Abstract
Microbial contaminations are responsible for many chronic, healthcare, persistent microbial infections and illnesses in the food sector, therefore their control is an important public health challenge. Over the past few years, essential oils (EOs) have emerged as interesting alternatives to synthetic antimicrobials as they are biodegradable, extracted from natural sources and potent antimicrobials. Through their multiple mechanisms of actions and target sites, no microbial resistance has been developed against them till present. Although extensive documentation has been reported on the antimicrobial activity of EOs, comparisons between the use of whole EOs or their active components alone for an antimicrobial treatment are less abundant. It is also essential to have a good knowledge about EOs to be used as alternatives to the conventional antimicrobial products such as chemical disinfectants. Moreover, it is important to focus not only on planktonic vegetative microorganisms, but to study also the effect on more resistant forms like spores and biofilms. The present article reviews the current knowledge on the mechanisms of antimicrobial activities of EOs and their active components on microorganisms in different forms. Additionally, in this review, the ultimate advantages of encapsulating EOs or combining them with other hurdles for enhanced antimicrobial treatments are discussed.
Keywords: Essential oils, Antimicrobial activity, Encapsulation, Hurdle technology, Disinfection
Essential oils; Antimicrobial activity; Encapsulation; Hurdle technology; Disinfection.
1. Introduction
The persistence of different types of microbial contaminations including vegetative, viable but nonculturable, spores, planktonic and sessile cells, remains a serious public health concern in food, industrial, biomedical and environmental fields despite the different prevention and control measures. These contaminations and their subsequent consequences result in an increase of occurrence of food-borne diseases, outbreaks, reduced shelf-life of food products, costly socio-economical losses, along with healthcare-associated infections. The centers for disease control (CDC) estimates that annually, around 48 million Americans suffer from foodborne illnesses resulting in 3000 deaths and 128,000 hospitalizations (Scharff, 2012). The European Food Safety Authority (EFSA) and the European Center for Disease Prevention and Control (ECDC) reported in 2018 a total of 5079 food-borne and water-borne outbreaks in the European Union (EU) (EFSA and ECDC, 2018). Along the health losses, foodborne illnesses have created economic burdens from which, illness costs are estimated to be annually between $60.9 and $90.2 billion in the United States (Scharff, 2018).
Several synthetic antimicrobials and control measures have been adopted to overcome microbial contaminations. Nevertheless, due to the recent negative perception of consumers towards synthetic antimicrobials, the resistance developed by certain microorganisms towards them, their possible side toxic effects along with their low biodegradability and negative environmental impacts, increased efforts are urgently needed to find some new effective control measures to limit microbial contaminations (Campana et al., 2017; Calo et al., 2015).
In recent years, biosourced antimicrobials and particularly essential oils (EOs) and their active components have received remarkable attention and emerged as vigorous and convenient natural alternatives for synthetic antimicrobials. For many years, EOs have been used in therapies, pharmaceuticals, perfumes, cosmetics and in food industries. They are environmental-friendly, biodegradable into non-toxic products with potent antimicrobial activity against different types of microbial contaminations (Ju et al., 2019; Teixeira et al., 2022). Additionally, several EO active components have been recognized as GRAS (Generally Recognized as Safe) by the US Food and Drug Administration (FDA) and have been accepted by the European Commission as flavoring agents in food products (Gutiérrez-del-Río et al., 2018). Till present, most of literature studies demonstrated no particular antimicrobial resistance to EOs as they exhibit several modes of action against multiple target sites in bacterial cells (García-Salinas et al., 2018; Vidács et al., 2018). Few studies reported a variable sensitivity of bacterial strains to EOs and/or an emergence of an antimicrobial resistance (Becerril et al., 2012; Marotta et al., 2016). However, further exploration is still required to have a strong evidence about the potential resistance that could be developed by the different bacterial strains against EOs. Different EOs (as thyme, tea tree, peppermint, clove, lemon, bergamot, lavender, mint), in addition to active EOs components (as carvacrol, thymol and eugenol) have been reported to exert significant antimicrobial activities against different microorganisms such as Salmonella spp., Staphylococcus aureus, Candida sp., Listeria monocytogenes., Escherichia coli, Klebsiella sp., Proteus sp., and Pseudomonas aeruginosa (Ben Hsouna et al., 2017; Çakmak et al., 2020; Ed-Dra et al., 2021; El-Darier et al., 2018; Orlo et al., 2021; Rajkowska et al., 2016; Valková et al., 2021). EOs were initially applied in their free form, nevertheless, they presented several limitations as their strong odors and flavors, low stability, poor water solubility, high volatility, high degradability and low bioavailability that limited their different applications (Radünz et al., 2018). Therefore, recent micro- and nano-encapsulation methods have been developed to overcome these challenges and ensure protection of free EO molecules from the external environmental conditions. In addition, encapsulation has been reported to mask EOs negative organoleptic properties, enhance their antimicrobial activity and ensure a controlled release (da Silva Gündel et al., 2018; Granata et al., 2018; Popiolski et al., 2016; Shetta et al., 2019). Another strategy adopted for the application of EOs was their combination with other hurdles. In most cases, combining EOs with other treatments exerted more pronounced anti-microbial activity and reduced the amounts of EOs being used, thus minimizing production costs, lowering environmental and sensorial impacts, and reducing any potential risk of developing antimicrobial resistance (Duarte et al., 2012; Moosavy et al., 2008; Yamazaki et al., 2004; Yoon et al., 2011).
This review provides an overview of the published data and current knowledge on EOs and their active components as potent antimicrobial tools used to fight different forms of microbial contaminations along with their mechanisms of action. Moreover, this review highlights the strategies used to improve EOs antimicrobial activity by micro- and nano-encapsulation and by their combination with other hurdles.
2. Search strategies
In this review, the specialized databases Scopus, ScienceDirect, PubMed and Web of Science were used for the literature search using different combinations of the following keywords: essential oils, antimicrobial, hurdle technology, and encapsulation.
2.1. Vegetative cells
Vegetative cells are the most common form of microbial contaminations that are easily detectable and generally inhibited by conventional treatments. Under harsh and stressful conditions as temperature, osmotic stress, desiccation and/or regular cleaning and disinfection procedures, some vegetative cells may adapt by implementing physiological, structural and/or molecular mechanisms making them more resistant (Esbelin et al., 2018; Potts, 2001). Sporulation and biofilm formation are the most common resistance mechanisms developed by microbial cells to survive stressful conditions. In addition, vegetative cells may enter the viable but not culturable physiological state as a response for the stressful conditions.
2.2. Viable but non culturable (VBNC) cells
VBNC cells are viable bacterial cells that have low levels of metabolic activity. Several non-spore forming microorganisms and pathogens could enter the VBNC state as E. coli, E. coli O 157:H7, L. monocytogenes, S. aureus, H. pylori, P. aeruginosa, Salmonella spp., Enterococcus faecalis, Yersinia, Bacillus coagulans, Micrococcus luteus, Lactobacillus brevis and Vibrio spp. (Boehnke et al., 2017; Casasola-Rodríguez et al., 2018; Chang and Lin, 2017; Doležalová et al., 2016; Han et al., 2018; Highmore et al., 2018; Jiang et al., 2015; Kibbee and Ormeci, 2017; Liu et al., 2018; Majeed et al., 2018; Mukamolova et al., 2018; Orta et al., 2017; Zhang et al., 2015; Zhao et al., 2016; Zhong and Zhao, 2018). Despite being in a stressed state, VBNC cells maintain a persistent cellular biology and structure with normal gene expression (Fakruddin et al., 2013). However, they are not culturable into colonies on standard bacteriological culture media and are unfortunately not detected by classical microbiological techniques. Alternative microscopic and molecular detection methods are thus required to detect them (Dong et al., 2020). With appropriate conditions, VBNC cells are resuscitated, repaired and become again culturable and metabolically active (Dong et al., 2020). Thus, they remain a public health concern with their ability to cause severe infections and diseases (Schottroff et al., 2018).
2.3. Microbial spores
Under unfavorable growth conditions, some microorganisms including mainly Clostridia and Bacilli may form metabolically dormant spores as an adaptive survival strategy (Setlow, 2014). Due to their unique structure and components, spores exhibit a better resistance state than their vegetative forms against several environmental, chemical and physical stresses (Trunet et al., 2017). They are able to escape the destruction by different types of treatments even the most severe ones as mechanical disruption, heating and/or the exposure to a variety of chemicals (Burgess et al., 2010). Spores are able to adhere tightly to both abiotic and biotic surfaces and spread to contaminate other surfaces, individuals or appliances (Mallozzi et al., 2010). Under proper conditions, dormant spores are able to recover by germination and return back into sensitive vegetative cells that are responsible for severe food contaminations, intoxications and illnesses, along with nosocomial infections (Mallozzi et al., 2010; Wells-Bennik et al., 2016). Moreover, microbial cells can communicate through small diffusible chemical signal molecules known as the autoinducers that regulate the quorum sensing (QS) mechanism involved in the coordination of the community activities and in monitoring their population density (Afonso et al., 2020; Palla et al., 2018). QS was shown to regulate spore germination, the metabolite productions as well as the development of an antimicrobial resistance (Narla et al., 2021; Preda and Sãndulescu, 2019). Studies have demonstrated that the inhibition of QS signaling molecules suppressed the initiation of sporulation as well as spores' germination (Vesty et al., 2020; Wang et al., 2018; Xiong et al., 2021).
2.4. Molds and mycotoxins
Molds are responsible for the production of prominent amounts of mycotoxins that have become an important global issue. The main mycotoxin producers are Penicillium, Aspergillus, Fusarium and Alternaria (Ramirez et al., 2018). The Food and Drug Organization estimates that more than 25% of food produced worldwide are contaminated to a certain level with mycotoxins (Magnoli et al., 2019). Mycotoxins can be responsible for acute and/or chronic toxicity, depending on the type and dose of the toxin, as well as the health and age of the exposed individual or animal. They pose great threats to the quality of food products and to the global food security (Guo et al., 2023; Singh et al., 2021). Moreover, due to their greater stability and as they are unavoidable, their prevention and/or elimination remains very challenging (Guo et al., 2023). Studies have shown that QS mechanisms with a variety of signaling molecules, play a major role in the regulation of molds and mycotoxins processes such as the growth of molds, hyphae formation, and mycotoxins production (Barriuso et al., 2018; Huang et al., 2020). Therefore, convenient control strategies that target QS mechanism should be adopted to control molds and their associated mycotoxins.
2.5. Planktonic cells and biofilms
Biofilms are complex communities of single or multiple microbial species able to attach and colonize different types of abiotic and biotic surfaces in food and water processing plants, industrial, clinical and biomedical fields (Bazargani and Rohloff, 2016; Khelissa et al., 2017). If the conditions are favorable, adherent cells to surfaces will multiply and secrete an extracellular matrix of organic polymers in which they become embedded. The matrix acts a protective barrier against different harmful and/or stressful conditions, provides nutrients, favors horizontal gene transfer and metabolic cooperation between bacterial species (Khelissa et al., 2017; Sandasi et al., 2011). Moreover, increased cell densities in biofilm structure, favors the QS signaling that could control tightly the expression of many genes promoting biofilm formation, as well as the transfer of genetic material between cells (Afonso et al., 2020; Li and Tian, 2012; Narla et al., 2021).
Thus, once biofilms are firmly established, it becomes very difficult to remove and control them as microorganisms grow as communities that associate in different layers. Over time, biofilms have shown potent resistance against different conventional commercial disinfectants (as peroxyacetic acid, quaternary ammonium and chlorine compounds) and have become up to 1000 fold more resistant than their planktonic microbial cells (Abdallah et al., 2014). Biofilms resistance is multifactorial and can be linked to the diffusion limitations of antimicrobials, phenotypic adaptations and genetic mutations (Bridier et al., 2011). Therefore they remain an important public health issue as they could disperse and revert into their planktonic form, colonizing thus new habitats and resulting in cross-contaminations, transmission of diseases, reduced products shelf-life and may possibly restart biofilm formation (Donlan, 2002; Srey et al., 2013; Valeriano et al., 2012). Biofilms could be wet or dry in relation to their growth environment. Wet biofilms are formed in hydrated environments whereas, dry biofilms are formed on hard and soft dry environmental surfaces (Almatroudi et al., 2015). Wet biofilms identified on medical devices with the presence of liquid and/or moisture are responsible for 65% of healthcare-associated infections (Ledwoch et al., 2018). Some studies showed that dry biofilms have a more pronounced heat resistance compared to wet biofilms.
For example, upon heating up to 121 °C for 20 min, dry S. aureus dry biofilms have shown higher heat resistance than wet biofilms and planktonic cells with a reduction of 2, 7 and 8 logs, respectively (Almatroudi et al., 2018). In fact, dry biofilms acquired a cross protection and an increased ability to withstand extreme conditions as they were exposed to sub-lethal periodic dehydrations that led to osmotic stress (Almatroudi et al., 2018). In another study, Bacillus cereus spores formed in dry biofilms on stainless steel and polystyrene surfaces were less heat resistant than spores formed in wet biofilms except for dry spores from B. cereus NIZO 4088 (Hayrapetyan et al., 2016). Drying biofilms by air exposure, increased spores formation and accelerated their germination and release from mother cells making them less heat resistant (Hayrapetyan et al., 2016).
3. Essential oils and their active components as potent antimicrobials
3.1. Essential oils composition
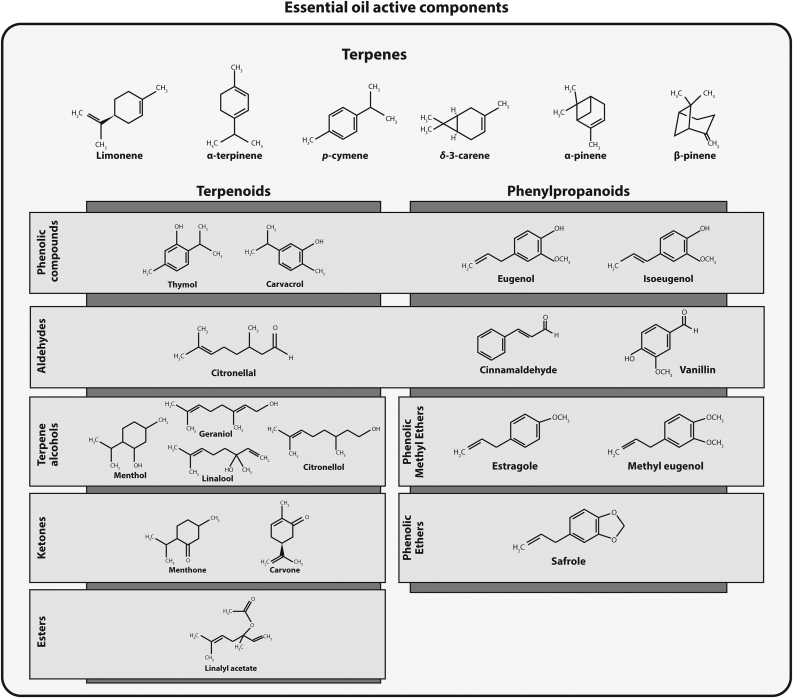
EOs are natural complex antimicrobials constituted of different major active components that some can be found at relatively high concentrations (between 20 and 70%) and others in trace amounts (Bakkali et al., 2008). The chemical composition and amount of extracted EOs depend on the harvest period, climate, soil type, plant and the extraction technique (García-Salinas et al., 2018). EOs active constituents are generated by different biosynthetic pathways and predict EOs biological properties. The different active compounds can be classified into terpene compounds with their oxidative derivatives terpenoids or into phenylpropanoids along with their aromatic derivatives (Figure 1) (Dhifi et al., 2016). More than 4000 different terpenes and only around 50 phenylpropanoids have been discovered till present (Omonijo et al., 2018).
Figure 1.
Chemical structure and function of some of the most common essential oils active components.
Terpenes are hydrocarbon compounds of general formula (C5H8) n where “n” represents the number of isoprene units (5-carbon blocks) (El Asbahani et al., 2015). Depending on “n”, terpenes could be classified as monoterpenes (C10H16), sesquiterpenes (C15H24), diterpenes (C20H32) and triterpenes (C30H40) (Swamy et al., 2016). Monoterpenes (as p-cymene, α-pinene, limonene, β-pinene, α-terpinene and δ-3-carene) constitute 90% of EOs composition but have shown no remarkable antimicrobial activity when used alone as they have a benzene ring with no functional groups on their side chains (Bakkali et al., 2008; Hyldgaard et al., 2012; Dhifi et al., 2016).
The biochemical modifications of terpenes by adding oxygen molecules, and removing or moving methyl groups via enzymes results in the formation of terpenoids (as carvacrol, thymol, citronellal, menthol, linalool, geraniol and linalyl acetate) (Bakkali et al., 2008; Hyldgaard et al., 2012). Terpenoids could be divided according to their functionalities into aldehydes, ketones, phenols, alcohols, esters, ethers, acids and epoxides (Chouhan et al., 2017). Their antimicrobial activity is mainly linked to the presence of functional groups.
Some EOs contain other oxygenated molecules known as phenylpropanoids (Dima and Dima, 2015). They are less frequent than terpenes and the mostly studied ones were eugenol, safrole, isoeugenol, cinnamaldehyde and vanillin (Hyldgaard et al., 2012). Aromatic compounds derived from phenylpropanoids may comprise phenolic acids, aldehydes, alcohols, ketones, esters, amines, epoxides and sulfides (Calo et al., 2015).
The greatest antimicrobial activity was reported for phenolic compounds mainly carvacrol, thymol and eugenol (Al-Maqtari et al., 2021; Tariq et al., 2019). This is related to the presence of an acidic hydroxyl group and delocalized electrons, followed by aldehydes (cinnamaldehyde, citronellal), terpene alcohols (geraniol, citronellol, menthol, linalool, α-terpineol, terpinen-4-ol) and then EOs containing esters (cedryl acetate) or ketones (menthone, carvone, camphor, thujone) (Al-Maqtari et al., 2021; Dhifi et al., 2016; Hyldgaard et al., 2012; Kalemba and Kunicka, 2003; Omonijo et al., 2018).
3.2. Antimicrobial mechanisms of essential oils and their active components
The activity of EOs and their active components has been reported against several types of bacteria, yeasts, molds and was found to be related to EOs chemical composition and structure, in addition to the type and structure of targeted microorganisms (Al-Maqtari et al., 2021; Kalagatur et al., 2018).
Against yeasts and molds, EOs were found to disrupt their membranes and damage their endomembrane system (Trindade et al., 2015; Hu et al., 2019). Additionally, EOs were reported to interfere with biofilms 3D structure reducing extracellular polymeric substances, to inhibit biofilms metabolic activity and respiration with subsequent adverse effects on mitochondria (Almeida et al., 2016; Braga et al., 2008; Hu et al., 2019).
Most studies reported that EOs are slightly more efficient against Gram-positive bacteria than Gram-negative bacteria (Brenes and Roura, 2010; Vidács et al., 2018; Aiemsaard et al., 2011; Bazargani and Rohloff, 2016; de Medeiros Barbosa et al., 2016). The presence of lipophilic ends of lipoteichoic acid in cell membranes of Gram-positive bacteria makes them more susceptible for EOs penetration (Chouhan et al., 2017). Moreover, Gram-negative bacteria are more resistant to the action of EOs due to their rigid and complex outer membrane rich in lipopolysaccharides providing a barrier and restricting the penetration of hydrophobic molecules (Bhargava et al., 2015; Bazargani and Rohloff, 2016). However, some studies reported that Gram negative bacteria are more sensitive to EOs than Gram positives. For example, Gram-negative such as Acinetobacter baumannii and Vibrio parahaemolyticus were the most sensitive microorganisms to Eucalyptus camaldulensis EO (Aleksic Sabo and Knezevic, 2019). Similarly, Gram-negative enteropathogenic E. coli biofilms were more sensitive than Gram-positive L. monocytogenes sessile cells to cinnamon EO and cinnamaldehyde (de Oliveira et al., 2012). In addition, it was reported that Gram-negative Salmonella were found more susceptible to both oregano and thyme EOs than L. monocytogenes (El Moussaoui et al., 2013). Studies have also shown that different strains of the same bacterium may exert highly variable responses to EOs (Sales et al., 2017; Marotta et al., 2016). It remains thus of utmost importance to evaluate the antimicrobial activity of EOs and their active components against different bacteria as well as against different strains of the same bacterium.
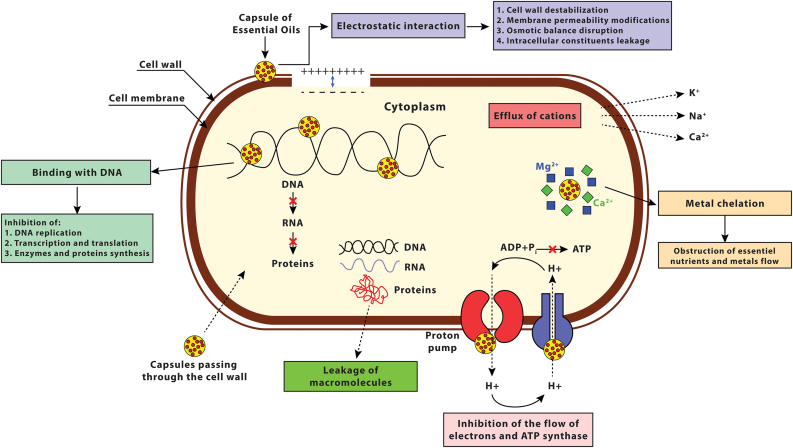
Depending on their chemical composition and structure, different mechanisms have been proposed for the antimicrobial activity of EOs and their active components (Figure 2). Originally, their mechanisms of action were limited to the destruction of cells cytoplasmic membranes, but additional mechanisms have been proposed recently.
Figure 2.
Different antimicrobial mechanisms of action of encapsulated essential oils.
The mechanism of action of several EOs and their phenolic compounds was mainly linked to their attachment and destabilization of bacterial cellular membranes. Owing to their lipophilic nature, EOs and their active components were reported to penetrate and accumulate easily in the lipid bilayer of cytoplasmic membranes. They align between the fatty acid chains of cell membranes causing destabilization and degradation of its different layers through an inactivation of enzymatic mechanisms or a destruction of electron transport system (Bhavaniramya et al., 2019; Burt, 2004; Dhifi et al., 2016; de Oliveira et al., 2010; Swamy et al., 2016). Subsequently, this leads to the breakdown of the integrity of the phospholipid bilayer and an increase in its permeability. The enhanced permeability favors the penetration of antimicrobial agents, disrupts the normal cellular function and results in a leakage of the vital intracellular content (ions, proteins, nucleic acids), a disruption of the proton motive force (PMF), a reduction in the membrane potential and a depletion of adenosine triphosphate (ATP) (Burt, 2004; Pathania et al., 2018; Zhang et al., 2017). The loss of intracellular components is tolerated to a certain amount with no loss of viability (Ju et al., 2019). However, the greater the contact time between EOs and microorganisms the higher is the loss of intracellular content that further leads to cell death by necrosis or apoptosis (Bakkali et al., 2008; Valeriano et al., 2012).
In literature studies, Salvia sclarea EO, carvacrol and eugenol were able to disrupt the membrane integrity and increase the permeability of L. monocytogenes, S. aureus and E. coli cellular membranes (Gill and Holley, 2006; Khan et al., 2017; Cui et al., 2015). This was confirmed by the dissipation of PMF, release of DNA and proteins, and the decrease in intracellular ATP with subsequent increase in extracellular ATP (Gill and Holley, 2006; Khan et al., 2017; Cui et al., 2015). The permeability of E. coli and S. aureus membranes was also altered after exposure to terpenes and phenylpropanoids. This alteration in permeability allows the relatively large molecules to pass easily through the membrane (Nogueira et al., 2021). Exposure of methicillin-resistant S. aureus (MRSA), E. coli and B. subtilis to Amomum villosum Lour and Origanum compactum EOs induced a leakage of macromolecular molecules such as DNA, RNA and proteins (Bouyahya et al., 2019; Tang et al., 2020). The leakage of vital intracellular constituents such as ions and proteins from S. Enteritidis and E. coli cells was also reported after exposure to carvacrol and thymol (Yammine et al., 2022). Also, the application of 0.2% v/v mustard EO caused a significant loss of intracellular ATP of both E. coli O157:H7 and Salmonella typhi (Turgis et al., 2009). Similar results were reported when applying 0.006–0.025% oregano EO against L. monocytogenes and E. coli 0157:H7 (Caillet et al., 2005; Caillet and Lacroix, 2006). Following treatment with carvacrol, oregano and eugenol, leakages of phosphate and potassium ions were reported from S. aureus, P. aeruginosa and E. coli bacterial membranes (Lambert et al., 2001; Walsh et al., 2003; Bouhdid et al., 2009). In Gram-negative bacteria, carvacrol and thymol have been reported to break down the outer membrane, releasing lipopolysaccharides and thus destabilizing and increasing the permeability of cytoplasmic membranes with loss of ATP (Pérez-Conesa et al., 2011; Turgis et al., 2009). Moreover, other studies reported that sub-inhibitory concentrations of citral and Thymus vulgaris EOs provoked increased sub-lethally injured Listeria and Salmonella bacterial cells, respectively (Ed-Dra et al., 2021; Silva-Angulo et al., 2015). Injured bacterial cells had a higher sensitivity to unfavorable conditions enhancing thus the effectiveness of other preservation methods added as salt.
Other mechanisms of action have been proposed for EOs phenolic compounds as their interference with specific proteins and their inhibition of flagella and toxins synthesis. An overnight exposure of E. coli O157:H7 to 1 mM (approximately 0.015%) carvacrol induced significant production of heat shock proteins 60 (which are usually secreted by bacteria under stressful and toxic conditions) and inhibited significantly the synthesis of flagella (Burt et al., 2007). Another effect reported of EOs on cell membranes was the inhibition of toxins production as the case reported when carvacrol and oregano inhibited the release of B. cereus and S. aureus toxins, respectively (Ultee and Smid, 2001). The mechanism of action to limit toxins production was probably related to the insufficient PMF or ATP energy to export the secreted toxins out of the bacterial cells or to the little energy left for toxins production as cells conserve the available energy for survival functions (Ultee and Smid, 2001). It has been also established that eugenol inhibited the activity of several enzymes such as amylase, ATPase, protease and histidine decarboxylase (Hyldgaard et al., 2012). An additional mechanism of action has been proposed for carvacrol that acted as a transmembrane carrier due to the presence of phenolic hydroxyl group and a system of delocalized electrons (Ultee et al., 2002). Carvacrol carried its hydroxyl group into the cytoplasm through the cytoplasmic membrane and carried back out a cation (mainly potassium) from the cytoplasm to the external environment (Hyldgaard et al., 2012).
Additionally, some phenolic compounds and aldehydes have shown the ability to suppress the expression of bacterial genes related to biofilm formation, to interfere with (QS) signaling and to prevent cell division and thus lead to cell death without causing a damage to bacterial cell membranes (Amalaradjou and Venkitanarayanan, 2011; Brackman et al., 2008; Domadia et al., 2007; Kwon et al., 2003). EOs and their active components have shown to breakdown the QS communication mechanism by interacting with bacterial cell wall receptors, reducing thus the reception of signaling molecules and degrading this cell-to-cell communication that eventually inhibits biofilms formation (Maurya et al., 2021). Luciardi et al. (2016) reported an inhibitory effect of Citrus reticulata EO on QS signaling and on the production of autoinducers and elastase enzymes that subsequently inhibited the formation of P. aeruginosa biofilms. Epigallocatechin gallate, a polyphenol found in tea extracts, was also found to down regulate a quorum sensing gene (agrA) in L. monocytogenes biofilms (Du et al., 2018). Cinnamaldehydes which are considered the aldehydes with the strongest antimicrobial activity, were able in different studies to bind to the FtsZ cell division regulating protein, inhibiting thus the process of cell division (Domadia et al., 2007; Hemaiswarya et al., 2011). Also, aldehydes functional groups were found to bind covalently with DNA of bacterial cells, thereby interfering with the normal translation and transcription functions with a subsequent blockage of proteins synthesis (Hyldgaard et al., 2012).
3.3. Free essential oils versus their active components
Several free EOs and their active components have been screened for their potent antimicrobial activity against a broad range of microorganisms. They were reported to induce significant reductions or inhibitions of microbial contaminations (Adukwu et al., 2012; Almeida et al., 2016; Amaral et al., 2015; Bazargani and Rohloff, 2016; Braga et al., 2008; de Medeiros Barbosa et al., 2016; García-Salinas et al., 2018; Kwieciński et al., 2009; Rattanachaikunsopon and Phumkhachorn, 2010; Scotti et al., 2021), as well as inhibitions of spore germination, mycelial growth and mycotoxins secretion (Hu et al., 2019; Xu et al., 2021) (Table 1). However, the use of EO as a whole or using its active major components alone to control microbial contaminations is still a debatable issue.
Table 1.
In vitro antimicrobial activity of free EOs and their active components against different types of microbial contaminations.
| Target microorganisms | EOs and active components | MICa | Exposure concentration, time | Antimicrobial activity | References |
|---|---|---|---|---|---|
| Gram-positive bacteria | |||||
| S. aureus | Cymbopogon flexuosus | 0.06% | 0.03–4%, 24 h | Complete inhibition at MIC | Adukwu et al. (2012) |
| Origanum compactum | 0.031–1% | MIC and 1.5MIC, 0–120 min | Immediately significant reductions at 1-1.5MIC | Bouhdid et al. (2009) | |
| Melaleuca alternifolia | 0.12–0.50% | 0–8%, 15–60 min | Significant reduction with 0.5% EO after 60 min | Kwieciński et al. (2009) | |
| Origanum vulgare L., Carvacrol | 2.5–5 μl/ml | 0.31–2.5 μl/ml, 24 h | No decrease in microbial counts | dos Santos Rodrigues et al. (2017) | |
| Carvacrol, CIN, Thymol | 0.2–0.4 mg/ml | 0.01–4 mg/ml, 24 h | Significant inhibition at MIC Around 3–5 log reductions with 0.5–1 mg/ml EO |
García-Salinas et al. (2018) | |
| Salvia officinalis, Mentha spicata | 1.25% | 2.5–5%, 6 min | 2.3–3.1 log reductions | Vetas et al. (2017) | |
| S. aureus (Biofilms) | Cymbopogon flexuosus | 0.06–0.12% | 0.06–4%, 24 h | Prevention of biofilm formation at MIC and total loss of viability at 0.125–4% | Adukwu et al. (2012) |
| Melaleuca alternifolia | 0.12–0.50% | 0–8%, 0–120 min | Complete eradication and inhibition of metabolism with 1% EO after 120 min | Kwieciński et al. (2009) | |
| Origanum vulgare L., Carvacrol | 5–10 μl/ml | 0.31–2.5 μl/ml, 360 h | Decrease in counts after 216 h exposure to 1.25–2.5 μl/ml | dos Santos Rodrigues et al. (2017) | |
| Carvacrol, CIN, Thymol | N/A | 0.25–1 mg/ml, 24 h | Significant inhibition at MIC Around 3–5 log reductions with 0.5–1 mg/ml EO |
García-Salinas et al. (2018) | |
| Origanum vulgare L. | 10 μl/ml | 5–10 μl/ml, 10–15 min | Complete removal of S. aureus LPMA63 but not S. aureus LPMA11 biofilms | Dos Santos Rodrigues et al. (2018) | |
| Carvacrol | 5 μl/ml | 2.5–5 μl/ml, 10–15 min | Complete removal of all biofilms | ||
| Salvia officinalis, Mentha spicata | 0.63–1.25% | 5–15%, 6 min | 0.8–3 log reductions | Vetas et al. (2017) | |
| S. epidermidis spp. |
Mutellina purpurea, α-pinene |
0.31–0.625 mg/ml | 0.15–2.5 mg/ml, 24 h | Complete inhibition at 0.312–0.625 mg/ml | Sieniawska et al. (2013) |
| Cinnamomum burmannii | 0.5–1% | MIC, 24 h | Complete inhibition | Nuryastuti et al. (2009) | |
| S. epidermidis spp. (Biofilms) |
Mutellina purpurea, α-pinene |
0.62–1.25 mg/ml | 2.5–10 mg/ml, 24 h | Concentrations >10 mg/ml were needed to eradicate biofilms | Sieniawska et al. (2013) |
| Cinnamomum burmannii | 1–2% | 0.01–2%, 1–24 h | Complete loss of metabolic activity after 3–24 h exposure with detachment of biofilms | Nuryastuti et al. (2009) | |
| Enterococcus spp. | Cymbopogon flexuosus, Thymus vulgaris | 0.47–15 mg/ml | MIC, 24 h | Complete inhibition | Quendera et al. (2019) |
| Enterococcus spp., (Biofilms) | Cymbopogon flexuosus, Thymus vulgaris | N/A | 1.9–30 mg/ml, 30 min-1 h | Higher levels of eradication were reported for Aeromonas spp. at 15 and 30 mg/ml for all treatment times | |
| L. monocytogenes | Corydothymus capitatus | 0.02% | 0.06–0.02%, N/A | At 0.025%, significant effect on murein composition and reduction of intracellular ATP with an increase in extracellular ATP | Caillet et al. (2005); Caillet and Lacroix (2006) |
| Origanum vulgare L., Rosmarinus officinalis L. | 0.6–10 μl/ml | MIC, 24 h | Significant reduction after 2–8 h exposure | de Medeiros Barbosa et al. (2016) | |
| L. monocytogenes, (Biofilms) | Thyme, Tea tree |
0.01–0.09% | MIC and 0.1%, 2 h | Significant 1.4–3.3 log reductions of sessile cells when treated with MIC and 0.1%. | Sadekuzzaman et al. (2018) |
| Thyme, Oregano, Carvacrol | N/A | 0.10–0.50%, 24h | Complete inactivation with 0.25% for 24 h | Desai et al. (2012) | |
| Gram-negative bacteria | |||||
| E. coli | Eugenol, CINb | 1 μg/ml | 0.5–2 μg/ml, 0–90 min | Complete inhibition at MIC after 60–75 min | Ali et al. (2005) |
| Carvacrol, Thymol | 200 mg/l | MIC, 12 h | No complete inhibition but significant reductions | Xu et al. (2008) | |
| Carvacrol, CIN, Thymol | 0.2–0.4 mg/ml | 0.01–4 mg/ml, 24h | Significant inhibition at MIC Around 3–5 log reductions with 0.5–1 mg/ml EO |
García-Salinas et al. (2018) | |
| Black pepper | 1.0 μl/ml | MIC and 2MIC, 24 h | Significant inhibitory effect of 96.73% after 24 h exposure to 2MIC | Zhang et al. (2017) | |
| Origanum vulgare L., Rosmarinus officinalis L. | 0.6–10 μl/ml | MIC, 24 h | Significant reduction after 2–8 h exposure | de Medeiros Barbosa et al. (2016) | |
| Thymbra capitata | 0.05% | 1%, 30–60 s | Inhibition of approximately 8 logs after 30–60 s | Falcó et al. (2019) | |
| E. coli, (Biofilms) | Thymbra capitata | N/A | 0.25–2.5%, 1–10 min | Reduction of >3 logs after 10 min exposure to 2.5% | Falcó et al. (2019) |
| E. coli O157:H7 | Carvacrol | N/A | 1 mM = 0.015%, overnight | Significant inhibition of flagella synthesis and production of heat shock proteins 60 | Burt et al. (2007) |
| p-cymene | N/A | 1–10 Mm, overnight | No significant activity | ||
| Corydothymus capitatus | 0.02% | 0.06–0.02%, N/A | At 0.025%, significant effect on murein composition and reduction of intracellular ATP with an increase in extracellular ATP | Caillet et al. (2005); Caillet and Lacroix (2006) | |
| Mustard | 0.2% | 0–0.2%, 30 min | At 0.1 and 0.2%, a significant increase in both extracellular ATP and cell constituents' release. | Turgis et al. (2009) | |
| E. coli O157:H7 (Biofilms) | Thyme, Tea tree |
0.01–0.09% | MIC and 0.1%, 2 h | Significant 1.4–3.3 log reductions of sessile cells when treated with MIC and 0.1%. | Sadekuzzaman et al. (2018) |
| Salmonella spp. | Origanum vulgare L., Rosmarinus officinalis L. | 0.6–10 μl/ml | MIC, 24 h | Significant reduction after 2–8 h exposure | de Medeiros Barbosa et al. (2016) |
| Thymbra capitata | 0.05% | 1%, 30–60 s | Inhibition of approximately 8 logs after 30–60 s | Falcó et al. (2019) | |
| Mustard | 0.2% | 0–0.2%, 30 min | At 0.1 and 0.2%, a significant increase in both extracellular ATP and cell constituents' release. | Turgis et al. (2009) | |
| Thyme, Oregano, Carvacrol | 0.025% | 0.006–0.1%, 24 h | Complete inhibition at MIC | Soni et al. (2013) | |
| Salmonella spp. (Biofilms) | Thymbra capitata | N/A | 0.25–2.5%, 1–10 min | Around 1 log reduction after 10 min exposure to 2.5% | Falcó et al. (2019) |
| Carvacrol, Thymol | 156–312 μg/ml | 16–624 μg/ml, 1 h | Reduced established biofilms but no complete inhibition | Amaral et al. (2015) | |
| Thyme, Tea tree |
0.01–0.09% | MIC and 0.1%, 2 h | Significant 1.4–3.3 log reductions of sessile cells when treated with MIC and 0.1%. | Sadekuzzaman et al. (2018) | |
| Thyme, Oregano, Carvacrol | N/A | 0.006–0.1%, 24 h | Significant reduction after 1 h exposure to 0.05 or 0.1% | Soni et al. (2013) | |
| Mentha piperita, Cymbopogon citratus | 7.8 μl/ml | MIC, 10–40 min | Significant reductions of 4.03–4.20 logs after 10 min contact and complete inhibition after 20 min | Valeriano et al. (2012) | |
| P. aeruginosa | Origanum compactum | 0.031–1% | MIC and 1.5MIC, 0–120 min | Immediately significant reductions at 1-1.5MIC | Bouhdid et al. (2009) |
| P. aeruginosa (Biofilms) | Carvacrol, Thymol | 0.02–0.05% | 1/16 –2MIC, 24 h | Inhibited up to 97% at 2MIC | El Abed et al., 2011 |
| Aeromonas spp. | Cymbopogon flexuosus, Thymus vulgaris | 0.47–15 mg/ml | MIC, 24 h | Complete inhibition | Quendera et al. (2019) |
| Aeromonas spp. (Biofilms) | Cymbopogon flexuosus, Thymus vulgaris | N/A | 1.9–30 mg/ml, 30 min-1 h | Higher levels of eradication were reported for Aeromonas spp. at 15 and 30 mg/ml for all treatment times | Quendera et al. (2019) |
| Thymus vulgaris, Cymbopogon citratus | 31–62 μl/ml | MIC, 15 min | Significant reduction of 3.84–4.51 log CFU/cm2 | Millezi et al. (2013) | |
| Fungi | |||||
| C. albicans | Cymbopogon winterianus, Cinnamon cassia | 65.5–250 μg/ml | 7.8–1000 μg/ml, 24–48 h | Complete inhibition at MIC | Almeida et al. (2016) |
| C. albicans (Biofilms) | N/A | 1 mg/ml,0–48 h | Significant reductions but no complete inhibition and no prevention of biofilm regrowth | ||
| Thymol | 125 μg/ml | 1/8MIC-MIC, up to 24 h | Significant reductions at MIC after 1 h and almost a complete destruction of 3D structure | Braga et al. (2008) | |
MIC: Minimum Inhibitory Concentration.
CIN: Cinnamaldehyde.
Few studies reported a better antimicrobial activity of whole EOs as lemongrass, Cymbopogon nardus and cinnamon compared to that of their major components used alone (Trindade et al., 2015; Chang et al., 2001; Aiemsaard et al., 2011) (Table 2). This was mainly explained by the interaction between the different components present in EOs that may have a synergistic or additive antimicrobial activity (Bazargani and Rohloff, 2016; Marino et al., 2001). In these cases, active components (citronellal, citral, geraniol and cinnamaldehyde) were mainly terpenoids with alcohol and aldehyde functions, which explains their weak antimicrobial activity when used alone. In addition, myrcene active component belonging to the terpene group possessed no antimicrobial activity when used alone against several microorganisms in comparison to the whole EO lemongrass (Aiemsaard et al., 2011).
Table 2.
Comparison of MIC values between free EOs and their active components against different types of microbial contaminations.
| Microorganism | Whole EO |
EO Active Components |
References | ||
|---|---|---|---|---|---|
| Type | MICa | Type | MIC | ||
| Gram-positive bacteria | |||||
| S. aureus | Oregano | 0.06–0.12%b | Carvacrol Thymol |
0.01–0.03% 0.03–0.06% |
Nostro et al. (2007) |
| Oregano | 5 μl/ml | Carvacrol | 2.5 μl/ml | dos Santos Rodrigues et al. (2017) | |
| Oregano | 575 mg/l | Carvacrol Thymol |
175 mg/l 140 mg/l |
Lambert et al. (2001) | |
| Lemongrass | 0.54 μl/ml | Citral Geraniol Myrcene |
0.62–1.25 μl/mlc 0.62–1.25 μl/ml >17.5 μl/ml |
Aiemsaard et al. (2011) | |
| S. aureus (Biofilms) | Oregano | 0.25–0.50% | Carvacrol Thymol |
0.03–0.12% 0.06–0.12% |
Nostro et al. (2007) |
| Oregano | 10 μl/ml | Carvacrol | 5 μl/ml | dos Santos Rodrigues et al. (2017) | |
| S. epidermidis | Oregano | 0.06–0.12% | Carvacrol Thymol |
0.01–0.03% 0.03–0.06% |
Nostro et al. (2007) |
| S. epidermidis (Biofilms) | Oregano | 0.12–0.50% | Carvacrol Thymol |
0.03–0.12% 0.03–0.12% |
Nostro et al. (2007) |
| L. monocytogenes | Oregano Thyme |
0.05% 0.05% |
Carvacrol | 0.05% | Desai et al. (2012) |
| Streptococcus agalactiae | Lemongrass | 0.27–0.54 μl/ml | Citral Geraniol Myrcene |
0.31–0.62 μl/ml 0.31 μl/ml >17.5 μl/ml |
Aiemsaard et al. (2011) |
| Gram-negative bacteria | |||||
| E. coli | Thyme | 1.60 mg/ml | Carvacrol Thymol |
0.20 mg/ml 0.20 mg/ml |
Vidács et al., (2018) |
| Cinnamon | 0.40 mg/ml | CINd | 0.20 mg/ml | ||
| Cinnamon | 250 μg/ml | CINd | 500 μg/ml | Chang et al. (2001) | |
| Lemongrass | 0.54–1.09 μl/ml | Citral Geraniol Myrcene |
1.25–2.5 μl/ml 1.25–2.5 μl/ml >17.5 μl/ml |
Aiemsaard et al. (2011) | |
| S. Typhimurium | Oregano Thyme |
0.05–0.10% 0.05–0.10% |
Carvacrol | 0.02% | Soni et al. (2013) |
| P. aeruginosa | Oregano | 1648 mg/l | Carvacrol Thymol |
450 mg/l 385 mg/l |
Lambert et al. (2001) |
| Cinnamon | 250 μg/ml | CINd | 1000 μg/ml | Chang et al. (2001) | |
| K. pneumoniae | Cinnamon | 500 μg/ml | CINd | 1000 μg/ml | Chang et al. (2001) |
| B. cereus | Lemongrass | 0.13 μl/ml | Citral Geraniol Myrcene |
0.15 μl/ml 0.31 μl/ml >17.5 μl/ml |
Aiemsaard et al. (2011) |
| Fungi | |||||
| C. albicans | C. nardus | 64 μg/ml | Citronellal | 512 μg/ml | Trindade et al. (2015) |
MIC: Minimum Inhibitory Concentration.
MIC values were determined in a range as they were tested on several strains.
MIC values were determined in a range as the experiments were done in triplicates.
CIN: Cinnamaldehyde.
Whereas, most of the other studies reported that active EO components had a similar or better antimicrobial activity against different types of microorganisms when compared to the activity of the whole EO (Desai et al., 2012; dos Santos Rodrigues et al., 2017; Nostro et al., 2007; Soni et al., 2013; Vidács et al., 2018). This was related to the presence of some active components, mainly phenolic compounds, such as carvacrol and thymol that form a fraction of EO but are mainly responsible for the wide and strong antimicrobial activity of the whole EO. Thus, higher amounts of EO will be required to exert the same antimicrobial activity as that of the main active component used alone against different types of microbial cells (dos Santos Rodrigues et al., 2017). The lower concentrations of active components used will also reduce the cost, potential toxicity and negative impacts of EOs on the sensory attributes of food products (Calo et al., 2015). Additionally, more reproducible and accurate standardization may be achieved when using active components alone as many factors could affect the chemical composition of whole EOs (de Oliveira et al., 2012).
All tested free EOs and their active components were effective against planktonic bacterial cells, except in two studies where p-cymene, oregano and carvacrol did not show significant antimicrobial activity against E. coli O157:H7 and S. aureus (Burt et al., 2007; dos Santos Rodrigues et al., 2017). Compared to their planktonic counterparts, biofilms required much higher concentrations of EOs or their active components to reach equivalent microbial reductions or not even (Adukwu et al., 2012; Almeida et al., 2016; Karpanen et al., 2008; Kwieciński et al., 2009; Quendera et al., 2019; Sieniawska et al., 2013; Somrani et al., 2021). In several studies, there was no complete eradication of biofilms even when EOs or their active components were used at 2 times their minimum inhibitory concentrations (MIC) determined on planktonic cells or even at higher concentrations (Almeida et al., 2016; El Abed et al., 2011; Falcó et al., 2019; Jadhav et al., 2013; Sandasi et al., 2011; Vetas et al., 2017; Vidács et al., 2018; Amaral et al., 2015; Quendera et al., 2019). Additionally, biofilms grown for longer time required higher concentrations of EOs and prolonged exposure time to be eliminated (Desai et al., 2012; Braga et al., 2008).
The intrinsic composition of food may influence as well the bacterial sensitivity and decrease EOs antimicrobial activity. It has been found that greater concentrations of free EO or their active components were needed to achieve an equivalent antimicrobial effect in food when compared to in vitro assays due to the interaction of EOs with food components (Cava et al., 2007; Gutierrez et al., 2008; Rattanachaikunsopon and Phumkhachorn, 2010; Singh et al., 2003). This may develop unpleasant organoleptic impacts and decrease the overall acceptability of food products (Jiménez et al., 2018; Radünz et al., 2018). EOs or their active components were found to be more effective in zero or low fat food compared to food with high fat levels (Rattanachaikunsopon and Phumkhachorn, 2010; Cava et al., 2007; Singh et al., 2003). Also, the antimicrobial activity of EOs may be related to some external determinants as pH, temperature and oxygen presence. Generally, at low pH, the antimicrobial activity of several EOs was enhanced due to their increased hydrophobicity and easier dissolution in bacterial cell membranes (Ali et al., 2005; Cava et al., 2007; Gutierrez et al., 2008; Nostro et al., 2012). The temperature effect on the antimicrobial activity of EOs was contradictory in some studies. Lower temperatures (7 °C compared to 35 °C) were found to enhance the antimicrobial activity of EOs or their active components due to the increase in unsaturated phospholipids in cytoplasmic membranes composition with a subsequent increase in membranes' fluidity (Cava et al., 2007). The increased fluidity weakens the membrane attachment and enables an easier dissolution of EOs into it (Cava et al., 2007). On the contrary, the antimicrobial activity of carvacrol combined with cymene was found better at 25 °C compared to their activities at 15 and 4 °C (Rattanachaikunsopon and Phumkhachorn, 2010). There was no clear explanation for the decreased sensitivity of bacterial cells to antimicrobial agents at lower temperatures but it could be attributed to changes in membrane’s properties and/or fluidity, or to low temperatures that may affect the synthesis of target sites in both EOs and bacterial cytoplasmic membranes which affects the sensitivity of microorganisms to EOs (Rattanachaikunsopon and Phumkhachorn, 2010). Also, the antimicrobial activity of EOs was found to be higher in the presence of low oxygen (vacuum packaging and modified atmosphere packaging) as compared to aerobic conditions (Skandamis et al., 2002; Tsigarida and Nychas, 2001).
In most published studies, the required time to eliminate planktonic or sessile cells by free EOs was between 1 and 216 h, which was considered to be neither practical nor economical for industrial applications. For example, at MIC concentrations, oregano, Cymbopogon nardus, eugenol and cinnamaldehyde required 2–8 h, 1 h, 9 h and 12 h to reduce significantly (up to 6 log reductions) planktonic and sessile microorganisms of L. monocytogenes, E. coli, S. enterica and H. pylori, respectively (Ali et al., 2005; de Medeiros Barbosa et al., 2016; de Oliveira et al., 2010). In other studies, higher concentrations than MIC detected on planktonic cells and a prolonged exposure time were required to induce significant reductions. In lab cultures, Cymbopogon flexuosus and Thymus vulgaris, thyme, oregano and carvacrol, and black pepper were needed at least at 2 MIC concentrations for a minimum of 1 h to induce significant reductions (up to 7 logs) in Aeromonas spp. and Enterococcus spp., Salmonella and E. coli (Quendera et al., 2019; Soni et al., 2013; Zhang et al., 2017). In few other studies, lower exposure times (10–20 min) to 5–10 μl of oregano and carvacrol (dos Santos Rodrigues et al., 2018), and to the MIC of peppermint and lemongrass (Valeriano et al., 2012) reduced significantly S. aureus and S. enterica sessile cells, respectively. Thymbra capitata was the only EO reported to induce more than 8 log reductions in both E. coli and S. enterica in maximum 60 s, but with 20 times its MIC value (Falcó et al., 2019).
3.4. Essential oils and their active components versus synthetic antimicrobials
In recent years, EOs and their active components have received particular attention and emerged as effective biosourced candidates to fight the different forms of microbial contaminations and replace the synthetic antimicrobials. Beside the numerous advantages of EOs, many studies showed that their antimicrobial activity could be even better or equivalent to that of synthetic antimicrobials (dos Santos Rodrigues et al., 2018; Vidács et al., 2018). For example, no sessile (<1 log CFU/cm2) S. aureus LPMA63 biofilms were detected on stainless steel after 15 min exposure to EOs active components oregano (10 μl/ml) and carvacrol (5 μl/ml), whereas 15 min exposure to 250 mg/L of a synthetic antimicrobial (sodium hypochlorite) was ineffective to eliminate biofilms and induced approximately 3.5 log CFU/cm2 reductions because of its limited diffusion (dos Santos Rodrigues et al., 2018). Cinnamon, thyme and marjoram EOs showed to have equivalent or better antimicrobial effect against E. coli and L. monocytogenes biofilms compared to chemical sanitizers conventionally used in food industries such as peracetic acid and sodium hypochlorite (Vidács et al., 2018). Also, in a similar study, cinnamon EO and cinnamaldehyde proved to have similar or greater antimicrobial activity against enteropathogenic E. coli (EPEC) and L. monocytogenes when compared to commercial synthetic sanitizers such as alkyl dimethyl benzyl ammonium chloride (ADBAC), sodium hypochlorite and hydrogen peroxide (de Oliveira et al., 2012).
A great number of synthetic antimicrobials has been widely applied to encounter microbial contaminations. Nevertheless, the long term exposure to sub-inhibitory concentrations of these antimicrobials induced microbial resistance and thus reduced their effectiveness (El Sayed Zaki et al., 2019). It was reported that 22 % of L. monocytogenes strains were resistant and 30% of S. aureus isolates have shown reduced susceptibility towards benzalkonium chloride (BAC) (Jiang et al., 2016; El Sayed Zaki et al., 2019). In another study, L. monocytogenes harboring quaternary ammonium compounds (QACs) resistance genes (qacH and bcrABC) were found to be prevalent in food processing environments (Møretrø et al., 2017). Several sanitizers such as acetic acid, NaOH, Na2SO4, glyceryl monolaurate, BAC and peracetic acid were ineffective to eliminate totally L. monocytogenes and S. aureus planktonic and sessile cells (Chavant et al., 2004; Ibusquiza et al., 2011; Khelissa et al., 2019). Synthetic antimicrobials were also reported to be ineffective in the total elimination of biofilms. An exposure for 10 min to sodium hypochlorite (1000–20,000 ppm) did not eliminate totally S. aureus dry biofilms (Charlebois et al., 2017). Also, L. monocytogenes biofilms showed resistance to chlorine followed by peroxyacetic acid and QACs (Belessi et al., 2011). C. perfringens biofilms were reported to be resistant to disinfectants commonly used in food processing environment and in farms like quaternary ammonium chloride, sodium hypochlorite, potassium monopersulfate, glutaraldehyde and hydrogen peroxide (Charlebois et al., 2017). QACs were very effective to kill more than 98% of L. monocytogenes planktonic cells but ineffective against 7 days old biofilms (Chavant et al., 2004).
Additionally, a potential risk of exposure to QAC disinfectants was reported as they are discharged from several sources and eventually reach freshwater and marine environments, soils, sediments and wastewater systems (Mulder et al., 2018). Despite being generally biodegradable under aerobic conditions, QACs adsorption into environmental surfaces and organisms cell membranes is faster than their degradation as they have long half-life degradation (Zhang et al., 2015). Thus, long-term exposure to residual QACs was found to have a great toxic effect not only on the environment but also on several organisms as aquatic species, plants, animals and humans (Ferk et al., 2007; Lavorgna et al., 2016; Mulder et al., 2018; Russo et al., 2018; Wu et al., 2011; Zhang et al., 2015). Moreover, some of QACs were considered endocrine disruptors and resulted in disturbances in the endocrine system of different organisms with subsequent alterations in reproduction and development (Melin et al., 2016; Sreevidya et al., 2018).
Due to these limitations and side effects of the conventional synthetic antimicrobial agents, the interest in biosourced antimicrobials as natural alternatives has increased remarkably. EOs are being favored as no particular resistance have been developed against them as they are composed of different active components, have multiple targets in bacterial cells and subsequently multiple modes of antimicrobial activity (Bakkali et al., 2008; García-Salinas et al., 2018; Kavanaugh and Ribbeck, 2012; Vidács et al., 2018). Additionally, as the chemical composition of each batch may be different, it would be difficult for microorganisms to develop a mechanism of resistance against EOs (Sieniawska et al., 2013). However, it should be pointed out that microbial strains could have different sensitivities to EOs (Marotta et al., 2016) and they might show as well a decreased susceptibility to some EOs due to the repeated exposure (Becerril et al., 2012). Therefore, further studies should explore the possible antimicrobial tolerance or resistance mechanisms that could be developed against EOs over time.
4. Combined essential oils and their active components with other treatments
4.1. Micro- and nano-encapsulated essential oils and their active components
Several strategies have been adopted to encounter the different limitations and improve the antimicrobial activity of free EOs including the development of new delivery systems as micro- and nano-capsules. The different encapsulation systems entrapping EOs were able to reduce or to eliminate microorganisms in different states as vegetative (Esmaeili and Asgari, 2015; Granata et al., 2018; Guarda et al., 2011; Jamil et al., 2016; Shetta et al., 2019), and biofilms (Giongo et al., 2016; Mechmechani et al., 2022; Rossi et al., 2017; Yammine et al., 2022). Moreover, encapsulated EOs reduced the viability of spores, inhibited molds growth as well as the formation of mycotoxins (Jiang et al., 2023; López-Meneses et al., 2018; Meng et al., 2022; Wang et al., 2018). Until present, and despite remaining a public health concern, no studies as to our knowledge have yet reported the antimicrobial activity of encapsulated EOs against VBNC cells.
The enhanced antimicrobial activity of the encapsulated EOs against the different types of microbial contaminations could be mainly linked to the reduced size of the particles and their increase in surface to volume ratio, which facilitates the diffusion of EOs into microbial cells (Cui et al., 2016; Granata et al., 2018). Also, the diffusion of EOs to cell membranes is facilitated by the encapsulation process as free EOs have low water solubility and thus a difficulty to interact with membranes whereas encapsulated EOs have an enhanced solubility (Moghimi et al., 2016). Encapsulation protects also EOs from degradation and volatilization which could be additionally related to a possible enhancement of their antimicrobial activity (da Silva Gündel et al., 2018).
4.1.1. Against vegetative cells
Several studies attempted to reduce considerably vegetative bacterial cells using encapsulated EOs. The encapsulation of Carum copticum EO enhanced its antimicrobial activity against S. epidermidis, S. aureus, B. cereus, E. coli, S. Typhimurium and P. vulgaris (Esmaeili and Asgari, 2015). Granata et al. (2018) reported a bactericidal activity of encapsulated Thymus capitata and Origanum vulgare EOs against pathogenic bacteria, namely S. aureus, E. coli, P. aeruginosa and L. monocytogenes. The encapsulation of EOs active components carvacrol and thymol promoted the growth inhibition of a broad spectrum of microorganisms such as S. aureus, E. coli O157:H7, L. innocua and S. cerevisiae (Guarda et al., 2011). Nanoencapsulated cardamon, green tea and peppermint EOs showed similar trends by exhibiting excellent antimicrobial potencies against E. coli and S. aureus (Jamil et al., 2016; Shetta et al., 2019). Moreover, several studies investigated the susceptibility of Gram-negative and Gram-positive bacteria to encapsulated EOs. Gram-positive bacteria were found to be more susceptible to the antimicrobial activity of several encapsulated EOs as compared to the Gram-negative bacteria (Esmaeili and Asgari, 2015; Granata et al., 2018; Guarda et al., 2011). This could be due to the additional outer membrane containing lipopolysaccharides in Gram-negative bacteria that obstructs the penetration of EOs.
Moreover, most of literature studies showed an enhanced antimicrobial activity of EOs when encapsulated into different carrier systems with lower MIC values compared to their free forms. Exceptionally, some EOs or their active components showed equivalent MIC values in their free and encapsulated forms, like Cymbopogon flexuosus, peppermint and carvacrol against S. aureus, E. coli and E. faecalis (Table 3). Lower EOs concentrations used in the encapsulation process achieved equivalent or better antimicrobial activity against several microorganisms when compared to the free EOs (da Silva Gündel et al., 2018; Granata et al., 2018; Mechmechani et al., 2022; Moghimi et al., 2018; Shetta et al., 2019; Yammine et al., 2022). This will additionally reduce sensorial impacts on food products, economic costs, toxic side effects and any potential resistance that may be developed by microorganisms against EOs. Also, an improved long-term antimicrobial activity of encapsulated EOs was demonstrated. For example, the antimicrobial activity of eugenol and thymol was prolonged for 24 h against E. coli O157:H7 and L. monocytogenes when encapsulated (Chen et al., 2015). Cardamom EO antimicrobial activity was also prolonged for 7 days against E. coli and methicillin resistant S. aureus with encapsulation (Jamil et al., 2016). Free cinnamon, clove and thyme EOs presented an antimicrobial activity for maximum 2 days, while encapsulating them prolonged their activity to 8 days (Soliman et al., 2013). However, most of EOs encapsulation studies lack a direct application in food or other types of matrices with their subsequent sensory evaluations.
Table 3.
Comparison of MIC values between free and encapsulated EOs and their active components against different types of microorganisms.
| Microorganisms (in planktonic state) | EOs or active EO components | MIC approximate range (mg/ml) |
Reference | |
|---|---|---|---|---|
| Free | Encapsulated | |||
| Gram-positive bacteria | ||||
| E. faecalis | Carvacrol | 0.62 | 0.62 | Mechmechani et al. (2022) |
| M. fortuitum | Cymbopogon flexuosus | 0.87 | 0.35 | Rossi et al. (2017) |
| M. massiliense | 3.50 | 0.35 | ||
| M. abscessus | 3.50 | 0.35 | ||
| S. aureus | Mentha piperita | 1.36 | 1.11 | Shetta et al. (2019) |
| Camellia sinensis | 5.44 | 0.57 | Shetta et al. (2019) | |
| Origanum vulgare | 4 | 0.5 | Granata et al. (2018) | |
| Thymus capitatus | 2 | 0.25 | Granata et al. (2018) | |
| Cymbopogon flexuosus | 0.58 | 0.58 | Rossi et al. (2017) | |
| Cardamon | 4.4 | 0.27 | Nahr et al. (2018) | |
| L. monocytogenes | Origanum vulgare | 2 | 1 | Granata et al. (2018) |
| L. delbrueckii | D-limonene | >25 | 10 | Donsì et al. (2011) |
| Gram-negative bacteria | ||||
| S. Enteritidis | Carvacrol | 1.25 | 0.31 | Yammine et al. (2022) |
| Thymol | 1.25 | 0.62 | ||
| P. aeruginosa | Carvacrol | 5 | 1.25 | Mechmechani et al. (2022) |
| Cymbopogon flexuosus | -∗ | >11.33 | Rossi et al. (2017) | |
| E. coli | Thymus daenensis | 4.0 | 0.4 | Moghimi et al. (2016) |
| Mentha piperita | 2.72 | 2.72 | Shetta et al. (2019) | |
| Camellia sinensis | 5.44 | 1.15 | Shetta et al. (2019) | |
| Origanum vulgare | 4 | 0.5 | Granata et al. (2018) | |
| Thymus capitatus | 2 | 0.25 | Granata et al. (2018) | |
| Cardamon | 4.4 | 0.27 | Nahr et al. (2018) | |
| D- limonene | >25 | 10 | Donsì et al., 2011 | |
| A. baumannii | Thymus daenensis | 0.06–0.08 | 0.03–0.04 | Moghimi et al. (2018) |
| Fungi | ||||
| C. albicans | Cymbopogon flexuosus | 1.22 | 0.28 | Rossi et al. (2017) |
| C. grubii | 0.58 | 0.28 | ||
| S. cerevisiae | D- limonene | >25 | 10 | Donsì et al., 2011 |
-∗No inhibitory activity detected at all tested concentrations.
4.1.2. Against microbial spores
Encapsulated EOs showed prominent activity against microbial spores by inhibiting mainly spore germination or the spore-forming microorganisms. For example, the germination of both Fusarium graminearum and Phomopsis sp. were inhibited once exposed to encapsulated cinnamon and lemon EOs, respectively (Meng et al., 2022; Wu et al., 2019). Moreover, encapsulated clove EO suppressed the activity of Penicillium digitatum spores' germination as well as the elongation of the germ tube (Wang et al., 2018). The antimicrobial activity of encapsulated EOs was also tested against spore-forming bacteria and results have shown that encapsulation of Mānuka EO inactivated 4 logs of spore-forming B. cereus as compared to only 1 log reduction induced by the non-encapsulated EO (Liu et al., 2021). Moreover, B. cereus were reduced significantly in rice samples with up to 81.88% release of intracellular DNA, proteins and ATP after exposure to encapsulated curry plant EO (Cui et al., 2017). Despite presenting high risks, studies on the activity of encapsulated EOs against bacterial spores are limited compared to their antimicrobial activity against the other types of microbial contaminations.
4.1.3. Against molds and mycotoxins
Encapsulated EOs exhibited also a remarkable activity against molds and their associated mycotoxins. Results showed that the encapsulation of several EOs together promoted their antifungal activity against Aspergillus flavus as well as their anti-aflatoxigenic potency by downregulating ver-1 and omt-A genes function (Kumar et al., 2019). Other studies showed that encapsulation of cinnamon and hop EOs enhanced their antifungal activities against Fusarium graminearum by inhibiting mycelial growth, and by remarkably suppressing the production of the deoxynivalenol mycotoxin in rice samples (Jiang et al., 2023; Wu et al., 2019). The growth of Aspergillus parasiticus was inhibited after exposure to nanoencapsulated Schinus molle L. EO with a 59% reduction in Aflatoxin production (López-Meneses et al., 2018). In beef patties samples, yeasts and mold counts were reduced to up to 3.16 logs after exposure to encapsulated cinnamon EO (Ghaderi-Ghahfarokhi et al., 2017). Moreover, Aflatoxin B1 levels were effectively reduced in Salvia hispanica seeds once treated with nanoencapsulated Zingiber zerumbet EO (Deepika et al., 2021). Lemon EO nanoemulsions significantly inhibited mycelial growth of Phomopsis sp. preventing rot development and postharvest decay of fresh kiwi fruits. This inhibitory effect was ascribed to the enhanced activities of the intracellular antioxidant enzymes, to the accumulation of reactive oxygen species, and to cell apoptosis (Meng et al., 2022). Wang et al. (2018), and Hasheminejad et al. (2019) reported a superior ability of encapsulated clove EO to control Penicillium digitatum green molds developed on Navel oranges, as well as the mycelial growth of Aspergillus niger in pomegranate, respectively. The activity of encapsulated EOs on molds and their associated mycotoxins was related to several factors including the type and concentrations of EOs used as well as the food matrix components.
4.1.4. Against biofilms
Increasing number of studies showed the promising antibiofilm activity of encapsulated EOs. They have reported damages to the biofilm matrix and even an eradication of biofilms. As reported by Yammine et al. (2022), nanoencapsulation of carvacrol and thymol inhibited totally S. Enteritidis biofilms after an exposure for 15 min at 2 MIC. While, free carvacrol and thymol at the same exposure time and concentration reduced up to 4.27 logs of biofilm cells. Similarly, nanoencapsulation of geranium EO inhibited significantly Candida spp. biofilm formation as compared to lower activity induced by free EO (Giongo et al., 2016). Mechmechani et al., 2022a, Mechmechani et al., 2022b demonstrated the higher antibiofilm activity of encapsulated carvacrol against P. aeruginosa and E. faecalis biofilms as compared to free EOs components. Nanoencapsulated Cymbopogon flexuosus showed a complete eradication of several strains of Mycobacteria biofilms (Rossi et al., 2017). Moreover, scanning electron microscopy images confirmed the morphological damages induced to bacterial cells recovered from biofilms after exposure to encapsulated EOs (Yammine et al., 2022). This proves that encapsulated EO could penetrate and disrupt the biofilms complex matrix and subsequently induce damages and death to bacterial cells.
4.2. Combining essential oils and their active components with other treatments
Strategies to control microbial contaminations are currently oriented toward multiple-hurdle technology such as the combination of EOs with other treatments (Calo et al., 2015; He et al., 2021; Mechmechani et al., 2022; Prakash et al., 2018). The main purpose of this combination is to observe a synergistic effect that provides a combined effect greater than the sum of individual effects. When combining EOs with other hurdles, synergism may be due to: i) the different modes of actions of the treatments applied and thus their different target sites, ii) to the interaction of one antimicrobial with cell membrane or cell wall leading to an increase uptake of other agents, or iii) to the inhibition of a series of steps in a biochemical pathway (Hyldgaard et al., 2012). Recent studies explored the combination of free and encapsulated EOs with other hurdles as heat, ultra-sound (US), high pressure processing (HPP), irradiation, pulsed electric field (PEF), vacuum packaging (VP), modified atmosphere packaging (MAP), antibiotics or other antimicrobial agents (Table 4). All the different combinations with EOs showed a significant synergistic effect except when coriander EO was combined with some antibiotics, an additive effect was observed and when oregano EO was combined with PEF, no synergism was noticed (Duarte et al., 2012; Clemente et al., 2020).
Table 4.
Antimicrobial activity of EOs and their active components in combination with other hurdles.
| Target microorganisms | EOs or active EO components (Concentration) | Combined treatment | Exposure time, Application | Antimicrobial activity |
References | |
|---|---|---|---|---|---|---|
| EO or other treatment alone | Combined treatment | |||||
| Free | ||||||
|
E. coli O157:H7, L. monocytogenes |
Citrus sinensis (0.2 μl), Citrus lemon (0.2 μl), Citrus reticulata (0.2 μl) | Mild heat (54 °C) | 10 min, Lab culture | No inactivation | More than 5 log cycles inactivation | Espina et al. (2011) |
| Cronobacter sakazakii (Desiccated and non-desiccated) | Citral (0.3–0.9%) | Mild heat (25–55 °C) | 2 h, Infant formula | N/Aa | Complete inhibition | Shi et al. (2017) |
| Total viable counts, Pseudomonas spp., Lactic acid bacteria, E. coli, Total coliforms, B. thermosphacta, Yeasts and Molds |
Thymol (0.4–0.8%) + Carvacrol (0.4–0.8%) | VPb | 21 days, Marinated chicken and beef (Shawarma) | 0.5–2.9 log reductions | 0.8–3.1 log reductions | Karam et al. (2019), Karam et al. (2020) |
| Total mesophilic counts | Cymbopogon citratus (400 μl) | MAPc (CO2 100%) | 21 days, Cabbage and radish sprouts | 1.55–2.26 log reductions | Complete inactivation (<1 log) after 14 days | Hyun et al. (2015) |
| Salmonella | Thyme (0.3–0.9%) | VP, MAP (CO2 50%) | 15 days, Minced pork meat | Up to 2.9 log reductions in the first 3 days | 1.69–4.05 log reductions in 15 days | Boskovic et al. (2017) |
| Total aerobic count, B. cereus, C. perfringens |
Origanum vulgare (400–32000 ppm) | High pressure CO2 (100 bar/80 °C) | 15 min-48 h, Paprika spice | Around 0.3 log reductions and no inactivation of spore population | Significant reductions and spores inactivation | Casas et al. (2016) |
| Acinetobacter baumannii | Coriandrum sativum L. (0–6 μl/ml) | Chloramphenicol (32–64 μl/ml) | 16–20 h, Lab culture | MIC ranged between 1 and 4 μl/ml. | MIC of EO and antibiotic decreased by up to 62.5 times | Duarte et al. (2012) |
| Acinetobacter baumannii | Myrtus communis L. (0.03–1 MICd) | Polymixin B, Ciprofloxacine (0.03–4 MIC) | 24 h, Lab culture | MIC ranged between 0.25 and 4 μl/ml | MIC of antibiotics and EO reduced by up to 1/8 MIC with complete inhibition of microbial counts after 6 h | Aleksic et al. (2014) |
|
L. monocytogenes, E. coli O157:H7 |
Oregano (0.01%), Lemongrass (0.01%) | Gamma irradiation (0.5 or 1 kGy) | 14 days, Fresh cut cauliflower | 1.16–3.29 log reductions | Undetectable up to 14 days at 0.5–1 kGy | Tawema et al. (2016) |
| Yeasts and molds | 0.52–2.61 log reductions | Around 2–3 log reductions | ||||
| Fusarium graminearum | Cananga odorata (0–5 mg/g) | Gamma irradiation (0–10 kGy) | 14 days, Maize | Complete inhibition at 3.9 mg/g | Significant reductions at 2.5 mg/g EO combined with 4 kGy irradiation | Kalagatur et al. (2018) |
|
A. niger, P. chrysogenum |
Ocimum basilicum (2%) | Irradiation (0–4 kGy) | 14 days, Rice | 0.42–1.18 log reductions | Up to 5 log reductions | Hossain et al. (2014) |
| S. Typhimurium | Clove (1.2 mg/ml) | Ultraviolet light-C | N/A, Lab culture | 1.8–2.9 log reductions | 6.8 log reductions | Silva-Espinoza et al. (2020) |
| S. Typhimurium | Cinnamomum verum (0–5%) | PEFe (10–30 kV/cm) | 60–3000 μs, Pasteurized skim milk | No bactericidal effect | Up to 1.97 log reductions | Pina-Pérez et al. (2012) |
| Campylobacter jejuni | Oregano (1/4 and 1/2 MIC = 15.625 and 31.25 ppm) | PEF (1–20 kV/cm) | 20 μs, Liquids and raw chicken | 1.64–3.32 log reductions with varied PEF treatments | EO applied following PEF induced further inactivation of 1.2 log cycles | Clemente et al. (2020) |
| L. monocytogenes | Thymus vulgaris L. (<0.06–0.50%) | HPP (200–300 MPa) | 15 min, Fresh cheese | Around 3.5 log reductions | More than 5 log reductions | Bleoancă et al. (2016) |
|
L. monocytogene, L. innocua |
Mentha piperita (0.05 and 0.1 ml/100 ml) | HPP (600 MPa) | 300 s, Yogurt drink | More than 5 log reductions | Additional 1 log reduction and reduced HPP treatment | Evrendilek and Balasubramaniam (2011) |
|
S. Typhimurium, L. monocytogenes |
Cinnamomum zeylanicum (0.625 μL/mL) | US (24 kHz, 400 W) | 0–6 days, Milk | 0.7–3.0 log reductions | 2.7–4.5 log reductions | Mortazavi and Aliakbarlu (2019) |
|
S. Typhimurium, S. aureus |
Zataria multiflora Boiss. (15–30 μl/100 ml) | Nisin (0–0.5 μg/ml at 8 and 25 °C) | 21 days, Barley soup | Complete inhibition in 2–12 days | Complete inhibition | Moosavy et al. (2008) |
| L. monocytogenes | Origanum vulgare L., Thymus vulgaris L., Rosmarinus officinalis L. (50–300 ppm) | Lactic acid (50 ppm) | 24 h, Lab culture | 32.0–83.48 % inhibition | 60.33–99.08% inhibition | Dimitrijevic et al. (2007) |
| S. epidermidis | Thymol (0.06–16 g/l), Tea tree (0.25–64 g/l), Eucalyptus (0.25–64 g/l) | Chlorhexidine digluconate (0.125–16 mg/l) | 24 h, Lab culture | MIC: 0.5–16 g/l | Reduced MIC to 0.5–1 g/l | Karpanen et al. (2008) |
| S. epidermidis (Biofilms) | MIC: 0.5–64 g/l | Reduced MIC to 0.25–16 g/l | ||||
| Salmonella enterica serotype Newport | Origanum vulgare, Carvacrol (0.1–0.5%) | Ozonized water (0.01–0.1 mg O3/L) | 60–120 min, Iceberg lettuce | 1.76–2.09 log reductions | Complete inhibition | Dev Kumar and Ravishankar (2019) |
| Nano-encapsulated | ||||||
|
E. coli O157:H7 Sakai, L. monocytogenes EGD-e |
Thymbra capitate nanoparticles (0.1 μl) | Heat (53 °C) | 12 min, Lab culture | Up to 0.5 log reductions | Up to 5 log reductions | Merino et al. (2019) |
|
A. niger, A. flavus, A.parasiticus, P. chrysogenum |
Thymus vulgaris + Origanum compactum nanocrystals (0.13 and 0.19%) | Irradiation (750 kGy) | 8 weeks, Rice | 1.19–2.87 log reductions | 3.7–4.93 log reductions | Hossain et al. (2019) |
| E. coli O157:H7 | Thyme nanoemulsions (0.375 mg/ml) | USf (127–255 W/cm2) | 3–9 min, Lab culture | 3.28–4.13 log reductions | 5.14–7.42 log reductions | Guo et al. (2020) |
| S. enterica | Oregano and thyme nanoemulsions (0.025%) | US (continuous and pulsed 200 W) | 5–25 min, Lettuce leaves | Up to 2.23 log reductions | Complete inactivation | Millan-Sango et al. (2016) |
N/A: Not Applicable.
VP: Vacuum Packaging.
MAP: Modified Atmosphere Packaging.
MIC: Minimum Inhibitory Concentration.
PEF: Pulsed Electric Field.
US: Ultrasound.
When EOs were combined with mild heating (53–55 °C), synergism was reported on the reduction and/or elimination of E. coli O157:H7, L. monocytogenes and C. sakazakii bacterial populations in lab cultures and in infant formulas (Espina et al., 2011; Merino et al., 2019; Shi et al., 2017).
Another promising intervention involves the combination of EOs with VP and MAP, which has been assessed for the packaging of different food products. Combination of EOs with VP and/or MAP showed a synergistic effect with an enhanced reduction of spoilage and/or pathogenic bacteria in marinated chicken samples (Karam et al., 2019), in paprika spices (Casas et al., 2016), in marinated beef (Karam et al., 2020), and in minced pork meat packages (Boskovic et al., 2017). Populations of total mesophilic counts in cabbage and radish sprouts were completely inactivated when lemongrass EO was combined with MAP (Hyun et al., 2015).
MIC of different EOs were reduced when combining them with conventional antibiotics such as Chloramphenicol, Ciprofloxacin, Gentamicin, Tetracycline and Polymixin B, in lab cultures (Aleksic et al., 2014; Duarte et al., 2012). Moreover, a synergistic antibiofilm activity of several EOs was reported when combined with common antibiotics as Norfloxacin, Gentamicin, Oxacillin, Tetracycline and Ampicillin against different strains of Gram-positive and Gram-negative biofilms (El Baaboua et al., 2015; Rosato et al., 2020). This combination reduced the amounts of both EOs and antibiotics used to control microbial contamination and biofilms. However, another study reported an additive effect against Acinetobacter baumannii when coriander EO was combined with Cefoperazone and Piperacillin antibiotics (Duarte et al., 2012).
An enhanced antimicrobial activity of EOs was also reported when combined with irradiation against L. monocytogenes, E. coli O157:H7, yeasts and molds, A. niger, Aspergillus flavus, Aspergillus parasiticus and Penicillium chrysogenum in cauliflower and rice (Hossain et al. 2014, 2019; Tawema et al., 2016). Additionally, a complete inhibition of S. Typhimurium biofilms, Fusarium graminearum mycotoxins and an absence of intracellular ATP within L. monocytogenes and E. coli O157:H7 populations were observed in lab cultures as well as in maize samples (Caillet et al., 2005; Caillet and Lacroix, 2006; Kalagatur et al., 2018; Silva-Espinoza et al., 2020).
Similarly, when EOs were combined with PEF, a synergistic effect was observed against S. Typhimurium and E. coli O157:H7 populations in pasteurized skim milk and in different juices, respectively (Ait-Ouazzou et al., 2013; Pina-Pérez et al., 2012). However, when oregano was combined with PEF, the antimicrobial activity of oregano did not improve against C. jejuni in liquids and on raw chicken, except when following a sequential combination approach and applying EO after the PEF treatment, a significant increase in inactivation levels was reported (Clemente et al., 2020).
When applying HPP in combination with EOs, an enhanced antimicrobial activity was observed against L. monocytogenes and L. innocua in fresh cheese and yogurt drink, respectively (Bleoancă et al., 2016; Evrendilek and Balasubramaniam, 2011). This combination resulted in a reduction of pressure severity and impacts on food products.
The application of US treatment in combination with EOs also resulted in enhanced antimicrobial activity against E. coli 0157:H7 and a reduction of S. enterica populations below detection limits in lettuce leaves (Guo et al., 2020; Millan-Sango et al., 2016). Also, in low and high fat milk samples, cinnamon EO combined with US treatment induced further significant reductions in S. Typhimurium and L. monocytogenes populations compared to individual treatments (Mortazavi and Aliakbarlu, 2019).
Additionally, when combined with other antimicrobial agents (such as nisin, lactic acid, diglycerol monolaurate or chlorhexidine digluconate), EOs showed remarkable synergistic effects against L. monocytogenes, B. subtilis and, S. epidermidis (Dimitrijevic et al., 2007; Ettayebi et al., 2000; Karpanen et al., 2008; Yamazaki et al., 2004; Yoon et al., 2011).
The combination of an optimized mixture of EOs with other hurdles showing synergistic effect may reduce the concentrations required to yield the same antimicrobial activity when compared to EOs used alone. This may result in lower economic costs and lower sensorial impacts on food products, while maintaining their microbiological safety (Hyldgaard et al., 2012). Also, combining EOs with conventional antibiotics may reduce the latter used concentrations and thus decrease the probability of developing resistance towards them and minimize their side effects.
5. Conclusions
In this review, the advances and major trends in the use of EOs as potent tools to fight microbial contaminations were addressed. Additionally, the functional role and mechanisms of action of the different active EO components were discussed. Among the different active EO components, phenolic compounds were found to exhibit the greatest antimicrobial activity by acting principally on the disruption and increase in permeability of cell membranes, inducing a leakage of vital intracellular contents and eventually leading to microorganisms' inactivation and death. In most studies, the antimicrobial activity of active compounds was more pronounced than that of whole EOs, thus minimizing the amounts of EOs being used while achieving more reproducibility and standardization. EOs and their active components were effectively used in their free, micro- and nano-encapsulated forms and combined with other hurdles for antimicrobial purposes. Generally, encapsulation of EOs and their combination with other hurdles provided an enhanced antimicrobial activity when compared to free EO applications, with lower amounts being used. This could be promising technologies to control the different types of microbial contaminations and achieve microbiological safety. Further research studies should explore: i) the antimicrobial mode of action of EOs combined with other hurdles as one strategy could promote or modify the action of the other; ii) the combination of encapsulated EOs with other hurdles to determine their potential antimicrobial efficiencies as it is expected to have a synergistic or additive effect; and iii) the impact of the strong odor of EOs on the sensorial properties of food products. Additional studies should also assess the activities of EOs encapsulated or combined with other hurdles against microorganisms in different states as biofilms, viable but not culturable cells, spores and molds. Moreover, robust national and international frameworks and regulations should be set towards the use of EOs and their active components before being applied in diverse applications and fields.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the Partenariat Hubert Curien (PHC)- Cèdre program [42281SD] and the open access funding was provided by the Qatar National Library.
Data availability statement
No data was used for the research described in the article.
Declaration of interest’s statement
The authors declare no competing interests.
Additional information:
No additional information is available for this paper.
References
- Abdallah M., Benoliel C., Drider D., Dhulster P., Chihib N.E. Biofilm Formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014;196(7):453–472. doi: 10.1007/s00203-014-0983-1. [DOI] [PubMed] [Google Scholar]
- Adukwu E.C., Allen S.C.H., Phillips C.A. The anti-biofilm activity of lemongrass (Cymbopogon flexuosus) and Grapefruit (Citrus Paradisi) essential oils against five strains of Staphylococcus aureus. J. Appl. Microbiol. 2012;113(5):1217–1227. doi: 10.1111/j.1365-2672.2012.05418.x. [DOI] [PubMed] [Google Scholar]
- Afonso T.B., Simões L.C., Lima N. Effect of quorum sensing and quenching molecules on inter-kingdom biofilm formation by Penicillium expansum and bacteria. Biofouling. 2020;36(8):965–976. doi: 10.1080/08927014.2020.1836162. [DOI] [PubMed] [Google Scholar]
- Aiemsaard J., Aiumlamai S., Aromdee C., Taweechaisupapong S., Khunkitti W. The effect of lemongrass oil and its major components on clinical isolate mastitis pathogens and their mechanisms of action on Staphylococcus aureus DMST 4745. Res. Vet. Sci. 2011;91(3):e31–e37. doi: 10.1016/j.rvsc.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Ait-Ouazzou A., Espina L., García-Gonzalo D., Pagán R. Synergistic combination of physical treatments and carvacrol for Escherichia coli O157:H7 inactivation in apple, mango, orange, and tomato juices. Food Control. 2013;32(1):159–167. [Google Scholar]
- Aleksic Sabo V., Knezevic P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. Plant extracts and essential oils: a review. Ind. Crop. Prod. 2019;132:413–429. doi: 10.1016/j.indcrop.2019.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksic V., Mimica-Dukic N., Simin N., Nedeljkovic N., Knezevic P. Synergistic effect of myrtus communis L. Essential oils and conventional antibiotics against multi-drug resistant Acinetobacter baumannii wound isolates. Phytomedicine. 2014;21(12):1666–1674. doi: 10.1016/j.phymed.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Ali S., Khan A., Ahmed I., Musaddiq M., Ahmed K., Polasa H., Venkateswar Rao L., Habibullah C., Sechi L., Ahmed N. Antimicrobial activities of eugenol and cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005;4(1):20. doi: 10.1186/1476-0711-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Maqtari Q.A., Rehman A., Mahdi A.A., Al-Ansi W., Wei M., Yanyu Z., Phyo H.M., Galeboe O., Yao W. Application of essential oils as preservatives in food systems: challenges and future prospectives – a review. Phytochemistry Rev. 2021;295:33–40. [Google Scholar]
- Almatroudi A., Hu H., Deva A., Gosbell I.B., Jacombs A., Jensen S.O., Whiteley G., Glasbey T., Vickery K. A new dry-surface biofilm model: an essential tool for efficacy testing of hospital surface decontamination procedures. J. Microbiol. Methods. 2015;117:171–176. doi: 10.1016/j.mimet.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Almatroudi A., Shamaila T., Honghua H., Durdana C. Staphylococcus aureus dry-surface biofilms are more resistant to heat treatment than traditional hydrated biofilms. J. Hosp. Infect. 2018;98(2):161–167. doi: 10.1016/j.jhin.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Almeida L.D.F.D.D., Paula J.F.D., de Almeida R.V.D., Williams D.W., Hebling J., Cavalcanti Y.W. Efficacy of citronella and cinnamon essential oils on Candida albicans biofilms. Acta Odontol. Scand. 2016;74(5):393–398. doi: 10.3109/00016357.2016.1166261. [DOI] [PubMed] [Google Scholar]
- Amalaradjou M.A.R., Venkitanarayanan K. Effect of trans-cinnamaldehyde on inhibition and inactivation of Cronobacter sakazakii biofilm on abiotic surfaces. J. Food Protect. 2011;74(2):200–208. doi: 10.4315/0362-028X.JFP-10-296. [DOI] [PubMed] [Google Scholar]
- Amaral V.C.S., Santos P.R., da Silva A.F., dos Santos A.R., Machinski M., Graton Mikcha J.M. Effect of carvacrol and thymol on Salmonella spp. Biofilms on polypropylene. Int. J. Food Sci. Technol. 2015;50(12):2639–2643. [Google Scholar]
- Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils – a review. Food Chem. Toxicol. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Barriuso J., Hogan D.A., Keshavarz T., Martínez M.J. Role of quorum sensing and chemical communication in fungal biotechnology and pathogenesis. FEMS Microbiol. Rev. 2018;42(5):627–638. doi: 10.1093/femsre/fuy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazargani M.M., Rohloff J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control. 2016;61:156–164. [Google Scholar]
- Becerril R., Nerín C., Gómez-Lus R. Evaluation of bacterial resistance to essential oils and antibiotics after exposure to oregano and cinnamon essential oils. Foodborne Pathog. Dis. 2012;9(8):699–705. doi: 10.1089/fpd.2011.1097. [DOI] [PubMed] [Google Scholar]
- Belessi C.E.A., Gounadaki A.S., Psomas A.N., Skandamis P.N. Efficiency of different sanitation methods on Listeria monocytogenes biofilms formed under various environmental conditions. Int. J. Food Microbiol. 2011;145:S46–S52. doi: 10.1016/j.ijfoodmicro.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Ben Hsouna A., Ben Halima N., Smaoui S., Hamdi N. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16:146. doi: 10.1186/s12944-017-0487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava K., Conti D.S., da Rocha S.R.P., Zhang Y. Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 2015;47:69–73. doi: 10.1016/j.fm.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Bhavaniramya S., Vishnupriya S., Al-Aboody M.S., Vijayakumar R., Baskaran D. Role of essential oils in food safety: antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019;2(2):49–55. [Google Scholar]
- Bleoancă I., Saje K., Mihalcea L., Oniciuc E.A., Smole-Mozina S., Nicolau A.I., Borda D. Contribution of high pressure and thyme extract to control Listeria monocytogenes in fresh cheese - a hurdle approach. Innovat. Food Sci. Emerg. Technol. 2016;38:7–14. [Google Scholar]
- Boehnke K.F., Eaton K.A., Fontaine C., Brewster R., Wu J., Eisenberg J.N.S., Valdivieso M., Baker L.H., Xi C. Reduced infectivity of waterborne viable but nonculturable Helicobacter pylori strain SS1 in mice. Helicobacter. 2017;22(4) doi: 10.1111/hel.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskovic M., Djordjevic J., Ivanovic J., Janjic J., Zdravkovic N., Glisic M., Glamoclija N., Baltic B., Djordjevic V., Baltic M. Inhibition of Salmonella by thyme essential oil and its effect on microbiological and sensory properties of minced pork meat packaged under vacuum and modified atmosphere. Int. J. Food Microbiol. 2017;258:58–67. doi: 10.1016/j.ijfoodmicro.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Bouhdid S., Abrini J., Zhiri A., Espuny M.J., Manresa A. Investigation of functional and morphological changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Origanum compactum essential oil. J. Appl. Microbiol. 2009;106(5):1558–1568. doi: 10.1111/j.1365-2672.2008.04124.x. [DOI] [PubMed] [Google Scholar]
- Bouyahya A., Abrini J., Dakka N., Bakri Y. Essential oils of Origanum Compactum increase membrane permeability, disturb cell membrane integrity, and suppress quorum-sensing phenotype in bacteria. J. Pharm. Anal. 2019;9(5):301–311. doi: 10.1016/j.jpha.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackman G., Defoirdt T., Miyamoto C., Bossier P., Van Calenbergh S., Nelis H., Coenye T. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 2008;8(1):149. doi: 10.1186/1471-2180-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga P.C., Culici M., Alfieri M., Dal Sasso M. Thymol inhibits Candida albicans biofilm formation and mature biofilm. Int. J. Antimicrob. Agents. 2008;31(5):472–477. doi: 10.1016/j.ijantimicag.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Brenes A., Roura E. Essential oils in poultry nutrition: main effects and modes of action. Anim. Feed Sci. Technol. 2010;158(1–2):1–14. [Google Scholar]
- Bridier A., Briandet R., Thomas V., Dubois-Brissonnet F. Resistance of bacterial biofilms to disinfectants: a review. Biofouling. 2011;27(9):1017–1032. doi: 10.1080/08927014.2011.626899. [DOI] [PubMed] [Google Scholar]
- Burgess S.A., Lindsay D., Flint S.H. Thermophilic Bacilli and their importance in dairy processing. Int. J. Food Microbiol. 2010;144(2):215–225. doi: 10.1016/j.ijfoodmicro.2010.09.027. [DOI] [PubMed] [Google Scholar]
- Burt S.A., van der Zee R., Koets A.P., de Graaff A.M., van Knapen F., Gaastra W., Haagsman H.P., Veldhuizen E.J.A. Carvacrol induces heat shock protein 60 and inhibits synthesis of flagellin in Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007;73(14):4484–4490. doi: 10.1128/AEM.00340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Caillet S., Lacroix M. Effect of gamma radiation and oregano essential oil on murein and ATP concentration of Listeria monocytogenes. J. Food Protect. 2006;69(12):2961–2969. doi: 10.4315/0362-028x-69.12.2961. [DOI] [PubMed] [Google Scholar]
- Caillet S., Shareck F., Lacroix M. Effect of gamma radiation and oregano essential oil on murein and ATP concentration of Escherichia coli O157:H7. J. Food Protect. 2005;68(12):2571–2579. doi: 10.4315/0362-028x-68.12.2571. [DOI] [PubMed] [Google Scholar]
- Çakmak H., Özselek Y., Turan O.Y., Firatligil E., Karbancioglu-Güler F. Whey protein isolate edible films incorporated with essential oils: antimicrobial activity and barrier properties. Polym. Degrad. Stabil. 2020;179 [Google Scholar]
- Calo J.R., Crandall P.G., O’Bryan C.A., Ricke S.C. Essential oils as antimicrobials in food systems – a review. Food Control. 2015;54:111–119. [Google Scholar]
- Campana R., Casettari L., Fagioli L., Cespi M., Bonacucina G., Baffone W. Activity of essential oil-based microemulsions against Staphylococcus aureus biofilms developed on stainless steel surface in different culture media and growth conditions. Int. J. Food Microbiol. 2017;241:132–140. doi: 10.1016/j.ijfoodmicro.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Casas J., Tello J., Gatto F., Calvo L. Microbial inactivation of paprika using oregano essential oil combined with high-pressure CO2. J. Supercrit. Fluids. 2016;116:57–61. [Google Scholar]
- Casasola-Rodríguez B., Ruiz-Palacios G.M., Pilar R.C., Losano L., Ignacio M.R., Orta de Velásquez M.T. Detection of VBNC Vibrio cholera by RT-real time PCR based on differential gene expression analysis. FEMS Microbiol. Lett. 2018;365(15) doi: 10.1093/femsle/fny156. [DOI] [PubMed] [Google Scholar]
- Cava R., Nowak E., Taboada A., Marin-Iniesta F. Antimicrobial activity of clove and cinnamon essential oils against Listeria monocytogenes in pasteurized milk. J. Food Protect. 2007;70(12):2757–2763. doi: 10.4315/0362-028x-70.12.2757. [DOI] [PubMed] [Google Scholar]
- Chang C.W., Lin M.H. Optimization of PMA-qPCR for Staphylococcus aureus and determination of viable bacteria in indoor air. Indoor Air. 2017;28:64–72. doi: 10.1111/ina.12404. [DOI] [PubMed] [Google Scholar]
- Chang S.T., Chen P.F., Chang S.C. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum Osmophloeum. J. Ethnopharmacol. 2001;77(1):123–127. doi: 10.1016/s0378-8741(01)00273-2. [DOI] [PubMed] [Google Scholar]
- Charlebois A., Jacques M., Boulianne M., Archambault M. Tolerance of Clostridium perfringens biofilms to disinfectants commonly used in the food industry. Food Microbiol. 2017;62:32–38. doi: 10.1016/j.fm.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Chavant P., Gaillard-Martinie B., Hébraud M. Antimicrobial effects of sanitizers against planktonic and sessile Listeria monocytogenes cells according to the growth phase. FEMS Microbiol. Lett. 2004;236(2):241–248. doi: 10.1016/j.femsle.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Chen H., Zhang Y., Zhong Q. Physical and antimicrobial properties of spray-dried Zein–Casein nanocapsules with Co-encapsulated eugenol and thymol. J. Food Eng. 2015;144(1):93–102. [Google Scholar]
- Chouhan S., Sharma K., Guleria S. Antimicrobial activity of some essential oils—present status and future perspectives. Medicines. 2017;4(3):58. doi: 10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente I., Condón-Abanto S., Pedrós-Garrido S., Whyte P., Lyng J.G. Efficacy of pulsed electric fields and antimicrobial compounds used alone and in combination for the inactivation of Campylobacter jejuni in liquids and raw chicken. Food Control. 2020;107(1) [Google Scholar]
- Cui H., Li W., Li C., Vittayapadung S., Lin L. Liposome containing cinnamon oil with antibacterial activity against methicillin-resistant Staphylococcus aureus biofilm. Biofouling. 2016;32(2):215–225. doi: 10.1080/08927014.2015.1134516. [DOI] [PubMed] [Google Scholar]
- Cui H., Li W., Lin L. Antibacterial activity of liposome containing curry plant essential oil against Bacillus cereus in rice. J. Food Saf. 2017;37(2) [Google Scholar]
- Cui H., Zhang X., Zhou H., Zhao C., Lin L. Antimicrobial activity and mechanisms of Salvia sclarea essential oil. Bot. Stud. 2015;56(1):16. doi: 10.1186/s40529-015-0096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Gündel S., de Souza M.E., Quatrin P.M., Klein B., Wagner R., Gündel A., de Almeida Vaucher R., Santos R.C.V., Ferreira Ourique A. Nanoemulsions containing cymbopogon flexuosus essential oil: development, characterization, stability study and evaluation of antimicrobial and antibiofilm activities. Microb. Pathog. 2018;118(5):268–276. doi: 10.1016/j.micpath.2018.03.043. [DOI] [PubMed] [Google Scholar]
- Deepika S.A., Chaudhari A.K., Das S., Dubey N.K. Zingiber zerumbet L. Essential oil based chitosan nanoemulsion as an efficient green preservative against fungi and Aflatoxin B1 contamination. J. Food Sci. 2021;86(1):149–160. doi: 10.1111/1750-3841.15545. [DOI] [PubMed] [Google Scholar]
- de Medeiros Barbosa I., da Costa Medeiros J.A., de Oliveira K.Á.R., Gomes-Neto N.J., Tavares J.F., Magnani M., de Souza E.L. Efficacy of the combined application of oregano and rosemary essential oils for the control of Escherichia coli, Listeria monocytogenes and Salmonella enteritidis in leafy vegetables. Food Control. 2016;59(1):468–477. [Google Scholar]
- de Oliveira M.M.M., Brugnera D.F., do Nascimento J.A., Batista N.N., Hilsdorf Piccoli R. Cinnamon essential oil and cinnamaldehyde in the control of bacterial biofilms formed on stainless steel surfaces. Eur. Food Res. Technol. 2012;234(5):821–832. [Google Scholar]
- de Oliveira M.M.M., Brugnera D.F., Cardoso M.D.G., Alves E., Piccoli R.H. Disinfectant action of cymbopogon sp. Essential oils in different phases of biofilm formation by Listeria monocytogenes on stainless steel surface. Food Control. 2010;21(4):549–553. [Google Scholar]
- Desai M.A., Soni K.A., Nannapaneni R., Schilling M.W., Silva J.L. Reduction of Listeria monocytogenes biofilms on stainless steel and polystyrene surfaces by essential oils. J. Food Protect. 2012;75(7):1332–1337. doi: 10.4315/0362-028X.JFP-11-517. [DOI] [PubMed] [Google Scholar]
- Dev Kumar G., Ravishankar S. Ozonized water with plant antimicrobials: an effective method to inactivate Salmonella enterica on iceberg lettuce in the produce wash water. Environ. Res. 2019;171(4):213–217. doi: 10.1016/j.envres.2018.11.023. [DOI] [PubMed] [Google Scholar]
- Dhifi W., Bellili S., Jazi S., Bahloul N., Mnif W. Essential oils’ chemical characterization and investigation of some biological activities: a critical review. Medicines. 2016;3(4):25. doi: 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima C., Dima S. Essential oils in foods: extraction, stabilization, and toxicity. Curr. Opin. Food Sci. 2015;5(10):29–35. [Google Scholar]
- Dimitrijevic S.I., Mihajlovski K.R., Antonovic D.G., Milanovic-Stevanovic M.R., Mijin D.Z. A study of the synergistic antilisterial Effects of a sub-lethal dose of lactic acid and essential oils from Thymus vulgaris L., Rosmarinus Officinalis L. and Origanum vulgare L. Food Chem. 2007;104(2):774–782. [Google Scholar]
- Doležalová E., Prukner V., Lukeš P., Šimek M. Stress response of Escherichia coli induced by surface streamer discharge in humid air. J. Phys. Appl. Phys. 2016;49 [Google Scholar]
- Domadia P., Swarup S., Bhunia A., Sivaraman J., Dasgupta D. Inhibition of bacterial cell division protein FtsZ by cinnamaldehyde. Biochem. Pharmacol. 2007;74(6):831–840. doi: 10.1016/j.bcp.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Dong K., Pan H., Yang D., Rao L., Zhao L., Wang Y., Liao X. Induction, detection, formation and resuscitation of viable but non-culturable state microorganisms. Compr. Rev. Food Sci. Food Saf. 2020;19(1):149–183. doi: 10.1111/1541-4337.12513. [DOI] [PubMed] [Google Scholar]
- Donlan R.M. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 2002;8(9):881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsì F., Annunziata M., Sessa M., Ferrari G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2011;44(9):1908–1914. [Google Scholar]
- dos Santos Rodrigues J.B., de Souza N.T., Scarano J.O.A., de Sousa J.M., Lira M.C., de Figueiredo R.C.B.Q., de Souza E.L., Magnani M. Efficacy of using oregano essential oil and carvacrol to remove young and mature Staphylococcus aureus biofilms on food-contact surfaces of stainless steel. LWT. 2018;93(7):293–299. [Google Scholar]
- dos Santos Rodrigues J.B., de Carvalho R.J., de Souza N.T., de Sousa Oliveira K., Franco O.L., Schaffner D., de Souza E.L., Magnani M. Effects of oregano essential oil and carvacrol on biofilms of Staphylococcus aureus from food-contact surfaces. Food Control. 2017;73(3):1237–1246. [Google Scholar]
- Du W., Zhou M., Liu Z., Chen Y., Li R. Inhibition effects of low concentrations of epigallocatechin gallate on the biofilm formation and hemolytic activity of Listeria monocytogenes. Food Control. 2018;85(3):119–126. [Google Scholar]
- Duarte A., Ferreira S., Silva F., Domingues F.C. Synergistic activity of coriander oil and conventional antibiotics against Acinetobacter baumannii. Phytomedicine. 2012;19(3–4):236–238. doi: 10.1016/j.phymed.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Ed-Dra A., Nalbone L., Filali F.R., Trabelsi N., El Majdoub Y.O., Bouchrif B., et al. Comprehensive evaluation on the use of Thymus vulgaris essential oil as natural additive against different serotypes of Salmonella enterica. Sustainability. 2021;13(8):4594. [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Center for Disease Prevention and Control) The European summary report on trends and sources of Zoonoses, Zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018;16(12):5500. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Abed S., Ibnsouda K.S., Latrache H., Zineb G., Mouradi H., Remmal A. Carvacrol and thymol components inhibiting Pseudomonas aeruginosa adherence and biofilm formation. Afr. J. Microbiol. Res. 2011;5(20):3229–3232. [Google Scholar]
- El Asbahani A., Miladi K., Badri W., Sala M., Aït Addi E.H., Casabianca H., El Mousadik A., et al. Essential oils: from extraction to encapsulation. Int. J. Pharm. 2015;483(1–2):220–243. doi: 10.1016/j.ijpharm.2014.12.069. [DOI] [PubMed] [Google Scholar]
- El Baaboua A., Maadoudi M., Bouyahya A., Belmehdi O., Kounnoun A., Cheyadmi S., Sanae O., Senhaji N.S., Abrini J. Evaluation of the combined effect of antibiotics and essential oils against Campylobacter multidrug resistant strains and their biofilm formation. South Afr. J. Bot. 2015;150:451–465. [Google Scholar]
- El Darier S.M., El-Ahwany A., Elkenany E.T., Abdeldaim A.A. An in vitro study on antimicrobial and anticancer potentiality of thyme and clove oils. Rendiconti Lincei. Sci. Fis. Nat. 2018;29(1):131–139. [Google Scholar]
- El Moussaoui N., Sanchez G., Khay E.O., Idaomar M., Ibn Mansour A., Abrini J., Aznar R. Antibacterial and antiviral activities of essential oils of northern Moroccan plants. Br. Biotechnol. J. 2013;3(3):318–331. [Google Scholar]
- El Sayed Zaki M., Bastawy S., Montasser K. Molecular study of resistance of Staphylococcus aureus to antiseptic quaternary ammonium compounds. J. Glob. Antimicrob. Resist. 2019;17(6):94–97. doi: 10.1016/j.jgar.2018.11.022. [DOI] [PubMed] [Google Scholar]
- Esbelin J., Santos T., Hébraud M. Desiccation: an environmental and food industry stress that bacteria commonly face. Food Microbiol. 2018;69(2):82–88. doi: 10.1016/j.fm.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Esmaeili A., Asgari A. In vitro release and biological activities of Carum copticum essential oil (CEO) loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2015;81(11):283–290. doi: 10.1016/j.ijbiomac.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Espina L., Somolinos M., Lorán S., Conchello P., García D., Pagán R. Chemical composition of commercial Citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food Control. 2011;22(6):896–902. [Google Scholar]
- Ettayebi K., El Yamani J., Rossi-Hassani B.D. Synergistic effects of nisin and thymol on antimicrobial activities in Listeria monocytogenes and Bacillus subtilis. FEMS Microbiol. Lett. 2000;183(1):191–195. doi: 10.1111/j.1574-6968.2000.tb08956.x. [DOI] [PubMed] [Google Scholar]
- Evrendilek G.A., Balasubramaniam V.M. Inactivation of Listeria monocytogenes and Listeria innocua in yogurt drink applying combination of high pressure processing and mint essential oils. Food Control. 2011;22(8):1435–1441. [Google Scholar]
- Fakruddin M.D., Bin Mannan K.S., Andrews S. Viable but nonculturable bacteria: food safety and public health perspective. ISRN Microbiol. 2013;2013:1–6. doi: 10.1155/2013/703813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcó I., Verdeguer M., Aznar R., Sánchez G., Randazzo W. Sanitizing food contact surfaces by the use of essential oils. Innovat. Food Sci. Emerg. Technol. 2019;51(1):220–228. [Google Scholar]
- Ferk F., Misik M., Hoelzl C., Uhl M., Fuerhacker M., Grillitsch B., Parzefall W., et al. Benzalkonium chloride (BAC) and dimethyldioctadecyl-ammonium bromide (DDAB), two common quaternary ammonium compounds, cause genotoxic effects in mammalian and plant cells at environmentally relevant concentrations. Mutagenesis. 2007;22(6):363–370. doi: 10.1093/mutage/gem027. [DOI] [PubMed] [Google Scholar]
- García-Salinas S., Elizondo-Castillo H., Arruebo M., Mendoza G., Irusta S. Evaluation of the antimicrobial activity and cytotoxicity of different components of natural origin present in essential oils. Molecules. 2018;23(6):1399. doi: 10.3390/molecules23061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaderi-Ghahfarokhi M., Barzegar M., Sahari M.A., Ahmadi Gavlighi H., Gardini F. Chitosan cinnamon essential oil nano-formulations: application as a novel additive for controlled release and shelf life extension of beef patties. Int. J. Biol. Macromol. 2017;102:19–28. doi: 10.1016/j.ijbiomac.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Gill A.O., Holley R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006;108(1):1–9. doi: 10.1016/j.ijfoodmicro.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Giongo J.L., de Almeida Vaucher R., Fausto V.P., Quatrin P.M., Lopes L.Q.S., Santos R.C.V., Gündel A., Gomes P., Steppe M. Anti- Candida activity assessment of Pelargonium graveolens oil free and nanoemulsion in biofilm formation in hospital medical supplies. Microb. Pathog. 2016;100(11):170–178. doi: 10.1016/j.micpath.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Granata G., Stracquadanio S., Leonardi M., Napoli E., Consoli G.M.L., Cafiso V., Stefani S., Geraci C. Essential oils encapsulated in polymer-based nanocapsules as potential candidates for application in food preservation. Food Chem. 2018;269(12):286–292. doi: 10.1016/j.foodchem.2018.06.140. [DOI] [PubMed] [Google Scholar]
- Guarda A., Rubilar J.F., Miltz J., Galotto M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011;146(2):144–150. doi: 10.1016/j.ijfoodmicro.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Guo J., He Z., Ma C., Li W., Wang J., Lin F., Liu X., Ling L. Evaluation of cold plasma for decontamination of molds and mycotoxins in rice grain. Food Chem. 2022;402 doi: 10.1016/j.foodchem.2022.134159. [DOI] [PubMed] [Google Scholar]
- Guo M., Zhang L., He Q., Arabi S.A., Zhao H., Chen W., Ye X., Liu D. Synergistic antibacterial effects of Ultrasound and thyme essential oils nanoemulsion against Escherichia coli O157:H7. Ultrason. Sonochem. 2020;66 doi: 10.1016/j.ultsonch.2020.104988. [DOI] [PubMed] [Google Scholar]
- Gutierrez J., Barry-Ryan C., Bourke P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008;124(1):91–97. doi: 10.1016/j.ijfoodmicro.2008.02.028. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-del-Río I., Fernández J., Lombó F. Plant nutraceuticals as antimicrobial agents in food preservation: terpenoids, polyphenols and thiols. Int. J. Antimicrob. Agents. 2018;52(3):309–315. doi: 10.1016/j.ijantimicag.2018.04.024. [DOI] [PubMed] [Google Scholar]
- Han D., Hung Y.C., Wang L. Evaluation of the antimicrobial efficacy of neutral electrolyzed water on pork products and the formation of viable but nonculturable (VBNC) pathogens. Food Microbiol. 2018;73:227–236. doi: 10.1016/j.fm.2018.01.023. [DOI] [PubMed] [Google Scholar]
- Hasheminejad N., Khodaiyan F., Safari M. Improving the antifungal activity of clove essential oil encapsulated by Chitosan nanoparticles. Food Chem. 2019;275:113–122. doi: 10.1016/j.foodchem.2018.09.085. [DOI] [PubMed] [Google Scholar]
- Hayrapetyan H., Abee T., Groot M.N. Sporulation dynamics and spore heat resistance in wet and dry biofilms of Bacillus cereus. Food Control. 2016;60(2):493–499. [Google Scholar]
- He Q., Guo M., Jin T.Z., Arabi S.A., Liu D. Ultrasound improves the decontamination effect of thyme essential oil nanoemulsions against Escherichia coli O157:H7 on cherry tomatoes. Int. J. Food Microbiol. 2021;337 doi: 10.1016/j.ijfoodmicro.2020.108936. [DOI] [PubMed] [Google Scholar]
- Hemaiswarya S., Soudaminikkutty R., Narasumani M.L., Doble M. Phenylpropanoids inhibit protofilament formation of Escherichia coli cell division protein FtsZ. J. Med. Microbiol. 2011;60(9):1317–1325. doi: 10.1099/jmm.0.030536-0. [DOI] [PubMed] [Google Scholar]
- Highmore C.J., Warner J.C., Rothwell S.D., Wilks S.A., Keevil C.W. Viable-but-Nonculturable Listeria monocytogenes and Salmonella enterica serovar thompson induced by chlorine stress remain infectious. mBio. 2018;9 doi: 10.1128/mBio.00540-18. e00540-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain F., Follett P., Salmieri S., Vu K.D., Fraschini C., Lacroix M. Antifungal activities of combined treatments of irradiation and essential oils (EOs) encapsulated chitosan nanocomposite films in in vitro and in situ conditions. Int. J. Food Microbiol. 2019;295(4):33–40. doi: 10.1016/j.ijfoodmicro.2019.02.009. [DOI] [PubMed] [Google Scholar]
- Hossain F., Follett P., Vu K.D., Salmieri S., Senoussi C., Lacroix M. Radiosensitization of Aspergillus niger and Penicillium chrysogenum using basil essential oil and ionizing radiation for food decontamination. Food Control. 2014;45(11):156–162. [Google Scholar]
- Hu F., Tu X.F., Thakur K., Hu F., Li X.L., Zhang Y.S., Zhang J.G., Wei Z.J. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem. Toxicol. 2019;134(12) doi: 10.1016/j.fct.2019.110821. [DOI] [PubMed] [Google Scholar]
- Huang C., Qian Y., Viana T., Siegumfeldt H., Arneborg N., Larsen N., Jespersen L. The quorum-sensing molecule 2-phenylethanol impaired conidial germination, hyphal membrane integrity and growth of Penicillium expansum and Penicillium nordicum. J. Appl. Microbiol. 2020;129(2):278–286. doi: 10.1111/jam.14621. [DOI] [PubMed] [Google Scholar]
- Hyldgaard M., Mygind T., Meyer R.L. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012;3:12. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun J.E., Bae Y.M., Yoon J.H., Lee S.Y. Preservative effectiveness of essential oils in vapor phase combined with modified atmosphere packaging against spoilage bacteria on fresh cabbage. Food Control. 2015;51(5):307–313. [Google Scholar]
- Ibusquiza P.S., Herrera J.J.R., Cabo M.L. Resistance to benzalkonium chloride, peracetic acid and nisin during formation of mature biofilms by Listeria monocytogenes. Food Microbiol. 2011;28(3):418–425. doi: 10.1016/j.fm.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Jadhav S., Shah R., Bhave M., Palombo E.A. Inhibitory activity of yarrow essential oil on Listeria planktonic cells and biofilms. Food Control. 2013;29(1):125–130. [Google Scholar]
- Jamil B., Abbasi R., Abbasi S., Imran M., Khan S.U., Ihsan A., Javed S., Bokhari H., Imran M. Encapsulation of cardamom essential oil in chitosan nano-composites: in-vitro efficacy on antibiotic-resistant bacterial pathogens and cytotoxicity studies. Front. Microbiol. 2016;7:1580. doi: 10.3389/fmicb.2016.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Zhong S., Schwarz P., Chen B., Rao J. Antifungal activity, mycotoxin inhibitory efficacy, and mode of action of hop essential oil nanoemulsion against Fusarium graminearum. Food Chem. 2022;400 doi: 10.1016/j.foodchem.2022.134016. [DOI] [PubMed] [Google Scholar]
- Jiang X., Yu T., Liang Y., Ji S., Guo X., Ma J., Zhou L. Efflux pump-mediated benzalkonium chloride resistance in Listeria monocytogenes isolated from retail food. Int. J. Food Microbiol. 2016;217(1):141–145. doi: 10.1016/j.ijfoodmicro.2015.10.022. [DOI] [PubMed] [Google Scholar]
- Jiang Y.T., Yan P.F., Liang J.P. Biological changes of Enterococcus faecalis in the viable but nonculturable state. Genet. Mol. Res. 2015;14:14790–14801. doi: 10.4238/2015.November.18.44. [DOI] [PubMed] [Google Scholar]
- Jiménez M., Domínguez J.A., Pascual-Pineda L.A., Azuara E., Beristain C.I. Elaboration and characterization of O/W cinnamon (Cinnamomum Zeylanicum) and black pepper (Piper Nigrum) emulsions. Food Hydrocolloids. 2018;77(4):902–910. [Google Scholar]
- Ju J., Chen X., Xie Y., Yu H., Guo Y., Cheng Y., Qian H., Yao W. Application of essential oil as a sustained release preparation in food packaging. Trends Food Sci. Technol. 2019;92(10):22–32. [Google Scholar]
- Kalagatur N.K., Mudili V., Kamasani J.R., Siddaiah C. Discrete and combined effects of ylang-ylang (cananga odorata) essential oil and gamma irradiation on growth and mycotoxins production by Fusarium graminearum in maize. Food Control. 2018;94(12):276–283. [Google Scholar]
- Kalemba D., Kunicka A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003;10(10):813–829. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- Karam L., Chehab R., Osaili T.M., Savvaidis I.N. Antimicrobial effect of thymol and carvacrol added to a vinegar-based marinade for controlling spoilage of marinated beef (shawarma) stored in air or vacuum packaging. Int. J. Food Microbiol. 2020;332(11) doi: 10.1016/j.ijfoodmicro.2020.108769. [DOI] [PubMed] [Google Scholar]
- Karam L., Roustom R., Abiad M.G., El-Obeid T., Savvaidis I.N. Combined effects of thymol, carvacrol and packaging on the shelf-life of marinated chicken. Int. J. Food Microbiol. 2019;291(2):42–47. doi: 10.1016/j.ijfoodmicro.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Karpanen T.J., Worthington T., Hendry E.R., Conway B.R., Lambert P.A. Antimicrobial efficacy of chlorhexidine digluconate alone and in combination with Eucalyptus oil, tea tree oil and thymol against planktonic and biofilm cultures of Staphylococcus epidermidis. J. Antimicrob. Chemother. 2008;62(5):1031–1036. doi: 10.1093/jac/dkn325. [DOI] [PubMed] [Google Scholar]
- Kavanaugh N.L., Ribbeck K. Selected antimicrobial essential oils eradicate Pseudomonas spp. and Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 2012;78(11):4057–4061. doi: 10.1128/AEM.07499-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I., Bahuguna A., Kumar P., Bajpai V.K., Kang S.C. Antimicrobial potential of carvacrol against uropathogenic Escherichia coli via membrane disruption, depolarization, and reactive oxygen species generation. Front. Microbiol. 2017;8(12):2421. doi: 10.3389/fmicb.2017.02421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelissa S.O., Abdallah M., Jama C., Faille C., Chihib N.E. Bacterial contamination and biofilm formation on abiotic surfaces and strategies to overcome their persistence. J. Mater. Environ. Sci. 2017;8(9):3326–3346. [Google Scholar]
- Khelissa S.O., Abdallah M., Jama C., Gharsallaoui A., Chihib N.E. Comparative study of growth temperature impact on the susceptibility of biofilm-detached and planktonic Staphylococcus aureus cells to benzalkonium chloride. Ann. Microbiol. 2019;69(3):291–298. [Google Scholar]
- Kibbee R.J., Ormeci B. Development of a sensitive and false-positive free PMA-qPCR viability assay to quantify VBNC Escherichia coli and evaluate disinfection performance in wastewater effluent. J. Microbiol. Methods. 2017;132:139–147. doi: 10.1016/j.mimet.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Kumar A., Kujur A., Yadav A., Pratap S., Prakash B. Optimization and mechanistic investigations on antifungal and Aflatoxin B1 inhibitory potential of nanoencapsulated plant-based bioactive compounds. Ind. Crop. Prod. 2019;131:213–223. [Google Scholar]
- Kwieciński J., Eick S., Wójcik K. Effects of tea tree (melaleuca alternifolia) oil on Staphylococcus aureus in biofilms and stationary growth phase. Int. J. Antimicrob. Agents. 2009;33(4):343–347. doi: 10.1016/j.ijantimicag.2008.08.028. [DOI] [PubMed] [Google Scholar]
- Kwon J.A., Yu C.B., Park H.D. Bacteriocidal effects and inhibition of cell separation of cinnamic aldehyde on Bacillus cereus. Lett. Appl. Microbiol. 2003;37(1):61–65. doi: 10.1046/j.1472-765x.2003.01350.x. [DOI] [PubMed] [Google Scholar]
- Lambert R.J.W., Skandamis P.N., Coote P.J., Nychas G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001;91(3):453–462. doi: 10.1046/j.1365-2672.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- Lavorgna M., Russo C., D’Abrosca B., Parrella A., Isidori M. Toxicity and genotoxicity of the quaternary ammonium compound benzalkonium chloride (BAC) using Daphnia magna and Ceriodaphnia dubia as model systems. Environ. Pollut. 2016;210(3):34–39. doi: 10.1016/j.envpol.2015.11.042. [DOI] [PubMed] [Google Scholar]
- Ledwoch K., Dancer S.J., Otter J.A., Kerr K., Roposte D., Rushton L., Weiser R., Mahenthiralingam E., Muir D.D., Maillard J.Y. Beware biofilm! Dry biofilms containing bacterial pathogens on multiple healthcare surfaces; a multi-centre study. J. Hosp. Infect. 2018;100(3):e47–e56. doi: 10.1016/j.jhin.2018.06.028. [DOI] [PubMed] [Google Scholar]
- Li Y.H., Tian X. Quorum sensing and bacterial social interactions in biofilms. Sensors. 2012;12(3):2519–2538. doi: 10.3390/s120302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Deng Y., Soteyome T., Li Y., Su J., Li L., Li B., Shirtliff M., Xu Z., Peters B. Induction and recovery of the viable but nonculturable state of hop-resistance Lactobacillus brevis. Front. Microbiol. 2018;9:2017. doi: 10.3389/fmicb.2018.02076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Tao M., Huang K. Encapsulation of Mānuka essential oil in yeast microcarriers for enhanced thermal stability and antimicrobial activity. Food Bioprocess Technol. 2021;14(12):2195–2206. [Google Scholar]
- López-Meneses A.K., Plascencia-Jatomea M., Lizardi-Mendoza J., Fernández-Quiroz D., Rodríguez-Félix F., Mourińo-Pérez R.R., Cortez-Rocha M.O. Schinus molle L. Essential oil-loaded chitosan nanoparticles: preparation, characterization, antifungal and anti-aflatoxigenic properties. LWT. 2018;96:597–603. [Google Scholar]
- Luciardi M.C., Blázquez M.A., Cartagena E., Bardón A., Arena M.E. Mandarin essential oils inhibit quorum sensing and virulence factors of Pseudomonas aeruginosa. LWT- Food Sci. Technol. 2016;68:373–380. doi: 10.1177/1082013219883465. [DOI] [PubMed] [Google Scholar]
- Magnoli A.P., Poloni V.L., Cavaglieri L. Impact of mycotoxin contamination in the animal feed industry. Curr. Opin. Food Sci. 2019;29:99–108. [Google Scholar]
- Majeed M., Majeed S., Nagabhushanam K., Punnapuzha A., Philip S., Mundkur L. Rapid assessment of viable but nonculturable Bacillus coagulans MTCC 5856 in commercial formulations using flow cytometry. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallozzi M., Viswanathan V.K., Vedantam G. Spore-forming Bacilli and Clostridia in human disease. Future Microbiol. 2010;5(7):1109–1123. doi: 10.2217/fmb.10.60. [DOI] [PubMed] [Google Scholar]
- Marino M., Bersani C., Comi G. Impedance measurements to study the antimicrobial activity of essential oils from lamiaceae and compositae. Int. J. Food Microbiol. 2001;67(3):187–195. doi: 10.1016/s0168-1605(01)00447-0. [DOI] [PubMed] [Google Scholar]
- Marotta S.M., Giarratana F., Parco A., Neri D., Ziino G., Giuffrida A., Panebianco A. Evaluation of the antibacterial activity of bergamo essential oils on different Listeria monocytogenes strains. Int. J. Food Saf. 2016;5(4):6176. doi: 10.4081/ijfs.2016.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya A., Prasad J., Das S., Dwivedy A.K. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021;5 [Google Scholar]
- Mechmechani S., Gharsallaoui A., Fadel A., Omari K.E., Khelissa S., Hamze M., Nour-Eddine N.E. Microencapsulation of carvacrol as an efficient tool to fight Pseudomonas aeruginosa and Enterococcus faecalis biofilms. PLoS One. 2022;17(7) doi: 10.1371/journal.pone.0270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechmechani S., Khelissa S., Gharsallaoui A., Omari K.E., Hamze M., Nour-Eddine N.E. Hurdle Technology using encapsulated enzymes and essential oils to fight bacterial biofilms. Appl. Microbiol. Biotechnol. 2022;106:2311–2335. doi: 10.1007/s00253-022-11875-5. [DOI] [PubMed] [Google Scholar]
- Melin V.E., Melin T.E., Dessify B.J., Nguyen C.T., Shea C.S., Hrubec T.C. Quaternary ammonium disinfectants cause subfertility in mice by targeting both male and female reproductive processes. Reprod. Toxicol. 2016;59(1):159–166. doi: 10.1016/j.reprotox.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Meng F.B., Gou Z.Z., Li Y.C., Zou L.H., Chen W.J., Liu D.Y. The efficiency of lemon essential oil-based nanoemulsions on the inhibition of Phomopsis sp. and reduction of postharvest decay of kiwifruit. Foods. 2022;11(10):1510. doi: 10.3390/foods11101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino N., Berdejo D., Bento R., Salman H., Lanz M., Maggi F., Sánchez-Gómez S., García-Gonzalo D., Pagán R. Antimicrobial efficacy of thymbra capitata (L.) cav. Essential oil loaded in self-assembled Zein nanoparticles in combination with heat. Ind. Crop. Prod. 2019;133(7):98–104. [Google Scholar]
- Millan-Sango D., Garroni E., Farrugia C., Van Impe J.F.M., Valdramidis V.P. Determination of the efficacy of Ultrasound combined with essential oils on the decontamination of Salmonella inoculated lettuce leaves. LWT. 2016;73(11):80–87. [Google Scholar]
- Millezi A.F., das Graças Cardoso M., Alves E., Piccoli R.H. Reduction of Aeromonas hidrophyla biofilm on stainless stell surface by essential oils. Braz. J. Microbiol. 2013;44(1):73–80. doi: 10.1590/S1517-83822013005000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghimi R., Aliahmadi A., Rafati H., Abtahi H.R., Amini S., Feizabadi M.M. Antibacterial and anti-biofilm activity of nanoemulsion of Thymus daenensis oil against multi-drug resistant Acinetobacter baumannii. J. Mol. Liq. 2018;265(9):765–770. [Google Scholar]
- Moghimi R., Ghaderi L., Rafati H., Aliahmadi A., McClements D.J. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem. 2016;194(3):410–415. doi: 10.1016/j.foodchem.2015.07.139. [DOI] [PubMed] [Google Scholar]
- Moosavy M.H., Basti A.A., Misaghi A., Salehi T.Z., Abbasifar R., Mousavi H.A.E., Alipour M., Razavi N.E., Gandomi H., Noori N. Effect of Zataria multiflora boiss. Essential oil and nisin on Salmonella typhimurium and Staphylococcus aureus in a food model system and on the bacterial cell membranes. Food Res. Int. 2008;41(10):1050–1057. [Google Scholar]
- Møretrø T., Schirmer B.C.T., Heir E., Fagerlund A., Hjemli P., Langsrud S. Tolerance to quaternary ammonium compound disinfectants may enhance growth of Listeria monocytogenes in the food industry. Int. J. Food Microbiol. 2017;241(1):215–224. doi: 10.1016/j.ijfoodmicro.2016.10.025. [DOI] [PubMed] [Google Scholar]
- Mortazavi N., Aliakbarlu J. Antibacterial effects of Ultrasound, cinnamon essential oil, and their combination against Listeria monocytogenes and Salmonella typhimurium in milk. J. Food Sci. 2019;84(12):3700–3706. doi: 10.1111/1750-3841.14914. [DOI] [PubMed] [Google Scholar]
- Mukamolova G.V., Yanopolskaya N.D., Kell D.B., Kaprelyants A.S. On resuscitation from the dormant state of Micrococcus luteus. Antonie Leeuwenhoek. 2018;73:237–243. doi: 10.1023/a:1000881918216. [DOI] [PubMed] [Google Scholar]
- Mulder I., Siemens J., Sentek V., Amelung W., Smalla K., Jechalke S. Quaternary ammonium compounds in soil: implications for antibiotic resistance development. Rev. Environ. Sci. Biotechnol. 2018;17(1):159–185. [Google Scholar]
- Nahr F.K., Ghanbarzadeh B., Hamishehkar H., Kafil H.S. Food grade nanostructured lipid carrier for cardamom essential oil: preparation, characterization and antimicrobial activity. J. Funct.Foods. 2018;40(1):1–8. [Google Scholar]
- Narla A.V., Borenstein D.B., Wingreen N.S. A biophysical limit for quorum sensing in biofilms. Proc. Natl. Acad. Sci. U. S. A. 2021;118(21) doi: 10.1073/pnas.2022818118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira J.O., Campolina G.A., Batista L.R., Alves E., Caetano A.R.S., Brandão R.M., Nelson D.L., Cardoso M.D.G. Mechanism of action of various terpenes and phenylpropanoids against Escherichia coli and Staphylococcus aureus. FEMS Microbiol. Lett. 2021;368(9):52. doi: 10.1093/femsle/fnab052. [DOI] [PubMed] [Google Scholar]
- Nostro A., Cellini L., Zimbalatti V., Blanco A.R., Marino A., Pizzimenti F., Di Giulio M., Bisignano G. Enhanced activity of carvacrol against biofilm of Staphylococcus aureus and Staphylococcus epidermidis in an acidic environment. APMIS. 2012;120(12):967–973. doi: 10.1111/j.1600-0463.2012.02928.x. [DOI] [PubMed] [Google Scholar]
- Nostro A., Procopio F., Pizzimenti F.C., Cannatelli M.A., Bisignano G., Marino A., Blanco A.R., Cioni P.L., Roccaro A.S. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007;56(4):519–523. doi: 10.1099/jmm.0.46804-0. [DOI] [PubMed] [Google Scholar]
- Nuryastuti T., van der Mei H.C., Busscher H.J., Iravati S., Aman A.T., Krom B.P. Effect of cinnamon oil on IcaA expression and biofilm formation by Staphylococcus epidermidis. Appl. Environ. Microbiol. 2009;75(21):6850–6855. doi: 10.1128/AEM.00875-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omonijo F.A., Ni L., Gong J., Wang Q., Lahaye L., Yang C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018;4(2):126–136. doi: 10.1016/j.aninu.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlo E., Russo C., Nugnes R., Lavorgna M., Isidori M. Natural methoxyphenol compounds: antimicrobial activity against foodborne pathogens and food spoilage bacteria, and role in antioxidant processes. Food. 2021;10(8):1807. doi: 10.3390/foods10081807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orta M.T., Noguez I., Casasola Rodriguez B., Roman Roman P.I. Effects of ozone and chlorine disinfection on VBNC Helicobacter pylori by molecular techniques and FESEM images. Environ. Technol. 2017;38:744–753. doi: 10.1080/09593330.2016.1210680. [DOI] [PubMed] [Google Scholar]
- Palla M., Battini F., Cristani C., Giovannetti M., Squartini A., Agnolucci M. Quorum sensing in rhizobia isolated from the spores of the mycorrhizal symbiont rhizophagus intraradices. Mycorrhiza. 2018;28(8):773–778. doi: 10.1007/s00572-018-0847-7. [DOI] [PubMed] [Google Scholar]
- Pathania R., Khan H., Kaushik R., Khan M.A. Essential oil nanoemulsions and their antimicrobial and food applications. Curr. Res. Nutr. Food Sci. J. 2018;6(3):626–643. [Google Scholar]
- Pérez-Conesa D., Cao J., Chen L., Mclandsborough L., Weiss J. Inactivation of Listeria monocytogenes and Escherichia coli O157:H7 biofilms by micelle-encapsulated eugenol and carvacrol. J. Food Protect. 2011;74(1):55–62. doi: 10.4315/0362-028X.JFP-08-403. [DOI] [PubMed] [Google Scholar]
- Pina-Pérez M.C., Martínez-López A., Rodrigo D. Cinnamon antimicrobial effect against Salmonella typhimurium cells treated by pulsed electric fields (PEF) in pasteurized skim milk beverage. Food Res. Int. 2012;48(2):777–783. [Google Scholar]
- Preda V.G., Sãndulescu O. Communication is the key: biofilms, quorum sensing, formation and prevention. Discoveries. 2019;7(3) doi: 10.15190/d.2019.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popiolski T.M., Otsuka I., Halila S., Muniz E.C., Soldi V., Borsali R. Preparation of polymeric micelles of poly(ethylene oxide-b-lactic acid) and their encapsulation with lavender oil. Mater. Res. 2016;19(6):1356–1365. [Google Scholar]
- Potts M. Desiccation tolerance: a simple process? Trends Microbiol. 2001;9(11):553–559. doi: 10.1016/s0966-842x(01)02231-4. [DOI] [PubMed] [Google Scholar]
- Prakash A., Baskaran R., Paramasivam N., Vadivel V. Essential oil based nanoemulsions to improve the quality of minimally processed fruits and vegetables: Q review. Food Res. Int. 2018;111:509–523. doi: 10.1016/j.foodres.2018.05.066. [DOI] [PubMed] [Google Scholar]
- Quendera A.P., Barreto A.S., Semedo-Lemsaddek T. Antimicrobial activity of essential oils against foodborne multidrug-resistant enterococci and aeromonads in planktonic and biofilm state. Food Sci. Technol. Int. 2019;25(2):101–108. doi: 10.1177/1082013218799027. [DOI] [PubMed] [Google Scholar]
- Radünz M., Helbig E., Borges C.D., Gandra T.V., Gandra E.A. A mini-review on encapsulation of essential oils. J. Anal. Pharm. Res. 2018;7(1) [Google Scholar]
- Rajkowska K., Nowak A., Kunicka-Styczyńska A., Siadura A. Biological effects of various chemically characterized essential oils: investigation of the mode of action of Candida albicans and HeLa cells. RSC Adv. 2016;6(99):97199–97207. [Google Scholar]
- Ramirez M.L., Cendoya E., Nichea M.J., Zachetti V.G.L., Chulze S.N. Impact of toxigenic fungi and mycotoxins in chickpea: a review. Curr. Opin. Food Sci. 2018;23:32–37. [Google Scholar]
- Rattanachaikunsopon P., Phumkhachorn P. Assessment of factors influencing antimicrobial activity of carvacrol and cymene against Vibrio cholerae in food. J. Biosci. Bioeng. 2010;110(5):614–619. doi: 10.1016/j.jbiosc.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Rosato A., Sblano S., Salvagno L., Carocci A., Clodovea M.L., Corbo F., Fracchiolla G. Anti-biofilm inhibitory synergistic effects of combinations of essential oils and antibiotics. Antibiotics. 2020;9(10):637. doi: 10.3390/antibiotics9100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G.G., Guterres K.B., Bonez P.C., da Silva Gundel S., Aggertt V.A., Siqueira F.S., Ourique A.F., Wagnerd R., Klein B., Santos R.C.V., de Campos M.M.A. Antibiofilm activity of nanoemulsions of cymbopogon flexuosus against rapidly growing Mycobacteria. Microb. Pathog. 2017;113(12):335–341. doi: 10.1016/j.micpath.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Russo C., Kundi M., Lavorgna M., Parrella A., Isidori M. Benzalkonium chloride and anticancer drugs in binary mixtures: reproductive toxicity and genotoxicity in the freshwater Crustacean Ceriodaphnia dubia. Arch. Environ. Contam. Toxicol. 2018;74(4):546–556. doi: 10.1007/s00244-017-0473-y. [DOI] [PubMed] [Google Scholar]
- Sadekuzzaman M., Mizan M.F.R., Kim H.S., Yang S., Ha S.D. Activity of thyme and tea tree essential oils against selected foodborne pathogens in biofilms on abiotic surfaces. LWT. 2018;89(3):134–139. [Google Scholar]
- Sales A.J., Bagherizadeh Y., Malekzadeh P., Ahmadi B., Bonab F.R. Evaluation of the antimicrobial effects of essential oil of reseda lutea L. On pathogenic bacteria: Staphylococcus aureus, Staphylococcus epidermidis, and Escherichia coli. Arch. Clin. Microbiol. 2017;8(3) [Google Scholar]
- Sandasi M., Leonard C.M., Van Vuuren S.F., Viljoen A.M. Peppermint (mentha piperita) inhibits microbial biofilms in vitro. South Afr. J. Bot. 2011;77(1):80–85. [Google Scholar]
- Scharff R.L. Economic burden from health losses due to foodborne illness in the United States. J. Food Protect. 2012;75(1):123–131. doi: 10.4315/0362-028X.JFP-11-058. [DOI] [PubMed] [Google Scholar]
- Scharff R.L. Springer; Cham: 2018. The economic burden of foodborne illness in the United States; pp. 123–142. (Food Safety Economics). [DOI] [PubMed] [Google Scholar]
- Schottroff F., Frohling A., Zunabovic-Pichler M., Krottenthaler A., Schluter O., Jager H. Sublethal injury and viable but non-culturable (VBNC) state in microorganisms during preservation of food and biological materials by non-thermal processes. Front. Microbiol. 2018;9:2773. doi: 10.3389/fmicb.2018.02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti R., Stringaro A., Nicolini L., Zanellato M., Boccia P., Maggi F., Gabbianelli R. Effects of essential oils from cymbopogon spp. and cinnamomum verum on biofilm and virulence properties of Escherichia coli O157:H7. Antibiotics. 2021;10(2):113. doi: 10.3390/antibiotics10020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Germination of spores of Bacillus species: what we know and do not know. J. Bacteriol. 2014;196(7):1297–1305. doi: 10.1128/JB.01455-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetta A., Kegere J., Mamdouh W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2019;126(4):731–742. doi: 10.1016/j.ijbiomac.2018.12.161. [DOI] [PubMed] [Google Scholar]
- Shi C., Jia Z., Sun Y., Chen Y., Guo D., Liu Z., Wen Q., Guo X., Ma L., Yang B., Baloch A.B., Xia X. Inactivation of nondesiccated and desiccated Cronobacter sakazakii in reconstituted infant formula by combination of citral and mild heat. J. Food Protect. 2017;80(7):1193–1197. doi: 10.4315/0362-028X.JFP-16-451. [DOI] [PubMed] [Google Scholar]
- Sieniawska E., Los R., Baj T., Malm A., Glowniak K. Antimicrobial efficacy of mutellina purpurea essential oil and α-pinene against Staphylococcus epidermidis grown in planktonic and biofilm cultures. Ind. Crop. Prod. 2013;51(11):152–157. [Google Scholar]
- Silva-Angulo A.B., Zanini S.F., Rosenthal A., Rodrigo D., Klein G., Martínez A. Comparative study of the effects of citral on the growth and injury of Listeria innocua and Listeria monocytogenes cells. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0114026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Espinoza B.A., Palomares-Navarro J.J., Tapia-Rodriguez M.R., Cruz-Valenzuela M.R., González-Aguilar G.A., Silva-Campa E., Pedroza-Montero M., Almeida-Lopes M., Miranda R., Ayala-Zavala J.F. Combination of ultraviolet light-C and clove essential oil to inactivate Salmonella typhimurium biofilms on stainless steel. J. Food Saf. 2020;40(3) [Google Scholar]
- Singh A., Singh R.K., Bhunia A.K., Singh N. Efficacy of plant essential oils as antimicrobial agents against Listeria monocytogenes in hotdogs. LWT - Food Sci. Technol. 2003;36(8):787–794. [Google Scholar]
- Singh B.K., Tiwari S., Dubey N.K. Essential oils and their nanoformulations as green preservatives to boost food safety against mycotoxin contamination of food commodities: a review. J. Sci. Food Agric. 2021;101(12):4879–4890. doi: 10.1002/jsfa.11255. [DOI] [PubMed] [Google Scholar]
- Skandamis P., Tsigarida E., E Nychas G.J. The effect of oregano essential oil on survival/death of Salmonella typhimurium in meat stored at 5°C under aerobic, VP/MAP conditions. Food Microbiol. 2002;19(1):97–103. [Google Scholar]
- Soliman E.A., El-Moghazy A.Y., Mohy El-Din M.S., Massoud M.A. Microencapsulation of essential oils within alginate: formulation and in vitro evaluation of antifungal activity. J. Encapsulation Adsorpt. Sci. 2013;3(1):48–55. [Google Scholar]
- Somrani M., Debbabi H., Palop A. Antibacterial and antibiofilm activity of essential oil of clove against Listeria monocytogenes and Salmonella enteritidis. Food Sci. Technol. Int. 2021;5 doi: 10.1177/10820132211013273. [DOI] [PubMed] [Google Scholar]
- Soni K.A., Oladunjoye A., Nannapaneni R., Schilling M.W., Silva J.L., Mikel B., Bailey R.H. Inhibition and inactivation of Salmonella typhimurium biofilms from polystyrene and stainless steel surfaces by essential oils and phenolic constituent carvacrol. J. Food Protect. 2013;76(2):205–212. doi: 10.4315/0362-028X.JFP-12-196. [DOI] [PubMed] [Google Scholar]
- Sreevidya V.S., Lenz K.A., Svoboda K.R., Ma H. Benzalkonium chloride, benzethonium chloride, and chloroxylenol - three replacement antimicrobials are more toxic than triclosan and triclocarban in two model organisms. Environ. Pollut. 2018;235(4):814–824. doi: 10.1016/j.envpol.2017.12.108. [DOI] [PubMed] [Google Scholar]
- Srey S., Jahid I.K., Ha S.D. Biofilm Formation in food industries: a food safety concern. Food Control. 2013;31(2):572–585. [Google Scholar]
- Swamy M.K., Akhtar M.S., Sinniah U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid. base Compl. Alternative Med. 2016;2016:1–21. doi: 10.1155/2016/3012462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C., Chen J., Zhang L., Zhang R., Zhang S., Ye S., Zhao Z., Yang D. Exploring the antibacterial mechanism of essential oils by membrane permeability, apoptosis and biofilm formation combination with proteomics analysis against mecithillin-resistant Staphylococcus aureus. Int. J. Med. Microbiol. 2020;310(5) doi: 10.1016/j.ijmm.2020.151435. [DOI] [PubMed] [Google Scholar]
- Tariq S., Wani S., Rasool W., Shafi K., Bhat M.A., Prabhakar A., et al. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019;134 doi: 10.1016/j.micpath.2019.103580. [DOI] [PubMed] [Google Scholar]
- Tawema P., Han J., Vu K.D., Salmieri S., Lacroix M. Antimicrobial effects of combined UV-C or gamma radiation with natural antimicrobial formulations against Listeria monocytogenes, Escherichia coli O157: H7, and total yeasts/molds in fresh cut cauliflower. LWT - Food Sci. Technol. 2016;65(1):451–456. [Google Scholar]
- Teixeira R.F., Filho C.A.B., Borges C.D. Essential oils as natural antimicrobials for application in edible coatings for minimally processed apple and melon: a review on antimicrobial activity and characteristics of food models. Food Packag. Shelf Life. 2022;31(3) [Google Scholar]
- Trindade L.A., Oliveira J.D.A., de Castro R.D., Lima E.D.O. Inhibition of adherence of C. albicans to dental implants and cover screws by cymbopogon nardus essential oil and citronellal. Clin. Oral Invest. 2015;19(9):2223–2231. doi: 10.1007/s00784-015-1450-3. [DOI] [PubMed] [Google Scholar]
- Trunet C., Carlin F., Coroller L. Investigating germination and outgrowth of bacterial spores at several scales. Trends Food Sci. Technol. 2017;64(6):60–68. [Google Scholar]
- Tsigarida E., Nychas G.J.E. Ecophysiological attributes of a Lactobacillus sp. and a Pseudomonas sp. on sterile beef fillets in relation to storage temperature and film permeability. J. Appl. Microbiol. 2001;90(5):696–705. doi: 10.1046/j.1365-2672.2001.01292.x. [DOI] [PubMed] [Google Scholar]
- Turgis M., Han J., Caillet S., Lacroix M. Antimicrobial activity of mustard essential oil against Escherichia coli O157:H7 and Salmonella typhi. Food Control. 2009;20(12):1073–1079. [Google Scholar]
- Ultee A., Bennik M.H.J., Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002;68(4):1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultee A., Smid E.J. Influence of carvacrol on growth and toxin production by Bacillus cereus. Int. J. Food Microbiol. 2001;64(3):373–378. doi: 10.1016/s0168-1605(00)00480-3. [DOI] [PubMed] [Google Scholar]
- Valeriano C., de Oliveira T.L.C., de Carvalho S.M., Cardoso M.D.G., Alves E., Piccoli R.H. The sanitizing action of essential oil-based solutions against Salmonella enterica serotype enteritidis S64 biofilm formation on AISI 304 stainless steel. Food Control. 2012;25(2):673–677. [Google Scholar]
- Valková V., Dúranová H., Galovičová L., Vukovic N.L., Vukic M., Kačániová M. In vitro antimicrobial activity of lavender, mint, and rosemary essential oils and the effect of their vapours on growth of Penicillium spp. in a bread model system. Molecules. 2021;26(13):3859. doi: 10.3390/molecules26133859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesty E.F., Whitbread E., Needs A.L., Tanko S., Jones W., Halliday K., Ghaderiardakani F., Xiaoguang L., Cámara M., Coates J. Cross-kingdom signalling regulates spore germination in the moss physcomitrella patens. Sci. Rep. 2020;10(1):1–13. doi: 10.1038/s41598-020-59467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetas D., Dimitropoulou E., Mitropoulou G., Kourkoutas Y., Giaouris E. Disinfection efficiencies of sage and spearmint essential oils against planktonic and biofilm Staphylococcus aureus cells in comparison with sodium hypochlorite. Int. J. Food Microbiol. 2017;257(9):19–25. doi: 10.1016/j.ijfoodmicro.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Vidács A., Kerekes E., Rajkó R., Petkovits T., Alharbi N.S., Khaled J.M., Vágvölgyi C., Krisch J. Optimization of essential oil-based natural disinfectants against Listeria monocytogenes and Escherichia coli biofilms formed on polypropylene surfaces. J. Mol. Liq. 2018;255(4):257–262. [Google Scholar]
- Walsh S.E., Maillard J.Y., Russell A.D., Catrenich C.E., Charbonneau D.L., Bartolo R.G. Activity and mechanisms of action of selected biocidal agents on gram-positive and -negative bacteria. J. Appl. Microbiol. 2003;94(2):240–247. doi: 10.1046/j.1365-2672.2003.01825.x. [DOI] [PubMed] [Google Scholar]
- Wang L., Xia Q., Li Y. Label free-based proteomic analysis of proteins in Bacillus cereus spores regulated by high pressure processing and slightly acidic electrolyzed water treatment. Food Control. 2018;90:392–400. [Google Scholar]
- Wells-Bennik M.H.J., Eijlander R.T., den Besten H.M.W., Berendsen E.M., Warda A.K., Krawczyk A.O., Nierop Groot M.N., Xiao Y., Zwietering M.H., Kuipers O.P., Abee T. Bacterial spores in food: survival, emergence, and outgrowth. Annu. Rev. Food Sci. Technol. 2016;7(1):457–482. doi: 10.1146/annurev-food-041715-033144. [DOI] [PubMed] [Google Scholar]
- Wu D., Lu J., Zhong S., Schwarz P., Chen B., Rao J. Influence of nonionic and ionic surfactants on the antifungal and mycotoxin inhibitory efficacy of cinnamon oil nanoemulsions. Food Funct. 2019;10(5):2817–2827. doi: 10.1039/c9fo00470j. [DOI] [PubMed] [Google Scholar]
- Wu H., Zhang H., Wang C., Wu Y., Xie J., Jin X., Yang J., Ye J. Genoprotective effect of hyaluronic acid against benzalkonium chloride-induced DNA damage in human corneal epithelial cells. Mol. Vis. 2011;17:3364. [PMC free article] [PubMed] [Google Scholar]
- Xiong Q., Zhang H., Shu X., Sun X., Feng H., Xu Z., Kovács A., Yunpeng L., Zhang R. Quorum sensing signal autoinducer-2 inhibits sporulation of Bacillus by interacting with RapC and functions across species. bioRxiv. 2021;11 [Google Scholar]
- Xu D., Wei M., Peng S., Mo H., Huang L., Yao L., Hu L. Cuminaldehyde in cumin essential oils prevents the growth and Aflatoxin B1 biosynthesis of Aspergillus flavus in peanuts. Food Control. 2021;125 [Google Scholar]
- Xu J., Zhou F., Ji B.P., Pei R.S., Xu N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008;47(3):174–179. doi: 10.1111/j.1472-765X.2008.02407.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki K., Yamamoto T., Kawai Y., Inoue N. Enhancement of antilisterial activity of essential oil constituents by nisin and diglycerol fatty acid ester. Food Microbiol. 2004;21(3):283–289. [Google Scholar]
- Yammine J., Gharsallaoui A., Fadel A., Mechmechani S., Karam L., Ismail A., E Chihib N. Enhanced antimicrobial, antibiofilm and ecotoxic activities of nanoencapsulated carvacrol and thymol as compared to their free counterparts. Food Control. 2022;143 [Google Scholar]
- Yoon J.I., Bajpai V.K., Kang S.C. Synergistic effect of nisin and cone essential oil of metasequoia glyptostroboides miki ex Hu against Listeria monocytogenes in milk samples. Food Chem. Toxicol. 2011;49(1):109–114. doi: 10.1016/j.fct.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Zhang C., Cui F., Zeng G.M., Jiang M., Yang Z.Z., Yu Z.G., Zhu M.Y., Shen L.Q. Quaternary ammonium compounds (QACs): a review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015;518–519(6):352–362. doi: 10.1016/j.scitotenv.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Zhang J., Ye K.P., Zhang X., Pan D.D., Sun Y.Y., Cao J.X. Antibacterial activity and mechanism of action of black pepper essential oil on meat-borne Escherichia coli. Front. Microbiol. 2017;7(1):2094. doi: 10.3389/fmicb.2016.02094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Wang Y., An H., Hao Y., Hu X., Liao X. New insights into the formation of viable but nonculturable Escherichia coli O157:H7 induced by high-pressure CO2. mBio. 2016;7 doi: 10.1128/mBio.00961-16. e00961-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J., Zhao X. Detection of viable but non-culturable Escherichia coli O157:H7 by PCR in combination with propidium monoazide. Biotech. 2018;8(28) doi: 10.1007/s13205-017-1052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.