Abstract
Objective:
The efficacy of naltrexone (Revia, Vivitrol) for the treatment of alcohol dependence exhibits a high degree of heterogeneity. The aim of the current study was to evaluate the extent to which variability in patient adherence to treatment contributed to the range of clinical responses observed during naltrexone treatment.
Method:
A systematic review was conducted of efficacy trials of naltrexone for the treatment of alcohol dependence to evaluate the level of adherence monitoring.
Results:
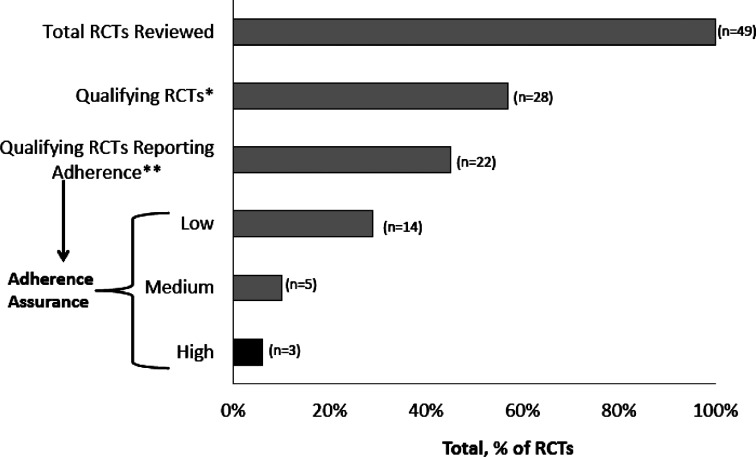
Of 49 identified trials, 22 (49%) met the inclusion criteria of being randomized, double-blind, placebo-controlled trials that reported adherence. The “adherence-assurance score” of these trials was calculated as a function of the frequency with which “low,” “moderate,” or “high” confidence levels of adherence monitoring were used. Of these 22 randomized, controlled trials, only 3 (14%) met criteria for high levels of adherence assurance, 5 (23%) met medium adherence-assurance criteria, and 14 (64%) met low adherence criteria. Of the three high-assurance studies, one used direct supervision of thrice-weekly oral dosing of naltrexone, and two used extended-release injectable formulations of naltrexone administered once per month. The Spearman correlation between risk ratios for return to heavy drinking (for naltrexone vs. placebo) and the level of adherence assurance (low vs. medium vs. high) was significant (r = -.62, p = .025).
Conclusions:
These findings suggest that the modest effect sizes for naltrexone reported in systematic reviews and meta-analyses may be attributable, at least in part, to variability in naltrexone adherence rates. High-assurance adherence strategies should be standard practice in clinical trials of medications being evaluated for the treatment of alcohol dependence.
Medication adherence (sometimes called “compliance”) can be defined as taking a medication according to the recommended dose and schedule. Medication nonadherence is a common problem and is associated with medication variables (presence of side effects, need for frequent dosing, use of oral medication), illness variables (chronicity, current symptom severity, presence of comor-bid illnesses requiring additional treatment), and patient variables (cognitive impairment, lack of insight, presence of comorbid depression and other psychological problems; Cramer and Rosenheck, 1998; DiMatteo, 2004; Osterberg and Blaschke, 2005; Pettinati et al., 2006; Substance Abuse and Mental Health Services Administration [SAMHSA], 2006). All of these factors are applicable to patients with alcohol dependence, and they contribute to the unusually high medication-nonadherence rates that have been reported in the medication treatment of alcohol-dependent patients (Cramer and Rosenheck, 1998; DiMatteo, 2004; Osterberg and Blaschke, 2005; Pettinati et al., 2006; SAMHSA, 2006).
Naltrexone is an opioid antagonist medication with high affinity for the μ-opioid receptor. Oral naltrexone (Revia) was granted Food and Drug Administration (FDA) approval in the United States in 1994 for the treatment of alcohol dependence. An injectable extended-release form of naltrexone (Vivitrol) received FDA approval for alcohol dependence treatment in 2004. Systematic reviews and meta-analyses (Bouza et al., 2004; Kranzler and Van Kirk, 2001; Pettinati et al., 2006; Roozen et al., 2006; Rösner et al., 2010; Srisurapanont and Jarusuraisin, 2005; Streeton and Whelan, 2001) have found oral naltrexone treatment to be efficacious for the treatment of alcohol dependence but with relatively small effect sizes. In post hoc subgroup analyses, medication non-adherence has been shown to contribute to the reduced effect sizes observed for oral naltrexone (Chick et al., 2000; Krystal et al., 2001; Pettinati et al., 2000). Similarly, Baros et al. (2007) reported a notably larger effect size (.58) in patients who received stringent medication monitoring (medication event monitoring system [MEMS] + urine riboflavin adherence monitoring) compared with the modest effect size (.18) observed in the overall intent-to-treat population. Analyses of prescription databases confirm a low level of persistence on oral naltrexone treatment, with the majority of patients discontinuing medication by 1 month (Harris et al., 2004; McCarty et al., 2009) and more than 75% discontinuing by 6 months (Chalk et al., 2011; Hermos et al., 2004; Kranzler et al., 2008).
Because of the significant impact of nonadherence on clinical outcome in alcohol-dependent patients treated with naltrexone, it is important to accurately measure adherence. In clinical trials, several methods are commonly used to monitor medication adherence, including patient self-report; counts of returned pills; use of blister packs; electronic monitoring of pill bottle opening (MEMS caps); the use of a biochemical marker, such as riboflavin, that is consumed along with the medication; supervised dosing to ensure consumption; monitoring of medication blood levels; and injection of once-monthly extended-release formulations.
We report here the results of a systematic review of how rigorously adherence was monitored in published naltrexone clinical trials, and how the level of adherence monitoring correlated with treatment effect size.
Method
Clinical trials of naltrexone were identified through four meta-analyses (Bouza et al., 2004; Kranzler and Van Kirk, 2001; Srisurapanont and Jarusuraisin, 2005; Streeton and Whelan, 2001) and three systematic reviews (Pettinati et al., 2006; Roozen et al., 2006; Rösner et al., 2010) of pharmacological treatment for alcohol dependence. In addition, a search was performed in PubMed using the search terms naltrexone and alcohol dependence. The search was limited to double-blind, placebo-controlled efficacy studies with a duration of 12 weeks or longer in human subjects with alcohol dependence or abuse, published in the English language. Studies involving patients with comorbid substance dependencies or psychiatric conditions were included.
The study applied a metric “adherence-monitoring score” to the methods used to monitor naltrexone treatment adherence in each of the qualifying trials. First, the adherence-monitoring method was assigned a “low,” “medium,” or “high” confidence level, based on its susceptibility to circumvention by the patient. Study methods that lacked direct supervision (i.e., self-report, pill count, blister pack) were assigned a low confidence level. Measurement of a moderately reliable analyte (i.e., riboflavin tracer in urine [Del Boca et al., 1996]) and methods that would require considerable patient effort to circumvent (i.e., MEMS caps; Feinn et al., 2003) were assigned a medium confidence level. Supervised administration of medication by a treatment provider (i.e., supervised dosing and use of extended-release injectable formulations [Gastfriend, 2011]) or measurement of a highly reliable analyte in the blood or urine (i.e., 6β-naltrexol [Cone et al., 1974; Meyer et al., 1984]) were assigned a high confidence level.
Second, an adherence-assurance score for each qualifying study using a single adherence-monitoring method was calculated using the following formula:
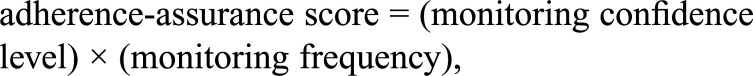 |
where monitoring confidence levels have been assigned a numerical value of 1 (low), 2 (medium), or 3 (high), and monitoring frequency equals the percentage of dosing days on which the monitoring method was used.
For studies using multiple monitoring methods, the following formula was used:
 |
Each positive test for 6β-naltrexol in plasma or urine or for riboflavin in urine was considered to provide confirmation for only a single dosing day because of the complex nature of inferring multiple administrations from an analyte concentration at a single time point. For trials that reported adherence rates and lacked a specific monitoring method frequency, adherence assurance was calculated as a lower limit, based on the available information. When multiple monitoring methods were used concurrently, scoring for that time interval was based on the highest confidence level. No additional assurance was allotted when methods having the same confidence level (e.g., pill counts and self-reports) were used concurrently. Calculated raw scores were normalized to 100%. Analyzed trials were ranked by adherence-assurance scores and assigned an adherence-assurance rating of high (3), medium (2), or low (1), based on their rank.
The relationship between the adherence-assurance score and treatment efficacy was evaluated with a Spearman correlation analysis. Efficacy was examined in terms of the risk ratio for return to heavy drinking (i.e., percentage of naltrexone-treated patients who returned to heavy drinking divided by the percentage of placebo-treated patients who returned to heavy drinking). Risk ratios were obtained from a Cochrane meta-analysis (Rösner et al., 2010) supplemented by additional values calculated from data from the original articles in the cases where the studies were not included in the Cochrane meta-analysis (Rösner et al., 2010). The results of two trials were not reported in a format that permitted calculation of heavy drinking risk ratios (Garbutt et al., 2005; Monterosso et al., 2001). The adherence-assurance ratings were converted into a 3-point scale (3 = high; 2 = medium; 1 = low), and a Spearman correlation was calculated relating the heavy drinking risk ratios to the adherence-assurance rating scale.
Results
A total of 49 trials assessing naltrexone efficacy for alcohol dependence were identified for this review. Twenty-eight (57%) of these were randomized, double-blind, placebo-controlled trials of at least 12 weeks’ duration and published in English. Of these 28 “qualifying” trials, 22 (79%) met the additional criterion of reporting adherence results and were included in the analysis. Six qualifying trials did not report adherence and thus were excluded from the analysis. The 22 analyzed studies spanned the period from 1992 to 2006, had sample sizes ranging from 31 to 618 patients treated with active naltrexone, had treatment durations of 12 to 52 weeks, and used doses ranging from 50 to 100 mg/day for oral naltrexone and from 150 to 380 mg/month for extended-release naltrexone. Twenty-one (43%) of the 49 identified efficacy studies did not meet the qualifying criteria. The reasons for exclusion included study design (seven open-label trials and six single-label trials), durations of less than 12 weeks (seven trials), and publication in a language other than English (one trial). A detailed summary of the qualifying trials and of the studies that were included and excluded is available on request from the first author.
High adherence-assurance scores were obtained for only a small fraction of the trials under consideration. Of the 22 qualifying trials reporting adherence, only 3 (14%) displayed high ratings for adherence assurance (Table 1 and Figure 1). These 3 represented only 6% of the 49 efficacy trials of naltrexone for alcohol dependence identified in the present study (Figure 1). An additional 5 trials (23% of 22) displayed medium ratings for adherence assurance, whereas the remaining 14 trials (64% of 22) displayed low ratings for adherence assurance (Table 1 and Figure 1).
Table 1.
Adherence-assurance rankings for qualifying studies (n = 22 studies)
| Monitoring method A |
Monitoring method B |
||||||||||
| Study | Method | Confidencea (%) | Frequency (%) | Subscore | Method | Confidence (%) | Frequency (%) | Subscore | Raw score (%) | Normal score (%) | Adherence-assurance rating |
| Garbutt et al., 2005b | Extended release | 3 | 100 | 300 | – | – | – | – | 300 | 100 | High |
| Kranzler et al., 2004c | Extended release | 3 | 100 | 300 | – | – | – | – | 300 | 100 | High |
| Oslinetal., 1997 | Direct supervision | 3 | 100 | 300 | – | – | – | – | 300 | 100 | High |
| Krystal et al., 2001 | 6β-naltrexol (plasma) | 3 | 1 | 3 | MEMS caps | 2 | 99 | 198 | 201 | 67 | Medium |
| O'Malley et al., 2003 (Trial 2) | MEMS caps | 2 | 100 | 200 | – | – | – | – | 200 | 67 | Medium |
| O'Malley et al., 2003 (Trial 3) | MEMS caps | 2 | 100 | 200 | – | – | – | – | 200 | 67 | Medium |
| Petrakis et al., 2005 | MEMS caps | 2 | 100 | 200 | – | – | – | – | 200 | 67 | Medium |
| Guardia et al., 2002 | Intermittent direct supervision | 3 | 43 | 129 | Pill counts | 1 | 57 | 57 | 186 | 62 | Medium |
| Monti et al., 2001 | Riboflavin | 2 | 10 | 20 | Pill counts | 1 | 90 | 90 | 110 | 37 | Low |
| Chick et al., 2000 | 6β-naltrexol (urine) | 3 | N.R. | Unknown | Pill counts | 1 | 100–N.R. | <100 | >100 | >33 | Low |
| Gastpar et al., 2002 | 6β-naltrexol (urine) | 3 | N.R. | Unknown | Pill counts | 1 | 100–N.R. | <100 | >100 | >33 | Low |
| Anton et al., 2006 | Blister packs | 1 | 100 | 100 | – | – | – | – | 100 | 33 | Low |
| Balldin et al., 2003 | Pill counts | 1 | 100 | 100 | – | – | – | – | 100 | 33 | Low |
| Kiefer et al.,2003 | Pill counts | 1 | 100 | 100 | – | – | – | – | 100 | 33 | Low |
| Monterosso et al., 2001 | Blister packs | 1 | 100 | 100 | – | – | – | – | 100 | 33 | Low |
| Morley et al., 2006 | Pill counts | 1 | 100 | 100 | Self-report | 1 | N.R. | 0 | 100 | 33 | Low |
| Morris et al., 2001 | Pill counts | 1 | 100 | 100 | – | – | – | – | 100 | 33 | Low |
| Petrakis et al., 2004 | Pill counts | 1 | 100 | 100 | – | – | – | – | 100 | 33 | Low |
| Volpicelli et al., 1997 | Pill counts | 1 | 100 | 100 | Self-report | 1 | 100 | 0 | 100 | 33 | Low |
| Schmitz et al., 2004 | Direct supervision | 3 | 24 | 72 | Riboflavin | 2 | N.R. | Unknown | >72 | >24 | Low |
| Anton etal., 1999 | Riboflavin | 2 | 14 | 28 | – | – | – | – | 28 | 9 | Low |
| O'Malley et al., 1992 | Riboflavin | 2 | 7 | 14 | – | – | – | – | 14 | 5 | Low |
Notes: N.R. = not reported.
Confidence: 1 = low (self-report, pill counts, blister packs); 2 = medium (MEMS [Medication Event Monitoring System], riboflavin testing); 3 = high (supervision, extended-release formulation);
Garbutt et al. (2005) used the extended-release formulation developed by Alkermes, Inc., Cambridge, MA;
Kranzler et al. (2004) used the extended-release formulation developed by DrugAbuse Sciences, Inc., Hayward, CA.
Figure 1.
Adherence assurance of efficacy trials with naltrexone. RCTs = randomized controlled trials. *Randomized, double-blind, placebo-controlled trials of ≥ 12 weeks’ duration; **adherence rate reported with results.
Trials reviewed here used diverse adherence-monitoring strategies. As shown in Table 1, trials that received a high rating for adherence assurance used high-confidence monitoring methods on a daily basis throughout. Several trials received medium or low ratings for adherence assurance despite the use of high-confidence methods (typically 6β-naltrexol measurement) because such methods were used at low (or unreported) frequencies (Table 1). Of the 22 qualifying trials reporting adherence, 8 (36%) used multiple methods to monitor adherence; nevertheless, in 6 of these, the adherence-assurance rating was still low.
The Spearman correlation between risk ratios for return to heavy drinking (for naltrexone vs. placebo) and level of adherence assurance (low vs. medium vs. high) was statistically significant (r = -.62, p = .025).
Discussion
A fundamental precondition for determining the efficacy of any treatment is confirmation that the treatment was, in fact, administered as prescribed. This systematic review of naltrexone randomized controlled trials indicates that the vast majority of clinical trials (71%) either did not report adherence rates (21%) or used low assurance-monitoring strategies (50%). Given the consensus regarding the negative impact of nonadherence on naltrexone response (Bouza et al., 2004; Kranzler and Van Kirk, 2001; Pettinati et al., 2006; Roozen et al., 2006; Rösner et al., 2010; Srisurapanont and Jarusuraisin, 2005; Streeton and Whelan, 2001), this review raises questions about the reliability of current estimates of the efficacy of naltrexone. These estimates are largely based on meta-analyses and systematic reviews in which the majority of included studies leave open the question as to how much of the prescribed medication was ingested.
Baros et al. (2007) reported effect sizes that were progressively larger, not just for reduction in heavy drinking days but also for abstinence, in patient subgroups subjected to more rigorous adherence-monitoring strategies. This finding is noteworthy because it contrasts starkly with the results of the two most recent reviews of naltrexone efficacy, both of which concluded that naltrexone is not efficacious in promoting abstinence (Pettinati et al., 2006; Roozen et al., 2006). Interestingly, in an analysis of patients treated with an extended-release formulation of naltrexone, O'Malley et al. (2007) also found a strong abstinence effect in patients abstinent at treatment initiation.
The current systematic review confirmed the finding of Baros et al. (2007). There was a moderate and significant correlation (r = -.62, p = .025) between the adherence-assurance score and the risk ratios for return to heavy drinking for naltrexone, compared with placebo.
In community-based alcoholism-treatment programs, even in the most sophisticated programs, almost all of the strategies used to ensure adherence in the trials reviewed here are impractical. Direct supervision of oral-medication administration is generally unrealistic because of patient burden and staff cost. In the Oslin study, participants were required to come to treatment three times a week for observed dosing by a staff member (Oslin et al., 1997). Technological adherence-monitoring methods—such as MEMS caps, blister packs, ri-boflavin tracer, and blood and urine assays—also may not be realistic or cost-effective outside the clinical trial arena. We were able to identify only one community-based naltrexone trial that used MEMS caps, and the researchers found that only 51% of the medication was taken as prescribed (Killeen et al., 2004). However, at this time, we are unaware of any reports on the incorporation of these monitoring technologies into real-world practice.
Psychosocial methods have been used to foster adherence in clinical trials. These methods include medication management (Pettinati et al., 2005); BRENDA (Anton et al., 2006; Garbutt et al., 2005); behavioral family counseling (O'Farrell and Fals-Stewart, 2002); and contingency management (Carroll and Onken, 2005). In practice, these interventions have not been widely adopted in community settings—and other than family counseling, these methods do not provide inherent adherence assurance. The challenges associated with the transfer of research-based psychosocial therapies have been well-documented (Backer et al., 1995; Carroll et al., 2002; Miller et al., 2004; Morgenstern et al., 2001).
Another approach to improving medication adherence is to reduce the administration frequency through the use of long-acting formulations (Center for Substance Abuse Treatment, SAMHSA, 2007; Kleber et al., 2007; Weiss, 2004). The issue of noncompliance with oral naltrexone has been recognized in the substance use disorders field for more than 30 years (Willette, 1975), and indeed the National Institutes of Health called for the development of a long-acting form of naltrexone to increase adherence (Willette, 1975).
The two extended-release naltrexone studies included in this review both qualified as high adherence-assurance studies, because the pharmacokinetics of these formulations ensure medication exposure for at least 30 days and administration of injection by a health care professional provides verification that each dose was received (Garbutt et al., 2005; Kranzler et al., 2004). One of these formulations was approved by the Food and Drug Administration in April 2006 and may pharmacokinetically fully address the challenge of adherence and adherence monitoring (Center for Substance Abuse Treatment, SAMHSA, 2007). In real-world use, three retrospective insurance-claims data analyses have confirmed significantly longer mean refill persistency with extended-release naltrexone in comparison with approved oral agents (Chalk et al., 2011; Mark et al., 2010; McCarty et al., 2009).
One limitation of this review is the retrospective nature of the analysis. In addition, the analysis was limited to medication adherence and did not evaluate other study-design variables that might be relevant. Other limitations include the use of only one bibliographic database to identify studies and the use of a nonvalidated scoring algorithm to compare adherence methods. Notwithstanding such limitations, the results show the high degree of heterogeneity of adherence-reporting and adherence-monitoring methods and illustrate the challenges in formulating conclusions on the efficacy of pharmacologic agents, such as oral naltrexone, in the treatment of alcohol dependence.
Conclusions
Differing standards in adherence-assurance monitoring and reporting contribute to the variance in outcome in published clinical trials of naltrexone for the treatment of alcohol dependence. This structured review demonstrates that, to date, a preponderance of studies published on alcohol-dependence pharmacotherapy have used methods that do not provide high levels of adherence assurance. As a result, valid inferences cannot confidently be made concerning the efficacy of naltrexone in treating alcohol dependence. However, it is important to note that the use of rigorous adherence-monitoring methods, such as observed daily dosing, may increase the internal validity of a clinical trial but only at the expense of external validity, and thus the results may not generalize to “real-world” clinical practice settings. The availability of an extended-release injectable formulation of naltrexone may ensure treatment adherence in a manner that is relevant to actual clinical practice.
In conclusion, accurate quantitative determination of treatment adherence, and assurance of treatment adherence, would appear to be an essential requirement for valid research in the alcohol-dependent patient population. The current findings have implications for research design, grant review; editorial review; and, most important, clinical practice.
Footnotes
This research was supported by funding from Alkermes, Inc., Waltham, MA.
References
- Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: Results of a placebo-controlled trial. American Journal of Psychiatry . 1999;156:1758–1764. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM Zweben, A., & the COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study: A randomized controlled trial. Journal of the American Medical Association . 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Backer TE, David SL, Soucy G. Reviewing the behavioral knowledge base of technology transfer . Rockville, MD: National Institute on Drug Abuse; 1995. (Monograph No. 155, pp. 1–300). [PubMed] [Google Scholar]
- Balldin J, Berglund M, Borg S, Månsson M, Bendtsen P, Franck J, Willander A A 6-month controlled naltrexone study: Combined effect with cognitive behavioral therapy in outpatient treatment of alcohol dependence. Alcoholism: Clinical and Experimental Research . 2003;27:1142–1149. doi: 10.1097/01.ALC.0000075548.83053.A9. [DOI] [PubMed] [Google Scholar]
- Baros AM, Latham PK, Moak DH, Voronin K, Anton RF What role does measuring medication compliance play in evaluating the efficacy of naltrexone? Alcoholism: Clinical and Experimental Research . 2007;31:596–603. doi: 10.1111/j.1530-0277.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- Bouza C, Angeles M, Muñoz A, Amate JM Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: A systematic review. Addiction . 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Farentinos C, Ball SA, Crits-Christoph P, Libby B, Morgenstern J, Woody GE MET meets the real world: Design issues and clinical strategies in the Clinical Trials Network. Journal of Substance Abuse Treatment . 2002;23:73–80. doi: 10.1016/s0740-5472(02)00255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Onken LS Behavioral therapies for drug abuse. American Journal of Psychiatry . 2005;162:1452–1460. doi: 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration. Naltrexone for extended-release injectable suspension for treatment of alcohol dependence. Substance Abuse Treatment Advisory . 2007;6:1–6. Available at http://kap.samhsa.gov/products/manuals/pdfs/naltrexone.pdf. [Google Scholar]
- Chalk M, Baser O, Halpern R, Gastfriend DR. Healthcare cost outcomes for alcohol dependence: A comparison of four treatments . Washington, DC: Presented at American Society of Addiction Medicine 42nd Annual Medical-Scientific Conference; 2011, April. [Google Scholar]
- Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R, Ritson B A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol and Alcoholism . 2000;35:587–593. doi: 10.1093/alcalc/35.6.587. [DOI] [PubMed] [Google Scholar]
- Cone EJ, Gorodetzky CW, Yeh SY The urinary excretion profile of naltrexone and metabolites in man. Drug Metabolism and Disposition: The Biological Fate of Chemicals . 1974;2:506–512. [PubMed] [Google Scholar]
- Cramer JA, Rosenheck R Compliance with medication regimens for mental and physical disorders. Psychiatric Services . 1998;49:196–201. doi: 10.1176/ps.49.2.196. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcoholism: Clinical and Experimental Research . 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Medical Care . 2004;42:200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- Feinn R, Tennen H, Cramer J, Kranzler HR Measurement and prediction of medication compliance in problem drinkers. Alcoholism: Clinical and Experimental Research . 2003;27:1286–1292. doi: 10.1097/01.ALC.0000080670.59386.6E. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O'Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Ehrich EW the Vivitrex Study Group. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: A randomized controlled trial. Journal of the American Medical Association . 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Gastfriend DR Intramuscular extended-release naltrexone: Current evidence. Annals ofthe New York Academy ofSciences . 2011;1216:144–166. doi: 10.1111/j.1749-6632.2010.05900.x. [DOI] [PubMed] [Google Scholar]
- Gastpar M, Bonnet U, Böning J, Mann K, Schmidt LG, Soyka M, Croop R Lack of efficacy of naltrexone in the prevention of alcohol relapse: Results from a German multicenter study. Journal of Clinical Psychopharmacology . 2002;22:592–598. doi: 10.1097/00004714-200212000-00009. [DOI] [PubMed] [Google Scholar]
- Guardia J, Caso C, Arias F, Gual A, Sanahuja J, Ramírez M, Ca-sas M A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: Results from a multi-center clinical trial. Alcoholism: Clinical and Experimental Research . 2002;26:1381–1387. doi: 10.1097/01.ALC.0000030561.15921.A9. [DOI] [PubMed] [Google Scholar]
- Harris KM, DeVries A, Dimidjian K Datapoints: Trends in naltrexone use among members of a large private health plan. Psychiatric Services . 2004;55:221. doi: 10.1176/appi.ps.55.3.221. [DOI] [PubMed] [Google Scholar]
- Hermos JA, Young MM, Gagnon DR, Fiore LD Patterns of dispensed disulfiram and naltrexone for alcoholism treatment in a veteran patient population. Alcoholism: Clinical and Experimental Research . 2004;28:1229–1235. doi: 10.1097/01.alc.0000134234.39303.17. [DOI] [PubMed] [Google Scholar]
- Killeen TK, Brady KT, Gold PB, Simpson KN, Faldowski RA, Tyson C, Anton RF Effectiveness of naltrexone in a community treatment program. Alcoholism: Clinical and Experimental Research . 2004;28:1710–1717. doi: 10.1097/01.alc.0000145688.30448.2c. [DOI] [PubMed] [Google Scholar]
- Kleber HD, Weiss RD, Anton RF, George TP, Greenfield SF, Kosten TR, Regier D. American Psychiatric Association; 2007. Treatment of patients with substance use disorders, second edition. American Journal of Psychiatry, 164, Supplement 4, 5–123. [PubMed] [Google Scholar]
- Kranzler HR, Stephenson JJ, Montejano L, Wang S, Gastfriend DR Persistence with oral naltrexone for alcohol treatment: Implications for health-care utilization. Addiction . 2008;103:1801–1808. doi: 10.1111/j.1360-0443.2008.02345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Van Kirk J Efficacy of naltrexone and acam-prosate for alcoholism treatment: A meta-analysis. Alcoholism: Clinical and Experimental Research . 2001;25:1335–1341. [PubMed] [Google Scholar]
- Kranzler HR, Wesson DR, Billot L the DrugAbuse Sciences Naltrexone Depot Study Group. Naltrexone depot for treatment of alcohol dependence: A multicenter, randomized, placebo-controlled clinical trial. Alcoholism: Clinical and Experimental Research . 2004;28:1051–1059. doi: 10.1097/01.alc.0000130804.08397.29. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA the Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. The New England Journal of Medicine . 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Mark TL, Montejano LB, Kranzler HR, Chalk M, Gastfriend DR Comparison of healthcare utilization among patients treated with alcoholism medications. American Journal of Managed Care . 2010;16:879–888. [PMC free article] [PubMed] [Google Scholar]
- McCarty D, McConnell J, Leader D, Un H. Continued use of addiction medications and impacts on use of health care . San Francisco, CA: Presented at Addiction Health Services Research Conference; 2009, October. [Google Scholar]
- Meyer MC, Straughn AB, Lo MW, Schary WL, Whitney CC Bioequivalence, dose-proportionality, and pharmacokinetics of naltrexone after oral administration. Journal of Clinical Psychiatry . 1984;45(9 Pt 2):15–19. [PubMed] [Google Scholar]
- Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M A randomized trial of methods to help clinicians learn motivational interviewing. Journal of Consulting and Clinical Psychology . 2004;72:1050–1062. doi: 10.1037/0022-006X.72.6.1050. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O'Brien CP, Volpicelli JR Predicting treatment response to naltrexone: The influence of craving and family history. American Journal on Addictions . 2001;10:258–268. doi: 10.1080/105504901750532148. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Swift RM, Gulliver SB, Colby SM, Mueller TI, Asher MK Naltrexone and cue exposure with coping and communication skills training for alcoholics: Treatment process and 1-year outcomes. Alcoholism: Clinical and Experimental Research . 2001;25:1634–1647. [PubMed] [Google Scholar]
- Morgenstern J, Morgan TJ, McCrady BS, Keller DS, Carroll KM Manual-guided cognitive-behavioral therapy training: A promising method for disseminating empirically supported substance abuse treatments to the practice community. Psychology of Addictive Behaviors . 2001;15:83–88. [PubMed] [Google Scholar]
- Morley KC, Teesson M, Reid SC, Sannibale C, Thomson C, Phung N, Haber PS Naltrexone versus acamprosate in the treatment of alcohol dependence: A multi-centre, randomized, double-blind, placebo-controlled trial. Addiction . 2006;101:1451–1462. doi: 10.1111/j.1360-0443.2006.01555.x. [DOI] [PubMed] [Google Scholar]
- Morris PL, Hopwood M, Whelan G, Gardiner J, Drummond E Naltrexone for alcohol dependence: A randomized controlled trial. Addiction . 2001;96:1565–1573. doi: 10.1046/j.1360-0443.2001.961115654.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell TJ, Fals-Stewart W Behavioral couples and family therapy for substance abusers. Current Psychiatry Reports . 2002;4:371–376. doi: 10.1007/s11920-002-0085-7. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Froehlich JC Advances in the use of naltrexone: An integration of preclinical and clinical findings. Recent Developments in Alcoholism . 2003;16:217–245. [PubMed] [Google Scholar]
- O'Malley SS, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR Efficacy of extended-release naltrexone in alcohol-dependent patients who are abstinent before treatment. Journal of Clinical Psycho-pharmacology . 2007;27:507–512. doi: 10.1097/jcp.0b013e31814ce50d. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Archives of General Psychiatry . 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Rounsaville BJ, Farren C, Namkoong K, Wu R, Robinson J, O'Connor PG Initial and maintenance naltrexone treatment for alcohol dependence using primary care vs specialty care: A nested sequence of 3 randomized trials. Archives of Internal Medicine . 2003;163:1695–1704. doi: 10.1001/archinte.163.14.1695. [DOI] [PubMed] [Google Scholar]
- Oslin D, Liberto JG, O'Brien J, Krois S, Norbeck J Naltrexone as an adjunctive treatment for older patients with alcohol dependence. American Journal of Geriatric Psychiatry . 1997;5:324–332. doi: 10.1097/00019442-199700540-00007. [DOI] [PubMed] [Google Scholar]
- Osterberg L, Blaschke T Adherence to medication. The New England Journal of Medicine . 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, O'Malley S, Rounsaville B, Poling J, McHugh-Strong C, Krystal JH the VA Naltrexone Study Collaboration Group. Naltrexone augmentation of neuroleptic treatment in alcohol abusing patients with schizophrenia. Psychopharmacology . 2004;172:291–297. doi: 10.1007/s00213-003-1658-9. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Rounsaville B the VA New England VISN I MIRECC Study Group. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biological Psychiatry . 2005;57:1128–1137. doi: 10.1016/j.biopsych.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, O'Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA The status of naltrexone in the treatment of alcohol dependence: Specific effects on heavy drinking. Journal of Clinical Psychopharmacology . 2006;26:610–625. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Pierce JD, O'Brien CP Improving naltrexone response: An intervention for medical practitioners to enhance medication compliance in alcohol dependent patients. Journal of Addictive Diseases . 2000;19:71–83. doi: 10.1300/J069v19n01_06. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Weiss RD, Dundon W, Miller WR, Donovan D, Ernst DB, Rounsaville BJ A structured approach to medical management: A psychosocial intervention to support pharma-cotherapy in the treatment of alcohol dependence. Journal of Studies on Alcohol, Supplement . 2005;15:170–178. doi: 10.15288/jsas.2005.s15.170. [DOI] [PubMed] [Google Scholar]
- Roozen HG, de Waart R, van der Windt DAWM, van den Brink W, de Jong CAJ, Kerkhof AJFM A systematic review of the effectiveness of naltrexone in the maintenance treatment of opioid and alcohol dependence. European Neuropsychopharmacology . 2006;16:311–323. doi: 10.1016/j.euroneuro.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Rösner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M Opioid antagonists for alcohol dependence. Coch-rane Database of Systemic Reviews . 2010;12 doi: 10.1002/14651858.CD001867.pub3. Art. No. CD001867. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Sayre SL, DeLaune KA, Grabowski J Treatment of cocaine-alcohol dependence with naltrexone and relapse prevention therapy. American Journal on Addictions . 2004;13:333–341. doi: 10.1080/10550490490480982. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, Jarusuraisin N Naltrexone for the treatment of alcoholism: A meta-analysis of randomized controlled trials. The International Journal of Neuropsychopharmacology . 2005;8:267–280. doi: 10.1017/S1461145704004997. [DOI] [PubMed] [Google Scholar]
- Streeton C, Whelan G Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: A meta-analysis of randomized controlled trials. Alcohol and Alcoholism . 2001;36:544–552. doi: 10.1093/alcalc/36.6.544. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Substance Abuse: Clinical Issues in Intensive Outpatient Treatment . 2006. Treatment Improvement Protocol (TIP) Series 47. DHHS Publication No. (SMA) 06-4182. Rockville, MD: Author. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK14448. [PubMed] [Google Scholar]
- Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O'Brien CP Naltrexone and alcohol dependence. Role of subject compliance. Archives of General Psychiatry . 1997;54:737–742. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- Weiss RD Adherence to pharmacotherapy in patients with alcohol and opioid dependence. Addiction . 2004;99:1382–1392. doi: 10.1111/j.1360-0443.2004.00884.x. [DOI] [PubMed] [Google Scholar]
- Willette RE Narcotic antagonists: The search for long-acting preparations: Introduction. National Institute on Drug Abuse Research Monograph Series . 1975;4:1–5. [PubMed] [Google Scholar]