Abstract
Background and aim:
Small fiber neuropathy (SFN) may present as complication in sarcoidosis. SFN can potentially result into a large range of symptoms with a high impact on quality of life. Although treatment of the underlying disease of SFN is paramount, little research has been performed to investigate SFN improvement as consequence of sarcoidosis treatment. This retrospective study investigates whether there is an association between the anti-inflammatory effects of infliximab and SFN-symptoms
Methods:
The Small Fiber Neuropathy Screening List (SFNSL) was used to measure changes in SFN symptoms during infliximab treatment. Maximal standardized uptake value (SUVmax) from Fluordeoxyglucose Positron Emission Tomography (FDG-PET) was used as a measure for inflammatory activity
Results:
36 sarcoidosis patients were eligible for analysis. SFNSL-score showed a mean decrease of -1,9 points (p = 0.446). SUVmax did improve with a mean of -3.7 (p<0.001). No correlation between a decrease of SUVmax and SFNSL screening list could be found (p=0.610)
Conclusions:
Our data reveal no association between anti-inflammatory effect of infliximab and SFN-related symptoms in patients with sarcoidosis, which contradicts previous case-reports and case-series. Given the major negative impact of SFN-related symptoms on the quality of life in patients with sarcoidosis, it is necessary that the possible beneficial effect of anti-inflammatory therapy will be further addressed in future prospective studies.
Keywords: small fiber neuropathy, sarcoidosis, infliximab
Introduction
Sarcoidosis is a systemic granulomatous disease of unknown cause that most often affects the lungs and intrathoracic lymph nodes, but many other organs and tissues can also be involved (1,2). Clinical presentation and treatment of sarcoidosis depends on the organs affected. Pharmacological treatment of sarcoidosis is mostly initiated to either prevent specific organ damage or alleviate symptoms and includes corticosteroids, disease modifying anti rheumatic drugs (DMARDs) or anti-tumor necrosis factor alpha (anti-TNF-α) inhibitors like infliximab (3,4).
Many symptoms in patients with sarcoidosis, however, are not organ specific and include fatigue, cognitive failure or pain which can be caused by small fiber neuropathy (SFN) (5,6). Prevalence of SFN in patients suffering from sarcoidosis ranges between 60-86% (5,7) and may contribute to a poor health-related quality of life (HRQL) (5,8,9).
The pathogenesis of SFN in sarcoidosis is unknown, but seems not related to direct granulomatous inflammation of small nerve fibers. However, some case reports demonstrate improvement of SFN related symptoms during anti-inflammatory treatment (10,11). Furthermore, the positive effects of ARA290, which can mitigate inflammation via innate immunity receptors, also suggest a possible relationship between sarcoidosis inflammation and SFN related symptoms (12). This prompted us to further investigate a possible relation between inflammatory activity in sarcoidosis and SFN-related symptoms. Our hypothesis was that decrease in inflammation results in improvement of SFN-related symptoms in patients with sarcoidosis. Compared to biomarkers such as angiotensin converting enzyme (ACE) and soluble interleukin 2 receptor (sIL-2R), FDG-PET has the highest sensitivity for demonstrating inflammatory activity in patients with sarcoidosis (13,14). In our local infliximab treatment protocol FDG-PET as well as the SFNSL are incorporated as baseline and during follow up. Therefore, it was possible to compare the course of SFN-related complaints with the reduction of inflammation during treatment with infliximab in a well-defined cohort of patients with sarcoidosis.
Methods
All sarcoidosis patients treated with infliximab at the St. Antonius Hospital between January 2010 – Oct 2021 were screened for inclusion. All patients signed informed consent (via Biobank informed consent, R05-08A). Sarcoidosis is diagnosed based on the criteria of the American Thoracic Society/European Respiratory Society (15). From these patients, start and stop dates of infliximab treatment were noted. The maximal Standardized Uptake Value (SUVmax) from FDG-PET was used to define sarcoidosis activity. The Small Fiber Neuropathy Screening List (SFNSL) was used to identify the amount and changes in SFN related symptoms during treatment.
The complete SFNSL questionnaire consists of 21 questions, with a score per question ranging between 0 for no complaints and 4 for maximal complaints. The SFNSL was correlated with temperature threshold testing (TTT) in an important reference paper by Hoitsma et al. (16). According to this paper, an SFNSL score below 11 suggest the absence of SFN based on the fact that no abnormal TTT was found in those patients. A score above 48 suggests the presence of SFN based on the fact that all patients with a score above 48 had an abnormal TTT. Eighty percent of the sarcoidosis patients with an SFNSL score between 11-48, showed an abnormal TTT suggesting presence of SFN. Furthermore, according to the SFNSL validation study, it is very likely for the group with a score between 11-48 to develop SFN within 2 years.
Inclusion criteria:
- Sarcoidosis with infliximab treatment for at least 3 months
- At least two SFNSL scores with at least 3 months in between
- Two or more SFNSL-scores within one treatment period
- SFNSL score up to 14 days around start date of treatment
- SUVmax data available 90 days before or 14 days after treatment initiation
- SFNSL-score ≥ 11
Exclusion criteria:
- No infliximab treatment
- Only one SFNSL-score within a treatment period
- SFNSL-score at start < 11
- PET-scan date difference of >90 days with SFNSL-score date
SFNSL questionnaires during treatment periods were collected. In case of multiple treatment periods, the longest period is used. In order to examine the effects of infliximab on SFN related symptoms, the SFNSL-score within 14 days around start and the most recent score were selected. The minimal important difference with clinical relevance on the SFNSL-score, is set on 3.5 points (17).
SUVmax was collected in order to investigate the response of sarcoidosis activity on infliximab treatment. Only values from the longest treatment period were included, within 90 days before or 14 days after initiation and the most recent from that period. Moreover, SUVmax had to be available 90 days around the most recent SFNSL-score.
Statistical analysis was performed with IBM SPSS 26.0 Statistics software. Since data was not normally distributed, Wilcoxon signed rank test was used to calculate significance of difference between first and last scores.
Results
A cohort of 392 patients with sarcoidosis was screened. They all received infliximab treatment for at least 3 months at the St. Antonius Hospital. 261 sarcoidosis patients were excluded due to lack of a PET-scan in more than 14 days after or 90 days before treatment initiation. Another 33 sarcoidosis patients were excluded due to less than 2 SFNSL-scores within one treatment period. 16 patients were excluded due to an SFNSL-score below 11 around treatment initiation. In addition, 46 patients were excluded due to a gap of more than 14 days between treatment initiation and SFNSL-score. Finally, 36 sarcoidosis patients were eligible for analysis, depicted in Figure 1.
Figure 1.
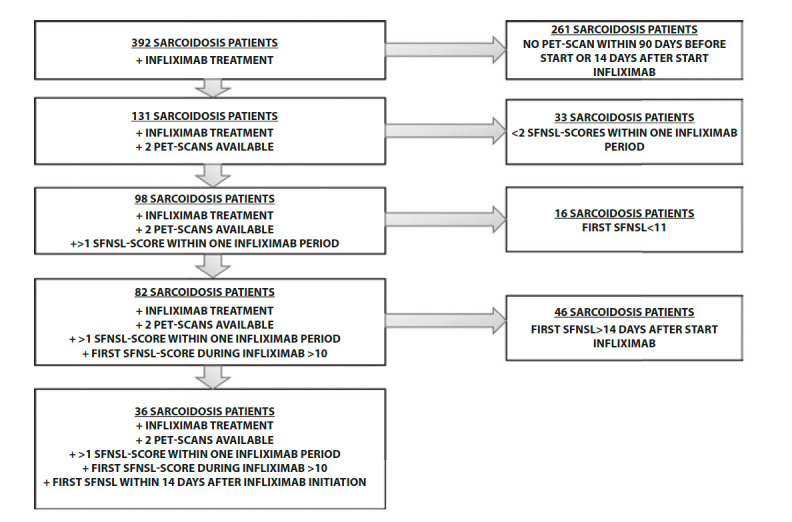
Results of exclusion criteria.
SFNSL scores were collected with a mean of 2 days (±6.9) after treatment initiation and the most recent score with a mean of 832 days (±735). When analyzing the relation between time and SFNSL change, no correlation could be found (p=0.508; R=0.114). SUVmax data were collected with a mean of 31 days (±25) before treatment initiation and the most recent data with a mean of 878 days (±662) after treatment initiation. An overview of baseline characteristics of participants is displayed in Table 1.
Table 1.
Baseline characteristics.
| SFNSL >11 | ||
|---|---|---|
| Group size | 36 | |
| Sex (male), n | 58% (21) | |
| Ethnicity (caucasian), n | 94% (34) | |
| Age (mean years, ±sd) | 57 (±10) | |
| Patients with suspected SFN by neurologist | 19% (7) | |
| Duration of sarcoidosis symptoms (years, ±sd) | 15 (±4) | |
| Duration of neurologic symptoms (years, ±sd) | 9 (±3) | |
| Duration of neurologic symptoms in suspected SFN (years, ±sd) | 10 (±5) | |
| Sarcoidosis diagnosis with biopsy, n (%) | 83% (26) | |
| Other risk factors for SFN | Diabetes Mellitus Hypothyroidism Fibromyalgia Vitamin B12 deficiency Trauma |
17% (6) 6% (2) 6% (2) 3% (1) 3% (1) |
| Smoking status, n (%) | Never | 44% (16) |
| Current | 0% (0) | |
| Former | 39% (14) | |
| Unknown | 17% (6) | |
| Scadding stage, n (%) | stage 0 | 8% (3) |
| stage 1 | 31% (11) | |
| stage 2 | 17% (6) | |
| stage 3 | 8% (3) | |
| stage 4 | 36% (13) | |
| Main treatment indication, n (%) | Pulmonary | 44 % (16) |
| Cardiac | 14% (5) | |
| Neurologic Manifestations | 11% (4) | |
| Other | 30% (11) | |
| Medication use during Inflximab | Corticosteroids | 69% (25) |
| Methotrexate | 83% (30) | |
| Azathioprine | 14% (5) | |
| Hydroxychloroquine | 8% (3) | |
| Deceased | 11% (4) |
In our study cohort, inflammatory activity defined by SUVmax improved with a mean of -3.7 (p=<0.001) during treatment, see Figure 2 A. When comparing SFNSL-scores at the start and the end of treatment, a difference in the mean of -1.9 points was found (p = 0.446), see Figure 2 B. The change of SFNSL-score seems not to correlate with the change in sarcoidosis activity measured by FDG-PET, as can be seen in figure 3. Only half of the population showed a decrease of more than 3.5 points on the SFNSL questionnaire.
Figure 2.
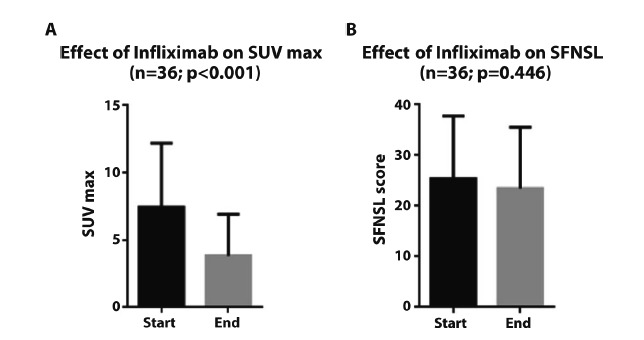
Effect of infliximab on SFNSL-score.
Figure 3.
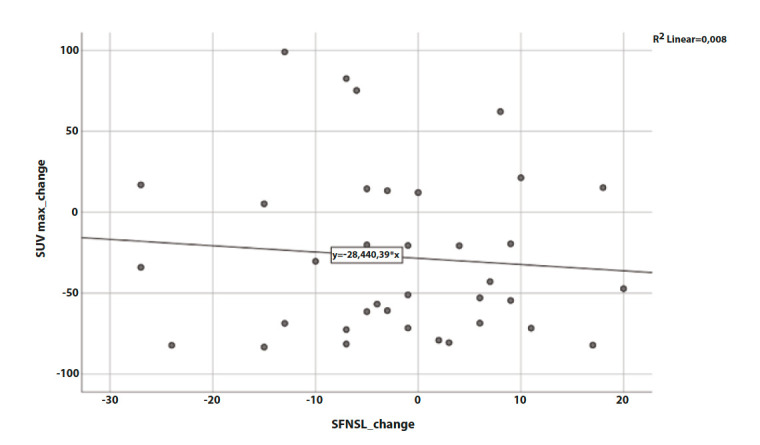
Correlation between SUVmax change and SFNSL change during Infliximab treatment.
Discussion
In this study a possible relationship between inflammatory activity and SFN-related symptoms was investigated in patients with sarcoidosis. In our opinion, the data are of special interest due to the fact that inflammatory activity was measured both at baseline and during treatment with FDG-PET, the most sensitive biomarker for inflammatory activity of sarcoidosis at present (18,19). This enabled us to investigate the relationship between the amount of inflammatory activity and SFN-related symptoms as accurately as possible. In concordance with previous studies, treatment with infliximab resulted in a significant decrease of inflammatory activity in patients with sarcoidosis (13,20–22). However, we did not find a relationship between decrease of inflammatory activity and improvement of SFN-related symptoms in patients with sarcoidosis. Therefore, we were not able to find support for our initial hypothesis.
Previous studies in pulmonary sarcoidosis demonstrated a relationship between the burden of inflammatory activity present in the lungs at baseline and the amount of pulmonary function improvement after 6 months of treatment with infliximab (22). More recently, in cardiac sarcoidosis, a relationship between the burden of inflammatory activity in the myocardium measured by FDG-PET correlated with left ventricular recovery after treatment with immunosuppressive therapy (23). Based on the fact that the degree of inflammation has decreased significantly in our patients, it is tempting to speculate that SFN-related symptoms can be seen as irreversible damage to the small fibers and not malfunction based on an active inflammatory process.
Our findings are not in line with other studies which found a positive relation between anti-TNF-α treatment and reduction of SFN symptoms (20,21). Note that although overall infliximab therapy could not improve SFN-associated symptoms, 50% of patients did show a decrease of more than 3.5 points on the SFNSL questionnaire. Therefore, this group of patients did meet the criteria of minimal important difference with clinical relevance on the SFNSL-score. As mentioned before, ARA290 showed positive effects on inflammation mitigation via innate immunity receptors. The patients who showed a decrease of more than 3.5 points on the SFNSL questionnaire, might gain advantage from immune modulation via innate immunity receptors rather than reduction of total burden of granulomatous inflammation due to infliximab.
There are several limitations in this study. First of all, the retrospective design resulted in only a small cohort of patients to be studied. We had substantial loss of data due to exceeded time lapse between treatment initiation, date of PET-scan and the date of SFNSL questionnaires. Secondly, we used the SFNSL to assess symptoms but did not accurately diagnose SFN in our patients. Therefore, it could be that we assessed SFN-related symptoms in patients who did not have an actual diagnosis of small fiber neuropathy.
Strength of the thesis however is the strong defined group of sarcoidosis patients. As stated before, FDG-PET is highly sensitive for demonstrating inflammatory activity in patients with sarcoidosis. It is actually the first to compare the change in SUVmax with SFN or SFN related symptoms defined by the SFNSL questionnaire.
Although our data reveal no association between anti-inflammatory effect of infliximab and SFN-related symptoms in patients with sarcoidosis, which contradicts previous case-reports and case-series, further studies are warranted. Given the major negative impact of SFN-related symptoms on the quality of life in patients with sarcoidosis, it is necessary that the possible beneficial effect of anti-inflammatory therapy will be addressed in future prospective studies. A large study cohort with clear distribution of SFN phenotypes might give novel insights in effectiveness of anti-TNF-α treatment.
Acknowledgements:
Funding: ZonMW TopZorg 842002001
Abbreviations:
ACE: Angiotensin converting enzyme; Anti-TNF-α: Anti-tumor necrosis factor alpha; DMARDs: Disease modifying anti rheumatic drugs; FDG-PET: Fluordeoxyglucose Positron Emission Tomography; HRQL: Health related quality of life; SFN: Small fiber neuropathy; SFNSL: Small fiber neuropathy screening list; sIL-2R: Soluble interleukin 2 receptor; SUVmax: Maximal standardized uptake value; TTT: Temperature threshold test.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Grunewald J, Grutters JC, Arkema E V, Saketkoo LA, Moller DR, Müller-Quernheim J. Sarcoidosis. Nat Rev Dis Prim. 2019;5(1) doi: 10.1038/s41572-019-0096-x. [DOI] [PubMed] [Google Scholar]
- 2.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet P-Y, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;103:527–534. doi: 10.1016/S0140-6736(13)60680-7. [DOI] [PubMed] [Google Scholar]
- 3.Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Hear Rhythm. 2014;11(7):1304–23. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 4.Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006 Oct 1;174(7):795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 5.Voortman M, Hendriks CMR, Elfferich MDP, et al. The Burden of Sarcoidosis Symptoms from a Patient Perspective. Lung [Internet] 2019;197(2):155–61. doi: 10.1007/s00408-019-00206-7. Available from: http://link.springer.com/10.1007/s00408-019-00206-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voortman M, Fritz D, Vogels OJM, et al. Small fiber neuropathy: A disabling and underrecognized syndrome. Curr Opin Pulm Med. 2017;23(5):447–57. doi: 10.1097/MCP.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 7.Gavrilova N, Starshinova A, Zinchenko Y, et al. Small fiber neuropathy in sarcoidosis. Pathophysiology. 2021;28(4):544–50. doi: 10.3390/pathophysiology28040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox CE, Donohue JF, Brown CD, Kataria YP, Judson MA. Health-related quality of life of persons with sarcoidosis. Chest [Internet] 2004;125(3):997–1004. doi: 10.1378/chest.125.3.997. Available from: http://dx.doi.org/10.1378/chest.125.3.997 . [DOI] [PubMed] [Google Scholar]
- 9.De Vries J, Wirnsberger RM. Fatigue, quality of life and health status in sarcoidosis. Sarcoidosis. 2010:92–104. [Google Scholar]
- 10.Wijnen PA, Cremers JP, Nelemans PJ, et al. Association of the TNF-α G-308A polymorphism with TNF-inhibitor response in sarcoidosis. Eur Respir J. 2014;43(6):1730–9. doi: 10.1183/09031936.00169413. [DOI] [PubMed] [Google Scholar]
- 11.Tavee JO, Karwa K, Ahmed Z, Thompson N, Parambil J, Culver DA. Respiratory Medicine. Elsevier Ltd: 2017. Sarcoidosis-associated small fiber neuropathy in a large cohort: Clinical aspects and response to IVIG and anti-TNF alpha treatment [Internet]. Vol. 126; pp. 135–138. Available from: http://dx.doi.org/10.1016/j.rmed.2017.03.011 . [DOI] [PubMed] [Google Scholar]
- 12.Van Velzen M, Heij L, Niesters M, et al. Ara 290 for treatment of small fiber neuropathy in sarcoidosis. Expert Opin Investig Drugs. 2014;23(4):541–50. doi: 10.1517/13543784.2014.892072. [DOI] [PubMed] [Google Scholar]
- 13.Vorselaars ADM, Crommelin HA, Deneer VHM, et al. Effectiveness of infliximab in refractory FDG PET-positive sarcoidosis. Eur Respir J [Internet] 2015;46(1):175–85. doi: 10.1183/09031936.00227014. Available from: http://dx.doi.org/10.1183/09031936.00227014 . [DOI] [PubMed] [Google Scholar]
- 14.Keijsers RG, Verzijlbergen FJ, Oyen WJ, et al. 18F-FDG PET, genotype-corrected ACE and sIL-2R in newly diagnosed sarcoidosis. Eur J Nucl Med Mol Imaging. 2009;36(7):1131–7. doi: 10.1007/s00259-009-1097-x. [DOI] [PubMed] [Google Scholar]
- 15.Costabel U, Hunninghake GW, Statement S. EDITORIAL ATS / ERS / WASOG statement on sarcoidosis. Eur Respir J. 1999;14(4):735–7. doi: 10.1034/j.1399-3003.1999.14d02.x. [DOI] [PubMed] [Google Scholar]
- 16.Hoitsma E, De Vries J, Drent M. The small fiber neuropathy screening list: Construction and cross-validation in sarcoidosis. Respir Med [Internet] 2011;105(1):95–100. doi: 10.1016/j.rmed.2010.09.014. Available from: http://dx.doi.org/10.1016/j.rmed.2010.09.014 . [DOI] [PubMed] [Google Scholar]
- 17.Voortman M, Beekman E, Drent M, Hoitsma E, De Vries J. Determination of the smallest detectable change (SDC) and the minimal important difference (MID) for the small fiber neuropathy screening list (SFNSL) in sarcoidosis. Sarcoidosis Vasc Diffus Lung Dis. 2018;35(4):333–41. doi: 10.36141/svdld.v35i4.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keijsers RGM, Van Den Heuve DAF, Grutters JC. Imaging the inflammatory activity of sarcoidosis. Eur Respir J. 2013;41(3):743–51. doi: 10.1183/09031936.00088612. [DOI] [PubMed] [Google Scholar]
- 19.Adams H, Keijsers RG, Korenromp IHE, Grutters JC. FDG PET for gauging of sarcoid disease activity. Semin Respir Crit Care Med. 2014;35(3):352–61. doi: 10.1055/s-0034-1376866. [DOI] [PubMed] [Google Scholar]
- 20.Hoitsma E, Faber C, Santen-Hoeufft M van, de Vries J, Reulen J, Drent M. Improvement of small fiber neuropathy in a sarcoidosis patient after treatment with infliximab. Sarcoidosis Vasc Diffus Lung Dis. 2006;23:73–7. [PubMed] [Google Scholar]
- 21.Parambil JG, Tavee JO, Zhou L, Pearson KS, Culver DA. Efficacy of intravenous immunoglobulin for small fiber neuropathy associated with sarcoidosis. Respir Med. 2011 Jan;105(1):101–5. doi: 10.1016/j.rmed.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Schimmelpennink MC, Vorselaars ADM, van Beek FT, et al. Efficacy and safety of infliximab biosimilar Inflectra® in severe sarcoidosis. Respir Med. 2018;138(November 2017):S7–13. doi: 10.1016/j.rmed.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Fazelpour S, Sadek MM, Nery PB, et al. Corticosteroid and immunosuppressant therapy for cardiac sarcoidosis: A systematic review. J Am Heart Assoc. 2021;10(17) doi: 10.1161/JAHA.121.021183. [DOI] [PMC free article] [PubMed] [Google Scholar]


