Abstract
Trauma, burns, and diabetes result in nonhealing wounds that can cause bone or tendon exposure, a significant health threat. The use of an artificial regeneration template combined with skin grafting as an alternative method to highly invasive flap surgery has been shown to be an effective way to cover full‐thickness skin defects with bone or tendon exposure for both functional and aesthetic recovery. However, artificial regeneration templates, such as Pelnac, are overwhelmingly expensive, limiting their clinical use. Here, we demonstrate for the first time that polyurethane film combined with absorbable gelatine sponge, affordable materials widely used for haemostasis, are effective for dermal reconstruction in wounds with bone or tendon exposure. The absorbable gelatine sponge combined with polyurethane film was applied to eight patients, all resulting in adequate granulation that fully covered the exposed bone or tendon. The outcome of absorbable gelatine sponge combined with polyurethane film application indicates that this approach is a potential novel and cost‐effective dermal reconstruction strategy for the treatment of severe wounds with bone or tendon exposure.
Keywords: absorbable gelatine sponge, artificial dermis, full‐thickness skin defect, polyurethane film, wound healing
1. INTRODUCTION
Full‐thickness skin defects due to trauma, diabetes, and cutaneous tumour excision, especially chronic nonhealing wounds, are becoming a significant health problem. It is estimated that between 15% and 20% of patients with diabetes will develop chronic nonhealing foot wounds. 1 Recent epidemiological data showed that approximately 1 to 3 million patients in the US suffered from chronic pressure ulcers annually. 2 Among these full‐thickness skin injuries, wounds with bone or tendon exposure that cannot be closed directly remain a challenge, and the development of effective dermal regeneration templates has become a research focus.
Wounds with bone or tendon exposure often require flap surgery, which can cause more damage and is limited by the position and area of the wound site. The artificial dermis is a useful biomaterial for reconstructing skin defects with bone or tendon exposure after extensive burns, 3 acute trauma, 4 and reconstructive surgery. 5 Classically, a double layer of the artificial dermis, such as Integra and Pelnac, is transplanted onto the wound site for 2 to 3 weeks until adequate vascularization occurs and followed by a skin graft for eventual wound healing. 6 , 7 , 8 , 9 , 10 The double‐layer structure comprises an upper layer of silicone membrane and a collagen sponge lower layer. The upper layer can prevent drying and infection, and protect the wound. The lower layer is a porous three‐dimensional scaffold structure that allows the extension and growth of the granulation tissue. This material can be used to cover wounds with exposed bone or tendon in the early stage of trauma, provide gradual coverage of granulation tissue without further necrosis, and requires only a secondary skin graft to cover the wound. 11 , 12 This approach causes less surgical trauma than skin flap transplantation, and no bloated appearance occurs. However, dermal regeneration templates such as Pelnac are comparatively expensive in China (approximately USD 2000 for 82 × 120 mm), especially for large‐area wounds, limiting their clinical use. Inspired by this, we designed a new double‐layer dermis dressing using polyurethane film and the absorbable gelatine sponge.
In this study, we are the first to demonstrate the usefulness of a haemostasis material, absorbable gelatine sponge (AGS), combined with polyurethane film as a low‐cost (approximately USD 10 for 82 × 120 mm) reconstructive surgery approach for severe skin defects with bone or tendon exposure.
2. MATERIAL AND METHODS
2.1. Patients
The hospital ethics committee approved this study, and all patients provided written informed consent to the treatment, healing process, and use of their pictures.
Six patients were male, and two were female. Their ages ranged from 48 to 63 years. The patients were transferred from different primary hospitals for surgical treatment at the Department of Burn and Plastic Surgery, Jinling Hospital, Nanjing University, from 2020 to 2021.
2.2. Preparation of the double‐layer AGS template
In this study, the absorbable gelatine sponge (Jinling Pharmaceutical Company, Ltd, Nanjing, China) and polyurethane film dressing (3M Tegaderm) were utilised. This bilayer, cost‐effective regeneration template was comprised of an upper viscous polyurethane film dressing at a thickness of 0.05 mm and a lower absorbable gelatine sponge at a thickness of 5 mm. The materials were pressed together gently to form the double‐layer dressing. The price and composition of the AGS template were compared with those of two other double‐layer artificial dermis materials commonly used in China.
2.3. Surgical procedure
All surgical procedures were performed under aseptic conditions. After debridement and haemostasis, bone or tendon exposure and absence of periosteum or aponeurosis could be seen. The wound was rinsed with hydrogen peroxide, 5% povidone‐iodine, and saline. Then, the wound was covered by a double‐layer AGS template as follows: (a) The AGS template was trimmed with scissors to fit the shape and size of the wound; (b) the double‐layer AGS template was fixed to the skin defect with sutures (No. 1/0 silk thread); (c) small drainage holes were made in the upper film to facilitate exudation. A pressure dressing was applied for 5 or 7 days depending on the extent of infection and the area of the skin defect; subsequently, the dressing was changed every 2 days. The upper film was not removed from the wound until the AGS was adequately vascularized. If haematomas were present under the artificial dermis or the gelatine sponge dissolved, the haematomas were removed and the AGS template was replaced. After the adequate growth of granulation tissue at the wound site, autologous skin grafting was performed. Patients with wounds positive for bacterial infections were administered appropriate antibiotics by intravenous drip.
2.4. Microstructural examination of the AGS template
The prepared bilayer AGS template was examined using scanning electron microscopy (SEM, HITACHI SU8100). Geometrical measurements of AGS microstructures were performed by SEM, and the pore size was further measured using ImageJ software.
2.5. Histological examination
Skin samples containing the wound edge and newly formed dermal tissue were collected from patient 2 in the third postoperative week, and the specimen was immersed in 10% formalin. The sample was dehydrated using an ethanol gradient and embedded in paraffin. Micrometre‐level thin tissue slices were prepared, and Haematoxylin & Eosin, Masson's trichrome staining were performed. Photomicrographs were collected using a Leica DFC295 microscope.
3. RESULTS
3.1. Clinical observations
Of the eight patients in this study, four had deep dermal burns (flame, chemical, and electric burns), one had a diabetic foot lesion, one had a crush injury, one had an open fracture, and one had osteomyelitis. The site of reconstructive surgery was the lower limb in five patients and the upper limb in three patients. The wound secretions of five patients were positive for bacterial infection, while the other three patients had negative results (Table 1, Figures 3, 4, 5 & S1). Reconstruction with a double‐layer AGS template was performed for wounds with bone or tendon exposure in which wound closure by secondary intention was considered challenging due to inadequate local blood supply. Complete wound closure was achieved in patient 3 after full‐extent epithelialization in the regenerated dermis‐like tissue with AGS. The second surgery involved full‐thickness skin grafting in two patients, medium‐thickness skin grafting in four patients, and thin split‐skin grafting in one patient. The mean duration of adequate vascularization of the dermis‐like tissues was 15.9 ± 6.3 days (Table 1). Wound closure was achieved in all patients. During the 3‐month follow‐up period, no wound infection, recurrent skin ulceration, or exposure to bone or tendon were observed. In this study, no patients experienced allergic reactions to the materials, restricted range of motion from scar contracture or disfigurement. Moreover, the price of the AGS template is considerably lower than that of other double‐layer artificial dermis materials in China (Table 2).
TABLE 1.
Clinical cases treated with the double‐layer absorbable gelatine sponge template
| Case | Age | Sex | Location | Skin defect size (cm2) | Wound infection | Cause | Exposed bone or tendon | Underlying disease | Adequate granulation time |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | Male | Left foot | 12 × 6 | Acinetobacter baumannii | Diabetic foot | Tendon and bone exposure; partly necrosis | Diabetes | 18 d |
| 2 | 52 | Male | Right tibia | 8 × 3 | Klebsiella pneumoniae and Candida | Burn third‐degree 75% TBSA | Bone exposure | None | 17 d |
| 3 | 60 | Male | Left middle finger | 1.5 × 1 | Negative | Hydrofluoric acid burn | Bone exposure; partly necrosis | None | 12 d |
| 4 | 51 | Female | Right index and middle finger | 2 × 1; 1 × 1 | Negative | Hot crush injury | Tendon exposure | None | 7 d |
| 5 | 55 | Male | Left forearm and elbow | 25 × 5 | Gram positive coccus | Crush injury | Tendon and bone exposure; partly necrosis | None | 20 d |
| 6 | 48 | Male | Right foot | 4 × 3 | Negative | Electric injury | Tendon exposure | None | 10 d |
| 7 | 56 | Male | Right tibia anterior | 3 × 1 | Gram positive coccus; Pseudomonas aeruginosa | Osteomyelitis | Bone exposure | Arteritis | 27 d |
| 8 | 63 | Female | Right tibia anterior | 3 × 3 | Gram positive coccus | Trauma bone fracture | Steel plate and bone exposure | Diabetes | 16 d |
FIGURE 3.
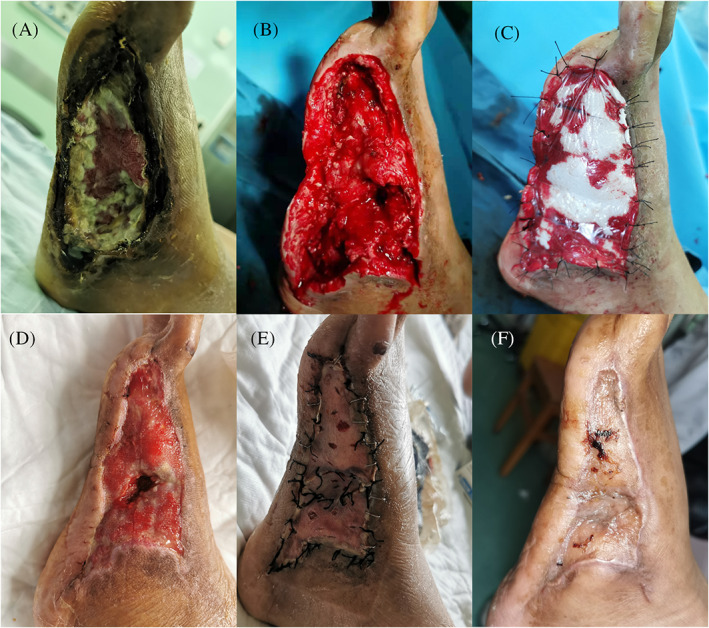
Case 1 (Table 1) (A) A 58‐year‐old man with a left diabetic foot lesion, (B) debridement leading to partial exposure of the metatarsal bone, cuboid bone, and partial flexor digitorum longus tendon, (C) the absorbable gelatine sponge template was applied to the wound, (D) adequate granulation was achieved when the 3 M film and sutures were removed, (E) medium‐thickness skin grafting was performed, and (F) one month after skin grafting surgery, the wound showed good resurfacing
FIGURE 4.
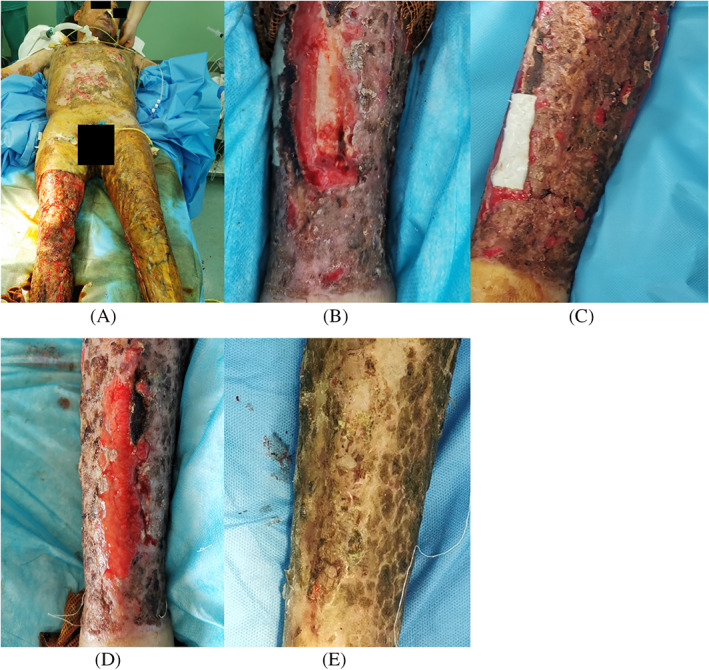
Case 2 (Table 1) (A) A 52‐year‐old man with a large‐area full‐thickness burn, (B) resection of necrotic tissue leads to an 80 × 30 mm skin defect in the right tibia region with partially exposed shin bone, (C) the absorbable gelatine sponge (AGS) template was applied to the surface of the exposed shin bone, (D) adequate granulation covered the whole wound area when the top layer of the AGS template was removed 17 days after surgery, and (E) 18 days after split‐thickness skin grafting
FIGURE 5.
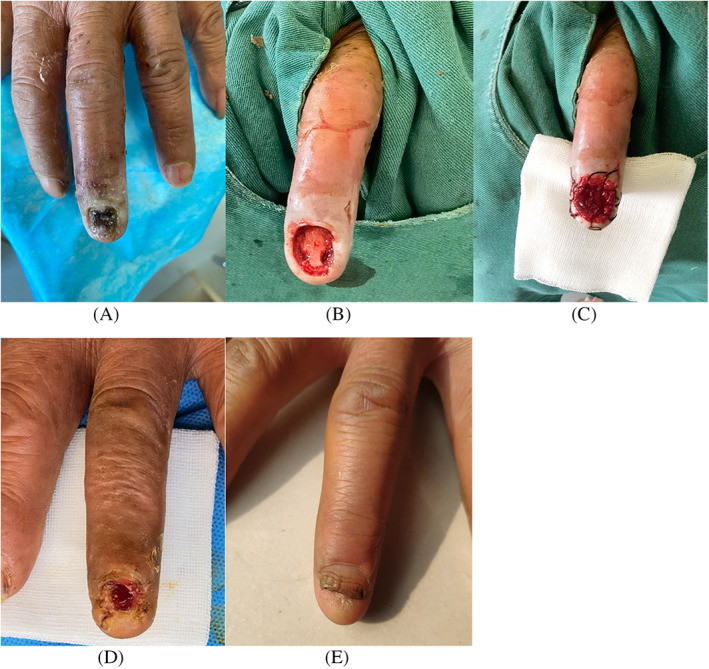
Case 3 (Table 1) (A) A 60‐year‐old man with a deep hydrofluoric acid burn on his left middle finger, (B) extensive debridement leading to the exposure of the phalangeal bone, (C) the absorbable gelatine sponge template was applied and secured with silk thread, (D) when the polyurethane sheet was removed on POD 12, the distal phalanx was covered with firm granulation tissue and partially epithelialized, and (E) full epithelialization and nail regeneration were observed 5 months after surgery
TABLE 2.
Comparison of double‐layer artificial dermal templates used in China
| Brand | Structure | Top sheet | Thickness | Size | Price |
|---|---|---|---|---|---|
| Pelnac | Bilayer | Silicone sheet | Top layer: 0.15 mm; Scaffold layer: 3 mm | 20 × 30 to 82 × 120 mm2 | 24.76 USD/cm2 |
| Lando | Bilayer | Silicone sheet | Top layer: 0.25 mm; Scaffold layer: 2 mm | 20 × 30 to 80 × 120 mm2 | 23.11 USD/cm2 |
| Absorbable gelatine sponge template | Bilayer | Polyurethane sheet | Top layer: 0.05 mm; Scaffold layer: 5 mm | 20 × 60 mm2 | 0.12 USD/cm2 |
3.2. Microstructural characterisation of the AGS template
Absorbable gelatine sponges have porous and interconnected network structures with a wide range of pore sizes ranging from 40 to 360 μm in diameter (Figure 1). The full structure of a bilayer wound dressing comprises a thin polyurethane film (PU) attached to the AGS. The polyurethane film did not affect the porous structure of the gelatine sponge, and the two layers adhered firmly to each other.
FIGURE 1.
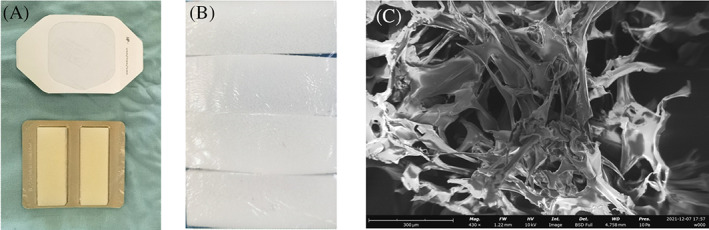
Macro and scanning electron microscope images of the absorbable gelatine sponge (AGS) template used in this study: (A) polyurethane film dressing (3M Tegaderm) and the absorbable gelatine sponge (Jinling Pharmaceutical Company, Ltd, Nanjing, China), (B) the polyurethane film and AGS were bonded together and trimmed according to the size of the wound, and (C) scanning electron microscopy images show the internal structure of the AGS template (scale bar: 300 μm)
3.3. Histological findings
Seventeen days after the application of the AGS wound dressing, the newly generated dermis tissue was adequately vascularized. The collagen fibres, fibroblasts, and infiltrated inflammatory cells were present. The collagen fibres appeared to be parallel to the surface and less organised than those in the normal skin dermis. In addition, a thin layer of epidermal tissue was observed in the dermal tissue generated by the AGS template. This epidermal tissue was thought to be epithelized from the normal tissue around the wound. Detailed information is shown in Figure 2.
FIGURE 2.
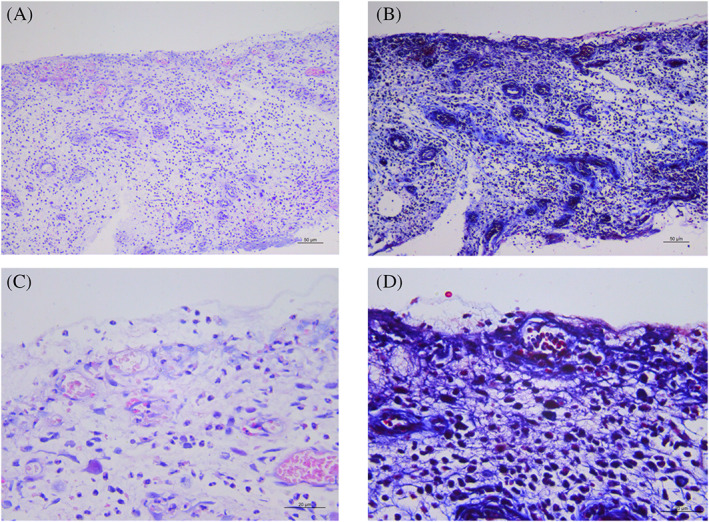
Histological examination of the absorbable gelatine sponge template graft covering the right shin bone of patient 2 on POD 17: (A,B) Haematoxylin and Eosin staining with scale bars of 50 μm (×100) and 20 μm (×400), respectively, and (C,D) Masson's trichrome staining with scale bars of 50 μm (×100) and 20 μm (×400), respectively
3.4. Clinical cases
3.4.1. Case 1
The patient in Case 1 was a 58‐year‐old man. He had an 8‐year history of type II diabetes with poor blood sugar level control and presented with skin ulcers on his left foot that had persisted for 1 year. The fourth and fifth toes of the patient's left foot had been amputated at a primary hospital. An arterial stent was placed into his anterior tibial artery to guarantee an adequate blood supply to the patient's left foot, and he was subsequently transferred to our department. A cutaneous ulcer with adherent black necrotic tissue 120 × 60 mm in size was present on the metatarsal bone and cuboid bone, and the patient was diagnosed with an infected diabetic foot lesion (Figure 3A). Debridement was performed under general anaesthesia to remove the necrotic tissue, leading to the partial exposure of the metatarsal bone, cuboid bone, and partial flexor digitorum longus tendon, and we further removed the cortical layer of bone to the level of healthy tissue (Figure 3B). After adequate haemostasis was achieved, the area was rinsed with hydrogen peroxide, 5% povidone‐iodine, and saline. Because the wound was deep, first, a layer of gelatine sponge was applied, and the double‐layer AGS template was subsequently used to cover the area (Figure 3C) and further secured with a negative pressure wound therapy (NPWT) device with the appropriate suction pressure. The polyurethane film of the AGS template was removed on postoperative day (POD) 18, and the exposed bones and tendon were completely covered by a firm layer of dermis‐like tissue (Figure 3D). Because a high‐friction area of the patient's foot was affected, medium‐thickness skin grafting was then performed with donor skin taken from the right thigh region (Figure 3E). Subsequent treatment was administered conservatively with Vaseline gauze, and complete wound closure was achieved 31 days after the surgery (Figure 3F).
3.4.2. Case 2
The patient in Case 2 was a 52‐year‐old man. The patient experienced a 3‐minute‐long flame exposure while working in a factory, causing a full‐thickness burn covering 75% of his total body surface area (Figure 4A). The right lower leg was debrided in our department with the patient under general anaesthesia. After resection of the necrotic tissue and repeated autologous thin split‐skin grafting, an 80 × 30 mm skin defect remained in the right tibia region with partially exposed shin bone (Figure 4B). The skin defect was covered with a double layer AGS template (Figure 4C). When the polyurethane film was removed from the AGS template on POD 17, satisfactory proliferation of dermis‐like tissue was observed, and the right tibia was completely covered (Figure 4D). Minimal curettage of the dermis‐like tissue was performed on POD 20, and thin split‐skin grafting at a thickness of 0.3 mm using autologous skin taken from the scalp region was performed, secured with the NPWT device. The NPWT device was removed on POD 5, and extremely favourable skin graft adherence was observed. All the skin grafts remained viable, and complete wound closure was observed 18 days after the second surgery (Figure 4E).
3.4.3. Case 3
The patient in Case 3 was a 60‐year‐old man whose left hand was exposed to hydrofluoric acid while working, resulting in skin and nail necrosis that extended to the bone surface of the distal phalanx of the left middle finger (Figure 5A). Extensive debridement of necrotic tissue, including partial bone cortex, and irrigation of the exposed phalangeal bone were performed under infiltration anaesthesia. Then, the area was covered with a 15 × 10 mm double‐layer AGS template secured to the wound with silk thread, and the template was subsequently covered with Vaseline gauze (Figure 5B,C). When the polyurethane film was removed on POD 12, satisfactory proliferation of dermis‐like tissue was confirmed, and the distal phalanx was covered in granulation tissue and partially epithelialized (Figure 5D). Conservative treatment with Vaseline gauze was continued, and complete epithelialization of the wound was observed on POD 22. No phalangeal bone exposure, digital motor dysfunction, or digital apex pain were observed 5 months postoperatively, and the clinical course was favourable (Figure 5E).
4. DISCUSSION
The regeneration template consisting of AGS and polyurethane film was useful for treating wounds with bone or tendon exposure, promoting the regeneration of firm dermis‐like tissue. Achieving adequate granulation in wounds with bone or tendon exposure is challenging due to their poor angiogenic ability. 13 Treating such wounds required highly invasive surgery in the past, such as free flap transplantation. 14 Patients with wounds with bone or tendon exposure have often experienced severe trauma that renders the surrounding tissue to be unfit for a standard graft. Flap surgery can also be contraindicated in cases where there is a lack of adequate flap donor sites or poor general clinical conditions, such as in patients with large burn areas. Therefore, biologic double‐layer scaffolds that are capable of covering a wide variety of soft tissue defects have been developed for both functional and cosmetic purposes. 1
Ideal skin substitutes and wound dressings provide mechanical protection and an optimum environment for moist wound healing, protecting the wound from dehydration, and should be flexible, convenient, and non‐toxic to healthy tissue. 15 Here, we successfully achieved favourable outcomes after performing reconstructive surgery with a hybrid artificial dermis consisting of AGS and polyurethane film for treating wounds with bone or tendon exposure. The double‐layer AGS template was used as a simple regenerative device and allowed surgeons to perform a minimally invasive treatment procedure and achieve adequate granulation in an average of 2 weeks. Biological templates with sponge structures can serve as scaffolds for the regeneration of volumetric dermal or lower tissue loss without the tissue depression formation at the wound site. 16 Several in vivo experiments in animals indicated that wound dressings with sponge structures effectively promote the healing of full‐thickness skin wounds. 16 , 17 , 18 , 19 , 20 , 21 Some studies have indicated that sponge structures can promote wound healing more effectively than gauze or traditional Chinese medicine. 22 In addition, the results of a previous study showed that a biological wound dressing with a composite sponge structure derived from sheep's small intestinal submucosa adhered uniformly to the wound and absorbed more wound exudation than polyurethane film. 15 Collagens in biological wound dressings are natural derivatives and have excellent biocompatibility; nevertheless, these materials degenerate rapidly. However, in the AGS template adopted in this study, the chemical crosslinking of gelatine was found to improve the mechanical strength and slow down the degradation rate, 23 extending the wound scaffolding duration. Of note, in this study, the AGS template was also successfully applied to all five patients with wounds positive for bacterial infection, indicating its potential as a biological scaffold for the treatment of infected skin defects.
The traditional artificial double‐layer dermis products Integra and Pelnac contain an outer layer of silicone sheet, which plays an important role in successful dermal regeneration. 24 , 25 This silicone sheet reinforces the mechanical strength of the regeneration template while having an air permeability level close to that of normal skin. 26 , 27 The Tegaderm (3M) polyurethane film used in this study is waterproof and breathable and was previously reported to be effective in the treatment of burns. 28 The other component of the AGS regeneration template, the absorbable gelatine sponge, is considered to have an ideal pore size because of its controllable structure, which is conducive to the growth of granulation tissue. Studies have shown that a small pore size scaffold (<200 μm) is conducive to the formation of a small vessel network with high density, but the penetration depth is low. 29 A large pore size scaffold (>200 μm) is conducive to the formation of a large blood vessel network with low density but high penetration depth. 29 The pore size of the scaffold is the key factor in blood vessel growth and pore sizes >250 μm promote faster growth. 30 The AGS template pore size ranged from approximately 40 to 360 μm in diameter in this study. The pathological results confirmed the formation of good granulation tissue. We have previously attempted to use a single layer of absorbable gelatine sponge for bone or tendon exposed wounds, but that approach failed. The double‐layer AGS template used in this study combines the advantages of synthetic and biological wound dressings and shows good effects. Two or more layers of AGS can be used for wounds with a depth of more than 5 mm to improve the appearance of the repaired wound.
The NPWT device was used for some patients in this study, which is mainly conducive to the drainage of wound secretions and blood, reducing the bacterial burden and ensuring that the artificial dermis fits more closely with the wound; additionally, there is no need to change dressings frequently. All patients in this study had periosteal or aponeurosis defects and the direct application of the NPWT device on wounds with these defects was ineffective. The NPWT device can increase the blood flow to wounds. However, some studies have shown that the application of NPWT devices on artificial dermis does not significantly accelerate the vascularization process. 31 Further research is needed to confirm the necessity of using NPWT devices with the AGS template.
In addition to effectively treating wounds with bone or tendon exposure, the AGS template used in this study has another distinct advantage, which is the low price. Pelnac is the most widely used artificial dermal product approved for wound treatment by the China Food and Drug Administration in China. 6 However, Pelnac is expensive, costing approximately USD 2000 for an 82 × 120 mm treatment. In contrast, the AGS template used in this study only costs approximately USD 10 for the same size. Thus, the AGS template could be a potential cost‐reducing alternative dermal regeneration template. All eight patients in this study were transferred from different primary hospitals at which no double‐layer artificial dermal dressings were administered, resulting in delayed wound treatment. These patients were unable to afford Pelnac or other current double‐layer dermis products. Due to its low price and easy harvesting, the double‐layer AGS template is beneficial as an affordable tool for treating acute and chronic wounds with bone or tendon exposure in primary hospitals.
In conclusion, this is the first report to prove that polyurethane film combined with the absorbable gelatine sponge is safe and cost‐effective for repairing wounds with exposed bone or tendons. Since this study was limited to a strictly defined patient population, further studies involving more patients are required to validate our findings.
CONFLICT OF INTEREST
All authors hereby declare that they have no conflicts of interest.
Supporting information
Figure S1 Findings during AGS template treatment of the remaining 5 patients involved in this study
ACKNOWLEDGEMENT
The Medical Technology Foundation (No. CLB20J022) supported this study.
Yu P, Hong N, Chen M, Zou X. Novel application of absorbable gelatine sponge combined with polyurethane film for dermal reconstruction of wounds with bone or tendon exposure. Int Wound J. 2023;20(1):18‐27. doi: 10.1111/iwj.13832
Pan Yu Nan Hong and Min Chen contributed equally to this work and should share the first co‐authorship.
Funding information Medical Technology Foundation, Grant/Award Number: CLB20J022
Contributor Information
Pan Yu, Email: yp52@163.com.
Xianbiao Zou, Email: xbzou@126.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Turner NJ, Badylak SF. The use of biologic scaffolds in the treatment of chronic nonhealing wounds. Adv Wound Care. 2015;4(8):490‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harrison T, Kindred J, Marks‐Maran D. Reducing avoidable harm caused by pressure ulcers. Br J Nurs. 2013;22(6):S4 S6, S8 passim. [DOI] [PubMed] [Google Scholar]
- 3. Heimbach D, Luterman A, Burke J, et al. Artificial dermis for major burns. A multi‐center randomized clinical trial. Ann Surg. 1988;208(3):313‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeong EK, Yu YC, Chan ZH, Roan TL. Is artificial dermis an effective tool in the treatment of tendon‐exposed wounds? J Burn Care Res. 2013;34(1):161‐167. [DOI] [PubMed] [Google Scholar]
- 5. Soejima K, Nozaki M, Sasaki K, Takeuchi M, Negishi N. Reconstruction of burn deformity using artificial dermis combined with thin split‐skin grafting. Burns. 1997;23(6):501‐504. [DOI] [PubMed] [Google Scholar]
- 6. Lou X, Xue H, Li G, et al. One‐stage Pelnac reconstruction in full‐thickness skin defects with bone or tendon exposure. Plast Reconstr Surg Glob Open. 2018;6(3):e1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamashita Y, Hashimoto I, Matsuo S, Abe Y, Ishida S, Nakanishi H. Two‐stage surgery for hidradenitis suppurativa: staged artificial dermis and skin grafting. Dermatol Surg. 2014;40(2):110‐115. [DOI] [PubMed] [Google Scholar]
- 8. Nicoletti G, Brenta F, Bleve M, et al. Long‐term in vivo assessment of bioengineered skin substitutes: a clinical study. J Tissue Eng Regen Med. 2015;9(4):460‐468. [DOI] [PubMed] [Google Scholar]
- 9. Yeong EK, Chen SH, Tang YB. The treatment of bone exposure in burns by using artificial dermis. Ann Plast Surg. 2012;69(6):607‐610. [DOI] [PubMed] [Google Scholar]
- 10. Adani R, Rossati L, Tarallo L, Corain M. Use of integra artificial dermis to reduce donor site morbidity after pedicle flaps in hand surgery. J Hand Surg Am. 2014;39(11):2228‐2234. [DOI] [PubMed] [Google Scholar]
- 11. Clerici G, Caminiti M, Curci V, Quarantiello A, Faglia E. The use of a dermal substitute to preserve maximal foot length in diabetic foot wounds with tendon and bone exposure following urgent surgical debridement for acute infection. Int Wound J. 2010;7(3):176‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee LF, Porch JV, Spenler W, Garner WL. Integra in lower extremity reconstruction after burn injury. Plast Reconstr Surg. 2008;121(4):1256‐1262. [DOI] [PubMed] [Google Scholar]
- 13. Matsumine H, Fujimaki H, Takagi M, et al. Full‐thickness skin reconstruction with basic fibroblast growth factor‐impregnated collagen‐gelatin sponge. Regen Ther. 2019;11:81‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shimizu M, Matsumine H, Takeuchi M. Reconstruction of Chopart's amputation stump using artificial dermis combined with free anterolateral thigh flap. Plast Reconstr Surg Glob Open. 2015;3(11):e558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim MS, Hong KD, Shin HW, et al. Preparation of porcine small intestinal submucosa sponge and their application as a wound dressing in full‐thickness skin defect of rat. Int J Biol Macromol. 2005;36(1–2):54‐60. [DOI] [PubMed] [Google Scholar]
- 16. Magden GK, Vural C, Bayrak BY, Ozdogan CY, Kenar H. Composite sponges from sheep decellularized small intestinal submucosa for treatment of diabetic wounds. J Biomater Appl. 2021;36(1):113‐127. [DOI] [PubMed] [Google Scholar]
- 17. Huang S, Deng T, Wu H, Chen F, Jin Y. Wound dressings containing bFGF‐impregnated microspheres. J Microencapsul. 2006;23(3):277‐290. [DOI] [PubMed] [Google Scholar]
- 18. Jinno C, Morimoto N, Ito R, et al. A comparison of conventional collagen sponge and collagen‐gelatin sponge in wound healing. Biomed Res Int. 2016;2016:4567146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kanda N, Morimoto N, Takemoto S, et al. Efficacy of novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor for dermis‐like tissue regeneration. Ann Plast Surg. 2012;69(5):569‐574. [DOI] [PubMed] [Google Scholar]
- 20. Koshinuma S, Murakami S, Noi M, et al. Comparison of the wound healing efficacy of polyglycolic acid sheets with fibrin glue and gelatin sponge dressings in a rat cranial periosteal defect model. Exp Anim. 2016;65(4):473‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ogino S, Morimoto N, Sakamoto M, et al. Efficacy of the dual controlled release of HGF and bFGF impregnated with a collagen/gelatin scaffold. J Surg Res. 2018;221:173‐182. [DOI] [PubMed] [Google Scholar]
- 22. Wu PZ, Zhou J, Zhang YW. Gelatin sponge microparticles for the treatment of the spontaneous rupture of hepatocellular carcinoma hemorrhage. Exp Ther Med. 2016;12(4):2201‐2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Carvalho RA, Grosso CRF. Characterization of gelatin based films modified with transglutaminase, glyoxal and formaldehyde. Food Hydrocoll. 2004;18(5):717‐726. [Google Scholar]
- 24. Suzuki S, Matsuda K, Isshiki N, Tamada Y, Yoshioka K, Ikada Y. Clinical evaluation of a new bilayer "artificial skin" composed of collagen sponge and silicone layer. Br J Plast Surg. 1990;43(1):47‐54. [DOI] [PubMed] [Google Scholar]
- 25. Suzuki S, Matsuda K, Maruguchi T, Nishimura Y, Ikada Y. Further applications of "bilayer artificial skin". Br J Plast Surg. 1995;48(4):222‐229. [DOI] [PubMed] [Google Scholar]
- 26. Lamke LO, Liljedahl SO. Evaporative water loss from burns, grafts and donor sites. Scand J Plast Reconstr Surg. 1971;5(1):17‐22. [DOI] [PubMed] [Google Scholar]
- 27. Suzuki S, Kawai K, Ashoori F, Morimoto N, Nishimura Y, Ikada Y. Long‐term follow‐up study of artificial dermis composed of outer silicone layer and inner collagen sponge. Br J Plast Surg. 2000;53(8):659‐666. [DOI] [PubMed] [Google Scholar]
- 28. Adly OA, Moghazy AM, Abbas AH, Ellabban AM, Ali OS, Mohamed BA. Assessment of amniotic and polyurethane membrane dressings in the treatment of burns. Burns. 2010;36(5):703‐710. [DOI] [PubMed] [Google Scholar]
- 29. Choi SW, Zhang Y, Macewan MR, Xia Y. Neovascularization in biodegradable inverse opal scaffolds with uniform and precisely controlled pore sizes. Adv Healthc Mater. 2013;2(1):145‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laschke MW, Harder Y, Amon M, et al. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng. 2006;12(8):2093‐2104. [DOI] [PubMed] [Google Scholar]
- 31. Stiefel D, Schiestl CM, Meuli M. The positive effect of negative pressure: vacuum‐assisted fixation of Integra artificial skin for reconstructive surgery. J Pediatr Surg. 2009;44(3):575‐580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Findings during AGS template treatment of the remaining 5 patients involved in this study
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


