Abstract
Objectives:
To evaluate the ability of MRI-targeted biopsy combined with systematic biopsy (MRI-biopsy) to reduce negative biopsies and detect clinically significant prostate cancer compared to systematic biopsy (SB) alone in the confirmatory biopsy setting using matched cohorts.
Patients and Methods:
Patients were identified from an active surveillance (AS) database who had a previously positive transrectal ultrasound (TRUS) guided SB followed by a confirmatory biopsy at a single institution between 2006–2019. Patients were divided into two cohorts based on confirmatory biopsy technique, either a SB alone or MRI-biopsy (which included MRI-targeted and systematic biopsies). Cohorts were then matched on age, PSA, number of positive cores on initial biopsy and initial biopsy Gleason grade group (GG). Logistic regression was performed to identify associations with confirmatory biopsy upgrading.
Results:
After matching, 514 patients were identified (257 per cohort). PSA, prostate volume, and PSA density prior to initial biopsy in addition to total number of initial biopsy positive cores and GG were similar between the matched cohorts. After confirmatory biopsy, 118/257 (45.9%) within the MRI-biopsy cohort were upgraded compared to 46/257 (17.9%) within the SB cohort (p<0.001). The rate of negative confirmatory biopsy was 32/257 (12.5%) compared to 97/257 (37.7%) among the MRI-biopsy and SB cohorts, respectively (p<0.001). Confirmatory MRI-biopsy was associated with greater odds of confirmatory biopsy upgrade from GG1 to ≥GG2 compared to SB alone (OR 3.62, 95% CI 1.97–6.63, p<0.001).
Conclusion:
The addition of MRI-targeted biopsies to systematic biopsy in the confirmatory biopsy setting among men with previously detected prostate cancer resulted in fewer negative confirmatory biopsies and detection of more clinically significant prostate cancer compared to systematic biopsy alone.
Keywords: biopsy, prostate cancer, magnetic resonance imaging, active surveillance, transrectal ultrasound
INTRODUCTION
Active surveillance (AS) is currently a standard conservative therapy for men with low risk prostate cancer and select men with intermediate risk prostate cancer (1, 2). The ability to accurately identify which patients have higher grade disease prior to AS enrollment is necessary to treat those with high risk disease while avoiding overtreatment of low risk disease. Traditionally, 12-core transrectal ultrasound (TRUS)-guided systematic biopsy (SB) was used as the gold standard technique for prostate cancer diagnosis. This technique is limited by significant sampling error and false negative results leading to undetected clinically significant disease that may be amenable to early treatment (3, 4).
Integration of multiparametric magnetic resonance imaging (MRI) into routine prostate cancer care and fusion technologies enabling targeted biopsy techniques has rapidly changed the detection and diagnosis of prostate cancer (4). Based on the results of two randomized trials, MRI-targeted prostate biopsy has improved the ability to detect clinically significant cancer in multiple patient groups, while reducing the rate of false negative biopsies (5–7). Among biopsy naïve men or men with previously negative biopsies, MRI of the prostate can detect clinically significant disease in the range of 44–87%, which is significantly higher than rates for SB (8–11). Given the improvement in detection of clinically significant disease, MRI has been incorporated into the diagnostic algorithm in different ways; however, the correct timing of when an MRI-targeted biopsy should be incorporated is not yet known. One area of particular interest is in men with low risk prostate cancer that was detected on SB who may be eligible for AS. Compared to MRI-targeted biopsy, under-grading and risk of grade progression at subsequent surveillance biopsies is higher among SB patients; thus, integration of MRI into the confirmatory biopsy setting may more accurately stage prostate cancer patients (12–14). Current European Association of Urology guidelines recommend MRI incorporation in the confirmatory biopsy setting (15).
The majority of previous randomized trials comparing MRI targeted biopsy to SB involved biopsy naïve patients or those with previously negative biopsies (5, 16–22). Notably, one randomized clinical trial has compared SB to MRI targeted biopsy in the confirmatory setting following prostate cancer diagnosis, reporting no significant improvement in the rate of clinically significant (Gleason grade group [GG] 2 or higher) cancer detection among MRI guided biopsies compared to standard SB. However, given aforementioned data about the sensitivity of MRI targeted biopsy, in addition to further data regarding variations in MRI performance, including quality and variability in interpretation (23–26), we hypothesized that MRI targeted biopsy may be associated with improved detection of GG2 disease in the confirmatory setting compared to SB. In order to test this hypothesis, we performed a matched cohort analysis comparing men who underwent MRI-targeted and SB following initial SB-based diagnosis of prostate cancer at our institution.
METHODS
Patient Selection
The study was approved by the institutional review board and all patients signed informed consent. Patients from two separate cohorts were included in the study. The first cohort of men were considered for enrollment on a prospective AS protocol that began in 2006. The study was conducted by a multidisciplinary team of urologic surgeons, radiation oncologists, and medical oncologists as previously described (27) and was registered at clinicaltrials.gov (trial number NCT00490763). Men underwent a diagnostic biopsy at either an outside institution or UT MD Anderson Cancer Center. Prior to AS protocol enrollment, the men underwent a confirmatory, systematic 11-core TRUS-guided biopsy within 6 months of initial diagnostic biopsy as previously described (28).
The second cohort consisted of men enrolled on a prospectively maintained database of MRI-targeted biopsies beginning in 2014. Men were included from this database who had an initial TRUS-guided SB performed for a history of an elevated PSA and/or abnormal digital rectal exam prior to prostate cancer diagnosis. In addition, all included men underwent a confirmatory biopsy using the Artemis™ system (Elgen, California) at our institution, including both targeted ultrasound fusion biopsies and systematic cores. Prior to biopsy, a prostate MRI was performed with endorectal coil at 1.5T. Multiparametric MRI sequences performed included T1-weighted, T2-weighted, diffusion-weighted imaging including calculation of the apparent diffusion co-efficient maps, and dynamic contrast enhancement. Images were reviewed by dedicated genitourinary radiologists. Suspicious lesions were graded on a Likert scale from 1–5 and any lesion graded ≥3 was targeted for biopsy. For targeted, ultrasound fusion biopsies, all biopsies were performed with monitored IV conscious sedation as previously described (29). One or two cores were obtained from suspicious lesions. In the same setting, all patients underwent additional SBs obtained from regions of an extended sextant, 12-region biopsy plan that were not previously sampled through a targeted biopsy. For this paper, MRI-biopsy will refer to patients receiving both MRI-targeted and SBs in the same setting.
All confirmatory biopsies in both cohorts were completed within 24 months of the original prostate cancer diagnosis. Any diagnostic biopsies performed outside of our institution were re-reviewed by a dedicated genitourinary pathologist. After confirmatory biopsy, men with localized prostate cancer and GG 1 or 2 disease were eligible for AS. AS was extended to patients with GG3 in rare situations based on physician discretion. Patients enrolled on the AS protocol listed above underwent biannual evaluation with digital rectal examination, serum PSA and testosterone. Additionally, surveillance SBs were repeated every 1 to 2 years. Patients with increasing tumor volume or Gleason score were recommended to undergo treatment; however, patients could choose to stay on AS if approved by the physician (30). MRI-biopsy patients who elected AS underwent a similar schedule but were not dictated by the AS protocol; thus, the two confirmatory biopsy cohorts may differ slightly in AS schedule.
Cohort matching
Baseline characteristics were compared between the two cohorts including age, race, PSA prior to the initial biopsy, prostate volume as measured on TRUS, and calculated PSA density. Prostate volume was calculated using the formula volume= length (cm) × width (cm) × height (cm) × π/6. Pathologic characteristics were collected for both the initial biopsy and confirmatory biopsy including number of cores taken, number of positive cores, sum of the total tumor length among all cores, maximum tumor length in a single core (27), and GG. Patients were categorized by risk group defined by the AUA guidelines after initial biopsy and confirmatory biopsy (1). Patients who underwent confirmatory MRI-biopsy were then matched to patients from the AS cohort based on age, pre-diagnostic PSA, diagnostic GG, and number of positive cores.
Statistical analysis
Descriptive statistics evaluated differences in baseline characteristics between cohorts. Wilcoxon rank sum and Fisher’s exact test were used for continuous and categorical variables, respectively. To correct for biases in confirmatory biopsy selection and confounding baseline characteristics, we matched the cohorts using 1:1 matching. Nearest neighbor matching was performed for age and PSA prior to initial biopsy, and exact matching was performed for initial biopsy GG and number of positive cores in the initial biopsy, as these variables may influence the biopsy detection rate (9). Multivariable logistic regression was used to determine the association between biopsy upgrading and covariates. Our primary outcome of interest was evaluating the effect of confirmatory biopsy type on probability of upgrading between the matched cohorts. Upgrading was defined as any increase in GG from the initial prostate biopsy to the confirmatory biopsy. We performed additional analyses to identify predictors of upgrading from GG1 to ≥GG2, GG1 to ≥GG3 and GG1 or 2 to ≥GG3. Covariates included in the multivariable models included biopsy cohort, patient age, PSA density as a continuous variable, initial biopsy GG, initial PSA as a continuous variable, number of initial biopsy positive cores, initial maximum single core tumor length, and total number of confirmatory biopsy cores. Given the limited number of upgrading events from GG1 to ≥GG3, we a priori only included biopsy cohort, patient age, PSA density, and total number of confirmatory biopsy cores in our model. We additionally applied these multivariable models to the unmatched population. Statistical significance was considered if p≤0.05. Statistical analysis was performed using Stata/SE version 15.1 statistical software (Stata Corp. LP, College Station, TX) and R studio (Version 1.1.383 - © 2009–2017 RStudio, Inc.).
RESULTS
A total of 1026 patients from two cohorts were identified between January 2006 and April 2019, of which 691 patients underwent a confirmatory SB and 335 underwent a confirmatory MRI-biopsy (including both an MRI-targeted biopsy and SB). The median time between initial biopsy and confirmatory biopsy was 4.2 months (IQR 2.8–5.8 months). The median time between MRI and MRI-biopsy was 1.7 months (IQR 0.8–1.9 months). Supplementary Table 1 lists the baseline characteristics by confirmatory biopsy cohort prior to matching. Prior to matching, significant differences existed between SB and MRI-biopsy cohorts including initial PSA (median 4.1 vs 5.7ng/mL, p<0.001), prostate volume (median 38.1 vs 41cm3, p=0.004), and PSA density (median 0.1 vs 0.14, p<0.001, respectively).
After matching, 257 patients remained in each confirmatory biopsy cohort (Table 1). Age was lower for the MRI-biopsy cohort at median 61 years-old compared to 64 years-old for the SB cohort (p<0.001). Clinical T-stage, PSA prior to initial biopsy, prostate volume and PSA density were no longer significantly different between cohorts. While statistically significant, the differences in initial biopsy characteristics between the two cohorts were clinically similar. The median total tumor length was 2mm (IQR 1–5mm) for the SB cohort vs 3mm (IQR 1.5–6mm) for the MRI-biopsy cohort (p<0.001). The maximum single core tumor length after initial biopsy was similar between cohorts, at a median of 1.5mm (IQR 1–3mm) for the TRUS-biopsy cohort vs 2.5mm (IQR 1–4mm) for the MRI-biopsy cohort (p=0.001). As expected after matching, no differences were noted between cohorts for the number of initial biopsy positive cores or initial biopsy GG. After initial biopsy, the majority of patients were diagnosed with GG1 (77.8%) or GG2 (21.4%) prostate cancer. Similar initial risk group classification was found between the matched cohorts, with the majority of patients having low risk disease (p=0.9) (Table 1).
Table 1.
Baseline characteristics after matching.
| Variable | Systematic confirmatory biopsy (n=257) | MRI confirmatory biopsy* (n=257) | p-value | |
|---|---|---|---|---|
|
| ||||
| Clinical Characteristics | Median age, years (IQR) | 64 (58–69) | 61 (56–66) | <0.001 |
| Race, n (%) | 0.96 | |||
| White | 202 (78.6) | 204 (79.4) | ||
| Black | 22 (8.6) | 22 (8.6) | ||
| Other | 33 (12.8) | 31 (12.1) | ||
| Clinical T stage, n (%) | 0.3 | |||
| T1-T2a | 253 (98.4) | 248 (96.5) | ||
| T2b-T2c | 2 (0.8) | 3 (1.2) | ||
| Unknown | 2 (0.8) | 6 (2.3) | ||
| Median PSA prior to biopsy, ng/mL (IQR) | 5.3 (4.1–7.3) | 5.3 (4.2–7.7) | 0.4 | |
| Median prostate volume, cm3 (IQR) | 38.8 (29.8–52.3) | 38 (31–53) | 0.6 | |
| Median PSA density, (IQR) | 0.13 (0.09–0.20) | 0.14 (0.09–0.20) | 0.2 | |
|
| ||||
| Initial Biopsy Characteristics | Median number of cores, (IQR) | 12 (12–12) | 12 (12–12) | 0.02 |
| Median total tumor length, mm (IQR) | 2 (1–5) | 3 (1.5–6) | <0.001 | |
| Median maximum single core length, mm (IQR) | 1.5 (1–3) | 2.5 (1–4) | 0.001 | |
| Median number positive cores, (IQR) | 2 (1–2) | 2 (1–2) | 0.8 | |
| Grade Group, n (%) | 1 | |||
| 1 | 200 (77.8) | 200 (77.8) | ||
| 2 | 55 (21.4) | 55 (21.4) | ||
| 3 | 2 (0.78) | 2 (0.78) | ||
| 4–5 | 0 | 0 | ||
| Risk Group, n (%) | 0.9 | |||
| Low | 179 (69.7) | 176 (68.5) | ||
| Intermediate - Favorable | 70 (27.2) | 71 (27.6) | ||
| Intermediate - Unfavorable | 5 (2) | 5 (2) | ||
| Unknown | 3 (1.2) | 5 (1.9) | ||
|
| ||||
| Confirmatory Biopsy Characteristics | Median number of cores, (IQR) | 11 (11–11) | 13 (12–14) | <0.001 |
| Median total tumor length, mm (IQR) | 4 (1.5–8.3) | 13.5 (4–21) | <0.001 | |
| Median maximum single core length, mm (IQR) | 3 (1.5–4.8) | 6 (2.5–9) | <0.001 | |
| Median number positive cores, (IQR) | 2 (1–3) | 3 (1–5) | 0.005 | |
| Grade Group, n (%) | <0.001 | |||
| Benign | 97 (37.7) | 32 (12.5) | ||
| 1 | 85 (33.1) | 77 (30.0) | ||
| 2 | 62 (24.1) | 105 (40.9) | ||
| 3 | 8 (3.1) | 27 (10.5) | ||
| 4–5 | 5 (1.9) | 16 (6.2) | ||
| Risk Group (excluding benign biopsies), n (%) | 0.008 | |||
| Low | 76 (47.5) | 74 (32.9) | ||
| Intermediate - Favorable | 65 (40.6) | 98 (43.6) | ||
| Intermediate - Unfavorable | 14 (8.8) | 35 (15.6) | ||
| High | 5 (3.1) | 13 (5.8) | ||
| Unknown | 0 | 5 (2.2) | ||
| Risk Group Change, n (%) | <0.001 | |||
| Increase | 45 (17.5) | 113 (44) | ||
| Decrease | 106 (41.3) | 46 (17.9) | ||
| Equal to initial risk group | 103 (40.1) | 93 (36.2) | ||
IQR = interquartile range
MRI-biopsy consisted of MRI-targeted biopsies and systematic biopsies
Among the matched cohort, confirmatory biopsy pathologic characteristics demonstrated a significantly higher total tumor length among the MRI-biopsy cohort compared to the SB cohort (median 13.5mm vs. 4mm, p<0.001). There was also a significantly higher maximum single core tumor length (median 6mm vs 3mm, p<0.001) and number of positive cores (median 3 vs. 2, p=0.005) for the MRI-biopsy cohort compared to the SB cohort (Table 1).
Figure 1 demonstrates the change in GG after confirmatory biopsy compared to initial biopsy GG among the matched cohorts. Significantly more patients in the confirmatory MRI-biopsy cohort were upgraded from GG1 to ≥GG2 (107/257, 41.6%) compared to the SB cohort (42/257, 16.3%) (p<0.001). Only 32/257 (12.5%) in the MRI-biopsy cohort had a negative confirmatory biopsy compared to 97/257 (37.7%) of the SB cohort (p<0.001). Among MRI-biopsy cohort, 118/257 (45.9%) had any degree of upgrading, 42/257 (16.3%) increased from GG1–2 to ≥GG3, and 31/257 (12.1%) increased from GG1 to ≥GG3. Among the SB cohort, 46/257 (17.9%) had any degree of upgrading, 12/257 (4.7%) increased from GG1-2 to ≥GG3, and 8/257 (3.1%) increased from GG1 to ≥GG3. After confirmatory biopsy, a greater number of patients in the MRI-biopsy cohort increased their risk group classification compared to the SB cohort (44% vs 17.5% respectively, p<0.001) (Table 1).
Figure 1.
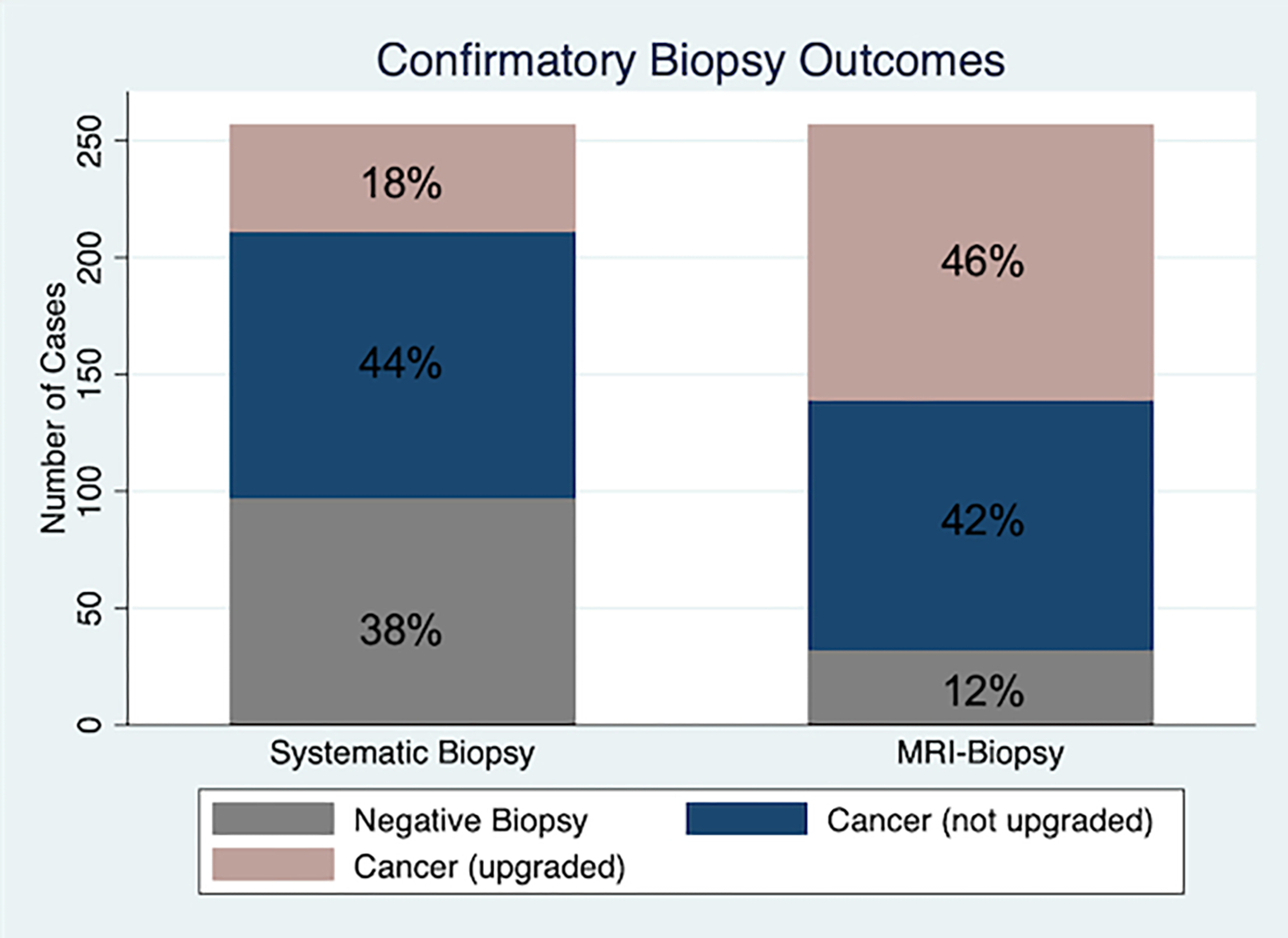
Confirmatory biopsy outcomes by confirmatory biopsy type using matched cohorts. Upgrading is determined based on comparing results between initial biopsy grade group and confirmatory biopsy grade group. Multivariable logistic regression demonstrated MRI-guided confirmatory biopsy is associated with greater odds of upgrading compared to standard systematic TRUS biopsy (OR 4.76, 95% CI 2.90–7.82, p<0.001).
Multivariable logistic regression was performed to identify variables associated with upgrading between the initial and confirmatory biopsy. After adjusting for covariates, MRI-biopsy was independently associated with an increased odds of upgrading (OR 4.76, 95% CI 2.90–7.82, p<0.001) (Table 2). Age, PSA density, and initial biopsy GG were also associated with confirmatory biopsy upgrading (p≤0.001 for each). Additional multivariable models demonstrated MRI-guided biopsy to be an independent predictor of upgrading from GG1 to ≥GG2 (OR 3.62, 95% CI 1.97–6.63, p<0.001), GG1 to ≥GG3 (OR 5.47, 95% CI 2.23–13.4, p<0.001) and GG1–2 to ≥GG3 (OR 5.07, 95% CI 2.38–10.8, p<0.001). Multivariable analysis was performed using the cohorts prior to matching which demonstrated similar findings (Supplementary Table 2).
Table 2.
Multivariable logistic regression for upgrading using matched cohorts.
| Any upgrading | OR | 95% CI | p-value |
|
| |||
| Confirmatory Biopsy cohort | |||
| Systematic biopsy | Ref | ||
| MRI-biopsy | 4.76 | 2.90–7.82 | <0.001 |
| Age | 1.05 | 1.02–1.09 | 0.001 |
| PSA density | 4.00 | 2.42–6.62 | <0.001 |
| Initial biopsy GG | |||
| GG1 | Ref | ||
| GG2 | 0.14 | 0.07–0.28 | <0.001 |
| Initial PSA | 0.97 | 0.91–1.03 | 0.3 |
| Number of initial biopsy positive cores | 1.08 | 0.90–1.28 | 0.4 |
| Initial maximum single core tumor length | 1.09 | 0.97–1.23 | 0.2 |
| Confirmatory biopsy total number of cores | 1.01 | 0.92–1.10 | 0.9 |
|
| |||
| Grade increase from GG1 to ≥GG2 | OR | 95% CI | p-value |
|
| |||
| Confirmatory Biopsy cohort | |||
| Systematic biopsy | Ref | ||
| MRI-biopsy | 3.62 | 1.97–6.63 | <0.001 |
| Age | 1.07 | 1.03–1.11 | <0.001 |
| PSA density | 4.04 | 2.16–7.55 | <0.001 |
| Initial PSA | 0.96 | 0.88–1.05 | 0.4 |
| Number of initial biopsy positive cores | 0.97 | 0.76–1.22 | 0.8 |
| Initial maximum single core tumor length | 1.05 | 0.91–1.22 | 0.5 |
| Confirmatory biopsy total number of cores | 1.00 | 0.88–1.13 | 0.98 |
|
| |||
| Grade increase from GG1 to ≥GG3 | OR | 95% CI | p-value |
|
| |||
| Confirmatory Biopsy cohort | |||
| Systematic biopsy | Ref | ||
| MRI-biopsy | 5.47 | 2.23–13.4 | <0.001 |
| Age | 1.10 | 1.05–1.17 | <0.001 |
| PSA density | 5.43 | 2.85–10.3 | <0.001 |
| Confirmatory biopsy total number of cores | 1.01 | 0.90–1.12 | 0.9 |
|
| |||
| Grade increase from GG1-2 to ≥GG3 | OR | 95% CI | p-value |
|
| |||
| Confirmatory Biopsy cohort | |||
| Systematic biopsy | Ref | ||
| MRI-biopsy | 5.07 | 2.38–10.8 | <0.001 |
| Age | 1.08 | 1.03–1.13 | 0.001 |
| PSA density | 7.42 | 3.43–16.02 | <0.001 |
| Initial PSA | 0.94 | 0.88–1.01 | 0.06 |
| Number of initial biopsy positive cores | 1.02 | 0.79–1.32 | 0.9 |
| Initial maximum single core tumor length | 0.91 | 0.75–1.10 | 0.3 |
| Confirmatory biopsy total number of cores | 1.02 | 0.93–1.13 | 0.6 |
GG=grade group, OR = Odds ratio, CI = confidence interval
MRI-biopsy consisted of MRI-targeted biopsies and systematic biopsies
Since confirmatory MRI-biopsies consist of both a targeted biopsy (TB) as well as SB, we evaluated among the MRI-biopsies that detected cancer, whether the cancer was detected by the SB, TB or both. Figure 2 demonstrates the results among matched patients. Among the 225 patients with a positive MRI-biopsy, 92/225 (40.9%) patients had only TB cores positive compared to 26/225 (11.6%) patients with only SB cores positive (p<0.001). Among 107 patients with both positive SB and TB cores, 48% had the same GG in both the SB and TB cores, 46% had higher GG in the TB cores, and only 6% had higher GG in the SB cores. Supplementary Figure 1 demonstrates similar findings prior to matching.
Figure 2.
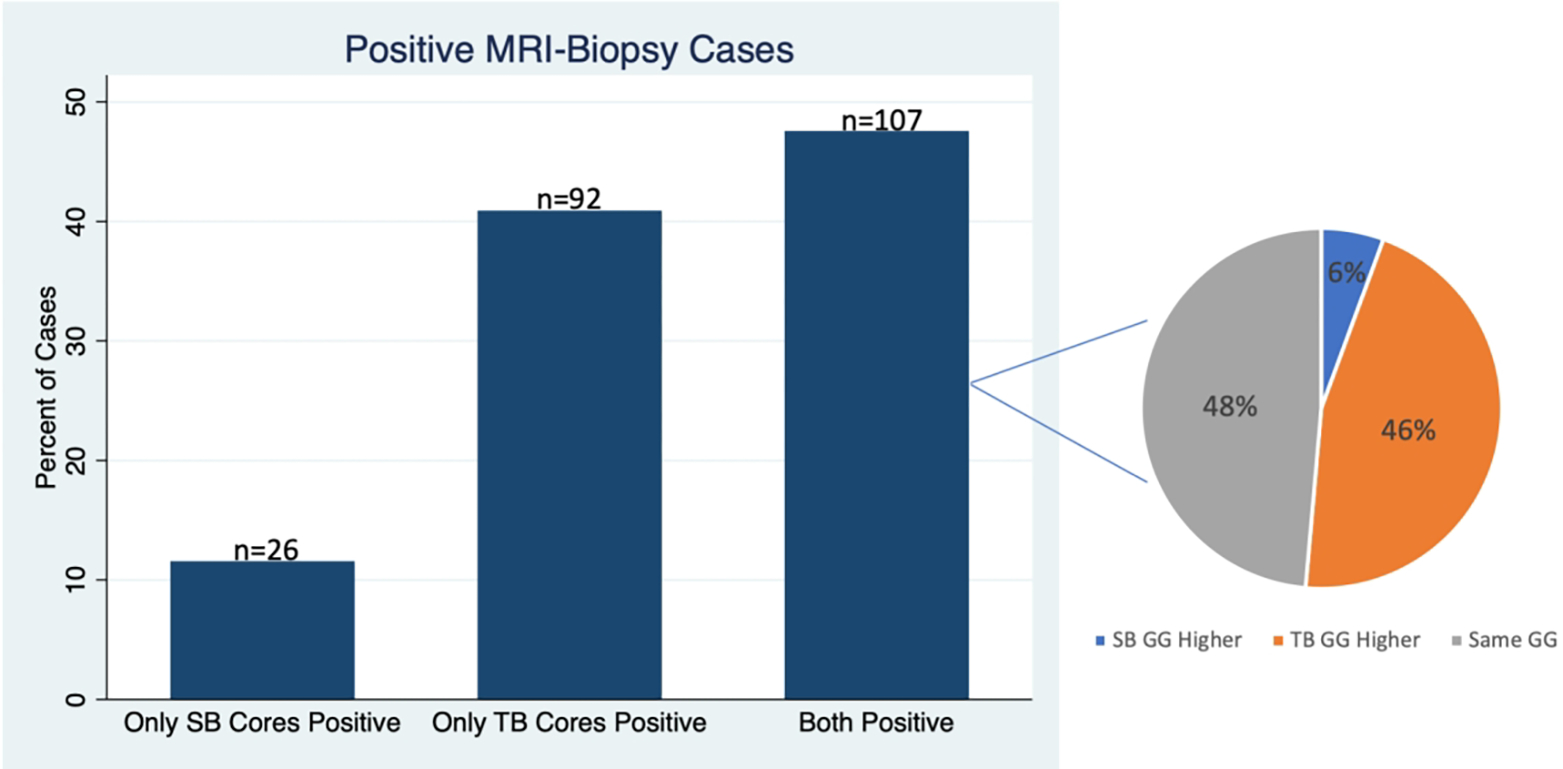
Matched patients undergoing confirmatory MRI-Biopsy (consisting of both a targeted biopsy [TB] and zonal systematic biopsy [SB]). Bar graph demonstrates among confirmatory MRI-biopsies showing cancer, what proportion had only positive SB cores, only positive TB cores or had both positive SB and TB cores. The pie chart demonstrates among the cases with positive SB and TB cores, what fraction had a higher Grade Group (GG) in the SB cores, in the TB cores, or that had the same GG in the SB and TB cores.
Supplementary Figure 2 demonstrates confirmatory MRI-biopsy GG outcomes by MRI score. Among the matched confirmatory MRI-biopsy population, 72/257 (28%), 129 /257 (50.2%) and 56/257 (21.8%) had an MRI score of 3, 4, or 5, respectively. Increasing MRI score was associated with fewer negative biopsies and increasing GG in both the total MRI-biopsy population and the matched MRI-biopsy population. Among the matched MRI-biopsy population with an MRI score of 5, 44/56 (78.6%) had ≥GG2 after confirmatory biopsy compared to patients with an MRI score of 3 in which only 27/72 (37.5%) had ≥GG2 confirmatory biopsy (p<0.001). Similar results occurred prior to matching.
DISCUSSION
Prior to enrollment in active surveillance for low risk prostate cancer, accurate staging and grading of prostate cancer is necessary to avoid under detection of clinically significant cancer while reducing the number of false negative tests. The results of this study demonstrate that patients undergoing a confirmatory MRI-biopsy, which included targeted biopsies and SB, were less likely to have a negative confirmatory biopsy compared to a SB alone. Additionally, MRI-biopsy was associated with an increase in the rate of upgrading from initial biopsy to confirmatory biopsy compared to SB alone. This study demonstrates that the addition of MRI-targeted biopsies to SB can increase the detection of clinically significant cancer (≥GG2 or GG3) to aid in identifying appropriate AS candidates. This is necessary as confirmatory SB alone can result in a negative biopsy rate between 21–50% and risk of grade progression with subsequent surveillance biopsy can range from 22–30% (12, 13).
This study validates the limited existing literature regarding MRI-biopsy utilization in the confirmatory biopsy setting. Among the biopsy-naïve population, previous trials have demonstrated improved detection rates of clinically significant cancer (defined as ≥GG2) by utilizing MRI targeted biopsies (5, 6). Less is understood in the confirmatory biopsy setting, and existing studies have been small, retrospective studies and have not considered baseline differences in the biopsy cohorts being compared (11, 31–36). The present study is strengthened by evaluating patients after matching on baseline characteristics that are known to impact biopsy detection rates (9, 37). A pooled analysis of existing studies that included an MRI-targeted confirmatory biopsy cohort demonstrated a 27% rate of cancer upgrading from GG1 to ≥GG2 with the combination of MRI-targeted biopsy and SB compared to 20% of SB alone (31). Among our study’s matched population, we found a similarly low rate of confirmatory biopsy upgrading (GG1 to ≥GG2) among patients undergoing SB (16.3%) compared to much higher rate of upgrading among the MRI-biopsy cohort (41.6%) than previously reported. Improvement in detection rate within the present study is likely multifactorial. The MRI-biopsy technique in this study included both targeted biopsies as well as a systematic biopsies, which may improve the detection rate compared to targeted biopsy alone (4, 38). Additionally, both the radiologists interpreting the MRI and the urologists performing the biopsy have significant experience, which has previously been shown to improve prostate cancer detection on the MRI and targeted biopsies (25, 26).
The Active Surveillance Magnetic Resonance Imaging Study (ASIST) was a prospective multicenter randomized open label trial that evaluated the effectiveness of MRI-targeted biopsies with SB versus SB alone in identifying higher grade prostate cancer on active surveillance (39). Similar to the present study, this trial enrolled patients prior to confirmatory biopsy after an initial diagnostic biopsy demonstrating the presence of low risk (GG1) prostate cancer; however, this trial did so in a randomized fashion. The results of this trial demonstrated that 23% of SB patients were upgraded from GG1 at initial biopsy to ≥GG2 at the time of confirmatory biopsy compared to upgrading in only 21% of patients in the MRI targeted arm (p=0.7) (39). The authors noted that MRI-biopsies were performed in centers early during their learning curve. The three centers included in the trial had significant differences in the rate of upgrading after MRI-biopsies, and the most experienced center achieved a higher rate of upgrading in the MRI-biopsy cohort. The present study had a markedly higher rate of upgrading from GG1 to ≥GG2 (41.6%) compared to the results of the ASIST trial, and may in part be explained by biopsy experience. Furthermore, the variability in MRI quality, interpretation, and fusion-biopsy technology is well established and affects biopsy outcomes (23–26). Compared to the ASIST trial, which had a negative biopsy rate of 27% in the MRI-targeted biopsy cohort, only 12% within the present study had a negative biopsy.
We additionally identified that increasing MRI suspicion scores are associated with fewer negative biopsies as well as increasing detection of higher-grade lesions (≥GG2). This is supported by previous studies demonstrating the positive correlation between increasing MRI positivity and subsequent targeted biopsy upgrading (6, 40). While the present study used a Likert scale for grading of MRI lesions (as opposed to the Prostate Imaging-Reporting and Data System [PI-RADS]), prior studies have demonstrated similar performance of the Likert and PI-RADS scoring systems, both being subject to the experience of the radiologist evaluating the MRI (4). The MRIs in this study were evaluated at a single center by radiologists with significant experience, which may limit reproducibility in centers with less experience. Additionally, interobserver variability could not be accounted for in this study. A recent study evaluated the inter-reader variability by comparing the area under the curve (AUC) for individual radiologists for detecting prostate cancer on MRI. While not compared with statistical testing, the study showed significant variation in the AUC between 8 radiologists at a single institution for detecting clinically significant prostate cancer, ranging from AUC of 0.63 to 0.85 (41). Despite this, our findings appear consistent with previous reports that have demonstrated increasing MRI score associated with detection of more clinically significant prostate cancer.
Patients with low GG biopsies as seen in this cohort, will likely have a higher negative predictive value (NPV) compared to higher risk populations with more advanced disease. Additional biomarkers have been considered to further improve the NPV of MRI-biopsy and possibly avoid unnecessary confirmatory biopsies. In the present study, increasing PSA density was found to be independently predictive of upgrading. This is supported by a study by Falagario et al which demonstrated that stratifying men based on their PSA density was able to improve the NPV of MRI-biopsy (42). Patients with a negative multiparametric MRI would forgo biopsy if their PSA density was <0.10. Another recent study utilized genomic testing and MRI findings to help select among favorable intermediate risk patients who was most likely to have adverse pathology on radical prostatectomy specimens (43). Incorporating additional biomarkers to MRI may help further improve the positive and NPV of MRI-biopsy by selecting patients most likely to harbor significant disease. Further prospective validation will be necessary to confirm the results of these retrospective series.
Given that MRI targeted biopsies improve the detection of clinically significant disease and decrease the detection of benign and clinically insignificant disease, we are likely to see risk inflation among the increasing number of patients undergoing MRI targeted biopsies. Some advocate for new risk models to reduce the artifactual rise in risk attributed to targeted biopsy (44). Examples of these strategies include developing models independent of number of positive cores or incorporating the amount of Gleason 4 disease, since targeted biopsies more accurately detect this quantity. While MRI targeted biopsies may result in risk inflation, the information gathered from these biopsies may be more useful to patients in determining which treatment options to pursue given the reduced risk of undergrading their cancer (44).
Due to the low rate of cancer progression and cancer specific mortality among men undergoing active surveillance prior to the implementation of MRI and MRI-targeted biopsies, the question remains whether the addition of MRI to confirmatory biopsy improves cancer specific survival outcomes. The Prostate testing for cancer and Treatment (ProtecT) trial randomized 1643 men to active monitoring, radical prostatectomy or radical radiotherapy, and after a 10-year median follow-up, the approximate overall mortality was 1% suggesting safety of active monitoring. Recent evaluation of patients enrolled in the ProtecT trial demonstrated that while only 2% of GG1 patients developed prostate cancer metastasis or death, 7% and 11% of GG2 and ≥GG3 patients developed metastasis or prostate cancer death, respectively (45). Thus, the ability to accurately detect higher grade cancer is important to avoid the small but significant progression of disease. Within our study, the ability to detect more higher-grade cancers with fewer negative biopsies may allow for improved counseling and patient selection prior to AS enrollment.
This study focused on the question of incorporating MRI-biopsy at the time of confirmatory biopsy. Other potential areas of incorporation of MRI-biopsy are at the time of initial diagnostic biopsy in both previously biopsied and biopsy naïve patients. Current randomized data support the use of MRI targeted biopsies among biopsy naïve men given higher detection of clinically significant prostate cancer when combined with systematic biopsy (5, 6). This is also true among men with clinical suspicion of prostate cancer and a previously negative TRUS-guided systematic biopsy (46). Determination of how to best utilize MRI and targeted biopsy technology in the prostate cancer diagnostic algorithm to minimize cost while maximizing diagnostic efficacy remains to be determined. Our study demonstrates that among patients considering AS, MRI-biopsy will more effectively identify clinically significant cancer and reduce non-diagnostic biopsies to more effectively inform treatment decisions.
This study is subject to the limitations of a retrospective design. We attempted to correct for biases and confounding among the cohorts by performing matching on variables known to impact biopsy results including age, PSA, and initial GG, and adjusted for covariates in multivariable models; however, unmeasured confounders likely still exist. This study was performed at a single large referral center with significant experience among radiologists, pathologists and urologists, which may limit the generalizability of our findings to other centers. Additionally, given that we are primarily a referral center, we may have incomplete information regarding biopsy history prior to a patient’s initial diagnostic biopsy. Patients who were previously biopsy naïve versus those with a multiple prior negative biopsy may have different disease reclassification rates which were not accounted for in this study. Patients with a negative MRI were not included in the MRI-biopsy cohort; thus, we do not know the rate of negative MRI in this population. Biopsy findings were not compared to final radical prostatectomy pathologic specimens; thus, the true differences in GG between the cohorts could not be determined. Previous studies, however, have identified a high degree of Gleason grade concordance (90%) between MRI-targeted biopsy and prostatectomy specimens at experienced centers (47). The study was conducted over a relatively long time period, and biopsy techniques and active surveillance protocols in the general population have likely changed over this time. However, the active surveillance protocol and biopsy techniques described here were standardized at our institution and did not vary over the period of investigation. Also, since MRI-targeted biopsy technology has only more recently been routinely incorporated into clinical practice, this limited our ability to match on the date of biopsy. Lastly, patient oncologic outcomes were not evaluated within this study, and the impact of MRI-biopsy on treatment selection and survival could not be determined. However, these data offer significant evidence for the role of MRI-targeted biopsy in combination with SB in men with a positive MRI after a diagnostic biopsy and enrollment onto AS.
CONCLUSION
The addition of MRI-targeted biopsies to systematic biopsy in the confirmatory biopsy setting among men with previously detected prostate cancer resulted in fewer negative confirmatory biopsies and detection of more clinically significant prostate cancer compared to systematic biopsy alone. MRI-targeted biopsies should be incorporated into the confirmatory biopsy setting, especially in the presence of a positive MRI.
Supplementary Material
Footnotes
Disclosures: None
References
- 1.Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J Urol. 2018;199(3):683–90. [DOI] [PubMed] [Google Scholar]
- 2.Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(5):479–505. [DOI] [PubMed] [Google Scholar]
- 3.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375(15):1415–24. [DOI] [PubMed] [Google Scholar]
- 4.Stabile A, Giganti F, Rosenkrantz AB, Taneja SS, Villeirs G, Gill IS, et al. Multiparametric MRI for prostate cancer diagnosis: current status and future directions. Nat Rev Urol. 2020;17(1):41–61. [DOI] [PubMed] [Google Scholar]
- 5.Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med. 2018;378(19):1767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815–22. [DOI] [PubMed] [Google Scholar]
- 7.Nzenza T, Murphy DG. PRECISION delivers on the PROMIS of mpMRI in early detection. Nat Rev Urol. 2018;15(9):529–30. [DOI] [PubMed] [Google Scholar]
- 8.Futterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A, et al. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur Urol. 2015;68(6):1045–53. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqui MM, Rais-Bahrami S, Truong H, Stamatakis L, Vourganti S, Nix J, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64(5):713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valerio M, Donaldson I, Emberton M, Ehdaie B, Hadaschik BA, Marks LS, et al. Detection of Clinically Significant Prostate Cancer Using Magnetic Resonance Imaging-Ultrasound Fusion Targeted Biopsy: A Systematic Review. Eur Urol. 2015;68(1):8–19. [DOI] [PubMed] [Google Scholar]
- 11.Filson CP, Natarajan S, Margolis DJ, Huang J, Lieu P, Dorey FJ, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer. 2016;122(6):884–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dall’Era MA, Albertsen PC, Bangma C, Carroll PR, Carter HB, Cooperberg MR, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62(6):976–83. [DOI] [PubMed] [Google Scholar]
- 13.Bul M, van den Bergh RC, Rannikko A, Valdagni R, Pickles T, Bangma CH, et al. Predictors of unfavourable repeat biopsy results in men participating in a prospective active surveillance program. Eur Urol. 2012;61(2):370–7. [DOI] [PubMed] [Google Scholar]
- 14.Goel S, Shoag JE, Gross MD, Al Hussein Al Awamlh B, Robinson B, Khani F, et al. Concordance Between Biopsy and Radical Prostatectomy Pathology in the Era of Targeted Biopsy: A Systematic Review and Meta-analysis. Eur Urol Oncol. 2019. [DOI] [PubMed] [Google Scholar]
- 15.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2017;71(4):618–29. [DOI] [PubMed] [Google Scholar]
- 16.Park BK, Park JW, Park SY, Kim CK, Lee HM, Jeon SS, et al. Prospective evaluation of 3-T MRI performed before initial transrectal ultrasound-guided prostate biopsy in patients with high prostate-specific antigen and no previous biopsy. AJR Am J Roentgenol. 2011;197(5):W876–81. [DOI] [PubMed] [Google Scholar]
- 17.Arsov C, Rabenalt R, Blondin D, Quentin M, Hiester A, Godehardt E, et al. Prospective randomized trial comparing magnetic resonance imaging (MRI)-guided in-bore biopsy to MRI-ultrasound fusion and transrectal ultrasound-guided prostate biopsy in patients with prior negative biopsies. Eur Urol. 2015;68(4):713–20. [DOI] [PubMed] [Google Scholar]
- 18.Baco E, Rud E, Eri LM, Moen G, Vlatkovic L, Svindland A, et al. A Randomized Controlled Trial To Assess and Compare the Outcomes of Two-core Prostate Biopsy Guided by Fused Magnetic Resonance and Transrectal Ultrasound Images and Traditional 12-core Systematic Biopsy. Eur Urol. 2016;69(1):149–56. [DOI] [PubMed] [Google Scholar]
- 19.Panebianco V, Barchetti F, Sciarra A, Ciardi A, Indino EL, Papalia R, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol. 2015;33(1):17.e1–.e7. [DOI] [PubMed] [Google Scholar]
- 20.Porpiglia F, Manfredi M, Mele F, Cossu M, Bollito E, Veltri A, et al. Diagnostic Pathway with Multiparametric Magnetic Resonance Imaging Versus Standard Pathway: Results from a Randomized Prospective Study in Biopsy-naive Patients with Suspected Prostate Cancer. Eur Urol. 2017;72(2):282–8. [DOI] [PubMed] [Google Scholar]
- 21.Taverna G, Bozzini G, Grizzi F, Seveso M, Mandressi A, Balzarini L, et al. Endorectal multiparametric 3-tesla magnetic resonance imaging associated with systematic cognitive biopsies does not increase prostate cancer detection rate: a randomized prospective trial. World J Urol. 2016;34(6):797–803. [DOI] [PubMed] [Google Scholar]
- 22.Tonttila PP, Lantto J, Paakko E, Piippo U, Kauppila S, Lammentausta E, et al. Prebiopsy Multiparametric Magnetic Resonance Imaging for Prostate Cancer Diagnosis in Biopsy-naive Men with Suspected Prostate Cancer Based on Elevated Prostate-specific Antigen Values: Results from a Randomized Prospective Blinded Controlled Trial. Eur Urol. 2016;69(3):419–25. [DOI] [PubMed] [Google Scholar]
- 23.Meng X, Rosenkrantz AB, Huang R, Deng FM, Wysock JS, Bjurlin MA, et al. The Institutional Learning Curve of Magnetic Resonance Imaging-Ultrasound Fusion Targeted Prostate Biopsy: Temporal Improvements in Cancer Detection in 4 Years. J Urol. 2018;200(5):1022–9. [DOI] [PubMed] [Google Scholar]
- 24.Kasabwala K, Patel N, Cricco-Lizza E, Shimpi AA, Weng S, Buchmann RM, et al. The Learning Curve for Magnetic Resonance Imaging/Ultrasound Fusion-guided Prostate Biopsy. Eur Urol Oncol. 2019;2(2):135–40. [DOI] [PubMed] [Google Scholar]
- 25.Bjurlin MA, Rosenkrantz AB, Taneja SS. MRI-fusion biopsy: the contemporary experience. Transl Androl Urol. 62017. p. 483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latchamsetty KC, Borden LS Jr., Porter CR, Lacrampe M, Vaughan M, Lin E, et al. Experience improves staging accuracy of endorectal magnetic resonance imaging in prostate cancer: what is the learning curve? Can J Urol. 2007;14(1):3429–34. [PubMed] [Google Scholar]
- 27.Gregg JR, Davis JW, Reichard C, Wang X, Achim M, Chapin BF, et al. Determining Clinically Based Factors Associated With Reclassification in the Pre-MRI Era using a Large Prospective Active Surveillance Cohort. Urology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babaian RJ, Toi A, Kamoi K, Troncoso P, Sweet J, Evans R, et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol. 2000;163(1):152–7. [PubMed] [Google Scholar]
- 29.Altok M, Kim B, Patel BB, Shih YT, Ward JF, McRae SE, et al. Cost and efficacy comparison of five prostate biopsy modalities: a platform for integrating cost into novel-platform comparative research. Prostate Cancer Prostatic Dis. 2018;21(4):524–32. [DOI] [PubMed] [Google Scholar]
- 30.Gregg JR, Lopez DS, Reichard C, Zheng J, Wu W, Ye Y, et al. Coffee, Caffeine Metabolism Genotype and Disease Progression in Patients with Localized Prostate Cancer Managed with Active Surveillance. J Urol. 2019;201(2):308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoots IG, Nieboer D, Giganti F, Moore CM, Bangma CH, Roobol MJ. Is magnetic resonance imaging-targeted biopsy a useful addition to systematic confirmatory biopsy in men on active surveillance for low-risk prostate cancer? A systematic review and meta-analysis. BJU Int. 2018;122(6):946–58. [DOI] [PubMed] [Google Scholar]
- 32.Da Rosa MR, Milot L, Sugar L, Vesprini D, Chung H, Loblaw A, et al. A prospective comparison of MRI-US fused targeted biopsy versus systematic ultrasound-guided biopsy for detecting clinically significant prostate cancer in patients on active surveillance. J Magn Reson Imaging. 2015;41(1):220–5. [DOI] [PubMed] [Google Scholar]
- 33.Walton Diaz A, Shakir NA, George AK, Rais-Bahrami S, Turkbey B, Rothwax JT, et al. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol. 2015;33(5):202.e1–.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Recabal P, Assel M, Sjoberg DD, Lee D, Laudone VP, Touijer K, et al. The Efficacy of Multiparametric Magnetic Resonance Imaging and Magnetic Resonance Imaging Targeted Biopsy in Risk Classification for Patients with Prostate Cancer on Active Surveillance. J Urol. 2016;196(2):374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alberts AR, Roobol MJ, Drost FH, van Leenders GJ, Bokhorst LP, Bangma CH, et al. Risk-stratification based on magnetic resonance imaging and prostate-specific antigen density may reduce unnecessary follow-up biopsy procedures in men on active surveillance for low-risk prostate cancer. BJU Int. 2017;120(4):511–9. [DOI] [PubMed] [Google Scholar]
- 36.Ma TM, Tosoian JJ, Schaeffer EM, Landis P, Wolf S, Macura KJ, et al. The Role of Multiparametric Magnetic Resonance Imaging/Ultrasound Fusion Biopsy in Active Surveillance. Eur Urol. 2017;71(2):174–80. [DOI] [PubMed] [Google Scholar]
- 37.Freedland SJ, Kane CJ, Amling CL, Aronson WJ, Terris MK, Presti JC Jr., Upgrading and downgrading of prostate needle biopsy specimens: risk factors and clinical implications. Urology. 2007;69(3):495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marenco J, Orczyk C, Collins T, Moore C, Emberton M. Role of MRI in planning radical prostatectomy: what is the added value? World J Urol. 2019;37(7):1289–92. [DOI] [PubMed] [Google Scholar]
- 39.Klotz L, Loblaw A, Sugar L, Moussa M, Berman DM, Van der Kwast T, et al. Active Surveillance Magnetic Resonance Imaging Study (ASIST): Results of a Randomized Multicenter Prospective Trial. Eur Urol. 2019;75(2):300–9. [DOI] [PubMed] [Google Scholar]
- 40.Tran GN, Leapman MS, Nguyen HG, Cowan JE, Shinohara K, Westphalen AC, et al. Magnetic Resonance Imaging-Ultrasound Fusion Biopsy During Prostate Cancer Active Surveillance. Eur Urol. 2017;72(2):275–81. [DOI] [PubMed] [Google Scholar]
- 41.Wajswol E, Winoker JS, Anastos H, Falagario U, Okhawere K, Martini A, et al. A cohort of transperineal electromagnetically tracked magnetic resonance imaging/ultrasonography fusion-guided biopsy: assessing the impact of inter-reader variability on cancer detection. BJU Int. 2019. [DOI] [PubMed] [Google Scholar]
- 42.Falagario UG, Martini A, Wajswol E, Treacy PJ, Ratnani P, Jambor I, et al. Avoiding Unnecessary Magnetic Resonance Imaging (MRI) and Biopsies: Negative and Positive Predictive Value of MRI According to Prostate-specific Antigen Density, 4Kscore and Risk Calculators. Eur Urol Oncol. 2019. [DOI] [PubMed] [Google Scholar]
- 43.Falagario UG, Beksac AT, Martini A, Cumarasamy S, Gupta A, Prasad S, et al. Defining Prostate Cancer at Favorable Intermediate Risk: The Potential Utility of Magnetic Resonance Imaging and Genomic Tests. J Urol. 2019;202(1):102–7. [DOI] [PubMed] [Google Scholar]
- 44.Robertson NL, Hu Y, Ahmed HU, Freeman A, Barratt D, Emberton M. Prostate cancer risk inflation as a consequence of image-targeted biopsy of the prostate: a computer simulation study. Eur Urol. 2014;65(3):628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bryant RJ, Oxley J, Young GJ, Lane JA, Metcalfe C, Davis M, et al. The ProtecT trial: analysis of the patient cohort, baseline risk stratification and disease progression. BJU Int. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenkrantz AB, Verma S, Choyke P, Eberhardt SC, Eggener SE, Gaitonde K, et al. Prostate Magnetic Resonance Imaging and Magnetic Resonance Imaging Targeted Biopsy in Patients with a Prior Negative Biopsy: A Consensus Statement by AUA and SAR. J Urol. 2016;196(6):1613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baco E, Ukimura O, Rud E, Vlatkovic L, Svindland A, Aron M, et al. Magnetic resonance imaging-transectal ultrasound image-fusion biopsies accurately characterize the index tumor: correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur Urol. 2015;67(4):787–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


