Abstract
Introduction:
The high prevalence of multiple chronic conditions (MCC), multimorbidity, and frailty may affect treatment and outcomes for older adults with cancer. The goal of this study was to use three conceptually distinct measures of morbidity to examine the association between these measures and mortality.
Materials and Methods:
Using Medicare claims data linked with the 2012–2016 Ohio Cancer Incidence Surveillance System we identified older adults with incident primary cancer sites of breast, colorectal, lung, or prostate (n=29,140). We used claims data to identify their Elixhauser comorbidities, Multimorbidity-Weighted Index (MWI), and Claims Frailty Index (CFI) as measures of MCC, multimorbidity, and frailty, respectively. We used Cox proportional hazard models to examine the association between these measures and survival time since diagnosis.
Results:
Lung cancer patients had the highest levels of MCC, multimorbidity, and frailty. There was a positive association between all three measures and a greater hazard of death after adjusting for age, sex (colorectal and lung only), and stage. Breast cancer patients with 5+ comorbidities had an adjusted hazard ratio (aHR) of 1.63 (95% confidence interval [CI]: 1.38, 1.93), and those with mild frailty had an aHR of 3.38 (95% CI; 2.12, 5.41). The C statistics for breast cancer were 0.79, 0.78, and 0.79 for the MCC, MWI, and CFI respectively. Similarly, lung cancer patients who were moderately or severely frail had an aHR of 1.82 (95% CI: 1.53, 2.18) while prostate cancer patients had an aHR of 3.39 (95% CI: 2.12, 5.41) and colorectal cancer patients had an aHR of 4.51 (95% CI: 3.23, 6.29). Model performance was nearly identical across the MCC, multimorbidity, and frailty models within cancer type. The models performed best for prostate and breast cancer, and notably worse for lung cancer. The frailty models showed the greatest separation in unadjusted survival curves.
Discussion:
The MCC, multimorbidity, and frailty indices performed similarly well in predicting mortality among a large cohort of older cancer patients. However, there were notable differences by cancer type. This work highlights that although model performance is similar, frailty may serve as a clearer indicator in risk stratification of geriatric oncology patients than simple MCCs or multimorbidity.
Keywords: Multiple chronic conditions, multimorbidity, frailty, older adults
Introduction
Cancer is a disease of aging, with an estimated 70% of all cancers expected to occur in adults 65 years of age or older by 2030.1–3 The high prevalence of multiple chronic conditions (MCC), functional limitations, geriatric syndromes, and/or frailty in older adults may result in a reduced ability to tolerate cancer treatment, highlighting the importance of multidimensional evaluation of their health and all factors that might affect treatment success.4–9 MCC is often used to refer to the presence of two or more concurrent chronic conditions.10–12 This same definition is frequently used for multimorbidity, although a recent study has incorporated functional limitations and geriatric syndromes into the conceptualization of complex multimorbidity.13,14 Finally, frailty is commonly defined as a vulnerability to poor outcomes, with respect to chronological age, and is generally focused on specific domains including weight loss, exhaustion, inactivity, slowness, and weakness as well aggregating age-associated health decrements/losses.15–18 Research has shown that both comorbidities and frailty can predict long-term life expectancy.19 Although these measures — MCC, multimorbidity, and frailty —have been shown to influence health outcomes and have some elements that overlap, they are, at their core, meant to be conceptually distinct.19 Given this, it is worth investigating the potentially divergent implications each approach has for cancer care, delivery, and outcomes. One clear, important, and frequently used measure of cancer care is survival from time of diagnosis. Indeed, there have been numerous studies which have associated MCCs, multimorbidity, and frailty with poor tolerance of treatment and outcomes, which in turn affect survival.20–26 However, these studies are often in different patient populations using varying measures of MCC, multimorbidity, and frailty.
Therefore, the goal of this study was two-fold: first, to compare the performance of three different measures of patient morbidity (MCC, multimorbidity, and frailty) in modeling mortality, a commonly used outcome, and second to examine if these relationships varied across cancer types. We focused this analysis on the four most common cancers: breast cancer, colorectal cancer, lung cancer, and prostate cancer.27
Methods
Data Source
The data in this study come from two distinct sources, linked at the individual level. The first is the 2012–2016 Ohio Cancer Incidence Surveillance System (OCISS), which serves as the cancer incidence database, with required reporting under state law, for all residents of Ohio, except for in situ cervical cancer, and basal and squamous cell cancers of the skin.28 The OCISS data includes information such as demographics (sex, age, race/ethnicity, residence, etc.) as well as cancer- and treatment-related variables such as primary site, date of diagnosis, stage at diagnosis, and initial treatment information. The second data source is Medicare claims, including carrier claims, durable medical equipment, and inpatient and outpatient claims for residents of Ohio in the years 2011 through 2016. The Centers for Medicare and Medicaid Services matched OCISS data to Medicare beneficiaries using a deterministic matching algorithm based on individual’s Social Security number, date of birth, and sex (see Supplemental Figure 1). We focused our study on those patients diagnosed with cancer from 2012 to 2016, with Medicare enrollment and claims data spanning from 2011 to 2016.
Inclusion Criteria
We limited our study population to those older (66+ years) Medicare beneficiaries, who were enrolled in fee-for-service, and had at least one valid claim (see Supplemental Figure 1). For this study we focused on the four most prevalent cancers, and therefore restricted our study population to those individuals whose primary site was either (female) breast, colorectal, lung, or prostate.27 We further removed in situ cancers, and limited to those patients with valid follow-up data and who survived at least one month to exclude those cancers which may have been diagnosed upon death. These exclusions yielded a final study cohort of 29,140. For the analyses using the Multimorbidity Weighted Index (MWI), we restricted to only those patients who were diagnosed in 2015 or earlier, as the MWI has only been published for ICD-9 data, and those with a non-missing MWI, meaning they had at least one of the conditions of interest (n = 18,747). In supplemental analyses we ran all models, as well as partial likelihood ratio tests to directly compare models, on this sub-cohort of 18,747 and observed the same pattern of results (see Supplemental Tables 1 and 2).
Multiple Chronic Conditions, Multimorbidity, and Frailty Measures
Although many would argue that MCCs and multimorbidity are synonymous, we posit that MCCs are simply the co-occurrence of chronic conditions10,29, which are typically captured in counts. Multimorbidity, on the other hand, may involve weighting and incorporating conditions that are associated with functional decline or that are multifactorial in nature, as is the case with MWI. Simply, our three measures move from a count representing multiple chronic conditions to a weighted index representing multimorbidity to an index that incorporates measures of function and chronic conditions as a measure of frailty. For our measure of MCCs, we identified the Elixhauser comorbidities and then summed the individual number that a patient had, grouping them as 0, 1 or 2, 3 or 4, and 5+.30 For multimorbidity, we used the MWI31–33, an index of 81 different chronic diseases weighted by their impact on the validated Short Form-36 physical functioning scale and summed to produce a final weighted index.31,32,34 The MWI was designed to be a continuous measure that also corresponds to the Short Form-36 units, so was examined continuously. However, in supplemental analyses, we also categorized it into quartiles (Q1: 0.0, 2.7; Q2: 2.7, 5.9; Q3: 5.9, 11.0; Q4: 11.0, 55.9). Finally, to measure frailty, we used the Claims Frailty Index (CFI) developed by Kim et al.,17 which used both diagnosis codes as well as Common Procedural Terminology and Healthcare Common Procedure Coding System codes to establish a frailty index ranging from 0 to 1.17,35 Consistent with studies by Kim et al. we then created a categorical variable from the raw score where < 0.10 were non-frail, 0.10 to < 0.20 were pre-frail, 0.20 to < 0.30 were mildly frail, 0.30 to < 0.40 were moderately frail, and 0.40 and above were severely frail.17,35 Due to small frequencies, moderately and severely frail were combined into one category. Similar to the MWI, in supplementary analyses we categorized the CFI into quartiles. We also compared the overlap of these measures by creating cross-tabulations between each of the categories. For all of these measures, we limited our claims to the one year prior to cancer diagnosis, excluding the 30 days immediately prior to diagnosis to limit to conditions preceding cancer diagnosis.
Outcomes of Interest and Statistical Analyses
This study’s main outcome of interest was overall mortality after diagnosis, which was calculated using the provided months of survival and vital status at last follow-up. Kaplan-Meier curves and Cox proportional hazards models were used to examine the association between each measure (MCC, multimorbidity, and frailty), and survival, while adjusting for age, sex (colorectal and lung only), and stage at diagnosis (local, regional, distant, unstaged). These models were stratified across the four cancer types ([female] breast cancer, colorectal cancer, lung cancer, and prostate cancer). Harrell’s statistic was generated for survival analysis models as a measure of model performance. The goal of this paper was not prediction, but rather to compare the performance, measured via Harrell’s statistic, of the models across the cancer types. In supplemental analyses, we detail the overlap of these three measures and the Kaplan-Meier curves with MWI and CFI split into quartiles (see Supplemental Table 5 and Supplemental Figures 2 and 3). Additionally, in supplemental analysis we treated each of these measures as continuous as well as standardized each measure to a mean of 0 and a standard deviation of 1 (see Supplemental Tables 3 and 4). Finally, we tested the proportional hazards assumption and found that it did not hold for breast cancer using the MWI or CFI, and all three lung cancer models. Supplementary analyses (not shown) suggest this was due, in part, to the smaller number of individuals at longer follow-up times with more severe MWI or CFI. Therefore, our estimated hazard ratios should be interpreted as the average effect, or weighted mean, over time given this non-proportionality.36 Data cleaning and analysis was conducted in SAS version 9, with analysis and visualization conducted in R.
This study was approved by the Case Western Reserve University Institutional Review Board (#20120107), the Ohio Department of Health Institutional Review Board (#201805), and the Centers for Medicare and Medicaid Services Privacy board (DUA: 2012–23469)
Results
Of the 29,140 patients, there were 7,680 (female) patients with breast cancer, 5,462 patients with colorectal cancer, 8,817 patients with lung cancer, and 7,181 patients with prostate cancer. As these data focused on older adults on Medicare, it was unsurprising that 27.9% of patients with breast cancer, 37.3% of patients with colorectal cancer, 25.0% of patients with lung cancer, and 13.4% of patients with prostate cancer were 80 years old or older (Table 1). The majority of patients were non-Hispanic White, including approximately 91% for breast, colorectal, and lung cancers, and 85% for prostate cancer (Table 1). Stage at diagnosis varied across cancer types, with 69% of patients with breast cancer being diagnosed with local-stage disease, compared to 37.6% of patients with colorectal cancer, 26.4% of patients with lung cancer, and 70.8% of patients with prostate cancer (Table 1).
Table 1.
Characteristics of the study population, stratified by cancer type
| Cancer Type | ||||
|---|---|---|---|---|
| Breast n = 7,680 |
Colorectal n = 5,462 |
Lung n = 8,817 |
Prostate n = 7,181 |
|
| Age, n (%) | ||||
| 66 – 69 | 1,996 (26.0) | 1,112 (20.4) | 2,082 (23.6) | 2,637 (36.7) |
| 70 – 74 | 1,994 (26.0) | 1,211 (22.2) | 2,568 (29.1) | 2,262 (31.5) |
| 75 – 79 | 1,548 (20.2) | 1,101 (20.2) | 1,964 (22.3) | 1,320 (18.4) |
| 80+ | 2,142 (27.9) | 2,038 (37.3) | 2,203 (25.0) | 962 (13.4) |
| Sex, n (%) | ||||
| Male | - | 2,658 (48.7) | 4,598 (52.2) | - |
| Female | - | 2,804 (51.3) | 4,219 (47.9) | - |
| Race/Ethnicity, n (%) | ||||
| Non-Hispanic White | 6,983 (90.9) | 5,007 (91.7) | 8,048 (91.3) | 6,103 (85.0) |
| Non-Hispanic Black | 621 (8.1) | 357 (6.5) | 689 (7.8) | 739 (10.3) |
| All Other | 76 (1.0) | 98 (1.8) | 80 (0.9) | 339 (4.7) |
| Stage, n (%) | ||||
| Local | 5,300 (69.0) | 2,051 (37.6) | 2,324 (26.4) | 5,087 (70.8) |
| Regional | 1,804 (23.5) | 2,101 (38.5) | 2,334 (26.5) | 893 (12.4) |
| Distant | 447 (5.8) | 928 (17.0) | 3,803 (43.1) | 557 (7.8) |
| Unstaged/Unknown | 129 (1.7) | 382 (7.0) | 356 (4.0) | 644 (9.0) |
| Died, n (%) | 1,068 (13.9) | 1,692 (31.0) | 5,470 (62.0) | 754 (10.5) |
| Follow-up time in months, median (IQR) | 23 (12 – 36) | 18 (8 – 32) | 10 (4 – 20) | 23 (12 – 37) |
| Elixhauser Comorbidity Count, n (%) | ||||
| Mean (Std. Dev) | 2.56 (2.59) | 2.72 (2.92) | 2.99 (2.91) | 1.83 (2.17) |
| Median (IQR) | 2 (0 – 4) | 2 (0 – 4) | 2 (0 – 5) | 1 (0 – 3) |
| 0 | 2,006 (26.1) | 1,585 (29.0) | 2,246 (25.5) | 2,643 (36.8) |
| 1 or 2 | 2,461 (32.0) | 1,560 (28.6) | 2,321 (26.3) | 2,486 (34.6) |
| 3 or 4 | 1,765 (23.0) | 1,137 (20.8) | 2,011 (22.8) | 1,276 (17.8) |
| 5+ | 1,448 (18.9) | 1,180 (21.6) | 2,239 (25.4) | 776 (10.8) |
| Claims Frailty Index, n (%) | ||||
| Mean (Std. Dev) | 0.15 (0.06) | 0.16 (0.06) | 0.16 (0.07) | 0.13 (0.05) |
| Median (IQR) | 0.14 (0.10 – 0.18) | 0.14 (0.10 – 0.18) | 0.15 (0.10 – 0.20) | 0.12 (0.10 – 0.15) |
| Non-Frail | 483 (6.3) | 296 (5.4) | 359 (4.1) | 909 (12.7) |
| Pre-Frail | 5,843 (76.1) | 4,109 (75.2) | 6,339 (71.9) | 5,657 (78.8) |
| Mildly Frail | 1,093 (14.2) | 825 (15.1) | 1,689 (19.2) | 532 (7.4) |
| Moderately/Severely Frail | 261 (3.4) | 232 (4.3) | 430 (4.9) | 83 (1.2) |
| Multimorbidity Weighted Index | n = 5,121 | n = 3,528 | n = 5,741 | n = 4,357 |
| Mean (Std. Dev) | 7.36 (6.2) | 8.20 (6.88) | 10.19 (7.73) | 6.19 (5.67) |
| Median (IQR) | 5.86 (2.63 – 10.27) | 6.38 (2.96 – 11.81) | 8.52 (4.32 – 14.62) | 4.92 (1.62 – 8.81) |
The distribution of MCC, the MWI, and the CFI varied across cancer types. Notably, patients with prostate cancer had lower counts of MCC, a greater percent were non-frail, and had the lowest mean MWI among all four cancers (Table 1). Patients with lung cancer had the highest levels of MCC, multimorbidity, and frailty with 25.4% having five or more Elixhauser comorbidities, a mean MWI of 10.2, and 19.2% being mildly frail with 4.9% being moderately or severely frail (Table 1). Even among patients with breast and colorectal cancer, 18.9% and 21.6%, respectively, had five or more Elixhauser comorbidities and 3.4% and 4.3%, respectively, were considered moderately or severely frail (Table 1).
Interestingly, the three measures only showed a moderate amount of overlap between them, with the CFI having a distribution skewed towards lower levels when compared to the Elixhauser comorbidities or the MWI (see Supplemental Table 5 and Supplemental Figure 2). For example, among those with five or more Elixhauser comorbidities, 34% were considered pre-frail and among those who were pre-frail only 14% were in the second quartile of MWI. However, we see that 94% of those who were moderately or severely frail were in the fourth quartile of the MWI, suggesting high overlap between the most frail and multimorbid individuals. The effect of this overlap can be seen in that the Kaplan-Meier curves for the Elixhauser comorbidity count and MWI are similar, while the CFI shows greater separation (Figures 1 – 4). However, when CFI is split into quartiles, instead, we see it far more comparable to the Elixhauser comorbidity count and MWI (see Supplemental Figure 3).
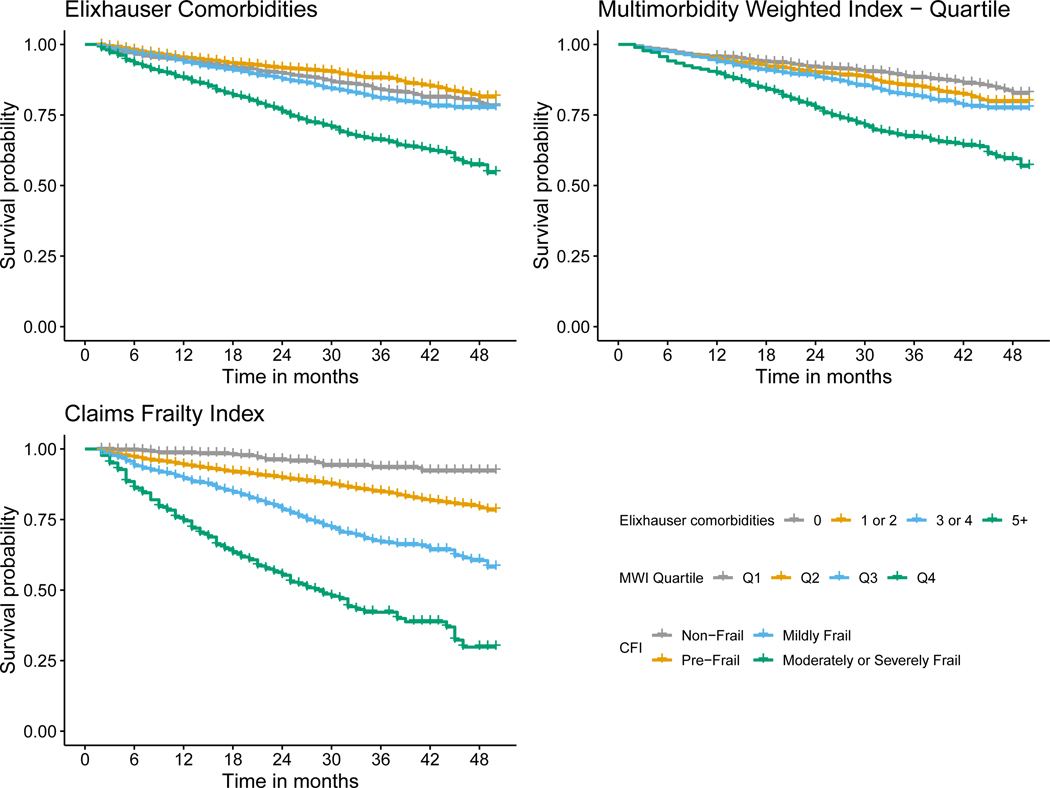
Figure 1.
Kaplan-Meier survival curve for breast cancer stratified by Elixhauser comorbidity count, Multimorbidity Weighted Index (MWI) quartile, Claims Frailty Index (CFI).
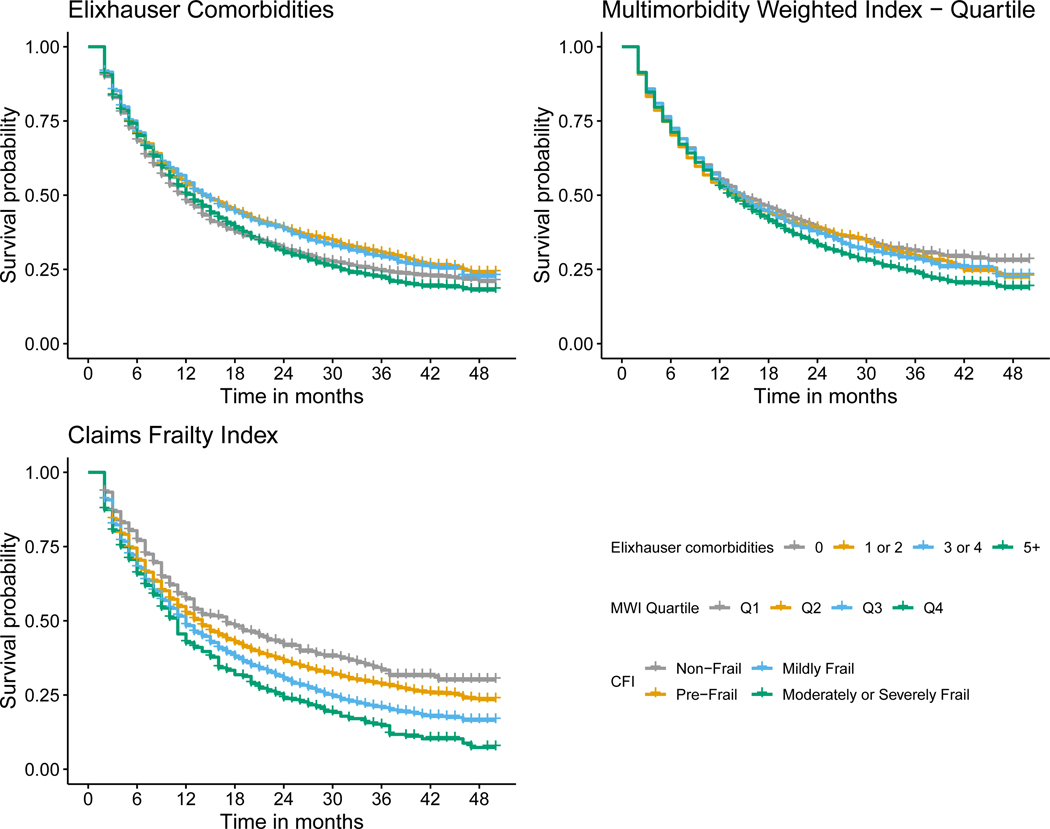
Figure 4.
Kaplan-Meier survival curve for prostate cancer stratified by Elixhauser comorbidity count, Multimorbidity Weighted Index (MWI) quartile, Claims Frailty Index (CFI).
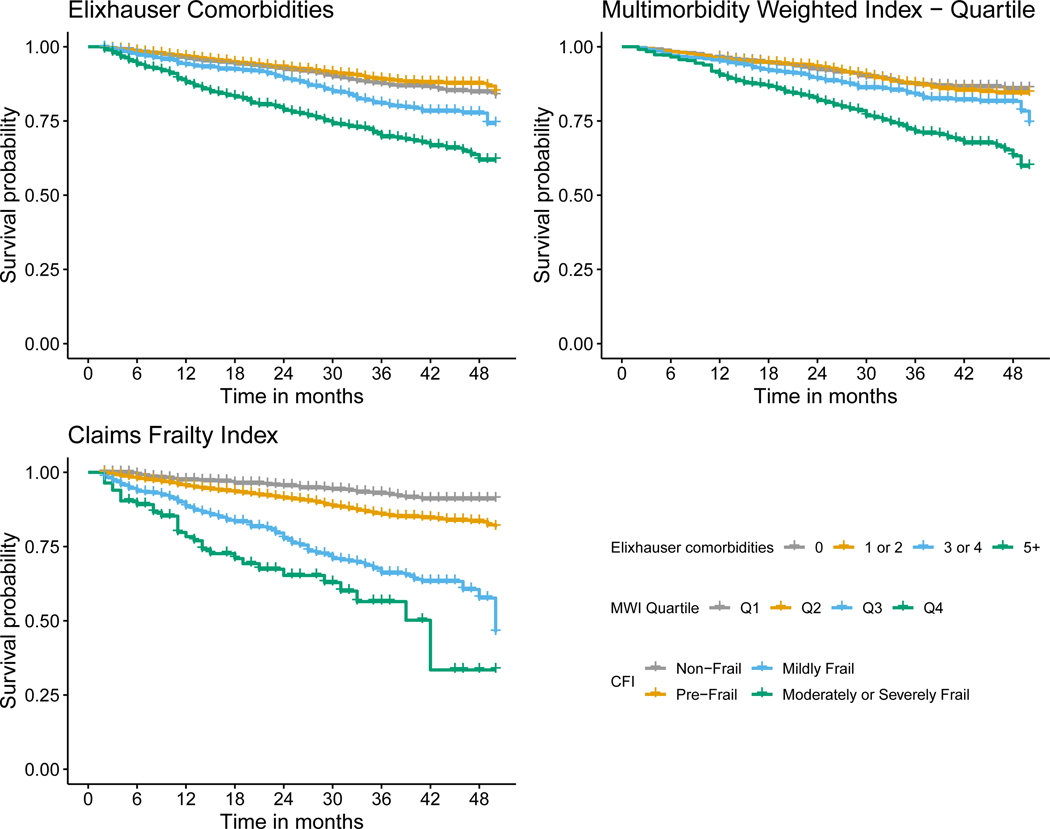
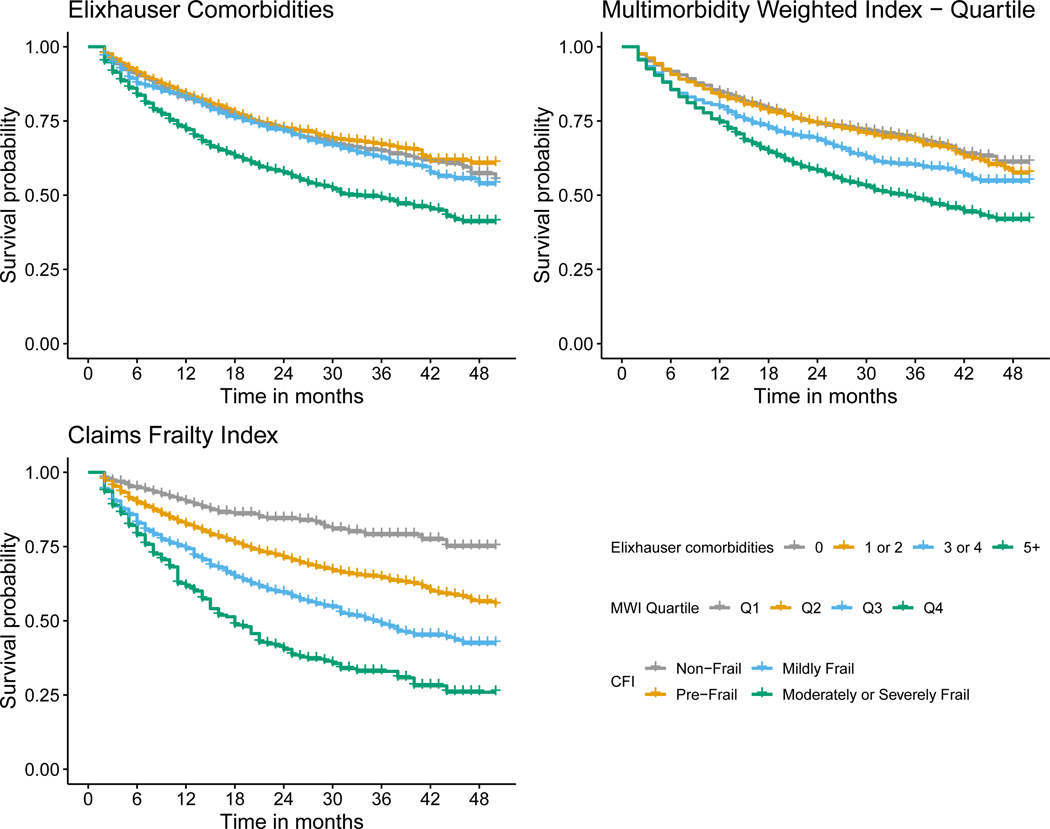
MCC, multimorbidity, and frailty were each associated with a greater hazard of death across all cancer types (Table 2 and Figures 1, 2, 3, and 4). Overall, mortality was highest among patients with lung cancer, with 62% dying during the study period, and a median survival time of ten months (Table 1). For patients with breast cancer, those with five or more Elixhauser comorbidities had a hazard ratio of 1.63 (95% confidence interval [CI]: 1.38, 1.93) compared to those patients with breast cancer without any comorbidities (Table 2, Figure 1). We saw a similar pattern across colorectal, lung, and prostate cancer, although there was a weaker association for patients with lung cancer (Table 2, Figures 2 – 4). The MWI was similarly associated with mortality, with a single point increase in MWI associated with a 1 to 6% increase in the hazard of mortality, although it varied by cancer type (Table 2, Figures 1 – 4). When evaluating frailty, on the other hand, we saw a much stronger association between those patients with moderate or severe frailty and mortality than when using MCCs. For patients with breast cancer who were moderately or severely frail there was a hazard ratio of 6.89 (95% CI: 4.24, 11.22) compared to those who were non-frail. This extended to patients with colorectal cancer, lung cancer, and prostate cancer, with hazard ratios of 4.51 (3.23, 6.29), 1.82 (1.53, 2.18), and 3.39 (2.12, 5.41), respectively (Table 2, Figures 1 – 4). This association of increased hazard for mortality was observed even for those patients who were considered pre-frail when compared to those who were non-frail (Table 2). Finally, we observed similar Harrell’s statistic across all three measures, although supplemental analyses suggested perhaps stronger performance by the CFI models, although this was not uniform (Table 2, see also Supplemental Table 2).
Table 2.
Hazard ratio and 95% confidence interval for mortality, stratified by cancer type and frailty or multimorbidity measure.
| Breast | Colorectal | Lung | Prostate | |
|---|---|---|---|---|
| Elixhauser Comorbidities | ||||
| Age | 1.08 (1.07, 1.09) | 1.06 (1.05, 1.06) | 1.03 (1.03, 1.03) | 1.08 (1.07, 1.10) |
| Sex (ref: Male) | - | 0.95 (0.86, 1.04) | 0.88 (0.84, 0.93) | - |
| Stage (ref: Localized) | - | - | - | - |
| Regional | 1.98 (1.72, 2.29) | 1.67 (1.46, 1.92) | 2.02 (1.84, 2.20) | 1.53 (1.18, 1.99) |
| Distant | 9.18 (7.83, 10.76) | 7.82 (6.83, 8.95) | 4.60 (4.25, 4.98) | 7.40 (6.17, 8.87) |
| Unstaged/Unknown | 4.32 (3.28, 5.68) | 3.97 (3.32, 4.73) | 3.88 (3.39, 4.44) | 1.79 (1.41, 2.27) |
| Elixhauser (ref: 0 comorbidities) | - | - | - | - |
| 1 or 2 Comorbidities | 0.66 (0.54, 0.79) | 0.84 (0.74, 0.97) | 0.85 (0.79, 0.92) | 0.78 (0.64, 0.94) |
| 3 or 4 Comorbidities | 0.88 (0.73, 1.06) | 0.97 (0.84, 1.13) | 0.91 (0.84, 0.99) | 1.09 (0.89, 1.34) |
| 5+ Comorbidities | 1.63 (1.38, 1.93) | 1.49 (1.31, 1.70) | 1.05 (0.97, 1.13) | 1.61 (1.31, 1.98) |
| Harrell’s statistic | 0.79 | 0.75 | 0.69 | 0.81 |
| Multimorbidity-Weighted Index | ||||
| Age | 1.08 (1.07, 1.09) | 1.05 (1.05, 1.06) | 1.03 (1.02, 1.03) | 1.09 (1.08, 1.10) |
| Sex (ref: Male) | - | 0.96 (0.86, 1.07) | 0.91 (0.85, 0.97) | - |
| Stage (ref: Localized) | - | - | - | - |
| Regional | 2.04 (1.74, 2.39) | 1.64 (1.41, 1.91) | 2.00 (1.80, 2.21) | 1.71 (1.27, 2.31) |
| Distant | 9.03 (7.48, 10.90) | 7.50 (6.42, 8.75) | 4.47 (4.08, 4.90) | 6.22 (5.01, 7.72) |
| Unstaged/Unknown | 4.28 (3.15, 5.81) | 3.68 (3.02, 4.49) | 4.14 (3.55, 4.82) | 1.73 (1.34, 2.24) |
| MWI, per 1 point increase | 1.06 (1.05, 1.07) | 1.04 (1.03, 1.05) | 1.01 (1.01, 1.02) | 1.04 (1.02, 1.05) |
| Harrell’s statistic | 0.78 | 0.74 | 0.69 | 0.80 |
| Claims Frailty Index | ||||
| Age | 1.07 (1.06, 1.08) | 1.05 (1.05, 1.06) | 1.03 (1.02, 1.03) | 1.08 (1.07, 1.09) |
| Sex (ref: Male) | - | 0.90 (0.81, 0.99) | 0.87 (0.82, 0.92) | - |
| Stage (ref: Localized) | - | - | - | - |
| Regional | 1.95 (1.69, 2.25) | 1.68 (1.47, 1.93) | 2.03 (1.86, 2.22) | 1.52 (1.17, 1.97) |
| Distant | 9.08 (7.75, 10.63) | 8.00 (6.99, 9.16) | 4.66 (4.30, 5.04) | 7.23 (6.03, 8.67) |
| Unstaged/Unknown | 3.84 (2.92, 5.06) | 3.81 (3.19, 4.55) | 3.86 (3.37, 4.42) | 1.80 (1.42, 2.28) |
| CFI (ref: Non-Frail) | - | - | - | - |
| Pre-Frail | 2.00 (1.27, 3.17) | 1.73 (1.30, 2.32) | 1.24 (1.07, 1.43) | 1.62 (1.19, 2.20) |
| Mildly Frail | 3.38 (2.12, 5.41) | 2.51 (1.85, 3.40) | 1.46 (1.25, 1.70) | 2.59 (1.83, 3.67) |
| Moderately or Severely Frail | 6.89 (4.24, 11.22) | 4.51 (3.23, 6.29) | 1.82 (1.53, 2.18) | 3.39 (2.12, 5.41) |
| Harrell’s statistic | 0.79 | 0.75 | 0.69 | 0.81 |
Figure 2.
Kaplan-Meier survival curve for colorectal cancer stratified by Elixhauser comorbidity count, Multimorbidity Weighted Index (MWI) quartile, Claims Frailty Index (CFI).
Figure 3.
Kaplan-Meier survival curve for lung cancer stratified by Elixhauser comorbidity count, Multimorbidity Weighted Index (MWI) quartile, Claims Frailty Index (CFI).
While there was variation in the statistics across cancer type, with the models often performing best for breast cancer and worst for lung cancer, the performance within cancer type was nearly identical across the Elixhauser comorbidity, MWI, and CFI models. Supplemental analysis using continuous and standardized measures for all three showed similar results (see Supplemental Tables 3 and 4).
Discussion
When applying three different measures of a patient’s health state—one for multiple chronic conditions (MCC), one for multimorbidity, and one for frailty—to older patients with cancer we found that regardless of measure, MCC, multimorbidity, and frailty were all associated with a greater hazard for mortality. This confirms the abundance of literature which has identified the association between MCC, multimorbidity, and frailty and care and outcomes, such as survival, in separate studies.21,37–40 This study, however, conducting a comparison of these three measures on the same patients, elucidates important differences in the role that MCC, multimorbidity, and frailty play across cancer types. To our knowledge, this is first time such a head-to-head comparison has been performed.
We observed that the association between MCC, multimorbidity, and frailty and mortality varied across cancer types. Specifically, we saw that the level of MCC, multimorbidity, and frailty seemed to be least associated with mortality for patients with lung cancer, as all levels of MCC, multimorbidity, and frailty had near identical survival curves (Figure 3). This is not surprising, given that most patients with lung cancer are diagnosed with late-stage disease, and that prognosis is driven by the underlying lung cancer diagnosis rather than other causes of death. Conversely, it appeared for the other three cancers, and most substantially breast and prostate cancer, that the highest levels of MCC, multimorbidity, and frailty were associated with shortened survival (Figures 1, 2, and 4). It is also worth noting that across all three measures and four cancers, the lower levels of MCC, multimorbidity, and frailty tended to cluster. Overall, these specific findings underscore the heterogeneity that MCC, multimorbidity, and frailty have on mortality depending on cancer type. As there were meaningful differences in the magnitude of the effects and model performance across cancer types, future studies should examine the mechanism of these associations in a cancer-specific context.
Taken together, these findings suggest that these measures are either capturing different components of health or, more likely, there are non-linear relationships and specific subsets of patients that carry a given combination of factors that may be more highly weighted in one of the measures and not the others. We see clearly in each of the figures that the CFI categories have more spread and may better elucidate the sub-groups who are at a higher risk for mortality. While this could certainly be mirrored adjusting the categories of Elixhauser and MWI, the CFI has established categories that conveniently separate the population into clinically interpretable groups which clearly are associated with mortality—giving it a notable strength over the other two measures. It is important to acknowledge that the quartile grouping of the MWI was arbitrarily chosen to roughly match the distribution of the Elixhauser comorbidities. Other published approaches using the MWI have utilized restricted cubic splines, although these will vary based on outcome and the sample population.41,42 Despite this limitation, it calls attention to the need to establish a validated approach for grouping both the Elixhauser comorbidities and the MWI in a clinically-meaningful approach similar to the CFI.
Although these measures of MCC, multimorbidity, and frailty performed similarly according to Harrell’s statistic, it was nonetheless notable that the higher levels of frailty had a substantially higher hazard of mortality than either MCC or multimorbidity. This reinforces recent work which emphasizes the importance of geriatric assessments in older patients with cancer.43 When considering which measure to use, given these findings, it is important to recognize that there are advantages to each that may make choosing one more appealing. First, the Elixhauser comorbidities are the most flexible and easy to implement across datasets that may vary in their structure. This measure is a discrete list of specific comorbidities that are simpler to document and extract. The biggest limitation to the Elixhauser comorbidities is that they likely do not capture the totality of one’s health through its limited inventory of conditions and may be missing important components of health such as function-related measures (e.g., use of walking aids) — a gap that the CFI fills. The CFI weights not only diagnosis codes, but procedures codes as well, meaning it can capture utilization of mobility-related services (e.g., using a walker or wheelchair) that are informative in making treatment decisions. This frailty measure, at its outset, may not be too informative as it is unclear what the raw CFI value means, however, using the categorical conversion eases the interpretation. Finally, as the CFI ranges from 0 to 1, with a sub-maximum around 0.7, it may be hard to extrapolate what small increases in the score represent.35 The third measure used, the MWI, has similar issues of interpretability. However, as developed, a 1-point increase in the MWI conveniently calibrates to a 1-point decrease in physical functioning, where declines of 2–3 points are clinically significant.41 Furthermore, as it has a wider distribution including less left skew than Elixhauser and no ceiling like the CFI, tracking a patient’s or population’s changes in multimorbidity overtime may be highly efficient when using the MWI. The MWI is also among the most comprehensive measures, including 81 prevalent and rare but impactful chronic conditions.32
Given all of this, it becomes clear that the similar model performance that we observed, as measured via the statistic, should not outweigh the conceptual and practical advantages of each of these measures. Similar statistics may mask important nuances that would help us to better understand and risk stratify these populations. As these indices encapsulate many different conditions—and did not completely overlap in categorizing patient’s morbidity (see Supplemental Table 5, Supplemental Figures 2 and 3), they need to be unpacked to identify specific combinations, or phenotypes, that are associated with not only mortality but also other specific adverse outcomes of importance to patients, such as functional decline, disability, and poor health-related quality of life. However, it is worth acknowledging that if one’s outcome and focus is simply model performance for predicting mortality, then the Elixhauser comorbidities, as a measure of MCCs, may be just as effective as more complex methods. Although, this may be missing important information – especially for the highest risk group – as evidenced by the greater separation of the survival curves we observed for CFI.
This study is limited by the usual aspects of administrative data, including the lack of clinical context. This is particularly important in this study as relevant aspects of health may not be captured or documented via diagnosis or procedure codes. Nonetheless, each of these measures has been independently tested and validated, with CFI and MWI performing similarly well to self-reported health and outcomes.17,32 Additionally, a few of our models violated the assumption of non-proportionality, which suggests we should interpret these hazard ratios as an average effect over the study period. Another important limitation is that these measures were developed in a population of adults who may not necessarily have cancer. Therefore, it is worth examining how the various weights assigned may or may not differ for older patients with cancer specifically. A substantial limitation of this work was not including detailed information on treatment in our models. It is possible that those patients with a higher MCC, multimorbidity, or frailty burden may receive less aggressive treatment, which leads to poorer prognosis and faster mortality. Treatment and other aspects of cancer care and outcomes, including quality of life, were outside the scope of this paper but provide a clear direction for future work. Finally, future work should continue to describe the mechanisms of these differences and continue to refine interventions that can target care and improve outcomes for older adults with concomitant cancer and complex health states.
Conclusions
Multiple chronic conditions, multimorbidity, and frailty are all associated with poor survival among older patients with cancer. However, we observed variation in these relationships across cancer types and across measures. Notably, while model performance was similar, it was clear that the highest levels of frailty had the strongest associations with mortality. Taken together, the findings of this study suggest that in order to achieve precision geriatric oncology we must consider the use of these indices in a cancer-specific context and continue to identify ways to improve their accuracy.
Supplementary Material
Acknowledgements
This study was funded by the Case Comprehensive Cancer Center (P30 CA043703).
Disclosures
Mr. Bensken is supported by a grant from the National Institute of Minority Health and Health Disparities (1F31MD015681).
Dr. Schiltz reports no disclosures.
Dr. Warner was supported by grants from the National Cancer Institute, Case Comprehensive Cancer Center (P30 CA043703) and the Centers for Disease Control and Prevention (3 U48 DP006404-03S7). This research was also supported in part by the Center for Family and Demographic Research, Bowling Green State University, which has core funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P2CHD050959).
Dr. Kim is supported by grants R01AG071809, R01AG062713, R01AG056368, and R21AG060227 from the National Institute on Aging. Dr. Kim receives personal fees from Alosa Health and VillageMD.
Dr. Wei was supported by the National Institutes of Health, National Institute on Aging (grant K23AG056638).
Dr. Quiñones reports no disclosures.
Dr. Ho is supported by the Clinical and Translational Science Collaborative of Cleveland, KL2TR002547 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research.
Dr. Kelley is supported by the National Institute on Aging (NIA K24 AG062785).
Dr Owusu is supported by grants from the National Institute of Minority Health and Health Disparities (R01 MD009699) and the National Cancer Institute (R25 CA22178A, 1P20CA233216-01).
Dr. Kent reports no disclosures.
Dr. Koroukian, in the past 36 months, was supported by grants from the: National Cancer Institute, Case Comprehensive Cancer Center (P30 CA043703); Centers for Disease Control and Prevention, U48 DP005030-05S1 and U48 DP006404-03S7; Ohio Medicaid Technical Assistance and Policy Program (MEDTAPP); National Institutes of Health (R15 NR017792, and UH3-DE025487); The American Cancer Society ( 132678-RSGI-19-213-01-CPHPS and RWIA-20-111-02 RWIA); and by contracts from Cleveland Clinic Foundation, including a subcontract from Celgene Corporation. Dr. Koroukian’s spouse has stock ownership in American Renal Associates, Inc.
References
- 1.Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94(10):2766–2792. [DOI] [PubMed] [Google Scholar]
- 2.Balducci L, Ershler WB. Cancer and ageing: a nexus at several levels. Nature reviews Cancer. 2005;5(8):655–662. [DOI] [PubMed] [Google Scholar]
- 3.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–2765. [DOI] [PubMed] [Google Scholar]
- 4.Repetto L, Balducci L. A case for geriatric oncology. Lancet Oncol. 2002;3(5):289–297. [DOI] [PubMed] [Google Scholar]
- 5.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition--multimorbidity. JAMA. 2012;307(23):2493–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162(20):2269–2276. [DOI] [PubMed] [Google Scholar]
- 7.Lochner KA, Cox CS. Prevalence of multiple chronic conditions among Medicare beneficiaries, United States, 2010. Prev Chronic Dis. 2013;10:E61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lochner KA, Goodman RA, Posner S, Parekh A. Multiple chronic conditions among Medicare beneficiaries: state-level variations in prevalence, utilization, and cost, 2011. Medicare & medicaid research review. 2013;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.United States Department of Health and Human Services. Multiple Chronic Conditions — A Strategic Framework: Optimum Health and Quality of Life for Individuals with Multiple Chronic Conditions. Washington, DC2010. [Google Scholar]
- 11.Anderson G. Chronic Care: Making the Case for Ongoing Care. Princeton, NJ: Robert Wood Johnson Foundation;2010. [Google Scholar]
- 12.Parekh AK, Goodman RA, Gordon C, Koh HK, Conditions HHSIWoMC. Managing multiple chronic conditions: a strategic framework for improving health outcomes and quality of life. Public Health Rep. 2011;126(4):460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koroukian SM, Warner DF, Owusu C, Given CW. Multimorbidity redefined: prospective health outcomes and the cumulative effect of co-occurring conditions. Prev Chronic Dis. 2015;12:E55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho VP, Bensken WP, Warner DF, et al. Association of Complex Multimorbidity and Long-term Survival After Emergency General Surgery in Older Patients With Medicare. JAMA Surg. 2022. [DOI] [PMC free article] [PubMed]
- 15.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 16.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. TheScientificWorldJournal. 2001;1:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockwood K, Howlett SE. Fifteen years of progress in understanding frailty and health in aging. BMC Med. 2018;16(1):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoenborn NL, Blackford AL, Joshu CE, Boyd CM, Varadhan R. Life expectancy estimates based on comorbidities and frailty to inform preventive care. J Am Geriatr Soc. 2022;70(1):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross CP, Guo Z, McAvay GJ, Allore HG, Young M, Tinetti ME. Multimorbidity and survival in older persons with colorectal cancer. J Am Geriatr Soc. 2006;54(12):1898–1904. [DOI] [PubMed] [Google Scholar]
- 21.Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26(6):1091–1101. [DOI] [PubMed] [Google Scholar]
- 22.Koroukian SM. Assessment and interpretation of comorbidity burden in older adults with cancer. J Am Geriatr Soc. 2009;57 Suppl 2:S275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koroukian SM, Xu F, Beaird H, Diaz M, Murray P, Rose JH. Complexity of care needs and unstaged cancer in elders: a population-based study. Cancer Detect Prev. 2007;31(3):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luque-Fernandez MA, Goncalves K, Salamanca-Fernandez E, et al. Multimorbidity and short-term overall mortality among colorectal cancer patients in Spain: A population-based cohort study. Eur J Cancer. 2020;129:4–14. [DOI] [PubMed] [Google Scholar]
- 25.Warner DF, Koroukian SM, Schiltz NK, et al. Complex Multimorbidity and Breast Cancer Screening Among Midlife and Older Women: The Role of Perceived Need. Gerontologist. 2019;59(Suppl 1):S77–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warner DF, Schiltz NK, Stange KC, et al. Complex multimorbidity and health outcomes in older adult cancer survivors. Fam Med Community Health. 2017;5(2):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Cancer Institute. Statistics at a Glance: The Burden of Cancer in the United States. National Institutes of Health. https://www.cancer.gov/about-cancer/understanding/statistics. Published 2020. Accessed 16 August, 2021. [Google Scholar]
- 28.Ohio Department of Health. Ohio Cancer Incidence Surveillance System (OCISS). https://odh.ohio.gov/wps/portal/gov/odh/know-our-programs/ohio-cancer-incidence-surveillance-system. Published 2021. Accessed 11 October, 2021.
- 29.Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis. 2014;11:E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 31.Wei MY, Kabeto MU, Langa KM, Mukamal KJ. Multimorbidity and Physical and Cognitive Function: Performance of a New Multimorbidity-Weighted Index. J Gerontol A Biol Sci Med Sci. 2018;73(2):225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei MY, Ratz D, Mukamal KJ. Multimorbidity in Medicare Beneficiaries: Performance of an ICD-Coded Multimorbidity-Weighted Index. J Am Geriatr Soc. 2020;68(5):999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei MY, Kawachi I, Okereke OI, Mukamal KJ. Diverse Cumulative Impact of Chronic Diseases on Physical Health-Related Quality of Life: Implications for a Measure of Multimorbidity. Am J Epidemiol. 2016;184(5):357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ware J, Snow K, Kosinski M, B G, & New England Medical Center. SF-36 Health Survey: Manual and Interpretation Guide. Massachusetts: The Health Institute, New England Medical Center; 1997. [Google Scholar]
- 35.Kim DH, Gautam N. SAS Programs - Claims-Based Frailty Index. In. NIA, trans. V10 ed: Harvard Dataverse; 2020. [Google Scholar]
- 36.Stensrud MJ, Hernan MA. Why Test for Proportional Hazards? JAMA. 2020;323(14):1401–1402. [DOI] [PubMed] [Google Scholar]
- 37.Cespedes Feliciano EM, Hohensee C, Rosko AE, et al. Association of Prediagnostic Frailty, Change in Frailty Status, and Mortality After Cancer Diagnosis in the Women’s Health Initiative. JAMA Netw Open. 2020;3(9):e2016747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duthie K, Strohschein FJ, Loiselle CG. Living with cancer and other chronic conditions: Patients’ perceptions of their healthcare experience. Can Oncol Nurs J. 2017;27(1):43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guy GP Jr., Yabroff KR, Ekwueme DU, Rim SH, Li R, Richardson LC Economic Burden of Chronic Conditions Among Survivors of Cancer in the United States. J Clin Oncol. 2017;35(18):2053–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loeppenthin K, Dalton SO, Johansen C, et al. Total burden of disease in cancer patients at diagnosis-a Danish nationwide study of multimorbidity and redeemed medication. Br J Cancer. 2020;123(6):1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei MY, Mukamal KJ. Multimorbidity, Mortality, and Long-Term Physical Functioning in 3 Prospective Cohorts of Community-Dwelling Adults. Am J Epidemiol. 2018;187(1):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei MY, Mukamal KJ. Multimorbidity and Mental Health-Related Quality of Life and Risk of Completed Suicide. J Am Geriatr Soc. 2019;67(3):511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohile SG, Mohamed MR, Xu H, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. The Lancet. 2021;398(10314):1894–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.