Abstract
Insecticide resistance threatens recent progress on malaria control in Africa. To characterize pyrethroid resistance in Uganda, Anopheles gambiae (s.s.) and Anopheles arabiensis were analyzed from 11 sites with varied vector control strategies. Mosquito larvae were collected between May 2018 and December 2020. Sites were categorized as receiving no indoor-residual spraying (‘no IRS’, n = 3); where IRS was delivered from 2009 to 2014 and in 2017 and then discontinued (‘IRS stopped’, n = 4); and where IRS had been sustained since 2014 (‘IRS active’, n = 4). IRS included bendiocarb, pirimiphos methyl and clothianidin. All sites received long-lasting insecticidal nets (LLINs) in 2017. Adult mosquitoes were exposed to pyrethroids; with or without piperonyl butoxide (PBO). Anopheles gambiae (s.s.) and An. arabiensis were identified using PCR. Anopheles gambiae (s.s.) were genotyped for Vgsc-995S/F, Cyp6aa1, Cyp6p4-I236M, ZZB-TE, Cyp4j5-L43F and Coeae1d, while An. arabiensis were examined for Vgsc-1014S/F. Overall, 2753 An. gambiae (s.l.), including 1105 An. gambiae (s.s.) and 1648 An. arabiensis were evaluated. Species composition varied by site; only nine An. gambiae (s.s.) were collected from ‘IRS active’ sites, precluding species-specific comparisons. Overall, mortality following exposure to permethrin and deltamethrin was 18.8% (148/788) in An. gambiae (s.s.) and 74.6% (912/1222) in An. arabiensis. Mortality was significantly lower in An. gambiae (s.s.) than in An. arabiensis in ‘no IRS’ sites (permethrin: 16.1 vs 67.7%, P < 0.001; deltamethrin: 24.6 vs 83.7%, P < 0.001) and in ‘IRS stopped’ sites (permethrin: 11.3 vs 63.6%, P < 0.001; deltamethrin: 25.6 vs 88.9%, P < 0.001). When PBO was added, mortality increased for An. gambiae (s.s.) and An. arabiensis. Most An. gambiae (s.s.) had the Vgsc-995S/F mutation (95% frequency) and the Cyp6p4-I236M resistance allele (87%), while the frequency of Cyp4j5 and Coeae1d were lower (52% and 55%, respectively). Resistance to pyrethroids was widespread and higher in An. gambiae (s.s.). Where IRS was active, An. arabiensis dominated. Addition of PBO to pyrethroids increased mortality, supporting deployment of PBO LLINs. Further surveillance of insecticide resistance and assessment of associations between genotypic markers and phenotypic outcomes are needed to better understand mechanisms of pyrethroid resistance and to guide vector control.
Keywords: Anopheles gambiae, Anopheles arabiensis, Pyrethroid resistance, Triple mutation, Piperonyl butoxide (PBO)
Graphical abstract
Highlights
-
•
Low mortality defined the pyrethroid resistance phenotype.
-
•
Underlying resistance marker genotypes partially explained the resistance phenotype.
-
•
Addition of piperonyl butoxide (PBO) increased mosquito mortality.
-
•
Triple mutation was associated with mosquito survival to permethrin plus PBO.
-
•
Cytochrome p450 Cyp4j5 was associated with survival to deltamethrin plus PBO.
1. Introduction
Remarkable progress in malaria control has been achieved over the past two decades following the scale-up of vector control interventions including long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) (Bhatt et al., 2015; Cibulskis et al., 2016; WHO, 2021). Nearly 70% of clinical malaria cases averted between 2000 and 2015 were attributed to use of LLINs (Bhatt et al., 2015). LLINs have been shown to reduce parasite prevalence, malaria morbidity, and malaria mortality in children (Kleinschmidt et al., 2018; Pryce et al., 2018); more recently, use of LLINs in early childhood has been associated with better survival outcomes through adulthood (Fink et al., 2022). In Uganda, LLINs serve as the backbone of malaria control, and mass campaigns are conducted every 3–4 years to distribute LLINs nationwide, supplemented by a targeted IRS program (Uganda National Malaria Control Division, 2019). IRS conducted in high-transmission areas has also been very effective (Katureebe et al., 2016; Nankabirwa et al., 2020; Namuganga et al., 2021). Various non-pyrethroid insecticides have been deployed, including bendiocarb (a carbamate), pirimiphos-methyl (an organophosphate) and clothianidin (a neonicotinoid), all with differing modes of action found to be suitable alternatives to pyrethroids (Akogbéto et al., 2010; Agossa et al., 2014; Fongnikin et al., 2020). However, the substantial benefits of LLINs and IRS are threatened by widespread insecticide resistance in Uganda (Mawejje et al., 2013; Mulamba et al., 2014; Okia et al., 2018; Tchouakui et al., 2021), and elsewhere (Ochomo et al., 2013, 2014; Yipmo et al., 2022).
The long-term application of insecticides for public health (WHO, 2021) and control of agricultural pests (Nkya et al., 2014) has increased selection pressure on malaria vectors (Lines, 1988; Nauen, 2007; Mathias et al., 2011; Ranson & Lissenden, 2016), driving the development and spread of insecticide resistance (Mathias et al., 2011; Ranson et al., 2011; Ranson & Lissenden, 2016; Hancock et al., 2020; Wat’senga et al., 2020). Conventional LLINs prequalified by the World Health Organization (WHO) rely on pyrethroid insecticides, including permethrin and deltamethrin, which are favored because of low mammalian toxicity (WHO, 1999), excito-repellency (Elliott et al., 1978; WHO, 2011), and relatively low cost compared to alternative insecticides (Hancock et al., 2020). Mosquitoes with relevant resistance mutations are more likely to survive if exposed to insecticides, thus extending their lifespan and the likelihood of transmitting malaria parasites (Verhaeghen et al., 2010; Kabula et al., 2016). Pyrethroid resistance has been shown to compromise vector control (Kigozi et al., 2012; Toé et al., 2014; Hargreaves et al., 2000), although the impact of insecticide resistance on malaria metrics is less conclusive (Kleinschmidt et al., 2018). Widespread resistance to pyrethroids has been reported across sub-Saharan Africa (Hancock et al., 2020; Lissenden et al., 2021), including in Uganda (Verhaeghen et al., 2006, 2010; Ramphul et al., 2009; Mawejje et al., 2013; Okia et al., 2013, 2018; Katureebe et al., 2016). To combat the spread of pyrethroid resistance, newer generation LLINs have been developed, which incorporate additional chemicals into the nets, such as piperonyl butoxide (PBO), a synergist (WHO, 2017; Protopopoff et al., 2018; Staedke et al., 2020; Gleave et al., 2021), pyriproxyfen, an insect growth regulator (Tiono et al., 2018; Ngufor et al., 2020), and chlorfenapyr, a pyrrole insecticide (Mosha et al., 2022). Initial studies of these dual active-ingredient nets are promising (Mosha et al., 2022). Current WHO guidelines on malaria control (WHO, 2022) recommend deployment of PBO-LLINs in areas with pyrethroid resistance and strategic co-deployment of LLINs and non-pyrethroid IRS, as a strategy to limit insecticide resistance (WHO, 2014, 2015, 2022). Further evidence of the impact of combining LLINs with IRS using non-pyrethroid insecticides on malaria burden and the selection for pyrethroid resistance is needed.
Resistance to pyrethroids is primarily mediated by changes in the voltage-gated sodium channel (Vgsc) (Ranson & Lissenden, 2016), which serves as the target site for these insecticides, and through metabolic mechanisms (Donnelly et al., 2009). Non-synonymous point mutations in Vgsc, commonly referred to as knockdown resistance (kdr) (Martinez-Torres et al., 1998), most commonly involve either an L995S (Ranson et al., 2000) or L995F (Martinez-Torres et al., 1998) mutation (numbering for An. gambiae (s.s.); the orthologous codon in An. arabiensis is 1014). Both mutations have been described previously in Uganda, with the L995S mutation at greater frequency (Verhaeghen et al., 2006; Mawejje et al., 2013; Okia et al., 2013, 2018; Lynd et al., 2019). Metabolic resistance in An. gambiae (s.s.) is often associated with changes in cytochrome p450 enzymes that potentially increase insecticide detoxification; in Uganda these include Cyp4j5 (Weetman et al., 2018), Cyp6p4 and an associated ‘Zanzibar-like’ transposable element (ZZB-TE) (Njoroge et al., 2021), and the Cyp6aa1/Cyp6aap duplication (Lucas et al., 2019; Njoroge et al., 2021). A carboxylesterase gene (Coeae1d) (Weetman et al., 2018) has also been associated with pyrethroid resistance in Uganda and Kenya. Previous analysis of An. gambiae (s.s.) mosquitoes collected from Uganda and Kenya (Weetman et al., 2018) showed that marker polymorphisms in Cyp4j5 and Coeae1d were found at relatively high frequency (0.61 and 0.53, respectively) and were associated with pyrethroid resistance. In Uganda and parts of the Democratic Republic of the Congo, the Cyp6aa1 duplication, Cyp6p4 point mutation and ZZB-TE insertion are found at high frequency as a triple-mutant (Njoroge et al., 2021), with the two p450 genes shown to be capable of metabolizing pyrethroids in vitro in An. gambiae (s.s.) None of these mechanisms are known to be associated with resistance to the insecticides (bendiocarb, pirimiphos-methyl and clothianidin) used for recent IRS in Uganda. To further characterize pyrethroid resistance in Uganda and explore patterns associated with non-pyrethroid IRS, we collected An. gambiae (s.s.) and An. arabiensis from 11 districts around Uganda under conditions of varying malaria control, including sites with and without IRS programmes, and analysed them using both phenotypic and genotypic assays.
2. Materials and methods
2.1. Study site characteristics
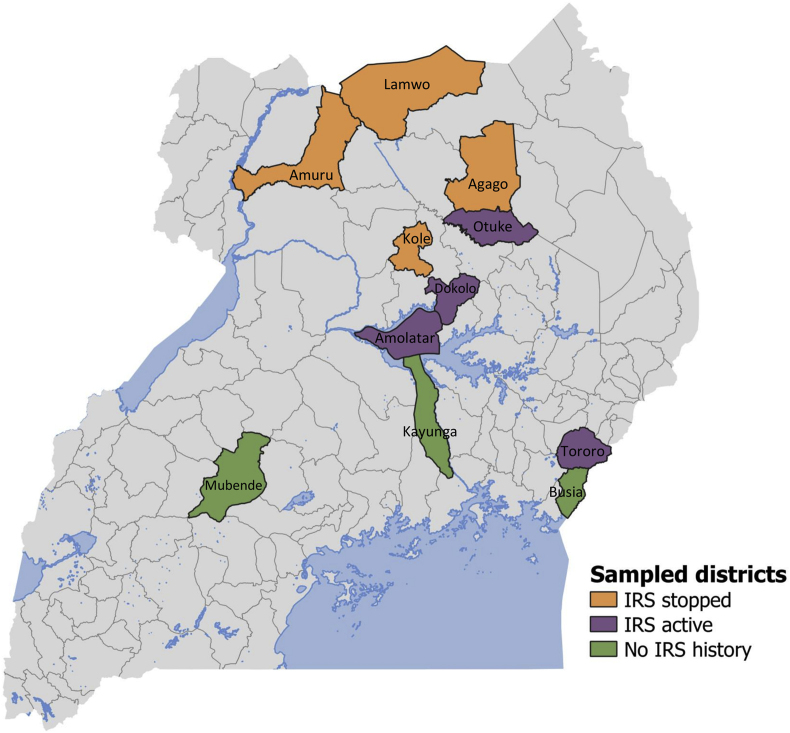
This study was conducted in 11 districts across Uganda (Fig. 1). Mubende and Kayunga districts are located in the central region (North Buganda sub-region), characterised by forest-savannah mosaic vegetation (Roberts & Ocaya, 2009); prevalence of malaria parasitemia in children aged 0–59 months, as measured by microscopy, was 9% in the 2019 Malaria Indicator Survey (MIS) (Uganda National Malaria Control Division, 2019). Kole, Otuke, Dokolo and Amolatar districts are located in the Lango sub-region of northern Uganda, which is characterised by short grassland vegetation (Roberts & Ocaya, 2009), and a regional parasite prevalence of 13% in 2019 (Uganda National Malaria Control Division, 2019). Amuru, Lamwo and Agago districts are located in Acholi sub-region, also in northern Uganda, bordering South Sudan, with a parasite prevalence of 12% in 2019. Busia and Tororo districts are located in Bukedi sub-region in eastern Uganda, bordering western Kenya. This area is characterized by moist savannah vegetation (Roberts & Ocaya, 2009), and parasite prevalence of 3% in 2019 (Uganda National Malaria Control Division, 2019). Previous meteorological data demonstrated that districts in the central and eastern regions experience bimodal rainfall with two peaks, one in March-May and the second in September-December (MOH, 2014), whilst the northern region receives less rainfall, with only one rainy season between March and October (MOH, 2014).
Fig. 1.
Map of study sites showing the location of sampled districts, and stratification by vector control measures. Abbreviations: IRS, indoor residual spraying; LLINs, long-lasting insecticidal nets. Key: green, No IRS (LLINs only); orange, IRS stopped (+ LLINs); purple, IRS active (+ LLINs).
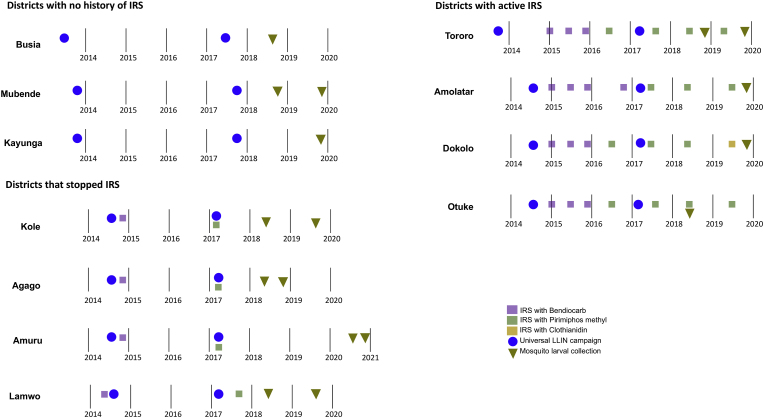
Study sites were stratified by vector control status. In all 11 districts, two mass campaigns were conducted to deliver conventional (pyrethroid only) LLINs in 2013–2014 and in 2017 (Fig. 2). ‘No IRS’ sites (Busia, Mubende and Kayunga) received LLINs only; the Ministry of Health did not implement IRS in these areas. ‘IRS stopped’ sites (Kole, Amuru, Lamwo and Agago) received LLINs plus annual rounds of IRS from 2009 to 2014, followed by a single round of IRS in 2017. ‘IRS active’ sites (Otuke, Tororo, Dokolo and Amolatar) received LLINs plus routine IRS from 2014 to 2019 (active at the time of larval sampling). Details of insecticides used are provided in Fig. 2 and have also been described elsewhere (Namuganga et al., 2021). Briefly, both ‘IRS stopped’ and ‘IRS active’ districts received IRS with two insecticide compounds, namely bendiocarb followed by pirimiphos methyl. Dokolo received IRS with clothianidin, rather than pirimiphos methyl, in 2019 (illustrated in Fig. 2).
Fig. 2.
Timeline of vector control measures and mosquito larval collections in study sites, stratified by IRS status. Abbreviations: IRS, indoor residual spraying; LLINs, long-lasting insecticidal nets. Key: purple, IRS with bendiocarb; green, IRS with pirimiphos methyl (Actellic); gold, IRS with Sumishield 50W (clothianidin); blue circle, LLINs distributed nationwide by Uganda’s Ministry of Health through the 2017–2018 universal coverage campaign; inverted triangles, mosquito larval collection.
2.2. Mosquito collections and identification
Mosquito larvae were collected between May 2018 and December 2020 (Fig. 2) using the dipping method (Service, 1993) from a range of breeding sites including man-made pits to excavate sand, brick, or murram, cow watering holes, tyre tracks, stagnant roadside pools, rice fields, and harvested gardens. Larvae were transported to the medical entomology insectary at the Central Public Health Laboratories in Kampala and were raised to adults using finely ground Tetramin fish food. Resultant adult mosquitoes were identified morphologically using keys (Gillies & De Meillon, 1968; Gillies & Coetzee, 1987) and classified as members of the Anopheles gambiae (sensu lato) species complex. Subsequent identification of sibling species was done using standard polymerase chain reaction (PCR) protocols (Scott et al., 1993).
2.3. Insecticide susceptibility tests
Assessment of insecticide susceptibility was performed using standard WHO tube bioassays (WHO, 1998, 2016). Adult non-blood-fed female An. gambiae (s.l.), aged 3–5 days-old were exposed to permethrin or deltamethrin at WHO diagnostic concentrations of 0.75% and 0.05%, respectively. Four replicates of 20–25 mosquitoes were exposed per insecticide for 1 h under temperatures ranging from 23.3 °C to 26.7 °C and relative humidity between 80% and 95%. Mortality was scored 24-h post-insecticide exposure. Mosquito samples were stored individually and preserved using desiccant silica gel for subsequent molecular analysis. For quality control, each assay was run with a control tube of 20–25 mosquitoes containing (standard pyrethroid control) silicone oil papers. Phenotypic data from larvae collected at different sampling points (Fig. 2) were pooled within each study site to improve test power.
2.4. Synergist bioassays
To further investigate underlying mechanisms of pyrethroid resistance via the synergist PBO, which acts primarily to block detoxification by cytochrome P450 monooxygenases, adult female An. gambiae (s.l.) were exposed to WHO insecticide papers treated with PBO (4%) for 1 h followed by permethrin or deltamethrin exposure for an additional diagnostic period of 1 h. Mortality was scored after 24 h. In control samples, PBO control papers were used prior to pyrethroid control paper exposure. Mosquito samples were stored singly over silica gel for further molecular analysis.
2.5. Molecular analysis
Genomic DNA was extracted from whole mosquitoes using the DNeasy kit (Qiagen, Hilden, Germany) and used as a template for molecular analyses. The Vgsc genotype at codon 1014 (995 using An. gambiae (s.s.) numbering) (The Anopheles gambiae 1000 Genomes Consortium, 2017) were determined using a locked nucleic allele (LNA) assay, which detects wild type and kdr mutants serine or phenylalanine (Lynd et al., 2018). The triple mutation with Cyp6aa1 duplication, Cyp6p4-I236M and ZZB-TE (cytochrome p450-linked ‘Zanzibar-like’ transposable element) was assessed using three independent LNA assays (Njoroge et al., 2021). All assays were run on AriaMx Real-Time PCR machine (Agilent, Santa Clara, USA). TaqMan assays were used to genotype Cyp4j5 and Coeae1d (Weetman et al., 2018). TaqMan assays used a primer/probe mix in addition to 1× sensimix (Bioline) and DNA template (1 μl) in a 10 μl volume reaction with denaturing for 5 min at 95 °C, followed by 40 cycles of denaturing for 15 s at 92 °C and annealing for 1 min at 60 °C. The TaqMan assays were performed on an Agilent MX3005P Real-Time PCR machine.
2.6. Statistical analysis
Statistical analysis using Stata (version 14.2, Stata Corp, College Station, TX, USA) generated measures of association (odds ratios) using mixed effects logistic regression, adjusting for repeated observations from the same study site. Key exposure variables were insecticide exposure, IRS status and species status. The primary outcome was mosquito phenotype, assessing whether changes in the exposure resulted in mortality or survival. To examine associations between genotypic markers of resistance and phenotypic outcomes, a logistic regression model was used. The nonsynonymous point mutation Cyp6p4 was selected as the marker of reference in the triple mutant haplotype due to the high level of correlation. Data were pooled by site and categorized by IRS status to improve the statistical power of the model. Pyrethroid resistance markers included in the model were Vgsc-L995S, Vgsc-L995F, Cyp6p4-I236M, Cyp4j5-L43F and Coeae1d.
3. Results
3.1. Species composition
Overall, 2753 An. gambiae (s.l.) adults were raised from larvae collected in 11 sites were phenotyped for pyrethroid resistance and speciated, including 1105 An. gambiae (s.s.) and 1648 An. arabiensis (Table 1). In the ‘no IRS’ sites, where vector control was limited to LLINs, the proportion of mosquitoes identified as An. gambiae (s.s.) ranged between 33.6 and 83.8%, while An. arabiensis ranged between 16.2 and 66.4%. In the sites where IRS was stopped 1.8–3.8 years prior to completing larval collections, most mosquitoes were identified as An. gambiae (s.s.) at 3 sites (76.5–99.4%), but at one site (Agago) 100% of mosquitoes were An. arabiensis. In the four IRS-active sites, in which IRS had been sustained for at least 3.5 years prior to larval collection, nearly all mosquitoes were identified as An. arabiensis (98–100%); only nine An. gambiae (s.s.) were collected from sites with active IRS, and these were excluded from subsequent analyses due to the small sample size.
Table 1.
Mosquitoes tested using phenotypic assays stratified by species, insecticide exposure, study site and vector control measures.
| Species | Insecticide exposure | No IRS |
IRS stopped |
IRS active |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Busia | Mubende | Kayunga | Kole | Amuru | Lamwo | Agago | Otuke | Tororo | Dokolo | Amolatar | ||
| An. gambiae (s.s.) | Total | 58 | 171 | 135 | 241 | 169 | 322 | 0 | 0 | 0 | 6 | 3 |
| Permethrin | 32 | 66 | 26 | 113 | 89 | 82 | 0 | 0 | 0 | 2 | 1 | |
| Permethrin + PBO | 0 | 16 | 39 | 0 | 39 | 75 | 0 | 0 | 0 | 1 | 0 | |
| Deltamethrin | 26 | 74 | 22 | 128 | 41 | 81 | 0 | 0 | 0 | 3 | 2 | |
| Deltamethrin + PBO | 0 | 15 | 48 | 0 | 0 | 84 | 0 | 0 | 0 | 0 | 0 | |
| An. arabiensis | Total | 71 | 33 | 267 | 74 | 1 | 81 | 158 | 112 | 365 | 293 | 193 |
| Permethrin | 34 | 15 | 78 | 41 | 0 | 16 | 72 | 57 | 119 | 86 | 96 | |
| Permethrin + PBO | 0 | 1 | 49 | 0 | 1 | 15 | 0 | 0 | 70 | 73 | 0 | |
| Deltamethrin | 37 | 17 | 93 | 33 | 0 | 16 | 86 | 55 | 111 | 74 | 97 | |
| Deltamethrin + PBO | 0 | 0 | 47 | 0 | 0 | 34 | 0 | 0 | 65 | 60 | 0 | |
3.2. Phenotypic bioassay results stratified by IRS categories, mosquito species, and insecticides
Anopheles gambiae (s.s.) and An. arabiensis were exposed to diagnostic concentrations of permethrin and deltamethrin, and mortality was measured (Supplementary Table S1). Overall, mortality of An. gambiae (s.s.) following exposure to pyrethroids was low, indicating high prevalence of resistance: 12.9% (53/411) for permethrin and 25.2% (95/377) for deltamethrin. Mortality of An. arabiensis was higher, indicating greater susceptibility to pyrethroids: 65.5% (402/614) for permethrin and 82.4% (510/619) for deltamethrin. Phenotypic assay results were pooled and compared between IRS category, species, and insecticide (Table 2). When different IRS category sites were compared, no significant difference in mortality was observed after exposure to either permethrin and deltamethrin, for either An. gambiae (s.s.) or An. arabiensis. When mosquito species were compared, mortality after exposure to both permethrin and deltamethrin was significantly lower for An. gambiae (s.s.) than An. arabiensis in both ‘no IRS’ (16.1 vs 67.7%, P < 0.001 for permethrin; 24.6 vs 83.7%, P < 0.001 for deltamethrin) and ‘IRS stopped’ sites (11.3 vs 63.6%, P < 0.001 for permethrin; 25.6 vs 88.9%, P < 0.001 for deltamethrin). In the ‘IRS active’ sites, the limited number of An. gambiae (s.s.) precluded species-specific comparisons. When the two pyrethroids were compared, An. gambiae (s.s.) mortality was significantly lower following exposure to permethrin than to deltamethrin in ‘IRS stopped’ sites (11.3 vs 25.6%, P = 0.001), but not in ‘no IRS’ sites (16.1 vs 24.6%, P = 0.10). For An. arabiensis, mortality was significantly lower following exposure to permethrin than to deltamethrin in ‘no IRS’ sites (67.7 vs 83.7%, P = 0.002), ‘IRS stopped’ sites (63.6 vs 88.9%, P < 0.001), and ‘IRS active’ sites (65.4 vs 79.2%, P < 0.001).
Table 2.
Mosquito mortality after exposure to pyrethroid insecticides using phenotypic assays, stratified by species, IRS category and insecticide.
| Comparison between IRS category, stratified by species | |||||||
|---|---|---|---|---|---|---|---|
| Species | IRS category | Permethrin |
Deltamethrin |
||||
| Mortality (%) | Odds ratio (95% CI) | P-value | Mortality (%) | Odds ratio (95% CI) | P-value | ||
| An. gambiae (s.s.) | No IRS | 20/124 (16.1) | Reference | 30/122 (24.6) | Reference | ||
| IRS stopped | 32/284 (11.3) | 0.64 (0.15–2.71) | 0.55 | 64/250 (25.6) | 0.66 (0.19–2.33) | 0.52 | |
|
An. arabiensis |
No IRS | 86/127 (67.7) | Reference | 123/147 (83.7) | Reference | ||
| IRS stopped | 82/129 (63.6) | 0.90 (0.28–2.94) | 0.86 | 120/135 (88.9) | 1.37 (0.51–3.64) | 0.53 | |
| IRS active |
234/358 (65.4) |
1.39 (0.47–4.10) |
0.55 |
267/337 (79.2) |
0.83 (0.37–1.87) |
0.66 |
|
| Comparison between mosquito species, stratified by IRS category | |||||||
| IRS category |
Species |
Permethrin | Deltamethrin | ||||
| Mortality (%) |
Odds ratio (95% CI) |
P-value |
Mortality (%) |
Odds ratio (95% CI) |
P-value |
||
| No IRS | An. arabiensis | 86/127 (67.7) | Reference | 123/147 (83.7) | Reference | ||
| An. gambiae (s.s.) | 20/124 (16.1) | 0.10 (0.05–0.19) | < 0.001 | 30/122 (24.6) | 0.06 (0.03–0.12) | < 0.001 | |
| IRS stopped |
An. arabiensis | 82/129 (63.6) | Reference | 120/135 (88.9) | Reference | ||
|
An. gambiae (s.s.) |
32/284 (11.3) |
0.20 (0.10–0.38) |
< 0.001 |
64/250 (25.6) |
0.08 (0.03–0.18) |
< 0.001 |
|
| Comparison between insecticides, stratified by IRS category | |||||||
| IRS category |
Insecticide |
An. gambiae (s.s.) | An. arabiensis | ||||
| Mortality (%) |
Odds ratio (95% CI) |
P-value |
Mortality (%) |
Odds ratio (95% CI) |
P-value |
||
| No IRS | Deltamethrin | 30/122 (24.6) | Reference | 123/147 (83.7) | Reference | ||
| Permethrin | 20/124 (16.1) | 0.59 (0.31–1.11) | 0.10 | 86/127 (67.7) | 0.40 (0.22–0.72) | 0.002 | |
| IRS stopped | Deltamethrin | 64/250 (25.6) | Reference | 120/135 (88.9) | Reference | ||
| Permethrin | 32/284 (11.3) | 0.44 (0.27–0.71) | 0.001 | 82/129 (63.6) | 0.21 (0.11–0.41) | < 0.001 | |
| IRS active | Deltamethrin | Insufficient An. gambiae (s.s.) collected | 267/337 (79.2) | Reference | |||
| Permethrin | 234/358 (65.4) | 0.48 (0.34–0.68) | < 0.001 | ||||
3.3. Synergist bioassays with piperonyl butoxide
Overall, when An. gambiae (s.s.) were exposed to the synergist PBO, mortality to both pyrethroids increased (Supplementary Table S1); for permethrin from 12.9% (53/411) to 56.5% (96/170), and for deltamethrin from 25.2% (95/377) to 68.7% (101/147). In An. arabiensis, mortality following PBO exposure also increased, from 65.5% (402/614) to 93.3% (195/209) for permethrin, and from 82.4% (510/619) to 89.8% (185/206) for deltamethrin. In the ‘no IRS’ sites, mortality of An. gambiae (s.s.) was significantly higher when PBO was added compared to that with the pyrethroid alone (permethrin: 54.5 vs 16.1%, P < 0.001; deltamethrin: 55.6 vs 24.6%, P < 0.001), indicating at least partial restoration of susceptibility to both permethrin and deltamethrin by PBO (Table 3). Similar results were observed in the ‘IRS stopped’ sites (permethrin: 57.0 vs 11.3%, P < 0.001; deltamethrin: 78.6 vs 25.6%, P < 0.001). When An. arabiensis from the ‘no IRS’ sites were exposed to PBO, mortality increased slightly, but not significantly, with permethrin (82.0 vs 67.7%, P = 0.36). Unexpectedly, mortality following exposure to PBO and deltamethrin was significantly lower compared to that with deltamethrin alone (66.0 vs 83.7%, P = 0.01). When An. arabiensis from the ‘IRS stopped’ sites were exposed to PBO, mortality increased to 100% for both permethrin and deltamethrin, but statistical significance could not be determined because all An. arabiensis died and comparisons could not be made. In the ‘IRS active’ sites, mortality of An. arabiensis increased significantly when PBO was added to both permethrin (96.5 vs 65.4%, P < 0.001) and deltamethrin (96.0 vs 79.2%, P < 0.001).
Table 3.
Mosquito mortality after exposure to pyrethroid insecticides with and without piperonyl butoxide, by species and IRS category.
| IRS category | Insecticide |
An. gambiae (s.s.) |
An. arabiensis |
||||
|---|---|---|---|---|---|---|---|
| Mortality (%) | Odds ratio (95% CI) | P-value | Mortality (%) | Odds ratio (95% CI) | P-value | ||
| No IRS | Permethrin | 20/124 (16.1) | Reference | 86/127 (67.7) | Reference | ||
| Permethrin + PBO | 30/55 (54.5) | 6.81 (3.08–15.1) | < 0.001 | 41/50 (82.0) | 1.52 (0.62–3.70) | 0.36 | |
| Deltamethrin | 30/122 (24.6) | Reference | 123/147 (83.7) | Reference | |||
| Deltamethrin + PBO | 35/63 (55.6) | 3.83 (2.01–7.31) | < 0.001 | 31/47 (66.0) | 0.38 (0.18–0.80) | 0.01 | |
| IRS stopped | Permethrin | 32/284 (11.3) | Reference | 82/129 (63.6) | Reference | ||
| Permethrin + PBO | 65/114 (57.0) | 15.0 (7.23–31.2) | < 0.001 | 16/16 (100) | Omitted because of collinearity | ||
| Deltamethrin | 64/250 (25.6) | Reference | 120/135 (88.9) | Reference | |||
| Deltamethrin + PBO | 66/84 (78.6) | 18.1 (8.36–39.3) | < 0.001 | 34/34 (100) | Omitted because of collinearity | ||
| IRS Active | Permethrin | Insufficient An. gambiae (s.s.) collected | 234/358 (65.4) | Reference | |||
| Permethrin + PBO | 138/143 (96.5) | 16.1 (6.31–41.2) | < 0.001 | ||||
| Deltamethrin | 267/337 (79.2) | Reference | |||||
| Deltamethrin + PBO | 120/125 (96.0) | 7.37 (2.82–19.3) | < 0.001 | ||||
3.4. Molecular markers of insecticide resistance in An. gambiae (s.s.)
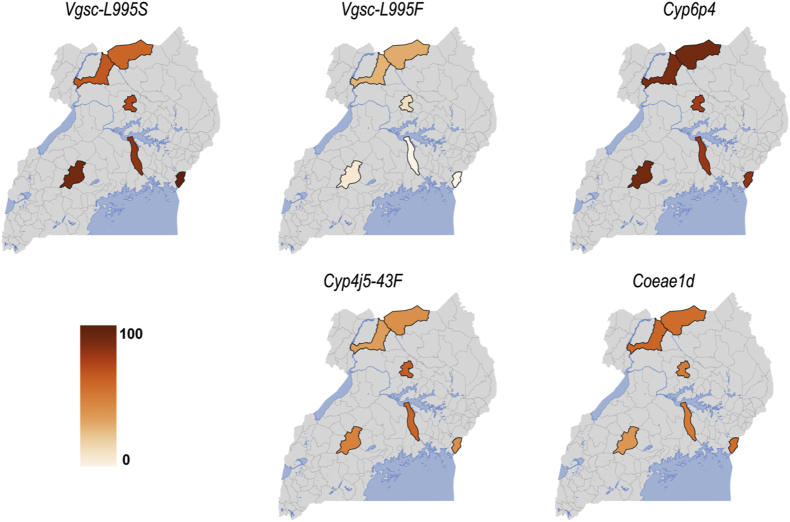
A subset of An. gambiae (s.s.) (Supplementary Table S2) were genotyped for molecular markers associated with pyrethroid resistance, including the kdr target site mutations Vgsc-L995S and Vgsc-L995F, and Cyp6aa1, Cyp6p4, ZZB-TE, Cyp4j5 and Coeae1d, associated with metabolic resistance. The frequency of the Vgsc-L995S resistance allele was high in the ‘no IRS’ sites, ranging from 83% in Kayunga to 96% in Busia, but was low in the ‘IRS stopped’ sites, ranging from 62% in Lamwo to 74% in Kole (Fig. 3, Supplementary Table S3). The frequency of the Vgsc-L995F resistance allele was low in An. gambiae (s.s.) but was highest in the northern ‘IRS stopped’ sites, ranging from 15% in Kole to 37% in Lamwo (Fig. 3, Supplementary Table S3; a summary of Vgsc genotypes in An. gambiae (s.s.) is shown in Supplementary Table S7). Comparison of resistance allele frequencies showed significantly higher Vgsc-L995F frequency in the ‘IRS stopped’ compared to ‘no IRS’ sites (28.40 vs 3.43, Fisherʼs exact test, P = 0.02). There was no significant difference in Vgsc-L995S resistance allele frequencies between the ‘IRS stopped’ and ‘no IRS’ sites (Supplementary Table S4). A high level of agreement was found between the metabolic resistance markers Cyp6aa1, Cyp6p4 and ZZB-TE (Spearman’s rank correlation = 0.72 for Cyp6p4 and 0.74 for ZZB-TE relative to Cyp6aa1). Thus, analyses were restricted to Cyp6p4. The frequency of the Cyp6p4-I236M resistance allele was very high in An. gambiae (s.s.) from all sites regardless of IRS status, ranging from 80% in Kayunga to 93% in Mubende, while the frequency of Cyp4j5 and Coeae1d ranged from 42% in Amuru to 65% in Kole and from 44% in Mubende to 62% in Amuru, respectively (Fig. 3, Supplementary Table S3).
Fig. 3.
Heatmaps showing the frequencies of target site mutations Vgsc-995S and Vgsc-995F, the triple mutant (represented by Cyp6P4), a cytochrome p450 Cyp4j5-L43F and carboxylesterase Coeae1d, associated with resistance to pyrethroids in An. gambiae (s.s.). The color scale ranges from white (0%) to dark orange (100%); the darker the shade, the higher the resistant allele frequency.
3.5. Molecular markers of insecticide resistance in An. arabiensis
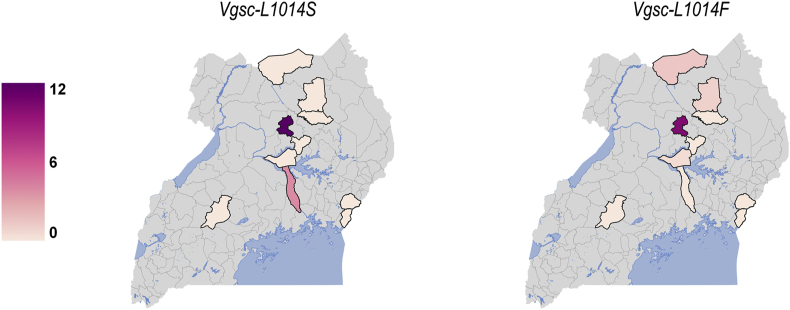
For An. arabiensis, only target-site resistance mutations (Vgsc-1014S and Vgsc-1014F) were genotyped (Supplementary Tables S5 and S6). Anopheles arabiensis were predominantly wild type (Vgsc-1014L) for kdr (Fig. 4); Vgsc-1014S was found only in Kayunga (3%) and in Kole (11%), while Vgsc-1014F was found in Agago (1%), Lamwo (2%) and Kole (9%). Vgsc-1014S was not detected in An. arabiensis from the ‘IRS active’ sites. However, Vgsc-1014F was found in a single An. arabiensis mosquito in Tororo and in one other An. arabiensis mosquito from Amolatar (Supplementary Table S5). A summary of Vgsc genotypes in An. arabiensis is provided in Supplementary Table S8).
Fig. 4.
Heatmaps showing the frequency of target site mutation Vgsc-L1014S and Vgsc-L1014F in An. arabiensis. The color scale ranges from white (0%) to dark purple (12%); the darker the shade, the higher the resistant allele frequency.
3.6. Association between genotypic resistance markers and phenotypic assays in An. gambiae (s.s.)
Analysis of the associations between genotypic resistance markers and phenotypic results in An. gambiae (s.s.) from ‘no IRS’ sites (Table 4) revealed significant associations between the target site mutations, Vgsc-L995 S/F, and survival when exposed to deltamethrin (odds ratio, OR: 3.44; 95% CI: 1.02–11.57; P = 0.046) and between Cyp4j5 and survival when exposed to deltamethrin + PBO (OR: 2.27; 95% CI: 1.08–4.80; P = 0.031). In ‘IRS stopped’ sites (Table 5), significant associations were found between Cyp6p4 and survival when exposed to permethrin + PBO (OR: 3.19; 95% CI: 1.16–8.80; P = 0.025) and when exposed to deltamethrin (OR: 2.27; 95% CI: 1.02–5.05, P = 0.045). All other measures of association were found to be non-significant in the ‘no IRS’ sites (Table 4) and ‘IRS stopped’ sites (Table 5).
Table 4.
Associations between resistant alleles and mosquito survival in An. gambiae (s.s.) mosquitoes following exposure to pyrethroid insecticides with and without piperonyl butoxide in sites with no IRS.
| Resistant alleles | Resistant allele frequency n/N (%) | Wild type alleles-survived n/N (%) | Resistant alleles-survived n/N (%) | Odds ratio (95% CI) | P-value | Resistant allele frequency n/N (%) | Wild type alleles-survived n/N (%) | Resistant alleles-survived n/N (%) | Odds ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Permethrin | Permethrin + PBO | |||||||||
| Vgsc-L995 S/F | 214/218 (98.2) | 4/4 (100) | 180/214 (84.1) | 0.62 (0.17–2.26) | 0.46 | 94/106 (88.7) | 2/12 (16.7) | 44/94 (46.8) | 1.17 (0.23–5.94) | 0.85 |
| Cyp6P4 (triple mutant) | 204/226 (90.3) | 19/22 (86.4) | 173/204 (84.8) | 0.93 (0.23–3.67) | 0.91 | 87/106 (82.1) | 6/19 (31.6) | 40/87 (46.0) | 1.22 (0.27–5.58) | 0.80 |
| Cyp4j5 | 113/216 (52.3) | 83/103 (80.6) | 99/113 (87.6) | 1.85 (0.80–4.29) | 0.15 | 60/106 (56.6) | 22/46 (47.8) | 24/60 (40.0) | 0.84 (0.33–2.16) | 0.72 |
| Coeae1d | 107/218 (49.1) | 90/111 (81.1) | 94/107 (87.9) | 1.97 (0.82–4.77) | 0.13 | 51/106 (48.1) | 25/55 (45.5) | 21/51 (41.2) | 0.85 (0.32–2.25) | 0.74 |
| Deltamethrin | Deltamethrin + PBO | |||||||||
| Vgsc-L995 S/F | 271/278 (97.5) | 2/7 (28.6) | 206/271 (76.0) | 3.44 (1.02–11.6) | 0.046 | 102/125 (81.6) | 14/23 (60.9) | 42/102 (41.2) | 1.41 (0.40–4.97) | 0.59 |
| Cyp6P4 (triple mutant) | 248/278 (89.2) | 20/30 (66.7) | 188/248 (75.8) | 0.82 (0.31–2.21) | 0.70 | 95/126 (75.4) | 15/31 (48.4) | 41/95 (43.2) | 0.94 (0.32–2.80) | 0.91 |
| Cyp4j5 | 147/278 (52.9) | 102/131 (77.9) | 106/147 (72.1) | 0.82 (0.46–1.47) | 0.51 | 75/134 (56.0) | 15/59 (25.4) | 41/75 (54.7) | 2.27 (1.08–4.80) | 0.03 |
| Coeae1d | 141/278 (50.7) | 103/137 (75.2) | 105/141 (74.5) | 0.98 (0.50–1.89) | 0.94 | 63/124 (50.8) | 30/61 (49.2) | 26/63 (41.3) | 0.65 (0.27–1.55) | 0.33 |
Table 5.
Associations between resistant alleles and mosquito survival in An. gambiae (s.s.) mosquitoes following exposure to pyrethroid insecticides with and without piperonyl butoxide in sites where IRS was stopped.
| Resistant alleles | Resistant allele frequency n/N (%) | Wild type alleles-survived n/N (%) | Resistant alleles-survived n/N (%) | Odds ratio (95% CI) | P-value | Resistant allele frequency n/N (%) | Wild type alleles-survived n/N (%) | Resistant alleles-survived n/N (%) | Odds ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Permethrin | Permethrin + PBO | |||||||||
| Vgsc-L995 S/F | 424/432 (98.2) | 6/8 (75) | 370/424 (87.2) | 1.40 (0.85–2.30) | 0.18 | 199/202 (98.5) | 0/3 (0) | 100/199 (50.3) | 1.25 (0.77–2.03) | 0.37 |
| Cyp6P4 (triple mutant) | 400/440 (90.9) | 25/40 (62.5) | 351/400 (87.8) | 1.87 (0.86–4.09) | 0.12 | 175/198 (88.4) | 6/23 (26.1) | 92/175 (52.6) | 3.19 (1.16–8.80) | 0.025 |
| Cyp4j5 | 214/430 (49.8) | 189/216 (87.5) | 185/214 (86.4) | 0.75 (0.22–2.57) | 0.64 | 95/200 (47.5) | 49/105 (46.7) | 51/95 (53.7) | 1.35 (0.80–2.28) | 0.27 |
| Coeae1d | 254/432 (58.8) | 157/178 (88.2) | 219/254 (86.2) | 0.78 (0.42–1.48) | 0.45 | 119/202 (58.9) | 45/83 (54.2) | 55/119 (46.2) | 0.70 (0.37–1.32) | 0.27 |
| Deltamethrin | Deltamethrin + PBO | |||||||||
| Vgsc-L995 S/F | 270/306 (88.2) | 6/36 (16.7) | 190/270 (70.4) | 1.64 (0.99–2.71) | 0.056 | 72/72 (100) | 0/0 (0) | 36/72 (50) | 1.60 (0.67–3.83) | 0.30 |
| Cyp6P4 (triple mutant) | 256/306 (83.7) | 13/50 (26.0) | 183/256 (71.5) | 2.27 (1.02–5.05) | 0.045 | 67/70 (95.7) | 0/3 (0) | 36/67 (53.7) | – | – |
| Cyp4j5 | 204/306 (66.7) | 71/102 (69.6) | 125/204 (61.3) | 1.0 (0.52–1.91) | 0.99 | 7/70 (10.0) | 30/63 (47.6) | 4/7 (57.1) | 1.19 (0.21–6.74) | 0.85 |
| Coeae1d | 164/306 (53.6) | 84/142 (59.2) | 112/164 (68.3) | 1.55 (0.83–2.88) | 0.17 | 42/72 (58.3) | 14/30 (46.7) | 22/42 (52.4) | 2.07 (0.64–6.66) | 0.22 |
4. Discussion
Resistance to pyrethroid insecticides threatens the effectiveness of malaria vector control. To further characterize pyrethroid resistance in Uganda, we collected An. gambiae (s.l.) from 11 districts implementing different IRS-based vector control strategies. We found high levels of pyrethroid resistance, particularly in An. gambiae (s.s.), but in settings where IRS was active, An. arabiensis dominated and almost no An. gambiae were identified. Combining PBO with a pyrethroid increased mortality for An. gambiae (s.s.), as well as An. arabiensis in some settings, indicating partial restoration of pyrethroid susceptibility and supporting the use of PBO LLINs in Uganda. The underlying genotypes only partially explained the resistance phenotype in An. gambiae (s.s.), while An. arabiensis were predominantly wild type for the target site resistance mutation.
In this study, resistance to permethrin and deltamethrin was widespread. Mortality in phenotypic assays was significantly lower in An. gambiae (s.s.) than An. arabiensis in sites without ongoing IRS. Mortality following exposure to permethrin was significantly lower than to deltamethrin for An. gambiae (s.s.) in sites where IRS had been stopped (but not in ‘no IRS’ sites), and for An. arabiensis in all sites, suggesting greater resistance to permethrin (a type I pyrethroid) than to deltamethrin (type II). Most An. gambiae (s.s.) had Vgsc-995 target site mutations, while these mutations were uncommon in An. arabiensis. The Cyp6p4-I236M resistance allele, a marker of metabolic resistance, was also common in An. gambiae (s.s.), while Cyp4j5 and Coeae1d were less common, present in just over half of An. gambiae (s.s.) tested. Some associations between genotypic markers of resistance and phenotypic outcomes were observed in An. gambiae (s.s.), although results were inconsistent, suggesting mechanisms of pyrethroid resistance are complex and insufficiently explained by currently recognized resistance markers.
The target site resistance mutation Vgsc-995S was found at very high frequency in An. gambiae (s.s.), consistent with prior observations in Uganda and Kenya (Okia et al., 2018; Lynd et al., 2019). The presence of the Vgsc-995F mutation, which has been associated with a strong resistance phenotype (Reimer et al., 2008), suggests pyrethroid selection pressure, in the study sites. The Vgsc-995F mutation has also been noted to confer greater resistance to type I (permethrin) than type II (deltamethrin) pyrethroids (Reimer et al., 2008), which may partially account for the significantly lower An. gambiae (s.s.) mortality to permethrin compared to deltamethrin observed in the ‘IRS stopped’ but not in the ‘no IRS’ sites. However, the very low frequency of this mutation (Vgsc-L1014F alternative) in An. arabiensis, suggests that the observed difference in insecticide specific mortality may be driven by other resistance mechanisms. The prevalence of the Vgsc-995F mutation seems to be increasing in Uganda, since the first report of this mutation at very low frequency in An. gambiae (s.s.) approximately 15 years ago (Verhaeghen et al., 2006). We found kdr mutations (Vgsc-995S and Vgsc-995F) within the same sample, particularly in An. gambiae (s.s.) The presence of both mutations (F/S heterozygotes) within the same mosquito is associated with a strong pyrethroid resistance phenotype, similar to that of F/F homozygotes. In An. arabiensis, both kdr mutations (L1014S and L1014F) were at relatively low frequency, with most individuals wild type homozygotes, akin to findings elsewhere in Uganda (Mawejje et al., 2013; Lynd et al., 2019). Nevertheless, kdr mutations (Vgsc-L1014S) in An. arabiensis have been found at frequencies as high as 63% in mosquitoes from Western Kenya (Hemming-Schroeder et al., 2018), neighboring Tororo (IRS active) and Busia (No IRS) districts, and as high as 89.5% in An. arabiensis from Dakar, Senegal (Dia et al., 2018).
The recently described mutants Cyp6aa1, Cyp6p4 and ZZB-TE (Njoroge et al., 2021) were found to be strongly correlated in An. gambiae (s.s.), indicating strong, though imperfect linkage disequilibrium and a high frequency of the triple mutant haplotype. The triple-mutant (represented by Cyp6p4) suggested strong positive selection in geographically distinct An. gambiae (s.s.) and was found at a frequency ranging from 80 to 93% in the target sites. This is consistent with observations of An. gambiae (s.s.) collected in Busia, Uganda and in Kenya (Njoroge et al., 2021). The Cyp6p4 mutation was associated with resistance to deltamethrin, similar to findings from western Kenya described by Njoroge et al. (2021). However, the association between the triple-mutant and mosquito survival following exposure to permethrin and PBO observed in this study has not previously been described and is unexpected given the expected blocking effects of PBO on P450 enzyme activity (Farnham, 1999). However, Njoroge et al. (2021) found that PBO LLINs were effective against a pyrethroid-resistant colony (from Busia, Uganda) with a triple-mutant frequency of 29.7%. The association between the Cyp4j5 P450 marker, and mosquito survival following exposure to deltamethrin plus PBO is another novel finding and similarly unexpected, although previous reports have found significant association between Cyp4j5 and deltamethrin (as well as permethrin) resistance (Weetman et al., 2018) and to our knowledge the marker association’s relationship with PBO has not previously been assessed.
Cluster-randomized trials in Uganda (Staedke et al., 2020) and Tanzania (Protopopoff et al., 2018) demonstrated significant declines in mosquito density and parasite prevalence associated with PBO LLINs, supported by the recently revised Cochrane review on PBO LLINs (Gleave et al., 2021). The WHO’s Vector Control Advisory Group concluded that PBO LLINs are more effective than pyrethroid-only LLINs in settings of high-level pyrethroid resistance, and the WHO now recommends PBO LLINs for the prevention and control of malaria in areas where malaria vectors demonstrate substantial pyrethroid resistance (WHO, 2022). As PBO LLINs are scaled-up, surveillance of markers of metabolic resistance will be essential.
We observed differences in the distribution of An. gambiae (s.s.) and An. arabiensis relative to IRS status. In sites with ‘no IRS’, An. gambiae (s.s.) and An. arabiensis were fairly evenly distributed, in contrast with the predominance of An. gambiae (s.s.) in ‘IRS stopped’ sites (apart from Agago) and An. arabiensis in ‘IRS active’ sites. Observed differences in species composition suggested an impact of IRS on malaria vectors, similar to other reports from this region (Musiime et al., 2019). Sustained vector control has previously been associated with changes in Anopheles mosquito species composition whereby highly anthropophagic An. gambiae (s.s.) is replaced by the less anthropophagic An. arabiensis (Bayoh et al., 2010; Mwangangi et al., 2013; Mawejje et al., 2021) potentially arising from the tendency of An. arabiensis to rest outdoors (Mahande et al., 2007), and behavioral patterns limiting contact with indoor based vector control interventions (Yohannes & Boelee, 2012). Similarly, a study in Tororo (one of the ‘IRS active’ sites) showed predominant An. gambiae (s.s.) (up to 77% abundance) prior to IRS, being replaced by An. arabiensis after IRS (Musiime et al., 2019). Stopping vector control has been associated with a rebound of primary vector species in some settings (Hargreaves et al., 2000; McCann et al., 2014). Pyrethroid-resistant primary vectors (such as An. gambiae (s.s.) and An. funestus) may have a selective advantage enabling them to overcome pyrethroid-based vector control or less effective non-pyrethroid IRS, resulting in a resurgence of malaria morbidity (Hargreaves et al., 2000). In the ‘IRS stopped’ district of Agago, in which we recorded predominantly An. arabiensis, it is plausible that there were spillover effects from sustained IRS (Namuganga et al., 2021) in the neighboring district of Otuke (Fig. 1), with the ‘invasion’ of An. gambiae (s.s.) in this district limited by IRS activity in Otuke. The absence of historical data on species composition pre-vector control implementation in the ‘IRS stopped’ area, however, limits interpretation of the impact of IRS on malaria vector-species composition. This noted, the consequences of stopping IRS in this region on malaria epidemiology have been associated with a rapid resurgence of the disease to pre-IRS levels (Raouf et al., 2017; Namuganga et al., 2021).
Highly anthropophilic and endophilic mosquitoes (An. gambiae (s.s.) and An. funestus) (Mwangangi et al., 2003) are more likely than zoophilic species (White et al., 1972; Molineaux et al., 1980) to be exposed to LLINs and IRS (Russell et al., 2010). Sympatric populations of An. gambiae (s.s.) and An. arabiensis or An. funestus, and zoophilic An. rivulorum (Kawada et al., 2012) have often revealed differential levels of mortality to insecticides in An. gambiae (s.s.) or An. funestus compared to An. arabiensis (Ochomo et al., 2014) or An. rivulorum (Kawada et al., 2012), respectively. In addition, the mechanisms mediating resistance in An. gambiae (s.s.) and An. funestus are more widespread and established (Kawada et al., 2011; Ranson et al., 2011; Mulamba et al., 2014; Ranson & Lissenden, 2016). Here, An. gambiae (s.s.) was significantly more resistant to pyrethroids than An. arabiensis, similar to reports from elsewhere (Ochomo et al., 2013). The significantly higher levels of pyrethroid resistance observed in An. gambiae (s.s.) in the ‘IRS stopped’ sites suggest that halting IRS interventions which have a different target site may open a population to selection by insecticides used for public health and/or agricultural purposes.
This study had several limitations. First, the findings are limited by the cross-sectional sampling done in only 11 districts. This may have introduced bias; however, sampling from several districts provided a snapshot of pyrethroid resistance in geographically distinct areas. Second, the definitions of insecticide resistance are based on WHO cut-offs using diagnostic concentrations of permethrin (0.75%) and deltamethrin (0.05%). Pyrethroid intensity assays to determine the operational significance of insecticide resistance were not conducted due to sample size limitations. Third, sample size limitations may have reduced the statistical power available to adequately test genotype-phenotype associations. Small sample sizes may result in a type II error and failure to reject the null hypothesis, due to an underestimation of the true effect. Nonetheless significant associations between target site/metabolic resistance markers with pyrethroid resistance were found in this analysis. Fourth, the concentration of PBO used was 4.0% which may not be directly comparable to the concentration of PBO on LLINs. In a study of PBO LLINs distributed by the Ugandan Ministry of Health in 2017–2018, the concentration of PBO at baseline was 26.81 g/kg in PermaNet 3.0, and 8.17 g/kg in Olyset Plus (Mechan et al., 2022) which may not be equivalent to the concentration included in the WHO tube assay. Finally, the absence of historical data before LLIN and/or IRS implementation limited the inferences that could be made on the development and spread of pyrethroid resistance mutations. Metabolic resistance mechanisms were not explored in An. arabiensis due to resource limitations and a lack of DNA-based markers for assessing metabolic resistance in this species.
5. Conclusions
Resistance to pyrethroids was widespread across Uganda, underscoring the importance of insecticide resistance management strategies targeting both An. gambiae (s.s.) and An. arabiensis. Adding PBO to pyrethroids improved mosquito mortality in both species, supporting the WHO’s new recommendation to deploy PBO LLINs for vector control in settings of pyrethroid resistance. Whilst target site resistance marker Vgsc 995S seems to be approaching fixation in An. gambiae (s.s.), the moderate frequency of Vgsc 995F in the ‘IRS stopped’ sites suggests intense insecticide selection pressure in northern Uganda. Our results also suggest an association between metabolic resistance variants (the triple-mutant-Cyp6p4 and Cyp4j5) and An. gambiae (s.s.) survival following exposure to PBO and pyrethroids underscoring the need for further research on the relationship between markers of metabolic resistance and PBO. Further surveillance of insecticide resistance and assessment of correlations between genotypic markers and phenotypic outcomes are needed to better understand mechanisms of pyrethroid resistance as PBO LLINs are scaled-up and to guide vector control measures.
Funding
This research was made possible through funding from the National Institutes of Allergy and Infectious Diseases (NIAID) as part of the International Centres of Excellence in Malaria Research (ICEMR) programme (U19AI089674), Fogarty International Centre (FIC) of the National Institutes of Health under Award Number D43TW7375 and Award Number D43TW010526, and GFATM NFM-2 The content presented herein is solely the responsibility of the authors and does not necessarily represent the official views of FIC or NIH or GFATM. All authors held final responsibility for the decision to submit this manuscript for publication. The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Ethical approval
Mosquito collections for this study were approved by the Makerere University College of Health Sciences, School of Medicine research ethics committee (Ref: 2018-066), Uganda National Council of Science and Technology (Ref: SS 4586), and London School of Hygiene and Tropical Medicine Ethics Committee (LSHTM Ethics Ref: 14584) under protocol study title “Investigating spatial and localized interactions between insecticide resistance, insecticidal malaria vector control and malaria transmission in Anopheles mosquitoes from Uganda” and by the School of Biomedical Sciences Research and Ethics Committee (Ref: SBS-HDREC-669) and Uganda National Council of Science and Technology (Ref: HS 2629) under study title “Entomological surveillance of vector behaviour, vector density and insecticide resistance to inform malaria vector control in Uganda.”
CRediT author statement
Henry Ddumba Mawejje, Sarah G. Staedke, David Weetman, Grant Dorsey, Amy Lynd, and Martin James Donnelly: conceptualisation. Henry Ddumba Mawejje, David Weetman, Amy Lynd and Martin James Donnelly: methodology. Henry Ddumba Mawejje, David Weetman, Amy Lynd, Martin James Donnelly: investigation. Henry Ddumba Mawejje, Sarah G. Staedke, Grant Dorsey, Martin James Donnelly, Amy Lynd, Philip J. Rosenthal, Adrienne Epstein, Jonathan Lines, Catherine Maiteki-Sebuguzi, Jimmy Opigo, Moses Kamya, and David Weetman: writing - review & editing. Grant Dorsey, Henry Ddumba Mawejje, Martin James Donnelly and David Weetman: formal analysis. Philip Rosenthal, Moses Kamya, Grant Dorsey, Jimmy Opigo, Catherine Maiteki-Sebuguzi: funding acquisition. Henry Ddumba Mawejje and Sarah G. Staedke: writing - original draft. All authors read and approved the final manuscript.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Given their role as Co-Editor, David Weetman had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Editor-in-Chief Aneta Kostadinova.
Acknowledgments
We thank Brian Kigozi Leetakubulidde, Francis Nyangabakye, Kilama Maxwell and Lilian Namuli Kayondo who supported the phenotypic tests and species identification. We acknowledge the central public health laboratory (CPHL)-Butabika, Kampala for providing a conducive research environment. We also acknowledge the communities/districts from where mosquito collections were done.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2022.100106.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
The data supporting the conclusions of this article are included within the article and its supplementary files.
References
- Agossa F.R., Aïkpon R., Azondékon R., Govoetchan R., Padonou G.G., Oussou O., et al. Efficacy of various insecticides recommended for indoor residual spraying: Pirimiphos methyl, potential alternative to bendiocarb for pyrethroid resistance management in Benin, West Africa. Trans. R. Soc. Trop. Med. Hyg. 2014;108:84–91. doi: 10.1093/trstmh/trt117. [DOI] [PubMed] [Google Scholar]
- Akogbéto M.C., Padonou G.G., Gbénou D., Irish S., Yadouleton A. Bendiocarb, a potential alternative against pyrethroid resistant Anopheles gambiae in Benin, West Africa. Malar. J. 2010;9:204. doi: 10.1186/1475-2875-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoh M.N., Mathias D.K., Odiere M.R., Mutuku F.M., Kamau L., Gimnig J.E., et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar. J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S., Weiss D., Cameron E., Bisanzio D., Mappin B., Dalrymple U., et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibulskis R.E., Alonso P., Aponte J., Aregawi M., Barrette A., Bergeron L., et al. Malaria: Global progress 2000–2015 and future challenges. Inf. Dis. Poverty. 2016;5:61. doi: 10.1186/s40249-016-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dia A.K., Guèye O.K., Niang E.A., Diédhiou S.M., Sy M.D., Konaté A., et al. Insecticide resistance in Anopheles arabiensis populations from Dakar and its suburbs: Role of target site and metabolic resistance mechanisms. Malar. J. 2018;17:116. doi: 10.1186/s12936-018-2269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly M.J., Corbel V., Weetman D., Wilding C.S., Williamson M.S., Black IV W.C. Does kdr genotype predict insecticide-resistance phenotype in mosquitoes? Trends Parasitol. 2009;25:213–219. doi: 10.1016/j.pt.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Elliott M., Janes N., Potter C. The future of pyrethroids in insect control. Annu. Rev. Entomol. 1978;23:443–469. [Google Scholar]
- Farnham A.W. In: Piperonyl butoxide. The insecticide synergist. Jones D.G., editor. Elsevier; NY: 1999. The mode of action of piperonyl butoxide with reference to studying pesticide resistance. [Google Scholar]
- Fink G., Mrema S., Abdulla S., Kachur S.P., Khatib R., Lengeler C., et al. Mosquito net use in early childhood and survival to adulthood in Tanzania. N. Engl. J. Med. 2022;386:428–436. doi: 10.1056/NEJMoa2112524. [DOI] [PubMed] [Google Scholar]
- Fongnikin A., Houeto N., Agbevo A., Odjo A., Syme T., N’Guessan R., Ngufor C. Efficacy of Fludora® Fusion (a mixture of deltamethrin and clothianidin) for indoor residual spraying against pyrethroid-resistant malaria vectors: Laboratory and experimental hut evaluation. Parasites Vectors. 2020;13:466. doi: 10.1186/s13071-020-04341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies M., Coetzee M. A supplement to the anophelinae of Africa South of the Sahara. Publ. S. Afr. Inst. Med. Res. 1987;55:1–143. [Google Scholar]
- Gillies M.T., De Meillon B. 1968. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region). South African Institute for Medical Research, Johannesburg, South Africa. [Google Scholar]
- Gleave K., Lissenden N., Chaplin M., Choi L., Ranson H. Piperonyl butoxide (PBO) combined with pyrethroids in insecticide-treated nets to prevent malaria in Africa. Cochrane Database Syst. Rev. 2021;5:1465–1858. doi: 10.1002/14651858.CD012776.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock P.A., Hendriks C.J., Tangena J.-A., Gibson H., Hemingway J., Coleman M., et al. Mapping trends in insecticide resistance phenotypes in African malaria vectors. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K., Koekemoer L., Brooke B., Hunt R., Mthembu J., Coetzee M. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med. Vet. Entomol. 2000;14:181–189. doi: 10.1046/j.1365-2915.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- Hemming-Schroeder E., Strahl S., Yang E., Nguyen A., Lo E., Zhong D., et al. Emerging pyrethroid resistance among Anopheles arabiensis in Kenya. Am. J. Trop. Med. Hyg. 2018;98:704–709. doi: 10.4269/ajtmh.17-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabula B., Tungu P., Rippon E.J., Steen K., Kisinza W., Magesa S., et al. A significant association between deltamethrin resistance, Plasmodium falciparum infection and the Vgsc-1014S resistance mutation in Anopheles gambiae highlights the epidemiological importance of resistance markers. Malar. J. 2016;15:289. doi: 10.1186/s12936-016-1331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katureebe A., Zinszer K., Arinaitwe E., Rek J., Kakande E., Charland K., et al. Measures of malaria burden after long-lasting insecticidal net distribution and indoor residual spraying at three sites in Uganda: A prospective observational study. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H., Dida G.O., Ohashi K., Komagata O., Kasai S., Tomita T., et al. Multimodal pyrethroid resistance in malaria vectors, Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus s.s. in western Kenya. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H., Dida G.O., Sonye G., Njenga S.M., Mwandawiro C., Minakawa N. Reconsideration of Anopheles rivulorum as a vector of Plasmodium falciparum in western Kenya: Some evidence from biting time, blood preference, sporozoite positive rate, and pyrethroid resistance. Parasites Vectors. 2012;5:230. doi: 10.1186/1756-3305-5-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigozi R., Baxi S.M., Gasasira A., Sserwanga A., Kakeeto S., Nasr S., et al. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt I., Bradley J., Knox T.B., Mnzava A.P., Kafy H.T., Mbogo C., et al. Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: A WHO-coordinated, prospective, international, observational cohort study. Lancet Infect. Dis. 2018;18:640–649. doi: 10.1016/S1473-3099(18)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lines J. Do agricultural insecticides select for insecticide resistance in mosquitoes? A look at the evidence. Parasitol. Today. 1988;4:S17–S20. doi: 10.1016/0169-4758(88)90083-x. [DOI] [PubMed] [Google Scholar]
- Lissenden N., Kont M.D., Essandoh J., Ismail H.M., Churcher T.S., Lambert B., et al. Review and meta-analysis of the evidence for choosing between specific pyrethroids for programmatic purposes. Insects. 2021;12:826. doi: 10.3390/insects12090826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas E.R., Miles A., Harding N.J., Clarkson C.S., Lawniczak M.K., Kwiatkowski D.P., et al. Whole-genome sequencing reveals high complexity of copy number variation at insecticide resistance loci in malaria mosquitoes. Genome Res. 2019;29:1250–1261. doi: 10.1101/gr.245795.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd A., Gonahasa S., Staedke S.G., Oruni A., Maiteki-Sebuguzi C., Dorsey G., et al. LLIN Evaluation in Uganda Project (LLINEUP): A cross-sectional survey of species diversity and insecticide resistance in 48 districts of Uganda. Parasites Vectors. 2019;12:94. doi: 10.1186/s13071-019-3353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd A., Oruni A., Van’t Hof A.E., Morgan J.C., Naego L.B., Pipini D., et al. Insecticide resistance in Anopheles gambiae from the northern Democratic Republic of Congo, with extreme knockdown resistance (kdr) mutation frequencies revealed by a new diagnostic assay. Malar. J. 2018;17:412. doi: 10.1186/s12936-018-2561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahande A., Mosha F., Mahande J., Kweka E. Feeding and resting behaviour of malaria vector, Anopheles arabiensis with reference to zooprophylaxis. Malar. J. 2007;6:100. doi: 10.1186/1475-2875-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Torres D., Chandre F., Williamson M., Darriet F., Bergé J.B., Devonshire A.L., et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol. Biol. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- Mathias D.K., Ochomo E., Atieli F., Ombok M., Nabie Bayoh M., Olang G., Muhia D., et al. Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in western Kenya. Malar. J. 2011;10:10. doi: 10.1186/1475-2875-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawejje H.D., Kilama M., Kigozi S.P., Musiime A.K., Kamya M., Lines J., et al. Impact of seasonality and malaria control interventions on Anopheles density and species composition from three areas of Uganda with differing malaria endemicity. Malar. J. 2021;20:138. doi: 10.1186/s12936-021-03675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawejje H.D., Wilding C.S., Rippon E.J., Hughes A., Weetman D., Donnelly M.J. Insecticide resistance monitoring of field-collected Anopheles gambiae s.l. populations from Jinja, eastern Uganda, identifies high levels of pyrethroid resistance. Med. Vet. Entomol. 2013;27:276–283. doi: 10.1111/j.1365-2915.2012.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann R.S., Ochomo E., Bayoh M.N., Vulule J.M., Hamel M.J., Gimnig J.E., et al. Reemergence of Anopheles funestus as a vector of Plasmodium falciparum in western Kenya after long-term implementation of insecticide-treated bed nets. Am. J. Trop. Med. Hyg. 2014;90:597–604. doi: 10.4269/ajtmh.13-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechan F., Katureebe A., Tuhaise V., Mugote M., Oruni A., Onyige I., et al. LLIN Evaluation in Uganda Project (LLINEUP): The fabric integrity, chemical content and bioefficacy of long-lasting insecticidal nets treated with and without piperonyl butoxide across two years of operational use in Uganda. Curr. Res. Parasitol. Vector Borne Dis. 2022;2 doi: 10.1016/j.crpvbd.2022.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOH . 2014. The Uganda Malaria Reduction Strategic Plan 2014–2020, Ministry of Health.https://www.health.go.ug/cause/the-uganda-malaria-reduction-strategic-plan-2014-2020/ Kampala, Uganda. [Google Scholar]
- Molineaux L., Gramiccia G., Who . World Health Organization; Geneva: 1980. The Garki project: Research on the epidemiology and control of malaria in the Sudan savanna of West Africa.https://apps.who.int/iris/handle/10665/40316 [Google Scholar]
- Mosha J.F., Kulkarni M.A., Lukole E., Matowo N.S., Pitt C., Messenger L.A., et al. Effectiveness and cost-effectiveness against malaria of three types of dual-active-ingredient long-lasting insecticidal nets (LLINs) compared with pyrethroid-only LLINs in Tanzania: A four-arm, cluster-randomised trial. Lancet. 2022;399:1227–1241. doi: 10.1016/S0140-6736(21)02499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulamba C., Riveron J.M., Ibrahim S.S., Irving H., Barnes K.G., Mukwaya L.G., et al. Widespread pyrethroid and DDT resistance in the major malaria vector Anopheles funestus in East Africa is driven by metabolic resistance mechanisms. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiime A.K., Smith D.L., Kilama M., Rek J., Arinaitwe E., Nankabirwa J.I., et al. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo, Uganda. Malar. J. 2019;18:445. doi: 10.1186/s12936-019-3076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwangangi J.M., Mbogo C.M., Nzovu J.G., Githure J.I., Yan G., Beier J.C. Blood-meal analysis for anopheline mosquitoes sampled along the Kenyan coast. J. Am. Mosq. Control Assoc. 2003;19:371–375. [PubMed] [Google Scholar]
- Mwangangi J.M., Mbogo C.M., Orindi B.O., Muturi E.J., Midega J.T., Nzovu J., et al. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar. J. 2013;12:13. doi: 10.1186/1475-2875-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namuganga J.F., Epstein A., Nankabirwa J.I., Mpimbaza A., Kiggundu M., Sserwanga A., et al. The impact of stopping and starting indoor residual spraying on malaria burden in Uganda. Nat. Commun. 2021;12:2635. doi: 10.1038/s41467-021-22896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nankabirwa J.I., Arinaitwe E., Rek J., Kilama M., Kizza T., Staedke S.G., et al. Malaria transmission, infection, and disease following sustained indoor residual spraying of insecticide in Tororo, Uganda. Am. J. Trop. Med. Hyg. 2020;103:1525–1533. doi: 10.4269/ajtmh.20-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Manag. Sci. 2007;63:628–633. doi: 10.1002/ps.1406. [DOI] [PubMed] [Google Scholar]
- Ngufor C., Agbevo A., Fagbohoun J., Fongnikin A., Rowland M. Efficacy of Royal Guard, a new alpha-cypermethrin and pyriproxyfen treated mosquito net, against pyrethroid-resistant malaria vectors. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-69109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njoroge H., Vanʼt Hof A., Oruni A., Pipini D., Nagi S.C., Lynd A., et al. 2021. Identification of a rapidly-spreading triple mutant for high-level metabolic insecticide resistance in Anopheles gambiae provides a real-time molecular diagnostic for anti-malarial intervention deployment.https://onlinelibrary.wiley.com/doi/epdf/10.1111/mec.16591 bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkya T.E., Poupardin R., Laporte F., Akhouayri I., Mosha F., Magesa S., et al. Impact of agriculture on the selection of insecticide resistance in the malaria vector Anopheles gambiae: A multigenerational study in controlled conditions. Parasites Vectors. 2014;7:480. doi: 10.1186/s13071-014-0480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochomo E., Bayoh M., Brogdon W., Gimnig J., Ouma C., Vulule J., Walker E. Pyrethroid resistance in Anopheles gambiae s.s. and Anopheles arabiensis in western Kenya: Phenotypic, metabolic and target site characterizations of three populations. Med. Vet. Entomol. 2013;27:156–164. doi: 10.1111/j.1365-2915.2012.01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochomo E., Bayoh N.M., Kamau L., Atieli F., Vulule J., Ouma C., et al. Pyrethroid susceptibility of malaria vectors in four districts of western Kenya. Parasites Vectors. 2014;7:310. doi: 10.1186/1756-3305-7-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okia M., Hoel D.F., Kirunda J., Rwakimari J.B., Mpeka B., Ambayo D., et al. Insecticide resistance status of the malaria mosquitoes: Anopheles gambiae and Anopheles funestus in eastern and northern Uganda. Malar. J. 2018;17:157. doi: 10.1186/s12936-018-2293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okia M., Ndyomugyenyi R., Kirunda J., Byaruhanga A., Adibaku S., Lwamafa D.K., Kironde F. Bioefficacy of long-lasting insecticidal nets against pyrethroid-resistant populations of Anopheles gambiae s.s. from different malaria transmission zones in Uganda. Parasites Vectors. 2013;6:130. doi: 10.1186/1756-3305-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopoff N., Mosha J.F., Lukole E., Charlwood J.D., Wright A., Mwalimu C.D., et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: A cluster, randomised controlled, two-by-two factorial design trial. Lancet. 2018;391:1577–1588. doi: 10.1016/S0140-6736(18)30427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce J., Richardson M., Lengeler C. Insecticide-treated nets for preventing malaria. Cochrane Database Syst. Rev. 2018;11:CD000363. doi: 10.1002/14651858.CD000363.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphul U., Boase T., Bass C., Okedi L.M., Donnelly M.J., Müller P. Insecticide resistance and its association with target-site mutations in natural populations of Anopheles gambiae from eastern Uganda. Trans. R. Soc. Trop. Med. Hyg. 2009;103:1121–1126. doi: 10.1016/j.trstmh.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Ranson H., Jensen B., Vulule J., Wang X., Hemingway J., Collins F. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol. Biol. 2000;9:491–497. doi: 10.1046/j.1365-2583.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- Ranson H., Lissenden N. Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–196. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Ranson H., N’guessan R., Lines J., Moiroux N., Nkuni Z., Corbel V. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Raouf S., Mpimbaza A., Kigozi R., Sserwanga A., Rubahika D., Katamba H., et al. Resurgence of malaria following discontinuation of indoor residual spraying of insecticide in an area of Uganda with previously high-transmission intensity. Clin. Infect. Dis. 2017;65:453–460. doi: 10.1093/cid/cix251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer L., Fondjo E., Patchoké S., Diallo B., Lee Y., Ng A., et al. Relationship between kdr mutation and resistance to pyrethroid and DDT insecticides in natural populations of Anopheles gambiae. J. Med. Entomol. 2008;45:260–266. doi: 10.1603/0022-2585(2008)45[260:rbkmar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Roberts R., Ocaya R. 2009. Agricultural Finance Yearbook 2009. Kampala, Uganda: Bank of Uganda and Plan for the Modernization of Agriculture. [Google Scholar]
- Russell T.L., Lwetoijera D.W., Maliti D., Chipwaza B., Kihonda J., Charlwood J.D., et al. Impact of promoting longer-lasting insecticide treatment of bed nets upon malaria transmission in a rural Tanzanian setting with pre-existing high coverage of untreated nets. Malar. J. 2010;9:187. doi: 10.1186/1475-2875-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J.A., Brogdon W.G., Collins F.H. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- Service M. Mosquito Ecology. Springer; Dordrecht: 1993. Sampling the larval population; pp. 75–209. [DOI] [Google Scholar]
- Staedke S.G., Gonahasa S., Dorsey G., Kamya M.R., Maiteki-Sebuguzi C., Lynd A., et al. Effect of long-lasting insecticidal nets with and without piperonyl butoxide on malaria indicators in Uganda (LLINEUP): A pragmatic, cluster-randomised trial embedded in a national LLIN distribution campaign. Lancet. 2020;395:1292–1303. doi: 10.1016/S0140-6736(20)30214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchouakui M., Mugenzi L.M., D Menze B., Khaukha J.N., Tchapga W., Tchoupo M., et al. Pyrethroid resistance aggravation in Ugandan malaria vectors is reducing bednet efficacy. Pathogens. 2021;10:415. doi: 10.3390/pathogens10040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Anopheles gambiae 1000 Genomes Consortium Natural diversity of the malaria vector Anopheles gambiae. Nature. 2017;552:96–100. doi: 10.1038/nature24995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiono A.B., Ouédraogo A., Ouattara D., Bougouma E.C., Coulibaly S., Diarra A., et al. Efficacy of Olyset Duo, a bednet containing pyriproxyfen and permethrin, versus a permethrin-only net against clinical malaria in an area with highly pyrethroid-resistant vectors in rural Burkina Faso: A cluster-randomised controlled trial. Lancet. 2018;392:569–580. doi: 10.1016/S0140-6736(18)31711-2. [DOI] [PubMed] [Google Scholar]
- Toé K.H., Jones C.M., N’Fale S., Ismail H.M., Dabiré R.K., Ranson H. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg. Inf. Dis. 2014;20:1691–1696. doi: 10.3201/eid2010.140619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uganda National Malaria Control Division . Uganda National Malaria Control Division; Kampala, Uganda, and Rockville, Maryland, USA: 2019. 2018–2019 Uganda Malaria Indicator Survey Atlas of Key Indicators.https://dhsprogram.com/pubs/pdf/ATR21/ATR21.pdf [Google Scholar]
- Verhaeghen K., Van Bortel W., Roelants P., Backeljau T., Coosemans M. Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis. Malar. J. 2006;5:16. doi: 10.1186/1475-2875-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen K., Van Bortel W., Roelants P., Okello P.E., Talisuna A., Coosemans M. Spatio-temporal patterns in kdr frequency in permethrin and DDT resistant Anopheles gambiae s.s. from Uganda. Am. J. Trop. Med. Hyg. 2010;82:566–573. doi: 10.4269/ajtmh.2010.08-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wat’senga F., Agossa F., Manzambi E.Z., Illombe G., Mapangulu T., Muyembe T., et al. Intensity of pyrethroid resistance in Anopheles gambiae before and after a mass distribution of insecticide-treated nets in Kinshasa and in 11 provinces of the Democratic Republic of Congo. Malar. J. 2020;19:169. doi: 10.1186/s12936-020-03240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weetman D., Wilding C.S., Neafsey D.E., Müller P., Ochomo E., Isaacs A.T., et al. Candidate-gene based GWAS identifies reproducible DNA markers for metabolic pyrethroid resistance from standing genetic variation in East African Anopheles gambiae. Sci. Rep. 2018;8:2920. doi: 10.1038/s41598-018-21265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G., Magayuka S.A., Boreham P. Comparative studies on sibling species of the Anopheles gambiae Giles complex (Dipt., Culicidae): Bionomics and vectorial activity of species A and species B at Segera, Tanzania. Bull. Entomol. Res. 1972;62:295–317. [Google Scholar]
- World Health Organization; Geneva: 1998. Test procedures for insecticide resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticides on treated surfaces: Report of the WHO informal consultation, 28–30 September 1998.https://apps.who.int/iris/bitstream/handle/10665/64879/WHO_CDS_CPC_MAL_98.12.pdf?sequence=1&isAllowed=y [Google Scholar]
- WHO . World Health Organization; Geneva: 1999. Safety of pyrethroid-treated mosquito nets: Fact sheet.https://www.who.int/publications-detail-redirect/who-cds-cpe-whopes-99.5 [Google Scholar]
- WHO . World Health Organization; Geneva: 2011. The technical basis for coordinated action against insecticide resistance: Preserving the effectiveness of modern malaria vector control. Meeting Report.https://apps.who.int/iris/bitstream/handle/10665/44526/9789241501095_eng.pdf [Google Scholar]
- WHO . World Health Organization; Geneva: 2014. Guidance for countries on combining indoor residual spraying and long-lasting Insecticidal nets.https://apps.who.int/iris/bitstream/handle/10665/338635/WHO-HTM-GMP-MPAC-2014.2-eng.pdf?sequence=1&isAllowed=y [Google Scholar]
- WHO . World Health Organization; Geneva: 2015. Indoor residual spraying: An operational manual for indoor residual spraying (IRS) for malaria transmission control and elimination.https://www.who.int/publications/i/item/9789241508940 [Google Scholar]
- WHO . World Health Organization; Geneva: 2016. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes.https://apps.who.int/iris/bitstream/handle/10665/250677/9789241511575-eng.pdf [Google Scholar]
- WHO . World Health Organization; Geneva: 2017. Conditions for deployment of mosquito nets treated with a pyrethroid and piperonyl butoxide: Recommendations.https://apps.who.int/iris/bitstream/handle/10665/258939/WHO-HTM-GMP-2017.17-eng.pdf [Google Scholar]
- WHO . World Health Organization; Geneva: 2021. World malaria report 2021.https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 [Google Scholar]
- WHO . World Health Organization; Geneva: 2022. WHO guidelines for malaria, 3 June 2022.https://reliefweb.int/report/world/who-guidelines-malaria-3-june-2022#:∼:text=The%20WHO%20global%20malaria%20strategy,residual%20foci%20of%20malaria%20transmission [Google Scholar]
- Yipmo E.S., Tchouakui M., Menze B.D., Mugenzi L.M., Njiokou F., Wondji C.S. Reduced performance of community bednets against pyrethroid-resistant Anopheles funestus and Anopheles gambiae, major malaria vectors in Cameroon. Parasites Vectors. 2022;15:230. doi: 10.1186/s13071-022-05335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohannes M., Boelee E. Early biting rhythm in the Afro-tropical vector of malaria, Anopheles arabiensis, and challenges for its control in Ethiopia. Med. Vet. Entomol. 2012;26:103–105. doi: 10.1111/j.1365-2915.2011.00955.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the conclusions of this article are included within the article and its supplementary files.