Abstract
Cancer is a heterogeneous disease with multifaceted drug resistance mechanisms (e.g., tumour microenvironment [TME], tumour heterogeneity, and immune evasion). Natural products are interesting repository of bioactive molecules, especially those with anticancer activities. Prodigiosin, a red pigment produced by Serratia marcescens, possesses inherent anticancer characteristics, showing interesting antitumour activities in different cancers (e.g., breast, gastric) with low or without harmful effects on normal cells. The present review discusses the potential role of prodigiosin in modulating and reprogramming the metabolism of the various immune cells in the TME, such as T and B lymphocytes, tumour-associated macrophages (TAMs), natural killer (NK) cells, and tumour-associated dendritic cells (TADCs), and myeloid-derived suppressor cells (MDSCs) which in turn might introduce as an immunomodulator in cancer therapy.
Keywords: Prodigiosin, TME, NK Cells, T Cells, B Cells, TAMs, TADCs, MDSCs
Introduction
Cancer is the leading cause of death in 135 countries according to the World Health Organisation (WHO) global health estimates in 2019 [1]. The Global Burden of Cancer (GLOBOCAN) also reported ~ 19 million new cancer cases in 2020 that are anticipated to increase by 47% (~ 28 million) in 2040 [2]. Understanding tumour biology has facilitated the development of targeted therapies; however, tumours display multidrug resistance (MDR) as a significant clinical burden due to heterogeneity [3–6]. Natural bioactive compounds from various sources (e.g., plants, microbes) have emerged as immunomodulators in diseases, such as diabetes, cardiovascular diseases (CVDs), inflammation, and cancer [7–9].
Research deems the use of natural compounds as ‘immunomodulators’ alongside the advanced understanding of the complex interactions between cancer and the immune system [10]. Immunomodulators boost the immune defences against threats (e.g., infections) or quench the abnormal immune response in immune-related disorders [11]. Natural compounds are proven to affect immune cells and to enhance anticancer immune responses in vitro and in patients. For example, berries—which contain multiple chemopreventive compounds—enhance the function of natural killer (NK) cells and decrease the number of infiltrating neutrophils in colorectal cancer (CRC) [12–14]. Epigallocatechin gallate (EGCG), resveratrol, all-trans retinoic acid (ATRA), curcumin, polysaccharide K (PSK), β-glucans, and carotenoids are also immunomodulators (e.g., elevate NK cells and inhibit myeloid-derived suppressor cells [MDSCs]) [15–19]. Notably, bacteria-based cancer immunotherapy has lured attention thanks to its distinctive and ample components, mechanisms, and benefits to stimulate the host immunity against cancer [20].
Prodigiosin is a secondary metabolite anticancer red pigment that belongs to the “prodiginines” family, and is produced by the Gram-negative bacteria Serratia marcescens (Fig. 1) [21]. It inhibits the mammalian target of rapamycin (mTOR) pathway and angiogenesis, and induces cycle arrest and apoptosis in cancer cells with minimal or without observed cytotoxicity on healthy cells [22]. Inherent toxicity is one of the major issues with immunosuppressants that prompted researchers to use combined regimens, especially in oncology. Prodigiosin offers interesting possibilities for a combinatorial applications, acting synergistically with cyclosporin A and additively with rapamycin, confirming its distinctiveness and the potential for further development as immunosuppressants [23–25]. Of note, prodigiosin analogues have demonstrated a good safety profile without genotoxicity in clinical trials for the treatment of chronic lymphocytic leukaemia (CLL) [26]. Although prodigiosin is a well-established anticancer molecule (Table 1), its immunomodulating and metabolic reprogramming activities were not studied─data are only available for a related compound, prodigiosin 25-C [27–29]. Therefore, the current review discusses a compendium of possible immunomodulating and metabolic reprogramming activities of prodigiosin on specific immune cells and their cytokines in cancer. The present review also provides a comprehensive list of target biomarkers for prodigiosin in the tumour microenvironment (TME) (Fig. 2) [30–39].
Fig. 1.
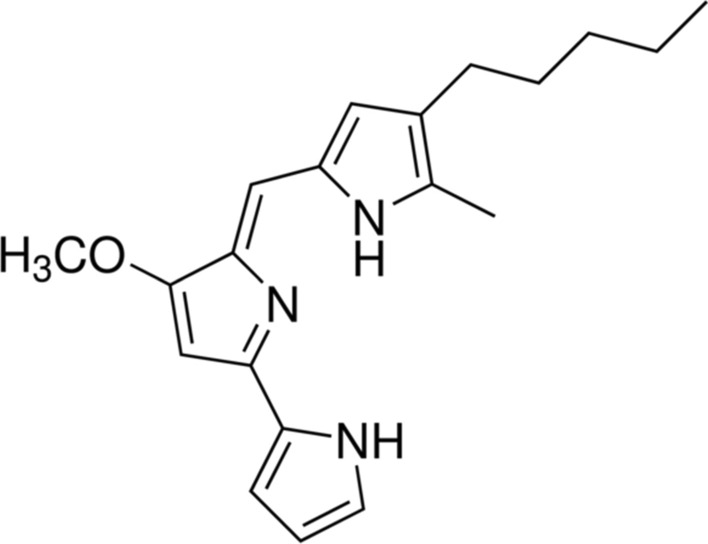
Structure of prodigiosin (2-methyl-3-pentyl-6-methoxyprodiginine)
Table 1.
Evidence-based anticancer effects of prodigiosin
| Cancer(s) type | Study model(s) | Anticancer effect(s) | Reference(s) |
|---|---|---|---|
| Ovarian | A2780RCIS (MRP1, 2 overexpressing cell line) |
↓ Cell viability Acts regardless of the BCRP, MDR1, or MRP transporter |
[40] |
| Gastric |
EPG85-257RNOV EPG85-257RDB |
||
| HGT-1 cell line |
↑ Apoptosis ↓ Cell viability Cell shrinkage Cell detachment from the culture substrate |
[41] | |
| Glioblastoma |
U87MG cell line GBM8401 cell line |
Stimulates stress markers of ER (e.g., BiP/GRP78, CHOP, and sXBP1) ↑ Autophagic cell death Activates the JNK pathway ↓ Decreasing the AKT/mTOR pathway ↑ Caspase 3 levels ↑ PARP cleavage ↑ LC3-II/LC3-I ↑ Bax/β-Actin ratio ↓ p62 |
[42] |
| Neuroblastoma |
LAN-1 IMR-2 SK-N-AS SH-SY5Y |
Uncouples the protons of the ETC to mitochondrial ATP synthase ↓ ATP production |
[43] |
| Colorectal cancer (CRC) | HT-29 cell line |
↓ G2/M ↑ Blockage in the G1 phase ↓ Number of viable cells ↓ Survivin mRNA levels ↑ Caspase 3 levels ↑ Bax mRNA levels ↑ Bad mRNA levels ↑ P53 protein levels ↓ Bcl-2 mRNA levels |
[44–46] |
|
DLD-1 cells SW-620 cells |
↑ Apoptosis ↑ Caspases levels Acts irrespective of p53 status (mutant or absent) PARP cleavage |
[47] | |
| DLD-1 cells |
↑ P53 protein levels ↑ Apoptosis ↑ Lysosomal pH |
[46] | |
| WiDr cells | ↑ Anticancer activity | [48] | |
|
NRK normal cells Swiss-3T3 normal cells |
No significant decrease in viable cells No apoptosis No toxicity |
[47] | |
|
HCT116 cells SW480 cells HT-29 cells N87 cells AGS cells LoVo cells Nude BALB/c male mice |
Accumulation of LC3B-II and SQSTM ↓ Lysosomal activity by accumulating EGFP-LC3 puncta Triggers autophagy ↑ LC3-II/LC3-I ↑ Caspase 3 levels ↑ in-vitro sensitivity to 5-FU ↑ in-vivo 5-FU efficacy |
[49] | |
|
SW480 cells HCT116 cells DLD1 cells Athymic nu/nu mice |
Restores P53 Activates P73 Prevents formation of colonosphere irrespective of p53 ↓ Viability of self-renewing 5-fluorouracil-resistant Aldefluor( +) CRCSCs ↓ Growth of xenograft tumours initiated with Aldefluor( +) cells without toxic effects and limits their tumourigenesis Activates a p53-responsive luciferase reporter in colonospheres ↓ Levels of the oncogenic N-terminally truncated isoform ΔNp73 in Aldefluor( +) cells ↑ Levels of the transcription factor c-Jun |
[50] | |
| P53 mutant SW480 cells |
Rescues a deficient P53 pathway ↑ Antitumour effects via disruption of the mutant P53/P73 complex and P73 upregulation |
[51] | |
| Breast | T47D cell line |
No effect on cell cycle ↑ Signature ER stress markers (i.e., CHOP and GRP78) ↑ Caspase 3 levels ↑ Bax expression levels ↑ Bak expression levels ↓ Bcl-2 expression levels ↓ Survivin transcription levels ↑ Apoptosis ↓ RAD51 mRNA expression ↑ JNK signalling pathway ↑ P38 MAPK signalling pathway |
[44, 52–55] |
| MCF-7 cell line |
↑ Signature ER stress markers (i.e., CHOP and GRP78) ↑ Apoptosis ↓ RAD51 mRNA expression ↑ JNK signalling pathway ↑ P38 MAPK signalling pathway Activates the IRE1–JNK pathway Activation of GSK3β Accumulation of P53 protein ↑ Bak expression levels ↑ Caspase 3 levels ↑ Caspase 7 levels ↓ Bcl-2 expression levels ↓ Survivin transcription levels ↑ Bax expression levels Activates NAG-1 Activates the PERK–eIF2α pathway ↑ P53 protein levels ↑ PUMA protein levels Arrests cell cycle at G1 phase |
[52–58] | |
| MDA-MB-231 cell line |
↓ Bcl-2 transcription and expression levels ↓ Cell viability ↓ Proliferation ↓ Phosphorylated LRP6 ↓ Phosphorylated DVL2 ↑ Apoptosis ↑ Caspase 3 levels ↑ Caspase 8 levels ↑ Caspase 9 levels No effect on Raf-1 ↑ Bak expression levels ↓ Survivin transcription and expression levels ↓ HSP90ɑ mRNA and protein levels ↑ Bax mRNA levels ↓ RAD51 mRNA expression ↑ JNK signalling pathway ↑ P38 MAPK signalling pathway ↓ mTOR expression levels ↓ EGFR expression levels ↓ VEGF expression levels PARP cleavage Blocked Wnt/β-Catenin signalling ↓ CDK1 levels ↓ phosphorylated GSK3β ↓ β-catenin gene expression Supports normal breast cell proliferation or growth* Prevents tumour locoregional recurrence in vivo* Causes significant breast cancer cell death* |
[53, 55, 56, 59–63] | |
|
KPL-1 cell line MKL-F cell line |
↑ Apoptosis ↑ Bak expression levels Activates caspase 3 ↓ Bcl-2 expression levels ↑ Bax expression levels |
[53] | |
| MDA-MB-468 cell line |
↓ Cell viability ↓ Proliferation ↑ Apoptosis Blocked Wnt/β-Catenin ssignalling ↓ Phosphorylated LRP6 ↓ Phosphorylated DVL2 ↓ phosphorylated GSK3β ↓ β-catenin gene expression |
[60] | |
|
MDA-MB-231 xenografts MMTV-Wnt1 transgenic mice |
↓ Tumour progression ↓ Ser9 phosphorylated GSK3β ↓ Wnt/β-Catenin ssignalling ↓ CDK levels ↓ Phosphorylated LRP6 ↓ Phosphorylated and unphosphorylated DVL2 ↓ Active β-catenin |
||
| Haematopoietic |
Acute human T cell leukaemia cells (Jurkat clone E6-1) NSO myeloma cells HL-60 human promyelocytic leukaemia cells Human Burkitt lymphoma cells (Ramos) |
↓ Number of viable cells ↑ Apoptosis Acts in absence of p53 ↓ Vacuolar ATPase No significant toxicity, apoptosis, or decrease in normal cells |
[64] |
| Acute human T cell leukaemia cells (Jurkat clone E6-1) | ↑ Phosphorylation of p38-MAPK | [65] | |
| Wt-p53Molt-4 cells (T-ALL) |
↓ Survivin protein levels ↑ Caspase 3 levels ↑ accumulation of P53 Less uniform cells without membrane integrity ↓ Number of viable cells Diminishes metabolic activity ↓ Rate of proliferation |
[66] | |
| CCRF-CEM cells |
↓ Proliferation rate ↓ Viable cell number ↓ Survivin mRNA and protein levels ↓ MMP-9 mRNA and protein levels ↑ Caspase 3 ↑ Apoptosis |
[67] | |
| B and T cells |
↑ Apoptosis ↑ Caspase 3 levels ↑ Caspase 9 levels |
[68] | |
| HCC |
HepG2 WiDr cells |
Changes cellular morphology to apoptotic types Disrupts cell connections ↓ Cell proliferation ↓ Metabolic activity Activates caspase 3 ↓ survivin expression ↑ Apoptotic rate ↑ Anticancer activity |
[48, 69] |
| Pancreatic | H8898 cell line |
↓ Cell proliferation ↑ mitotic arrest ↑ ROS levels Cell death DNA fragmentation ↑ Apoptosis |
[70] |
| Lung |
Doxorubicin-sensitive A549 cell line Doxorubicin-resistant anti-Dox-A549 cell line |
↑ Cytotoxicity ↑ Anticancer activity ↑ PARP cleavage ↑ Apoptosis Activates autophagy ↓ Autophagic inhibitor expression Activates non-PI3K-Class III/Beclin-1 inducer expression ↓ PI3K-p85/AKT/mTOR signalling pathways |
[48, 71, 72] |
| Doxorubicin-sensitive- and -resistant-bearing C57BL/6 mice |
No acute toxicity ↓ Tumor cell accumulation around the trachea |
||
|
A549 cell line HSAEC cells (i.e., an immortalised healthy cell line) |
No cytotoxic effect on healthy cells ↓ Cell viability Changes morphology ↓ DNA replication ↑ Metabolic rewiring |
[72] | |
|
A549 cells CL1-5 cells H23 cells 293 T cells |
↑ p27KIP1 expression Stabilises p27KIP1 through transcriptional repression of SKP2 ↓ E2F1 ↓ PKB levels |
[73] | |
| 95-D cells |
↓ RhoA gene expression and protein levels ↓ MMP-2 ↓ Metastasis and invasion ↑ Cell aggregation |
[74] | |
| GLC cell line |
↑ Mitochondrial apoptosis via caspase-dependent and independent manner ↑ Cytochrome c and AIF release into the cytoplasm |
[75] | |
| GLC4/ADR cell line |
↑ Cytochrome c release Activates caspase cascade ↑ PARP cleavage |
[76] | |
| Urothelial | CNE2 cells |
↓ Cell proliferation ↓ Cell migration ↓ Cell invasion Interrupts the cell cycle in G0/G1 phase |
[77] |
| Nasopharyngeal |
Cisplatin-sensitive or resistant cells J82 253 J T24 RT-112 |
Blocked autophagy Resensitised cisplatin-resistant cells to apoptotic cell death In combination with cisplatin, prodigiosin sensitised both cisplatin-sensitive and -resistant cell lines to cisplatin ↓ Activities of cathepsin B and L Alters lysosomal function |
[22] |
| Choriocarcinoma | JEG3 cell line |
↓ IAP family, including XIAP, cIAP-1 and cIAP-2 ↓ Cell growth ↑ Apoptosis ↑ Caspase 3 levels ↑ Caspase 9 levels ↑ PARP cleavage ↑ P53 expression level ↑ Bax/Bcl-2 expression level |
[78, 79] |
| Prostate cancer |
PC3 cell line PC3 and JEG3 tumour-bearing nude mice |
↓ Cell and tumour growth ↑ Bax/Bcl-2 expression level ↑ Apoptosis ↑ PARP cleavage ↑ Caspase 3 levels ↑ Caspase 9 levels ↑ P53 expression level ↓ IAP family, including XIAP, cIAP-1 and cIAP-2 |
[78] |
| Melanoma | The substrain B16BL6 of mouse melanoma B16 cells |
↓ Metastasis and invasion ↑ Mouse survival rate |
[74] |
| SK-MEL-5 cell line |
Activates the mitochondrial apoptotic pathway Disrupts MCL-1/BAK complexes ↓ mTORC1 protein levels ↓ mTORC2 protein levels Loss of AKT phosphorylation |
[80, 81] | |
| SK-MEL-28 cell line |
Cell cycle arrest at G0/G1 phase ↑ Apoptosis ↑ DNA damage ↓ Survivin protein levels ↓ Clonogenic capacity in survivin knockdown cells ↓ mTORC1 protein levels ↓ mTORC2 protein levels Loss of AKT phosphorylation |
[81, 82] | |
| SK-Mel-19 cell line |
Cell cycle arrest at G0/G1 phase ↑ Apoptosis ↑ DNA damage ↓ Survivin protein levels ↓ Clonogenic capacity in survivin knockdown cells |
[82] |
↑denotes overexpression, upregulation, overactivation, or induction, whereas ↓ expresses reduced activity, suppression, or downregulation
*According to the in-vitro and in-vivo results of an experimental study of prodigiosin-encapsulated scaffolds using blended FDA-approved polymers (polylactic-co-glycolic acid [PLGA], polyethylene glycol [PEG] and polycaprolactone [PCL])
5-FU, 5-fluorouracil; ADR, adriamycin-resistant; AIF, apoptosis-inducing factor; ALL, acute lymphocytic leukaemia; ATP, adenosine triphosphate; ATPase, adenosine triphosphatase; Bax, Bcl-2-associated X protein; Bad, Bcl-2-associated death promoter; Bcl-2, B-cell lymphoma-2; Bak, Bcl2 antagonist/killer; B-CLL, B-Cell chronic lymphocytic leukaemia; BCRP, breast cancer resistance protein; BiP/GRP78, binding immunoglobulin protein-glucose-regulated protein 78; CDK1, cyclin dependent kinase 1; CHOP, C/EBP homologous protein; cIAP-1, cellular inhibitor of apoptosis protein-1; cIAP-2, cellular inhibitor of apoptosis protein-2; CRC, colorectal cancer; CRCSCs, colorectal cancer stem cells; DVL2, dishevelled segment polarity protein 2; E2F1, E2F transcription factor 1; EGFP-LC3, enhanced green fluorescent protein-microtubule-associated protein 1A/1B-light chain 3; EGFR, epidermal growth factor receptor; ER, endoplasmic reticulum; ETC, electron transport chain; GRP78, glucose-regulated protein 78; GSK3β, glycogen synthase kinase 3 beta; HSAEC, human primary small airway epithelial cells; HSP90ɑ, heat shock protein 90 alpha; IAP, inhibitor of apoptosis protein; IRE1–JNK, inositol requiring enzyme 1-c-Jun NH2-terminal kinase; LRP6, low-density lipoprotein receptor-related protein 6; MAPK, mitogen-activated protein kinase; MCL-1/BAK, myeloid-cell leukaemia 1-Bcl2 antagonist/killer MDCK, madindarby canine kidney; MDR1, multidrug resistance 1; MKL, megakaryoblastic leukaemia 1; MMP-2, matrix metalloproteinase-2; MMP-9, matrix metalloproteinase-9; MRP, multidrug resistance-associated protein; MMTV-Wnt1, mice transgenic for mouse mammary tumour virus-Wnt1; mTOR, mammalian target of rapamycin; NAG-1, nonsteroidal anti-inflammatory drug-activated gene-1; PARP, poly (ADP-ribose) polymerase; PERK–eIF2α, protein kinase R (PKR)-like endoplasmic reticulum kinase-eukaryotic translation initiation factor 2A; PI3K, phosphoinositide 3-kinase; PKB, protein kinase B; PUMA, P53 upregulated modulator of apoptosis; ROS, reactive oxygen species; SCLC, small cell lung cancer; SKP2, S-phase kinase associated protein 2; SQSTM, sequestosome; sXBP1, spliced X-box binding protein 1; VEGF, vascular endothelial growth factor; XIAP, X-linked inhibitor of apoptosis
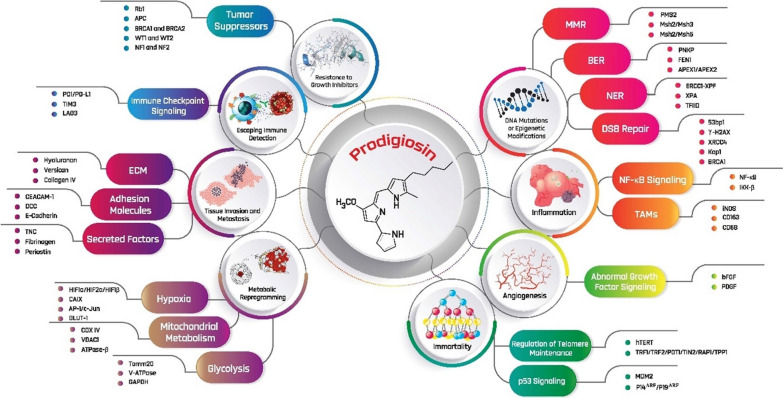
Fig. 2.
Cancer biomarkers where prodigiosin may exert anticancer actions. APC, adenomatous polyposis coli; APEX, AP DNA endonuclease; ATPase-β, adenosine triphosphatase-beta; BER, base excision repair; bFGF, basic fibroblast growth factor; BRCA1, breast cancer 1; BRCA2, breast cancer 2; CAIX, carbonic anhydrase IX; CD68, cluster of differentiation 68; CD163, cluster of differentiation 163; CEACAM-1, CEA cell adhesion molecule-1; COX IV, cytochrome C oxidase subunit IV; DCC, deleted in colorectal carcinoma; DSB, double-strand break; ECM, extracellular matrix; ERCC1-XPF, excision repair cross complementing protein 1-xeroderma pigmentosum group F; FEN1, flap endonuclease 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GLUT-1, glucose transporter protein type 1; HIF1α, hypoxia-inducible factor 1-alpha; HIF1β, hypoxia-inducible factor 1-beta; hTERT, human telomerase reverse transcriptase; IKK-β, inhibitor of nuclear factor kappa-B kinase; iNOS, inducible nitric oxide synthase; Kap1, kruppel-associated box (KRAB)-associated protein 1; LAG3, lymphocyte-activation gene 3; MDM2, mouse double minute 2; MMR, mismatch repair; Msh, MutS homolog 2; NER, nucleotide excision repair; NF-κB, nuclear factor kappa B; NF1, neurofibromatosis 1; PD1/PD-L1, programmed cell death 1/programmed death ligand 1; PDGF, platelet-derived growth factor; PMS2, PMS1 homolog 2; PNKP, polynucleotide kinase 3'-phosphatase; POT1, protection of telomeres 1; PTEN, Phosphatase and TENsin; V-ATPase, vacuolar proton-translocating adenosine triphosphatase; RAP1, repressor activator protein 1; Rb1, retinoblastoma protein 1; TAMs, tumor-associated macrophages; TFIID, transcription factor II D; TIN2, TRF1-interacting protein 2; TIM3, T-cell immunoglobulin mucin-3; Tomm20, translocase of outer mitochondrial membrane 20; TNC, tenascin C; TPP1, tripeptidyl peptidase 1; TRF, telomere restriction fragment; VDAC1, voltage-dependent anion-selective channel 1; WT1, Wilms' tumor 1; WT2, Wilms' tumor 2; XPA, xeroderma pigmentosum group A; XRCC4, X-ray repair cross complementing 4
The possible role of prodigiosin as an ‘immunomodulator’ in cancer
Prodigiosin might improve the efficacy of immunotherapy by regulating multiple immune cells (e.g., T cells) and other proteins in the TME (e.g., programmed death ligand-1 [PD-L1]) [83–87]. Genetic mutations that occur during DNA replication and increased genetic instability in tumours, create neoantigens that evoke an immune response [88]. Failure of immune surveillance facilitates tumour growth and progression despite the expression of immunogenic target expression [89].
The role of prodigiosin on immune-associated molecules
PD-L1
Immune checkpoint inhibitors provide durable clinical response and have become an important anticancer strategy versus standard-of-care (SOC) [90]. Targeting PD-1/PD-L1 by antibodies is minimally effective in several cancers, including renal cell carcinoma (RCC) and non-small cell lung cancer (NSCLC) [90, 91]. However, research has reported that failure of immune checkpoint inhibition is attributed to an increased mTOR activity [92]. Inhibition of mTORC1 decreased PD-L1 levels in NSCLC cell lines [93]; although, such inhibition increased PD-L1 levels in other tumour models. For example, everolimus upregulated PD-L1 expression in RCC cell lines and in xenografted tumour tissues [94]. These results indicate that PD-L1 expression levels following mTOR inhibition vary based on tumour types. Hence, using prodigiosin as an effective mTOR inhibitor in different cancer types in vitro and in vivo might explain why mTOR inhibition effects PD-L1 expression levels differently.
Heat shock protein 90 (HSP90)
Heat shock proteins are stress hallmarks that are abnormally regulated in cancer to prevent cell degradation and death and preserve the protein structure in a stressful environment [86]. They are essential for the immune system regulatory function in healthy cells; although, cancer cells are drug-resistant due to elevated expression levels of HSP90 [95]. For example, combining bortezomib with HSP90 inhibition improved survival and delayed disease progression in mouse models, and suppressed tumour growth in multiple myeloma (MM) cell culture [96, 97]. Moreover, anti-HSP90 treatment improved T-cell killing in melanoma cell lines, and significantly sustained responses with a better safety profile in relapsed/refractory MM (RRMM) patients [96, 98–101]. Recently, a combination of prodigiosin and the HSP inhibitor, PU-H71, decreased the levels of HSP90α in MDA-MB-231 cells [102]. Among other HSPs, HSP90 stimulates Tregs and T helper 1 (Th1) and Th2 cells that support other cells in the immune system. Inhibition of HSP90 using prodigiosin may have the potential to modify Tregs and enhance tumour therapy [59].
The godfather of tumour suppressors, p53, as a hallmark of inflammation and the immune system function
The P53 protein regulates the immune system and cellular processes, and it is one of the most frequently altered genes that drive malignancy, chemo and radioresistance, and disease progression [103–108]. Wild type P53 is involved in inflammatory and autoimmune disorders by inducing Tregs differentiation. For example, systemic lupus erythematosus (SLE) patients have an inhibited P53 function because the P53 C-terminal domain are bound to autoimmune antibodies [109]. Mice lacking P53 also had autoimmune lesions in liver, lungs, and kidneys. They also had a few number of Tregs with impaired differentiation versus p53-expressing mice. Additionally, lung- and pancreatic-deficient P53 tumours exhibited immune tolerance by recruiting both Tregs and monocyte/macrophage lineage cells [110].
There may be a functional HSP90─P53 relationship that impacts P53 function, where compounds that disrupt such association would enhance tumour targeting. For example, prodigiosin inhibited HSP90 and rescued P53 in triple-negative breast cancer (TNBC) and P53-deficient CRC cells, respectively, inhibiting tumour growth and leading to tumour cell death [51, 59]. Heat shock protein 90 is a signal protein that controls the function of survivin where HSP90 inhibition dissociates the HSP90-survivin complex, initiating mitochondrial apoptosis and suppressing metastasis [111]. Prodigiosin led to the accumulation of P53, decreased survivin levels, and increased capsase-3 expression levels in chemoresistant acute lymphoblastic leukaemia (ALL) [112]. Notably, prodigiosin initiates selective apoptosis in malignant breast cancer cell lines regardless of MDR or the P53 status [44, 113]. In contrast, prodigiosin did not accumulate P53 in human CLL cells versus doxorubicin-treated cells [68].
The role of prodigiosin on immune cells in the TME
Tregs
Cancer immunotherapy relies partly on the immunomodulating actions mediated by conventional (Tconv) T cells and Tregs [114]. Regulatory T cells maintain the proper function of the adaptive immune system; however, they can also suppress anticancer immunity and lead to poor disease prognosis (e.g., NSCLC, breast cancer) [115–118]. Patients at high risk of ovarian and breast carcinomas are expected to have reduced survival rates due to elevated levels of Tregs [119, 120]. Heat shock proteins facilitate the immunosuppressive function, division and growth, and cytokine release of Tregs [99]. A second-generation HSP90 inhibitor (i.e., ganetespib) reduced the number of Tregs in skin cancer in vitro and in vivo. Similarly, the notion that prodigiosin inhibited HSP90α expression levels in TNBC cells supports that it may prevent Tregs-mediated immunosuppression in the TME. Prodigiosin may also decrease Tregs numbers and enhance their antitumour functions by suppressing HSP90 and survivin as well as activating p53 simultaneously. Moreover, prodigiosin may modulate Tregs differentiation and prevent immune tolerance because of its effect on P53 irrespective of its status.
T lymphocytes
Despite their antigen-directed cancer cytotoxicity, overstimulation of T cells (i.e., T cell exhaustion) causes T cell senescence with defects in effector functions and proliferation, preventing tumour control [121–123]. Persistent antigen exposure helps tumours evade the immune surveillance and causes T cell dysfunction, where dysfunctional T cells have multiple inhibitory receptors such as PD-1 [124]. Prodigiosin selectively suppressed the proliferation and immune functions of T cells but not B-cells in vitro and in vivo [125]. However, data are insufficient to confirm whether prodigiosin directly or indirectly inhibited the immune functions of T cells. Prodigiosin also suppressed IL-2Rα expression in the IL-2/IL-2R signalling to block T-cell activation, inhibiting graft versus host disease (GvHD) and delayed the progression of autoimmune diabetes without toxicity in mice [126]. Prodigiosin 25-C (a related compound) directly attacked the activated CD8+ T cells by inhibiting the acidification of intracellular organelles needed for cytotoxic T lymphocytes (CTLs) functions [127]. Prodigiosin represents an effective molecule in an immunosuppressive TME characterised by dysfunctional T cells, and might be an important molecule for immunologic studies on T cells [126]. Studying the extent of T-cell inhibition after treatment with prodigiosin is noteworthy, because deficient T-cell inhibition causes autoimmune diseases, whereas cancers arise due to excessive T-cell inhibition [128].
B lymphocytes
Regardless of the available consensus about the immunosuppressive role of prodigiosin on T-cell proliferation, little is known about its effects on B cells [129, 130]. B cells constitute ~ 25% of all cells in some cancers and 40% of tumour-infiltrating lymphocytes (TILs) in breast cancer patients [131–133]. B cells destruct tumours by increasing T cell responses and support tumour growth by favouring immunosuppression via complement activation or immune complex formation [134]. Prodigiosin inhibited polyclonal B-cell proliferation and immortalisation in human peripheral blood lymphocytes (PBLs) and Epstein Barr virus (EBV) [130]. The differential response of prodigiosin on T and B cells might be attributed to the source of the cells used in the experiments. For instance, human cells demonstrate selective inhibition of T-cell proliferation compared to mouse cells [23, 129, 130, 135]. Moreover, B cells are heterogeneous and diverse, and might increase T-cell anticancer activities or facilitate carcinogenesis through angiogenesis, inflammation, and immunosuppression [134].
Tumour-associated macrophages (TAMs)
Existing data support the interesting role of prodigiosin in modulating tumour-associated macrophages (TAMs). Recruited macrophages into the TME are converted into TAMs, certain types of immunosuppressive macrophages that promote “tumour tolerance” by suppressing the generation and function of antitumour T cells [136, 137]. Solid tumours (e.g., breast, prostate) had accumulations of TAMs that confer poor disease prognosis [138–140]. Prodigiosin is proapoptotic and effective against both epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF). It might also prevent the growth of malignant tumours by inhibiting the induction of TAM infiltration and M2 polarisation (Fig. 3) [102, 141, 142]. For example, less M2-polarised TAMs exist in CRC mouse models after EGFR signal disruption by gene knockout (KO) or cetuximab [18, 143]. Involvement of the phosphatidylinositol 3-kinase (PI3K/Akt) pathway in TAM regulation and the inhibitory effect of prodigiosin on this pathway, suggest that prodigiosin might prevent TAM recruitment and initiate tumour-necrosis factor (TNF)-related apoptosis [26, 144–146]. TAMs-induced matrix metalloproteinase-9 (MMP-9) and VEGF also mediate metastasis in a TNBC mouse model and primary lung cancer tissues [147, 148]. Prodigiosin inhibited matrix metalloproteinase-9 (MMP-9) that is argued to release VEGF to regulate TAM-driven tumour growth and angiogenesis (Fig. 3) [67, 149–152].
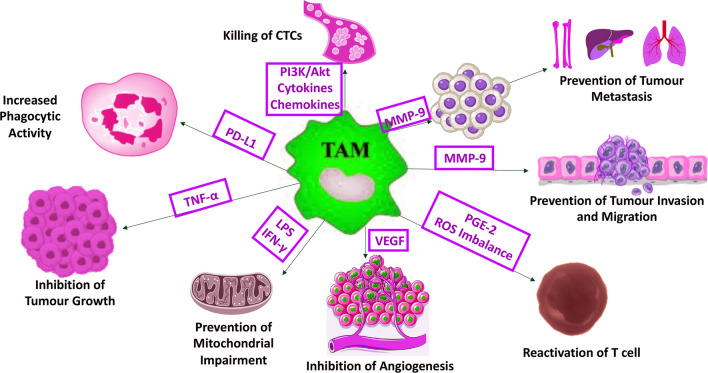
Fig. 3.
A proposed model for the immunomodulatory effect of prodigiosin on TAM-mediated immunosuppression via ROS imbalance and inhibition of MMP-9, PGE-2, VEGF, TNF-α, PI3K/Akt, LPS, IFN-γ, cytokines, and chemokines. CTCs, circulating tumour cells; IFN-γ, interferon-gamma; LPS, lipopolysaccharide; MMP-9, matrix metalloproteinase-9; PD-L1, programmed death-ligand 1; PGE-2, prostaglandin E2; PI3K/Akt, phosphoinositide-3-kinase–protein kinase/Akt; ROS, reactive oxygen species; TAM, tumour-associated macrophages; TNF-α, tumour necrosis factor-alpha; VEGF, vascular endothelial growth factor
Prodigiosin might interfere with TAMs-secreted nicotinamide adenine dinucleotide phosphate oxidase (NOX) function or suppress its upregulation in the TME, preventing oxidative stress-induced carcinogenesis. Tumour-associated macrophages secrete chemokines and cytokines (e.g., interleukins [ILs], prostaglandin E2 [PGE2], and TNF-α) that facilitate carcinogenesis, and express NOX2 that maintains immunological tolerance, tumourigenesis, and metastasis [153–156]. Prodigiosin analogue inhibited NOX activation by affecting the translocations of p47phox and Rac protein to the plasma membrane in a mouse macrophage cell line [157]. Targeting NOX2 by prodigiosin to reduce metastasis warrants further investigation, considering reactive oxygen species (ROS) source, tumour cells’ susceptibility to ROS toxicity, cancer progression stage, and effector cells’ sensitivity to ROS‐induced immunosuppression.
Prodigiosin might modulate the immune response of TAMs by inhibiting TNF-α, IL-2, and interferon-gamma (IFN-γ), reducing TAMs-mediated immunosuppression. Cuevas et al., recently showed that prodigiosin modulated the immune response and stabilised atherosclerotic lesions by inhibiting circulating TNF-α, IL-2, and IFN-γ in vivo (Fig. 3) [158]. Activation of M1 macrophages via IFN-γ is essential in immune function and contributes to tissue damage by proinflammatory cytokines [136]. For example, IFN-γ switched the immunosuppressive TAMs into immunostimulatory cells, potentiating the efficacy of antitumour immunotherapies by generating effector T cells in ovarian cancer [137]. Nevertheless, IFN-γ also conditioned protumourigenic effects in solid tumours and induced lung colonisation and enhanced expression of class I major histocompatibility complex (MHC I)-related antigens [159, 160]. Prodigiosin also inhibited the onset and progression of autoimmune diabetes in non-obese diabetic mice, and reduced IL-2, IFN-γ, and TNF-α mRNA levels in prodigiosin-treated group without side effects [161]. Nonetheless, prodigiosin did not inhibit the secretion of IL-2 in vitro but inhibited the mitogenic signalling from IL-2, suggesting an unusual mechanism of action [135]. It is important to consider the negative effect of prodigiosin on IL-2, because it is among the most potent inducers of antitumour activity in preclinical studies [162].
Solid tumours are characterised by suppressed antitumour immunity due to high PGE2 levels that reduce apoptosis, and increase tumour growth, invasion, metastasis, and angiogenesis [163–166]. There is a TAMs-PGE2 reciprocal relationship where TAMs secrete PGE2 that directly inhibits CD4+ and CD8+ T cells’ effector function, while PGE2 regulates macrophage polarisation into M2 TAMs [166–168]. Activation of the COX-2/PGE2 pathway also stimulates PD-L1 expression via TAMs to inhibit the immune response and promote immune tolerance by modulating T-cell activity and facilitating cancer immune escape [155, 169]. Accordingly, it is wise to consider that prodigiosin-related mTOR inhibition may interfere indirectly with the COX-2/PGE2 pathway by decreasing PD-L1 levels (Fig. 4) [93]. However, mTOR inhibition simultaneously upregulates PD-L1 expression in some circumstances such as in xenografted tumour tissues and in RCC cell lines [94].
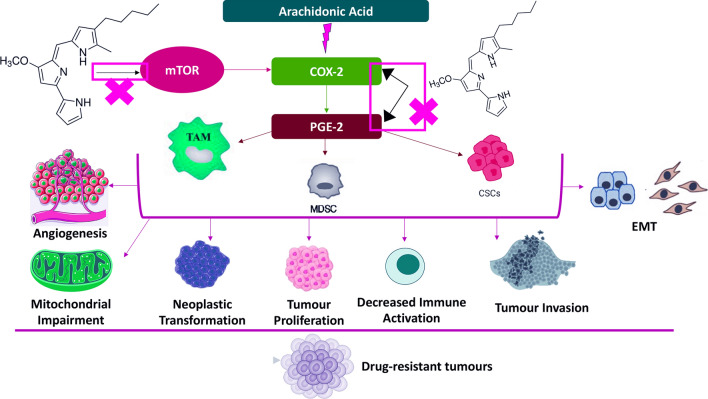
Fig. 4.
The inhibitory effect of prodigiosin on mTOR and COX-2/PGE-2 pathways that further sensitise tumour cells to drugs. COX-2, cyclooxygenase-2; CSCs, cancer stem cells; EMT, epithelial-mesenchymal transition; PGE-2, prostaglandin E2; MDSCs, myeloid-derived suppressor cells; mTOR, mammalian target of rapamycin; TAM, tumour-associated macrophages
Tumour-associated dendritic cells (TADCs)
Similar to TAMs, prodigiosin might modulate TADCs immune functions via PGE2. Although DCs initiate T-cell anticancer immune response, malignant tumours possess other types of DCs with reduced migration and accumulation in lymphoid organs that lead to immunosuppressive T cells [170]. High PGE2 levels shift the immunostimulatory DCs into immunosuppressive cells to reduce the proliferation of anticancer T cells by upregulating PD-L1 [170]. Prostaglandin E2 inhibits MHC II expression and upregulates IL-10 via EP2 and EP4 receptors, suppressing DCs’ antigen presentation mediated via the COX-2/EP3 signalling [171, 172]. The immunomodulatory actions of prodigiosin on TAMs discussed earlier denote that it may affect DCs in the TME. Prodigiosin might reverse TAM-mediated attenuation of tumouricidal and tumour antigen-presenting behaviours occurring to DCs due to the established metabolic crosstalk [142].
NK cells
Activated NK cells eliminate tumours via death receptor-mediated killing, granule exocytosis, and cytokine production (i.e., IFN-γ) that stimulates other immune cells [173]. Nevertheless, PGE2 per se trades off NK cell activities (e.g., tumour lysis) for metastases development via activated EP2 and EP4 receptors [171, 174]. Prostaglandin E2 suppresses the function of NK cells by multiple mechanisms, such as inhibiting IFN-γ production and ILs–induced IFN-γ expression in NK cells via EP2 receptor or downregulating NK receptors through the cAMP/PKA pathway [171]. EP4 antagonist inhibited PGE2-mediated NK cell suppression by protecting IFN-γ production by NK cells, inhibiting breast and lung tumour metastases [175–177]. Prodigiosin might have an immunomodulatory role since the reciprocal NK-DCs crosstalk is inhibited by PGE2 through chemokine and cytokine modulation (Fig. 5) [178].
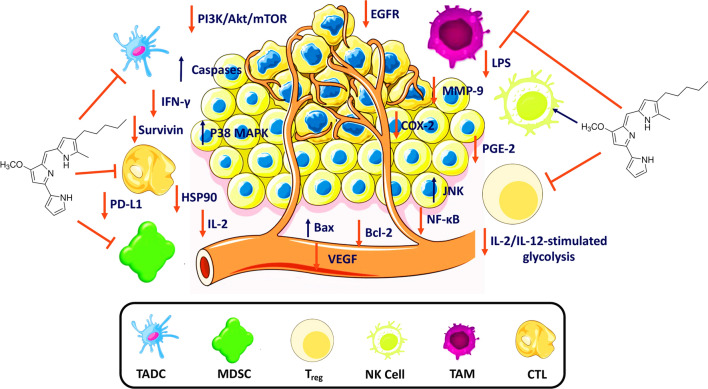
Fig. 5.
The potential role of prodigiosin as an immunomodulator in the TME. COX-2, cyclooxygenase-2; CTL, cytotoxic T lymphocyte; HSP90, heat shock protein 90; IFN-γ, interferon-gamma; IL, interleukin; JNK, c-Jun N-terminal kinases; LPS, lipopolysaccharide; MAPK; mitogen-activated protein kinase; MDSC, myeloid-derived suppressor cell; MMP-9, matrix metalloproteinase-9; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa B; NK, natural killer; PD-L1, programmed death-ligand 1; PGE-2, prostaglandin E2; PI3K/Akt, phosphoinositide-3-kinase–protein kinase/Akt; TADC, tumour-associated dendritic cell; TAM, tumour-associated macrophage; Treg; regulatory T cell; VEGF, vascular endothelial growth factor
MDSCs
Tumours maintain an immunosuppressive TME through high levels of heterogeneous immature myeloid cells, referred to as MDSCs [179]. It is mandatory to target myeloid populations that stop anticancer immunity or activate stimulatory cells that promote antitumour immunity. In addition to ILs and VEGF, PGE2-rich tumoural exosomes induce MDSCs activation and migration and promote MDSCs-dependent tumour growth [179–182]. Prostaglandin E2 controls MDSCs differentiation and increases their levels, enhancing the stemness of cervical cancer cells in vitro and in vivo (Fig. 4). For example, MDSCs express the four PGE2 receptors (i.e., EP1-4) in tumour-bearing mice [183, 184].
COX-2 inhibitors reduce MDSCs levels and delay the burden of primary carcinoma tumour, because PGE2-induced COX-2 activates the secretion of endogenous MDSCs-related PGE2 (Fig. 4) [185]. Production of PGE2 in lung and ovarian cancers is correlated with COX-2 expression, promoting recruitment and retention of MDSCs [185, 186]. COX-2 inhibitors reduce PGE2 production resulting in decreased levels of MDSC-attracting C-C Motif Chemokine Ligand 2 (CCL2) in vivo, suggesting that blocking COX-2 impedes the development and accumulation of MDSCs [187]. In–silico molecular docking analysis revealed that prodigiosin inhibited COX-2 effectively and could be assessed as an antiinflammatory compound in further research [188]. Inflammation is characterised by high levels of PGE2 through COX-2. Moreover, overexpression of COX-2 (also a downstream target of mTORC1) promoted proliferation and growth of several cancers. Downregulation of COX-2 exerts a protective effect against hyperactivated mTORC1-mediated tumourigenesis caused by loss of tuberous sclerosis complex (TSC) in TSC-null cell [189]. Likewise, the ability of prodigiosin to inhibit the mTOR pathway might prevent COX-2-mediated tumourigenesis via increased PGE2 production (Fig. 4).
Production of PGE2 by MDSCs increases PD-L1 expression in ovarian cancer through the mTOR signalling pathway (Fig. 4). Bone marrow (BM) cells cultured with bladder cancer cells showed significant PD-L1 expression in monocytic MDSCs [183]. Tumour-infiltrating PD-L1+ cells also showed high expression levels of both COX-2 and PGE2 synthase 1 (mPGES1) in tumour-bearing mice. Inhibition of mPGES1/COX-2 by prodigiosin may reduce the expression of MDSCs-related PD-L1, arguing that reprogramming PGE2 metabolism enhances tumour sensitivity to immunotherapy.
The role of prodigiosin in the metabolic reprograming of immune cells
The interesting anticancer and immunomodulatory actions of prodigiosin (Table 1) prompt us to further discuss whether it is involved in the metabolic reprogramming of TME-related immune cells (Fig. 5) [190]. Interaction of the principal metabolic pathways in immune cells (e.g., pentose phosphate pathway [PPP], fatty acid oxidation [FAO], Krebs’ cycle, and glycolysis), provides energy and nutrients to maintain their activity. Both metabolites and metabolic activity regulate autophagy, apoptosis, and posttranslational modifications, and pro and antiinflammatory effects [191–194].
NK cells
The mTOR/PI3K pathway is sensitive to a high number of extracellular signals and is a key regulator of cellular growth, proliferation, and metabolism. Cancer is characterised by an aberrant mTOR signalling that supports tumour proliferation, survival, metabolic programming, and drug resistance [195]. The mTOR signalling pathway enhances glycolysis and mitochondrial function to regulate the metabolism of NK cells. Inhibition of mTOR by rapamycin reduced both IL-2/IL-12-stimulated glycolysis and IL-2-stimulated levels in mouse NK cells and human NK cell glycolysis, respectively (Fig. 5) [196, 197]. For instance, higher glucose transporter 1 (GLUT1) levels that absorb glucose exist following the upregulation of both CD71 and CD98 [198, 199]. The anti-mTOR activity of prodigiosin in Table 1 highlight that it might have a pivotal role in the metabolic reprogramming of NK cells [59].
TAMs
Macrophages with different polarisation states are also different in glycometabolism. Anaerobic glycolysis provides instant energy to help proinflammatory M1 macrophages eliminate pathogens, whereas mitochondrial oxidative phosphorylation (OXPHOS) generates energy for antiinflammatory M2 macrophages [200]. Therefore, mitochondrial dysfunction impedes M2 repolarisation that inhibits regulatory immune signals, and facilitates tumour angiogenesis, migration, and metastasis [166, 201].
Prodigiosin suppresses inflammatory responses induced by lipopolysaccharide (LPS) in activated murine macrophage, by inhibiting the activation of p38 mitogen-activated kinase (MPAK), c-Jun N-terminal kinase (JNK), and nuclear factor kappa B (NF-κB) (Fig. 5). Stimulation of LPS and IFN-γ reduces OXPHOS levels, impairing the mitochondrial function required for M2 repolarisation with the accumulation of hypoxia-inducible factor-1α (HIF-1α) and metabolites of Krebs’ cycle (e.g., succinate) [202]. Hypoxia-inducible factor-1α regulates GLUT1 to affect the polarisation and functions of macrophages via metabolic reprogramming of PPP and anaerobic glycolysis [203]. In this regard, prodigiosin might prevent mitochondrial impairment (Fig. 3) and facilitate ‘M2 polarisation’. Namely, inhibition of LPS and IFN-γ increases OXPHOS levels (Figs. 3, 5), implicating a mechanism by which prodigiosin be immunosuppressive where M2 macrophages prevail to favour tumour progression and tissue repair.
T lymphocytes
Naïve T cells preserve a resting state using OXPHOS in contrast to activated T cells that grow via glucose and lipid metabolism. Proliferating cells exhibit higher aerobic glycolysis rate, referred to as the Warburg effect where the principal driver of aerobic glycolysis is ‘mitochondrial dysfunction’ [204, 205]. Imbalance of ROS production due to mitochondrial dysfunction destroys cell membranes and DNA, disrupts cell proliferation, induces apoptosis, and inhibits autophagy [206–210]. Prodigiosin fosters the protecting antioxidative function of nuclear factor erythroid 2-related factor 2 (Nrf2) and scavenges ROS in hepatocellular carcinoma (HCC) cells [211, 212]. However, it upregulates ROS levels and suppressed proliferation and autophagy in leukaemia cell line. These data suggest that prodigiosin might interfere with be the metabolic reprogramming of T cells via ROS, considering its ROS stimulatory and scavenging roles (Fig. 3).
The mTOR pathway is crucial in upregulating GLUT1 expression in naïve T cells to promote glucose absorption and to improve the immune response [213–215]. It also helps Th2 cells’ differentiation via the OXPHOS–aerobic glycolysis metabolic transition. Mammalian target of rapamycin complex 1 (mTORC1) is essential for Th1 cells while mammalian target of rapamycin complex 2 (mTORC2) regulates OXPHOS and glycolysis in Th2 cells [216–218]. Moreover, the mTOR pathway regulates the production and memory differentiation of CD8+ T cells [219–223]. Consistent with the inhibition of the PI3K/Akt/mTOR pathway by prodigiosin in cancer, it might be involved in the metabolic reprogramming of Th cells and CD8+ T cells [42, 48, 71, 72]. However, these data should be used cautiously because the ability of prodigiosin to suppress the immune functions of T cells has not been confirmed yet.
TADCs
Metabolic reprogramming (i.e., decreased OXPHOS and an increased glycolysis) is essential for activation and functions of DCs [224]. Stimulated LPS helps DCs regulate the mTOR signal, stabilise HIF1-α, and increase inducible nitric oxide synthase (iNOS) expression [225, 226]. Since prodigiosin inhibits the mTOR pathway and reduces iNOS expression by inhibiting LPS-triggered inflammatory responses, it might prevent the manipulation of the metabolic processes that affect the activation and functions of DCs (Fig. 5) [72, 227]. However, the metabolic environment where DCs compete with neighbouring cells for nutrition is difficult to simulate and measure both in vitro and in vivo [203].
Given these, does prodigiosin have possible immunomodulatory actions on TAMs, TADCs, NK cells, and MDSCs via COX-2/PGE2 in cancer?
The crosstalk between immune cells outlines the potential of prodigiosin to interfere with the COX-2/PGE2 pathway (Fig. 4) and decrease the immunological tolerance mediated by TAMs, TADCs, NK cells, and MDSCs. Nonetheless, the opposite effects of PGE2 (suppressive/protective) due to disease course (e.g., cancer, gastric lesions) and status of the immune system, should be considered while developing cancer-specific treatments. For instance, PGE2 protected rats from HCl/ethanol-induced gastric lesions by reducing the levels of antioxidants, apoptotic biomarkers, and inflammatory mediators [228]. Prodigiosin prevented apoptosis in the gastric mucosa by downregulating the expression of COX-2, caspase-3, IL1-β, Bax, and TNF-α, while upregulating Bcl-2 expression; hence, increasing PGE2 production [228]. However, these conditions favour tumour growth and proliferation. These data are confirmed by the idea that reducing PGE2 levels might prevent tumour initiation, inhibit tumour growth and metastasis, reprogram antitumour immunity, and increase the efficacy of immunotherapies.
Tumour mutation burden (TMB)
Analysis of ~ 5000 mutations from ~ 7000 cancers highlighted that tumour mutation burden (TMB) status in cancer cells—the number of somatic mutations/megabase of the genome encoding tumours—successfully predicted the efficacy of immune checkpoint blockade [229]. Studies demonstrated that PI3K/mTOR pathway mutations are correlated with TMB status in NSCLC and nasopharyngeal carcinoma [230, 231]. Treatment with an mTOR inhibitor (i.e., everolimus) led to tumour shrinkage and disease stabilisation in patients with NSCLC [230]. Prodigiosin may have an interesting role on TMB by inhibiting PI3K/mTOR pathway and P53 (Table 1) [50].
Conclusion
The current review demonstrated the compelling immunomodulatory and metabolic reprogramming activities of prodigiosin on TME-related immune cells (Fig. 5). Particularly, the crosstalk between the immune cells, the involvement of the mTOR pathway, and expression of PGE-2 and COX-2, dictate the potential of prodigiosin as an immunomodulator in the TME. Using prodigiosin to inhibit the PI3K/mTOR pathway may clarify its hypothesised effects on TMB, especially because the TMB status associated with PI3K/mTOR pathway gene mutations is still unclear.
Future perspectives
Further research may confirm whether prodigiosin has a compensatory mechanism that overcomes cancer resistance, and whether it renders B cells pro or antitumourigenic. More data are required to examine the controversial role of prodigiosin in blocking T-cell activation by suppressing IL-2Rα expression in the IL-2/IL-2R signalling. It is also important to focus on prodigiosin encapsulation in nanoparticles because limited research demonstrated that it might be an excellent alternative in cancer treatment. Based on the crosstalk between immune cells, using prodigiosin in cancer immunotherapy elicits outstanding inquiries such as:
Could prodigiosin be used as a tool to identify the point at which immune tolerance occurs?
What is the dominant role (immunostimulatory/immunosuppressive) of prodigiosin on every immune cell in the TME?
How could prodigiosin modulate NK-DC crosstalk and strengthen both DC- and NK-cell-mediated immune response?
Acknowledgements
Not applicable.
Abbreviations
- ALL
Acute lymphoblastic leukaemia
- ATRA
All-trans retinoic acid
- CLL
Chronic lymphoblastic leukaemia
- COX
Cyclooxygenase
- CRC
Colorectal cancer
- CTLs
Cytotoxic T lymphocytes
- CVD
Cardiovascular disease
- EBV
Epstein Barr virus
- EGCG
Epigallocatechin gallate
- EGFR
Epidermal growth factor receptor
- FAO
Fatty acid oxidation
- GLOBOCAN
Global burden of cancer
- GLUT1
Glucose transporter 1
- GvHD
Graft versus host disease
- HCC
Hepatocellular carcinoma
- HIF-1α
Hypoxia-inducible factor-1α
- HSP90
Heat shock protein 90
- IFN
Interferon
- ILs
Interleukins
- iNOS
Inducible nitric oxide synthase
- JNK
C-Jun N-terminal kinase
- KO
Knock out
- LPS
Lipopolysaccharide
- MDR
Multidrug resistance
- MDSCs
Myeloid-derived suppressor cells
- MHCI
Class I major histocompatibility complex
- MM
Multiple myeloma
- MAPK
P38 Mitogen-activated kinase
- mTOR
Mammalian target of rapamycin
- NF-κB
Nuclear factor kappa B
- NK
Natural killer
- NOX
Nicotinamide adenine dinucleotide phosphate oxidase
- NSCLC
Non-small cell lung cancer
- OXPHOS
Oxidative phosphorylation
- PBLs
Peripheral blood lymphocytes
- PD-1
Programmed death protein 1
- PD-L1
Programmed death ligand 1
- PGE2
Prostaglandin E2
- PGES1
Prostaglandin E2 synthase 1
- PI3K
Phosphatidylinositol 3-kinase
- PPP
Pentose phosphate pathway
- PSK
Polysaccharide K
- RCC
Renal cell carcinoma
- ROS
Reactive oxygen species
- RRMM
Relapsed/refractory multiple myeloma
- SLE
Systemic lupus erythematosus
- SOC
Standard-of-care
- TADCs
Tumour-associated dendritic cells
- TAMs
Tumour-associated macrophages
- TILs
Tumour-infiltrating lymphocytes
- TMB
Tumour mutation burden
- TME
Tumour microenvironment
- TNBC
Triple-negative breast cancer
- TNF
Tumour-necrosis factor
- Tregs
Regulatory T cells
- TSC
Tuberous sclerosis complex
- VEGF
Vascular endothelial growth factor
- WHO
World Health Organisation
Author contributions
MMA conceptualised the idea of the manuscript and developed the first draft. MMA, CA, and AS contributed to methodology and data curation. MMA, CA, and NMH edited the manuscript and prepared the figures. AS supervised the whole process. All authors contributed to editing, reviewing, and approving the final version of the manuscript. All authors have read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammed Moustapha Anwar, Email: igsr.dr.mohamedanwar@alexu.edu.eg.
Nadia M. Hamdy, Email: nadia_hamdy@pharma.asu.edu.eg
Ahmed S. Sultan, Email: dr_asultan@alexu.edu.eg
References
- 1.(WHO) WHO. Global health estimates: Leading causes of death [Internet]. Available from: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri B. Cancer 2021: new therapeutic approaches for the treatment of cancer—building on advances in cancer biology and the molecular genetics of cancer. Curr Opin Pharmacol. 2021;60:341–5. doi: 10.1016/j.coph.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Gmeiner WH. Recent advances in our knowledge of mCRC tumor biology and genetics: a focus on targeted therapy development. Onco Targets Ther. 2021;14:2121–30. doi: 10.2147/OTT.S242224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shlyakhtina Y, Moran KL, Portal MM. Genetic and non-genetic mechanisms underlying cancer evolution. Cancers (Basel) 2021;13(6):1380. doi: 10.3390/cancers13061380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward RA, Fawell S, Floc’h N, Flemington V, McKerrecher D, Smith PD. Challenges and opportunities in cancer drug resistance. Chem Rev. 2021;121(6):3297–351. doi: 10.1021/acs.chemrev.0c00383. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Cadena A, Barreto A, Fioretino S, Jandus C. Immune system activation by natural products and complex fractions: a network pharmacology approach in cancer treatment. Cell Stress. 2020;4(7):154–66. doi: 10.15698/cst2020.07.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gangwar V, Garg A, Lomore K, Korla K, Bhat SS, Rao RP, et al. Immunomodulatory effects of a concoction of natural bioactive compounds-mechanistic insights. Biomedicines. 2021;9(11):1522. doi: 10.3390/biomedicines9111522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moody R, Wilson K, Jaworowski A, Plebanski M. Natural compounds with potential to modulate cancer therapies and self-reactive immune cells. Cancers (Basel) 2020;12(3):673. doi: 10.3390/cancers12030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan P, Huang Y-W, Oshima K, Yearsley M, Zhang J, Arnold M, et al. The immunomodulatory potential of natural compounds in tumor-bearing mice and humans. Crit Rev Food Sci Nutr. 2019;59(6):992–1007. doi: 10.1080/10408398.2018.1537237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Sotto A, Vitalone A, Di Giacomo S. Plant-derived nutraceuticals and immune system modulation: an evidence-based overview. Vaccines. 2020;8(3):468. doi: 10.3390/vaccines8030468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan P, Kang S, Wang Y, Liu K, Oshima K, Huang Y-W, et al. Black raspberries enhance natural killer cell infiltration into the colon and suppress the progression of colorectal cancer. Front Immunol. 2017;8:997. doi: 10.3389/fimmu.2017.00997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan P, Skaer C, Yu J, Zhao H, Ren H, Oshima K, et al. Berries and other natural products in the pancreatic cancer chemoprevention in human clinical trials. J berry Res. 2017;7(3):147–61. doi: 10.3233/JBR-170159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan P, Skaer CW, Stirdivant SM, Young MR, Stoner GD, Lechner JF, et al. Beneficial regulation of metabolic profiles by black raspberries in human colorectal cancer patients. Cancer Prev Res (Phila) 2015;8(8):743–50. doi: 10.1158/1940-6207.CAPR-15-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furugaki K, Pokorna K, Le Pogam C, Aoki M, Reboul M, Bajzik V, et al. DNA vaccination with all-trans retinoic acid treatment induces long-term survival and elicits specific immune responses requiring CD4+ and CD8+ T-cell activation in an acute promyelocytic leukemia mouse model. Blood. 2010;115(3):653–6. doi: 10.1182/blood-2007-08-109009. [DOI] [PubMed] [Google Scholar]
- 16.Zou JY, Su CH, Luo HH, Lei YY, Zeng B, Zhu HS, et al. Curcumin converts Foxp3+ regulatory T cells to T helper 1 cells in patients with lung cancer. J Cell Biochem. 2018;119(2):1420–8. doi: 10.1002/jcb.26302. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh D-S, Wang H, Tan S-W, Huang Y-H, Tsai C-Y, Yeh M-K, et al. The treatment of bladder cancer in a mouse model by epigallocatechin-3-gallate-gold nanoparticles. Biomaterials. 2011;32(30):7633–40. doi: 10.1016/j.biomaterials.2011.06.073. [DOI] [PubMed] [Google Scholar]
- 18.Ma X, Wu D, Zhou S, Wan F, Liu H, Xu X, et al. The pancreatic cancer secreted REG4 promotes macrophage polarization to M2 through EGFR/AKT/CREB pathway. Oncol Rep. 2016;35(1):189–96. doi: 10.3892/or.2015.4357. [DOI] [PubMed] [Google Scholar]
- 19.Habtemariam S. Trametes versicolor (Synn. Coriolus versicolor) polysaccharides in cancer therapy: targets and efficacy. Biomedicines. 2020;8(5):135. doi: 10.3390/biomedicines8050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang X, Pan J, Xu F, Shao B, Wang Y, Guo X, et al. Bacteria-based cancer immunotherapy. Adv Sci (Weinheim) Baden-Wurttemberg Ger. 2021;8(7):2003572. doi: 10.1002/advs.202003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yip C-H, Mahalingam S, Wan K-L, Nathan S. Prodigiosin inhibits bacterial growth and virulence factors as a potential physiological response to interspecies competition. PLoS ONE. 2021;16(6):e0253445. doi: 10.1371/journal.pone.0253445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berning L, Schlütermann D, Friedrich A, Berleth N, Sun Y, Wu W, et al. Prodigiosin sensitizes sensitive and resistant urothelial carcinoma cells to cisplatin treatment. Molecules. 2021;26(5):1294. doi: 10.3390/molecules26051294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han S-B, Lee CW, Yoon YD, Kang JS, Lee KH, Yoon WK, et al. Effective prevention of lethal acute graft-versus-host disease by combined immunosuppressive therapy with prodigiosin and cyclosporine A. Biochem Pharmacol. 2005;70(10):1518–26. doi: 10.1016/j.bcp.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Pandey R, Chander R, Sainis KB. Prodigiosins: a novel family of immunosuppressants with anti-cancer activity. Indian J Biochem Biophys. 2007;44(5):295–302. [PubMed] [Google Scholar]
- 25.Stepkowski SM, Erwin-Cohen RA, Behbod F, Wang M-E, Qu X, Tejpal N, et al. Selective inhibitor of Janus tyrosine kinase 3, PNU156804, prolongs allograft survival and acts synergistically with cyclosporine but additively with rapamycin. Blood. 2002;99(2):680–9. doi: 10.1182/blood.V99.2.680. [DOI] [PubMed] [Google Scholar]
- 26.Suryawanshi RK, Koujah L, Patil CD, Ames JM, Agelidis A, Yadavalli T, et al. Bacterial pigment prodigiosin demonstrates a unique antiherpesvirus activity that is mediated through inhibition of prosurvival signal transducers. J Virol. 2020;94(13):e00251–e320. doi: 10.1128/JVI.00251-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metcalfe S, Ashley N, Chen Z, Calne RY. Prodigiosin 25C: effect in in vitro models for T cell activation and T cell cycling and in vivo for rat heart allografts. Int Arch Allergy Immunol. 1993;101(2):132–5. doi: 10.1159/000236510. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura A, Magae J, Tsuji RF, Yamasaki M, Nagai K. Suppression of cytotoxic T cell induction in vivo by prodigiosin 25-C. Transplantation. 1989;47(6):1013–6. doi: 10.1097/00007890-198906000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Tsuji RF, Magae J, Yamashita M, Nagai K, Yamasaki M. Immunomodulating properties of prodigiosin 25-C, an antibiotic which preferentially suppresses induction of cytotoxic T cells. J Antibiot (Tokyo) 1992;45(8):1295–302. doi: 10.7164/antibiotics.45.1295. [DOI] [PubMed] [Google Scholar]
- 30.Vidal AF, Ferraz RS, El-Husny A, Silva CS, Vinasco-Sandoval T, Magalhães L, et al. Comprehensive analysis of germline mutations in northern Brazil: a panel of 16 genes for hereditary cancer-predisposing syndrome investigation. BMC Cancer. 2021;21(1):363. doi: 10.1186/s12885-021-08089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbreath C, Ma S, Yu L, Sonavane R, Roggero CM, Devineni A, et al. Dynamic differences between DNA damage repair responses in primary tumors and cell lines. Transl Oncol. 2020;14(1):100898. doi: 10.1016/j.tranon.2020.100898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosaka K, Yang Y, Seki T, Du Q, Jing X, He X, et al. Therapeutic paradigm of dual targeting VEGF and PDGF for effectively treating FGF-2 off-target tumors. Nat Commun. 2020;11(1):3704. doi: 10.1038/s41467-020-17525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan H-C, Chang F-W, Tsai J-D, Lin K-M, Chen C-M, Lin S-Z, et al. Telomeres and cancer. Life (Basel) 2021;11(12):1405. doi: 10.3390/life11121405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deregowska A, Wnuk M. RAP1/TERF2IP-A Multifunctional player in cancer development. Cancers (Basel) 2021;13(23):5970. doi: 10.3390/cancers13235970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cilluffo D, Barra V, Di Leonardo A. P14(ARF): the absence that makes the difference. Genes (Basel) 2020;11(7):824. doi: 10.3390/genes11070824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landry JP, Schertz KL, Chiang Y-J, Bhalla AD, Yi M, Keung EZ, et al. Comparison of cancer prevalence in patients with neurofibromatosis type 1 at an academic cancer center vs in the general population from 1985 to 2020. JAMA Netw open. 2021;4(3):e210945. doi: 10.1001/jamanetworkopen.2021.0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nunes R, Sella T, Treuner K, Atkinson JM, Wong J, Zhang Y, et al. Prognostic utility of breast cancer index to stratify distant recurrence risk in invasive lobular carcinoma. Clin Cancer Res. 2021;27(20):5688–96. doi: 10.1158/1078-0432.CCR-21-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bikas A, Jensen K, Patel A, Costello J, Reynolds SM, Mendonca-Torres MC, et al. Cytochrome C oxidase subunit 4 (COX4): a potential therapeutic target for the treatment of medullary thyroid cancer. Cancers (Basel) 2020;12(9):2548. doi: 10.3390/cancers12092548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ibrahim SA, Kulshrestha A, Katara GK, Riehl V, Sahoo M, Beaman KD. Cancer-associated V-ATPase induces delayed apoptosis of protumorigenic neutrophils. Mol Oncol. 2020;14(3):590–610. doi: 10.1002/1878-0261.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elahian F, Moghimi B, Dinmohammadi F, Ghamghami M, Hamidi M, Mirzaei SA. The anticancer agent prodigiosin is not a multidrug resistance protein substrate. DNA Cell Biol. 2013;32(3):90–7. doi: 10.1089/dna.2012.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Díaz-Ruiz C, Montaner B, Pérez-Tomás R. Prodigiosin induces cell death and morphological changes indicative of apoptosis in gastric cancer cell line HGT-1. Histol Histopathol. 2001;16(2):415–21. doi: 10.14670/HH-16.415. [DOI] [PubMed] [Google Scholar]
- 42.Cheng S-Y, Chen N-F, Kuo H-M, Yang S-N, Sung C-S, Sung P-J, et al. Prodigiosin stimulates endoplasmic reticulum stress and induces autophagic cell death in glioblastoma cells. Apoptosis. 2018;23(5):314–28. doi: 10.1007/s10495-018-1456-9. [DOI] [PubMed] [Google Scholar]
- 43.Francisco R, Pérez-Tomás R, Gimènez-Bonafé P, Soto-Cerrato V, Giménez-Xavier P, Ambrosio S. Mechanisms of prodigiosin cytotoxicity in human neuroblastoma cell lines. Eur J Pharmacol. 2007;572(2):111–9. doi: 10.1016/j.ejphar.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 44.Dalili D, Fouladdel S, Rastkari N, Samadi N, Ahmadkhaniha R, Ardavan A, et al. Prodigiosin, the red pigment of Serratia marcescens, shows cytotoxic effects and apoptosis induction in HT-29 and T47D cancer cell lines. Nat Prod Res. 2012;26(22):2078–83. doi: 10.1080/14786419.2011.622276. [DOI] [PubMed] [Google Scholar]
- 45.Hassankhani R, Sam MR, Esmaeilou M, Ahangar P. Prodigiosin isolated from cell wall of Serratia marcescens alters expression of apoptosis-related genes and increases apoptosis in colorectal cancer cells. Med Oncol. 2015;32(1):1–8. doi: 10.1007/s12032-014-0366-0. [DOI] [PubMed] [Google Scholar]
- 46.Castillo-Ávila W, Abal M, Robine S, Pérez-Tomás R. Non-apoptotic concentrations of prodigiosin (H+/Cl−symporter) inhibit the acidification of lysosomes and induce cell cycle blockage in colon cancer cells. Life Sci. 2005;78(2):121–7. doi: 10.1016/j.lfs.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 47.Montaner B, Pérez-Tomás R. Prodigiosin-induced apoptosis in human colon cancer cells. Life Sci. 2001;68(17):2025–36. doi: 10.1016/S0024-3205(01)01002-5. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen VB, Chen S-P, Nguyen TH, Nguyen MT, Tran TTT, Doan CT, et al. Novel efficient bioprocessing of marine chitins into active anticancer prodigiosin. Mar Drugs. 2019;18(1):15. doi: 10.3390/md18010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao C, Qiu S, He J, Peng Y, Xu H, Feng Z, et al. Prodigiosin impairs autophagosome-lysosome fusion that sensitizes colorectal cancer cells to 5-fluorouracil-induced cell death. Cancer Lett. 2020;481:15–23. doi: 10.1016/j.canlet.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Prabhu VV, Hong B, Allen JE, Zhang S, Lulla AR, Dicker DT, et al. Small-molecule prodigiosin restores p53 tumor suppressor activity in chemoresistant colorectal cancer stem cells via c-Jun-mediated ΔNp73 inhibition and p73 Activation. Cancer Res. 2016;76(7):1989–99. doi: 10.1158/0008-5472.CAN-14-2430. [DOI] [PubMed] [Google Scholar]
- 51.Hong B, Prabhu VV, Zhang S, van den Heuvel APJ, Dicker DT, Kopelovich L, et al. Prodigiosin rescues deficient p53 signaling and antitumor effects via upregulating p73 and disrupting its interaction with mutant p53. Cancer Res. 2014;74(4):1153–65. doi: 10.1158/0008-5472.CAN-13-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan M-Y, Shen Y-C, Lu C-H, Yang S-Y, Ho T-F, Peng Y-T, et al. Prodigiosin activates endoplasmic reticulum stress cell death pathway in human breast carcinoma cell lines. Toxicol Appl Pharmacol. 2012;265(3):325–34. doi: 10.1016/j.taap.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto C, Takemoto H, Kuno K, Yamamoto D, Nakai K, Baden T, et al. Cycloprodigiosin hydrochloride, a H+/Cl- symporter, induces apoptosis in human colon cancer cell lines in vitro. Oncol Rep. 2001;8(4):821–4. doi: 10.3892/or.8.4.821. [DOI] [PubMed] [Google Scholar]
- 54.Ho TF, Peng YT, Chuang SM, Lin SC, Feng BL, Lu CH, et al. Prodigiosin down-regulates survivin to facilitate paclitaxel sensitization in human breast carcinoma cell lines. Toxicol Appl Pharmacol. 2009;235(2):253–60. doi: 10.1016/j.taap.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Lu CH, Lin SC, Yang SY, Pan MY, Lin YW, Hsu CY, et al. Prodigiosin-induced cytotoxicity involves RAD51 down-regulation through the JNK and p38 MAPK pathways in human breast carcinoma cell lines. Toxicol Lett. 2012;212(1):83–89. doi: 10.1016/j.toxlet.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Akpan UM, Pellegrini M, Obayemi JD, Ezenwafor T, Browl D, Ani CJ, et al. Prodigiosin-loaded electrospun nanofibers scaffold for localized treatment of triple negative breast cancer. Mater Sci Eng C. 2020;114:110976. doi: 10.1016/j.msec.2020.110976. [DOI] [PubMed] [Google Scholar]
- 57.Soto-Cerrato V, Viñals F, Lambert JR, Kelly JA, Pérez-Tomás R. Prodigiosin induces the proapoptotic gene <em>NAG-1</em> via glycogen synthase kinase-3β activity in human breast cancer cells. Mol Cancer Ther. 2007;6(1):362–9. doi: 10.1158/1535-7163.MCT-06-0266. [DOI] [PubMed] [Google Scholar]
- 58.Abd el hameid MK. Design, synthesis, and screening of 5-Aryl-3-(2-(pyrrolyl)thiophenyl)-1,2,4-oxadiazoles as potential antitumor molecules on breast cancer MCF-7 cell line. Chem Pharm Bull. 2018;66(12):1181–95. doi: 10.1248/cpb.c18-00636. [DOI] [PubMed] [Google Scholar]
- 59.Anwar MM, Shalaby M, Embaby AM, Saeed H, Agwa MM, Hussein A. Prodigiosin/PU-H71 as a novel potential combined therapy for triple negative breast cancer (TNBC): preclinical insights. Sci Rep. 2020;10(1):14706. doi: 10.1038/s41598-020-71157-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Z, Li B, Zhou L, Yu S, Su Z, Song J, et al. Prodigiosin inhibits Wnt/β-catenin signaling and exerts anticancer activity in breast cancer cells. Proc Natl Acad Sci U S A. 2016;113(46):13150–5. doi: 10.1073/pnas.1616336113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dozie-Nwachukwu SO, Danyuo Y, Obayemi JD, Odusanya OS, Malatesta K, Soboyejo WO. Extraction and encapsulation of prodigiosin in chitosan microspheres for targeted drug delivery. Mater Sci Eng C. 2017;71:268–78. doi: 10.1016/j.msec.2016.09.078. [DOI] [PubMed] [Google Scholar]
- 62.Obayemi JD, Jusu SM, Salifu AA, Ghahremani S, Tadesse M, Uzonwanne VO, et al. Degradable porous drug-loaded polymer scaffolds for localized cancer drug delivery and breast cell/tissue growth. Mater Sci Eng C. 2020;112:110794. doi: 10.1016/j.msec.2020.110794. [DOI] [PubMed] [Google Scholar]
- 63.Jusu SM, Obayemi JD, Salifu AA, Nwazojie CC, Uzonwanne V, Odusanya OS, et al. Drug-encapsulated blend of PLGA-PEG microspheres: in vitro and in vivo study of the effects of localized/targeted drug delivery on the treatment of triple-negative breast cancer. Sci Rep. 2020;10(1):1–23. doi: 10.1038/s41598-020-71129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Montaner B, Navarro S, Piqué M, Vilaseca M, Martinell M, Giralt E, et al. Prodigiosin from the supernatant of Serratia marcescens induces apoptosis in haematopoietic cancer cell lines. Br J Pharmacol. 2000;131(3):585–93. doi: 10.1038/sj.bjp.0703614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Montaner B, Pérez-Tomás R. The cytotoxic prodigiosin induces phosphorylation of p38-MAPK but not of SAPK/JNK. Toxicol Lett. 2002;129(1–2):93–8. doi: 10.1016/S0378-4274(01)00477-5. [DOI] [PubMed] [Google Scholar]
- 66.Sam MR, Pourpak RS. Regulation of p53 and survivin by prodigiosin compound derived from Serratia marcescens contribute to caspase-3-dependent apoptosis in acute lymphoblastic leukemia cells. Hum Exp Toxicol. 2017;37(6):608–17. doi: 10.1177/0960327117718052. [DOI] [PubMed] [Google Scholar]
- 67.Sam MR, Ghoreishi S. Prodigiosin produced by Serratia marcescens inhibits expression of MMP-9 and survivin and promotes caspase-3 activation with induction of apoptosis in acute lymphoblastic leukaemia cells. J Appl Microbiol. 2018;125(4):1017–29. doi: 10.1111/jam.13949. [DOI] [PubMed] [Google Scholar]
- 68.Campàs C, Dalmau M, Montaner B, Barragán M, Bellosillo B, Colomer D, et al. Prodigiosin induces apoptosis of B and T cells from B-cell chronic lymphocytic leukemia. Leukemia. 2003;17(4):746–50. doi: 10.1038/sj.leu.2402860. [DOI] [PubMed] [Google Scholar]
- 69.Yenkejeh RA, Sam MR, Esmaeillou M. Targeting survivin with prodigiosin isolated from cell wall of Serratia marcescens induces apoptosis in hepatocellular carcinoma cells. Hum Exp Toxicol. 2017;36(4):402–11. doi: 10.1177/0960327116651122. [DOI] [PubMed] [Google Scholar]
- 70.Zhang J, Liu J, Shen Y, Wei D, Li K. Inhibitive effect of prodigiosin on the proliferation of human malignant pancreatic cancer cells. Med Chem Res. 2005;14(4):181–97. doi: 10.1007/s00044-005-0133-z. [DOI] [Google Scholar]
- 71.Chiu W-J, Lin S-R, Chen Y-H, Tsai M-J, Leong M, Weng C-F. Prodigiosin-emerged PI3K/Beclin-1-independent pathway elicits autophagic cell death in doxorubicin-sensitive and -resistant lung cancer. J Clin Med. 2018;7(10):321. doi: 10.3390/jcm7100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davient B, Ng JPZ, Xiao Q, Li L, Yang L. Comparative transcriptomics unravels prodigiosin’s potential cancer-specific activity between human small airway epithelial cells and lung adenocarcinoma cells. Front Oncol. 2018;8:573. doi: 10.3389/fonc.2018.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsieh H-Y, Shieh J-J, Chen C-J, Pan M-Y, Yang S-Y, Lin S-C, et al. Prodigiosin down-regulates SKP2 to induce p27(KIP1) stabilization and antiproliferation in human lung adenocarcinoma cells. Br J Pharmacol. 2012;166(7):2095–108. doi: 10.1111/j.1476-5381.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang J, Shen Y, Liu J, Wei D. Antimetastatic effect of prodigiosin through inhibition of tumor invasion. Biochem Pharmacol. 2005;69(3):407–14. doi: 10.1016/j.bcp.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 75.Llagostera E, Soto-Cerrato V, Montaner B, Pérez-Tomás R. Prodigiosin induces apoptosis by acting on mitochondria in human lung cancer cells. Ann N Y Acad Sci. 2003;1010(1):178–81. doi: 10.1196/annals.1299.030. [DOI] [PubMed] [Google Scholar]
- 76.The International Agency for Research on Cancer (IARC) High cytotoxic sensitivity of the human small cell lung doxorubicin-resistant carcinoma (GLC4/ADR) cell line to prodigiosin through apoptosis activation. Anticancer Drugs. 2005;16(4):393–9. doi: 10.1097/00001813-200504000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y, Zhou H, Ma X, Lin C, Lu L, Liu D, et al. Prodigiosin inhibits proliferation, migration, and invasion of nasopharyngeal cancer cells. Cell Physiol Biochem. 2018;48(4):1556–62. doi: 10.1159/000492278. [DOI] [PubMed] [Google Scholar]
- 78.Li D, Liu J, Wang X, Kong D, Du W, Li H, et al. Biological potential and mechanism of prodigiosin from serratia marcescens subsp. Lawsoniana in human choriocarcinoma and prostate cancer cell lines. Int J Mol Sci. 2018;19(11):3465. doi: 10.3390/ijms19113465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao K, Li D, Cheng G, Zhang B, Han J, Chen J, et al. Targeted delivery prodigiosin to choriocarcinoma by peptide-guided dendrigraft poly-l-lysines nanoparticles. Int J Mol Sci. 2019;20(21):5458. doi: 10.3390/ijms20215458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hosseini A, Espona-Fiedler M, Soto-Cerrato V, Quesada R, Pérez-Tomás R, Guallar V. Molecular interactions of prodiginines with the BH3 domain of anti-apoptotic Bcl-2 family members. PLoS ONE. 2013;8(2):e57562. doi: 10.1371/journal.pone.0057562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Espona-Fiedler M, Soto-Cerrato V, Hosseini A, Lizcano JM, Guallar V, Quesada R, et al. Identification of dual mTORC1 and mTORC2 inhibitors in melanoma cells: prodigiosin vs. obatoclax. Biochem Pharmacol. 2012;83(4):489–96. doi: 10.1016/j.bcp.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 82.Branco PC, Pontes CA, Rezende-Teixeira P, Amengual-Rigo P, Alves-Fernandes DK, Maria-Engler SS, et al. Survivin modulation in the antimelanoma activity of prodiginines. Eur J Pharmacol. 2020;888:173465. doi: 10.1016/j.ejphar.2020.173465. [DOI] [PubMed] [Google Scholar]
- 83.Ghosh M, Saha S, Bettke J, Nagar R, Parrales A, Iwakuma T, et al. Mutant p53 suppresses innate immune signaling to promote tumorigenesis. Cancer Cell. 2021;39(4):494–508.e5. doi: 10.1016/j.ccell.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Albrecht T, Brinkmann F, Albrecht M, Lonsdorf AS, Mehrabi A, Hoffmann K, et al. Programmed death ligand-1 (PD-L1) is an independent negative prognosticator in western-world gallbladder cancer. Cancers (Basel) 2021;13(7):1682. doi: 10.3390/cancers13071682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Briukhovetska D, Dörr J, Endres S, Libby P, Dinarello CA, Kobold S. Interleukins in cancer: from biology to therapy. Nat Rev Cancer. 2021;21(8):481–99. doi: 10.1038/s41568-021-00363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Albakova Z, Siam MKS, Sacitharan PK, Ziganshin RH, Ryazantsev DY, Sapozhnikov AM. Extracellular heat shock proteins and cancer: new perspectives. Transl Oncol. 2021;14(2):100995. doi: 10.1016/j.tranon.2020.100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Principe DR, Chiec L, Mohindra NA, Munshi HG. Regulatory T-cells as an emerging barrier to immune checkpoint inhibition in lung cancer. Front Oncol. 2021;11:684098. doi: 10.3389/fonc.2021.684098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ye Z, Shi Y, Lees-Miller SP, Tainer JA. Function and molecular mechanism of the DNA damage response in immunity and cancer immunotherapy. Front Immunol. 2021;12:5155. doi: 10.3389/fimmu.2021.797880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21(6):345–59. doi: 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ju X, Zhang H, Zhou Z, Wang Q. Regulation of PD-L1 expression in cancer and clinical implications in immunotherapy. Am J Cancer Res. 2020;10(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- 91.Casey SC, Tong L, Li Y, Do R, Walz S, Fitzgerald KN, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352(6282):227–31. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Donnell JS, Massi D, Teng MWL, Mandala M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin Cancer Biol. 2018;48:91–103. doi: 10.1016/j.semcancer.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 93.Lastwika KJ, Wilson W, Li QK, Norris J, Xu H, Ghazarian SR, et al. Control of PD-L1 expression by oncogenic activation of the AKT–mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76(2):227–38. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 94.Hirayama Y, Gi M, Yamano S, Tachibana H, Okuno T, Tamada S, et al. Anti-PD-L1 treatment enhances antitumor effect of everolimus in a mouse model of renal cell carcinoma. Cancer Sci. 2016;107(12):1736–44. doi: 10.1111/cas.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5(10):761–72. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 96.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Kung AL, Davies FE, et al. Antimyeloma activity of heat shock protein-90 inhibition. Blood. 2006;107(3):1092–100. doi: 10.1182/blood-2005-03-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sydor JR, Normant E, Pien CS, Porter JR, Ge J, Grenier L, et al. Development of 17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride (IPI-504), an anti-cancer agent directed against Hsp90. Proc Natl Acad Sci. 2006;103(46):17408–13. doi: 10.1073/pnas.0608372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mbofung RM, McKenzie JA, Malu S, Zhang M, Peng W, Liu C, et al. HSP90 inhibition enhances cancer immunotherapy by upregulating interferon response genes. Nat Commun. 2017;8(1):451. doi: 10.1038/s41467-017-00449-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brenu EW, Staines DR, Tajouri L, Huth T, Ashton KJ, Marshall-Gradisnik SM. Heat shock proteins and regulatory T cells. Autoimmune Dis. 2013;2013:813256. doi: 10.1155/2013/813256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumaraguru U, Pack CD, Rouse BT. Toll-like receptor ligand links innate and adaptive immune responses by the production of heat-shock proteins. J Leukoc Biol. 2003;73(5):574–83. doi: 10.1189/jlb.0902470. [DOI] [PubMed] [Google Scholar]
- 101.Facciponte JG, MacDonald IJ, Wang X-Y, Kim H, Manjili MH, Subjeck JR. Heat shock proteins and scavenger receptors: role in adaptive immune responses. Immunol Invest. 2005;34(3):325–42. doi: 10.1081/IMM-200064505. [DOI] [PubMed] [Google Scholar]
- 102.Anwar MM, Shalaby M, Embaby AM, Saeed H, Agwa MM, Hussein A. Prodigiosin/PU-H71 as a novel potential combined therapy for triple negative breast cancer (TNBC): preclinical insights. Sci Rep. 2020;10(1):1–15. doi: 10.1038/s41598-020-71157-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen F, Wang W, El-Deiry WS. Current strategies to target p53 in cancer. Biochem Pharmacol. 2010;80(5):724–730. doi: 10.1016/j.bcp.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 104.Prabhu VV, Allen JE, Hong B, Zhang S, Cheng H, El-Deiry WS. Therapeutic targeting of the p53 pathway in cancer stem cells. Expert Opin Ther Target. 2012;16(12):1161–74. doi: 10.1517/14728222.2012.726985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kressner U, Inganäs M, Byding S, Blikstad I, Påhlman L, Glimelius B, et al. Prognostic value of p53 genetic changes in colorectal cancer. J Clin Oncol. 1999;17(2):593. doi: 10.1200/JCO.1999.17.2.593. [DOI] [PubMed] [Google Scholar]
- 106.Katkoori VR, Jia X, Shanmugam C, Wan W, Meleth S, Bumpers H, et al. Prognostic significance of p53 codon 72 polymorphism differs with race in colorectal adenocarcinoma. Clin Cancer Res. 2009;15(7):2406–16. doi: 10.1158/1078-0432.CCR-08-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9(10):701–13. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- 108.Kastenhuber ER, Lowe SW. Putting p53 in Context. Cell. 2017;170(6):1062–78. doi: 10.1016/j.cell.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kawashima H, Takatori H, Suzuki K, Iwata A, Yokota M, Suto A, et al. Tumor suppressor p53 inhibits systemic autoimmune diseases by inducing regulatory T cells. J Immunol. 2013;191(7):3614–23. doi: 10.4049/jimmunol.1300509. [DOI] [PubMed] [Google Scholar]
- 110.Blagih J, Zani F, Chakravarty P, Hennequart M, Pilley S, Hobor S, et al. Cancer-specific loss of p53 leads to a modulation of myeloid and T cell responses. Cell Rep. 2020;30(2):481–496.e6. doi: 10.1016/j.celrep.2019.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fortugno P, Beltrami E, Plescia J, Fontana J, Pradhan D, Marchisio PC, et al. Regulation of survivin function by Hsp90. Proc Natl Acad Sci U S A. 2003;100(24):13791–6. doi: 10.1073/pnas.2434345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sam MR, Pourpak RS. Regulation of p53 and survivin by prodigiosin compound derived from Serratia marcescens contribute to caspase-3-dependent apoptosis in acute lymphoblastic leukemia cells. Hum Exp Toxicol. 2018;37(6):608–17. doi: 10.1177/0960327117718052. [DOI] [PubMed] [Google Scholar]
- 113.Ho T-F, Ma C-J, Lu C-H, Tsai Y-T, Wei Y-H, Chang J-S, et al. Undecylprodigiosin selectively induces apoptosis in human breast carcinoma cells independent of p53. Toxicol Appl Pharmacol. 2007;225(3):318–28. doi: 10.1016/j.taap.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 114.Ahrends T, Borst J. The opposing roles of CD4(+) T cells in anti-tumour immunity. Immunology. 2018;154(4):582–92. doi: 10.1111/imm.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rocamora-Reverte L, Melzer FL, Würzner R, Weinberger B. The complex role of regulatory T cells in immunity and aging. Front Immunol. 2021;11:616949. doi: 10.3389/fimmu.2020.616949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127(4):759–67. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 117.Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020;19(1):116. doi: 10.1186/s12943-020-01234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: can treg cells be a new therapeutic target? Cancer Sci. 2019;110(7):2080–9. doi: 10.1111/cas.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–80. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 120.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 121.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35(2):51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–99. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang J, He T, Xue L, Guo H. Senescent T cells: a potential biomarker and target for cancer therapy. EBioMedicine. 2021;68:103409. doi: 10.1016/j.ebiom.2021.103409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Han SB, Kim HM, Kim YH, Lee CW, Jang E-S, Son KH, et al. T-cell specific immunosuppression by prodigiosin isolated from Serratia marcescens. Int J Immunopharmacol. 1998;20(1):1–13. doi: 10.1016/S0192-0561(97)00062-3. [DOI] [PubMed] [Google Scholar]
- 126.Han SB, Park SH, Jeon YJ, Kim YK, Kim HM, Yang KH. Prodigiosin blocks T cell activation by inhibiting interleukin-2Ralpha expression and delays progression of autoimmune diabetes and collagen-induced arthritis. J Pharmacol Exp Ther. 2001;299(2):415–25. [PubMed] [Google Scholar]
- 127.Lee M-H, Kataoka T, Magae J, Nagai K. Prodigiosin 25-C suppression of cytotoxic T cells in vitro and in vivo similar to that of concanamycin B, a specific inhibitor of vacuolar type H+-ATPase. Biosci Biotechnol Biochem. 1995;59(8):1417–21. doi: 10.1271/bbb.59.1417. [DOI] [PubMed] [Google Scholar]
- 128.Das S, Ariizumi K, Cruz PDJ. T-cell inhibitors: a bench-to-bedside review. Dermat contact atopic Occup drug. 2012;23(5):195–202. doi: 10.1097/DER.0b013e31826e43ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pandey R, Chander R, Sainis KB. A novel prodigiosin-like immunosuppressant from an alkalophilic Micrococcus sp. Int Immunopharmacol. 2003;3(2):159–67. doi: 10.1016/S1567-5769(02)00114-5. [DOI] [PubMed] [Google Scholar]
- 130.Songia S, Mortellaro A, Taverna S, Fornasiero C, Scheiber EA, Erba E, et al. Characterization of the new immunosuppressive drug undecylprodigiosin in human lymphocytes: retinoblastoma protein, cyclin-dependent kinase-2, and cyclin-dependent kinase-4 as molecular targets. J Immunol. 1997;158(8):3987–95. [PubMed] [Google Scholar]
- 131.Chin Y, Janseens J, Vandepitte J, Vandenbrande J, Opdebeek L, Raus J. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. 1992;12(5):1463–6. [PubMed] [Google Scholar]
- 132.Marsigliante S, Biscozzo L, Marra A, Nicolardi G, Leo G, Lobreglio GB, et al. Computerised counting of tumour infiltrating lymphocytes in 90 breast cancer specimens. Cancer Lett. 1999;139(1):33–41. doi: 10.1016/S0304-3835(98)00379-6. [DOI] [PubMed] [Google Scholar]
- 133.Coronella-Wood JA, Hersh EM. Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother. 2003;52(12):715–38. doi: 10.1007/s00262-003-0409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fridman WH, Petitprez F, Meylan M, Chen TW-W, Sun C-M, Roumenina LT, et al. B cells and cancer: To B or not to B? J Exp Med. 2021 doi: 10.1084/jem.20200851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mortellaro A, Songia S, Gnocchi P, Ferrari M, Fornasiero C, D’Alessio R, et al. New immunosuppressive drug PNU156804 blocks IL-2-dependent proliferation and NF-κB and AP-1 activation. J Immunol. 1999;162(12):7102–9. [PubMed] [Google Scholar]
- 136.Zhou J, Tang Z, Gao S, Li C, Feng Y, Zhou X. Tumor-associated macrophages: recent insights and therapies. Front Oncol. 2020;10:188. doi: 10.3389/fonc.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Duluc D, Corvaisier M, Blanchard S, Catala L, Descamps P, Gamelin E, et al. Interferon-gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int J cancer. 2009;125(2):367–73. doi: 10.1002/ijc.24401. [DOI] [PubMed] [Google Scholar]
- 138.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat rev Cancer. 2004 doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 139.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 140.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196(3):254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 141.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 142.Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12(1):76. doi: 10.1186/s13045-019-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang W, Chen L, Ma K, Zhao Y, Liu X, Wang Y, et al. Polarization of macrophages in the tumor microenvironment is influenced by EGFR signaling within colon cancer cells. Oncotarget. 2016;7(46):75366–78. doi: 10.18632/oncotarget.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell. 2011;20(6):701–14. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen Q, Zhang XHF, Massagué J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20(4):538–49. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zheng P, Luo Q, Wang W, Li J, Wang T, Wang P, et al. Tumor-associated macrophages-derived exosomes promote the migration of gastric cancer cells by transfer of functional Apolipoprotein E. Cell Death Dis. 2018;9(4):434. doi: 10.1038/s41419-018-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Steenbrugge J, Breyne K, Demeyere K, De Wever O, Sanders NN, Van Den Broeck W, et al. Anti-inflammatory signaling by mammary tumor cells mediates prometastatic macrophage polarization in an innovative intraductal mouse model for triple-negative breast cancer. J Exp Clin Cancer Res. 2018;37(1):191. doi: 10.1186/s13046-018-0860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang R, Zhang J, Chen S, Lu M, Luo X, Yao S, et al. Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression. Lung Cancer. 2011;74(2):188–96. doi: 10.1016/j.lungcan.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 149.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114(5):623–33. doi: 10.1172/JCI200422087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2(10):737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67(11):5064–6. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 152.Lin EY, Li J-F, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66(23):11238–46. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 153.Ouyang W, O’Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity. 2019;50(4):871–91. doi: 10.1016/j.immuni.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 154.Weyemi U, Redon CE, Parekh PR, Dupuy C, Bonner WM. NADPH oxidases NOXs and DUOXs as putative targets for cancer therapy. Anticancer Agents Med Chem. 2013;13(3):502–14. [PMC free article] [PubMed] [Google Scholar]
- 155.Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci U S A. 2017;114(5):1117–22. doi: 10.1073/pnas.1612920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Holla S, Ghorpade DS, Singh V, Bansal K, Balaji KN. Mycobacterium bovis BCG promotes tumor cell survival from tumor necrosis factor-α-induced apoptosis. Mol Cancer. 2014;13:210. doi: 10.1186/1476-4598-13-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nakashima T, Iwashita T, Fujita T, Sato E, Niwano Y, Kohno M, et al. A prodigiosin analogue inactivates NADPH oxidase in macrophage cells by inhibiting assembly of p47phox and Rac. J Biochem. 2007;143(1):107–115. doi: 10.1093/jb/mvm196. [DOI] [PubMed] [Google Scholar]
- 158.Cuevas A, Saavedra N, Salazar LA, Cavalcante MF, Silva JC, Abdalla DSP. Prodigiosin modulates the immune response and could promote a stable atherosclerotic lession in C57bl/6 Ldlr-/- Mice. Int J Mol Sci. 2020;21(17):6417. doi: 10.3390/ijms21176417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Garbe C, Krasagakis K, Zouboulis CC, Schröder K, Krüger S, Stadler R, et al. Antitumor activities of interferon alpha, beta, and gamma and their combinations on human melanoma cells in vitro: changes of proliferation, melanin synthesis, and immunophenotype. J Invest Dermatol. 1990;95(6 Suppl):231S–7S. doi: 10.1111/1523-1747.ep12875837. [DOI] [PubMed] [Google Scholar]
- 160.Taniguchi K, Petersson M, Höglund P, Kiessling R, Klein G, Kärre K. Interferon gamma induces lung colonization by intravenously inoculated B16 melanoma cells in parallel with enhanced expression of class I major histocompatibility complex antigens. Proc Natl Acad Sci U S A. 1987;84(10):3405–9. doi: 10.1073/pnas.84.10.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim HM, Kang JS, Lim J, Park S-K, Lee K, Yoon YD, et al. Inhibition of human ovarian tumor growth by cytokine-induced killer cells. Arch Pharm Res. 2007;30(11):1464–70. doi: 10.1007/BF02977372. [DOI] [PubMed] [Google Scholar]
- 162.Weiss JM, Subleski JJ, Wigginton JM, Wiltrout RH. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther. 2007;7(11):1705–21. doi: 10.1517/14712598.7.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cai Y, Yousef A, Grandis JR, Johnson DE. NSAID therapy for PIK3CA-altered colorectal, breast, and head and neck cancer. Adv Biol Regul. 2020;75:100653. doi: 10.1016/j.jbior.2019.100653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Harris RE, Beebe-Donk J, Doss H, Burr DD. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review) Oncol Rep. 2005;13(4):559–83. [PubMed] [Google Scholar]
- 165.Drew DA, Schuck MM, Magicheva-Gupta MV, Stewart KO, Gilpin KK, Miller P, et al. Effect of low-dose and standard-dose aspirin on PGE(2) biosynthesis among individuals with colorectal adenomas: a randomized clinical trial. Cancer Prev Res (Phila) 2020;13(10):877–88. doi: 10.1158/1940-6207.CAPR-20-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Finetti F, Travelli C, Ercoli J, Colombo G, Buoso E, Trabalzini L. Prostaglandin E2 and cancer: insight into tumor progression and immunity. Biology (Basel) 2020 doi: 10.3390/biology9120434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26(1):78. doi: 10.1186/s12929-019-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Oshima H, Hioki K, Popivanova BK, Oguma K, Van Rooijen N, Ishikawa T-O, et al. Prostaglandin E2 signaling and bacterial infection recruit tumor-promoting macrophages to mouse gastric tumors. Gastroenterology. 2011;140(2):596–607.e7. doi: 10.1053/j.gastro.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 169.Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727–42. [PMC free article] [PubMed] [Google Scholar]
- 170.Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209(3):495–506. doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang D, DuBois RN. Role of prostanoids in gastrointestinal cancer. J Clin Invest. 2018;128(7):2732–42. doi: 10.1172/JCI97953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ogawa F, Amano H, Eshima K, Ito Y, Matsui Y, Hosono K, et al. Prostanoid induces premetastatic niche in regional lymph nodes. J Clin Invest. 2014;124(11):4882–94. doi: 10.1172/JCI73530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fulton AM, Chong YC. Prostaglandin E2 receptor activity and susceptibility to natural killer cells. J Leukoc Biol. 1992;51(2):176–80. doi: 10.1002/jlb.51.2.176. [DOI] [PubMed] [Google Scholar]
- 175.Holt DM, Ma X, Kundu N, Collin PD, Fulton AM. Modulation of host natural killer cell functions in breast cancer via prostaglandin E2 receptors EP2 and EP4. J Immunother. 2012;35(2):179–88. doi: 10.1097/CJI.0b013e318247a5e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Holt D, Ma X, Kundu N, Fulton A. Prostaglandin E(2) (PGE (2)) suppresses natural killer cell function primarily through the PGE(2) receptor EP4. Cancer Immunol Immunother. 2011;60(11):1577–86. doi: 10.1007/s00262-011-1064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kundu N, Ma X, Holt D, Goloubeva O, Ostrand-Rosenberg S, Fulton AM. Antagonism of the prostaglandin E receptor EP4 inhibits metastasis and enhances NK function. Breast Cancer Res Treat. 2009;117(2):235–42. doi: 10.1007/s10549-008-0180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Harizi H. Reciprocal crosstalk between dendritic cells and natural killer cells under the effects of PGE2 in immunity and immunopathology. Cell Mol Immunol. 2013;10(3):213–21. doi: 10.1038/cmi.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32(1):19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Travelli C, Consonni FM, Sangaletti S, Storto M, Morlacchi S, Grolla AA, et al. Nicotinamide phosphoribosyltransferase acts as a metabolic gate for mobilization of myeloid-derived suppressor cells. Cancer Res. 2019;79(8):1938–51. doi: 10.1158/0008-5472.CAN-18-1544. [DOI] [PubMed] [Google Scholar]
- 181.Porta C, Consonni FM, Morlacchi S, Sangaletti S, Bleve A, Totaro MG, et al. Tumor-derived prostaglandin E2 promotes p50 NF-κB-dependent differentiation of monocytic MDSCs. Cancer Res. 2020;80(13):2874–88. doi: 10.1158/0008-5472.CAN-19-2843. [DOI] [PubMed] [Google Scholar]
- 182.Xiang X, Poliakov A, Liu C, Liu Y, Deng Z, Wang J, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J cancer. 2009;124(11):2621–33. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67(9):4507–13. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 184.Kuroda H, Mabuchi S, Yokoi E, Komura N, Kozasa K, Matsumoto Y, et al. Prostaglandin E2 produced by myeloid-derived suppressive cells induces cancer stem cells in uterine cervical cancer. Oncotarget. 2018;9(91):36317–30. doi: 10.18632/oncotarget.26347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118(20):5498–505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yang F, Wei Y, Cai Z, Yu L, Jiang L, Zhang C, et al. Activated cytotoxic lymphocytes promote tumor progression by increasing the ability of 3LL tumor cells to mediate MDSC chemoattraction via Fas signaling. Cell Mol Immunol. 2015;12(1):66–76. doi: 10.1038/cmi.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71(7):2664–74. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Krishna PS, Vani K, Prasad MR, Samatha B, Bindu NSVSSSLH, Charya MAS, et al. In–silico molecular docking analysis of prodigiosin and cycloprodigiosin as COX-2 inhibitors. Springerplus. 2013;2(1):172. doi: 10.1186/2193-1801-2-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li H, Jin F, Jiang K, Ji S, Wang L, Ni Z, et al. mTORC1-mediated downregulation of COX2 restrains tumor growth caused by TSC2 deficiency. Oncotarget. 2016 doi: 10.18632/oncotarget.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Thiele K, Diao L, Arck PC. Immunometabolism, pregnancy, and nutrition. Semin Immunopathol. 2018;40(2):157–74. doi: 10.1007/s00281-017-0660-y. [DOI] [PubMed] [Google Scholar]
- 191.Murphy MP, O’Neill LAJ. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell. 2018;174(4):780–4. doi: 10.1016/j.cell.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 192.Fan J, Krautkramer KA, Feldman JL, Denu JM. Metabolic regulation of histone post-translational modifications. ACS Chem Biol. 2015;10(1):95–108. doi: 10.1021/cb500846u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Voss K, Larsen SE, Snow AL. Metabolic reprogramming and apoptosis sensitivity: defining the contours of a T cell response. Cancer Lett. 2017;408:190–6. doi: 10.1016/j.canlet.2017.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Williams NC, O’Neill LAJ. A role for the Krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front Immunol. 2018;9:141. doi: 10.3389/fimmu.2018.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Popova NV, Jücker M. The role of mTOR signaling as a therapeutic target in cancer. Int J Mol Sci. 2021;22(4):1743. doi: 10.3390/ijms22041743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Slattery K, Woods E, Zaiatz-Bittencourt V, Marks S, Chew S, Conroy M, et al. TGFβ drives NK cell metabolic dysfunction in human metastatic breast cancer. J Immunother cancer. 2021 doi: 10.1136/jitc-2020-002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Donnelly RP, Loftus RM, Keating SE, Liou KT, Biron CA, Gardiner CM, et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J Immunol. 2014;193(9):4477–84. doi: 10.4049/jimmunol.1401558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Keating SE, Zaiatz-Bittencourt V, Loftus RM, Keane C, Brennan K, Finlay DK, et al. Metabolic reprogramming supports IFN-γ production by CD56bright NK cells. J Immunol. 2016;196(6):2552–60. doi: 10.4049/jimmunol.1501783. [DOI] [PubMed] [Google Scholar]
- 199.Zheng X, Qian Y, Fu B, Jiao D, Jiang Y, Chen P, et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat Immunol. 2019;20(12):1656–67. doi: 10.1038/s41590-019-0511-1. [DOI] [PubMed] [Google Scholar]
- 200.Rodríguez-Prados J-C, Través PG, Cuenca J, Rico D, Aragonés J, Martín-Sanz P, et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185(1):605–14. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 201.Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17(3):684–96. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 202.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238–42. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.O’Neill LAJ, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016;213(1):15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–70. doi: 10.1126/science.124.3215.269. [DOI] [PubMed] [Google Scholar]
- 205.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 206.Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, et al. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta Mol Cell Res. 2012;1823(12):2297–310. doi: 10.1016/j.bbamcr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 207.Murphy MP. Mitochondrial dysfunction indirectly elevates ROS production by the endoplasmic reticulum. Cell Metab. 2013;18(2):145–6. doi: 10.1016/j.cmet.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 208.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48(2):158–67. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ji S, Sun R, Xu K, Man Z, Ji J, Pu Y, et al. Prodigiosin induces apoptosis and inhibits autophagy via the extracellular signal-regulated kinase pathway in K562 cells. Toxicol Vitr. 2019;60:107–15. doi: 10.1016/j.tiv.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 210.Lin SR, Fu YS, Tsai MJ, Cheng H, Weng CF. Natural compounds from herbs that can potentially execute as autophagy inducers for cancer therapy. Int J Mol Sci. 2017 doi: 10.3390/ijms18071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen J, Li Y, Liu F, Hou D-X, Xu J, Zhao X, et al. Prodigiosin promotes Nrf2 activation to inhibit oxidative stress induced by microcystin-LR in HepG2 cells. Toxins (Basel) 2019;11(7):403. doi: 10.3390/toxins11070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lu H, Lei X, Zhang Q. Moderate activation of IKK2-NF-kB in unstressed adult mouse liver induces cytoprotective genes and lipogenesis without apparent signs of inflammation or fibrosis. BMC Gastroenterol. 2015;15(1):94. doi: 10.1186/s12876-015-0325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, Aghvanyan A, et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol. 2010;185(2):1037–44. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014;20(1):61–72. doi: 10.1016/j.cmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Verbist KC, Guy CS, Milasta S, Liedmann S, Kamiński MM, Wang R, et al. Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature. 2016;532(7599):389–93. doi: 10.1038/nature17442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yang J-Q, Kalim KW, Li Y, Zhang S, Hinge A, Filippi M-D, et al. RhoA orchestrates glycolysis for TH2 cell differentiation and allergic airway inflammation. J Allergy Clin Immunol. 2016;137(1):231–245.e4. doi: 10.1016/j.jaci.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12(4):295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9(8):563–75. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and eomesodermin. Immunity. 2010;32(1):67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–12. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang L, Tschumi BO, Lopez-Mejia IC, Oberle SG, Meyer M, Samson G, et al. Mammalian target of rapamycin complex 2 controls CD8 T cell memory differentiation in a foxo1-dependent manner. Cell Rep. 2016;14(5):1206–17. doi: 10.1016/j.celrep.2015.12.095. [DOI] [PubMed] [Google Scholar]
- 223.Pollizzi KN, Patel CH, Sun I-H, Oh M-H, Waickman AT, Wen J, et al. mTORC1 and mTORC2 selectively regulate CD8+ T cell differentiation. J Clin Invest. 2015;125(5):2090–108. doi: 10.1172/JCI77746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Brombacher EC, Everts B. Shaping of dendritic cell function by the metabolic micro-environment. Front Endocrinol. 2020 doi: 10.3389/fendo.2020.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lawless SJ, Kedia-Mehta N, Walls JF, McGarrigle R, Convery O, Sinclair LV, et al. Glucose represses dendritic cell-induced T cell responses. Nat Commun. 2017;8:15620. doi: 10.1038/ncomms15620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Everts B, Amiel E, van der Windt GJW, Freitas TC, Chott R, Yarasheski KE, et al. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120(7):1422–31. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Huh J-E, Yim J-H, Lee H-K, Moon E-Y, Rhee D-K, Pyo S. Prodigiosin isolated from Hahella chejuensis suppresses lipopolysaccharide-induced NO production by inhibiting p38 MAPK, JNK and NF-kappaB activation in murine peritoneal macrophages. Int Immunopharmacol. 2007;7(13):1825–33. doi: 10.1016/j.intimp.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 228.Abdelfattah MS, Elmallah MIY, Ebrahim HY, Almeer RS, Eltanany RMA, Abdel Moneim AE. Prodigiosins from a marine sponge-associated actinomycete attenuate HCl/ethanol-induced gastric lesion via antioxidant and anti-inflammatory mechanisms. PLoS ONE. 2019;14(6):e0216737. doi: 10.1371/journal.pone.0216737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–21. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hu J, Shang Y, Shi X, Zhang S, Shi J, Yao M, et al. Characterization of genomic alterations and the significance of PI3K/mTOR pathway mutations and tumor mutational burden in non-small cell lung cancer. Oncol Rep. 2020;43(6):2053–61. doi: 10.3892/or.2020.7559. [DOI] [PubMed] [Google Scholar]
- 231.Dong Z-Y, Zhong W-Z, Zhang X-C, Su J, Xie Z, Liu S-Y, et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017;23(12):3012–24. doi: 10.1158/1078-0432.CCR-16-2554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.