Abstract
In light of the increased use of communication technologies, the harm caused by continuous exposure to emitted radiation on pregnancy and developing newborns is among the public concerns. Using Sprague-Dawley rats, our study investigates the effects of 24 h/day prenatal and postnatal 900 MHz radiofrequency electromagnetic radiation (RF-EMR) exposure of female rats on liver oxidative stress (OS) and other hepatic parameters at postnatal days (PND) 1, 9, and 21. Our results showed that RF-EMR exposure led to an increase in oxidative stress status as indicated by a significant elevation in MDA level at PND9 and PND21, a decrease in catalase (CAT) activity at all ages, a reduction (PND1 and PND9) in catalase amounts and mRNA expression, in addition to a decrease in GPx activity at PND21 in the exposed group. Current findings also showed a significant increase in cytoSOD at PND9 and 21 and a reduction in mitoSOD at PND21 in the exposed groups compared to the control groups. However, significant increases in glutathione peroxidase (GPx) level and mitoSOD activity were observed at all studied ages. Furthermore, cytoSOD activity showed a significant reduction in PND1, whereas in PND9 the value of this parameter increased compared to the non-exposed group. Moreover, while SOD1 mRNA expression increased at PND1, it decreased at PND9 and 21. However, GPx1 expression was shown to be always decreased in the exposed group. In addition, at PND1 and 9, exposed rats showed a similar response on Akt1, nuclear factor erythroïd 2-related factor 2 (Nrf-2), and intercellular adhesion molecule-1 (ICAM-1) expression. Therefore, an increased oxidative stress status produced from a continuous (24 h/day) GSM-modulated 900 MHz radiofrequency electromagnetic radiation (RF-EMR) exposure during the prenatal and postnatal periods may result in adverse health effects during future life stages.
Keywords: GSM, Oxidative stress, Rat, Nrf2, ICAM-1
Highlights
-
•
A 24 h/day GSM-EMR prenatal and postnatal exposure creates age dependent effects in female rat liver.
-
•
Pre- and post-natal EMR exposure increases oxidative stress at early development.
-
•
Continuous EMR exposure increases MDA level in female rat liver after day 9.
-
•
A 24 h/day EMR exposure decreases CAT, GPx expression and activation in new rats.
-
•
EMR exposure reduces hepatic Nrf-2, Akt1, ICAM-1 expression in female neonates.
GSM; Oxidative stress; Rat; Nrf2; ICAM-1.
1. Introduction
Humans are daily exposed to different types of electromagnetic radiations (EMR) from different sources such as wireless, power transmission, electric devices, mobile phones, portable computers, tablets, and others. These sources have a direct contribution to biosphere pollution (Belyaev et al., 2016; Santini et al., 2018). Cell phones are considered among the major sources of EMR and thus a cause of environmental pollution that affects human health (Schuz et al., 2006; Sultangaliyeva et al., 2020). Moreover, people that live near base stations for mobile phones have higher potential exposures to radiofrequency electromagnetic radiation (RF-EMR). So, there is a major concern about the effects of these radiations on human health (Lu et al., 2012). According to World Health Organization (WHO), electromagnetic field pollution (EMP) of the environment was defined as one of the primary issues for humans (Sultangaliyeva et al., 2020). Studies have shown that exposure to EMR can affect the liver, kidneys, and nervous system (Kim et al., 2019; Sabban et al., 2018; Sultangaliyeva et al., 2020). EMR exposure can affect reproductive functions (Santini et al., 2018), sperm quality (Yu et al., 2021), different cellular pathways, gene expression processes in isolated human mononuclear cells by exposure to 900 MHz global system for mobile communications radiofrequency electromagnetic field (GSM RF-EMF) at a specific absorption rate (SAR) of 0.4 W/kg for 1, 2, 4, 6, and 8 h (Lu et al., 2012), deregulate the cell cycle (Liu et al., 2012; Santini et al., 2018), and induce apoptosis (Lu et al., 2012), moderate hyperemia, inflammation of liver lobules in addition to occasional necrosis of hepatocytes (Holovská et al., 2015). Moreover, one of the interesting outcomes of the recent studies concluded that there is a relationship between exposure to EMR radiations and oxidative stress (OS) in many body tissues (Kıvrak et al., 2017).
Oxidative stress is the outcome of an imbalance in the capacity of the redox defense system to avoid the effects of free radicals in many body tissues (Kıvrak et al., 2017). Furthermore, there is experimental evidence that free radicals and oxidative stress increase with stages of development and pregnancy (Jauniaux et al., 2000).
Studies carried out in humans and animals have shown that exposure to electromagnetic waves (EMW) during pregnancy and/or their offspring during their neonatal life may exhibit an increased oxidative stress status in the liver with a significant elevation in malondialdehyde (MDA) level and a significant reduction in the total antioxidant system (Martínez-Sámano et al., 2010). According to the literature, exposure of dams during their pregnancy period and their offspring to EMFs induces hepatic oxidative stress status in rat offspring. The severity of this status can be related to many factors such as the duration of exposure, frequency of emitted waves, species, gender, and age of neonates such as in pregnant rats and newborns after exposure to 2.45GHz Wi-Fi-induced EMR for 1 h per day for 5 days per week from pregnancy to 3 weeks of age, in rats and their offspring after exposure to mobile phone (900 and 1800 MHz)-induced EMR for 60 min/d from pregnancy to 6 weeks of age, in rats after 950 MHz ultra-high-frequency electromagnetic radiation (UHF EMR) exposure for half an hour per day for 21 days of gestation and 6, 15 or 30 days of postnatal life, for SAR ranged from 1.3-1.0 W/kg, and in male rat pups on the postnatal day 21 after 900-MHz EMF exposure for 1 h daily during days 13–21 of gestation period (Çelik et al., 2016; Çetin et al., 2014; Furtado-Filho et al., 2014; Topal et al., 2015).
According to our knowledge, this report is the first study that showed the effects of continuous exposure to global system for mobile communications electromagnetic waves (GSM-EMW) produced by mobile phone base stations antenna from pregnancy till 3 weeks after birth period on the liver of female offspring rats at postnatal day 1, 9 and 21. In addition, we have provided an overview of the correlation between oxidative stress, continuous radiofrequency electromagnetic radiation (RF-EMR) exposure, and some molecular changes in neonatal rat liver. Therefore, this work investigated the effects of these (RF-EMR) on oxidative stress, inflammatory and apoptotic parameters in the liver of newborn rats such as the MDA level, the amount, relative activity and gene expression of the most important anti-oxidation enzymes (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and nuclear factor erythroïd 2-related factor 2 (Nrf-2)) in addition to the expression changes in protein kinase (Akt) and intercellular adhesion molecule-1 (ICAM-1).
2. Materials and methods
2.1. Animal preparation
The animal protocol was approved by the IRB committee at the Beirut Arab University, Beirut, Lebanon, and all animal procedures were carried out based on the NIH guidelines (https://osp.od.nih.gov/wp-content/uploads/NIH_Guidelines.pdf).
Sprague-Dawley pregnant female rats were obtained after several series of assays by placing four female rats and one male rat in cages and by examining the vaginal plug (The next day between 8:30 and 10:30 am) as a sign of pregnancy (Azemi et al., 2012). The size of the litter was adjusted to obtain litters of the equivalent size and only female rats were used in this study. On (PND) 1, the second day after birth, the litters were reduced to 4 female pups per dam, for a total of 16 female pups per each postnatal age per each group (4 female pups/cage x 4 cages/each postnatal age/each control or exposed group). Throughout the preparation period, all animals were placed in plexiglass cages and housed in a controlled chamber for a cycle of light-dark 12h: 12h and room temperature (22 °C). The plexiglass cages were 55 cm long, 35 cm wide, and 15 cm high, and each dam or female rat was housed with their four female pups per cage (1 female rat/litter/cage).
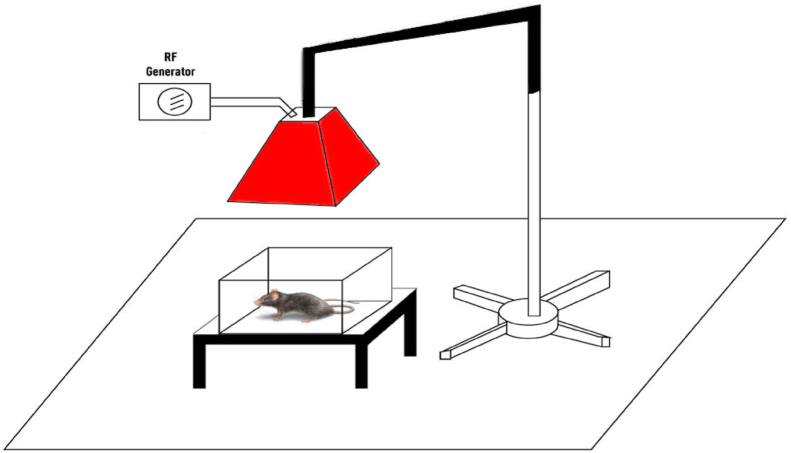
2.2. GSM-EMF exposure
The whole body exposure to this radiation was continuous for 24 h per day from gestational day 1 until their pups were weaned (on post-natal day (PND) 21). The whole body of animals was exposed to GSM-EMW as shown by Ramadan et al. (2015). Briefly, animals were exposed to radiation emitted by a device similar to the antennas of mobile phone stations with a frequency equal to 900 MegaHertz. This device generally consists of a radiofrequency signal (RF) generator (model RFS 900–64, RFPA, Artigues-près-Bordeaux, France) accompanied with RF-EMF antennas (local made) supported by a stand, and placed horizontally 100 cm above the cages containing rats (Figure 1). The generator’s power was set to obtain a field intensity of 25 ± 0.4 V/m. A radiofrequency probe (PMM EP600, Narda Safety Test Solution, Hauppauge, NY, USA) monitored with computer software (Win EP 600, Narda Safety Test Solution) was used to check the level of RF-EMF exposure at 3 different periods (gestational day 1, PND1 and PND21). The liver-specific absorption rate (SAR) (0.783 W/kg) was calculated using the equation:
Figure 1.
Global system for mobile communications electromagnetic waves (GSM-EMW) exposure system.
SAR = σ∗E2/ρ, where (E) is the magnitude of the electric field (25 V/m) (σ) is the conductivity 1.34 S/m and (ρ) is the mass density of the tissue-equivalent media 1 070 kg/m3 (Means and Chan, 2001). Control animals were housed in the same environment conditions without radiation exposure.
2.3. Animal groups
The resulting pregnant rats are divided equally into control and (RF-EMR) exposed groups. Forty-eight female pups were obtained and analyzed from the control group of pregnant rats as well as the same number was obtained from the exposed one. In both the control and exposed groups, the total number of female pups was divided into three subgroups PND1, PND9, and PND21 (16; 16; 16). PND1 is the first day after birth. PND9 is the midpoint or half-period (1–16 postpartum) during which the pup can breathe but is still suckling with its eyelids remaining sealed and external ear ducts plugged with periderm. The third selected age is during the period 17 + postpartum where periderm seals of ears and eyelids vanish, the PND21. During this age, the pup is weanling (within the next three days after the beginning of the 17 + postpartum period). Moreover, these specific pre-puberty ages have been chosen to study for the first time the effects of a permanent exposure of 24 h per day to RF-EMR on the early postnatal period in rats. Rats’ body weights were measured every week and just before their sacrifice (Table I and Figure I). At the end of the preparation period, rats were euthanized with an intraperitoneal sodium pentobarbital overdose (1 ml/kg; 200 mg/ml solution). The liver was removed, weighed, frosted in liquid nitrogen, and stored at -80 °C for later experimental usage.
2.4. Biochemical and molecular analysis
2.4.1. Determination of the lipid peroxidation product (MDA) levels
The quantity of lipid peroxidation product: Malondialdehyde (MDA) in the livers of female rats, was measured using the commercial kit 'The Lipid peroxidation (MDA) Colorimetric/Fluorometric Assay Kit ' (Biovision, USA, 100 assays, Catalog number K739-100) according to the specified instructions. This measurement was made colorimetrically at a wavelength equal to 532 nm using the SP-830 Plus spectrophotometer after MDA forms a complex with the TBA (Thiobarbituric Acid) reagent provided in the kit.
2.4.2. Measurement of superoxide dismutase (SOD) absolute activity
The mitochondrial and cytosol SOD fractions were extracted using the Mitochondrial/Cytosol Fractionation Kit from BioVision (Catalog number: K256-25). The absolute activity (in U/ml) of SOD was estimated using the Superoxide Dismutase Activity Assay kit (Biovision, USA, 100 Rnx, catalog number K335-100) for a colorimetric reading at a wavelength of 450 nm using a microplate Elisa reader (Mindray MR-96A) according to the specified instructions provided by the seller.
2.4.3. Measurement of catalase (CAT) absolute activity
After homogenization of the hepatic tissues by using the assay buffers obtained from the commercial Catalase Activity Colorimetric/Fluorometric Assay kit (Biovision, USA, 100 Rnx, catalog number K773 -100), the absolute activity (in mU/ml or nmol/min/ml) of CAT, a second antioxidant enzyme, was estimated according to instructions supplied by the vendor.
2.4.4. Measurement of glutathione peroxidase (GPx) absolute activity
Supernatants were obtained by cold centrifugation after homogenization of the hepatic tissues using buffer acquired by the Glutathione Peroxidase Activity Colorimetric Assay kit (Biovision, USA, 100 Rnx, catalog number K762-100). The absolute GPx activity (in mU/ml or nmol/min/ml) was measured according to the instructions provided by the supplier.
2.4.5. Estimation of the relative activity of enzymes
First, to quantify each studied antioxidant enzyme (superoxide dismutase (SOD) in its two mitochondrial and cytosol fractions, catalase (CAT) and glutathione peroxidase (GPx)), samples were taken from the supernatants obtained after cold centrifugation of the homogenized liver tissues, extracted from rats, with the specific assay buffer to isolate each enzyme. These buffers were provided in the previous used commercial kits: the Mitochondrial/Cytosol Fractionation Kit (Biovision, USA, Catalog number: K256-25) to obtain the mitochondrial and cytosolic SOD fractions in the liver tissues of rats, in Catalase Activity Colorimetric/Fluorometric Assay kit (Biovision, USA, 100 Rnx, Catalog number K773-100) and Glutathione Peroxidase Activity Colorimetric Assay kit (Biovision, USA, 100 Rnx, Catalog number K762-100) to obtain catalase and GPx protein samples, respectively. All control and exposed samples were included.
The quantification of each of the enzymes cited above was done using the Bradford reagent (Sigma ALDRICH, B 6916-500 ml). The absorbance measurement was made at a wavelength equal to 595 nm. The quantity of each antioxidant enzyme was determined in mg/ml of the initial sample and then in mg/g of the mass of the studied liver tissue. The relative activities (in mU/min/mg or μmol/min/mg of proteins) were estimated by the calculation determining the ratio between their absolute activities and the protein levels of the enzymes. The relative enzyme activity in μmol/min/mg of protein (as a common unit), was obtained by dividing this factor in mU/min/mg by 1000.
2.4.6. Determination of SOD1, CAT, GPx1, Nrf-2, ICAM-1, and Akt1 mRNA levels using q-RT- PCR
The iTaq™ Universal SYBR ® Green Supermix (Bio-Rad Laboratories, USA, catalog number MLL4801) was used for the quantitative real time-PCR (q-RT-PCR) determination of DNA sequences (mRNA levels) obtained by reverse transcription of RNA molecules extracted from the frozen liver tissue of exposed and control rats, using the Quick-RNA TM MiniPrep Plus Kit (Zymo Research, catalog nos. R1057 and R1058) and then transcribed to cDNA molecules by the iScript ™ cDNA Synthesis Kit (Bio-RAD Laboratories, catalog number: 1708891). Each RT-PCR quantification was carried out in triplicates by using a thermal cycler with a CFX Connect Real-Time PCR Detection System (BIO-RAD, catalog number: 1855200). The specific genes’ primers, purchased from Macrogen, Korea, are shown in Table 1. Data, expressed as relative fold change in studied gene expression, was calculated using 2−ΔΔCt after being normalized to reference gene beta-actin (ß- Actin) and TATA box binding protein (TBP).
Table 1.
Specific forward and reverse primers (from Macrogen) designed for oxidative stress, inflammation and apoptosis-related genes (SOD1, GPx1, CAT, Nrf-2, ICAM-1 and Akt1), and for reference endogenous genes (ß- Actin and TBP) in rat liver.
| Symbols | Genes Rattus norvegicus | Primer Sequences (Forward and Reverse):5’→3’ |
|---|---|---|
| SOD1 | Superoxide dismutase 1 | F: CCACTGCAGGACCTCATTTT R: CACCTTTGCCCAAGTCATCT |
| GPx1 | Glutathione peroxidase 1 | F: ATAGAAGCCCTGCTGTCCAA R: GAAACCGCCTTTCTTTAGGC |
| CAT | Catalase | F: ACATGGTCTGGGACTTCTGG R: CAAGTTTTTGATGCCCTGGT |
| Nrf-2 | Nuclear factor-erythroid derived 2-like 2 | F: CCTAAAGCACAGCCAACACA R: GCCTCTAATCGGCTTGAATG |
| Akt1 | Protein kinase B | F: CCTCAAGAATGATGGCACCT R: TTTGAGTCCATCAGCCACAG |
| ICAM-1 | Intercellular adhesion molecule 1 | F: AGGTATCCATCCATCCCACA R: GCCACAGTTCTCAAAGCACA |
| ß-Actin | ß-actin | F: GGGTATGGAATCCTGTGGCATCC R: GCTCAGGAGGAGCAATGATCTTGA |
| TBP | TATA box binding protein | F: GACTCCTGTCTCCCCTACCC R: CTCAGTGCAGAGGAGGGAAC |
2.5. Western blot analysis of Nrf-2
Hepatic tissue of female rats at PND9 was homogenized in a lysis extraction buffer and extracted proteins were quantified with Lowry protein assay. Western blots were performed as described in previous studies (Fusco et al., 2020). Briefly, total proteins were separated on sodium dodecyl sulfate (SDS) acrylamide and electrotransferred to nitrocellulose membranes. Then, membranes are gently shacked with primary antibodies anti- Nrf-2 (ab31163, Abcam USA) and anti- GAPDH as a reference protein. Protein bands were revealed using the Clarity™ Western ECL Substrate Kit (Bio-rad, United States, 200 ml, cat # 170–5060): After that, the primary antibodies were directed to the target proteins, a secondary detection reagent which is a secondary antibody conjugated to a horseradish peroxidase (HRP) enzyme will recognize and bind to the first ones. The conjugated enzyme then converts the substrate into light (by chemiluminescence) which was detected by an imager. Clarity substrate provides a big sensitivity, a long signal duration through an enhancer, very low background levels, and thus clear images. Protein bands were then quantified by the Image-J. analysis software, and standardized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) levels.
2.6. Data analysis
Results were the presented as means ± standard error of the mean (SEM). Normality was tested using Kolmogorov-Smirnov test. The Mann-Whitney U test or t-test was applied for the comparison of differences between the two studied groups at the different ages of testing. Statistical analysis was performed in SPSS software (version 20, SPSS Inc., Chicago, Illinois, U.S.A.). The significance level was set at p < 0.05.
3. Results
3.1. Effect of GSM-EMW exposure on hepatic MDA level
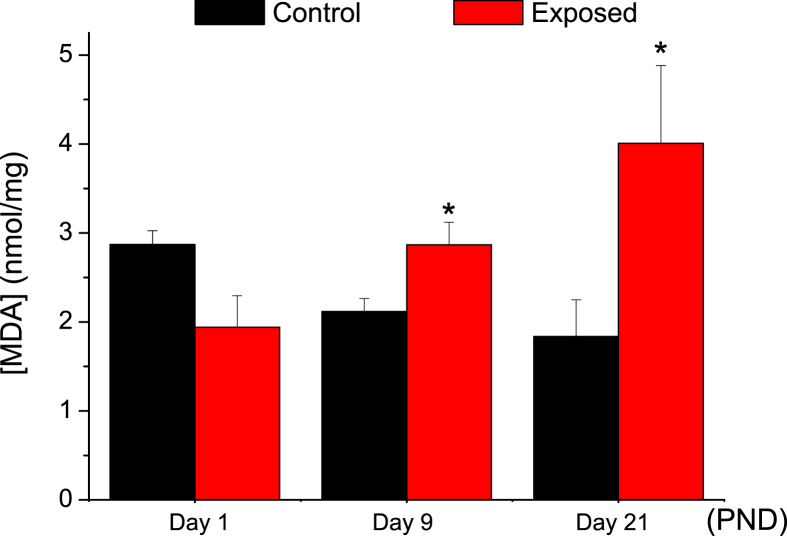
MDA is one of the lipid peroxidation natural products that should be quantified during the measurement of oxidative damage in pathophysiological processes. In comparison with control rats at same ages, mean hepatic MDA level in exposed group increased by nearly 35% and 118% at PND9 (2.12 ± 0.15, n = 12; 2.87 ± 0.25, n = 10; P = 0.006) and PND21 (1.84 ± 0.41, n = 12; 4.01 ± 0.87, n = 12; P = 0.039) respectively, while no significant changes were observed at PND1 (2.87 ± 0.15, n = 12; 1.94 ± 0.35, n = 12) (Figure 2). Other oxidative stress biomarkers can be also studied to assess a certain oxidative stress status such as antioxidant enzymes: SOD, CAT, and GPx.
Figure 2.
Effects of RF-EMR exposure on the level of malondialdehyde (MDA) product (as mean ± SEM) in nmol/mg of liver tissue in rats aged of 1 (ctrl: n = 12; exp: n = 12), 9 (ctrl: n = 12; exp: n = 10), and 21 (ctrl: n = 12; exp: n = 12) of their postnatal days. (P-value <0.05 (∗) represents a significant change in results for exposed vs. control group).
3.2. Effect of GSM-EMW exposure on hepatic mitochondrial and cytosol SOD
SOD is one of the most essential antioxidant enzymes that can be studied. It catalyzes the dismutation process of superoxide anions into hydrogen peroxide (H2O2) and molecular water. Moreover, in comparison with the control, although no significant change in hepatic mitoSOD fraction was observed in exposed rats at PND1 and PND9, a significant decrease by 67% was observed at PND21 (Table 2).
Table 2.
Amounts of mitochondrial superoxide dismutase (SOD) protein fraction, as mean ± SEM (in mg/g of liver mass) in both control (Ctrl) and exposed (Exp) groups of rats aged of 1, 9, and 21 of their postnatal days (PND) (P-value <0.05 (∗) represents a significant change in results for exposed vs. control group).
| Group | Age | N | SOD mitochondrial protein fraction (mg/g of liver mass) |
|---|---|---|---|
| Ctrl | PND1 | 18 | 1.02 ± 0.22 |
| Exp | PND1 | 12 | 0.35 ± 0.08 |
| Ctrl | PND9 | 12 | 0.66 ± 0.08 |
| Exp | PND9 | 12 | 0.40 ± 0.05 |
| Ctrl | PND21 | 24 | 2.83 ± 0.40 |
| Exp | PND21 | 18 | 0.93 ± 0.09∗ |
Moreover, after exposure to GSM-EMW, and in comparison with the control group, it was marked a significant elevation in hepatic SOD cytosolic fraction by ∼200% (P = 0.026) and ∼133% (P = 0.013) at PND9 and PND21 respectively, where no significant changes were detected at PND1 between control and exposed groups (Table 3). H2O2 is one of the reaction products as a result of SOD activity, and can be then converted by other antioxidant enzymes: CAT and GPx.
Table 3.
Amounts of cytosolic superoxide dismutase (SOD) protein fraction, as mean ± SEM (in mg/g of liver mass) in both control (Ctrl) and exposed (Exp) groups of rats aged of 1, 9, and 21 of their postnatal days (PND) (P-value <0.05 (∗) represents a significant change in results for exposed vs. control group).
| Group | Age | N | SOD cytosolic protein fraction (mg/g of liver mass) |
|---|---|---|---|
| Ctrl | PND1 | 24 | 0.030 ± 0.002 |
| Exp | PND1 | 30 | 0.040 ± 0.002 |
| Ctrl | PND9 | 24 | 0.020 ± 0.003 |
| Exp | PND9 | 24 | 0.062 ± 0.009∗ |
| Ctrl | PND21 | 24 | 0.030 ± 0.006 |
| Exp | PND21 | 24 | 0.071 ± 0.007∗ |
3.3. Effect of GSM-EMW exposure on hepatic CAT and GPx protein levels
CAT is another important antioxidant enzyme that is present in nearly all living organisms. It helps in the decomposition process of H2O2 produced by SOD into other harmless products such as water and oxygen. After exposure to RF-EMR, and in comparison with the control group, the t-test showed a significant reduction in the mean hepatic catalase protein level of 61% and 26% at PND1 (P < 0.001) and PND9 (P = 0.027) respectively, with no significant changes at PND21 (Table 4). Free H2O2 can also be reduced to water by another antioxidant enzyme that is GPx.
Table 4.
Measurement of catalase (CAT) protein level in mg/g of liver mass (as mean ± SEM) in female rats aged of 1, 9, and 21 of their postnatal days (PND) in the absence (Ctrl) and the presence of radiations (Exp) (P-value <0.05 (∗) represents a significant change in results for exposed vs. control group).
| Group | Age | N | CAT protein level (mg/g of liver mass) |
|---|---|---|---|
| Ctrl | PND1 | 19 | 32.21 ± 5.74 |
| Exp | PND1 | 24 | 12.50 ± 4.54∗ |
| Ctrl | PND9 | 21 | 19.07 ± 8.70 |
| Exp | PND9 | 22 | 14.15 ± 5.92∗ |
| Ctrl | PND21 | 24 | 14.57 ± 4.49 |
| Exp | PND21 | 24 | 15.96 ± 6.98 |
GPx is a family of enzymes that play an important role in the prevention of oxidative damage, and the protection of organisms. Low levels of GPx have been correlated with free radical-related disorders. GPx is one of the antioxidant enzymes that help also in the conversion of reduced glutathione (GSH) to oxidized glutathione (GSSG), in the reduction process of lipid hydroperoxide to alcohols, and free hydrogen peroxide to water. Moreover, in comparison to the control group, and by applying the t-test, RF-EMR exposure was shown to significantly increase the mean hepatic GPx protein level by nearly 30% (P = 0.018), 35% (P < 0.001), and 28% (P < 0.001) at all tested ages PND1, PND9, and PND21, respectively (Table 5).
Table 5.
Measurement of glutathione peroxidase (GPx) protein level in mg/g of liver mass (as mean ± SEM) in female rats aged of 1, 9, and 21 of their postnatal days (PND) in the absence (Ctrl) and the presence of radiations (Exp) (P-value <0.05 (∗) represents a significant change in results for exposed vs. control group).
| Group | Age | N | GPx protein level (mg/g of liver mass) |
|---|---|---|---|
| Ctrl | PND1 | 24 | 15.61 ± 3.22 |
| Exp | PND1 | 24 | 20.26 ± 8.29∗ |
| Ctrl | PND9 | 24 | 9.97 ± 2.79 |
| Exp | PND9 | 24 | 13.44 ± 2.41∗ |
| Ctrl | PND21 | 22 | 15.66 ± 3.57 |
| Exp | PND21 | 22 | 20.12 ± 3.31∗ |
3.4. Effect of GSM-EMW exposure on relative mitSOD and cytSOD activities
Compared to the control group, RF-EMR exposure induced a significant increase (P < 0.001) in SOD relative mitochondrial activity by 379%, 279%, and 1402% for all postnatal tested ages (PND1, PND9, and PND21, respectively) (Table 6).
Table 6.
Measurement of superoxide dismutase (SOD) relative activity in μmol/min/mg of protein (as mean ± SEM) in the mitochondrial fraction of hepatic tissues, in both control (Ctrl) and exposed (Exp) groups of female rats aged of 1, 9, and 21 of their postnatal days (PND) (P-value <0.05 (∗) represents a significant change in results for exposed vs. control group).
| Group | Age | N | SOD relative mitochondrial activity (μmol/min/mg of protein) |
|---|---|---|---|
| Ctrl | PND1 | 36 | 9.30 ± 8.32 |
| Exp | PND1 | 32 | 44.56 ± 35.86∗ |
| Ctrl | PND9 | 36 | 17.63 ± 10.69 |
| Exp | PND9 | 32 | 66.78 ± 42.33∗ |
| Ctrl | PND21 | 48 | 2.77 ± 2.35 |
| Exp | PND21 | 36 | 41.61 ± 22.04∗ |
On the other hand, after exposure to RF-EMR, and in comparison with the control group, the mean of hepatic SOD relative cytosolic activity showed a significant reduction of 48% (P = 0.015) while a significant increase of 60% (P = 0.001) at PND1 and PND9 respectively. No significant effect was noticed (P = 0.256) at PND21 (Table 7).
Table 7.
Measurement of superoxide dismutase (SOD) relative activity in μmol/min/mg of protein (as mean ± SEM) in the cytosolic fraction of hepatic tissues, in both control (Ctrl) and exposed (Exp) groups of female rats aged of 1, 9, and 21 of their postnatal days (PND) (P-value <0.05 (∗) represents a significant change in results for exposed vs. control group).
| Group | Age | N | SOD relative cytosolic activity (μmol/min/mg of protein) |
|---|---|---|---|
| Ctrl | PND1 | 19 | 5.36 ± 1.15 |
| Exp | PND1 | 24 | 2.78 ± 0.31∗ |
| Ctrl | PND9 | 28 | 231.35 ± 17.51 |
| Exp | PND9 | 32 | 369.98 ± 36.01∗ |
| Ctrl | PND21 | 18 | 10.09 ± 1.65 |
| Exp | PND21 | 18 | 12.76 ± 1.61 |
3.5. Effect of GSM-EMW exposure on CAT and GPx activities
Compared to the control group, GSM-EMW exposure was shown to significantly reduce by 94.54%, 75%, and 65% the mean hepatic catalase relative activity at all postnatal tested ages PND1 (P = 0.001), PND9 (P < 0.001) and PND21 (P < 0.001), respectively (Table 8).
Table 8.
Measurements of catalase relative activity (in μmol/min/mg of protein) (as mean ± SEM) in the hepatic tissues of both control (Ctrl) and exposed (Exp) groups of female rats aged of 1, 9, and 21 of their postnatal days (PND) (P-value <0.05 (∗) represents a significant change in results for exposed vs. control group).
| Group | Age | N | CAT relative activity (μmol/min/mg of protein) |
|---|---|---|---|
| Ctrl | PND1 | 48 | 0.115 ± 0.030 |
| Exp | PND1 | 48 | 0.006 ± 6.755E-4∗ |
| Ctrl | PND9 | 48 | 0.041 ± 0.003 |
| Exp | PND9 | 48 | 0.013 ± 0.001∗ |
| Ctrl | PND21 | 48 | 0.021 ± 0.001 |
| Exp | PND21 | 48 | 0.007 ± 9.614E-4∗ |
Moreover, in comparison with control rats, although no significant changes in mean hepatic GPx relative activity was observed in exposed ones at PND1 and PND9, a significant decrease (P < 0.001) by nearly 88% was evident in rats at PND21 (Table 9).
Table 9.
Measurements of glutathione peroxidase (GPx) relative activity (in μmol/min/mg of protein) (as mean ± SEM) in the hepatic tissues of both control (Ctrl) and exposed (Exp) groups of female rats aged of 1, 9, and 21 of their postnatal days (PND) (P-value <0.05 (∗) represents a significant change in results for exposed vs. control group).
| Group | Age | N | GPx relative activity (μmol/min/mg of protein) |
|---|---|---|---|
| Ctrl | PND1 | 48 | 0.0006 ± 0.0002 |
| Exp | PND1 | 48 | 0.0006 ± 0.0002 |
| Ctrl | PND9 | 48 | 0.0007 ± 0.0003 |
| Exp | PND9 | 48 | 0.0007 ± 0.0001 |
| Ctrl | PND21 | 48 | 0.0006 ± 0.0003 |
| Exp | PND21 | 48 | 0.00007 ± 0.00003∗ |
3.6. Effect of GSM-EMW exposure on SOD1, catalase, GPx1, and Nrf-2 mRNA expression
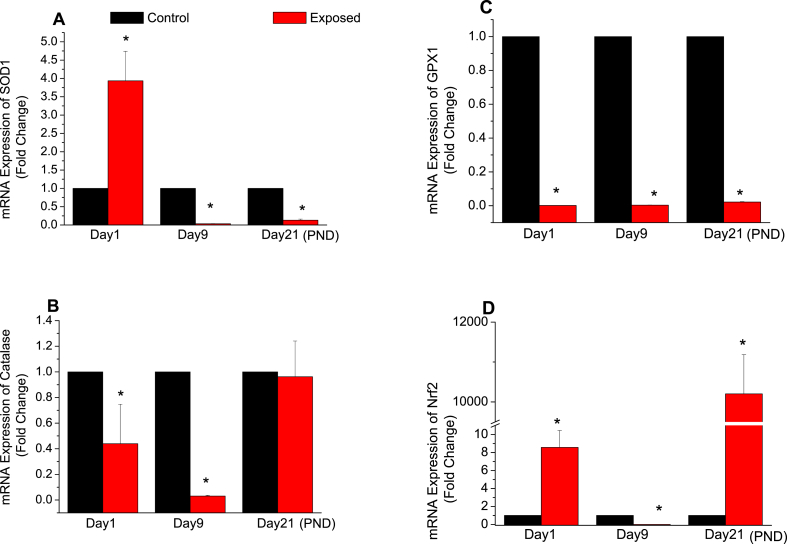
By applying the 2−ΔΔCt method to calculate the relative mRNA expression of genes related to oxidative stress defense in treated samples, by using ß-Actin and Tbp as housekeeping genes for normalization and control samples as calibrators (2−ΔΔCt = 1): it was noticed that, by comparing to control group, SOD1 mRNA expression significantly increased in exposed samples about four times (3.937 ± 0.803) at PND1, before decreasing significantly to about 3% (0.035 ± 0.004), and 13% (0.132 ± 0.030) at PND9 and PND21 respectively (Figure 3A, p < 0.05). Moreover, compared to the control rats, the mRNA expression of catalase was significantly decreased in the liver of the exposed animals to nearly half at PND1 (0.44 ± 0.308), 3% at PND9 (0.0315 ± 0.0056) with no significant difference at PND21 (0.962 ± 0.278) (Figure 3B, p < 0.05). For GPx1, and always in comparison with the control group, RF-EMR exposure was showed to significantly decrease the mRNA expression during all the studied postnatal period: 0.1% at PND1 (0.0015 ± 3.107E-4), 0.3% at PND9 (0.004 ± 5.714E-4) and 2% at PND21 (0.022 ± 0.002) (Figure 3C, p < 0.05). However, Nrf-2 mRNA expression showed a significant increase in exposed samples, where it was elevated by at least eight times from the control group at PND1 (8.561 ± 1.876) and ten thousand times at PND21 (10201.57 ± 982.93) with a big significant decrease to 4.678E-5 ± 8.739E-6 at PND9 (Figure 3D, p < 0.05).
Figure 3.
Effects of radiofrequency electromagnetic radiation (RF-EMR) exposure on the mRNA expression (fold change) of antioxidant enzymes (A) superoxide dismutase 1 (SOD1) (B) catalase (Cat) (C) glutathione peroxidase 1 (GPx1) and (D) the nuclear factor erythroid 2–related factor 2 (Nrf-2) in the liver of rats aged of 1, 9, and 21 of their postnatal days (n = 9). (P-value <0.05 (∗) was considered significant by comparing results of exposed vs. control group).
3.7. Effect of GSM-EMW exposure on Akt1 and ICAM-1 mRNA expression
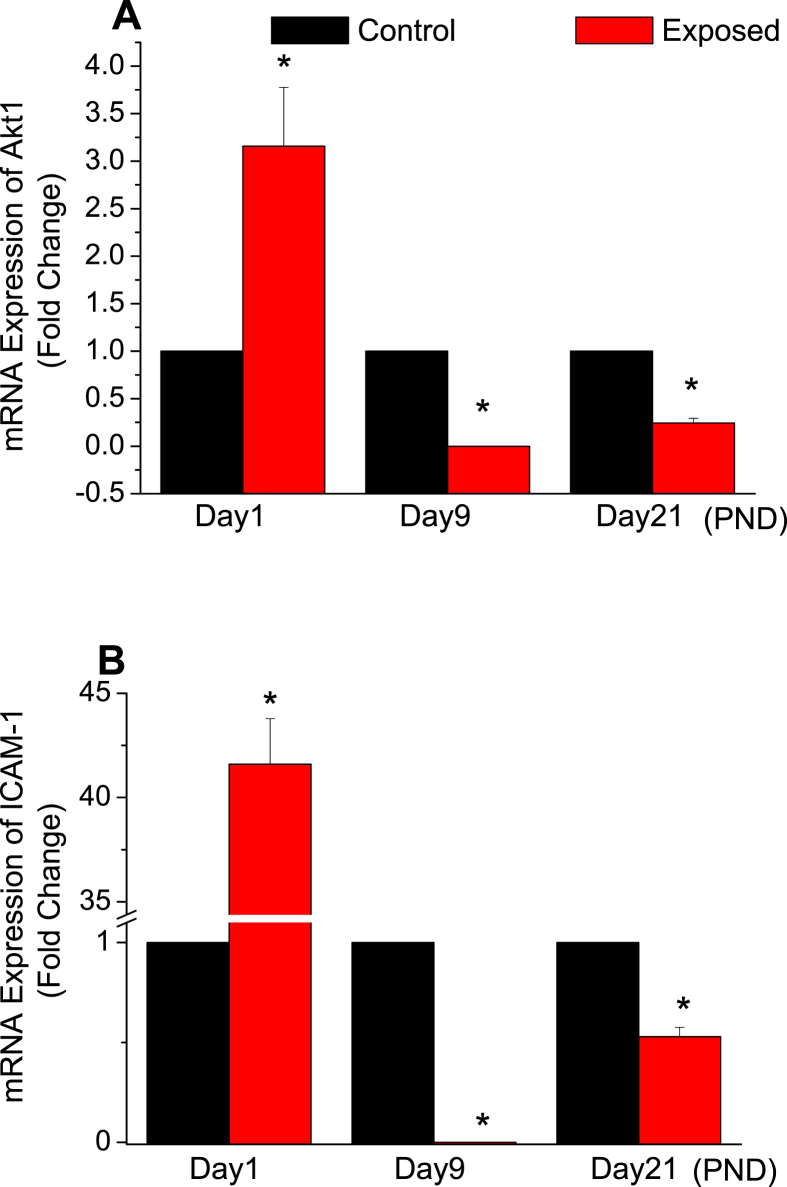
For genes related to apoptosis and inflammation processes, the expression of Akt1 and ICAM-1 mRNA was studied in the liver of control and exposed rats during their postnatal days. With comparison to the control group, Akt1 mRNA expression was significantly increased in exposed samples about three times at PND1 to be 3.157 ± 0.62, before decreasing significantly during the remaining period of study to be 3.442E-5 ± 8.221E-6 at PND9, and 0.244 ± 0.048 at PND21 (Figure 4A, p < 0.05). Similarly, compared to the control group, ICAM-1 mRNA expression significantly increased in the exposed group samples by at least forty one times at PND1 to be 41.593 ± 2.192 before decreasing significantly to 3.999E-5 ± 6.256E-6 at PND9, and to about half value at PND21 to be equal 0.528 ± 0.047 (Figure 4B, p < 0.05).
Figure 4.
Effects of radiofrequency electromagnetic radiation (RF-EMR) exposure on the mRNA expression (fold change) of the apoptotic parameter (A) protein kinase B (Akt1) and inflammatory parameter (B) the intracellular adhesion molecule 1 (ICAM-1) in the liver of rats aged of 1, 9, and 21 of their postnatal days (n = 9). (P-value <0.05 (∗) was considered significant by comparing results of exposed vs. control group.
3.8. Effect of GSM-EMW exposure on Nrf-2 protein expression
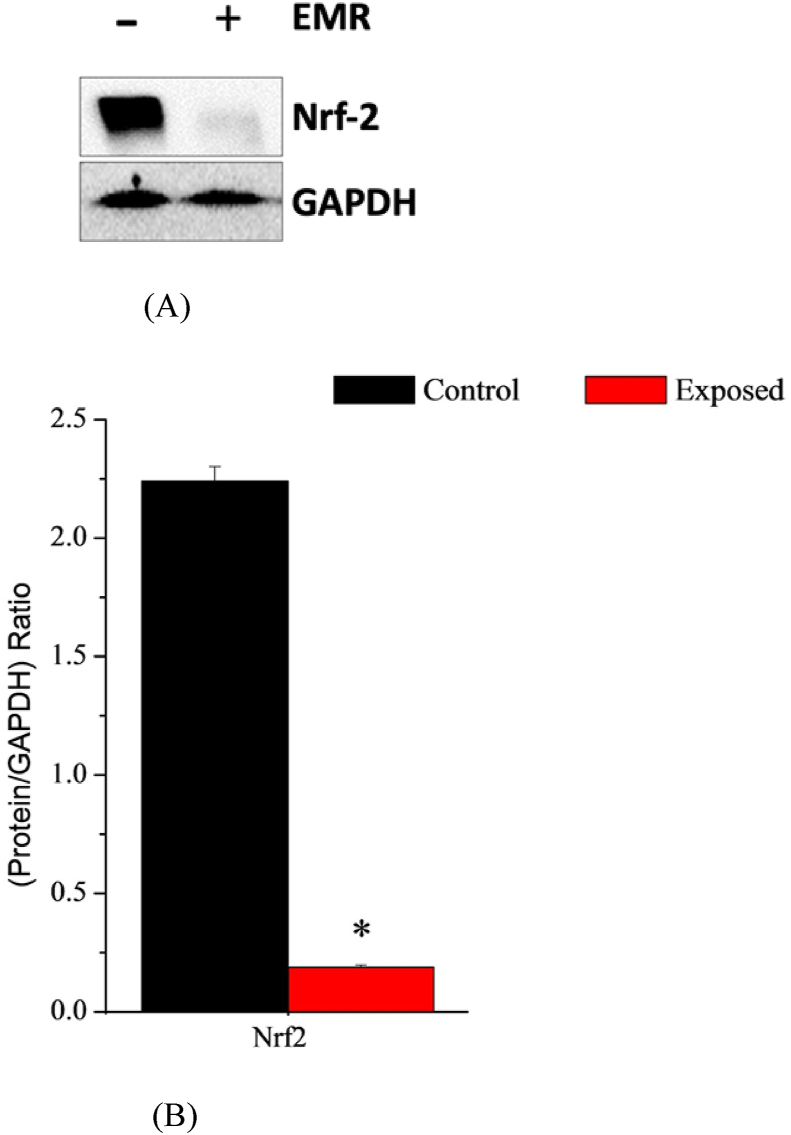
The effects of exposure to electromagnetic radiation on Nrf-2 expression in the liver of newborns were detected by western blotting. The PND9 was chosen as the midpoint of the first stage after birth. The relative expression of protein bands was quantified by using Image J. analysis software and standardized to GAPDH levels (ratio of protein/GAPDH). Results showed that RF-EMR significantly down-regulated Nrf-2 expression (0.187 ± 0.01; P = 3.58 × 10-11) in hepatic tissues of female newborn rats at PND9 when compared to the control ones at the same age (For Nrf-2: 2.24 ± 0.06) (Figures 5A and Fig. 5B) (Figures 6A and Fig. 6B).
Figure 5.
Effects of radiofrequency electromagnetic radiation (RF-EMR) exposure on Nrf-2 expression. (A): western blotting results for Nrf-2 and the GAPDH standard protein in the liver of newborn rats (n = 3) at the PND9 in both control (-) and exposed groups (+). (B): schematic presentation of the relative protein expression of Nrf-2 (ratio: protein/GAPDH). The values presented are the means ± SEM (P-value <0.05 (∗) represents a significant change in results for exposed vs. control group). (N.B: Full original western blotting gels results for nuclear factor erythroid-2-related factor 2 (Nrf-2) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were added as Figure 6 (A and B) in the supplementary material section).
4. Discussion
The daily wide exposure to EMR increases by many sources. Various studies have assessed the effects of these radiations, especially on human health based on their intensity, time, distance of exposure (Sabban et al., 2018), and frequency (Ragy, 2015). The influence of exposure to radiation emitted from mobile phone stations represents a major concern, especially for people living near these stations. In fact, EMR sources, as well as mobile phones devices and stations’ waves, permanently contribute to environmental pollution which therefore harms human health (Durusoy et al., 2017). Mobile phones communicate with base stations through 900 MHz microwaves and the exposure to radiation emitted from mobile phones can increase reactive oxygen species (ROS) and causes an oxidative stress status in some organs, especially in the liver (Ragy, 2015), by an imbalance between the generation of oxidants and their elimination (Kivrak et al., 2017). The hepatotoxic effects of 900 MHz GSM-EMW exposure can be shown by the evaluation of the antioxidant status of liver enzymes, and the level of lipid peroxidation and ROS accumulation. These toxic effects may be most likely affected by the duration of radiation exposure (permanent exposure). This study aimed to determine the effects of permanent exposure of female rats to GSM-EMFs radiation of 900 MHz, for 24 h/day, during 22 days of prenatal development period to the first day after birth (PND1) and up to three weeks of their postnatal life period (PND21) where pups were weaned. Female rats were only studied to have more accurate scientific findings by focusing on one sex of offspring, and to obtain new different results compared to other previous reports that have only studied the male sex rats in similar scenarios (Odacı et al., 2015; Topal et al., 2015; Türedi et al., 2015). The duration of exposure to radiation per day is determined due to the premise of housing or the permanent presence of pregnant females with their offspring during their early postnatal period in specific exposed areas. The effects on the liver were only detected in this study because it is one of the most affected organs by EMR, and many adverse effects may be produced in its tissues after exposure to these radiations. In addition, the different types of hepatic cells are generally very sensitive to oxidative stress, and the hepatic DNA, lipids, and proteins are primarily affected by ROS production. Moreover, the fetal liver is considered the main hematopoietic organ during the development period (Soares-da-Silva et al., 2020), and is important for prenatal and postnatal survival. The liver’s daily normal functions represent generally the origin of the body’s permanent homeostasis.
Increased oxidative stress could be caused by internal physiological and/or physiopathological conditions, such as growth (Li et al., 2015) and many external environmental pollutants such as exposure to EMR (Li et al., 2015). MDA is one of the lipid peroxidation end products and its quantification is useful in the assessment of oxidative damage (Yarijani et al., 2019). It was shown that higher MDA level was observed in the first stages of breast cancer (Didziapetriene et al., 2014), in autoimmune diseases such as Rheumatoid arthritis (Mateen et al., 2016), in the case of hyperbilirubinemia (Altuner Torun et al., 2017), after endurance exercise (Jablan et al., 2017), by consumption of sodium benzoate generally found in beverages (Anjum et al., 2018), after bisphenol A (BPA) exposure (Zhang et al., 2022) and in neonates born (Bandyopadhyay et al., 2017). Moreover, it has been reported that the MDA level measured in many organs such as kidneys, brain, and liver (Martínez-Sámano et al., 2010) was increased (Özorak et al., 2013; Topal et al., 2015) due to short (Özorak et al., 2013) or long period of exposure (Dasdag et al., 2008) of offspring. Similarly, our results showed that, in comparison with the control group, a significant increase in hepatic MDA level was detected in exposed animals at PND9 and PND21 only, accompanied by a decrease in antioxidant enzymatic activity.
Additionally, it was reported that gene expression is negatively correlated to the protein production rate. This expression is shown to be at a low level when the protein production rate is high (Arvas et al., 2011). This relationship is observed in our study for cytoSOD and GPx enzymes at all ages. However, RF-EMR exposure induces not only an insignificant effect or decrease in the expression of CAT enzyme but also in their level and relative activity. This observation is not a surprise since a directly proportional relationship is between the enzyme’s level and its activity (Khan Academy, 2021). Thus, the significant increase of hepatic MDA level in the exposed group after the PND1 could be related to the significant decrease of the total hepatic antioxidants level in rats possibly by directly affecting their gene expression, protein amounts, and relative activities and consequently increasing the level of free radicals and lipid peroxidation (MDA level) in the liver of exposed rats.
Previous studies have shown that the effect of EMR exposure in newborn rats on the different antioxidant enzymes levels, activities, and expressions is controversial. In fact, previous results reported an increase (Li et al., 2015; Topal et al., 2015), a decrease (Masoumi et al., 2018; Odacı et al., 2015), or even a non-significant effect (Demirel et al., 2012) in the level and/or activities of total SOD, CAT, and GPx enzymes. In general, these studies were influenced by factors such as the species, gender, age of animals, duration, frequency, intensity of radiation, and studied organs. All the above-mentioned issues make it hard to compare our results with those in the literature. Nevertheless, it is possible that GSM-EMR exposure induce a reduction in the CAT enzymes activity, protein amount, and gene expression which may lead to an increase in oxidative stress status. These results were similar to previous findings showing a decrease in liver CAT of neonates rats after exposure to EMR (Furtado-Filho et al., 2014).
Exposure to EMR may represent a continuous source of stress that deregulate the cell cycle (Liu et al., 2012; Santini et al., 2018) and lead to many changes in the levels of gene expression, apoptosis in different animal cells types (Kim et al., 2019; Sultangaliyeva et al., 2020) and to a chronic inflammation inducing many chronic diseases (Fusco et al., 2020; Kim et al., 2020). Nrf-2 is a transcription activator normally present in the cytoplasm of various types of cells such as renal, muscular, cardiac, hepatic, and brain cells (Costilla et al., 2019; Niture et al., 2014). In oxidative stress, Nrf-2 gets translocated to the nucleus to up-regulate the expression of genes involved in processes such as cellular protection, detoxification, antioxidant defense, and inflammatory responses (Costilla et al., 2019; Niture et al., 2014). Despite the significant decrease in Nrf-2 level and mRNA expression at PND9, our study showed that an increase in their expression after RF-EMR exposure was observed at PND1 and PND21. It has been revealed that Nrf-2 expression was induced through the up-regulation of a serine or threonine-specific protein kinase (Akt) (Zhang et al., 2017) that plays an essential role in various cellular processes such as glucose metabolism, apoptosis, cell proliferation, and others. There are three main Akt/PKB isoforms in mammalian genomes: Akt1 (PKBα), Akt2 (PKBβ), and Akt3 (PKBγ) (Manning and Toker, 2017). In general, Akt can make BAD of the Bcl-2 family lose its pro-apoptotic function through its phosphorylation and dissociation, or lead to the transcription of pro-survival genes through the regulation of Ikk and the activation of NF-kB (Singh et al., 2018). Akt has been evaluated among many other apoptosis-related genes because of its role in the regulation and activation of Nrf-2. Nrf-2 is the transcriptional factor that possesses an important role in the cell protection process, antioxidant defense, and other responses in case of oxidative stress, the status that we aim to detect in this study. The increase of Akt1 expression (involved in cellular survival pathways by inhibiting the apoptosis process) at PND1 is positively correlated with an increase in Nrf-2 expression. However, surprisingly, at PND21, a decrease in the expression in the Akt1 was observed which suggests the increase in Nrf-2 expression at this age is not induced through the up-regulation of Akt but the decrease in Akt expression could result in accelerated apoptosis.
Moreover, it was reported that increased Nrf-2 leads to an increase in pro-inflammatory mediators’ transcription such as ICAM-1. This is a transmembrane glycoprotein from the Ig superfamily and is expressed by many cell types such as leukocytes and endothelial cells (Figenschau et al., 2018). Despite the increase in ICAM-1 expression at PND1, the RF-EMR exposure induces a decrease in their expression at PND9 and PND21. This could decrease the immune response since the ICAM-1 plays a role in the recruitment of leucocytes to sites of inflammation, and in the activation of lymphocytes by interacting with LFA-1 (Figenschau et al., 2018).
The current study provided the mRNA expression changes of some of the most important antioxidant enzymes such as SOD, GPx, and CAT, in addition to Nrf-2 which is responsible for the transcription of many antioxidant and pro-inflammatory responses-related genes such as the previously mentioned enzymes and ICAM-1. The mRNA expression of another apoptosis-related gene, which may have an important role in the activation of Nrf-2, was also assessed. Thus, the related expressed and/or activated parameters during an expected anti-oxidative defense, a pro-inflammatory response, or other activating pathways have been studied by real time PCR.
5. Conclusion
Our results show an age-dependent effect of 24 h of radiofrequency electromagnetic radiation (RF-EMR) emitted from a global system for mobile communication (GSM) phone relay antenna on oxidative stress status, antioxidant enzymes level, activity, and expression in the female neonate and young rats. The continuous prenatal and postnatal exposure of rats to GSM-EMR may increase the hepatic production of free radicals, and present an increased oxidative stress (OS) status resulting from a significant elevation of the malondialdehyde (MDA) level with a significant decrease in gene expression and activation of some important antioxidant enzymes such as catalase and glutathione peroxidase after the postnatal day 9 (PND9). Moreover, a significant reduction of hepatic nuclear factor erythroid-2-related factor 2 (Nrf-2) expression at this stage could create more complications represented by an initiation of a pro-apoptotic process plus an immune response perturbation through a significant decrease in hepatic protein kinase B (Akt1) and intercellular adhesion molecule 1 (ICAM-1) expression respectively. These complications in addition to the imbalance between the reactive oxygen species (ROS) production and the antioxidant defense may lead to several chronic diseases such as diabetes, cancer, and others. Thus, further studies are required to study the effect of this persistent exposure and its consequences on the health and hepatic function of newborns and young rats in order to conserve their bodies’ permanent homeostasis and to prevent other dangerous implications.
Declarations
Author contribution statement
Sukaina Zeitoun-Ghandour: Analyzed and interpreted the data; Wrote the paper.
Lina Sabra; Lina Ismail; Ali Bazzi: Contributed reagents, materials, analysis tools or data.
Ahmad Daher: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Mahmoud Khalil: Conceived and designed the experiments; Wrote the paper.
Wissam H. Joumaa: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the National Council for Scientific Research in Lebanon CNRS-L; and Lebanese University (7-595).
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article.
References
- Altuner Torun Y., Ertural U., Ergul A.B., et al. Reduction in serum paraoxonase level in newborns with hyperbilirubinemia as a marker of oxidative stress. J. Matern. Fetal Neonatal Med. 2017;30:2297–2300. doi: 10.1080/14767058.2016.1247154. [DOI] [PubMed] [Google Scholar]
- Anjum I., Jaffery S.S., Fayyaz M., et al. Sugar beverages and dietary sodas impact on brain health: a mini literature review. Cureus. 2018;10 doi: 10.7759/cureus.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvas M., Pakula T., Smit B., et al. Correlation of gene expression and protein production rate - a system wide study. BMC Genom. 2011;12:616. doi: 10.1186/1471-2164-12-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azemi M.E., Namjoyan F., Khodayar M.J., et al. The Antioxidant capacity and Anti-diabetic effect of Boswellia serrata Triana and Planch aqueous extract in Fertile Female Diabetic rats and the possible effects on reproduction and histological changes in the liver and kidneys. Jundishapur J. Nat. Pharm. Prod. 2012;7:168–175. [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay T., Bhatia B.D., Khanna H.D. A study of oxidative stress in neonates delivered through meconium-stained amniotic fluid. Eur. J. Pediatr. 2017;176:317–325. doi: 10.1007/s00431-016-2845-0. [DOI] [PubMed] [Google Scholar]
- Belyaev I., Dean A., Eger H., et al. Europaem EMF guideline 2016 for the prevention, diagnosis and treatment of EMF-related health problems and illnesses. Rev. on. Environ. Health. 2016;31:363–397. doi: 10.1515/reveh-2016-0011. [DOI] [PubMed] [Google Scholar]
- Çelik Ö., Kahya M.C., Nazıroğlu M. Oxidative stress of brain and liver is increased by Wi-Fi (2.45GHz) exposure of rats during pregnancy and the development of newborns. J. Chem. Neuroanat. 2016;75:134–139. doi: 10.1016/j.jchemneu.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Çetin H., Nazıroğlu M., Çelik Ö., et al. Liver antioxidant stores protect the brain from electromagnetic radiation (900 and 1800 MHz)-induced oxidative stress in rats during pregnancy and the development of offspring. J. Matern. Fetal Neonatal Med. 2014;27:1915–1921. doi: 10.3109/14767058.2014.898056. [DOI] [PubMed] [Google Scholar]
- Costilla M., Delbono R.M., Klecha A., et al. Oxidative stress produced by Hyperthyroidism status induces the Antioxidant enzyme transcription through the activation of the Nrf-2 factor in lymphoid tissues of Balb/c mice. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/7471890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasdag S., Bilgin H.M., Akdag M.Z., et al. Effect of long term mobile phone exposure on Oxidative-Antioxidative processes and Nitric Oxide in rats. Biotechnol. Biotechnol. Equip. 2008;22:992–997. [Google Scholar]
- Demirel S., Doganay S., Turkoz Y., et al. Effects of third generation mobile phone-emitted electromagnetic radiation on oxidative stress parameters in eye tissue and blood of rats. Cutan. Ocul. Toxicol. 2012;31:89–94. doi: 10.3109/15569527.2012.657725. [DOI] [PubMed] [Google Scholar]
- Didziapetriene J., Smailyte G., Bublevic J., et al. Relationship of MDA plasma concentrations to Long-Term survival of breast cancer patients. Tumori. 2014;100:333–337. doi: 10.1700/1578.17220. [DOI] [PubMed] [Google Scholar]
- Durusoy R., Hassoy H., Özkurt A., et al. Mobile phone use, school electromagnetic field levels and related symptoms: a cross-sectional survey among 2150 high school students in Izmir. Environ. Health. 2017;16:51. doi: 10.1186/s12940-017-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figenschau S.L., Knutsen E., Urbarova I., et al. ICAM1 expression is induced by Proinflammatory cytokines and associated with TLS formation in aggressive breast cancer subtypes. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-29604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado-Filho O.V., Borba J.B., Dallegrave A., et al. Effect of 950 MHz UHF electromagnetic radiation on biomarkers of oxidative damage, metabolism of UFA and antioxidants in the livers of young rats of different ages. Int. J. Radiat. Biol. 2014;90:159–168. doi: 10.3109/09553002.2013.817697. [DOI] [PubMed] [Google Scholar]
- Fusco R., Cordaro M., Siracusa R., et al. Consumption of anacardium occidentale L. (Cashew nuts) inhibits oxidative stress through modulation of the Nrf2/HO−1 and NF-kB pathways. Molecules. 2020;25:4426. doi: 10.3390/molecules25194426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holovská K., Almášiová V., Cigánková V., et al. Structural and Ultrastructural study of rat liver influenced by electromagnetic radiation. J. Toxicol. Environ. Health A. 2015;78:353–356. doi: 10.1080/15287394.2014.979272. [DOI] [PubMed] [Google Scholar]
- Jablan J., Inić S., Stosnach H., et al. Level of minerals and trace elements in the urine of the participants of mountain ultra-marathon race. J. Trace Elem. Med. Biol. 2017;41:54–59. doi: 10.1016/j.jtemb.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Jauniaux E., Watson A.L., Hempstock J., et al. Onset of maternal arterial blood flow and placental oxidative stress. Am. J. Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Academy. 2021. Enzymes Review, Unit: Cellular Energetics.https://www.khanacademy.org/science/ap-biology/cellular-energetics [place unknown]: AP®/College Biology. [Google Scholar]
- Kim J.H., Lee J.K., Kim H.G., et al. Possible effects of radiofrequency electromagnetic field exposure on central nerve system. Biomol. Ther. 2019;27:265–275. doi: 10.4062/biomolther.2018.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Indu Viswanath A.N., Park J.H., et al. Nrf2 activator via interference of Nrf2 Keap1 interaction has Antioxidant and Anti-inflammatory properties in Parkinson’s disease animal model. Neuropharmacology. 2020;167 doi: 10.1016/j.neuropharm.2020.107989. [DOI] [PubMed] [Google Scholar]
- Kıvrak E.G., Yurt K.K., Kaplan A.A., et al. Effects of electromagnetic fields exposure on the Antioxidant defense system. J. Microsc. Ultrastruct. 2017;5:167–176. doi: 10.1016/j.jmau.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Tan H.–Y., Wang N., et al. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015;16:26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.X., Tai J.L., Li G.Q., et al. Exposure to 1950-MHz TD-SCDMA electromagnetic fields affects the apoptosis of astrocytes via caspase-3-dependent pathway. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.S., Huang B.T., Huang Y.X. Reactive oxygen species formation and apoptosis in human peripheral blood mononuclear cell induced by 900MHz mobile phone radiation. Oxid. Med. Cell. Longev. 2012 doi: 10.1155/2012/740280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning B.D., Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Sámano J., Torres-Durán P.V., Juárez-Oropeza M.A., et al. Effects of Acute electromagnetic field exposure and movement restraint on Antioxidant system in liver, heart, kidney and plasma of Wistar rats: a preliminary report. Int. J. Radiat. Biol. 2010;86:1088–1094. doi: 10.3109/09553002.2010.501841. [DOI] [PubMed] [Google Scholar]
- Masoumi A., Karbalaei N., Mortazavi S.M.J., Shabani M. Radiofrequency radiation emitted from Wi-Fi (2.4 GHz) causes impaired insulin secretion and increased oxidative stress in rat pancreatic islets. Int. J. Radiat. Biol. 2018;94:850–857. doi: 10.1080/09553002.2018.1490039. [DOI] [PubMed] [Google Scholar]
- Mateen S., Moin S., Khan A.Q., et al. Increased reactive oxygen species formation and oxidative stress in Rheumatoid Arthritis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means D., Chan K.W. Office of Engineering and Technology Federal Communications Commission; Washington: 2001. Evaluating Compliance with FCC Guidelines for Human Exposure to Radiofrequency Electromagnetic fields: Additional Information for Evaluating Compliance of mobile and Portable Devices with FCC Limits for Human Exposure to Radiofrequency Emissions. [Google Scholar]
- Niture S.K., Khatri R., Jaiswal A.K. Regulation of nrf2—an update. Free Radic. Biol. Med. 2014;66:36–44. doi: 10.1016/j.freeradbiomed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odacı E., Ünal D., Mercantepe T., et al. Pathological effects of prenatal exposure to a 900 MHz electromagnetic field on the 21-day-old male rat kidney. Biotech. Histochem. 2015;90:93–101. doi: 10.3109/10520295.2014.947322. [DOI] [PubMed] [Google Scholar]
- Özorak A., Nazıroğlu M., Çelik O., et al. Wi-Fi (2.45 GHz)- and mobile phone (900 and 1800 MHz)-induced risks on oxidative stress and elements in kidney and testis of rats during pregnancy and the development of offspring. Biol. Trace Elem. Res. 2013;156:221–229. doi: 10.1007/s12011-013-9836-z. [DOI] [PubMed] [Google Scholar]
- Ragy M.M. Effect of exposure and withdrawal of 900-MHz-electromagnetic waves on brain, kidney and liver oxidative stress and some biochemical parameters in male rats. Electromagn. Biol. Med. 2015;34:279–284. doi: 10.3109/15368378.2014.906446. [DOI] [PubMed] [Google Scholar]
- Ramadan W., Khachfe H., Esteve E., et al. Global System for Mobile communications (GSM) electromagnetic waves affect the activity, morphology, and structure of skeletal muscles in adult male rats. Adv. Life Sci. 2015;7:1–9. [Google Scholar]
- Sabban I.F., Pangesti G., Saragih H.T. Effects of exposure to electromagnetic waves from 3G mobile phones on oxidative stress in fetal rats. Pak. Vet. J. 2018;38:384–388. [Google Scholar]
- Santini S.J., Cordone V., Falone S., et al. Role of mitochondria in the oxidative stress induced by electromagnetic fields: focus on reproductive systems. Oxid. Med. Cell. Longev. 2018 doi: 10.1155/2018/5076271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuz J., Jacobsen R., Olsen J.H., et al. Cellular telephone use and cancer risk: update of a Nationwide Danish cohort study. J. Natl. Cancer Inst. 2006;98:1707–1713. doi: 10.1093/jnci/djj464. [DOI] [PubMed] [Google Scholar]
- Singh R., Chaudhary P., Arya R. Role of IGF-1R in ameliorating apoptosis of GNE deficient cells. Sci. Rep. 2018;8:7323. doi: 10.1038/s41598-018-25510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-da-Silva F., Peixoto M., Cumano A., et al. Crosstalk between the hepatic and hematopoietic systems during embryonic development. Front. Cell Dev. Biol. 2020;8:612. doi: 10.3389/fcell.2020.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultangaliyeva I., Beisenova R., Tazitdinova R., et al. The influence of electromagnetic radiation of cell phones on the behavior of animals. Vet. World. 2020;13:549–555. doi: 10.14202/vetworld.2020.549-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topal Z., Hanci H., Mercantepe T., et al. The effects of prenatal long-duration exposure to 900-MHz electromagnetic field on the 21-day-old newborn male rat liver. Turk. J. Med. Sci. 2015;45:291–297. doi: 10.3906/sag-1404-168. [DOI] [PubMed] [Google Scholar]
- Türedi S., Hancı H., Topal Z., et al. The effects of prenatal exposure to a 900-MHz electromagnetic field on the 21-day-old male rat heart. Electromagn. Biol. Med. 2015;34:390–397. doi: 10.3109/15368378.2014.952742. [DOI] [PubMed] [Google Scholar]
- Yarijania Z.M., Najafia H., Shackebaeia D., et al. Amelioration of renal and hepatic function, oxidative stress, inflammation and histopathologic damages by Malva sylvestris extract in gentamicin induced renal toxicity. Biomed. Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108635. [DOI] [PubMed] [Google Scholar]
- Yu G., Bai Z., Song C., et al. Current progress on the effect of mobile phone radiation on sperm quality: an updated systematic review and meta-analysis of human and animal studies. Environ. Pollut. 2021;282 doi: 10.1016/j.envpol.2021.116952. [DOI] [PubMed] [Google Scholar]
- Zhang H., Yang R., Shi W., et al. The association between bisphenol A exposure and oxidative damage in rats/mice: a systematic review and meta-analysis. Environ. Pollut. 2022;292 doi: 10.1016/j.envpol.2021.118444. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wei Z., Liu W., et al. Melatonin protects against Arsenic trioxide-induced liver injury by the upregulation of Nrf2 expression through the activation of PI3K/AKT pathway. Oncotarget. 2017;8:3773–3780. doi: 10.18632/oncotarget.13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.