Abstract
Background & Aim:
Numerous studies have linked air pollution with cardiovascular diseases. Fewer studies examined the associations at low concentration levels or assessed potential modifiers. Some investigations only examined hospitalizations, which can miss incident cases. This study aims to address these gaps through a nationwide cohort study of Medicare enrollees.
Methods:
Our study cohort comprise all Medicare enrollees (≥65 years old) continuously enrolled in the fee-for-service program and both Medicare part A and B across the contiguous U.S. from 2000 to 2016. We examined the associations of population-weighted ZIP code-level annual average PM2.5, NO2, and warm-season O3 (May-October), with the first diagnoses of atrial fibrillation (AF), congestive heart failure (CHF), and stroke. We fit multi-pollutant Cox proportional hazards models adjusted for individual demographic characteristics and area-level covariates. We further examined these associations at low pollutant concentration levels and the potential effect modifications by race/ethnicity and comorbidities (diabetes, hypertension, hyperlipidemia).
Results:
Elevated PM2.5 and NO2 levels were associated with increased incidence of AF, CHF, and stroke. For each 1 μg/m3 increase in annual PM2.5, hazard ratios (HRs) were 1.0059 (95%CI: 1.0054, 1.0064), 1.0260 (95%CI: 1.0256, 1.0264), and 1.0279 (95%CI: 1.0274, 1.0284), respectively. For each1 ppb increase in annual NO2, HRs are 1.0057 (95%CI: 1.0056, 1.0059), 1.0112 (95%CI: 1.0110, 1.0113), and 1.0095 (95%CI: 1.0093, 1.0096), respectively. For warm-season O3, each 1 ppb increase was associated with increased incidence of CHF (HR=1.0035, 95%CI: 1.0033–1.0037) and stroke (HR=1.0026, 95%CI: 1.0023–1.0028). Larger magnitudes of HRs were observed when restricted to pollutants levels lower than NAAQS standards. Generally higher risks were observed for Black people and diabetics.
Conclusions:
Long-term exposure to PM2.5, NO2, and warm-season O3 were associated with increased incidence of cardiovascular diseases, even at low pollutant concentration levels. Black people and people with diabetes were found to be vulnerable populations.
Keywords: PM2.5, NO2, warm-season O3, atrial fibrillation, congestive heart failure, stroke
1. Introduction
More than 877,500 people die from cardiovascular diseases (CVDs) in the United States every year1. Atrial fibrillation (AF), congestive heart failure (CHF), and stroke are three common CVDs in the U.S. Both AF and CHF are linked with an elevated risk of stroke, which is the fifth leading cause of death in the U.S. according to the Centers for Disease Control and Prevention (CDC)1,2.
Previous studies have examined the associations between air pollution and AF, CHF, and stroke. PM2.5, NO2, and O3 are three pollutants of major interest. A cohort study conducted in Ontario, Canada reported significant associations between 5-year moving average PM2.5, NO2, and O3 and stroke and AF, and the associations were observed even at concentration levels lower than U.S. NAAQS standards3. Another cohort study examining Medicare participants also reported significant associations between 1-year average PM2.5 and O3 with the hospital admissions for stroke and heart failure4. There are also several significant associations reported from other studies5–9. However, many of these findings were from single-pollutant models, while associations in multiple-pollutant models were less assessed3,5,6. Due to correlations between different air pollutants, the results from single-pollutant models could be confounded by other co-existing pollutants. Moreover, many previous studies have used hospitalization as the primary measure of morbidity, which may only capture severe cases and assess exacerbation rather than incidence of the outcomes5,10–15. In particular, the incidence of heart failure or atrial fibrillation is rarely accompanied by a hospital admission. In addition, the potential modification of these associations by race/ethnicity and different comorbidities, as well as potential differing associations at low vs. high pollutant concentration levels, are also worth further examination given the current limited and heterogeneous evidence3,5,8,16–19.
To address these knowledge gaps, we investigated the associations between long-term exposure to PM2.5, NO2, warm-season O3 and the first diagnoses of AF, CHF, and stroke in a nationwide cohort of Medicare enrollees (≥65 years old) across the contiguous U.S. We utilized the Medicare Chronic Conditions Data Warehouse (CCW) database to capture the first diagnoses of these outcomes and high spatiotemporal resolution exposure models to assess the pollutant concentrations at the ZIP code-level. In addition, we assessed potential modification by race/ethnicity and comorbidities such as hypertension, hyperlipidemia, and diabetes. The associations at low pollutant concentration levels have also been investigated separately.
2. Methodology
2.1. Study Population & Outcomes Data
Our study population included all Medicare enrollees (≥65 years old) with continuous enrollment in the fee-for-service (FFS) program and both Medicare part A (hospital insurance) and part B (medical insurance) from 2000 to 2016 in the contiguous U.S. The Medicare denominator file included enrollment records of age, self-reported sex, self-reported race/ethnicity (classified as White, Black, Asian, Hispanic, Native American, and others), Medicaid eligibility, date of death, and the residential ZIP code for each Medicare participant. Residential ZIP code, Medicaid eligibility, and age are updated annually. We restricted our study population to the aforementioned programs because the CCW database relied on these programs to identify incident cases20. The CCW database provides the date of the first occurrence of a disease identified using an algorithm that incorporates information across all available Medicare claims (such as inpatient and outpatient claims, the Carrier File (primarily physician visits), skilled nursing facility, and home health-care claims)20.
There were three outcomes of interest in this study: atrial fibrillation (AF), congestive heart failure (CHF), and stroke. We created three separate open cohorts for these three outcomes. The participants entered the cohort on January 1st of the year after they enrolled in Medicare for each calendar year until the first occurrence of the outcome, end of enrollment in either of the mentioned programs above, death, or the end of the study in 2016, whichever came first. To better estimate incidence, we required a 2-year clean period for each cohort: only the participants who were free of the outcome of the interest, in any Medicare claim as noted above, in the first two years after their enrollment were included and the follow-up would only start after the 2-year clean period.
The Medicare denominator file and the CCW database were both obtained from the Centers for Medicare and Medicaid Services (CMS). They were stored, processed, and analyzed in the Rollins High-Performance Computing (HPC) Cluster at Emory University, with Health Insurance Portability and Accountability Act (HIPAA) compliance. This study was conducted with approval from the institutional review boards of Emory and the Harvard School of Public Health.
2.2. Exposure Assessment
We obtained ambient daily PM2.5 (24-hour mean), NO2 (1-hour maximum), and O3 (8-hour maximum) level predictions at 1 km×1 km spatial resolution from 2000 to 2016 for the contiguous U.S.21–23 Concentrations were estimated from spatial-temporal ensemble models integrating three machine learning algorithms (neural networks, random forest, gradient boosting). The predictor variables included satellite data, meteorological variables, land-use variables, elevation, chemical transport model predictions, etc24–26. These ensemble models have demonstrated strong prediction performance with an average annual cross-validation R2 value of 0.89, 0.84, and 0.86 for PM2.5, NO2, and O3, respectively24–26.
Based on the daily predictions, we calculated the annual average PM2.5 and NO2 concentrations and warm-season O3 concentration (defined as the average exposure from May to October) for each 1 km×1 km grid from 2000 to 2016. Warm-season O3 is commonly used to capture the O3 exposure in studies examining the associations between air pollution and health because cold-season O3 concentration is very low and negatively correlated with NO2. We may assume that the health risk associated with O3 in the cold seasons is negligible.27 The definition of warm season for ozone as May to October has also been widely used in previous studies conducted in the U.S28–30. Gridded 30 second (~1 km) population data were retrieved from the NASA Socioeconomic Data and Applications Center (SEDAC) for the years 2000, 2005, 2010, 2015, and 2020, and further linearly interpolated for each year from 2000 to 201631. We then calculated the population-weighted average annual PM2.5 and NO2 concentrations and warm-season O3 concentrations at the ZIP code-level.
We linked these ZIP code-level population-weighted average pollutant concentrations to the study cohorts by year and residential ZIP code to assign exposure to each enrollee in each year. Giving proportionately greater weight to the air pollution exposure in densely populated areas helps in better capturing individual exposures. The main exposure metrics used in this study are the annual average population-weighted concentrations of the concurrent year.
2.3. Covariates
We obtained individual-level age, self-reported sex, self-reported race/ethnicity, and Medicaid eligibility (as a proxy for individual-level socioeconomic status) from the Medicare denominator file and obtained the first occurrence records of hyperlipidemia, hypertension, and diabetes from the CCW database. The pre-existences of these conditions were included in the analysis as potential confounders and potential modifiers.
In addition, we included neighborhood-level demographic, socioeconomic, healthcare-access-related and meteorological covariates for analysis. The following covariates were chosen because they might be associated with both the air pollution and health outcomes of interest in our study.
The demographic and socioeconomic variables were obtained from the U.S. Decennial Census (2000, 2010) and the annual American Community Survey (2011–2016) including percent of the population age ≥65; percentages of the population self-reported as non-Hispanic White, non-Hispanic Black, Asian, or Hispanic, separately; percentages of the adult population with high school education or greater; percentages of the adult population with income below the poverty line; percent of households receiving public assistance; percent of housed individuals renting their property; percent of residences with at least three housing units in the structure; population density; and median household income32. All the demographic and socioeconomic variables were calculated at ZCTA level and translated into ZIP codes. Linear interpolation was used to fill in years 2001–2009.
Healthcare access-related variables included the distance to the nearest hospital (non-time-varying for the year 2010) and the average annual percent of Medicare enrollees having at least one ambulatory visit to a primary care clinician obtained from Dartmouth Health Atlas Access-to-care variables33. We also included the percent of population who have ever been smokers and the mean body mass index (BMI) from BRFSS and the total number of active medical doctors per 1000 people, total number of hospitals, and the number of beds regularly maintained for inpatients from Area Health Resources Files (AHRF) of U.S. Health Resources & Services Administration (HRSA)34. All these variables were matched to the ZIP code-level and the data for possible missing years were filled using linear interpolation and extrapolation.
We also obtained the 1 km × 1 km gridded monthly maximum and minimum temperature data from Daymet and the 4 km × 4 km gridded daily maximum and minimum relative humidity data from gridMET35,36. We then calculated the annual average relative humidity, summer (June, July, August) mean temperature and winter (consecutive months - December, January, February) mean temperature at ZIP code-level. There is evidence suggesting a U-shape relationship between daily temperature and human health, where both extreme cold and extreme hot weather having harmful effects. This suggests that the relationships between seasonal temperature and health may have different shapes in winter and summer37. Therefore, we have included both summer mean temperature and winter mean temperature for confounding control in our study.
2.4. Statistical Analysis
In the main analyses, we fit tri-pollutant stratified Cox proportional hazards models to estimate the impacts of long-term exposures to PM2.5, NO2, and warm-season O3 on AF, CHF, and stroke incidence, separately. The exposure metrics used in our study are the annual average population-weighted concentrations of the concurrent year. For each outcome, we fit a separate model controlling for individual-level Medicaid eligibility, pre-existing comorbidities (hypertension, hyperlipidemia, diabetes), and the ZIP code-level covariates mentioned in the previous section. Each model was stratified by sex and race/ethnicity, and age was the time scale. Three air pollutants of interest were included in the models simultaneously. The formula of the Cox proportional hazards models could be presented as:
where hk(t) was the expected hazard at time t for stratum k; h0k(t) was the baseline hazard for stratum k representing the hazard when all the covariates are equal to 0; β1·PM2.5, β2·NO2 and β 3·warm O3 were the exposure metrics and corresponding coefficients; were the covariates included for confounding control and the corresponding coefficients. The strata represented by k were for sex and race/ethnicity, and the covariates included all individual-level and neighborhood- level variables mentioned before.
To explore potential modification by race/ethnicity and pre-existing comorbidities, we repeated the main analyses models with additional interaction terms between exposures and potential modifiers. The interactions were separately examined for the three air pollutants. With three exposures (PM2.5, NO2, warm-season O3) and four potential modifiers (race/ethnicity, hypertension, hyperlipidemia, diabetes), 12 additional Cox models were fit for each outcome.
We also examined the associations at low pollutant concentration levels by restricting the cohorts to enrollees who lived in areas with pollutant concentrations below a certain threshold throughout the study period. Members of EPA’s Clean Air Scientific Advisory Board have suggested that lowering the current NAAQS standards for annual PM2.5 to between 8 and 10 μg/m3 could protect the general public and at-risk groups, EPA is currently considering lowering its standard to within that range38. Therefore, we set our thresholds for PM2.5 as these two values. For NO2 and warm-season O3, the concentrations in our study were quite well below the current NAAQS standards, so we set our thresholds for these two pollutants as plausible targets for further reduction by consulting the median values of our study. Hence, we examined two thresholds for each exposure: 10 μg/m3 and 8 μg/m3 for PM2.5, 25 ppb and 18 ppb for NO2, 50 ppb and 40 ppb for warm-season O3. Therefore, six additional sub-cohorts and additional Cox models were fit for each outcome. All of these thresholds are below the U.S. NAAQS and EU standards. However, for PM2.5 and NO2, these thresholds are still higher than the 2021 WHO Air Quality Guidelines39–41.
Three sets of sensitivity analyses were conducted to assess the robustness of the main results. First, we repeated the main analyses with a clean period of 3 years instead of the original 2-year clean period, that is only the participants free of the outcome of the interest in the first three years after enrollment were included in the follow-up, and the follow-up would only start after this 3-year period, to better capture the actual first diagnosis of the outcomes at the cost of fewer person-years of follow-up and cases. Second, we repeated the main analyses with 3-year average pollutants concentrations as exposure metrics (concurrent year and two previous years) instead of the 1-year exposure windows (only concurrent year) to estimate the associations between long-term exposures and outcomes. Then, we repeated the main analyses and estimated the associations with additional quadratic terms for the meteorological variables (summer mean temperature, winter mean temperature, relative humidity). These sensitivity analyses were conducted for each outcome. Results of the sensitivity analyses can be found in the supplementary materials.
We also conducted a regional analysis on 4 U.S. Census Regions (Midwest, Northeast, West, South) to see if the associations varied across different regions42. The interaction terms between exposures and categorical regional variables were included. We found that Blacks and diabetics had higher risks, but we know that diabetes is more prevalent among Blacks43(pp2011−2016). To ensure that the effect measure modifications we identified were not confounded by this overlap, we conducted additional sets of analysis assessing the effect measure modifications by race among the non-diabetics and the effect measure modifications by diabetes among the White racial group. To see whether the modification by race/ethnicity could be confounded by different Census regions, we also conducted analysis examining potential modification by race/ethnicity controlling for categorical regional variables and the interaction terms between exposure and regions.
Moreover, to control for the autocorrelation of exposures and outcomes, we used a generalized estimating equation with clusters at ZIP code-level throughout all the models conducted44,45. All data work and statistical analyses were run on the Rollins HPC Cluster at Emory University using R 4.0.246.
3. Results
Table 1 summarizes the demographic characteristics and exposure of the study cohorts. For each cohort we have included over 30 million Medicare enrollees with an average follow-up time of approximately 7 years. The cohorts were mainly White (over 88%) and female (over 58%), with an average age at the beginning of follow-up of approximately 73 years old. We observed 4,939,663 cases of AF, 8,234,206 cases of CHF, and 4,865,640 cases of stroke from these cohorts during the follow-up periods. The mean concentrations for the pollutants of interest were: 10.2 μg/m3 for PM2.5, 19.8 ppb for NO2, and 42.9 ppb for warm-season O3. The correlation coefficients for the three pollutants range from 0.2 to 0.4 (Table S1 in Supplementary Materials).
Table 1.
Demographic characteristics and exposure summary of study cohorts
| Characteristics | AF Cohort | CHF Cohort | Stroke Cohort |
|---|---|---|---|
|
| |||
| Follow-up Start Age, Mean (S.D.) | 73.1 (6.9) | 72.7 (6.6) | 73.1 (6.9) |
| Female Sex, n (%) | 18,977,901 (59.0) | 17,597,195 (58.4) | 18,955,470 (58.4) |
| Race/ethnicity, n (%) | |||
| White | 28,327,456 (88.1) | 26,756,246 (88.8) | 28,736,911 (88.6) |
| Black | 2,363,489 (7.4) | 2,035,968 (6.8) | 2,265,406 (7.0) |
| Asian | 398,340 (1.2) | 366,135 (1.2) | 392,072 (1.2) |
| Hispanic | 434,323 (1.6) | 377,991 (1.3) | 419,639 (1.3) |
| Native American | 111,238 (0.3) | 100,946 (0.3) | 109,894 (0.3) |
| Others | 518,409 (1.6) | 495,662 (1.6) | 520,442 (1.6) |
| Average Follow-up Years | 6.8 | 6.5 | 6.8 |
| Case Numbers | 4,939,663 | 8,234,206 | 4,865,640 |
| Total Person-years | 218,957,061 | 194,885,797 | 219,978,768 |
| PM2.5 Concentration (μg/m3), Mean (S.D.) | 10.2 (2.8) | 10.2 (2.8) | 10.2 (2.8) |
| NO2 Concentration (ppb), Mean (S.D.) | 19.8 (9.2) | 19.7 (9.1) | 19.8 (9.2) |
| Warm-season O3 Concentration (ppb), Mean (S.D.) | 42.9 (5.8) | 42.9 (5.8) | 42.9 (5.8) |
Abbreviations: AF for atrial fibrillation; CHF for congestive heart failure; S.D. for standard deviation.
Table 2 presents the main results from the full cohorts and low-pollutant-concentration-level sub-cohorts. In the full cohorts, we found that the exposures to PM2.5 and NO2 were positively associated with increased incidence of AF, CHF, and stroke. For each 1 μg/m3 increase in annual PM2.5, hazard ratios (HR) were 1.0059 (95% CI: 1.0054–1.0064), 1.0260 (95% CI: 1.0256–1.0264), and 1.0279 (95% CI: 1.0274–1.0284) for the three corresponding outcomes, respectively. For each 1 ppb increase in annual NO2, HRs were 1.0057 (95% CI: 1.0056–1.0059), 1.0112 (95% CI: 1.0110–1.0113), and 1.0095 (95% CI: 1.0093–1.0096), respectively. The exposure to warm-season O3 was only associated with increased incidence of CHF and stroke, with HRs of 1.0035 (95% CI: 1.0033–1.0037) and 1.0026 (95% CI: 1.0023–1.0028) respectively.
Table 2.
Associations between air pollution and atrial fibrillation (AF), congestive heart failure (CHF) and stroke incidence: results from the full cohorts and low-pollutant-concentration-level sub-cohorts analyses, presented as hazard ratios (HR) and 95% confidence intervals per 1 μg/m3 increase in PM2.5, 1 ppb increase in NO2, and 1 ppb increase in warm-season O3
| Pollutant | HR for AF | HR for CHF | HR for Stroke |
|---|---|---|---|
|
| |||
| PM2.5 | 1.0059 (1.0049, 1.0069) | 1.0260 (1.0245, 1.0275) | 1.0279 (1.0264, 1.0293) |
| PM2.5 (<10 μg/m3) | 1.0689 (1.0637, 1.0741) | 1.0959 (1.0891, 1.1027) | 1.1011 (1.0946, 1.1077) |
| PM2.5 (<8 μg/m3) | 1.1503 (1.1358, 1.1650) | 1.1731 (1.1564, 1.1900) | 1.1878 (1.1698, 1.2061) |
| NO2 | 1.0057 (1.0054, 1.0061) | 1.0112 (1.0106, 1.0117) | 1.0095 (1.0089, 1.0100) |
| NO2 (<25 ppb) | 1.0128 (1.0120, 1.0135) | 1.0200 (1.0189, 1.0210) | 1.0193 (1.0184, 1.0203) |
| NO2 (<18 ppb) | 1.0233 (1.0217, 1.0249) | 1.0275 (1.0254, 1.0297) | 1.0304 (1.0284, 1.0324) |
| Warm-season O3 | 0.9998 (0.9994, 1.0003) | 1.0035 (1.0028, 1.0043) | 1.0026 (1.0019, 1.0032) |
| Warm-season O3 (<50 ppb) | 1.0134 (1.0124, 1.0144) | 1.0181 (1.0168, 1.0195) | 1.0190 (1.0177, 1.0204) |
| Warm-season O3 (<40 ppb) | 1.0553 (1.0499, 1.0607) | 1.0589 (1.0533, 1.0645) | 1.0649 (1.0587, 1.0711) |
The sample size of the low-pollutant-concentration-level sub-cohorts could be found in Table S2 in Supplementary Materials. In the first sets of low-pollutant-concentration-level sub-cohorts (PM2.5<10 μg/m3, NO2<25 ppb, warm-season O3<50 ppb), the exposures were all found to be associated with increased incidence of AF, CHF, and stroke, and the HRs were higher than in the full cohorts (Table 2). Moreover, when we further restricted the sub-cohorts to a lower threshold (PM2.5<8 μg/m3, NO2<18 ppb, warm-season O3<40 ppb), the associations persisted and the HRs became even stronger than the first sets of sub-cohorts (Table 1).
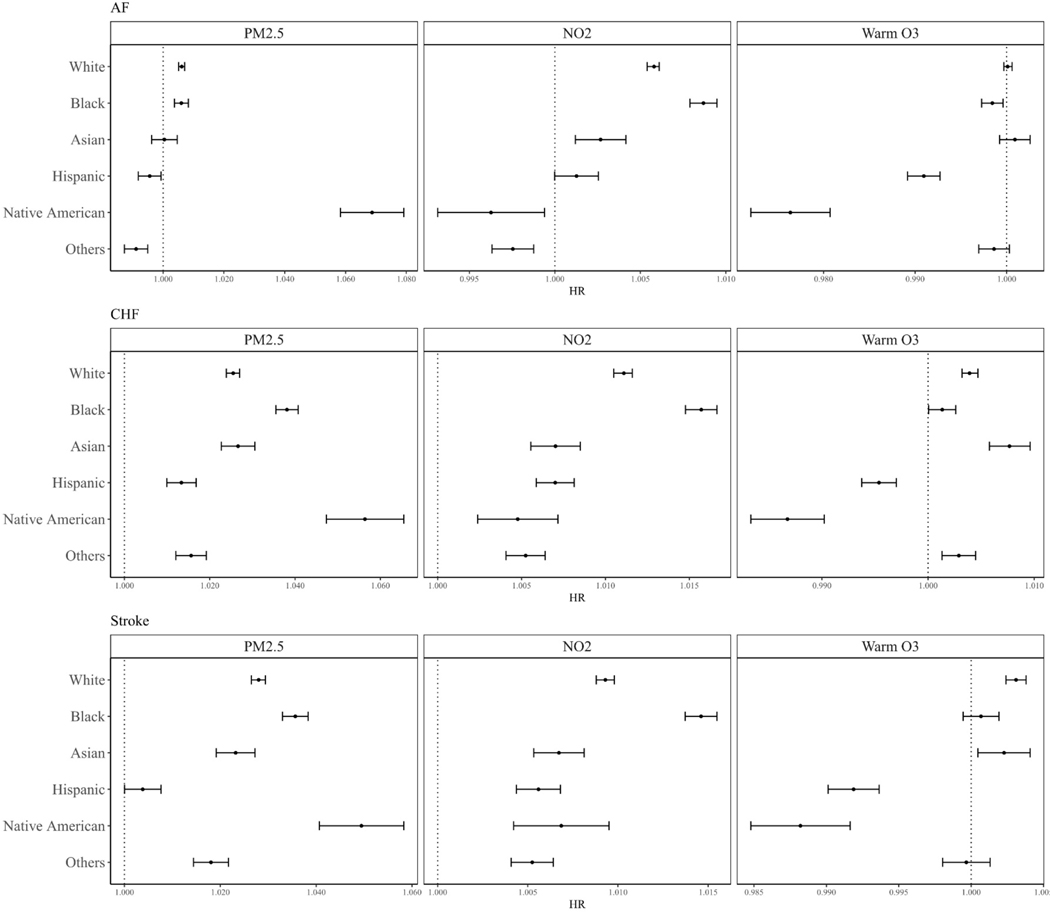
The results of potential modifications by race/ethnicity are presented in Figure 1. We found that PM2.5 and NO2 had higher HRs on all three outcomes for Black people vs. White people, while warm-season O3 had lower HRs for Black people than for White people. For Native Americans, the HRs of PM2.5 were generally larger, while for NO2 and warm-season O3, the HRs were lower than for White people. For Asians, all the HRs were lower or not significantly different compared to White people. Similar trends were also observed for Hispanic people, with all HRs were of lower magnitudes than White people.
Figure 1.
Potential effect modifications by race/ethnicity
Abbreviations: AF for atrial fibrillation; CHF for congestive heart failure.
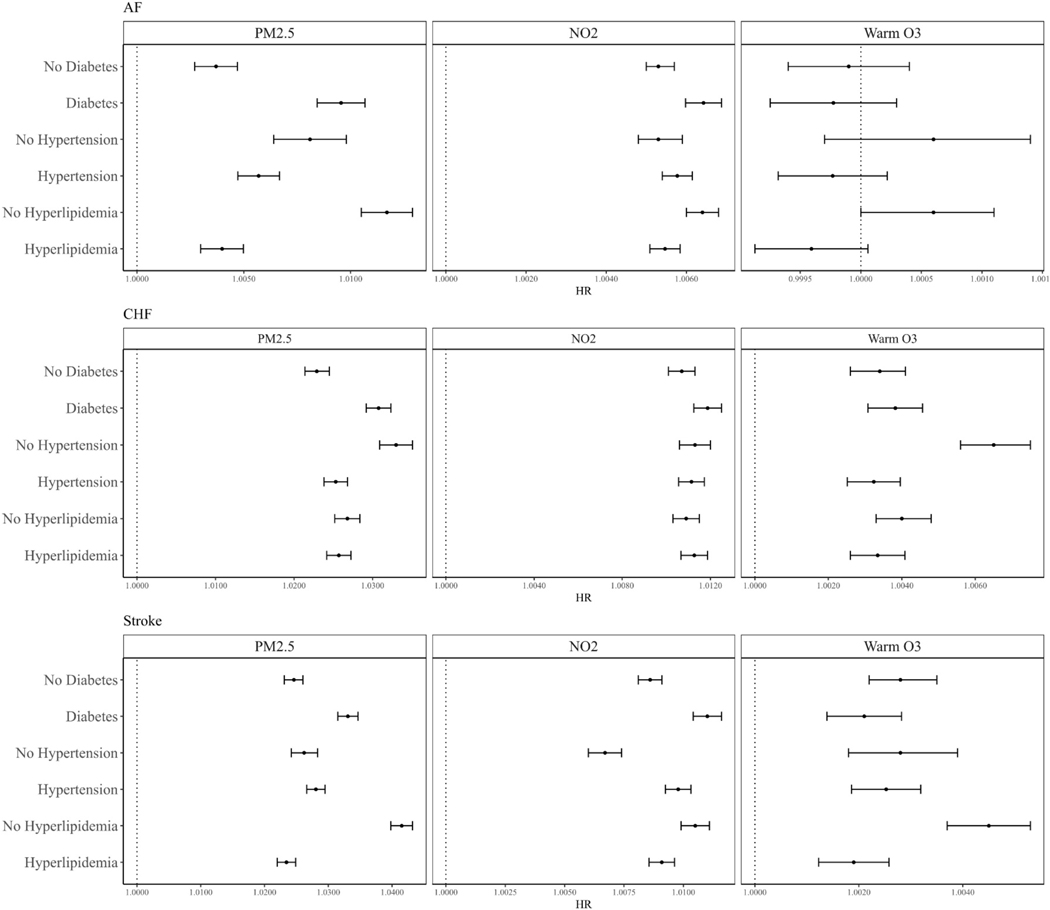
Figure 2 summarizes the estimated modification by the three pre-existing comorbidities: diabetes, hypertension, and hyperlipidemia. The HRs between PM2.5 and NO2 and all three outcomes were found to be of higher magnitudes for people with diabetes, while no clear modification pattern was observed for warm-season O3. For people with hypertension, we found a higher HR between NO2 and stroke, while all other estimates were of lower magnitudes or not significantly different compared to people without hypertension. Moreover, we found lower HRs between all exposures and all three outcomes for people with hyperlipidemia than for those without.
Figure 2.
Potential effect modifications by comorbidities (hypertension, hyperlipidemia, diabetes)
Abbreviations: AF for atrial fibrillation; CHF for congestive heart failure.
The results of the sensitivity analyses are presented in Table S3, S4, and S5 of Supplementary Materials. First, when assessing exposures as 3-year moving average of air pollutants, we still found positive associations between PM2.5 and NO2 and AF, CHF, and stroke, and positive associations between warm-season O3 and CHF and stroke (Table S3). The general pattern that the HRs of PM2.5 and NO2 were of lower magnitudes for AF and higher for CHF and stroke persisted in the results of sensitivity analyses. Second, for the three sub-cohorts with a more stringent clean period excluding anyone who had a diagnosis for the outcome in their first 3 years of follow-up, the models also yielded similar results to the main analyses (Table S4). Third, with additional quadratic terms of meteorological variables, we still observed similar association estimates with the main analyses (Table S5), which indicated that the linear terms included in the main analyses should be adequate to control the confounding by meteorological factors. In general, the results of the main analyses were robust to these three sets of sensitivity analyses we have conducted.
Results of the additional regional analysis and effect measure modification analysis are presented in Figure S1, S2, S3, and S4 of Supplementary Materials. Among the four U.S. Census Regions, people living in the West region had lower HRs associated with PM2.5 and NO2 across all three outcomes compared to people living in other three regions. For the additional effect measure modification analysis, we found that among the non-diabetics, the Black people still had higher HRs associated with PM2.5 and NO2 across all three outcomes, and among the White people, the diabetics also still had higher risks associated with PM2.5 and NO2 across all three outcomes. This indicated that the pre-existing diabetes and race/ethnicity could modify the associations without the intersectionality between these two factors. We also found that after controlling for regional variables, the general patterns of the potential modification by race/ethnicity is consistent with what we observed from the models without regional variables.
4. Discussion
In this study, we have examined the association between PM2.5, NO2, and warm-season O3 and the incidences of AF, CHF, and stroke in the Medicare population. Associations between PM2.5, NO2 and the first incidences of AF, CHF, and stroke, as well as associations between warm-season O3 and first incidences of CHF and stroke have been found from the multi-pollutant models. When we restricted the data to low pollutant concentration levels, we still found associations between all pollutants and all outcomes, and these associations were even stronger than those in the full range of exposure. We also found that these associations were modified by race/ethnicity and comorbidities, with evidence that Black people and people with pre-existing diabetes are more susceptible to PM2.5 and NO2. All the associations were observed from models controlling for the individual-level characteristics, neighborhood-level socioeconomic and demographic factors, and meteorological variables. Both summer and winter mean temperatures were included because of the U-shape relationship observed between daily temperature and human health indicated that warmer temperature is protective in the winter but harmful in the summer37.
There are several different methods to capture the incidence of non-fatal cardiovascular diseases that have been used previously. Many previous studies examining both short-term and long-term associations have used hospitalization data to capture the outcomes5,10–14,47. However, for AF and CHF, hospitalizations generally do not mark the onset of the diseases, but rather an exacerbation. Some studies try to include more records like emergency department visits, outpatient records, and physician diagnoses to better approximate the incidence of the outcomes3,6,7,16,48–51. Other incident outcome assessment methods, such as self-reported information, interviews, and hospital discharge data, have also been used17–19. However, most of these methods are still not accurate enough or the studies are conducted in non-representative cohorts not immediately generalizable to the broader population.
In our study, we have utilized the CCW database to systematically capture the incidence of the outcomes among all Medicare enrollees continuously enrolled in the FFS program and both Medicare part A and part B across the contiguous U.S. from 2000 to 2016. The CCW database utilizes algorithms incorporating claims indicating an individual received service for specific conditions. The algorithms combine all the records from inpatient, outpatient, skilled nursing facilities, home health claims, and carrier claims (primarily doctor visits)20. Compared to the hospital database used by many previous studies, this database, together with our two-year washout period, better captures the incidence of the outcomes. Compared to other cohort studies, we observed far more events, used a more generalizable population of people aged 65+, and did not rely on self-reports. We believe this is one of the largest nationwide studies that can accurately capture the first incidence of health outcomes to examine the association between air pollution and cardiovascular diseases.
Larger magnitudes of hazard ratios at lower concentrations were observed in our study, which is also common among previous studies of air pollution. For example, a meta-regression of 135 coefficients from studies linking long-term PM2.5 and mortality reported that the slope of the concentration-response curve increased continuously as the focus shifted to studies with lower concentrations52. Another recent paper also confirmed that for PM2.5 and other pollutants like NO2 and O3, similar increases at lower concentrations were reported53. Most of those deaths reported in previous studies were from cardiovascular disease, which is consistent with our findings for the CVD incidences. Opinions on why larger effect sizes were observed at lower concentrations are differing. There is one opinion that if the sensitivity of humans to air pollution were normally distributed, then the concentration-response associations would be expected to look like a cumulative normal curve, which does level off at higher exposures.
Evidence for potential modification by race/ethnicity were mixed for warm-season O3 in our study. However, for PM2.5 and NO2, we found strong evidence of effect modification for Black people with higher hazard ratios across all outcomes compared to White, despite controlling for multiple measures of socioeconomic status. This suggests that those assessed metrics do not adequately capture all the factors that make Blacks more susceptible to air pollution. For example, some studies have shown that psychosocial stress modifies the effects of air pollution54,55. Some unmeasured spatial components may also increase the vulnerability of the Black racial group. Moreover, our study also found that the associations between all three pollutants and all three outcomes were generally at a lower magnitude for Asians and Hispanics compared to White people. The reasons for the lower risks found for Asians and Hispanics are worth more explorations in the future studies. It may also be due to the psychosocial stress since the higher individual incomes for Asian and the supportive family structure for Hispanic may lead to lower stress for these groups. Moreover, the relatively small sample size of Asian (1.2%), Hispanic (1.6%), and Native American (0.3%) population suggests caution for result interpretation, especially for the Native American population whose confidence intervals were much wider than other race/ethnicity categories. Previous studies examining potential modification by race/ethnicity in associations between air pollution and cardiovascular diseases have yielded quite heterogeneous results, there was not a general consistent trend on whether a specific racial group was having higher or lower risks compared to the other groups8,56,57. Our study added to the evidence of greater susceptibility to the adverse effects of air pollution among the Black population. This also echoed the needs for the focus on environmental justice since we found that Blacks are more susceptible to the potential harmful effects from environmental pollutions. With literature indicating that people of color in the U.S. are exposed to higher levels of air pollution, this could be a double disadvantage for the Black racial group who have higher exposure levels and are more likely to be harmed by air pollution58.
In addition to race/ethnicity, we also examined the potential modifications by pre-existing comorbidities including hypertension, hyperlipidemia, and diabetes. For the interaction terms of these comorbidities, we found strong evidence that people with diabetes were more susceptible to air pollution than people without diabetes. However, for people with hypertension, the results were mixed across different air pollutants and outcomes, and for people with hyperlipidemia, there was strong evidence suggesting that they are less susceptible than people without hyperlipidemia. One possible reason for the “protective effects” of hyperlipidemia may be that the medications (particularly statins) used to treat hyperlipidemia could block the association between air pollution and cardiovascular diseases59,60. Several mechanisms have been proposed on how air pollution could induce cardiovascular effects. One mechanism is through air pollution-induced inflammation, and there are already many studies demonstrating that air pollutants are associated with an increased inflammatory response56,61–63. HMG-CoA reductase inhibitors, also known as statins, are a class of medications widely used in the treatment of hyperlipidemia, and have been found to inhibit inflammatory responses and demonstrate a protective effect against air pollution64,65. There is epidemiological evidence suggesting that people taking statins may be less susceptible to air pollution than people not taking statins62,63. Hence, participants with hyperlipidemia in our study may be protected against air pollution by way of treatment. Some previous studies have also examined the potential modifications by diabetes, hypertension, and hyperlipidemia of the associations between air pollution and cardiovascular diseases in both short-term and long-term time scales. Diabetes and hypertension have been studied in greater detail, with mixed findings reporting higher risks, lower risks, and also no signs of significant interactions for people with comorbidities across different air pollutants and cardiovascular events6,7,12–14,16,18,49,66. Hyperlipidemia as a modifier has been less studied previously. One case-crossover study on AF hospitalizations reported that patients with hyperlipidemia may be more susceptible to short-term exposure to PM 12. Unfortunately, there is no medication records in the Medicare denominator file and CCW data we have, so we are unable to conduct further analysis on this possible explanation, and we believe it is a potential research direction worth more exploration in the future.
Several previous studies have reported evidence of associations between air pollution and cardiovascular diseases. For the Medicare population, there are other studies reporting significant associations between PM2.5, NO2, and O3 and stroke and heart failure4,9. A pooled analysis based on the ELAPSE (Effects of Low-Level Air Pollution: A Study in Europe) project reported significant associations between annual average PM2.5 and NO2 and stroke, but null association for warm-season O3 19. A separate study based on the Danish Nurse Cohort reported a positive but non-significant association between 1-year average PM2.5 and AF and a significant association between 3-year average PM2.5 and AF, with null findings for NO2 50. There are also two cohort studies conducted in Ontario, Canada reporting significant associations between the long-term exposures to all three air pollutants (PM2.5, NO2, O3) and all three outcomes of interests3,16. Furthermore, some other studies have reported mixed results5,7,18,48,66. Our study is generally consistent with findings of associations for all air pollutants and outcomes except for O3, which is also more heterogeneous in previous studies. Moreover, our findings of consistent and even stronger associations between air pollution and cardiovascular diseases at lower pollutant concentration levels are also consistent with several previous studies3,8,17,19. However, heterogeneity still exists in the shape of associations at low pollutant concentration levels in many other studies3,5,16. Our study provides evidence that there is no sign of a threshold of air pollution’s health effects and that there are greater marginal benefits to further reducing air pollution.
Besides the traditional air pollutants being examined by previous literature, some studies also noted that noise could be a potential important confounder for the health-air pollution relationship, and noise itself could also become an exposure of interest. Based on Danish Nurse Cohort, there are two studies suggesting that noise could be an important co-pollutant with air pollution related to mortality and stroke incidence, and they also reported similar direction of effect modifications for diabetics and no effect modification for persons with hypertension for road traffic noise, which is typically highly correlated with pollutants from traffic emissions such as NO267,68. We think these findings are somewhat supportive of our results. In our study, we did not have noise as an exposure, and this is a limitation of our study. However, the exploration of the role of noise in the associations between air pollution and cardiovascular diseases incidence could be a potential research direction in the future.
Our study examined the associations between PM2.5, NO2, warm-season O3 and incident AF, CHF, and stroke in tri-pollutant models. As one of the largest studies examining the associations between air pollution and cardiovascular diseases, our study incorporated nearly 20 million participants with hundreds of millions of follow-up years and millions of incidence cases for analyses. This large population-based cohort across the whole contiguous U.S. provided sufficient statistical power, even for the subgroup and effect measure modification analyses, which is a main advantage of this study compared to previously published research. Another important advantage of our study is the use of CCW database to better capture the first incidence of the outcomes. This is a huge improvement compared to previous studies using hospitalization records, since the CCW database has included more medical records rather than only the hospitalizations. Many incidences occur well before the hospitalizations, and many incidences do not result in hospitalization. Therefore, with more medical records covered by CCW database, we could better capture the actual incidence rather than the exacerbation of the outcomes. Moreover, our study has also examined the associations at low pollutant concentration levels and the potential modifications by race/ethnicity and comorbidities. These analyses provided us more thorough understanding of the associations between air pollution and cardiovascular disease.
Our study also has some limitations. First, we assumed a linear relationship between the exposures, covariates, and the outcome, which may result in some residual confounding, especially for the associations between exposures and outcomes. In our study, we do see steeper slopes at lower concentrations, which is indicative of non-linearity. More detailed examinations of the concentration-response curves should be examined in future research. Second, although we used high-resolution air pollution spatiotemporal models with strong validation metrics, there is still potential for measurement error in our analysis from lack of indoor air pollution measurements, commuting across different places, lack of detailed residential and activity diaries, and the residual prediction error of exposure models. However, we would expect most of these errors to be non-differential. We also think commuting and workplace exposures are of less concern in a population that is mostly retired. Further, with the National Human Activity Pattern Survey in the U.S. reporting that U.S. adults spent 69% of their time at home and 8% of the time immediately outside their home69, it is reasonable to use the residential air pollution to capture the exposure. However, the lack of indoor air pollution measurement may be a concern, since there is study reporting different effect sizes adjusting for time spent indoor70. We believe this could be a worthwhile potential research direction with more adequate data on activity diaries and time spending indoor. Third, the usage of concurrent year concentrations as exposure metrics may also introduce some exposure measurement errors because in the years of diseases incidence or censoring, these exposure metrics may cover some periods of the time that the participants didn’t actually experience. However, our sensitivity analysis using 3-year average exposure produced similar results.
5. Conclusions
We found positive associations of long-term exposure to PM2.5 and NO2 with atrial fibrillation, congestive heart failure, and stroke, and positive associations of warm-season O3 with congestive heart failure and stroke. These associations were consistent and even stronger when we restricted to pollutants concentrations levels below U.S. and EU standards, which suggests no sign of threshold effects and greater marginal benefits to further reduce air pollution. In particular, we observe strong associations when restricted to PM2.5 concentrations below those the U.S. EPA is considering for revised standards. Moreover, we also found that some of these associations could be modified by race/ethnicity and comorbidities. Greater susceptibilities to PM2.5 and NO2 were observed for Black people and people with diabetes, indicating potential vulnerable populations.
Supplementary Material
A nationwide Medicare cohort study was conducted across the contiguous U.S.
Associations were examined using co-pollutant models and at low pollution levels
Potential effect modifications by race/ethnicity and comorbidities were assessed
Air pollution is found associated with incidence of CVD even at low concentration
People who identify as Black or have diabetes are more susceptible to air pollution
Acknowledgement
We want to specially thank the Centers for Medicare & Medicaid Services for giving us access to the Medicare claims to conduct this study.
Funding:
This study was supported by the United States Environmental Protection Agency (grant number RD-83587201 received by JS, https://www.epa.gov/) and the National Institutes of Health (grant number R01 ES032418 received by JS, R01 AG074357 received by LS, R21 ES032606 received by LS, https://www.nih.gov/). The contents of this publication are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
Footnotes
Declaration of Competing Interests
I have read the journal’s policy and the authors of this manuscript have the following competing interests: Joel Schwartz has been an expert witness for the US Department of Justice in cases involving Clean Air Act violations.
Tingfan Jin: Methodology, Formal Analysis, Data Curation, Writing – Original Draft, Writing – Review & Editing; Qian Di: Data Curation, Writing – Review & Editing; Weeberb J. Réquia: Data Curation, Writing – Review & Editing; Mahdieh Danesh Yazdi: Data Curation, Writing – Review & Editing; Edgar Castro: Data Curation, Writing – Review & Editing; Tszshan Ma: Data Curation, Writing – Review & Editing; Yifan Wang: Data Curation, Writing – Review & Editing; Haisu Zhang: Data Curation, Writing – Review & Editing; Liuhua Shi: Data Curation, Writing – Review & Editing, Funding Acquisition; Joel Schwartz: Conceptualization
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability
The rules governing the Medicare dataset prohibit any sharing by us of the health datasets being used for our epidemiologic research. Restricted by our Data Use Agreement with the U.S. Centers for Medicare & Medicaid Services, the Medicare data that support the findings of this study are neither sharable nor publicly available from us. However, Academic and non-profit researchers who are interested in using Medicare data should contact the US Centers for Medicare & Medicaid Services directly to obtain their own datasets upon completion of a Data Use Agreement. The air pollution data used in this study are publicly available on NASA’s SEDAC website.
References
- 1.Heart Disease and Stroke | CDC. Published March 7, 2022. Accessed May 9, 2022. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/heart-disease-stroke.htm
- 2.WOLF PA, ABBOTT RD, KANNEL WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke (1970). 1991;22(8):983–988. doi: 10.1161/01.STR.22.8.983 [DOI] [PubMed] [Google Scholar]
- 3.Shin S, Burnett RT, Kwong JC, et al. Ambient Air Pollution and the Risk of Atrial Fibrillation and Stroke: A Population-Based Cohort Study. Environmental health perspectives. 2019;127(8):87009–87009. doi: 10.1289/EHP4883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danesh Yazdi M, Wang Y, Di Q, Zanobetti A, Schwartz J. Long-term exposure to PM2.5 and ozone and hospital admissions of Medicare participants in the Southeast USA. Environment international. 2019;130:104879-104879. doi: 10.1016/j.envint.2019.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olaniyan T, Pinault L, Li C, et al. Ambient air pollution and the risk of acute myocardial infarction and stroke: A national cohort study. Environmental research. 2022;204(Pt A):111975–111975. doi: 10.1016/j.envres.2021.111975 [DOI] [PubMed] [Google Scholar]
- 6.Lim YH, Jorgensen JT, So R, et al. Long-Term Exposure to Air Pollution, Road Traffic Noise, and Heart Failure Incidence: The Danish Nurse Cohort. Journal of the American Heart Association. 2021;10(20):e021436-e021436. doi: 10.1161/JAHA.121.021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim IS, Yang PS, Lee J, et al. Long-term exposure of fine particulate matter air pollution and incident atrial fibrillation in the general population: A nationwide cohort study. International journal of cardiology. 2019;283:178–183. doi: 10.1016/j.ijcard.2018.12.048 [DOI] [PubMed] [Google Scholar]
- 8.Danesh Yazdi M, Wang Y, Di Q, et al. Long-Term Association of Air Pollution and Hospital Admissions Among Medicare Participants Using a Doubly Robust Additive Model. Circulation (New York, NY). 2021;143(16):1584–1596. doi: 10.1161/CIRCULATIONAHA.120.050252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma T, Yazdi MD, Schwartz J, et al. Long-term air pollution exposure and incident stroke in American older adults: A national cohort study. Global Epidemiology. 2022;4:100073-. doi: 10.1016/j.gloepi.2022.100073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang C, Chen A, Chen R, et al. Acute effect of ambient air pollution on heart failure in Guangzhou, China. International journal of cardiology. 2014;177(2):436–441. doi: 10.1016/j.ijcard.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 11.Saifipour A, Azhari A, Pourmoghaddas A, et al. Association between ambient air pollution and hospitalization caused by atrial fibrillation. ARYA atherosclerosis. 2019;15(3):106–112. doi: 10.22122/arya.v15i3.1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HH, Pan SC, Chen BY, Lo SH, Guo YL. Atrial fibrillation hospitalization is associated with exposure to fine particulate air pollutants. Environmental health. 2019;18(1):117–117. doi: 10.1186/s12940-019-0554-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, Liu X, Wang X, Qu W, Li W, Dong L. Effect of air pollution on hospitalization for acute exacerbation of chronic obstructive pulmonary disease, stroke, and myocardial infarction. Environmental science and pollution research international. 2019;27(3):3384–3400. doi: 10.1007/s11356-019-07236-x [DOI] [PubMed] [Google Scholar]
- 14.LiH, TiaY, SonJ, et al. Effect of Ambient Air Pollution on Hospitalization for Heart Failure in 26 of China’s Largest Cities. The American journal of cardiology. 2018;121(5):628–633. doi: 10.1016/j.amjcard.2017.11.039 [DOI] [PubMed] [Google Scholar]
- 15.Shah AS, Langrish JP, Nair H, et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. The Lancet (British edition). 2013;382(9897):1039–1048. doi: 10.1016/S0140-6736(13)60898-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai L, Shin S, Burnett RT, et al. Exposure to ambient air pollution and the incidence of congestive heart failure and acute myocardial infarction: A population-based study of 5.1 million Canadian adults living in Ontario. Environment international. 2019;132:105004–105004. doi: 10.1016/j.envint.2019.105004 [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Zhou T, Song Y, et al. Joint exposure to various ambient air pollutants and incident heart failure: a prospective analysis in UK Biobank. European heart journal. 2021;42(16):1582–1591. doi: 10.1093/eurheartj/ehaa1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stafoggia M, Cesaroni G, Peters A, et al. Long-Term Exposure to Ambient Air Pollution and Incidence of Cerebrovascular Events: Results from 11 European Cohorts within the ESCAPE Project. Environmental health perspectives. 2014;122(9):919–925. doi: 10.1289/ehp.1307301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf K, Hoffmann B, Andersen ZJ, et al. Long-term exposure to low-level ambient air pollution and incidence of stroke and coronary heart disease: a pooled analysis of six European cohorts within the ELAPSE project. The Lancet Planetary health. 2021;5(9):e620–e632. doi: 10.1016/S2542-5196(21)00195-9 [DOI] [PubMed] [Google Scholar]
- 20.Categories Condition. Chronic Conditions Data Warehouse. Accessed June 10, 2022. https://www2.ccwdata.org/condition-categories
- 21.Di Q, Wei Y, Shtein A, et al. Daily and Annual PM2.5 Concentrations for the Contiguous United States, 1- km Grids, v1 (2000 – 2016). Published online 2021. 10.7927/0rvr-4538 [DOI]
- 22.Di Q, Wei Y, Shtein A, et al. Daily and Annual NO2 Concentrations for the Contiguous United States, 1-km Grids, v1 (2000 – 2016). Published online 2022. 10.7927/f8eh-5864 [DOI]
- 23.Requia WJ, Wei Y, Shtein A, et al. Daily 8-Hour Maximum and Annual O3 Concentrations for the Contiguous United States, 1-km Grids, v1 (2000 – 2016). Published online 2021. 10.7927/a4mb-4t86 [DOI]
- 24.Requia WJ, Di Q, Silvern R, et al. An Ensemble Learning Approach for Estimating High Spatiotemporal Resolution of Ground-Level Ozone in the Contiguous United States. Environmental science & technology. 2020;54(18):11037–11047. doi: 10.1021/acs.est.0c01791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Q, Amini H, Shi L, et al. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environment international. 2019;130:104909–104909. doi: 10.1016/j.envint.2019.104909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Q, Amini H, Shi L, et al. Assessing NO2 Concentration and Model Uncertainty with High Spatiotemporal Resolution across the Contiguous United States Using Ensemble Model Averaging. Environmental science & technology. 2020;54(3):1372–1384. doi: 10.1021/acs.est.9b03358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Wei Y, Fang Z. Ozone Pollution: A Major Health Hazard Worldwide. Frontiers in immunology. 2019;10:2518–2518. doi: 10.3389/fimmu.2019.02518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robichaud A, Menard R. Multi-year objective analyses of warm season ground-level ozone and PM2.5 over North America using real-time observations and Canadian operational air quality models. Atmospheric chemistry and physics. 2014;14(4):1769–1800. doi: 10.5194/acp-14-1769-2014 [DOI] [Google Scholar]
- 29.Shi L, Rosenberg A, Wang Y, et al. Low-Concentration Air Pollution and Mortality in American Older Adults: A National Cohort Analysis (2001–2017). Environmental science & technology. 2022;56(11):7194–7202. doi: 10.1021/acs.est.1c03653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi L, Steenland K, Li H, et al. A national cohort study (2000–2018) of long-term air pollution exposure and incident dementia in older adults in the United States. Nature communications. 2021;12(1):6754–6754. doi: 10.1038/s41467-021-27049-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Center for International Earth Science Information Network - CIESIN - Columbia University. Gridded Population of the World, Version 4 (GPWv4): Population Density, Revision 11. Published online 2018. 10.7927/H49C6VHW [DOI]
- 32.Manson S, Schroeder J, Van Riper D, Kugler T, Ruggles S. IPUMS National Historical Geographic Information System: Version 16.0 [dataset]. Published online 2021. 10.18128/D050.V16.0 [DOI] [Google Scholar]
- 33.The Center for the Evaluative Clinical Sciences DMS. The Dartmouth Atlas of Health Care. Chicago, Ill. : American Hospital Publishing, [1996] ©1996; 1996. https://search.library.wisc.edu/catalog/999781019802121 [Google Scholar]
- 34.Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Data. Published online 2018. [Google Scholar]
- 35.Abatzoglou JT. Development of gridded surface meteorological data for ecological applications and modelling. International journal of climatology. 2013;33(1):121–131. doi: 10.1002/joc.3413 [DOI] [Google Scholar]
- 36.Thornton MM, Shrestha R, Wei Y, Thornton PE, Kao S, Wilson BE. Daymet: Daily Surface Weather Data on a 1-km Grid for North America, Version 4. Published online 2020. doi: 10.3334/ORNLDAAC/1840 [DOI] [Google Scholar]
- 37.Curriero FC, Heiner KS, Samet JM, Zeger SL, Strug L, Patz JA. Temperature and Mortality in 11 Cities of the Eastern United States. American journal of epidemiology. 2002;155(1):80–87. doi: 10.1093/aje/155.1.80 [DOI] [PubMed] [Google Scholar]
- 38.Frey HC, Adams PJ, Adgate JL, et al. The Need for a Tighter Particulate-Matter Air-Quality Standard. The New England journal of medicine. 2020;383(7):680–683. doi: 10.1056/NEJMsb2011009 [DOI] [PubMed] [Google Scholar]
- 39.US EPA O. NAAQS Table. Published April 10, 2014. Accessed September 12, 2022. https://www.epa.gov/criteria-air-pollutants/naaqs-table
- 40.What are the WHO Air quality guidelines? Accessed September 12, 2022. https://www.who.int/news-room/feature-stories/detail/what-are-the-who-air-quality-guidelines
- 41.Air quality standards — European Environment Agency. Accessed September 12, 2022. https://www.eea.europa.eu/themes/air/air-quality-concentrations/air-quality-standards
- 42.United States Bureau of the Census. Geographic Areas Reference Manual. U.S. Dept. of Commerce, Economics and Statistics Administration, Bureau of the Census; 1994. [Google Scholar]
- 43.Cheng YJ, Kanaya AM, Araneta MRG, et al. Prevalence of Diabetes by Race and Ethnicity in the United States, 2011–2016. JAMA. 2019;322(24):2389–2398. doi: 10.1001/jama.2019.19365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Q, Wang Y, Zanobetti A, et al. Air Pollution and Mortality in the Medicare Population. The New England journal of medicine. 2017;376(26):2513–2522. doi: 10.1056/NEJMoa1702747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee EW, Wei LJ, Amato DA, Leurgans S. Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations. In: Survival Analysis: State of the Art. Nato Science. Springer; Netherlands; :237–247. doi: 10.1007/978-94-015-7983-4_14 [DOI] [Google Scholar]
- 46.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. https://www.R-project.org/ [Google Scholar]
- 47.de Aguiar Pontes Pamplona Y, Arbex MA, Braga ALF, Pereira LAA, Martins LC. Relationship between air pollution and hospitalizations for congestive heart failure in elderly people in the city of São Paulo. Environmental science and pollution research international. 2020;27(15):18208–18220. doi: 10.1007/s11356-020-08216-2 [DOI] [PubMed] [Google Scholar]
- 48.Kwon OK, Kim SH, Kang SH, et al. Association of short- and long-term exposure to air pollution with atrial fibrillation. European journal of preventive cardiology. 2019;26(11):1208–1216. doi: 10.1177/2047487319835984 [DOI] [PubMed] [Google Scholar]
- 49.Amini H, Dehlendorff C, Lim YH, et al. Long-term exposure to air pollution and stroke incidence: A Danish Nurse cohort study. Environment international. 2020;142:105891–105891. doi: 10.1016/j.envint.2020.105891 [DOI] [PubMed] [Google Scholar]
- 50.Andersen ZJ, Cramer J, Jorgensen JT, et al. Long-Term Exposure to Road Traffic Noise and Air Pollution, and Incident Atrial Fibrillation in the Danish Nurse Cohort. Environmental health perspectives. 2021;129(8):87002–87002. doi: 10.1289/EHP8090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.HarE, HohenseC, LadeF, et al. Long-Term Exposures to Air Pollution and the Risk of Atrial Fibrillation in the Women’s Health Initiative Cohort. Environmental health perspectives. 2021;129(9). doi: 10.1289/EHP7683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vodonos A, Awad YA, Schwartz J. The concentration-response between long-term PM2.5 exposure and mortality; A meta-regression approach. Environmental research. 2018;166:677–689. doi: 10.1016/j.envres.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 53.Wei Y, Wang Y, Wu X, et al. Causal Effects of Air Pollution on Mortality Rate in Massachusetts. American journal of epidemiology. 2020;189(11):1316–1323. doi: 10.1093/aje/kwaa098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clougherty JE, Levy JI, Kubzansky LD, et al. Synergistic Effects of Traffic-Related Air Pollution and Exposure to Violence on Urban Asthma Etiology. Environmental health perspectives. 2007;115(8):1140–1146. doi: 10.1289/ehp.9863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiu YHM, Coull BA, Sternthal MJ, et al. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. Journal of allergy and clinical immunology. 2013;133(3):713–722.e4. doi: 10.1016/j.jaci.2013.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamanaka RB, Mutlu GM. Particulate Matter Air Pollution: Effects on the Cardiovascular System. Frontiers in endocrinology (Lausanne). 2018;9:680–680. doi: 10.3389/fendo.2018.00680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erqou S, Clougherty JE, Olafiranye O, et al. Particulate Matter Air Pollution and Racial Differences in Cardiovascular Disease Risk. Arteriosclerosis, thrombosis, and vascular biology. 2018;38(4):935–942. doi: 10.1161/ATVBAHA.117.310305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tessum CW, Paolella DA, Chambliss SE, Apte JS, Hill JD, Marshall JD. PM2.5 polluters disproportionately and systemically affect people of color in the United States. Science advances. 2021;7(18). doi: 10.1126/sciadv.abf4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz J, Park SK, O’Neill MS, et al. Glutathione-S-Transferase M1, Obesity, Statins, and Autonomic Effects of Particles: Gene-by-Drug-by-Environment Interaction. American journal of respiratory and critical care medicine. 2005;172(12):1529–1533. doi: 10.1164/rccm.200412-1698OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeka A, Sullivan JR, Vokonas PS, Sparrow D, Schwartz J. Inflammatory markers and particulate air pollution: characterizing the pathway to disease. International journal of epidemiology. 2006;35(5):1347–1354. doi: 10.1093/ije/dyl132 [DOI] [PubMed] [Google Scholar]
- 61.Mannucci PM, Harari S, Franchini M. Novel evidence for a greater burden of ambient air pollution on cardiovascular disease. Haematologica (Roma). 2019;104(12):2349–2357. doi: 10.3324/haematol.2019.225086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Neill MS, Veves A, Sarnat JA, et al. Air pollution and inflammation in type 2 diabetes: a mechanism for susceptibility. Occupational and environmental medicine (London, England). 2007;64(6):373–379. doi: 10.1136/oem.2006.030023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ostro B, Malig B, Broadwin R, et al. Chronic PM2.5 exposure and inflammation: Determining sensitive subgroups in mid-life women. Environmental research. 2014;132:168–175. doi: 10.1016/j.envres.2014.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tasat DR, Yakisich JS. Expanding the pleiotropic effects of statins: attenuation of air pollution-induced inflammatory response. American journal of physiology Lung cellular and molecular physiology. 2012;303(8):L640–L641. doi: 10.1152/ajplung.00280.2012 [DOI] [PubMed] [Google Scholar]
- 65.Ferraro SA, Yakisich JS, Gallo FT, Tasat DR. Simvastatin pretreatment prevents ambient particle-induced lung injury in mice. Inhalation toxicology. 2011;23(14):889–896. doi: 10.3109/08958378.2011.623195 [DOI] [PubMed] [Google Scholar]
- 66.Yue C, Yang F, Li F, Chen Y. Association between air pollutants and atrial fibrillation in general population: A systematic review and meta-analysis. Ecotoxicology and environmental safety. 2021;208:111508– 111508. doi: 10.1016/j.ecoenv.2020.111508 [DOI] [PubMed] [Google Scholar]
- 67.Cole-Hunter T, Dehlendorff C, Amini H, et al. Long-term exposure to road traffic noise and stroke incidence: a Danish Nurse Cohort study. Environmental health. 2021;20(1):115-115. doi: 10.1186/s12940-021-00802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cole-Hunter T, So R, Amini H, et al. Long-term exposure to road traffic noise and all-cause and cause-specific mortality: a Danish Nurse Cohort study. The Science of the total environment. 2022;820:153057–153057. doi: 10.1016/j.scitotenv.2022.153057 [DOI] [PubMed] [Google Scholar]
- 69.Klepeis NE, Nelson WC, Ott WR, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. Journal of exposure analysis and environmental epidemiology. 2001;11(3):231–252. doi: 10.1038/sj.jea.7500165 [DOI] [PubMed] [Google Scholar]
- 70.Cole-Hunter T, de Nazelle A, Donaire-Gonzalez D, et al. Estimated effects of air pollution and space-time- activity on cardiopulmonary outcomes in healthy adults: A repeated measures study. Environment international. 2018;111:247–259. doi: 10.1016/j.envint.2017.11.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The rules governing the Medicare dataset prohibit any sharing by us of the health datasets being used for our epidemiologic research. Restricted by our Data Use Agreement with the U.S. Centers for Medicare & Medicaid Services, the Medicare data that support the findings of this study are neither sharable nor publicly available from us. However, Academic and non-profit researchers who are interested in using Medicare data should contact the US Centers for Medicare & Medicaid Services directly to obtain their own datasets upon completion of a Data Use Agreement. The air pollution data used in this study are publicly available on NASA’s SEDAC website.