Abstract
The role of interleukin-5 (IL-5) during Toxoplasma gondii infection was investigated by comparing disease progression in IL-5 gene deficient (IL-5−/−) mice and their wild-type (WT) counterparts on a C57BL/6 background. IL-5−/− mice infected orally with T. gondii were less susceptible to infection than WT mice as demonstrated by reduced mortality rates. Consistent with this data, orally infected IL-5−/− mice had less severe pathological changes in their small intestines than WT mice at 8 days postinfection. At this time, splenocytes and mesenteric lymph node cells derived from IL-5−/− mice produced levels of IL-12, interferon-γ (IFN-γ), IL-4, IL-10, and nitric oxide (measured as nitrite) similar to those derived from WT mice when stimulated with Toxoplasma lysate antigen. However, peak serum IL-12 and IFN-γ levels (at days 6 and 8, respectively) were significantly higher in IL-5−/− mice than in WT mice. In addition, WT mice but not IL-5−/− mice had raised levels of eosinophils in their peripheral blood between days 5 and 8 following infection. Oral administration of Nω-nitro-l-arginine methyl (from day 4 postinfection) increased mortality rates in both IL-5−/− and WT mice, indicating a protective role for nitric oxide during the early stages of oral T. gondii infection. In comparison with oral infection, no difference in mortality was observed between IL-5−/− and WT mice following intraperitoneal infection with T. gondii, with all mice surviving until 35 days postinfection. Similarly, no significant differences were observed in the severity of the meningitis, perivascular cuffing, or number of microglial nodules or parasites in the brains of intraperitoneally infected mice. Together, these results demonstrate a detrimental role for IL-5 during the early stage of oral infection with T. gondii which is associated with increased small-intestine pathology, eosinophilia, and reduced plasma IL-12 and IFN-γ levels.
The susceptibility of different mouse strains to infection with Toxoplasma gondii varies dramatically depending on the route of infection (3, 6). Mouse strains that are susceptible to oral infection (such as strain C57BL/6), unlike those that are resistant via this route of infection, have been shown to develop severe necrotic lesions in their small intestines (21, 22). While treatment of susceptible mice with antibody against interferon-γ (IFN-γ) or CD4 cells before oral infection results in their increased mortality, such treatment commencing at 4 days postinfection has increased their survival (21, 22). This implies that the regulation of CD4 cells and IFN-γ production during the early stages of orally initiated T. gondii infection is of critical importance in determining the outcome of disease. Nevertheless, protection during both oral and parenteral infection has been associated with IFN-γ-producing CD8+ T cells (8, 13). However, additional studies using neutralizing monoclonal antibodies and gene-deficient mice have made it clear that, irrespective of infection route, resistance to early T. gondii infection is dependent on a delicate balance between the production of proinflammatory cytokines, which control parasite growth, and regulatory cytokines, which limit host pathology (2, 10). Thus while a number of studies have shown that Th2 cytokines can have detrimental roles (11, 14), mice deficient in the Th2 cytokines interleukin-4 (IL-4) or IL-10 have been shown to exhibit increased susceptibility to early T. gondii infection (15, 27). Furthermore, a role for Th2 cells in limiting inflammation specifically in the gut has been suggested by the work of Chardes et al. (9). These workers demonstrated that despite a predominant Th1 response in the spleen of mice infected with T. gondii, a Th2 bias was present in the mesenteric lymph nodes of these mice.
IL-5, a product of both Th2 and mast cells, has been shown to play an influential role in mucosal immunity. IL-5 is associated primarily with the ability to induce eosinophilia, and as a consequence this cytokine has been shown to have an important role in the induction of a Th2 response through the ability of eosinophils to release IL-4 early in infection (28). Notably, IL-5 has been shown not only to act in synergy with IL-2 and IL-4 to induce antibody production by B cells, but in addition, IL-5 together with transforming growth factor β can enhance immunoglobulin A production in mucosal lymphoid tissues; anti-T. gondii immunoglobulin A has been demonstrated to inhibit parasite invasion of enterocytes (24). A recent study, which examined IL-5 gene-deficient mice infected with T. gondii by the parenteral route, demonstrated a protective role for IL-5 during chronic stages, although not in the early stages, of disease (34). However, this study did not examine the role of IL-5 following oral infection and as a consequence did not address the potential role of IL-5 at the gut mucosal surface or associated lymphoid tissue. The following study was, therefore, undertaken using C57BL/6, IL-5−/−, and wild-type (WT) mice to determine whether IL-5 plays a significant role following oral infection. We have identified a counter-protective role for IL-5, because mice deficient in this cytokine were more resistant to infection as determined by survival and the extent of small-intestine pathology. The resistance of IL-5−/− mice compared with WT mice was associated with increased levels of IL-12 and IFN-γ in their peripheral blood and an inability to mount an eosinophilia.
MATERIALS AND METHODS
Mice.
IL-5-deficient C57BL/6 mice (IL-5−/−) (17) were bred and maintained at the University of Strathclyde from stock obtained from the Max Planck Institut for Immunobiology, Freiburg, Germany. Age-matched WT mice of the same strain combination obtained from the same source were used as controls in all of the experiments.
Toxoplasma gondii.
The RRA (Beverley) strain of T. gondii, an avirulent cyst-forming strain, was used for all experimental infections as previously described (26). This strain was maintained in the Department of Immunology, University of Strathclyde, by continual passage of infective brain homogenate in outbred University of Strathclyde albino mice.
Infections.
Brains from mice infected 17 to 21 weeks previously were harvested and homogenized in 2 ml of phosphate-buffered saline (PBS [pH 7.4]) by six passages through a 21-gauge needle. A 15-μl aliquot of brain suspension was placed on a glass microscope slide and mounted with a coverslip. The entire preparation was scanned microscopically at ×100 magnification, and the number of cysts was counted. Experimental mice were infected either by gavage orally or by intraperitoneal injection, as previously stated, with 200-μl of the brain homogenate containing 10 cysts.
Preparation of Toxoplasma lysate antigen.
Tachyzoites, grown in the peritoneum of cotton rats, were washed in PBS (pH 7.4) (by centrifugation at 1,200 rpm for 5 min at 4°C). The pellet was resuspended in the appropriate volume of reverse-osmosis water and mixed thoroughly by vortex. The suspension was passed five times through a 25-gauge needle and disrupted by freezing (at −70°C) and thawing (at 37°C) three times. The concentration of solution was adjusted to physiological levels by adding the appropriate volume of 10× PBS. Following centrifugation at 3,000 rpm at 4°C for 5 min, the supernatant was filtered through a 0.22-μm-pore-size filter, and the protein concentration was determined by Bradford assay (5).
Monitoring infections and histopathological analysis and collection of peripheral blood.
Mice were monitored daily for mortality, and finally they were sacrificed by terminal anesthesia. The small intestines from orally infected mice and the brains from intraperitoneally infected mice were removed for histopathological processing. Tissues were fixed in 0.1 M phosphate buffer (pH 7.4) containing 4% formaldehyde. Sections were cut from wax-embedded tissues and stained with hematoxylin and eosin. The spleens and mesenteric lymph nodes from groups of infected mice were also removed aseptically for in vitro spleen cell proliferation and cytokine production assays as described in detail below. Blood was collected from mice into heparinized capillary tubes preinfection and on days 4, 6, and 8 postinfection, via their tail veins. Following centrifugation, plasma was collected and stored at −70°C for determination of cytokine levels as described below.
Cell proliferation/stimulation assay.
Cell proliferation assays were carried out as described by Roberts et al. (26). Groups of five male C57BL/6 (B6) and B6 IL-5−/− mice, infected with T. gondii either orally (8 days previously) or intraperitoneally (35 days previously), were used in each experiment as stated. Their spleens and mesenteric lymph nodes were removed aseptically and placed in 5 ml of RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, and 0.05 mM β-mercaptoethanol (Gibco, Paisley, United Kingdom). Spleen and mesenteric lymph node cell suspensions were also prepared from similar groups of uninfected mice. Cell suspensions were centrifuged at 200 × g at 4°C for 5 min. The supernatant was decanted, and the erythrocytes were lysed by resuspension of the pellet in 3 ml of Boyle's solution (0.17 M Tris–0.6 M ammonium chloride; BDH Ltd., Dorset, United Kingdom) for 3 min at 37°C. Following two washes in RPMI 1640 medium, the cells were resuspended in 2 ml of RPMI 1640 supplemented as above, and viable cells were counted by trypan blue exclusion in a hemocytometer. Cell suspensions were adjusted to 5 × 106 cells per ml, and aliquots of 100 μl each, containing 5 × 105 cells, were added to the wells of 96-well flat-bottomed tissue culture plates (Costar, Cambridge, Mass.) which contained either 100 μl of TLA per well at concentrations of 40 and 2 μg/ml, RPMI 1640 medium alone, or ConA (10 μg/ml; Sigma, Poole, United Kingdom) (thus, final concentrations of Toxoplasma lysate antigen [TLA] were 20 μg/ml and 1 μg/ml and that of concanavalin A [ConA] was 5 μg/ml). Spleens and mesenteric lymph nodes were examined individually from each mouse in triplicate. The cultures were incubated for 60 h at 37°C and 5% CO2, after which each well was pulsed with 0.25 μCi of tritiated thymidine (specific activity, 35 Ci/mmol; ICN/Flow, High Wycombe, United Kingdom). At this time, supernatants were collected from parallel cultures and stored at −70°C for cytokine quantification. After a further incubation for 12 h at 37°C and 5% CO2, cells were harvested onto filter paper (ICN/Flow) by using a cell harvester (Skatronis, Lier, Norway). Thymidine incorporation was measured by liquid scintillation on a β-counter (Pharmacia LKB Biotech, Milton Keynes, United Kingdom), using 1 ml of Optiscint (Pharmacia Biosystems, Herts, United Kingdom) added to the filter disks in 3-ml vials (Hughes and Hughes, Somerset, United Kingdom).
The stimulation index was calculated for each individual mouse as the median count per minute for triplicate stimulated cultures divided by the mean count per minute for triplicate nonstimulated cultures. The mean stimulation index for each group of five mice was then calculated.
Analysis of supernatants and measurement of plasma cytokine levels.
Cytokine (IFN-γ, IL-4, IL-10, and IL-12p40) levels were measured in serum and in the supernatants of TLA-stimulated (20 and 1 μg/ml) and nonstimulated cultures by capture enzyme-linked immunosorbent assay (ELISA) using the antibody pairs at predetermined concentrations as described by Roberts et al. (26). Microtiter plates were coated overnight at 4°C with an appropriate capture antibody (rat anti-mouse IFN-γ, 2 μg/ml; rat anti-mouse IL-4, 2 μg/ml; rat anti-mouse IL-10, 2 μg/ml [Pharmingen]; or rat anti-mouse IL-12, 1 μg/ml [Genzyme]) in PBS (pH 9.0). Following three washes in PBS (pH 7.4) containing 0.05% Tween (Sigma, Pool, United Kingdom), plates were blocked for 1 h at 37°C with PBS (pH 7.0) containing 10% fetal calf serum and then washed three times. Samples were applied in duplicate, at 1/2 to 1/30 dilutions for IFN-γ and were undiluted when applied for IL-4, IL-10, and IL-12, along with serial dilutions of standards consisting of the appropriate murine recombinant cytokine (IFN-γ, IL-4, IL-10 [Pharmingen], or IL-12 [Genzyme]) in PBS (pH 7.0) containing 10% fetal calf serum. Plates were incubated for 2 h at 37°C. After a further four washes, biotinylated detecting antibodies (rat anti-mouse IFN-γ, rat anti-mouse IL-4, or rat anti-mouse IL-10 [Pharmingen]; or rat anti-mouse IL-12 [Genzyme]) were added at a concentration of 1 μg/ml in PBS (pH 7.0) containing 10% fetal calf serum. The plates were incubated for 1 h at 37°C before a further five washes. Streptavidin-alkaline phosphatase (at 1/1,000 dilution) (Pharmingen) or streptavidin-horseradish peroxidase (at 1/500) for IL-4 (Pharmingen) was added to each well, and plates incubated at 37°C for 30 to 45 min. After this, plates were washed six times. Binding was visualized with substrate consisting of p-nitrophenyl phosphate (Sigma) (1 mg/ml) in glycine buffer or tetramethylbenzidine (1%) in sodium acetate buffer (pH 5.5) (for IL-4). Absorbances were measured at 405 nm or 450 nm (for IL-4) on a Titertek Multiscan plate reader (Flow Laboratories, Irvine, Ayrshire, United Kingdom) after 1 to 3 h of incubation. Cytokine concentrations were determined from the appropriate standard curve.
Cell culture supernatants were analyzed for the production of NO using the Griess reaction assay, which measures the concentration of nitrite, a stable product of the reaction of NO with O2 (12). Supernatants (100 μl) were incubated with 50 μl of 0.7% sulfanilimide (Sigma) in 14% HCl plus 50 μl of 0.09% napthylethylenediamine (Sigma) in distilled water for 5 to 10 min at room temperature. The absorbance was read at 540 nm with sodium nitrate (Sigma) as standard (10−4 to 10−7 M) using a Titertek Multiscan plate reader (Flow Laboratories).
Measurement of eosinophil levels in the peripheral blood of mice.
Blood was collected from the tail vein before infection and on days 6 and 8 postinfection, (between 9:00 and 12:00 a.m. because the number of eosinophils in the peripheral blood is known to show diurnal variation). Freely flowing blood was diluted with Turk's solution (100 μg of gentian violet per ml in 2% acetic acid) and Dunger's solution (0.2% eosin in 10% acetone). The total white blood cells (WBCs) and eosinophils, which stained pink, were counted on a hemocytometer. Percentages of eosinophils against total WBCs were calculated.
Administration of L-NAME to mice.
Four days after oral infection with T. gondii, groups of 5 to 7 mice were treated with Nω-nitro-l-arginine methyl (L-NAME) (Sigma), the competitive inhibitor of NOS enzyme, (dose equivalent, 200 mg/kg/day) in their drinking water (1 g/liter) for 1 week (23). Noninfected mice were also L-NAME treated to serve as controls.
Statistical analyses and experimental design.
Statistical analyses were performed using the Mann-Whitney U test for the comparison of survival data, stimulation indices, cytokine production, and pathology data. All experiments were performed at least twice with similar findings.
RESULTS
Mortality.
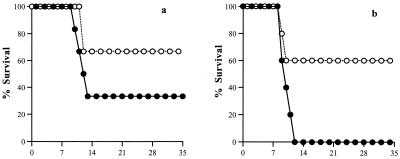
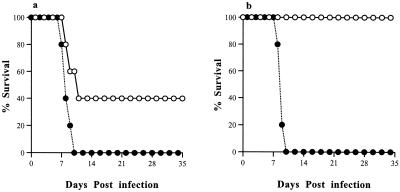
In a series of four experiments with 5 to 7 mice in each group, IL-5−/− mice infected orally with T. gondii had significantly (P = 0.025) greater survival rates than their WT counterparts. This pattern was observed in both male and female mice, although male mice had lower mortality rates than females (P < 0.05). All the mice infected intraperitoneally (both male and female) survived during the 35-day course of study (data not shown). In a representative experiment (Fig. 1a and b), oral infection resulted in a 67% mortality rate in male WT mice, compared with a 33% mortality rate in male IL-5−/− mice. Similarly, in females, oral infection led to 100% mortality in WT mice compared with only 40% mortality in IL-5−/− mice. All deaths occurred within 9 to 13 days postinfection.
FIG. 1.
Percent survival of male (a) and female (b) WT (closed circles) and IL-5−/− (open circles) mice infected with T. gondii. Mice were infected with 10 T. gondii cysts by the oral route (n = 5 to 7 per group). IL-5−/− mice infected by the oral route had significantly greater survival than WT mice infected by the same route irrespective of gender, although females had greater mortality rates than males. No deaths occurred in either male or female WT or IL-5−/− mice infected by the intraperitoneal route (data not shown). Results represent five replicate experiments. Numbers along the x axes indicate the number of days postinfection.
Histopathology.
Sections of small intestine and liver from male WT and IL-5−/− mice infected orally with T. gondii were examined for histopathological changes at days 6 and 8 postinfection. At day 6 postinfection, no significant differences in intestinal or liver pathology were observed between WT and IL-5−/− mice. No significant difference was noted in liver pathology between WT and IL-5−/− mice at day 8 postinfection (data not shown). However, at day 8 postinfection immediately preceding the time in which deaths had occurred in previous experiments, different degrees of pathological changes including villus blunting and fibrin thrombi of various sizes were found in the intestines of both WT and IL-5−/− mice (Table 1 and Figure 2). In general, pathological changes were more severe in the WT than in the IL-5−/− mice. Intestines of WT mice showed significantly (P < 0.01) greater degrees of necrosis and villus blunting than those of IL-5−/− mice where pathological changes were absent or less severe. There were, however, no significant differences in the numbers of fibrin thrombi in the blood vessels of their small intestines between the two groups of mice.
TABLE 1.
Histopathology of the small intestine in WT and IL-5−/− mice infected orally with T. gondiia
| Mouse strain | Histologic scoreb for:
|
Fibrin thrombi (n) | |
|---|---|---|---|
| Necrosis | Villus blunting | ||
| Wild type | 0 | 1 | 2 |
| 3 | 2 | 0 | |
| 3 | 3 | 1 | |
| 3 | 3 | 2 | |
| 2 | 3 | 2 | |
| 3 | 3 | 0 | |
| 2 | 3 | 0 | |
| 3 | 3 | 2 | |
| IL-5−/− | 0 | 1 | 3 |
| 1 | 2 | 2 | |
| 0 | 1 | 2 | |
| 2 | 1 | 1 | |
| 2 | 2 | 6 | |
| 0 | 2 | 4 | |
| 0 | 1 | 2 | |
| 2 | 2 | 11 | |
| 1 | 2 | 2 | |
Mice were killed 8 days postinfection, and the entire length of the small intestine was examined histologically and scored for necrosis and villus blunting. Fibrin thrombi were counted in each section. Necrosis and villus blunting were more severe in WT than IL-5−/− mice (P < 0.01), although no statistically significant difference in the number of fibrin thrombi was found.
0, absent; 1, mild; 2, moderate; 3, severe.
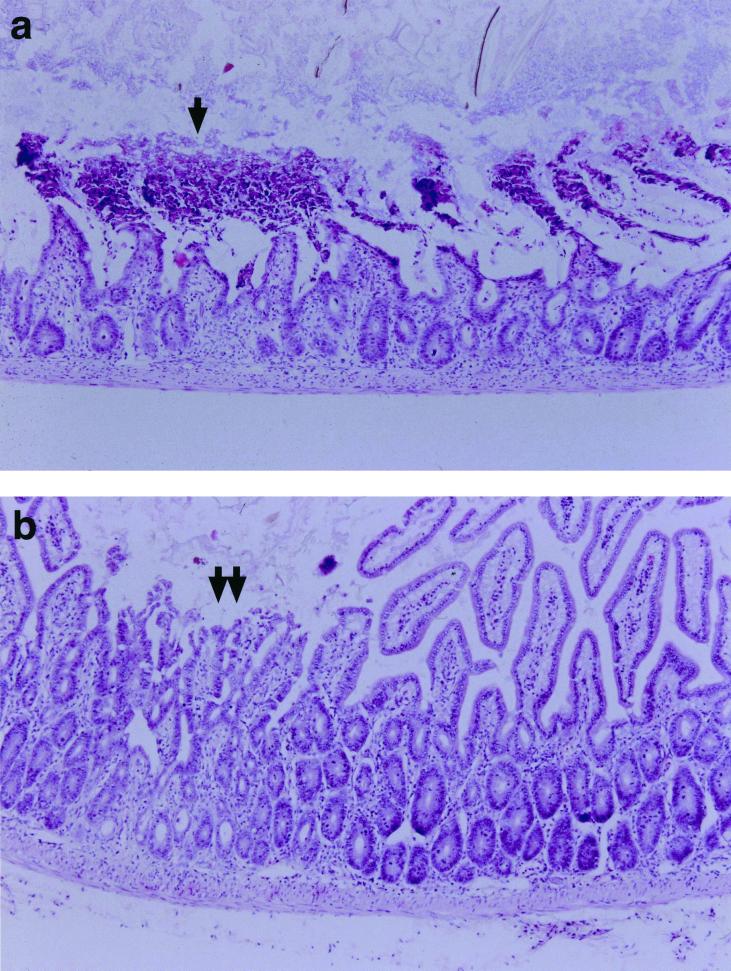
FIG. 2.
Representative hematoxylin and eosin (H&E)-stained sections from the small intestines of (a) WT and (b) IL-5−/− male mice. Mice were infected with 10 T. gondii cysts by the oral route, and their intestines were removed for examination at 8 days postinfection. There was extensive necrosis of the small intestinal villi of WT mice (a, arrow). In contrast the small intestine of the IL-5−/− mice showed mild blunting of the villus architecture and focal necrosis (b, double arrow).
At day 35 postinfection, the brains of both WT and IL-5−/− mice infected intraperitoneally with T. gondii showed some histopathological changes including moderate to severe meningitis, cuffing of the blood vessels by inflammatory cells, and microglial nodules. Cysts were present in all brains irrespective of mouse strain. There were no significant differences in any of these parameters between either group of mice (data not shown).
Comparison of T. gondii-specific splenocyte responses from WT and IL-5−/− mice after oral infection.
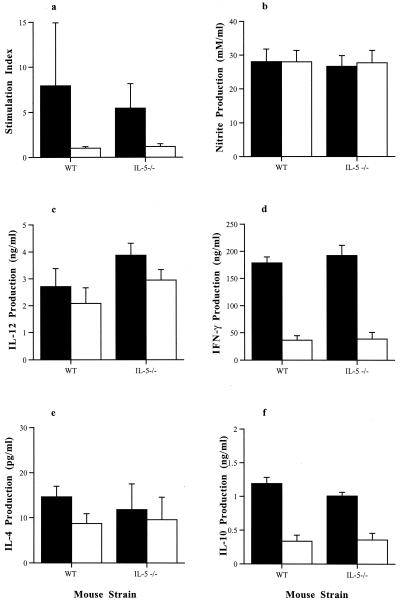
The ability of spleen cells from both immunocompetent and IL-5-deficient mice infected orally with T. gondii to proliferate in response to TLA stimulation was assessed on days 6 and 8 postinfection (Fig. 3a). A marked proliferation was noted in the TLA-stimulated splenocyte cultures derived from both infected WT and IL-5−/− mice compared with those from noninfected WT and IL-5−/− mice. There were no significant differences between the stimulation indices of lymphocytes from infected WT mice compared with those from infected IL-5−/− mice.
FIG. 3.
Comparison of TLA-induced splenocyte responses of male WT and IL-5−/− mice infected with 10 T. gondii cysts by the oral route. Splenocytes were isolated from mice at 8 days postinfection and stimulated with 20 μg of TLA (closed bars) per ml or 1 μg of TLA (open bars) per ml for 96 h. Proliferation was assessed over the final 18 h by incorporation of tritiated thymidine (a). Supernatants were collected from parallel cultures after 72 h and analyzed for nitrite (b), IL-12 (c), IFN-γ (d), IL-4 (e), and IL-10 (f). No statistically significant differences were found (by Mann-Whitney U test) between the responses of WT and IL-5−/− mice. Results represent three replicate experiments.
The supernatants of spleen cell cultures derived from IL-5−/− and WT mice infected orally with T. gondii were examined for cytokine production in response to TLA stimulation at day 8 postinfection. Cell culture supernatants from IL-5−/− mice were found to contain quantities of IFN-γ, IL-12, IL-4, and IL-10 similar to those from WT mice when stimulated with either 1 or 20 μg of TLA per ml. Similarly, stimulation of spleen cells with either 1 or 20 μg of TLA per ml resulted in similar levels of NO production as measured by nitrite in the supernatants of cultures. Only results from day 8 postinfection are shown (Fig. 3).
Comparison of T. gondii-specific mesenteric lymph node cell responses from WT and IL-5−/− mice after oral infection.
In response to TLA, considerable proliferation was noted in mesenteric lymph node (MLN) cell cultures from 6- and 8-day-infected WT and IL-5−/− mice compared with noninfected mice (Fig. 4). However, no significant differences were observed between the stimulation indices of lymphocytes from infected WT mice and those from infected IL-5−/− mice.
FIG. 4.
Comparison of TLA-induced mesenteric lymph node (MLN) cell responses of male WT and IL-5−/− mice infected with 10 T. gondii cysts by the oral route. MLN cells were isolated from mice at 8 days postinfection and stimulated with 20 μg of TLA (closed bars) per ml or 1 μg of TLA (open bars) per ml for 96 hours. Proliferation was assessed over the final 18 h by incorporation of tritiated thymidine (a). Supernatants were collected from parallel cultures after 72 h and analyzed for nitrite (b), IL-12 (c), IFN-γ (d), IL-4 (e), and IL-10 (f). No statistically significant differences were found (by Mann-Whitney U test) between the responses of WT and IL-5−/− mice. Results represent three replicate experiments.
When supernatants of MLN cell cultures from both infected WT and IL-5−/− mice were examined, no significant differences in IFN-γ, IL-12, IL-4, IL-10, or nitrite levels were found following stimulation with either 1 or 20 μg of TLA per ml between the two groups of mice. Only results from day 8 postinfection are shown (Fig. 4).
Comparison of IL-12, IFN-γ, and IL-10 levels in the plasma of WT and IL-5−/− mice after oral infection.
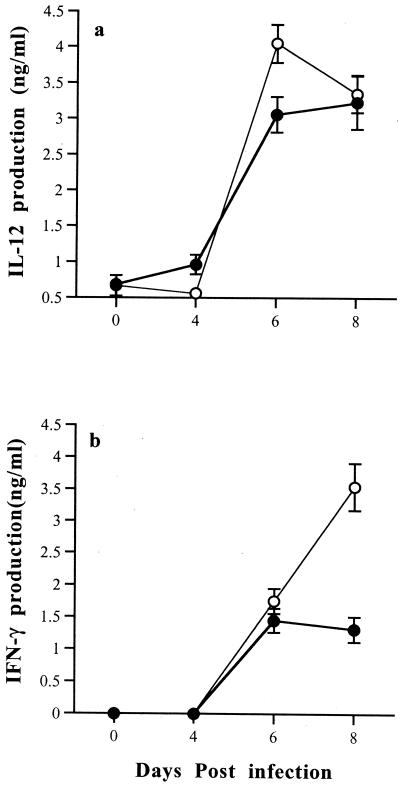
As IL-5 is associated with enhancing Th2 responses, plasma IL-12, IFN-γ, and IL-10 levels were also measured in orally infected WT and IL-5−/− mice. IL-12 and IFN-γ levels were raised in the serum of infected mice on days 6 and 8 postinfection. IL-12 levels peaked at day 6 in IL-5−/− mice, at which time they were significantly higher than in WT mice (P < 0.05). IFN-γ levels peaked at day 8 in IL-5−/− mice, again at significantly higher levels than in WT mice (P < 0.05) (Fig. 5).
FIG. 5.
Comparison of plasma IL-12 (a) and IFN-γ (b) responses of male WT mice (closed circles) and IL-5−/− mice (open circles) infected with 10 T. gondii cysts by the oral route. IL-12 levels were significantly greater in the plasma of IL-5−/− mice at day 6 postinfection (P < 0.05), and IFN-γ levels were significantly higher in the plasma of IL-5−/− mice at day 8 postinfection (P < 0.05). IL-10 could not be detected in the plasma of WT or IL-5−/− mice at any of the time points examined (data not shown).
Comparison of T. gondii-specific splenocyte responses from WT and IL-5−/− mice infected intraperitoneally at 35 days postinfection.
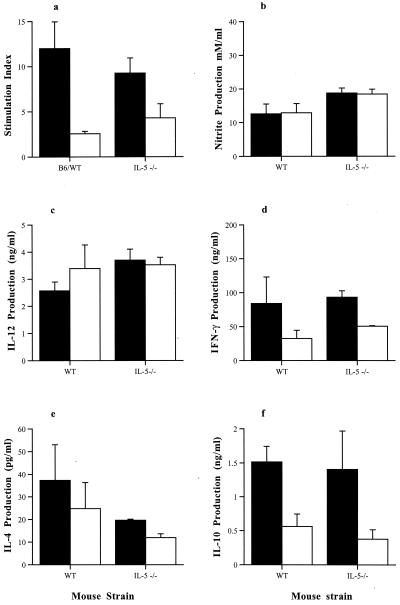
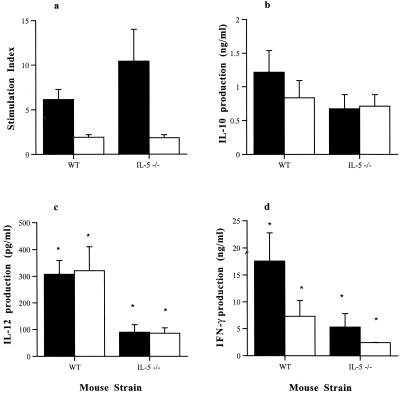
T. gondii-specific splenocyte proliferative responses from both WT and IL-5−/− mice infected intraperitoneally with T. gondii were assessed on day 35 postinfection (Fig. 6). Splenocytes derived from both infected WT and IL-5−/− mice showed a marked proliferation in response to TLA, compared with those from noninfected WT and IL-5−/− mice. However, there was no significant difference between the stimulation indices of lymphocytes derived from infected WT and IL-5−/− mice.
FIG. 6.
Comparison of TLA-induced splenocyte responses of male WT and IL-5−/− mice infected with 10 T. gondii cysts by the introperitoneal route. Splenocytes were isolated from mice at 35 days postinfection and stimulated with 20 μg of TLA (closed bars) per ml or 1 μg of TLA (open bars) per ml for 96 h. Proliferation was assessed over the final 18 h by incorporation of tritiated thymidine (a). Supernatants were collected from parallel cultures after 72 h and analyzed for IL-10 (b), IL-12 (c), and IFN-γ (d). Statistically significant differences (by Mann-Whitney U test) between the responses of WT and IL-5−/− mice are marked (∗). Results represent two replicate experiments.
Cytokine levels were measured in the supernatants of splenocyte cultures derived from WT mice and IL-5−/−mice infected intraperitoneally 35 days previously and stimulated with TLA (Fig. 5). Splenocytes derived from WT mice produced significantly (P < 0.05) more IL-12 than those from IL-5−/− mice when stimulated with either 1 or 20 μg of TLA per ml. Similarly IFN-γ production was also greater in WT splenocyte cultures compared with IL-5−/− cultures whether stimulated with 1 or 20 μg of TLA (P < 0.05) per ml. In contrast no significant differences in IL-10 production were observed between groups when the spleen cells were stimulated with 1 or 20 μg of TLA per ml. (Fig. 6).
Peripheral blood eosinophil levels in WT and IL-5−/− mice.
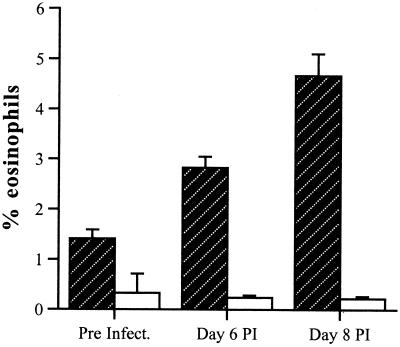
As IL-5-induced eosinophilia is associated not only with raised Th2 responses but with mucosal pathology, eosinophil levels were compared for WT and IL-5−/− mice. Preinfection levels of eosinophils were low in both WT and IL-5−/− mice although WT mice had significantly greater numbers of eosinophils than IL-5−/− mice (P < 0.05). The percentage (± standard deviation) of eosinophils present in the blood of WT mice increased significantly (P < 0.05) over the 8-day period, from 1.141 ± 0.18 to 4.66 ± 0.44. During this time, the percentage of eosinophils in IL-5−/− mice remained relatively constant at less than 1% (Fig. 7).
FIG. 7.
Peripheral blood eosinophil levels in WT (hatched bars) and IL-5−/− (open bars) male mice infected with T. gondii. Mice were infected with 10 T. gondii cysts by the oral route. Blood was collected before infection and on days 6 and 8 postinfection. The total white blood cells and eosinophils were counted as described in Materials and Methods. The percentage of eosinophils present in the blood of WT mice increased significantly over the 8-day period from 1.141 ± 0.18 to 4.66 ± 0.44 (P < 0.05). No significant increases in eosinophil levels were evident in IL-5−/− mice.
The effect of L-NAME on survival of WT and IL-5−/−mice.
As eosinophils produce NO, which has been implicated in mucosal pathology during T. gondii infection, the role of NO was investigated in WT and IL-5−/− mice. Inhibition of NO production by daily oral administration of L-NAME (days 4 through 8 postinfection) in infected male WT and IL-5−/− mice resulted in significantly increased mortality compared with control mice, which received water (P < 0.05) (Fig. 8). Whereas both gene-deficient and WT L-NAME-treated animals showed 100% mortality by 9 days postinfection, control survival following infection was 40% and 10%, respectively. L-NAME treatment had no detrimental effect on noninfected animals as measured by mortality.
FIG. 8.
Percent survival of control and L-NAME-treated (a) WT and (b) IL-5−/− male mice infected with T. gondii. Mice were infected with 10 T. gondii cysts by the oral route. Treated groups (closed circles) received approximately 2 mg of L-NAME per kg of body weight per day (1g/liter in their drinking water) from day 4 postinfection. Untreated mice received normal drinking water (open circles). Both WT and IL-5−/− mice treated with L-NAME had significantly reduced survival compared with their untreated controls.
DISCUSSION
Immunity during the early stages of T. gondii infection is largely dependent on NK cell and macrophage interactions that result in the production of IFN-γ, tumor necrosis factor-α (TNF-α), IL-12, and NO (reviewed in reference 2). However, numerous studies have shown that in the absence of Th2-associated cytokines, such as IL-4 and IL-10, mice infected with T. gondii have greatly increased both production of proinflammatory cytokines and mortality (15, 27). On the other hand, long-term protection against toxoplasmic encephalitis is critically dependent on class I restricted CD8+ T cells and the production of IFN-γ (7, 31). The role of Th2-associated cytokines during this later stage of infection is, however, controversial. Thus in separate studies mice deficient in IL-4 have been reported to have both increased and decreased disease severity (27, 32). Mice deficient in IL-5, another Th2-associated cytokine closely linked to IL-4 production, have been shown to have increased susceptibility to T. gondii infection compared with their WT counterparts, manifesting as increased weight loss and higher numbers of parasites in their brains (34). However, because the mice in this earlier study were infected intraperitoneally, the role of IL-5 in mucosal immunity to T. gondii infection was not addressed. This is significant as IL-5 has been demonstrated in other experimental systems to have a major influence on the development of mucosal pathology. In the present study we have demonstrated that IL-5 plays a detrimental role, mediating intestinal pathology, during the early phase of oral infection with T. gondii.
IL-5 is the major cytokine regulating eosinophil functions, including their production of substances associated with cytotoxic activity such as eosinophil cationic protein, major basic protein, and reactive nitrogen intermediates. Thus in various helminth infections the use of IL-5-deficient mice has demonstrated a protective function for IL-5 and consequently also eosinophils (25, 33). However, further studies have indicated that IL-5 can, under certain circumstances, be detrimental to the host as a result of eosinophil-mediated tissue damage. Thus pulmonary eosinophilia is reduced in IL-5−/− compared with WT mice infected with Brugia maylayi (16), and the extent of lung pathology is also reduced in IL-5−/− compared with WT mice infected with Strongyloides ratti (25). Similarly we find decreased susceptibility of IL-5−/− compared with WT mice following oral infection with T. gondii. This manifests as decreased mortality in IL-5−/− mice compared with WT mice in the first 10 days of infection, associated with less severe pathological lesions, including necrosis, in their small intestines. Consistent with a detrimental role for eosinophils, following infection WT mice developed a peripheral blood eosinophilia which was absent in IL-5−/− mice.
The susceptibility of C57BL/6 mice to oral infection with T. gondii has been associated with CD4+ T-cell-dependent IFN-γ production and necrosis of the small intestine (22). In addition, small-intestine necrosis following T. gondii infection has also been directly related to the level of NO induced by IFN-γ and TNF-α (21). Thus, treating such mice systemically with aminoguanidine reduced intestinal NO production and increased survival. However, it has recently been demonstrated in Trichnella spiralis infections that Th2-dependent responses can also mediate enteropathology and, moreover, that this is also through NO production as a consequence of IL-4-mediated TNF-α production (19, 20). Thus as IL-5 can promote IL-4 production and a Th2 response and eosinophils themselves are potent NO producers, we examined the role of NO in IL-5−/− and WT mice infected orally with T. gondii. In contrast with Liesenfeld et al. (21), who used intraperitoneally administered aminoguanidine, we found that treatment orally with the NO inhibitor L-NAME resulted in rapid and 100% mortality in both IL-5−/− and WT mice. This would clearly suggest a protective rather than an exacerbative function for NO in the small intestine during acute T. gondii infection in C57BL/6 mice. The definitive role of NO in immunity to T. gondii continues to be contentious. It is well established that IFN-γ, with TNF-α, mediates the antiparasitic effects of NO in the murine model primarily by up-regulating the expression of nitric oxide synthase and the production of NO (18, 30). NO is not only directly toxoplasmacidal, but it can also drive tachyzoite to bradyzoite conversion (4). Nevertheless, following intraperitoneal infection, control of early parasite growth in mice appears to be independent of NO production (29). Thus, mice deficient in nitric oxide synthase or tumor necrosis factor receptor 1 are able to control early T. gondii growth although they ultimately succumb to infection (1, 29). Differences between the present study and the Liesenfeld et al. study (21) may reflect the different routes of drug administration used in each study. NO has widespread regulatory functions, and systemic administration of aminoguanidine as in the study by Liesenfeld and coworkers is more likely to have far-reaching immunological consequences than local administration. Nevertheless, while our study does not preclude that IL-5 may mediate pathology in the intestines by eliciting excess NO production, it clearly shows that NO is essential to control early T. gondii infection in the small intestine.
Previous studies have shown IL-5-mediated eosinophilia to be a major source of Th2 cytokine production. We did not observe any difference in TLA-induced splenocyte or MLN production of IL-12, IFN-γ, IL-4, or IL-10 between WT and IL-5−/− mice at 6 or 8 days post-oral infection. However, plasma IL-12 and IFN-γ were significantly raised in both WT and IL-5−/− mice at days 6 and 8 postinfection with IL-5−/− mice having significantly higher levels of IL-12 and IFN-γ than WT mice at days 6 and 8 postinfection, respectively. Previous studies have indicated that, in mice infected with T. gondii, a Th2 bias is present in the mesenteric lymph node cells compared with splenocytes (9). While we do not see this bias, the absence of IL-5 does not enhance IFN-γ production in the mesenteric lymph node cell cultures. These results suggest a possible detrimental effect of eosinophils possibly through alteration of Th1-Th2 balance. Conversely at day 35 postinfection in intraperitoneally infected mice, we found a reduced Th1 response in IL-5−/− mice, demonstrated by decreased splenocyte production of IL-12 and IFN-γ. These immunological differences were not associated with any difference in disease progression as determined by the extent of pathological lesions or cyst burdens found in the brains of IL-5−/− and WT mice. While our studies of intraperitoneal infections had to be terminated at week 5 postinfection due to a general deterioration in the health of the mice, similar studies by Zhang and Denkers were continued for 28 weeks (34). During the course of these studies, Zhang and Denkers, at day 63 postinfection, found similar differences to those reported here, in splenocyte IFN-γ and IL-12 production. The differences observed between IL-5−/− and WT mice, following intraperitoneal infection, have been attributed to IL-5-dependent B-cell-induced IL-12 production. In support of this, depletion of B cells from splenocyte cultures derived from both IL-5−/− and WT mice was sufficient to reduce IL-12 production in WT splenocyte cultures to the equivalent levels measured in IL-5−/− splenocyte cultures (34). Moreover, Zhang and Denkers (34) found that IL-5−/− mice had increased mortality and weight loss over WT mice, although not clearly evident until week 14 postinfection. It is likely that the shorter duration of the present study did not give sufficient time for these differences to manifest.
Finally, the present study clearly illustrates a detrimental role for IL-5 in the early mucosal immune response during T. gondii infection. Furthermore, our results not only indicate different roles for IL-5 in different tissues, but together with the work of Zhang and Denkers (34) suggest different roles for IL-5 in influencing the immune response and disease progression during acute and chronic T. gondii infection.
ACKNOWLEDGMENTS
M.B.N. is in receipt of a scholarship from MCHE of Iran. F.B. is a Wellcome Trust Senior Fellow. This work was funded in part by a Fogarty International Research Collaborative Award. J.A. is on Wellcome Trust-funded research leave. C.W.R. was a Glaxo-Jack Research Lecturer.
REFERENCES
- 1.Alexander J, Jebbari H, Bluethmann H, Satoskar A, Roberts C W. Immunological control of Toxoplasma gondii and appropriate vaccine design. Curr Top Microbiol Immunol. 1996;219:183–195. doi: 10.1007/978-3-642-51014-4_17. [DOI] [PubMed] [Google Scholar]
- 2.Alexander J, SchartonKersten T M, Yap G, Roberts C W, Liew F Y, Sher A. Mechanisms of innate resistance to Toxoplasma gondii infection. Philos Trans R Soc London Ser B. 1997;352:1355–1359. doi: 10.1098/rstb.1997.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackwell J M, Roberts C W, Alexander J. Influence of genes within the MHC on mortality and brain cyst development in mice infected with Toxoplasma gondii: kinetics of immune regulation in BALB H-2 congenic mice. Parasite Immunol. 1993;15:317–324. doi: 10.1111/j.1365-3024.1993.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 4.Bohne W, Heesemann J, Gross U. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect Immun. 1994;62:1761–1767. doi: 10.1128/iai.62.5.1761-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Brown C, McLeod R. Mechanisms of survival of mice during acute and chronic Toxoplasma gondii infection. Parasitol Today. 1994;10:290–292. doi: 10.1016/0169-4758(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 7.Brown C R, Hunter C A, Estes R G, Beckmann E, Forman J, David C, Remington J S, McLeod R. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology. 1995;85:419–428. [PMC free article] [PubMed] [Google Scholar]
- 8.Chardes T, BuzoniGatel D, Lepage A, Bernard F, Bout D. Toxoplasma gondii oral infection induces specific cytotoxic CD8alpha/beta + Thy-1 + gut intraepithelial lymphocytes, lytic for parasite-infected enterocytes. J Immunol. 1994;153:4596–4603. [PubMed] [Google Scholar]
- 9.Chardes T, VelgeRoussel F, Mevelec P, Mevelec M N, BuzoniGatel D, Bout D. Mucosal and systemic cellular immune responses induced by Toxoplasma gondii antigens in cyst orally infected mice. Immunology. 1993;78:421–429. [PMC free article] [PubMed] [Google Scholar]
- 10.Denkers E Y. T lymphocyte-dependent effector mechanisms of immunity to Toxoplasma gondii. Microbes Infect. 1999;1:699–708. doi: 10.1016/s1286-4579(99)80071-9. [DOI] [PubMed] [Google Scholar]
- 11.Denkers E Y, Zhang Y. Early IL-4 induction by Toxoplasma gondii contributes to host susceptibility during acute infection. J Allergy Clin Immunol. 1997;99:1562. [Google Scholar]
- 12.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 13.Gazzinelli R T, Hakim F T, Hieny S, Shearer G M, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 14.Gazzinelli R T, Oswald I P, James S L, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992;148:1792–1796. [PubMed] [Google Scholar]
- 15.Gazzinelli R T, Wysocka M, Hieny S, SchartonKersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma, and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 16.Hall L R, Mehlotra R K, Higgins A W, Haxhiu M A, Pearlman E. An essential role for interleukin-5 and eosinophils in helminth-induced airway hyperresponsiveness. Infect Immun. 1998;66:4425–4430. doi: 10.1128/iai.66.9.4425-4430.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopf M, Brombacher F, Hodgkin P D, Ramsay A J, Milbourne E A, Dai W J, Ovington K S, Behm C A, Kohler G, Young I G, Matthaei K I. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 18.Langermans J A M, Vander Hulst M E B, Nibbering P H, Hiemstra P S, Fransen L, Van F R. IFN-gamma-induced L-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. J Immunol. 1992;148:568–574. [PubMed] [Google Scholar]
- 19.Lawrence C E, Paterson J C M, Higgins L M, MacDonald T T, Kennedy M W, Garside P. IL-4 regulated enteropathy in an intestinal nematode infection. Eur J Immunol. 1998;28:2672–2684. doi: 10.1002/(SICI)1521-4141(199809)28:09<2672::AID-IMMU2672>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence C E, Paterson J C M, Wei X-Q, Liew F-Y, Garside P, Kennedy M W. Nitric oxide mediates intestinal pathology but not immune expulsion during Trichinella spiralis infection in mice. J Immunol. 2000;164:4229–4234. doi: 10.4049/jimmunol.164.8.4229. [DOI] [PubMed] [Google Scholar]
- 21.Liesenfeld O, Kang H, Park D, Nguyen T A, Parkhe C V, Watanabe H, Abo T, Sher A, Remington J S, Suzuki Y. TNF-alpha, nitric oxide and IFN-gamma are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 1999;21:365–376. doi: 10.1046/j.1365-3024.1999.00237.x. [DOI] [PubMed] [Google Scholar]
- 22.Liesenfeld O, Kosek J, Remington J S, Suzuki Y. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mabbott N A, Sternberg J M. Bone marrow nitric oxide production and development of anemia in Trypanosoma brucei-infected mice. Infect Immun. 1995;63:1563–1566. doi: 10.1128/iai.63.4.1563-1566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mack D G, McLeod R. Human Toxoplasma gondii-specific secretory immunoglobulin A reduces T. gondii infection of enterocytes in vitro. J Clin Investig. 1992;90:2585–2592. doi: 10.1172/JCI116153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ovington K S, McKie K, Matthaei K I, Young I G, Behm C A. Regulation of primary Strongyloides ratti infections in mice: a role for interleukin-5. Immunology. 1998;95:488–493. doi: 10.1046/j.1365-2567.1998.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts C W, Cruickshank S M, Alexander J. Sex-determined resistance to Toxoplasma gondii is associated with temporal differences in cytokine production. Infect Immun. 1995;63:2549–2555. doi: 10.1128/iai.63.7.2549-2555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts C W, Ferguson D J P, Jebbari H, Satoskar A, Bluethmann H, Alexander J. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect Immun. 1996;64:897–904. doi: 10.1128/iai.64.3.897-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabin E A, Kopf M A, Pearce E J. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J Exp Med. 1996;184:1871–1878. doi: 10.1084/jem.184.5.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SchartonKersten T M, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sibley L D, Adams L B, Fukutomi Y, Krahenbuhl J L. Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J Immunol. 1991;147:2340–2345. [PubMed] [Google Scholar]
- 31.Suzuki Y, Conley F K, Remington J S. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol. 1989;143:2045–2050. [PubMed] [Google Scholar]
- 32.Suzuki Y, Yang Q, Yang S M, Nguyen N, Lim S, Liesenfeld O, Kojima T, Remington J S. IL-4 is protective against development of toxoplasmic encephalitis. J Immunol. 1996;157:2564–2569. [PubMed] [Google Scholar]
- 33.Vallance B A, Blennerhassett P A, Deng Y, Matthaei K I, Young I G, Collins S M. IL-5 contributes to worm expulsion and muscle hypercontractility in a primary T. spiralis infection. Am J Physiol Gastrointest Liver Physiol. 1999;277:G400–G408. doi: 10.1152/ajpgi.1999.277.2.G400. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Denkers E Y. Protective role for interleukin-5 during chronic Toxoplasma gondii infection. Infect Immun. 1999;67:4383–4392. doi: 10.1128/iai.67.9.4383-4392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]