Abstract
Host genes are thought to determine the immune response to malaria infection and the outcome. Cytophilic antibodies have been associated with protection, whereas noncytophilic antibodies against the same epitopes may block the protective activity of the protective ones. To assess the contribution of genetic factors to immunoglobulin G (IgG) subclass responses against conserved epitopes and Plasmodium falciparum blood-stage extracts, we analyzed the isotypic distribution of the IgG responses in 366 individuals living in two differently exposed areas in Burkina Faso. We used one-way analysis of variance and pairwise estimators to calculate sib-sib and parent-offspring correlation coefficients, respectively. Familial patterns of inheritance of IgG subclass responses to defined antigens and P. falciparum extracts appear to be similar in the two areas. We observed a sibling correlation for the IgG, IgG1, IgG2, IgG3, and IgG4 responses directed against ring-infected-erythrocyte surface antigen, merozoite surface protein 1 (MSP-1), MSP-2, and P. falciparum extract. Moreover, a parent-offspring correlation was found for several IgG subclass responses, including the IgG, IgG1, IgG2, IgG3, and IgG4 responses directed against conserved MSP-2 epitopes. Our results indicated that the IgG subclass responses against P. falciparum blood-stage antigens are partly influenced by host genetic factors. The localization and identification of these genes may have implications for immunoepidemiology and vaccine development.
Plasmodium falciparum malaria affects more than 2 million people and remains a major public health problem in many developing countries. Host immune responses are critical to strategies for the control of both infection and pathology. In particular, antibody-dependent cellular mechanisms are thought to be central in the elimination of the parasite (1, 2, 5), and increased proinflammatory immune response is associated with severe malaria (11). Immunoglobulin G1 (IgG1) and IgG3 are considered cytophilic and protective against P. falciparum, whereas IgG4 is thought to be neither and to block protective mechanisms. Furthermore, IgG2, which binds the H131 allelic form of FcγRIIA, may be involved in human resistance to malarial infection (2).
In this context, several investigators have sought to determine whether genetic factors control host immune responses to P. falciparum antigens. Taylor et al. reported that antibody responses to merozoite surface protein 1 (MSP-1), MSP-2, and Pfs260/230 were similar in identical and nonidentical twins and proposed that antibody responses to malaria antigens in immune individuals result from clonal imprinting (30). However, antibody responses to ring-infected-erythrocyte surface antigen (RESA) were found to be more concordant within monozygotic twin pairs than in dizygotic twin pairs (26). Similarly, Jepson et al. obtained evidence for genetic control of cell-mediated immune responses and levels of IgG antibody to various P. falciparum antigens (12). Furthermore, familial correlation of some IgG responses against RESA and MSP-2 was found in Papua New Guinea (28). Therefore, human immune responses to P. falciparum antigens appear to be, at least in part, genetically regulated.
HLA class II-associated nonresponsiveness has been reported for the candidate malaria vaccine Spf66 (19). In contrast, antibody responses induced by natural exposure to malaria infection show little association with HLA expression (25, 30, 32). Twin studies indicate that the genetic contribution of non-HLA genes to the human immune responses to P. falciparum antigens exceeds that of the HLA genes (12, 26).
Immunogenetic polymorphisms likely affect susceptibility to malaria infection or disease, and the identification of genes controlling human immune responses to malaria is of major interest. In urban subjects in Burkina Faso, we recently detected a linkage between blood infection levels and chromosome 5q31-q33, which contains numerous genes encoding immunological molecules (22). In the same population, moreover, we observed an association between high IgG2 and low IgG4 levels on the one hand and resistance to P. falciparum malaria on the other (2).
In this study, we focused on the genetic control of the IgG subclass responses to specific P. falciparum antigens by investigating 75 families from two differently exposed areas in Burkina Faso. We evaluated the degree of resemblance among family members with respect to the levels of antibody directed against RESA, MSP-1, and MSP-2 conserved epitopes and crude P. falciparum antigens. We present here the correlations among sibling pairs and parent-offspring pairs.
MATERIALS AND METHODS
Study area, subjects, and plasma samples.
The study population lived for more than 20 years in an urban district of Bobo-Dioulasso, Burkina Faso, and in a rural area southwest of the city. The population structure and the area of parasite exposure were described extensively elsewhere (21, 31). Informed consent for multiple immunoparasitological and clinical surveys was obtained individually from all participants. The Medical Authority of Burkina Faso approved the study protocol. Malaria transmission was assessed by determining the number of infective bites per person per year at different capture sites during 2 years (31). Three and four capture sites were chosen in the urban district and in the rural area, respectively. The numbers of infective bites per person per year calculated in the three urban capture sites were similar, and only slight differences among the four rural capture sites were recorded. The number of infective bites per person per year was less than 30 in the urban area and more than 230 in the village (21).
Seventy-five informative families, which had at least two available sibs each, were selected for immunoanalyses; 34 and 41 nuclear families were from the urban area and the rural area, respectively. Blood samples were taken from 366 individuals by venipuncture in July 1994 (n = 273), at the end of the dry season (P1), and in December 1994 (n = 334), at the end of the rainy season (P2). The distributions of available sibship sizes were as follows: 3, 11, 7, 11, and 2 sibships from the urban area contained 2, 3, 4, 5, and 6 sibs, respectively, and 23, 15, 2, and 1 sibships from the rural area contained 2, 3, 4, and 5 sibs, respectively. The descriptive statistics for the study subjects are in Table 1.
TABLE 1.
Descriptive statistics of study subjects
| Area and characteristic | Fathers | Mothers | Offspring |
|---|---|---|---|
| Urban | |||
| No. | 24 | 32 | 134 |
| Age (yrs)a | 54 ± 9 | 42 ± 8 | 12 ± 6 |
| Rural | |||
| No. | 32 | 40 | 104 |
| Age (yrs)a | 44 ± 8 | 38 ± 9 | 11 ± 6 |
Ages are means ± standard deviations.
P. falciparum blood-stage extract and peptides.
The P. falciparum W2 strain (Southeast Asia) was maintained and synchronized as previously described (15). When the parasitemia reached 10%, schizont-infected red blood cells were treated with 0.15% saponin. Isolated schizonts were sonicated on ice in phosphate-buffered saline containing protease inhibitors (50 μM phenylmethylsulfonyl fluoride, 50 μg of aprotinin/ml, 1 μM pepstatin, 20 μg of leupeptin/ml, 10 μg of α2-macroglobulin/ml). Sonicates were centrifuged, and the supernatants were filtered through a 0.22-μm-pore-size membrane. These P. falciparum crude extracts were aliquoted and stored at −70°C until use.
Five synthetic peptides corresponding to highly conserved B-cell epitopes were used: (i) the epitope (EENV)4 of the C-terminal part of RESA (20), which is immunodominant and antibodies to which were associated with resistance to clinical malaria (23); (ii) the epitope KLYQAQYDLSF, representing amino acids 277 to 287 of the N-terminal conserved part of MSP-1 (MSP-1-Nt) (18), antibodies to which were significantly associated with clinical protection (24); (iii) the epitope KAASNTFINNA, representing amino acids 27 to 34 of the N-terminal conserved region of MSP-2 (MSP-2-Nt); (iv) the epitope AAAQHGHMHGS, representing amino acids 199 to 206 of the C-terminal conserved region of MSP-2 (MSP-2-Ct1); and (v) the epitope AAANTSDSQKE, representing amino acids 213 to 220 of the C-terminal conserved region of MSP-2 (MSP-2-Ct2) (13).
Specific IgG isotype titration by ELISA.
Enzyme-linked immunosorbent assay (ELISA) plates (Nunc) were coated either with 1 μg of P. falciparum extract/ml in sodium carbonate buffer (100 mM, pH 9.6) or with 10 μg of peptides conjugated to glutaraldehyde-activated poly-l-lysine/ml (3). Plates were saturated with 3% bovine serum albumin in phosphate-buffered saline. Serum dilutions were incubated for 16 h at 4°C (1:20 for IgG2 and IgG4, 1:100 for IgG1 and IgG3, and 1:400 for IgG). The following monoclonal antibodies were used: anti-IgG1 (clone 8c/6-39; The Binding Site), IgG2 and IgG3 (clone HP 6002 and HP 6050; Clinisciences), and IgG4 (clone RJ4; Immunotech). Total IgG was detected using a goat F(ab′)2 anti-human IgG (Jackson Laboratories). The anti-IgG1 and -IgG were conjugated to alkaline phosphatase, the anti-IgG2 and -IgG3 were biotinylated, and the anti-IgG4 was unlabeled. F(ab′)2 anti-mouse IgG conjugated to alkaline phosphatase was used for IgG4 detection. Signal amplification was performed for IgG2 and IgG3 detection by using streptavidin and biotinylated alkaline phosphatase (Pierce); the sensitivity of the assay was 30-fold higher than that of the assay using the same monoclonal antibodies conjugated to alkaline phosphatase. After 2 h of incubation at room temperature, enzymatic activities were revealed by p-nitrophenyl phosphate (Sigma) (1 mg/ml) in Tris buffer (pH 9.6). The optical densities were read at 405 nm using a DIAS automatic plate reader (Dynex Technology).
Thirty negative reference sera were used to determine the detection threshold; competition experiments using IgG1, IgG2, IgG3, and IgG4 purified from myeloma were performed to check the specificity and sensitivity of ELISAs. No cross-reaction was observed. A pool of 200 samples was used to draw standard curves. All tests were done in duplicate, and antibody levels were calculated by using the standard curve and were expressed as arbitrary units (AU). To allow for zero values in further analyses, we applied a logarithmic transformation based on log(1+AU) (LAU) to the AU.
Data adjustment and phenotype of interest.
We first compared the antibody levels measured at P1 and at P2. The correlation was assessed by Spearman's rank test and linear regression analysis. As previously described, we detected significant variation of some antibody levels between P1 and P2 using the paired Student's t test (2). We therefore took into account the influence of the date of bleeding in further calculations. The mean LAU and standard deviation were calculated at P1 and at P2. To correct the individual LAU for the visit effect, the LAU was standardized at P1 and at P2, and the mean of adjusted LAU (MALAU) was calculated for each subject.
The influence of the covariates on MALAU was assessed by analysis of variance (ANOVA) for categorical variables and by Spearman's rank correlation and polynomial regression for age. The factors tested were sex, ethnic group in the urban area, which was classified as one of four major groups (Mossi, Dafing, Bissa, and others), and age, which was expressed in years and considered a quantitative variable. Covariates with a significant effect on MALAU were retained for adjustment. The standardized residual was used to estimate familial correlations.
Familial correlations.
To assess whether the antibody response to defined B-cell epitopes and crude antigens was correlated within families, we estimated sibling-sibling and parent-offspring correlation coefficients. The methods for measuring such correlations are detailed elsewhere (8, 9). Briefly, we used the ANOVA estimator for inferences concerning sibling correlations and the pairwise estimators for inferences concerning parent-offspring correlations. Families with sibships having only one member were excluded from the analysis.
(i) Estimator of sibling correlations.
The phenotype, defined above as the age-adjusted and standardized residual score from regression analysis, was used to compute a one-way ANOVA. The observations were grouped by family to provide estimates for the variability within families and the variability between families. The mean squared between-family variance (MSB) and the mean squared within-family variance (MSW) were calculated, and the ratio of these variance components was used to measure sibling resemblance. If the siblings are similar with respect to the phenotype, MSW is small and MSB is high. The intraclass correlation coefficient Rss was computed as previously recommended (9): (MSB − MSW)/(MSB − MSW + koMSW). ko is calculated as
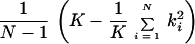 |
where N is the number of families, K is the total number of observations, and ki is the number of sibs in the ith family. We tested the significance for Rss through the usual ANOVA F statistic.
(ii) Estimators of parent-offspring correlations.
We used the pairwise estimator (Rpo) and the family-weighted pairwise estimator (Rfw). The former is obtained by computing the standard Pearson product-moment correlation over all possible parent-offspring pairs that can be formed from the sample data. The family-weighted pairwise estimator is obtained by computing the standard Pearson product-moment correlation between the parent phenotype and the average phenotype over all children in a family. This estimator was more suitable when sibling correlation was fairly large (Rss > 0.5). The estimators of parent-offspring correlation (Rpo and Rfw) were tested for their statistical significance by computing the ratio (Z) of Rpo and Rfw by their estimated large-sample standard error (8, 9). The resulting test statistic that takes into account the sibling correlation is given by an approximate standard normal deviate (8, 9) as follows:
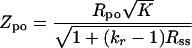 |
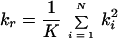 |
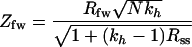 |
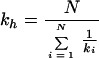 |
RESULTS
Presentation of the data and calculation of the phenotypes.
In the rural area, the IgG subclass levels in July (P1) and in December (P2) were correlated (data not shown). In the urban area, antibody responses were also correlated between P1 and P2, except for the IgG4 responses against RESA, MSP-1-Nt, and MSP-2-Ct1. As previously described (2), the date of bleeding influenced the IgG subclass levels. We therefore adjusted the IgG subclass levels for the bleeding effect, and we used the mean of the adjusted IgG subclass levels in further analyses. In addition, this calculation made the phenotypes in the two areas comparable.
In the rural and urban areas, there was no significant effect of sex or ethnic group on the IgG subclass levels whatever the antigen used (data not shown). In contrast, age had a significant effect on anti-P. falciparum extract IgG levels of all subclasses (Table 2). Moreover, age was positively correlated with anti-RESA, -MSP-1, and -MSP-2 IgG, IgG2, and IgG3 levels in the rural and urban areas (Table 2). Age significantly influenced anti-RESA, -MSP-1, and -MSP-2 IgG4 levels in the rural area. Where appropriate, age was retained for adjustment and calculation of the phenotypes.
TABLE 2.
Antibody responses to P. falciparum blood-stage epitopes correlated with agea
| Antigen and antibody | Urban area
|
Rural area
|
||
|---|---|---|---|---|
| ρ | P | ρ | P | |
| RESA | ||||
| IgG1 | 0.08 | NS | 0.14 | 0.0412 |
| IgG2 | 0.68 | <10–4 | 0.51 | <10–4 |
| IgG3 | 0.25 | 0.0005 | 0.41 | <10–4 |
| IgG4 | 0.03 | NS | 0.29 | <10–4 |
| IgG | 0.30 | <10–4 | 0.46 | <10–4 |
| MSP-1-Nt | ||||
| IgG1 | 0.05 | NS | 0.06 | NS |
| IgG2 | 0.63 | <10–4 | 0.37 | <10–4 |
| IgG3 | 0.25 | 0.0006 | 0.28 | <10–4 |
| IgG4 | 0.02 | NS | 0.15 | 0.0342 |
| IgG | 0.22 | 0.0019 | 0.39 | <10–4 |
| MSP-2-Nt | ||||
| IgG1 | 0.03 | NS | 0.03 | NS |
| IgG2 | 0.63 | <10–4 | 0.56 | <10–4 |
| IgG3 | 0.22 | 0.002 | 0.14 | 0.0439 |
| IgG4 | −0.04 | NS | 0.19 | 0.0053 |
| IgG | 0.22 | 0.0028 | 0.39 | <10–4 |
| MSP-2-Ct1 | ||||
| IgG1 | 0.16 | 0.0319 | 0.09 | NS |
| IgG2 | 0.60 | <10–4 | 0.60 | <10–4 |
| IgG3 | 0.20 | 0.006 | 0.29 | <10–4 |
| IgG4 | −0.11 | NS | 0.16 | 0.0175 |
| IgG | 0.17 | 0.0185 | 0.44 | <10–4 |
| MSP-2-Ct2 | ||||
| IgG1 | 0.05 | NS | 0.19 | 0.007 |
| IgG2 | 0.63 | <10–4 | 0.51 | <10–4 |
| IgG3 | 0.19 | 0.0095 | 0.18 | 0.0085 |
| IgG4 | −0.13 | NS | 0.13 | NS |
| IgG | 0.15 | 0.033 | 0.37 | <10–4 |
| Extract | ||||
| IgG1 | 0.22 | 0.0028 | 0.20 | 0.0032 |
| IgG2 | 0.69 | <10–4 | 0.56 | <10–4 |
| IgG3 | 0.47 | <10–4 | 0.60 | <10–4 |
| IgG4 | 0.26 | 0.0004 | 0.32 | <10–4 |
| IgG | 0.49 | <10–4 | 0.59 | <10–4 |
ρ, Spearman's rank correlation coefficient. NS, not significant.
The IgG subclass responses of sibs are correlated.
Sibling correlation coefficients are presented in Table 3. In the urban area, significant sib-sib correlations were found for most of the IgG subclass responses, except for the IgG3 response against RESA and the IgG2 and IgG responses against crude extract. The IgG responses against RESA, MSP-1-Nt, MSP-2-Nt, MSP-2-Ct1, and MSP-2-Ct2 were correlated between sibs. In the rural area, most of the IgG subclass responses of sibs were also correlated. Interestingly, the IgG subclass responses, the sibling correlation of which was not significant, were not the same in the urban and rural areas. If the two populations are included in the analysis, all the IgG responses of sibs were correlated, and the correlation coefficients ranged from 0.13 to 0.39. Furthermore, we also observed sib-sib correlations when selecting individuals under 7 years old (n = 78), who are of special interest when the outcome of infection and protective immunity are being studied. For example, the IgG1, IgG2, IgG3, and IgG4 responses against MSP-1-Nt, MSP-2-Nt, MSP-2-Ct1, and MSP-2-Ct2 were correlated between sibs (P < 0.05).
TABLE 3.
Sib-sib correlation coefficients for the IgG subclass responses to defined and crude P. falciparum antigensa
| Antigen and antibody | Urban area
|
Rural area
|
Urban and rural areas
|
|||
|---|---|---|---|---|---|---|
| Rss | P | Rss | P | Rss | P | |
| RESA | ||||||
| IgG1 | 0.23 | 0.0016 | 0.35 | 0.0010 | 0.25 | 0.0001 |
| IgG2 | 0.23 | 0.0020 | 0.23 | 0.0210 | 0.21 | 0.0006 |
| IgG3 | 0.12 | NS | 0.23 | 0.0234 | 0.14 | 0.0165 |
| IgG4 | 0.14 | 0.0032 | 0.46 | <10–4 | 0.30 | <10–4 |
| IgG | 0.22 | 0.0023 | 0.17 | NS | 0.20 | 0.0012 |
| MSP-1-Nt | ||||||
| IgG1 | 0.20 | 0.0054 | 0.30 | 0.0044 | 0.24 | 0.0001 |
| IgG2 | 0.22 | 0.0023 | 0.17 | NS | 0.21 | 0.0008 |
| IgG3 | 0.29 | 0.0001 | 0.31 | 0.0034 | 0.31 | <10–4 |
| IgG4 | 0.14 | 0.0290 | 0.39 | 0.0003 | 0.26 | 0.0001 |
| IgG | 0.28 | 0.0002 | 0.19 | 0.0450 | 0.24 | 0.0002 |
| MSP-2-Nt | ||||||
| IgG1 | 0.26 | 0.0004 | 0.23 | 0.0210 | 0.25 | 0.0001 |
| IgG2 | 0.14 | 0.0280 | 0.19 | 0.0457 | 0.21 | 0.0009 |
| IgG3 | 0.19 | 0.0071 | 0.28 | 0.0078 | 0.24 | 0.0001 |
| IgG4 | 0.32 | <10–4 | 0.24 | 0.0183 | 0.29 | <10–4 |
| IgG | 0.28 | 0.0002 | 0.34 | 0.0014 | 0.30 | <10–4 |
| MSP-2-Ct1 | ||||||
| IgG1 | 0.17 | 0.0120 | 0.13 | NS | 0.15 | 0.0105 |
| IgG2 | 0.46 | <10–4 | 0.27 | 0.0081 | 0.39 | <10–4 |
| IgG3 | 0.13 | 0.0470 | 0.22 | 0.0260 | 0.18 | 0.0032 |
| IgG4 | 0.24 | 0.0010 | 0.16 | NS | 0.21 | 0.0007 |
| IgG | 0.32 | <10–4 | 0.19 | 0.0480 | 0.26 | <10–4 |
| MSP-2-Ct2 | ||||||
| IgG1 | 0.18 | 0.0097 | 0.18 | NS | 0.18 | 0.0037 |
| IgG2 | 0.20 | 0.0057 | 0.22 | 0.0257 | 0.22 | 0.0006 |
| IgG3 | 0.25 | 0.0007 | 0.24 | 0.0163 | 0.26 | 0.0001 |
| IgG4 | 0.35 | <10–4 | 0.10 | NS | 0.26 | 0.0001 |
| IgG | 0.32 | <10–4 | 0.14 | NS | 0.25 | 0.0001 |
| Extract | ||||||
| IgG1 | 0.18 | 0.0110 | 0.36 | 0.0008 | 0.24 | 0.0002 |
| IgG2 | 0.12 | NS | 0.30 | 0.0040 | 0.19 | 0.0020 |
| IgG3 | 0.16 | 0.0207 | 0.27 | 0.0094 | 0.19 | 0.0018 |
| IgG4 | 0.21 | 0.0030 | 0.19 | 0.0490 | 0.21 | 0.0009 |
| IgG | 0.10 | NS | 0.26 | 0.0117 | 0.13 | 0.0230 |
The intraclass correlation coefficients were calculated as previously described (9), and the ANOVA F statistic was used to calculate the levels of significance.
Parent-offspring correlations.
Table 4 shows the parent-offspring correlations in antibody isotype responses. First, we computed the standard Pearson product-moment correlation over all possible parent-offspring pairs. In each area, most of the parent-offspring correlations were not significant; the mother-offspring correlations were nevertheless frequently significant. When the individuals from the two populations were analyzed, the mother-offspring correlations were significant, except for the IgG1, IgG2, IgG3, and IgG4 responses against RESA, the IgG2 and IgG responses against MSP-1, and the IgG1 and IgG responses against crude extract. The father-offspring correlations were less frequently significant, but they generally confirmed most of the mother-offspring correlations. This difference of significance may be explained by the smaller number of fathers (n = 56) than mothers (n = 72).
TABLE 4.
Parent-offspring correlation coefficients for the IgG subclass responses to defined and crude P. falciparum antigensa
| Antigen and antibody | Urban area
|
Rural area
|
Urban and rural areas
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rmo | P | Rfo | P | Rmo | P | Rfo | P | Rmo | P | Rfo | P | |
| RESA | ||||||||||||
| IgG1 | 0.04 | NS | 0.02 | NS | 0.15 | NS | 0.00 | NS | 0.09 | NS0.00NS | 0.00 | NS |
| IgG2 | 0.00 | NS | 0.06 | NS | 0.35 | 0.0014 | 0.20 | 0.0451 | 0.12 | NS | 0.12 | NS |
| IgG3 | 0.02 | NS | 0.10 | NS | 0.02 | NS | 0.16 | NS | 0.02 | NS | 0.12 | NS |
| IgG4 | 0.00 | NS | 0.13 | NS | 0.20 | NS | 0.40 | 0.0011 | 0.09 | NS | 0.22 | 0.0047 |
| IgG | 0.22 | 0.0267 | 0.11 | NS | 0.07 | NS | 0.24 | 0.0169 | 0.16 | 0.0239 | 0.17 | 0.0161 |
| MSP-1-Nt | ||||||||||||
| IgG1 | 0.20 | 0.0383 | 0.20 | 0.0358 | 0.10 | NS | 0.02 | NS | 0.16 | 0.0299 | 0.10 | NS |
| IgG2 | 0.00 | NS | 0.00 | NS | 0.24 | 0.0144 | 0.11 | NS | 0.05 | NS | 0.03 | NS |
| IgG3 | 0.27 | 0.0123 | 0.12 | NS | 0.21 | 0.0442 | 0.39 | 0.0007 | 0.24 | 0.0026 | 0.21 | 0.0085 |
| IgG4 | 0.22 | 0.0176 | 0.34 | 0.0006 | 0.08 | NS | 0.15 | NS | 0.15 | 0.0396 | 0.24 | 0.0023 |
| IgG | 0.24 | 0.0234 | 0.02 | NS | 0.00 | NS | 0.08 | NS | 0.10 | NS | 0.04 | NS |
| MSP-2-Nt | ||||||||||||
| IgG1 | 0.20 | 0.0454 | 0.30 | 0.0049 | 0.17 | NS | 0.18 | NS | 0.19 | 0.0124 | 0.24 | 0.0018 |
| IgG2 | 0.00 | NS | 0.00 | NS | 0.46 | <10–4 | 0.13 | NS | 0.22 | 0.0026 | 0.03 | NS |
| IgG3 | 0.20 | 0.0338 | 0.00 | NS | 0.27 | 0.0116 | 0.44 | 0.0001 | 0.21 | 0.0064 | 0.13 | NS |
| IgG4 | 0.35 | 0.0021 | 0.35 | 0.0023 | 0.16 | NS | 0.14 | NS | 0.27 | 0.0008 | 0.26 | 0.0014 |
| IgG | 0.22 | 0.0311 | 0.00 | NS | 0.15 | NS | 0.05 | NS | 0.19 | 0.0126 | 0.01 | NS |
| MSP-2-Ct1 | ||||||||||||
| IgG1 | 0.14 | NS | 0.15 | NS | 0.20 | 0.0294 | 0.11 | NS | 0.17 | 0.0144 | 0.12 | NS |
| IgG2 | 0.20 | NS | 0.00 | NS | 0.44 | 0.0001 | 0.21 | 0.0418 | 0.31 | 0.0003 | 0.10 | NS |
| IgG3 | 0.16 | NS | 0.00 | NS | 0.32 | 0.0025 | 0.37 | 0.0007 | 0.24 | 0.0012 | 0.04 | NS |
| IgG4 | 0.33 | 0.0023 | 0.32 | 0.0026 | 0.20 | 0.0328 | 0.13 | NS | 0.27 | 0.0004 | 0.23 | 0.0025 |
| IgG | 0.18 | NS | 0.00 | NS | 0.12 | NS | 0.12 | NS | 0.16 | 0.0314 | 0.04 | NS |
| MSP-2-Ct2 | ||||||||||||
| IgG1 | 0.20 | 0.0307 | 0.25 | 0.0111 | 0.15 | NS | 0.04 | NS | 0.18 | 0.0125 | 0.16 | 0.0190 |
| IgG2 | 0.00 | NS | 0.22 | 0.0219 | 0.43 | 0.0001 | 0.25 | 0.0165 | 0.18 | 0.0116 | 0.23 | 0.0023 |
| IgG3 | 0.28 | 0.0074 | 0.10 | NS | 0.44 | 0.0001 | 0.39 | 0.0005 | 0.36 | <10–4 | 0.20 | 0.0095 |
| IgG4 | 0.45 | 0.0002 | 0.33 | 0.0042 | 0.21 | 0.0271 | 0.22 | 0.0182 | 0.33 | <10–4 | 0.26 | 0.0008 |
| IgG | 0.21 | 0.0430 | 0.09 | NS | 0.18 | NS | 0.18 | NS | 0.20 | 0.0079 | 0.14 | 0.0500 |
| Extract | ||||||||||||
| IgG1 | 0.06 | NS | 0.16 | NS | 0.03 | NS | 0.12 | NS | 0.06 | NS | 0.14 | 0.0400 |
| IgG2 | 0.18 | 0.0413 | 0.00 | NS | 0.41 | 0.0003 | 0.22 | 0.0347 | 0.29 | 0.0001 | 0.10 | NS |
| IgG3 | 0.24 | 0.0106 | 0.09 | NS | 0.01 | NS | 0.27 | 0.0118 | 0.13 | 0.0498 | 0.13 | NS |
| IgG4 | 0.24 | 0.0164 | 0.29 | 0.0055 | 0.08 | NS | 0.03 | NS | 0.15 | 0.0277 | 0.18 | 0.0118 |
| IgG | 0.03 | NS | 0.05 | NS | 0.09 | NS | 0.19 | 0.0497 | 0.05 | NS | 0.10 | NS |
Rmo (Rfo) is the Pearson product-moment correlation coefficient between the mother's antibody response (father's antibody response) and the offspring's antibody response.
We also evaluated the family-weighted estimator by pairing the mean of the offspring phenotypes with the phenotype of their fathers or mothers and by computing the Pearson product-moment correlation over the resulting pairs (data not shown). We confirmed all the parent-offspring correlations found using the pairwise method. We also found additional parent-offspring correlations. For example, in the rural area, there were mother-offspring correlations for the IgG4 responses against RESA and MSP-2-Nt and father-offspring correlations for the IgG4 response against MSP-2-Nt and MSP-2-Ct1.
The IgG1, IgG2, IgG3, and IgG responses of spouses were not correlated except for the IgG1 response against MSP-2-Nt and the IgG response against RESA. Strikingly, the IgG4 responses of spouses were correlated (data not shown).
DISCUSSION
Since particular IgG subclass responses were found to be associated with resistance to malaria (1, 4, 6, 10, 14, 17, 29), genes controlling such responses may affect the resistance or the susceptibility to malarial infection or disease in humans. In this study, we evaluated familial correlation of IgG subclass responses against RESA, MSP-1, and MSP-2 conserved B epitopes and crude P. falciparum antigens in two populations living in differently exposed areas in Burkina Faso. To overcome the confounding effect of the antigen polymorphisms, we deliberately focused on conserved protein sequences commonly expressed by parasites, and we used a P. falciparum strain from Asia to prepare crude extract. We estimated sib-sib correlations and parent-offspring correlations through the variance analysis and the pairwise methods, respectively (9). We took into account covariates that significantly influenced the phenotype.
Age had a strong influence on parasite-specific IgG2 and IgG3 levels in the rural and urban areas. The influence of age on IgG1 or IgG4 was less clear. Because the cytophilic IgG2 and IgG3 have been correlated with resistance to P. falciparum malaria (1, 2, 17), of particular interest is the correlation between age on the one hand and IgG2 and IgG3 levels on the other. This is consistent with a slow development of protective immune responses (2, 7).
No difference between ethnic groups was found, and in particular, IgG subclass levels for the most represented group in the urban area (the Mossi) were similar to those for the other groups. This result is consistent with our previous report showing no interethnic difference in parasitemia in the same area (21). Modiano et al. (16) reported that the Mossi and Rimaïbe showed similar levels of antibody against RESA and Pf332, whereas the Fulani living in the same area displayed higher levels of antibody against both antigens and were also less parasitized. This suggested a genetic control of the capacity to mount protective immune responses and emphasized the need to analyze genetic control of IgG subclass responses against malarial antigens.
We obtained strong evidence for genetic regulation of the IgG subclass responses against several malarial antigens. There was sib-sib correlation for the IgG responses and the IgG subclass responses directed against RESA, MSP-1-Nt, MSP-2-Nt, MSP-2-Ct1, MSP-2-Ct2, and P. falciparum crude extracts. Moreover, parent-offspring correlations were also found for the IgG subclass responses. In particular, for the whole population, there were mother-offspring correlations for the IgG responses and the IgG subclass responses against MSP-2-Nt, MSP-2-Ct1, and MSP-2-Ct2 (Table 4). Father-offspring correlation coefficients were also significant for all the IgG subclass responses against MSP-2-Ct2. Similarly, Stirnadel et al. (27), in Papua New Guinea, observed significant heritability for the IgG, IgG1, IgG2, and IgG3 responses against MSP-2. Unfortunately, the IgG4 response was not analyzed in that study. Interestingly, the variance of some IgG subclass responses was partly explained by sharing of HLA class II genotypes. However, the heritability was low, and segregation analyses indicated that genetic control in IgG subclass responses was complex. These results are consistent with previous analyses of genetic control in the IgG responses to malarial antigens (12, 26). Those analyses suggested that the genetic contribution of MHC genes is lower than that of non-MHC genes. This pointed out the possible role of genes which control B-cell function, such as those encoding interleukin 4 (IL-4), IL-13, or IL-5, and which are located on chromosome 5q31-q33. In addition, chromosome 5q31-q33 is linked to blood infection levels (22). Genes located on chromosome 5q31-q33 might influence immunological parameters involved in protection, including some IgG subclass responses to malarial antigens.
Nevertheless, familial correlations may result partly from shared environmental factors or shared households, although the malaria transmission intensities were found to be homogeneous within each study area. The measure of spouse-spouse resemblance was used to estimate the influence of shared household on the phenotype. The IgG4 responses of spouses were correlated, suggesting that shared household influences the IgG4 responses. However, the absence of correlation among the IgG, IgG1, IgG2, and IgG3 responses of spouses argues against a strong influence of shared environmental effect. It should be stressed that members of nuclear families tend to occupy the same residence. When using methods for inferences concerning familial correlation, we cannot estimate the relative contribution of shared household and genetic components in explaining interfamily variations. Interestingly, sib pair linkage analyses compare only children from the same nuclear family and overcome such theoretical problems. Further linkage analyses are required to confirm the genetic control of IgG subclass responses and to localize genes involved.
In summary, we observed sibling correlations in families from two differently exposed populations living in Burkina Faso. We found substantial heritability for most of the IgG subclass responses directed against RESA, MSP-1, and MSP-2 conserved B epitopes and crude P. falciparum antigens. These results show that linkage and association studies should be done to localize and identify genes regulating IgG subclass responses. The identification of such genes may help in determining the genetic control of susceptibility to malarial infection and disease. Moreover, polymorphisms of these genes would have to be considered in the development of vaccines.
ACKNOWLEDGMENTS
We thank all volunteer families of Bobo-Dioulasso and Logoforousso for their participation. We thank the medical authority of Burkina Faso “Ministère de la Santé,” Ouagadougou, and “Direction provinciale de la Santé,” Bobo-Dioulasso, for encouragement during this work. We thank Alain Bourgois for critical reading of the manuscript and A. S. Traoré and F. Tall for their encouragement.
This work was supported by research grants from the French Ministry of Research and Technology, from the AUF LAF 303, and from the Fondation pour la Recherche Médicale. C.A. was supported by a studentship from the Fondation pour la Recherche Médicale.
REFERENCES
- 1.Aribot G, Rogier C, Sarthou J L, Trape J F, Balde A T, Druilhe P, Roussilhon C. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, west Africa) Am J Trop Med Hyg. 1996;54:449–457. doi: 10.4269/ajtmh.1996.54.449. [DOI] [PubMed] [Google Scholar]
- 2.Aucan C, Traore Y, Tall F, Nacro B, Traore L T, Fumoux F, Rihet P. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect Immun. 2000;68:1252–1258. doi: 10.1128/iai.68.3.1252-1258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball J M, Henry N L, Montelaro R C, Newman M J. A versatile synthetic peptide-based ELISA for identifying antibody epitopes. J Immunol Methods. 1994;171:37–44. doi: 10.1016/0022-1759(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 4.Beck H P, Felger I, Genton B, Alexander N, al-Yaman F, Anders R F, Alpers M. Humoral and cell-mediated immunity to the Plasmodium falciparum ring-infected erythrocyte surface antigen in an adult population exposed to highly endemic malaria. Infect Immun. 1995;63:596–600. doi: 10.1128/iai.63.2.596-600.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chumpitazi B F, Lepers J P, Simon J, Deloron P. IgG1 and IgG2 antibody responses to Plasmodium falciparum exoantigens correlate inversely and positively, respectively, to the number of malaria attacks. FEMS Immunol Med Microbiol. 1996;14:151–158. doi: 10.1111/j.1574-695X.1996.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 7.Day K P, Marsh K. Naturally acquired immunity to Plasmodium falciparum. Immunol Today. 1991;12:A68–A71. doi: 10.1016/s0167-5699(05)80020-9. [DOI] [PubMed] [Google Scholar]
- 8.Donner A. The use of correlation and regression in the analysis of family resemblance. Am J Epidemiol. 1979;110:335–342. doi: 10.1093/oxfordjournals.aje.a112819. [DOI] [PubMed] [Google Scholar]
- 9.Donner A, Eliasziw M. Methodology for inferences concerning familial correlations: a review. J Clin Epidemiol. 1991;44:449–455. doi: 10.1016/0895-4356(91)90084-m. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira U M, Kimura E A, Katzin A M, Santos-Neto L L, Ferrari J O, Villalobos J M, de Carvalho M E. The IgG-subclass distribution of naturally acquired antibodies to Plasmodium falciparum, in relation to malaria exposure and severity. Ann Trop Med Parasitol. 1998;92:245–256. doi: 10.1080/00034989859807. [DOI] [PubMed] [Google Scholar]
- 11.Grau G E, Taylor T E, Molyneux M E, Wirima J J, Vassalli P, Hommel M, Lambert P H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 12.Jepson A, Banya W, Sisay-Joof F, Hassan-King M, Nunes C, Bennett S, Whittle H. Quantification of the relative contribution of major histocompatibility complex (MHC) and non-MHC genes to human immune responses to foreign antigens. Infect Immun. 1997;65:872–876. doi: 10.1128/iai.65.3.872-876.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones G L, Edmundson H M, Lord R, Spencer L, Mollard R, Saul A J. Immunological fine structure of the variable and constant regions of a polymorphic malarial surface antigen from Plasmodium falciparum. Mol Biochem Parasitol. 1991;48:1–9. doi: 10.1016/0166-6851(91)90158-3. [DOI] [PubMed] [Google Scholar]
- 14.Kitua A Y, Urassa H, Wechsler M, Smith T, Vounatsou P, Weiss N A, Alonso P L, Tanner M. Antibodies against Plasmodium falciparum vaccine candidates in infants in an area of intense and perennial transmission: relationships with clinical malaria and with entomological inoculation rates. Parasite Immunol. 1999;21:307–317. doi: 10.1046/j.1365-3024.1999.00230.x. [DOI] [PubMed] [Google Scholar]
- 15.Lambros C, Vanderberg J P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 16.Modiano D, Chiucchiuini A, Petrarca V, Sirima B S, Luoni G, Perlmann H, Esposito F, Coluzzi M. Humoral response to Plasmodium falciparum Pf155/ring-infected erythrocyte surface antigen and Pf332 in three sympatric ethnic groups of Burkina Faso. Am J Trop Med Hyg. 1998;58:220–224. doi: 10.4269/ajtmh.1998.58.220. [DOI] [PubMed] [Google Scholar]
- 17.Oeuvray C, Theisen M, Rogier C, Trape J F, Jepsen S, Druilhe P. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect Immun. 2000;68:2617–2620. doi: 10.1128/iai.68.5.2617-2620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patarroyo E M, Romero P, Torres M L, Clavijo P, Moreno A, Martinez A, Rodriguez R, Guzman F, Cabezas E. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature. 1987;328:629–632. doi: 10.1038/328629a0. [DOI] [PubMed] [Google Scholar]
- 19.Patarroyo E M, Vinasco J, Amador R, Espejo F, Silva Y, Moreno A, Rojas M, Mora A L, Salcedo M, Valero V, et al. Genetic control of the immune response to a synthetic vaccine against Plasmodium falciparum. Parasite Immunol. 1991;13:509–516. doi: 10.1111/j.1365-3024.1991.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 20.Perlmann H, Perlmann P, Berzins K, Wahlin B, Troye-Blomberg M, Hagstedt M, Andersson I, Hogh B, Petersen E, Bjorkman A. Dissection of the human antibody response to the malaria antigen Pf155/RESA into epitope specific components. Immunol Rev. 1989;112:115–132. doi: 10.1111/j.1600-065x.1989.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 21.Rihet P, Abel L, Traoré Y, Traoré-Leroux T, Aucan C, Fumoux F. Human malaria: segregation analysis of blood infection levels in a suburban area and a rural area in Burkina Faso. Genet Epidemiol. 1998;15:435–450. doi: 10.1002/(SICI)1098-2272(1998)15:5<435::AID-GEPI1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Rihet P, Traoré Y, Abel L, Aucan C, Traoré-Leroux T, Fumoux F. Malaria in humans: Plasmodium falciparum blood infection levels are linked to chromosome 5q31–q33. Am J Hum Genet. 1998;63:498–505. doi: 10.1086/301967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riley E M, Allen S J, Troye B M, Bennett S, Perlmann H, Andersson G, Smedman L, Perlmann P, Greenwood B M. Association between immune recognition of the malaria vaccine candidate antigen Pf155/RESA and resistance to clinical disease: a prospective study in a malaria-endemic region of west Africa. Trans R Soc Trop Med Hyg. 1991;85:436–443. doi: 10.1016/0035-9203(91)90207-f. [DOI] [PubMed] [Google Scholar]
- 24.Riley E M, Allen S J, Wheeler J G, Blackman M J, Bennett S, Takacs B, Schonfeld H J, Holder A A, Greenwood B M. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992;14:321–337. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 25.Riley E M, Olerup O, Bennett S, Rowe P, Allen S J, Blackman M J, Troye-Blomberg M, Holder A A, Greenwood B M. MHC and malaria: the relationship between HLA class II alleles and immune responses to Plasmodium falciparum. Int Immunol. 1992;4:1055–1063. doi: 10.1093/intimm/4.9.1055. [DOI] [PubMed] [Google Scholar]
- 26.Sjoberg K, Lepers J P, Raharimalala L, Larsson A, Olerup O, Marbiah N T, Troye B M, Perlmann P. Genetic regulation of human anti-malarial antibodies in twins. Proc Natl Acad Sci USA. 1992;89:2101–2104. doi: 10.1073/pnas.89.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stirnadel H A, Beck H P, Alpers M P, Smith T A. Genetic analysis of IgG subclass responses against RESA and MSP2 of Plasmodium falciparum in adults in Papua New Guinea. Epidemiol Infect. 2000;124:153–162. doi: 10.1017/s0950268899003325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stirnadel H A, Beck H P, Alpers M P, Smith T A. Heritability and segregation analysis of immune responses to specific malaria antigens in Papua New Guinea. Genet Epidemiol. 1999;17:16–34. doi: 10.1002/(SICI)1098-2272(1999)17:1<16::AID-GEPI2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Taylor R R, Allen S J, Greenwood B M, Riley E M. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am J Trop Med Hyg. 1998;58:406–413. doi: 10.4269/ajtmh.1998.58.406. [DOI] [PubMed] [Google Scholar]
- 30.Taylor R R, Egan A, McGuinness D, Jepson A, Adair R, Drakely C, Riley E. Selective recognition of malaria antigens by human serum antibodies is not genetically determined but demonstrates some features of clonal imprinting. Int Immunol. 1996;8:905–915. doi: 10.1093/intimm/8.6.905. [DOI] [PubMed] [Google Scholar]
- 31.Traoré Y, Rihet P, Traoré-Leroux T, Aucan C, Gazin P, Coosemans M, Smith A, Abel L, Tall F, Nacro B, Traoré A, Fumoux F. Analysis of the genetic factors controlling malarial infection in man. Sante. 1999;9:53–59. [PubMed] [Google Scholar]
- 32.Troye-Blomberg M, Olerup O, Larsson A, Sjoberg K, Perlmann H, Riley E, Lepers J P, Perlmann P. Failure to detect MHC class II associations of the human immune response induced by repeated malaria infections to the Plasmodium falciparum antigen Pf155/RESA. Int Immunol. 1991;3:1043–1051. doi: 10.1093/intimm/3.10.1043. [DOI] [PubMed] [Google Scholar]


