses were not seen.
TABLE 4.
Immune response to C. difficile toxoid vaccine
| Assay | No. of subjects with response/total (geometric mean peak postvaccination assay result)
|
|||||
|---|---|---|---|---|---|---|
| 6.25 μg of toxoid
|
25 μg of toxoid
|
100 μg of toxoid
|
||||
| Toxoid | Toxoid-alum | Toxoid | Toxoid-alum | Toxoid | Toxoid-alum | |
| Neutralizing antibody | ||||||
| Anti-toxin A | 4/5 (80)a | 5/5 (269) | 5/5 (134) | 5/5 (604) | 5/5 (339) | 5/5 (243) |
| Anti-toxin B | 4/5 (368) | 4/5 (80) | 5/5 (368) | 5/5 (1,513) | 5/5 (485) | 5/5 (2,560) |
| Serum antibody ELISA | ||||||
| Anti-toxin A | ||||||
| IgA | 4/5 (238)b | 5/5 (785) | 5/5 (1,622) | 5/5 (5,272) | 5/5 (6,369) | 5/5 (1,548) |
| IgG | 5/5 (275) | 5/5 (1,217) | 5/5 (422) | 5/5 (2,345) | 5/5 (1,060) | 5/5 (1,260) |
| Anti-toxin B | ||||||
| IgA | 4/5 (13) | 3/5 (4) | 3/5 (13) | 5/5 (39) | 5/5 (29) | 5/5 (23) |
| IgG | 4/5 (56) | 5/5 (64) | 3/5 (161) | 5/5 (488) | 5/5 (183) | 5/5 (342) |
| ASC ELISPOT | ||||||
| Anti-toxin A | ||||||
| IgA | 4/5 (12)c | 3/5 (6.7) | 5/5 (23) | 5/5 (84) | 5/5 (179) | 5/5 (89) |
| IgG | 4/5 (5.2) | 2/5 (2.8) | 5/5 (30) | 4/5 (79) | 4/5 (38) | 5/5 (63) |
| Anti-toxin B | ||||||
| IgA | 3/5 (6.6) | 5/5 (5.7) | 5/5 (60) | 5/5 (86) | 5/5 (134) | 5/5 (115) |
| IgG | 5/5 (5.4) | 5/5 (1.8) | 5/5 (54) | 5/5 (170) | 5/5 (42) | 5/5 (123) |
| Fecal antibody ELISA | ||||||
| Anti-toxin A IgA | 5/5 (2,219)d | 4/5 (5,275) | 1/4 (907) | 0/5 (779) | 2/4 (329) | 2/3 (210) |
| Anti-toxin B IgA | 2/5 (43) | 3/5 (60) | 2/4 (170) | 2/5 (127) | 3/4 (157) | 3/3 (95) |
Reciprocal titer.
103 EU/ml.
Number of ASC per 106 PBMC.
Nanograms per milliliter.
FIG. 1.
Geometric mean (GM) serum anti-toxin A and anti-toxin B neutralizing antibody titers among volunteers who were inoculated with C. difficile toxoid vaccine at a dose of either 6.25 (A), 25 (B), or 100 (C) μg. At each dose, five subjects received soluble toxoid vaccine (broken line) and five received alum-adjuvanted toxoid (solid line). Each subject received a dose of vaccine on days 1, 8, 30, and 60 (arrowheads).
(ii) Serum antibody responses determined by ELISA.
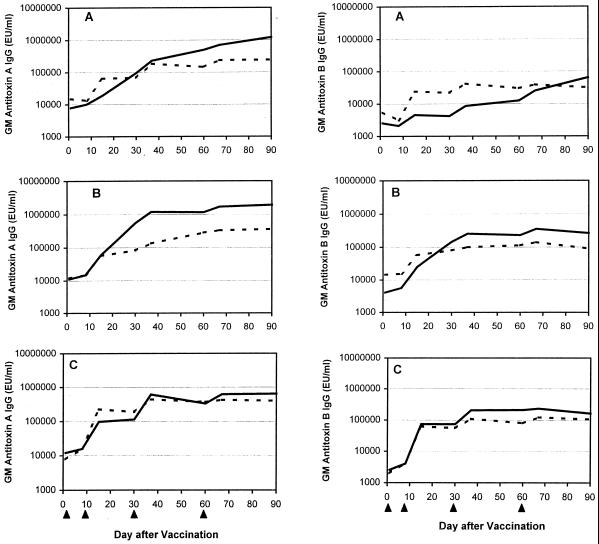
Serum anti-toxin A IgA and IgG antibody responses were seen in all but one subject (who received 6.25 μg of soluble toxoid; Table 4). The magnitude of the anti-toxin A responses was high (14 of 30 or 47% of IgA responses and 17 of 30 or 57% of IgG responses were at least 106 EU/ml). Among recipients of soluble toxoid, the antitoxin A and B responses increased with the dose (although not significantly); however, responses among the toxoid-alum group appeared to plateau after 25 μg (Table 4 and Fig. 2). Accordingly, the serum antibody responses following toxoid-alum administration generally exceeded those following soluble-toxoid administration for the 6.25- and 25-μg dose groups but not for the 100-μg group, although no significant effect of formulation on the magnitude of the response was revealed by multivariate ANOVA.
FIG. 2.
Geometric mean (GM) serum anti-toxin A and anti-toxin B IgG responses, as measured by ELISA, among volunteers who were inoculated with C. difficile toxoid vaccine at a dose of either 6.25 (A), 25 (B), or 100 (C) μg. At each dose, five subjects received soluble toxoid vaccine (broken line) and five received alum-adjuvanted toxoid (solid line). Each subject received a dose of vaccine on days 1, 8, 30, and 60 (arrowheads).
(iii) Kinetics of serum antibody responses.
Although a rise in antibody titer generally followed each injection of vaccine, the fourth dose did not substantially enhance the overall anti-toxin A antibody levels in the two higher-dose groups (Fig. 1 and 2). For anti-toxin B, the fourth dose elicited a booster effect on IgG antibody in a neutralizing assay (Fig. 1) but not in an ELISA (Fig. 2). There was a strong correlation between levels of serum neutralization, which is the assay likely to predict levels of physiologic antibody, and serum anti-toxin A IgG, which has been shown to be a correlate of protective immunity (r = 0.83, P < 0.001) (19).
To explore the feasibility of preventing primary or relapsing CDAD by administering this vaccine to patients upon admission to the hospital or after an episode of CDAD, we determined the proportion of subjects whose anti-toxin A IgG antibody levels rose at least fourfold by day 8 (1 week after administration of the first dose) and day 15 (1 week after administration of two doses). Only two subjects (both received 100 μg of toxoid-alum) mounted a fourfold rise in antibody by day 8. Whereas all five subjects who received 100 μg of toxoid-alum seroconverted by day 15, with the fold rise ranging from 13 to 403, a 4-fold response occurred in only two or three subjects in each of the other dose groups. Seroconversion to toxin B was also infrequent by day 8 (three subjects) but common by day 15, occurring in all of the subjects who received 100 μg of toxoid (10- to 196-fold rises), in four of five subjects who received 100 μg of toxoid-alum formulation (30- to 107-fold rises), and in two to four subjects in each of the other dose groups (4- to 42-fold rises). Early neutralizing antibody seroconversions were less common (data not shown).
(iv) ASC responses.
Vaccination elicited an IgA or IgG ASC response to toxin A in 27 (90%) and 24 (80%) subjects and to toxin B in 28 (93%) and 30 (100%) subjects, respectively (Table 4). All but one subject in the two highest-dose groups responded in all four assays. There was a trend toward increasing magnitude of response with increasing doses in each of the assays, which achieved statistical significance only for anti-toxin A IgA (P = 0.02) and anti-toxin B IgG (P < 0.01). The height of the response was not significantly different between the two formulations (P > 0.47).
(v) Fecal antibody responses determined by ELISA.
Fecal IgA responses occurred less frequently than did the other immune responses measured (Table 4). There were no significant differences between the groups or the two formulations.
DISCUSSION
We found that a C. difficile vaccine containing formalin-inactivated toxoids A and B was safe, without serious adverse reactions, and highly immunogenic in healthy volunteers, even at doses as low as 6.25 μg of total protein (containing 1.5 μg of toxoid A and 1.1 μg of toxoid B). Vaccination elicited systemic antibody responses in nearly all subjects, in both binding (ELISA) and physiologic (neutralization) assays. The dose-response studies indicated that the maximum response occurred after administration of 25 μg of the toxoid-alum formulation and after administration of 100 μg of toxoid alone. Three doses of vaccine (days 1, 8, and 30) seem to be sufficient, since the fourth dose did not appear to substantially boost serum IgG or neutralizing antibody.
Considerable evidence supports the role of antibody-mediated anti-toxic immunity in protection and recovery from C. difficile-associated disease. In animal models of C. difficile infection, parenteral and mucosal administration of specific toxin-neutralizing monoclonal and polyclonal antibodies prevents experimentally induced C. difficile-associated enterotoxicity (3, 6, 16, 23). Furthermore, treatment with toxin-neutralizing oral IgY preparations (derived from egg yolk harvested from immunized hens) effectively averts CDAD (16). In addition, active immunization with toxoid preparations, delivered either parenterally or by a combination of the parenteral and mucosal routes, protects animals against diarrhea and death following a challenge with C. difficile (5, 6, 32).
Data from humans also support a role for anti-toxic immunity in protection against CDAD. In a prospective study of hospitalized patients who were receiving antibiotics, Kyne et al. showed that patients who developed diarrhea after becoming colonized with C. difficile had significantly lower levels of serum anti-toxin A IgG at the time of colonization compared with patients who remained asymptomatic (19). In another study, patients with low anti-toxin A titers in serum (IgG) and feces (IgA) were more likely to experience prolonged and relapsing CDAD (37). Several case reports have described patients with severe, persistent, or relapsing CDAD who were effectively treated with preparations of intravenous gamma globulin containing C. difficile antitoxin (20, 30, 36).
In light of these observations that serum anti-toxin A IgG antibody levels correlate with protection against CDAD (16, 19), it has been proposed that this measure be used as a surrogate marker of vaccine efficacy (19). Thus, it is important to note that in our trial, vaccination elicited a serum IgG antibody response to toxin A in all of the subjects and that the functional activity of these antibodies could be demonstrated by neutralization of toxin-induced cytotoxicity in all but one subject (who belonged to the lowest-dose group). Furthermore, the responses were strong, with the median fold increases in anti-toxin A antibody levels 90 days after administration of the first dose of vaccine ranging from 32 to 43 for neutralization and from 42 to 92 for IgG. It is likely that many of these responses were anamnestic, since most of the subjects had measurable baseline serum IgG antibody levels. Other studies have detected serum antibodies to C. difficile toxins in approximately 60% of the children and adults tested in the United States (34), presumably as a result of asymptomatic colonization during infancy and early childhood (18, 35).
Whereas serum anti-toxin A IgG in the ELISA has been correlated with immunity, the protective function of other immune responses is less clear. In the hamster model, protection against death following a challenge with C. difficile correlates more closely with in vitro neutralization of toxins A and B in a cell-rounding (cytotoxicity) assay than with anti-toxin antibody levels measured by ELISA (6). The relative importance of anti-toxin A and B antibody in preventing intestinal damage is also not known. Immunological data which show an inverse correlation between anti-toxin A antibodies and disease expression (19) suggest that toxin A is the principal culprit, while studies of toxin binding to human colonic epithelium indicate that toxin B mediates disease and must be targeted for immunoprotection (27). Stimulation or transfer of antibodies to both toxins should therefore be a goal for vaccination and passive immunotherapy.
Mucosal antibody responses, although less common than systemic antibody responses, were also present in about half of the vaccinees. A possible role for mucosal immunity in protection from and treatment of CDAD has been postulated. The presence of secretory IgA antibodies in colonic aspirates from patients which inhibit binding of toxin A to its glycoprotein receptor in rabbit ileal intestinal brush border membranes suggests a potential mechanism of action (12). Further support for a role of secretory IgA in immunity to C. difficile comes from observations of protective efficacy conferred by orally administered antitoxic immunoglobulin preparations in animals (15, 16), the ability of bovine colostral concentrate containing antibody to toxins A and B to neutralize the biologic effects of C. difficile toxins (cytotoxicity in cultured human fibroblasts, binding of toxin A to its enterocyte receptor, increased fluid secretion and mannitol permeability in rat ileal loops) (13), and the clinically milder illness seen in patients who have high fecal anti-toxin A IgA titers (37). The enterotoxicity of C. difficile toxins has been attributed to their ability to disrupt the actin cytoskeleton and enhance tight-junction permeability in human intestinal epithelial cell monolayers (7), since in vivo, these effects could result in transudation of fluid into the intestinal lumen and watery diarrhea. This pathogenesis may explain the protective activity of parenterally administered vaccines and antibodies, since access of serum antibodies to the intestinal lumen would be an early event in infection.
Several potential uses can be envisioned for a C. difficile vaccine. Plasma donors could be immunized to generate hyperimmune globulin for passive immunoprophylaxis of selected high-risk subjects, such as hospitalized patients; those with a high (circa 17%) risk of CDAD can be identified, based on factors such as age, anticipated hospital stay, antibiotic treatment, admission to a ward where C. difficile is endemic, and severity of underlying illness (19). Passive immunoprophylaxis might also benefit patients with multiple relapses of CDAD, particularly since data suggest that they have an underlying inability to mount an effective humoral immune response to infection (37). Hyperimmune globulin as a potential treatment of patients with established CDAD is another strategy which warrants further investigation. Compared with the large doses of IVIG that have been used to treat chronic relapsing CDAD (20, 30, 36), hyperimmune globulin would allow optimization of the antibody dose using a lower volume of product.
If the immune response to vaccination were rapid, then active vaccination of high-risk subjects upon admission to the hospital might be feasible. The decision to administer the first two doses of vaccine within a 1-week interval was made with this approach in mind. Unfortunately, few subjects mounted responses within 1 week, although all recipients of 100 μg of the toxoid-alum formulation mounted at least a 13-fold increase in serum IgG anti-toxin A antibody within 14 days of receiving the first vaccination.
Primary prevention of CDAD by vaccination of groups with a high incidence of disease, such as adults 65 years of age or older, is another potential application. In Sweden, for example, the incidence of CDAD in adults between 60 and 99 years of age is 121 to 526 per 100,000 population (10). Interestingly this compares with 11 to 16 per 100,000 for invasive pneumococcal disease among the elderly in Sweden (2). With this approach, vaccine would be administered when individuals are in a relatively healthy state and more likely to respond immunologically. Furthermore, prevention of community-acquired illness would be possible. Population-based analyses indicate that a substantial proportion of CDAD occurs in the community. In a Swedish study, 28% of CDAD cases were community acquired, yielding an annual incidence of 20 per 100,000 population (with 56% of patients requiring hospitalization) (10), whereas in a Boston health maintenance organization, the incidence was 7.7 per 100,000 participants (with 18% of subjects hospitalized) (8).
For any of these clinical applications, the vaccine must be well tolerated, as well as immunogenic. In our study, occasional systemic reactions (abdominal pain, arthralgia, and diarrhea) occurred but were minimally bothersome and, in the absence of a placebo control, could not be definitively attributed to vaccination. Local reactions, particularly arm pain, were more common among recipients of alum and were usually mild. Of note is the occurrence of itching at the site of injection in six subjects. This appeared to represent an arthus-type response (presumably to the toxoid antigen), rather than type 1 immediate-type hypersensitivity, because the onset was delayed (≥1 day after immunization in five of six subjects), inflammation was limited to the site of injection without urticaria or signs of anaphylaxis, and there was no predisposition for recipients of alum-containing formulations (alum is known to drive IgE-mediated reactions). Arthus reactions are common after parenteral vaccine administration and result when immune complexes deposited at the site of injection stimulate vasoactive amines. Since these reactions occur in the presence of pre-existing antibody to the injected antigen, this would explain why itching did not occur after administration of the first dose of vaccine. Nonetheless, a skin biopsy would have been required for definitive diagnosis.
We conclude that C. difficile toxoid vaccine is safe and immunogenic in healthy volunteers. Further development of this preparation as a prophylactic vaccine and as an immunostimulatory agent for producing hyperimmune globulin could lead to promising strategies for the prevention and treatment of C. difficile-associated diseases.
ACKNOWLEDGMENTS
We are indebted to the volunteers who participated in this study. We thank Elizabeth Peddicord for coordinating the recruitment and evaluation of volunteers; JoAnna Becker, Kathleen Palmer, and the nurses at the Center for Vaccine Development for providing clinical care; Mardi Reymann for technical assistance; Linda Moore and Karen McCarthy for help with data management; Linda Rosendorf for regulatory support; and Regina Rabinovich and Dennis Lang for helpful suggestions.
This work was supported by a contract from the National Institute of Allergy and Infectious Diseases to Myron Levine (NO1-AI-45251).
REFERENCES
- 1.Barbut F, Leluan P, Antoniotti G, Collignon A, Sedallian A, Petit J C. Value of routine stool cultures in hospitalized patients with diarrhea. Eur J Clin Microbiol Infect Dis. 1995;14:346–349. doi: 10.1007/BF02116530. [DOI] [PubMed] [Google Scholar]
- 2.Burman L A, Norrby R, Trollfors B. Invasive pneumococcal infections: incidence, predisposing factors, and prognosis. Rev Infect Dis. 1985;7:133–142. doi: 10.1093/clinids/7.2.133. [DOI] [PubMed] [Google Scholar]
- 3.Corthier G, Muller M C, Wilkins T D, Lyerly D, L'Haridon R. Protection against experimental pseudomembranous colitis in gnotobiotic mice by use of monoclonal antibodies against Clostridium difficile toxin A. Infect Immun. 1991;59:1192–1195. doi: 10.1128/iai.59.3.1192-1195.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeMarais P L, Gertzen J, Weinstein R A. Nosocomial infections in human immunodeficiency virus-infected patients in a long-term-care setting. Clin Infect Dis. 1997;25:1230–1232. doi: 10.1086/516076. [DOI] [PubMed] [Google Scholar]
- 5.Fernie D S, Thomson R O, Batty I, Walker P D. Active and passive immunization to protect against antibiotic associated caecitis in hamsters. Dev Biol Stand. 1983;53:325–332. [PubMed] [Google Scholar]
- 6.Giannasca P J, Zhang Z X, Lei W D, Boden J A, Giel M A, Monath T P, Thomas W D., Jr Serum antitoxin antibodies mediate systemic and mucosal protection from Clostridium difficile disease in hamsters. Infect Immun. 1999;67:527–538. doi: 10.1128/iai.67.2.527-538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hecht G, Pothoulakis C, LaMont J T, Madara J L. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of the cultured human. J Clin Investig. 1988;82:1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirschhorn L R, Trnka Y, Onderdonk A, Lee M L, Platt R. Epidemiology of community-acquired Clostridium difficile-associated diarrhea. J Infect Dis. 1994;169:127–133. doi: 10.1093/infdis/169.1.127. [DOI] [PubMed] [Google Scholar]
- 9.Johnson S, Gerding D N. Clostridium difficile-associated diarrhea. Clin Infect Dis. 1998;26:1027–1034. doi: 10.1086/520276. [DOI] [PubMed] [Google Scholar]
- 10.Karlstrom O, Fryklund B, Tullus K, Burman L G. A prospective nationwide study of Clostridium difficile-associated diarrhea in Sweden. The Swedish C. difficile Study Group. Clin Infect Dis. 1998;26:141–145. doi: 10.1086/516277. [DOI] [PubMed] [Google Scholar]
- 11.Kelly C P, Pothoulakis C, LaMont J T. Clostridium difficile colitis. N Engl J Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 12.Kelly C P, Pothoulakis C, Orellana J, LaMont J T. Human colonic aspirates containing immunoglobulin A antibody to Clostridium difficile toxin A inhibit toxin A-receptor binding. Gastroenterology. 1992;102:35–40. doi: 10.1016/0016-5085(92)91781-x. [DOI] [PubMed] [Google Scholar]
- 13.Kelly C P, Pothoulakis C, Vavva F, Castagliuolo I, Bostwick E F, O'Keane J C, Keates S, LaMont J T. Anti-Clostridium difficile bovine immunoglobulin concentrate inhibits cytotoxicity and enterotoxicity of C. difficile toxins. Antimicrob Agents Chemother. 1996;40:373–379. doi: 10.1128/aac.40.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kent K C, Rubin M S, Wroblewski L, Hanff P A, Silen W. The impact of Clostridium difficile on a surgical service: a prospective study of 374 patients. Ann Surg. 1998;227:296–301. doi: 10.1097/00000658-199802000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim P H, Iaconis J P, Rolfe R D. Immunization of adult hamsters against Clostridium difficile-associated ileocecitis and transfer of protection to infant hamsters. Infect Immun. 1987;55:2984–2992. doi: 10.1128/iai.55.12.2984-2992.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kink J A, Williams J A. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect Immun. 1998;66:2018–2025. doi: 10.1128/iai.66.5.2018-2025.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knoop F C, Owens M, Crocker I C. Clostridium difficile: clinical disease and diagnosis. Clin Microbiol Rev. 1993;6:251–265. doi: 10.1128/cmr.6.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotloff K L, Wade J C, Morris J G., Jr Lack of association between Clostridium difficile toxin and diarrhea in infants. Pediatr Infect Dis J. 1988;7:662–663. doi: 10.1097/00006454-198809000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Kyne L, Warny M, Qamar A, Kelly C P. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342:390–397. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 20.Leung D Y, Kelly C P, Boguniewicz M, Pothoulakis C, LaMont J T, Flores A. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J Pediatr. 1991;118:1–7. doi: 10.1016/s0022-3476(05)83393-1. [DOI] [PubMed] [Google Scholar]
- 21.Lima N L, Guerrant R L, Kaiser D L, Germanson T, Farr B M. A retrospective cohort study of nosocomial diarrhea as a risk factor for nosocomial infection. J Infect Dis. 1990;161:948–952. doi: 10.1093/infdis/161.5.948. [DOI] [PubMed] [Google Scholar]
- 22.Losonsky G A, Rennels M B, Lim Y, Krall G, Kapikian A Z, Levine M M. Systemic and mucosal immune responses to rhesus rotavirus vaccine MMU 18006. Pediatr Infect Dis J. 1988;7:388–393. doi: 10.1097/00006454-198806000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Lyerly D M, Bostwick E F, Binion S B, Wilkins T D. Passive immunization of hamsters against disease caused by Clostridium difficile by use of bovine immunoglobulin G concentrate. Infect Immun. 1991;59:2215–2218. doi: 10.1128/iai.59.6.2215-2218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McFarland L V. Epidemiology of infectious and iatrogenic nosocomial diarrhea in a cohort of general medicine patients. Am J Infect Control. 1995;23:295–305. doi: 10.1016/0196-6553(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 25.McFarland L V, Surawicz C M, Rubin M, Fekety R, Elmer G W, Greenberg R N. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol. 1999;20:43–50. doi: 10.1086/501553. [DOI] [PubMed] [Google Scholar]
- 26.McNulty C, Logan M, Donald I P, Ennis D, Taylor D, Baldwin R N, Bannerjee M, Cartwright K A. Successful control of Clostridium difficile infection in an elderly care unit through use of a restrictive antibiotic policy. J Antimicrob Chemother. 1997;40:707–711. doi: 10.1093/jac/40.5.707. [DOI] [PubMed] [Google Scholar]
- 27.Riegler M, Sedivy R, Pothoulakis C, Hamilton G, Zacherl J, Bischof G, Cosentini E, Feil W, Schiessel R, LaMont J T. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Investig. 1995;95:2004–2011. doi: 10.1172/JCI117885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riley T V. Antibiotic-associated diarrhoea. A costly problem. Pharmacoeconomics. 1996;10:1–3. doi: 10.2165/00019053-199610010-00001. [DOI] [PubMed] [Google Scholar]
- 29.Roghmann M C, McCarter R J, Jr, Brewrink J, Cross A S, Morris J G., Jr Clostridium difficile infection is a risk factor for bacteremia due to vancomycin-resistant enterococci (VRE) in VRE-colonized patients with acute leukemia. Clin Infect Dis. 1997;25:1056–1059. doi: 10.1086/516112. [DOI] [PubMed] [Google Scholar]
- 30.Salcedo J, Keates S, Pothoulakis C, Warny M, Castagliuolo I, LaMont J T, Kelly C P. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut. 1997;41:366–370. doi: 10.1136/gut.41.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spencer R C. Clinical impact and associated costs of Clostridium difficile-associated disease. J Antimicrob Chemother. 1998;41(Suppl. C):5–12. doi: 10.1093/jac/41.suppl_3.5. [DOI] [PubMed] [Google Scholar]
- 32.Torres J F, Lyerly D M, Hill J E, Monath T P. Evaluation of formalin-inactivated Clostridium difficile vaccines administered by parenteral and mucosal routes of immunization in hamsters. Infect Immun. 1995;63:4619–4627. doi: 10.1128/iai.63.12.4619-4627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van de Verg L, Herrington D A, Murphy J R, Wasserman S S, Formal S B, Levine M M. Specific immunoglobulin A-secreting cells in peripheral blood of humans following oral immunization with a bivalent Salmonella typhi-Shigella sonnei vaccine or infection by pathogenic S. sonnei. Infect Immun. 1990;58:2002–2004. doi: 10.1128/iai.58.6.2002-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viscidi R, Laughon B E, Yolken R, Bo-Linn P, Moench T, Ryder R W, Bartlett J G. Serum antibody response to toxins A and B of Clostridium difficile. J Infect Dis. 1983;148:93–100. doi: 10.1093/infdis/148.1.93. [DOI] [PubMed] [Google Scholar]
- 35.Viscidi R, Willey S, Bartlett J G. Isolation rates and toxigenic potential of Clostridium difficile isolates from various patient populations. Gastroenterology. 1981;81:5–9. [PubMed] [Google Scholar]
- 36.Warny M, Denie C, Delmee M, Lefebvre C. Gamma globulin administration in relapsing Clostridium difficile-induced pseudomembranous colitis with a defective antibody response to toxin A. Acta Clin Belg. 1995;50:36–39. doi: 10.1080/17843286.1995.11718419. [DOI] [PubMed] [Google Scholar]
- 37.Warny M, Vaerman J P, Avesani V, Delmee M. Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection. Infect Immun. 1994;62:384–389. doi: 10.1128/iai.62.2.384-389.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilcox M H. Cleaning up Clostridium difficile infection. Lancet. 1996;348:767–768. doi: 10.1016/S0140-6736(05)65204-X. [DOI] [PubMed] [Google Scholar]
- 39.Zollinger W D, Boslego J W. A general approach to standardization of the solid-phase radioimmunoassay for quantitation of class-specific antibodies. J Immunol Methods. 1981;46:129–140. doi: 10.1016/0022-1759(81)90130-7. [DOI] [PubMed] [Google Scholar]