Abstract
Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium are antigenically and genetically very similar organisms; however, they differ markedly in their virulence for cattle. We evaluated the capacity of bovine macrophages infected with M. avium subsp. paratuberculosis or M. avium subsp. avium to express major histocompatibility complex (MHC) class I and class II antigens on their surface and to interact with primed autologous lymphocytes. Our results indicate that infection of bovine macrophages with M. avium subsp. paratuberculosis promoted the downregulation of MHC class I and class II molecules on the macrophage surface within 24 and 12 h, respectively. Alternatively, MHC class II expression by M. avium subsp. avium-infected macrophages was not detected until 24 h after infection, and the magnitude of the decrease was smaller. Decreased MHC class I expression by M. avium subsp. avium-infected macrophages was not detected. Unlike M. avium subsp. paratuberculosis-infected macrophages, M. avium subsp. avium-infected macrophages upregulated MHC class I and class II expression after activation by gamma interferon or tumor necrosis factor alpha. Further, M. avium subsp. avium-infected macrophages were lysed by primed autologous lymphocytes, whereas M. avium subsp. paratuberculosis-infected macrophages were not. Overall, the results support the hypothesis that the difference in the virulence of M. avium subsp. paratuberculosis and M. avium subsp. avium for cattle is dependent on a difference in the capacity of the organisms to suppress mycobacterial antigen presentation to T lymphocytes.
Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium are antigenically and genetically very similar organisms (8, 21). Despite considerable study, clear antigenic differences between the organisms have not been identified; at the nucleotide level, the two organisms are 99.6% conserved (14, 21). However, a few insertion sequences, including IS900, IS1311, and F57, appear to be unique to M. avium subsp. paratuberculosis (10, 16, 19). Despite the similarity, the virulence of the two organisms for cattle is markedly different. M. avium subsp. paratuberculosis organisms localize in the intestinal lamina propria and submucosa and produce a chronic granulomatous enteritis termed Johne's disease. Intestinal lesions develop over several months after infection and are characterized by loose aggregates of epithelioid macrophages and giant cells (7). Conspicuously absent from lesions are lymphocytes and neutrophils, and tubercle formation does not occur.
Unlike M. avium subsp. paratuberculosis, M. avium subsp. avium is relatively nonpathogenic for cattle (11). Organisms appear to enter through the intestinal tract and localize in lymph nodes. Cattle typically mount an effective systemic immune response, form caseous granulomas, and eliminate the organisms (11).
Some cattle appear to eliminate M. avium subsp. paratuberculosis infections, while others develop chronic disease, although this phenomenon has not been completely studied. Currently, the immune mechanisms responsible for susceptibility or resistance to infection are poorly characterized. Both cell-mediated and humoral immune responses have been reported to occur in the subclinical stage of Johne's disease (3, 25). However, considerable individual variations in cell-mediated immune responsiveness appear to occur, and immune responses may not occur until late in the subclinical stage of disease (6, 25).
To better understand components of the immune response that determine resistance or susceptibility to M. avium subsp. paratuberculosis, we used an in vitro model to study major histocompatibility complex (MHC) class I and class II antigen expression by macrophages infected with M. avium subsp. paratuberculosis. The results were compared to those for macrophages infected with M. avium subsp. avium.
MATERIALS AND METHODS
Bacteria.
M. avium subsp. paratuberculosis strain 19698 was obtained from the American Type Culture Collection. The strain was isolated from the feces of a cow with naturally acquired Johne's disease. This strain has been extensively characterized (17) and has been adapted to growth in 7H9 broth supplemented with OADC (Difco Laboratories, Detroit, Mich.), Tween 80, and mycobactin J (Allied Laboratories, Ames, Iowa). The organisms were grown to approximately 108 per ml, washed, and resuspended in 7H9 broth containing OADC, Tween 80, mycobactin J, and 5% fetal bovine serum. M. avium subsp. avium strain 35716, isolated from a cow, was obtained from the American Type Culture Collection and grown as described above for M. avium subsp. paratuberculosis. Both organisms were stored at 4°C for up to 3 months. The viability of organisms was assessed at least once per month by use of standard colony-counting assays.
Cell culture procedure.
Heparinized blood was obtained from five adult dairy cows from a Johne's disease-free herd at the University of Minnesota. Mononuclear cells were isolated by use of Ficoll-Hypaque density gradient centrifugation, washed, and resuspended at 3 × 106 cells/ml. For preparation of monocyte-derived macrophages, 5 × 106 mononuclear cells were allowed to adhere to a tissue culture plate for 90 min, and nonadherent cells were washed off. Adherent cells were cultured for 6 days at 37°C in 5% CO2 in RPMI 1640 tissue culture medium supplemented with 10% fetal bovine serum. Monocyte-derived macrophages were infected with M. avium subsp. paratuberculosis or M. avium subsp. avium (10 bacilli/macrophage) on day 7 of culturing. At 4, 12, 24, or 48 h after the addition of organisms, culture plates were cooled to 4°C; macrophages were scraped off, washed, and resuspended in 100 μl of Dulbecco's phosphate-buffered saline containing 1% sheep serum and 2 mM sodium azide. Negative controls consisted of macrophages to which no bacteria were added. Positive controls consisted of macrophages incubated with bovine gamma interferon (100 U/ml; a gift from Novartis Animal Health) and heat-killed Staphylococcus intermedius organisms (10 organisms/macrophage).
Macrophages from each experiment were stained with Ziehl-Neelsen carbolfuchsin stain, which specifically stained mycobacterial organisms (9). The percentage of macrophages containing organisms was determined by use of light microscopy.
Macrophage MHC class I and class II expression.
Macrophage cell suspensions were incubated with anti-bovine MHC class I antibody MCA 81 (Serotec USA, Washington, D.C.), anti-bovine MHC class II antibody MCA 1085 (Serotec USA), anti-bovine CD18 antibody R15.7 (provided by Mark Jutila, Montana State University), or BNI 80.44 (provided by Mark Jutila), an antibody that recognizes an unknown, constitutively expressed molecule on bovine macrophages. After incubation on ice for 1 h, cells were washed and resuspended in 100 μl of Dulbecco's phosphate-buffered saline containing 1% sheep serum and 2 mM sodium azide. Twenty microliters of a 1:20 dilution of fluorescein-labeled sheep anti-mouse immunoglobulin G was added and incubated for 30 min in the dark on ice. Thereafter, samples were diluted to 0.5 ml and analyzed by flow cytometry (FACS Calibur; Becton Dickenson Co.). Propidium iodide was added just before analysis to identify dead cells. Cells were displayed as forward-angle versus side-angle light scatter plots and by scatter plots that analyzed log green fluorescence intensity on the x axis and log red fluorescence intensity on the y axis. Propidium iodide-positive cells were gated out, and the median fluorescence intensity of the remaining macrophage population was determined.
Macrophage activation.
To determine the capacity of bovine macrophages to upregulate MHC class I or class II expression, we incubated macrophages either with bovine gamma interferon (100 U/ml), with or without the addition of heat-killed S. intermedius organisms (10 organisms/macrophage), or with human tumor necrosis factor alpha (TNF-α; 100 U/ml). These concentrations have been reported to activate bovine monocyte-derived macrophages in vitro (20, 26).
Macrophage killing by primed lymphocytes.
Bovine monocyte-derived macrophages were cultured on coverslips for 7 days. Macrophages were infected with M. avium subsp. paratuberculosis or M. avium subsp. avium (10 bacilli/macrophage) on day 7 of culturing. After 2 h of incubation, uningested organisms were washed off. Thereafter, autologous, primed lymphocytes were added at a ratio of 10 lymphocytes per macrophage, and incubation was continued for 4 days. Primed lymphocytes consisted of blood mononuclear cells incubated with a lysate of M. avium subsp. paratuberculosis or M. avium subsp. avium for 4 days (4). After incubation, primed lymphocytes were washed off, and the percentage of live macrophages was determined by use of the trypan blue exclusion technique. Negative controls consisted of macrophages to which no bacteria were added.
Statistical analysis.
All data were determined in duplicate or triplicate, and results were averaged and reported as the mean and standard error (SE). Data from at least three experiments were analyzed by use of a factorial analysis of variance (ANOVA). The means of interest were compared by use of the Bonferroni-Dunn F test.
RESULTS
Phagocytosis of mycobacterial organisms by bovine macrophages.
Of macrophages incubated with M. avium subsp. paratuberculosis or M. avium subsp. avium for 24 or 48 h, greater than 95% contained one or more organisms. Of macrophages incubated with M. avium subsp. paratuberculosis for 4 or 12 h, 48% ± 15% or 87% ± 13% contained one or more organisms, respectively. Of macrophages incubated with M. avium subsp. avium for 4 or 12 h, 46% ± 9% and 88% ± 17% contained one or more organisms, respectively. All organisms appeared to be intact.
MHC class I and class II expression by monocyte-derived macrophages.
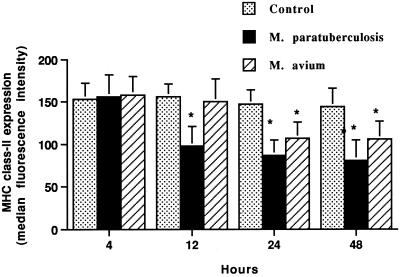
Surface expression of MHC class I and class II molecules by monocyte-derived macrophages was evaluated after 4, 12, 24, and 48 h of incubation with or without the addition of M. avium subsp. paratuberculosis or M. avium subsp. avium. Macrophages incubated with M. avium subsp. paratuberculosis for 12, 24, and 48 h had a consistent reduction (P < 0.01) in MHC class II expression (Fig. 1 and 2). The reduction in the median fluorescence intensity of M. avium subsp. paratuberculosis-incubated macrophages approached 50% at 24 and 48 h of incubation. The percentage of macrophages with decreased fluorescence intensity at 24 h of incubation was 62% ± 18%. Alternatively, macrophages incubated with M. avium subsp. avium had no reduction in MHC class II expression at 12 h and less reduction at 24 and 48 h than M. avium subsp. paratuberculosis-incubated macrophages. The percentage of macrophages with decreased fluorescence intensity was 21% ± 9%.
FIG. 1.
Incubation of bovine macrophages with M. avium subsp. paratuberculosis (M. paratuberculosis) or M. avium subsp. avium (M. avium) decreases surface expression of MHC class II antigens, as detected by flow cytometry. Bovine monocyte-derived macrophages (106) were cultured for various times with or without M. avium subsp. paratuberculosis or M. avium subsp. avium added. The mean and SE for four separate experiments are shown. Asterisks indicate statistically significant differences relative to control values (P < 0.05).
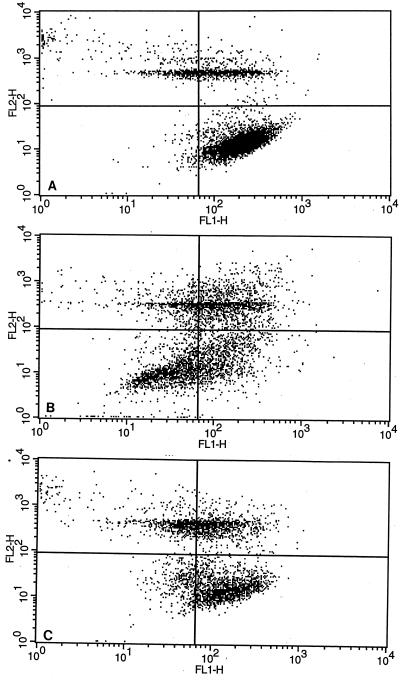
FIG. 2.
Flow cytometric scatter plots of MHC class II expression by bovine monocyte-derived macrophages. Just before analysis, 100 μl of propidium iodide was added to identify dead cells. Cells were displayed as log green fluorescence intensity (FL1-H; i.e. a measure of surface MHC class II expression) versus log red fluorescence intensity (FL2-H; a measure of propidium iodide uptake). Quadrant gates were set to eliminate dead cells (upper right and left quadrants) and to identify cells with decreased MHC class II expression compared to that of uninfected macrophages (lower left quadrant). (A) Uninfected macrophages incubated for 24 h. (B) Macrophages infected with M. avium subsp. paratuberculosis organisms for 24 h. (C) Macrophages infected with M. avium subsp. avium organisms for 24 h.
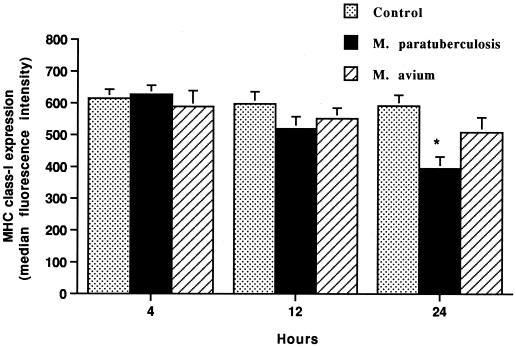
Macrophages incubated with M. avium subsp. paratuberculosis for 24 h but not for 4 or 12 h had a consistent reduction (P < 0.05) in MHC class I expression (Fig. 3). Macrophages incubated with M. avium subsp. avium had only a slight reduction in MHC class I expression at 24 h.
FIG. 3.
Incubation of bovine macrophages with M. avium subsp. paratuberculosis (M. paratuberculosis) but not M. avium subsp. avium (M. avium) decreases surface expression of MHC class I antigens, as detected by flow cytometry. Bovine monocyte-derived macrophages (106) were cultured for various times with or without M. avium subsp. paratuberculosis or M. avium subsp. avium added. The mean and SE for three separate experiments are shown. The asterisk indicates a statistically significant difference relative to the control value (P < 0.05).
Expression of other adhesion molecules and constitutively expressed surface molecules.
To determine if the decrease in MHC class I and class II expression was a nonspecific response to infection, we determined the effects of M. avium subsp. paratuberculosis or M. avium subsp. avium infection on the expression of cell surface adhesion molecule CD18 by use of a monoclonal antibody and by labeling with a monoclonal antibody that recognizes a poorly characterized constitutively expressed molecule on bovine macrophages. Little change in the expression of this molecule was noted when macrophages were incubated with M. avium subsp. paratuberculosis or M. avium subsp. avium for 12 or 24 h (Table 1).
TABLE 1.
Effects of M. avium subsp. paratuberculosis or M. avium subsp. avium on the expression of CD18 and a constitutively expressed molecule on bovine monocyte-derived macrophagesa
| Additive | Surface marker | Mean fluorescence intensity at the following time (h):
|
||
|---|---|---|---|---|
| 0 | 12 | 24 | ||
| None (control) | CD18 | 254 ± 72 | 228 ± 58 | 266 ± 67 |
| BNI 80.44 | 68 ± 4 | 65 ± 3 | 58 ± 5 | |
| M. avium subsp. paratuberculosis | CD18 | 234 ± 61 | 268 ± 48 | 278 ± 56 |
| BNI 80.44 | 67 ± 3 | 63 ± 7 | 66 ± 5 | |
| M. avium subsp. avium | CD18 | 251 ± 44 | 263 ± 48 | 271 ± 38 |
| BNI 80.44 | 58 ± 7 | 62 ± 6 | 60 ± 6 | |
Data are reported as the mean and SE for two separate experiments.
MHC class I and class II expression by activated macrophages.
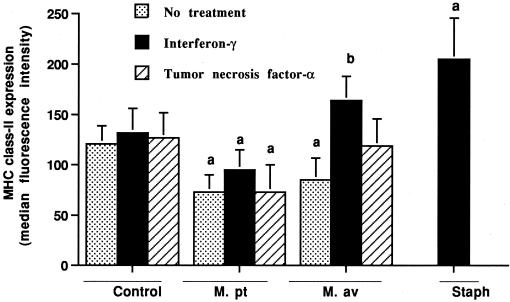
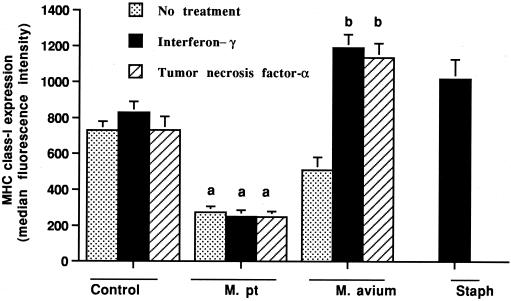
Incubation of control macrophages with gamma interferon for 24 h only slightly upregulated the surface expression of MHC class I and class II molecules (Fig. 4 and 5). In contrast, when gamma interferon and heat-killed S. intermedius organisms were added, the expression of both MHC class I and class II molecules was consistently upregulated (P < 0.01). The addition of TNF-α had no effect on MHC class I or class II expression by uninfected macrophages.
FIG. 4.
Effects of gamma interferon and TNF-α on MHC class II expression by bovine macrophages incubated for 24 h with or without M. avium subsp. paratuberculosis (M. pt) or M. avium subsp. avium (M. av). Unlike M. avium subsp. paratuberculosis-infected macrophages, M. avium subsp. avium-infected macrophages upregulated MHC class II molecules in response to gamma interferon. As a positive control, uninfected macrophages were incubated with gamma interferon and killed S. intermedius (Staph) organisms. The mean and SE for three separate experiments are shown. a, statistically significant differences relative to control values (P < 0.05); b, statistically significant differences relative to samples incubated with M. avium subsp. avium organisms alone (P < 0.05).
FIG. 5.
Effects of gamma interferon and TNF-α on MHC class I expression by bovine macrophages incubated for 24 h with or without M. avium subsp. paratuberculosis (M. pt) or M. avium subsp. avium. Unlike M. avium subsp. paratuberculosis-infected macrophages, M. avium subsp. avium-infected macrophages upregulated MHC class I molecules in response to gamma interferon and TNF-α. As a positive control, uninfected macrophages were incubated with gamma interferon and killed S. intermedius (Staph) organisms. The mean and SE for three separate experiments are shown. a, statistically significant differences relative to control values (P < 0.05); b, statistically significant differences relative to samples incubated with M. avium subsp. avium organisms alone (P < 0.05).
The responses of macrophages infected with M. avium subsp. paratuberculosis or M. avium subsp. avium to activation stimuli were very different. The addition of gamma interferon to cells incubated with M. avium subsp. paratuberculosis for 4 h resulted in only a slight upregulation of MHC class II expression and no change in MHC class I expression when assessed 12 or 24 h later (P < 0.05) (Fig. 4 and 5). Alternatively, the addition of gamma interferon to M. avium subsp. avium-infected macrophages resulted in a marked upregulation of the expression of both MHC class I and class II molecules (P < 0.05). As for control macrophages, the addition of TNF-α to M. avium subsp. paratuberculosis-infected macrophages had no effect on MHC class I or class II expression. In contrast, the addition of TNF-α to M. avium subsp. avium-infected macrophages markedly upregulated MHC class I expression (P < 0.05) and tended to upregulate MHC class II expression.
Effects of killed organisms and culture supernatants on MHC class I and class II expression.
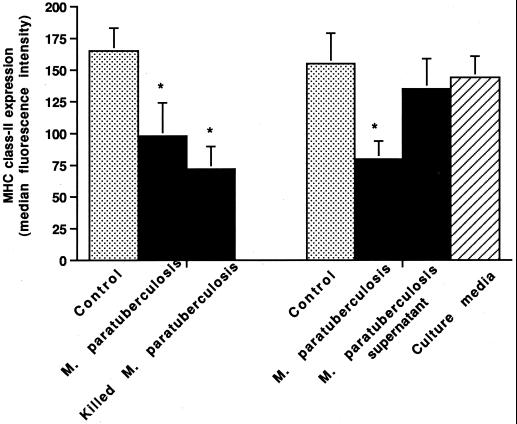
The addition of heat-killed M. avium subsp. paratuberculosis organisms to macrophages resulted in a greater decrease in mean MHC class I (76 versus 61%) and class II (68 versus 44%) expression than did the addition of live organisms (Fig. 6) (data for MHC class I not shown).
FIG. 6.
Effects of live versus killed M. avium subsp. paratuberculosis (M. paratuberculosis) organisms and culture supernatants on MHC class II expression by bovine macrophages. Bovine monocyte-derived macrophages (106) were incubated for 24 h with or without the addition of live or heat-killed M. avium subsp. paratuberculosis organisms or culture supernatant. The mean and SE for two separate experiments are shown. Asterisks indicate statistically significant differences relative to control values (P < 0.05).
To determine if a soluble factor was involved in the suppression of MHC expression, fresh culture media and supernatants from M. avium subsp. paratuberculosis and M. avium subsp. avium cultures were incubated with macrophages for 24 h (Fig. 6). Neither fresh media nor culture supernatants substantially reduced macrophage MHC class I or class II expression.
Macrophage killing by primed lymphocytes.
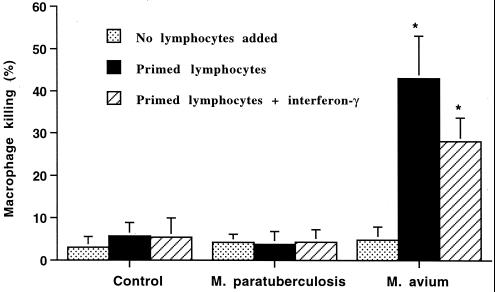
Incubation of macrophages with M. avium subsp. paratuberculosis or M. avium subsp. avium for 24 h resulted in only a slight increase in the mean number of nonviable macrophages (Fig. 7). Incubation of uninfected macrophages or macrophages infected with M. avium subsp. paratuberculosis with autologous primed lymphocytes resulted in a minimal change in the number of nonviable macrophages. Alternatively, incubation of M. avium subsp. avium-infected macrophages with autologous primed lymphocytes resulted in a marked increase in the number of nonviable macrophages. The addition of gamma interferon did not further increase macrophage killing.
FIG. 7.
Effects of autologous primed lymphocytes on killing of macrophages incubated with or without M. avium subsp. paratuberculosis (M. paratuberculosis) or M. avium subsp. avium (M. avium) added. Bovine monocyte-derived macrophages (106) were incubated with autologous primed lymphocytes (107) and with or without gamma interferon. The mean and SE for three separate experiments are shown. Asterisks indicate statistically significant differences relative to control values and M. avium subsp. paratuberculosis values (P < 0.05).
DISCUSSION
These results demonstrate that infection of bovine macrophages with M. avium subsp. paratuberculosis induces the downregulation of MHC class I and class II molecule expression on the macrophage surface. The effects of M. avium subsp. avium on MHC expression were substantially different. M. avium subsp. paratuberculosis downregulated MHC class II molecules by 12 h after the addition of organisms, whereas with M. avium subsp. avium, downregulation was not detected until 24 h. Since both organisms were phagocytized by macrophages at similar rates, the differences in MHC class I and class II expression did not appear to be determined by the rate of uptake of the organisms. Further, M. avium subsp. paratuberculosis prevented the subsequent upregulation of MHC class I and class II molecules by gamma interferon and TNF-α. Alternatively, macrophages incubated with M. avium subsp. avium readily upregulated MHC class I and class II expression when exposed to gamma interferon or TNF-α. The observed differences in MHC class I and class II expression in vitro may explain, at least in part, differences in the virulence of the organisms. The presence of gamma interferon or TNF-α at sites of infection would result in the upregulation of MHC class I and class II expression by M. avium subsp. avium-infected macrophages but not by M. avium subsp. paratuberculosis-infected macrophages. This process could result in enhanced antigen presentation to T lymphocytes by M. avium subsp. avium-infected macrophages and initiation of an effective immune response. The lack of MHC upregulation by M. avium subsp. paratuberculosis-infected macrophages may attenuate or delay the immune response. This hypothesis is supported by our observation that M. avium subsp. avium-infected macrophages but not M. avium subsp. paratuberculosis-infected macrophages are lysed by primed autologous lymphocytes. The addition of exogenous gamma interferon did not further enhance macrophage killing; however, primed lymphocytes may have produced enough gamma interferon to induce the upregulation of MHC molecules.
The mechanism by which MHC class I and class II expression is suppressed in infected macrophages was not resolved by these studies. The failure of an M. avium subsp. paratuberculosis cell culture supernatant to suppress macrophage MHC expression indicates that the suppressive factor is not secreted by the organisms. The capacity of killed M. avium subsp. paratuberculosis organisms to suppress MHC expression indicates that the factor(s) is not synthesized by organisms after phagocytosis. Further, the enhanced capacity of killed versus live organisms to suppress MHC expression suggests that the factor(s) involved may be derived from the bacterial cell wall (which may be more readily degraded when organisms are heat killed).
The results of previous studies indicate that a cell wall component of many mycobacteria, lipoarabinomannan, attenuates the capacity of mouse macrophages to become activated in response to gamma interferon (18). Lipoarabinomannan is a major component of the cell wall of M. avium subsp. paratuberculosis and has properties comparable to those of lipopolysaccharide of gram-negative bacteria (5, 23). Lipopolysaccharide also has been reported to downregulate MHC class II expression by macrophages (12). Our data, in combination with those of previous studies, suggest that the downregulation of MHC class II expression may be a nonspecific event in macrophages phagocytizing mycobacteria and gram-negative bacteria and may be mediated by lipopolysaccharide-like bacterial cell membrane components. However, macrophage infection by M. avium subsp. paratuberculosis is distinct in that MHC class I and class II expression by infected macrophages cannot be upregulated by gamma interferon. This inability to upregulate antigen-presenting molecules on infected cells may attenuate or delay the cellular immune response in Johne's disease (22). This attenuated immune response may provide time for the organisms to proliferate and overwhelm the capacity of the immune response to destroy the organisms in some cattle. These observations are consistent with observations that most infected cattle develop a cellular immune response during the subclinical stages of Johne's disease and that some cattle eliminate the organisms before developing clinical disease (7).
Decreased MHC class II expression is not unique to macrophages infected with Mycobacterium spp. (24). Previous studies have reported that Leishmania spp., Toxoplasma gondii, and murine cytomegalovirus are able to inhibit gamma interferon-induced MHC class II expression by macrophages (13, 15). The mechanism by which organisms downregulate MHC class II expression is poorly understood. Leishmania amazonesis and L. mexicana amastigotes have been shown to degrade MHC class II molecules within parasitophorous vacuoles (1, 2). Degradation appears to be mediated by cysteine proteases. Murine cytomegalovirus appears to interfere with the expression of transcription factors involved in I-Aβ gene expression (13).
Overall, the results presented here support the hypothesis that the differences in virulence and in lesions induced in cattle by M. avium subsp. paratuberculosis and M. avium subsp. avium may be dependent on the capacity of infected macrophages to effectively present mycobacterial antigens to T lymphocytes. The differences may be the result, at least in part, of downregulation of MHC class I and class II expression by M. avium subsp. paratuberculosis organisms. Further studies are needed to define the microbial factors that mediate these changes.
ACKNOWLEDGMENT
This work was supported by a grant from the Minnesota Agricultural Experiment Station.
REFERENCES
- 1.Antoine J C, Prima E, Lang T, Courret N. The biogenesis and properties of the parasitophorous vacuoles that harbor Leishmania in murine macrophages. Trends Microbiol. 1998;6:392–401. doi: 10.1016/s0966-842x(98)01324-9. [DOI] [PubMed] [Google Scholar]
- 2.Antoine J C, Lang T, Prima E, Courret N, Hellio R. H-2M molecules, like MHC class II molecules, are targeted to parasitophorous vacuoles of Leishmania-infected macrophages and internalized by amastigotes of L. amazonensis and L. mexicana. J Cell Sci. 1999;112:2559–2570. doi: 10.1242/jcs.112.15.2559. [DOI] [PubMed] [Google Scholar]
- 3.Bassey E O, Collins M T. Study of T-lymphocyte subsets of healthy and Mycobacterium avium subsp. paratuberculosis-infected cattle. Infect Immun. 1997;65:4869–4872. doi: 10.1128/iai.65.11.4869-4872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonecini-Almeida M G, Chitale S, Boutsikakis I, Geng J, Doo H, He S, Ho J L. Induction of in vitro human macrophage anti-Mycobacterium tuberculosis activity: requirement for INF-γ and primed lymphocytes. J Immunol. 1998;160:4490–4499. [PubMed] [Google Scholar]
- 5.Brennan P L, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 6.Chiodini R J. Immunology: resistance to paratuberculosis. Vet Clin N Am Food Anim Pract. 1996;12:313–343. doi: 10.1016/s0749-0720(15)30409-6. [DOI] [PubMed] [Google Scholar]
- 7.Clarke C J. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J Comp Pathol. 1997;116:217–261. doi: 10.1016/s0021-9975(97)80001-1. [DOI] [PubMed] [Google Scholar]
- 8.Cobb A J, Frothingham R. The GroES antigens of Mycobacterium avium and Mycobacterium paratuberculosis. Vet Microbiol. 1999;67:31–35. doi: 10.1016/s0378-1135(99)00019-x. [DOI] [PubMed] [Google Scholar]
- 9.Coles E H. Veterinary clinical pathology. 2nd ed. Philadelphia, Pa: The W. B. Saunders Co.; 1974. pp. 589–590. [Google Scholar]
- 10.Collins D M, Gabric D M, de Lisle G W. Identification of a repetitive DNA sequence specific to Mycobacterium paratuberculosis. FEMS Microbiol Lett. 1989;60:175–178. doi: 10.1016/0378-1097(89)90503-x. [DOI] [PubMed] [Google Scholar]
- 11.de Lisle G W, Yates G F, Joyce M A. Case report and DNA characterization of Mycobacterium avium isolated from multiple animals with lesions in a beef cattle herd. J Vet Diagn Med. 1998;10:283–284. doi: 10.1177/104063879801000311. [DOI] [PubMed] [Google Scholar]
- 12.Desai S D, Birdi T J, Antia N H. Correlation between macrophage activation and bactericidal function and Mycobacterium leprae antigen presentation in macrophages of leprosy patients and normal individuals. Infect Immun. 1989;57:1311–1317. doi: 10.1128/iai.57.4.1311-1317.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heise M T, Connick M, Virgin H W T. Murine cytomegalovirus inhibits interferon-γ-induced antigen presentation to CD4 T cells by macrophages via regulation of expression of major histocompatibility complex class-II-associated genes. J Exp Med. 1998;187:1037–1046. doi: 10.1084/jem.187.7.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurley S S, Splitter G A, Welch R A. Deoxyribonucleic acid relatedness of Mycobacterium paratuberculosis to other members of the family Mycobacteriaceae. Int J Syst Bacteriol. 1988;38:143–146. [Google Scholar]
- 15.Luder C G T, Lang T, Beuerrle B, Gross H G. Down-regulation of MHC class II molecules and inability to up-regulate class I molecules in murine macrophages after infection with Toxoplasma gondii. Clin Exp Immunol. 1998;112:308–317. doi: 10.1046/j.1365-2249.1998.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsh I, Whittington R, Cousins D. PCR-restriction endonuclease analysis for identification and strain typing of Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium based on polymorphisms in IS1311. Mol Cell Probes. 1999;13:115–126. doi: 10.1006/mcpr.1999.0227. [DOI] [PubMed] [Google Scholar]
- 17.Merkal R S, Curran B. Growth and metabolic characterization of Mycobacterium paratuberculosis. Appl Microbiol. 1974;28:276–279. doi: 10.1128/am.28.2.276-279.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neill M A, Klebaboff S J. The effects of phenolic glycolipid-1 from Mycobacterium leprae on the antimicrobial activity of human macrophages. J Exp Med. 1988;167:30–42. doi: 10.1084/jem.167.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poupart P, Coene M, Van Heuversyn H, Cocito C. Preparation of a specific RNA probe for detection of Mycobacterium paratuberculosis and diagnosis of Johne's disease. J Clin Microbiol. 1993;31:1601–1605. doi: 10.1128/jcm.31.6.1601-1605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stabel J R. Production of γ-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of subclinical paratuberculosis. J Vet Diagn Investig. 1996;8:345–350. doi: 10.1177/104063879600800311. [DOI] [PubMed] [Google Scholar]
- 21.Stevenson K, Sharp J M. The contribution of molecular biology to Mycobacterium avium subspecies paratuberculosis research. Vet J. 1997;153:269–286. doi: 10.1016/s1090-0233(97)80062-7. [DOI] [PubMed] [Google Scholar]
- 22.Tweardy D J, Schacter B Z, Ellner J J. Association of altered dynamics of monocyte surface expression of human leukocyte antigen DR with immunosuppression in tuberculosis. J Infect Dis. 1984;149:31–37. doi: 10.1093/infdis/149.1.31. [DOI] [PubMed] [Google Scholar]
- 23.Valentin-Weigand P, Goethe R. Pathogenesis of Mycobacterium avium subspecies paratuberculosis infections in ruminants: still more questions than answers. Microb Infect. 1999;1:1121–1127. doi: 10.1016/s1286-4579(99)00203-8. [DOI] [PubMed] [Google Scholar]
- 24.Wadee A A, Kuschke R H, Dooms T G. The inhibitory effects of Mycobacterium tuberculosis on MHC class II expression by monocytes activated with riminophenazines and phagocyte stimulants. Clin Exp Immunol. 1995;100:434–446. doi: 10.1111/j.1365-2249.1995.tb03718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waters W R, Stabel J R, Sacco R E, Harp J A, Pesch B A, Wannemuehler M J. Antigen-specific B-cell unresponsiveness induced by chronic Mycobacterium avium subsp. paratuberculosis infection of cattle. Infect Immun. 1999;67:1593–1598. doi: 10.1128/iai.67.4.1593-1598.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zurbrick B G, Follett D M, Czuprynski C J. Cytokine regulation of the intracellular growth of Mycobacterium paratuberculosis in bovine monocytes. Infect Immun. 1988;56:1692–1697. doi: 10.1128/iai.56.7.1692-1697.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]