INTRODUCTION
Melanoma is an aggressive form of skin cancer that, when localized, is highly curable with a simple surgical excision. However, as it grows in the span of just a few millimeters, its lethality increases markedly. More so than many other cancers, the management of melanoma is governed by phase III randomized trials in almost every aspect of treatment. Over the last 10 years, not only have there been several landmark surgical trials but also major advances in systemic treatments using targeted and immunologic approaches to metastatic disease. The availability of effective systemic therapies has led to rapid improvements in survival as well as significant alterations in the traditional approaches to managing this disease. This monograph is organized into 6 sections that provide focused updates on the advances in various aspects of melanoma care. These sections incorporate the latest findings and will help with the management of individual patients. In addition, we also aim to integrate all the approaches to melanoma to help providers better understand the evolving complexity of an integrated multidisciplinary care approach to optimize the care of these patients.
MELANOMA EPIDEMIOLOGY AND GENETICS
Incidence and Mortality
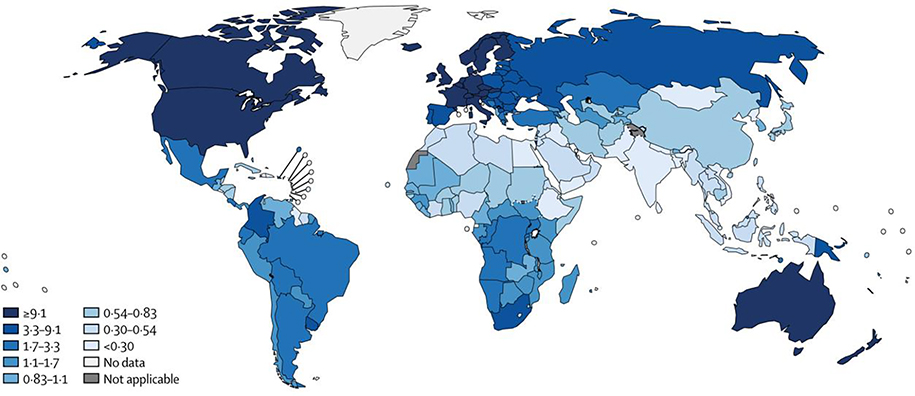
Cutaneous malignant melanoma (CMM) is the fifth most common malignancy in the United States, and it is by far the deadliest cutaneous cancer. The highest-incidence regions worldwide are North America, Australia, and Western Europe (Figure 1).1 Since the mid-1990s, the yearly incidence in the United States has increased by approximately 0.4% per year (Figure 2).2 For most of that period, overall mortality remained the same, with only a slight decline in the last 5 years, from 2.7% to 2.1%. Other nationwide databases in high-incidence areas have demonstrated similar trends,3,4 which are thought to reflect increased surveillance and detection with some recent improvement in mortality for advanced melanoma. Because most patients are diagnosed with curable stage I disease, 5-year overall survival (OS) for melanoma patients is 92%. However, approximately 6,850 Americans will die from melanoma in 2020, reflecting the deadly nature of advanced melanoma2. Despite recent improvements in chemotherapy and immunotherapy, advanced disease remains deadly and carries a 5-year OS of only 19%.5
Figure 1.
Melanoma incidence by country (per 100,000 person-years) (with permission from Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet. 2018;392(10151):971–984).
Figure 2.
Melanoma incidence and mortality in the United States by sex compared to other cancers 1975 to 2017 (with permission from Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7–33).
Predisposition
Cumulative research into CMM epidemiology has identified a variety of factors that modify a person’s risk. Risk increases with age, with a notable inflection in incidence rates for both men and women after age 60 years. Men are more likely to be affected overall, an effect that is more pronounced at older ages. By age 65 years, men are 1.55 times more likely to develop melanoma. Perhaps as a result, the average age at diagnosis is lower for women.2, 5
Host Factors
Phenotype
The most prominent phenotypic factors that are associated with development of melanoma are skin pigmentation and presence or absence of melanocytic nevi. Skin pigmentation is largely determined by melanocyte synthesis of eumelanin (ie, dark complexion) or pheomelanin (ie, lighter complexion, blue eyes, and red hair). A pheomelanin-predominant pigmentation phenotype (rather than eumelanin) is associated with increased risk for melanoma. The features of this phenotype and the relative risk (RR) of melanoma are summarized in Table 1.6
Table 1.
Relative risk for melanoma associated with phenotypic factors.
| Relative Risk for Melanoma | |
|---|---|
| Phenotypic Factor | (95% Confidence Interval) |
|
| |
| Family history | RR = 1.74, 1.41–2.14 |
| Fitzpatrick skin type (I vs. IV) | RR = 2.09, 1.67–2.58 |
| High density of freckles | RR = 2.10, 1.80–2.45 |
| Skin color (fair vs. dark) | RR = 2.06, 1.68–2.52 |
| Eye color (blue vs. dark) | RR = 1.47, 1.28–1.69 |
| Hair color (red vs. dark) | RR = 3.64, 2.56–5.37 |
Adapted from Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41(14):2040–2059.
Pheomelanin-predominant phenotypes are also associated with sunburn of the skin when exposed to ultraviolet (UV) radiation (UVR), which causes DNA damage. Eumelanin is protective against UVR-mediated sunburn. A personal history of sunburn, a reported tendency to sunburn, and especially a tendency for blistering sunburn are all associated with increased melanoma risk.7, 8 Additionally, estimates of total lifetime UV radiation exposure, especially when intermittent, have been shown to be associated with increased risk.9
Regarding melanocytic nevi, both the number and quality of nevi seem to convey risk.10, 11 There is a steady increase in CMM risk that is proportional to total body nevus count; RR reaches as high as 6.9 for those with more than 100 total nevi.10, 11 The characteristics of cutaneous nevi are also relevant. “Atypical” or “clinically dysplastic” are terms used to refer to large (>5 mm), flat nevi with abnormal clinical features, such as poorly defined borders, variegated color, uneven contour, or erythema. Patients with a single atypical nevus have a 2-fold increase in melanoma risk.12 The presence of at least 10 atypical nevi carries a RR of 12.12 On the other hand, congenital nevi do not seem to predispose to melanoma.12
Medical History
Risk for development of melanoma has also been related to factors in a patient’s personal and medical history. Medical factors, especially prior cutaneous or noncutaneous malignancy, have been described. Also, personal factors such as environmental exposure have been studied in melanoma epidemiology.
History of skin damage predisposes to melanoma. Actinic skin changes are a long-term effect of UVR-mediated DNA damage and are a precursor to skin cancers. Increased risk for melanoma is seen in those with a history of actinic damage alone (RR = 2.02, 95% CI 1.24–3.29), and those with a history of premalignant or malignant nonmelanoma skin lesions (RR = 4.28, 95% CI 2.80–6.55).6
Personal history of noncutaneous cancer has also been shown to be a risk factor. Melanoma is an important cause of second primary malignancy in patients who have recovered from common childhood cancers.13 Significantly, childhood cancer survivors who develop melanoma are more likely to die of their disease than other primary melanoma patients (hazard ratio = 2.57; 95% CI, 1.55–4.27).14 In addition to childhood cancer, adult hematologic malignancies such as chronic lymphocytic leukemia (CLL)15 or non-Hodgkin’s lymphoma16 are associated with subsequent development of melanoma, possibly due to disruption of normal immune function.
Melanoma is an immunogenic cancer, and a variety of immune system impairments have been associated with increased risk. Specifically, the solid organ transplant (SIR = 2.20, 95%CI 2.01–2.39)17, 18 and hematopoietic stem cell transplant (hazard ratio = 5.5, 95% CI, 1.7–17.7)19 populations have been found to be at risk. Solid organ transplant recipients who develop melanoma have an increased mortality risk of approximately 3–4 times other melanoma patients.17, 18 Human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) patients were previously shown to be a high-risk population;20 however, this finding has been disputed.21
Genetic Risk
A patient’s genetic risk should also be considered. Familial melanoma is discussed in detail in a subsequent section, but familial cases can often be linked to: (1) a small number of high-penetrance loci (eg, CDKN2A, CDK4); (2) an increasingly large number of low-penetrance loci; (3) a familial cancer syndrome that includes melanoma and other cancers (Cowden syndrome, Li-Fraumeni syndrome, BRCA2); or (4) rare genodermatoses such as xeroderma pigmentosum.22
Environmental Factors
Environmental exposures can modify patients’ risk for melanoma. These factors invariably relate to lifetime UVR exposure. The UV radiation that is responsible for skin damage is primarily from the UVA (315–400 nm) and UVB (280–315 nm) spectra. UVB radiation is absorbed directly by DNA macromolecules, and primarily causes damage via pyridine cyclodimerization, often affecting adjacent thymine residues.23 This damage is repaired by the nucleotide excision repair apparatus. UVA radiation represents approximately 95% of sunlight UV radiation and is the primary source of radiation in tanning beds. UVA also creates thymine dimers, but additionally can cause indirect oxidative damage to DNA.24 The characteristic point mutation from UV damage is T->C.
Perhaps the most extensively studied environmental factor is tanning bed use. Once theorized to be potentially protective against UVR-mediated sun damage by inducing melanin production, it is now known that any exposure to tanning beds carries a measurable RR of melanoma.25 Risk appears to be higher among those with many tanning bed exposures, and those who use them at a younger age.26, 27 Notably, one study found that those with early-onset melanoma (prior to age 40 years) were much more likely to have used tanning beds at a young age.26
Lifetime sun exposure is also an important factor. Those who live in sunnier climates or low latitudes are at increased risk.4, 28 It follows that patients who are exposed to sunlight in their professional or leisure activities would also be at increased risk. This effect has been observed in airline pilots and crew.29
Different patterns in sun exposure may predispose to different molecular pathways for melanoma development. For example, BRAF-mutant cutaneous melanomas are more closely associated with intermittent, intense sun exposure (especially in younger years), and more melanocytic nevi.30 It has been observed that melanoma arising within nevi have the same activating oncogene mutations as adjacent benign nevus cells. Transformation may be the result of acquisition of additional mutations, such as CDKN2A homozygous deletion, resulting in subsequent clonal expansion.31, 32 In contrast, TP53-mutant cutaneous melanoma is more often associated with chronic sun exposure, actinic skin damage, and fewer nevi.33, 34
Melanoma Genetics
Molecular Oncogenesis of Melanoma
Melanoma has a very high mutational burden compared to other cancers, reflecting its status as a disease of DNA damage.35 Mutations detected in melanoma average more than 100 per Mb, the highest among common malignancies. The diverse mutational landscape places emphasis on the distinction between driver and bystander mutations. There are 3 main categories of mutation that drive melanoma molecular oncogenesis: 1) driver mutations in cell growth and survival signaling; 2) disruption of canonical cell cycle regulation mechanisms; and 3) pigmentation genes.
Driver mutations in cell growth and survival signaling.
Gain of function mutations in cell growth signaling proteins are common in melanoma. Most external growth factors signal through receptor tyrosine kinases, activating intracellular pathways such as the mitogen-activated protein kinase (MAPK) pathway (Figure 3).36 An activating BRAF mutation, V600E, is present in approximately one half of all melanoma cases.37 Because of its central function in growth signaling, BRAFV600E is an important therapeutic target for melanoma.38 Other key drivers of cell growth in melanoma are NRAS and PTEN (Figure 3).
Figure 3.
Melanoma oncogenes that promote constitutive cell growth. Most cellular growth factors activate mitogen-activated protein kinase (MAPK) signaling pathways (green) and AKT signaling (yellow) through receptor tyrosine kinases. Variants in BRAF or NRAS (red) are able to function in the absence of external stimuli to activate MAPK signaling. Through another pathway, PTEN inactivation (red) causes accumulation of PIP3, which mediates recruitment of AKT and its activating kinases. AKT activation strongly promotes cell growth. These growth pathways are overactive in melanoma. RAS, rat sarcoma virus (v-Ras) homologs such as KRAS, NRAS, HRAS; RAF, rat accelerated fibrosarcoma (v-RAF) homologs such as BRAF; MEK, MAPK/Erk kinase 1; Erk, extracellular signal-related kinase 1; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homolog; PIP2/PIP3, phosphatidylinositol 4,5-bisphosphate or 3,4,5-trisphosphate; PDK1, 3-phosphoinositide dependent kinase 1; mTOR, mammalian target of rapamycin; AKT, v-Akt murine thymoma homolog or protein kinase B.
Disruption of canonical cell cycle regulation mechanisms.
Melanoma growth is dependent on escape from typical growth arrest pathways.36 The CDKN2A locus, perhaps the most frequently mutated in melanoma, codes for 2 tumor suppressor genes that regulate cell growth, p16INK4a and p14ARF. The essential anticancer function of these proteins is summarized in Figure 4.
Figure 4.
Canonical cell cycle regulatory pathways that are disrupted by CDKN2A mutation. Transition from the G1 phase of the cell cycle to S phase is controlled by retinoblastoma protein (Rb). Phosphorylation of Rb is required for progression. p16INK4a controls this tightly regulated transition by inhibiting Rb phosphorylation. CDKN2A encodes a second tumor suppressor protein on an alternate reading frame, hence the name ARF. p14ARF regulates the function of MDM2 and MDM4, which inhibit p53 and promote its ubiquitin-mediated degradation. Without p14ARF, p53 is unable to function as a critical regulator of cell proliferation and DNA damage repair. Rb, retinoblastoma; CDK4/6, cyclin dependent protein kinases 4 and 6; INK4a, inhibitor of cyclin-dependent kinase 4; MDM2/4, mouse double minute 2 homolog and mouse double minute 4 homolog.
In summary, the collective function of TP53, MDM2/4, and p14ARF allow the cell to arrest proliferation in the setting of DNA damage. TP53 inactivating mutations are found in only 20% of melanomas; however, most melanomas overexpress MDM2/MDM4, which are downregulators of p53 via ubiquitin-mediated degradation.39 Loss of p53 may be a late event in melanoma biology and may be present in more advanced tumors. As depicted in Figure 4, p16INK4a, Cyclin D, CDK4, and Rb tightly regulate the G1-S cell cycle transition. Dysregulation of this mechanism can lead to unrestricted progression of the cell cycle and is observed in 90% to 100% of melanomas.40, 41
Pigmentation Genes.
MC1R codes for the melanocortin receptor on melanocytes and is a key source of variability in pigmentation between individuals. It is no surprise that MC1R variants are highly associated with melanoma risk.36 Studies have shown that the effect of MC1R extends beyond just pigmentation and photoprotection and may also lead to oncogenesis by non-pigmentation routes.42 Additionally, other genes related to melanocyte differentiation and function have been identified as recurrently mutated in melanoma. For example, MITF, a transcription factor required for melanocyte development, may serve as a “lineage survival oncogene” in melanoma, promoting growth by expressing proteins that are important during cell differentiation.43 Dependence on lineage-specific survival mechanisms has been described in melanoma and may convey sensitivity to certain therapeutics.44 Transcription factors associated with melanocyte differentiation can be used to subcategorize melanoma and suggest that melanoma can be phenotypically dynamic in response to therapy.
Molecular Genetics of Melanoma Subtypes
Acral lentiginous melanoma and mucosal melanoma have a somewhat different genetic signature than other histological variants of cutaneous melanoma.36 Each represents less than 2% of melanoma cases, but they are notable because they are associated with poorer outcomes. In both subtypes, KIT mutations are particularly common, which may provide a clinically actionable target in the future.45 Of note, KIT-mutated tumors usually do not have coexisting BRAF or NRAS mutations. Finally, desmoplastic melanoma is much more likely to harbor an NF1 mutation than other melanoma subtypes.
Modern molecular techniques have also allowed melanoma to be subcategorized by level of differentiation, as detected using expression of transcription factors.44 In response to therapy, some tumors develop a de-differentiated phenotype, resembling their neural crest predecessors. This phenomenon may happen in a stepwise pattern with distinct phenotypic progression. Future research will be directed to understanding the genetic and epigenetic events that mediate benign and malignant melanocyte differentiation.
Uveal melanoma is a rare and deadly melanoma subtype that occurs in the eye. Uveal melanoma is associated with mutations in the Gαq (G protein-coupled receptor) signal transduction pathway. Mutations in the BAP1, SF3B1, and EIF1AX genes are associated with aggressive disease. Interestingly, each locus is associated with a different pattern of aneuploidy in chromosomes 3, 6, and 8 that can convey high risk of aggressive disease. Gene expression profiling (GEP) is commonly used to stratify prognosis in uveal melanoma.46
Familial Melanoma
Approximately 5% to 12% cases of melanoma occur in a familial pattern.47, 48 Almost one half of these cases can be traced to a small number of high-penetrance loci, and 20% to 40% are specifically due to CDKN2A variants. However, 45% of familial cases are linked to low-penetrance variants or do not have an identified cause. Genome-side association studies have begun to elucidate some of the lower-frequency loci.
The CDKN2A locus is responsible for the most famous and well-studied familial melanoma syndrome, familial atypical mole malignant melanoma (FAMMM) syndrome, also known as B-K mole syndrome or dysplastic nevus syndrome (Table 2). However, only 2% of new melanoma diagnoses will harbor a germline CDKN2A variant. Guidelines are available to help clinicians identify high-risk patients who would most benefit from a referral to a genetic counselor. One well-described method, called the “Rule of Twos and Threes” was designed to identify patients with a 10% or higher risk of harboring a CDKN2A germline mutation (Table 3).49 Of note, these rules were developed in the Australian melanoma population and should be adapted by clinicians based on local population risk and on clinical suspicion.
Table 2.
Summary of commonly tested genes for familial melanoma.
| Gene Locus | Associated Cancers | Syndromes |
|---|---|---|
|
| ||
| CDKN2A (NCCN) | CMM, pancreatic cancer, others | FAMMM syndrome |
| TP53 (NCCN) | Osteosarcoma, CMM, breast cancer, many others | Li-Fraumeni syndrome |
| CDK4 (NCCN) | CMM | Familial CMM |
| BAP1 (NCCN) | Uveal melanoma, renal cell cancer, CMM, mesothelioma | |
| MITF (NCCN) | CMM, renal cell cancer | |
| POT1 | CMM, glioma, CLL, colorectal cancer | |
| PTEN | Breast, thyroid, uterine, renal, colorectal cancers. CMM. Hamartomatous disease including tricholemmoma and polyps | Cowden Syndrome |
| RB1 | Retinoblastoma, CMM, others | |
| BRCA2 | Breast and ovarian cancer, CMM, others | Hereditary breast and ovarian cancer (HBOC) |
|
| ||
| Other Notable Genes | ||
|
| ||
| MCRR (NCCN) | Associated with CMM risk | |
| BRCA1 | Breast & ovarian cancer, others. | HBOC |
| Possibly melanoma | ||
| TERT (NCCN) | Mutated in many cancers | |
| CHEK2 | Sarcoma, breast cancer, CMM | |
The 13 gene loci that are most commonly included in familial melanoma testing panels. NCCN is used to denote the 7 gene loci that are suggested by the National Comprehensive Cancer Network (NCCN) Guidelines. CDKN2A, cyclin dependent kinase inhibitor 2a; TP53, tumor suppressor p53; CDK4, cyclin-dependent kinase 4; BAP1, BRCA-associated protein 1; MITF, microphthalmia-associated transcription factor; POT1, protection of telomeres protein 1; PTEN, phosphatase and tensin homolog; RB1, retinoblastoma; BRCA, familial breast cancer 2; MC1R, melanocortin receptor 1; BRCA1, familial breast cancer 1. TERT, telomerase reverse transcriptase; CHEK2, checkpoint kinase 2; CMM, cutaneous malignant melanoma; CLL, chronic lymphocytic leukemia.
Table 3.
Rule of two’s and three’s for familial melanoma.
| • Any patient with three primary melanomas |
| • A patient with one primary melanoma and two 1st or 2nd degree relatives (same side) with a diagnosis of melanoma or pancreatic cancer |
With increasing availability of panel testing, suspicious probands can be tested for a variety of cancer susceptibility syndromes. Some of these feature melanomas as a “subordinate” cancer phenotype. Examples include Cowden syndrome, Lynch syndrome, Li-Fraumeni syndrome, and BRCA 2. More complex guidelines are available to assist the clinician in managing a proband with a dense cancer pedigree.50 Referral to a genetic counselor is recommended if there is suspicion of a familial cancer syndrome.
Modern screening panels for germline melanoma predisposition are usually composed of between 7 and 13 high-risk gene loci. A summary of the most commonly tested loci is presented in Figure 2. Genes that are suggested by the National Comprehensive Cancer Network (NCCN) Guidelines are: CDKN2A, CDK4, MC1R, MC1R, TERT, MITF, BRCA2, and BAP1 (especially for uveal melanoma).
Screening for familial melanoma patients should be tailored to each patient’s estimated risk. Patient education is essential because self-examination is the most powerful tool for identifying suspicious lesions. Screening examinations are performed at 3- to 12-month intervals. Those with large numbers of nevi are examined more frequently. Adding images to the medical record helps with tracking of nevi in evolution. Of course, appropriate screening for other malignancies should also be performed if the patient is at risk.
Summary
Melanoma is the deadliest cutaneous malignancy and is a common cause of cancer mortality in North America, Australia, and Western Europe. Those with lighter complexion, increased sun exposure, and immunosuppression are at higher risk. Oncogenesis is dependent on DNA damage from UV radiation, and those with impaired DNA damage repair are predisposed to melanoma. Familial melanoma syndromes have been described and can be detected by targeted gene panels.
MELANOMA EVALUATION AND STAGING
Clinical Suspicion for Melanoma
The diagnosis of melanoma begins with clinical suspicion. Often, patients or their clinicians will notice an abnormal skin lesion on examination. Several methods have been developed to rapidly identify pigmented lesions that should be referred to a specialist for additional evaluation or biopsy. The characteristics of each lesion should be measured within the context of a patient’s overall risk. The ABCDE method51 (Figure 5) has been well-described and prospectively validated.52 Additional methods include the Glasgow 7-point checklist,53 and the “Ugly Duckling” method, which emphasizes cutaneous nevi that differ from others on the patient. The advantage of these criteria is that they can be utilized by dermatologists, other clinicians, and laypersons alike, increasing early detection of disease.54, 55 Figure 6 presents a clinical example of an atypical nevus that was later confirmed to contain melanoma.34 Routine screening of average-risk patients is uncommon, but some studies have suggested that it could be cost-effective.56 Screening for familial cases is discussed in the section on genetics. Finally, deep convolutional neural networks have been designed to employ machine learning methods for melanoma diagnosis. These interesting new techniques can parse melanoma from other skin lesions based on high-resolution images alone and may play a role in the future of melanoma surveillance.57
Figure 5.
American Academy of Dermatology ABCDE criteria (with permission from American Academy of Dermatology Ad Hoc Task Force for the ABCDEs of Melanoma, Tsao H, Olazagasti JM, et al. Early detection of melanoma: reviewing the ABCDEs. J Am Acad Dermatol. 2015;72(4):717–723).
Figure 6.
Melanoma arising within a nevus. Note the characteristics of this lesion, such as variegated color, irregular borders, and distinct appearance compared to neighboring nevi (with permission from Rivers JK. Is there more than one road to melanoma? Lancet 2004;363(9410):728–730).
Evaluation of Suspicious Lesions
Excisional Biopsy
Diagnostic excisional biopsy is the standard of care for suspicious pigmented skin lesions.58 Preferred techniques include deep saucerization encompassing the entirety of the lesion, taking care not to transect the deep margin. Full-thickness excisions and punch biopsies are becoming less common because less invasive methods have been shown to be noninferior.59 Shallow shave biopsies are appropriate if melanoma is considered unlikely. A summary of biopsy techniques is provided in Table 3. Peripheral margins of 1 to 3 mm of clinically uninvolved skin are preferred to facilitate histopathologic interpretation. Pre-biopsy photographs are particularly helpful if more than 1 area is biopsied. Axial orientation of biopsy is less likely to disrupt lymphatic drainage of the area and future lymphoscintigraphy. If lesion size or location precludes excisional biopsy, full-thickness incisional or punch biopsy may be appropriate. Multiple representative biopsies can be considered for large, pigmented lesions.
Some melanoma diagnoses are made from incisional, shave, or punch biopsies with a positive margin. In these cases, repeat biopsy may be considered if there is suspicion that the tumor thickness was not adequately sampled. Rates of upstaging (increasing T-category) with repeat biopsy range from 6% to 14% depending on original biopsy type. Acral lentiginous and desmoplastic melanoma are most likely to be upstaged. The superficial spreading subtype has only a 1.4% risk of upstage.60
If a specimen contains dysplastic melanocytes without melanoma, studies have not shown a benefit to proceeding with surgical excision. In one study, less than 2% of these excisions resulted in a significant change in diagnosis.61 Some clinicians will discuss excision with the patient if there is high-grade dysplasia.
Evaluation of Biopsies
Biopsy specimens are systematically catalogued and evaluated in keeping with American Academy of Dermatology, the College of American Pathologists (CAP), and the American Joint Committee on Cancer (AJCC).58, 62–65 An example of a CAP Synoptic Report is included as a supplementary figure (Figure S1). For histopathologic staging, the essential characteristics of the specimen are the Breslow thickness, reported to 0.1 mm, and the presence or absence of ulceration.66 New to the 8th Edition of the AJCC guidelines, the binary measure of mitotic rate greater than or less than 1 mitosis per mm2 is no longer considered for histopathologic staging, but remains an important prognostic factor across all thickness categories. Margin status should always be reported. Microsatellitosis, when present, conveys important prognostic information and must be reported.67, 68 Other histologic features that may provide prognostic value are the presence of vertical growth phase, angiolymphatic invasion, neurotropism/perineural invasion, regression, and tumor infiltrating lymphocytes (TILs).69–71 Melanoma has several distinct histologic subtypes; the prognostic importance of subtype has been disputed, with some exceptions.72 The lentigo maligna subtype is associated with greater subclinical horizontal spread.73 The desmoplastic subtype is locally aggressive and often has decreased/absent pigmentation. It is associated with increased sensitivity to immunotherapy and decreased risk of lymph node metastasis.74 If desmoplastic histology is described, further description of “pure” or “mixed” desmoplastic characteristics provides additional clinical information. Patients with “pure” desmoplastic tumors have an unusually low risk for regional lymph node metastasis and improved 5-year survival.75
Initial Evaluation
The initial evaluation for a new diagnosis of primary melanoma should include: (1) a detailed history, with review of environmental and genetic risk factors for melanoma and nonmelanoma skin cancer; (2) physical examination, with attention to local and regional lymph node basins; and (3) a complete skin examination. Abnormal findings should prompt additional investigation as appropriate. For melanoma of unknown primary, an anogenital/pelvic examination and detailed ophthalmologic examination are essential.
Laboratory and Imaging Evaluation
Baseline blood tests are only indicated if there is appropriate clinical suspicion. Routine laboratory studies are not cost-effective. As an example, routine serum lactate dehydrogenase (LDH) is insensitive for metastatic melanoma.75 Routine use of chest radiograph (CXR) is also discouraged.76–79 Regarding advanced imaging techniques, positron emission tomography-computed tomography (PET-CT) and nodal ultrasound (US) have increased sensitivity for metastasis when compared to physical examination, but the routine use of these imaging modalities in melanoma patients leads to high rates of false positive findings. Therefore, cross-sectional imaging has not been universally adopted as part of the initial evaluation for cutaneous melanoma, and nodal US is not a substitute for sentinel lymph node biopsy (SLNB) or sampling of clinically positive nodes.80–84 Advanced imaging is appropriate for patients with an examination concerning for metastasis.
Cross-sectional imaging has a comparatively higher detection rate of occult distant metastasis in select patients with thick, ulcerated primary melanomas or bulky nodal disease, and may obviate the need for large surgical resection in these patients.37, 85–87 Using these criteria as requisites for advanced imaging may increase the detection rate for occult metastasis to 16%.86 For thick primary lesions with ulceration (at least T3b), some institutions recommend imaging prior to surgical excision.
Increased understanding of the molecular genetics of melanoma has resulted in the development of molecular testing to identify patients who might benefit from targeted therapeutics. Commonly available tests for melanoma include immunohistochemistry (IHC), GEP analysis via next-gen sequencing, and others.
Specific gene targets for molecular testing include BRAF, NRAS, KIT, and IHC for PD-L1. BRAF, a serine/threonine kinase which is central to cell growth signaling, is the most commonly mutated proto-oncogene in melanoma.88 Missense mutation of BRAF at codon V600 is responsible for 80% of activating BRAF mutations and conveys sensitivity to small molecule inhibitors such as vemurafenib and dabrafenib.88 IHC and sequencing tests for BRAFV600 are widely available, and some clinicians choose to include these tests as a part of the initial evaluation for patients who are considered at high risk for recurrence, such as those with category T2 or T3 primary tumors. Mutations in KIT, a receptor tyrosine kinase, are rare in cutaneous melanoma but present in 10% to 15% of mucosal and acral lentiginous melanomas. KIT mutants are detectable with molecular testing and provide a potential drug target for patients with these unusual but deadly melanoma subtypes.45 IHC positivity for PD-L1 greater than 1% is correlated with response to immune checkpoint blockade, but has poor predictive performance in melanoma.89 Thus, many clinicians will choose a trial of checkpoint blockade immunotherapy even in patients with low PD-L1 IHC staining.
GEP tests, for example DecisionDX-Melanoma (Castle Biosciences, Friendswood, Texas), classify melanoma patients as low- or high-risk based on a panel of transcripts detected in the tumor.90 It has been suggested that GEP tests can be used at diagnosis to supplement risk-stratification for early-stage patients with a negative SLNB. Because most new melanoma diagnoses happen at an early stage, and more than one half of deaths occur in patients who initially had a negative SLNB, identification of high-risk patients would be valuable.91 However, GEP tests are limited by low positive predictive value and a lack of strong evidence in large cohorts of patients to support their routine use at this time.92, 93 Currently, GEP tests are not recommended for treatment decisions in average-risk patients with cutaneous melanoma.94 They may be used in patients who are judged to be at high risk for recurrence or in the context of a clinical trial.
Staging
Complete histopathologic staging after wide local excision (WLE) +/− SLNB +/− completion lymph node dissection (CLND) is recommended. The AJCC staging method involves determination of the T, N, and M category of each tumor. The principles of TNM categorization for melanoma are depicted in Tables 4, 5, and 6. Tables 7 and 8 describe the assignment of stage based on TNM category. All recommendations reflect the AJCC 8th edition guidelines on melanoma, published in 2017.63, 64
Table 4.
Definition of N category for American Joint Committee on Cancer (AJCC) TNM staging.
| Extent of Regional Lymph Node and/or Lymphatic Metastasis |
||
|---|---|---|
| N Category | No. of Tumor-Involved Regional Lymph Nodes | Presence of In-transit, Satellite, and/or Microsatellite Metastases |
|
| ||
| NX | Regional nodes not assessed (ie, SLNB not performed, regional nodes previously removed for another reason); Exception: pathological N category is not required for T1 melanomas, use clinical N information |
No |
|
| ||
| N0 | No regional metastases detected | No |
|
| ||
| N1 | One tumor-involved node or any number of in-transit, satellite, and/or microsatellite metastases with no tumor-involved nodes | No |
| N1a | One clinically occult (ie, detected by SLNB) | No |
| N1b | One clinically detected | No |
| N1c | No regional lymph node disease | Yes |
|
| ||
| N2 | Two or 3 tumor-involved nodes or any number of in-transit, satellite, and/or microsatellite metastases with one tumor-involved node | |
| N2a | Two or 3 clinically occult (ie, detected by SLNB) | No |
| N2b | Two or 3, at least 1 of which was clinically detected | No |
| N2c | One clinically occult or clinically detected | Yes |
|
| ||
| N3 | Four or more tumor-involved nodes or any number of in-transit, satellite, and/or microsatellite metastases with 2 or more tumor-involved nodes, or any number of matted nodes without or with in-transit, satellite, and/or microsatellite metastases | |
| N3a | Four or more clinically occult (ie, detected by SLNB) | No |
| N3b | Four or more, at least 1 of which was clinically detected, or the presence of any number of matted nodes | No |
| N3c | Two or more clinically occult or clinically detected and/or presence of any number of matted nodes | Yes |
Adapted from Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual, 8th ed., Springer International Publishing; 2017:563–585.
SLNB, sentinel lymph node biopsy.
Table 5.
Definition of T category for American Joint Committee on Cancer (AJCC) TNM staging.
| T Category | Thickness | Ulceration Status |
|---|---|---|
|
| ||
| TX: Primary tumor thickness cannot be assessed (eg, diagnosis by curettage) | Not applicable | Not applicable |
|
| ||
| T0: No evidence of primary tumor (eg, unknown primary or completely regressed melanoma) | Not applicable | Not applicable |
|
| ||
| Tis (melanoma in situ) | Not applicable | Not applicable |
|
| ||
| T1 | ≤1.0 mm | Unknown or unspecified |
| T1a | <0.8 mm | Without ulceration |
| T1b | <0.8 mm | With ulceration |
| 0.8–1.0 mm | With or without ulceration | |
|
| ||
| T2 | >1.0–2.0 mm | Unknown or unspecified |
| T2a | >1.0–2.0 mm | Without ulceration |
| T2b | >1.0–2.0 mm | With ulceration |
|
| ||
| T3 | >2.0–4.0 mm | Unknown or unspecified |
| T3a | >2.0–4.0 mm | Without ulceration |
| T3b | >2.0–4.0 mm | With ulceration |
|
| ||
| T4 | >4.0 mm | Unknown |
| T4a | >4.0 mm | Without ulceration |
| T4b | >4.0 mm | With ulceration |
With permission from Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge SB, Greene FL, et al., eds. AJCC Cancer Staging Manual, 8th ed. Springer International Publishing; 2017:563–585.
Table 6.
Definition of M category for the American Joint Committee on Cancer (AJCC) TNM staging.
| M Category | M Criteria |
|
|---|---|---|
| Anatomic Site | LDH Level | |
|
| ||
| M0 | No evidence of distant metastasis | Not applicable |
|
| ||
| M1 | Evidence of distant metastasis | See below |
|
| ||
| M1a | Distant metastasis to skin, soft tissue including muscle, and/or nonregional lymph node | Not recorded or unspecified |
| M1a(0) | Not elevated | |
| M1a(1) | Elevated | |
|
| ||
| M1b | Distant metastasis to lung with or without M1a sites of disease | Not recorded or unspecified |
| M1b(0) | Not elevated | |
| M1b(1) | Elevated | |
|
| ||
| M1c | Distant metastasis to non-CNS visceral sites with or without M1a or M1b sites of disease | Not recorded or unspecified |
| M1c(0) | Not elevated | |
| M1c(1) | Elevated | |
|
| ||
| M1d | Distant metastasis to CNS with or without M1a or M1c sites of disease | Not recorded or unspecified |
| M1d(0) | Not elevated | |
| M1d(1) | Elevated | |
With permission from Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual 8th ed. Springer International Publishing; 2017:563–585.
LDH, lactate dehydrogenase; CNS, central nervous system.
Table 7.
Assignment of stage based on T, N, and M category: clinical stage group.
| When T is… | And N is… | And M is… | Then the clinical stage group is… |
|---|---|---|---|
|
| |||
| Tis | N0 | M0 | 0 |
| T1a | N0 | M0 | IA |
| T1b | N0 | M0 | IB |
| T2a | N0 | M0 | IB |
| T2b | N0 | M0 | IIA |
| T3a | N0 | M0 | IIA |
| T3b | N0 | M0 | IIB |
| T4a | N0 | M0 | IIC |
| ANY T, TIS | ≥N1 | M0 | III |
| ANY T | ANY N | M1 | IV |
With permission from Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual 8th ed. Springer International Publishing; 2017:563–585.
Table 8.
Assignment of stage based on T, N, and M category: pathological group.
| When T is… | And N is… | And M is… | Then the pathological stage group is… |
|---|---|---|---|
|
| |||
| Tis | N0b | M0 | 0 |
| T1a | N0 | M0 | IA |
| T1b | N0 | M0 | IA |
| T2a | N0 | M0 | IB |
| T2b | N0 | M0 | IIA |
| T3a | N0 | M0 | IIA |
| T3b | N0 | M0 | IIB |
| T4a | N0 | M0 | IIB |
| T4b | N0 | M0 | IIC |
| T0 | N1b, n1c | M0 | IIIB |
| T0 | N2b, N2c, N3b or N3c | M0 | IIIC |
| T1a/b-T2a | N1a or N2a | M0 | IIIA |
| T1a/b-T2a | N1b/c or N2b | M0 | IIIB |
| T2b/T3a | N1a-N2b | M0 | IIIB |
| T1a-T3a | N2c or N3a/b/c | M0 | IIIC |
| T3b/T4a | Any N >N1 | M0 | IIIC |
| T4b | N1a-N2c | M0 | IIIC |
| T4b | N3a/b/c | M0 | IIID |
| Any T, Tis | Any N | M1 | IV |
With permission from Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual 8th ed. Springer International Publishing; 2017:563–585.
Summary of AJCC Staging
Stages IA through IIC are principally determined by the T category of the primary lesion (ie, Breslow thickness and ulceration). Mitotic rate is no longer a criterion for T category but has prognostic value across all tumor thicknesses. T1 lesions (traditional “thin melanoma”) are up to 1 mm thick. T2 refers to 1 to 2 mm thickness, T3 to 2 to 4 mm thickness, and T4 to “thick melanoma” of Breslow thickness greater than 4 mm. T categories are modified with “a” or “b” to reflect the presence or absence of ulceration. Any nodal disease places the patient in stage III.
Stages IIIA-IIID refer to node-positive disease of any T category. N1 refers to one positive node, N2 to 2 or 3 nodes, and N3 to 4 or more nodes with detectable melanoma. The NXa & NXb designation denotes nodal disease that is clinically occult (“a” designation) or clinically evident (“b” designation). NXc denotes micro/macrosatellites or in-transit metastasis in addition to tumor-involved lymph nodes. Stage III is divided into 4 subcategories based on differential outcomes (Table 9). As usual, stage IV is reserved for distant metastasis. M categories all result in stage IV designation but are subdivided based on the anatomic location of the metastasis. M1a refers to skin/soft tissue and nonregional lymph nodes, M1b refers to lung metastasis, M1c refers to non-central nervous system (CNS) visceral metastasis, and finally M1d refers to CNS metastasis. Each M category is subdivided based on presence/absence of elevated serum LDH, which is an independent adverse predictor of survival across all M categories. LDH is also a useful indicator of disease response and recurrence in select patients.
Table 9.
Description of stage III subgroups.

|
With permission from Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge SB, Greene FL, et al, ed. AJCC Cancer Staging Manual 8th Ed. Springer International Publishing; 2017:563–585.
Stage-Specific Survival and Treatment Applications
Stage I melanoma has an excellent prognosis, with 5-year disease-specific survival (DSS) of 99% and 97% for stages IA and IB. Stage II is subdivided to IIA, IIB, and IIC, which have disease-specific 5-year survival of 94%, 87%, and 82%, respectively. Stage IIIA survival is 93%, which is higher than stages IIB and IIC. Patient reclassification with the 8th edition of the AJCC Guidelines resulted in an increase in survival for both of these groups, possibly due to the Will Rogers phenomenon95 (ie, when more accurate staging causes “stage migration” of a subset of patients, the average survival of both stages is increased). Stage IV survival is not subdivided, but new M category designations were designed to facilitate future research into the heterogeneity of metastatic melanoma.
Stage-specific disease management is a topic of current investigation. Although resection remains the standard for curable disease, there has been increasing interest in neoadjuvant therapy for patients presenting with stage III melanoma.96 Additional research is needed, but neoadjuvant therapy will be a central question in the future of melanoma management. Adjuvant therapies such as BRAF/MEK inhibitors (for BRAF-positive tumors) and immune checkpoint blockade are currently approved for stage IIIB/C/D patients and resected stage IV patients. Some stage IIIA patients are also eligible. These treatment modalities are also being studied in stage IIB/C disease, considering the poor 5-year survival in these patients.97 Intratumoral therapies are also an area of active research.98
CURRENT CONSIDERATIONS IN THE MANAGEMENT OF EARLY-STAGE MELANOMA
Early stage melanoma includes stage 0, I, and II melanomas as defined by the AJCC 8th edition staging system.99 These are essentially melanomas of any T classification with a negative lymphatic basin and no systemic metastasis. Early stage melanoma has a generally favorable prognosis, with 10-year survival ranging from 98% for stage IA disease to 75% for stage IIC disease.100 The mainstay of treatment is surgery, which includes resection of the primary tumor with adequate margins, and an evaluation of the tumor’s lymphatic basin using SLNB. The necessity and extent of each of these surgical procedures is guided by the histopathological evaluation of a biopsy taken from these lesions. Here, we review the current considerations and debates regarding each of these elements of treatment, from the biopsy itself, to the mode of excision and its necessary margins, as well as the indications and implications of a SLNB. We also briefly discuss novel molecular techniques and their potential role in these decisions.
Technique of Biopsy
The Breslow thickness of a melanoma has been shown to be the strongest predictor of prognosis.101, 102 Since the AJCC staging manual’s 6th edition, when the anatomical Clark level was abandoned, Breslow thickness has become the dominant variable, together with ulceration, defining the T classification.103 As a result, an accurate assessment of melanoma thickness is central to deciding on the clinical and surgical management of patients. Current NCCN guidelines, as well as the American Academy of Dermatology guidelines, recommend an excisional biopsy or at least a full thickness incisional biopsy (punch biopsy) to accurately assess Breslow thickness. They discourage the use of shave biopsy, as it can limit the assessment of depth, but allow its consideration in cases where the index of suspicion is low or for the assessment of lentigo maligna.104, 105
Shave biopsies have been proven to understage the Breslow thickness more than any other biopsy technique. Zager and colleagues59 show that residual tumor can be found upon final excision in up to 22% of cases. This residual tumor, left behind during shave biopsy, will cause an upgrading of the T classification upon final excision in 3% to 12.5% of cases. But interestingly enough, studies have shown that this upgrading rarely influences surgical recommendations and it does not seem to affect tumor recurrence or DSS.59, 106–108 Consequently, there have been calls for a change in biopsy recommendations.59 These calls have been fortified by data showing that almost two thirds (62.6%) of lesions diagnosed as melanoma are biopsied by dermatologists; and although the preferred mode of sampling for surgeons is an excisional biopsy and that of primary care physicians is a punch biopsy, dermatologists, as shown by both retrospective analyses and surveys, categorically prefer shave biopsies, rendering shave biopsy the leading means of melanoma diagnosis.106, 109 This understanding has led many, including NCCN panelists, to comment that “any diagnosis is better than none even if microstaging may not be complete,” vindicating the use of shave biopsy in the diagnosis of melanoma.105 Further discussion of the evaluation of malignant melanoma, including indications for systemic evaluation, can be found elsewhere in this monograph.
Excision of the Primary Tumor
WLE of the primary tumor has been the standard of care in melanoma surgery since the beginning of the 20th century. The aim of excision is removal of the tumor in its entirety with sufficiently clear microscopic margins to ensure the best chances for cure and local, regional, and systemic control. The historically accepted surgical margins, advocated by Handley110 in 1907, were 5 cm. These generous excision margins often led to the need for skin grafts and complex reconstruction, considerable wound complications, and eventual long-term morbidity. Led by the motivation to preserve function as well as aesthetics, and avoid the disfiguring nature of these procedures, a series of trials were published in the 1980s challenging this 5 cm paradigm.110, 111 The major randomized controlled trials (RCTs) that influence current margin recommendations are presented in Table 10. They can be generally divided into trials addressing excision margins in melanomas with a Breslow thickness of up to 2 mm and those which addressed thicker melanomas. Most of these trials exclude melanomas of the hands, feet, head, and neck, and the anogenital areas, as wider excisions in these anatomical locations are more morbid and less practiced.
Table 10.
Randomized controlled trials investigating thin resection margins for invasive melanoma.
| Group | Number of patients | Melanoma thickness | Excision margins | Follow up | Survival | Recurrence | Excluded | Comments | |
|---|---|---|---|---|---|---|---|---|---|
| Veronesi et al., NEJM, 1988120 Veronesi et al., Arch Surg, 1991119 |
WHO melanoma program trial 10 | 612 | ≤2 mm | 1 vs 3 cm | 90 months | 8-year DFS 81.6% vs 84.4% 8-year OS 89.6% vs 90.3% |
14% vs 13% Local 1.3% vs 0% Lymphatic 6.9% vs 7.8% Distant 5.6% vs 4.6% |
Facial, fingers, toes | No local recurrence when thickness <1 mm |
| Ringborg et al., Cancer, 1996113 Cohn-Cedermark et al., Cancer, 2000112 |
1st Swedish melanoma group trial | 989 | 0.8–2 mm | 2 vs 5 cm | 11 years | 10-year DFS 81% v. 83% 10-OS 79% vs 76% |
21% vs 19% Local 0.6% vs 1% Lymphatic 15% vs 12% Distant 15% vs 14% |
Head & neck, hands, feet, vulva | |
| Khayat et al., Cancer, 2003114 | French cooperative group trial | 337 | <2.1 mm | 2 vs 5 cm | 16 years | 10-year DFS 85% vs 83% 10-year OS 87% vs. 86% |
Median time to recurrence 43 vs. 37.6 months 13.6% vs 20% recurrence Local 0.6% vs 2.4%, Lymphatic 8.1% vs 6.7% Distant 2.5% vs 6.1% |
Toe, nail, finger, acral-lentiginous | LND not performed |
| Balch et al., Ann Surg, 1993116 Balch et al., Ann Surg Oncol, 2001115 |
Intergroup melanoma surgical trial | 468 | 1–4 mm | 2 vs 4 cm | 72 months | 5-year OS 79.5% vs 83.7% 10-year OS 70% vs 77% |
Local 2.1% vs 2.6% Distant 10.9% vs 8.5% |
Primary closure in 89% vs 54% (p<.001) | |
| Gillgren et al., Lancet, 2011365 Utjés et al., Lancet, 2019366 |
2nd Swedish melanoma group trial (Scandinavian Baltic trial) | 936 | >2 mm | 2 vs 4 cm | 19.6 years | Melanoma specific mortality 41% vs 43.5% Overall mortality 65% vs 67% |
41.7% vs 42.5% Local 4.3% vs 1.9 Regional 4.1% vs 3.2% Lymphatic 21.5% vs 24.2% Distal 8.2% vs 11.5% |
hands, feet, head & neck, anogenital region | 9% underwent SLNB |
| Thomas et al., NEJM, 2004118 Hayes et al., Lancet Oncol, 2016117 |
UK Melanoma Study Group, the British Association of Plastic Surgeons and the Scottish Cancer Therapy Network | 900 | >2 mm | 1 vs 3 cm | 8.8 years | Melanoma specific mortality 42.8% vs 36.9% (p=0.041). Overall mortality 55.8% vs 53.9% (p=0.14) |
Local 3.3% vs 2.9% Locoregional 37% vs 31.8% (p=0.05) Distal 8.4% vs 6.7% |
General anesthesia in 32.1% vs 66.4% of patients |
DFS, disease-free survival; OS, overall survival; WHO, World Health Organization; LND, lymph node dissection; SLNB, sentinel lymph node biopsy.
Questioning the 5 cm margin, the Swedish Melanoma Group trial and the French Cooperative Group trial compared a 2 cm excision to a 5 cm excision in melanomas of up to 2 mm thickness. The Swedish study, including 769 patients, published its first findings in 1996, demonstrating a regional lymph node recurrence rate of 12.1% versus 8.3%, favoring the wider 5 cm excision. Nevertheless, both groups were comparable in overall recurrence rates (20.4% versus 20.5%); but more importantly, it showed a comparable 5-year OS of 86.4% and 88.7%, that was preserved upon long-term follow-up, with a 10-year OS of 79% and 76%, for a 2 cm versus 5 cm margin, respectively.112, 113 The French Cooperative Group trial, performing a second randomization of its 337 patients, assigning them to receive adjuvant isoprinosine or no adjuvant therapy, demonstrated similar findings. After a follow-up period of 19 years, the authors reported a recurrence rate of 13.6% versus 20% and a mortality rate of 19.8% versus 17.5% for 2 cm and 5 cm margins, respectively.114 These findings helped to establish a 2 cm margin as adequate for melanomas of up to 2 mm thickness.
This 2 cm margin was further investigated and shown to be adequate in melanomas of greater than 2 mm thickness. The Intergroup Melanoma Surgical Trial, led by Charles Balch, dealt with intermediate thickness melanomas of 1 to 4 mm, and randomized 486 patients to either a 2 cm or 4 cm excision margin. It showed comparable results, in terms of recurrence rates and 5- and 10-year OS, between the 2 groups; and although a narrow resection margin did not confer a negative prognostic effect, the authors demonstrated that both tumor thickness (RR=1.74, p=0.0008) and ulceration (RR=2.21, p=0.0095) conferred a statistically significant negative effect on survival. Moreover, they were able to demonstrate the negative effect a wider excision had on patient outcomes, in terms of a longer hospital stay (7.0 ± 5.1 versus 5.2 ± 4.5 days) and the ability to perform primary closure of the surgical site (54% versus 89%).115, 116
Noticing that the mean thickness of melanomas in the Intergroup trial was 1.96 mm, 2 groups investigated the feasibility of narrow margins in melanomas greater than 2 mm thickness. The 2nd Swedish Melanoma Group trial randomized 936 patients to a 2 cm versus 4 cm excision margin. With a follow-up of more than 19 years, they were able to show comparable recurrence rates (41.7% vs 42.5%), melanoma specific mortality (41% vs 43.5%), and overall mortality rates (55.8% vs 53.9%) between the 2 groups. The United Kingdom Melanoma Study group, together with the British Association of Plastic Surgery and the Scottish Cancer Therapy Network challenged the 2 cm margin, randomizing 900 patients with melanomas of more than 2 mm thickness to a 1 cm excision margin versus a 3 cm margin. They demonstrated similar overall mortality rates of 55.8% versus 53.9% between the 2 groups. Nevertheless, patients undergoing a narrow excision with 1 cm margins exhibited a higher locoregional recurrence rate (37% vs 31.8%, p=0.05) and worse melanoma specific survival (MSS, 42.8% vs 36.9%, 0=0.041).117, 118 These findings helped to establish the recommended margins for T3 and T4 melanomas as 2 cm.
As for thinner melanomas (≤2 mm), the World Health Organization (WHO) trial, led by Veronesi and Cascinelli, showed a narrow 1 cm excision margin to be equivalent to a wide 3 cm excision margin, in terms of 8-year OS (89.6% vs 90.3%). Disease-free survival (DFS), as well as recurrence rates, were likewise comparable between the 2 groups, with a recurrence rate of 14% and 13%, respectively. The authors were also able to show superior results in terms of DFS and OS in patients with 0.1 to 1 mm melanomas compared to those with 1.1 to 2 mm lesions, and that no patient with a melanoma less than 1 mm thickness recurred locally after excision with a 1 cm margin.119, 120 These results, in conjunction with findings showing that a margin narrower than 1 cm in these thin (≤1 mm) melanomas confers higher recurrence rates,121 established a margin of 1 cm as the recommended margin for up to 1 mm thick melanomas. These left melanomas of a 1 to 2 mm thickness in a controversial gray zone, with an ambiguous recommended margin of 1 to 2 cm. The MelmarT trial, an international multicenter RCT aims to answer this question, and is randomizing patients with melanomas of more than 1 mm thickness into 1 cm versus 2 cm resection margins.122 It is estimated to be completed in 2026 and will provide further guidance.
There are no similar RCTs for melanoma in situ, with the recommended excision margins being 0.5 to 1 cm.58, 105 Most authorities recognize a 0.5 mm margin as adequate for most melanoma in situ cases, but others advocate a wider excision. 58, 105, 123–125 One prospective database analysis showed, using Mohs micrographic surgery (MMS), that a 6 mm excision margin confers a 86% clear margin rate, while a 9 mm margin allows for a 98.9% margin rate.124 Several other studies show similar results for both melanoma in situ and lentigo maligna, advocating margins up to 1.2 cm for head and neck tumors. Agreement does exist regarding the need for a wider margin than 0.5 cm for lentigo maligna, a subtype of melanoma that has a tendency for subclinical peripheral tumor extension.126 Table 11 summarizes the current recommendations for margins in primary cutaneous melanoma.
Table 11.
Recommended surgical margins for primary cutaneous melanoma.
Surgical margins are considered as measured during surgery from the edge of the lesion or post-excisional biopsy site.
For large or poorly defined melanoma in situ, lentigo maligna type, a surgical margin or more than 0.5 cm can be considered.
Consider histologic margin assessment prior to reconstruction and closure (Adapted from the Cutaneous Melanoma NCCN Guidelines, Version 4.2020).105
Surgical Technique
Modern surgical techniques and excision margins usually allow for primary wound closure. More complex reconstructive techniques, such as skin grafting or tissue flaps, may be required for larger defects or those in challenging areas such as the face, scalp, or neck. In these cases, it is recommended to use grafts from the contralateral side in order to minimize the risk of local recurrence due to microscopic in-transit disease.111 It is recommended that excision is carried down to the depth of the underlying muscular fascia, but not necessarily including the fascia.58 No randomized trials have addressed the question of depth of resection or whether excision to the deep adipose layer is adequate.
The use of MMS in melanoma surgery, commonly complemented by melanoma antigen recognized by T-cells 1 (MART-1) immunostaining, is advocated by some authorities as an alternative for WLE, mostly in the dermatological literature. It is advocated not only for melanoma in situ and lentigo maligna, but also for invasive melanoma of up to 1 mm thickness. Several retrospective studies show MMS to be equivalent to WLE in terms of OS, MSS, and local recurrence in these patients, with some studies claiming superiority in terms of survival, especially in head and neck melanomas.127–130 It has also been shown that MMS is used more frequently than WLE in the face as well as in the scalp and neck areas, and several authors advocate its use in these areas where tissue conservation is critical.130, 131
Lymph Node Evaluation
The subject of immediate elective dissection of regional lymph nodes (ELND), in clinically node-negative melanoma patients, was an issue of controversy throughout the 1960s and 1970s. Although most authorities agreed that dissection was not necessary for patients with thin tumors with low-risk features, the definitions of thin and low-risk were debated. A prospective clinical trial conducted by the WHO, comparing ELND with follow-up and lymphatic dissection upon clinically detectable nodal involvement failed to show a difference in survival between the 2 groups, deeming observation a viable option in node-negative patients. This study also showed that only 20% of patients had lymphatic involvement, with lymph node involvement being directly related to melanoma thickness. This finding stressed that 80% of patients were unnecessarily exposed to the potential morbidity of lymphatic dissection, most notably lymphedema, with no benefit to their survival.132–134 Nevertheless, a survival advantage of 11% was found with ELND in the subgroup of patients with intermediate Breslow thickness lesions who had lymphatic involvement, either discovered upon ELND or diagnosed clinically during observation.134, 135
The need to identify this subset of patients with occult lymphatic disease led to the implementation of SLNB into the management of stage I and II melanoma. The technique, introduced by Morton and colleagues135 in 1992, incorporated preoperative planar lymphoscintigraphy with technetium-labeled dextran in ambiguously located tumors, such as the trunk, to designate the target lymphatic draining basin. Intraoperatively, blue dye was injected into the area of the tumor and the lymphatic channels were followed using meticulous dissection in the expected lymphatic basin until the sentinel lymph nodes were identified and excised. Later, the use of 99mTc-labeled albumin for intraoperative radiolymphoscintigraphy was added to the technique, enabling dual tracer identification of sentinel nodes. Morton was able to show the accuracy of the technique, with a false negative rate of 1%, establishing SLNB as a central part of the management of stage I and II melanoma. The technique was incorporated into the AJCC staging system in 2001.103, 135
The first Multicenter Selective Lymphadenectomy Trial (MSLT-1) investigated the potential contribution of SLNB to the detection of occult lymph node metastases in patients with intermediate thickness melanoma (1.2 to 3.5 mm). This multicenter trial randomized patients to undergo WLE with SLNB or WLE with observation. In the SLNB arm, if the biopsy was positive, the patient underwent CLND. In the observation arm, if the patient developed clinically evident nodal disease, they then also returned to the operating room for CLND. The 5-year DFS rate was higher in the SLNB group (78.3% vs 73.1%, respectively, p=0.009), with similar 5-year MSS rates (87.1% vs 86.6%, respectively), findings that persisted in the final analysis. Nevertheless, much like the WHO trial, within the subgroup of patients with nodal metastases, patients who underwent SLNB with CLND had improved 5-year survival compared to those who had lymph node dissection (LND) upon clinical recurrence (72.3% vs 52.4%, respectively, p=0.004). In addition, SLNB was shown to be an important staging modality, as patients with nodal metastases upon SLNB were restaged as stage III and had significantly worse survival compared to those with a negative SLNB (72.3% vs 90.2%, respectively, p<0.001).136 Thick (>3.5 mm, in this study) melanomas were also evaluated, demonstrating similar 10-year DFS (50.7% vs 40.5%, in the SLNB+CLND vs. observation, p=0.03). Again, patients with positive SLNB had worse 10-year MSS compared to negative SLNB (62.1 vs 85.1%, p<0.001), further reinforcing the role of SLNB for staging purposes.137
These findings led international clinical and surgical oncology societies to recommend the use of SLNB in intermediate thickness melanomas, as well as in thick melanomas.138–141 The utility of SLNB in thin melanomas (≤1 mm) remains controversial. Although the incidence of nodal involvement in this group is low (approximately 5%), the majority (70%) of melanomas diagnosed in the United States are thin. As a result, a considerable number of melanoma deaths occur in patients who originally were diagnosed with thin melanoma. Subgroup analysis has demonstrated that the main predictor of SLNB positivity in thin melanoma is tumor thickness. Among patients with a tumor thickness of less than 0.75 mm, the rate of SLNB positivity is 2.7%, whereas for those with a thickness of 0.75 to 1 mm, it is 6.2%.142 Other risk factors do not predict sentinel node positivity as reliably. Among these risk factors are ulceration, mitotic rate, lymphovascular invasion (LVI), Clark level, tumor vertical growth phase, TILs, tumor regression, age, and others. The recommendation of most professional societies is to consider SLNB in melanomas 0.8 to 1 mm thick and those less than 0.8 mm thick with high-risk features, including ulceration, high mitotic rate and LVI, especially in younger patients.58, 105, 140, 141 Table 12 describes the recommendations for SLNB.
Table 12.
Recommendations for sentinel lymph node biopsy.
| Breslow thickness + other characteristics | NCCN recommendation* | ASCO + SSO recommendations** | % positive SLN |
|---|---|---|---|
| Stage IA (T1a): <0.8 with no ulceration | Not recommended, unless uncertain about adequacy of biopsy staging | Not recommded | <5% |
| Stage IB (T1b): <0.8 with ulceration or 0.8–1 mm with no ulceration T1a with adverse features (high mitotic index≥2/mm2 [in setting or young age], LVI, or a combination) |
Discuss with patient and consider SLNB | Discuss with patient and consider (does not include T1a tumors with adverse features) | 5–10% |
| Stage IB (T2a) or II: >1 mm | Offer SLNB (unless non-mitogenic or older patients, with a lower probability of positive SLNB) | Recommended for T2 and T3 tumors For T4 tumors, may be recommended |
>10% |
SLN, sentinel lymph node; SLNB, sentinel lymph node biopsy; LVI, lymphovascular invasion; NCCN, National Comprehensive Cancer Network; ASCO, American Society of Clinical Oncology; SSO Society of Surgical Oncology.
If patient is unfit or unwilling to act based on SLNB findings, reasonable to forgo SLNB.
Adapted from the Cutaneous Melanoma NCCN Guidelines, Version 4.2020.105
Adapted from the ASCO and SSO clinical practice guideline update.140
Completion Lymph Node Dissection in Patients with a Positive Sentinel Lymph Node Biopsy
With the implementation of SLNB in the management of stage I and II melanoma, 5% to 40% of patients will consequently be upgraded to stage III disease. MSLT-I demonstrated that these patients benefited from a SLNB complemented by CLND more than they did from observation. However, within the MSLT-I cohort, 88% of patients who had a single positive sentinel lymph node had no additional positive nodes upon CLND.49
In light of these findings, 2 major randomized trials set out to understand the value of CLND after a positive SLNB. First was the German Dermatologic Cooperative Oncology Group DeCOG-SLT trial, which was ended early due to low accrual and low event rate. It randomized 483 patients with at least 1 mm thick melanomas and a positive SLNB to undergo CLND or observation. Its results, published in 2016, showed a similar 3-year distant metastasis-free survival (DMFS) between the 2 groups (77.0% in the observation group and 74.9% in the CLND group, p=0.87). Three-year OS and recurrence-free survival (RFS) were likewise similar. Furthermore, although the observation group had no serious adverse events, 14% of the CLND group suffered from grade 3 or 4 events. Therefore, the authors concluded that CLND can be omitted in these patients; but as 66% of the cohort had only up to 1 mm micrometastases in the sentinel lymph node, the authors limited their recommendations to such patients.143
The second such trial was the MSLT-II trial, which also randomized patients with a positive SLNB (including micrometastatic disease identified with molecular techniques) to CLND or observation with serial nodal US. Intention-to-treat analysis results were similar to those of the DeCOG-SLT trial, with comparable 3-year MSS of 86% in both groups. The authors noted that CLND conferred a favorable DFS, related to an improved rate of disease control in the regional nodes, but lymphedema rates were considerably higher in the CLND arm (24.1% vs 6.3%). The authors concluded that although CLND improves regional control and provides additional staging information, it comes at the cost of an increased lymphedema rate, and confers no benefit in MSS. Therefore, observation with serial nodal US in SLNB-positive patients is a safe option for patients who can reliably undergo such surveillance. One caveat to these findings is that CLND identified additional positive nodes in 11.5% of patients, a finding that was a strong predictor of recurrence. This subset of patients derives benefit from CLND.144 The results of these 2 trials led to the overwhelming acceptance by both the NCCN and American Society of Clinical Oncology (ASCO)/Society of Surgical Oncology (SSO) of observation as an acceptable treatment option for low-risk patients with a positive SLNB.105, 141 Patients potentially excluded from these recommendations are those with what can be considered as high-risk features, such as extracapsular extension of their lymph node metastases, significant tumor burden in the sentinel lymph node exceeding 10 mm, microsatellitosis of the primary tumor, more than 3 involved nodes, more than 2 involved nodal basins, and patients on immunosuppression.
Adjuvant Therapy
Surgery remains the mainstay of treatment in early-stage cutaneous melanoma. However, patients with stage IIA through IIC disease are noted to have a 10-year MSS of 88% to 75%. In comparison, stage IIIA melanoma has a 10-year MSS of 88%, and stage IIIB a 77% MSS.64 With the tremendous effect that the introduction of immune checkpoint inhibitors and BRAF-directed therapy has had on the treatment paradigms and outcomes of metastatic melanoma and stage III disease, the role of these novel agents in the adjuvant setting for stage II melanoma is being investigated.145–148 Several RCTs are currently investigating this question. The largest is KEYNOTE-716 (Safety and Efficacy of Pembrolizumab Compared to Placebo in Resected High-Risk Stage II Melanoma), an international, randomized, phase III trial testing the efficacy of 1 year of pembrolizumab in stage IIB and IIC patients.97, 149
Gene Expression Profiling in Melanoma
In recent years, several commercial GEP tests have become available. Much like the genomic assays for breast cancer, they aim to differentiate low clinical risk patients into low and high genomic risk patients, helping to more accurately discern these patients’ actual risk of recurrence and using that information to guide treatment and surveillance recommendations. The 2 available GEP tests are the 31-gene expression profile DecisionDx-Melanoma™ (Castle Biosciences; TX, USA), which is the most commonly available assay in the United States, and the 8-gene expression profile MelaGenix (NelaCare; Koln, Germany). There are several other GEPs which are still in clinical development.
The DecisionDx-Melanoma uses 28 signature genes and 3 control genes to classify tumors into low-risk (class 1) or high-risk (class 2) for recurrence, with a sub-classification into A and B subgroups.150 Several studies have demonstrated the association between a class 2(B) score and worse RFS and DMFS, compared to a class 1(A) score.151–154 This association has been shown to be statistically significant and independent of the traditional clinical variables of Breslow thickness, ulceration, mitotic rate, and SLNB status. One trial has gone further and shown an association between DecisionDx score and SLNB-positivity, showing 55 to 64 and ≥65-year-old patients with a class 1A score to have 4.9% and 1.6% rate of SLNB-positivity, respectively, and class 2B patients to have 30.8% and 11.9% SLNB-positivity, respectively. These findings point to the potential of GEPs to affect treatment decisions, identifying patients in which SLNB might be avoided or recommended.155
In spite of these early findings, the NCCN and the American Academy of Dermatology discourage the use of any GEP outside of a clinical trial.58, 105 Nevertheless, an estimated of 5% to 10% of cutaneous melanomas are already being subjected to GEP testing.150 One survey shows that more than 20% of “pigmented-lesion experts” are using one of the GEPs, with 63% indicating that their management of patients is affected by the results.156 Questioning this premature use of GEPs, a meta-analysis conducted by Marchetti and colleagues157 points to the limited ability of both DecisionDx-Melanoma and MelaGenix to predict recurrence in localized, stage I and II melanoma. It demonstrates that the GEPs were able to accurately classify patients with recurrence in 76% to 82% of stage II and only 29% to 32% of stage I cases.157 Another criticism regards the clinical application of some of these recent findings regarding DecisionDx, emphasizing the low sensitivity and positive predictive value of the test for T1 disease, resulting in only 1% of T1 patients potentially benefiting from the test, while 13% will be falsely identified as either low- or high-risk.92 Many authorities further question the clinical implication of GEP results, even for accurately identified high-risk patients, pointing to the limitation of diagnostic imaging in detecting pre-symptomatic recurrence, the unproven benefit of treating an asymptomatic recurrence, and the lack of evidence regarding benefit of adjuvant systemic treatment in these patients.
Nevertheless, with time and further research, these assays have the potential to help identify the 2% of T1a patients who will relapse and die of their disease, as well as the 5% of patients with a negative SLNB who will experience a recurrence. They might eventually assist in management decisions regarding these patients, allowing for escalation or de-escalation of treatment according the GEP score, guiding wider or narrower margins for excision, deciding upon the necessity of SLNB, as well as informing decisions concerning adjuvant systemic treatments and patient surveillance.
In summary, there have been great advances in the surgical treatment of early-stage melanoma in recent decades. There has been considerable de-escalation both in the extent of surgery for the primary tumor, with the narrowing of recommended resection margins, as well as in the extent of surgery of the lymphatic basin, with the introduction of SLNB and the selective omission of CLND from a subset of lymph node positive patients. Recommendations continue to evolve as further subsets of patients are identified whose risk for recurrence calls for more or less aggressive surgical and/or systemic therapy. Promising adjuncts to this process include the emerging GEP tests, as well as tumor molecular testing. By characterizing tumors on the molecular level, we might be able to better identify patients who will benefit from further escalation or de-escalation of treatment, as well as to assign targeted therapies to selected patients.
TREATMENT OF REGIONALLY ADVANCED MELANOMA
The hallmark of stage III melanoma is nodal involvement. Previously divided into 3 groups, the 8th edition of the AJCC staging for melanoma now classifies stage III melanoma into 4 subsets (IIIA, IIIB, IIIC, and IIID) based on histopathologic factors: the nodal involvement (number of nodes and microscopic versus macroscopic disease) and tumor characteristics (depth and the presence of ulceration).99 Stage III disease represents a heterogeneous group of patients with regional microscopic or macroscopic nodal disease with or without in-transit or satellite metastasis. As a result of this marked heterogeneity of disease, the outcomes also encompass a wide range of DSS, from 88% 10-year DSS in stage IIIA to 24% for stage IIID disease.64 Stage III subcategorization introduced by the AJCC 8th edition now allows for more accurate evaluation of outcomes and prognosis for patients with stage III disease. Appropriate risk stratification is essential, because patients with macroscopic nodal disease had very poor outcomes prior to the introduction of modern systemic therapy. For example, these patients previously had a greater than 70% chance of recurrence or death at 5 years, and a 9% 5-year OS rate.101, 115, 158
Stage IIIA encompasses non-ulcerated tumors less than 2 mm thick or ulcerated tumors less than 1 mm thick, with microscopic nodal disease in 3 or fewer lymph nodes. Stage IIIB disease includes non-ulcerated tumors less than 4 mm thick, ulcerated tumors less than 2 mm thick with nodal disease in 3 or fewer lymph nodes with at least 1 clinically palpable node, or satellite or in-transit tumors. Stage IIIC disease is comprised of non-ulcerated tumors less than 4 mm thick or ulcerated tumors less than 4 mm thick with either: in-transit disease plus microscopic nodal disease, microscopic nodal disease in 4 or more nodes, 2 or more palpable lymph nodes, or clumped lymph nodes. Stage IIID disease involves ulcerated tumors greater than 4 mm thick with lymph node involvement in more than 3 nodes, clumped lymph nodes, palpable lymphatic disease in 2 or more nodes, or nodal disease plus in-transit disease.
Therapeutic Lymph Node Dissection
The 8th edition of the AJCC staging breaks down lymph nodes into microscopic and macroscopic (ie, occult versus clinically detectible). This distinction is important since the management of the 2 entities is diverging. Although it was previously believed that CLND improved survival and outcomes for all node positive patients, more recent studies have shown that in the setting of microscopic disease there is no survival benefit to CLND.143, 144 However, those patients with high-risk features such as primary tumor microsatellitosis, extracapsular extension, greater than 2 lymph node basins involved, and greater than 3 involved nodes, potentially should be considered for CLND to help with locoregional disease control.141 In summary, CLND may be used selectively based on risk stratification in patients with micrometastatic disease.
For patients with microscopic disease only, 2 recent trials—the German Dermatologic Cooperative Oncology Group (DeCOG-SLT) and the Multicenter Selective Lymphadenectomy Trial-II (MSLT-II)—have been crucial in the movement from CLND to observation alone.143, 144 The DeCOG-SLT is a multicenter, randomized, phase III clinical trial that looked at survival in CLND versus observation alone in patients with positive SLNB.143, 159 At median follow-up of 72 months, there was no significant difference in the 5-year RFS, DMFS, OS.159 Similarly, the MSLT-II trial, an international, randomized, phase III trial, also evaluated the 3-year MSS, DFS, OS, and DMFS between CLND and observation alone.144 The MSLT-II trial showed no significant differences in mean 3-year MSS, DMFS, or OS between the 2 groups. However, unlike the DeCOG-SLT trial, there was a significant difference in the 3-year DFS (68% in CLND group vs. 63% in the observation group, p=0.05). Furthermore, the investigators also noted a higher rate of non-sentinel lymph node (NSLN) recurrence in patients in the observation arm, and further analysis revealed this to be an independent prognostic factor for melanoma-related death.144 As such, MSLT-II supports the role of CLND for diagnosing NSLN metastasis, the presence of which serves as a prognostic factor for melanoma-related death. It does not, however, show any additional survival benefit in those patients diagnosed with NSLN disease (despite achieving a 70% relative decrease in NSLN recurrence). In short, for patients with metastatic disease identified on SLNB, there is a role for observation alone, involving frequent clinical follow-up with visits every 4 months for 2 years, then every 6 months between years 3 and 5, with annual US nodal evaluation for 5 years. However, the option for observation in lieu of CLND is not recommended for those who are unable to keep this intensive follow-up regimen, or those who have clinically evident nodal disease. Of note, both DeCOG-SLT and MSLT-II trials excluded patients with in-transit disease, satellite disease, and locoregional metastasis.
For those patients with clinically evident nodal disease usually consisting of a tumor deposit of 10 mm or greater, CLND is still recommended as standard practice, although this recommendation is in evolution given some early promising results with neoadjuvant treatment approaches in this group of patients (see below). For patients with positive axillary nodes, the recommendation is excision of levels 1 through 3. For positive inguinal lymph nodes, there remains some controversy about superficial versus deep groin dissection. A 2011 retrospective study by van der Ploeg and colleagues160 showed no difference in DFS between patients undergoing superficial versus deep groin dissection, with greater incidence of chronic lymphedema in the deep groin dissection group. In this study, CT had high negative predictive value for the detection of pelvic nodal involvement (91%). Thus, the current recommendation is for superficial groin dissection for those patients without pathologic iliac nodes on CT. Deep inguinal dissection should be reserved for patients with positive deep nodes on CT or multiple positive nodes on superficial dissection.
Neoadjuvant Therapy—Systemic Immunotherapy
Stage III disease can be conceptually divided into resectable or unresectable disease. For those with resectable disease, the current treatment guidelines include surgical resection with or without adjuvant therapy consisting of immunotherapy and/or targeted kinase inhibitors in patients with BRAF-mutant disease.161 Recently, there have been several studies suggesting that a neoadjuvant treatment approach with immunotherapy and/or targeted therapies may improve outcomes. The use of neoadjuvant therapy in melanoma may provide several advantages, including reducing surgical morbidity by reducing the disease burden, as well as providing the opportunity to evaluate pathologic response to therapy, which can be predictive recurrence risk. Several trials have shown changes in expression of PD-1, PD-L1, and CD8 that are associated with tumor response to therapy and recurrence.162–165 Furthermore, the use of immunotherapy prior to resection of the tumor (and its associated neoantigens) may potentially induce a stronger tumor-specific T-cell response.
Several phase I studies have demonstrated the potential efficacy of neoadjuvant therapy in stage III melanoma using pembrolizumab, nivolumab, and ipilimumab, either alone or in combination.163–165 The OpACIN phase Ib trial randomized patients with stage III melanoma to adjuvant or neoadjuvant therapy using combination ipilimumab and nivolumab.165 Of the 10 patients in the neoadjuvant treatment arm, 7 patients had a pathologic response (3 with pathologic complete response [pCR], 4 with partial response). Of note, none of the patients who achieved a pCR had recurred by 30 months, whereas the 2 patients in the neoadjuvant arm who did not have a pCR had recurrent disease. At 30 months, the RFS was 80% in the neoadjuvant group and 60% in the adjuvant group, with a 30-month OS of 90% for neoadjuvant therapy and 67% for adjuvant therapy. Adverse events were common in this study, with 18 of 20 (90%) patients experiencing 1 or more grade 3 or 4 toxicity events.163
The success of early studies paved the way for subsequent phase II studies, which have also shown promising results with use of neoadjuvant therapy in stage III melanoma.162, 166–168 In a phase II randomized study, Amaria and colleagues162 showed that neoadjuvant immune checkpoint blockade with combination ipilimumab and nivolumab resulted in high response rates, with pCR of 45%. Single agent therapy with nivolumab resulted in pCR of 25%, but with fewer adverse events. As with the OpACIN phase Ib study, there was a high rate of toxicity with combination therapy, with 73% of patients experiencing grade 3 or higher events, as opposed to the 8% seen with single agent therapy.162 Based on the high rate of toxicity observed in their initial studies,163 the OpACIN-neo trial, a multicenter, phase II RCT identified the optimal dosing and schedule of ipilimumab plus nivolumab to provide effective but less toxic therapy.168 This study identified the regimen of ipilimumab 1 mg/kg and nivolumab 3 mg/kg as optimal with regard to response rates and rates of adverse events.168
In addition to studying the use of neoadjuvant immunotherapy, there are several studies that have evaluated the use of neoadjuvant targeted kinase inhibitors. Two trials looking at the use of neoadjuvant dabrafenib (BRAF-inhibitor) and trametinib (MEK-inhibitor) in patients with BRAF-mutated melanoma have shown positive outcomes.
First, NeoCombi, a single-arm phase II study, reported outcomes of 35 patients with BRAF-mutated melanoma receiving combination dabrafenib plus trametinib for 12 weeks followed by resection and subsequent 40 weeks of adjuvant therapy. At the time of surgery, all 35 patients had some pathologic response, with 49% having pCR. The 1-year RFS was 82% in those patients who achieved a pCR, compared to 63% in those who did not achieve pCR.169
In a second single center, phase II randomized trial, Amaria and colleagues170 compared the use of neoadjuvant and adjuvant combination dabrafenib plus trametinib with the standard of care (surgery followed by adjuvant therapy). In the neoadjuvant group, patients received 8 weeks of neoadjuvant therapy followed by surgery and then up to 44 weeks of adjuvant therapy. This trial was stopped early after the interim safety analysis revealed significantly longer survival in patients who received neoadjuvant therapy, and then continued as a single arm study. At a median follow-up of 18.6 months, 71% of the 14 patients treated with neoadjuvant therapy were alive without disease progression, and none of the 7 patients treated with standard of care were alive.170
To better characterize the impact of neoadjuvant therapies, the International Neoadjuvant Melanoma Consortium performed a meta-analysis of 6 trials of neoadjuvant therapy (4 with immunotherapy, and 2 with targeted therapy). After pooling the data, there was a significant difference in outcomes seen with those patients who achieved pCR versus those who did not; there was a 12-month RFS of 95% in those with pCR versus 62% in those without, and 2-year RFS of 89% in those with pCR versus 48% in those without. Overall, the response associated with neoadjuvant immunotherapy appeared to be more durable than that seen with targeted therapy. Although a similar percentage of patients achieved pCR (38% with immunotherapy and 47% with targeted therapy), patients with pCR following targeted therapy had a 25% recurrence rate at 2 years, whereas those with immunotherapy had 0% recurrence. Furthermore, RFS was superior in patients treated with immunotherapy compared to targeted therapy, at 83% versus 45% at 2 years, respectively.171 In summary, this study identified pCR as the most important prognostic factor after neoadjuvant therapy. Responses with immunotherapy were more durable than those achieved with targeted therapy.
Treatment of In-transit Disease and Locally Advanced Unresectable Disease
Some patients with stage III melanoma have unresectable disease, thus presenting a unique therapeutic challenge. The prognosis of patients with non-nodal locoregional metastasis is similar to that of patients with clinically detectable nodes.64 In-transit disease, which occurs in 3% to 10% of melanomas, is disease that occurs between the primary site and the regional nodal basins.172 It most commonly manifests as multiple tumor nodules but may also be present as a single site of disease. In-transit disease is most common in the extremities, but also occurs on the trunk, head, and neck. Location, depth of invasion, and ulceration are all factors that are known to contribute to the risk of development of in-transit disease. For example, the lower extremity is a common location.173
The approach to treatment must consider the overall condition of the patient, the anatomic site, extent of locoregional disease, and the presence or absence of disseminated metastases. There are several options for the treatment of in-transit disease that range from surgical excision to regional treatment and now also include several effective systemic therapies. Part of the challenge is that the definition of resectable disease had not been clearly established. Many surgeons feel that the presence of 3 or more lesions or resection of a single lesion after which the skin cannot be closed primarily should merit consideration of nonsurgical therapy as a first step. Given the significant advances and effectiveness of systemic therapy, the role of regional therapy and surgery in this group of patients is rapidly changing. Although regional treatments such as limb perfusion and limb infusion were once destination therapies, they have now been replaced as front-line treatments by systemic and/or intralesional treatments. Intralesional therapy may be conceived of as an adjunct to systemic therapy, with the aim to help attain locoregional control and/or to alter the regional tumor microenvironment to help augment systemic immunotherapy. Isolated limb perfusion (ILP) and isolated limb infusion (ILI) are currently reserved for salvage therapy or for patients with no other treatment options.
Surgery
Surgery can have a role up front to remove small volume lesions if morbidity is felt to be minimal. Most of these patients should then be considered for adjuvant systemic therapy in the form of immunotherapy based on the results of the checkmate 238 trial148 or targeted BRAF/MEK inhibition based on the results of the COMBI-AD trial.147 Surgery may also have a role in the removal of refractory metastatic lesions when there is evidence of response to systemic therapy elsewhere in the body. Palliative procedures may be considered for a lesion that is large, necrotic, and draining fluid.174, 175
When regional in-transit disease is deemed to be unresectable or recurrent, multidisciplinary discussion is indicated to consider optimal sequencing of systemic therapy versus regional chemotherapy (ILP or ILI) or intralesional treatment (talimogene laherparepvec [T-VEC]). Increasingly, a systemic neoadjuvant approach is being applied to these patients based on preliminary studies that suggest high response rates for both targeted therapies170 and immunotherapies.162, 176 Use of regional chemotherapy with either ILP or ILI using melphalan requires a center with adequate expertise in this technique. There are data to suggest that a combination of regional therapy followed at some point by checkpoint blockade immunotherapy has better regional and systemic disease control than regional chemotherapy alone.177, 178
Isolated Limb Perfusion
One of the first therapies for the treatment of in-transit disease was hyperthermic isolated limb perfusion. First described in 1958 by Creech and colleagues,179 ILP involves the dissection and cannulation of the major vessels in either the upper or lower extremity, followed by isolation of the extremity from systemic circulation using a tourniquet and the instillation of heated chemotherapy. The heated chemotherapy (most often melphalan) is then circulated through the extremity at 38° to 42° C at a rate of 300 to 500 L/min.180 A meta-analysis from 2010 evaluated the outcomes in 2,018 hyperthermic ILPs performed between 1990 and 2008. This analysis showed clinical response rates between 64% and 100% (median, 90%), with complete response (CR) rates ranging from 25% to 89% (median, 58%), with a limb salvage rate of 95%. Patients in this study had a median OS of 36.7 months, with a median 5-year survival of 36.5%.181 Additional European studies showed improved responses with the addition of TNF-alpha to melphalan, however a 2006 phase III trial (ACO-SOG Trial Z0020) showed no significant difference between melphalan alone and melphalan plus TNF-alpha in either overall response rate (ORR) or CR at 3 months, but did show a higher complication rate with the addition of TNF-alpha.182 Therefore, TNF-alpha is not routinely used in the United States. Although highly effective, hyperthermic ILP does carry significant toxicity as measured on the Wieberdink toxicity scale (toxicity ranges from grade I - no change in the affected limb, to grade V - severe tissue damage requiring amputation). Although the rates of severe toxicity (grade IV or higher) are relatively low (with limb loss reported in <3.3% of patients), the long-term morbidity including limb edema, muscle atrophy, stiffness, and functional impairment, is as high as 44%.183–186
Isolated Limb Infusion
Similar to hyperthermic ILP, ILI also involves isolation of the limb with a tourniquet followed by infusion of hot melphalan. However, unlike hyperthermic ILP, it does not involve dissection of the vessels, and does not involve perfusion of the limb. Instead, it is performed without an oxygenator and, as such, the extremity becomes acidotic and hypoxic during drug circulation. First described by Thompson and colleagues187 in 1998, ILI has been shown to have less toxicity than ILP, with studies showing 97% of patients having grade III toxicity or less, with comparable response rates.188 A multicenter analysis performed by Miura and colleagues189 in 2019 evaluated long-term outcomes for patients with stage IIIB or IIIC melanoma who underwent first-time ILI, across 9 different Australian and US tertiary centers, between 1992 and 2018. Out of the 687 first time ILIs performed during the study period, only 3.9% of patients developed grade IV toxicity and there were no grade V toxicities noted. Favorable response to ILI was associated with significantly longer progression-free survival (PFS) (53.6 vs. 12.7 months; p<0.0001) and OS of 46.5 vs. 24.4 months.189 Furthermore, because it does not involve dissection of the vessels, ILI can be repeated multiple times, unlike ILP. For patients with recurrence within 3 months of ILI, ILP is recommended as an escalation of care. For patients with recurrence more than 3 months after ILI, either repeat ILI or ILP are acceptable options.190
Intralesional Therapy
Although ILI and ILP are useful for extremity lesions, in-transit and locally advanced disease can also occur on the trunk, head, and neck, where isolation of the vasculature is not feasible. Intralesional therapy is useful for disease not located on an extremity and for patients who are ineligible for operative resection. There are multiple different intralesional therapies that have been used over the years to treat in-transit disease and this review is by no means inclusive. One of the first intralesional therapies was bacillus Calmette-Guerin (BCG). First described by Zbar and colleagues191 in 1971 as having an effect on tumor growth, this live, attenuated strain of M. bovis was one of the most commonly used treatments for in-transit disease. Early studies by Morton and colleagues192 showed that 90% of lesions injected with BCG had a CR, and reported regression in 17% of uninjected lesions, suggesting an abscopal response.193 Although early studies reported the efficacy of BCG injections in the treatment of melanoma,193–195 later studies did not show the same promise. The Eastern Cooperative Oncology Group (ECOG) phase III randomized trial (E1673) in 2004 looked at the effect of BCG on resected stage I, II, and III melanoma and found no significant difference in DFS or OS.196 In addition to questionable impact on OS, BCG injections carry a 12% complication rate and the use of BCG injections requires close monitoring and pre-injection prophylaxis with isoniazid. The toxicity of BCG ranges from induration at the injection site to severe sepsis and even death.197 For these reasons, BCG is now rarely used.
The history of intralesional therapy for melanoma also includes injection of IL-2. A glycoprotein affecting growth, proliferation, and activation of cytotoxic T lymphocytes, NK, and lymphokine-activated killer cells, IL-2 was originally approved for systemic therapy in metastatic renal cell carcinoma and melanoma.198, 199 However, systemic administration of IL-2 resulted in significant toxicities and the response rates only ranged from 10% to 15%.198, 200 Intralesional use of IL-2 was based on several phase II trials201, 202 and a subsequent systematic review,203 which showed CR rates in 62% to 70% of injected lesions, with a durable response of at least 6 months with minimal toxic effects. There was, however, no abscopal effect observed with intralesional IL-2.
PV-10 (Rose Bengal) is another intralesional therapy that has been used for the treatment of in-transit disease. A xanthene dye most commonly used for detection of ophthalmic injury, it was first used for melanoma in a phase I trial in 2008 by Thompson and colleagues204 In this small study of 11 patients, injection of melanoma lesions with PV-10 resulted in CR in 36% of injected lesions and 15% of non-injected lesions. A subsequent phase II study in patients with refractory metastatic melanoma showed a CR rate of 26% and a partial response rate of 25% after 4 injections, with 33% of patients experiencing an abscopal response in untreated lesions.205
The newest therapy for intralesional injections is talimogene laherparepvec (T-VEC), the only oncolytic viral therapy approved by the FDA for treatment of locoregionally advanced melanoma. Derived from a genetically modified herpes simplex virus (HSV-1), it has 2 putative mechanisms of action: selectively infecting tumor cells and promoting immune-mediated destruction.206 Intratumoral injection of T-VEC in a phase II trial comprised of patients with stage IIIC/IV melanoma showed an ORR of 26% in both injected and non-injected lesions, with a durable response of 7 to 31 months in 92% of patients who responded.207 The OPTiM trial, a phase III trial by Andtbacka and colleagues,208 compared intratumoral injections of T-VEC and granulocyte-macrophage colony-stimulating factor (GM-CSF). This trial showed a higher durable response rate in the T-VEC group compared to the GM-CSF group, with a longer median OS (23.3 months vs. 18.9 months, p=0.051).208
Adjuvant Therapy
Immunotherapy
Following resection, the current standard of care involves the use of adjuvant therapy in stage IIIA melanoma and higher. Similar to neoadjuvant therapy, adjuvant therapy involves the use of immunotherapy and targeted therapies. Ipilimumab, nivolumab, and pembrolizumab are all FDA approved for use in resected stage III melanoma, as is combination dabrafenib and trametinib for patients with BRAF-mutant disease. Ipilimumab, an anti-CTLA-4 monoclonal antibody, was the first to be approved, following the completion of the international phase III European Organisation for Research and Treatment of Cancer (EORTC) 18071 trial, which compared adjuvant high-dose ipilimumab to placebo in patients with completely resected stage III melanoma. In this study, they showed a significant difference in median RFS of 26.1 versus 17.1 months with ipilimumab versus placebo, as well as a difference in 3-year RFS (46.5% in ipilimumab group vs. 34.8% in placebo group).209 Further analysis showed significantly improved RFS and OS at 5 years in the ipilimumab group compared to placebo (40.8% vs. 30.3%, p<0.001 for RFS and 65.4% vs. 54.4%, p=0.0013 for OS).210
Nivolumab and pembrolizumab are anti-PD-1 antibodies also approved for use in the adjuvant setting in stage III and IV melanoma. Several studies have been performed confirming the efficacy of nivolumab in the adjuvant setting, including the CheckMate 066 trial, which evaluated the OS in patients treated with nivolumab or dacarbazine. At 1 year, there was a significant difference in OS between the nivolumab and dacarbazine groups (73% vs. 42%, p<0.001).211 However, nivolumab was not approved for use until after the CheckMate 238 trial, a randomized, double-blind, phase III trial with a primary endpoint of RFS. This study compared patients with high-risk resected stage IIIb, IIIC, or IV melanoma treated with either ipilimumab or nivolumab in the adjuvant setting and followed for up to 1 year or until disease recurrence. Their 12-month RFS was 70.5% versus 60.8% for nivolumab and ipilimumab, respectively, with lower rates of grade III or IV adverse events in the nivolumab group.148 Pembrolizumab is the most recent immunotherapy approved for adjuvant use in stage III melanoma. Approval was granted following the European Organisation for Research and Treatment of Cancer (EORTC) 1325/KEYNOTE-054 trial, a phase III randomized trial comparing pembrolizumab to placebo in patients with high-risk stage III melanoma. There was a significant difference in 1-year RFS between pembrolizumab and placebo (75.4% vs. 61%, HR 0.57, CI 95%, 0.43–0.74, p<0.001).145 Similar to nivolumab, pembrolizumab has also been found to be superior to ipilimumab with regard to response rates and toxicity.212 There have also been several studies looking at the combination of CTLA-4 and PD-1 inhibitors, demonstrating significant improvements in PFS and OS. The CheckMate 067 trial is what led to the approval of combination ipilimumab plus nivolumab for use in unresectable or metastatic disease. Patients treated with combination therapy had median PFS of 11.5 months, compared with 2.9 months with ipilimumab alone, and 6.9 months with nivolumab alone. Patients with PD-L1 positive tumors had median PFS of 14 months with nivolumab monotherapy; however, in those with PD-L1 negative tumors the response was superior with combination therapy (11.2 months vs. 5.3 months with combination vs. nivolumab monotherapy). Combination therapy resulted in a higher percentage of grade III or IV adverse events (55% in combination vs. 16.3% in nivolumab monotherapy or 27.3% in ipilimumab monotherapy).213 In summary, combination checkpoint blockade therapy improves survival but results in more adverse events.
Targeted Therapies
Targeted therapies have also been approved for adjuvant therapy in stage III melanoma. There are 3 combinations of BRAF and MEK inhibitors currently approved for the adjuvant setting: vemurafenib and cobimetinib, dabrafenib and trametinib, and encorafenib and binimetinib. The COMBI-AD trial is a double-blind, placebo-controlled phase III study in patients with resected BRAF-mutated stage III melanoma comparing RFS in those treated with dabrafenib plus trametinib versus placebo. Initial results showed a significant difference in RFS and 3-year OS between groups. The RFS was 58% versus 39% in combination versus placebo at a median follow-up of 2.8 years, with higher rates of DMFS and RFS in the combination therapy group.147 More recently, the investigators reported the RFS at a median follow-up of 44 months. The current 3- and 4-year RFS rates were 59% versus 38% in the dabrafenib plus trametinib and placebo groups, respectively. Subgroup analysis performed at that time also favored the combination of dabrafenib plus trametinib over placebo irrespective of baseline factors including stage, nodal disease burden, and tumor ulceration.146
Combination Immunotherapy and Intralesional Therapy
More recently, there have been studies looking at the combination of immunotherapy with intratumoral therapies, specifically T-VEC. It is believed that T-VEC can help expose tumor-associated antigens and thus prime the anti-tumor immune response.214 Several studies have been conducted looking at the combination of T-VEC with ipilimumab as well as pembrolizumab.
Chesney and colleagues215 evaluated the combination of T-VEC plus ipilimumab versus ipilimumab alone in a patient with unresectable stage IIIB-IV melanoma. They found an objective response in 38 of 98 (39%) patients treated with T-VEC plus ipilimumab versus 18 of 100 (18%) patients treated with ipilimumab alone, without any significance difference in adverse events.215 The MASTERKEY-265 trial, an open-label single-arm phase Ib trial, focused on the combination of T-VEC plus pembrolizumab in patients with unresectable stage IIIB-IV melanoma. At a median follow-up of 36.8 months, the ORR was 67% with a CR of 43% (9/21 patients).216, 217 The larger randomized phase III trial (KEYNOTE-034, NCT02263508) comparing T-VEC plus pembrolizumab versus pembrolizumab alone is currently underway.
Radiation Therapy
For patients who are high risk for recurrence, radiation therapy can be useful to help prevent local recurrence, but has not been shown to have an effect on overall long-term survival.218 The ANZMTG trial, a prospective multicenter phase III study evaluated the difference in locoregional recurrence in patients treated with regional nodal basin radiation versus observation following lymphadenectomy for a palpable lymph node field relapse. Patients were treated with adjuvant radiotherapy (48Gy in 20 fractions given over a maximum of 30 days) or observation. Radiation did significantly reduce the risk of locoregional recurrence. At a median follow-up of 73 months, there was a significantly lower rate of recurrence in the adjuvant group compared to the observation group (21% vs. 36%, p=0.023). However, OS and RFS did not differ.219 Thus, current recommendations are for the use of radiation therapy in patients considered high risk for locoregional recurrence and for patients after failure of previous regional LNDs as salvage therapy. Radiation is also useful as salvage therapy for those patients who are not good surgical candidates. In this situation, radiation therapy is aimed at symptom relief and management.
Conclusions
The heterogeneity of stage III melanoma lends itself to multiple different avenues of therapy. The mainstay remains surgical resection, however newer systemic therapies including immunotherapy and targeted therapies provide benefit in both the neoadjuvant and adjuvant settings. As such, all patients with stage III disease would benefit from presentation at a multidisciplinary tumor board to make sure the optimal treatment strategy is developed based on the patient’s individual situation and the treatment portfolio of the treating institution.
METASTATIC MELANOMA
The last decade has seen a revolution in the treatment of metastatic melanoma. After a quarter century of stagnation and limited therapeutic options that conferred minimal survival benefit, a new understanding of the molecular behavior of melanoma has led to multiple breakthroughs. The development of immunotherapy and targeted therapy has resulted in an improved outlook for patients with stage IV melanoma. Surgery continues to provide a benefit for patients with resectable metastases, and the addition of the new systemic agents in the neoadjuvant or adjuvant setting has shown initial promise. New therapeutic modalities under investigation are exploring ways to harness the power of the immune system through vaccination, novel cytokines, and the administration of autologous tumor-specific immune cells. Multiple ongoing trials seek to build on the promise of the last decade.
Presentation and Prognosis
Cutaneous melanoma is considered to have metastasized and become systemic when it has spread beyond regional lymph node basins. The presence of distant metastases is classified as stage IV disease.220 The most recent edition of the AJCC staging guidelines for melanoma breaks down metastatic disease into substages based on location of distant metastasis. M1a disease includes metastasis to distant skin, subcutaneous tissue, and non-regional lymph nodes. M1b disease represents lung metastases. M1c disease includes visceral metastases outside of the CNS. Finally, M1d disease represents metastatic disease in the CNS.64 The LDH level has proven to be a significant historical prognostic factor in patients with metastatic disease and is again included in the staging system.221 The rates of 1- and 2-year OS in patients with normal LDH levels are twice that of patients with elevated LDH (65% and 40%, vs 32% and 18%, respectively).95 The prognostic effect of LDH in patients with stage IV melanoma has persisted even after treatment with modern systemic therapy.222, 223 In the most recent AJCC staging, M-stage disease has been further amended within each substage to specify patients with baseline (0) or elevated (1) LDH.64 See Table 6 for a summary of the AJCC 8th edition M-staging criteria.
Of all patients who are diagnosed with cutaneous melanoma, roughly 4% have metastatic disease at the time of presentation.224 Of those who present with local disease, 11% will ultimately develop distant metastases.225 The median time to development of distant metastases is just over 2 years.225, 226 The only variable that has an influence on survival in those who develop distant metastases is the stage of the primary tumor, suggesting that prognosis and the behavior of the disease is driven by the underlying biology of the primary, even after complete resection.227 For patients who ultimately go on to develop metastases after presenting with stage I disease, median OS after discovery of metastasis is 29.5 months. The outlook is markedly worse for patients who initially presented with stage II or stage III disease, as their median OS after diagnosis of distant metastases is 15 months.225 Unlike in other malignancies, the time interval to distant recurrence does not affect survival.226
For patients who developed metastatic melanoma in the latter half of the 20th century, the outlook was grim. Of those who qualified for clinical trials, only 12% had a response, and the median OS was 6 to 7 months.228, 229 A long-term survey of patients with metastatic melanoma showed that historic site-specific median OS for metastases to the skin, distant lymph nodes, and the gastrointestinal (GI) tract was 12.5 months, with 14% of patients surviving 5 years. This was in agreement with other analyses of national-level data, which showed 5-year OS of 16% for stage IV disease.230 The prognosis was worse for those who presented with pulmonary disease, with a median OS of 8.3 months and 5-year OS of just 4%. Patients with liver, brain, or bone metastases fared even worse, with a median OS of 4.4 months and 5-year OS rate of 3%. These figures did not change significantly over the 22-year period of study from 1971 to 1993.228 The most recent sub-stage-specific analysis performed by the AJCC, for the 7th edition of their staging manual, showed 1-year OS of 62% for patients with M1a disease. Patients with M1b and M1c disease (defined per AJCC 7th edition staging as non-pulmonary visceral metastases with normal serum LDH or distant metastases at any site with an elevated serum LDH) had 1-year survival of 53% and 33%, respectively.95
The revolution in systemic therapy for melanoma that occurred in the second decade of the 21st century has rearranged the map for patients with stage IV disease. Although data from individual studies is incredibly promising and it is clear that the development of immunotherapy and targeted therapy improve DFS and OS in patients with metastatic melanoma, the long-term survival data are not yet mature, making it difficult to calculate meaningful stage-specific prognostic information and to chart a course for patients diagnosed with stage IV disease. A broad analysis of national-level survival data recently found that between 2013 and 2016 mortality due to melanoma decreased by 17.9%, due almost entirely to the impact of new systemic therapies that have become available since 2011.231 See Figure 7 for the striking change in mortality rate that occurred shortly after the introduction of immunotherapy. In the context of an incidence rate that has been rising steadily over the last several decades,232 such a sharp decline in mortality represents one of the great breakthroughs in the history of oncology. So profoundly has the paradigm shifted that for the most recent edition of the AJCC staging manual for melanoma, the AJCC opted not to update their analysis for patients with metastatic disease, given that the results would not be reflective of the therapeutic options currently available and the stage-specific survival data would not reflect the impact of these newer agents.64 The stage-specific survival figures from the AJCC 7th edition95 were retained, with the caveat that the continued adoption and proliferation of modern systemic therapy will markedly improve the prognosis for patients with stage IV disease.
Figure 7.
Trends in mortality rate for cutaneous melanoma. (With permission from Berk-Krauss J, Stein JA, Weber J, Polsky D, Geller AC. New Systematic Therapies and Trends in Cutaneous Melanoma Deaths Among US Whites, 1986–2016. Am J Public Health. 2020;110(5):731–733).
Evaluation
Patients who present with concern for metastatic disease should be thoroughly evaluated in concordance with the most recent guidelines.94 Given its prognostic importance for stage IV disease, a serum LDH should be drawn. A biopsy should be obtained for confirmation of the diagnosis. An excisional biopsy is preferred. For a distant skin recurrence, punch biopsy is acceptable if an excisional biopsy would be anatomically prohibitive.58 If molecular testing for BRAF V600 mutant status has not already been performed on the primary tumor, this should be assessed to determine the patient’s eligibility for targeted therapy. Immunohistochemistry is a readily available method that can be utilized with a high degree of sensitivity and specificity.233 Additional genetic testing for BRAF mutations other than V600E should be done with PCR-based methods (eg, for V600K, which also responds to targeted therapy) and for KIT mutations for acral and mucosal melanomas, given the availability of targeted therapy for these otherwise less immunotherapy-responsive melanoma subtypes. Imaging should be obtained to assess for the full extent of distant metastatic disease. No specific imaging modality is recommended, but a meta-analysis has shown that PET-CT is more sensitive and specific than CT alone in the evaluation of metastatic melanoma.234 Imaging should be driven by most likely sites of metastasis and evaluation of symptoms. PET-CT covering skull vertex to mid-thigh is sufficient except in the case of melanomas arising in the distal lower extremity.
Treatment
Upon completion of a thorough, guideline-directed evaluation, the patient with metastatic melanoma may proceed down one of several treatment pathways depending on the burden and location of the metastases.
Patients with disease that is not amenable to surgical resection should be initiated on systemic therapy. It is developments within this modality that has revolutionized metastatic melanoma over the last decade. Prior to 2011, the primary drug was the traditional chemotherapy agent dacarbazine. It was initially approved in 1975 and persisted not because of its effectiveness (in fact, it confers no long-term response or survival benefit235) but rather because of the lack of success finding a better alternative.
Immunotherapy
The first wave of immunotherapy began with approval of the cytokine interferon in 1996. The use of this agent has been associated with a marginal RFS benefit in several trials in the adjuvant setting but has limited evidence of improving OS. The high rate of serious side effects has also been a limiting factor in its widespread adoption.236 IL-2 is a cytokine that was approved by the FDA in 1998. Although there was no difference in ORR compared to cytotoxic chemotherapy, high-dose IL-2 did result in a single-digit percentage of durable responses with improvement in long-term DFS.237, 238 Although associated with a high rate of toxicity events, the small possibility of durable CR made this risk more palatable.
A new era of systemic therapy for metastatic melanoma began in early 2011 when the CTLA-4 inhibitor ipilimumab was approved. CTLA-4 is a transmembrane protein that downregulates T-cell activity after binding its ligand B7 and inhibits the immune system’s response to foreign epitopes, such as those associated with cancer cells. When bound by a checkpoint inhibitor like ipilimumab, the inhibitory activity generated by CTLA-4-mediated signaling is lost, thus activating T-cells and allowing them to proliferate and wage a prolonged response against the encroaching malignant cells.239 Ipilimumab improves survival in metastatic melanoma compared to dacarbazine,240 with a meta-analysis showing 3-year OS of 22%.241 A phase III trial comparing ipilimumab monotherapy to both the gp100 tumor-associated antigen vaccine and ipilimumab vaccine combination therapy demonstrated that, in patients with unresectable stage III or IV melanoma that had failed previous therapy, ipilimumab monotherapy resulted in superior 1-year and 2-year OS (45.6% and 23.5%, respectively), with a median OS of 10 months.242 The response rate was low (11%), but 60% of the patients who responded maintained their response for at least 2 years. The overall toxicity rate was high (60%), but only 15% of the adverse events were grade III or higher. A unique feature of ipilimumab’s pharmacodynamic profile is a relatively delayed tumor response, sometimes taking more than 3 to 4 months to show its effects. If the patient undergoes an imaging study within that interval, the mass may actually look to have initially progressed due to an influx of inflammatory cells that have been recruited to the area (termed “pseudoprogression”), before later shrinking.243 This prolonged development of a response makes ipilimumab a suboptimal choice for patients who present with symptomatic metastases (eg, biliary or bowel obstruction).
Programmed cell death protein 1 (PD-1), a T-cell surface protein, also downregulates the immune system when it binds the ligand PDL-1, a protein that is found on many normal cells as well as expressed by multiple human cancers as a mechanism to evade immune defenses.244 The mechanism of action of pembrolizumab, similar to other checkpoint inhibitors, involves binding PD-1, thereby allowing the immune response to continue and proliferate against cancer cells. Pembrolizumab was approved by the FDA in 2014 for patients who had failed ipilimumab and BRAF mutants who had progressive disease on a BRAF inhibitor. The KEYNOTE-006 trial compared pembrolizumab with ipilimumab in patients with unresectable stage III-IV melanoma. Pembrolizumab was superior across all categories, with a response rate of 33.6% (compared to 11.9% for ipilimumab), PFS at 6 months of 47.3% (compared to 26.5%), 12-month OS of 74.1% (compared to 58.2%), and a rate of grade III or higher adverse events of 13.3% (compared to 19.9%).212
Nivolumab, another checkpoint inhibitor that acts on PD-1, was approved shortly after pembrolizumab. In a phase III trial comparing nivolumab and dacarbazine in treatment-naïve patients with metastatic BRAF wild-type melanoma, nivolumab demonstrated an improved response rate compared to dacarbazine (40% vs 13.9%), superior DFS of 5.1 months (vs 2.2 months), superior 1-year OS of 72.9% (vs 42.1%), and a decreased rate of grade III or higher adverse events at 11.7% (vs 17.6%).211
Combination therapy with ipilimumab and nivolumab has been shown to provoke objective responses in more than 50% of patients245 and to improve PFS (median 11.5 months) compared to nivolumab (6.9 months) or ipilimumab (2.9 months) monotherapy.213 This, however, is accompanied by a greatly increased incidence of grade III or IV adverse events, at 55.0% (vs 16.3% and 27.3%, respectively). Five-year follow-up results of the CheckMate 067 trial show ipilimumab/nivolumab combination therapy continuing to outperform either of the drugs alone in unresectable stage III or stage IV disease. OS was 52% in the combination therapy group, 44% for the nivolumab group, and 26% for the ipilimumab group. Patients with BRAF mutations who received combination therapy had a 5-year OS rate of 60%, compared to 48% for patients with BRAF wild-type disease. There were fewer substantial discrepancies between BRAF mutant and BRAF wild-type patients in the nivolumab and ipilimumab monotherapy groups. Median OS has not been reached in the combination therapy group at 5 years follow-up, whereas it was 36.9 months and 19.9 months for the nivolumab and ipilimumab groups, respectively. Combination therapy was associated with 5-year PFS of 36%, compared to 29% for nivolumab and 8% for ipilimumab. Median PFS was superior in the combination therapy group as well, at 11.5 months, compared to 6.9 months for nivolumab and just 2.9 months for ipilimumab. The rate of grade III or IV adverse events was considerably higher than in either of the monotherapy groups, at 59% for nivolumab with ipilimumab, 28% for ipilimumab, and 22% for nivolumab.246
Targeted Therapy
In cutaneous melanoma, as well as many other human cancers, the mitogen-activated protein kinase pathway is a key contributor to the loss of cell-cycle control and the development of malignancy.247 Refer to Figure 8 for a schematic drawing of this pathway, with the points of action for targeted therapy agents indicated.248 A gene that produces a protein component of this pathway—the BRAF gene—is the most commonly mutated gene in melanoma, with reported frequency of mutation between 42% and 66%.37, 249–251 Of the BRAF mutations, the overwhelming majority are of the V600E type. There is also a not insignificant frequency of V600K mutation. Patients with either mutation are candidates for treatment with BRAF-inhibitor therapy. BRAF-inhibitors exert their effect by inhibiting the mutant B-raf protein, interrupting the transmission of signals down the MAPK pathway, thereby halting the replication of malignant cells. The first drug in this category became available in 2011, with the approval of vemurafenib. In the phase III, randomized BRIM-3 trial, which compared vemurafenib and dacarbazine in treatment-naïve patients with BRAF-mutant unresectable stage III and stage IV melanoma, vemurafenib was associated with a 73% relative reduction in the risk of disease progression or death compared to dacarbazine. The response rate was nearly 50% in those patients with V600E mutations, compared to just 5% in the dacarbazine group. Six-month OS was 84% in the vemurafenib group, compared to 64% in the dacarbazine group.252 Extended follow-up of this study demonstrated a median OS of 13.6 months in the vemurafenib group, compared to 9.7 months in the dacarbazine group. However, the survival benefit diminished over time, as 18-month OS was 39% in the vemurafenib group and 34% in the dacarbazine group. Significant rates of secondary skin malignancies were noted in the vemurafenib group, with 19% of patients developing squamous cell carcinomas and 10% developing keratoacanthomas. This effect, interestingly, is abrogated with the addition of MEK inhibitors, as detailed later in this section.253
Figure 8.
The MAP kinase pathway. Points of action for current targeted therapy agents are indicated. (With permission from Kainthla R, Kim KB, Falchook GS. Dabrafenib for treatment of BRAF-mutant melanoma. Pharmgenomics Pers Med. 2014;7:21–29).
The development of dabrafenib, another BRAF-inhibitor, closely followed vemurafenib, and this drug was approved by the FDA in 2013. Dabrafenib and vemurafenib induce similar response rates, however there are different rates of adverse events that accompany each drug. Dabrafenib is associated with an increased rate of pyrexia, but a much lower incidence of secondary cutaneous skin cancers compared to vemurafenib.254 A randomized phase III trial (BREAK-3) comparing dabrafenib and dacarbazine showed a greatly increased response rate with dabrafenib (50%) compared to dacarbazine (6%), as well as increased PFS with dabrafenib (median PFS, 6.7 months) compared to dacarbazine (2.9 months). Only 6% of patients in the dabrafenib arm developed squamous cell carcinomas or keratoacanthomas, which represents a substantial decrease compared to the incidence of these lesions in vemurafenib trials.255 Using a standardized health-related quality of life measurement tool, patients in the dabrafenib arm of the trial reported improved quality of life metrics over the course of the treatment period.256 However, the dramatic effect of dabrafenib appears to be only temporary. Long-term follow-up of patients on dabrafenib trials shows 5-year OS rates just above 20%, essentially equivalent to dacarbazine.257 The pattern of successful initial response, followed by eventual relapse and return to the mean suggests the prospect of acquired resistance. Indeed, a later review showed that approximately one half of patients developed resistance to BRAF-inhibitors after less than a year of therapy.258
Acting on the premise that the MAP kinase pathway was circumventing the BRAF blockade and re-engaging downstream to reactivate the pathway via alternative signaling pathways, the MEK-inhibitor trametinib was introduced in an attempt to combat this phenomenon. Trametinib monotherapy offered superior response and survival benefit compared to historical monotherapy protocols. For instance, in comparison with dacarbazine or paclitaxel, the response rate was 22% with trametinib (vs. 8%). Median PFS was 4.8 months in the trametinib group (vs. 1.5 months), and 6-month OS was 81% (vs. 67%).259 On the basis of this finding and other data, trametinib was approved in early 2013. Unfortunately, despite acting further down the MAPK pathway, trametinib had a lower response rate and worse PFS compared to BRAF-inhibitors.259 However, the true value of trametinib is due to its synergistic relationship with dabrafenib. A phase II trial of dabrafenib/trametinib combination therapy demonstrated increased response rate with combination therapy versus monotherapy (76% vs. 54%). Median PFS was also significantly improved compared to monotherapy (9.4 months vs. 5.8 months). Adverse event profiles were also notable, with just 7% of patients on combination therapy compared to 19% of patients on monotherapy developing additional malignant cutaneous lesions. A quarter of patients on monotherapy developed pyrexia, compared to 71% of patients on combination therapy. However, only 5% of patients developed grade III pyrexia.260 Multiple phase III trials have demonstrated superior objective response rates compared to monotherapy (64–68% vs. 45–51%).211, 259–264 A review of long-term outcomes shows that combination therapy provides improved OS at 5 years (34%) compared to monotherapy. For those patients who experienced a CR, this was associated with a dramatic increase in 5-year OS, to 71%.259, 262, 263, 265, 266 Combination therapy was associated with lower rates of secondary cutaneous malignancies than monotherapy.261, 262, 264 The combination of dabrafenib and trametinib for metastatic melanoma with BRAF V600 mutation was approved by the FDA in January, 2014. Hope remains that among the population of patients who present with low metastatic disease burden and normal LDH, some patients may be in effect cured by combination targeted therapy, as 55% of patients in this group are alive at 5 years.265 Recently published long-term data on a cohort of patients with BRAF-mutant stage III disease status after resection and 12 months of dabrafenib trametinib further reinforces the near-curative effect of combination therapy in some patients, as 52% of the cohort were disease-free at 5 years and 65% had not had distant recurrence.267
An additional option for metastatic disease that is unresectable but anatomically accessible is intralesional therapy. Most prominent is talimogene laherparepvec (T-VEC), a modified herpes virus which, when injected into a melanoma lesion, generates a local response by increasing the population of dendritic cells, resulting in increased presentation of tumor antigens to T-cells. Distant metastases that are not injected have also been noted to respond to T-VEC therapy in more than 30% of cases, indicating a systemic effect of this local intervention.268
Symptomatic but unresectable metastatic melanoma may be treated with surgical debulking or with radiation therapy. Larger doses per fraction have been associated with increased likelihood of durable response.269
Brain Metastasis
Patients with melanoma that has metastasized to the CNS are categorized as having M1d disease. These patients have historically been excluded from many clinical trials, leaving physicians with limited evidence upon which to base their decisions.270 An analysis of patients excluded from phase III clinical trials reports outcomes not dissimilar from other patients with metastatic disease. For patients with normal LDH levels, ECOG status 0–1, and asymptomatic brain metastases, median OS was 16.4 months, with 35.1% of patients surviving 3 years. For patients with the same characteristics, but LDH 250–500 U/L, median OS drops to 9.9 months and 3-year OS to 21.0%. The prognosis becomes incrementally worse with declining ECOG status, rising LDH, and the presence of symptoms related to the metastases. Patients with ECOG scores of 2 or greater, with LDH greater than 500 U/L, and symptomatic brain metastases only have a median OS of 3.4 months, with just 0.3% of patients alive at 3 years. BRAF mutation status was not associated with survival in this cohort. The conclusion of this study was that the presence of brain metastases did not preclude long-term survival in select patients with aggressive treatment and that patients with brain metastasis could benefit from inclusion in phase III trials.271
The treatment of all patients with brain metastases should begin with discussion at a multidisciplinary conference involving representatives from surgical oncology, medical oncology, radiation oncology, and neurosurgery. Although all disciplines may not be involved in the patient’s care at the outset, their input is often needed eventually upon progression of disease.
If patients present with asymptomatic disease or symptomatic brain metastases in the setting of considerable extracranial disease burden, it is recommended they initiate systemic therapy. Combination therapy with ipilimumab and nivolumab has been shown to be safe and to generate reasonable response rates272, 273 that are superior to ipilimumab and nivolumab monotherapy.274 Pembrolizumab has also been shown to generate a response, however less than ipilimumab/nivolumab combination therapy.275 Patients with a BRAF mutation who are not candidates for immunotherapy may benefit from combination BRAF/MEK inhibition. A phase II trial showed response rates of 44% to 58% in patients with asymptomatic brain lesions, although these responses were short-lived, as the median PFS interval was just under 6 months.276
In the scenario of a patient with known brain metastases being treated with systemic therapy, the patient should undergo serial brain magnetic resonance imaging (MRI) every 6 to 8 weeks, per NCCN guidelines, to monitor for progression of disease. If the disease progresses on systemic therapy, alternate regimens or modalities of therapy should be considered.
Should the patient present with symptomatic brain metastases, administration of steroids is a prudent temporizing measure to decrease inflammation and minimize symptoms. If the metastasis is large, singular, and resectable, neurosurgical intervention is recommended.
Systemic therapy or radiation may be considered in the adjuvant setting if the patient is free of residual disease. Stereotactic radiosurgery is the modality of choice, as it improves local control, although it does not improve OS compared to observation.277 Although whole-brain radiation (WBRT) provides a slight advantage in local control, there is no OS benefit, and WBRT is associated with significant negative effects on cognitive function.278
Resectable Disease
For patients who present with metastases amenable to complete surgical excision, resection is recommended. Despite the recent development of significantly more effective systemic therapy, surgical resection still offers a DSS benefit compared to systemic therapy alone.175 Those patients who have small volume disease with the potential for complete resection, and who are stable on systemic therapy, should be taken to the operating room.279 Even in patients who develop recurrent disease after metastasectomy, serial resection confers a survival benefit.280
Metastasis to distant skin, subcutaneous tissue, muscle, or lymph nodes (stage M1a disease) represents 20% of metastases.281 Aggressive resection with 2 cm margins is indicated for what is often symptomatic disease, and in cohorts that undergo complete resection, median OS can reach 60 months.282
The lung is the most common site of distant metastasis in melanoma. Pulmonary metastases are usually asymptomatic and diagnosed via routine imaging during the follow-up period. Patients from the MSLT-I trial who developed pulmonary metastases, followed by metastasectomy, experienced a clinically significant, although not statistically significant, improvement in survival (median OS, 17.9 months) compared to patients treated with systemic therapy alone (9.1 months).283 Stereotactic body radiation therapy (SBRT) is also an option for nonsurgical candidates to treat a limited number of small pulmonary metastases in the appropriate clinical setting.284
Patients with non-pulmonary visceral metastasis (stage M1c disease) also benefit from surgical resection. Again reviewing MSLT-I patients, those with resected visceral metastases had a median OS of 15.0 months, compared to 6.3 months in those who received only systemic therapy.283 Forty percent of patients with M1c disease will have liver metastases.285 A systematic review reported median interval of 54 months to the development of liver metastases.286 Two single institution reviews demonstrated OS beyond 2 years after metastasectomy, far superior to 8 months in patients who received systemic therapy alone.287, 288 Radiofrequency ablation of hepatic lesions is an alternative for patients who are not surgical candidates.289 Of those with stage M1c disease, 20% will have metastases to the GI tract, primarily the small bowel.285 Metastasis to other abdominal organs, including the adrenals,290 spleen,291 and pancreas292, is less common, but all benefit from surgical resection compared to nonoperative management.285
Patients who undergo complete resection may initiate adjuvant therapy. For patients who are BRAF wild-type, data from a phase III RCTs suggest that nivolumab is associated with improved DFS at 1 year (63.0%) compared to ipilimumab (57.5%), with substantially fewer adverse events necessitating the cessation of therapy (9.5% vs 42.6%).148 Given pembrolizumab’s superiority to ipilimumab,212 pembrolizumab may also be used in the adjuvant setting. However, if the patient has previously failed PD-1 inhibitors, then ipilimumab may be used. More than 40% of patients who develop metastatic disease harbor BRAF V600 mutations,293 and these patients may be treated with combination BRAF/MEK inhibitors for 1 year or, as above, with immunotherapy with a PD-1 checkpoint inhibitor for 1 year, depending on comorbidities (eg, preexisting autoimmune disease may preclude the use of immunotherapy) and clinician discretion in the absence of a head-to-head comparison of these agents.
Patients with residual disease after metastasectomy have, by definition, disseminated disease not amenable to resection and should be treated with systemic therapy per NCCN guidelines. Details of systemic therapy regimens are discussed earlier in this section.
Neoadjuvant Therapy for Metastatic Melanoma
In parallel with the evolution of neoadjuvant therapy for other malignancies, the administration of neoadjuvant systemic therapy prior to resection of melanoma metastases has come to the fore in recent years. One phase II study (NeoCombi) investigating neoadjuvant dual BRAF/MEK inhibition in resectable stage IIIB and IIIC melanoma may have relevance to stage IV patients. The authors reported 46% of patients with a CR, 40% with a partial response, and no patients with progression of disease. Of these patients, nearly 50% had a cPR.169 A randomized phase II trial comparing neoadjuvant dabrafenib trametinib combination therapy followed by surgery, with surgery subsequently followed by adjuvant targeted therapy in patients with resectable metastatic melanoma, was halted early due to a statistically significant survival benefit conferred by neoadjuvant therapy. At the point of cessation, PFS was 19.7 months in the neoadjuvant group compared to 2.9 months in the surgery-first group,170 making a convincing argument for neoadjuvant systemic therapy prior to resection of oligometastatic disease.
Metastasis from Unknown Primary
A curious problem in cutaneous melanoma is the discovery of distant metastatic disease in the absence of an identifiable primary lesion. Of all patients with cutaneous melanoma, 3.2% are diagnosed with a metastasis with unknown primary (MUP).294 Although often disconcerting to the patient in not knowing the initial origin of the tumor, patients with nodal disease from an unknown primary who undergo therapeutic LND have a prognosis that is superior to that of patients with stage III disease. Multiple studies report 5-year OS of 55% to 58% for MUP compared to 40% to 42% for stage III disease with a known primary.295, 296 Superior survival is also observed for patients with visceral metastases from an unknown primary compared to those patients with a known primary that has metastasized to visceral organs.297 As a result of the positive prognostic effect conferred by MUP, an aggressive surgical and systemic approach with curative intent is indicated.
There are multiple hypotheses as to the development of MUP and the mechanism responsible for its relatively good prognosis. Perhaps the most agreed upon is that a previously extant primary lesion was eradicated by the patient’s immune system.296–298 A robust immune response would also suggest better control of distant metastasis, contributing to relatively improved survival even when distant disease was present. Another hypothesis is that melanoma arising de novo in the axillary nodal tissue does not necessarily represent loss of local control of a cutaneous primary, but rather the presence of ectopic nevus cells in a nodal basin that develop into a primary malignancy.297
Patients who present with a metastatic lesion and no known primary should have their history taken followed by a complete examination, including commonly overlooked locations such as the eyes, oral cavity, nasopharynx, anus, rectum, and interdigital webbing. A whole-body PET-CT is also indicated to screen for additional foci of metastatic disease. Finally, although still investigational, commercially available tumor genome sequencing can provide clues as to the origin of the tumor if a characteristic mutation is present (eg, BRAF V600E mutation would essentially exclude a uveal primary, whereas a GNAQ/11 mutation would strongly support a uveal primary).
Future Directions and Ongoing Clinical Trials
After a decade of discovery that has revolutionized the landscape of melanoma, there is a great deal of optimism and excitement for what the next decade holds. Although it is unlikely that quantum leaps of equivalent magnitude to the development of immunotherapy and targeted therapy will occur again, several areas offer promise for continued improvements in the care of patients with cutaneous melanoma.
Given the success of immunotherapy for melanoma, it is logical to use an immune checkpoint backbone with additional agents in attempts to engender an increased response. Current trials pursuing this line of inquiry are summarized in Table 13.
Table 13.
Clinical trials investigating combination therapy with a backbone of BRAF/MEK inhibition.
| Clinicaltrials.gov identifier | Study name | Phase / Status | Description of trial |
|---|---|---|---|
| NCT03595683 367 | Pembrolizumab and EDP1503 in Advanced Melanoma | 2 – Active, not recruiting | Investigation of effect of biologic agent (EDP1503) on response to pembrolizumab in patients with advanced melanoma. |
| NCT04493203 368 | Nivolumab Plus Axitinib in Patients with Anti-PD1 Refractory Advanced Melanoma | 2 – Not yet recruiting | Combination PD1-inhibition and tyrosine kinase inhibitor therapy in patients with unresectable stage III or IV melanoma that have failed on previous PD1-inhibition and/or CTLA4-inhibitor therapy. |
| NCT02965716 369 | Talimogene Laherparepvec and Pembrolizumab in Treating Patients with Stage III-IV Melanoma | 2 – Recruiting | Investigation of synergistic effect of intralesional anti-tumor vaccine and PD1-inhibitor in patients with stage III-IV melanoma. |
| NCT03897881 309 | Personalized Cancer Vaccine mRNA-4157 and Pembrolizumab Versus Pembrolizumab Alone After Complete Resection of High-Risk Melanoma (KEYNOTE-942) | 2 – Recruiting | Phase 2 study comparing adjuvant immunotherapy with pembrolizumab / personalized vaccine and pembrolizumab alone after resection of high-risk melanoma. |
A similar path is being followed with targeted therapy. Combination BRAF and MEK inhibition led to significant survival benefit in patients with advanced melanoma, and now ongoing trials are investigating the addition of PD-1 inhibitors and other agents. See Table 14 for ongoing trials of combination therapy with a BRAF/MEK inhibitor backbone.
Table 14.
Clinical trials investigating combination therapy with a backbone of immunotherapy.
| Clinicaltrials.gov | Study name | Phase / Status | Description of trial |
|---|---|---|---|
| NCT02967692 370 | A Study of the Anti-PD1 Antibody PDR001, in Combination with Dabrafenib and Trametinib in Advanced Melanoma [COMBI-I trial] | 3 – Active, not recruiting | Randomized, double-blinded trial comparing combination BRAF/MEK inhibitor therapy with BRAF/MEK inhibition and the addition of PD1-inhibition in patients with advanced melanoma. |
| NCT04527549 371 | Testing the Combination of Dabrafenib and Trametinib With or Without Hydroxychloroquine in Patients with Stage IIIC or IV BRAF V600E/K Melanoma | 2 – Not yet recruiting | Determining if hydroxychloroquine-induced autophagy improves survival when that agent is added to BRAF/MEK inhibitor therapy for patients with BRAF-mutant stage IIIC or IV melanoma. |
Neoadjuvant therapy, as discussed previously, is a topic under intense investigation for patients with nodal or systemic disease that may be amenable to resection.299 There are multiple ongoing trials in this realm, and a group has coalesced around the subject—the International Neoadjuvant Melanoma Consortium (INMC). This group has published guidelines for trial design in an attempt to streamline the many investigative efforts underway.96 Several representative clinical trials investigating neoadjuvant therapy are summarized in Table 15.
Table 15.
Neoadjuvant clinical trials.
| Clinicaltrials.gov identifier | Study name | Phase / Status | Description of trial |
|---|---|---|---|
| NCT02036086 372 | Neoadjuvant Vemurafenib Plus Cobimetinib for BRAF Mutant Melanoma with Palpable Lymph Node Metastases | 2 – Active, not recruiting | Neoadjuvant vemurafenib/cobimetinib in patients with BRAF V600 mutant stage IIIB-C melanoma |
| NCT01972347 373 | Neoadjuvant Dabrafenib/Trametinib for Stage IIIB-C BRAF V600 Mutation Positive Melanoma | 2 – Active, not recruiting | Phase 2 study of neoadjuvant dabrafenib/trametinib in stage IIIB-C BRAF V600 mutant melanoma. |
| NCT04495010 374 | Neoadjuvant Nivolumab plus Ipilimumab Followed by Adjuvant Nivolumab or Neoadjuvant Nivolumab plus Ipilimumab Followed by Adjuvant Observation Compared with Adjuvant Nivolumab in Treatment-Naïve High-risk Melanoma (CheckMate 7UA) | 2 – Not yet recruiting | Neoadjuvant nivolumab/ipilimumab followed by adjuvant nivolumab versus neoadjuvant nivolumab/ipilimumab followed by observation versus adjuvant nivolumab in treatment-naïve high-risk melanoma |
The concept of using autologous tumor-specific immune cells to attack malignant cells has been around for several decades, but there has been increased interest in TILs in recent years. The immunogenic nature of melanoma makes it an ideal candidate for the generation of immune cells that recognize melanoma-specific antigens, collection, ex vivo proliferation, and subsequent reintroduction into the patient. 300 Although the collection and proliferation process are complex with imperfect yield, and few centers have TIL programs, those patients who successfully receive this therapy have high rates of response (approaching 50% in several studies301, 302) and these responses are often durable. A current line of investigation involves TIL administration after a course of modern immunotherapy.303, 304
Cytokines such as IL-2 were some of the first agents that ushered in the era of immunotherapy for melanoma. Efforts continue to develop novel cytokine equivalents with anti-tumor activity. IL-15 acts by upregulating the activity of natural killer cells, which act directly against tumor cells and also amplify other cytokine responses.305 IL-21 plays a key role in potentiating the proliferation of memory T-cell precursors.306 The implications of this activity in developing memory T-cells for adoptive cellular therapy, such as TIL therapy mentioned previously in this section, may be significant.
Other attempts to direct the power of the immune system against melanoma include vaccination with custom peptides (NEO-PV-01 trial)307 or mRNAs (KEYNOTE-942 trial)308, 309, representing predicted immunogenic neoantigens from a given specific patient’s tumor exome. Another approach involves using dendritic cells harvested from the patient, matured and proliferated ex vivo, then reinfused to the patient. Studies have shown dendritic cell vaccines to induce a response, improve survival, and to do so with acceptable toxicity rates.310 The administration of dendritic cell vaccines in concert with PD1-inhibitors has increased the response to those agents in mice,311 offering the prospect of synergistic combinations of vaccines with modern systemic therapy regimens.
Conclusions
Advances in systemic therapy have defined the last decade in melanoma and have irrevocably changed the horizon for both patients with metastatic disease and the researchers and clinicians who seek to help them. Improved prospects for long-term survival created by developments in immunotherapy and targeted therapy encourage more ambition in the pursuit of new therapies and justify optimism that the development and implementation of novel modalities can again revolutionize the field.
STAGE-SPECIFIC FOLLOW-UP FOR CUTANEOUS MELANOMA
Once a patient with a primary cutaneous melanoma undergoes successful resection and there is no evidence of residual disease, they enter into a period of surveillance. After the usual initial postoperative visits to monitor healing of the surgical wound, the patient returns to clinic regularly over the next several years in order to monitor for the development of recurrent disease, or the presentation of a second primary skin cancer. For patients with a previous melanoma, the risk of developing a second skin cancer is 2 to 3 times that of the general population. As each patient’s risk of recurrence is related to the stage of their primary disease, guidelines for frequency and duration of follow-up visits differ by stage. Beyond a thorough history and physical examination, the components of a follow-up clinic visit also may differ depending on the stage of the patient’s primary tumor, as some patients will undergo serial imaging for a period of time after resection. A prolonged period of surveillance also allows for the provision of emotional support to patients as they navigate various survivorship issues. This long-term relationship facilitates an ongoing dialogue between the patient and their oncologists wherein questions and concerns can be addressed, and positive behaviors reinforced.
Rationale for Follow-up After Treatment of Primary Cutaneous Melanoma
There are multiple objectives in the follow-up period after a patient has completed initial therapy for a primary cutaneous melanoma. Of chief importance is the detection of recurrent disease or a second primary skin cancer.
Recurrence
It is well-documented that the diagnosis of a primary melanoma at an earlier stage is associated with an improved prognosis.95 There also appears to be a survival benefit associated with earlier detection of metastatic disease,139, 312 and this benefit has been shown to be independent of lead-time bias.313 As therapeutic options for metastatic melanoma have become more effective, the timing of detection of metastases has gained additional relevance. Patients with a lower burden of metastatic disease at presentation are more likely to be candidates for complete surgical resection and those with a smaller tumor burden may have a better response to systemic therapy,314 although this is an extrapolation from the literature on primary melanoma.
Of all patients diagnosed with a cutaneous melanoma who underwent resection, overall recurrence rates have been reported between 5.6% and 22.9%.312, 315–318 However, this rate is very heterogeneous depending on the stage of the primary lesion and on certain high-risk features that confer a higher risk of recurrence. These features include the presence of ulceration in the primary lesion, a primary located on the head or neck, mitotic rate greater than 3 per square millimeter, and thick primary melanomas.315, 317, 318
The overall rate of recurrence for patients with stage I melanoma is 6.8%.319 Patients with stage IA primary lesions have reported recurrence rates of 5.2%, with stage IB patients developing recurrent disease in 18.4% of cases.320 Stage II disease has an overall recurrence rate of approximately 29%,321, 322 but with a substantially increased risk of recurrence from stage IIA (28.7%) compared to stage IIB (40.6%) and IIB disease (44.3%).320 Please see Table 16 for a summary of stage-specific recurrence rates.
Table 16.
Stage-specific recurrence rates for cutaneous melanoma.
| Stages | Recurrence rate158, 323–325, 331, 344, 346, 348, 349 |
|---|---|
|
| |
| Stage I | 6.8% overall |
| IA | 5.2% |
| IB | 18.4% |
|
| |
| Stage II | 30 – 47% overall |
| IIA | 21.6 – 28.7% |
| IIB | 23.4 – 40.6% |
| IIC | 44.3 – 46% |
|
| |
| Stage III | 48.2 – 62.2% overall |
| IIIA | 40.0 – 48% |
| IIIB | 38.6 – 71% |
| IIIC | 50 – 85% |
Range of stage-specific recurrence rates reported across multiple studies.
In patients who recur, locoregional recurrence is most common in those with a primary of lower stage, occurring in 70.2% to 76% of cases.318, 323 These recurrences are distributed between local/in-transit recurrence (20.8–30%)158, 315, 323, 324 and regional nodal recurrence (21–46%).158, 315, 318, 323, 324 The rate of initial recurrence manifesting as distant disease increases with stage of the primary. Rates of distant recurrence have been reported in the range of 24% to 30% across all stages of disease.315, 318, 323 Park and colleagues325 report that in stage II disease, 50% of first recurrences were distant, whereas two thirds of first recurrences were distant in patients with a stage III primary. First recurrence as distant metastasis is associated with worse outcomes.158 The most common methods by which recurrences are detected align with stage-specific patterns of recurrence. Patients with stage IIA and IIB disease are more likely to suffer locoregional recurrence, with nearly 80% of recurrences detected on history and physical examination.326 Distant metastasis becomes more common with increasing stage of the primary lesion, and more than 60% of recurrences in patients with stage III disease are asymptomatic and detected on surveillance imaging.327, 328
The vast majority of recurrences happen within the first 2 to 4 years after the primary diagnosis.329 Romano, and colleagues158 report that the rate of locoregional recurrence after 3 years in stage IIIA disease is less than 5%. For stage IIIB and IIIC disease, the rate of locoregional recurrence drops below 5% at 2 years and 7 months, respectively, after initial diagnosis. The rate of distant metastasis follows a similar pattern, with the rate dropping below 5% after 32 months for stage IIIA disease, 40 months for stage IIIB disease, and 21 months for stage IIIC disease. In a subset of patients, recurrence can occur after a very short postoperative interval. For instance, Bloemendal and colleagues330 published data from a cohort in which 18% of patients with stage IIIB and IIIC disease developed recurrence within 12 weeks of resection, with a median time to recurrence of 7.4 weeks. In a similar population, Lim and colleagues reported a median time to recurrence of 10.1 months.328 Some have interpreted these results as an argument for neoadjuvant therapy in patients with high-risk disease in an attempt to select those who will obtain long-term benefit from an operation.331 In contrast, for other patients recurrence can occur over a decade after initial diagnosis. Faries and colleagues332 detail a cohort of patients in which 6.9% had a recurrence more than 10 years after a primary lesion was treated. These recurrences are more likely to be distant.
Second Primary
Patients who have been diagnosed with a primary cutaneous melanoma have an increased risk for developing a second primary skin cancer and merit an increased intensity of surveillance on these grounds. Recent analyses of United States and Swedish national databases, respectively, have reported rates of 3.6%333 (for patients with stage I and II primary melanoma) and 4.5%334 (no staging breakdown mentioned) for the development of a second primary melanoma. Zimmer and colleagues335 reported a 5.9% rate of second primary at 10 years in a cohort of patients with stage III and IV primary melanomas. This is in comparison to a lifetime risk of just more than 2% for the entire United States population.232 A deeper analysis of the United States cohort seems to show the benefit of rigorous follow-up and patient education as, of those diagnosed with a second primary, almost all had a lesion at the same stage or lower than their initial primary. Forty-eight percent had a second primary with a lower stage than their first, and 50% were diagnosed at an equivalent stage.333 Similar findings have been reported from a cohort of patients with multiple primary melanomas from Memorial Sloan Kettering Cancer Center, with an 11.4% risk of developing a second primary within 5 years.336 Sixty percent of the patients in that cohort developed the second primary within 1 year of their initial diagnosis. The Swedish study showed an increasing rate of second primary development over the last half century. For patients initially diagnosed in the 2000s, 6.4% of women and 7.9% of men in this cohort developed a second primary melanoma within 10 years of their initial diagnosis. An increasing incidence of developing additional primary melanomas after the initial diagnosis has implications for follow-up and may lead to recommendations for increased intensity of surveillance in future guidelines.
Psychosocial Support
In addition to monitoring for recurrence or the onset of a new primary lesion, follow-up clinic visits can be utilized as an opportunity to address other patient-related issues that may be dealt with most effectively in a face-to-face manner. For instance, reinforcing the importance of behaviors that minimize sun exposure, and encouraging sun protection, including the use of sunscreen, which has been shown by a RCT to reduce the incidence of melanoma.337 The demonstration of self-examination techniques for the skin and lymph node basins can also be effectively accomplished in the clinic setting. Assuring adequate time for patients to ask questions can help ease anxiety and can be an opportunity for the physician to clarify or correct information the patient may have encountered in their own searches but is confusing or incorrect. Multiple studies have demonstrated that patients value psychosocial support regarding cancer diagnosis and treatment, and significant patient-reported rates of anxiety (12.1%) and depression (11.6%) exist in a large German study population. However, in that same cohort, documented rates of anxiety (0.2%) and depression (1.7%) were discrepant, indicating an unaddressed need.338 There are few validated survey instruments with which clinicians may reliably collect quality-of-life data pertinent to patients with melanoma.339 This knowledge gap may contribute to clinicians’ inability to identify and address areas of concern in patients being followed after the diagnosis and treatment of a cutaneous melanoma. A more deliberate approach to the evaluation of a patient’s psychosocial well-being could help clinicians more effectively address these issues.340 Although cancer surgeons may be well placed to help inform, guide, and reassure patients about clinical matters in the follow-up period, referral to ancillary specialists may be more appropriate for other issues in which other team members have more expertise. Lacking appropriate pathways down which to direct patients may result in physicians ordering superfluous and non-indicated laboratory tests or imaging studies in attempts to relieve patient anxiety.341, 342 Clinicians may also feel the need to schedule more frequent follow-up appointments with certain patients, beyond the frequency recommended by clinical guidelines, in an attempt to deal with psychosocial issues surrounding cancer survivorship.343 This is not only an inefficient use of resources, but also merely a temporary solution to a psychosocial problem that will persist without appropriate management. Faced with both an aging population with a larger pool of people at an age typical for development of disease, and a rising incidence of melanoma, oncologists may face unmanageable clinical volumes. To offset these volumes, it may be helpful for a subset of patients with low-risk disease but with psychosocial needs necessitating frequent office visits beyond what is indicated for clinical surveillance to be directed to more appropriate providers in order to address the psychosocial component of their recovery.343 Other health systems may provide more readily accessible resources for the melanoma patient in the follow-up period. For example, in Germany melanoma patients are routinely routed to counselors or physical therapists depending on their needs.344 In Scotland, a RCT showed that melanoma follow-up visits with a general practitioner were given positive reviews by the patients involved.345
These programs may be more difficult to implement in the United States where private insurers offer differing levels of access and coverage and a declining number of Americans maintain a relationship with a primary care provider.
Components of Follow-up After Treatment of Primary Cutaneous Melanoma
For all patients who are in the surveillance period after resection of a primary cutaneous melanoma, the NCCN94 has baseline recommendations for what should be included in a follow-up visit, regardless of the nature of the primary lesion.
History and Physical Examination
A thorough history should be taken, with a focus on new skin lesions, changing skin lesions, or the development of systemic symptoms worrying for metastasis. A complete physical examination should be performed, including interdigital spaces, the anogenital region, the oral cavity, and lymph nodes. For patients with stage IIB-IV melanomas, it is recommended that these follow-up visits occur every 3 to 6 months for the first 2 years after resection, then every 3 to 12 months until the patient is 5 years out from resection. At that point, the patient should return to care at least annually for surveillance, as there remains a risk of delayed recurrence and they are also at increased risk of developing another primary skin cancer. Patients with melanoma in situ may be followed annually, while patients with stage IA-IIA disease should be seen every 6 to 12 months during the first 5 years after resection before transitioning to annual follow-up. Although routine to all physicians, the history and physical examination can be a powerful tool, with studies showing that 47% of all recurrences were detected on history and physical examination, compared to just 21% of all recurrences detected via imaging.346 As the majority of primary melanomas are lower stage lesions, which tend to recur locally, this increased proportion of recurrences detected on examination is consistent with the preponderance of local recurrence in early-stage disease. As the stage of the primary increases, the likelihood of distant recurrence also increases. The findings of Kurtz and colleagues326 reflect this, with their paper reporting 79% of recurrences in patients with stage IIA or IIB primaries detected on examination while 50% of recurrences in stage IIC through IIIC patients were asymptomatic and discovered on imaging. At our institution, after the initial postoperative visit, the patient’s surgeon and the patient’s dermatologist will alternate staggered biannual visits, so the patient is being seen every 3 months. After 2 years, the frequency of the visits will decrease to every 6 months, with the surgeon and the dermatologist each seeing the patient once per year. After 5 years, patients may stop seeing the surgeon but are encouraged to follow up with their dermatologist annually for the rest of their lives. Throughout the follow-up period, the importance of sun-safe behavior is emphasized, and proper techniques for self-examination are reinforced with encouragement to perform self-examinations regularly.
The European Society for Medical Oncology (ESMO) recommends post-resection follow-up but did not reach a consensus position on the frequency or duration of such visits.347 Australian guidelines recommend a 10-year duration of follow-up, citing a less than 1% rate of recurrence beyond that period. For stage I disease, they recommend annual visits. Patients with stage IIA disease are recommended to return for clinic visits every 6 months for 2 years, before returning annually for the remainder of the 10-year follow-up period. Patients with stage IIB-IIC disease should be seen in follow-up every 3 to 4 months for the first 2 years postoperatively, then every 6 months for 1 year, before transitioning to annual visits. Patients with stage III disease are recommended to return for follow-up every 3 months for 2 years, every 6 months for 1 year, then annually for the remaining 5 years.348
Imaging
The primary role of imaging is to investigate the suspicion of distant metastasis. Should the patient report symptoms concerning for visceral metastasis, relevant CT images should be obtained with intravenous (IV) contrast. In patients with stage IIC disease and above, routine imaging is indicated even in the absence of symptoms. A cost analysis of postoperative surveillance imaging concluded that biannual CT of the chest, abdomen, and pelvis is cost-effective over the first 4 years of follow-up in patients with stage IIC and stage III disease, and for the first 3 post-resection years in patients with stage IIB disease.349 Chest radiographs have been used historically to screen for pulmonary metastasis but have proven to be ineffectual and that practice has been discarded. If there is concern for metastasis to the brain, the patient should undergo MRI of the brain. Romano and colleagues158 from Memorial Sloan Kettering Cancer Center published data showing that 36% of patients with a first recurrence in the brain presented with seizures. They proposed that routine brain MRI, at least in the first year after resection, would prevent some number of seizures in this group of patients. The addition of immunotherapy and targeted therapy to the armamentarium and the associated survival benefit for patients with metastatic disease has enhanced the value of routine imaging during the follow-up period. Having an efficacious treatment modality increases the importance of early diagnosis of distant metastatic disease. According to NCCN guidelines, patients with stage IIB or greater primary melanomas should undergo serial imaging to screen for recurrence. This may be performed every 3 to 12 months for the first 2 years post-resection, and then every 6 to 12 months up until 5 years post-resection, at the physician’s discretion. As most recurrences occur within the first 2 to 4 years postoperatively, empiric imaging should not be continued after 5 years.94 The recommendation for routine surveillance imaging for the first several years after treatment of a primary melanoma in otherwise asymptomatic patients is justified by several studies which show that many recurrences are both early and asymptomatic, discovered by surveillance imaging alone. Lim and colleagues328 report that in a cohort of patients with primary stage IIIB and IIIC melanomas who experienced a recurrence, the majority (66%) were asymptomatic and had a short median time to recurrence. Lewin and colleagues350 reported similar rates of asymptomatic recurrence, detected only by imaging. Their study also highlighted the role of PET-CT in screening for recurrent disease, as did a study by Leon-Ferre and colleagues,327 which reported 60% of all recurrences were detected by PET-CT within the first year after resection. Although PET-CT has been shown to effectively detect distant recurrence in high-risk stage IIB-IIIB disease before symptoms arise, this early detection is not associated with a survival benefit.351 Several other studies report high rates (66–96.5%) of distant recurrence detected initially by imaging.325, 328, 338, 350, 352, 353 Despite the intuitive appeal of initiating systemic therapy while the burden of metastatic disease is low, there is no evidence that early discovery through surveillance and subsequent intervention for distant recurrence is associated with a survival benefit.354–356
For those patients with a positive sentinel lymph node who did not undergo completion LND, US surveillance of the regional lymph node basin has equivalent distant RFS143 and MSS,144 according to 2 recent multicenter trials. If the patient elects to pursue observation, the NCCN recommends US every 4 months for the first 2 years, then every 6 months until 5 years post-resection. Serial US examination may also be used in the surveillance of regional lymphatic basins in higher risk patients who were unlikely to tolerate general anesthesia and were deemed poor candidates for SLNB. Although regional control suffers with this approach, there is no impact on distant RFS or DSS.357 US can also be utilized in the setting of a patient who develops worrisome regional adenopathy during the follow-up period. US has been shown to be superior to other imaging modalities in the evaluation of lymphatic basins, with high sensitivity (96%) and specificity (99%).358, 359
Australian guidelines mirror United States guidelines, recommending routine imaging every 3 to 12 months for the first 3 years post-resection of stage IIC and stage III melanoma.348 ESMO did not reach consensus on imaging recommendations, deferring to national guidelines, but noted that serial imaging in high-risk patients could lead to earlier detection of recurrence, although a survival benefit has yet to be borne out.347
Laboratory Tests
Laboratory tests are only relevant in melanoma in the setting of LDH holding prognostic value in stage IV disease. A representative study showed that recurrent disease was only identified through blood tests in 2.5% of cases.353 Laboratory tests are not recommended for inclusion in follow-up protocols.
Patient Education
Follow-up visits should be used to demonstrate self-examination skills that patients can utilize at home, including monitoring of cutaneous lesions using the “ABDCE” criteria and monitoring of regional lymph node basins. The importance of minimizing sun exposure should be emphasized. Patients should be counseled on avoiding sun exposure during peak hours, wearing protective clothing if they anticipate prolonged exposure, and using a broad-spectrum, high sun protection factor (SPF) sunblock when they are outdoors for prolonged periods. This suite of measures is consistently endorsed in recent guidelines released by major professional bodies across the United States, Europe, and Australia. Please refer to Table 17 for a comparison of guidelines between the NCCN (United States), ESMO (Europe), and Cancer Council Australia.
Table 17.
Comparison between US, European, and Australian guidelines for follow-up in patients with resected cutaneous melanoma.
| National Comprehensive Cancer Network (NCCN)63 | European Society for Medical Oncology (ESMO)66 | Cancer Council Australia67 | |
|---|---|---|---|
|
| |||
| History and physical examination | -Stage 0: annually -Stage IA-IIA: every 6–12 mo for 5 y, then annually -Stage IIB-IV: every 3–6 mo for 2 y, then every 3–12 mo for 3 y, then annually -Consider office-based imaging technologies in patients with numerous moles and/or atypical nevi |
No consensus, but follow-up is generally recommended. Defer to national guidelines. Range of recommendations: -every 3 mo for 3 y, then every 6–12 mo -return visits as needed |
-Stage I: annually for 10 y -Stage IIA: every 6 mo for 2 y, then annually for 8 y -Stage IIB-C: every 3–4 mo for 2 y, then every 6 mo for 1 y, then annually for 5 y -Stage IIIA-C: every 3 mo for 2 y, then every 6 mo for 1 y, then annually for 5 y -Those with risk factors (eg, family history, dysplastic nevus syndrome) may continue follow-up beyond 10 y |
| Imaging | Indications: -Equivocal lymph node examination -Positive SLN, but did not undergo CLND: every 4 mo for 2 y, then every 6 mo for 3 y -Stage IIB-IV disease: every 3–12 mo for 2 y, then every 6–12 mo for 3 y |
-Not recommended for patients with thin melanomas -No consensus, but patients with high-risk disease may undergo serial imaging to aid early identification of recurrence, although no survival benefit |
-Stage I-IIB: do not recommend routine imaging -Stage IIC-III: every 3–12 mo for 3 y -CT/MRI when indicated by clinical findings |
| Genetic testing | Consider additional genetic testing/genetic counselling if family history of multiple melanomas, or combination of melanoma, astrocytoma, pancreatic cancer | Test for actionable mutations at time of diagnosis No recommendations on further testing | Consider genetic testing for CDKN2A mutations/genetic counselling if family history of 3 or more melanomas in first- or second-degree relatives with high-risk features (eg, early onset, multiple primaries, history of pancreatic cancer) |
| Laboratory testing | Routine labs not recommended. | Routine labs not recommended | Routine labs not recommended. |
| Patient education | -Sun-safe behavior (avoiding exposure during peak hours, protective clothing, sunscreen) -Self-examination techniques |
-Sun-safe behavior (avoid sunburns, avoid extended unprotected exposure) -Self-examinations for life -Educate regarding increased risk to family members |
-Sun-safe behavior -Self-examination techniques |
CLND, completion lymph node dissection; CT, computed tomography; MRI, magnetic resonance imaging; SLN, sentinel lymph node.
Future Directions
Recent advances in systemic therapy have resulted in significant improvements in survival for patients with melanoma, ensuring larger populations of patients who will need to be followed after successful treatment of their primary lesion. Moving forward, approaches to follow-up may place a greater emphasis on factors unique to individual patients—characteristics of the primary tumor, personal and family history, and genetic characteristics of the primary disease, not simply blanket recommendations based on tumor stage.
GEP holds promise for the more accurate placement of patients into high- or low-risk groups, and postoperative imaging and follow-up can be planned accordingly. However, at this point, the implications of test results and the appropriate response based on these results remain to be determined.360 Multiple reports evaluating a commercially available GEP tool verify its efficacy as a predictor of recurrence, distant metastasis, and survival.361 Studies suggest that the combination of tumor characteristics and information gleaned from GEP can be synergistic in the creation of a follow-up regimen. Ferris and colleagues362 report on a cohort in which 21% of patients had discrepant risk stratification between an AJCC prediction tool and that generated by GEP. Of the deaths in this discordant group, 85% were predicted to be high-risk by GEP but were classified as low risk per AJCC prediction tool. Using these tools in concert may improve identification of high-risk patients, allowing for intensified surveillance.
Measurement of circulating cell-free tumor DNA has been shown to indicate the development of progressive disease before it becomes apparent clinically, offering a potential tool for early detection of recurrence.363
Experiences with telemedicine during the SARS-CoV-2 pandemic have illuminated a possible role for telemedicine in the follow-up of early-stage melanoma patients. As the majority of recurrences in these patients are locoregional, and many are detected by patients on self-examination, it may be reasonable to incorporate video visits in an attempt to minimize clinical volume for the physician and travel burden for the patient. Other technology-based interventions include smartphone apps that leverage artificial intelligence to analyze photos of lesions submitted by patients. When a lesion is classified as worrisome, the patient is encouraged to see a physician. Although success rates at identifying lesions that eventually turned out to be malignant were only between 20% and 30%,364 this concept holds promise for earlier detection of skin cancers and increasing access to expert evaluation for patients who live in rural areas or may otherwise face barriers to attending office-based consultations.
Conclusions
After the diagnosis and resection of a primary cutaneous melanoma, patients are at risk for recurrence as well as the development of a second primary skin cancer. The risk of recurrence increases with increasing stage of the primary lesion, and intensity of follow-up is augmented accordingly. As the majority of recurrences occur within the first several years after diagnosis and resection of the primary, clinical examinations and imaging studies are performed with decreasing frequency over the period of follow-up. The occurrence of one primary melanoma is associated with an increased risk of a developing another primary skin cancer. This risk persists and, as such, patients are followed for life. In the future, genetic testing will help clinicians to more precisely characterize a patient’s risk for recurrence and will contribute to the development of personalized follow-up protocols.
Supplementary Material
Figure S1. Synoptic report according to College of American Pathologists (CAP) standards. This is an example of a synoptic report for reporting on a skin biopsy of a melanoma. It includes all criteria that are reported according to CAP standards (with permission from Shon W, Frishberg DP, Gershenwald JE, et al. Protocol for examination of excision specimens from patients with melanoma of the skin. College of American Pathologists website. https://documents.cap.org/protocols/cp-skin-melanoma-excision-19-4100.pdf.
ACKNOWLEDGEMENTS
Keenan J. Robbins, MBBS, was supported by the Washington University School of Medicine Surgical Oncology Basic Science and Translational Research Training Program grant (National Cancer Institute, T32CA009621). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ken Newcomer, Barnes-Jewish Hospital, Washington University, St. Louis, Missouri.
Keenan J. Robbins, Washington University, St. Louis, Missouri.
Jennifer Perone, University of Texas Medical Branch, Galveston, Texas.
Fernando Lambreton Hinojosa, University of Texas Medical Branch, Galveston, Texas.
David Chen, Washington University, St. Louis, Missouri.
Susan Jones, Department of Pediatrics, Washington University, St. Louis, Missouri.
Charles K. Kaufman, Washington University, St. Louis, Missouri.
Roi Weiser, University of Texas Medical Branch, Galveston, Texas.
Ryan C. Fields, Chief, Section of Surgical Oncology, Associate Program Director, Surgery Residency Program, Washington University, St. Louis, Missouri.
Douglas S. Tyler, University of Texas Medical Branch, Galveston, Texas.
REFERENCES
- 1.Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet. 2018;392(10151):971–984. [DOI] [PubMed] [Google Scholar]
- 2.SEER Cancer Statistics Review (CSR) 1975–2017. National Cancer Institute website. https://seer.cancer.gov/csr/1975_2017/ based on November 2019 SEER data submission. Accessed July 29, 2020. [Google Scholar]
- 3.Australian Institute of Health and Welfare. Cancer data in Australia. June 2020. website. https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia. Accessed July 29, 2020. [Google Scholar]
- 4.MacKie RM, Hauschild A, Eggermont AM. Epidemiology of invasive cutaneous melanoma. Ann Oncol. 2009;20 Suppl 6:vi1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. [DOI] [PubMed] [Google Scholar]
- 6.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41(14):2040–2059. [DOI] [PubMed] [Google Scholar]
- 7.Chen ST, Geller AC, Tsao H. Update on the epidemiology of melanoma. Curr Dermatol Rep. 2013;2(1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S, Han J, Laden F, Qureshi AA. Long-term ultraviolet flux, other potential risk factors, and skin cancer risk: a cohort study. Cancer Epidemiol Biomarkers Prev. 2014;23(6):1080–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41(1):45–60. [DOI] [PubMed] [Google Scholar]
- 10.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer. 2005;41(1):28–44. [DOI] [PubMed] [Google Scholar]
- 11.Rigel DS, Rivers JK, Kopf AW, et al. Dysplastic nevi. Markers for increased risk for melanoma. Cancer. 1989;63(2):386–389. [DOI] [PubMed] [Google Scholar]
- 12.Tucker MA, Halpern A, Holly EA, et al. Clinically recognized dysplastic nevi. A central risk factor for cutaneous melanoma. JAMA. 1997;277(18):1439–1444. [PubMed] [Google Scholar]
- 13.Pappo AS, Armstrong GT, Liu W, et al. Melanoma as a subsequent neoplasm in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Pediatr Blood Cancer. 2013;60(3):461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown AL, Arroyo VM, Agrusa JE, Scheurer ME, Gramatges MM, Lupo PJ. Survival disparities for second primary malignancies diagnosed among childhood cancer survivors: A population-based assessment. Cancer. 2019;125(20):3623–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsen CM, Lane SW, Green AC. Increased risk of melanoma in patients with chronic lymphocytic leukaemia: systematic review and meta-analysis of cohort studies. Melanoma Res. 2016;26(2):188–194. [DOI] [PubMed] [Google Scholar]
- 16.Lam CJ, Curtis RE, Dores GM, et al. Risk factors for melanoma among survivors of non-hodgkin lymphoma. J Clin Oncol. 2015;33(28):3096–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbins HA, Clarke CA, Arron ST, et al. Melanoma risk and survival among organ transplant recipients. J Invest Dermatol. 2015;135(11):2657–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Arcy ME, Coghill AE, Lynch CF, et al. Survival after a cancer diagnosis among solid organ transplant recipients in the United States. Cancer. 2019;125(6):933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omland SH, Gniadecki R, Haedersdal M, Helweg-Larsen J, Omland LH. Skin cancer risk in hematopoietic stem-cell transplant recipients compared with background population and renal transplant recipients: a population-based cohort study. JAMA Dermatol. 2016;152(2):177–183. [DOI] [PubMed] [Google Scholar]
- 20.Olsen CM, Knight LL, Green AC. Risk of melanoma in people with HIV/AIDS in the pre- and post-HAART eras: a systematic review and meta-analysis of cohort studies. PLoS One. 2014;9(4):e95096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanik EL, Hernandez-Ramirez RU, Qin L, et al. Brief report: cutaneous melanoma risk among people with HIV in the United States and Canada. J Acquir Immune Defic Syndr. 2018;78(5):499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraemer KH, Lee MM, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987;123(2):241–250. [DOI] [PubMed] [Google Scholar]
- 23.Shah P, He YY. Molecular regulation of UV-induced DNA repair. Photochem Photobiol. 2015;91(2):254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douki T, Reynaud-Angelin A, Cadet J, Sage E. Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation. Biochemistry. 2003;42(30):9221–9226. [DOI] [PubMed] [Google Scholar]
- 25.International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review. Int J Cancer. 2007;128(10):2425–2435. [DOI] [PubMed] [Google Scholar]
- 26.Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128(10):2425–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghiasvand R, Rueegg CS, Weiderpass E, Green AC, Lund E, Veierod MB. Indoor tanning and melanoma risk: long-term evidence from a prospective population-based cohort study. Am J Epidemiol. 2017;185(3):147–156. [DOI] [PubMed] [Google Scholar]
- 28.Richards TB, Johnson CJ, Tatalovich Z, et al. Association between cutaneous melanoma incidence rates among white US residents and county-level estimates of solar ultraviolet exposure. J Am Acad Dermatol. 2011;65(5 Suppl 1):S50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003;95(24):1878–1890. [DOI] [PubMed] [Google Scholar]
- 31.Shitara D, Tell-Marti G, Badenas C, et al. Mutational status of naevus-associated melanomas. Br J Dermatol. 2015;173(3):671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lozada JR, Geyer FC, Selenica P, et al. Massively parallel sequencing analysis of benign melanocytic naevi. Histopathology. 2019;75(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiteman DC, Watt P, Purdie DM, Hughes MC, Hayward NK, Green AC. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95(11):806–812. [DOI] [PubMed] [Google Scholar]
- 34.Rivers JK. Is there more than one road to melanoma? Lancet. 2004;363(9410):728–730. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy BY, Miller DM, Tsao H. Somatic driver mutations in melanoma. Cancer. 2017;123(S11):2104–2117. [DOI] [PubMed] [Google Scholar]
- 37.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. [DOI] [PubMed] [Google Scholar]
- 38.Zhang T, Dutton-Regester K, Brown KM, Hayward NK. The genomic landscape of cutaneous melanoma. Pigment Cell Melanoma Res. 2016;29(3):266–283. [DOI] [PubMed] [Google Scholar]
- 39.Lee B, Sandhu S, McArthur G. Cell cycle control as a promising target in melanoma. Curr Opin Oncol. 2015;27(2):141–150. [DOI] [PubMed] [Google Scholar]
- 40.Bartkova J, Lukas J, Guldberg P, et al. The p16-cyclin D/Cdk4-pRb pathway as a functional unit frequently altered in melanoma pathogenesis. Cancer Res. 1996;56(23):5475–5483. [PubMed] [Google Scholar]
- 41.Bennett DC. How to make a melanoma: what do we know of the primary clonal events? Pigment Cell Melanoma Res. 2008;21(1):27–38. [DOI] [PubMed] [Google Scholar]
- 42.Raimondi S, Sera F, Gandini S, et al. MC1R variants, melanoma and red hair color phenotype: a meta-analysis. Int J Cancer. 2008;122(12):2753–2760. [DOI] [PubMed] [Google Scholar]
- 43.Garraway LA, Widlund HR, Rubin MA, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005;436(7047):117–122. [DOI] [PubMed] [Google Scholar]
- 44.Tsoi J, Robert L, Paraiso K, et al. Multi-stage differentiation defines melanoma subtypes with differential vulnerability to drug-induced iron-dependent oxidative stress. Cancer Cell. 2018;33(5):890–904 e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA. 2011;305(22):2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smit KN, Jager MJ, de Klein A, Kili E. Uveal melanoma: Towards a molecular understanding. Prog Retin Eye Res. 2020;75:100800. [DOI] [PubMed] [Google Scholar]
- 47.Ransohoff KJ, Jaju PD, Tang JY, Carbone M, Leachman S, Sarin KY. Familial skin cancer syndromes: Increased melanoma risk. J Am Acad Dermatol. 2016;74(3):423–434; quiz 435–436. [DOI] [PubMed] [Google Scholar]
- 48.Read J, Wadt KA, Hayward NK. Melanoma genetics. J Med Genet. 2016;53(1):1–14. [DOI] [PubMed] [Google Scholar]
- 49.Leachman SA, Carucci J, Kohlmann W, et al. Selection criteria for genetic assessment of patients with familial melanoma. J Am Acad Dermatol. 2009;61(4):677 e671–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leachman SA, Lucero OM, Sampson JE, et al. Identification, genetic testing, and management of hereditary melanoma. Cancer Metastasis Rev. 2017;36(1):77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.American Academy of Dermatology Ad Hoc Task Force for the AoM, Tsao H, Olazagasti JM, et al. Early detection of melanoma: reviewing the ABCDEs. J Am Acad Dermatol. 2015;72(4):717–723. [DOI] [PubMed] [Google Scholar]
- 52.Thomas L, Tranchand P, Berard F, Secchi T, Colin C, Moulin G. Semiological value of ABCDE criteria in the diagnosis of cutaneous pigmented tumors. Dermatology. 1998;197(1):11–17. [DOI] [PubMed] [Google Scholar]
- 53.Healsmith MF, Bourke JF, Osborne JE, Graham-Brown RA. An evaluation of the revised seven-point checklist for the early diagnosis of cutaneous malignant melanoma. Br J Dermatol. 1994;130(1):48–50. [DOI] [PubMed] [Google Scholar]
- 54.Carli P, De Giorgi V, Palli D, et al. Self-detected cutaneous melanomas in Italian patients. Clin Exp Dermatol. 2004;29(6):593–596. [DOI] [PubMed] [Google Scholar]
- 55.Barnhill RL, Roush GC, Ernstoff MS, Kirkwood JM. Interclinician agreement on the recognition of selected gross morphologic features of pigmented lesions. Studies of melanocytic nevi V. J Am Acad Dermatol. 1992;26(2 Pt 1):185–190. [DOI] [PubMed] [Google Scholar]
- 56.Adamson AS, Jarmul JA, Pignone MP. Screening for melanoma in men: a cost-effectiveness analysis. J Gen Intern Med. 2020;35(4):1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esteva A, Kuprel B, Novoa RA, et al. Corrigendum: Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;546(7660):686. [DOI] [PubMed] [Google Scholar]
- 58.Swetter SM, Tsao H, Bichakjian CK, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80(1):208–250. [DOI] [PubMed] [Google Scholar]
- 59.Zager JS, Hochwald SN, Marzban SS, et al. Shave biopsy is a safe and accurate method for the initial evaluation of melanoma. J Am Coll Surg. 2011;212(4):454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doolan BJ, Robinson AJ, Wolfe R, et al. Accuracy of partial biopsies in the management of cutaneous melanoma. Australas J Dermatol. 2019;60(3):209–213. [DOI] [PubMed] [Google Scholar]
- 61.Reddy KK, Farber MJ, Bhawan J, Geronemus RG, Rogers GS. Atypical (dysplastic) nevi: outcomes of surgical excision and association with melanoma. JAMA Dermatol. 2013;149(8):928–934. [DOI] [PubMed] [Google Scholar]
- 62.Scolyer RA, Balamurgan T, Busam K, et al. Invasive melanoma, histopathology reporting guide, 2nd edition. International Collaboration on Cancer Reporting; Sydney, Australia: 2019:ISBN: 978-971-925687-925632-925683. [Google Scholar]
- 63.Gershenwald JE, Scolyer RA, Hess KR. Melanoma of the skin. In: Amin MB, Edge SB, Greene FL, et al. , eds. AJCC Cancer Staging Manual. 8th ed. Chicago: Springer International Publishing; 2017:563–585. [Google Scholar]
- 64.Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shon W, Frishberg DP, Gershenwald JE, et al. Protocol for examination of excision specimens from patients with melanoma of the skin. college of american pathologists. website. https://documents.cap.org/protocols/cp-skin-melanoma-excision-19-4100.pdf. Accessed July 29, 2020. [Google Scholar]
- 66.Eigentler TK, Buettner PG, Leiter U, Garbe C, Central Malignant Melanoma Registry of the German Dermatological S. Impact of ulceration in stages I to III cutaneous melanoma as staged by the American Joint Committee on Cancer Staging System: an analysis of the German Central Malignant Melanoma Registry. J Clin Oncol. 2004;22(21):4376–4383. [DOI] [PubMed] [Google Scholar]
- 67.Nagore E, Oliver V, Botella-Estrada R, Moreno-Picot S, Insa A, Fortea JM. Prognostic factors in localized invasive cutaneous melanoma: high value of mitotic rate, vascular invasion and microscopic satellitosis. Melanoma Res. 2005;15(3):169–177. [DOI] [PubMed] [Google Scholar]
- 68.Shaikh L, Sagebiel RW, Ferreira CM, Nosrati M, Miller JR, 3rd, Kashani-Sabet M. The role of microsatellites as a prognostic factor in primary malignant melanoma. Arch Dermatol. 2005;141(6):739–742. [DOI] [PubMed] [Google Scholar]
- 69.Clemente CG, Mihm MC, Jr., Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77(7):1303–1310. [DOI] [PubMed] [Google Scholar]
- 70.Paek SC, Griffith KA, Johnson TM, et al. The impact of factors beyond Breslow depth on predicting sentinel lymph node positivity in melanoma. Cancer. 2007;109(1):100–108. [DOI] [PubMed] [Google Scholar]
- 71.Straume O, Akslen LA. Independent prognostic importance of vascular invasion in nodular melanomas. Cancer. 1996;78(6):1211–1219. [DOI] [PubMed] [Google Scholar]
- 72.Levi F, Randimbison L, La Vecchia C, Te VC, Franceschi S. Prognostic factors for cutaneous malignant melanoma in Vaud, Switzerland. Int J Cancer. 1998;78(3):315–319. [DOI] [PubMed] [Google Scholar]
- 73.Anderson KW, Baker SR, Lowe L, Su L, Johnson TM. Treatment of head and neck melanoma, lentigo maligna subtype: a practical surgical technique. Arch Facial Plast Surg. 2001;3(3):202–206. [DOI] [PubMed] [Google Scholar]
- 74.Ochoa CE, Joseph RW. Desmoplastic melanoma: a brief review and the efficacy of immunotherapy. Expert Rev Anticancer Ther. 2019;19(3):205–207. [DOI] [PubMed] [Google Scholar]
- 75.Hawkins WG, Busam KJ, Ben-Porat L, et al. Desmoplastic melanoma: a pathologically and clinically distinct form of cutaneous melanoma. Ann Surg Oncol. 2005;12(3):207–213. [DOI] [PubMed] [Google Scholar]
- 76.Wang TS, Johnson TM, Cascade PN, Redman BG, Sondak VK, Schwartz JL. Evaluation of staging chest radiographs and serum lactate dehydrogenase for localized melanoma. J Am Acad Dermatol. 2004;51(3):399–405. [DOI] [PubMed] [Google Scholar]
- 77.Miranda EP, Gertner M, Wall J, et al. Routine imaging of asymptomatic melanoma patients with metastasis to sentinel lymph nodes rarely identifies systemic disease. Arch Surg. 2004;139(8):831–836; discussion 836–837. [DOI] [PubMed] [Google Scholar]
- 78.Fogarty GB, Tartaguia C. The utility of magnetic resonance imaging in the detection of brain metastases in the staging of cutaneous melanoma. Clin Oncol (R Coll Radiol). 2006;18(4):360–362. [DOI] [PubMed] [Google Scholar]
- 79.Singh B, Ezziddin S, Palmedo H, et al. Preoperative 18F-FDG-PET/CT imaging and sentinel node biopsy in the detection of regional lymph node metastases in malignant melanoma. Melanoma Res. 2008;18(5):346–352. [DOI] [PubMed] [Google Scholar]
- 80.Starritt EC, Uren RF, Scolyer RA, Quinn MJ, Thompson JF. Ultrasound examination of sentinel nodes in the initial assessment of patients with primary cutaneous melanoma. Ann Surg Oncol. 2005;12(1):18–23. [DOI] [PubMed] [Google Scholar]
- 81.Ho Shon IA, Chung DK, Saw RP, Thompson JF. Imaging in cutaneous melanoma. Nucl Med Commun. 2008;29(10):847–876. [DOI] [PubMed] [Google Scholar]
- 82.Bafounta ML, Beauchet A, Chagnon S, Saiag P. Ultrasonography or palpation for detection of melanoma nodal invasion: a meta-analysis. Lancet Oncol. 2004;5(11):673–680. [DOI] [PubMed] [Google Scholar]
- 83.Machet L, Nemeth-Normand F, Giraudeau B, et al. Is ultrasound lymph node examination superior to clinical examination in melanoma follow-up? A monocentre cohort study of 373 patients. Br J Dermatol. 2005;152(1):66–70. [DOI] [PubMed] [Google Scholar]
- 84.Johnson TM, Fader DJ, Chang AE, et al. Computed tomography in staging of patients with melanoma metastatic to the regional nodes. Ann Surg Oncol. 1997;4(5):396–402. [DOI] [PubMed] [Google Scholar]
- 85.Kuvshinoff BW, Kurtz C, Coit DG. Computed tomography in evaluation of patients with stage III melanoma. Ann Surg Oncol. 1997;4(3):252–258. [DOI] [PubMed] [Google Scholar]
- 86.Gold JS, Jaques DP, Busam KJ, Brady MS, Coit DG. Yield and predictors of radiologic studies for identifying distant metastases in melanoma patients with a positive sentinel lymph node biopsy. Ann Surg Oncol. 2007;14(7):2133–2140. [DOI] [PubMed] [Google Scholar]
- 87.Krug B, Crott R, Lonneux M, Baurain JF, Pirson AS, Vander Borght T. Role of PET in the initial staging of cutaneous malignant melanoma: systematic review. Radiology. 2008;249(3):836–844. [DOI] [PubMed] [Google Scholar]
- 88.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morrison C, Pabla S, Conroy JM, et al. Predicting response to checkpoint inhibitors in melanoma beyond PD-L1 and mutational burden. J Immunother Cancer. 2018;6(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cook RW, Middlebrook B, Wilkinson J, et al. Analytic validity of DecisionDx-Melanoma, a gene expression profile test for determining metastatic risk in melanoma patients. Diagn Pathol. 2018;13(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gerami P, Cook RW, Russell MC, et al. Gene expression profiling for molecular staging of cutaneous melanoma in patients undergoing sentinel lymph node biopsy. J Am Acad Dermatol. 2015;72(5):780–785 e783. [DOI] [PubMed] [Google Scholar]
- 92.Marchetti MA, Bartlett EK, Dusza SW, Bichakjian CK. Use of a prognostic gene expression profile test for T1 cutaneous melanoma: Will it help or harm patients? J Am Acad Dermatol. 2019;80(6):e161–e162. [DOI] [PubMed] [Google Scholar]
- 93.Grossman D, Okwundu N, Bartlett EK, et al. Prognostic gene expression profiling in cutaneous melanoma: Identifying the knowledge gaps and assessing the clinical benefit. JAMA Dermatol. 2020;156(9):1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.National Comprehensive Cancer Network. Cutaneous melanoma (version 3.2020). NCCN guidelines. website. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma_blocks.pdf. Accessed August 29, 2020. [Google Scholar]
- 95.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amaria RN, Menzies AM, Burton EM, et al. Neoadjuvant systemic therapy in melanoma: recommendations of the International Neoadjuvant Melanoma Consortium. Lancet Oncol. 2019;20(7):e378–e389. [DOI] [PubMed] [Google Scholar]
- 97.Poklepovic AS, Luke JJ. Considering adjuvant therapy for stage II melanoma. Cancer. 2020;126(6):1166–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bommareddy PK, Silk AW, Kaufman HL. Intratumoral approaches for the treatment of melanoma. Cancer J. 2017;23(1):40–47. [DOI] [PubMed] [Google Scholar]
- 99.Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th ed. Chicago: Springer International Publishing; 2017. [Google Scholar]
- 100.Gershenwald JE, Scolyer RA. Melanoma Staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Ann Surg Oncol. 2018;25(8):2105–2110. [DOI] [PubMed] [Google Scholar]
- 101.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–3634. [DOI] [PubMed] [Google Scholar]
- 102.Breslow A, Cascinelli N, van der Esch EP, Morabito A. Stage I melanoma of the limbs: assessment of prognosis by levels of invasion and maximum thickness. Tumori. 1978;64(3):273–284. [DOI] [PubMed] [Google Scholar]
- 103.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19(16):3635–3648. [DOI] [PubMed] [Google Scholar]
- 104.Bichakjian CK, Halpern AC, Johnson TM, et al. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol. 2011;65(5):1032–1047. [DOI] [PubMed] [Google Scholar]
- 105.National Comprehensive Cancer Network. Cutaneous melanoma (version 4.2020). NCCN guidelines. website. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf. Accessed October 27, 2020.
- 106.Kaiser S, Vassell R, Pinckney RG, Holmes TE, James TA. Clinical impact of biopsy method on the quality of surgical management in melanoma. J Surg Oncol. 2014;109(8):775–779. [DOI] [PubMed] [Google Scholar]
- 107.Mills JK, White I, Diggs B, Fortino J, Vetto JT. Effect of biopsy type on outcomes in the treatment of primary cutaneous melanoma. Am J Surg. 2013;205(5):585–590; discussion 590. [DOI] [PubMed] [Google Scholar]
- 108.Saco M, Thigpen J. A retrospective comparison between preoperative and postoperative Breslow depth in primary cutaneous melanoma: how preoperative shave biopsies affect surgical management. J Drugs Dermatol. 2014;13(5):531–536. [PubMed] [Google Scholar]
- 109.Farberg AS, Rigel DS. A comparison of current practice patterns of US dermatologists versus published guidelines for the biopsy, initial management, and follow up of patients with primary cutaneous melanoma. J Am Acad Dermatol. 2016;75(6):1193–1197 e1191. [DOI] [PubMed] [Google Scholar]
- 110.Handley W The pathology of melanotic growths in relation to their operative treatment. Lancet. 1907;1:927–935. [Google Scholar]
- 111.Testori AAE, Blankenstein SA, van Akkooi ACJ. Primary melanoma: from history to actual debates. Curr Oncol Rep. 2019;21(12):112. [DOI] [PubMed] [Google Scholar]
- 112.Cohn-Cedermark G, Rutqvist LE, Andersson R, et al. Long term results of a randomized study by the Swedish Melanoma Study Group on 2-cm versus 5-cm resection margins for patients with cutaneous melanoma with a tumor thickness of 0.8–2.0 mm. Cancer. 2000;89(7):1495–1501. [PubMed] [Google Scholar]
- 113.Ringborg U, Andersson R, Eldh J, et al. Resection margins of 2 versus 5 cm for cutaneous malignant melanoma with a tumor thickness of 0.8 to 2.0 mm: randomized study by the Swedish Melanoma Study Group. Cancer. 1996;77(9):1809–1814. [DOI] [PubMed] [Google Scholar]
- 114.Khayat D, Rixe O, Martin G, et al. Surgical margins in cutaneous melanoma (2 cm versus 5 cm for lesions measuring less than 2.1-mm thick). Cancer. 2003;97(8):1941–1946. [DOI] [PubMed] [Google Scholar]
- 115.Balch CM, Soong SJ, Smith T, et al. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1–4 mm melanomas. Ann Surg Oncol. 2001;8(2):101–108. [DOI] [PubMed] [Google Scholar]
- 116.Balch CM, Urist MM, Karakousis CP, et al. Efficacy of 2-cm surgical margins for intermediate-thickness melanomas (1 to 4 mm). Results of a multi-institutional randomized surgical trial. Ann Surg. 1993;218(3):262–267; discussion 267–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hayes AJ, Maynard L, Coombes G, et al. Wide versus narrow excision margins for high-risk, primary cutaneous melanomas: long-term follow-up of survival in a randomised trial. Lancet Oncol. 2016;17(2):184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thomas JM, Newton-Bishop J, A’Hern R, et al. Excision margins in high-risk malignant melanoma. N Engl J Med. 2004;350(8):757–766. [DOI] [PubMed] [Google Scholar]
- 119.Veronesi U, Cascinelli N. Narrow excision (1-cm margin). A safe procedure for thin cutaneous melanoma. Arch Surg. 1991;126(4):438–441. [DOI] [PubMed] [Google Scholar]
- 120.Veronesi U, Cascinelli N, Adamus J, et al. Thin stage I primary cutaneous malignant melanoma. Comparison of excision with margins of 1 or 3 cm. N Engl J Med. 1988;318(18):1159–1162. [DOI] [PubMed] [Google Scholar]
- 121.MacKenzie Ross AD, Haydu LE, Quinn MJ, et al. the association between excision margins and local recurrence in 11,290 thin (T1) primary cutaneous melanomas: A case-control study. Ann Surg Oncol. 2016;23(4):1082–1089. [DOI] [PubMed] [Google Scholar]
- 122.NIH: U.S. National Library of Medicine. Melanoma margins trial investigating 1cm v 2cm wide excision margins for primary cutaneous melanoma (MelmarT). ClinicalTrials.gov. website. https://clinicaltrials.gov/ct2/show/results/NCT02385214. Accessed November 2, 2020.
- 123.Kunishige JH, Doan L, Brodland DG, Zitelli JA. Comparison of surgical margins for lentigo maligna versus melanoma in situ. J Am Acad Dermatol. 2019;81(1):204–212. [DOI] [PubMed] [Google Scholar]
- 124.Kunishige JH, Brodland DG, Zitelli JA. Surgical margins for melanoma in situ. J Am Acad Dermatol. 2012;66(3):438–444. [DOI] [PubMed] [Google Scholar]
- 125.Siscos SM, Neill BC, Seger EW, Hooton TA, Hocker TLH. The current state of Mohs surgery for the treatment of melanoma: A nationwide cross-sectional survey of Mohs surgeons. Dermatol Surg. 2020;46(10):1267–1271. [DOI] [PubMed] [Google Scholar]
- 126.Akhtar S, Bhat W, Magdum A, Stanley PR. Surgical excision margins for melanoma in situ. J Plast Reconstr Aesthet Surg. 2014;67(3):320–323. [DOI] [PubMed] [Google Scholar]
- 127.Cheraghlou S, Christensen SR, Agogo GO, Girardi M. Comparison of survival after Mohs micrographic surgery vs wide margin excision for early-stage invasive melanoma. JAMA Dermatol website. https://www.ncbi.nlm.nih.gov/pubmed/31553403. Accessed September 25, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Demer AM, Hanson JL, Maher IA, Liszewski W. Association of Mohs micrographic surgery vs wide local excision with overall survival outcomes for patients with melanoma of the trunk and extremities. JAMA Dermatol website. https://www.ncbi.nlm.nih.gov/pubmed/33084853. Accessed October 21, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hanson J, Demer A, Liszewski W, Foman N, Maher I. Improved overall survival of melanoma of the head and neck treated with Mohs micrographic surgery versus wide local excision. J Am Acad Dermatol. 2020;82(1):149–155. [DOI] [PubMed] [Google Scholar]
- 130.Nosrati A, Berliner JG, Goel S, et al. Outcomes of melanoma in situ treated with Mohs micrographic surgery compared with wide local excision. JAMA Dermatol. 2017;153(5):436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Whalen J, Leone D. Mohs micrographic surgery for the treatment of malignant melanoma. Clin Dermatol. 2009;27(6):597–602. [DOI] [PubMed] [Google Scholar]
- 132.Gonzalez A Sentinel lymph node biopsy: past and present implications for the management of cutaneous melanoma with nodal metastasis. Am J Clin Dermatol. 2018;19(Suppl 1):24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Veronesi U, Adamus J, Bandiera DC, et al. Inefficacy of immediate node dissection in stage 1 melanoma of the limbs. N Engl J Med. 1977;297(12):627–630. [DOI] [PubMed] [Google Scholar]
- 134.Veronesi U, Adamus J, Bandiera DC, et al. Delayed regional lymph node dissection in stage I melanoma of the skin of the lower extremities. Cancer. 1982;49(11):2420–2430. [DOI] [PubMed] [Google Scholar]
- 135.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392–399. [DOI] [PubMed] [Google Scholar]
- 136.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355(13):1307–1317. [DOI] [PubMed] [Google Scholar]
- 137.Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Coit DG, Thompson JA, Albertini MR, et al. Cutaneous melanoma, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(4):367–402. [DOI] [PubMed] [Google Scholar]
- 139.Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline - Update 2016. Eur J Cancer. 2016;63:201–217. [DOI] [PubMed] [Google Scholar]
- 140.Wong SL, Balch CM, Hurley P, et al. Sentinel lymph node biopsy for melanoma: American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guideline. Ann Surg Oncol. 2012;19(11):3313–3324. [DOI] [PubMed] [Google Scholar]
- 141.Wong SL, Faries MB, Kennedy EB, et al. Sentinel lymph node biopsy and management of regional lymph nodes in melanoma: American Society of Clinical Oncology and Society of Surgical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2018;36(4):399–413. [DOI] [PubMed] [Google Scholar]
- 142.Andtbacka RH, Gershenwald JE. Role of sentinel lymph node biopsy in patients with thin melanoma. J Natl Compr Canc Netw. 2009;7(3):308–317. [DOI] [PubMed] [Google Scholar]
- 143.Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(6):757–767. [DOI] [PubMed] [Google Scholar]
- 144.Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–1801. [DOI] [PubMed] [Google Scholar]
- 146.Hauschild A, Dummer R, Schadendorf D, et al. Longer follow-up confirms relapse-free survival benefit with adjuvant Dabrafenib plus Trametinib in patients with resected BRAF V600-mutant stage III melanoma. J Clin Oncol. 2018;36(35):3441–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Long GV, Hauschild A, Santinami M, et al. Adjuvant Dabrafenib plus Trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–1823. [DOI] [PubMed] [Google Scholar]
- 148.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835. [DOI] [PubMed] [Google Scholar]
- 149.NIH: U.S. National Library of Medicine. Safety and efficacy of Pembrolizumab compared to placebo in resected high-risk stage II melanoma (MK-3475-716/KEYNOTE-716). ClinicalTrials.gov. website. https://clinicaltrials.gov/ct2/show/NCT03553836. Accessed November 2, 2020.
- 150.Grossman D, Kim CC, Hartman RI, et al. Prognostic gene expression profiling in melanoma: necessary steps to incorporate into clinical practice. Melanoma Manag. 2019;6(4):MMT32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gastman BR, Gerami P, Kurley SJ, Cook RW, Leachman S, Vetto JT. Identification of patients at risk of metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J Am Acad Dermatol. 2019;80(1):149–157 e144. [DOI] [PubMed] [Google Scholar]
- 152.Greenhaw BN, Zitelli JA, Brodland DG. Estimation of prognosis in invasive cutaneous melanoma: An independent study of the accuracy of a gene expression profile test. Dermatol Surg. 2018;44(12):1494–1500. [DOI] [PubMed] [Google Scholar]
- 153.Keller J, Schwartz TL, Lizalek JM, et al. Prospective validation of the prognostic 31-gene expression profiling test in primary cutaneous melanoma. Cancer Med. 2019;8(5):2205–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zager JS, Gastman BR, Leachman S, et al. Performance of a prognostic 31-gene expression profile in an independent cohort of 523 cutaneous melanoma patients. BMC Cancer. 2018;18(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1-T2 melanoma using gene expression profiling. Future Oncol. 2019;15(11):1207–1217. [DOI] [PubMed] [Google Scholar]
- 156.Varedi A, Gardner LJ, Kim CC, et al. Use of new molecular tests for melanoma by pigmented-lesion experts. J Am Acad Dermatol. 2020;82(1):245–247. [DOI] [PubMed] [Google Scholar]
- 157.Marchetti MA, Coit DG, Dusza SW, et al. Performance of gene expression profile tests for prognosis in patients with localized cutaneous melanoma: A systematic review and meta-analysis. JAMA Dermatol. 2020;156(9):953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Romano E, Scordo M, Dusza SW, Coit DG, Chapman PB. Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. J Clin Oncol. 2010;28(18):3042–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Leiter U, Stadler R, Mauch C, et al. Final analysis of DeCOG-SLT Trial: No survival benefit for complete lymph node dissection in patients with melanoma with positive sentinel node. J Clin Oncol. 2019;37(32):3000–3008. [DOI] [PubMed] [Google Scholar]
- 160.van der Ploeg APT, van Akkooi ACJ, Schmitz PIM, et al. Therapeutic surgical management of palpable melanoma groin metastases: superficial or combined superficial and deep groin lymph node dissection Ann Surg Oncol. 2011;18(12):3300–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.National Comprehensive Cancer Network. Insights - Melanoma (version 3.2016). NCCN guidelines. website. https://education.nccn.org/melanoma-accred-review. Accessed December 21, 2020. [DOI] [PubMed] [Google Scholar]
- 162.Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655–1661. [DOI] [PubMed] [Google Scholar]
- 164.Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25(3):454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Najjar Y, McCurry D, Lin H, et al. A phase I study of neoadjuvant combination immunotherapy in locally/regionally advanced melanoma. J Clin Oncol. 2019;37(suppl 15):9586. doi: 9510.1200/JCO.2019.9537.9515_suppl.9586 [Google Scholar]
- 166.Reijers I, Rozeman EA, Menzies AM, et al. Personalized response-driven adjuvant therapy after combination ipilimumab and nivolumab in high-risk resectable stage III melanoma: PRADO trial. J Clin Oncol website. https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl.TPS9605. Accessed November 30, 2020. [Google Scholar]
- 167.Rozeman EA, Menzies AM, Krijgsman O, et al. 18-months relapse-free survival (RFS) and biomarker analyses of OpACIN-neo: A study to identify the optimal dosing schedule of neoadjuvant (neoadj) ipilimumab (IPI) + Nivolumab (NIVO) in stage III melanoma. Ann Oncol. 2019;30(suppl 5):v851–v934. [Google Scholar]
- 168.Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20(7):948–960. [DOI] [PubMed] [Google Scholar]
- 169.Long GV, Saw RPM, Lo S, et al. Neoadjuvant dabrafenib combined with trametinib for resectable, stage IIIB-C, BRAF(V600) mutation-positive melanoma (NeoCombi): a single-arm, open-label, single-centre, phase 2 trial. Lancet Oncol. 2019;20(7):961–971. [DOI] [PubMed] [Google Scholar]
- 170.Amaria RN, Prieto PA, Tetzlaff MT, et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: a single-centre, open-label, randomised, phase 2 trial. Lancet Oncol. 2018;19(2):181–193. [DOI] [PubMed] [Google Scholar]
- 171.Menzies AM, Rozeman EA, Amaria RN, et al. Pathological response and survival with neoadjuvant therapy in melanoma: A pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). J Clin Oncol website. https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl.9503. Accessed November 30, 2020. [DOI] [PubMed] [Google Scholar]
- 172.Grunhagen DJ, de Wilt JH, van Geel AN, Eggermont AM. Isolated limb perfusion for melanoma patients--a review of its indications and the role of tumour necrosis factor-alpha. Eur J Surg Oncol. 2006;32(4):371–380. [DOI] [PubMed] [Google Scholar]
- 173.Pawlik TM, Ross MI, Johnson MM, et al. Predictors and natural history of in-transit melanoma after sentinel lymphadenectomy. Ann Surg Oncol. 2005;12(8):587–596. [DOI] [PubMed] [Google Scholar]
- 174.Bello DM, Panageas KS, Hollmann T, et al. Survival outcomes after metastasectomy in melanoma patients categorized by response to checkpoint blockade. Ann Surg Oncol. 2020;27(4):1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nelson DW, Fischer TD, Graff-Baker AN, et al. Impact of effective systemic therapy on metastasectomy in stage IV melanoma: A matched-pair analysis. Ann Surg Oncol. 2019;26(13):4610–4618. [DOI] [PubMed] [Google Scholar]
- 176.Song Y, Straker RJ 3rd, Xu X, et al. Neoadjuvant versus adjuvant immune checkpoint blockade in the treatment of clinical stage III melanoma. Ann Surg Oncol. 2020;27(8):2915–2926. [DOI] [PubMed] [Google Scholar]
- 177.Ariyan CE, Brady MS, Siegelbaum RH, et al. Robust antitumor responses result from local chemotherapy and CTLA-4 blockade. Cancer Immunol Res. 2018;6(2):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jiang BS, Beasley GM, Speicher PJ, et al. Immunotherapy following regional chemotherapy treatment of advanced extremity melanoma. Ann Surg Oncol. 2014;21(8):2525–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Creech O Jr., Krementz ET, Ryan RF, Winblad JN Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann Surg. 1958;148(4):616–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fraker DL. Management of in-transit melanoma of the extremity with isolated limb perfusion. Curr Treat Options Oncol. 2004;5(3):173–184. [DOI] [PubMed] [Google Scholar]
- 181.Moreno-Ramirez D, de la Cruz-Merino L, Ferrandiz L, Villegas-Portero R, Nieto-Garcia A. Isolated limb perfusion for malignant melanoma: systematic review on effectiveness and safety. Oncologist. 2010;15(4):416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cornett WR, McCall LM, Petersen RP, et al. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol. 2006;24(25):4196–4201. [DOI] [PubMed] [Google Scholar]
- 183.Moller MG, Lewis JM, Dessureault S, Zager JS. Toxicities associated with hyperthermic isolated limb perfusion and isolated limb infusion in the treatment of melanoma and sarcoma. Int J Hyperthermia. 2008;24(3):275–289. [DOI] [PubMed] [Google Scholar]
- 184.Olieman AF, Schraffordt Koops H, Geertzen JH, Kingma H, Hoekstra HJ, Oldhoff J. Functional morbidity of hyperthermic isolated regional perfusion of the extremities. Ann Surg Oncol. 1994;1(5):382–388. [DOI] [PubMed] [Google Scholar]
- 185.Vrouenraets BC, in’t Veld GJ, Nieweg OE, van Slooten GW, van Dongen JA, Kroon BB. Long-term functional morbidity after mild hyperthermic isolated limb perfusion with melphalan. Eur J Surg Oncol. 1999;25(5):503–508. [DOI] [PubMed] [Google Scholar]
- 186.Vrouenraets BC, Klaase JM, Kroon BB, van Geel BN, Eggermont AM, Franklin HR. Long-term morbidity after regional isolated perfusion with melphalan for melanoma of the limbs. The influence of acute regional toxic reactions. Arch Surg. 1995;130(1):43–47. [DOI] [PubMed] [Google Scholar]
- 187.Thompson JF, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol. 1998;14(3):238–247. [DOI] [PubMed] [Google Scholar]
- 188.Kroon HM, Moncrieff M, Kam PC, Thompson JF. Outcomes following isolated limb infusion for melanoma. A 14-year experience. Ann Surg Oncol. 2008;15(11):3003–3013. [DOI] [PubMed] [Google Scholar]
- 189.Miura JT, Kroon HM, Beasley GM, et al. Long-term oncologic outcomes after isolated limb infusion for locoregionally metastatic melanoma: An international multicenter analysis. Ann Surg Oncol. 2019;26(8):2486–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chai CY, Deneve JL, Beasley GM, et al. A multi-institutional experience of repeat regional chemotherapy for recurrent melanoma of extremities. Ann Surg Oncol. 2012;19(5):1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zbar B, Bernstein ID, Rapp HJ. Suppression of tumor growth at the site of infection with living Bacillus Calmette-Guerin. J Natl Cancer Inst. 1971;46(4):831–839. [PubMed] [Google Scholar]
- 192.Morton DL, Eilber FR, Holmes EC, et al. BCG immunotherapy of malignant melanoma: summary of a seven-year experience. Ann Surg. 1974;180(4):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Storm FK, Sparks FC, Morton DL. Treatment for melanoma of the lower extremity with intralesional injection of bacille Calmette Guerin and hyperthermic perfusion. Surg Gynecol Obstet. 1979;149(1):17–21. [PubMed] [Google Scholar]
- 194.Mastrangelo MJ, Bellet RE, Berkelhammer J, Clark WH Jr., Regression of pulmonary metastatic disease associated with intralesional BCG therapy of intracutaneous melanoma metastases. Cancer. 1975;36(4):1305–1308. [DOI] [PubMed] [Google Scholar]
- 195.Mastrangelo MJ, Sulit HL, Prehn LM, Bornstein RS, Yarbro JW, Prehn RT. Intralesional BCG in the treatment of metastatic malignant melanoma. Cancer. 1976;37(2):684–692. [DOI] [PubMed] [Google Scholar]
- 196.Agarwala SS, Neuberg D, Park Y, Kirkwood JM. Mature results of a phase III randomized trial of bacillus Calmette-Guerin (BCG) versus observation and BCG plus dacarbazine versus BCG in the adjuvant therapy of American Joint Committee on Cancer Stage I-III melanoma (E1673): a trial of the Eastern Oncology Group. Cancer. 2004;100(8):1692–1698. [DOI] [PubMed] [Google Scholar]
- 197.Miura JT, Zager JS. Intralesional therapy as a treatment for locoregionally metastatic melanoma. Expert Rev Anticancer Ther. 2018;18(4):399–408. [DOI] [PubMed] [Google Scholar]
- 198.Eklund JW, Kuzel TM. A review of recent findings involving interleukin-2-based cancer therapy. Curr Opin Oncol. 2004;16(6):542–546. [DOI] [PubMed] [Google Scholar]
- 199.Pointer DT Jr., Zager JS. Management of locoregionally advanced melanoma. Surg Clin North Am. 2020;100(1):109–125. [DOI] [PubMed] [Google Scholar]
- 200.Temple-Oberle CF, Byers BA, Hurdle V, Fyfe A, McKinnon JG. Intra-lesional interleukin-2 therapy for in transit melanoma. J Surg Oncol. 2014;109(4):327–331. [DOI] [PubMed] [Google Scholar]
- 201.Radny P, Caroli UM, Bauer J, et al. Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases. Br J Cancer. 2003;89(9):1620–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Weide B, Derhovanessian E, Pflugfelder A, et al. High response rate after intratumoral treatment with interleukin-2: results from a phase 2 study in 51 patients with metastasized melanoma. Cancer. 2010;116(17):4139–4146. [DOI] [PubMed] [Google Scholar]
- 203.Byers BA, Temple-Oberle CF, Hurdle V, McKinnon JG. Treatment of in-transit melanoma with intra-lesional interleukin-2: a systematic review. J Surg Oncol. 2014;110(6):770–775. [DOI] [PubMed] [Google Scholar]
- 204.Thompson JF, Hersey P, Wachter E. Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res. 2008;18(6):405–411. [DOI] [PubMed] [Google Scholar]
- 205.Thompson JF, Agarwala SS, Smithers BM, et al. Phase 2 Study of Intralesional PV-10 in Refractory Metastatic Melanoma. Ann Surg Oncol. 2015;22(7):2135–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kohlhapp FJ, Kaufman HL. Molecular pathways: Mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin Cancer Res. 2016;22(5):1048–1054. [DOI] [PubMed] [Google Scholar]
- 207.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27(34):5763–5771. [DOI] [PubMed] [Google Scholar]
- 208.Andtbacka RHI, Collichio F, Harrington KJ, et al. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma. J Immunother Cancer. 2019;7(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. [DOI] [PubMed] [Google Scholar]
- 210.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. [DOI] [PubMed] [Google Scholar]
- 212.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 213.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109–1119 e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label phase ii study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol. 2018;36(17):1658–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Long GV, Dummer R, Andtbacka RH. Follow-up analysis of MASTERKEY-265 phase 1b (ph1b) study of talimogene laherparepvec (T-VEC) in combination (combo) with pembrolizumab (pembro) in patients (pts) with unresectable stage IIIB-IVM1c melanoma (MEL). Pigment Cell Melanoma Res. 2019:doi: 10.1111/pcmr12738. [DOI] [Google Scholar]
- 217.Long GV, Dummer R, Ribas A, et al. A Phase I/III, multicenter, open-label trial of talimogene laherparepvec (T-VEC) in combination with pembrolizumab for the treatment of unresected, stage IIIb-IV melanoma (MASTERKEY-265). J Immunother Cancer website. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4645515/. Accessed November 30, 2020. [Google Scholar]
- 218.Burmeister BH, Henderson MA, Ainslie J, et al. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol. 2012;13(6):589–597. [DOI] [PubMed] [Google Scholar]
- 219.Henderson MA, Burmeister BH, Ainslie J, et al. Adjuvant lymph-node field radiotherapy versus observation only in patients with melanoma at high risk of further lymph-node field relapse after lymphadenectomy (ANZMTG 01.02/TROG 02.01): 6-year follow-up of a phase 3, randomised controlled trial. Lancet Oncol. 2015;16(9):1049–1060. [DOI] [PubMed] [Google Scholar]
- 220.American Joint Committee on Cancer. Melanoma of the skin staging, 7th edition. website. https://cancerstaging.org/references-tools/quickreferences/documents/melanomasmall.pdf. Accessed August 17, 2020. [Google Scholar]
- 221.Bedikian AY, Johnson MM, Warneke CL, et al. Prognostic factors that determine the long-term survival of patients with unresectable metastatic melanoma. Cancer Invest. 2008;26(6):624–633. [DOI] [PubMed] [Google Scholar]
- 222.Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17(12):1743–1754. [DOI] [PubMed] [Google Scholar]
- 223.Nosrati A, Tsai KK, Goldinger SM, et al. Evaluation of clinicopathological factors in PD-1 response: derivation and validation of a prediction scale for response to PD-1 monotherapy. Br J Cancer. 2017;116(9):1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Enewold L, Sharon E, Harlan LC. Metastatic melanoma: Treatment and survival in the US after the introduction of ipilimumab and vemurafenib. Oncol Res Treat. 2017;40(4):174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wilson MA, Zhong J, Rosenbaum BE, et al. Impact of initial stage on metastatic melanoma survival. Melanoma Res. 2019;29(3):281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Vallet A, Oriano B, Mortier L, et al. Association of time from primary diagnosis to first distant relapse of metastatic melanoma with progression of disease and survival. JAMA Dermatol. 2019;155(6):673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Luen S, Wong SW, Mar V, et al. Primary tumor thickness is a prognostic factor in stage IV melanoma: A retrospective study of primary tumor characteristics. Am J Clin Oncol. 2018;41(1):90–94. [DOI] [PubMed] [Google Scholar]
- 228.Barth A, Wanek LA, Morton DL. Prognostic factors in 1,521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181(3):193–201. [PubMed] [Google Scholar]
- 229.Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18(22):3782–3793. [DOI] [PubMed] [Google Scholar]
- 230.Cronin KA, Lake AJ, Scott S, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer. 2018;124(13):2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Berk-Krauss J, Stein JA, Weber J, Polsky D, Geller AC. New systematic therapies and trends in cutaneous melanoma deaths among US Whites, 1986–2016. Am J Public Health. 2020;110(5):731–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.NIH: National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer stat facts: Melanoma of the skin. website. https://seer.cancer.gov/statfacts/html/melan.html. Accessed August 18, 2020.
- 233.Anwar MA, Murad F, Dawson E, Abd Elmageed ZY, Tsumagari K, Kandil E. Immunohistochemistry as a reliable method for detection of BRAF-V600E mutation in melanoma: a systematic review and meta-analysis of current published literature. J Surg Res. 2016;203(2):407–415. [DOI] [PubMed] [Google Scholar]
- 234.Gao G, Gong B, Shen W. Meta-analysis of the additional value of integrated 18FDG PET-CT for tumor distant metastasis staging: comparison with 18FDG PET alone and CT alone. Surg Oncol. 2013;22(3):195–200. [DOI] [PubMed] [Google Scholar]
- 235.Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology (Williston Park). 2009;23(6):488–496. [PMC free article] [PubMed] [Google Scholar]
- 236.Di Trolio R, Simeone E, Di Lorenzo G, Buonerba C, Ascierto PA. The use of interferon in melanoma patients: a systematic review. Cytokine Growth Factor Rev. 2015;26(2):203–212. [DOI] [PubMed] [Google Scholar]
- 237.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–2116. [DOI] [PubMed] [Google Scholar]
- 238.Tarhini AA, Agarwala SS. Interleukin-2 for the treatment of melanoma. Curr Opin Investig Drugs. 2005;6(12):1234–1239. [PubMed] [Google Scholar]
- 239.O’Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110(12):2614–2627. [DOI] [PubMed] [Google Scholar]
- 240.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. [DOI] [PubMed] [Google Scholar]
- 241.Schadendorf D, Hodi FS, Robert C. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in metastatic or locally advanced, unresectable melanome. European Cancer Congress Annual Meeting. Amsterdam, The Netherlands: 2013. [Google Scholar]
- 242.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kirkwood JM, Tarhini AA, Panelli MC, et al. Next generation of immunotherapy for melanoma. J Clin Oncol. 2008;26(20):3445–3455. [DOI] [PubMed] [Google Scholar]
- 244.Munhoz RR, Postow MA. Clinical development of PD-1 in advanced melanoma. Cancer J. 2018;24(1):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. [DOI] [PubMed] [Google Scholar]
- 247.Inamdar GS, Madhunapantula SV, Robertson GP. Targeting the MAPK pathway in melanoma: why some approaches succeed and other fail. Biochem Pharmacol. 2010;80(5):624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kainthla R, Kim KB, Falchook GS. Dabrafenib for treatment of BRAF-mutant melanoma. Pharmacogenomics Pers Med. 2014;7:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–2147. [DOI] [PubMed] [Google Scholar]
- 250.Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23(3):529–545, ix. [DOI] [PubMed] [Google Scholar]
- 251.Rubinstein JC, Sznol M, Pavlick AC, et al. Incidence of the V600K mutation among melanoma patients with BRAF mutations, and potential therapeutic response to the specific BRAF inhibitor PLX4032. J Transl Med. 2010;8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15(3):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Menzies AM, Long GV, Murali R. Dabrafenib and its potential for the treatment of metastatic melanoma. Drug Des Devel Ther. 2012;6:391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. [DOI] [PubMed] [Google Scholar]
- 256.Grob JJ, Amonkar MM, Martin-Algarra S, et al. Patient perception of the benefit of a BRAF inhibitor in metastatic melanoma: quality-of-life analyses of the BREAK-3 study comparing dabrafenib with dacarbazine. Ann Oncol. 2014;25(7):1428–1436. [DOI] [PubMed] [Google Scholar]
- 257.Hauschild A, Ascierto PA, Schadendorf D, et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib monotherapy: Analysis from phase 2 and 3 clinical trials. Eur J Cancer. 2020;125:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tanda ET, Vanni I, Boutros A, et al. Current state of target treatment in BRAF mutated melanoma. Front Mol Biosci. 2020;7:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lugowska I, Kosela-Paterczyk H, Kozak K, Rutkowski P. Trametinib: a MEK inhibitor for management of metastatic melanoma. Onco Targets Ther. 2015;8:2251–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367(18):1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–1876. [DOI] [PubMed] [Google Scholar]
- 262.Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371(20):1877–1888. [DOI] [PubMed] [Google Scholar]
- 263.Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386(9992):444–451. [DOI] [PubMed] [Google Scholar]
- 264.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39. [DOI] [PubMed] [Google Scholar]
- 265.Robert C, Grob JJ, Stroyakovskiy D, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381(7):626–636. [DOI] [PubMed] [Google Scholar]
- 266.Schadendorf D, Amonkar MM, Stroyakovskiy D, et al. Health-related quality of life impact in a randomised phase III study of the combination of dabrafenib and trametinib versus dabrafenib monotherapy in patients with BRAF V600 metastatic melanoma. Eur J Cancer. 2015;51(7):833–840. [DOI] [PubMed] [Google Scholar]
- 267.Dummer R, Hauschild A, Santinami M, et al. Five-year analysis of adjuvant dabrafenib plus trametinib in Stage III melanoma. N Engl J Med. 2020;383(12):1139–1148. [DOI] [PubMed] [Google Scholar]
- 268.Vidovic D, Giacomantonio C. Insights into the molecular mechanisms behind intralesional immunotherapies for advanced melanoma. Cancers (Basel). 2020;12(5):1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Vuong W, Lin J, Wei RL. Palliative radiotherapy for skin malignancies. Ann Palliat Med. 2017;6(2):165–172. [DOI] [PubMed] [Google Scholar]
- 270.Donia M, Kimper-Karl ML, Hoyer KL, Bastholt L, Schmidt H, Svane IM. The majority of patients with metastatic melanoma are not represented in pivotal phase III immunotherapy trials. Eur J Cancer. 2017;74:89–95. [DOI] [PubMed] [Google Scholar]
- 271.van Zeijl MCT, Ismail RK, de Wreede LC, et al. Real-world outcomes of advanced melanoma patients not represented in phase III trials. Int J Cancer. 2020;147(12):3461–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–465. [DOI] [PubMed] [Google Scholar]
- 273.Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672–681. [DOI] [PubMed] [Google Scholar]
- 275.Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18(7):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Enomoto LM, Levine EA, Shen P, Votanopoulos KI. Role of surgery for metastatic melanoma. Surg Clin North Am. 2020;100(1):127–139. [DOI] [PubMed] [Google Scholar]
- 280.Ollila DW, Hsueh EC, Stern SL, Morton DL. Metastasectomy for recurrent stage IV melanoma. J Surg Oncol. 1999;71(4):209–213. [DOI] [PubMed] [Google Scholar]
- 281.Deutsch GB, Kirchoff DD, Faries MB. Metastasectomy for stage IV melanoma. Surg Oncol Clin N Am. 2015;24(2):279–298. [DOI] [PubMed] [Google Scholar]
- 282.Leung AM, Hari DM, Morton DL. Surgery for distant melanoma metastasis. Cancer J. 2012;18(2):176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Howard JH, Thompson JF, Mozzillo N, et al. Metastasectomy for distant metastatic melanoma: analysis of data from the first Multicenter Selective Lymphadenectomy Trial (MSLT-I). Ann Surg Oncol. 2012;19(8):2547–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Franceschini D, Franzese C, De Rose F, et al. Role of extra cranial stereotactic body radiation therapy in the management of Stage IV melanoma. Br J Radiol. 2017;90(1077):20170257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Deutsch GB, Flaherty DC, Kirchoff DD, et al. Association of surgical treatment, systemic therapy, and survival in patients with abdominal visceral melanoma metastases, 1965–2014: Relevance of surgical cure in the era of modern systemic therapy. JAMA Surg. 2017;152(7):672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Hameed AM, Ng EE, Johnston E, et al. Hepatic resection for metastatic melanoma: a systematic review. Melanoma Res. 2014;24(1):1–10. [DOI] [PubMed] [Google Scholar]
- 287.Doussot A, Nardin C, Takaki H, et al. Liver resection and ablation for metastatic melanoma: A single center experience. J Surg Oncol. 2015;111(8):962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Faries MB, Leung A, Morton DL, et al. A 20-year experience of hepatic resection for melanoma: is there an expanding role? J Am Coll Surg. 2014;219(1):62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Shashank A, Shehata M, Morris DL, Thompson JF. Radiofrequency ablation in metastatic melanoma. J Surg Oncol. 2014;109(4):366–369. [DOI] [PubMed] [Google Scholar]
- 290.Mittendorf EA, Lim SJ, Schacherer CW, et al. Melanoma adrenal metastasis: natural history and surgical management. Am J Surg. 2008;195(3):363–368; discussion 368–369. [DOI] [PubMed] [Google Scholar]
- 291.de Wilt JH, McCarthy WH, Thompson JF. Surgical treatment of splenic metastases in patients with melanoma. J Am Coll Surg. 2003;197(1):38–43. [DOI] [PubMed] [Google Scholar]
- 292.Reddy S, Wolfgang CL. The role of surgery in the management of isolated metastases to the pancreas. Lancet Oncol. 2009;10(3):287–293. [DOI] [PubMed] [Google Scholar]
- 293.Ekedahl H, Cirenajwis H, Harbst K, et al. The clinical significance of BRAF and NRAS mutations in a clinic-based metastatic melanoma cohort. Br J Dermatol. 2013;169(5):1049–1055. [DOI] [PubMed] [Google Scholar]
- 294.Kamposioras K, Pentheroudakis G, Pectasides D, Pavlidis N. Malignant melanoma of unknown primary site. To make the long story short. A systematic review of the literature. Crit Rev Oncol Hematol. 2011;78(2):112–126. [DOI] [PubMed] [Google Scholar]
- 295.Cormier JN, Xing Y, Feng L, et al. Metastatic melanoma to lymph nodes in patients with unknown primary sites. Cancer. 2006;106(9):2012–2020. [DOI] [PubMed] [Google Scholar]
- 296.Lee CC, Faries MB, Wanek LA, Morton DL. Improved survival after lymphadenectomy for nodal metastasis from an unknown primary melanoma. J Clin Oncol. 2008;26(4):535–541. [DOI] [PubMed] [Google Scholar]
- 297.van der Ploeg AP, Haydu LE, Spillane AJ, et al. Melanoma patients with an unknown primary tumor site have a better outcome than those with a known primary following therapeutic lymph node dissection for macroscopic (clinically palpable) nodal disease. Ann Surg Oncol. 2014;21(9):3108–3116. [DOI] [PubMed] [Google Scholar]
- 298.Lee CC, Faries MB, Wanek LA, Morton DL. Improved survival for stage IV melanoma from an unknown primary site. J Clin Oncol. 2009;27(21):3489–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Helmink B, Wargo JA. Neoadjuvant therapy for melanoma: is it ready for prime time? Lancet Oncol. 2019;20(7):892–894. [DOI] [PubMed] [Google Scholar]
- 300.Lee S, Margolin K. Tumor-infiltrating lymphocytes in melanoma. Curr Oncol Rep. 2012;14(5):468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Besser MJ, Shapira-Frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16(9):2646–2655. [DOI] [PubMed] [Google Scholar]
- 302.Radvanyi LG, Bernatchez C, Zhang M, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2012;18(24):6758–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.NIH: U.S. National Library of Medicine. National Cancer Institute. Immunotherapy using tumor infiltrating lymphocytes for patients with metastatic melanoma. website. https://clinicaltrials.gov/ct2/show/NCT01993719. NLM identifier: NCT01993719. Accessed September 4, 2020.
- 304.NIH: U.S. National Library of Medicine. H. Lee Moffitt Cancer Center and Research Institute. Combining PD-1 blockade, CD137 agonism and adoptive cell therapy for metastatic melanoma. website. https://clinicaltrials.gov/ct2/show/NCT02652455. NLM identifier: NCT02652455. Accessed September 4, 2020.
- 305.Vacca P, Pietra G, Tumino N, Munari E, Mingari MC, Moretta L. Exploiting human NK cells in tumor therapy. Front Immunol. 2019;10:3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Chen Y, Yu F, Jiang Y, et al. Adoptive transfer of interleukin-21-stimulated human CD8+ T memory stem cells efficiently inhibits tumor growth. J Immunother. 2018;41(6):274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Ott PA, Govindan R, Naing A, et al. A personal neoantigen vaccine, NEO-PV-01, with anti-PD1 induces broad de novo anti-tumor immunity in patients with metastatic melanoma, NSCLC, and bladder cancer. Ann Oncol, 2018. website. https://www.annalsofoncology.org/article/S0923-7534(19)49584-7/fulltext. Accessed September 4, 2020. [Google Scholar]
- 308.Burris HA, Patel MR, Cho DC, et al. A phase I multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in patients with resected solid tumors and in combination with pembrolizumab in patients with unresectable solid tumors. J Clin Oncol. 2019;37(15):2523–2523. [Google Scholar]
- 309.NIH: U.S. National Library of Medicine. ModernaTX, Inc. A phase 2 randomized study of adjuvant immunotherapy with the personalized cancer vaccine mRNA-4157 and pembrolizumab versus pembrolizumab alone after complete resection of high-risk melanoma (KEYNOTE-942). website. https://clinicaltrials.gov/ct2/show/NCT03897881. NLM identifier: NCT03897881. Accessed September 13, 2020.
- 310.Payandeh Z, Yarahmadi M, Nariman-Saleh-Fam Z, et al. Immune therapy of melanoma: Overview of therapeutic vaccines. J Cell Physiol. 2019:doi: 10.1002/jcp.28181. [DOI] [PubMed] [Google Scholar]
- 311.Yazdani M, Gholizadeh Z, Nikpoor AR, et al. Vaccination with dendritic cells pulsed ex vivo with gp100 peptide-decorated liposomes enhances the efficacy of anti PD-1 therapy in a mouse model of melanoma. Vaccine. 2020;38(35):5665–5677. [DOI] [PubMed] [Google Scholar]
- 312.Garbe C A rational approach to the follow-up of melanoma patients. Recent Results Cancer Res. 2002;160:205–215. [DOI] [PubMed] [Google Scholar]
- 313.Leiter U, Buettner PG, Eigentler TK, Forschner A, Meier F, Garbe C. Is detection of melanoma metastasis during surveillance in an early phase of development associated with a survival benefit? Melanoma Res. 2010;20(3):240–246. [DOI] [PubMed] [Google Scholar]
- 314.Joseph RW, Elassaiss-Schaap J, Kefford R, et al. Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin Cancer Res. 2018;24(20):4960–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Blakely AM, Comissiong DS, Vezeridis MP, Miner TJ. Suboptimal compliance with National Comprehensive Cancer Network Melanoma Guidelines: Who is at risk? Am J Clin Oncol. 2018;41(8):754–759. [DOI] [PubMed] [Google Scholar]
- 316.Robinson AV, Keeble C, Lo MCI, et al. The neutrophil-lymphocyte ratio and locoregional melanoma: a multicentre cohort study. Cancer Immunol Immunother. 2020;69(4):559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Verver D, van Klaveren D, Franke V, et al. Development and validation of a nomogram to predict recurrence and melanoma-specific mortality in patients with negative sentinel lymph nodes. Br J Surg. 2019;106(3):217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.von Schuckmann LA, Hughes MCB, Ghiasvand R, et al. Risk of melanoma recurrence after diagnosis of a high-risk primary tumor. JAMA Dermatol. 2019;155(6):688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Bajaj S, Donnelly D, Call M, et al. Melanoma prognosis: Accuracy of the American Joint Committee on Cancer Staging Manual eighth edition. J Natl Cancer Inst. 2020;112(9):921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Francken AB, Accortt NA, Shaw HM, et al. Follow-up schedules after treatment for malignant melanoma. Br J Surg. 2008;95(11):1401–1407. [DOI] [PubMed] [Google Scholar]
- 321.Berger AC, Ollila DW, Christopher A, et al. Patient symptoms are the most frequent indicators of recurrence in patients with American Joint Committee on cancer stage II melanoma. J Am Coll Surg. 2017;224(4):652–659. [DOI] [PubMed] [Google Scholar]
- 322.Lee AY, Droppelmann N, Panageas KS, et al. Patterns and timing of initial relapse in pathologic stage II melanoma patients. Ann Surg Oncol. 2017;24(4):939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Francken AB, Shaw HM, Accortt NA, Soong SJ, Hoekstra HJ, Thompson JF. Detection of first relapse in cutaneous melanoma patients: implications for the formulation of evidence-based follow-up guidelines. Ann Surg Oncol. 2007;14(6):1924–1933. [DOI] [PubMed] [Google Scholar]
- 324.Francken AB, Accortt NA, Shaw HM, et al. Prognosis and determinants of outcome following locoregional or distant recurrence in patients with cutaneous melanoma. Ann Surg Oncol. 2008;15(5):1476–1484. [DOI] [PubMed] [Google Scholar]
- 325.Park TS, Phan GQ, Yang JC, et al. Routine computer tomography imaging for the detection of recurrences in high-risk melanoma patients. Ann Surg Oncol. 2017;24(4):947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kurtz J, Beasley GM, Agnese D, et al. Surveillance strategies in the follow-up of melanoma patients: too much or not enough? J Surg Res. 2017;214:32–37. [DOI] [PubMed] [Google Scholar]
- 327.Leon-Ferre RA, Kottschade LA, Block MS, et al. Association between the use of surveillance PET/CT and the detection of potentially salvageable occult recurrences among patients with resected high-risk melanoma. Melanoma Res. 2017;27(4):335–341. [DOI] [PubMed] [Google Scholar]
- 328.Lim KHJ, Spain L, Barker C, et al. Contemporary outcomes from the use of regular imaging to detect relapse in high-risk cutaneous melanoma. ESMO Open. 2018;3(2):e000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Dicker TJ, Kavanagh GM, Herd RM, et al. A rational approach to melanoma follow-up in patients with primary cutaneous melanoma. Scottish Melanoma Group. Br J Dermatol. 1999;140(2):249–254. [DOI] [PubMed] [Google Scholar]
- 330.Bloemendal M, van Willigen WW, Bol KF, et al. Early recurrence in completely resected IIIB and IIIC melanoma warrants restaging prior to adjuvant therapy. Ann Surg Oncol. 2019;26(12):3945–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Ollila DW, Meyers MO. Time may heal all wounds, but while it does, melanoma marches on. Ann Surg Oncol. 2019;26(12):3800–3802. [DOI] [PubMed] [Google Scholar]
- 332.Faries MB, Steen S, Ye X, Sim M, Morton DL. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217(1):27–34; discussion 34–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.DiFronzo LA, Wanek LA, Elashoff R, Morton DL. Increased incidence of second primary melanoma in patients with a previous cutaneous melanoma. Ann Surg Oncol. 1999;6(7):705–711. [DOI] [PubMed] [Google Scholar]
- 334.Helgadottir H, Isaksson K, Fritz I, et al. Multiple primary melanoma incidence trends over five decades, a nation-wide population-based study. J Natl Cancer Inst. 2020: Jun 24:djaa088. doi: 010.1093/jnci/djaa1088. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Zimmer L, Haydu LE, Menzies AM, et al. Incidence of new primary melanomas after diagnosis of stage III and IV melanoma. J Clin Oncol. 2014;32(8):816–823. [DOI] [PubMed] [Google Scholar]
- 336.Ferrone CR, Ben Porat L, Panageas KS, et al. Clinicopathological features of and risk factors for multiple primary melanomas. JAMA. 2005;294(13):1647–1654. [DOI] [PubMed] [Google Scholar]
- 337.Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29(3):257–263. [DOI] [PubMed] [Google Scholar]
- 338.Livingstone E, Krajewski C, Eigentler TK, et al. Prospective evaluation of follow-up in melanoma patients in Germany - results of a multicentre and longitudinal study. Eur J Cancer. 2015;51(5):653–667. [DOI] [PubMed] [Google Scholar]
- 339.Winstanley JB, White EG, Boyle FM, Thompson JF. What are the pertinent quality-of-life issues for melanoma cancer patients? Aiming for the development of a new module to accompany the EORTC core questionnaire. Melanoma Res. 2013;23(2):167–174. [DOI] [PubMed] [Google Scholar]
- 340.Stamataki Z, Brunton L, Lorigan P, Green AC, Newton-Bishop J, Molassiotis A. Assessing the impact of diagnosis and the related supportive care needs in patients with cutaneous melanoma. Support Care Cancer. 2015;23(3):779–789. [DOI] [PubMed] [Google Scholar]
- 341.Mijnhout GS, Teule GJ, Hoekstra OS, Pijpers R, Borgstein PJ, Meijer S. [Follow-up after melanoma resection is more extensive than recommended in the practice guidelines, primarily for reassurance of the patient]. Ned Tijdschr Geneeskd. 1999;143(19):997–1001. [PubMed] [Google Scholar]
- 342.Rychetnik L, McCaffery K, Morton R, Irwig L. Psychosocial aspects of post-treatment follow-up for stage I/II melanoma: a systematic review of the literature. Psychooncology. 2013;22(4):721–736. [DOI] [PubMed] [Google Scholar]
- 343.Francken AB, Hoekstra HJ. Follow-up of melanoma patients: the need for evidence-based protocols. Ann Surg Oncol. 2009;16(4):804–805. [DOI] [PubMed] [Google Scholar]
- 344.Garbe C, Hauschild A, Volkenandt M, et al. Evidence and interdisciplinary consense-based German guidelines: diagnosis and surveillance of melanoma. Melanoma Res. 2007;17(6):393–399. [DOI] [PubMed] [Google Scholar]
- 345.Murchie P, Nicolson MC, Hannaford PC, Raja EA, Lee AJ, Campbell NC. Patient satisfaction with GP-led melanoma follow-up: a randomised controlled trial. Br J Cancer. 2010;102(10):1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Hofmann U, Szedlak M, Rittgen W, Jung EG, Schadendorf D. Primary staging and follow-up in melanoma patients--monocenter evaluation of methods, costs and patient survival. Br J Cancer. 2002;87(2):151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U, clinicalguidelines@esmo.org EGCEa. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. 2019;30(12):1884–1901. [DOI] [PubMed] [Google Scholar]
- 348.Cancer Council Australia: Clinical Guidelines Network. Clinical practice guidelines for the diagnosis and management of melanoma. website. https://wiki.cancer.org.au/australia/Guidelines:Melanoma. Accessed September 12, 2020.
- 349.Podlipnik S, Moreno-Ramirez D, Carrera C, et al. Cost-effectiveness analysis of imaging strategy for an intensive follow-up of patients with American Joint Committee on Cancer stage IIB, IIC and III malignant melanoma. Br J Dermatol. 2019;180(5):1190–1197. [DOI] [PubMed] [Google Scholar]
- 350.Lewin J, Sayers L, Kee D, et al. Surveillance imaging with FDG-PET/CT in the post-operative follow-up of stage 3 melanoma. Ann Oncol. 2018;29(7):1569–1574. [DOI] [PubMed] [Google Scholar]
- 351.Koskivuo I, Kemppainen J, Giordano S, et al. Whole body PET/CT in the follow-up of asymptomatic patients with stage IIB-IIIB cutaneous melanoma. Acta Oncol. 2016;55(11):1355–1359. [DOI] [PubMed] [Google Scholar]
- 352.Madu MF, Timmerman P, Wouters M, van der Hiel B, van der Hage JA, van Akkooi ACJ. PET/CT surveillance detects asymptomatic recurrences in stage IIIB and IIIC melanoma patients: a prospective cohort study. Melanoma Res. 2017;27(3):251–257. [DOI] [PubMed] [Google Scholar]
- 353.Podlipnik S, Carrera C, Sanchez M, et al. Performance of diagnostic tests in an intensive follow-up protocol for patients with American Joint Committee on Cancer (AJCC) stage IIB, IIC, and III localized primary melanoma: A prospective cohort study. J Am Acad Dermatol. 2016;75(3):516–524. [DOI] [PubMed] [Google Scholar]
- 354.Bhutiani N, Egger ME, McMasters KM. Optimizing follow-up assessment of patients with cutaneous melanoma. Ann Surg Oncol. 2017;24(4):861–863. [DOI] [PubMed] [Google Scholar]
- 355.Fields RC, Coit DG. Evidence-based follow-up for the patient with melanoma. Surg Oncol Clin N Am. 2011;20(1):181–200. [DOI] [PubMed] [Google Scholar]
- 356.Freeman M, Laks S. Surveillance imaging for metastasis in high-risk melanoma: importance in individualized patient care and survivorship. Melanoma Manag. 2019;6(1):MMT12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Ipenburg NA, Thompson JF, Uren RF, Chung D, Nieweg OE. Focused ultrasound surveillance of lymph nodes following lymphoscintigraphy without sentinel node biopsy: A useful and safe strategy in elderly or frail melanoma patients. Ann Surg Oncol. 2019;26(9):2855–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Ulrich J, van Akkooi AJ, Eggermont AM, Voit C. New developments in melanoma: utility of ultrasound imaging (initial staging, follow-up and pre-SLNB). Expert Rev Anticancer Ther. 2011;11(11):1693–1701. [DOI] [PubMed] [Google Scholar]
- 359.Xing Y, Cromwell KD, Cormier JN. Review of diagnostic imaging modalities for the surveillance of melanoma patients. Dermatol Res Pract. 2012;2012:941921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Aderhold K, Wilson M, Berger AC, Levi S, Bennett J. Precision medicine in the treatment of melanoma. Surg Oncol Clin N Am. 2020;29(1):1–13. [DOI] [PubMed] [Google Scholar]
- 361.Knackstedt RW, Knackstedt T, Gastman B. Gene expression profiling in melanoma: past results and future potential. Future Oncol. 2019;15(7):791–800. [DOI] [PubMed] [Google Scholar]
- 362.Ferris LK, Farberg AS, Middlebrook B, et al. Identification of high-risk cutaneous melanoma tumors is improved when combining the online American Joint Committee on Cancer Individualized Melanoma Patient Outcome Prediction Tool with a 31-gene expression profile-based classification. J Am Acad Dermatol. 2017;76(5):818–825 e813. [DOI] [PubMed] [Google Scholar]
- 363.Knuever J, Weiss J, Persa OD, et al. The use of circulating cell-free tumor DNA in routine diagnostics of metastatic melanoma patients. Sci Rep. 2020;10(1):4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies. BMJ. 2020;368:m127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Gillgren P, Drzewiecki KT, Niin M, et al. 2-cm versus 4-cm surgical excision margins for primary cutaneous melanoma thicker than 2 mm: a randomised, multicentre trial. Lancet. 2011;378(9803):1635–1642. [DOI] [PubMed] [Google Scholar]
- 366.Utjes D, Malmstedt J, Teras J, et al. 2-cm versus 4-cm surgical excision margins for primary cutaneous melanoma thicker than 2 mm: long-term follow-up of a multicentre, randomised trial. Lancet. 2019;394(10197):471–477. [DOI] [PubMed] [Google Scholar]
- 367.NIH: U.S. National Library of Medicine. University of Chicago. Pembrolizumab and EDP1503 in advanced melanoma. website. https://clinicaltrials.gov/ct2/show/NCT03595683. NLM identifier: NCT03595683. Accessed September 4, 2020.
- 368.NIH: U.S. National Library of Medicine. University of Pittsburg. Nivolumab plus axitinib in patients with anti-PD1 refractory advanced melanoma. website. https://clinicaltrials.gov/ct2/show/NCT04493203. NLM identifier: NCT04493203. Accessed September 4, 2020.
- 369.NIH: U.S. National Library of Medicine. National Cancer Institute. Talimogene laherparepvec and pembrolizumab in treating patients with stage III-IV melanoma. website. https://clinicaltrials.gov/ct2/show/NCT02965716. NLM identifier: NCT02965716. Accessed September 4, 2020.
- 370.NIH: U.S. National Library of Medicine. Novartis Pharmaceuticals. A study of the anti-PD1 antibody PDR001, in combination with dabrafenib and trametinib in advanced melanoma (COMBI-i). website. https://clinicaltrials.gov/ct2/show/NCT02967692. NLM identifier: NCT02967692. Accessed September 4, 2020.
- 371.NIH: U.S. National Library of Medicine. Eastern Cooperative Oncology Group (ECOG-ACRIN Cancer Research Group). Testing the combination of dabrafenib and trametinib with or without hydroxychloroquine in patients with stage IIIc or IV BRAF V600E/K melanoma. website. https://clinicaltrials.gov/ct2/show/NCT04527549. NLM identifier: NCT04527549. Accessed September 4, 2020.
- 372.NIH: U.S. National Library of Medicine. Sunnybrook Health Sciences Centre. A pilot study of the neoadjuvant use of vemurafenib plus cobimetinib (GDC-0973) in patients with BRAF mutant melanoma with palpable lymph node metastases. website. https://clinicaltrials.gov/ct2/show/NCT02036086. NLM identifier: NCT02036086. Accessed September 13, 2020.
- 373.NIH: U.S. National Library of Medicine. Melanoma Institute Australia. Neoadjuvant dabrafenib plus trametinib for stage IIIB-C BRAF V600 mutation positive melanoma website. https://clinicaltrials.gov/ct2/show/NCT01972347. NLM identifier: NCT01972347. Accessed September 13, 2020.
- 374.NIH: U.S. National Library of Medicine. Bristol-Myers Squibb. Neoadjuvant nivolumab plus ipilimumab followed by adjuvant nivolumab or neoadjuvant nivolumab plus ipilimumab followed by adjuvant observation compared with adjuvant nivolumab in treatment-naïve high-risk melanoma participants (CheckMate 7UA). website. https://clinicaltrials.gov/ct2/show/NCT04495010. NLM identifier: NCT04495010. Accessed September 13, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Synoptic report according to College of American Pathologists (CAP) standards. This is an example of a synoptic report for reporting on a skin biopsy of a melanoma. It includes all criteria that are reported according to CAP standards (with permission from Shon W, Frishberg DP, Gershenwald JE, et al. Protocol for examination of excision specimens from patients with melanoma of the skin. College of American Pathologists website. https://documents.cap.org/protocols/cp-skin-melanoma-excision-19-4100.pdf.