Abstract
We have previously described a 104-kDa protein termed Pet (for plasmid-encoded toxin) secreted by some strains of enteroaggregative Escherichia coli (EAEC). Through an unknown mechanism, this toxin (i) raises transepithelial short-circuit current (Isc) and decreases the electrical resistance of rat jejunum mounted in the Ussing chamber, (ii) causes cytoskeletal alterations in HEp-2 cells and HT29/C1 cells, and (iii) is required for histopathologic effects of EAEC on human intestinal mucosa. Pet is a member of the autotransporter class of secreted proteins and together with Tsh, EspP, EspC, ShMu, and SepA proteins comprises the SPATE subfamily. Here, we show that Pet is internalized by HEp-2 cells and that internalization appears to be required for the induction of cytopathic effects. Evidence supporting Pet internalization includes the facts that (i) the effects of Pet on epithelial cells were inhibited by brefeldin A, which interferes with various steps of intracellular vesicular transport; (ii) immunoblots using anti-Pet antibodies detected Pet in the cytoplasmic fraction of intoxicated HEp-2 cells; (iii) Pet was detected inside HEp-2 cells by confocal microscopy; and (iv) a mutant in the passenger domain cleavage site, which prevents Pet release from the bacterial outer membrane, did not produce cytopathic effects on epithelial cells, whereas the release of mutant Pet from the outer membrane with trypsin yielded active toxin. We have also shown that the Pet serine protease motif is required to produce cytopathic effects but not for Pet secretion. Our results suggest an intracellular mode of action for the Pet protease and are consistent with we our recent report suggesting an intracellular mode of action for Pet (J. M. Villaseca, F. Navarro-García, G. Mendoza-Hernández, J. P. Nataro, A. Cravioto, and C. Eslava, Infect. Immun. 68:5920–5927, 2000).
Enteroaggregative Escherichia coli (EAEC) has been associated with persistent pediatric diarrhea, especially in developing countries (1, 2, 3, 19; H. R. Smith, T. Cheasty, and B. Rowe, Letter, Lancet 350:814–815, 1997). Supernatants from EAEC outbreak strains contain two high molecular weight proteins of 104 and 109-kDa which, when injected into rat ileal loops, induce fluid accumulation and cytotoxic effects on the mucosa (C. Eslava, J. Villaseca, R. Morales, A. Navarro, and A. Cravioto, Abstr. 93rd Gen. Meet. Am. Soc. Microbiol. 1993, abstr. B-105). We have previously cloned and sequenced the gene encoding the 104-kDa protein and have found that it that bears nucleotide homology to a class of serine protease autotransporter proteins (SPATEs) from E. coli and Shigella spp. (7). We have shown that the 104-kDa EAEC protein, termed Pet (plasmid-encoded toxin), raises the Isc and decreases the electrical resistance of rat jejunum mounted in the Ussing chamber, an effect that is accompanied by mucosal damage, increased mucus release, exfoliation of cells, and development of crypt abscesses (20). We have also shown that Pet is required for EAEC-induced damage to human intestinal mucosa (6).
While investigating its mode of action, we found that Pet induces temperature-, time-, and dose-dependent cytopathic effects on HEp-2 cells and HT29/C1 cells and appears to be a cytoskeleton-altering toxin since it induces contraction of the cytoskeleton, loss of actin stress fibers, and release of the cellular focal contacts in cell monolayers, followed by complete cell rounding and detachment (21). We have also shown that Pet cytotoxicity and enterotoxicity depend on Pet serine protease activity, since both effects are inhibited by phenylmethylsulfonyl fluoride and are not induced by Pet S260I, which is mutated in a predicted serine protease motif and thereby lacks in vitro protease activity (21).
Although the Pet serine protease motif is required for the cytopathic effects on cultured epithelial cells, the cytopathic effect of Pet is different from that induced by other proteases, such as trypsin, which acts on the cell surface (21). The effects of Pet occur after 2 h of exposure, and cell damage is irreversible; incubation at 37°C is necessary to observe the cytopathic effects (21). Here, we show that Pet enters the eukaryotic cell and that trafficking through the vesicular system appears to be required for the induction of cytopathic effects.
MATERIALS AND METHODS
Strains and plasmids.
The minimal Pet clone pCEFN1 described previously was constructed by cloning the pet gene of EAEC strain 042 into the BamHI/KpnI site of pSPORT1 and expressed in E. coli HB101 (5). HB101(pCEFN1) was used to obtain Pet protein, and supernatant proteins from the HB101(pSPORT1) was used as a control. The strains were maintained on L agar or L broth containing ampicillin (100 μg/ml).
Toxin preparation.
Broth cultures from HB101(pCEFN1) or the Pet mutants were incubated overnight at 37°C and then centrifuged at 7,000 × g for 15 min. The culture supernatant was filtered throughout 0.22-μm-pore-size cellulose acetate membrane filters (Corning, Cambridge, Mass.), concentrated 100-fold in an ultrafree centrifugal filter device with a 100-kDa cutoff (Millipore, Bedford, Mass.), filter sterilized again, and stored at −20°C for up to 3 months. One hundred milliliters of HB101(pCEFN1) overnight culture produced about 1 mg of Pet protein.
Cell culture.
HEp-2 cells were propagated in humidified 5% CO2–95% air at 37°C in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum (Hyclone, Logan, Utah), 1% nonessential amino acids, 5 mM L-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml). The subcultures were serially propagated after harvesting with 10 mM EDTA and 0.25% trypsin (GIBCO BRL, Grand Island, N.Y.) in phosphate-buffered saline (PBS) solution (pH 7.4). For experimental use, subconfluent HEp-2 cells were resuspended with EDTA-trypsin, plated into four-well LabTek slides (VWR, Bridgeport, N.J.), and allowed to grow to ca. 60% confluence (about 2 days).
Tissue culture assay.
For all experiments, Pet-containing concentrated filtrates were diluted directly into tissue culture medium (without antibiotics or serum) and added to the target cells at a final volume of 500 μl per well (for four-well LabTek slides). Following the specified incubation times in a humidified atmosphere of 10% CO2–90% air at 37°C, the medium was aspirated and the cells were washed twice with PBS and processed by the following three methods.
(i) Giemsa.
The cells were fixed with 70% methanol for 10 min and stained with 10% Giemsa for 10 min (Sigma Chemical Co., St. Louis, Mo.) and the slides were read at a magnification of ×100 with standard bright-field light microscopy. For the cell intoxication assay, toxic activity (defined as altered HEp-2 cell morphology) was scored on a scale previously described (21). A score of 1 indicated the presence of elongated or rounded cells greater than that for the control (but <50% of cells affected); 2 indicated that >50% of the cells were rounded but detachment was <50%; 3 indicated that >50% of the cells were detached and all remaining cells were rounded; and 4 indicated that all (or nearly all) cells were detached from the glass.
(ii) FAS assay.
The cells were fixed with 2% formalin–PBS, washed, permeabilized by adding 0.1% Triton X-100–PBS, and stained with 0.05 μg of fluorescein isothiocyanate (FITC)-phalloidin per ml (12). Slides were mounted with 90% glycerol, covered with a glass cover slide, and examined at a magnification of ×400 under epifluorescent microscopy.
(iii) Immunostaining.
The cells were fixed with 2% formalin–PBS, washed, permeabilized by adding 0.1% Triton X-100–PBS and incubated with anti-Pet antibodies. The preparations were immunostained using fluorescein-labeled goat anti-rabbit immunoglobulin G (IgG). Slides were mounted with 90% glycerol, covered with a glass cover slide, and examined under a Zeiss LSM410 confocal microscope. In some cases both FAS assay and immunostaining techniques were simultaneously used.
For vesicular trafficking inhibition experiments, cells were preincubated for 40 min at 37°C in culture medium containing the inhibitory agent being tested: NH4Cl (15, 30, and 45 mM), brefeldin A (7.5, 15, 30, and 60 μM), or chloroquine (0.05, 0.1, and 0.2 mM) (all from Sigma Chemical). The original medium was then replaced with medium containing Pet protein (10 μg/ml) in addition to the inhibitor.
Western immunoblot.
For cell fractionation experiments, control and Pet-treated HEp-2 cells were washed three times, pooled by scraping, and lysed by sonication in buffer containing protease inhibitors (2 mM Tris [pH 7.6], 1 mM EDTA, 2 mM MgCl2, and the Complete reagent [Boehringer, Mannheim, Germany]). Lysed cells were ultracentrifuged at 4°C for 60 min at 100,000 × g. The proteins from either cytoplasm or membrane fractions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the protein bands obtained were transferred to nitrocellulose membranes (23). Finally, the membranes were probed with anti Pet rabbit polyclonal antibodies (21). Pet protein was also visualized with goat anti-rabbit antibodies conjugated with alkaline phosphatase (Kirkegaard & Perry Laboratories, Gaithersburg, Md.).
Site-directed mutagenesis.
Site-directed mutagenesis was performed using the QuikChange site-directed mutagenesis kit from Stratagene (La Jolla, Calif.). The mutagenic oligonucleotides encoded a glycine and isoleucine to replace the two asparagines at residues 1018 and 1019, the cleavage site of the passenger domain (accession no. AF056581). Directed mutagenesis was performed on the minimal clone pCEFN1 according to the manufacturer's instructions using PfuTurbo DNA polymerase. After recovering plasmid DNA from several transformants, we confirmed the mutagenized DNA sequences on an Applied Biosystems model 373A automated sequencer via dye terminator cycle sequencing with Taq polymerase (Perkin-Elmer Corp., Norwalk, Conn.), according to the manufacturer's instructions. Sequencing was performed in the Biopolymer Laboratory, Department of Microbiology and Immunology, University of Maryland School of Medicine.
RESULTS
Role of serine protease motif on Pet secretion and its cytopathic effects.
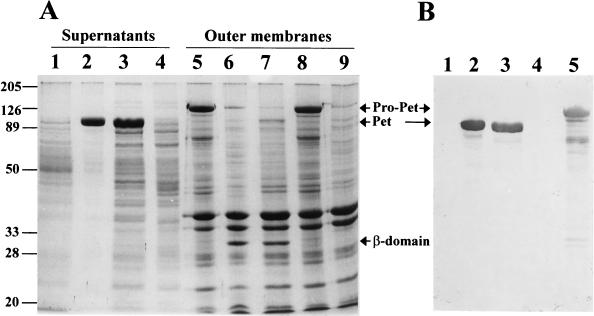
The Pet serine protease mutant was previously constructed by substituting isoleucine for the predicted catalytic serine residue 260 (21). Like native Pet (5), the S260 mutant secreted a 104-kDa species and left the 30-kDa β domain in the bacterial outer membrane (Fig. 1). We had previously reported that the Pet S260I mutant no longer induces cytopathic or enterotoxic effects (21). However, it is possible that the mutant could be released via cleavage by alternative membrane proteases. In addition, the mutant may have undergone structural changes which reduced activity. To address these issues we determined the N-terminal amino acid sequence (1019NLNKRMGDLR …) of the β domains of Pet and S260I and found that they are identical, which suggests that the serine protease motif is not required for cleavage of the β barrel. The peptide profiles of S260I and Pet degraded with proteinase K were similar; moreover, both proteins were eluted from Sephacryl S-300 exclusion columns at the same fraction and showed the same chromatographic profile (data not shown).
FIG. 1.
Effect of the serine protease motif on secretion of Pet and its mutants. (A) SDS-PAGE of supernatants or outer membranes from Pet clone pCEFN1 and its mutants in HB101. Shown are supernatants from HB101(pSPORT1) (lane 1), Pet clone (lane 2), Pet S260I mutant (lane 3), and Pet N1018G-N1019I mutant (lane 4) as well as outer membranes from Pet N1018G-N1019I mutant (lanes 5 and 8), Pet clone (lane 6), Pet S260I mutant (lane 7), and HB101(pSPORT1) (lane 9). The supernatants were concentrated 100-fold in an ultrafree centrifugal filter device with a 100-kDa cutoff (Millipore). Values to the left of the panel are molecular masses (in kilodaltons). (B) Detection of Pet by Western blot. The lanes are as described for panel A. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The reaction was visualized using rabbit anti-Pet and anti-rabbit antibodies conjugated with alkaline phosphatase. The proprotein (pro-Pet), mature Pet, and the β domain are indicated.
These results suggest that although the serine protease motif is not required for Pet processing, it is essential for the cytopathic and enterotoxic effects. These results also indicate that another protease may be used for Pet secretion; however, we have shown that normal processing of the Pet precursor occurs in the absence of the DegP, OmpP, and OmpT proteases and in the absence of the DsbA isomerase (5), suggesting that other endogenous membrane-associated enzymes are involved in Pet export.
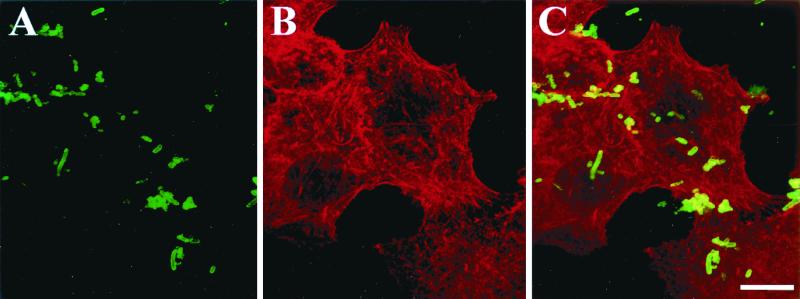
Based on the sequence of the C-terminal β domain in the outer membrane, the cleavage site for Pet was predicted to be between N1018 and N1019 (5). To investigate whether Pet is initially processed at this site upon secretion from the bacterium, we constructed a Pet mutant by changing the asparagines to glycine and isoleucine, respectively. Pet N1018G-N1019I-expressing bacteria no longer processed or secreted Pet protein into the supernatant of HB101, but a 134-kDa species was found in lysed bacteria by Pet immunoblotting (Fig. 1). Confocal microscopy of N1018G-N1019I-expressing bacteria immunolabeled with anti-Pet antibodies suggested that the Pet passenger domain was exported through the outer membrane transporter but that Pet remained exposed on the bacterial surface (Fig. 2A). Preparations containing Pet N1018G-N1019I (as bacterial lysates or whole bacteria) did not produce cytoskeletal effects on epithelial cells (Fig. 2B), suggesting that Pet protein must to be secreted and processed to be fully functional.
FIG. 2.
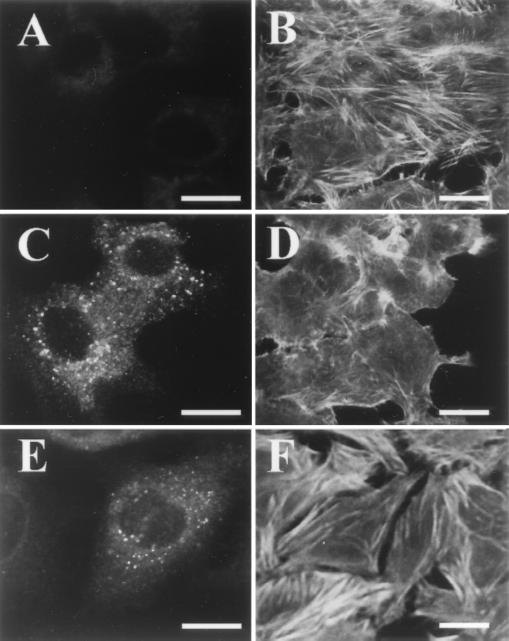
Pet N1018G-N1019I-expressing bacteria do not intoxicate HEp-2 cells. HEp-2 cells were incubated with Pet N1018G-N1019I-expressing bacteria, which had been killed by UV irradiation. After incubation the cells were fixed and stained simultaneously with rhodamine-phalloidin and anti-Pet antibodies by using fluorescein-labeled anti-rabbit antibodies; slides were observed by confocal microscope. (A) Immunolocalization of mutant Pet on the bacterial surface; (B) visualization of the cellular actin filaments; (C) merging of both channels (red and green). Cells appear similar to the negative control. Bar = 20 μm.
Effects of cellular trafficking inhibitors on Pet activity.
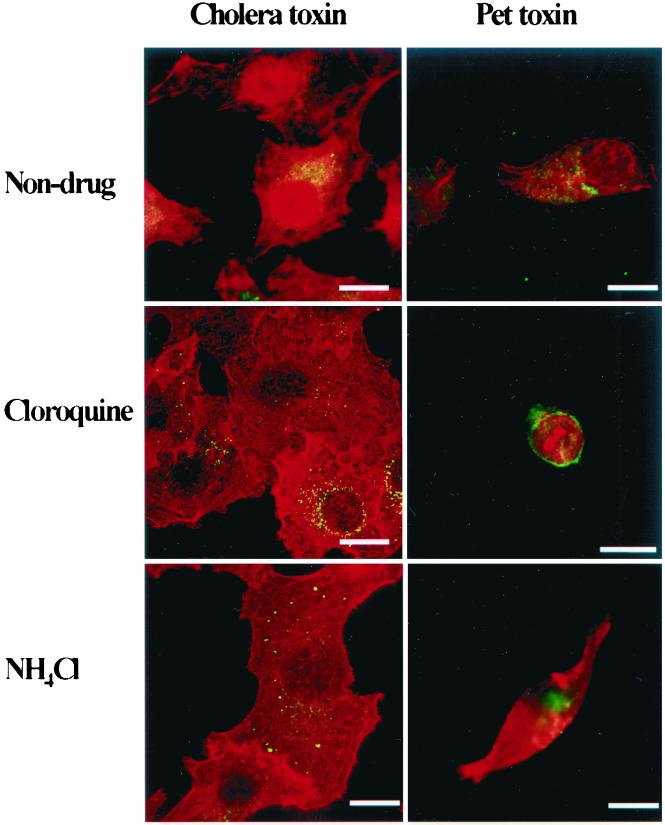
The serine protease motif of Pet plays a key role in the induction of epithelial cell damage. In addition, Pet toxicity is completely inhibited at 4°C, indicating either that the catalytic activity is temperature dependent or that intracellular trafficking of the toxin is required. To investigate whether intracellular uptake of Pet is necessary for the intoxication of HEp-2 cells, the effects of three inhibitors of cellular trafficking were tested: chloroquine, ammonium chloride, and brefeldin A. Brefeldin A induced a clear dose-dependent inhibition of cell detachment (Table 1). In contrast, chloroquine did not inhibit cytopathic effects induced by Pet, whereas NH4Cl produced a very slight inhibition (Fig. 3). Cholera toxin (CT) was used as a control for these studies, and as previously reported, inhibition of CT trafficking was demonstrated for chloroquine and NH4Cl (14), since both agents arrested the CT within vesicles (Fig. 3).
TABLE 1.
Dose-dependent inhibition of cell detachment by brefeldin A
| Brefeldin A concn (μM) | Morphologic effectsa |
|---|---|
| 0 | 3.6 ± 0.48 |
| 7.5 | 2.4 ± 0.48 |
| 15 | 1.4 ± 0.48 |
| 30 | 0.2 ± 0.4 |
| 60 | 0 |
The morphologic changes of HEp-2 cells produced by Pet are shown as means ± SD standard deviation (n = 5 wells) from previously reported scores (see Materials and Methods section): 1, elongated cells; 2, rounded cells; 3, 50% detachment of the cells; 4, 95% detachment of cells.
FIG. 3.
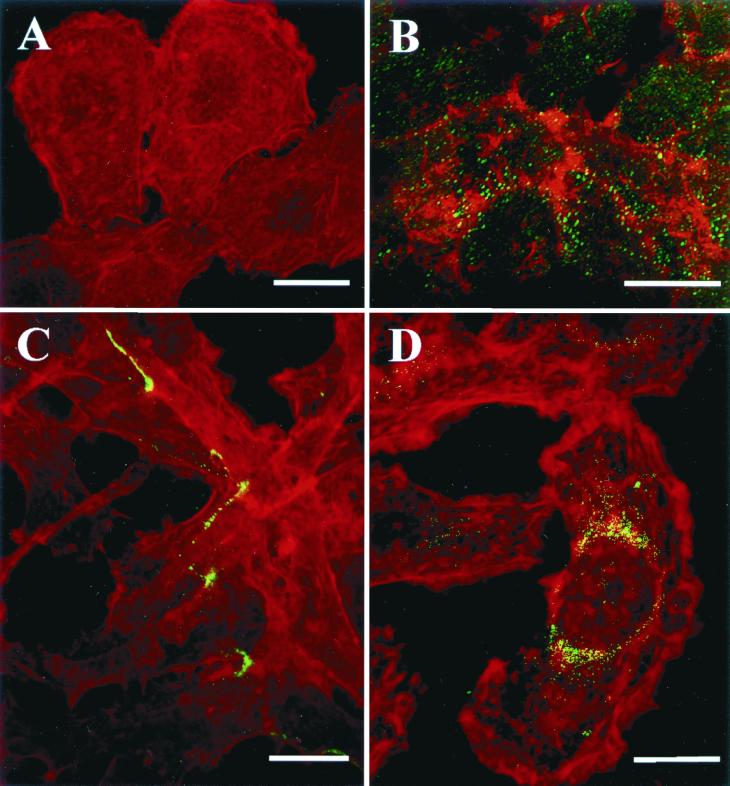
Effects of chloroquine and NH4Cl on CT and Pet intoxication. Pet protein (10 μg/ml) or CT (1 μg/ml) was added to cell cultures, which were preincubated with 100 μM chloroquine or 40 mM NH4Cl. After 3 h of incubation the cells were fixed, stained simultaneously with rhodamine-phalloidin and anti-Pet or anti-CT antibodies and fluorescein-labeled anti-rabbit antibodies. Slides were observed by confocal microscopy. Chloroquine and NH4Cl arrested CT within vesicles but not Pet. Bar = 20 μm.
When HEp-2 cells were pretreated with brefeldin A at various doses 30 min prior to the addition of Pet (to a final concentration of 10 μg/ml), inhibition of Pet effects was first apparent at a brefeldin A concentration of 12.5 μM. At concentrations of 30 μM and above, Pet activity was abolished. In control studies, brefeldin A alone did not induce cytopathic effects at doses of up to 100 μM. For the sake of comparison, brefeldin A pretreated cells were incubated with trypsin (a serine protease that acts extracellularly) at 25 μg per ml; brefeldin pretreatment did not inhibit the loosening of cells from the substratum induced by trypsin (data not shown).
To assess the time course of brefeldin A inhibition, HEp-2 cells were pretreated with brefeldin A at a concentration of 50 μM for various times before and after Pet addition. The inhibitory effects of brefeldin A were apparent when the compound was added at any time before or simultaneously with Pet (at 10 μg/ml). Addition of brefeldin A 5 min after the addition of Pet did not produce significant inhibitory effects (data not shown).
Internalization of Pet protein into epithelial cells.
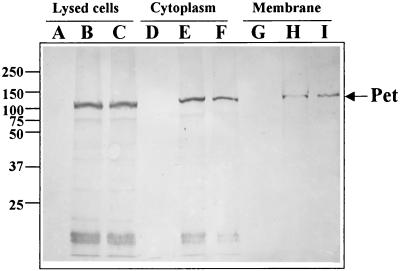
Brefeldin A data suggest that Pet must enter the epithelial cell to produce its cytopathic effects. In order to demonstrate Pet internalization directly, we performed cellular fractionation of HEp-2 cells treated with Pet or Pet S260I protein and performed immunoblotting with anti-Pet polyclonal antibodies. Both Pet and Pet S260I proteins were found within intoxicated and lysed HEp-2 cells, and cellular fractionation showed that both proteins were located mainly in the cytoplasmic fraction; a sparse Pet-reactive band was seen in the membrane fraction (Fig. 4).
FIG. 4.
Detection of Pet and Pet S260I in cytoplasm and membrane fractions from HEp-2 cells. HEp-2 cells treated with Pet or Pet S260I were fractionated into cytoplasm and membrane fractions. These fractions were separated by SDS-PAGE and transferred to nitrocellulose membranes. The reaction of anti-Pet antibodies was visualized using anti-rabbit antibodies conjugated with alkaline phosphatase. Whole untreated HEp-2 cells (lane A), cells treated with Pet (lane B), or cells treated with Pet S260I (lane C); cytoplasm fraction from untreated HEp-2 cells (lane D), cells treated with Pet (lane E), or treated with Pet S260I (lane F); and membrane fraction from untreated HEp-2 cells (lane G), cells treated with Pet (lane H), or cells treated with Pet S260I (lane I) are shown. Values to the left of the panel are molecular masses (in kilodaltons).
To further visualize Pet internalization, HEp-2 cells treated with either Pet or Pet S260I for 1 to 5 h were fixed, permeabilized, and incubated with anti-Pet polyclonal antibodies and then with fluorescein-labeled goat anti-rabbit IgG, and observed under confocal microscopy. These experiments showed that during the first 3 h of incubation with the toxin, the antitoxin antibodies react with small, round intracellular structures consistent in appearance with endosomes (Fig. 5). However, despite apparent entry of both Pet and S260I into HEp-2 cells, only Pet produced cytoskeletal rearrangement (Fig. 5).
FIG. 5.
Detection of Pet within epithelial cells by confocal microscopy. HEp-2 cells were treated with Pet or Pet S260I for 3 h. After treatment the cells were fixed and processed for FAS and immunostaining. Shown are untreated HEp-2 cells stained with anti-Pet (A) or FITC phalloidin (B); HEp-2 cells treated with Pet and stained with anti-Pet (C) or FITC phalloidin (D); HEp-2 cells treated with Pet S260I and stained with anti-Pet (E) or FAS (F). Note that both Pet and Pet S260I are internalized, but only Pet produces cytoskeletal damage. Bar = 20 μm.
We hypothesized that if the effect of brefeldin A on Pet intoxication was due to its inhibition of Pet trafficking through the vesicular system, then our confocal images in the presence of brefeldin A would reveal Pet uptake but persistence of Pet in some cellular compartment. As shown in Fig. 6, we found that in the absence of brefeldin A, Pet-containing endosomes disappeared after 3 h of intoxication, accompanied by increased diffuse staining of the cell cytoplasm with Pet antibody. However, in the presence of brefeldin A at 30 μM, we observed continued presence of Pet-containing endosomes. These endosomes were frequently clustered at the plasma membrane or around the nuclei.
FIG. 6.
Immunolocalization of Pet into brefeldin A-treated HEp-2 cells. HEp-2 cells were treated with brefeldin A (30 μM) and Pet (10 μg/ml) simultaneously and then incubated for 3 h. After this time, the cells were fixed, stained simultaneously for Pet (green) and the actin cytoskelton (red), and then observed by confocal microscopy. (A) Untreated cells stained with rhodamine-phalloidin. (B) Merge of both channels (red and green) showing effects of Pet on the actin cytosketon. (C and D) Visualization of cytoskeleton (red) and Pet (green) in brefeldin A-treated HEp-2 cells. Note that Pet in brefeldin A-treated HEp-2 cells is localized in endosomes at the internal face of the membrane (C) or around the nucleus (D). Bar = 20 μm.
We also tested mutant Pet N1018G-N1019I to provide further evidence that Pet must be internalized to produce the cytoskeletal effects. Pet N1018G-N1019I bacteria were grown overnight and killed by exposure to UV light, and the bacteria were incubated with HEp-2 cells as described previously (21). In contrast to the effects observed when the cells were treated with native Pet protein, HEp-2 cells treated with Pet N1018G-N1019I showed a normal cytoskeleton, despite the demonstration of Pet at the surface of the epithelial cells (Fig. 2C). Confocal microscopy did not reveal Pet inside of the epithelial cells or cytoskeletal damage. Moreover, neither Pet internalization nor cytoskeletal damage was seen when lysed Pet N1018G-N1019I-expressing bacteria were incubated with epithelial cells (Fig. 2C). However, when the UV-treated Pet N1018G-N1019I bacteria were preincubated with trypsin, which cleaved Pet protein from the membrane, HEp-2 cell cytopathic effects were induced. These effects were not seen with those cells treated only with trypsin (data not shown). These results suggest that when Pet is bound to the bacterial membrane, it is unable to produce cell damage.
DISCUSSION
EAEC has been associated characteristically with persistent diarrhea among infants, particularly in the developing world (1, 2, 3, 19, 26). However, recent outbreaks and volunteer studies suggest that EAEC strains are virulent in adults (17); Smith et al., Letter) and have a global distribution (8, 9). Previous data from our laboratories in animal models (24), cultured cells (18), intestinal segments mounted in Ussing chamber (20), in vitro organ culture (IVOC) (6), rat ideal loops and autopsy specimens from infected patients (Eslava et al., Abstr. 93rd Gen. Meet. Am. Soc. Microbiol. 1993) have shown that a plausible explanation for the persistent nature of EAEC disease involves intestinal mucosal damage. However, the mechanism of this mucosal damage is not fully characterized.
We have shown that the EAEC Pet toxin elicits Isc increases in rat mucosa mounted in the Ussing chamber, an effect which is accompanied by a decrease in tissue resistance and damage to the tissue when examined under light microscopy (20). Furthermore, Pet is able to intoxicate HEp-2 and HT29 cells after 2 h at 37°C; these effects are dose and time dependent and are characterized by cell elongation, followed by rounding and ultimately release from the substratum. Our data also reveal contraction of the cytoskeleton and loss of actin stress fibers. However, cytoskeleton-altering drugs such as taxol, colchicine, phallacidin, or cytochalasin D do not prevent the cytoskeletal damage (21). Furthermore, only 10 min of exposure to Pet followed by incubation for another 2 h at 37°C is sufficient to elicit the same morphologic changes. These data indicate that the intoxication of HEp-2 cells is irreversible and that an irreversible step occurs between 10 min and 2 h.
We suspected that the observed lag time in Pet action might represent the time required for cellular entry and trafficking. Certain chemical inhibitors of receptor-mediated endocytosis and vesicular trafficking, which either alkalinize certain intracellular compartments (NH4Cl and chloroquine) or disrupt the vesicular system and Golgi apparatus (brefeldin A), have been shown previously to inhibit the intoxication of intestinal cell lines by other enterotoxins that require cellular uptake (4, 11, 13, 15, 22). For Pet, chloroquine had no effect, and NH4Cl produced a very slight inhibition. It is well known that endocytosed toxins that are translocated into the cytosol from an endosomal compartment depend on the low pH of the endosomal compartment (14). These data suggest that Pet is not translocated from endosomes and its mechanism perhaps is similar to those of other toxins, including Pseudomonas exotoxin A, Shiga toxin, and ricin (16). Indeed, as with these toxins, brefeldin A induced a clear dose-dependent inhibition of Pet-induced cytopathic effects.
In further support of the hypothesis that Pet acts intracellularly, we observed Pet in cellular vacuoles by confocal microscopy and in the cytoplasmic fraction of cells by Western immunoblotting. An inhibitory effect of brefeldin A suggests that Pet entry requires transit through the Golgi apparatus; alternatively the receptor or target of Pet may require Golgi processing. In support of the former hypothesis, confocal microscopy of brefeldin A-pretreated cells exposed to Pet demonstrated uptake of the toxin but arrest of trafficking at the endosomal level. These data place the brefeldin blockade either at the level of the Golgi apparatus itself or, conceivably, at the level of the endosome after initial internalization. Of note, the Western immunoblot data reveal that the complete Pet toxin is internalized and does not appear to be processed or degraded intracellularly as occurs for some other toxins (16).
Brefeldin A induces multiple derangements of the cellular vesicular transport system, and inhibition by brefeldin A is associated classically with toxins whose internalization pathways involve retrograde transport from the Golgi to the endoplasmic reticulum. Notably, however, the Pet-deduced amino acid sequence does not exhibit a KDEL (or RDEL) retrieval motif, which is known to mediate the retention of eukaryotic proteins in the endoplasmic reticulum and to be involved in retrograde transport from the Golgi apparatus (14–16). These data suggest that Pet may use an alternative transport signal, as does Shiga toxin (10).
Data suggesting Pet internalization are consistent with our recent report (25). Pet toxin induces degradation of the membrane cytoskeletal protein fodrin (nonerythroid spectrin). Disruption of the membrane skeleton could account for the cytopathic effects that we have described. However, even though Pet cleaves fodrin in vitro and in vivo, we have yet to establish that this is the essential first step in Pet intoxication, and this question is currently being addressed in our laboratories. Cleavage of fodrin would be a novel mechanism of cellular intoxication for a bacterial toxin.
Controversy exists over how cleavage of the passenger domain from the β domain occurs for many autotransporters, especially whether cleavage is a result of a membrane-bound protease or an autoproteolytic event. At least some of the autotransporters (for instance, IgA1 protease and Hap from Haemophilus influenzae) are capable of autoprocessing via their serine protease active site (7). Moreover, passenger domains may be processed at several sites to permit release from the outer membrane (7). Indeed, if the primary auto-cleavage site of Hap is mutated, the protein will be cleaved at alternative sites (7). Here we have shown that Pet protein is not autoprocessed by its serine protease active site, since a Pet serine protease mutant secretes the normal passenger domain (mature protein) and the N-terminal sequence of the β domain of this mutant was the same as that of the native protein, indicating that the cleavage occurs at the same site (between N1018 and N1019). Also, when these amino acids were changed to glycine and isoleucine (Pet N1018G-N1019I) the mature protein was no longer secreted and was found decorating the bacterial surface as the proprotein of 134 kDa.
Pet shares homology with members of the SPATE subfamily (serine protease autotransporters of the family Enterobacteriaceae), which have a serine protease motif (consensus, GDSGSP) at a similar position in the E. coli proteins Tsh, EspP, and EspC (5), and with the Shigella proteins Pic and SepA (7). Therefore, Pet protein may be prototypical of this family, and as we have shown here, Pet protein acts through a novel mechanism, including internalization. The Pet serine protease motif is needed to produce the cytoskeletal effects but it is apparently not needed to allow the secretion of Pet nor internalization into the host cell.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant A143615 and TW00499 (from the Fogarty Center) to J.P.N., and F.N.G. was supported with an installing grant from the Consejo Nacional de Ciencia y Tecnología de México (CONACYT, I3004M).
We thank to Rocio Huerta for her technical help.
REFERENCES
- 1.Bhan M K, Khoshoo V, Sommerfelt H, Raj P, Sazawal S, Srivastava R. Enteroaggregative Escherichia coli and Salmonella associated with nondysenteric persistent diarrhea. Pediatr Infect Dis J. 1989;8:499–502. doi: 10.1097/00006454-198908000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Bhan M K, Raj P, Levine M M, Kaper J B, Bhandari N, Srivastava R, Kumar R, Sazawal S. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J Infect Dis. 1989;159:1061–1064. doi: 10.1093/infdis/159.6.1061. [DOI] [PubMed] [Google Scholar]
- 3.Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet. 1991;337:262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- 4.Donta S T, Beristain S, Tomicic T K. Inhibition of heat-labile cholera and Escherichia coli enterotoxins by brefeldin A. Infect Immun. 1993;61:3282–3286. doi: 10.1128/iai.61.8.3282-3286.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eslava C, Navarro-Garcia F, Czeczulin J R, Henderson I R, Cravioto A, Nataro J P. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66:3155–3163. doi: 10.1128/iai.66.7.3155-3163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson I R, Hicks S, Navarro-Garcia F, Elias W P, Philips A D, Nataro J P. Involvement of the enteroaggregative Escherichia coli plasmid-encoded toxin in causing human intestinal damage. Infect Immun. 1999;67:5338–5344. doi: 10.1128/iai.67.10.5338-5344.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson I R, Navarro-Garcia F, Nataro J P. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:370–378. doi: 10.1016/s0966-842x(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 8.Huppertz H I, Rutkowski S, Aleksic S, Karch H. Acute and chronic diarrhoea and abdominal colic associated with enteroaggregative Escherichia coli in young children living in western Europe. Lancet. 1997;349:1660–1662. doi: 10.1016/S0140-6736(96)12485-5. [DOI] [PubMed] [Google Scholar]
- 9.Itoh Y, Nagano I, Kunishima M, Ezaki T. Laboratory investigation of enteroaggregative Escherichia coli O untypeable: H10 associated with a massive outbreak of gastrointestinal illness. J Clin Microbiol. 1997;35:2546–2550. doi: 10.1128/jcm.35.10.2546-2550.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson M E, Simpson J C, Girod A, Pepperkok R, Roberts L M, Lord J M. The KDEL retrieval system is exploited by Pseudomonas exotoxin A, but not by Shiga-like toxin-1, during retrograde transport from the Golgi complex to the endoplasmic reticulum. J Cell Sci. 1999;112:467–475. doi: 10.1242/jcs.112.4.467. [DOI] [PubMed] [Google Scholar]
- 11.Keusch G T, Jacewicz M. Primary amines and chloroquine inhibit cytotoxic responses to Shigella toxin and permit late antibody rescue of toxin treated cells. Biochem Biophys Res Commun. 1984;121:69–76. doi: 10.1016/0006-291x(84)90689-2. [DOI] [PubMed] [Google Scholar]
- 12.Knutton S, Shaw R K, Bhan M K, Smith H R, McConnell M M, Cheasty T, Williams P H, Baldwin T J. Ability of enteroaggregative Escherichia coli strains to adhere in vitro to human intestinal mucosa. Infect Immun. 1992;60:2083–2091. doi: 10.1128/iai.60.5.2083-2091.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lencer W I, de Almeida J B, Moe S, Stow J L, Ausiello D A, Madara J L. Entry of cholera toxin into polarized human intestinal epithelial cells. Identification of an early brefeldin A sensitive event required for A1-peptide generation. J Clin Investig. 1993;92:2941–2951. doi: 10.1172/JCI116917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lencer W I, Hirst T R, Holmes R K. Membrane traffic and the cellular uptake of cholera toxin. Biochim Biophys Acta. 1999;1450:177–190. doi: 10.1016/s0167-4889(99)00070-1. [DOI] [PubMed] [Google Scholar]
- 15.Lencer W I, Strohmeier G, Moe S, Carlson S L, Constable C T, Madara J L. Signal transduction by cholera toxin: processing in vesicular compartments does not require acidification. Am J Physiol. 1995;269:G548–557. doi: 10.1152/ajpgi.1995.269.4.G548. [DOI] [PubMed] [Google Scholar]
- 16.Lord J M, Roberts L M. Toxin entry: retrograde transport through the secretory pathway. J Cell Biol. 1998;140:733–736. doi: 10.1083/jcb.140.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nataro J P, Deng Y, Cookson S, Cravioto A, Savarino S J, Guers L D, Levine M M, Tacket C O. Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J Infect Dis. 1995;171:465–468. doi: 10.1093/infdis/171.2.465. [DOI] [PubMed] [Google Scholar]
- 18.Nataro J P, Hicks S, Phillips A D, Vial P A, Sears C L. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect Immun. 1996;64:4761–4768. doi: 10.1128/iai.64.11.4761-4768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nataro J P, Steiner T, Guerrant R L. Enteroaggregative Escherichia coli. Emerg Infect Dis. 1998;4:251–261. doi: 10.3201/eid0402.980212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarro-García F, Eslava C, Villaseca J M, López-Revilla R, Czeczulin J R, Srinivas S, Nataro J P, Cravioto A. In vitro effects of a high-molecular-weight heat-labile enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66:3149–3154. doi: 10.1128/iai.66.7.3149-3154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarro-García F, Sears C, Eslava C, Cravioto A, Nataro J P. Cytoskeletal effects induced by Pet, the serine protease enterotoxin of enteroaggregative Escherichia coli. Infect Immun. 1999;67:2184–192. doi: 10.1128/iai.67.5.2184-2192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orlandi P A, Curran P K, Fishman P H. Brefeldin A blocks the response of cultured cells to cholera toxin. Implications for intracellular trafficking in toxin action. J Biol Chem. 1993;268:12010–12016. [PubMed] [Google Scholar]
- 23.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vial P A, Robins-Browne R, Lior H, Prado V, Kaper J B, Nataro J P, Maneval D, Elsayed A, Levine M M. Characterization of enteroadherent-aggregative Escherichia coli, a putative agent of diarrheal disease. J Infect Dis. 1988;158:70–79. doi: 10.1093/infdis/158.1.70. [DOI] [PubMed] [Google Scholar]
- 25.Villaseca J M, Navarro-García F, Mendoza-Hernández G, Nataro J P, Cravioto A, Eslava C. Pet toxin from enteroaggregative Escherichia coli produces cellular damage associated with fodrin disruption. Infect Immun. 2000;68:5920–5927. doi: 10.1128/iai.68.10.5920-5927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wanke C A, Schorling J B, Barrett L J, Desouza M A, Guerrant R L. Potential role of adherence traits of Escherichia coli in persistent diarrhea in an urban Brazilian slum. Pediatr Infect Dis J. 1991;10:746–751. doi: 10.1097/00006454-199110000-00006. [DOI] [PubMed] [Google Scholar]