Abstract

In this work, new heterogeneous Mo-containing catalysts based on sulfonic titanium dioxide were developed for the oxidation of sulfur-containing model feed. The synergistic effect of molybdenum and sulfonic group modifiers allows for enhancing catalytic activity in dibenzothiophene oxidative transformation, and a strong interaction between support and active component for thus obtained catalysts provides increased stability for leaching. For the selected optimal conditions, the Mo/TiO2-SO3H catalyst exhibited 100% DBT conversion for 10 min (1 wt % catalyst, molar ratio of H2O2:DBT, 2:1; 80 °C). Complete oxidation of DBT in the presence of the synthesized catalyst is achieved when using a stoichiometric amount of oxidizing agent, which indicates its high selectivity. The enhanced stability for metal leaching was proved in recycling tests, where the catalyst was operated for seven oxidation cycles without regeneration with retainable activity in DBT-containing model feed oxidation with hydrogen peroxide under mild reaction conditions. In 30 min of the reaction (H2O2:S = 2:1 (mol), 0.5% catalyst, 5 mL of acetonitrile, 80 °C), it was possible to reduce the content of sulfur compounds in the diesel fraction by 88% (from 5600 to 600 ppm).
1. Introduction
The problem of reducing the content of sulfur compounds in fuels is particularly relevant today since sulfur compounds present in motor fuels are sources of acid rain, harm the environment, and human health.1,2 In this regard, the requirements for the sulfur content in gasoline and diesel fuels are being tightened all over the world, in particular, the European Parliament and the Council of the European Union introduced in 2009 a limit on the sulfur content in motor fuels of no more than 10 ppm.3
The most common industrial method of purification of oil fractions from sulfur is hydrotreating.4,5 This method can reduce the amount of sulfur in the gasoline and diesel fractions by more than 99%, but the process is carried out under harsh conditions, which requires high energy and operating costs, as well as a source of cheap hydrogen. In this regard, alternative hydrogen-free desulfurization methods are becoming an urgent subject of research, among which oxidative desulfurization due to its advantages (lack of hydrogen, low energy consumption, environmental friendliness, low costs) is of the greatest interest.6,7
Oxidative desulfurization combines the process of oxidation of sulfur-containing compounds followed by the purification of the resulting feed from sulfones by adsorption or extraction methods.8 Peracids,9 organic peroxides,10 ozone11 and air oxygen,12 and hydrogen peroxide13 can act as oxidizing agents. Hydrogen peroxide is an environmentally friendly oxidant (the only by-product is water), and unlike oxygen, does not require high temperatures during the oxidation process, which contributes to its widespread use.2
Transition-metal salts,8,14 polyoxometallates,15,16 molybdenum, and tungsten-containing ionic liquids17,18 are widely used as catalysts since transition metals such as molybdenum, tungsten, and vanadium are capable of forming active peroxo complexes in the presence of oxidants.19−21 At the same time, liquid-phase homogeneous catalysts have significant disadvantages: a small phase contact area and the complexity of regeneration. These problems can be solved by immobilizing the active component to heterogeneous supports.22−25 In addition, the synergistic effect that occurs between molybdenum active sites and supports contributes to an increase in the efficiency of the oxidation rate, and as a result, milder reaction conditions.26,27
Titanium oxide is of particular interest among carriers due to its unique physicochemical properties, non-toxic nature, relative cheapness, and accessibility, as well as due to its resistance to ultraviolet irradiation and stability for aggressive oxidant treatment.28,29 The application of titanium oxide with various additives as a catalyst for oxidative desulfurization under ultraviolet irradiating is known.30−34 Despite the high activity at low temperatures, such catalysts have significant drawbacks when introduced to industry—the use of light for hydrogen peroxide decomposition. Approaches for the production of titanium-containing catalysts that do not require UV radiation are known in the literature.35−41 These catalytic systems require a sufficiently long time of oxidation, and therefore the task is to develop catalysts in the presence of which oxidation will take place without UV and in a minimum contact time.
The reaction time can be reduced by synthesizing bifunctional catalysts containing an acidic component and a transition metal atom capable of forming active peroxo complexes. The literature describes molybdenum–titanium catalytic systems such as MoO3–TiO2@MCM-22 and Mo/MTP-350,42,43 which allow the oxidation of DBT in a short time. However, the synthesis of the above catalysts requires the use of expensive templates, which greatly increases the cost of the catalyst and, as a consequence, the cost of the oxidative desulfurization process.
In this work, a preliminary modification of the titanium oxide surface with sulfo groups was carried out, followed by the application of ammonium heptamolybdate. Such modification by sulfo groups allowed us to solve several tasks, such as increasing the activity of the catalyst due to the contribution of sulfo groups, which can act as catalysts; to increase the stability of the catalyst due to more efficient retention of molybdenum compounds by sulfo groups.
2. Experimental Methods
2.1. Materials and Reagents
Titanium (IV) oxide (anatase, 99.5%, Sigma-Aldrich), chlorosulfonic acid ClSO3H (99%, Sigma-Aldrich), dichloromethane (Prime Chemicals Group), (NH4)6Mo7O24·4H2O (98%, Prime Chemicals Group), hydrogen peroxide H2O2 (50 wt %, “Prime Chemicals Group”), dodecane n-C12H26 (99%, “Labteh”), and dibenzothiophene (DBT) C12H8S (98%, Sigma-Aldrich) were used as received. As a real raw material, a sample of a straight-run diesel fraction of 240–320 °C, previously extracted with dimethylformamide, with a total sulfur content of 5250 ppm, was used in this work.
2.2. Synthesis of Catalysts
To synthesize TiO2-SO3H, chlorosulfonic acid (0.3 mL, 13.2 mmol) was added to a suspension of powdered TiO2 (0.5 g) in dry CH2Cl2 (15 mL) for 5 min, while the mixture was slowly stirred in an ice bath. After that, the mixture was stirred for 2 h at room temperature. Then, the resulting mixture was centrifuged and the solid powder was washed with dichloromethane (2 × 10 mL) and dried at 70 °C first on the rotor, then 80–110 °C in the air for 16 h to remove water. The product was obtained in the form of a white solid powder, which was stored in a closed bottle.
Modification of TiO2-SO3H and TiO2 was carried out by impregnation with (NH4)6Mo7O24·4H2O dissolved in distilled water. Thus, 0.5 g of the carrier was immersed in a solution of ammonium heptamolybdate, stirred for 2 h on a rotary evaporator at a temperature of 40 °C, then at a temperature of 60 °C to a constant mass of the catalyst. After that, the synthesized materials were dried for 16 h at 80–120 °C in air.
2.3. Material Characterization Methods
The number of sulfo groups was determined by acid–base titration. The catalyst (0.05 g) was dissolved in 5 mL of NaOH (0.1045 M) and stirred for 5 min. Then 3–4 drops of the Methyl Orange indicator were added to the flask. The resulting mixture was titrated with 0.1040 M HCl solution until the color of the solution changed from yellow to red. The experiment was conducted at least three times with an error of less than 1%.
The Fourier transform infrared spectroscopy (FTIR) spectra were recorded using a Nicolet IR2000 (Thermo Scientific) spectrometer equipped with a multi-reflection prefix for horizontal attenuated total reflection with a ZnSe crystal. The spectra were obtained using multiple distortions of the total internal reflection method in the range of 4000–500 cm–1 with a resolution of 4 cm–1. All spectra were obtained by averaging 100 scans.
The elemental composition was studied by X-ray spectral fluorescence analysis (XFA) using an ARL Perform’X X-ray fluorescence wave spectrometer (Thermo Fisher Scientific, New Wave). Powdered samples were pressed into tablets on a substrate of boric acid and covered with a dacron film, which was pressed with a ring-shaped frame to the cuvette.
The characteristics of the porous structure of the samples were determined using the Gemini VII 2390 (V1.02 t) Micromeritics automatic surface analyzer according to the standard procedure. Before analysis, the samples were degassed at 120 °C for 12 h to a pressure of 3 × 10–3 atm. The nitrogen adsorption–desorption isotherm was removed at liquid nitrogen temperature. The range of relative pressure analysis is 0.05–0.99 P/P0. The characteristics of the porous structure were calculated using standard software. The specific surface area (SBET) was calculated using the BET (Brunauer–Emmet–Teller) model at a relative partial pressure P/P0 = 0.2. The total pore volume was measured at P/P0 0.99 using the Barrett–Joyner–Halenda (BJH) model.
X-ray phase analysis was performed on a Rigaku Rotaflex D/max-RC instrument using copper CuKα-radiation (λ = 0.154 nm). The diffraction pattern from the sample was registered in the angular range 2θ = 5–60° with a step of 0.02° and a recording rate of 2 deg/min.
The sample morphology was analyzed by scanning electron microscopy (SEM) using a JEOL JIB 4501 multibeam SEM-FIB system. Since the samples are insulators their fine powders were deposited onto the carbon tape and coated by carbon to avoid charging artifacts. The acceleration voltage was set to 11 kV. Elemental mapping was performed with the help of a SDD X-Maxn 50 EDS system.
TGA measurements were carried out on NETZSCH TG 209 F1 thermal weights equipped with an all-round holder with a protective screen and a type P temperature sensor. The device was calibrated according to the melting points of standard substances (Ag, Al, Bi, In, Sn, purity 99.99%). The error in determining the mass does not exceed 0.1% (determined by the CaC2O4·2H2O standard). The experiment was carried out in a standard open allund container (V = 85 mm3, d = 6.7 mm) in a stream (20 mL/min) of synthetic air in the temperature range of 30–800 °C at a heating rate of 10°/min. Experimental data were processed using the NETZSCH Proteus Analysis package according to the ISO/CD 11358 standard.
2.4. Oxidation of the Model Fuel
The model fuel of dibenzothiophene in dodecane, used to study the activity of catalysts, contained 500 ppm of sulfur. The volume of the model mixture in each reaction was 5 mL. Oxidation was carried out according to the following method: the model fuel was heated to the reaction temperature, then 0.0094–0.075 g of catalyst, 1 mL of acetonitrile, and the calculated amount of 50% hydrogen peroxide solution (based on the ratio H2O2:S = 2:1–20:1) was added at a stirring speed of 700 rpm.
After the reaction was completed, the fuel was separated from the catalyst and oxidant. The composition of the reaction products and control of the purity of the starting substances were carried out by gas chromatography on a chromatograph “Crystal-2000M” with a flame ionization detector, column—Zebron L = 30 m, d = 0.32 mm, liquid-phase ZB-1, with temperature programming from 100 to 250 °C. Chromatograms were recorded and analyzed on a computer using the Chromatek Analyst 1.5 program. The concentration was determined by the change in the relative area of the peaks of the substrate and products (in mass %).
Figures 4–9 show the average values of the three converging results. The error of the experiment is not more than 5%.
Figure 4.
Isotherms of low-temperature nitrogen adsorption/desorption (A) and IR spectra (B) of synthesized materials.
Figure 9.
Possible oxidation scheme.
2.5. Oxidation of Real Fuel
Diesel fraction (5 mL), 0.5 wt % of catalyst, and 90 μL of 50% H2O2 were added to the reactor equipped with a reverse refrigerator and a magnetic stirrer. The reaction was carried out at a temperature of 80 °C for 30 min in the presence of 5 mL of acetonitrile. The mixing speed was 700 rpm. After the end of the reaction, the oxidized fuel was separated from acetonitrile and the oxidative-catalytic mixture, washed with water and then 5 mL of dimethylformamide was added to extract the oxidized sulfur compounds. Further, the purified hydrocarbon phase was separated and analyzed for residual sulfur content. The fraction losses during heating were no more than 3%.
Determination of the sulfur content in the initial diesel fraction and oxidized mixtures after the reaction was carried out using an X-ray energy dispersion sulfur analyzer “ASE-2 JSC” (NPP “Burevestnik”). The relative error of the device is not more than 5%, the range of the determined concentration is from 5 to 50,000 ppm.
3. Results and Discussion
3.1. Characterization of Supports and Catalysts
The use of catalysts containing acid groups has become widespread in oxidative desulfurization both as homogeneous and heterogeneous catalysts.44,45 In this work, sulfo groups were used as an acidic component, since they are active in the oxidation of sulfur-containing compounds and are easily applied to the surface of titanium oxide.46,47
For increasing the activity of the catalyst, it was decided to synthesize bifunctional catalysts. Molybdenum was chosen as the second active center because it is a relatively accessible metal that forms peroxo complexes in the presence of hydrogen peroxide.
To understand the contribution of each functional group to the oxidation of organosulfur compounds, three catalysts (Mo/TiO2, TiO2-SO3H, Mo/TiO2-SO3H) based on titanium oxide were synthesized according to Figure 1. All these catalysts, as well as the support itself—anatase—were subjected to physicochemical research and used as catalysts for oxidative desulfurization of model fuels.
Figure 1.
Scheme of the synthesis of catalysts based on titanium oxide.
In the first stage, titanium oxide was subjected to interaction with chlorosulfonic acid to form TiO2-SO3H. According to titration and elemental analysis data, the content of sulfo groups was 22 wt %.
After sulfation, TiO2-SO3H and the initial anatase were impregnated with ammonium heptamolybdate to obtain Mo/TiO2-SO3H and Mo/TiO2, respectively. The amount of molybdenum in the synthesized materials was −5 wt %, which was confirmed by the method of elemental analysis. When modifying the carrier in various ways, a change in the color of the resulting catalysts was observed (Figure 2).
Figure 2.

Photo of the synthesized catalysts.
Figure 3A shows that it looks like the peak on X-ray diffraction (XRD) spectrum intensities for Mo/TiO2 and the sulfated analogues are practically the same. Based on this, it can be concluded that the modification of the surface of titanium oxide by sulfo groups does not affect its structure. Peaks were found in the structure of molybdenum-containing catalysts at 2θ values of 25.3, 37.9, 48.1, 54.0, and 55.0 corresponding to anatase,48 as well as 27.2, 36, and 41 corresponding to the structure of rutile,49 which was originally present in the initial titanium oxide (Figure 3). No sharp diffraction peak of Mo was detected in the obtained samples, which may be due to a similar ion radius of Mo6+ (0.59 Å) and Ti4+ (0.65 Å), which led to the fact that Mo6+ was well dispersed on a titanium dioxide carrier or was included in the titanium dioxide matrix in an amorphous form.48
Figure 3.
XRD patterns of Mo/TiO2 and Mo/TiO2-SO3H (A) and TGA curves of TiO2-SO3H and Mo/TiO2-SO3H (B).
TGA of catalysts (Figure 3B) shows an initial weight loss (2.5 wt % for TiO2-SO3H and 6 wt % for Mo/TiO2-SO3H) due to the desorption of water below 100 °C. This is followed by a second weight loss, corresponding to the loss of the covalently bound sulfonic group.50 It can also be noted that Mo/TiO2-SO3H is more thermally stable (up to 325 °C) compared to catalyst TiO2-SO3H.
The obtained nitrogen adsorption/desorption isotherms are characteristic of materials based on titanium oxide (Figure 4A). Sulfation of titanium oxide leads to a sharp decrease in the surface area, however, as follows from the XRD (Figure 3A), this is not due to the destruction of the structure during sulfation. Since a sufficiently strong decrease in the surface area is observed only when applying sulfo groups, this may be due to this stage of surface modification. As can be seen from the SEM (Figure 5) for molybdenum-containing catalysts, a slight tracking of catalyst particles is visible for the sulfated sample, which may negatively affect its surface area. At the same time, there is no strong difference in the structure of synthesized materials, which makes it possible to use the chosen method for the synthesis of these materials. The application of molybdenum compounds to the surface of materials practically does not affect the textural characteristics.
Figure 5.
SEM images of molybdenum-containing catalysts. (A), (B) for Mo/TiO2, (C), and (D) for Mo/TiO2-SO3H.
A distinct peak of stretching vibration of the Ti-O bond (710 cm–1) is visible on the IR spectra of all four samples (Figure 4B).51 For molybdenum-containing catalysts, 914 and 1410 cm–1 peaks appear, corresponding to the Mo–O bond52 and NH4+ vibrational band,53 respectively. Peaks of 1130, 1150, and 1590 cm–1 in TiO2-SO3H and Mo/TiO2-SO3H are characteristic of the sulfo group.54 Also, a plateau (2800–3660 cm–1), characteristic of water, appears on the IR spectra of these catalysts. This can be explained by the presence in the catalysts of water bound by hydrogen bonds with sulfo groups since this peak is not observed for non-sulfated materials.54
3.2. Oxidation of Model Sulfides
A solution of dibenzothiophene in dodecane was used as a model fuel. The choice of DBT is due to its relative inertness to oxidation reactions. Another reason for choosing DBT is the large amount of data in the literature on the activity of various titanium-containing catalysts in the oxidation of this substrate (Table 2). The oxidative desulfurization process was carried out with the addition of acetonitrile, which well dissolves the oxidation product, the corresponding dibenzothiophene sulfone. The results of the comparative activity of the synthesized catalysts at different reaction temperatures are shown in Figure 6A.
Table 2. Comparison of Titanium-Containing Catalytic Systems of Oxidative Desulfurization.
| oxidation
conditions |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| catalyst | substrate | oxidant | [O]/S | T (°C) | catalyst dosage (g/mLmodel fuel) | oxidation time (min) | UV | conversion (%) | reference | notes |
| TiO2 | DBT | H2O2 | not specified | 40 | 0.006 | 180 | yes | 91 | (30)Dedual 2014 | |
| anatase nano-TiO2 | DBT | H2O2 | 10 | 70 | 0.01 | 1 | yes | 100 | (31)Li 2016 | |
| amorphous TiO2 and [Bmim]BF4 | DBT | H2O2 | 2 | 30 | 0.001 + 0.2 mL of IL | 120 | yes | 96.6 | (33)Zhu 2014 | |
| nano-TiO2 in [Bmim]BF4 | DBT | O2 | RTa | 0.002 + 0.2 mL of IL | 600 | yes | 98.2 | (32)Wang 2012 | ||
| 40%-TiO2/g-C3N4 | DBT | H2O2 | 6 | RTa | 0.01 | 120 | yes | 98.9 | (34)Wang 2014 | |
| 7.5Ti-Al-SBA-15 | DBT | H2O2 | 20 | 70 | 0.003 | 360 | yes | 90 | (61)Pham 2020 | |
| TiO2-TBOT (TBOT—titanium (IV) tetrabutoxide) | DBT | H2O2 | 4 | 60 | 0.01 | 60 | no | 100 | (62)Zuo 2019 | |
| mesoporous TS-1 | DBT | cumene hydroperoxide (CHP) | 15 | 80 | 0.002 | 180 | no | 97 | (35)Yang 2012 | |
| TS-1 | DBT | H2O2 | 15 | 70 | 0.025 | 15 | no | 100 | (36)Shen 2015 | |
| hierarchical TS-1 | DBT | TBHP | 2 | 60 | 0.005 | 120 | no | 100 | (37)Lv 2018 | |
| hierarchical TS-1 | DBT | TBHP | 3 | 60 | 0.004 | 30 | no | 100 | (63)Wang 2022 | |
| 30%-Ti-MY (MY - hierarchical zeolite Y) | DBT | CHP | 10 | 50 | 0.005 | 120 | no | 97 | (64)Zhang 2018 | |
| Ti/C-ZSM-5–30 | DBT | TBHP | 6 | 60 | 0.005 | 20 | no | 100 | (65)Wang 2022 | |
| Ti-CMK-3 | DBT | H2O2 | 12 | 60 | 0.003 | 20 | no | 100 | (39)Rivoira 2018 | |
| hydrophobic Ti-silica | DBT | H2O2 | 2.5 | 60 | 0.0006 | 480 | no | > 99 | (38)Fraile 2016 | |
| mesoporous TiO2-SiO2 composite | DBT | TBHP | 2 | 60 | 0.005 | 25 | no | 100 | (40)Liu 2021 | |
| 2D-TiO2@MWCNT (multi-walled carbon nanotube nanocomposites) | DBT | H2O2 | 9 | 69 | 0.0012 | 46 | no | 100 | (41)Mahmoudabadi 2021 | |
| MoO3–TiO2@MCM-22 | DBT | CYHPO | 2 | 100 | 0.005 | 15 | no | 99.96 | (42)Luo 2019 | 10 mL acetonitrile as extraction solvent |
| Ti-SBA-15 | DBT | CHP | 2 | 80 | 0.01 | 10 | no | 100 | (66)Kim 2012 | |
| 5%Mo/MTP-350 (mesoporous titanium phosphonates) | DBT | TBHP | 3 | 40 | 0.002 | 30 | no | 100 | (43)Chen 2021 | |
| meso/macroporous Mo/TiO2 | DBT | H2O2 | 4 | 30 | 0.005 | 100 | no | 100 | (51)Du 2020 | |
| Mo/KIT-6-Ti | DBT | CHP | 4 | RT | 0.0075 | 40 | no | 100 | (27)Zou 2021 | |
| Mo/TiO2-SO3H | DBT | H2O2 | 2 | 80 | 0.007 | 10 | no | 100 | this work | 1 mL acetonitrile as an extraction solvent |
RT—room temperature.
Figure 6.
Comparison of the activity of catalysts (A) and effect of oxidation time on DBT conversion (B). Reaction conditions: H2O2:S = 2:1 (mol), 0.5% catalyst, 1 mL acetonitrile, 30 min, 80 °C.
According to the results obtained, titanium oxide itself exhibits insignificant activity in the oxidation of DBT, which is also known from the literature.30 The addition of molybdenum compounds to the catalyst composition improves the results, and this difference increases with increasing temperature and at 70 °C the DBT conversion in the presence of the Mo/TiO2 catalyst is 2 times higher than the conversion in the presence of anatase. The introduction of the sulfo group to titanium oxide (TiO2-SO3H) insignificantly improves the oxidation results, being inferior to the results obtained in the presence of the Mo/TiO2 catalyst at temperatures of 40 and 60 °C. However, the difference in the activity of the Mo/TiO2 and TiO2-SO3H catalysts decreases with increasing temperature, and at 80 °C sulfonic titanium oxide exhibits greater activity in comparison with the molybdenum-containing analogue. This seems to be related to the fact that the rate of formation of the corresponding per-sulfonic acid increases sharply at temperatures above 70 °C.55 The results obtained in the presence of a combined Mo/TiO2-SO3H catalyst containing both active sites (a compound of molybdenum and a sulfo group) show that the combination of two catalytic sites makes it possible to achieve better results than the total effect of each active site. This fact is apparently because molybdenum, which is capable of forming active peroxo complexes in a wide temperature range, can catalyze the formation of the corresponding peracid, increasing its activity in the oxidation of sulfur-containing substrates.56
The time-dependent DBT conversion is shown in Figure 6B. It should be noted that the Mo/TiO2-SO3H catalyst makes it possible to oxidize more than half of the substrate in just 10 min. The TiO2-SO3H catalyst shows better results over the entire kinetic curve compared to Mo/TiO2, which indicates a high activity of sulfo groups. Sulfo groups activity is comparable to or exceeds the results of well-studied molybdenum-containing catalysts. For further experiments, the process conditions were compared at a reaction time of 10 min.
Without an oxidizing agent, the DBT conversion is less than 15%, which is associated with the adsorption of the substrate in the pores of the catalyst and idle extraction with acetonitrile. It should be noted that a maximum is observed when the ratio of oxidant:substrate changes (Figure 7A). For the exhaustive oxidation of dibenzothiophene, a 2-fold excess of the oxidizing agent is sufficient. However, an increase in the amount of excess hydrogen peroxide to an oxidant:feed ratio of 5:1 lead to an increase in the rate of the oxidation reaction. A further increase in the ratio of oxidant:feedstock 10:1 and 20:1 leads to a significant decrease in the conversion of DBT. This probably can be explained by the adsorption of an excess amount of hydrogen peroxide on the catalyst surface, which can create difficulties for the diffusion of a non-polar substrate to active sites of the catalyst. Therefore, in further experiments, we used a molar ratio of hydrogen peroxide:DBT of 2:1, sufficient for oxidation to the corresponding sulfone.
Figure 7.
Effect of the amount of hydrogen peroxide (A), the amount of catalyst (B), and the amount of acetonitrile (C) on the conversion of DBT and the nature of the substrate (D). Reaction conditions: H2O2:S = 2:1 (mol), 0.5% catalyst (1.0% for (D)), 1 mL acetonitrile, 80 °C, 10 min.
An increase in the catalyst dosage from 0.25 to 2% by weight leads to an increase in the DBT conversion (Figure 7B). In this case, already 1% mass of the catalyst is enough for complete oxidation of DBT in 10 min, which is a very good indicator for a titanium-containing catalyst (Table 2). It is important to note that 100% DBT conversion is achieved with a stoichiometric amount of hydrogen peroxide, which indicates high selectivity of the synthesized Mo/TiO2-SO3H catalyst and the target consumption of the oxidant only for DBT oxidation. An increase in the amount of catalyst to 2% also makes it possible to achieve 100% conversion of DBT, which in turn indicates that the possible decomposition reactions of hydrogen peroxide in the presence of a catalyst are minimal.
As mentioned above, the reactions were carried out in the presence of acetonitrile, which extracts well the oxidation products, sulfones. Moreover, in the absence of acetonitrile, the conversion of DBT is significantly lower (Figure 7C). We assume that in the absence of acetonitrile, the particles of the polar catalyst aggregate upon the addition of hydrogen peroxide, which was shown earlier for the molybdenum-containing catalysts supported on polar support SBA-15.57 The aggregation of catalyst particles, in turn, reduces the effective contact area with the model fuel and complicates the diffusion of non-polar substrates. Therefore, carrying out the extraction–oxidative desulfurization is a more preferable process. It is important to note that a further increase in the amount of acetonitrile to 2 and 3 mL leads to a slight decrease in the conversion of DBT, which may be a consequence of a decrease in the concentration of hydrogen peroxide due to dilution. With a decrease in the concentration of hydrogen peroxide, all other things being equal, the conversion of DBT decreases, which was shown by us earlier.47
Since sulfur compounds of various structures are present in real raw materials, it is important to assess the effect of the nature of the substrate on its conversion. Benzo- and dibenzothiophene and their alkyl derivatives were selected as substrates. The activity of synthesized catalysts, depending on the structure of the substrate, falls in the series (Figure 7D): DBT > Me2DBT > MeDBT > BT > MeBT, which is consistent with the literature data.6,47 The lower conversion of benzothiophene is associated with its lower oxidizing ability, however, it should be noted that in the case of DBT, the presence of alkyl substituents practically does not affect the conversion, which opens up the possibility of using the developed catalysts for oxidative desulfurization of real fuels.
Since a sufficiently large amount of the active phase is present in the catalysts, it is possible to leach it into the liquid phase of the reaction mixture. In addition to the elemental analysis, a hot filtration test was conducted to check the absence of leaching. To do this, after 10 min of reaction, the catalyst was separated from the model mixture and acetonitrile, followed by a continuation of the reaction for up to 30 min. As can be seen from the obtained dependence (Figure 8A), in the case of Mo/TiO2-SO3H, the reaction stops and the DBT conversion does not change. In the case of Mo/TiO2, the DBT conversion increases slightly, which is due to a slight leaching of ammonium heptamolybdate from the surface of titanium oxide, which is also confirmed by an elemental analyzer (Table 1). Thus, additional modification of titanium oxide by sulfo groups avoids the leaching of molybdenum compounds from the carrier surface.
Figure 8.
Hot filtration test (A) and the effect of catalyst recycling (B). Reaction conditions: H2O2:S = 2:1 (mol), 0.5% catalyst, 1 mL of acetonitrile, 80 °C, 10 min.
Table 1. Textural Characteristics and Elemental Composition of Samples.
| textural
properties of various samples |
quantity
of sulfo groups (wt %) |
|||||
|---|---|---|---|---|---|---|
| catalyst | SBET (m2/g) | Vpore (cm3/g) | dpore (Å) | elemental analysis | titration method | quantity of molybdenum |
| TiO2 | 51 | 0.12 | 84 | |||
| Mo/TiO2 | 48 | 0.11 | 80 | 4.8 | ||
| TiO2-SO3H | 28 | 0.07 | 77 | 22.3 | 21.0 | |
| Mo/TiO2-SO3H | 18 | 0.06 | 72 | 21.5 | 20.1 | 4.9 |
| Mo/TiO2a | 45 | 0.10 | 78 | 4.0 | ||
| Mo/TiO2-SO3Ha | 17 | 0.06 | 71 | 21.3 | 20.2 | 4.9 |
Catalysts after 7 oxidation cycles, washed with acetone to remove sulfones and dried.
One of the key characteristics of a catalyst is its lifetime. Oxidation catalysts are used in polar solvents capable of dissolving molybdenum compounds in themselves. Therefore, recycling experiments were carried out to determine the stability of the catalyst operation. After each stage of oxidation, the phase containing the model fuel was separated, a fresh portion of the oxidizing agent was added to the acetonitrile solution, then a fresh portion of the model fuel was added, and oxidation was carried out under similar conditions. The results are shown in Figure 8A. It is important to note that the conversion of dibenzothiophene is maintained for at least 7 cycles when in the case of the Mo/TiO2 catalyst the conversion of DBT decreases after 6 cycles. Thus, it can be assumed that the active phase of the catalyst is tightly bound to the support surface and is stable to leaching processes under the conditions of the process.
Since titanium-containing catalysts can be sensitive to light, an oxidation reaction of DBT was carried out in the absence of light (dark glass, additionally coated with non-light-emitting foil). For the reaction, conditions were chosen in which the DBT conversion was 65%: H2O2:S = 2:1 (mol), 0.5% Mo/TiO2-SO3H, 1 mL of acetonitrile, 80 °C, 10 min. According to the results obtained, the conversion of DBT in dark glass was 64%, which indicates that the synthesized catalysts are not sensitive to light.
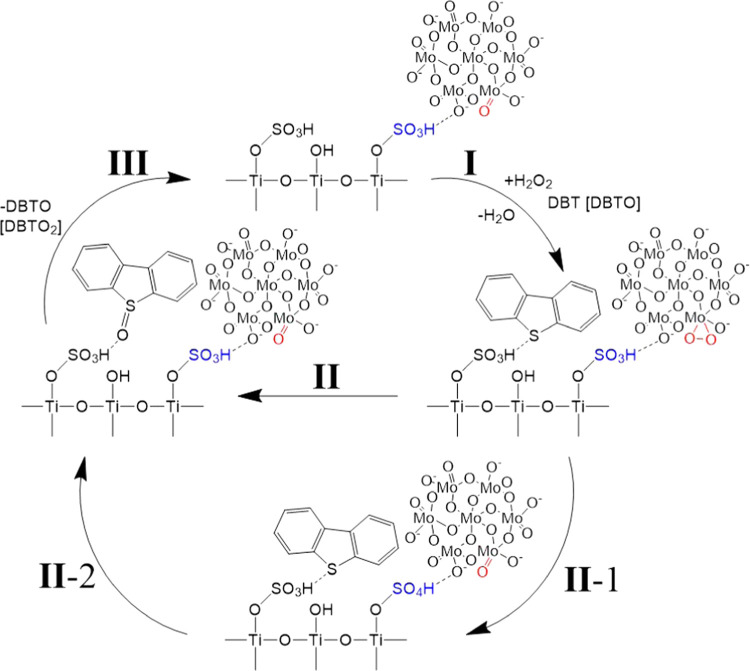
Based on the results obtained and the facts known from the literature, we suggested a possible scheme for the oxidation of dibenzothiophene with hydrogen peroxide in the presence of a Mo/TiO2-SO3H catalyst. Given that the catalyst contains two active sites, the reaction scheme also consists of two possible cycles, each of which can take place during oxidation. In the first stage (I), the peroxo complex is formed upon contact of the heptamolybdate anion with hydrogen peroxide.58,59 In this case, sulfur-containing compounds, which are Lewis bases, are coordinated on sulfo groups, which are Bronsted acids.21 In the second stage, direct oxidation of sulfur-containing substrates by the molybdenum peroxo complex is possible with the formation of an oxidation product and the transition of the catalyst to the initial state (II). Another possible direction of the reaction is the formation of peracid under the action of peroxo complexes (II-1).60 In the last stage (II-2), the peracid oxidizes the sulfur-containing substrate and is reduced to the original sulfo group. Thus, the reaction scheme proposed in Figure 9 illustrates how the heptamolybdate anion and sulfonic catalytic sites work, and also shows why the combination of the two sites produces better results.
A comparison of the results obtained in the oxidation of organosulfur substrates in the presence of a synthesized combined catalyst with titanium-containing catalysts known in the literature is given in Table 2. Using conventional titanium oxide for DBT-containing model feed oxidation, the DBT removal is not complete. It was shown by Li and co-authors that the anatase additive allows for complete DBT oxidation for 1 min.31 Zhu and Wang investigated the oxidation of DBT in the presence of amorphous and nano-titanium oxide and ionic liquids.32−34 Researchers have shown that the catalysts with the composition “TiO2 in [Bmim]BF4” allow oxidation with hydrogen peroxide and molecular oxygen at low temperatures (RT-30 °C) under ultrasonic exposure. To increase the activity of the photocatalyst, the authors34 synthesized titanium oxide deposited on a carbon substrate (g-C3N4), which made it possible to carry out oxidation at room temperature without using an ionic liquid. The authors of refs (35−37) have shown that the use of titanium-containing zeolite TS-1 as a catalyst makes it possible to completely oxidize the model mixture of DBT with hydrogen peroxide or organic peroxides without ultraviolet exposure. The synthesis of various titanium silicates and the deposition of titanium and its oxide on mesoporous supports also allow for oxidation without an ultraviolet source,38−41 while the authors of the study39 also managed to reduce the reaction time to 20 min.
The literature also describes molybdenum–titanium catalytic systems such as MoO3–TiO2@MCM-22 and Mo/MTP-350,42,43 which allow the oxidation of DBT in a short time. However, the synthesis of the above catalysts requires the use of expensive templates, which greatly increases the cost of the catalyst and, as a consequence, the cost of the oxidative desulfurization process.
The catalyst synthesized in this work is not only simply synthesized with high yields, but also it works effectively in a short oxidation time (10 min), due to the combination of two active centers in its composition—sulfo groups and molybdenum oxide.
The effectiveness of the most active catalyst Mo/TiO2-SO3H was also investigated in the oxidative desulfurization of the straight-run diesel fraction. Oxidation was carried out under the following conditions: H2O2:S = 2:1 (mol), 0.5% catalyst, 5 mL of acetonitrile, 80 °C, 30 min. In 30 min of reaction, it was possible to reduce the content of sulfur compounds in the diesel fraction by 88% (from 5600 to 600 ppm).
4. Conclusions
Sulfo-functionalized catalysts based on titanium oxide were used for the first time in the oxidative desulfurization process. The composition and structure of the obtained catalysts were confirmed by the methods of low-temperature adsorption/desorption of nitrogen, XRD, X-ray fluorescence (XRF), SEM, and FTIR. According to the data of low-temperature adsorption/desorption of nitrogen, the deposition of molybdenum on the surface of titanium oxide leads to an insignificant decrease in the surface area, while for modification with sulfo groups, a significant (almost 2 times) decrease in the surface area is observed. The data on the mass content of sulfo groups, determined by acid–base titration and elemental analysis, agree well, which indicates a successful modification of the titanium oxide surface.
The results on DBT conversion obtained in the presence of a combined Mo/TiO2-SO3H catalyst containing both active sites (a compound of molybdenum and a sulfo group) show that the combination of two catalytic sites makes it possible to achieve better results than the total effect of each active site. Already 1% by mass catalyst amount is enough for complete oxidation of DBT in 10 min, which is a very good indicator for a titanium-containing catalyst. Complete oxidation of DBT is achieved with a stoichiometric amount of hydrogen peroxide, which indicates a high selectivity of the synthesized Mo/TiO2-SO3H catalyst and the target consumption of the oxidant only for sulfur-containing substrate oxidation. The presence of sulfo groups increases the stability of the catalyst and minimizes the leaching of molybdenum, which allows the catalyst to operate for 7 oxidation cycles without the need for regeneration and without changing the activity.
Thus, the combination of two catalytic sites makes it possible to obtain not only a more active catalyst compared to analogues but also significantly increases the service life of such a catalyst, and the relative simplicity of its synthesis opens up opportunities for scaling up the oxidative desulfurization process using molybdenum-containing heterogeneous catalysts modified with sulfo groups based on titanium oxide.
Glossary
Abbreviations
- BJH
Barrett–Joyner–Halenda model
- BET
Brunauer–Emmet–Teller model
- CYHPO
cyclohexanone peroxide
- DBT
dibenzothiophene
- FT-IR
Fourier transform infrared spectroscopy
- SEM
scanning electron microscopy
- TBHP
tert-butyl hydroperoxide
- XFA
X-ray spectral fluorescence analysis
- XRD
X-ray diffraction
- XRF
X-ray fluorescence
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The study was financially supported by the Russian Science Foundation (project no. 21-79-10140, https://rscf.ru/ project/21-79-10140/).
The authors declare no competing financial interest.
References
- Timko M. T.; Wang J. A.; Burgess J.; Kracke P.; Gonzalez L.; Jaye C.; Fischer D. A. Roles of surface chemistry and structural defects of activated carbons in the oxidative desulfurization of benzothiophenes. Fuel 2016, 163, 223–231. 10.1016/j.fuel.2015.09.075. [DOI] [Google Scholar]
- Kampouraki Z. C.; Giannakoudakis D. A.; Triantafyllidis K. S.; Deliyanni E. A. Catalytic oxidative desulfurization of a 4,6-DMDBT containing model fuel by metal-free activated carbons: the key role of surface chemistry. Green Chem. 2019, 21, 6685–6698. 10.1039/C9GC03234G. [DOI] [Google Scholar]
- European Union emission inventory report 1990–2014 under the UNECE Convention on Long-Range Transboundary Air Pollution (LRTAP), European Environment Agency, 2016, 10.2800/18374. [DOI]
- Javadli R.; de Klerk A. Desulfurization of heavy oil. Appl. Petrochem. Res. 2012, 1, 3–19. 10.1007/s13203-012-0006-6. [DOI] [Google Scholar]
- Gutiérrez O. Y.; Singh S.; Schachtl E.; Kim J.; Kondratieva E.; Hein J.; Lercher J. A. Effects of the support on the performance and promotion of (Ni)MoS2 catalysts for simultaneous hydrodenitrogenation and hydrodesulfurization. ACS Catal. 2014, 4, 1487–1499. 10.1021/cs500034d. [DOI] [Google Scholar]
- Houda S.; Lancelot C.; Blanchard P.; Poinel L.; Lamonier C. Oxidative desulfurization of heavy oils with high sulfur content: a review. Catalysts 2018, 8, 344–369. 10.3390/catal8090344. [DOI] [Google Scholar]
- Sun L. L.; Su T.; Xu J. J.; Hao D. M.; Liao W. P.; Zhao Y. C.; Ren W. Z.; Deng C. L.; Lu H. Y. Aerobic oxidative desulfurization coupling of Co polyanion catalysts and p-TsOH-based deep eutectic solvents through a biomimetic approach. Green Chem. 2019, 21, 2629–2634. 10.1039/c8gc03941k. [DOI] [Google Scholar]
- Akopyan A. V.; Fedorov R. A.; Andreev B. V.; Tarakanova A. V.; Anisimov A. V.; Karakhanov E. A. Oxidative desulfurization of hydrocarbon feedstock. Russ. J. Appl. Chem. 2018, 91, 529–542. 10.1134/S1070427218040018. [DOI] [Google Scholar]
- Betiha M. A.; Rabie A. M.; Ahmed H. S.; Abdelrahman A. A.; El-Shahat M. F. Oxidative desulfurization using graphene and its composites for fuel containing thiophene and its derivatives: An update review. Egypt. J. Pet. 2018, 27, 715–730. 10.1016/j.ejpe.2017.10.006. [DOI] [Google Scholar]
- Abdullah W. N. W.; Ali R.; Bakar W. A. W. A. In depth investigation of Fe/MoO3–PO4/Al2O3 catalyst in oxidative desulfurization of Malaysian diesel with TBHP-DMF system. J. Taiwan Inst. Chem. Eng. 2016, 58, 344–350. 10.1016/j.jtice.2015.06.001. [DOI] [Google Scholar]
- Akopyan A. V.; Grigoriev D. A.; Polikarpova P. L.; Eseva E. A.; Litvinova V. V.; Anisimov A. V. Ozone-assisted oxidative desulfurization of light oil fractions. Pet. Chem. 2017, 57, 904–907. 10.1134/S0965544117100024. [DOI] [Google Scholar]
- Eseva E.; Akopyan A.; Schepina A.; Anisimov A.; Maximov A. Deep aerobic oxidative desulfurization of model fuel by Anderson-type polyoxometalate catalysts. Catal. Commun. 2021, 149, 106256 10.1016/j.catcom.2020.106256. [DOI] [Google Scholar]
- Hossain M. N.; Park H. C.; Choi H. S. A comprehensive review on catalytic oxidative desulfurization of liquid fuel oil. Catalysts 2019, 9, 229–241. 10.3390/catal9030229. [DOI] [Google Scholar]
- Rakhmanov E. V.; Tarakanova A. V.; Valieva T.; Akopyan A. V.; Litvinova V. V.; Maksimov A. L.; Anisimov A. V.; Vakarin S. V.; Semerikova O. L.; Zaikov Y. P. Oxidative desulfurization of diesel fraction with hydrogen peroxide in the presence of catalysts based on transition metals. Pet. Chem. 2014, 54, 48–50. 10.1134/S0965544114010101. [DOI] [Google Scholar]
- Taghizadeh M.; Mehrvarz E.; Taghipour A. Polyoxometalate as an effective catalyst for the oxidative desulfurization of liquid fuels: a critical review. Rev. Chem. Eng. 2020, 36, 831–858. 10.1515/revce-2018-0058. [DOI] [Google Scholar]
- Zou C. J.; Zhao P. W.; Ge J.; Qin Y. B.; Luo P. Y. Oxidation/adsorption desulfurization of natural gas by bridged cyclodextrins dimer encapsulating polyoxometalate. Fuel 2013, 104, 635–640. 10.1016/j.fuel.2012.06.096. [DOI] [Google Scholar]
- Jiang W.; Xiao J.; Dong L.; Wang C.; Li H.; Luo Y.; Zhu W.; Li H. Polyoxometalate-based poly(ionic liquid) as a precursor for superhydrophobic magnetic carbon composite catalysts toward aerobic oxidative desulfurization. ACS Sustainable Chem. Eng. 2019, 7, 15755–15761. 10.1021/acssuschemeng.9b04026. [DOI] [Google Scholar]
- Jiang W.; Dong L.; Liu W.; Guo T.; Li H. P.; Zhang M.; Zhu W. S.; Li H. M. Designing multifunctional SO3H-based polyoxometalate catalysts for oxidative desulfurization in acid deep eutectic solvents. RSC Adv. 2017, 7, 55318–55325. 10.1039/C7RA10125B. [DOI] [Google Scholar]
- Bryzhin A. A.; Gantman M. G.; Buryak A. K.; Tarkhanova I. G. Brønsted acidic SILP-based catalysts with H3PMo12O40 or H3PW12O40 in the oxidative desulfurization of fuels. Appl. Catal., B 2019, 257, 117938 10.1016/j.apcatb.2019.117938. [DOI] [Google Scholar]
- Lv G. J.; Deng S. L.; Yi Z.; Zhang X. B.; Wang F. M.; Li H. C.; Zhu Y. Q. One-pot synthesis of framework W-doped TS-1 zeolite with robust Lewis acidity for effective oxidative desulfurization. Chem. Commun. 2019, 55, 4885–4888. 10.1039/C9CC00715F. [DOI] [PubMed] [Google Scholar]
- Akopyan A.; Polikarpova P.; Gul O.; Anisimov A.; Karakhanov E. Catalysts based on acidic SBA-15 for deep oxidative desulfurization of model fuels. Energy Fuels. 2020, 34, 14611–14619. 10.1021/acs.energyfuels.0c02008. [DOI] [Google Scholar]
- Crucianelli M.; Bizzarri B. M.; Saladino R. SBA-15 anchored metal containing catalysts in the oxidative desulfurization process. Catalysts 2019, 9, 984–1013. 10.3390/catal9120984. [DOI] [Google Scholar]
- Cruz P.; Granados E. A.; Fajardo M.; del Hierro I.; Perez Y. Heterogeneous oxidative desulfurization catalysed by titanium grafted mesoporous silica nanoparticles containing tethered hydrophobic ionic liquid: A dual activation mechanism. Appl. Catal., A 2019, 587, 117241 10.1016/j.apcata.2019.117241. [DOI] [Google Scholar]
- Wang P. Y.; Jiang L. C.; Zou X. Q.; Tan H. Q.; Zhang P. P.; Li J. L.; Liu B. S.; Zhu G. S. Confining polyoxometalate clusters into porous aromatic framework materials for catalytic desulfurization of dibenzothiophene. ACS Appl. Mater. Int. 2020, 12, 25910–25919. 10.1021/acsami.0c05392. [DOI] [PubMed] [Google Scholar]
- Akopyan A.; Polikarpova P.; Vutolkina A.; Cherednichenko K.; Stytsenko V.; Glotov A. Natural clay nanotube supported Mo and W catalysts for exhaustive oxidative desulfurization of model fuels. Pure Appl. Chem. 2021, 93, 231–241. 10.1515/pac-2020-0901. [DOI] [Google Scholar]
- Zou J.; Lin Y.; Wu S.; Zhong Y.; Yang C. Molybdenum dioxide nanoparticles anchored on nitrogen-doped carbon nanotubes as oxidative desulfurization catalysts: role of electron transfer in activity and reusability. Adv. Funct. Mater. 2021, 31, 2100442 10.1002/adfm.202100442. [DOI] [Google Scholar]
- Zou J.; Lin Y.; Wu S.; Wu M.; Yang C. Construction of bifunctional 3-D ordered mesoporous catalyst for oxidative desulfurization. Sep. Purif. Technol. 2021, 264, 118434 10.1016/j.seppur.2021.118434. [DOI] [Google Scholar]
- Lu S. X.; Zhong H.; Mo D. M.; Hu Z.; Zhou H. L.; Yao Y. A H-titanate nanotube with superior oxidative desulfurization selectivity. Green Chem. 2017, 19, 1371–1377. 10.1039/c6gc03573f. [DOI] [Google Scholar]
- Rezvani M. A.; Shaterian M. Synthesis and characterization of new nanocomposite CTAB@POM@TiO2 as an efficient heterogeneous catalyst for oxidative desulfurization of gas oil. Inorg. Nano-Metal Chem. 2019, 49, 23–32. 10.1080/24701556.2019.1577259. [DOI] [Google Scholar]
- Dedual G.; MacDonald M. J.; Alshareef A.; Wu Z.; Tsang D. C. W.; Yip A. C. K. Requirements for effective photocatalytic oxidative desulfurization of a thiophene-containing solution using TiO2. J. Environ. Chem. Eng. 2014, 2, 1947–1955. 10.1016/j.jece.2014.08.012. [DOI] [Google Scholar]
- Li L. T.; Zhang J. S.; Shen C.; Wang Y. J.; Luo G. S. Oxidative desulfurization of model fuels with pure nano-TiO2 as catalyst directly without UV irradiation. Fuel 2016, 167, 9–16. 10.1016/j.fuel.2015.11.047. [DOI] [Google Scholar]
- Wang X. J.; Li F. T.; Liu J. X.; Kou C. G.; Zhao Y.; Hao Y. J.; Zhao D. S. Preparation of TiO2 in Ionic Liquid via Microwave Radiation and in Situ Photocatalytic Oxidative Desulfurization of Diesel Oil. Energy Fuels. 2012, 26, 6777–6782. 10.1021/ef301337y. [DOI] [Google Scholar]
- Zhu W. S.; Xu Y. H.; Li H. M.; Dai B. L.; Xu H.; Wang C.; Chao Y. H.; Liu H. Preparation of titanium dioxide/tungsten disulfide composite photocatalysts with enhanced photocatalytic activity under visible light. Korean J. Chem. Eng. 2014, 31, 211–217. 10.1039/c4ra01482k. [DOI] [Google Scholar]
- Hu X.; Deng L.; Ouyang H.; Wang H. Immobilization of g-C3N4 nanosheets on diatomite via electrostatic adsorption and their photocatalytic activity. RSC Adv. 2018, 8, 28032–28040. 10.1039/C8RA05408H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. T.; Jeong K. E.; Jeong S. Y.; Ahn W. S. Synthesis of mesoporous TS-1 using a hybrid SiO2-TiO2 xerogel for catalytic oxidative desulfurization. Mater. Res. Bull. 2012, 47, 4398–4402. 10.1016/j.materresbull.2012.09.041. [DOI] [Google Scholar]
- Shen C.; Wang Y. J.; Xu J. H.; Luo G. S. Synthesis of TS-1 on porous glass beads for catalytic oxidative desulfurization. Chem. Eng. J. 2015, 259, 552–561. 10.1016/j.cej.2014.08.027. [DOI] [Google Scholar]
- Lv G. J.; Deng S. L.; Zhai Y.; Zhu Y. Q.; Li H. C.; Wang F. M.; Zhang X. B. P123 lamellar micelle-assisted construction of hierarchical TS-1 stacked nanoplates with constrained mesopores for enhanced oxidative desulfurization. Appl. Catal., A 2018, 567, 28–35. 10.1016/j.apcata.2018.09.009. [DOI] [Google Scholar]
- Fraile J. M.; Gil C.; Mayoral J. A.; Muel B.; Roldan L.; Vispe E.; Calderon S.; Puente F. Heterogeneous titanium catalysts for oxidation of dibenzothiophene in hydrocarbon solutions with hydrogen peroxide: On the road to oxidative desulfurization. Appl. Catal., B 2016, 180, 680–686. 10.1016/j.apcatb.2015.07.018. [DOI] [Google Scholar]
- Rivoira L. P.; Ledesma B. C.; Juarez J. M.; Beltramone A. R. Novel and simple one-pot method for the synthesis of TiO2 modified-CMK-3 applied in oxidative desulfurization of refractory organosulfur compounds. Fuel 2018, 226, 498–507. 10.1016/j.fuel.2018.04.054. [DOI] [Google Scholar]
- Liu X.; Liu X.; Shan J.; Huai J.; Yang H.; Yan X.; Zheng Y.; Li H. Synthesis of amorphous mesoporous TiO2-SiO2 and its excellent catalytic performance in oxidative desulfurization. Inorg. Chem. Commun. 2021, 123, 108336 10.1016/j.inoche.2020.108336. [DOI] [Google Scholar]
- Mahmoudabadi Z. A.; Rashidi A.; Tavasoli A. Synthesis of two-dimensional TiO2@multi-walled carbon nanotube nanocomposites as smart nanocatalyst for ultra-deep oxidative desulfurization of liquid fuel: Optimization via response surface methodology. Fuel 2021, 306, 121635 10.1016/j.fuel.2021.121635. [DOI] [Google Scholar]
- Luo Q.; Zhou Q.; Lin Y.; Wu S. H.; Liu H. Y.; Du C.; Zhong Y. Y.; Yang C. P. Fast and deep oxidative desulfurization of dibenzothiophene with catalysts of MoO3–TiO2@MCM-22 featuring adjustable Lewis and Brønsted acid sites. Catal. Sci. Technol. 2019, 9, 6166–6179. 10.1039/C9CY01438A. [DOI] [Google Scholar]
- Chen L.; Hu Z. P.; Ren J. T.; Wang Z.; Yuan Z. Y. Efficient oxidative desulfurization over highly dispersed molybdenum oxides supported on mesoporous titanium phosphonates. Microporous Mesoporous Mater. 2021, 315, 110921 10.1016/j.micromeso.2021.110921. [DOI] [Google Scholar]
- Polikarpova P.; Akopyan A.; Shlenova A.; Anisimov A. New mesoporous catalysts with Brønsted acid sites for deep oxidative desulfurization of model fuels. Catal. Commun. 2020, 146, 106123 10.1016/j.catcom.2020.106123. [DOI] [Google Scholar]
- Akopyan A. V.; Plotnikov D. A.; Polikarpova P. D.; Kedalo A. A.; Egazar’yants S. V.; Anisimov A. V.; Karakhanov E. A. Deep purification of vacuum gas oil by the method of oxidative desulfurization. Pet. Chem. 2019, 59, 975–978. 10.1134/s0965544119090019. [DOI] [Google Scholar]
- Shirini F.; Abedini M.; Kiaroudi S. A. Introduction of titania sulfonic acid (TiO2-SO3H) as a new, efficient, and reusable heterogenous solid acid catalyst for the synthesis of biscoumarins. Phosphorus, Sulfur Silicon Relat. Elem. 2014, 189, 1279–1288. 10.1080/10426507.2013.858254. [DOI] [Google Scholar]
- Akopyan A. V.; Kulikov L. A.; Polikarpova P. D.; Shlenova A. O.; Anisimov A. V.; Maximov A. L.; Karakhanov E. A. Metal-free oxidative desulfurization catalysts based on porous aromatic frameworks. Ind. Eng. Chem. Res. 2021, 60, 9049–9058. 10.1021/acs.iecr.1c00886. [DOI] [Google Scholar]
- Chen J.; Pu G.; Li J. Oxidation of NOx using hydrogen peroxide vapor over Mo/TiO2. ACS Omega 2020, 5, 11784–11791. 10.1021/acsomega.0c01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy B. M.; Ratnam K. J.; Saikia P.; Thrimurthulu G. Influence of alkaline earth metal on acid–base characteristics of V2O5/MO–TiO2 (M = Ca, Sr and Ba) catalysts. J. Mol. Catal. A: Chem. 2007, 276, 197–204. 10.1016/j.molcata.2007.07.018. [DOI] [Google Scholar]
- Atghia S. V.; Beigbaghlou S. S. Nanocrystalline titania-based sulfonic acid (TiO2-Pr-SO3H) as a new, highly efficient and recyclable solid acid catalyst for the N-Boc protection of amines at room temperature. J. Organomet. Chem. 2013, 745-746, 42–49. 10.1016/j.jorganchem.2013.07.033. [DOI] [Google Scholar]
- Du Y.; Zhou L. N.; Liu Z. H.; Guo Z. R.; Wang X. Z.; Lei J. H. Designed formation of mesoscopical order of ionic liquid-based meso/macroporous Mo/TiO2 materials for high-performance oxidative desulfurization. Chem. Eng. J. 2020, 387, 124056 10.1016/j.cej.2020.124056. [DOI] [Google Scholar]
- Sikarwar P.; Kumar U. K. A.; Gosu V.; Subbaramaiah V. Catalytic oxidative desulfurization of DBT using green catalyst (Mo/MCM-41) derived from coal fly ash. J. Environ. Chem. Eng. 2018, 6, 1736–1744. 10.1016/j.jece.2018.02.021. [DOI] [Google Scholar]
- Xu Y.; Shu Y.; Liu S.; Huang J.; Guo X. Interaction between ammonium heptamolybdate and NH4ZSM-5 zeolite: the location of Mo species and the acidity of Mo/HZSM-5. Catal. Lett. 1995, 35, 233–245. 10.1007/BF00807179. [DOI] [Google Scholar]
- Cabrera-Munguia D. A.; Gonzalez H.; Tututi-Rios E.; Gutierrez-Alejandre A.; Rico J. L. Acid properties of M-SBA-15 and M-SBA-15-SO3H (M = Al, Ti) materials and their role on esterification of oleic acid. J. Mater. Res. 2018, 33, 3634–3645. 10.1557/jmr.2018.374. [DOI] [Google Scholar]
- Sun H.; Wang S. Catalytic oxidation of organic pollutants in aqueous solution using sulfate radicals. Catalysis 2015, 27, 209–247. 10.1039/9781782622697-00209. [DOI] [Google Scholar]
- Wang J.; Wang Z.; Cheng Y.; Cao L.; Bai F.; Yue S.; Xie P.; Ma J. Molybdenum disulfide (MoS2): A novel activator of peracetic acid for the degradation of sulfonamide antibiotics. Water Res. 2021, 201, 117291 10.1016/j.watres.2021.117291. [DOI] [PubMed] [Google Scholar]
- Akopyan A. V.; Polikarpova P. D.; Arzyaeva N. V.; Anisimov A. V.; Maslova O. V.; Senko O. V.; Efremenko E. N. Model Fuel Oxidation in the Presence of Molybdenum-Containing Catalysts Based on SBA-15 with Hydrophobic Properties. ACS Omega 2021, 6, 26932–26941. 10.1021/acsomega.1c03267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost B. M.; Masuyama Y. Molybdenum Catalyzed Reactions Selectivity in Oxidations with Hydrogen Peroxide and Ammonium Molybdate. Isr. J. Chem. 1984, 24, 134–143. 10.1002/ijch.198400023. [DOI] [Google Scholar]
- Mouheb L.; Dermeche L.; Mazari T.; Benadji S.; Essayem N.; Rabia C. Clean Adipic Acid Synthesis from Liquid-Phase Oxidation of Cyclohexanone and Cyclohexanol Using (NH4)xAyPMo12O40 (A: Sb, Sn, Bi) Mixed Heteropolysalts and Hydrogen Peroxide in Free Solvent. Catal. Lett. 2018, 148, 612–620. 10.1007/s10562-017-2263-6. [DOI] [Google Scholar]
- Sun H. Q.; Wang S. B. Catalytic oxidation of organic pollutants in aqueous solution using sulfate radicals. Catalysis 2015, 27, 209–247. 10.1039/9781782622697-00209. [DOI] [Google Scholar]
- Pham X. N.; Nguyen M. B.; Doan H. V. Direct synthesis of highly ordered Ti-containing Al-SBA-15 mesostructured catalysts from natural halloysite and its photocatalytic activity for oxidative desulfurization of dibenzothiophene. Adv. Powder Technol. 2020, 31, 3351–3360. 10.1016/j.apt.2020.06.028. [DOI] [Google Scholar]
- Zuo M.; Huang X.; Li J.; Chang Q.; Duan Y.; Yan L.; Xiao Z.; Mei S.; Lu S.; Yao Y. Oxidative desulfurization in diesel via a titanium dioxide triggered thermocatalytic mechanism. Catal. Sci. Technol. 2019, 9, 2923–2930. 10.1039/c9cy00298g. [DOI] [Google Scholar]
- Wang H.; Du G.; Chen S.; Su Z.; Sun P.; Chen T. Steam-assisted strategy to fabricate Anatase-free hierarchical titanium Silicalite-1 Single-Crystal for oxidative desulfurization. J. Colloid Interface Sci. 2022, 617, 32–43. 10.1016/j.jcis.2022.02.121. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Leng K.; Hou C.; Sun Y. Preparation of titanium-containing hierarchical zeolite Y and its catalytic performance in oxidative desulfurization of dibenzothiophene. Curr. Catal. 2018, 7, 122–128. 10.2174/2211544707666180126151745. [DOI] [Google Scholar]
- Wang Y.; Du F.; Wang C.; Zhao J.; Sun H.; Sun C. The synthesis and oxidation desulfurization performance of Ti-modified hierarchical cheese-like ZSM-5 zeolite. J. Chem. Res. 2022, 46, 174751982110686 10.1177/17475198211068663. [DOI] [Google Scholar]
- Kim T.-W.; Kim M.-J.; Kleitz F.; Nair M. M.; Guillet-Nicolas R.; Jeong K.-E.; Chae H.-J.; Kim C.-U.; Jeong S.-Y. Tailor-made mesoporous Ti-SBA-15 catalysts for oxidative desulfurization of refractory aromatic sulfur compounds in transport fuel. Chem. Cat. Chem. 2012, 4, 687–697. 10.1002/cctc.201100417. [DOI] [Google Scholar]