Abstract
Background
Nuts contain a number of nutritional attributes which may be cardioprotective. A number of epidemiological studies have shown that nut consumption may have a beneficial effect on people who have cardiovascular disease (CVD) risk factors. However, results from randomised controlled trials (RCTs) are less consistent.
Objectives
To determine the effectiveness of nut consumption for the primary prevention of CVD.
Search methods
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Web of Science Core Collection, CINAHL, Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment Database (HTA) and Health Economics Evaluations Database (HEED) up to 30 July 2015. We searched trial registers and reference lists of reviews for further studies. We did not apply any language restrictions.
Selection criteria
We included RCTs of dietary advice to increase nut consumption or provision of nuts to increase consumption lasting at least three months and including healthy adults or adults at moderate and high risk of CVD. The comparison group was no intervention or minimal intervention. The outcomes of interest were CVD clinical events and CVD risk factors.
Data collection and analysis
Two review authors independently selected trials for inclusion, abstracted the data and assessed the risk of bias in included trials.
Main results
We included five trials (435 participants randomised) and one ongoing trial. One study is awaiting classification. All trials examined the provision of nuts to increase consumption rather than dietary advice. None of the included trials reported on the primary outcomes, CVD clinical events, but trials were small and short term. All five trials reported on CVD risk factors. Four of these trials provided data in a useable format for meta‐analyses, but heterogeneity precluded meta‐analysis for most of the analyses. Overall trials were judged to be at unclear risk of bias.
There were variable and inconsistent effects of nut consumption on CVD risk factors (lipid levels and blood pressure). Three trials monitored adverse events. One trial reported an allergic reaction to nuts and three trials reported no significant weight gain with increased nut consumption. None of the included trials reported on other secondary outcomes, occurrence of type 2 diabetes as a major risk factor for CVD, health‐related quality of life and costs.
Authors' conclusions
Currently there is a lack of evidence for the effects of nut consumption on CVD clinical events in primary prevention and very limited evidence for the effects on CVD risk factors. No conclusions can be drawn and further high quality longer term and adequately powered trials are needed to answer the review question.
Plain language summary
Eating nuts to prevent cardiovascular disease
Review question
This Cochrane review aims to answer the question whether or not eating nuts can prevent cardiovascular disease.
Background
Cardiovascular diseases are a group of conditions that affect the heart and blood vessels. They are a major cause of death worldwide. The food we eat may influence the risk of getting cardiovascular disease. Nuts, if consumed regularly and at relatively high doses (50 g to 100 g), are believed to reduce total cholesterol and low‐density lipoprotein (LDL) cholesterol (bad cholesterol).
Study characteristics
This review includes randomised controlled trials, which lasted at least 12 weeks. Participants were between 37 to 54 years old on average. The evidence is current up to 30 July 2015.
Key results
We included five trials (435 participants), one of which had two treatment arms. All five trials investigated the effects of eating nuts. No studies were found which investigated the effect of giving advice to eat more nuts. None of the studies reported on deaths or cardiovascular events. None of the results show a clear effect on total cholesterol levels and blood pressure. One study reported one case of an allergic reaction to nuts. Three studies reported no significant weight gain with increased nut consumption. No other adverse events were reported.
Quality of the evidence
All included trials are small, with 60 to 100 participants, and have a high level of variation (heterogeneity). Therefore the results should be interpreted with caution. Overall we regarded the included trials as being at unclear risk of bias.
Background
Description of the condition
Cardiovascular diseases (CVD), which include coronary heart disease (CHD) and cerebrovascular disease, are a variety of conditions that affect the heart and blood vessels (WHO 2013). They are the leading cause of death worldwide, with over 17 million deaths per year attributed to CVD (WHO 2013). In 2011, CVD accounted for nearly 160,000 deaths in the UK (BHF 2014). Around 74,000 of these deaths were caused by CHD (BHF 2014). Low‐ and middle‐income countries (LMICs) are also affected by CVD. In 2001, three quarters of global deaths from CHD took place in LMICs (WHO 2013). According to Gaziano 2010, the rapid increase in CHD burden in LMICs is attributable to the acquisition of lifestyle‐related risk factors, socio‐economic changes and an increase in life span in these countries.
Dietary factors may play a vital role in the development of CVD and its risk factors, and may contribute to the geographic variability in CVD morbidity and mortality (Yusuf 2001; Scarborough 2011). These factors are important, not only because they have been linked to CVD development, but also because they can be modified. This makes them one of the main targets for interventions aimed at the primary prevention and management of CVD.
Description of the intervention
Nuts have been in the human diet for thousands of years. Indeed, records show that people ate pistachio nuts as far back as 7000 BC (King 2008). This pattern has continued today with nuts eaten globally in a variety of ways, including as ingredients in recipes and as snacks (King 2008). However, the amount of nuts consumed around the world differs. For instance, nut consumption in countries with a Mediterranean style diet is twice that of those with an American diet (Dreher 1996; Sabaté 2006).
In botanical terms, nuts are considered as a dry fruit with one seed that has a hard shell or pericarp (Sabaté 2006). Nuts are an energy dense food containing around 44% to 76% total fat (Sabaté 2010). Their saturated fatty acid content is low, being 4% to 16%, with almost half of their total fat content consisting of unsaturated fatty acids (Ros 2006). They also contain protein (around 10% to 30%) (Blomhoff 2006) and a number of micronutrients and minerals, such as folic acid, selenium, zinc and niacin (Brufau 2006). Many nuts also contain antioxidants (Vinson 2012). Almonds, for instance, contain a number of flavonoids such as catechins, flavonols and flavonones, whilst walnuts contain a variety of polyphenols and tocopherols (Blomhoff 2006). Raw walnuts and toasted almonds in particular have shown a high antioxidant efficacy (Vinson 2012). This may explain why almonds and walnuts are the main types of nuts studied in trials (based on 25 nut consumption trials included in Sabaté 2010).
How the intervention might work
A number of epidemiological studies show that nuts have a beneficial effect on CVD risk factors (Fraser 1992; Albert 2002). For example, in the Iowa Women's Health Study (IWHS) CVD mortality was lower in people who ate nuts/peanut butter five or more times per week (hazard ratio (HR) 0.67 (95% confidence interval (CI) 0.56 to 0.81; Blomhoff 2006). Evidence also comes from systematic reviews of observational studies. Mukuddem‐Petersen 2005 conducted a systematic review of observational studies looking at the effect of nuts on the lipid profile of normal and hyperlipidemic participants. They found that eating 1.5 to 3.5 servings of nuts five times or more per week along with a heart healthy diet significantly reduced low‐density lipoprotein cholesterol (LDL‐C) and total cholesterol. In particular, three studies of almond consumption showed that consuming 50 g to 100 g of almonds per day was associated with 4% to 17% lower total cholesterol and 7% to 19% lower LDL‐C in both hypercholesterolemia and non‐cholesterolemic participants. Randomised controlled trials (RCTs) also provide some evidence that nuts may be beneficial for people with CVD risk factors (Sabaté 1993; Jenkins 2002). One RCT comparing a recommended cholesterol‐lowering diet with walnuts to one without found that incorporating 84 g of walnuts on a daily basis for four weeks decreased serum levels of total cholesterol by 12% (Sabaté 1993).
The mechanisms by which nuts reduce CVD risk are not exactly known. However, nuts contain a number of nutritional attributes that have been linked to cardioprotection (Sabaté 2010). For instance, walnuts contain high levels of n‐3 fatty acids, which are known to be cardioprotective (Sabaté 2010). It is thought that the individual nutrients contained in nuts or the composite of cardioprotective nutrients that they contain, or both, may account for the beneficial effect of nuts on CVD and its risk factors (Kris‐Etherton 2008). Furthermore, the beneficial ratio of unsaturated fatty acids to saturated fatty acids may be an important factor in the health benefits associated with frequent nut intake (Sabaté 2010).
Why it is important to do this review
Despite the potential benefits from increased nut intake, there are few systematic reviews examining the effects of nut consumption on CVD prevention and, of those available, most include observational studies which are subject to bias and confounding (Mukuddem‐Petersen 2005; Afshin 2014; Zhou 2014). One systematic review of the effects of walnut consumption on lipid levels included only short term trials (average six weeks duration) so the sustained effects could not be established (Banal 2009). To our knowledge, there are currently no systematic reviews examining RCT evidence on CVD risk factors or clinical outcomes over the longer term (three months or more). To address this, this Cochrane review will examine evidence from RCTs of three months or longer duration on nut consumption for the primary prevention of CVD in the general population and in people at high risk of CVD.
Objectives
To determine the effectiveness of nut consumption for the primary prevention of CVD.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs, reported as full‐text articles, those published as abstract only, and unpublished data.
Types of participants
We included trials of adults (aged 18 years and older) from the worldwide population with and without CVD risk factors (e.g. hypertension, hyperlipidemia, overweight/obese). As this Cochrane review is interested in the primary prevention of CVD, we excluded people who experienced a myocardial infarction (MI), stroke, revascularization procedure (coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI)), and those with angina or angiographically defined CHD.
We excluded trials with > 25% of participants with diagnosed CVD or type 2 diabetes at baseline. Whilst type 2 diabetes is a major risk factor for CVD, interventions targeting specifically this patient group are covered by Cochrane reviews from the Cochrane Metabolic and Endocrine Disorders Group.
Types of interventions
We included trials comparing the provision of nuts or advice to increase nut consumption with no intervention or minimal intervention (e.g. leaflets with no face‐to‐face intervention or reinforcement). Trials were excluded in which the control group received an intervention which was not also given to the intervention group, e.g. particular foods other than nuts. We included trials of at least 12 weeks duration. Longer term studies are most informative in terms of behavioral change and sustained changes for public health interventions, with follow‐up being seen as the time elapsed since the start of the intervention.
We did not include multifactorial intervention studies, such as those including exercise or lifestyle interventions, in this review in order to avoid confounding.
Types of outcome measures
Primary outcomes
Cardiovascular mortality.
All‐cause mortality.
Non‐fatal endpoints such as MI, CABG, PCI, angina or angiographically defined CHD, stroke, carotid endarterectomy and peripheral arterial disease (PAD).
Secondary outcomes
Changes in blood pressure (systolic and diastolic) and blood lipids (total cholesterol, LDL‐C, high‐density lipoprotein (HDL) cholesterol and triglycerides).
Type 2 diabetes as a major CVD risk factor.
Health‐related quality of life (using any validated scale).
Costs.
Adverse effects (as defined by the authors of the included trials, e.g. weight gain, anaphylaxis).
Search methods for identification of studies
Electronic searches
We systematically searched the following bibliographic databases on 30 July 2015:
Cochrane Central Register of Controlled Trials (CENTRAL, Issue 6 of 12, 2015) on the Cochrane Library.
MEDLINE (Ovid, 1946 to July week 4 2015).
EMBASE Classic and EMBASE (Ovid, 1947 to 2015 July 29).
CINAHL (EBSCO, 1937 to 17 July 2015).
Web of Science Core Collection (Thomson Reuters, 1970 to 29 July 2015).
Database of Abstracts of Reviews of Effects (DARE, Issue 2 of 4, 2015), Health Technology Assessment Database (HTA, Issue 2 of 4, 2015) and Health Economics Evaluations Database (HEED, Issue 2 of 4, 2015) on the Cochrane Library.
The search strategies are presented in Appendix 1. We applied the Cochrane sensitivity‐maximizing RCT filter (Lefebvre 2011) to MEDLINE (Ovid) and adaptations of it to the other databases, except the Cochrane Library.
We did not apply any restrictions on language of publication.
Searching other resources
We searched ClinicalTrials.gov (www.ClinicalTrials.gov) and the WHO International Clinical Trials Registry Platform (WHO ICTRP) Search Portal (apps.who.int/trialsearch/) on 12 March 2015. Search terms used were 'cardiovascular and nuts'.
We contacted trial authors for additional information. We checked reference lists of included trials reports and review articles for additional references.
Data collection and analysis
Selection of studies
Two review authors (AA, RG, NM, KR) independently screened titles and abstracts for inclusion of all the potential studies identified by the searches and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publications. Two review authors (AA, RG, NM, KR) independently screened the full‐text articles and identified trials for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion. We identified and excluded duplicates and collated multiple reports of the same trial so that each trial, rather than each report, is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and Characteristics of excluded studies table.
1.
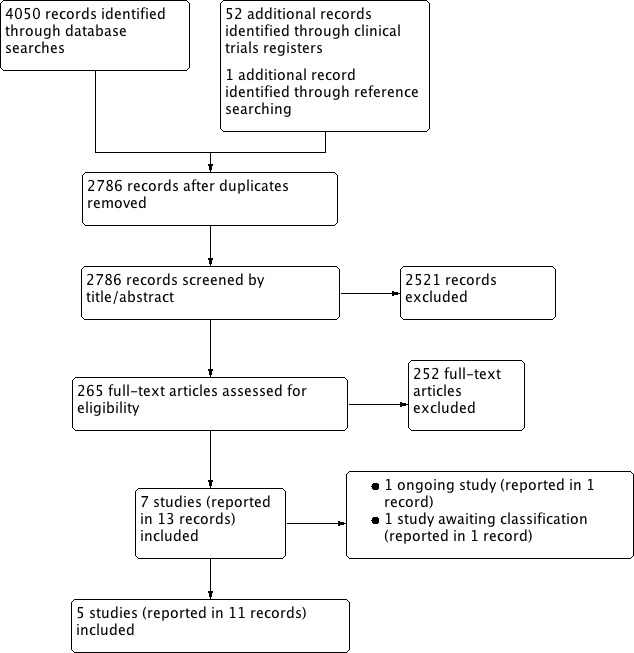
Study flow diagram.
Data extraction and management
We used a data collection form for trial characteristics and outcome data, which was piloted on at least one trial included in the review. Two review authors (AA, NM) extracted trial characteristics from included trials. We extracted the following trial characteristics:
Methods: trial design, total duration of trial, details of any 'run in' period, number of trial centres and location, trial setting, withdrawals and date of trial.
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant medications and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (AA, NM) independently extracted outcome data from included trials. We resolved disagreements by consensus or by involving a third review author (KR). One review author (NM) transferred data into the RevMan 2014 file. We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (KR) spot‐checked trial characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (AA, NM) independently assessed risk of bias for each included trial using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (KR). We assessed the risk of bias according to the following domains:
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias (e.g. industry funding).
We graded each potential source of bias as either 'high', 'low' or 'unclear' and provided a quote from the study report together with a justification for our judgment in the 'Risk of bias' section of the Characteristics of included studies table. We summarised the 'Risk of bias' judgements across different trials for each of the domains listed. Where information on risk of bias related to unpublished data or correspondence with a trial author, we noted this in the 'Risk of bias' section of the Characteristics of included studies table.
When considering treatment effects, we took into account the risk of bias for the trials that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted this Cochrane review according to the published protocol (Martin 2015) and reported any deviations from it in the Differences between protocol and review section.
Measures of treatment effect
We analysed continuous data as mean difference (MD) with 95% CIs. For continuous variables we present data for the change from baseline rather than end point data. We entered data presented as a scale with a consistent direction of effect, with the exception of HDL cholesterol where an increase in this outcome is a positive finding.
We planned to analyse dichotomous data as odds ratios or risk ratios with 95% CIs but that did not apply to any of our included analyses. Narratively describing skewed data reported as medians and interquartile ranges also did not apply to this review.
Unit of analysis issues
Studies with multiple intervention groups
Data for the control group have been used for each intervention group comparison. We reduced the weight assigned to the control group by dividing the control group N by the number of intervention groups analysed.
Cluster RCTs
We planned to analyse cluster RCTs using the unit of randomisation (cluster) as the number of observations. However, none of the included trials are cluster RCTs.
Cross‐over studies
For included cross‐over studies we only used the first period.
Dealing with missing data
We contacted trial authors or study sponsors in order to verify key trial characteristics and obtain missing numerical outcome data where possible (e.g. when a study is identified as abstract only). Where papers did not report results as change from baseline we calculated this. For the standard deviation differences we followed the methods presented in the Cochrane Handbook for Systematic Reviews of Interventions for imputing these (Section 16.1.3.2: Imputing standard deviations for changes from baseline; Higgins 2011), and assumed a correlation of 0.5 between baseline and follow‐up measures as suggested by Follman 1992.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. If no heterogeneity was present, a fixed‐effect meta‐analysis was performed. If we identified substantial heterogeneity (I² statistic > 50%) we reported it and explored possible causes by prespecified subgroup analysis if there were a sufficient number trials. If the heterogeneity could not be explained, we either provided a narrative overview or used a random‐effects model with appropriately cautious interpretation.
Assessment of reporting biases
If we had been able to pool more than 10 trials, we would have created and examined a funnel plot to explore possible small study biases for the primary outcomes. However, this did not apply to this Cochrane review.
Data synthesis
We performed statistical analysis using RevMan 2014. None of the included trials reported dichotomous data, which we would have added as events and the number of participants. We entered continuous data as means and standard deviations. We performed meta‐analysis if treatments and participants were similar enough. In the absence of substantial heterogeneity (> 50%) and provided that there are sufficient trials, we combined the results using a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses:
Type of nut.
Dosage.
Duration of the intervention.
Type of intervention (provision or advice).
Risk level of participants (presence of CVD risk factors versus no CVD risk factors).
We planned to use the formal test for subgroup interactions in RevMan 2014.
There were an insufficient number of trials included in this Cochrane review to perform these analyses.
Sensitivity analysis
We planned to carry out sensitivity analyses by only including trials at low risk of bias. However an insufficient number of trials met the inclusion criteria of the review to do this.
Results
Description of studies
Results of the search
The search of the main databases retrieved 4050 references, of which 1311 were duplicates, leaving 2739 to screen. The search of clinical trial registers retrieved 52 trial reports, of which 6 were duplicates, leaving 46 to screen. One reference was identified through reference checking. In total we screened 2786 references by title and abstract and excluded 2521. We retrieved and screened the full text of 265 references, which led to the exclusion of 252. Eleven references were eligible for inclusion and reported on five studies. We identified one ongoing study and one study is awaiting classification. A PRISMA flow chart (Figure 1) illustrates this process.
Included studies
This Cochrane review includes five trials (reported in 11 references) with a total of 435 participants (see Characteristics of included studies).
All studies were RCTs. Two studies were trials with two parallel treatment arms (Balci 2012; Abazarfard 2014). One trial had two relevant treatment arms of different amounts of hazelnuts consumed and are denoted Tey 2013 (30 g) and Tey 2013 (60 g) as they are entered separately in the analysis. One study had four treatment arms (Tey 2011) of which we were only interested in the nut intervention and control arm. One study, Sabaté 2005, was a cross‐over trial and we only used the first period, as planned and described in the Unit of analysis issues section.
Two trials were conducted in New Zealand (Tey 2011; Tey 2013 (30 g) and Tey 2013 (60 g)), one in USA (Sabaté 2005), one in Iran (Abazarfard 2014) and one does not provide the information (Balci 2012). None of the included RCTs investigated advice to increase nut consumption but instead they all provided nuts to the intervention groups. Almonds were studied in one trial (Abazarfard 2014, 50 g per day), walnuts in two trials (Balci 2012, 10 g per day; Sabaté 2005, 28 g to 56 g per day) and hazelnuts in two trials (Tey 2013 (30 g), 30 g per day; Tey 2013 (60 g), 60 g per day; Tey 2011, 42 g per day). Control groups were asked to follow their usual diet (Sabaté 2005; Tey 2011; Tey 2013 (30 g); Tey 2013 (60 g)) or to follow advice for a balanced diet (Abazarfard 2014) or healthy nutrition (Balci 2012). All participants, regardless of allocation to intervention or control group, were advised to maintain their usual activity habits (Sabaté 2005; Tey 2013 (30 g); Tey 2013 (60 g); Abazarfard 2014) or had their physical activity measured at baseline and during the intervention (Tey 2011). One trial did not provide information on physical activity (Balci 2012).
In all studies, recruitment took place via public advertisements from the general population. The number of randomised participants per trial ranged from 60 to 110 (N = 60 in Balci 2012; N = 110 in Tey 2013 (30 g) and Tey 2013 (60 g) combined; N = 108 in Abazarfard 2014; N = 94 in Sabaté 2005 and N = 63 in the two arms of interest in Tey 2011). The mean age of participants ranged from 37.4 to 54.3 years.
Studies varied in the types of participants recruited. One trial recruited only female participants (Abazarfard 2014) while the other trials included male and female participants with similar ratios (Balci 2012, 45% male; Tey 2011, 47% male; Tey 2013 (30 g) and Tey 2013 (60 g), 43% male; Sabaté 2005, 44% male). Abazarfard 2014 included pre‐menopausal women aged 20 to 55 years with a BMI ≥ 25 kg/m². Balci 2012 included participants with prediabetic metabolic syndrome. Tey 2013 (30 g) and Tey 2013 (60 g) included participants with BMI ≥ 25 kg/m², aged between 18 and 65 years. Tey 2011 also included participants aged 18 to 65 years with a BMI < 30 kg/m². Information for Sabaté 2005 differed between the two papers reporting this study regarding the inclusion of participants with BMI < 35 kg/m²/BMI ≤ 35 kg/m², aged between 30 and 72 years.
The duration of the intervention varied between the included trials. Two RCTs had a follow‐up of 12 weeks (Tey 2011; Tey 2013 (30 g) and Tey 2013 (60 g)), two RCTs of three months (Balci 2012; Abazarfard 2014) and one RCT for six months (Sabaté 2005).
We have provided details about one ongoing study, NCT01950806, in the Characteristics of ongoing studies table. This study investigates whether pecans are effective in reducing the risk of CVD or diabetes.
One study awaiting classification (Njike 2015) is a published conference abstract. We have contacted the study authors for further details and are awaiting a response (Characteristics of studies awaiting classification).
Excluded studies
We have listed 116 references (reporting 112 studies) of the 252 excluded references with reasons for exclusion in the Characteristics of excluded studies table, because they most closely missed the inclusion criteria. The studies were excluded because they were not randomised (N = 12), the intervention was not of interest (N = 9), the intervention for the control group was inappropriate (N = 23), studying the wrong type of participants (N = 12) or the follow‐up was less than 12 weeks (N = 56).
Risk of bias in included studies
We have presented details of the risk of bias in included trials as part of the Characteristics of included studies table, and a 'Risk of bias' graph and summary in Figure 2 and Figure 3, respectively.
2.
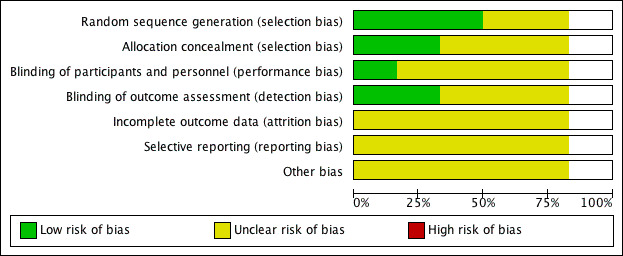
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
3.
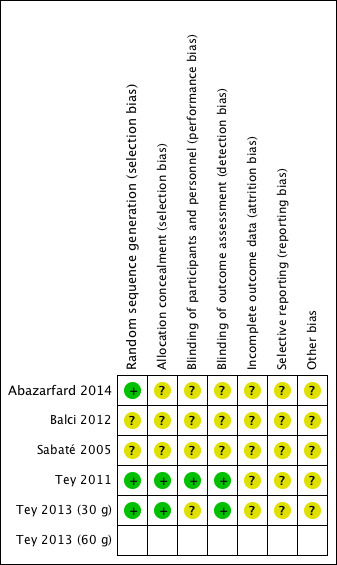
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study. One study had two intervention arms (Tey 2013 (30 g) and Tey 2013 (60 g)), and whilst the two arms were treated separately in the analysis, we assessed the risk of bias for the total trial. The risk of bias for the second arm of Tey 2013 (60 g) is the same as Tey 2013 (30 g).
Overall, the risk of selection bias was either at low risk of bias or unclear. Blinding of participants was judged to be of unclear risk of bias as participants cannot be blinded to the food they eat and it was unclear whether or not personnel were blinded. Blinding of the outcome assessors and incomplete outcome reporting were judged to be at low risk of bias in two studies and unclear risk of bias in the remaining three. Reporting bias and other sources of bias were judged to be unclear for all included studies. One trial had two intervention arms (Tey 2013 (30 g) and Tey 2013 (60 g)), and whilst the two arms were treated separately in the analysis, we assessed the risk of bias for the total trial.
Allocation
The methods of random sequence generation were unclear in two studies (Sabaté 2005; Balci 2012). Three of the five studies which stated the method of random sequence generation were judged to have a low risk of bias in this domain (Tey 2011; Tey 2013 (30 g) and Tey 2013 (60 g); Abazarfard 2014). The methods of allocation concealment were unclear in three studies (Sabaté 2005; Balci 2012; Abazarfard 2014) and judged to be of low risk in two studies (Tey 2011; Tey 2013 (30 g) and Tey 2013 (60 g)).
Blinding
Most trials were judged to be of unclear risk of bias in the domain blinding of participants and personnel as we consider it to be impossible to blind participants to the food they consume and it was unclear whether personnel were blinded or not. One trial's clinical trial registry entry stated it as double‐blinded (Abazarfard 2014) but because participants are unable to be blinded to food they eat, it was judged to be at unclear risk of bias. One trial, Tey 2011, revealed from communication with the author that the personnel were blinded and this trial was considered to be at low risk of bias for this domain.
Outcome assessors were said to have been blinded in two trials (Tey 2011; Tey 2013 (30 g) and Tey 2013 (60 g)). They have therefore been judged to be of low risk of bias for this domain.
Incomplete outcome data
One of the five included trials, Abazarfard 2014, reported losses to follow‐up with similar numbers of losses in the intervention and control arm but without an intention‐to‐treat (ITT) analysis. We judged this study to be of unclear risk of bias. Two studies could not be judged on attrition bias as no information was provided (Sabaté 2005; Balci 2012). Two trials conducted an ITT analysis but did not report on how missing data were dealt with (Tey 2011; Tey 2013 (30 g) and Tey 2013 (60 g)). We therefore judged them to be of unclear risk of bias.
Selective reporting
Selective reporting was judged to be an unclear risk of bias in all five studies (Sabaté 2005; Tey 2011; Balci 2012; Tey 2013 (30 g) and Tey 2013 (60 g); Abazarfard 2014) because of insufficient information to make this judgement.
Other potential sources of bias
For all included RCTs there was insufficient information to judge the risk of bias from other potential sources.
Effects of interventions
We found no studies investigating the effect of advice to increase nut consumption.
All five included RCTs investigated the effect of nut provision. None of the five trials reported on our primary outcomes: cardiovascular mortality, all‐cause mortality and non‐fatal endpoints such as MI, CABG, PCI, angina or angiographically defined CHD, stroke, carotid endarterectomy and PAD. Four of the five included trials provided usable data for analysis (Tey 2011; Balci 2012; Tey 2013 (30g) and Tey 2013 (60g); Abazarfard 2014). One trial did not provide any usable data and the trial author did not reply to our enquiry for the missing information (Sabaté 2005).
Changes in blood pressure (systolic and diastolic)
Three trials (267 participants) measured mean change in systolic and diastolic blood pressure (mmHg) from baseline to last follow‐up (Balci 2012; Tey 2013 (30g) and Tey 2013 (60g); Abazarfard 2014), which was three months for Abazarfard 2014 and Balci 2012 and 12 weeks for Tey 2013 (30 g) and Tey 2013 (60 g). For systolic blood pressure, heterogeneity was substantial between trials (I² statistic = 55%) and we used a random‐effects model. There was no statistically significant effect of nut consumption on systolic blood pressure (MD 3.22 mmHg, 95% CI ‐2.70 to 9.14; P = 0.29; Analysis 1.1). However, one trial was a clear outlier with an extremely large reduction in systolic blood pressure in the control arm during the trial period of 14 mmHg (Abazarfard 2014). Excluding this trial from the sensitivity analysis gave a MD of ‐0.28 mmHg (95% CI ‐5.79 to 5.23; P = 0.92).
For diastolic blood pressure, because of the substantial heterogeneity (I² statistic = 87%) the pooled effect estimate was suppressed and results from individual trials are plotted (Analysis 1.2). One trial showed a statistically significant reduction (Abazarfard 2014), the two arms of the Tey 2013 trial showed a statistically significant increase in diastolic blood pressure with the intervention (Tey 2013 (30g); Tey 2013 (60g)) and the remaining trial showed no effect of the intervention (Balci 2012).
Changes in blood lipids (total cholesterol, LDL‐C, HDL cholesterol and triglycerides)
Three trials (266 participants) measured mean change in total cholesterol (mmol/L), HDL cholesterol (mmol/L), LDL cholesterol (mmol/L) and triglycerides (mmol/L) from baseline to last follow‐up (Tey 2011; Tey 2013 (30g) and Tey 2013 (60g); Abazarfard 2014), which was three months for Abazarfard 2014 and 12 weeks for Tey 2011, Tey 2013 (30g) and Tey 2013 (60g).
For total cholesterol, there was substantial heterogeneity between the trials (I² statistic = 79%) which precluded meta‐analysis. Results for individual trials are plotted and the pooled effect estimate is suppressed (Analysis 1.3). One trial showed large and statistically significant reduction in total cholesterol with nut consumption (Abazarfard 2014), one a small and borderline significant reduction (Tey 2011), and the remaining two arms of the Tey 2013 trial showed no effect of the intervention on total cholesterol levels (Tey 2013 (30g); Tey 2013 (60g)).
For LDL cholesterol, there was low to moderate heterogeneity between the trials (I² statistic = 25%) and we used a fixed‐effect model. There was no statistically significant effect of nut consumption in lowering LDL cholesterol (MD ‐0.02, 95% CI ‐0.1 to 0.06; P = 0.57; Analysis 1.4).
For HDL cholesterol, there was no heterogeneity between the trials (I² statistic = 0%), and we used a fixed‐effect model for the analysis. There was no effect of nut consumption on HDL levels in the pooled analysis (MD ‐0.00, 95% CI ‐0.04 to 0.04; P = 0.82; Analysis 1.5).
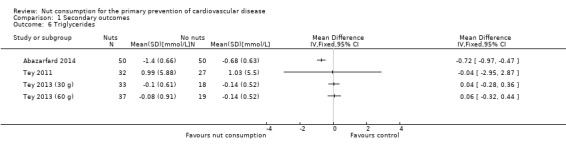
For triglycerides, there was substantial heterogeneity between trials (I² statistic = 84%) and we did not perform a meta‐analysis. We plotted individual trials and the pooled effect estimate is suppressed (Analysis 1.6). One trial showed a significant reduction in triglyceride levels with nut consumption (Abazarfard 2014), the remaining two trials showed no effect of the intervention on triglyceride levels (Tey 2011; Tey 2013 (30g); Tey 2013 (60g)).
Occurrence of type 2 diabetes as a major CVD risk factor
None of the included trials reported on type 2 diabetes as a major CVD risk factor.
Health‐related quality of life (using any validated scale)
None of the included trials reported on quality of life.
Costs
None of the included trials reported on costs.
Adverse effects (as defined by the authors of the included trials, e.g. weight gain, anaphylaxis)
One trial, Tey 2011, reported one adverse event (1/32, allergic reaction to nuts) but also found that "nuts can be incorporated into the diet without adversely affecting body weight". This was also found by two other trials. Sabaté 2005 reports a non‐significant weight gain when consuming walnuts daily (mean daily consumption 35 g) for six months. Tey 2013 (30g) and Tey 2013 (60g) noted "no significant weight change" for both nut groups over the study period.
Discussion
Summary of main results
We included five trials (435 participants randomised), one ongoing trial and one study is awaiting classification. All trials examined the provision of nuts to increase consumption rather than dietary advice. None of the included trials reported on our primary outcomes, CVD clinical events, but trials were small and short term. All five trials reported on CVD risk factors. Four of these trials provided data in a useable format for meta‐analyses, but heterogeneity precluded meta‐analysis for most of the analyses. Overall trials were judged to be at unclear risk of bias.
Heterogeneity was significant for most analyses of CVD risk factors with inconsistent effects seen for systolic blood pressure, diastolic blood pressure, total cholesterol and triglycerides. Heterogeneity was low for LDL and HDL cholesterol where there was no significant effect of nut consumption on these outcomes although the number of studies contributing to these analyses was small. Three trials monitored adverse events. One trial reported an allergic reaction to nuts and three trials no significant weight gain with increased nut consumption. None of the included trials reported our other secondary outcomes, occurrence of type 2 diabetes as a major risk factor for CVD, health‐related quality of life and costs.
Overall completeness and applicability of evidence
None of the included RCTs reported on our primary outcomes.
Five trials reported on CVD risk factors (lipid levels and blood pressure) with inconsistent findings. There were limitations in the available data as only four trials provided data in a useable format for meta‐analyses and for the remaining study we were unable to obtain additional information from the trial authors. The findings to date for these outcomes are inconclusive.
Quality of the evidence
Overall, the included RCTs were at unclear risk of bias for most of the 'Risk of bias' domains. Provison of nuts is a behavioural intervention and as such participants cannot be blinded to group allocation although trial personnel were blinded in two studies (Tey 2011; Abazarfard 2014). Outcome assessors were blind to group allocation in three trials (Tey 2011; Tey 2013 (30 g) and Tey 2013 (60 g); Abazarfard 2014).
All studies recruited small numbers of participants and small study bias is of particular concern for this review (Sterne 2000; Sterne 2001; Nüesch 2010). Due to the relatively small number of included studies we were unable to examine the effects of publication bias in funnel plots.
Potential biases in the review process
We performed a comprehensive search across major databases for interventions involving increased nut consumption for this Cochrane review. In addition, we screened the reference lists of systematic reviews and contacted study authors for information when needed. Two review authors independently performed all screening, inclusion and exclusion of articles, and extracted data from the included trials.
We only included trials with a minimum of 12 weeks duration as longer term studies are most informative in terms of behavioral change and sustained changes for public health interventions. However, this limited the number of trials eligible for inclusion and we excluded most studies because they were short term interventions.
Agreements and disagreements with other studies or reviews
In terms of our primary outcome, CVD clinical events, none of the included trials reported this outcome. Previous evidence for an association between nut consumption and clinical endpoints comes from observational studies which are subject to bias and confounding (Blomhoff 2006).
We were unable to determine the effectiveness of nut consumption on major CVD risk factors (lipid levels and blood pressure) with the trials included in the current review due to missing information, heterogeneity between trials and the limited number of trials available. There are few systematic reviews examining the effects of nut consumption on CVD prevention and most include observational studies which are subject to bias and confounding (Mukuddem‐Petersen 2005; Afshin 2014; Zhou 2014). One systematic review of the effects of walnut consumption on lipid levels included only short term trials (average six weeks) so the sustained effects could not be established (Banal 2009).
A recent systematic review, Mohammadifard 2015, examined the effects of nut consumption on blood pressure and found beneficial effects on systolic blood pressure in participants at high risk of CVD. However, it is unclear if these effects are sustained as the review included very short term trials.
The global burden of disease (GBD) study suggests that 40% of the disability‐adjusted life‐years from ischemic heart disease are attributable to diets low in nuts and seeds (Lim 2012). This systematic review shows no RCT evidence on the effect of nuts on mortality or heart disease, bringing into question the validity of the evidence on the effect of diet on heart disease. The primary source of data for nuts in the GBD study is from non‐randomised studies.
Authors' conclusions
Implications for practice.
Currently there is insufficient evidence to determine the effects of increased nut consumption for reducing CVD risk in healthy participants and in people at increased risk of CVD, and no recommendations can be made.
Implications for research.
Currently there is no evidence for the effects of nut consumption on CVD clinical events in primary prevention and very limited evidence on the effects of CVD risk factors. No conclusions can be drawn and further high quality longer term and adequately powered trials are needed to answer the review question. We will add the results of the ongoing trial to the evidence base when they become available and will incorporate the data into an update of this Cochrane review.
Acknowledgements
We are grateful to the Satellite of the Cochrane Heart Group, Chicago, USA for the support received. We also thank Marina Karanikolos, from the London School of Hygiene and Tropical Medicine, UK, for her invaluable help with one Russian paper. We thank Agnes Tey Siew Ling, the contact author of three included studies (Tey 2011; Tey 2013 (30 g); Tey 2013 (60 g)), and David Colquhoun, contact author of excluded study Colquhoun 1996, for their replies to our queries.
Appendices
Appendix 1. Search strategies
The Cochrane Library
#1 MeSH descriptor: [Nuts] this term only #2 nut or nuts #3 almond* or walnut* or peanut* or pecan* or hazelnut* or chestnut* or acorn* or pistachio* or cashew* or macadamia* or mongongo* or buckwheat* or coconut* #4 #1 or #2 or #3 #5 MeSH descriptor: [Cardiovascular Diseases] explode all trees #6 cardio* #7 cardia* #8 heart* #9 coronary* #10 angina* #11 ventric* #12 myocard* #13 pericard* #14 isch?em* #15 emboli* #16 arrhythmi* #17 thrombo* #18 atrial next fibrillat* #19 tachycardi* #20 endocardi* #21 (sick next sinus) #22 MeSH descriptor: [Stroke] explode all trees #23 (stroke or strokes) #24 cerebrovasc* #25 cerebral next vascular #26 apoplexy #27 (brain near/2 accident*) #28 ((brain* or cerebral or lacunar) near/2 infarct*) #29 MeSH descriptor: [Hypertension] explode all trees #30 hypertensi* #31 (peripheral next arter* next disease*) #32 ((high or increased or elevated) near/2 blood pressure) #33 MeSH descriptor: [Hyperlipidemias] explode all trees #34 hyperlipid* #35 hyperlip?emia* #36 hypercholesterol* #37 hypercholester?emia* #38 hyperlipoprotein?emia* #39 hypertriglycerid?emia* #40 MeSH descriptor: [Arteriosclerosis] explode all trees #41 MeSH descriptor: [Cholesterol] explode all trees #42 cholesterol #43 "coronary risk factor*" #44 MeSH descriptor: [Blood Pressure] this term only #45 "blood pressure" #46 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 #47 #4 and #46
MEDLINE
1. Nuts/ 2. (nut or nuts).tw. 3. almond*.tw. 4. walnut*.tw. 5. peanut*.tw. 6. pecan*.tw. 7. hazelnut*.tw. 8. chestnut*.tw. 9. acorn*.tw. 10. pistachio*.tw. 11. cashew*.tw. 12. macadamia*.tw. 13. mongongo*.tw. 14. buckwheat*.tw. 15. coconut*.tw. 16. (brazilnut* or (brazil adj nut*)).tw. 17. or/1‐16 18. exp Cardiovascular Diseases/ 19. cardio*.tw. 20. cardia*.tw. 21. heart*.tw. 22. coronary*.tw. 23. angina*.tw. 24. ventric*.tw. 25. myocard*.tw. 26. pericard*.tw. 27. isch?em*.tw. 28. emboli*.tw. 29. arrhythmi*.tw. 30. thrombo*.tw. 31. atrial fibrillat*.tw. 32. tachycardi*.tw. 33. endocardi*.tw. 34. (sick adj sinus).tw. 35. exp Stroke/ 36. (stroke or strokes).tw. 37. cerebrovasc*.tw. 38. cerebral vascular.tw. 39. apoplexy.tw. 40. (brain adj2 accident*).tw. 41. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 42. exp Hypertension/ 43. hypertensi*.tw. 44. peripheral arter* disease*.tw. 45. ((high or increased or elevated) adj2 blood pressure).tw. 46. exp Hyperlipidemias/ 47. hyperlipid*.tw. 48. hyperlip?emia*.tw. 49. hypercholesterol*.tw. 50. hypercholester?emia*.tw. 51. hyperlipoprotein?emia*.tw. 52. hypertriglycerid?emia*.tw. 53. exp Arteriosclerosis/ 54. exp Cholesterol/ 55. cholesterol.tw. 56. "coronary risk factor* ".tw. 57. Blood Pressure/ 58. blood pressure.tw. 59. or/18‐58 60. 17 and 59 61. randomized controlled trial.pt. 62. controlled clinical trial.pt. 63. randomized.ab. 64. placebo.ab. 65. drug therapy.fs. 66. randomly.ab. 67. trial.ab. 68. groups.ab. 69. 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 70. exp animals/ not humans.sh. 71. 69 not 70 72. 60 and 71
EMBASE
1. exp nut/ 2. (nut or nuts).tw. 3. almond*.tw. 4. walnut*.tw. 5. peanut*.tw. 6. pecan*.tw. 7. hazelnut*.tw. 8. chestnut*.tw. 9. acorn*.tw. 10. pistachio*.tw. 11. cashew*.tw. 12. macadamia*.tw. 13. mongongo*.tw. 14. buckwheat*.tw. 15. coconut*.tw. 16. or/1‐15 17. exp cardiovascular disease/ 18. cardio*.tw. 19. cardia*.tw. 20. heart*.tw. 21. coronary*.tw. 22. angina*.tw. 23. ventric*.tw. 24. myocard*.tw. 25. pericard*.tw. 26. isch?em*.tw. 27. emboli*.tw. 28. arrhythmi*.tw. 29. thrombo*.tw. 30. atrial fibrillat*.tw. 31. tachycardi*.tw. 32. endocardi*.tw. 33. (sick adj sinus).tw. 34. exp cerebrovascular disease/ 35. (stroke or strokes).tw. 36. cerebrovasc*.tw. 37. cerebral vascular.tw. 38. apoplexy.tw. 39. (brain adj2 accident*).tw. 40. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 41. exp hypertension/ 42. hypertensi*.tw. 43. peripheral arter* disease*.tw. 44. ((high or increased or elevated) adj2 blood pressure).tw. 45. exp hyperlipidemia/ 46. hyperlipid*.tw. 47. hyperlip?emia*.tw. 48. hypercholesterol*.tw. 49. hypercholester?emia*.tw. 50. hyperlipoprotein?emia*.tw. 51. hypertriglycerid?emia*.tw. 52. exp Arteriosclerosis/ 53. exp Cholesterol/ 54. cholesterol.tw. 55. "coronary risk factor*".tw. 56. Blood Pressure/ 57. blood pressure.tw. 58. or/17‐57 59. 16 and 58 60. random$.tw. 61. factorial$.tw. 62. crossover$.tw. 63. cross over$.tw. 64. cross‐over$.tw. 65. placebo$.tw. 66. (doubl$ adj blind$).tw. 67. (singl$ adj blind$).tw. 68. assign$.tw. 69. allocat$.tw. 70. volunteer$.tw. 71. crossover procedure/ 72. double blind procedure/ 73. randomized controlled trial/ 74. single blind procedure/ 75. 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 76. (animal/ or nonhuman/) not human/ 77. 75 not 76 78. 59 and 77
CINAHL
S49 S30 AND S48 S48 S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 S47 TX cross‐over* S46 TX crossover* S45 TX volunteer* S44 (MH "Crossover Design") S43 TX allocat* S42 TX control* S41 TX assign* S40 TX placebo* S39 (MH "Placebos") S38 TX random* S37 TX (doubl* N1 mask*) S36 TX (singl* N1 mask*) S35 TX (doubl* N1 blind*) S34 TX (singl* N1 blind*) S33 TX (clinic* N1 trial?) S32 PT clinical trial S31 (MH "Clinical Trials+") S30 S4 AND S29 S29 S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 S28 TI "Blood Pressure" OR AB "Blood Pressure" S27 (MH "Blood Pressure+") S26 TI "coronary risk factor*" OR AB "coronary risk factor*" S25 TI cholesterol OR AB cholesterol S24 (MH "Cholesterol+") S23 (MH "Arteriosclerosis+") S22 TI diabet* OR AB diabet* S21 (MH "Diabetes Mellitus+") S20 AB (hyperlipid* OR hyperlip?emia* OR hypercholesterol* OR hypercholester?emia* OR hyperlipoprotein?emia* OR hypertriglycerid?emia*) S19 TI (hyperlipid* OR hyperlip?emia* OR hypercholesterol* OR hypercholester?emia* OR hyperlipoprotein?emia* OR hypertriglycerid?emia*) S18 (MH "Hyperlipidemia+") S17 TI "high blood pressure" OR AB "high blood pressure" S16 AB (hypertensi* OR "peripheral arter* disease*") S15 TI (hypertensi* OR "peripheral arter* disease*") S14 (MH "Hypertension+") S13 TI (stroke OR strokes OR cerebrovasc* OR cerebral N2 vascular OR apoplexy OR brain N2 accident* OR brain N2 infarct*) S12 (MH "Stroke") S11 AB ("atrial fibrillat*" OR tachycardi* OR endocardi* OR sick N2 sinus) S10 TI ("atrial fibrillat*" OR tachycardi* OR endocardi* OR sick N2 sinus) S9 AB (pericard* OR isch?em* OR emboli* OR arrhythmi* OR thrombo*) S8 TI (pericard* OR isch?em* OR emboli* OR arrhythmi* OR thrombo*) S7 AB (cardio* OR cardia* OR heart* OR coronary* OR angina* OR ventric* OR myocard*) S6 TI (cardio* OR cardia* OR heart* OR coronary* OR angina* OR ventric* OR myocard*) S5 (MH "Cardiovascular Diseases+") S4 S1 OR S2 OR S3 S3 almond* or walnut* or peanut* or pecan* or hazelnut* or chestnut* or acorn* or pistachio* or cashew* or macadamia* or mongongo* or buckwheat* or coconut* S2 nut or nuts S1 (MH "Nuts+")
Web of Science
#10 #9 AND #8 9 TS=((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)) #8 #7 AND #3 #7 #6 OR #5 OR #4 #6 TS=(hypertensi* or peripheral arter* disease* or ((high or increased or elevated) near/2 ("blood pressure")) or hyperlipid* or hyperlip?emia* or hypercholesterol* or hypercholester?emia* or hyperlipoprotein?emia* or hypertriglycerid?emia*) #5 TS=((stroke or strokes) or cerebrovasc* or apoplexy or (brain near/2 accident*) or ((brain* or cerebral or lacunar) near/2 infarct*)) #4 TS=(cardio* or cardia* or heart* or coronary* or angina* or ventric* or myocard* or pericard* or isch?em*) #3 #2 OR #1 #2 TS=(almond* or walnut* or peanut* or pecan* or hazelnut* or chestnut* or acorn* or pistachio* or cashew* or macademia* or mongongo* or buckwheat* or coconut*) #1 TS=(nut or nuts)
Data and analyses
Comparison 1. Secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systolic blood pressure | 4 | 267 | Mean Difference (IV, Random, 95% CI) | 3.22 [‐2.70, 9.14] |
| 2 Diastolic blood pressure | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Total cholesterol | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 LDL cholesterol | 4 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| 5 HDL cholesterol | 4 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.04, 0.04] |
| 6 Triglycerides | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
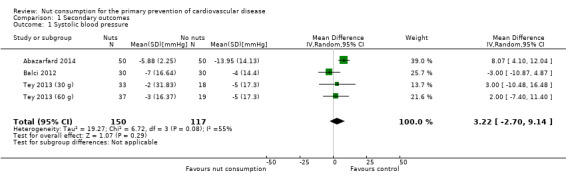
Comparison 1 Secondary outcomes, Outcome 1 Systolic blood pressure.
1.2. Analysis.
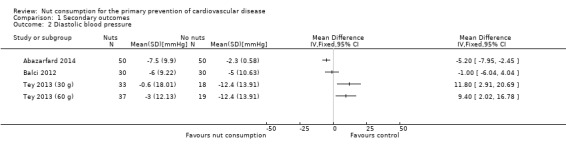
Comparison 1 Secondary outcomes, Outcome 2 Diastolic blood pressure.
1.3. Analysis.
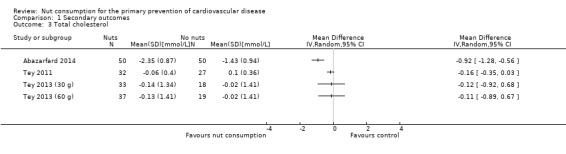
Comparison 1 Secondary outcomes, Outcome 3 Total cholesterol.
1.4. Analysis.
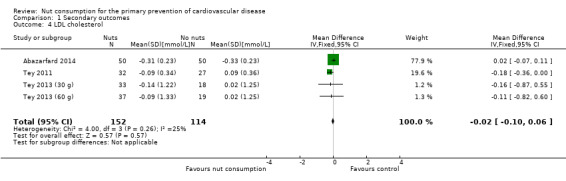
Comparison 1 Secondary outcomes, Outcome 4 LDL cholesterol.
1.5. Analysis.
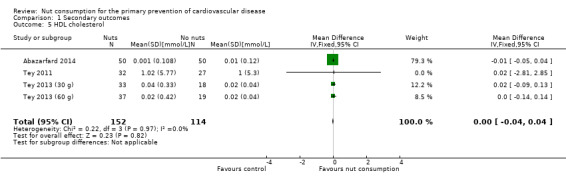
Comparison 1 Secondary outcomes, Outcome 5 HDL cholesterol.
1.6. Analysis.
Comparison 1 Secondary outcomes, Outcome 6 Triglycerides.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abazarfard 2014.
| Methods | Individual randomisation, parallel group design 3 months duration |
|
| Participants | Participants were recruited via public advertisement in Shiraz, Iran. Inclusion criteria: BMI ≥ 25 kg/m², 20 to 55 years old, no exercise, premenopausal women. Exclusion criteria: chronic illness, uncontrolled hypertension, on lipid‐lowering medication, taking vitamin supplements, inflammatory condition, diabetes, use of antihyperglycemic drugs, working night shifts, pregnant, lactating, smoking, alcohol consumption, known allergy or sensitivity to nuts, on weight control diets or any other specific diets, use of medication known to affect body weight or a weight loss of ≥ 5 kg in preceding 6 months. 100% female Mean age of intervention group: 42.36 ± 7.30 Mean age of control group: 42.94 ± 6.82 Enrolment: N = 108 Randomised to intervention group: N = 54 Randomised to control group: N = 54 Available at end of follow‐up in intervention group: N = 50 Available at end of follow‐up in control group: N = 50 |
|
| Interventions | Intervention group: follow designed balanced low calorie diet. Provision of 50 g raw almond in form of two snacks per day. Control group: follow designed balanced low calorie diet. No consumption of nuts. Same for both groups: instructed to maintain their usual activity habits with recommendation to walk with medium speed 30 minutes every day. Training about diet, healthy nutrition and self‐monitoring and stimulus control. Phone call every 15 days to ask about situations and diet compliance. Provision of suggestions to enhance compliance. Also 24 hr dietary recalls at baseline, end of study and at the end of each month. |
|
| Outcomes | Systolic blood pressure, diastolic blood pressure, total cholesterol, total cholesterol/HDL cholesterol ratio, HDL cholesterol, LDL cholesterol, triglycerides at baseline and 3 months follow‐up. | |
| Notes | Contacted trial author for data on primary outcomes cardiovascular mortality, all‐cause mortality, non‐fatal endpoints and secondary outcomes (type 2 diabetes, quality of life, costs and adverse events) as well as selection bias and detection bias. We did not receive any reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned into almond and nut‐free groups through the balanced block randomization method (block size of four)." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from IRCT entry: double blinded. However, participants cannot be blinded to food they eat and it is unclear whether this refers to personnel and outcome assessors. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | An equal number of participants (n = 4) were lost to follow‐up in each group but no ITT analysis was performed. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available, paper is an extract from a thesis. |
| Other bias | Unclear risk | No indications of further biases but information too limited to make judgement. |
Balci 2012.
| Methods | Individual randomisation, parallel group design. 3 months duration. |
|
| Participants | All participants had prediabetic metabolic syndrome diagnosed according to NCEP ATP III criteria. 45% male, 55% female Mean age: unknown Enrolment: N = 60 Randomised to intervention group: N = 30 Randomised to control group: N = 30 Available at end of follow‐up in intervention group: N = 30 Available at end of follow‐up in control group: N = 30 |
|
| Interventions | Intervention group: healthy nutrition with provision of 10 g walnuts per day Control group: healthy nutrition only |
|
| Outcomes | Systolic blood pressure, diastolic blood pressure, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides at baseline and 3 months follow‐up. | |
| Notes | Published conference abstract only. Could not identify trial author's email address to ask for missing data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "participants are randomly separated to two groups". Random sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants could not be blinded to food they eat and it is unclear whether or not personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | Not described. |
| Other bias | Unclear risk | No indications of further biases but information too limited to make judgement. |
Sabaté 2005.
| Methods | Individual randomisation, cross‐over design. 6 months duration. |
|
| Participants | Recruitment via advertisements in South‐East California, USA. Inclusion criteria: BMI > 25 kg/m² (=overweight), weight change < 1 kg during previous six months, BMI < 35 kg/m², an habitual diet including nuts less than once a week. Exclusion criteria: diagnosed metabolic disorder that can affect weight, i.e. diabetes, hypothyroidism, or aversion or known allergy to nuts 44.44% male, 55.56% female Mean age (range): 54.3 ± 10.6 (30 to 72 years) Enrolment: N = 94 Loss to follow‐up: n = 4 (not stated from which group or at which stage of trial) Available at end of follow‐up in intervention group: N = 49 Available at end of follow‐up in control group: N = 41 |
|
| Interventions | Intervention group: provision of walnuts that corresponded to approximately 12% of their daily energy intake. Daily energy intake of 1800 to 2300 kcal = 28 g walnuts; 2300 to 2800 kcal = 46 g walnuts; > 2800 kcal = 56 g walnuts. Walnut allotment was adjusted based on the daily energy intake reported in the 24 hr dietary recalls. Walnuts were distributed in portioned packs at each clinic visit every 2 months. They also got a large pack of walnuts for family members. They were requested to return any unconsumed portions. Control group: participants were asked to refrain from eating walnuts and substantial amounts of any other nuts. Same for both groups: participants were asked to follow their usual diet. Instructions to the change physical activity habits and not to attempt to lose weight while in the study. Seven 24h dietary recalls during 6 months intervention, unannounced and following a protocol, by phone. |
|
| Outcomes | Total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides. | |
| Notes | We contacted the trial authors of Sabaté 2005 and Torabian 2010 for further information. We did not receive any reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants cannot be blinded to food they eat and it is unclear whether personnel was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Unclear risk | Not described. |
| Other bias | Unclear risk | No indications of further biases but information too limited to make judgement. |
Tey 2011.
| Methods | Individual randomisation, parallel design with four arms (chocolate, crisps, nuts, control). 12 weeks duration. |
|
| Participants | Recruitment in New Zealand. Advertisement with flyers around University of Otago campus, supermarkets and university staff bulletin and papers. Interested people phoned/emailed investigator. Inclusion criteria: age between 18 and 65 years. Exclusion criteria: BMI ≥ 30 kg/m², asthma, pregnant or breastfeeding, chronic disease such as cancer, heart disease or diabetes, people with food allergies or food aversions. 47% male, 53% female Mean age (range): 37.4 ± 14.0 (18 to 65) Enrolment to nuts and control group: N = 63 Randomised to nut group: N = 32 Randomised to control group: N = 31 Available at end of follow‐up in nut group: N = 27 Available at end of follow‐up in control group: N = 27 |
|
| Interventions | Intervention group(s): 42 g hazelnuts, 50 g chocolate or 50 g potato crisps. Snacks were individually portioned into daily serving sized bags and participants were asked to collect them every 3 weeks. Participants were asked to return any snacks that were not eaten. Control group: no additional food. Same for all groups: all participants were asked to maintain their regular diet during a 2‐week run‐in period, then randomisation. Physical activity measured at baseline and during the intervention by wearing accelerometers for 7 days. Three‐day diet records were collected. |
|
| Outcomes | Total cholesterol, total cholesterol/HDL cholesterol ratio, HDL cholesterol, LDL cholesterol, triglycerides, adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by minimisation, block size of four, 8 strata. |
| Allocation concealment (selection bias) | Low risk | "Allocation within each strata was conducted by an off‐site statistician using blocks of size four". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants cannot be blinded to food they eat. Personnel blinded (from communication with author). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers assessing outcomes were blinded to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis conducted but unclear how missing data were dealt with. |
| Selective reporting (reporting bias) | Unclear risk | Not described. |
| Other bias | Unclear risk | No indications of further biases but information too limited to make judgement. |
Tey 2013 (30 g).
| Methods | Individual randomisation, 3‐arm parallel group design ‐ this comparison 30 g versus control. 12 weeks duration. |
|
| Participants | Recruitment from general public in Dunedin, New Zealand. Inclusion criteria: aged between 18 and 65 years inclusive, with BMI ≥ 30 kg/m². Exclusion criteria: asthma, allergies or aversion to nuts, familial hyperlipidemia, major chronic disease or inflammatory disease such as Crohn or celiac disease, current smokers, pregnant or breastfeeding women, people who were participating in weight‐loss programmes or taking medication known to affect inflammatory markers. Mean age: 42.5 ± 12.4 43% male, 57% female Enrolment: N = 110 Randomised to nut group (30 g): N = 35 Randomised to control group: N = 38 Available at end of follow‐up in nut group (30 g): N = 33 (excluded: n = 1 BMI < 25 kg/m², n = 1 pregnant) Available at end of follow‐up in control group: N = 37 (excluded: n = 1 BMI < 25 kg/m²), for analysis: N = 18 as per methods planned in protocol. |
|
| Interventions | Intervention group: hazelnuts were individually portioned into daily‐serving sized bags (30 g). Participants were asked to collect their nuts every three weeks and to return any nuts not eaten. Compliance was assessed by weighing the bags returned, by nut diary and by three‐day diet records. Control group: received no additional food. Same for both groups: 2‐week run in period with no nuts. Participants were encouraged to maintain their usual pattern of dietary habits and physical activity level and to maintain the same dosage of medications and supplements (if taken at baseline) throughout the study. All were asked to attend 6 clinic visits after a 12‐hr overnight fast, twice each at baseline, after 6 weeks and at the end of the 12‐week intervention. Physical activity was measured with accelerometers at baseline and at week 7. Participants were asked to wear the accelerometer for 7 days. |
|
| Outcomes | Systolic blood pressure, diastolic blood pressure, total cholesterol, total cholesterol/HDL cholesterol ratio, HDL cholesterol, LDL cholesterol, triglycerides | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation to balance groups by age, sex and BMI. |
| Allocation concealment (selection bias) | Low risk | "An off‐site statistician conducted group allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants cannot be blinded to food they eat and it is unclear whether personnel was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Those researchers who assessed the outcomes were blinded to the treatments." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "All analyses were performed by using a modified intention‐to‐treat principle." Unclear how missing data were dealt with. |
| Selective reporting (reporting bias) | Unclear risk | Not described. |
| Other bias | Unclear risk | No industry funding. |
Tey 2013 (60 g).
| Methods | Individual randomisation, 3‐arm parallel group design ‐ this comparison 60 g versus control. 12 weeks duration. |
|
| Participants | Recruitment from general public in Dunedin, New Zealand. Inclusion criteria: aged between 18 and 65 years inclusive, with BMI ≥ 30 kg/m². Exclusion criteria: asthma, allergies or aversion to nuts, familial hyperlipidemia, major chronic disease, or inflammatory disease such as Crohn or coeliac disease, current smokers, pregnant or breastfeeding women, people who were participating in weight‐loss programmes or taking medication known to affect inflammatory markers. Mean age: 42.5 ± 12.4 43% male, 57% female Enrolement: N = 110 Randomised to nut group (60 g): N = 37 Randomised to control group: N = 38 Available at end of follow‐up in nut group (60 g): N = 37 Available at end of follow‐up in control group: n = 37 (excluded: n = 1 BMI < 25 kg/m²), for analysis: N = 19 as per methods planned in protocol. |
|
| Interventions | Intervention group: hazelnuts were individually portioned into daily‐serving sized bags (60 g). Participants were asked to collect their nuts every 3 weeks and to return any nuts not eaten. Compliance was assessed by weighing the bags returned, by nut diary and by 3‐day diet records. Control group: received no additional food. Same for both groups: 2‐week run in period with no nuts. Participants were encouraged to maintain their usual pattern of dietary habits and physical activity level, and to maintain the same dosage of medications and supplements (if taken at baseline) throughout the study. All were asked to attend 6 clinic visits after a 12‐hr overnight fast, twice each at baseline, after 6 weeks and at the end of the 12‐week intervention. Physical activity was measured with accelerometers at baseline and at week 7. Participants were asked to wear the accelerometer for 7 days. |
|
| Outcomes | Systolic blood pressure, diastolic blood pressure, total cholesterol, total cholesterol/HDL cholesterol ratio, HDL cholesterol, LDL cholesterol, triglycerides. | |
| Notes | ||
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acharjee 2008 | Follow‐up was too short. |
| Acharya 2004 | Intervention was not of interest. |
| ACTRN12615000176561 | Inappropriate control. |
| Alper 2003 | Not randomised. |
| Anderson 2013 | Follow‐up period was too short. |
| Baer 2012 | Follow‐up period was too short. |
| Bakhtiary 2011 | Intervention was not of interest. |
| Bao 2013 | Not randomised. |
| Barbour 2014 | Not randomised. |
| Berryman 2010 | Inappropriate control. |
| Berryman 2011 | Inappropriate control. |
| Berryman 2013 | Inappropriate control. |
| Blumberg 2011 | Follow‐up period was too short. |
| Bunner 2014 | Intervention was not of interest. |
| Burns‐Whitmore 2014 | Follow‐up period was too short. |
| Canales 2011 | Follow‐up period was too short. |
| Casas 2011 | Intervention was not of interest. |
| Casas‐Agustench 2011 | Inappropriate control. |
| Chair 2003 | Intervention was not of interest. |
| Chen 2014 | Inappropriate control. |
| Cheng 2004 | Intervention was not of interest. |
| Chisholm 1998 | Follow‐up was too short. |
| Chisholm 2010 | Follow‐up was too short. |
| Choudhury 2014 | Not randomised. |
| Cohen 2011 | Inappropriate control. |
| Colquhoun 1996 | Follow‐up was too short. |
| Cominetti 2012 | Follow‐up was too short. |
| Coppell 2010 | Inappropriate participants ‐ all diabetic. |
| Costa 2011 | Follow‐up was too short. |
| Curb 2000 | Follow‐up was too short. |
| Davis 2007 | Follow‐up was too short. |
| Din 2011 | Follow‐up was too short. |
| Edwards 1999 | Follow‐up was too short. |
| Esfahani 2010 | Intervention was not of interest. |
| Foster 2012 | Intervention was not of interest. |
| Garg 2007 | Not randomised. |
| Gebauer 2008 | Follow‐up was too short. |
| Gebauer 2011 | Follow‐up was too short. |
| Ghadimi Nouran 2010 | Follow‐up was too short. |
| Gillen 2005 | Inappropriate control. |
| Griel 2008 | Follow‐up was too short. |
| Gulati 2011 | Inappropriate control. |
| Gulati 2014 | Inappropriate control. |
| Hargrove 2001 | Follow‐up was too short. |
| Hernández‐Alonso 2014 | Inappropriate control. |
| Holligan 2011 | Inappropriate control. |
| Holligan 2013 | Follow‐up was too short. |
| Holligan 2014 | Follow‐up was too short. |
| Hollis 2007 | Follow‐up was too short. |
| Hudthagosol 2011 | Follow‐up was too short. |
| Iwamoto 2002 | Follow‐up was too short. |
| Jaceldo‐Siegl 2011 | Not randomised. |
| Jambazian 2005 | Follow‐up was too short. |
| Jenkins 2008 | Follow‐up was too short. |
| Jenkins 2011 | Inappropriate control. |
| Jones 2014 | Inappropriate control. |
| Jonsson 2013 | Intervention was not of interest. |
| Katz 2012 | Follow‐up was too short. |
| Kay 2010 | Follow‐up was too short. |
| Kendall 2002 | Follow‐up was too short. |
| Kendall 2003 | Follow‐up was too short. |
| Kendall 2009 | Inappropriate control. |
| Kendall 2013 | Inappropriate control. |
| Kochar 2010 | Not randomised. |
| Kocyigit 2006 | Follow‐up was too short. |
| Li 2009 | Inappropriate control. |
| Li 2010 | Inappropriate control. |
| Li 2011 | Follow‐up was too short. |
| López‐Uriarte 2010 | Inappropriate control. |
| Maranhão 2011 | Inappropriate participants ‐ adolescents. |
| McKeown 2010 | Not randomised. |
| Mercanligil 2007 | Follow‐up was too short. |
| Mohammadifard 2012 | Not randomised. |
| Morgan 2000 | Follow‐up was too short. |
| Morgan 2002 | Follow‐up was too short. |
| Mukuddem‐Petersen 2007 | Follow‐up was too short. |
| Munoz 2001 | Follow‐up was too short. |
| Nishi 2014 | Inappropriate participants ‐ all diabetics. |
| Núñez 2004 | Follow‐up was too short. |
| O'Byrne 1997 | Not randomised. |
| Oliveira 2010 | Inappropriate participants ‐ adolescents. |
| Orem 2013 | Not randomised. |
| Petersen 2013 | Inappropriate participants ‐ all diabetics. |
| Pieters 2005 | Follow‐up was too short. |
| Rajaram 2001 | Follow‐up was too short. |
| Rajaram 2010 | Follow‐up was too short. |
| Reinsma 2010 | Inappropriate participants ‐ all diabetics. |
| Ricklefs 2013 | Inappropriate participants ‐ all diabetics. |
| Rismankarzadeh 2005 | Follow‐up was too short. |
| Ros 2004 | Follow‐up was too short. |
| Sabate 1993 | Follow‐up was too short. |
| Sabaté 2003 | Follow‐up was too short. |
| Sari 2010 | Not randomised. |
| Schutte 2006 | Follow‐up was too short. |
| Sheridan 2007 | Follow‐up was too short. |
| Somerset 2011 | Follow‐up was too short. |
| Somerset 2013 | Follow‐up was too short. |
| Storniolo 2013 | Inappropriate control. |
| Sweazea 2014 | Inappropriate participants ‐ all diabetics. |
| Tan 2013 | Follow‐up was too short. |
| Tapsell 2004 | Inappropriate participants ‐ all diabetics. |
| Tapsell 2009 | Inappropriate participants ‐ all diabetics. |
| Tapsell 2010 | Inappropriate participants ‐ all diabetics. |
| Wang 2012 | Inappropriate control. |
| West 2012 | Follow‐up was too short. |
| Wien 2010 | Inappropriate control. |
| Wien 2014 | Inappropriate participants ‐ all diabetics. |
| Wu 2010 | Inappropriate control. |
| Wu 2014 | Follow‐up was too short. |
| Zambón 1998 | Follow‐up was too short. |
| Zambón 2000 | Follow‐up was too short. |
| Zibaeenezhad 2005 | follow‐up too short |
Characteristics of studies awaiting assessment [ordered by study ID]
Njike 2015.
| Methods | RCT, parallel design, 6 months duration. |
| Participants | 112 participants. |
| Interventions | Participants randomised to ad libitum diet or calorie‐controlled diet, then randomised again in each of these two groups to walnut‐included and walnut‐excluded diets. |
| Outcomes | Diet quality, body mass index, percent body fat, percent body water, visceral fat, glucose, HbA1c, endothelial function, total cholesterol and LDL cholesterol. |
| Notes | We contacted the trial author for more information and are awaiting a response. |
Characteristics of ongoing studies [ordered by study ID]
NCT01950806.
| Trial name or title | The effect of pecans on biomarkers of risk for cardiovascular disease and diabetes. |
| Methods | Randomised crossover double blind trial. |
| Participants | Adults at risk of CVD/type 2 diabetes; enrolment: 25. |
| Interventions | Pecan‐containing diet versus nut‐free diet. |
| Outcomes | Change in biomarkers of oxidative stress, inflammation, endothelial function, antioxidant activity, insulin resistance, blood pressure, plasma lipid profile compared with control diet. |
| Starting date | 23 September 2013. |
| Contact information | Diane L. McKay (diane.mckay@tufts.edu), Jeffrey B. Blumberg (jeffrey.blumberg@tufts.edu). |
| Notes | Expected completion date: November 2015. |
Differences between protocol and review
Due to limited resources, we did not search Google Scholar or conduct citation searching as planned in the protocol (Martin 2015).
Contributions of authors
Nicole Martin searched for and screened articles, performed data abstraction and analyses, drafted the review and approved the final review version.
Roberta Germano screened articles, drafted the review and approved the final review version.
Louisa Hartley drafted the review and approved the final review version.
Alma Adler screened articles, performed data abstraction, drafted the review and approved the final manuscript version.
Karen Rees screened articles, checked the data and analyses, drafted the review and approved the final review version.
Sources of support
Internal sources
Warwick Medical School, University of Warwick, UK.
Department of Non‐communicable Disease Epidemiology, London School of Hygiene & Tropical Medicine, UK.
External sources
NIHR Cochrane Programme Grant, UK.
Karen Rees is also supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care West Midlands at University Hospitals Birmingham NHS Foundation Trust, UK.
Declarations of interest
Nicole Martin: none known.
Roberta Germano: none known.
Louisa Hartley: none known.
Alma Adler: none known.
Karen Rees: none known.
New
References
References to studies included in this review
Abazarfard 2014 {published data only}
- Abazarfard Z, Salehi M, Keshavarzi S. The effect of almonds on anthropometric measurements and lipid profile in overweight and obese females in a weight reduction program: A randomized controlled trial. Journal of Research in Medical Sciences 2014;19(5):457‐64. [PMC free article] [PubMed] [Google Scholar]
- IRCT2013062313751N1. The effect of low calorie balance diet with and without almond on weight reduction and Lipid profile in overwieght persons (BMI>25). http://www.irct.ir/searchresult.php?keyword=&id=13751&number=1&prt=5082&total=10&m=1 (accessed 15 December 2014).
Balci 2012 {published data only}
- Balci MK, Balci B, Hoda P. MON‐243: Metabolic effects of walnuts in patients with prediabetic metabolic syndrome. The Endocrine Society's 94th Annual Meeting and Expo; 2012 June 23‐26 June; Houston, TX. Endocrine Society. Washington, DC: Endocrine Society, 2012; Vol. 33 (3, Meeting Abstracts).
Sabaté 2005 {published data only}
- Sabaté J, Cordero‐MacIntyre Z, Siapco G, Torabian S, Haddad E. Does regular walnut consumption lead to weight gain?. British Journal of Nutrition 2005;94(5):859‐64. [DOI] [PubMed] [Google Scholar]
- Simon JA, Tanzman JS, Sabaté J. Lack of effect of walnuts on serum levels of prostate specific antigen: a brief report. Journal of the American College of Nutrition 2007;26(4):317‐20. [DOI] [PubMed] [Google Scholar]
- Torabian S, Haddad E, Cordero‐MacIntyre Z, Tanzman J, Fernandez ML, Sabate J. Long‐term walnut supplementation without dietary advice induces favorable serum lipid changes in free‐living individuals. European Journal of Clinical Nutrition 2010;64(3):274‐9. [DOI] [PubMed] [Google Scholar]
Tey 2011 {published data only}
- Tey S, Brown R, Gray A, Chisholm A, Delahunty C. Nuts assist with weight maintenance while improving diet quality. Annals of Nutrition and Metabolism 2011;58(Suppl 3):12. [Google Scholar]
- Tey SL, Brown R, Gray A, Chisholm A, Delahunty C. Nuts improve diet quality compared to other energy‐dense snacks while maintaining body weight. Journal of Nutrition and Metabolism 2011;2011:357350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tey SL, Brown RC, Gray AR, Chisholm AW, Delahunty CM. Long‐term consumption of high energy‐dense snack foods on sensory‐specific satiety and intake. American Journal of Clinial Nutrition 2012;95(5):1038‐47. [DOI] [PubMed] [Google Scholar]
Tey 2013 (30 g) {published data only}
- Tey ASL, Brown R, Gray A, Chisholm A, Delahunty C. Dose‐response effect of hazelnut consumption on body composition and inflammatory markers in overweight and obese individuals. FASEB Journal 2012;26(Meeting Abstract Supplement):1033.16. [Google Scholar]
- Tey SL, Gray AR, Chisholm AW, Delahunty CM, Brown RC. The dose of hazelnuts influences acceptance and diet quality but not inflammatory markers and body composition in overweight and obese individuals. Journal of Nutrition 2013;143(8):1254‐62. [DOI] [PubMed] [Google Scholar]
Tey 2013 (60 g) {published data only}
- Tey ASL, Brown R, Gray A, Chisholm A, Delahunty C. Dose‐response effect of hazelnut consumption on body composition and inflammatory markers in overweight and obese individuals. FASEB Journal 2012;26(Meeting Abstract Supplement):1033.16. [Google Scholar]
- Tey SL, Gray AR, Chisholm AW, Delahunty CM, Brown RC. The dose of hazelnuts influences acceptance and diet quality but not inflammatory markers and body composition in overweight and obese individuals. Journal of Nutrition 2013;143(8):1254‐62. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Acharjee 2008 {published data only}
- Acharjee S, Zhou JR, Welty F. Soy Nuts are More Effective in Lowering Blood Pressure, Triglycerides and Inflammation in Postmenopausal Women Who are Equol Producers, Regardless of Metabolic Syndrome Status. Circulation 2008;118:S1122, Abstract no: 3274. [Google Scholar]
Acharya 2004 {published data only}
- Acharya S, Maskarinec G, Williams AE, Oshiro C, Hebshi S, Murphy SP. Nutritional changes among premenopausal women undertaking a soya based dietary intervention study in Hawaii. Journal of Human Nutrition and Dietetics 2004;17(5):413‐9; quiz 421‐4. [DOI] [PubMed] [Google Scholar]
ACTRN12615000176561 {unpublished data only}
- ACTRN12615000176561. The effect of a 12 week intervention changing the composition bread, so its composition is either lower in sodium or contains nuts or beetroot, on markers of cardiovascular disease in comparison to a control bread amongst those with at least one characteristic of the metabolic syndrome. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12615000176561 (accessed 24 March 2015).
Alper 2003 {published data only}
- Alper CM, Mattes RD. Peanut consumption improves indices of cardiovascular disease risk in healthy adults. Journal of the American College of Nutrition 2003;22(2):133‐41. [DOI] [PubMed] [Google Scholar]
Anderson 2013 {published data only}
- Anderson AD, Anderson MM, Jacobson JL, Popko MR, Young JR, Limburg PJ, et al. Metabolic effects of bedtime pistachio consumption for 6 weeks in overweight persons. FASEB Journal 2013;27:1072.20. [Google Scholar]
Baer 2012 {published data only}
- Baer DJ, Gebauer SK, Novotny JA. Measured energy value of pistachios in the human diet. British Journal of Nutrition 2012;107(1):120‐5. [DOI] [PubMed] [Google Scholar]
Bakhtiary 2011 {published data only}
- Bakhtiary A, Yassin Z, Hanachi P, Rahmat A, Ahmad Z, Halalkhor S, et al. Evaluation of the oxidative stress and glycemic control status in response to soy in older women with the metabolic syndrome. Iranian Red Crescent Medical Journal 2011;13(11):795‐804. [Google Scholar]
Bao 2013 {published data only}
- Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, et al. Association of nut consumption with total and cause‐specific mortality. New England Journal of Medicine 2013;369(21):2001‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbour 2014 {published data only}
- Barbour JA, Howe PRC, Buckley JD, Bryan J, Coates AM. Nut consumption for vascular health and cognitive function. Nutrition Research Reviews 2014;27(1):131‐58. [DOI] [PubMed] [Google Scholar]
Berryman 2010 {published data only}
- Berryman CE, Bordi PL, West SG, Fleming JA, Kris‐Etherton PM. Effects of a diet rich in almonds on low‐density lipoprotein cholesterol (LDL‐C), LDL‐C particle size, abdominal adiposity and vascular health. FASEB Journal 2010;24(Meeting Abstract Supplement):721.4. [Google Scholar]
Berryman 2011 {published data only}
- Berryman CE, Bordi PL, Fleming JA, West SG, Hill AM, Kris‐Etherton PM. Does the addition of almonds to a Step I diet provide additional LDL‐C lowering?. FASEB Journal 2011;25(Meeting Abstract Supplement):971.26. [Google Scholar]
Berryman 2013 {published data only}
- Berryman CE, West SG, Chen CYO, Blumberg JB, Fleming JA, Preston AG, et al. Effects of polyphenolic‐rich dark chocolate/cocoa and almonds on established and emerging cardiovascular risk factors: Study design. FASEB Journal 2013;27:1078.13. [Google Scholar]
Blumberg 2011 {published data only}
- Blumberg J, Chen C, Vita J. Effect of almonds on vascular reactivity and inflammation in patients with coronary artery disease. Annals of Nutrition and Metabolism 2011;58:290. [Google Scholar]
Bunner 2014 {published data only}
- Bunner AE, Gonzalez J, Agarwal U, Valente F, Barnard ND. Nutrition intervention for diabetic neuropathy. Diabetes 2014;63:A578. [Google Scholar]
Burns‐Whitmore 2014 {published data only}
- Burns‐Whitmore B, Hall L, Bushnell A, Towne A, Roy S. Effects of pistachio consumption on body composition and blood lipids in healthy young women. FASEB Journal 2014;1:640.6. [Google Scholar]
Canales 2011 {published data only}
- Canales A, Sánchez‐Muniz FJ, Bastida S, Librelotto J, Nus M, Corella D, et al. Effect of walnut‐enriched meat on the relationship between VCAM, ICAM, and LTB4 levels and PON‐1 activity in ApoA4 360 and PON‐1 allele carriers at increased cardiovascular risk. European Journal of Clinical Nutrition 2011;65(6):703‐10. [DOI] [PubMed] [Google Scholar]
Casas 2011 {published data only}
- Casas R, Sacanella E, Romero E, Vinas C, Lamuela‐Raventos R, Ros E, et al. Inhibition of activation NF‐B Related to atherogenesis after one year of intervention with a Mediterranean diet. Annals of Nutrition and Metabolism 2011;58:318. [Google Scholar]
Casas‐Agustench 2011 {published data only}
- Casas‐Agustench P, López‐Uriarte P, Bulló M, Ros E, Cabré‐Vila JJ, Salas‐Salvadó J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutrition, Metabolism & Cardiovascular Diseases 2011;21(2):126‐35. [DOI] [PubMed] [Google Scholar]
Chair 2003 {published data only}
- Chair SY. An Indo Mediterranean diet was more effective than a conventional prudent diet in reducing coronary artery disease risk factors and events. Evidence Based Nursing 2003;6(3):79. [DOI] [PubMed] [Google Scholar]
Chen 2014 {published data only}
- Chen CM, Li HT, Liao YY, Liu JF, Chen CY. Effect of almonds on cardiovascular risk factors in Chinese patients with type 2 diabetes mellitus: study design. FASEB Journal 2014;28(1):1035.1. [Google Scholar]
Cheng 2004 {published data only}
- Cheng C, Graziani C, Diamond JJ. Cholesterol‐lowering effect of the Food for Heart Nutrition Education Program. Journal of the American Dietetic Association 2004;104(12):1868‐72. [DOI] [PubMed] [Google Scholar]
Chisholm 1998 {published data only}
- Chisholm A, Mann J, Skeaff M, Frampton C, Sutherland W, Duncan A, et al. A diet rich in walnuts favourably influences plasma fatty acid profile in moderately hyperlipidaemic subjects. European Journal of Clinical Nutrition 1998;52(1):12‐6. [DOI] [PubMed] [Google Scholar]
Chisholm 2010 {published data only}
- Chisholm A, Tey S, Brown R, Gray A, Delahunty C. Effects on CVD risk factors of adding ground, sliced and whole hazelnuts to customary diets of hypercholesterolaemic men and women. Atherosclerosis Supplements 2010;11(2):122. [Google Scholar]
Choudhury 2014 {published data only}
- Choudhury K, Clark J, Griffiths HR. An almond‐enriched diet increases plasma α‐tocopherol and improves vascular function but does not affect oxidative stress markers or lipid levels. Free Radical Research 2014;48(5):599‐606. [DOI] [PubMed] [Google Scholar]
Cohen 2011 {published data only}
- Cohen AE, Johnston CS. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A(1c) in individuals with well‐controlled type 2 diabetes mellitus. Metabolism: Clinical and Experimental 2011;60(9):1312‐7. [DOI] [PubMed] [Google Scholar]
Colquhoun 1996 {published data only}
- Colquhoun DM, Humphries JA, Moores D, Somerset SM. Effects of a macadamia nut enriched diet on serum lipids and lipoproteins compared to a low fat diet. Food Australia 1996;48(5):216‐21. [Google Scholar]
Cominetti 2012 {published data only}
- Cominetti C, Bortoli MC, Garrido AB Jr, Cozzolino SMF. Brazilian nut consumption improves selenium status and glutathione peroxidase activity and reduces atherogenic risk in obese women. Nutrition Research 2012;32(6):403‐7. [DOI] [PubMed] [Google Scholar]
Coppell 2010 {published data only}
- Coppell KJ, Kataoka M, Williams SM, Chisholm AW, Vorgers SM, Mann JI. Nutritional intervention in patients with type 2 diabetes who are hyperglycaemic despite optimised drug treatment ‐ Lifestyle Over and Above Drugs in Diabetes (LOADD) study: randomised controlled trial. BMJ 2010;341:c3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Costa 2011 {published data only}
- Costa NMB, Goncalves Oliveira Fialho C, Bressan J, Goncalves Alfenas RC, Mattes RD. The effects of whole or skinned peanut intake on body composition, lipid profile and fibrinogen in obese women on a low‐energy dietary intervention. FASEB Journal 2011;25:980.4. [Google Scholar]
Curb 2000 {published data only}
- Curb JD, Wergowske G, Dobbs JC, Abbott RD, Huang B. Serum lipid effects of a high‐monounsaturated fat diet based on macadamia nuts. Archives of Internal Medicine 2000;160(8):1154‐8. [DOI] [PubMed] [Google Scholar]
Davis 2007 {published data only}
- Davis L, Stonehouse W, Loots du T, Mukuddem‐Petersen J, Westhuizen FH, Hanekom SM, et al. The effects of high walnut and cashew nut diets on the antioxidant status of subjects with metabolic syndrome. European Journal of Nutrition 2007;46(3):155‐64. [DOI] [PubMed] [Google Scholar]
Din 2011 {published data only}
- Din JN, Aftab SM, Jubb AW, Carnegy FH, Lyall K, Sarma J, et al. Effect of moderate walnut consumption on lipid profile, arterial stiffness and platelet activation in humans. European Journal of Clinical Nutrition 2011;65(2):234‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 1999 {published data only}
- Edwards K, Kwaw I, Matud J, Kurtz I. Effect of pistachio nuts on serum lipid levels in patients with moderate hypercholesterolemia. Journal of the American College of Nutrition 1999;18(3):229‐32. [DOI] [PubMed] [Google Scholar]
Esfahani 2010 {published data only}
- Esfahani A, Jenkins DJ, Kendall CW. Session 4: CVD, diabetes and cancer: A dietary portfolio for management and prevention of heart disease. Proceedings of the Nutrition Society 2010;69(1):39‐44. [DOI] [PubMed] [Google Scholar]
Foster 2012 {published data only}
- Foster GD, Shantz KL, Vander Veur SS, Oliver TL, Lent MR, Virus A, et al. A randomized trial of the effects of an almond‐enriched, hypocaloric diet in the treatment of obesity. American Journal of Clinical Nutrition 2012;96(2):249‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garg 2007 {published data only}
- Garg ML, Blake RJ, Wills RBH, Clayton EH. Macadamia nut consumption modulates favourably risk factors for coronary artery disease in hypercholesterolemic subjects. Lipids 2007;42(6):583‐7. [DOI] [PubMed] [Google Scholar]
Gebauer 2008 {published data only}
- Gebauer SK, West SG, Kay CD, Alaupovic P, Bagshaw D, Kris‐Etherton PM. Effects of pistachios on cardiovascular disease risk factors and potential mechanisms of action: a dose‐response study. American Journal of Clinical Nutrition 2008;88(3):651‐9. [DOI] [PubMed] [Google Scholar]
Gebauer 2011 {published data only}
- Gebauer SK, Novotny JA, Baer DJ. Pistachios reduce LDL‐cholesterol when consumed as whole nuts as part of a controlled typical American diet in healthy normolipidemic individuals. FASEB Journal 2011;25(Meeting Abstract Supplement):971.35. [Google Scholar]
Ghadimi Nouran 2010 {published data only}
- Ghadimi Nouran M, Kimiagar M, Abadi A, Mirzazadeh M, Harrison G. Peanut consumption and cardiovascular risk. Public Health Nutrition 2010;13(10):1581‐6. [DOI] [PubMed] [Google Scholar]
Gillen 2005 {published data only}
- Gillen LJ, Tapsell LC, Patch CS, Owen A, Batterham M. Structured dietary advice incorporating walnuts achieves optimal fat and energy balance in patients with type 2 diabetes mellitus. Journal of the American Dietetic Association 2005;105(7):1087‐96. [DOI] [PubMed] [Google Scholar]
Griel 2008 {published data only}
- Griel AE, Cao Y, Bagshaw DD, Cifelli AM, Holub B, Kris‐Etherton PM. A macadamia nut‐rich diet reduces total and LDL‐cholesterol in mildly hypercholesterolemic men and women. Journal of Nutrition 2008;138(4):761‐7. [DOI] [PubMed] [Google Scholar]
Gulati 2011 {published data only}
- Gulati S, Misra A. Case control study for the evaluation of beneficial effect(S) of pistachio nut intake on cardiovascular risk factors in Asian Indians with the metabolic syndrome. FASEB Journal 2011;25(Meeting Abstract Supplement):971.15. [Google Scholar]
Gulati 2014 {published data only}
- Gulati S, Misra A, Pandey RM, Bhatt SP, Saluja S. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: a 24‐wk, randomized control trial. Nutrition 2014;30(2):192‐7. [DOI] [PubMed] [Google Scholar]
Hargrove 2001 {published data only}
- Hargrove RL, Etherton TD, Pearson TA, Harrison EH, Kris‐Etherton PM. Low fat and high monounsaturated fat diets decrease human low density lipoprotein oxidative susceptibility in vitro. Journal of Nutrition 2001;131(6):1758‐63. [DOI] [PubMed] [Google Scholar]
Hernández‐Alonso 2014 {published data only}
- Hernández‐Alonso P, Baldrich‐Mora M, Salas‐Salvadó J, Arcelin P, Palau‐Galindo A, Ciutat M, et al. Effect of pistachio Intake on Insulin Resistance and metabolic risk markers‐the EPIRDEM Study. Obesity Facts 2014;7:40. [Google Scholar]
- Hernández‐Alonso P, Salas‐Salvadó J, Baldrich‐Mora M, Juanola‐Falgarona M, Bulló M. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: a randomized clinical trial. Diabetes Care 2014;37(11):3098‐105. [DOI] [PubMed] [Google Scholar]
- Hernández‐Alonso P, Salas‐Salvadó J, Baldrich‐Mora M, Mallol R, Correig X, Bulló M. Effect of pistachio consumption on plasma lipoprotein subclasses in pre‐diabetic subjects. Nutrition, Metabolism & Cardiovascular Diseases 2015;25(4):396‐402. [DOI] [PubMed] [Google Scholar]
- Hernández‐Alonso P, Salas‐Salvadó J, Baldrich‐Mora M, Mallol R, Correig X, Bulló M. Regular consumption of pistachio modulates plasma lipoprotein subclasses in pre‐diabetic subjects. Obesity Facts 2015;8:72‐3. [DOI] [PubMed] [Google Scholar]
- NCT01441921. Effect of pistachio intake on insulin resistance and type 2 diabetes mellitus (EPIRDEM). https://clinicaltrials.gov/ct2/show/NCT01441921 (accessed 20 February 2015).
Holligan 2011 {published data only}
- Holligan S, Gebauer S, West S, Kay CD, Kris‐Etherton P. Effects of pistachios on emerging CVD risk factors in moderately hypercholesterolemic individuals. FASEB Journal 2011;25(Meeting Abstract Supplement):971.21. [Google Scholar]
Holligan 2013 {published data only}
- Holligan S, West SG, Gebauer SK, Kay CD, Kris‐Etherton PM. A moderate‐fat diet with pistachios lowers small‐dense LDL and improves markers of insulin sensitivity in subjects with moderately‐elevated cholesterol levels. FASEB Journal 2013;27(Meeting Abstract Supplement):1057.13. [Google Scholar]
Holligan 2014 {published data only}
- Holligan SD, West SG, Gebauer SK, Kay CD, Kris‐Etherton PM. A moderate‐fat diet containing pistachios improves emerging markers of cardiometabolic syndrome in healthy adults with elevated LDL levels. British Journal of Nutrition 2014;112(5):744‐52. [DOI] [PubMed] [Google Scholar]
Hollis 2007 {published data only}
- Hollis J, Mattes R. Effect of chronic consumption of almonds on body weight in healthy humans. British Journal of Nutrition 2007;98(3):651‐6. [DOI] [PubMed] [Google Scholar]
Hudthagosol 2011 {published data only}
- Hudthagosol C, Haddad EH, McCarthy K, Wang PW, Oda K, Sabaté J. Pecans acutely increase plasma postprandial antioxidant capacity and catechins and decrease LDL oxidation in humans. Journal of Nutrition 2011;141(1):56‐62. [DOI] [PubMed] [Google Scholar]
Iwamoto 2002 {published data only}
- Iwamoto M, Imaizumi K, Sato M, Hirooka Y, Sakai K, Takeshita A, et al. Serum lipid profiles in Japanese women and men during consumption of walnuts. European Journal of Clinical Nutrition 2002;56(7):629‐37. [DOI] [PubMed] [Google Scholar]
Jaceldo‐Siegl 2011 {published data only}
- Jaceldo‐Siegl K, Sabaté J, Batech M, Fraser GE. Influence of body mass index and serum lipids on the cholesterol‐lowering effects of almonds in free‐living individuals. Nutrition, Metabolism & Cardiovascular Diseases 2011;21(Suppl 1):S7‐13. [DOI] [PubMed] [Google Scholar]
Jambazian 2005 {published data only}
- Jambazian PR, Haddad E, Rajaram S, Tanzman J, Sabaté J. Almonds in the diet simultaneously improve plasma alpha‐tocopherol concentrations and reduce plasma lipids. Journal of the American Dietetic Association 2005;105(3):449‐54. [DOI] [PubMed] [Google Scholar]
Jenkins 2008 {published data only}
- Jenkins DJ, Kendall CW, Marchie A, Josse AR, Nguyen TH, Faulkner DA, et al. Effect of almonds on insulin secretion and insulin resistance in nondiabetic hyperlipidemic subjects: a randomized controlled crossover trial. Metabolism: Clinical and Experimental 2008;57(7):882‐7. [DOI] [PubMed] [Google Scholar]
Jenkins 2011 {published data only}
- Jenkins DJ, Kendall CW, Banach MS, Srichaikul K, Vidgen E, Mitchell S, et al. Nuts as a replacement for carbohydrates in the diabetic diet. Diabetes Care 2011;34(8):1706‐11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Jones 2014 {published data only}
- Jones JB, Provost M, Keaver L, Breen C, Ludy MJ, Mattes RD. A randomized trial on the effects of flavorings on the health benefits of daily peanut consumption. American Journal of Clinical Nutrition 2014;99(3):490‐6. [DOI] [PubMed] [Google Scholar]
Jonsson 2013 {published data only}
- Jonsson T, Granfeldt Y, Lindeberg S, Hallberg AC. Subjective satiety and other experiences of a Paleolithic diet compared to a diabetes diet in patients with type 2 diabetes. Nutrition Journal 2013;12:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 2012 {published data only}
- Katz DL, Davidhi A, Ma Y, Kavak Y, Bifulco L, Njike VY. Effects of walnuts on endothelial function in overweight adults with visceral obesity: a randomized, controlled, crossover trial. Journal of the American College of Nutrition 2012;31(6):415‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kay 2010 {published data only}
- Kay CD, Gebauer SK, West SG, Kris‐Etherton PM. Pistachios increase serum antioxidants and lower serum oxidized‐LDL in hypercholesterolemic adults. Journal of Nutrition 2010;140(6):1093‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kendall 2002 {published data only}
- Kendall CWC, Jenkins DJA, Marchie A, Parker T, Connelly PW, Spiller GA. Dose response to almonds in hyperlipidemia: a randomized controlled cross‐over trial. American Journal of Clinical Nutrition 2002;75:384S. [Google Scholar]
Kendall 2003 {published data only}
- Kendall CWC, Jenkins DJA, Marchie A, Ren YL, Ellis PR, Lapsley KG. Energy availability from almonds: Implications for weight loss and cardiovascular health. A randomized controlled dose‐response trial. FASEB Journal 2003;17(4):A339. [Google Scholar]
Kendall 2009 {published data only}
- Kendall CW, Wong JM, Sievenpiper JL, Parker TL, Mitchell S, Banach MS, et al. Nuts and blood lipids in type 2 diabetes. European Heart Journal 2009;30:237. [Google Scholar]
Kendall 2013 {published data only}
- Kendall CWC, Augustin LSA, Bashyam B, Nishi S, Jenkins DJA. Effect of nuts on coronary heart disease risk factors in type 2 diabetes. FASEB Journal 2013;27(Meeting Abstract Supplement):368.5. [Google Scholar]
Kochar 2010 {published data only}
- Kochar J, Gaziano JM, Djoussé L. Nut consumption and risk of type II diabetes in the Physicians' Health Study. European Journal of Clinical Nutrition 2010;64(1):75‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kocyigit 2006 {published data only}
- Kocyigit A, Koylu AA, Keles H. Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers. Nutrition, Metabolism & Cardiovascular Diseases 2006;16(3):202‐9. [DOI] [PubMed] [Google Scholar]
Li 2009 {published data only}
- Li Z, Song R, Nguyen C, Zerlin A, Naowamondhol K, Karp H, et al. Impact of pistachio nut consumption on triglycerides in obese subjects over 12 weeks. FASEB Journal 2009;23(Meeting Abstract Supplement):213.5. [Google Scholar]
Li 2010 {published data only}
- Li Z, Song R, Nguyen C, Zerlin A, Karp H, Naowamondhol K, et al. Pistachio nuts reduce triglycerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12‐week weight loss program. Journal of the American College of Nutrition 2010;29(3):198‐203. [DOI] [PubMed] [Google Scholar]
Li 2011 {published data only}
- Li SC, Liu YH, Liu JF, Chang WH, Chen CM, Chen CY. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism: Clinical and Experimental 2011;60(4):474‐9. [DOI] [PubMed] [Google Scholar]
López‐Uriarte 2010 {published data only}
- López‐Uriarte Patricia P, Nogués R, Saez G, Bulló M, Romeu M, Masana L, et al. Effect of nut consumption on oxidative stress and the endothelial function in metabolic syndrome. Clinical Nutrition 2010;29(3):373‐80. [DOI] [PubMed] [Google Scholar]
Maranhão 2011 {published data only}
- Maranhão PA, Kraemer‐Aguiar LG, Oliveira CL, Kuschnir MCC, Vieira YR, Souza MGC, et al. Brazil nuts intake improves lipid profile, oxidative stress and microvascular function in obese adolescents: a randomized controlled trial. Nutrition and Metabolism 2011;8(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKeown 2010 {published data only}
- McKeown PP, Logan K, McKinley MC, Young IS, Woodside JV. Session 4: CVD, diabetes and cancer ‐ Evidence for the use of the Mediterranean diet in patients with CHD. Proceedings of the Nutrition Society 2010;69(1):45‐60. [DOI] [PubMed] [Google Scholar]
Mercanligil 2007 {published data only}
- Mercanligil SM, Arslan P, Alasalvar C, Okut E, Akgül E, Pinar A, et al. Effects of hazelnut‐enriched diet on plasma cholesterol and lipoprotein profiles in hypercholesterolemic adult men. European Journal of Clinical Nutrition 2007;61(2):212‐20. [DOI] [PubMed] [Google Scholar]
Mohammadifard 2012 {published data only}
- Mohammadifard N, Sarrafzadegan N, Sajjadi F, Maghroun M, Alikhasi H. Nut consumption and serum lipids among iranian population: Isfahan healthy heart program. Circulation 2012;125(19):e911. [Google Scholar]
Morgan 2000 {published data only}
- Morgan WA, Clayshulte BJ. Pecans lower low‐density lipoprotein cholesterol in people with normal lipid levels. Journal of the American Dietetic Association 2000;100(3):312‐8. [DOI] [PubMed] [Google Scholar]
Morgan 2002 {published data only}
- Morgan JM, Horton K, Reese D, Carey C, Walker K, Capuzzi DM. Effects of walnut consumption as part of a low‐fat, low‐cholesterol diet on serum cardiovascular risk factors. International Journal for Vitamin and Nutrition Research 2002;72(5):341‐7. [DOI] [PubMed] [Google Scholar]
Mukuddem‐Petersen 2007 {published data only}
- Mukuddem‐Petersen J, Stonehouse Oosthuizen W, Jerling JC, Hanekom SM, White Z. Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: a controlled feeding trial. British Journal of Nutrition 2007;97(6):1144‐53. [DOI] [PubMed] [Google Scholar]
Munoz 2001 {published data only}
- Munoz S, Merlos M, Zambón D, Rodríguez C, Sabaté J, Ros E, et al. Walnut‐enriched diet increases the association of LDL from hypercholesterolemic men with human HepG2 cells. Journal of Lipid Research 2001;42(12):2069‐76. [PubMed] [Google Scholar]
Nishi 2014 {published data only}
- Nishi S, Kendall C, Bashyam B, Augustin L, Jenkins D. Effect of nuts on coronary heart disease and cancer risk in type 2 diabetes. FASEB Journal 2014;28(Suppl 1):825.8. [Google Scholar]
Núñez 2004 {published data only}
- Núñez I, Gilabert R, Pérez‐Heras A, Serra M, Casals E, Deulofeu R, et al. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Atherosclerosis Supplements 2004;5:139‐40. [DOI] [PubMed] [Google Scholar]
O'Byrne 1997 {published data only}
- O'Byrne DJ, Knauft DA, Shireman RB. Low fat‐monounsaturated rich diets containing high‐oleic peanuts improve serum lipoprotein profiles. Lipids 1997;32(7):687‐95. [DOI] [PubMed] [Google Scholar]
Oliveira 2010 {published data only}
- Oliveira C, Maranhão P, Koury J, Kraemer‐Aguiar LG, Kuschnir MC, Bouskela E. Effect of brazil nut consumption on the lipid profile, inflammatory marker, antioxidant capacity and skin nutritive microcirculatory patterns in obese female adolescents. Obesity Reviews 2010;11:165. [Google Scholar]
Orem 2013 {published data only}
- Orem A, Yucesan FB, Orem C, Akcan B, Kural BV, Alasalvar C, et al. Hazelnut‐enriched diet improves cardiovascular risk biomarkers beyond a lipid‐lowering effect in hypercholesterolemic subjects. Journal of Clinical Lipidology 2013;7(2):123‐31. [DOI] [PubMed] [Google Scholar]
Petersen 2013 {published data only}
- Petersen KN, Ricklefs K, Alanbagy SS, Johnston CS, Sweazea KL. Almond consumption reduces diastolic blood pressure in men with type 2 diabetes. FASEB Journal 2013;27(Meeting Abstract Supplement):lb422. [Google Scholar]
Pieters 2005 {published data only}
- Pieters M, Oosthuizen W, Jerling JC, Loots DT, Mukuddem‐Petersen J, Hanekom SM. Clustering of haemostatic variables and the effect of high cashew and walnut diets on these variables in metabolic syndrome patients. Blood Coagulation & Fibrinolysis 2005;16(6):429‐37. [DOI] [PubMed] [Google Scholar]
Rajaram 2001 {published data only}
- Rajaram S, Burke K, Connell B, Myint T, Sabaté J. A monounsaturated fatty acid‐rich pecan‐enriched diet favorably alters the serum lipid profile of healthy men and women. Journal of Nutrition 2001;131(9):2275‐9. [DOI] [PubMed] [Google Scholar]
Rajaram 2010 {published data only}
- Rajaram S, Connell KM, Sabaté J. Effect of almond‐enriched high‐monounsaturated fat diet on selected markers of inflammation: a randomised, controlled, crossover study. British Journal of Nutrition 2010;103(6):907‐12. [DOI] [PubMed] [Google Scholar]
Reinsma 2010 {published data only}
- Reinsma K, Heim L, Wien M, Oda K, Sabaté J. Effects of peanut and peanut butter consumption on waist circumference and body weight in adults with type 2 diabetes. FASEB Journal 2010;24(Meeting Abstract Supplement):564.18. [Google Scholar]
Ricklefs 2013 {published data only}
- Ricklefs K, Petersen K, Alanbagy S, Johnston CS, Sweazea KL. Investigating the effects of 12 week almond consumption in type 2 diabetes. FASEB Journal 2013;27(Meeting Abstract Supplement):lb424. [Google Scholar]
Rismankarzadeh 2005 {published data only}
- Rismankarzadeh M, Vosoughi AA, Rafieeyan M, Tamizifar B, Aminzade A. A low‐dose almond‐based diet decreases LDL‐C while preserving HDL‐C. Archives of Iranian Medicine 2005;8:45‐51. [Google Scholar]
Ros 2004 {published data only}
- Ros E, Núñez I, Pérez‐Heras A, Serra M, Gilabert R, Casals E, et al. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation 2004;109(13):1609‐14. [DOI] [PubMed] [Google Scholar]
Sabate 1993 {published data only}
- Sabaté J, Fraser GE, Burke K, Knutsen SF, Bennett H, Lindsted KD. Effects of walnuts on serum lipid levels and blood pressure in normal men. New England Journal of Medicine 1993;328(9):603‐7. [DOI] [PubMed] [Google Scholar]
Sabaté 2003 {published data only}
- Sabaté J, Haddad E, Tanzman JS, Jambazian P, Rajaram S. Serum lipid response to the graduated enrichment of a Step I diet with almonds: a randomized feeding trial. American Journal of Clinical Nutrition 2003;77(6):1379‐84. [DOI] [PubMed] [Google Scholar]
Sari 2010 {published data only}
- Sari I, Baltaci Y, Bagci C, Davutoglu V, Erel O, Celik H, et al. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: a prospective study. Nutrition 2010;26(4):399‐404. [DOI] [PubMed] [Google Scholar]
Schutte 2006 {published data only}
- Schutte AE, Rooyen JM, Huisman HW, Mukuddem‐Petersen J, Oosthuizen W, Hanekom SM, et al. Modulation of baroreflex sensitivity by walnuts versus cashew nuts in subjects with metabolic syndrome. American Journal of Hypertension 2006;19(6):629‐36. [DOI] [PubMed] [Google Scholar]
Sheridan 2007 {published data only}
- Sheridan MJ, Cooper JN, Erario M, Cheifetz CE. Pistachio nut consumption and serum lipid levels. Journal of the American College of Nutrition 2007;26(2):141‐8. [DOI] [PubMed] [Google Scholar]
Somerset 2011 {published data only}
- Somerset S, Graham L, Markwell K, Colquhoun D. Isoenergetic replacement of dietary saturated with monounsaturated fat via macadamia nuts can affect coronary risk in overweight subjects. Atherosclerosis Supplements 2011;12(1):143‐4. [Google Scholar]
Somerset 2013 {published data only}
- Somerset SM, Graham L, Markwell K. Isoenergetic replacement of dietary saturated with monounsaturated fat via macadamia nuts enhances endothelial function in overweight subjects. e‐SPEN Journal 2013;8(3):e113‐9. [Google Scholar]
Storniolo 2013 {published data only}
- Storniolo CE, Bullo M, Fito M, Saez GT, Toledo E, Estruch E, et al. Mediterranean diet with virgin olive oil or nuts modulate nitric oxide, endothelin‐1 and blood pressure in hypertensive women. Annals of Nutrition and Metabolism 2013;62:35. [Google Scholar]
Sweazea 2014 {published data only}
- Sweazea K, Johnston C, Ricklefs K, Petersen K, Alanbagy S. Almond supplementation without dietary advice significantly reduces C‐reactive protein in subjects with poorly‐controlled type 2 diabetes. FASEB Journal 2014;28(Suppl 1):830.24. [Google Scholar]
Tan 2013 {published data only}
- Tan SY, Mattes RD. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: a randomized, controlled trial. European Journal of Clinical Nutrition 2013;67(11):1205‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tapsell 2004 {published data only}
- Tapsell LC, Gillen LJ, Patch CS, Batterham M, Owen A, Baré M, et al. Including walnuts in a low‐fat/modified‐fat diet improves HDL cholesterol‐to‐total cholesterol ratios in patients with type 2 diabetes. Diabetes Care 2004;27(12):2777‐83. [DOI] [PubMed] [Google Scholar]
Tapsell 2009 {published data only}
- Tapsell LC, Batterham MJ, Teuss G, Tan SY, Dalton S, Quick CJ, et al. Long‐term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. European Journal of Clinical Nutrition 2009;63(8):1008‐15. [DOI] [PubMed] [Google Scholar]
Tapsell 2010 {published data only}
- Tapsell LC. Health Benefits of Walnut Consumption. ISHS Acta Horticulturae: Proceedings of the Sixth International Walnut Symposium; 2009 Feb 25‐27; Melbourne 2010;861:409‐15. [Google Scholar]
Wang 2012 {published data only}
- Wang X, Li Z, Liu Y, Lv X, Yang W. Effects of pistachios on body weight in Chinese subjects with metabolic syndrome. Nutrition Journal 2012;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2012 {published data only}
- West SG, Gebauer SK, Kay CD, Bagshaw DM, Savastano DM, Diefenbach C, et al. Diets containing pistachios reduce systolic blood pressure and peripheral vascular responses to stress in adults with dyslipidemia. Hypertension 2012;60(1):58‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wien 2010 {published data only}
- Wien M, Bleich D, Raghuwanshi M, Gould‐Forgerite S, Gomes J, Monahan‐Couch L, et al. Almond consumption and cardiovascular risk factors in adults with prediabetes. Journal of the American College of Nutrition 2010;29(3):189‐97. [DOI] [PubMed] [Google Scholar]
Wien 2014 {published data only}
- Wien M, Oda K, Sabaté J. A randomized controlled trial to evaluate the effect of incorporating peanuts into an American Diabetes Association meal plan on the nutrient profile of the total diet and cardiometabolic parameters of adults with type 2 diabetes. Nutrition Journal 2014;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2010 {published data only}
- Wu H, Pan A, Yu Z, Qi Q, Lu L, Zhang G, et al. Lifestyle counseling and supplementation with flaxseed or walnuts influence the management of metabolic syndrome. Journal of Nutrition 2010;140(11):1937‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2014 {published data only}
- Wu L, Piotrowski K, Rau T, Waldmann E, Broedl UC, Demmelmair H, et al. Walnut‐enriched diet reduces fasting non‐HDL‐cholesterol and apolipoprotein B in healthy Caucasian subjects: a randomized controlled cross‐over clinical trial. Metabolism: Clinical & Experimental 2014;63(3):382‐91. [DOI] [PubMed] [Google Scholar]
Zambón 1998 {published data only}
- Zambón D, Campero B, Pérez‐Heras A, Rodríguez‐Villar C, Ros E, Casals E, et al. Effects of walnuts on the serum lipid profile of hypercholesterolemic subjects: the Barcelona walnut trial. FASEB Journal 1998;12:A506. [Google Scholar]
Zambón 2000 {published data only}
- Zambón D, Sabaté J, Muñoz S, Campero B, Casals E, Merlos M, et al. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. (Erratum appears in Annals of Internal Medicine 2000;133(8):659). Annals of Internal Medicine 2000;132(7):538‐46. [DOI] [PubMed] [Google Scholar]
Zibaeenezhad 2005 {published data only}
- Zibaeenezhad MJ, Shamsnia SJ, Khorasani M. Walnut consumption in hyperlipidemic patients. Angiology 2005;56(5):581‐3. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Njike 2015 {published data only}
- Njike V, Ayettey R, Petraro P, Treu J, Katz D. Walnut ingestion in adults at risk for diabetes: effects on diet quality, body composition, and cardiac risk measures. FASEB Journal 2015;29(Suppl 1):747.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01950806 {published data only}
- NCT01950806. The effect of pecans on biomarkers of risk for cardiovascular disease and diabetes. http://ClinicalTrials.gov/show/NCT01950806 (accessed 15 December 2014).
Additional references
Afshin 2014
- Afshin A, Micha R, Khatibzadeh S, Mozaffarian D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: a systematic review and meta‐analysis. American Journal of Clinical Nutrition 2014;100(1):278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albert 2002
- Albert CM, Gaziano JM, Willett WC, Manson JE. Nut consumption and decreased risk of sudden death in the Physicians' Health Study. Archives of Internal Medicine 2002;162(12):1382‐7. [DOI] [PubMed] [Google Scholar]
Banal 2009
- Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta‐analysis and systematic review. American Journal of Clinical Nutrition 2009;90(1):56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
BHF 2014
- British Heart Foundation. Cardiovascular disease. http://www.bhf.org.uk/heart‐health/conditions/cardiovascular‐disease.aspx (accessed 17 March 2014).
Blomhoff 2006
- Blomhoff R, Carlsen MH, Andersen LF, Jacobs DR Jr. Health benefits of nuts: potential role of antioxidants. British Journal of Nutrition 2006;96(Suppl 2):S52‐60. [DOI] [PubMed] [Google Scholar]
Brufau 2006
- Brufau G, Boatella J, Rafecas M. Nuts: source of energy and macronutrients. British Journal of Nutrition 2006;96(Suppl 2):S24‐28. [DOI] [PubMed] [Google Scholar]
Dreher 1996
- Dreher ML, Maher CV, Kearney P. The traditional and emerging role of nuts in healthful diets. Nutrition Reviews 1996;54(8):241‐5. [DOI] [PubMed] [Google Scholar]
Follman 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [DOI] [PubMed] [Google Scholar]
Fraser 1992
- Fraser GE, Sabaté J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Archives of Internal Medicine 1992;152(7):1416‐24. [PubMed] [Google Scholar]
Gaziano 2010
- Gaziano TA, Bitton A, Anand S, Abrahams‐Gessel S, Murphy A. Growing epidemic of coronary heart disease in low‐ and middle‐income countries. Current Problems in Cardiology 2010;35(2):72‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jenkins 2002
- Jenkins DJ, Kendall CW, Marchie A, Parker TL, Connelly PW, Qian W, et al. Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low‐density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation 2002;106(11):1327‐32. [DOI] [PubMed] [Google Scholar]
King 2008
- King JC, Blumberg J, Ingwersen L, Jenab M, Tucker KL. Tree nuts and peanuts as components of a healthy diet. Journal of Nutrition 2008;138(9):1736S‐40S. [DOI] [PubMed] [Google Scholar]
Kris‐Etherton 2008
- Kris‐Etherton PM, Hu FB, Ros E, Sabaté J. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. Journal of Nutrition 2008;138(9):1746S‐51S. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lim 2012
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair‐Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2224‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohammadifard 2015
- Mohammadifard N, Salehi‐Abargouei A, Salas‐Salvadó J, Guasch‐Ferré M, Humphries K, Sarrafzadegan N. The effect of tree nut, peanut, and soy nut consumption on blood pressure: a systematic review and meta‐analysis of randomized controlled clinical trials. American Journal of Clinical Nutrition 2015;101(5):966‐82. [DOI] [PubMed] [Google Scholar]
Mukuddem‐Petersen 2005
- Mukuddem‐Petersen J, Oosthuizen W, Jerling JC. A systematic review of the effects of nuts on blood lipid profiles in humans. Journal of Nutrition 2005;135(9):2082‐9. [DOI] [PubMed] [Google Scholar]
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ros 2006
- Ros E, Mataix J. Fatty acid composition of nuts ‐ implications for cardiovascular health. British Journal of Nutrition 2006;96(Suppl 2):S29‐35. [DOI] [PubMed] [Google Scholar]
Sabaté 1993
- Sabaté J, Fraser GE, Burke K, Knutsen SF, Bennett H, Lindsted KD. Effects of walnuts on serum lipid levels and blood pressure in normal men. New England Journal of Medicine 1993;328(9):603‐7. [DOI] [PubMed] [Google Scholar]
Sabaté 2006
- Sabaté J, Ros E, Salas‐Salvadó J. Nuts: nutrition and health outcomes. British Journal of Nutrition 2006;96(Suppl 2):S1‐2. [DOI] [PubMed] [Google Scholar]
Sabaté 2010
- Sabaté J, Wien M. Nuts, blood lipids and cardiovascular disease. Asia Pacific Journal of Clinical Nutrition 2010;19(1):131‐6. [PubMed] [Google Scholar]
Scarborough 2011
- Scarborough P, Morgan RD, Webster P, Rayner M. Differences in coronary heart disease, stroke and cancer mortality rates between England, Wales, Scotland and Northern Ireland: the role of diet and nutrition. BMJ Open 2011;1(1):e000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2000
- Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. Journal of Clinical Epidemiology 2000;53(11):1119‐29. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta‐analysis. BMJ 2001;323(7304):101‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vinson 2012
- Vinson JA, Cai Y. Nuts, especially walnuts, have both antioxidant quantity and efficacy and exhibit significant potential health benefits. Food & Function 2012;3(2):134‐40. [DOI] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. Cardiovascular diseases (CVDs) Fact sheet number 317. http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed 13 March 2014).
Yusuf 2001
- Yusuf S, Reddy S, Ôunpuu S, Anand S. Global burden of cardiovascular diseases. Part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation 2001;104(23):2855‐64. [DOI] [PubMed] [Google Scholar]
Zhou 2014
- Zhou D, Yu H, He F, Reilly KH, Zhang J, Li S, et al. Nut consumption in relation to cardiovascular disease risk and type 2 diabetes: a systematic review and meta‐analysis of prospective studies. American Journal of Clinical Nutrition 2014;100(1):270–7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Martin 2015
- Martin N, Germanò R, Hartley L, Adler AJ, Rees K. Nut consumption for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD011583] [DOI] [PMC free article] [PubMed] [Google Scholar]


