Abstract
Vaccination suppresses Helicobacter pylori colonization but does not cure infection. Furthermore, postvaccination gastritis, likely induced by enhanced host response to residual colonization, may exacerbate disease. The goal of this study was to determine if adoptive transfer of C57BL/6 splenocytes to C57BL/6scid/scid (severe combined immunodeficient [SCID]) mice cures infection without exacerbating gastritis. H. pylori-infected and uninfected C57BL/6 mice and SCID recipients of normal splenocytes were killed at intervals between 5 and 51 weeks after infection. Colonization and gastritis were quantified, humoral immune responses were determined by enzyme-linked immunosorbent assay, and cellular immune responses were determined by delayed-type hypersensitivity response and by a proliferative response of cultured splenocytes to H. pylori sonicate. In infected C57BL/6 mice, gastritis developed gradually and bacterial colonization diminished but persisted throughout the experiment. In contrast, gastritis in infected recipient SCID mice developed rapidly and bacterial colonization decreased precipitously. Gastritis in those mice peaked 9 weeks after adoptive transfer, however, and began to resolve. By 45 weeks after transfer, gastritis had returned to background levels and bacteria were no longer detectable. Resolution of gastritis and elimination of infection were associated with a cellular but not humoral immune response to H. pylori antigens. These results demonstrate that although the host response fails to clear bacterial colonization in normal mice, enhanced cellular immune responses in recipient SCID mice are capable of clearing H. pylori infection and allowing resolution of gastritis. Thus, immune mechanisms of cure exist, and effective and safe vaccination protocols may be feasible.
The startling discovery made in 1983 that a bacterial organism, Helicobacter pylori, is the underlying cause of peptic ulcer disease (27, 39) led to several major changes in the therapy of gastric disease in recent years. First, the realization that peptic ulcer disease (and possibly some forms of gastric cancer [32, 33]) may be treatable or preventable with antibiotics resulted in rapid development of many excellent treatment protocols. Peptic ulcer disease is now considered an infectious disease and can be cured by elimination of the causative organism, markedly improving the prognosis for those whose infections are curable. It has become apparent, however, that H. pylori infection is not an easy foe. Once infected, people appear to remain infected for life, and even with medical intervention, the organism may persist or recur. In fact, it appears to be true of the gastric helicobacters in general that colonization persists in spite of a strong host immune response, and even bacteria that are susceptible to antibiotics in vitro may be resistant to them in vivo. Thus, we are faced with the task of eliminating a highly resistant pathogen that the normal host itself cannot eliminate.
Because of the difficulty of eliminating H. pylori infection by conventional means, vaccination protocols have been developed. Many of these have been shown to be at least partly effective in suppressing or eliminating colonization in animal models (2, 5, 6, 11, 12, 14, 17, 21, 22, 24, 25, 31), and human trials have shown some promise (28). Like treatment regimens, however, vaccine trials have been incompletely effective. Further, complications such as postimmunization gastritis, a phenomenon whereby gastritis actually worsens following vaccination of experimental animals (8, 14, 17), have raised concerns about the potential safety of vaccination of human patients.
For these reasons it is important to identify circumstances under which unvaccinated animals clear gastric H. pylori infection. Identification of host responses which lead to successful clearance will greatly strengthen our ability to determine the specific immune factors which lead to cure rather than exacerbation of disease. The purpose of the present study was to use a recently developed model of adoptive transfer of splenocytes to determine if enhanced immune responses can lead to elimination of infection and resolution of gastritis in an H. pylori-infected host.
MATERIALS AND METHODS
Mice.
C57BL/6 and C57BL/6scid/scid (severe combined immunodeficient [SCID]) mice, 4 to 6 weeks old, were purchased from Jackson Laboratories. All mice were free of detectable intestinal helicobacter species, based on health surveillance procedures at Jackson laboratories or on PCR-based detection of fecal helicobacter species in our own laboratory by the method of Shames et al. (36). Immune incompetence of SCID mice was verified by the absence of murine immunoglobulin G (IgG) as detected by enzyme-linked immunosorbent assay (15). Mice were kept in sterile microisolator cages in a barrier facility and were fed sterile water and Tek-lad laboratory chow ad libitum. A total of 197 mice were used in this study. All procedures involving animals were approved by the Ohio State University institutional laboratory animal care and use committee.
Bacteria.
H. pylori strain SS1, a mouse-adapted human isolate, was grown on 5% sheep blood agar plates (BBL, Cockeysville, Md.) or in brucella broth with 10% fetal calf serum. For preparation of sonicates and for mouse inoculation, bacteria were grown in broth overnight at 37°C in a microaerobic environment with gentle agitation. Bacterial sonicates for footpad injection were prepared as previously described (15).
Bacterial infection and adoptive transfer.
Details of the adoptive transfer mouse model used in this study have been previously published (15). Briefly, 106 splenocytes from normal, uninfected C57BL/6 mice were transferred to H. pylori-infected C587BL/6scid/scid mice by intraperitoneal injection. Adoptive transfer was performed 4 to 6 weeks after oral inoculation of SCID mice with 108 CFU of live, broth-cultured H. pylori. This protocol results in engraftment of B and T lymphocytes and development of serologic and cellular immune responses to H. pylori in the recipient mice. In contrast to infected normal C57BL/6 mice, which develop minimal to mild cellular immune responses and mild gastritis, recipient SCID mice develop strong cellular immune responses which result in delayed-type hypersensitivity responses to H. pylori antigens and severe gastritis within 2 to 4 weeks of adoptive transfer. Uninfected recipient mice do not develop either H. pylori-specific immune responses or gastritis.
Experimental design.
Groups of mice included the following: infected and uninfected C57BL/6 mice, infected and uninfected SCID mice (not given splenocytes by adoptive transfer), and infected and uninfected recipient SCID mice (SCID mice which were reconstituted with splenocytes from C57BL/6 mice). C57BL/6 and nonrecipient SCID mice were killed 5, 6, 8, 16, 36, or 51 weeks after bacterial inoculation, and recipient SCID mice were killed 1, 2, 4, 9, 31, or 45 weeks after adoptive transfer. The number of mice per group is given in Table 1. Data on some of the mice have been previously reported (15).
TABLE 1.
Number of mice per experimental group
| Mouse strain | Infection | Adoptive transfer | Sacrifice intervala
|
|||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| C57BL/6 | Yes | No | 5 | 5 | 9 | 7 | 5 | 5 |
| C57BL/6 | No | No | 5 | 5 | 5 | 7 | 5 | 5 |
| SCID | Yes | Yes | 5 | 5 | 9 | 5 | 5 | 4 |
| SCID | Yes | No | 5 | 5 | 10 | 5 | 4 | 5 |
| SCID | No | Yes | 5 | 5 | 5 | 6 | 4 | 5 |
| SCID | No | No | 5 | 5 | 5 | 7 | 5 | 5 |
Sacrifice intervals for C57BL/6 mice and nonrecipient SCID mice were 5, 6, 8, 16, 36, or 51 weeks after bacterial inoculation. Sacrifice intervals for recipient SCID mice were 1, 2, 4, 9, 31, or 45 weeks after adoptive transfer.
One day prior to sacrifice, mice were given 10 μg of H. pylori sonicate by injection into the hind footpad. The opposite footpad received sterile saline. Twenty-four hours later, footpad thickness was measured with a dial thickness gauge, and the difference in thickness between the control and sonicate-treated footpad was recorded. Foot swelling in response to antigen was determined in mice killed 5, 6, 8, and 36 weeks after bacterial inoculation or 1, 2, 4, and 31 weeks after adoptive transfer. At sacrifice, serum was collected and stored at −20°C. Stomachs were aseptically removed and bisected along the greater and lesser curvatures. One half of the stomach was homogenized, and bacterial colonization was determined by plating serial dilutions on blood agar plates to which a modified Skirrow's antibiotic supplement had been added (vancomycin, 100 μg/ml; polymyxin B, 3.3 μg/ml; trimethoprim lactate, 25 μg/ml; amphotericin B, 50 μg/ml; and naladixic acid, 10.1 μg/ml). The other half of the stomach was divided longitudinally into 1- to 2-mm-wide strips and immersed in formalin for histologic examination. In some experiments, splenocytes were isolated for determination of blastogenesis in response to H. pylori sonicate.
Enzyme-linked immunosorbent assay.
H. pylori-specific serum IgG and IgM were determined as previously described (15). Briefly, plates were coated with 100 μg of H. pylori SS1 sonicate/ml for 48 h at 4°C, washed, blocked with blocking buffer (1 g of gelatin/liter in 0.1 M phosphate-buffered saline plus 0.02% sodium azide), washed again, and incubated for 90 min at 37°C with terminal mouse sera diluted 1:50. Plates were then washed again, incubated with alkaline phosphatase-conjugated goat anti-mouse IgM or IgG (Bio-Rad), and washed, and alkaline phosphatase was detected with an alkaline phosphatase substrate kit (Bio-Rad) according to the manufacturer's instructions. Results are expressed as optical density at 450 nm.
Lymphocyte culture.
Splenocytes were isolated by disaggregation and hypotonic lysis (15). For blastogenesis studies, antigen (1 μg/ml) or mitogen (concanavalin A [ConA], 25 μg/ml) was added and cells were incubated for 5 days at 37°C in 5% CO2. [3H]thymidine was added (5 μCi/ml), and cells were harvested 16 to 18 h later. Uptake of radioactive label was determined by scintillation counting with a Packard model 3375 liquid scintillation counter. The labeling index was calculated as a percent maximal stimulation. For this, counts per minute (cpm) in ConA-stimulated and antigen-stimulated splenocytes were corrected by subtraction of background radioactivity (counts per minute in media alone). The labeling index was the ratio of the corrected counts per minute in sonicate-stimulated cells to the corrected counts per minute in ConA-stimulated cells, expressed as a percentage. Absolute counts for ConA-stimulated cells were up to 30,000 cpm. Counts of media were generally less than 1,000 cpm.
Histologic evaluation.
For quantification of gastritis, hematoxylin and eosin-stained sections were scored for lymphocytic and neutrophilic inflammation and gastric epithelial metaplasia as previously described (13, 15). Briefly, sections were examined under ×200 magnification, and the percentage of fields containing the following lesions was recorded: (i) polymorphonuclear leukocytes (neutrophilic inflammation); (ii) gastritis (inflammatory infiltrate [lymphocytes, macrophages, and/or neutrophils] sufficient to displace glands); and (iii) metaplasia (loss of normal fundic morphology with replacement by undifferentiated mucus-type glands). In this way the extent of inflammatory lesions, which correlates with severity, was quantified. Slides were evaluated blind, without prior knowledge of their source.
Statistics.
Group means were compared by nonparametric methods (Mann-Whitney U test) or by analysis of variance with Bonferroni's correction to compare individual groups. Values in the text are expressed as the mean ± the standard deviation. Error bars in graphs represent standard errors. Statistical significance was set at P < 0.05.
RESULTS
Gastritis.
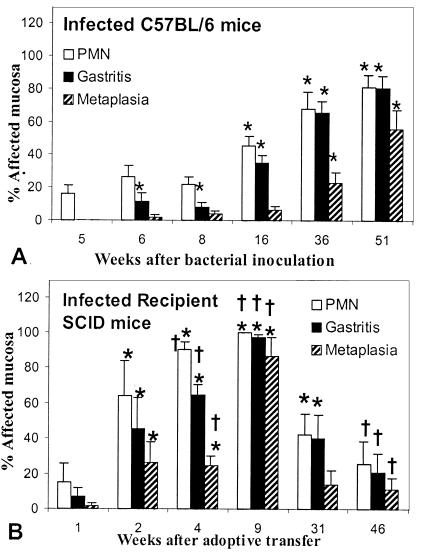
In H. pylori-infected C57BL/6 mice, gastritis gradually increased in extent over the 51-week course of the experiment (Fig. 1). By 16 weeks after inoculation, neutrophilic infiltrate (45.3% ± 15.6%) and overall gastritis (34.9% ± 13.1%) were easily discernible in the stomachs of most mice in this group and were significantly more extensive than in uninfected mice (P = 0.0027 and P = 0.0017, respectively). By 36 weeks after infection, gastric epithelial metaplasia became significantly more extensive than in uninfected mice (23.2% ± 14.1%; P = 0.009). All morphological indicators of gastritis persisted through the end of the experiment in C57BL/6 mice.
FIG. 1.
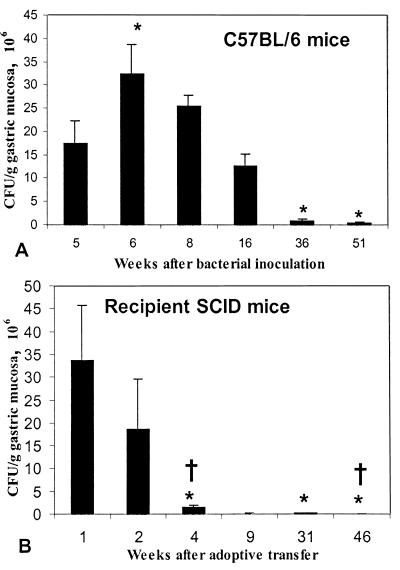
Change in gastric lesions in infected C57BL/6 mice (A) and recipient SCID mice (B) between 5 and 51 weeks after bacterial inoculation. In C57BL/6 mice, all lesions continued to increase in extent. In recipient SCID mice, lesions increased in extent until 9 weeks after adoptive transfer and then decreased in extent. By 45 weeks after transfer, lesions were not significantly different from those in uninfected mice. ∗, P < 0.05 compared to uninfected mice. †, P < 0.05 compared to C57BL/6 mice at the same postinfection interval (see Materials and Methods). PMN, polymorphonuclear leukocytes.
In contrast to wild-type mice, infected recipient SCID mice rapidly developed extensive gastritis. All morphological indicators of gastritis in these mice were significantly elevated by 2 weeks after transfer, and they continued to increase until 9 weeks after transfer (Fig. 1). Also in contrast to C57BL/6 mice, recipient SCID mice began to recover from gastritis by 31 weeks after transfer, and by 45 weeks after transfer, the extent of neutrophil infiltrate, gastritis, and metaplasia was not significantly different from that of uninfected recipient mice (P = 0.480, 0.216, and 0.157, respectively). Thus, infected recipient SCID mice differed from infected C57BL/6 wild-type mice in that gastritis in recipient SCID mice progressed rapidly, reached a peak, and resolved, while gastritis in C57BL/6 mice progressed slowly over the course of the 51-week experiment and did not resolve.
In all mice, gastritis depended on both H. pylori infection and functional immunocytes. Except for variable neutrophilic infiltration, uninfected C57BL/6 mice, uninfected recipient SCID mice, or infected nonrecipient SCID mice did not develop gastritis.
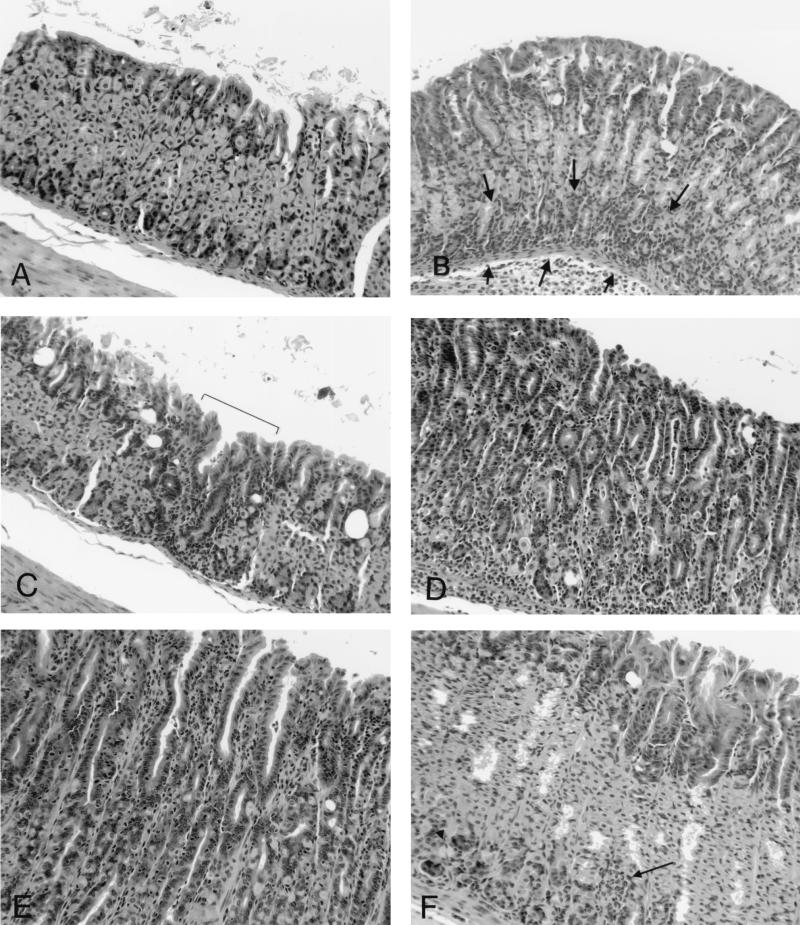
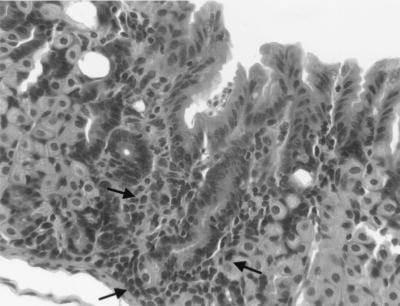
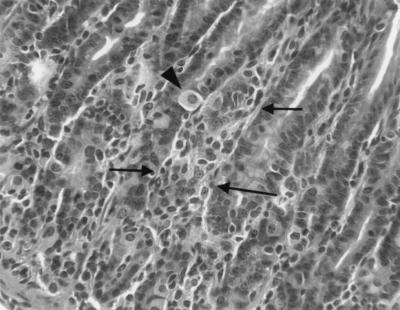
Figure 2 illustrates the histologic appearance of gastric lesions in infected C57BL/6 and infected recipient SCID mice. The character of the gastritis was similar in both groups. Five weeks after inoculation, lesions were absent in C57BL/6 mice and minimal in recipient SCID mice (Fig. 2A and B). Eight weeks after inoculation, lesions in C57BL/6 mice consisted mostly of lymphocytes with scattered plasma cells, and lesions were scattered widely within the gastric mucosa either in the superficial lamina propria or at the base of the glands (Fig. 2C and 3). In recipient SCID mice 8 weeks after inoculation, lesions were more extensive, the intensity of the infiltrate increased, and neutrophils became more prominent, often effacing glands or filling glandular lumina (Fig. 2D). The most extensively affected stomachs (infected recipient SCID mice killed 4 or 9 weeks after adoptive transfer) contained foci of widespread loss of glands with replacement by lymphocytic and neutrophilic infiltrate (Fig. 2D and 4). In remaining glands there was marked mucosal metaplasia characterized by loss of normal fundic gland morphology and replacement by straight glands lined with undifferentiated and mucus-type cells with only occasional remaining parietal or chief cells. Fifty-one weeks after inoculation, lesions in C57BL/6 mice had progressed (Fig. 2E) but were still less extensive than lesions in recipient SCID mice killed 4 to 9 weeks after transfer. By 45 weeks after transfer, lesions in recipient SCID mice had resolved (Fig. 2F). Uninfected mice and infected, nonrecipient mice had no gastric lesions.
FIG. 2.
Typical histologic appearance of gastric lesions in infected C57BL/6 and recipient SCID mice. (A) Five weeks after bacterial inoculation, lesions are absent in C57BL/6 mice. (B) Five weeks after inoculation (1 week after adoptive transfer), SCID mice have mild, widespread inflammation at the base of the gastric glands (arrows). (C) Eight weeks after infection, C57BL/6 mice develop scattered multifocal inflammatory infiltrate consisting mostly of lymphocytes (bracket). (D) Eight weeks after inoculation (4 weeks after transfer), SCID mice develop severe, widespread gastritis consisting of lymphocytes, plasma cells, and neutrophils, accompanied by destruction of gastric glands, gland abscesses (arrow), and loss of gastric gland structure. (E) Fifty-one weeks after inoculation, lesions in C57BL/6 mice are extensive and similar to, although less severe than, lesions in SCID mice 8 weeks after inoculation. (F) Fifty-one weeks after inoculation (45 weeks after transfer), lesions in SCID mice are largely resolved, with only scattered foci of inflammation (arrow) and with recovery of gastric epithelial gland structure.
FIG. 3.
Higher magnification of Fig. 2C. The focus of gastric inflammation is small and consists mostly of lymphocytes (arrows).
FIG. 4.
Higher magnification of Fig. 2D. Displacement of gastric glands by mixed inflammatory infiltrates (arrows) is accompanied by loss of normal fundic gland architecture (see text). The arrowhead indicates a single remaining parietal cell.
Bacterial colonization.
In C57BL/6 mice bacterial colonization began to decrease by 36 weeks after inoculation, and by 51 weeks, colonization density (3.34 × 105 ± 4.64 × 105 CFU/g) was significantly decreased compared to colonization density 5 weeks after inoculation (17.4 × 106 ± 10.8 × 106 CFU/g; P = 0.0090; Fig. 5). In recipient SCID mice, in contrast, bacterial colonization decreased rapidly. By 8 weeks after bacterial inoculation (4 weeks after adoptive transfer), colonization in recipient SCID mice was significantly less than colonization in C57BL/6 mice at that sacrifice interval (P = 0.0002) or colonization in recipient SCID mice at 5 weeks after inoculation (1 week after adoptive transfer; P = 0.0027). By 45 weeks after transfer, when inflammation had largely returned to background levels, bacteria were not detectable in any recipient SCID mouse. Bacterial colonization did not diminish in nonrecipient SCID mice. Five weeks after inoculation, mean colonization in these mice was 4.52 × 107 ± 1.23 × 107 CFU/g of gastric mucosa, and 51 weeks after inoculation, mean colonization had increased to 2.61 × 108 ± 0.53 × 108 CFU/g.
FIG. 5.
Bacterial colonization in infected C57BL/6 mice (A) and recipient SCID mice (B) between 5 and 51 weeks after bacterial inoculation. In C57BL/6 mice, colonization peaked 6 weeks after infection and did not decrease until 36 weeks after infection. In recipient SCID mice, bacterial colonization decreased rapidly, and by 8 weeks after inoculation it was significantly less than colonization at 5 weeks. ∗, P < 0.05 compared to 5 weeks after infection. †, P < 0.05 compared to C57BL/6 mice at the same postinfection interval.
Immune response to H. pylori antigen.
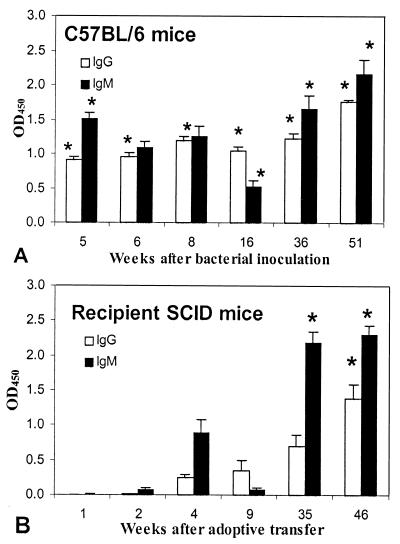
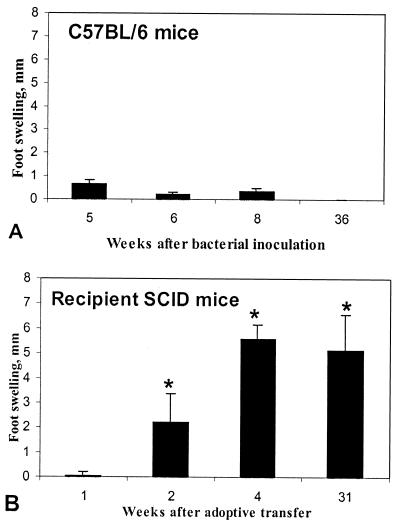
In infected C57BL/6 mice, significantly elevated serum IgG and IgM were present by 5 weeks after bacterial inoculation (P = 0.0235 and 0.0368, respectively, compared to uninfected mice [Fig. 6]), and both IgG and IgM remained elevated throughout the course of infection. In infected recipient SCID mice, however, H. pylori-specific IgM did not become significantly elevated until 31 weeks after transfer (P = 0.0086 compared to uninfected mice) and IgG did not become significantly elevated until 45 weeks after transfer (P = 0.0109). In contrast to serologic response, cellular immune response as reflected in delayed-type hypersensitivity and lymphocyte blastogenesis in response to H. pylori antigen was more pronounced in infected recipient SCID mice than in infected C57BL/6 mice. C57BL/6 mice did not develop significant footpad swelling in response to H. pylori antigen (Fig. 7). However, significant footpad swelling was evident in SCID mice by 2 weeks after transfer and was evident in mice killed 4, 9, or 31 weeks after inoculation (all SCID groups tested).
FIG. 6.
Serum H. pylori-specific IgG and IgM in C57BL/6 mice (A) and recipient SCID mice (B) between 5 and 51 weeks after bacterial inoculation. In C57BL/6 mice, both IgG and IgM were significantly elevated by 5 weeks after inoculation. In SCID mice, IgG and IgM did not become elevated until 51 and 36 weeks after inoculation, respectively. ∗, P < 0.05 compared to uninfected mice. OD450, optical density at 450 mm.
FIG. 7.
Delayed-type hypersensitivity (DTH) response to H. pylori antigens 5, 6, 8, and 36 weeks after bacterial inoculation in C57BL/6 (A) and SCID (B) mice. C57BL/6 mice did not develop a significant DTH response (as measured by footpad swelling). In SCID mice, footpad swelling was significantly elevated at 6 weeks after inoculation and remained elevated at the two later sacrifice intervals. ∗, P < 0.05 compared to uninfected mice.
Splenocytes from all groups of mice tested responded to H. pylori antigen by increased proliferation. However, the labeling index in infected recipient SCID mice (33.4% ± 16.0%) was significantly higher than in infected C57BL/6 mice (15.2% ± 15.4%) (P = 0.020). The labeling index was higher in infected C57BL/6 mice than in uninfected C57BL/6 mice (8.0 ± 6.35) but the difference was small and not statistically significant (P = 0.202).
DISCUSSION
The persistence of H. pylori in normal C57BL/6 mice in this study is consistent with extensive evidence in humans and other animals that the host immune response to gastric helicobacter fails to eliminate colonization (4, 7, 9, 10, 14, 15, 18–20, 23, 26, 29, 34, 35, 37, 38, 40). In spite of this failure, there remains some evidence that the host immune response can at least partly suppress colonization. First, we have shown that in normal mice, colonization diminishes as gastritis intensifies. In contrast, immunodeficient nonrecipient SCID mice which fail to develop gastritis support higher H. pylori colonization densities than do wild-type mice (15) (see above). Thus, the normal immune response has some protective effect. Second, stimulation of the immune response by vaccination in a variety of animal species markedly decreases colonization in response to bacterial challenge (1, 2, 5, 6, 11, 12, 14, 17, 25). In mice, vaccination also reduces the bacterial load in animals which are already infected (3, 21, 24). In spite of these promising results, however, it has become clear that while vaccination reduces bacterial colonization, it does not eliminate it. Furthermore, and of perhaps greater concern, increased severity of gastritis has been associated with vaccination in infected or challenged animals, even in the absence of detectable bacterial colonization (8, 14, 17). It has been suggested that this postimmunization gastritis is due to residual, low-level colonization because antibiotic treatment of affected hosts decreases the inflammation (17). However, there is evidence that gastritis due to H. pylori is at least in part due to cross-reactivity with host antigens (30), and the possibility remains that the increased severity of gastritis in immune hosts is due to an autoimmune-like phenomenon.
The present study was conceived based on the premise that successful vaccination is unlikely unless cure can be achieved in an unvaccinated host. The recent discovery that adoptive transfer of splenocytes leads to a rapid decrease in bacterial load in recipient SCID mice suggested that complete elimination of infection could occur (15). In that previous study it was shown that adoptive transfer of splenocytes from uninfected C57BL/6 mice to infected immunodeficient SCID recipients induces severe, rapidly progressive gastritis and rapidly decreasing colonization in the recipient. These changes were associated with preferential engraftment of CD4+ T cells, and later studies demonstrated that transfer of CD4+ cells alone is both necessary and sufficient for induction of gastritis and bacterial suppression (16). The previous studies were only 16 weeks in duration, however, and although bacterial colonization was suppressed in recipient mice, it was still detectable. Of greater concern, suppression of colonization in those studies was associated with severe widespread gastritis with epithelial proliferation and erosions. Thus, although it was clear from the short-term studies that a cellular immune response was associated with partial clearance, it was also associated with exacerbation of disease.
The results of the present long-term study demonstrate for the first time that adoptive transfer of splenocytes leads to elimination of H. pylori infection and resolution of gastritis in recipient SCID mice. This is important because it is the first example of spontaneous elimination of colonization in an animal model in the absence of either antimicrobial treatment or vaccination. Perhaps more significant is the fact that bacterial elimination was accompanied by virtually complete recovery of the normal gastric mucosal architecture. This was striking in view of the severe and widespread mucosal damage seen in recipient SCID mice 9 weeks after transfer and the previously cited concerns regarding exacerbation of gastritis in an immune host.
These results have three important implications. First, they demonstrate that the adaptive immune response is capable of eliminating infection by H. pylori. Identification of the immune elements that lead to this clearance will advance progress in the development of effective immunotherapies. Second, the results show that when bacteria are eliminated, complete recovery is possible even when gastritis is severe. Third, they support the suggestion that severe gastritis in immune hosts (i.e., postimmunization gastritis) is a response to bacterial antigen rather than an autoimmune response. The recipient SCID mice in this study developed a strong host response and extensive gastritis, but once bacterial antigens were gone, the gastritis returned to normal even in the face of the continued presence of host antigens. Thus, immune clearance of infection per se does not lead to residual disease, and effective immunotherapy, once developed, is likely to be safe.
In summary, our results show that the host response is in fact capable of eliminating infection and promoting complete recovery. The demonstration that recovery from both infection and gastritis can occur in an animal model is strong evidence that effective and safe vaccination protocols are feasible. It is likely that identification of the characteristics of this response in recipient SCID mice will allow development of vaccine protocols designed to stimulate H. pylori-specific cellular immunity and lead to the elimination of infection and resolution of gastritis in normal hosts.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grants NIH R01 AI43643 and NIH R29 DK-45340 from the National Institutes of Health.
We thank Susan Ringler for technical assistance and Steven Krakowka for critical reading of the manuscript.
REFERENCES
- 1.Batchelder M, Fox J G, Monath T, Yan L, Attardo L, Georgakopoulos K, Li X, Marini R, Shen Z, Pappo J, Lee C. Oral vaccination with recombinant urease reduces gastric Helicobacter pylori colonization in the cat. Gastroenterology. 1996;110:A58. [Google Scholar]
- 2.Chen M, Lee A, Hazell S. Immunisation against gastric helicobacter infection in a mouse/Helicobacter felis model. Lancet. 1992;339:1120–1121. doi: 10.1016/0140-6736(92)90720-n. [DOI] [PubMed] [Google Scholar]
- 3.Corthesy-Theulaz I, Porta N, Glauser M, Saraga E, Vaney A C, Haas R, Kraehenbuhl J P, Blum A L, Michetti P. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology. 1995;109:115–121. doi: 10.1016/0016-5085(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 4.Crabtree J E. Immune and inflammatory responses to Helicobacter pylori infection. Scand J Gastroenterol. 1996;31:3–10. [PubMed] [Google Scholar]
- 5.Cuenca R, Blanchard T G, Czinn S J, Nedrud J G, Monath T P, Lee C K, Redline R W. Therapeutic immunization against Helicobacter mustelae in naturally infected ferrets. Gastroenterology. 1996;110:1770–1775. doi: 10.1053/gast.1996.v110.pm8964402. [DOI] [PubMed] [Google Scholar]
- 6.Czinn S J, Cai A, Nedrud J G. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine. 1993;11:637–642. doi: 10.1016/0264-410x(93)90309-l. [DOI] [PubMed] [Google Scholar]
- 7.D'Elios M, Manghetti M, De Carli M, Costa F, Baldari C T, Burroni D, Telford J L, Romagnani S, Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 8.Dieterich C, Bouzourene H, Blum A L, Corthesy-Theulaz I E. Urease-based mucosal immunization against Helicobacter heilmannii infection induces corpus atrophy in mice. Infect Immun. 1999;67:6206–6209. doi: 10.1128/iai.67.11.6206-6209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiTommaso A, Xiang Z, Bugnoli M, Pileri P, Figura N, Bayeli P F, Rappuoli R, Abrignani S, DeMagistris M T. Helicobacter pylori-specific CD4+ T-cell clones from peripheral blood and gastric biopsies. Infect Immun. 1995;63:1102–1106. doi: 10.1128/iai.63.3.1102-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois A, Fiala N, Heman A L, Drazek E S, Tarnawski A, Fishbein W N, Perez P G, Blaser M J. Natural gastric infection with Helicobacter pylori in monkeys: a model for spiral bacteria infection in humans. Gastroenterology. 1994;106:1405–1417. doi: 10.1016/0016-5085(94)90392-1. [DOI] [PubMed] [Google Scholar]
- 11.Dubois A, Lee C K, Fiala N, Kleanthous H, Mehlman P T, Monath T. Immunization against natural Helicobacter pylori infection in nonhuman primates. Infect Immun. 1998;66:4340–4346. doi: 10.1128/iai.66.9.4340-4346.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton K, Ringler S, Krakowka S. Vaccination of gnotobiotic piglets against Helicobacter pylori. J Infect Dis. 1998;178:1399–1405. doi: 10.1086/314463. [DOI] [PubMed] [Google Scholar]
- 13.Eaton K A, Danon S J, Weisbrode S, Krakowka S. Tenth International Workshop on Campylobacter, Helicobacter, and Related Organisms. Baltimore: University of Maryland School of Medicine; 1999. A new grading system for reproducible inter-laboratory histologic quantification of gastritis in mice infected with Helicobacter pylori. [Google Scholar]
- 14.Eaton K A, Krakowka S. Chronic active gastritis due to Helicobacter pylori in immunized gnotobiotic piglets. Gastroenterology. 1992;103:1580–1586. doi: 10.1016/0016-5085(92)91181-3. [DOI] [PubMed] [Google Scholar]
- 15.Eaton K A, Ringler S R, Danon S J. Murine splenocytes induce severe gastritis and delayed-type hypersensitivity and suppress bacterial colonization in Helicobacter pylori-infected SCID mice. Infect Immun. 1999;67:4594–4602. doi: 10.1128/iai.67.9.4594-4602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eaton K A, Mefford M, Ringler S S. CD4+ T cells are necessary and sufficient for gastritis in Helicobacter pylori-infected SCID mice. Vet Pathol. 1999;36:510. [Google Scholar]
- 17.Ermak T H, Ding R, Ekstein B, Hill J, Myers G A, Lee C K, Pappo J, Kleanthous H K, Monath T P. Gastritis in urease-immunized mice after Helicobacter felis challenge may be due to residual bacteria. Gastroenterology. 1997;113:1118–1128. doi: 10.1053/gast.1997.v113.pm9322506. [DOI] [PubMed] [Google Scholar]
- 18.Ferrero R L, Thiberge J M, Huerre M, Labigne A. Immune responses of specific-pathogen-free mice to chronic Helicobacter pylori (strain SS1) infection. Infect Immun. 1998;66:1349–1355. doi: 10.1128/iai.66.4.1349-1355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox J G, Correa P, Taylor N S, Lee A, Otto G, Murphy J C, Rose R. Helicobacter mustelae-associated gastritis in ferrets. An animal model of Helicobacter pylori gastritis in humans. Gastroenterology. 1990;99:352–361. doi: 10.1016/0016-5085(90)91016-y. [DOI] [PubMed] [Google Scholar]
- 20.Fox J G, Perkins S, Yan L, Shen Z, Attardo L, Pappo J. Local immune response in Helicobacter pylori-infected cats and identification of H. pylori in saliva, gastric fluid and faeces. Immunology. 1996;88:400–406. doi: 10.1046/j.1365-2567.1996.d01-677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghiara P, Rossi M, Marchetti M, Ditommaso A, Vindigni C, Ciampolini F, Covacci A, Telford J L, Demagistris M T, Pizza M, Rappuoli R, Delgiudice G. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect Immun. 1997;65:4996–5002. doi: 10.1128/iai.65.12.4996-5002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guy B, Hessler C, Fourage S, Haensler J, Vialonlafay E, Rokbi B, Millet M J Q. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine. 1998;16:850–856. doi: 10.1016/s0264-410x(97)00258-2. [DOI] [PubMed] [Google Scholar]
- 23.Krakowka S, Ringler S S, Eaton K A, Green W B, Leunk R. Manifestations of the local gastric immune response in gnotobiotic piglets infected with Helicobacter pylori. Vet Immunol Immunopathol. 1996;52:159–173. doi: 10.1016/0165-2427(95)05547-9. [DOI] [PubMed] [Google Scholar]
- 24.Lee A. Therapeutic immunization against Helicobacter infection. Gastroenterology. 1996;110:2003–2006. doi: 10.1053/gast.1996.v110.agast962003. [DOI] [PubMed] [Google Scholar]
- 25.Lee A, Chen M. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit–whole-cell vaccine. Infect Immun. 1994;62:3594–3597. doi: 10.1128/iai.62.8.3594-3597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindholm C, Quidingjarbrink M, Lonroth H, Hamlet A, Svennerholm A M. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964–5971. doi: 10.1128/iai.66.12.5964-5971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- 28.Michetti P, Kreiss C, Kotloff K L, Porta N, Blanco J L, Bachmann D, Herranz M, Saldinger P F, Corthesytheulaz I, Losonsky G, Nichols R, Simon J, Stolte M, Ackerman S, Monath T P, Blum A L. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology. 1999;116:804–812. doi: 10.1016/s0016-5085(99)70063-6. [DOI] [PubMed] [Google Scholar]
- 29.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn S J. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 30.Negrini R, Lisato L, Zanella I, Cavazzini L, Gullini S, Villanacci V, Poiesi C, Albertini A, Ghielmi S. Helicobacter pylori infection induces antibodies cross-reacting with human gastric mucosa. Gastroenterology. 1991;101:437–445. doi: 10.1016/0016-5085(91)90023-e. [DOI] [PubMed] [Google Scholar]
- 31.Pappo J, Thomas W D, Kabok Z, Taylor N S, Murphy N S, Fox J G. Effect of oral immunization with recombinant urease on murine Helicobacter felis gastritis. Infect Immun. 1995;63:1246–1252. doi: 10.1128/iai.63.4.1246-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 33.Parsonnet J, Hansen S, Rodriguez L, Gelb A B, Warnke R A, Jellum E, Orentreich N, Vogelman J H, Friedman G D. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 34.Roth K, Kapadia S, Martin S, Lorenz R. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J Immunol. 1999;163:1490–1497. [PubMed] [Google Scholar]
- 35.Seidel K, Stolte M, Lehn N, Bauer J. Antibodies against Helicobacter felis in sera of cats and dogs. Zentbl Vetmed Reihe B. 1999;46:181–188. doi: 10.1046/j.1439-0450.1999.00219.x. [DOI] [PubMed] [Google Scholar]
- 36.Shames B, Fox J G, Dewhurst F, Yan L L, Shen Z L, Taylor N S. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J Clin Microbiol. 1995;33:2968–2972. doi: 10.1128/jcm.33.11.2968-2972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solnick J, Canfield D, Yang S, Parsonnet J. Rhesus monkey (Macaca mulatta) model of Helicobacter pylori: noninvasive detection and derivation of specific-pathogen-free monkeys. Lab Anim Sci. 1999;49:197–201. [PubMed] [Google Scholar]
- 38.Sugiyama T, Yabana T, Yachi A. Mucosal immune response to Helicobacter pylori and cytotoxic mechanism. Scand J Gastroenterol Suppl. 1995;208:30–32. [PubMed] [Google Scholar]
- 39.Warren J R. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273. [PubMed] [Google Scholar]
- 40.Wyatt J I, Rathbone B J. Immune response of the gastric mucosa to Campylobacter pylori. Scand J Gastroenterol Suppl. 1988;142:44–49. [PubMed] [Google Scholar]