Abstract
Purpose
We examine prevalence, characteristics, quality of life (QOL) assessments, and long-term effects of interventions for laryngeal dysfunction after recovery from COVID-19 infection.
Materials and methods
653 patients presenting to Yale's COVID clinic from April 2020 to August 2021 were identified retrospectively. Patients with PCR-positive COVID-19 who underwent evaluation by fellowship-trained laryngologists were included. Patient demographics, comorbidities, intubation/tracheostomy, strobolaryngoscopy, voice metrics, and management data were collected. Patient-reported QOL indices were Dyspnea Index (DI), Cough Severity Index (CSI), Voice Handicap Index-10 (VHI-10), Eating Assessment Tool-10 (EAT-10), and Reflux Symptom Index (RSI).
Results
57 patients met inclusion criteria: 37 (64.9 %) were hospitalized for COVID-19 infection and 24 (42.1 %) required intubation. Mean duration between COVID-19 diagnosis and presentation to laryngology was significantly shorter for patients who were intubated compared to non-intubated (175 ± 98 days versus 256 ± 150 days, respectively, p = 0.025). Dysphonia was diagnosed in 40 (70.2 %) patients, dysphagia in 14 (25.0 %) patients, COVID-related laryngeal hypersensitivity in 13 (22.8 %), and laryngotracheal stenosis (LTS) in 10 (17.5 %) patients. Of the 17 patients who underwent voice therapy, 11 (64.7 %) reported improvement in their symptoms and 2 (11.8 %) patients reported resolution. VHI scores decreased for patients who reported symptom improvement. 7 (70 %) patients with LTS required >1 procedural intervention before symptom improvement. Improvement across QOL indices was seen in patients with LTS.
Conclusions
Laryngeal dysfunction commonly presents and is persistent for months after recovery from COVID-19 in non-hospitalized and non-intubated patients. Voice therapy and procedural interventions have the potential to address post-COVID laryngeal dysfunction.
Keywords: Laryngeal dysfunction, COVID-19, Voice therapy, Dysphonia, Laryngeal hypersensitivity, Quality of life
1. Introduction
The systemic inflammatory response associated with COVID-19 infection poses significant multi-organ system risk and predisposes patients to developing persistent symptoms, so-called ‘long COVID’, ‘long-haul COVID’ or ‘post-COVID syndrome’ [1], [2], [3]. The pathologic basis of ‘long COVID’ are thought to be a consequence of an overly activated immune response or compensatory immunosuppressive states which can manifest as pulmonary dysfunction, cardiac injury, coagulopathic syndrome, and neurologic disturbances, among others [2], [4]. In Otolaryngology, COVID-19 pathologic processes and management (e.g. intubation) contribute to the development of laryngeal and esophageal disorders [5], [6], [7], [8], [9], [10]. As the COVID-19 pandemic evolves and much of the population recovers from mild to severe COVID-19 infection, the character, duration, and nature of such disorders remain to be better elucidated.
Intubation rates for respiratory failure due to COVID-19 have ranged from 5 to 88 % from reports worldwide [11], [12], [13]. In general, intubation, tracheostomy and airway procedures pose significant risk for acute glottic injury or longer-term disorders such as laryngotracheal stenosis (LTS) [8]. Complications such as vocal cord damage, which include ulcerations, granulomas, and edema, and dysphagia have been documented [8], [14]. Effects of intubation due to COVID-19 have also been reported, including glottic stenosis, vocal cord immobility or erythema, voice changes, cricoid chondronecrosis, and swallowing dysfunction [5], [15]. Furthermore, neurologic injury that is virally-induced or secondary to inflammatory responses may impair control of vocal and swallowing structures [16]. Notably, dysphonia has been reported in 10–66 % of patients who were intubated, with dysphagia in 37–41 % [7], [17], [18], [19].
In non-intubated patients with COVID-19 infection, vocal fatigue, dry throat, increased phlegm production, dysphonia, cough, rhinitis, and dysphagia have been reported as direct symptoms of the virus [18], [20], [21], [22]. Dysphonia has been reported in 22–44 % of patients who did not require hospitalization for COVID-19 infection and dysphagia in 17 % of these patients [18], [21]. However, there remains a paucity of data examining the pathophysiology of these symptoms, as well as the effect of intubation on the severity of COVID-19 sequelae. Furthermore, the management of these otolaryngological symptoms has not been well-studied and the need for effective treatments persists.
Overall, laryngeal disorders present a tremendous burden on patients' quality of life and can persist for months [7]. As the COVID-19 pandemic evolves, the character, duration, and nature of such disorders remain to be better elucidated in order to best manage the enduring sequelae of the disease. This study aims to quantify the prevalence and nature of long-term laryngeal dysfunction in patients with varying severity of COVID-19 infection, including patients who were and were not intubated and those who were and were not hospitalized. We also investigate the treatment and follow-up outcomes of these patients to understand the impact of COVID-19 on laryngology clinics and provide a foundation of knowledge to further investigate and optimize the treatment practices of otolaryngologists. This is reinforced by our incorporation of comprehensive patient-reported quality of life measures and the effects of interventions for these laryngeal sequelae.
2. Materials and methods
2.1. Study design and population
This study was deemed exempt by the Yale University Institutional Review Board and conducted in compliance with regulatory and ethical guidelines. All patients who presented to the Yale COVID clinic, a longitudinal care clinic for patients recovering from COVID-19 infection, between July 2020 and August 2021 were identified through retrospective chart review. Patients with a documented diagnosis of COVID-19 with a positive reverse transcription polymerase chain reaction (RT-PCR) test who were referred for laryngeal dysfunction were included for analysis. Patients were excluded if they: 1) had a history of laryngeal changes prior to their COVID-19 infection; 2) absence of laboratory diagnosis for COVID-19 by RT-PCR; and 3) did not have a diagnosis of laryngeal dysfunction after being evaluated by fellowship-trained laryngologists.
2.2. Data collection
Demographic, comorbidity, hospitalization, intubation, and tracheostomy status, laryngological diagnoses, stroboscopy and laryngoscopy findings, and clinical interventions data were collected across initial and follow-up visits. The diagnosis of COVID-related laryngeal hypersensitivity, a diagnosis of exclusion, was based on the presence of persistent symptoms (e.g. dyspnea of unexplained etiology, cough, frequent throat clearing) even with maximal medical therapy for laryngopharyngeal reflux as documented by laryngologists in the patient medical record. Data from follow-up visits through February 2022 for each patient were collected. Follow-up duration was defined as time between initial presentation to a laryngologist and the last documented follow-up visit. Race/ethnicity was encoded as Caucasian Non-Hispanic, African-American Non-Hispanic, Hispanic or Latino, and other. Insurance status was categorized as Private/Managed, Medicaid, Medicare, and Not Insured. Patient-reported quality of life (QOL) outcomes were assessed by the Dyspnea Index (DI), Cough Severity Index (CSI), Eating Assessment Tool-10 (EAT-10), Voice Handicap Index-10 (VHI-10), and Reflux Symptom Index (RSI). Clinician-perceived voice evaluations for grade, roughness, breathiness, asthenia, and strain were also recorded [23], [24]. For patients who underwent voice therapy, number of sessions were obtained from Speech Language Pathology (SLP) notes and improvements or persistence in voice symptoms reported by patients documented in laryngology visit notes were captured.
2.3. Statistical analyses
Data were analyzed and visualized with Rstudio Version 1.4.1717 using R software with the tidyverse package. Distributions of hospitalization characteristics (e.g. intubation), QOL scores and voice evaluations upon initial visit were investigated across clinical diagnoses and demographic characteristics such as sex, race/ethnicity, and insurance status. Median values with interquartile range (IQR) were reported for patient-reported QOL indices. Univariate chi-squared analysis, Fisher's exact, unpaired t-tests, and Mann-Whitney U tests were used to examine differences across categorical and continuous variables between intubated and non-intubated patients in our cohort.
3. Results
3.1. Patient overview
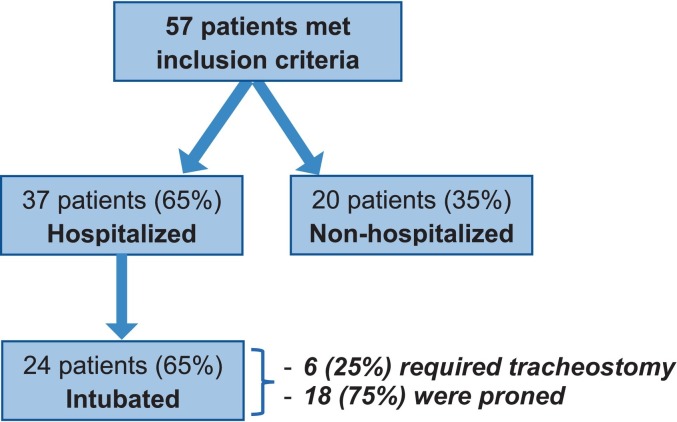
A total of 57 patients met inclusion criteria (Fig. 1 ). Of these, 37 (64.9 %) were hospitalized for COVID-19 infection. Among those who were admitted, 24 (64.9 %) required an ICU stay, 24 (64.9 %) were intubated, and 6 (16.2 %) required tracheostomy. Of the 24 patients who were intubated, 6 (25 %) underwent tracheostomy after a median intubation period of 19 days (IQR: 10–27 days) and 18 (75.0 %) were proned for a median duration of 3 days (IQR: 2–6 days).
Fig. 1.
Overview of patients in terms of hospitalization, intubation, tracheostomy, and pronation status.
Significant differences in age, sex, insurance status, and certain comorbidities (hypertension, type II diabetes mellitus, and cardiac conditions) were observed between intubated and non-intubated individuals (Table 1 ). The average time from COVID-19 infection to initial presentation to a laryngologist was significantly shorter for patients who were intubated compared to those who were not intubated (175 ± 98 days versus 256 ± 150 days, respectively, p = 0.025). Of the total cohort, 37 (64.9 %) patients had more than one visit with a laryngologist. Average follow-up duration for patients who were intubated was 268 ± 150 days and 188 ± 128 days for those who were not intubated (p = 0.103).
Table 1.
Baseline characteristics of patients who were intubated and were not intubated.
| All patients N = 57 |
Not intubated N = 33 |
Intubated N = 24 |
p-Value | |
|---|---|---|---|---|
| Age at COVID Diagnosis, Mean (SD) | 52.7 (13.3) | 47.2 (12.3) | 60.3 (10.8) | <0.001 |
| Sex | 0.001 | |||
| F | 37 (64.9) | 28 (84.8) | 9 (37.5) | |
| M | 20 (35.1) | 5 (15.2) | 15 (62.5) | |
| Race/ethnicity | 0.622 | |||
| Caucasian, non-Hispanic | 27 (47.4) | 18 (54.5) | 9 (37.5) | |
| African American, non-Hispanic | 15 (26.3) | 8 (24.2) | 7 (29.2) | |
| Hispanic or Latino | 13 (22.8) | 6 (18.2) | 7 (29.2) | |
| Other | 2 (3.5) | 1 (3.0) | 1 (4.2) | |
| Insurance status | 0.003 | |||
| Medicaid | 19 (33.3) | 11 (33.3) | 8 (33.3) | |
| Medicare | 12 (21.1) | 2 (6.1) | 10 (41.7) | |
| Private | 25 (43.8) | 19 (57.6) | 6 (25.0) | |
| Not insured | 1 (1.8) | 1 (3.0) | 0 (0) | |
| Tobacco use | 0.252 | |||
| Current | 2 (3.5) | 1 (3.0) | 1 (4.2) | |
| Former | 22 (38.6) | 12 (36.4) | 10 (41.7) | |
| Never | 33 (57.9) | 20 (60.6) | 13 (54.2) | |
| Average pack years, mean (SD) | 6.3 (15.4) | 3.2 (6.0) | 10.6 (22.7) | 0.082 |
| Comorbidities | 0.066 | |||
| Alcohol use disorder | 3 (5.3) | 2 (6.1) | 1 (4.2) | >0.999 |
| Asthma | 18 (31.6) | 13 (39.4) | 5 (20.8) | 0.161 |
| Cardiac | 15 (26.3) | 5 (15.2) | 10 (41.7) | 0.035 |
| COPD | 2 (3.5) | 0 (0) | 2 (8.3) | 0.173 |
| History of pneumonia or chest infection | 4 (7.0) | 1 (3.0) | 3 (12.5) | 0.300 |
| Hypertension | 26 (45.6) | 9 (27.3) | 15 (62.5) | 0.014 |
| Obstructive sleep apnea | 11 (19.3) | 6 (18.2) | 5 (20.8) | >0.999 |
| Type 2 diabetes mellitus | 15 (26.3) | 5 (15.2) | 10 (41.7) | 0.035 |
| No comorbidities | 12 (21.1) | 9 (27.3) | 3 (12.5) | 0.208 |
3.2. Laryngological diagnoses
The most common diagnosis was dysphonia, which was observed in 40 (70.2 %) patients. Dysphonia was attributed to muscle tension dysphonia (MTD), paresis/paralysis, and structural etiologies in 22, 20, and 11 patients, respectively (Table 2). Presentation, treatment, and outcome characteristics of patients with dysphonia (N = 40 total) are presented by severity of COVID-19 infection delineated by hospitalization, intubation, and tracheostomy status (Supplementary Table). Dysphagia was diagnosed in 14 (25.0 %) patients, with a slight preponderance in non-intubated individuals almost reaching statistical significance (p = 0.072). Laryngotracheal stenosis (LTS) was diagnosed in 10 (17.5 %) patients, laryngopharyngeal reflux (LPR) in 9 (15.8 %), and globus in 5 (8.8 %). A separate disease entity, COVID-related laryngeal hypersensitivity was seen in 13 (22.8 %) patients and significantly more prevalent in non-intubated patients compared to intubated patients (36.4 % vs. 4.2 %, p = 0.004). Additionally, diagnosis of dyspnea of unknown etiology was made in 6 (10.5 %) patients.
Table 2.
Laryngological presentation, diagnoses, and follow-up for patients who were intubated and those who were not intubated.
| All patients N = 57 |
Not intubated N = 33 |
Intubated N = 24 |
p-Value | |
|---|---|---|---|---|
| Days to initial presentation to laryngology, mean (SD) | 222 (136) | 256 (150) | 175 (98) | 0.025 |
| Diagnoses | <0.001 | |||
| COVID-related laryngeal hypersensitivity | 13 (22.8) | 12 (36.4) | 1 (4.2) | 0.004 |
| Dysphagia | 14 (24.6) | 11 (33.3) | 3 (12.5) | 0.072 |
| Dysphonia | 40 (70.2) | 20 (60.6) | 20 (83.3) | 0.083 |
| Muscle tension dysphonia | 22 (38.6) | 12 (36.4) | 10 (41.7) | |
| Paresis/paralysis | 20 (35.1) | 9 (27.3) | 11 (45.8) | |
| Structural | 21 (36.8) | 6 (18.2) | 5 (20.8) | |
| Dyspnea of unknown etiology | 6 (10.5) | 5 (15.2) | 1 (4.2) | 0.385 |
| Globus | 5 (8.8) | 4 (12.1) | 1 (4.2) | 0.385 |
| Laryngopharyngeal reflux (LPR) | 9 (15.8) | 6 (18.2) | 3 (12.5) | 0.72 |
| Laryngotracheal stenosis (LTS) | 10 (17.5) | 0 (0) | 10 (41.7) | <0.001 |
| Post-intubation phonatory insufficiency (PIPI) | 2 (3.5) | 0 (0) | 2 (8.3) | 0.173 |
| Follow-up time, mean (SD) N = 37 with >1 visit |
232 (144) | 188 (128) | 268 (150) | 0.103 |
3.3. Stroboscopy findings
Of the 39 patients who underwent stroboscopy, a normal stroboscopy exam was observed in 7 (17.9 %) patients. No significant differences were found in stroboscopy findings between patients who were and were not intubated (Fig. 2 ). The most common abnormality observed on stroboscopy exam was an asymmetric mucosal wave, which was seen in 15 (38.5 %) patients. With respect to other alterations, 11 (28.2 %) patients had unilateral vocal fold paresis, 9 (23.1 %) exhibited irregular periodicity, 8 (20.5 %) patients had decreased amplitude, and 4 (10.3 %) had incomplete glottic closure.
Fig. 2.
Stroboscopy findings for all patients (N = 39), who underwent stroboscopy during initial presentation to laryngology, categorized by intubation status.
3.4. Patient-reported quality of life
QOL indices were recorded for 45 (78.9 %) patients, of which 18 (40.0 %) had at least one follow-up appointment with QOL outcome survey data. On initial presentation to Otolaryngology, the median scores for VHI, DI and RSI were 11/40 (IQR: 5.5–24.0), 21/40 (IQR: 11.0–27.0), and 22/45 (IQR: 12–27). Median baseline scores of 11/40 (IQR: 5.0–21.0) and 4/40 (IQR: 1.0–12.0) were observed for CSI and EAT-10, respectively. Notably, the patients with dysphonia appeared to have higher baseline VHI scores and lower CSI scores when compared to patients without dysphonia (Fig. 3 ). Additionally. Patients with LTS were also observed to have considerably higher baseline QOL scores across all the indices, indicating worse QOL. Patients with COVID-related laryngeal hypersensitivity had comparable scores across QOL indices relative to patients without this diagnosis.
Fig. 3.
QOL index scores reported by patients during the initial visit across three diagnoses that were present in >10 patients in the cohort.
3.5. Clinician-perceived voice evaluations
During the initial presentation to laryngology, GRBAS scores were recorded for 36 (63.2 %) patients. Among these patients, 19 (52.8 %) had at least one follow-up appointment with GRBAS data. Median baseline GRBAS scores for patients were: 2.0 (IQR: 1.0–2.3) for grade, 1.0 (IQR: 0.0–2.0) for roughness, 0.0 (IQR: 0.0–0.0) for breathiness, 0.0 (IQR: 0.0–0.0) for asthenia, and 1.0 (IQR: 0.0–1.0) for strain.
3.6. Interventions and longitudinal outcomes
Patients in the cohort were recommended various interventions including voice therapy (42.1 %, n = 24), swallow function evaluation (29.8 %, n = 17), proton pump inhibitor (PPI) initiation (14.0 %, n = 8), and respiratory retraining therapy (8.8 %, n = 5) (Table 3 ). Ten patients (17.5 %) underwent procedural interventions such as dilations, steroid injections, excision of stenosis, and cordotomies.
Table 3.
Interventions recommended for patients who were and were not intubated.
| Intervention recommended | All patients N = 57 |
Not intubated N = 33 |
Intubated N = 24 |
p-Value |
|---|---|---|---|---|
| Cough suppression therapy | 2 (3.5) | 1 (3.0) | 1 (4.2) | >0.999 |
| Swallow function evaluationa | 17 (29.8) | 13 (39.4) | 4 (16.7) | 0.083 |
| Proton pump inhibitor | 8 (14.0) | 5 (15.2) | 3 (12.5) | >0.999 |
| Procedural intervention | 10 (17.5) | 1 (3.0) | 9 (37.5) | 0.001 |
| Reflux precautions | 7 (12.3) | 6 (18.2) | 1 (4.2) | 0.220 |
| Respiratory retraining therapy | 5 (8.8) | 5 (15.2) | 0 (0) | 0.067 |
| Speech therapy | 1 (1.8) | 1 (3.0) | 0 (0) | >0.999 |
| Vocal hygiene | 3 (5.3) | 2 (6.1) | 1 (4.2) | >0.999 |
| Voice therapy | 24 (42.1) | 14 (42.4) | 10 (41.7) | 0.111 |
| None | 1 (1.8) | 0 (0) | 1 (4.2) | 0.421 |
Denotes group of interventions comprising esophagram, modified barium swallow (MBS), esophagogastroduodenoscopy (EGD), or esophageal manometry (also called motility testing).
Of the 24 patients who were recommended for voice therapy, 17 patients (58.3 %) underwent voice therapy during the time frame captured by our dataset. Voice therapy status could not be assessed for 4 patients due to a single laryngology visit. Two patients declined voice therapy, and 1 patient had not begun voice therapy. Notably, of the 17 patients who underwent voice therapy, 11 (64.7 %) reported improvement in their symptoms and 2 (11.8 %) patients reported resolution. Among the patients reporting improvement or resolution, the median number of voice therapy sessions completed was 7 (IQR: 4 to 11). Three patients reported persistent symptoms while undergoing voice therapy with a median number of 2 (IQR: 2–3.5) completed sessions; these patients had additional diagnoses of PIPI, LPR, and COVID-related laryngeal hypersensitivity. One patient reported persistent dysphonia despite voice therapy completion (8 sessions); this patient had an additional diagnosis of COVID-related laryngeal hypersensitivity. VHI scores decreased for patients who reported improvement (Fig. 4 ). Clinician-perceived voice evaluations, measured by GRBAS scores, improved or remained stable for patients reporting improvement after voice therapy. QOL and GRBAS grade scores did not demonstrate other trends. Of the 5 patients who were recommended respiratory retraining therapy, 2 patients underwent this intervention. Both patients reported improvement in their dyspnea while undergoing respiratory retraining therapy.
Fig. 4.
Changes in VHI score (top) and grade (from GRABS; bottom) for patients who were recommended voice therapy.
Among the ten patients who were recommended and underwent procedural interventions, all ten (100 %) had a diagnosis of laryngotracheal stenosis and reported improvement in their symptoms. For these ten patients, the median time between presentation and last follow-up visit at which improvement was reported was 298 (IQR: 173–408) days. Seven (70 %) of these patients underwent more than one procedure prior to reported symptom improvement; median number of procedures was 3 (IQR: 2–5). Five of the ten patients had follow-up QOL index score data available which showed improvement across the indices: median QOL index score changes were −4 (IQR: −3 to −14) for the DI, −3 (IQR: −3 to −25) for the CSI, 0 for (IQR: 1 to −7) for the EAT-10, −4 (IQR: −2 to −9) for the VHI, and −6 (IQR: −1 to −17) for the RSI.
4. Discussion
Our findings underscore the considerable long-term burden of laryngeal dysfunction in patients after recovery from COVID-19 infection of varying severity. To our knowledge, our study is the first in these patients to provide clinical insights by incorporating data from quality of life (QOL) indices, voice evaluations, and long-term follow-up clinical outcomes data after various interventions. In our cohort of 57 patients, we found that patients initially presented 222 days (±136 days) after confirmation of COVID-19 infection, and all patients presented at least 90 days from COVID-19. Among the 37 (74.0 %) patients who were seen for follow-up, the average follow-up period was 232 days (±144 days).
Our results offer valuable insight and comparison to previous studies to highlight the variety and prolonged impact of laryngeal disorders that can produce profound effects on patients' QOL. Consistent with prior work, patients in our cohort who were intubated were significantly older [25]. Interestingly, the proportions of intubated patients that were male, had cardiac comorbidities, hypertension, or diabetes were significantly greater compared to non-intubated patients. COVID-related laryngeal hypersensitivity was significantly more prevalent in non-intubated patients; this is consistent with the findings of Allisan-Arrighi [25], who reported laryngeal hypersensitivity in four non-intubated patients, and our study marks a three-fold expansion in the number of patients with this diagnosis. The significantly longer time to laryngology presentation for non-intubated patients compared to intubated patients may suggest a need for greater awareness of laryngeal sequelae among general clinicians, who may attribute these laryngeal symptoms to other causes besides COVID-19 or as idiopathic. Greater awareness of laryngeal manifestations, such as laryngeal hypersensitivity, presenting long after COVID-19 recovery and consideration of key clinical and demographic characteristics, such as intubation status, sex, insurance, comorbidities, etc., that can impact these laryngological presentations can inform how otolaryngologists and general clinicians diagnose and care for these patients.
Our study identifies the considerable burden of laryngeal sequelae in patients who were not hospitalized or intubated. Our cohort differed from those of prior studies [15], [26] in a few ways including a smaller proportion of hospitalized and intubated patients, thus offering greater context for the long-term COVID-related effects for patients who were not subjected to airway trauma or severe systemic inflammation. Among their cohort of 24 patients, Neevel et al. reported that 20 (83 %) patients were hospitalized with 18 (75 %) intubated and 10 (42 %) with tracheostomy [26]. Their cohort presented earlier with a median of 107 days, compared to 222 days for our cohort, and similarly saw a preponderance of dysphonia (79 %), including muscle tension dysphonia observed in 67 % of non-intubated patients [26]. Ten percent of patients in our cohort presented to laryngology >1 year after COVID-19 infection, indicating the delayed onset, longer-term potential impact of COVID-19 and representing a growing population that may become important for clinicians to be aware of. In addition to the delayed onset and prolonged duration of symptoms captured in our study, our findings suggest that the quality and nature of complaints may have also shifted. For example, in this prior study [26], dyspnea was observed in 71 % of patients, compared to 10.5 % in our study. This difference may also be attributed to a more restrictive definition employed for ‘dyspnea of unknown etiology,’ which better reflects our understanding of the variable identifiable and iatrogenic etiologies of dyspnea in COVID-19 patients. Naunheim et al. investigated laryngeal complications in a prospective cohort of 20 patients, 65 % of whom were intubated for an average duration of 21.8 days; of those intubated, 69.2 % were proned [15]. While the rates of intubation and proning in our cohort are comparable to those of Naunheim, our cohort included a sizable proportion (n = 20, 35.1 %) of patients not hospitalized for COVID-19 infection presenting with laryngeal dysfunction. Given the milder forms of COVID-19 infection with newer variants, this group of non-hospitalized patients with laryngeal dysfunction represents an important growing population that may present to otolaryngologists in the coming years.
Direct airway trauma or prolonged inflammation from infectious responses can independently and synergistically produce voice and swallowing symptoms. Endotracheal intubation has been commonly associated with a variety of injuries: a systematic review of 9 studies, comprising 775 ICU patients observed laryngeal injuries in 83 % of cases, with the most common symptoms being dysphonia (76 %), pain (76 %), hoarseness (63 %), and dysphagia (49 %) [27]. Persistent dysphonia and dysphagia have been shown to correlate significantly among ICU patients after recovery [17], [28].
Vocal concerns also emerge from processes unrelated to intubation and clinical airway management. In a cohort of 160 patients who were not hospitalized for their COVID-19 infection, dysphonia was diagnosed in 44 % of these patients by physicians through telehealth visits [21]. Notably, the authors reported that dysphonia persisted over 2 weeks in 33/70 (47.1 %) patients, and >1 month in 11/70 (15.7 %); patients were followed for maximum of 3 months [21]. In our cohort, average time to initial presentation and follow-up time were 222 days (±136 days) and 232 days (±144 days), respectively, indicating that dysphonia and other laryngological symptoms can present and persist for many months after COVID-19 infection. For patient-reported outcomes, although our study was not powered to investigate statistical differences in QOL scores for various diagnoses, trends did emerge: for example, VHI scores were higher among patients with dysphonia compared to those without a diagnosis of dysphonia. This finding is consistent with those reported by Tahir et al. that found significantly higher VHI scores in patients with COVID-19-related dysphonia compared to healthy controls [29].
Beyond characterization of laryngeal sequelae, this study describes the long-term effects of interventions for these sequelae, focusing on voice therapy and procedural interventions. Of the 17 patients who underwent voice therapy, the majority of them reported improvement over the course of follow-up; mean follow-up time was 260 days (±136 days) for these patients. The literature on the efficacy of voice therapy for patients with laryngeal dysfunction due to COVID-19 is nascent. One case series examining vocal fold paresis and paralysis after COVID-19 infection reported that after referral for voice therapy or in-office injection, patients did not go on to require additional intervention [16]. However, patients in this case series did not return for follow-up, which the authors attributed to possible improvement with voice therapy or spontaneous resolution of paresis [16]. Our study provides evidence of improvement after voice therapy with follow-up visits and with a trend towards decreasing, therefore improving, VHI scores in those patients who reported improvement. Clinician-perceived voice evaluations captured by GRBAS scores also improved or remained stable for patients reporting improvement, providing both patient- and physician-reported data suggesting the benefit of voice therapy in these patients with long-term laryngeal dysfunction after COVID-19.
For patients who underwent procedural interventions, all ten had a diagnosis of LTS and 70 % of them required more than one procedure prior to reported symptom improvement. Similarly, in their case series, Scholfield et al. found that all 3 patients required multiple (2–4) endoscopic procedures for recurrent stenosis due to COVID-19 [30]. Watson et al. found that 4/14 patients, who had significant laryngeal injury post-intubation for COVID-19, required a second airway procedure for persisting symptoms [31]. While a greater proportion of our patients required more than one intervention, this may be explained by the longer follow-up time in our study as Watson et al. had a maximum follow-up of six weeks after intervention. Furthermore, for 5 patients with LTS who underwent procedures, we observed trends in improvement in scores across QOL indices at follow-up. Thus, our findings can be regarded as an initial step towards understanding the interventions needed to care for patients with long-term laryngeal sequelae after recovery from COVID-19 infection.
Our study has several limitations. Although a comprehensive chart review was performed for all patients in our cohort, our dataset was limited by missing data for certain key points including QOL indices and clinician-reported voice evaluations, among others. Even if fully represented among our datasets, the subjective nature of QOL surveys completed by patients may influence the reliability of these metrics in understanding patient symptom severity and improvement. While we systematically identified patients through our institution's established longitudinal COVID clinic, our dataset is limited by its moderate sample size and retrospective nature, which are partly explained by the recency of the COVID-19 pandemic. As a consequence, our study was not powered to detect statistical differences in QOL scores, stroboscopy findings, and interventions between distinct laryngological diagnoses or through stratification by critical hospitalization-related features such as presence of intubation, tracheostomy, or proning. Future studies are needed to continue investigating speech and swallow disorders with larger cohort sizes and over extended timelines, with an emphasis on post-intervention outcomes and more objective assessments of these outcomes.
5. Conclusion
This study comprehensively describes laryngeal dysfunction in patients who have recovered from varying severities of COVID-19 infection (e.g. intubated, non-intubated, non-hospitalized). Even among non-intubated and non-hospitalized patients, laryngeal dysfunction commonly presents and is persistent for months after recovery from COVID-19. Interventions such as procedures for laryngotracheal stenosis and voice therapy can be beneficial for these patients with post-COVID laryngeal sequelae. Future directions include assessing benefits of these interventions more objectively in larger cohorts.
The following is the supplementary data related to this article.
Presentation, treatment, and outcome characteristics of patients with a diagnosis of dysphonia (N = 40 total).
Funding sources
None.
Declaration of competing interest
None.
Footnotes
This project has been approved by the Yale University Institutional Review Board (IRB 2000031332) on 9/29/2021.
Meeting information: The abstract of this paper was accepted as an Oral Podium Presentation at the American Laryngological Association Combined Spring Meetings in Dallas, Texas, USA held April 27-May 1, 2022.
References
- 1.Mehandru S., Merad M. Pathological sequelae of long-haul COVID. Nat Immunol. 2022;23(2):194–202. doi: 10.1038/s41590-021-01104-y. 2022/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oronsky B., Larson C., Hammond T.C., et al. A review of persistent post-COVID syndrome (PPCS) Clin Rev Allergy Immunol. 2021 doi: 10.1007/s12016-021-08848-3. 2021/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang F., Kream R.M., Stefano G.B. Long-term respiratory and neurological sequelae of COVID-19. Med Sci Monit. 2020;26 doi: 10.12659/MSM.928996. e928996-e928996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osbeck Sandblom H., Dotevall H., Svennerholm K., Tuomi L., Finizia C. Characterization of dysphagia and laryngeal findings in COVID-19 patients treated in the ICU-an observational clinical study. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0252347. e0252347-e0252347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voisin N., Tringali S., Fieux M. Laryngeal dyspnoea and COVID-19. Eur Ann Otorhinolaryngol Head Neck Dis. 2021;138(5):415–416. doi: 10.1016/j.anorl.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regan J., Walshe M., Lavan S. Dysphagia, dysphonia, and dysarthria outcomes among adults hospitalized with COVID-19 across Ireland. Laryngoscope. 2021 doi: 10.1002/lary.29900. doi:10.1002/lary.29900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piazza C., Filauro M., Dikkers F.G., et al. Long-term intubation and high rate of tracheostomy in COVID-19 patients might determine an unprecedented increase of airway stenoses: a call to action from the european laryngological society. Eur Arch Otorhinolaryngol. 2021;278(1):1–7. doi: 10.1007/s00405-020-06112-6. 2021/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lechien J.R., Circiu M.P., Crevier-Buchman L., Hans S. Post-COVID-19 paradoxical vocal fold movement disorder. Eur Arch Otorhinolaryngol. 2021;278(3):845–846. doi: 10.1007/s00405-020-06391-z. 2021/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miles A., McRae J., Clunie G., et al. An international commentary on dysphagia and dysphonia during the COVID-19 pandemic. Dysphagia. 2022 doi: 10.1007/s00455-021-10396-z. 2022/01/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohammadi M., Khafaee Pour Khamseh A., Varpaei H.A. Invasive airway "Intubation" in COVID-19 patients; statistics, causes, and recommendations: a review article. Review article. Anesth Pain Med. 2021;11(3) doi: 10.5812/aapm.115868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hur K., Price C.P.E., Gray E.L., et al. Factors associated with intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngol Head Neck Surg. 2020;163(1):170–178. doi: 10.1177/0194599820929640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishikimi M., Rasul R., Sison C.P. Intubated COVID-19 predictive (ICOP) score for early mortality after intubation in patients with COVID-19. Sci Rep. 2021;11(1):21124. doi: 10.1038/s41598-021-00591-1. 2021/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colice G.L., Stukel T.A., Dain B. Laryngeal complications of prolonged intubation. Chest. 1989;96(4):877–884. doi: 10.1378/chest.96.4.877. 1989/10/01/ [DOI] [PubMed] [Google Scholar]
- 15.Naunheim M.R., Zhou A.S., Puka E., et al. Laryngeal complications of COVID-19. Laryngoscope Investig Otolaryngol. 2020;5(6):1117–1124. doi: 10.1002/lio2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rapoport S.K., Alnouri G., Sataloff R.T., Woo P. Acute vocal fold paresis and paralysis after COVID-19 infection: a case series. Ann Otol Rhinol Laryngol. 2021 doi: 10.1177/00034894211047829. [DOI] [PubMed] [Google Scholar]
- 17.Leis-Cofiño C., Arriero-Sánchez P., González-Herranz R., Arenas-Brítez Ó., Hernández-García E., Plaza G. Persistent dysphonia in hospitalized COVID-19 patients. J Voice. 2021 doi: 10.1016/j.jvoice.2021.07.001. S0892-1997(21)00234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Ani R.M., Rashid R.A. Prevalence of dysphonia due to COVID-19 at Salahaddin General Hospital, Tikrit City, Iraq. Am J Otolaryngol. Sep-Oct 2021;42(5) doi: 10.1016/j.amjoto.2021.103157. 103157-103157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Printza A., Tedla M., Frajkova Z., Sapalidis K., Triaridis S. Dysphagia severity and management in patients with COVID-19. Curr Health Sci J. 2021;47(2):147–156. doi: 10.12865/CHSJ.47.02.01. Apr-Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dassie-Leite A.P., Gueths T.P., Ribeiro V.V., Pereira E.C., Martins P.D.N., Daniel C.R. Vocal signs and symptoms related to COVID-19 and risk factors for their persistence. J Voice. 2021 doi: 10.1016/j.jvoice.2021.07.013. S0892-1997(21)00253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantarella G., Aldè M., Consonni D. Prevalence of dysphonia in non hospitalized patients with COVID-19 in Lombardy, the Italian Epicenter of the Pandemic. J Voice. 2021 doi: 10.1016/j.jvoice.2021.03.009. S0892-1997(21)00108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azzam A.A.A., Samy A., Sefein I., ElRouby I. Vocal disorders in patients with COVID 19 in Egypt. Indian J Otolaryngol Head Neck Surg. 2021:1–7. doi: 10.1007/s12070-021-02663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karnell M.P., Melton S.D., Childes J.M., Coleman T.C., Dailey S.A., Hoffman H.T. Reliability of clinician-based (GRBAS and CAPE-V) and patient-based (V-RQOL and IPVI) documentation of voice disorders. J Voice. Sep 2007;21(5):576–590. doi: 10.1016/j.jvoice.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Hirano M., McCormick K.R. Clinical examination of voice by minoru hirano. J Acoust Soc Am. 1986;80(4) doi: 10.1121/1.393788. 1986/10/01. 1273-1273. [DOI] [Google Scholar]
- 25.Allisan-Arrighi A.E., Rapoport S.K., Laitman B.M., et al. Long-term upper aerodigestive sequelae as a result of infection with COVID-19. Laryngoscope Investig Otolaryngol. 2022;7(2):476–485. doi: 10.1002/lio2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neevel A.J., Smith J.D., Morrison R.J., Hogikyan N.D., Kupfer R.A., Stein A.P. Postacute COVID-19 laryngeal injury and dysfunction. OTO Open. 2021;5(3) doi: 10.1177/2473974X211041040. 2473974X211041040 2021/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brodsky M.B., Levy M.J., Jedlanek E., et al. Laryngeal injury and upper airway symptoms after Oral endotracheal intubation with mechanical ventilation during critical care: a systematic review. Crit Care Med. 2018;46(12):2010–2017. doi: 10.1097/CCM.0000000000003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagier A., Melotte E., Poncelet M., Remacle S., Meunier P. Swallowing function after severe COVID-19: early videofluoroscopic findings. Eur Arch Otorhinolaryngol. 2021;278(8):3119–3123. doi: 10.1007/s00405-020-06522-6. 2021/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tahir E., Kavaz E., Çengel Kurnaz S., Temoçin F., Atilla A. Patient reported voice handicap and auditory-perceptual voice assessment outcomes in patients with COVID-19. Logoped Phoniatr Vocol. 2021:1–10. doi: 10.1080/14015439.2021.2011958. [DOI] [PubMed] [Google Scholar]
- 30.Scholfield D.W., Warner E., Ahmed J., Ghufoor K. Subglottic and tracheal stenosis associated with coronavirus disease 2019. J Laryngol Otol. 2021;135(7):656–658. doi: 10.1017/S0022215121001134. [DOI] [PubMed] [Google Scholar]
- 31.Watson N.A., Karagama Y., Burnay V., Boztepe S., Warner S., Chevretton E.B. Effects of coronavirus disease-2019 on voice: our experience of laryngeal complications following mechanical ventilation in severe coronavirus disease-2019 pneumonitis and review of current literature. Curr Opin Otolaryngol Head Neck Surg. 2021;29(6):437–444. doi: 10.1097/MOO.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Presentation, treatment, and outcome characteristics of patients with a diagnosis of dysphonia (N = 40 total).