Abstract
An outline of the advantages, in terms of sustainability, of Deep Eutectic Solvents (DESs) is provided, by analyzing some of the most popular DESs, obtained by the combination of choline chloride, as a hydrogen bond acceptor, and six hydrogen bond donors. The analysis is articulated into four main issues related to sustainability, which are recurrently mentioned in the literature, but are often taken for granted without any further critical elaboration, as the prominent green features of DESs: their low toxicity, good biodegradability, renewable sourcing, and low cost. This contribution is intended to provide a more tangible, evidence-based evaluation of the actual green credentials of the considered DESs, to reinforce or question their supposed sustainability, also in mutual comparison with one another.
1. Introduction
One of the most relevant issues in green chemistry concerns the massive use of solvents, which are extensively employed in chemical processes at any level. Conventional organic solvents are mainly represented by volatile organic compounds (VOCs), which are generally regarded as non-sustainable, due to the hazards posed by their flammability and their toxicity toward human health and the environment, as well as to their non-renewable petrochemical production (with few exceptions).1 In the search for alternatives to VOCs, Deep Eutectic Solvents (DESs) have emerged at the beginning of the present century and have rapidly garnered the interest of the scientific community.2 Thanks to its peculiar and tunable properties, this class of solvents has found application in different fields, ranging from organic synthesis, including enzymatic catalysis, to drug dissolution and delivery, from biomass processing to gas capture, and from extraction processes to electrochemistry, including energy storage.3 A DES is a mixture of at least two components, one of which acts as Hydrogen Bond Acceptor (HBA) and one as Hydrogen Bond Donor (HBD). At a specific molar ratio of the components, the mixture shows a significant depression of the melting point (deep eutectic).4 Ever since the first reports, the potential of DESs as sustainable solvent systems has been pointed out.2a,5 Their components are usually small molecules, such as alcohols, short-chain carboxylic acids, amides, sugars, and abundant in nature, often as plant metabolites; the most common HBA, choline chloride (ChCl), is used as an additive for domestic animal feed.6 On this basis, there is a general claim on the sustainability of DESs, which is usually expressed in terms of “low toxicity, high biodegradability, low cost, sourcing from renewable feedstock”. However, this general claim is rather vague and rarely supported by solid evidence, as it is often uncritically taken for granted by papers reporting new scientific developments and applications of DESs. Moreover, the supposed green credentials may vary significantly according to the nature of the components of the eutectic mixture. The present contribution aims at providing a comparative evaluation of the sustainable features of DESs, by choosing ChCl as HBA and six commonly employed HBDs, reported in Figure 1: glycerol (Gly, HBD1), ethylene glycol (EG, HBD2), urea (U, HBD3), glucose (Glu, HBD4), malonic acid (MA, HBD5), and lactic acid (LA, HBD6).
Figure 1.
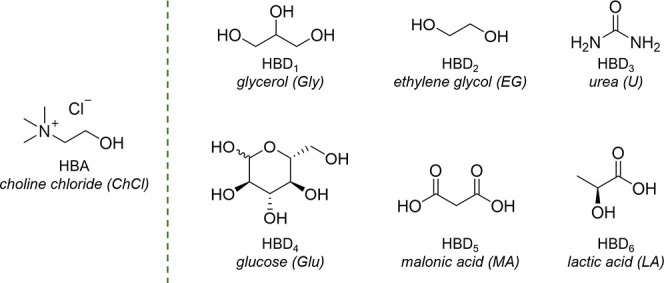
Hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD) components of the DESs considered in this review.
It is worth remembering that sustainability is a concept encompassing several aspects, as stated by the sustainable development goals, outlined by the United Nations in the 2030 Agenda for Sustainable Development.7 Therefore, this work is intended to critically discuss and address point by point the aforementioned claims, which have all contributed to define DESs as sustainable solvents, by discussing each of the “green” features (toxicity, biodegradability, renewable feedstocks, production) in separate sections. Moreover, the comparison among different DESs is meant to point out in which respect they are comparable and in which they differ from one another from the point of view of sustainability. It should be noted that there is an ongoing debate to define in a more rigorous way under which conditions an eutectic mixture can be rightfully called a deep eutectic solvent; this discussion also involves, but is not limited to, how “deep” should the eutectic be, particularly when one of the components is already liquid at room temperature.8 On this basis, not all the molar ratios of ChCl and HBD1–6 reported in the literature could be correctly defined as DESs. However, from an application perspective it seems reasonable to include in the present discussion studies performed on ChCl/HBD1–6 mixtures at several molar ratios and not only in the proper “eutectic” composition, considering that lots of physical properties (the simplest being the existence in the liquid state) are shared among many compositions. Yet, in so doing, it would be more correct to term such systems as “DES-like”.
2. Toxicity
The effects that the solvents used in industrial and technological applications induce on living organisms represent a concern for the environment, mainly through contamination of waters and soils, and ultimately for human health. Among the features that have raised interest around DESs as promising alternatives to classical solvents is their supposed low toxicity, which would represent an improvement not only over VOCs but also over common Ionic Liquids (ILs), such as imidazolium- or pyridinium-based ones.9 The debate on the toxicity of DESs started from the observation that their components are in most cases abundant in nature as environmentally friendly plant metabolites. As summarized in Table 1, safety data sheets of these compounds do not highlight particular concerns for human handling, apart from the well-known acute oral toxicity reported for EG. The ecotoxicity, usually assessed through the effects produced on fish or other aquatic organisms, is negligible as well (Table 1).
Table 1. Available Information, from Safety Data Sheet, on the Toxicity of ChCl and HBD1–6a.
| Component | CLP classificationb | Acute toxicity to rat (oral LD50c) | Toxicity to fish (LC50d) |
|---|---|---|---|
| ChCl (HBA) | Not a hazardous substance | LD50 = 3.40 g/kge | No data available |
| Gly (HBD1) | Not a hazardous substance | LD50 = 27.2 g/kg | LC50 = 54000 mg/L |
| Rainbow trout (Oncorhynchus mykiss) | |||
| Static test, 96 h | |||
| EG (HBD2) | Acute toxicity, oral (category 4) Specific target organ toxicity, repeated exposure, oral (category 2), kidney | No data available | LC50 = 72860 mg/L |
| Fathead minnow (Pimephales promelas) | |||
| Static test, 96 h | |||
| U (HBD3) | Not a hazardous substance | LD50 = 8.47 g/kg | No data available |
| Glu (HBD4) | Not a hazardous substance | LD50 = 25.8 g/kg | No data available |
| MA (HBD5) | Serious eye damage (category 1) | LD50 = 3.25 g/kg | LC50 = 95.4 mg/L |
| Japanese rice fish (Oryzias latipes) | |||
| Flow-through test, 96 h | |||
| LA (HBD6) | Skin irritation (category 2) | LD50 = 3.54 g/kg | LC50 = 130 mg/L, |
| Serious eye damage (category 1) | Rainbow trout (Oncorhynchus mykiss) | ||
| Static test, 96 h |
Safety data sheet available at Sigma-Aldrich Web site.
According to EC Regulation No. 1272/2008 (CLP/GHS). The definition of the categories for each class of hazard are included in the text of the Regulation.
Amount of substance that kills 50% of test animals in a single dose.
Concentration at which 50% mortality occurs.
Oral LD50 to mice: 3.90 g/kg.
However, it is now well established that synergistic effects of the components when combined in a DES could alter the properties of the mixture, because of the emerging interactions in the supramolecular structure of the eutectic mixture, characterized by a network of hydrogen bonds.10 The toxicity of a DES could then be different from that of its components: it thus becomes necessary to evaluate the safety profile of each system. To date, several studies have tried to shed light on the actual toxicity of various DESs, by investigating the effects on human and animal cell lines, microorganisms, including bacterial, yeast and fungi strains, and marine animal models. However, results from different works do not always point toward the same direction, and a lack of systematicity, also concerning the methods used to assess DESs toxicity, has recently been highlighted.11 In the present paragraph, the available data on ChCl/HBD1–6 are compared to define whether one or more among them could be able to meet the definition of an “environmentally friendly solvent”. Since microbial toxicity has recently been the object of a comprehensive review,11b hereafter the discussion will focus on toxicity toward animal models. In addition to that, two studies on the toxicity toward plant species (phytotoxicity) are included. Table 2 summarizes the available data concerning the effects induced by ChCl/HBD1–6 DESs on animals. In 2013, 10 years after DESs had made their way into the scientific debate,2a the first investigation to evaluate their actual ecotoxicological profile was conducted on the brine shrimp Artemia salina, an assay which evaluates the survival rate of the shrimp’s nauplii as a function of time after immersion in a solution of the potentially toxic agent.12 The results showed that solutions of the three tested DESs, ChCl/HBD1–3 in a 1/3 molar ratio, were harmful toward this organism, and their toxicity was significantly higher than that of solutions of both their components alone and of the simple mixture of their components (without the prior formation of the eutectic). It should be pointed out that the brine shrimp assay involves the monitoring of the survival rate of the shrimp’s nauplii after several hours or days,12b−12d while in this case all organisms were found dead after minutes or tents of minutes. Furthermore, the authors did not include a control group in the experiment, to exclude other possible reasons for the toxicity, and did not report the concentration of the DESs in the solutions used for the assay: thus, no reliable LC50 (lethal concentration, at which 50% mortality occurs) values could be provided. These data should then be considered as a preliminary assessment of the toxicity and a starting point for further investigations, suggesting that the synergistic effect could lead to a toxicity enhancement of the system, probably due to the involvement of ChCl and HBDs in the eutectic mixture. This point is of paramount importance to design suitable procedures for DES-containing waste treatment, for example, through dilution in aqueous medium and subsequent disruption of the supramolecular structure. Following efforts were focused on the toxicity toward other aquatic species, such as Hydra sinensis, a freshwater invertebrate.13 Again, DESs (or DES-like mixtures) composed of ChCl and HBD1–3 (in 1/1, 1/2, and 2/1 molar ratios) were tested (Table 2). In all cases, the exposition to a 10 mM concentration of the DESs caused significant shortenings in the survival times of the hydras, compared to the control group; in addition, before death, the morphology of the organisms underwent the changes typical of exposure to a toxic chemical. Interestingly, while the HBD1–3 solutions displayed limited toxicity, ChCl proved to be much more lethal than the DESs; the components ChCl and HBD1–3, mixed together in solution but without prior formation of the eutectic, were also as toxic as the choline salt alone. In this case, then, a synergistic effect of the DES appears to be able to mitigate the toxicity of the most harmful component by incorporating it in the eutectic structure, in an opposite way to that observed in the A. salina assay. However, it should be observed that the DES concentration in the solution into which the organisms were immersed was quite high (10 mM, i.e., in the range 2000–4000 mg/L depending on the considered DES): studies within a range of concentrations would be necessary to determine LC50 values. Among aquatic invertebrates, the toxicity of three DESs, ChCl/HBD1–3 in a 1/2 molar ratio, was studied also on the planktonic crustacean Daphnia magna. The test, conducted according to OECD Guideline No. 202, evaluated the immobilization of the organisms after 24 h of exposition to different concentrations of the considered DESs.14 According to the results, expressed as EC50 (half maximal effective concentration), the three eutectic mixtures could be classified as “relatively harmless”, with EC50 values higher than 1000 mg/L (Table 2). Noteworthy, significant differences were observed with different HBDs, as ChCl/Gly had an EC50 value more than twice greater than ChCl/U.14a The acute toxicity (expressed as LC50) of DESs composed of ChCl and HBD1–5 (in various molar ratios, see Table 2) on a fish model, the Eurasian carp (Cyprinius carpio), was investigated in two different papers.15 In sharp contrast with the experiments on A. salina and H. sinensis, and in accordance with that on D. magna, all tested DESs were practically harmless: the LC50 values were higher than 100 mg/L in all cases. Indeed, the tests were conducted according to the OECD Guideline No. 203 for fish acute toxicity, which poses 100 mg/L as a threshold above which it is not necessary to determine the exact LC50 figure.16 Nevertheless, the latter was calculated for three systems only, namely ChCl/Gly, ChCl/EG, and ChCl/U in 1/2 molar ratio, resulting in values in the 6000–10000 mg/L range.15a Similarly, the single components, as well as their mixtures without prior eutectic formation, showed no significant effects on the fishes, in line with low ecotoxicities reported in the safety data sheets (Table 1). The only exception was MA, which revealed to be slightly toxic (again, in line with what reported in Table 1) when not incorporated into a DES, highlighting a synergistic mitigation by the eutectic mixture.15b Finally, DESs composed by ChCl and HBD1–4 were tested for the toxicity toward mice.17 The LD50 (lethal dose, the amount of a solid or liquid material that kills 50% of test animals in a single dose) values after oral administration (Table 2) showed that the considered DESs are relatively toxic, with an approximative two-/four-fold decrease compared with the components alone. The authors observed a correlation between the toxicity and the ChCl/HBD molar ratio, as in the case of ChCl/U 1/3 the lethality was so fast that it did not allow the determination of the LD50, while the 1/2 ratio was revealed to be less toxic.17a It should as well be considered that, for ChCl/U, the 1/2 ratio is the one that identifies the “proper” DES composition, and its reduced toxicity could then arise from inherent synergistic effect in the deep eutectic mixture. The metabolomics analysis of the treated mice was also evaluated, revealing acute liver and kidney injury, associated with elevated oxidative stress.17a,17b Moreover, stress response to ammonia exposure was detected after the oral administration of ChCl/U 1/2, at a dose of 1.5 g/kg; the authors traced this back to ammonia impurities present in the urea used to produce the DES, hypothesizing that the absorption and toxicity of this chemical could be enhanced by the presence of the DES itself.17b On this basis, it is possible that ammonia stress is involved in the higher toxicity of ChCl/U 1/3, compared to ChCl/U 1/2 (Table 2). Apart from ChCl/HBD1–3, also the toxicity of the sugar-based ChCl/Glu 2/1 was tested.18 However, in the paper, there is a discordance between the materials and methods and the results sections, which poses doubts on whether the authors evaluated the toxicity upon oral or parenteral administration of the mixtures (the latter is more likely, according to the authors’ discussion). For this reason, the results are not included in Table 2. The investigation of the compatibility of DESs with parenteral administration is more focused on the possible use of the eutectics as drug delivery systems, rather than on their ecotoxicity.3d,3e
Table 2. Toxicity of ChCl/HBD1–5 DESs Towards Animal Modelsa.
| DES |
|||||
|---|---|---|---|---|---|
| Animal model | Components | Ratio | Results | Ref. | |
| Brine shrimp (Artemia salina) | ChCl/Gly | 1/3 | • All DESs were toxic | (12a) | |
| ChCl/EG | 1/3 | • Higher toxicity for DESs than for components alone or their mixture | |||
| ChCl/U | 1/3 | ||||
| Hydra (Hydra sinensis) | ChCl/Gly | Variousb | • All DESs were toxic | (13) | |
| ChCl/EG | Variousb | • Lower toxicity for DESs than for components alone (particularly ChCl) or their mixture | |||
| ChCl/U | Variousb | ||||
| Crustacean (Daphnia magna) | EC50 (mg/L) | (14a) | |||
| ChCl/Gly | 1/2 | 2530 | • All DESs were “relatively harmless” | ||
| ChCl/EG | 1/2 | 1870 | • The DES components alone were not tested | ||
| ChCl/U | 1/2 | 1100 | |||
| Fish (Cyprinius carpio) | LC50 (mg/L) | (15) | |||
| ChCl/Gly | 1/2, 1/3 | >100 | • All DESs were “practically harmless”, as well as their components alone or their mixture | ||
| ChCl/EG | 1/2, 1/3 | >100 | |||
| ChCl/U | 1/2 | >100 | • Among DES components, slight toxicity for MA solution (LC50 = 50 ± 15 mg/L) and ChCl + MA solution (LC50 = 55 ± 13 mg/L) | ||
| ChCl/Glu | 2/1 | >100 | |||
| ChCl/MA | 1/1 | >100 | |||
| Mice | LD50 (g/kg) | (17) | |||
| ChCl/Gly | 1/3 | 6.39 ± 0.53 | • All DESs were relatively toxic | ||
| 1/2 | 7.73c | • Higher toxicity for DESs than for components alone | |||
| ChCl/EG | 1/3 | 5.33 ± 0.49 | • Liver and kidney injury detected (oxidative stress) | ||
| ChCl/U | 1/3 | toxic | • Ammonia stress detected with ChCl/U 1/2 | ||
| 1/2 | 5.46 ± 0.36 | ||||
All other DESs studied in the considered papers are not reported.
1/1, 1/2, 2/1 ratios were tested, without significant differences from one another.
95% confidence interval: 7.13–8.39 g/kg.17c
Alongside studies on animals, the phytotoxicity of DESs was evaluated with testng garlic (Allium sativum)13b and wheat (Triticum aestivum)19 as specimen; the results are summarized in Table 3. In the case of garlic, the effect of ChCl/HBD1–3 1/1 DESs on germination from garlic cloves was evaluated by measuring the root growth inhibition after soaking in the DES solution. The results showed that all DESs were harmful toward the root growth, as well as their components, among which U was the less toxic, while ChCl and Gly had the worst effect: it is then particularly noteworthy that the ChCl/Gly DES displayed a synergistic effect such that its toxicity was significantly lower than both its two components.13b Regarding the T. aestivum experiment, three parameters were monitored after treatment with different concentrations of DESs: the seed germination, and the root and shoot growth of the seedling after germination. It was found that both ChCl/Gly 1/2 and ChCl/Glu 2/1 were practically harmless toward seed germination (EC50 > 20000 mg/L), while some root and shoot growth inhibition was observed (EC50 ≈ 800–3700 mg/L), with roots being more sensitive than shoots. This was explained by the direct contact of roots with the DES solutions. The study did not evaluate the toxicity of single DES precursors.19 It should be noted that another work by the same group revealed lower values of EC50 for imidazolium-based ILs, using barley (Hordeum vulgare) as a plant model, confirming the higher phytotoxicity of the ILs compared to the above-mentioned DESs.20
Table 3. Phytotoxicity of ChCl/HBD1–4 DESsa.
| DES |
||||
|---|---|---|---|---|
| Plant | Components | Ratio | Results | Ref. |
| Garlic (Allium sativum) | ChCl/Gly | 1/1 | • Root growth inhibition by DESs as well as their components | (13b) |
| ChCl/EG | 1/1 | • High toxicity of ChCl, mitigated in ChCl/U, but not in ChCl/EG | ||
| ChCl/U | 1/1 | • ChCl/Gly significantly less toxic than both its components | ||
| Wheat (Triticum aestivum) | ChCl/Gly | 1/2 | • Practically harmless toward seed germination | (19) |
| ChCl/Glu | 2/1 | • Relatively higher toxicity for shoot and root growth inhibition | ||
All other DESs studied in the considered papers are not reported.
The analysis of available information on the toxicity of DESs on animal and plant species reveals different possible scenarios, in which the hazard of the eutectic mixtures may be higher, lower, or equal compared to their components alone. It is now diffusely recognized that the initial assumption of DESs as generically “environmentally friendly”, based on the nature of their constituents, is somehow naïve. As a matter of fact, the studies reported in this chapter indicate that even these apparently harmless compounds may represent a concern for the environment, and the cases of ChCl toxicity toward hydra (Table 2) and garlic (Table 3) are illustrative in this regard. The matter could be particularly relevant in case DESs were used as solvents in large-scale applications, because of their amount in waste effluents, which could result in high concentrations in the environment. Furthermore, as suggested by some of the results presented in this chapter and by studies on microbial toxicity, the peculiar physicochemical properties of DESs, such as their viscosity, may have a role in altering their intrinsic chemical toxicity and their ability to constitute a hazard for the environment. Indeed, a rigorous evaluation of the safety of DESs should involve an effort not only in trying to apply standard procedures to produce comparable data, as remarkably done for example in the carp toxicity studies, but also in establishing new general guidelines to take into account the nature and the characteristics of these mixtures.11a,11b In conclusion, it seems premature to make a pronouncement on the actual toxicity of DESs. However, it is worth mentioning that the available data on ecotoxicity toward aquatic organisms are encouraging, with LC50 values in the order of 102–103 mg/L (Table 2). Among those considered in this review, the studies in which some differences were observed highlighted that MA could be a relatively more harmful HBD (the only one to display some toxicity in the C. carpio studies), as highlighted also in Table 1, while U appeared much safer than others in the A. sativum works (Table 3). One point that certainly identifies DESs as safe and environmentally friendly is their negligible vapor pressure, which excludes the possibility of atmospheric contamination and hazards deriving from inhalation.21
3. Biodegradability
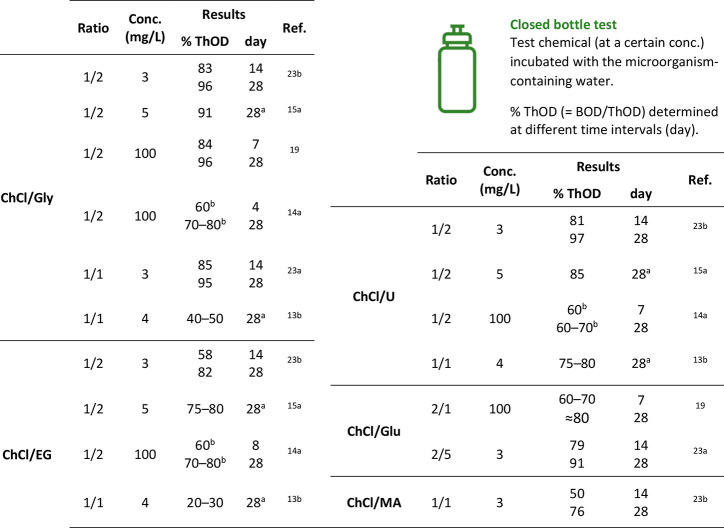
The discussion on the biodegradability of DESs takes its cues from the same assumptions that have been enunciated regarding their toxicity: since their components are plant metabolites, present in variable amounts in the environment and in biological systems, they should get easily degraded by naturally occurring microorganisms. The assessment of the persistence of a substance in the environment is once again particularly important to design suitable protocols for waste treatment, especially in the case of solvents, whose disposal may involve large amounts of substance. Under this point of view, it would be of course desirable to replace nonbiodegradable conventional solvents with “endogenous” ones. The DES components considered in this review are classified as “readily biodegradable” by their safety data sheet, except for malonic acid and urea, for which no data are available (anyway, they are classified as not persistent in the environment). As for toxicity, however, also the biodegradability of DESs cannot be taken for granted and should be evaluated on a case-by-case basis. Up to now, the topic has been investigated in some studies, all of which were conducted according to OECD Guideline No. 301 D (namely, the “closed bottle test”), that allows classifying a chemical as “readily biodegradable” or not in an aerobic aqueous medium.22 The experiment consists of adding the test chemical, previously dissolved into a saline solution, to a bottle containing an inoculum, i.e., a water sample collected from a secondary effluent of a wastewater treatment plant or from surface water (e.g., of a lake), diluted with deionized or distilled water. The bottle is closed and stored in the dark, and the amount of dissolved O2 is measured at different time intervals to determine the biochemical oxygen demand (BOD, the amount of O2 consumed by aerobic organisms to metabolize organic matter) by comparison with a blank solution, not containing the test chemical. The BOD value is then used to calculate the percentage of the theoretical oxygen demand (ThOD), which is based on the mass and molecular formula of the chemical employed. If this percentage reaches 60% in a 14-days window, starting from the day in which it has reached 10%, and not exceeding the 28th day from the beginning of the experiment, the test chemical can be considered as “readily biodegradable”. To validate the results, the experiment is also performed on a reference compound, which is known to meet the criteria for ready biodegradability: if such compound reaches the pass level (60% ThOD) within 14 days, the test is considered valid.22 The results from the different papers in which the biodegradability of ChCl/HBD1–5 DESs has been investigated are summarized in Table 4. First of all, it should be noted that the criteria for ready biodegradability are met in almost all cases, with high levels of % ThOD not only at 28 days from the beginning of the experiment but also after 14 or even 7 days. In one work only, ChCl/Gly and ChCl/EG DESs, both in a 1/1 molar ratio, failed to reach the pass level.13b The authors attributed this significant discrepancy to different experimental conditions and different source and concentration of the inoculum, even if their experiment was successfully validated with sodium benzoate as a reference compound, obtaining results comparable to other studies.23 The effects of microbial populations coming from different sites have also been pointed out by a study on pyridinium and imidazolium-based ILs.24
Table 4. Biodegradability of ChCl/HBD1–5 DESs.
Not explicitly stated in the paper.
Obtained through manometric respirometry (OECD 301 F) instead of a closed bottle test (OECD 301 D).22
Beyond the definition of “readily biodegradable”, that is common to all the considered DESs in Table 4, some differences can be highlighted, according to the nature of the HBD component. ChCl/Gly and ChCl/U show the highest biodegradability values: under the same experimental conditions, ChCl/Gly 1/2 and ChCl/U 1/2 (at a 3 mg/L concentration) showed % ThOD values of 96% and 97% respectively, while ChCl/EG 1/2 and ChCl/MA 1/1 were one step below, with 82% and 76% ThOD respectively, after 28 days. Remarkably, the difference was even greater in the first 14 days, with 83% and 81% for Gly- and U-based DESs, while ChCl/EG only reached 58%, similarly to the 50% ThOD for ChCl/MA (Table 4).23b A similar trend was observed with the same DESs at a 5 mg/L concentration (91% for ChCl/Gly, 85% for ChCl/U, 75–80% for ChCl/EG).15a These slight differences have been interpreted according to the Boethling’s rules of thumb for designing biodegradable small molecules,25 since a higher number of hydroxyl groups per mole (Gly vs EG) or the presence of carboxylic derivatives, such as acids, esters, or amides (as in the case of U) should improve the biodegradability profile. For MA-based DES, acidification of the aqueous medium was invoked to account for the less efficient biodegradation.15a,23b High values of % ThOD were obtained also with Glu as HBD: under the same experimental conditions, ChCl/Glu 2/5 and ChCl/Gly 1/1 (at a 3 mg/L concentration) reached 79% and 85% ThOD respectively after just 14 days, and more than 90% after 28 days (Table 4).23a It appears then that the issue on the biodegradability of DESs is less controversial than the one on toxicity: the available literature data point toward the definition of “readily biodegradable” for the considered ChCl/HBD1–5 DESs, in particular for Gly, U, and Glu as HBDs, with EG and MA just immediately following. To the best of our knowledge, no biodegradability studies were performed on LA-based DESs. Interestingly, this green feature is shared with a series of ILs composed of choline as the cation and 19 amino acids as the anion, in contrast with the often low biodegradability of more classical pyridinium- or imidazolium-based ILs.26 This suggests that the use of naturally occurring compounds, such as plant metabolites, may be enough to ensure a good biodegradability for their eutectic mixtures too. It should be noted that these encouraging results have all been obtained in an aqueous medium, i.e., in the most relevant conditions in the context of a possible industrial application of DESs. However, it would be interesting to investigate also the biodegradability in soil, another possible recipient of environmental pollution, as it was done for example for imidazolium-based ILs.27 Lastly, the mere fact that DESs are easily metabolized by wastewater microorganisms may represent just a part of the issue, as the nature of the products of such mineralization should be taken into account too. Looking at ChCl/HBD1–6 DESs, for example, the common HBA, ChCl, would most certainly release chloride anions in solution, which may represent a concern for the aquatic environment.28 On this regard, the chemical oxygen demand (COD, amount of oxygen needed to oxidize a given substance, including oxidizable inorganic matter), could be a better indicator than BOD. A complete picture of the environmental fate of DESs would therefore require the investigation and monitoring of the nature, concentration, and speciation of all their biodegradation products.
4. Feedstocks
A typical sentence that one could frequently read in the introduction of papers on DESs is that they “come from (potentially) renewable sources”. This claim is rooted into the fact that the most commonly employed DESs’ components are commodity chemicals, which are (or could be) sourced from biorenewable feedstocks, through fermentation processes or direct extraction from biomass. While this statement is in principle correct, its current validity ought to be verified by considering the actual feedstocks that are employed to produce each precursor on an industrial scale. The aim of the present section is to provide a picture of which shade of the “potentially” renewable definition has been currently achieved for the seven DES components reported in Figure 1. This approach would help classify the different DESs according to their real or only foreseeable renewable features. For this purpose, the Ullmann’s Encyclopedia of Industrial Chemistry was used as a reference for the relevant commercial-scale processes, and it was compared with recent literature on the topic.
The HBA salt of choline chloride (ChCl) is already a significant example in this regard, since it is produced through the reaction of trimethylamine, hydrochloric acid, and ethylene oxide (represented in Figure 2).6a,29 Among the building blocks used in the chemical process, trimethylamine is produced from methanol and ammonia, which are actually derived from fossil sources, and the synthesis of ammonia is also particularly energy demanding; ethylene oxide comes from a renowned petrochemical derivative, ethylene,30 but its synthesis from ethanol (and thus from renewable bioethanol) has also been proposed, as well as its replacement with glycolaldehyde.31 It should be pointed out that choline could theoretically be isolated from the different bioavailable sources (such as animal wastes, legumes...); yet, as far as we are aware, there are not scientific studies nor industrial processes dealing with its sustainable and/or cost-effective extraction. For these reasons, the renewable production of ChCl appears to be far from an actual implementation. Ethylene oxide is a substrate also for the industrial synthesis of HBD2, ethylene glycol (EG).32 In the case of EG, however, alternative biosynthetic routes from sugars and lignocellulosic biomass have been developed, which appear to be promising: at present, efforts are being devoted to improve the efficiency of such processes, in order to be economically competitive with the chemical synthesis, which means productivity (g/L/h), EG titer (g/L), and yield (g/g) values higher than 3.0 g/L/h, 100 g/L, and 0.5 g/g, respectively.33 Recently, an engineered Escherichia coli strain has been employed at laboratory scale to obtain results as high as 2.25 g/L/h, 108 g/L, and 0.36 g/g, respectively.34 EG may even be derived by HBD1, glycerol (Gly),35 which, despite being closely related to EG from a structural point of view, has a completely different sourcing. Indeed, Gly is the byproduct in the processes of hydrolysis or transesterification of triglycerides, among which the production of biodiesel is undoubtedly the most relevant, while the petrochemical synthesis from propylene has only seen some importance in the past century.36 Gly can thus be considered as a biorenewable raw material, even if it is well-known that the crop-growing for the biodiesel industry poses sustainability issues related to soil exploitation and biodiversity; on this matter, an interesting alternative is represented by the use of microalgae as biomass for biodiesel (and Gly) production.37 Another biorenewable HBD component is of course glucose (Glu), which is produced from starch via acid- or enzyme-catalyzed hydrolysis, the latter being the most relevant.38 Conversely, the production of the HBD3, urea (U), which is a major player in the fertilizer industry, depends on fossil sources: it is synthesized from ammonia (see above) and carbon dioxide, obtained during the process of ammonia synthesis.39 Recent studies have shown that a viable alternative could be represented by the exploitation of biomass, in particular, urban waste, through gasification to syngas and further conversion into urea; however, this appears still far from a commercial application.40
Figure 2.
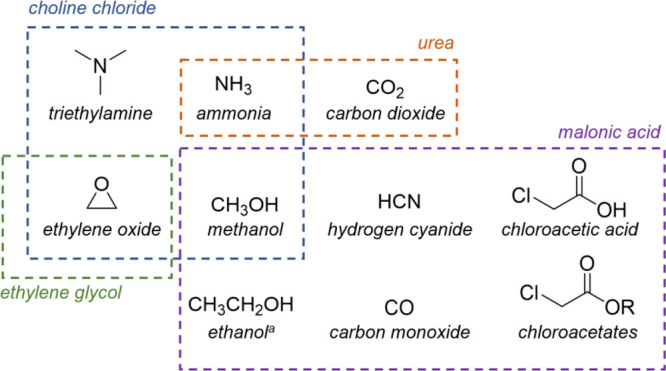
Fossil sources-derived chemicals involved in the industrial-scale production of the ChCl and HBDs. aEthanol should be excluded from this list when it is bioethanol.
Finally, the two acids HBD5–6, malonic acid and lactic acid, albeit structurally similar, are quite different from the point of view of renewability. Malonic acid (MA) is obtained from the hydrolysis of alkyl malonates, which derive from fossil sources. Indeed, they are produced either through the hydrogen cyanide process or the carbon monoxide process: in the first case, the building blocks are chloroacetic acid, hydrogen cyanide, and the appropriate alcohol, while the carbon monoxide process employs a chloroacetate, its corresponding alcohol and carbon monoxide (Figure 2).41 Alternative pathways may be represented by the thermocatalytic or enzymatic conversion of sugars and lignocellulosic biomass: on this matter, it should be noted that MA was inserted into the list of the top 30 value-added chemicals that can be produced from sugars, drawn up on behalf of the U.S. Department of Energy in 2004.42 Conversely, lactic acid (LA) has been predominantly obtained through fermentation processes, which also ensure a high degree of enantiomeric purity, since the 1990s.43 Nowadays, scientific and technological advances in the biorenewable production of LA are focused on lowering the cost of the feedstock, by exploitation of lignocellulosic biomass or food waste instead of more expensive starch or refined sugars.44
Some conclusions can be drawn from this brief survey. The first one is that, at the present time, no ChCl-based DES can be defined as totally renewable on a commercial scale, due to ChCl itself: indeed, several papers apply this definition, but they only justify it by the recyclability of the DES for the specific application, for a certain number of times, and by its biodegradability. Secondly, it is possible to sort ChCl/HBD1–6 DESs into three “shades of renewability”, based on the different HBD components. This comparative classification would see at the first place those HBDs whose production is currently detached from fossil sources, i.e., Gly, Glu, and LA, thus making their DESs the “most renewable” among those considered in this review. On the other hand, U seems the furthest one from a concrete renewable sourcing, while EG and MA stand in between, since they are not currently produced from biobased sources on a commercial scale, but the scientific progress on the matter appears to be in a more advanced state. It should also be considered that fermentation and biosynthetic pathways may afford the compounds of interest with lower purities compared to their chemical synthesis. For example, crude Gly produced from biodiesel industry has purities of 60–80%, and the price for refined Gly (96–99% or higher purity) fluctuated between 4 and 17 times higher than that for crude Gly, in the 2001–2009 period.45 The issue on purity of DESs components is then articulated in two different questions. The first one concerns whether it would be possible to form the eutectic mixtures even with lower-grade components, compared to those usually employed in research laboratories, where DESs have been formed and studied up to now. Second, assuming that the first point can be fulfilled, it would be necessary to assess which applications of DESs are more sensitive to contamination in the components: for example, these would probably be less impacting when the eutectic mixture is employed in extractive or biomass treatment processes, while it can be envisaged that applications of DESs in fine organic synthesis, drug delivery, or electrochemistry would require high-purity solvent media. Thus, the evaluation of renewable source-derived HBA and HBD components cannot ignore the costs (and the environmental impact) associated with their possibly indispensable purification processes.
5. Production
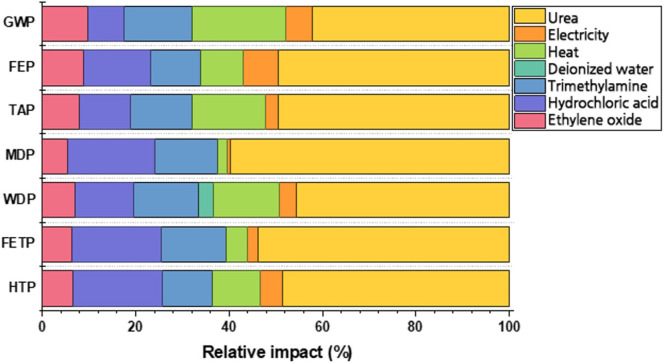
Alongside the renewability of their components, a notable feature that is often acknowledged to DESs is their cheap and environmentally friendly production. The claim is motivated both with the low cost of DESs components and the intrinsic simplicity of the eutectic mixture formation, since it involves the simple mixing of the components, through stirring at a suitable temperature, usually in the range 60–100 °C.46 As mentioned in the previous chapter, ChCl and HBD1–6 are commodity chemicals, produced in bulk by the petrochemical industry or from renewable sources, which would account for their low price. This aspect is particularly relevant in comparison with ILs, since the starting materials to produce the latter are usually specialty or fine chemicals, e.g. imidazole and alkyl iodides or bromides, for imidazole-based ILs. However, the actual cost is dramtically dependent on the required purity (as well as on the volumes involved) once more; therefore, a rigorous cost analysis should take into account the specific application for which the DES is intended. The aspects involved in the preparation of the DES from its components are most interesting because it is in principle very easy and intrinsically atom-economic. Compared to the synthesis of ILs, which generally involves alkylation reactions, performed in other solvents and in controlled atmosphere, with conventional workup and purification of the IL product, this represents a major improvement for what concerns sustainability. From an industrial point of view, however, the DES formation process is not trivial because of the issues related to the mixing of two solids, as well as to the handling of the viscous liquid produced. Furthermore, long heating times may result in partial decomposition of the DES components.47 Mechanochemical synthesis, through twin screw extrusion technology, was proposed as a possible solution to these problems, affording ChCl/U 1/2 (and two other DESs) with high efficiency, avoiding prolonged exposition to high temperature, to dramatically reduce the thermal degradation.48 The DES production through twin screw extrusion was recently taken as the reference method to analyze the environmental impact of ChCl/U 1/2 through cradle-to-gate (i.e., from the raw materials to the finished product) life-cycle assessment (LCA).49 The LCA approach considered both the synthesis of the components, ChCl and U, and the manufacturing of the eutectic mixture; seven indicators, including but not limited to the global warming potential or the water depletion potential, were used to compare the production of ChCl/U 1/2 with that of four VOCs, namely dichloromethane, ethyl acetate, methanol, and ethanol. The produced amount of the five solvents was normalized for a specific application, i.e., the use as solvents for the synthesis of 0.2 kg of acetophenone through oxidation of 1-phenylethanol.49 The study revealed that the DES had a lower environmental impact than dichloromethane and ethyl acetate for almost all the considered indicators, but it was overcome by methanol and ethanol, whose manufacturing was more environmentally friendly. Furthermore, the authors compared the production of 1 kg of ChCl/U 1/2 with 1 kg of other DESs, including ChCl/Gly, ChCl/EG, and ChCl/Glu, all in 1/2 molar ratio. The results showed that the different HBDs did not lead to dramatic changes in the output: with Gly, slightly higher global warming potential and terrestrial acidification potential were observed, this last indicator being higher than ChCl/U also for ChCl/Glu. Only in the case of water depletion potential, the value was significantly higher for ChCl/Gly, compared to the others. With EG as HBD, the results were substantially identical to ChCl/U.49 An interesting outcome of the LCA study is that the feedstocks and the synthesis of the two components, ChCl and U, account for the largest part of the burden on the seven indicators, as reported in Figure 3, while the actual production of the DES through mixing and heating impacts only to a limited extent. This provides a quantitative ground to the initial assumption that the preparation of DESs is simple and low-impacting, even at an industrial scale. In this framework, the environmental (and economical) impact of DESs production would be dumped mainly on the production of their components, thus orienting the direction for further improvement: indeed, increasing the environmental-friendly character of the synthesis of ChCl and HBDs would constitute a relevant part in addressing the whole issue. As shown by the LCA analysis, DESs production is already more desirable than that of two VOCs (at least for the specific application taken into account), but the target of overcoming also less impacting VOCs, such as methanol and ethanol, has not been met yet, and must be set in forthcoming development.
Figure 3.
Different contributions, in the production of ChCl/U 1/2, to the seven indicators employed in the LCA study: GWP = global warming potential, FEP = freshwater eutrophication potential, TAP = terrestrial acidification potential, MDP = metal depletion potential, WDP = water depletion potential, FETP = freshwater ecotoxicity potential, HTP = human toxicity potential.49 Adapted with permission from ref (49). Copyright 2022 The Royal Society of Chemistry.
6. Conclusion
A comparative, broad-spectrum survey on the sustainability of widely employed DESs (or DES-like mixtures), sharing ChCl as their common HBA component, has been depicted. Four main aspects, i.e., toxicity, biodegradability, renewable sourcing, and production have been considered and treated together for the first time, to provide a comprehensive outline. Toxicity and biodegradability have often been reported together (see references cited in Table 4); more in-depth analysis on the issues related to production has received attention only in recent times48,49 but assumptions on the renewability of DES components have never been tackled in detail. Overall, from our survey it appears that the “greenest” credentials could be attributed to ChCl/Gly mixtures, which show negligible toxicity and promising biodegradability profiles, together with current production of the HBD from renewable sources, even if the LCA gives a slightly worse outcome, compared to the others. On the other hand, ChCl/U DESs share with Gly-based ones good biodegradability and appear even able to surpass them in terms of reduced toxicity and a more environmental-friendly production, at least as stated by the LCA; however, our aggregate evaluation is burdened, under a medium- and long-term sustainability point of view, by the negative impact of the current absence of feasible synthetic pathways for U from other resources than fossil fuels. Therefore, in our opinion these mixtures should be placed one step behind ChCl/Gly. Also ChCl/EG DESs display comparatively interesting results in terms of toxicity, biodegradability, and production, while their renewable sourcing at an industrial scale is not established yet, and could be regarded as a “yellow street light”. EG-based mixtures could then be considered similar to U-based ones in terms of sustainability, of course always keeping in mind the inherent hazard of EG as a pure component (see Table 1). Less information is available on ChCl/Glu mixtures, particularly concerning their toxicity toward animals, apart from the encouraging results on fish (Table 2). However, they are biodegradable according to the studies published so far, and the production of Glu from biomass is well established. On this basis, it would be desirable to have more insights into the toxicity of these mixtures, in order to properly compare them with so far best performing ChCl/Gly DESs. Lack of information also affects the evaluation of LA-based DESs, in which the HBD component is remarkably obtained from renewable sources, but surprisingly no studies on biodegradability or animal toxicity were found. On the other hand, the other carboxylic acid HBD considered, MA, was revealed to be less convincing than its competitors under all point of views: it was the only one to show some toxicity in the fish study (Table 2), it had worse biodegradability performances (Table 4), even if not bad in absolute terms, and its industrial production from renewable sources has not been achieved yet. Even if also in this case there is no sufficient available information to provide a complete assessment, ChCl/MA mixtures appear to be the less sustainable ones, among those here considered.
Biographies

Dr. Stefano Nejrotti graduated in Chemistry from the University of Torino, in 2017, after spending a research period at the IRCOF in Rouen (France). He obtained his PhD at the University of Torino in 2021, working on gold-catalyzed synthetic methodologies, and on synthesis in unconventional solvents. In 2019, he spent a period as visiting PhD student in the group of Prof. A. M. Echavarren at the ICIQ in Tarragona (Spain). He is currently a postdoctoral fellow at the University of Torino, where he works on the characterization of unconventional solvents, their use in organic synthesis, and on the synthesis of fluorescent molecules for applications in lighting.

Dr. Achille Antenucci obtained his PhD from Sapienza University of Rome in 2019, defending a thesis focused on Brønsted and Lewis acidic catalysts for organic synthesis. In 2018, he spent a period at the Max-Planck-Institut für Kohlenforschung (Mülheim an der Ruhr, Germany) as a visiting PhD student in the group of Prof. Benjamin List. Afterwards, he moved to University of Torino (Italy), where he worked for two years as a postdoctoral research fellow. Currently, he is working as a researcher in the chemical industry. His research interests rely on sustainable synthesis and catalysis, particularly focused on the exploitation of renewable feedstocks.

Dr. Carlotta Pontremoli obtained her PhD in Materials Science and Technology at Politecnico di Torino working on innovative nanomatrices based on Mesoporous Bioactive Glasses for smart release and tissue regeneration. During her PhD, she was involved in different European Projects giving her the possibility to spend a period as visiting PhD student at Universidad Complutense Madrid (Spain) in the group of Prof. Maria Vallet-Regì and at Charité – Universitätsmedizin Berlin (Germany). After her PhD, she moved to the University of Torino in the Department of Chemistry as a postdoc fellow, where her work is mainly related to the synthesis and characterization of NIR-photosensitizers for photodynamic therapy and the development of biocatalysts based on the bioconjugation of enzymes within innovative polymeric materials.

Dr. Lorenzo Gontrani is Assistant Professor at the University of Rome “Tor Vergata”. He has a solid background in the computational description of complex chemical systems (ab initio, classical molecular dynamics). More recently, his current research interests are mainly focused on the synthesis of metal oxide nanoparticles in traditional and innovative media, like Deep Eutectic Solvents and Ionic Liquids, and on their experimental characterization with diffraction, spectroscopic, and microscopy techniques. He also deals with the calculation of electronic properties of dyes for Dye-Sensitized Solar Cells, in various environments. He is (co)author of 104 international peer-reviewed papers (h-index 30, >2800 citations)

Prof. Marilena Carbone is Associate Professor in Inorganic Chemistry at the University of Rome Tor Vergata. She specializes in innovative synthesis, and the characterization and application of nanomaterials to biomedicine, electrochemistry, and sensors. She is a promoter of research on tattoo inks and their removal and is member of the Commission for Tattoo Inks of the German Institute for Risk Assessment. She is (co)author of 122 publications (h-index 32, >2500 citations).

Prof. Nadia Barbero is Associate Professor in Organic Chemistry at the University of Torino, and her research interests are focused on the design and synthesis of new organic and hybrid materials for nonconventional and technological applications (nanotechnology, nanomedicine, and emerging photovoltaics). She is currently involved in the development of NIR photosensitizers for Dye-Sensitized Solar Cells, photodynamic therapy, and biobased LEDs. She is (co)author of 79 publications (h-index 26, >1800 citations) in international peer-reviewed journals.

Dr. Matteo Bonomo is Assistant Professor at the University of Torino, and his work is mainly related to the synthesis and characterization of innovative materials for emerging photovoltaics. More recently, he has been involved in the investigation of structural and electronic properties of Ionic Liquids and Deep Eutectic Solvents for energy-related application. He has received, among others awards, the Junior “ENERCHEM 2020” (for his innovative contribution in Chemistry for Renewable Energy) and the GIF Young Investigator Award 2022. He is (co)author of 69 publications (h-index 20, >1300 citations) in international peer-reviewed journals.
The authors declare no competing financial interest.
References
- a Clarke C. J.; Tu W.-C.; Levers O.; Brohl A.; Hallett J. P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118 (2), 747–800. 10.1021/acs.chemrev.7b00571. [DOI] [PubMed] [Google Scholar]; b Capello C.; Fischer U.; Hungerbühler K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9 (9), 927–934. 10.1039/b617536h. [DOI] [Google Scholar]; c Anastas P.; Eghbali N. Green chemistry: principles and practice. Chem. Soc. Rev. 2010, 39 (1), 301–312. 10.1039/B918763B. [DOI] [PubMed] [Google Scholar]
- a Abbott A. P.; Capper G.; Davies D. L.; Rasheed R. K.; Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, (1), 70–71. 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]; b Smith E. L.; Abbott A. P.; Ryder K. S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114 (21), 11060–11082. 10.1021/cr300162p. [DOI] [PubMed] [Google Scholar]; c Paiva A.; Craveiro R.; Aroso I.; Martins M.; Reis R. L.; Duarte A. R. C. Natural deep eutectic solvents–solvents for the 21st century. ACS Sustainable Chem. Eng. 2014, 2 (5), 1063–1071. 10.1021/sc500096j. [DOI] [Google Scholar]; d Ruß C.; König B. Low melting mixtures in organic synthesis–an alternative to ionic liquids?. Green Chem. 2012, 14 (11), 2969–2982. 10.1039/c2gc36005e. [DOI] [Google Scholar]; e Hansen B. B.; Spittle S.; Chen B.; Poe D.; Zhang Y.; Klein J. M.; Horton A.; Adhikari L.; Zelovich T.; Doherty B. W.; Gurkan B.; Maginn E. J.; Ragauskas A.; Dadmun M.; Zawodzinski T. A.; Baker G. A.; Tuckerman M. E.; Savinell R. F.; Sangoro J. R. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121 (3), 1232–1285. 10.1021/acs.chemrev.0c00385. [DOI] [PubMed] [Google Scholar]
- a Alonso D. A.; Baeza A.; Chinchilla R.; Guillena G.; Pastor I. M.; Ramón D. J. Deep eutectic solvents: the organic reaction medium of the century. Eur. J. Org. Chem. 2016, 2016 (4), 612–632. 10.1002/ejoc.201501197. [DOI] [Google Scholar]; b Cicco L.; Dilauro G.; Perna F. M.; Vitale P.; Capriati V. Advances in deep eutectic solvents and water: applications in metal-and biocatalyzed processes, in the synthesis of APIs, and other biologically active compounds. Org. Biomol. Chem. 2021, 19 (12), 2558–2577. 10.1039/D0OB02491K. [DOI] [PubMed] [Google Scholar]; c Guajardo N.; Müller C. R.; Schrebler R.; Carlesi C.; Dominguez de Maria P. Deep eutectic solvents for organocatalysis, biotransformations, and multistep organocatalyst/enzyme combinations. ChemCatChem. 2016, 8 (6), 1020–1027. 10.1002/cctc.201501133. [DOI] [Google Scholar]; d Emami S.; Shayanfar A. Deep eutectic solvents for pharmaceutical formulation and drug delivery applications. Pharm. Dev. Technol. 2020, 25 (7), 779–796. 10.1080/10837450.2020.1735414. [DOI] [PubMed] [Google Scholar]; e Pedro S. N.; Freire M. G.; Freire C. S.; Silvestre A. J. Deep eutectic solvents comprising active pharmaceutical ingredients in the development of drug delivery systems. Expert Opin. Drug Delivery 2019, 16 (5), 497–506. 10.1080/17425247.2019.1604680. [DOI] [PubMed] [Google Scholar]; f Tang X.; Zuo M.; Li Z.; Liu H.; Xiong C.; Zeng X.; Sun Y.; Hu L.; Liu S.; Lei T.; Lin L. Green processing of lignocellulosic biomass and its derivatives in deep eutectic solvents. ChemSusChem 2017, 10 (13), 2696–2706. 10.1002/cssc.201700457. [DOI] [PubMed] [Google Scholar]; g Kalhor P.; Ghandi K. Deep eutectic solvents for pretreatment, extraction, and catalysis of biomass and food waste. Molecules 2019, 24 (22), 4012. 10.3390/molecules24224012. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Chen Y.; Han X.; Liu Z.; Yu D.; Guo W.; Mu T. Capture of toxic gases by deep eutectic solvents. ACS Sustainable Chem. Eng. 2020, 8 (14), 5410–5430. 10.1021/acssuschemeng.0c01493. [DOI] [Google Scholar]; i Sarmad S.; Mikkola J. P.; Ji X. Carbon dioxide capture with ionic liquids and deep eutectic solvents: a new generation of sorbents. ChemSusChem 2017, 10 (2), 324–352. 10.1002/cssc.201600987. [DOI] [PubMed] [Google Scholar]; j de los Ángeles Fernández M.; Boiteux J.; Espino M.; Gomez F. J.; Silva M. F. Natural deep eutectic solvents-mediated extractions: The way forward for sustainable analytical developments. Anal. Chim. Acta 2018, 1038, 1–10. 10.1016/j.aca.2018.07.059. [DOI] [PubMed] [Google Scholar]; k Dai Y.; Van Spronsen J.; Witkamp G.-J.; Verpoorte R.; Choi Y. H. Ionic liquids and deep eutectic solvents in natural products research: mixtures of solids as extraction solvents. J. Nat. Prod. 2013, 76 (11), 2162–2173. 10.1021/np400051w. [DOI] [PubMed] [Google Scholar]; l Lu W.; Liu S. Choline chloride–based deep eutectic solvents (Ch-DESs) as promising green solvents for phenolic compounds extraction from bioresources: state-of-the-art, prospects, and challenges. Biomass Convers. Biorefin. 2022, 12, 2949–2962. 10.1007/s13399-020-00753-7. [DOI] [Google Scholar]; m Abbott A. P. Deep Eutectic Solvents and their application in electrochemistry. Curr. Opin. Green Sustain. Chem. 2022, 36, 100649. 10.1016/j.cogsc.2022.100649. [DOI] [Google Scholar]; n Obeten M.; Ugi B.; Alobi N. A review on electrochemical properties of choline chloride based eutectic solvent in mineral processing. J. Appl. Sci. Environ. Manag. 2017, 21 (5), 991–998. 10.4314/jasem.v21i5.29. [DOI] [Google Scholar]; o Wu J.; Liang Q.; Yu X.; Lü Q. F.; Ma L.; Qin X.; Chen G.; Li B. Deep eutectic solvents for boosting electrochemical energy storage and conversion: a review and perspective. Adv. Funct. Mater. 2021, 31 (22), 2011102. 10.1002/adfm.202011102. [DOI] [Google Scholar]
- a Francisco M.; van den Bruinhorst A.; Kroon M. C. Low-transition-temperature mixtures (LTTMs): A new generation of designer solvents. Angew. Chem., Int. Ed. 2013, 52 (11), 3074–3085. 10.1002/anie.201207548. [DOI] [PubMed] [Google Scholar]; b García G.; Atilhan M.; Aparicio S. An approach for the rationalization of melting temperature for deep eutectic solvents from DFT. Chem. Phys. Lett. 2015, 634, 151–155. 10.1016/j.cplett.2015.06.017. [DOI] [Google Scholar]
- Abbott A. P.; Harris R. C.; Ryder K. S.; D’Agostino C.; Gladden L. F.; Mantle M. D. Glycerol eutectics as sustainable solvent systems. Green Chem. 2011, 13 (1), 82–90. 10.1039/C0GC00395F. [DOI] [Google Scholar]
- a Atwater C.Choline. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley, 2001. [Google Scholar]; b Blusztajn J. K. Choline, a vital amine. Science 1998, 281 (5378), 794–795. 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations General Assembly, 2015.
- a Alizadeh V.; Malberg F.; Pádua A. A.; Kirchner B. Are there magic compositions in deep eutectic solvents? Effects of composition and water content in choline chloride/ethylene glycol from ab initio molecular dynamics. J. Phys. Chem. B 2020, 124 (34), 7433–7443. 10.1021/acs.jpcb.0c04844. [DOI] [PubMed] [Google Scholar]; b Bonomo M.; Gontrani L.; Capocefalo A.; Sarra A.; Nucara A.; Carbone M.; Postorino P.; Dini D. A combined electrochemical, infrared and EDXD tool to disclose Deep Eutectic Solvents formation when one precursor is liquid: Glyceline as case study. J. Mol. Liq. 2020, 319, 114292. 10.1016/j.molliq.2020.114292. [DOI] [Google Scholar]; c Agieienko V.; Buchner R. Is ethaline a deep eutectic solvent?. Phys. Chem. Chem. Phys. 2022, 24 (9), 5265–5268. 10.1039/D2CP00104G. [DOI] [PubMed] [Google Scholar]; d Martins M. A.; Pinho S. P.; Coutinho J. A. Insights into the nature of eutectic and deep eutectic mixtures. J. Solution Chem. 2019, 48 (7), 962–982. 10.1007/s10953-018-0793-1. [DOI] [Google Scholar]; e van den Bruinhorst A.; Costa Gomes M. Is there depth to eutectic solvents?. Curr. Opin. Green Sustain. Chem. 2022, 37, 100659. 10.1016/j.cogsc.2022.100659. [DOI] [Google Scholar]; f Cappelluti F.; Mariani A.; Bonomo M.; Damin A.; Bencivenni L.; Passerini S.; Carbone M.; Gontrani L. Stepping away from serendipity in Deep Eutectic Solvent formation: Prediction from precursors ratio. J. Mol. Liq. 2022, 367, 120443. 10.1016/j.molliq.2022.120443. [DOI] [Google Scholar]
- a Bubalo M. C.; Radošević K.; Redovniković I. R.; Halambek J.; Srček V. G. A brief overview of the potential environmental hazards of ionic liquids. Ecotoxicol. Environ. Saf. 2014, 99, 1–12. 10.1016/j.ecoenv.2013.10.019. [DOI] [PubMed] [Google Scholar]; b Petkovic M.; Seddon K. R.; Rebelo L. P. N.; Pereira C. S. Ionic liquids: a pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40 (3), 1383–1403. 10.1039/C004968A. [DOI] [PubMed] [Google Scholar]
- a Naseem Z.; Shehzad R. A.; Ihsan A.; Iqbal J.; Zahid M.; Pervaiz A.; Sarwari G. Theoretical investigation of supramolecular hydrogen-bonded choline chloride-based deep eutectic solvents using density functional theory. Chem. Phys. Lett. 2021, 769, 138427. 10.1016/j.cplett.2021.138427. [DOI] [Google Scholar]; b Zahn S.; Kirchner B.; Mollenhauer D. Charge spreading in deep eutectic solvents. ChemPhysChem 2016, 17 (21), 3354–3358. 10.1002/cphc.201600348. [DOI] [PubMed] [Google Scholar]
- a Torregrosa-Crespo J.; Marset X.; Guillena G.; Ramón D. J.; Martínez-Espinosa R. M. New guidelines for testing “Deep eutectic solvents” toxicity and their effects on the environment and living beings. Sci. Total Environ. 2020, 704, 135382. 10.1016/j.scitotenv.2019.135382. [DOI] [PubMed] [Google Scholar]; b Marchel M.; Cieśliński H.; Boczkaj G. Deep eutectic solvents microbial toxicity: Current state of art and critical evaluation of testing methods. J. Hazard. Mater. 2022, 425, 127963. 10.1016/j.jhazmat.2021.127963. [DOI] [PubMed] [Google Scholar]; c Chen Y.; Mu T. Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chem. Eng. 2021, 2 (2), 174–186. 10.1016/j.gce.2021.01.004. [DOI] [Google Scholar]
- a Hayyan M.; Hashim M. A.; Hayyan A.; Al-Saadi M. A.; AlNashef I. M.; Mirghani M. E.; Saheed O. K. Are deep eutectic solvents benign or toxic?. Chemosphere 2013, 90 (7), 2193–2195. 10.1016/j.chemosphere.2012.11.004. [DOI] [PubMed] [Google Scholar]; b Meyer B.; Ferrigni N.; Putnam J.; Jacobsen L.; Nichols D.; McLaughlin J. L. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 1982, 45 (05), 31–34. 10.1055/s-2007-971236. [DOI] [PubMed] [Google Scholar]; c Lewan L.; Andersson M.; Morales-Gomez P. The use of Artemia salina in toxicity testing. Altern. Lab. Anim. 1992, 20 (2), 297–301. 10.1177/026119299202000222. [DOI] [Google Scholar]; d Hartl M.; Humpf H.-U. Toxicity assessment of fumonisins using the brine shrimp (Artemia salina) bioassay. Food Chem. Toxicol. 2000, 38 (12), 1097–1102. 10.1016/S0278-6915(00)00112-5. [DOI] [PubMed] [Google Scholar]
- a Huang Z. L.; Wu B. P.; Wen Q.; Yang T. X.; Yang Z. Deep eutectic solvents can be viable enzyme activators and stabilizers. J. Chem. Technol. Biotechnol. 2014, 89 (12), 1975–1981. 10.1002/jctb.4285. [DOI] [Google Scholar]; b Wen Q.; Chen J.-X.; Tang Y.-L.; Wang J.; Yang Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 2015, 132, 63–69. 10.1016/j.chemosphere.2015.02.061. [DOI] [PubMed] [Google Scholar]
- a Lapeña D.; Errazquin D.; Lomba L.; Lafuente C.; Giner B. Ecotoxicity and biodegradability of pure and aqueous mixtures of deep eutectic solvents: glyceline, ethaline, and reline. Environ. Sci. Pollut. Res. 2021, 28 (7), 8812–8821. 10.1007/s11356-020-11144-w. [DOI] [PubMed] [Google Scholar]; b OECD, Test No. 202: Daphnia sp. Acute Immobilisation Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, 2004. [Google Scholar]
- a Juneidi I.; Hayyan M.; Hashim M. A. Evaluation of toxicity and biodegradability for cholinium-based deep eutectic solvents. RSC Adv. 2015, 5 (102), 83636–83647. 10.1039/C5RA12425E. [DOI] [Google Scholar]; b Juneidi I.; Hayyan M.; Mohd Ali O. Toxicity profile of choline chloride-based deep eutectic solvents for fungi and Cyprinus carpio fish. Environ. Sci. Pollut. Res. 2016, 23 (8), 7648–7659. 10.1007/s11356-015-6003-4. [DOI] [PubMed] [Google Scholar]
- OECD , Test No. 203: Fish, Acute Toxicity Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, 2019. [Google Scholar]
- a Hayyan M.; Looi C. Y.; Hayyan A.; Wong W. F.; Hashim M. A. In vitro and in vivo toxicity profiling of ammonium-based deep eutectic solvents. PLoS One 2015, 10 (2), e0117934. 10.1371/journal.pone.0117934. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jung D.; Jung J. B.; Kang S.; Li K.; Hwang I.; Jeong J. H.; Kim H. S.; Lee J. Toxico-metabolomics study of a deep eutectic solvent comprising choline chloride and urea suggests in vivo toxicity involving oxidative stress and ammonia stress. Green Chem. 2021, 23 (3), 1300–1311. 10.1039/D0GC03927F. [DOI] [Google Scholar]; c Chen J.; Wang Q.; Liu M.; Zhang L. The effect of deep eutectic solvent on the pharmacokinetics of salvianolic acid B in rats and its acute toxicity test. J. Chromatogr. B 2017, 1063, 60–66. 10.1016/j.jchromb.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Mbous Y. P.; Hayyan M.; Wong W. F.; Looi C. Y.; Hashim M. A. Unraveling the cytotoxicity and metabolic pathways of binary natural deep eutectic solvent systems. Sci. Rep. 2017, 7 (1), 1–14. 10.1038/srep41257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radošević K.; Bubalo M. C.; Srček V. G.; Grgas D.; Dragičević T. L.; Redovniković I. R. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. 10.1016/j.ecoenv.2014.09.034. [DOI] [PubMed] [Google Scholar]
- Bubalo M. C.; Hanousek K.; Radošević K.; Srček V. G.; Jakovljević T.; Redovniković I. R. Imidiazolium based ionic liquids: effects of different anions and alkyl chains lengths on the barley seedlings. Ecotoxicol. Environ. Saf. 2014, 101, 116–123. 10.1016/j.ecoenv.2013.12.022. [DOI] [PubMed] [Google Scholar]
- a Wu S.-H.; Caparanga A. R.; Leron R. B.; Li M.-H. Vapor pressure of aqueous choline chloride-based deep eutectic solvents (ethaline, glyceline, maline and reline) at 30–70 C. Thermochim. Acta 2012, 544, 1–5. 10.1016/j.tca.2012.05.031. [DOI] [Google Scholar]; b Xin K.; Roghair I.; Gallucci F.; van Sint Annaland M. Total vapor pressure of hydrophobic deep eutectic solvents: Experiments and modelling. J. Mol. Liq. 2021, 325, 115227. 10.1016/j.molliq.2020.115227. [DOI] [Google Scholar]
- OECD , Test No. 301: Ready Biodegradability. In OECD Guidelines for the Testing of Chemicals, Section 3; OECD Publishing: Paris, 1992. [Google Scholar]
- a Huang Y.; Feng F.; Jiang J.; Qiao Y.; Wu T.; Voglmeir J.; Chen Z.-G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. 10.1016/j.foodchem.2016.11.013. [DOI] [PubMed] [Google Scholar]; b Zhao B.-Y.; Xu P.; Yang F.-X.; Wu H.; Zong M.-H.; Lou W.-Y. Biocompatible deep eutectic solvents based on choline chloride: characterization and application to the extraction of rutin from Sophora japonica. ACS Sustainable Chem. Eng. 2015, 3 (11), 2746–2755. 10.1021/acssuschemeng.5b00619. [DOI] [Google Scholar]
- Docherty K. M.; Aiello S. W.; Buehler B. K.; Jones S. E.; Szymczyna B. R.; Walker K. A. Ionic liquid biodegradability depends on specific wastewater microbial consortia. Chemosphere 2015, 136, 160–166. 10.1016/j.chemosphere.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Boethling R.; Sommer E.; DiFiore D. Designing small molecules for biodegradability. Chem. Rev. 2007, 107 (6), 2207–2227. 10.1021/cr050952t. [DOI] [PubMed] [Google Scholar]
- a Hou X.-D.; Liu Q.-P.; Smith T. J.; Li N.; Zong M.-H. Evaluation of toxicity and biodegradability of cholinium amino acids ionic liquids. PLoS One 2013, 8 (3), e59145. 10.1371/journal.pone.0059145. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Coleman D.; Gathergood N. Biodegradation studies of ionic liquids. Chem. Soc. Rev. 2010, 39 (2), 600–637. 10.1039/b817717c. [DOI] [PubMed] [Google Scholar]; c Jordan A.; Gathergood N. Biodegradation of ionic liquids–a critical review. Chem. Soc. Rev. 2015, 44 (22), 8200–8237. 10.1039/C5CS00444F. [DOI] [PubMed] [Google Scholar]
- Modelli A.; Sali A.; Galletti P.; Samorì C. Biodegradation of oxygenated and non-oxygenated imidazolium-based ionic liquids in soil. Chemosphere 2008, 73 (8), 1322–1327. 10.1016/j.chemosphere.2008.07.012. [DOI] [PubMed] [Google Scholar]
- a De Boeck G.; Vlaeminck A.; Van der Linden A.; Blust R. The energy metabolism of common carp (Cyprinus carpio) when exposed to salt stress: an increase in energy expenditure or effects of starvation?. Physiol. Biochem. Zool. 2000, 73 (1), 102–111. 10.1086/316717. [DOI] [PubMed] [Google Scholar]; b Elphick J. R.; Bergh K. D.; Bailey H. C. Chronic toxicity of chloride to freshwater species: effects of hardness and implications for water quality guidelines. Environ. Toxicol. Chem. 2011, 30 (1), 239–246. 10.1002/etc.365. [DOI] [PubMed] [Google Scholar]; c Soucek D. J.; Linton T. K.; Tarr C. D.; Dickinson A.; Wickramanayake N.; Delos C. G.; Cruz L. A. Influence of water hardness and sulfate on the acute toxicity of chloride to sensitive freshwater invertebrates. Environ. Toxicol. Chem. 2011, 30 (4), 930–938. 10.1002/etc.454. [DOI] [PubMed] [Google Scholar]
- Ernst M.; Melder J.-P.; Berger F. I.; Koch C.. Ethanolamines and Propanolamines. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley, 2022. [Google Scholar]
- a Roose P.Methylamines. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley, 2015. [Google Scholar]; b Rebsdat S.; Mayer D., Ethylene Oxide. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley, 2001. [Google Scholar]
- a Lucky C.; Wang T.; Schreier M. Electrochemical Ethylene Oxide Synthesis from Ethanol. ACS Energy Lett. 2022, 7 (4), 1316–1321. 10.1021/acsenergylett.2c00265. [DOI] [Google Scholar]; b Faveere W. H.; Van Praet S.; Vermeeren B.; Dumoleijn K. N.; Moonen K.; Taarning E.; Sels B. F. Toward Replacing Ethylene Oxide in a Sustainable World: Glycolaldehyde as a Bio-Based C2 Platform Molecule. Angew. Chem., Int. Ed. 2021, 60 (22), 12204–12223. 10.1002/anie.202009811. [DOI] [PubMed] [Google Scholar]
- a Rebsdat S.; Mayer D.. Ethylene Glycol. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley, 2000;. [Google Scholar]; b Yang Q.; Yang Q.; Xu S.; Zhu S.; Zhang D. Technoeconomic and environmental analysis of ethylene glycol production from coal and natural gas compared with oil-based production. J. Cleaner Prod. 2020, 273, 123120. 10.1016/j.jclepro.2020.123120. [DOI] [Google Scholar]
- a Pang J.; Zheng M.; Sun R.; Wang A.; Wang X.; Zhang T. Synthesis of ethylene glycol and terephthalic acid from biomass for producing PET. Green Chem. 2016, 18 (2), 342–359. 10.1039/C5GC01771H. [DOI] [Google Scholar]; b Zhang Y.; Liu D.; Chen Z. Production of C2–C4 diols from renewable bioresources: new metabolic pathways and metabolic engineering strategies. Biotechnol. Biofuels 2017, 10 (1), 1–20. 10.1186/s13068-017-0992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Salusjärvi L.; Havukainen S.; Koivistoinen O.; Toivari M. Biotechnological production of glycolic acid and ethylene glycol: current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103 (6), 2525–2535. 10.1007/s00253-019-09640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae T. U.; Choi S. Y.; Ryu J. Y.; Lee S. Y. Production of ethylene glycol from xylose by metabolically engineered Escherichia coli. AIChE J. 2018, 64 (12), 4193–4200. 10.1002/aic.16339. [DOI] [Google Scholar]
- Kandasamy S.; Samudrala S. P.; Bhattacharya S. The route towards sustainable production of ethylene glycol from a renewable resource, biodiesel waste: a review. Catal. Sci. Technol. 2019, 9 (3), 567–577. 10.1039/C8CY02035C. [DOI] [Google Scholar]
- a Christoph R.; Schmidt B.; Steinberner U.; Dilla W.; Karinen R.. Glycerol. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley, 2006. [Google Scholar]; b Anneken D. J.; Both S.; Christoph R.; Fieg G.; Steinberner U.; Westfechtel A.. Fatty Acids. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley, 2006. [Google Scholar]; c Ciriminna R.; Pina C. D.; Rossi M.; Pagliaro M. Understanding the glycerol market. Eur. J. Lipid Sci. Technol. 2014, 116 (10), 1432–1439. 10.1002/ejlt.201400229. [DOI] [Google Scholar]
- a Tan H.; Aziz A. A.; Aroua M. Glycerol production and its applications as a raw material: A review. Renew. Sust. Ener. Rev. 2013, 27, 118–127. 10.1016/j.rser.2013.06.035. [DOI] [Google Scholar]; b Ennaceri H.; Fischer K.; Schulze A.; Moheimani N. R. Membrane fouling control for sustainable microalgal biodiesel production: A review. Renew. Sust. Ener. Rev. 2022, 161, 112335. 10.1016/j.rser.2022.112335. [DOI] [Google Scholar]
- a Schenck F. W.Glucose and Glucose-Containing Syrups. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley, 2006. [Google Scholar]; b BeMiller J. N.; Huber K. C.. Starch. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley, 2011. [Google Scholar]
- a Meessen J. H.Urea. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley, 2010. [Google Scholar]; b The Future of Petrochemicals; International Energy Agency, 2018. [Google Scholar]
- a Antonetti E.; Iaquaniello G.; Salladini A.; Spadaccini L.; Perathoner S.; Centi G. Waste-to-chemicals for a circular economy: the case of urea production (waste-to-urea). ChemSusChem 2017, 10 (5), 912–920. 10.1002/cssc.201601555. [DOI] [PubMed] [Google Scholar]; b Zhang H.; Wang L.; Van herle J.; Marechal F.; Desideri U. Techno-economic comparison of 100% renewable urea production processes. Appl. Energy 2021, 284, 116401. 10.1016/j.apenergy.2020.116401. [DOI] [Google Scholar]
- Strittmatter H.; Hildbrand S.; Pollak P.. Malonic Acid and Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley, 2007. [Google Scholar]
- a Wan Y.; Lee J.-M. Toward value-added dicarboxylic acids from biomass derivatives via thermocatalytic conversion. ACS Catal. 2021, 11 (5), 2524–2560. 10.1021/acscatal.0c05419. [DOI] [Google Scholar]; b Zhang Z.; Huber G. W. Catalytic oxidation of carbohydrates into organic acids and furan chemicals. Chem. Soc. Rev. 2018, 47 (4), 1351–1390. 10.1039/C7CS00213K. [DOI] [PubMed] [Google Scholar]; c Chae T. U.; Ahn J. H.; Ko Y.-S.; Kim J. W.; Lee J. A.; Lee E. H.; Lee S. Y. Metabolic engineering for the production of dicarboxylic acids and diamines. Metab. Eng. 2020, 58, 2–16. 10.1016/j.ymben.2019.03.005. [DOI] [PubMed] [Google Scholar]; d Werpy T.; Petersen G.. Top Value Added Chemicals from Biomass: Volume I--Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Richland, WA (US), Golden, CO (US), Pacific Northwest National Laboratory, National Renewable Energy Laboratory, 2004. [Google Scholar]
- a Starr J. N.; Westhoff G.. Lactic Acid. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley, 2014. [Google Scholar]; b Komesu A.; de Oliveira J. A. R.; da Silva Martins L. H.; Maciel M. R. W.; Maciel Filho R. Lactic acid production to purification: a review. Bioresources 2017, 12 (2), 4364–4383. 10.15376/biores.12.2.4364-4383. [DOI] [Google Scholar]
- a Abdel-Rahman M. A.; Tashiro Y.; Sonomoto K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: overview and limits. J. Biotechnol. 2011, 156 (4), 286–301. 10.1016/j.jbiotec.2011.06.017. [DOI] [PubMed] [Google Scholar]; b Ahmad A.; Banat F.; Taher H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Innov. 2020, 20, 101138. 10.1016/j.eti.2020.101138. [DOI] [Google Scholar]; c Wang Y.; Tashiro Y.; Sonomoto K. Fermentative production of lactic acid from renewable materials: Recent achievements, prospects, and limits. J. Biosci. Bioeng. 2015, 119 (1), 10–18. 10.1016/j.jbiosc.2014.06.003. [DOI] [PubMed] [Google Scholar]
- a Ayoub M.; Abdullah A. Z. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew. Sust. Ener. Rev. 2012, 16 (5), 2671–2686. 10.1016/j.rser.2012.01.054. [DOI] [Google Scholar]; b Xiao Y.; Xiao G.; Varma A. A universal procedure for crude glycerol purification from different feedstocks in biodiesel production: experimental and simulation study. Ind. Eng. Chem. Res. 2013, 52 (39), 14291–14296. 10.1021/ie402003u. [DOI] [Google Scholar]
- Liu Y.; Friesen J. B.; McAlpine J. B.; Lankin D. C.; Chen S.-N.; Pauli G. F. Natural deep eutectic solvents: properties, applications, and perspectives. J. Nat. Prod. 2018, 81 (3), 679–690. 10.1021/acs.jnatprod.7b00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Rodriguez Rodriguez N.; van den Bruinhorst A.; Kollau L. J.; Kroon M. C.; Binnemans K. Degradation of deep-eutectic solvents based on choline chloride and carboxylic acids. ACS Sustainable Chem. Eng. 2019, 7 (13), 11521–11528. 10.1021/acssuschemeng.9b01378. [DOI] [Google Scholar]; b Hayyan A.; Mjalli F. S.; AlNashef I. M.; Al-Wahaibi T.; Al-Wahaibi Y. M.; Hashim M. A. Fruit sugar-based deep eutectic solvents and their physical properties. Thermochim. Acta 2012, 541, 70–75. 10.1016/j.tca.2012.04.030. [DOI] [Google Scholar]
- Crawford D. E.; Wright L.; James S.; Abbott A. Efficient continuous synthesis of high purity deep eutectic solvents by twin screw extrusion. Chem. Commun. 2016, 52 (22), 4215–4218. 10.1039/C5CC09685E. [DOI] [PubMed] [Google Scholar]
- Zaib Q.; Eckelman M. J.; Yang Y.; Kyung D. Are deep eutectic solvents really green?: A life-cycle perspective. Green Chem. 2022, 24, 7924–7930. 10.1039/D2GC01752K. [DOI] [Google Scholar]