Abstract
Objective:
This article reviews research on post-acute alcohol withdrawal syndrome (PAWS) management.
Method:
We conducted a PRISMA (Preferred Reporting Items for Systematic Revision and Meta-Analyses)-guided scoping review of the published PAWS literature, searching six electronic databases (from their inception through December 2020) for English-language randomized and nonrandomized studies.
Results:
A total of 16 treatment studies met the inclusion criteria. The strength of evidence overall for pharmacologic treatments is low, with often only short-term results being reported, small treatment samples used, or inconsistent results found. However, for negative affect and sleep symptoms, more evidence supports using gabapentinoids (gabapentin and pregabalin) and anticonvulsants (carbamazepine and oxcarbazepine). Although preliminary data support acamprosate, there were no controlled trials. Despite an older treatment trial showing some positive data for amitriptyline for mood, the clinical measures used were problematic, and side effects and safety profile limit its utility. Finally, there is no evidence that melatonin and other agents (homatropine, Proproten-100) show PAWS symptoms.
Conclusions:
Although there is some evidence for targeted pharmacotherapy for treating specific PAWS symptoms, there are few recent, robust, placebo-controlled trials, and the level of evidence for treatment efficacy is low.
Individuals with alcohol use disorders (AUDs) frequently cycle between drinking and withdrawal states (GBD 2016 Alcohol and Drug Use Collaborators, 2018). The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5; American Psychiatric Association [APA], 2013), defines alcohol withdrawal syndrome (AWS) as the development of two or more of the following symptoms within hours to a few days of cessation of or reduction of heavy alcohol use: autonomic hyperactivity (sweating, fast pulse), increased hand tremor, insomnia, nausea and vomiting, transient hallucination or illusions, psychomotor agitation, anxiety, and grand mal seizures (APA, 2013). AWS symptoms are caused by increased central N-methyl-D-aspartate (NMDA) glutamate transmission with diminished intrinsic gamma-aminobutyric acid (GABA)-ergic neurotransmission (Huang et al., 2014). AWS treatment focuses on the relief of immediate symptoms, prevention of complications, and rehabilitation initiation (American Society of Addiction Medicine [ASAM], 2020; Heilig et al., 2010). Placebo-controlled trials suggest benzodiazepines, β-adrenergic receptor antagonists (β-blockers), calcium channel blockers, anticonvulsants, and clonidine improve AWS (Amato et al., 2011; ASAM, 2020; Berglund et al., 2003; Soyka et al., 2008).
Although acute AWS symptoms usually last for only a few days up to a week, some symptoms can persist, including anxiety, depression, irritability, cognitive dysfunction, cravings for alcohol, sleep disturbance, fatigue, and autonomic irregularities (Bokhan et al., 2003; De Soto et al., 1985; Stojek et al., 1990; Vik et al., 2004; Voltaire-Carlsson et al., 1996; Watanabe et al., 2001). These symptoms—termed post-acute withdrawal syndrome (PAWS)—were first described more than six decades ago (Satel et al., 1993). In 1954, Wellman described “late withdrawal symptoms” in abstinent alcoholic-dependent persons, which consisted of irritability, depression, insomnia, fatigue, restlessness, and distractibility, constituting a physical syndrome most severe during the first 6 months of abstinence (Wellman, 1954). Building on Wellman's findings, Segal and colleagues (1970) were the first to coin the term “protracted withdrawal syndrome” in 1960, describing neurovegetative and emotional instability symptoms persisting long after acute withdrawal had subsided. Following Segal, Kissin (1979) described several protracted alcohol abstinence syndrome cases in 1979, emphasizing their importance to relapse prevention.
PAWS has been a relatively neglected topic (De Soto et al., 1985), and few recent scientific studies support its existence. Consequently, the notion of PAWS remains highly controversial (Satel et al., 1993). Although it has not yet gained formal recognition by the DSM (APA, 2013) or the International Classification of Disease (ICD; Hughes, 1994), PAWS has been informally recognized as a high-risk interval for return to alcohol consumption following abstinence (Melemis, 2015). Accordingly, randomized controlled trials have shown that initiating AUD treatment following acute detoxification with acamprosate, carbamazepine, and trazodone (Beleslin, 1991; Le Bon et al., 2003; Mueller et al., 1997; Wilde & Wagstaff, 1997) or cognitive behavioral therapy (Hori et al., 2005) may reduce risk. However, these studies have not formally emphasized the notion of PAWS (Potgieter et al., 1999). Furthermore, as most extant AWS studies are limited to acute withdrawal treatment, further research remains needed regarding the post-acute withdrawal abstinent period (Williams & Mc-Bride, 1998).
Consequently, the goal of this article was to summarize the extant literature examining the treatment (pharmacological and nonpharmacological) of PAWS.
Method
Protocol and registration
We registered our study protocol with the PROSPERO database of reviews (CRD42020208946; National Institute for Health Research, 2019). We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Liberati et al., 2009) but made some adjustments because the review was a scoping review rather than a full systematic review. As a review of published data, we did not require ethics approval.
Definition of post-acute withdrawal
Although there are no consensus definitions of PAWS in the extant literature, the ASAM 2020 clinical practice guidelines describe “protracted alcohol withdrawal” as subacute symptoms of irritability, anxiety, and sleep disturbance that persist beyond 30 days from the start of acute withdrawal (ASAM, 2020). However, as this definition is relatively recent and inconsistent with the timelines of the symptoms considered by most articles that pertain to protracted withdrawal, we applied a more liberal definition, including any study that evaluated symptoms persisting beyond the acute withdrawal phase and without restriction to a particular cluster of symptoms.
Eligibility criteria
We restricted eligibility to human adult populations (ages ≥ 18 years), examining any pharmacological (e.g., medications) or nonpharmacological (e.g., psychotherapy) interventions for the treatment of PAWS. We restricted eligibility to English-language articles or those with an available English-language translation. We considered randomized controlled trials and nonrandomized intervention studies (e.g., pre–post studies). We excluded commentaries, reviews, editorials, and case reports; we did not restrict the study's data or location.
Information sources and search
In collaboration with a health sciences research librarian, we developed a comprehensive search strategy using combinations of terms related to “alcohol,” “post-acute,” “withdrawal,” and “protracted” in PubMed, MEDLINE, EMBASE, and PsycINFO from the date of their inception to December 2020. In addition, we supplemented the electronic database searches with manual searches of all eligible articles’ reference lists and previous reviews for additional studies.
Study selection
We reviewed studies for eligibility using Covidence, a web-based review manager, and Zotero citation manager (Roy Rosenzweig Center for History and New Media, 2018; Veritas Health Innovation, 2019). After removing duplicates, one reviewer (A.B.) independently selected the studies, reviewed the main reports and supplementary materials, extracted the relevant information from the included trials, and assessed the risk of bias; a second author (N.E.) reviewed excluded studies for erroneous selection. Any discrepancies were resolved by consensus.
Data collection process and data items
One reviewer (A.B.) extracted the following data from included studies, while another (D.C.) confirmed the extracted data for accuracy. Where necessary, we contacted corresponding authors to secure data. We used a standardized tool to extract information about authors, study objectives, sample characteristics, inclusion/exclusion criteria, study design, experimental processes, treatment protocols, outcome variables, and analytic strategy in Covidence, which we transferred to a Microsoft Excel spreadsheet (Veritas Health Innovation, 2019).
Study risk of bias assessment
We applied the Cochrane Risk of Bias Tool for randomized controlled trials (Higgins et al., 2011). In brief, this tool appraises the risk of bias in trials attributable to randomization, allocation concealment, blinding, participant attrition, selective reporting, and other sources of bias (e.g., unclear adherence to treatment, allegiance bias). One reviewer (A.B.) appraised the study's risk of bias, which was confirmed by the remaining reviewers (D.C. and N.E.). For coding purposes, studies receiving one high risk of bias rating in any individual domain or two unclear risks of bias ratings had a high overall risk of bias.
Summary measures
Although there was insufficient homogeneity to enable meta-analysis, we summarized findings across studies by describing their population, intervention, comparison, outcome, and design features as per previous descriptive reviews in addiction medicine (Bahji, 2019; Bahji & Bajaj, 2018, 2019; Bahji et al., 2021).
Results
Study selection
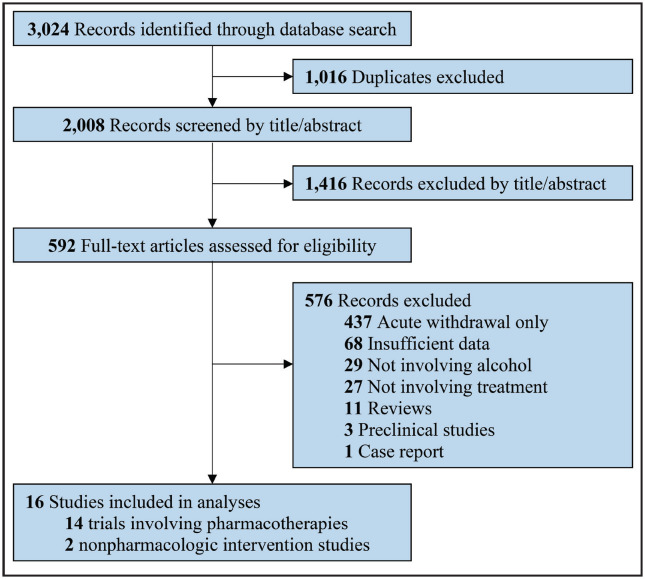
We screened 3,024 studies, from which 2,008 were unique citations and 1,016 were duplicate citations. From these, we excluded 1,416 records during the title and abstract screening phase, leaving 592 full-text articles for review. Subsequently, 16 treatment studies met the inclusion criteria (Figure 1). Fourteen were pharmacological trials, whereas two were nonpharmacological intervention studies. We did not find any additional articles through reviewing reference lists of identified articles.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow diagram
Pharmacological treatments
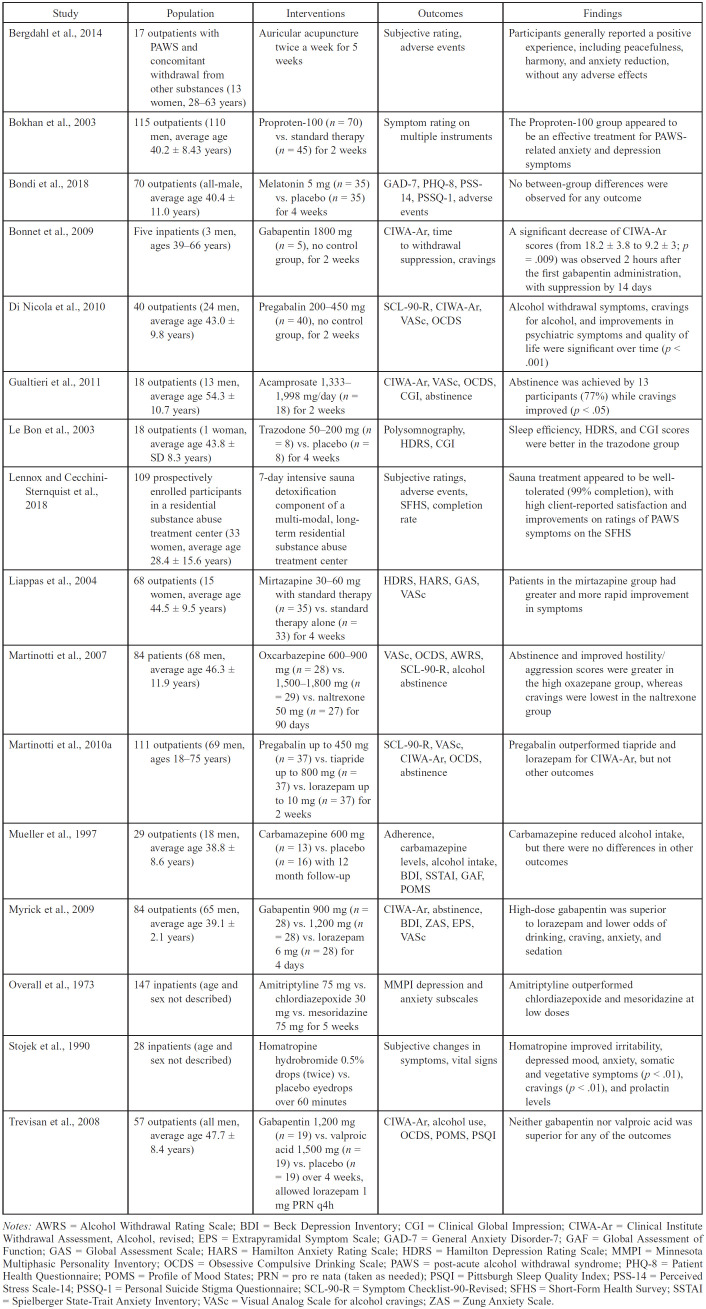
Pharmacological treatments involving antidepressants, sleep-promoting agents, anticonvulsants, gabapentinoids, and two novel therapies have been explored for therapeutic efficacy in PAWS management (Table 1), which we summarize here.
Table 1.
Characteristics of studies of pharmacologic treatments for post-acute alcohol withdrawal syndrome (n = 14)
| Study | Population | Interventions | Outcomes | Findings |
|---|---|---|---|---|
| Bergdahl et al., 2014 | 17 outpatients with PAWS and concomitant withdrawal from other substances (13 women, 28-63 years) | Auricular acupuncture twice a week for 5 weeks | Subjective rating, adverse events | Participants generally reported a positive experience, including peacefulness, harmony, and anxiety reduction, without any adverse effects |
| Bokhan et al., 2003 | 115 outpatients (110 men, average age 40.2 ± 8.43 years) | Proproten-100 (n = 70) vs. standard therapy (n = 45) for 2 weeks | Symptom rating on multiple instruments | The Proproten-100 group appeared to be an effective treatment for PAWS-related anxiety and depression symptoms |
| Bondi et al., 2018 | 70 outpatients (all-male, average age 40.4 ± 11.0 years) | Melatonin 5 mg (n = 35) vs. placebo (n = 35) for 4 weeks | GAD-7, PHQ-8, PSS-14, PSSQ-1, adverse events | No between-group differences were observed for any outcome |
| Bonnet et al., 2009 | Five inpatients (3 men, ages 39-66 years) | Gabapentin 1800 mg (n = 5), no control group, for 2 weeks | CIWA-Ar, time to withdrawal suppression, cravings | A significant decrease of CIWA-Ar scores (from 18.2 ± 3.8 to 9.2 ± 3; p = .009) was observed 2 hours after the first gabapentin administration, with suppression by 14 days |
| Di Nicola et al., 2010 | 40 outpatients (24 men, average age 43.0 ± 9.8 years) | Pregabalin 200-450 mg (n = 40), no control group, for 2 weeks | SCL-90-R, CIWA-Ar, VASc, OCDS | Alcohol withdrawal symptoms, cravings for alcohol, and improvements in psychiatric symptoms and quality of life were significant over time (p < .001) |
| Gualtieri et al., 2011 | 18 outpatients (13 men, average age 54.3 ± 10.7 years) | Acamprosate 1,3331,998 mg/day (n = 18) for 2 weeks | CIWA-Ar, VASc, OCDS, CGI, abstinence | Abstinence was achieved by 13 participants (77%) while cravings improved (p < .05) |
| Le Bon et al., 2003 | 18 outpatients (1 woman, average age 43.8 ± SD 8.3 years) | Trazodone 50-200 mg (n = 8) vs. placebo (n = 8) for 4 weeks | Polysomnography, HDRS, CGI | Sleep efficiency, HDRS, and CGI scores were better in the trazodone group |
| Lennox and Cecchini-Sternquist et al., 2018 | 109 prospectively enrolled participants in a residential substance abuse treatment center (33 women, average age 28.4 ± 15.6 years) | 7-day intensive sauna detoxification component of a multi-modal, longterm residential substance abuse treatment center | Subjective ratings, adverse events, SFHS, completion rate | Sauna treatment appeared to be well-tolerated (99% completion), with high client-reported satisfaction and improvements on ratings of PAWS symptoms on the SFHS |
| Liappas et al., 2004 | 68 outpatients (15 women, average age 44.5 ± 9.5 years) | Mirtazapine 30-60 mg with standard therapy (n = 35) vs. standard therapy alone (n = 33) for 4 weeks | HDRS, HARS, GAS, VASc | Patients in the mirtazapine group had greater and more rapid improvement in symptoms |
| Martinotti et al., 2007 | 84 patients (68 men, average age 46.3 ± 11.9 years) | Oxcarbazepine 600-900 mg (n = 28) vs. 1,500-1,800 mg (n = 29) vs. naltrexone 50 mg (n = 27) for 90 days | VASc, OCDS, AWRS, SCL-90-R, alcohol abstinence | Abstinence and improved hostility/aggression scores were greater in the high oxazepane group, whereas cravings were lowest in the naltrexone group |
| Martinotti et al., 2010a | 111 outpatients (69 men, ages 18-75 years) | Pregabalin up to 450 mg (n = 37) vs. tiapride up to 800 mg (n = 37) vs. lorazepam up to 10 mg (n = 37) for 2 weeks | SCL-90-R, VASc, CIWA-Ar, OCDS, abstinence | Pregabalin outperformed tiapride and lorazepam for CIWA-Ar, but not other outcomes |
| Mueller et al., 1997 | 29 outpatients (18 men, average age 38.8 ± 8.6 years) | Carbamazepine 600 mg (n = 13) vs. placebo (n = 16) with 12 month follow-up | Adherence, carbamazepine levels, alcohol intake, BDI, SSTAI, GAF, POMS | Carbamazepine reduced alcohol intake, but there were no differences in other outcomes |
| Myrick et al., 2009 | 84 outpatients (65 men, average age 39.1 ± 2.1 years) | Gabapentin 900 mg (n = 28) vs. 1,200 mg (n = 28) vs. lorazepam 6 mg (n = 28) for 4 days | CIWA-Ar, abstinence, BDI, ZAS, EPS, VASc | High-dose gabapentin was superior to lorazepam and lower odds of drinking, craving, anxiety, and sedation |
| Overall et al., 1973 | 147 inpatients (age and sex not described) | Amitriptyline 75 mg vs. chlordiazepoxide 30 mg vs. mesoridazine 75 mg for 5 weeks | MMPI depression and anxiety subscales | Amitriptyline outperformed chlordiazepoxide and mesoridazine at low doses |
| Stojek et al., 1990 | 28 inpatients (age and sex not described) | Homatropine hydrobromide 0.5% drops (twice) vs. placebo eyedrops over 60 minutes | Subjective changes in symptoms, vital signs | Homatropine improved irritability, depressed mood, anxiety, somatic and vegetative symptoms (p < .01), cravings (p < .01), and prolactin levels |
| Trevisan et al., 2008 | 57 outpatients (all men, average age 47.7 ± 8.4 years) | Gabapentin 1,200 mg (n = 19) vs. valproic acid 1,500 mg (n = 19) vs. placebo (n = 19) over 4 weeks, allowed lorazepam 1 mg PRN q4h | CIWA-Ar, alcohol use, OCDS, POMS, PSQI | Neither gabapentin nor valproic acid was superior for any of the outcomes |
Notes: AWRS = Alcohol Withdrawal Rating Scale; BDI = Beck Depression Inventory; CGI = Clinical Global Impression; CIWA-Ar = Clinical Institute Withdrawal Assessment, Alcohol, revised; EPS = Extrapyramidal Symptom Scale; GAD-7 = General Anxiety Disorder-7; GAF = Global Assessment of Function; GAS = Global Assessment Scale; HARS = Hamilton Anxiety Rating Scale; HDRS = Hamilton Depression Rating Scale; MMPI = Minnesota Multiphasic Personality Inventory; OCDS = Obsessive Compulsive Drinking Scale; PAWS = post-acute alcohol withdrawal syndrome; PHQ-8 = Patient Health Questionnaire; POMS = Profile of Mood States; PRN = pro re nata (taken as needed); PSQI = Pittsburgh Sleep Quality Index; PSS-14 = Perceived Stress Scale-14; PSSQ-1 = Personal Suicide Stigma Questionnaire; SCL-90-R = Symptom Checklist-90-Revised; SFHS = Short-Form Health Survey; SSTAI = Spielberger State-Trait Anxiety Inventory; VASc = Visual Analog Scale for alcohol cravings; ZAS = Zung Anxiety Scale.
Antidepressants. Given the significant affective disturbance seen in protracted withdrawal, three trials have explored antidepressants as PAWS treatments. In a 28-day trial of eight patients treated with trazodone 50 mg by Day 3 titrated to 150–200 mg by Day 28 compared with eight given placebo, the trazodone group had fewer depressive symptoms and enhanced sleep efficiency, the latter supported by polysomnographic findings (p = .041); however, the major limitation was the trial's small sample size (n = 16) (Le Bon et al., 2003). In a study of 68 outpatients, the addition of mirtazapine 30–60 mg (n = 35, M = 28.86, SD = 10.78 mg) for 4 weeks after the first week of standard detoxification led to more significant improvements in PAWS-related anxiety and depressive symptomatology compared with traditional detoxification alone (n = 33, p < .01; Liappas et al., 2004).
Although the two groups had similar baseline symptoms, the mirtazapine group consumed more alcohol per day, suggesting greater AUD severity (Liappas et al., 2004). Finally, an older study (Overall et al., 1973) of 146 inpatients compared amitriptyline 75 mg daily to chlordiazepoxide 30 mg daily or mesoridazine 75 mg daily over 5 weeks to treat depression and anxiety symptoms in recently detoxified individuals with AUD with decreased self-reported scores on subscales of the Minnesota Multiphasic Personality Inventory; chlordiazepoxide was significantly less effective than mesoridazine or amitriptyline.
Sleep-promoting agents. Although sleep disturbance is notable in PAWS, there is no evidence for using sleep-promoting agents other than the antidepressant trazodone. A trial of 70 persons in sober living examined the effect of adding 5 mg of melatonin (n = 35) versus placebo (n = 35). Still, there was no evidence that melatonin improved any measured outcomes (Bondi et al., 2018).
Anticonvulsants. Elevated glutamatergic neurotransmission through NMDA signaling appears to mediate PAWS and acute withdrawal, pointing to anticonvulsants’ role in restabilizing brain neurochemistry during protracted withdrawal. In a 12-month double-blind, placebo-controlled pilot study following acute detoxification from alcohol, carbamazepine demonstrated efficacy for decreased alcohol intake and improved mood; however, the trial was plagued by compliance difficulties and a sizable dropout rate (Mueller et al., 1997). Another trial compared naltrexone (50 mg, n = 27) to two doses of oxcarbazepine (1,500–1,800 mg, n = 27; 600–900 mg, n = 28) over 90 days (Martinotti et al., 2007). Abstinence was higher with high-dose oxcarbazepine (58.6%) than in the low-dose (42.8%) or naltrexone (40.7%) groups; cravings were lowest with naltrexone (p < .05).
Acamprosate. A small pilot open study confirmed the efficacy of acamprosate in maintaining abstinence and reducing PAWS in 18 recently detoxified alcohol-dependent outpatients (Gualtieri et al., 2011). Participants received either 1,332 mg/day or 1,998 mg/day, depending on their weight, for 30 days; however, there was no placebo control group (Gualtieri et al., 2011). Acamprosate was well tolerated, improving alcohol craving and relapses while reducing protracted withdrawal symptom severity measured using the Clinical Institute Withdrawal Assessment for Alcohol (Gualtieri et al., 2011).
Gabapentinoids. Gabapentinoids, like gabapentin and pregabalin, may target anxiety and sleep symptoms within PAWS. Gabapentin also improves negative affect and sleep symptoms of PAWS (Mason et al., 2018). However, as gabapentin does not suppress or prevent alcohol withdrawal seizures, it is not recommended as a stand-alone therapy for acute or protracted alcohol withdrawal (Hammond et al., 2015; Leung et al., 2015). In one trial, gabapentin appeared to outperform lorazepam during PAWS for abstinence, cravings, and tolerability (Myrick et al., 2009). However, Trevisan and colleagues (2008) did not replicate these findings when they compared 1,200 mg/day of gabapentin to valproic acid (1,500 mg/day or less) and placebo for PAWS. Pregabalin is a newer gabapentinoid with more rapid absorption and time to peak serum concentration (1 vs. 3 hours to reach peak levels) and a longer half-life elimination time, allowing twice-daily rather than thrice-daily dosing (Mason et al., 2018).
Pregabalin has shown efficacy for treating uncomplicated AWS and related negative affective symptoms in a 2-week open-label study (Di Nicola et al., 2010) and a 2-week multicenter trial versus tiapride and lorazepam (Martinotti et al., 2010b). These findings were replicated in a 16-week multicenter trial against naltrexone, which found that pregabalin was well tolerated, improving withdrawal symptoms as well as naltrexone (Martinotti et al., 2010a). However, some of pregabalin's pharmacokinetic improvements—such as quicker absorption and higher potency—have led to a concomitant increase in its abuse potential (Häkkinen et al., 2014; Schjerning et al., 2016).
Novel agents. In 2003, an open trial of 115 AUD outpatients investigated Proproten-100—an antibody preparation targeting S100 proteins in the hippocampus and hypothalamus—for PAWS (Bokhan et al., 2003). The experimental group (n = 70) received 5–8 tablets of Proproten-100 sublingually per day. The control group (n = 45) received a cocktail involving amitriptyline, piracetam, and three anxiolytics. Although 47% of the experimental group and 28% of the control group showed improved neurovegetative symptoms by Day 5, the differences were no longer significant by Day 10, suggesting that the agent was not effective for protracted withdrawal (Bokhan et al., 2003). In another trial (Stojek et al., 1990), Stojek and colleagues tested homatropine eye drops compared with placebo for treating PAWS symptoms among 28 AUD inpatients, reporting decreased irritability, depression, anxiety, somatic, and vegetative symptoms, and lower cravings for alcohol in the homatropine-treated group. However, because the therapeutic duration of homatropine is approximately 24 hours, the longer term efficacy is unknown (Stojek et al., 1990).
Pharmacotherapy summary. Although many agents have been investigated in PAWS treatment, anticonvulsants and gabapentinoids appear to have more evidence than the other categories of pharmacotherapies. However, the strength of evidence is relatively low for all medications, given the limited quality of the included studies and small sample sizes. Nonpharmacological treatments
We did not identify any psychotherapy studies for the treatment of PAWS. However, there were two nonpharmacological treatments of PAWS from two noncontrolled studies showing short-term subjective benefits. Participants generally reported a positive experience in one study of auricular acupuncture twice a week for 5 weeks for 17 outpatients with PAWS (and concomitant withdrawal from other substances), including peacefulness, harmony, and anxiety reduction, without any adverse effects (Bergdahl et al., 2014). Similarly, sauna detoxification appeared to be a well-tolerated regimen in another study, with high client-reported satisfaction and improvements in ratings of PAWS symptoms on the Short-Form Health Survey after 2 to 4 weeks following a 7-day intensive treatment phase (Lennox & Cecchini-Sternquist, 2018). However, the preliminary findings suggest that some methodological issues, such as a lack of control groups, objective measures, and longer term follow-up measures, limit the quality of the available evidence.
Risk of bias in individual studies
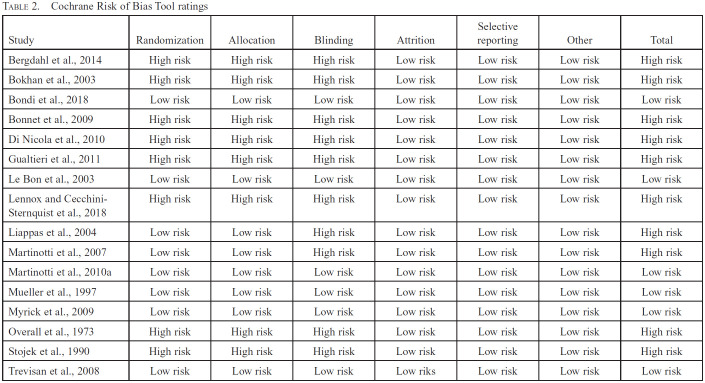
Using the Cochrane Risk of Bias tool ratings (Table 2), only 6 of the 16 studies received a low overall risk of bias rating. The most common reasons for the higher risk of bias ratings in the component studies were unclear randomization and blinding methods. On the whole, attrition across studies was low. Because most studies were at high risk of bias, we downgraded the overall strength of evidence.
Table 2.
Cochrane Risk of Bias Tool ratings
| Study | Randomization | Allocation | Blinding | Attrition | Selective reporting | Other | Total |
|---|---|---|---|---|---|---|---|
| Bergdahl et al., 2014 | High risk | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Bokhan et al., 2003 | High risk | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Bondi et al., 2018 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Bonnet et al., 2009 | High risk | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Di Nicola et al., 2010 | High risk | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Gualtieri et al., 2011 | High risk | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Le Bon et al., 2003 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Lennox and Cecchini-Sternquist et al., 2018 | High risk | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Liappas et al., 2004 | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | High risk |
| Martinotti et al., 2007 | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | High risk |
| Martinotti et al., 2010a | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Mueller et al., 1997 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Myrick et al., 2009 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Overall et al., 1973 | High risk | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Stojek et al., 1990 | High risk | High risk | High risk | Low risk | Low risk | Low risk | High risk |
| Trevisan et al., 2008 | Low risk | Low risk | Low risk | Low riks | Low risk | Low risk | Low risk |
Discussion
Summary of evidence
To our knowledge, this is the first scoping review to explore the treatment of PAWS, which ASAM defines as a syndrome with persistent, subacute symptoms of irritability, anxiety, and sleep disturbance (ASAM, 2020). There currently is a lack of controlled trials for nonpharmacological therapies for PAWS, so these cannot be recommended. The strength of evidence overall for pharmacologic treatments is low, with often only short-term results being reported, small treatment samples used, or inconsistent results found. However, for PAWS negative affect and sleep symptoms, more evidence supports using the gabapentinoids (gabapentin and pregabalin) and the anticonvulsants (carbamazepine and oxcarbazepine). Although acamprosate has some preliminary data, there were no controlled trials. Despite an older treatment trial showing some positive data for amitriptyline for mood, clinical measures used were problematic, and its side effects and safety profile limit its utility. Finally, there is a lack of evidence to support the efficacy of melatonin and other agents (homatropine, Proproten-100) for PAWS symptoms.
Implications of findings
In a review of protracted withdrawal by Satel and colleagues (1993), the authors concluded that symptoms extending beyond the period of acute withdrawal from alcohol—as well as opioids, for that matter—have been relatively consistently described but not conclusively demonstrated. Although it has been nearly 30 years since the publication of the Satel et al. review of protracted withdrawal syndromes, the PAWS field has not advanced remarkably apart from animal studies, which was not the present review's focus. Regrettably, PAWS has not received formal recognition as a disorder in any edition of the DSM or the ICD. It remains a relatively underestimated and ambiguously defined clinical condition that follows the acute stage of AWS (Caputo et al., 2020). Protracted withdrawal syndromes, in general, have not received prominent discussion, although they are clinically relevant. Likewise, whereas several trials have explored different PAWS treatments—as evidenced by those uncovered by the present review—few have been extensively studied since the 1990s, even though several of these agents showed promise in small pilot studies.
The lack of a shared, precise definition may partially explain why PAWS has not been widely adopted. The ASAM guidelines support the existence of PAWS, which they define as a syndrome with persistent, subacute symptoms of irritability, anxiety, and sleep disturbances (ASAM, 2020). Likely what is needed to define PAWS further is a specific timeline for symptom onset and persistence (i.e., the onset of symptoms within the first month after acute withdrawal that persists for greater than 1 month), specific symptoms that define PAWS (i.e., three or more of the following: irritability, depressed mood/anhedonia, anxiety, cravings, cognitive impairment, and sleep impairment), and its presence associated with functional impairment or predisposing to substance relapse. However, the larger topics of acute withdrawal and AUD maintenance may have absorbed PAWS where proposed PAWS treatments could have been lost amid the wave of naltrexone, acamprosate, and disulfiram. One of the other consequences of the relative lack of understanding of PAWS is the scarcity of published guidance on its management. For example, the ASAM 2020 Clinical Practice Guidelines on Alcohol Withdrawal Management identified protracted withdrawal as an area for future consideration (ASAM, 2020). Because there appears to be plausible neurobiological support for the basis of PAWS, impairment from its presence, and treatment consequences for identifying PAWS, PAWS must be more formally defined.
Although our review found limited, mixed-quality evidence for different pharmacotherapeutic classes in managing specific PAWS symptoms (such as sleep disruption, mood, or anxiety symptoms), there remains a need to enhance the evidence base for PAWS and its treatment. Consequently, one strategy for improving PAWS research is to recognize it formally. We hope that the present review's findings—by synthesizing literature across approximately four decades of research—may create a stronger argument for formalizing PAWS as a diagnostic entity. Furthermore, considering that PAWS symptoms are mainly related to the neuro-adaptive changes of GABA and NMDA systems, traditional treatments for AUD—such as naltrexone, nalmefene, and disulfiram—may not be able to suppress PAWS symptoms (Caputo et al., 2020).
Caputo and colleagues suggested that following the management of AWS, a more specifically designed pharmacological therapy able to suppress PAWS symptoms could perhaps be used earlier and help prevent the risk of alcohol relapse, which remains higher during the first months of abstinence (Caputo et al., 2020). Conversely, medications acting on GABA and NMDA neurotransmitter systems to counterbalance the up-regulation of NMDA and the down-regulation of GABA could be used in combination and started as soon as possible (Caputo et al., 2020). In addition, there is some evidence that acamprosate initiation following alcohol detoxification can mitigate relapse and PAWS (Gual & Lehert, 2001).
Limitations
There are a few limitations to discuss at the study and outcome level. The primary limitation is the high heterogeneity between studies owing to the nebulous nature of PAWS, the lack of a shared consensus definition, the variable durations of symptoms presented as components of PAWS, and the small sample sizes of the component studies. In addition, much of the literature on PAWS is dated, and there is a shortage of robust, randomized, controlled trials. Furthermore, there is a lack of standardization of PAWS across studies, and the extent of post-acute withdrawal abstinence was highly variable. Finally, as a scoping review, the search was limited to only a few databases and published literature. As a result, the review may have been vulnerable to publication bias. However, it is unclear if this significantly affected the overall conclusions. Unlike a traditional systematic review, only one author (A.B.) reviewed and identified the articles for inclusion, and the second reviewer only reviewed the excluded articles. With future studies, a more extensive systematic review or meta-analysis could be conducted.
Conclusion
Although there is some evidence for targeted pharmaco-therapy for treating specific PAWS symptoms, there are few recent, robust, placebo-controlled trials, and the level of evidence is low. In addition, as the presence of PAWS appears to contribute to relapse, there is a need for specific criteria for PAWS to be developed and tested and high-quality treatment studies done involving agents addressing the neurobiological underpinnings of symptoms.
Conflict-of-Interest Statement
Anees Bahji receives a small honorarium for teaching undergraduate and postgraduate medical trainees in the Cumming School of Medicine at the University of Calgary. In addition, Dr. Bahji is an unpaid member of the Canadian Network for Mood and Anxiety Treatments editorial committee, the International Society of Addiction Journal Editors, the Canadian Society of Addiction Medicine policy committee, and the Addiction Psychiatry section of the Canadian Psychiatric Association. Dr. Bahji is also an unpaid associate editor of the Canadian Journal of Addiction and a mental health educator for TED-Ed, where he receives a small honorarium for supporting online educational content. Finally, Dr. Bahji does not report any royalties, licenses, consulting fees, payment or honoraria for lectures or presentations, speaker's bureaus, manuscript writing, expert testimony, patents, or participation on other boards.
Acknowledgments
The authors acknowledge the University of Calgary Health Sciences Librarians for their support in developing our search strategy. We also recognize that our work takes place on historical and contemporary Indigenous lands, including the territories of Treaties 6, 7 & 8 and the homeland of the Métis. We also acknowledge the many Indigenous communities that have been forged in urban centers across Alberta.
Footnotes
All authors contributed to this study's design, the interpretation of the data, subsequent manuscript drafts (and revisions), and final approval for submission. Dr. Bahji wrote the initial draft of the work and managed revision feedback from the other authors.
Dr. Bahji has received grants to support addiction research training from the National Institutes of Health and the National Institute on Drug Abuse (NIDA) through the International Collaborative Addiction Medicine Research Fellowship (R25-DA037756) and the Research in Addiction Medicine Scholars Program (R25-DA033211), respectively. In addition, Dr. Bahji is a recipient of the 2020 Friends of Matt Newell Endowment from the University of Calgary Cumming School of Medicine. Dr. Bahji also received financial support from a 2020 Research Grant on the Impact of COVID-19 on Psychiatry by the American Psychiatric Association and the American Psychiatric Association Foundation. Currently, Dr. Bahji has been awarded doctoral studies research funding from the Canadian Institutes of Health Research (CIHR) Fellowship and the Harley N. Hotchkiss Graduate Scholarship in Neuroscience from the University of Calgary. Furthermore, Dr. Bahji has received research funding through the Calgary Health Trust. However, the content is solely the authors’ responsibility and does not represent the official views of NIDA, the University of Calgary, the CIHR, or the Calgary Health Trust.
Registration number: PROSPERO CRD42020208946.
References
- Amato L., Minozzi S., Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the Alcohol Withdrawal Syndrome. Cochrane Database of Systematic Reviews. 2011;6:CD008537. doi: 10.1002/14651858.CD008537.pub2. doi:10.1002/14651858.CD008537.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. 5th edition. Arlington, VA: Author; 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. Journal of Addiction Medicine. 2020;14(Supplement):1–72. doi: 10.1097/ADM.0000000000000668. doi:10.1097/ADM.0000000000000668. [DOI] [PubMed] [Google Scholar]
- Bahji A. An epidemic of incompetence: A critical review of addictions curriculum in Canadian residency programs. MedEdPublish. 2019;8:3. doi: 10.15694/mep.2019.000003.1. doi:10.15694/mep.2019.000003.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahji A., Bajaj N. Opioids on trial: A systematic review of interventions for the treatment and prevention of opioid overdose. Canadian Journal of Addiction. 2018;9:26–33. doi:10.1097/CXA.0000000000000013. [Google Scholar]
- Bahji A., Bajaj N. The value of hospitalization in the opioid epidemic: A scoping review. Canadian Journal of Addiction. 2019;10:6–17. doi:10.1097/CXA.0000000000000049. [Google Scholar]
- Bahji A., Kasurak E., Sterling M., Good L. Misuse and dependence of dimenhydrinate: A mixed studies systematic review. Journal of Psychiatric Research. 2021;136:581–588. doi: 10.1016/j.jpsychires.2020.10.032. doi:10.1016/j.jpsychires.2020.10.032. [DOI] [PubMed] [Google Scholar]
- Beleslin D. [Modern drug therapy in alcoholism] Medicinski Pregled. 1991;44:279–284. [PubMed] [Google Scholar]
- Bergdahl L., Berman A. H., Haglund K. Patients’ experience of auricular acupuncture during protracted withdrawal. Journal of Psychiatric and Mental Health Nursing. 2014;21:163–169. doi: 10.1111/jpm.12028. doi:10.1111/jpm.12028. [DOI] [PubMed] [Google Scholar]
- Berglund M., Thelander S., Salaspuro M., Franck J., Andréasson S., Öjehagen A. Treatment of alcohol abuse: An evidence-based review. Alcoholism: Clinical and Experimental Research. 2003;27:1645–1656. doi: 10.1097/01.ALC.0000090144.99832.19. doi:10.1097/01.ALC.0000090144.99832.19. [DOI] [PubMed] [Google Scholar]
- Bokhan N. A., Abolonin A. F., Krylov E. N., Vetlugina T. P., Ivanova S. A. Comparative efficiency of Proproten-100 during the therapy of patients with alcoholism in the stage of therapeutic remission. Bulletin of Experimental Biology and Medicine. 2003;135:171–175. doi: 10.1023/a:1024709014483. doi:10.1023/A:1024709014483. [DOI] [PubMed] [Google Scholar]
- Bondi C. D., Kamal K. M., Johnson D. A., Witt-Enderby P. A., Giannetti V. J. The effect of melatonin upon postacute withdrawal among males in a residential treatment program (M-PAWS): A randomized, double-blind, placebo-controlled trial. Journal of Addiction Medicine. 2018;12:201–206. doi: 10.1097/ADM.0000000000000386. doi:10.1097/ADM.0000000000000386. [DOI] [PubMed] [Google Scholar]
- Bonnet U., Specka M., Hamzavi Abedi R., Wiltfang J., Scherbaum N. Severe protracted alcohol withdrawal syndrome: Prevalence and pharmacological treatment at an inpatient detoxification unit—a naturalistic study. Pharmacopsychiatry. 2009;42:76–78. doi: 10.1055/s-0028-1103292. doi:10.1055/s-0028-1103292. [DOI] [PubMed] [Google Scholar]
- Caputo F., Cibin M., Loche A., De Giorgio R., Zoli G. The recognition and management of protracted alcohol withdrawal may improve and modulate the pharmacological treatment of alcohol use disorder. Journal of Psychopharmacology. 2020;34:1171–1175. doi: 10.1177/0269881120936483. doi:10.1177/0269881120936483. [DOI] [PubMed] [Google Scholar]
- De Soto C. B., O’Donnell W. E., Allred L. J., Lopes C. E. Symptomatology in alcoholics at various stages of abstinence. Alcoholism: Clinical and Experimental Research. 1985;9:505–512. doi: 10.1111/j.1530-0277.1985.tb05592.x. doi:10.1111/j.1530-0277.1985.tb05592.x. [DOI] [PubMed] [Google Scholar]
- Di Nicola M., Martinotti G., Tedeschi D., Frustaci A., Mazza M., Sarchiapone M., Janiri L. Pregabalin in outpatient detoxification of subjects with mild-to-moderate alcohol withdrawal syndrome. Human Psychopharmacology: Clinical and Experimental. 2010;25:268–275. doi: 10.1002/hup.1098. doi:10.1002/hup.1098. [DOI] [PubMed] [Google Scholar]
- GBD 2016 Alcohol and Drug Use Collaborators. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. The Lancet Psychiatry. 2018;5:987–1012. doi: 10.1016/S2215-0366(18)30337-7. doi:10.1016/S2215-0366(18)30337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gual A., Lehert P. Acamprosate during and after acute alcohol withdrawal: A double-blind placebo-controlled study in Spain. Alcohol and Alcoholism. 2001;36:413–418. doi: 10.1093/alcalc/36.5.413. doi:10.1093/alcalc/36.5.413. [DOI] [PubMed] [Google Scholar]
- Gualtieri I., Hatzigiakoumis D. S., De Vita O., Tedeschi D., Quatrale M., Guglielmo R., Janiri L. Use of acamprosate in the treatment of alcohol dependence: Efficacy in the reduction of craving, relapse prevention and protracted withdrawal in an Italian sample. Dipendenze Patologiche. 2011;1:9–14. [Google Scholar]
- Häkkinen M., Vuori E., Kalso E., Gergov M., Ojanperä I. Profiles of pregabalin and gabapentin abuse by postmortem toxicology. Forensic Science International. 2014;241:1–6. doi: 10.1016/j.forsciint.2014.04.028. doi:10.1016/j.forsciint.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Hammond C. J., Niciu M. J., Drew S., Arias A. J. Anticonvulsants for the treatment of alcohol withdrawal syndrome and alcohol use disorders. CNS Drugs. 2015;29:293–311. doi: 10.1007/s40263-015-0240-4. doi:10.1007/s40263-015-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M., Egli M., Crabbe J. C., Becker H. C. Acute withdrawal, protracted abstinence and negative affect in alcoholism: Are they linked? Addiction Biology. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. doi:10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. T., Altman D. G., Gøtzsche P. C., Jüni P., Moher D., Oxman A. D. Cochrane Bias Methods Group & Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. doi:10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T., Komiyama T., Harada S., Matsumoto T. Treatment of substance dependence by a bio-cognitive model based on behavioral pharmacology. Seishin Shinkeigaku Zasshi = Psychiatria Et Neurologia Japonica. 2005;107:1147–1158. [PubMed] [Google Scholar]
- Huang M.-C., Chen C.-H., Pan C.-H., Lin S.-K. Lack of efficacy of dextromethorphan in managing alcohol withdrawal: A preliminary report of a randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychopharmacology. 2014;34:149–152. doi: 10.1097/JCP.0000000000000052. doi:10.1097/JCP.0000000000000052. [DOI] [PubMed] [Google Scholar]
- Hughes J. R. Protracted withdrawal. American Journal of Psychiatry. 1994;151:785–786. doi: 10.1176/ajp.151.5.aj1515785. doi:10.1176/ajp.151.5.785-a. [DOI] [PubMed] [Google Scholar]
- Kissin B. Biological investigations in alcohol research. Journal of Studies on Alcohol. 1979;(Supplement 8):146–181. doi: 10.15288/jsas.1979.s8.146. doi:10.15288/jsas.1979.s8.146. [DOI] [PubMed] [Google Scholar]
- Le Bon O., Murphy J. R., Staner L., Hoffmann G., Kormoss N., Kentos M., Verbanck P. Double-blind, placebo-controlled study of the efficacy of trazodone in alcohol post-withdrawal syndrome: Polysomnographic and clinical evaluations. Journal of Clinical Psycho-pharmacology. 2003;23:377–383. doi: 10.1097/01.jcp.0000085411.08426.d3. doi:10.1097/01.jcp.0000085411.08426.d3. [DOI] [PubMed] [Google Scholar]
- Lennox R. D., Cecchini-Sternquist M. Safety and tolerability of sauna detoxification for the protracted withdrawal symptoms of substance abuse. Journal of International Medical Research. 2018;46:4480–4499. doi: 10.1177/0300060518779314. doi:10.1177/0300060518779314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J. G., Hall-Flavin D., Nelson S., Schmidt K. A., Schak K. M. The role of gabapentin in the management of alcohol withdrawal and dependence. Annals of Pharmacotherapy. 2015;49:897–906. doi: 10.1177/1060028015585849. doi:10.1177/1060028015585849. [DOI] [PubMed] [Google Scholar]
- Liappas J., Paparrigopoulos T., Malitas P., Tzavellas E., Christodoulou G. Mirtazapine improves alcohol detoxification. Journal of Psychopharmacology. 2004;18:88–93. doi: 10.1177/0269881104040241. doi:10.1177/0269881104040241. [DOI] [PubMed] [Google Scholar]
- Liberati A., Altman D. G., Tetzlaff J., Mulrow C., Gøtzsche P. C., Ioannidis J. P. A., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Medicine. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. doi:10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinotti G., Di Nicola M., Frustaci A., Romanelli R., Tedeschi D., Guglielmo R., Janiri L. Pregabalin, tiapride and lorazepam in alcohol withdrawal syndrome: A multi-centre, randomized, single-blind comparison trial. Addiction. 2010a;105:288–299. doi: 10.1111/j.1360-0443.2009.02792.x. doi:10.1111/j.1360-0443.2009.02792.x. [DOI] [PubMed] [Google Scholar]
- Martinotti G., Di Nicola M., Romanelli R., Andreoli S., Pozzi G., Moroni N., Janiri L. High and low dosage oxcarbazepine versus naltrexone for the prevention of relapse in alcohol-dependent patients. Human Psychopharmacology. 2007;22:149–156. doi: 10.1002/hup.833. doi:10.1002/hup.833. [DOI] [PubMed] [Google Scholar]
- Martinotti G., Di Nicola M., Tedeschi D., Andreoli S., Reina D., Pomponi M., Janiri L. Pregabalin versus naltrexone in alcohol dependence: A randomised, double-blind, comparison trial. Journal of Psychopharmacology. 2010b;24:1367–1374. doi: 10.1177/0269881109102623. doi:10.1177/0269881109102623. [DOI] [PubMed] [Google Scholar]
- Mason B. J., Quello S., Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opinion on Investigational Drugs. 2018;27:113–124. doi: 10.1080/13543784.2018.1417383. doi:10.1080/13543784.2018.1417383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melemis S. M. Relapse prevention and the five rules of recovery. Yale Journal of Biology and Medicine. 2015;88:325–332. [PMC free article] [PubMed] [Google Scholar]
- Mueller T. I., Stout R. L., Rudden S., Brown R. A., Gordon A., Solomon D. A., Recupero P. R. A double-blind, placebo-controlled pilot study of carbamazepine for the treatment of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1997;21:86–92. doi:10.1111/j.1530-0277.1997.tb03733.x. [PubMed] [Google Scholar]
- Myrick H., Malcolm R., Randall P. K., Boyle E., Anton R. F., Becker H. C., Randall C. L. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcoholism: Clinical and Experimental Research. 2009;33:1582–1588. doi: 10.1111/j.1530-0277.2009.00986.x. doi:10.1111/j.1530-0277.2009.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health Research. PROSPERO international prospective register of systematic reviews. 2019. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014006602 Retrieved from.
- Overall J. E., Brown D., Williams J. D., Neill L. T. Drug treatment of anxiety and depression in detoxified alcoholic patients. Archives of General Psychiatry. 1973;29:218–225. doi: 10.1001/archpsyc.1973.04200020052006. doi:10.1001/archpsyc.1973.04200020052006. [DOI] [PubMed] [Google Scholar]
- Potgieter A. S., Deckers F., Geerlings P. Craving and relapse measurement in alcoholism. Alcohol and Alcoholism. 1999;34:254–260. doi: 10.1093/alcalc/34.2.254. doi:10.1093/alcalc/34.2.254. [DOI] [PubMed] [Google Scholar]
- Roy Rosenzweig Center for History and New Media. George Mason University; 2018. www.zotero.org Zotero (5.0.96.2) [IOS] Retrieved from. [Google Scholar]
- Satel S. L., Kosten T. R., Schuckit M. A., Fischman M. W. Should protracted withdrawal from drugs be included in DSMIV? American Journal of Psychiatry. 1993;150:695–704. doi: 10.1176/ajp.150.5.695. doi:10.1176/ajp.150.5.695. [DOI] [PubMed] [Google Scholar]
- Schjerning O., Pottegård A., Damkier P., Rosenzweig M., Nielsen J. Use of Pregabalin - A nationwide pharmacoepidemiological drug utilization study with focus on abuse potential. Pharmacopsychiatry. 2016;49:155–161. doi: 10.1055/s-0042-101868. doi:10.1055/s-0042-101868. [DOI] [PubMed] [Google Scholar]
- Segal B. M., Kushnarev V. M., Urakov I. G., Misionzhnik E. U. Alcoholism and disruption of the activity of deep cerebral structures: Clinical-laboratory research. Quarterly Journal of Studies on Alcohol. 1970;31:587–601. doi:10.15288/qjsa.1970.31.587. [PubMed] [Google Scholar]
- Soyka M., Kranzler H. R., Berglund M., Gorelick D., Hesselbrock V., Johnson B. A., Möller H.-J., & the WFSBP Task Force on Treatment Guidelines for Substance Use Disorders 2008World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Substance Use and Related Disorders, Part 1: Alcoholism. World Journal of Biological Psychiatry 96–23.doi:10.1080/15622970801896390 [DOI] [PubMed] [Google Scholar]
- Stojek A., Madejski J., Dedelis E., Janicki K. [Correction of the symptoms of late substance withdrawal syndrome by intra-conjunctival administration of 5% homatropine solution (preliminary report)] Psychiatria Polska. 1990;24:195–201. [PubMed] [Google Scholar]
- Trevisan L., Ralevski E., Keegan K., Oville A., Vuppalapati D., Gonzalez G., Petrakis I. L. Alcohol detoxification and relapse prevention using valproic acid versus gabapentin in alcohol-dependent patients. Addictive Disorders & Their Treatment. 2008;7:119–128. doi:10.1097/ADT.0b013e31812e6a3c. [Google Scholar]
- Veritas Health Innovation. Covidence systematic review software [English] 2019 https://get.covidence.org/systematic-review-software?campaignid=15030045989&adgroupid=130408703002&gclid=CjwKCAjwxZqSBhAHEiwASr9n9B0h0iPnRZVSjzamEZOkcP1mrpvCcn3sA3iym194UEZ7pCJYp5RdmRoCr30QAvD_BwE Retrieved from. [Google Scholar]
- Vik P. W., Cellucci T., Jarchow A., Hedt J. Cognitive impairment in substance abuse. Psychiatric Clinics of North America. 2004;27:97–109. doi: 10.1016/S0193-953X(03)00110-2. doi:10.1016/S0193-953X(03)00110-2. [DOI] [PubMed] [Google Scholar]
- Voltaire-Carlsson A., Hiltunen A. J., Koechling U. M., Borg S. Effects of long-term abstinence on psychological functioning: A prospective longitudinal analysis comparing alcohol-dependent patients and healthy volunteers. Alcohol. 1996;13:415–421. doi: 10.1016/0741-8329(96)81678-8. doi:10.1016/0741-8329(96)81678-8. [DOI] [PubMed] [Google Scholar]
- Watanabe K.-I., Ogihara-Hashizume A., Kobayashi Y., Mitsushio H., Komiyama T. Impaired sleep during the post-alcohol withdrawal period in alcoholic patients. Addiction Biology. 2001;6:163–169. doi: 10.1080/13556210020040244. doi:10.1080/13556210020040244. [DOI] [PubMed] [Google Scholar]
- Wellman M. Management of the late withdrawal symptoms of alcohol. Canadian Medical Association Journal. 1954;71:360–365. [PMC free article] [PubMed] [Google Scholar]
- Wilde M. I., Wagstaff A. J. Acamprosate. A review of its pharmacology and clinical potential in the management of alcohol dependence after detoxification. Drugs. 1997;53:1038–1053. doi: 10.2165/00003495-199753060-00008. doi:10.2165/00003495-199753060-00008. [DOI] [PubMed] [Google Scholar]
- Williams D., McBride A. J. The drug treatment of alcohol withdrawal symptoms: A systematic review. Alcohol and Alcoholism. 1998;33:103–115. doi: 10.1093/oxfordjournals.alcalc.a008365. doi:10.1093/oxfordjournals.alcalc.a008365. [DOI] [PubMed] [Google Scholar]