Abstract
Anti-CD38 monoclonal antibodies (mAbs), daratumumab, and isatuximab have represented a breakthrough in the treatment of multiple myeloma (MM). Recently, CD38-based mAbs were expected to achieve increasing potential beyond MM, which encouraged us to develop new anti-CD38 mAbs to meet clinical needs. In this study, we developed a novel humanized anti-CD38 antibody, FTL004, which exhibited enhanced pro-apoptotic ability and negligible binding to red blood cells (RBCs). FTL004 presented a better ability to induce direct apoptosis independent of Fc-mediated cross-linking against lymphoma and MM cell lines as well as primary myeloma cells derived from MM patients. For instance, FTL004 induced RPMI 8226 cells with 55% early apoptosis cells compared with 20% in the isatuximab-treated group. Of interest, FTL004 showed ignorable binding to CD38 on human RBCs in contrast to tumor cells, even at concentrations up to 30 μg/mL. Furthermore, with an engineered Fc domain, FTL004 displayed stronger antibody-dependent cellular cytotoxicity (ADCC) against CD38+ malignant cells. In vivo MM and non-Hodgkin lymphoma tumor xenograft models showed that FTL004 possessed an effective anti-tumor effect. Cryo-electron microscopy structure resolved two epitope centers of FTL004 on CD38: one of which was unique while the other partly overlapped with that of isatuximab. Taken together, FTL004 distinguishes it from other CD38 targeting mAbs and represents a potential candidate for the treatment of MM and non-Hodgkin lymphoma.
Keywords: Multiple myeloma, Monoclonal antibodies, CD38, Red blood cells, Direct apoptosis
To the editor
CD38 is a type II transmembrane glycoprotein with multiple functions and expressed lower on normal blood cells and higher on hematologic tumor cells [1, 2]. Anti-CD38 mAbs were effective in some hematological malignancies, especially multiple myeloma (MM) [3]. Although both daratumumab and isatuximab have significantly improved the outcome of patients with MM, incomplete responses and on-target/off-tumor effects emerge during the treatment [4, 5]. Herein, we described FTL004, a novel humanized IgG1κ anti-CD38 mAb possessing novel properties.
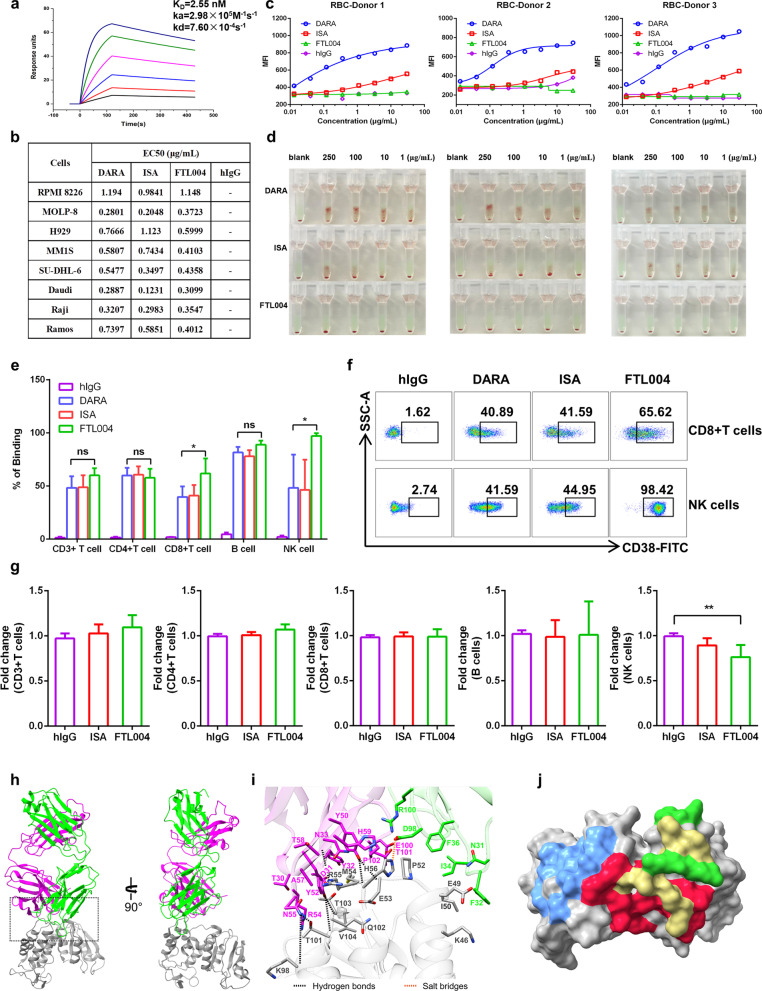
FTL004 exhibited similar affinities to CD38 (KD of 2.55, 3.84, and 1.2 nM, respectively) in surface plasmon resonance (Fig. 1a) and to CD38-positive cell lines in flow cytometry (Fig. 1b), compared with daratumumab or isatuximab. However, these antibodies bound differently to blood cells. Daratumumab or isatuximab bound to RBCs to different extents from healthy donors, while the binding of FTL004 to RBCs was virtually undetectable (Fig. 1c). This property should result in a more favorable pharmacokinetic of FTL004 by circumventing binding to CD38 on circulating RBCs [6] and a better safety profile by minimizing side effects associated with RBC binding. Moreover, FTL004 did not agglutinate RBCs in indirect antiglobulin tests (Fig. 1d), which could avoid the interferences with blood transfusion that are observed in other anti-CD38 mAbs [7, 8].
Fig. 1.
Distinct binding profile of FTL004 to CD38+ tumor cells, RBCs, and immune cells. a Affinity analysis of FTL004 to human CD38 as measured by Biacore 8 K (KD = 2.55 × 10–9 mol/L). b EC50 values for anti-CD38 mAbs binding to diverse cells. DARA, daratumumab; ISA, isatuximab. c Binding of FTL004, daratumumab or isatuximab to freshly isolated RBCs from healthy donors. d Indirect antiglobulin tests for CD38 mAbs to the same three healthy donors RBCs above. e The percentage of binding of CD38 mAbs to normal PBMCs (n = 4), including to CD45+ CD3+ T cells, CD3+ CD4+ T cells, CD3+ CD8+ T cells, CD45+ CD19+ B cells and CD3-CD56+ NK cells. Data show means ± SD and unpaired Student’s t tests were used to determine the statistical significance between daratumumab versus FTL004. *P < 0.05, **P < 0.01 and ***P < 0.001. f Flow cytometry dot plots showing the binding of anti-CD38 mAbs to NK cells and CD8+ T cells. g PBMCs from normal donors (n = 6) were pretreated with 1 μg/mL FTL004 or isatuximab for 3 days. Flow cytometry was used to determine the percentage of CD3+ T cells, CD4+ T cells, CD8+ T cells, B cells, and NK cells in lymphocytes. Data were then normalized to isotype controls and fold changes (means ± SD) are shown. Isotype cells were used as controls in settings. h The overall structure of the CD38/FTL004 Fab complex. CD38 and the heavy and light chains of FTL004 are colored gray, purple, and green, respectively. i Stereoscopic view of the direct hydrogen bonds, salt bridge, in the interface between CD38 and FTL004. j Comparison of the binding of FTL004, daratumumab, and isatuximab. Surface representation of the epitopes of FTL004 (green), daratumumab (blue), isatuximab (red) and superposition part of FTL004 and isatuximab (yellow) on CD38 (gray)
For other immune cells, FTL004 bound to most subpopulation cells of peripheral blood mononuclear cells (PBMCs) in a comparable intensity with daratumumab or isatuximab (Fig. 1e). Unexpectedly, FTL004 exhibited a slightly higher binding to CD8+ T cells and a much higher binding to NK cells (Fig. 1f). These events reflected that the epitope of FTL004 binding on CD38 may be distinct. Although anti-CD38 mAbs require a high threshold for CD38 expression to induce cell death, we must be wary of its possible off-target effects. PBMCs coculture assays showed that there was no depletion in T and B cells, indicating the limited off-tumor toxicity of FTL004 (Fig. 1g). But as expected, NK cells, with higher expression of CD38, were reduced to an acceptable level [9].
Then, the CD38/FTL004 complex structure was determined using Cryo-electron microscopy at 3.86 Å resolution (Fig. 1h, i). Of note, there seem to be two epitope centers of FTL004, one of them includes from Pro52 to His56 and the other one is 91TQTV94. Superimposition of the CD38/daratumumab (PDB code 7DHA) complex and CD38/isatuximab (PDB code 4CMH) complex onto the CD38/FTL004 complex revealed that the epitopes of FTL004 share no common residue of CD38 with daratumumab, whereas one of the epitopes (91TQTV94) of FTL004 partly overlaps with that of isatuximab (Fig. 1j).
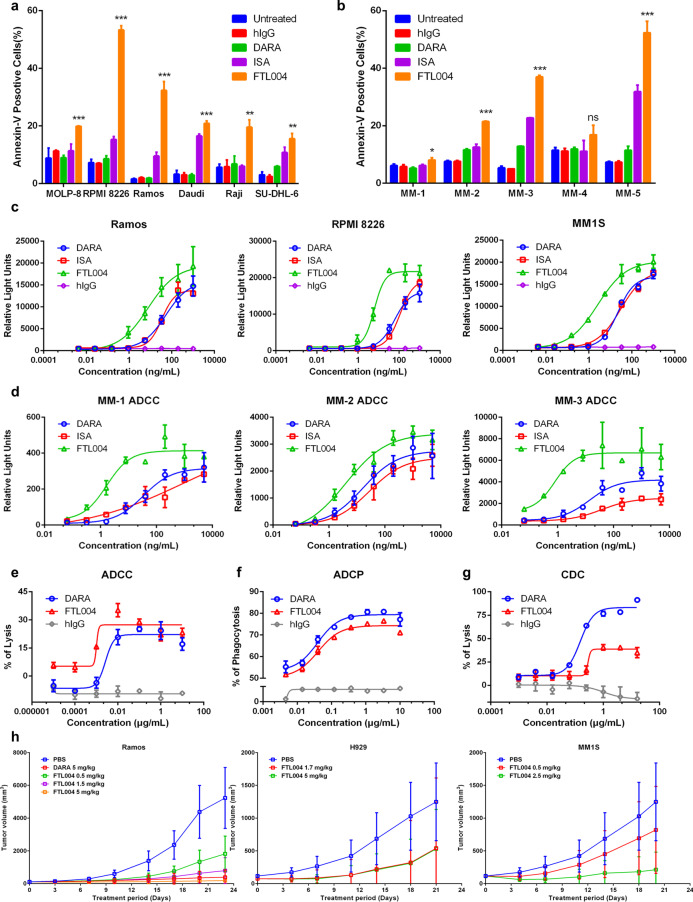
Next, to evaluate the tumor-killing capacity of FTL004, pro-apoptotic activity was first analyzed. We found that FTL004 had a superior ability to induce direct apoptosis independent of cross-linking reagents against CD38-positive cell lines (Fig. 2a) and primary MM cells (Fig. 2b) than isatuximab. Particularly, the highest apoptosis rate of FTL004 on primary MM cells was detected to be up to 60%. On the other hand, the Fc domain of FTL004 was engineered (S240D/I333E mutation) to elevate the affinity to human Fcγ receptors, leading to the enhancement of antibody-dependent cellular cytotoxicity (ADCC) [10]. In ADCC bioassays with Jurkat-Lucia-CD16a-NFAT cells [11], FTL004 showed a distinctly higher intensity of ADCC, with EC50 values of 2–8 ng/mL, compared with daratumumab and isatuximab (Fig. 2c). Consistent results were obtained in primary MM cells (Fig. 2d).
Fig. 2.
Anti-tumor efficacy of FTL004. a Induction of apoptosis by FTL004, daratumumab, or isatuximab in various cell lines, cells were treated with anti-CD38 mAbs 1.5 μg/mL for 24 h. Data represent means of triplicates and SD. Statistical significance between FTL004 and hIgG groups was determined by unpaired Student’s t tests. *P < 0.05; **P < 0.01, ***P < 0.001. b Apoptosis induction by FTL004 in primary MM cells. c, d ADCC reporter bioassays to various CD38+ tumor cell lines (c) and MM primary cells (d) of FTL004, daratumumab, or isatuximab. Data represent means of triplicates and SD. e–g Representative dose–response curve examples for ADCC activity (e), ADCP activity (f), and CDC activity (g) of FTL004 or daratumumab to CHO-CD38+ cells. Data show mean ± SD of three experiments. h Anti-tumor efficacy of FTL004 in lymphoma cell Ramos, MM cell H929, MM1S xenograft models. NOD-SCID mice bearing xenografts were treated with administration of PBS, daratumumab, or FTL004. Tumor burdens were monitored. Each point on the graph represents the average tumor volume
To investigate further, we examined the ADCC of FTL004 with healthy PBMCs. PBMCs exhibited enhanced ADCC activity against CHO-CD38+ cells in the presence of FTL004, with threefold higher than daratumumab (Fig. 2e). Meanwhile, FTL004 induced stronger ADCP with 74% phagocytosed CHO-CD38+ cells in an EC50 value of 40 ng/mL (Fig. 2f). However, FTL004 induced a lower CDC than daratumumab of CHO-CD38+ cells (Fig. 2g). In vivo studies were further carried out, and FTL004 was examined in Ramos, H929, and MM1S-bearing NOD-SCID mice (Fig. 2h). FTL004 treatment resulted in significant inhibition of tumor growth compared to PBS treatment.
In summary, FTL004 is a novel CD38 mAb that owns unique properties. Although the mechanism by which FTL004 binds differently to tumor cells versus RBCs remains unclear, it is expected that FTL004 owns limited on-target/off-tumor effects. The results above suggest that FTL004 is a therapeutic Ab with high potential for the treatment of MM and non-Hodgkin lymphoma.
Acknowledgements
The authors thank all of the patients and healthy donors who consented to this study. They also thank Yiran Tao (West China-California Research Center for Predictive Intervention Medicine, West China Hospital, Sichuan University) for FACS data acquisition and analysis.
Abbreviations
- mAb
Monoclonal antibody
- MM
Multiple myeloma
- RBCs
Red blood cells
- PBMCs
Peripheral blood mononuclear cells
- ADCC
Antibody-dependent cellular cytotoxicity
Author contributions
ZGB, GCY, ZXD, MFX, HY, LQ, GLT, and YJL were involved in study conception and design; ZGB, WY, LS, YZN, ZZX, JXH, and WYX contributed to the development of methodology; QW, LQH, YLL, CCX, YL, YHB, WY, and SZM participated in acquisition of data; LH, CPY, RZQ, YM, and QF were involved in analysis and interpretation of data; ZGB, WX, LY, ZSY, CXF, DH, and LY wrote and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81,872,907, 32,070,049, 32,222,040), Science and Technology Plan Project of Sichuan Province (Provincial Academy and Provincial University Cooperation Project) (2020YFSY0025), Sichuan Science and Technology Program (2021YJ0454, 2019YFS0003), Ministry of Science and Technology of China (MoST) (2021YFA1301900), Sichuan University start-up funding (20822041D4057), the CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-5-075), and the National Major Scientific and Technological Special Project for Significant New Drugs Development (2018ZX09201018-021, 2017ZX09302010).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The human subject studies were approved by the Ethnic Committee of West China Hospital of Sichuan University. All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
Qing Li, Ying Huang, Fanxin Ma, Xianda Zhang, Shuang Liu, Zhouning Yang, Pengyu Chen, Ziqing Ren, Meng Yu, and Feng Qu are employees at Sound Biopharmaceuticals Co., Ltd. No potential conflicts of interest were disclosed by the other authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guangbing Zhang, Cuiyu Guo, Yan Wang, and Xianda Zhang equally contributors
Ying Huang, Fanxin Ma, and Jinliang Yang contributed equally to the article as Co-senior authors
Contributor Information
Ying Huang, Email: huangy@soundbiopharma.com.
Fanxin Ma, Email: mafx@soundbiopharma.com.
Jinliang Yang, Email: jinliangyang@scu.edu.cn.
References
- 1.Chini CCS, Peclat TR, Warner GM, Kashyap S, Espindola-Netto JM, de Oliveira GC, et al. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD(+) and NMN levels. Nat Metab. 2020;2(11):1284–1304. doi: 10.1038/s42255-020-00298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta K, Shahid U, Malavasi F. Human CD38, a cell-surface protein with multiple functions. FASEB J Off Publ Fed Am Soc Exp Biol. 1996;10(12):1408–1417. doi: 10.1096/fasebj.10.12.8903511. [DOI] [PubMed] [Google Scholar]
- 3.van de Donk NW, Janmaat ML, Mutis T, van Lammerts Bueren JJ, Ahmadi T, Sasser AK, et al. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016;270(1):95–112. doi: 10.1111/imr.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatnagar V, Gormley NJ, Lola L, et al. FDA approval summary: daratumumab for treatment of multiple myeloma after one prior therapy. Oncologist. 2017 doi: 10.1634/theoncologist.2017-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ofran Y. Daratumumab: new indications revolving around “off-targets”. Haematologica. 2021;106(12):3032–3033. doi: 10.3324/haematol.2021.279487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fedyk ER, Idamakanti N, Chen J, Estevam J, Palumbo A. The binding of CD38 therapeutics to red blood cells and platelets subverts depletion of target cells. Blood. 2019;134(Supplement_1):3136. doi: 10.1182/blood-2019-128238. [DOI] [Google Scholar]
- 7.Werle E, Ziebart J, Wasmund E, Eske-Pogodda K. Daratumumab interference in pretransfusion testing is overcome by addition of daratumumab Fab fragments to patients’ plasma. Transfus Med Hemother. 2019 doi: 10.1159/000495773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lancman G, Arinsburg S, Jhang J, Cho HJ, Jagannath S, Madduri D, et al. Blood transfusion management for patients treated with anti-CD38 monoclonal antibodies. Front Immunol. 2018;9:2616. doi: 10.3389/fimmu.2018.02616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naeimi Kararoudi M, Nagai Y, Elmas E, de Souza Fernandes Pereira M, Ali SA, Imus PH, et al. CD38 deletion of human primary NK cells eliminates daratumumab-induced fratricide and boosts their effector activity. Blood. 2020;136(21):2416–27. doi: 10.1182/blood.2020006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jebamani P, Sriramulu DK, Jung ST, Lee S-G. Structural study on the impact of S239D/I332E mutations in the binding of Fc and FcγRIIIa. Biotechnol Bioprocess Eng. 2021;26(6):985–992. doi: 10.1007/s12257-021-0024-2. [DOI] [Google Scholar]
- 11.Hong Y, Guo H, Wei M, Zhang Y, Fang M, Cheng T, et al. Cell-based reporter assays for measurements of antibody-mediated cellular cytotoxicity and phagocytosis against SARS-CoV-2 spike protein. J Virol Methods. 2022;307:114564. doi: 10.1016/j.jviromet.2022.114564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.