Abstract
It is essential to investigate the apical surface properties of both M cells and dome enterocytes to understand the mechanisms involved in the binding of pathogens to M cells. In rabbit appendix tissue, monoclonal antibodies (MAbs) highlight differences between M cells (MAb 58) and dome enterocytes (MAb 214). Such antibodies ultimately recognized intestinal mucin-related epitopes. To further characterize these differences, the labeling patterns obtained with these MAbs were compared to those obtained with other antibodies to intestinal mucins on dissected domes from all gut-associated lymphoid tissues. A glycoprotein recognized by MAb 58 was purified on a CsCl isopycnic density gradient and microsequenced, and its mRNA expression was localized by in situ hybridization. It was identified as the rabbit homologue of human Muc2, i.e., the major mucin secreted in intestine tissue. Two other Muc2 carbohydrate epitopes were also expressed on M cells, although Muc2 mRNA was not detected. All results indicated that M cells express, on their apical membrane, glycoconjugates bearing at least three glycosidic epitopes from Muc2. MAb 214 and MAb 6G2, which recognized a partially characterized mucin expressed on dome enterocytes, were negative markers for M cells in rabbit gut-associated lymphoid tissues. We propose that the presence, on the surface of M cells, of carbohydrates also expressed on Muc2, together with the absence of an enterocyte-associated mucin, could favor pathogen attachment and accessibility to the M-cell luminal membrane.
The gut-associated lymphoid tissue (GALT) dispersed along the gastrointestinal tract is the main defense against pathogens, which can proliferate in this favorable environment. M cells are specialized GALT epithelial cells that select and transport pathogens across follicle-associated epithelium (FAE) to the lymphoid tissues in which the protective immune response takes place (for reviews, see references 15, 21, and 23). Why pathogens selectively gain access to and are trapped at the surface of M cells is still a matter of debate. Indeed, the rather poorly developed glycocalyx on the apical surface of M cells (compared to enterocytes) might constitute a small-sized selective barrier to particles, therefore facilitating the accessibility of antigens to M cells (8). However, to understand the mechanism of the initial binding of pathogens to M cells, it is important to characterize the molecules exposed at the surface of the different dome epithelial cells. β1 integrin is the only described protein that may serve as a specific binding site for Yersinia pseudotuberculosis invasin at the apical membrane of mouse M cells (3). However, other mechanisms should contribute to interactions since invasin-deficient Yersinia spp. still bind to M cells with lower affinity (18). It has been proposed that carbohydrates could have an important role in pathogen recognition by epithelial cells (for a review, see reference 6). Hence, M cells may display a specific apical glycosylation pattern. In this respect, several lectins have been found to interact rather specifically with M cells, depending on their gut location and species (4, 9, 12). Such specific properties have even been used to target antigens to lymphoid tissues (7, 11). Knowledge of the surface properties of M cells is thus important for designing oral vaccines.
Using a monoclonal antibody (MAb) strategy, we recently documented the differential expression of specific epitopes at the apex of M cells and dome enterocytes in rabbit appendix FAE (16). Such epitopes are also expressed on mucins, particularly M-cell-specific carbohydrates. This might be a highly significant observation since several pathogens are known to interact with the carbohydrate moiety of purified intestinal mucins (17, 26, 30). MAb 58 recognizes a carbohydrate epitope expressed on M-cell apical surfaces, as well as on endocytic vesicles and the Golgi complex of M cells (16). It also recognizes mucin in secretory granules and adherent mucus. It is not yet known whether the epitope expressed on M cells belongs to a membranous form of an unknown mucin or to another cross-reacting molecule. MAb 214 recognized a mucin peptidic epitope present on the apical surface of dome enterocytes.
In this study, we used MAbs to epitopes expressed on intestinal mucins and compared their distribution with that of MAb 58 and MAb 214 on dome epithelia in the different rabbit GALTs. We showed that three different carbohydrate epitopes from the apex of rabbit M cells were also expressed on the rabbit equivalent of human mucin Muc2, whereas a dome enterocyte membrane-associated mucin was always absent from M-cell glycocalyx.
MATERIALS AND METHODS
Animals.
New Zealand albino rabbits weighing 2 to 3 kg were obtained from the Institut National de la Recherche Agronomique, Montpellier, France. Animals were housed and cared for according to French (87–848) and European (EC-L358) regulations.
Reagents.
Cesium chloride was from Gibco-BRL (Paisley, Scotland); benzonase, and biotin-coupled lectins, Ulex europaeus agglutinin, Vicia villosa agglutinin, wheat germ agglutinin, and Abrus precatorius agglutinin were from Sigma Chemical Co. (St. Louis, Mo.); and streptavidin-peroxidase was from Pasteur Production (Marnes-la-Coquette, France). All other chemicals were reagent grade.
Antibodies.
Goat anti-mouse immunoglobulin G (IgG) coupled to horseradish peroxidase (HRP), fluorescein isothiocyanate, or tetramethyl rhodamine isothiocyanate was from Biosys (Compiègne, France); 10-nm gold-coupled protein A was from the Utrecht University School of Medicine (Utrecht, The Netherlands); rabbit anti-mouse IgG was from Dako (Glostrup, Denmark); and HRP-conjugated sheep antidigoxigenin was from Roche Diagnostics (Meylan, France). Monoclonal antibodies to rabbit mucins 6G2 (24), 5H7 (2), and 3A4 (20) were a gift from Suzanne Maroux, Faculté des Sciences de Saint Jérôme, Marseille, France. The monoclonal antibody WE9 against human Muc2 (25) was a gift from Daniel Podolsky, Massachusetts General Hospital, Boston, Mass.
Immunofluorescence microscopy.
Tissues were fixed for 2 h with 2% formaldehyde in 100 mM potassium phosphate buffer (pH 7.4). Single domes were microdissected with a scalpel under a stereo microscope and stained in droplets of MAb diluted in Tris buffer, followed by fluorescein isothiocyanate- or tetramethyl rhodamine isothiocyanate-coupled secondary antibodies, as previously described (16). Stained domes were mounted with Mowiol containing 1.4-diazabicyclo[2.2.2.]octane (DABCO) between slides and coverslips, using coverslip debris as a spacer, sealed with a mixture of lanolin-mineral oil-paraffin (1:1:1), and observed with a TCS 4D confocal microscope (Leica Mikroskopie, Wetzlar, Federal Republic of Germany) equipped with an argon-krypton laser.
Electron microscopy.
Tissues were fixed for 1 h at room temperature with 2% formaldehyde–1% glutaraldehyde in 100 mM phosphate buffer (pH 7.2), dehydrated in graded alcohol, and embedded in Epon or in Lowicryl K4M according to the manufacturer's instructions. Sections were obtained with an RMC (Tucson, Ariz.) MT7000 ultramicrotome and immunolabeled for 90 min with MAb 3A4, followed by a rabbit anti-mouse IgG and by protein A Gold 10. The sections were stained with uranyl acetate and lead citrate and observed on a Hitachi H7100 electron microscope.
Cell dissociation and membrane preparation.
The appendix epithelium was detached with 40 mM EDTA and a postnuclear supernatant (1,000 × g × 10 min) was prepared as previously described (16). The total membrane fraction was collected by centrifugation on a discontinuous sucrose gradient. The postnuclear supernatant was loaded over 2 ml of 0.3 M sucrose overlying 1 ml of 2 M sucrose in Tris-HCl buffer (20 mM [pH 7.4])–5 mM MgCl2–20 mM Na2HPO4 containing a cocktail of protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 μg of antipain per ml, 1 μg of pepstatin per ml, 0.5 μg of leupeptin per ml, and 15 μg of benzamidine per ml), and centrifuged for 1 h at 100,000 × g at 4°C (28,000 rpm in an SW40Ti rotor from Beckman Instruments, Inc., Palo Alto, Calif). The supernatant (S100K) was stored at −20°C. The membrane fraction was collected at the 0.3 M-2 M sucrose interface and treated for 1 h at room temperature with a mixture of benzonase (25 U/ml) and RNase (9 μg/ml). The mixture was then adjusted to 100 mM Na2CO3 containing antiproteases (pH 11), incubated in ice for 30 min, centrifuged, and collected as described above. The supernatant (Scarb) was stored at −20°C until further use.
Purification of the mucin form of antigen 58. (i) gdn-HCl solubilization.
Freshly prepared, carbonate-washed membranes were diluted 30-fold in 6 M guanidine hydrochloride (gdn-HCl)–10 mM sodium phosphate (pH 6.5), and incubated overnight at 4°C with continuous rotation. A supernatant (Sgdn) and pellet (Pgdn) were obtained after centrifugation at 100,000 × g in a Ti50 rotor (Beckman Instruments, Inc.).
(ii) Triton X-100 extraction.
Pgdn was incubated overnight at 4°C with 0.5% Triton X-100 in 6 M gdn-HCI–10 mM sodium phosphate (pH 6.5) and centrifuged at 100,000 × g for 1 h to obtain a supernatant (STX) and a pellet (PTX).
(iii) Insoluble mucin extraction by reduction of the pellet PTX.
The final pellet (PTX) was reduced with 10 mM dithiothreitol (DTT) in 100 mM Tris-HCl, (pH 8.0)–6 M gdn-HCl–5 mM EDTA for 5 h at 37°C, alkylated overnight with 25 mM iodoacetamide at 4°C (1), and centrifuged at 100,000 × g for 1 h to produce a supernatant (SDTT) and a pellet (PDTT).
(iv) Density gradient centrifugation.
S100K, Scarb, Sgdn, STX, and SDTT were adjusted to 35 ml with 6 M gdn-HCl–10 mM Na2HPO4 (pH 6.5). CsCl was added until a density of 1.42 was reached and the solutions were centrifuged at 206,000 × g for 36 h at 10°C (50,000 rpm in a Vti 50 rotor, Beckman Instrument Inc.); 1.1-ml fractions were then collected with an Autodensiflow fraction collector (Labconco, Kansas City, Mo.). The density of each fraction was measured by weighting 300 μl in a Pedersen pipette. The fractions were analyzed by dot blot analysis, as described below, and the fractions of interest were pooled and stored at 4°C until use. Pooled fractions were dialyzed overnight against 3 100-ml volumes of phosphate-buffered saline (PBS; 1/10) and concentrated 10-fold with a Speedvac apparatus.
Dot blot assay.
Ten microliters of each fraction was spotted on a prewetted Immobilon-P membrane (Millipore Corp., Bedford, Mass.) and analyzed by Coomassie blue and periodic acid-Schiff (PAS) staining. MAbs or biotinylated lectins were incubated with 2 μl of spotted fraction, followed by peroxidase-labeled anti-mouse antibodies or streptavidin conjugated to peroxidase and an enhanced chemiluminescence kit (Amersham, Buckinghamshire, United Kingdom). Signal intensity was evaluated with the ImageQuant program after scanning the spots with a Duoscan Agfa.
Immunoprecipitation of purified mucins.
Thirty microliters of the pooled fractions was diluted 100-fold in Tris-saline buffer containing 1% Triton X-100 and incubated overnight with 1 μg of purified MAb 58 or 1 μl of MAb 5H7 ascites and 10 μl of a 50% protein A-Sepharose slurry. Immunoprecipitates were washed five times with the incubation buffer before elution in Laemmli sample buffer and were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Gel electrophoresis and immunoblotting.
Membrane proteins solubilized in PBS were quantified by the bicinchoninic acid protein assay (BCA) kit (Pierce Chemical, Rockford, Ill.) using bovine serum albumin as the standard. Nonreduced or reduced samples (100 mM DTT in sample buffer, 3-min boiling) were run on an SDS-2 to 10% PAGE gradient with a 60/1 acrylamide/bisacrylamide ratio. Molecular weight markers were polymers of phosphorylase b (Sigma) with apparent molecular masses of 97, 195, 292, and 390 kDa. Separated proteins were electrotransferred from the gel to Immobilon-P membranes with a semidry apparatus. The membranes were stained with Coomassie blue or PAS, and antigens were detected using hybridoma culture supernatants or ascites (diluted 1/1,000) followed by goat anti-mouse antibody conjugated to HRP and then enhanced chemiluminescence.
Attempts to characterize a membrane glycoprotein form of antigen 58.
Membrane proteins were extracted from freshly prepared membranes with 1% Triton X-100 in Tris-HCl buffer (pH 7.4) at 4°C for one night. Alternatively, in an attempt to solubilize Muc2 and discard it from the membrane fraction, membranes were reduced overnight with 10 mM DTT in Tris-HCl buffer (pH 8.0), followed by alkylation with 25 mM iodoacetamide for 5 h.
Both preparations were centrifuged at 100,000 × g, and the fractions containing the membrane proteins, Triton X-100 supernatant, or DTT pellet were analyzed for the presence of MAb 58 epitope by immunoblotting as described above.
N-terminal amino acid sequence determination.
The 150-kDa band of the purified mucous form of antigen 58 was excised from Immobilon-P membranes and sequenced by the Centre Commun de Séquençage des Protéines (Lyon, France).
In situ hybridization procedure.
Freshly excised appendix and Peyer's patch tissues were frozen in embedding medium and stored at −80°C. Sections 8 to 10 μm thick were cut in a Reichert 2700 cryostat, collected on Superfrost/Plus Gold slides (Sigma), and stored at −80°C. Prior to hybridization, tissue sections were fixed in 4% paraformaldehyde in PBS for 10 min, rinsed twice for 10 min in PBS, treated for 15 min with 1% H2O2 in PBS to inactivate endogenous peroxidase, and rinsed twice for 10 min in 2× SSC (1× SSC is 150 mM NaCl plus 15 mM sodium citrate [pH 7]).
Anti-sense oligonucleotides corresponding to the N-terminal 15-amino-acid sequence of the human link peptide 5′-GCC-CTG-GTA-GCT-GTA-GTA-GAG-TCC-GTC-GAA-GGT-GAC-ATA-GTG-CGG-3′ and of the rat link peptide 5′-ACC-CTG-GTA-ACT-GTA-GTA-AAG-TCC-ATC-GAA-GGT-GAC- AAA-GTG-TGG-3′ were synthesized by Eurogentech (Seraing, Belgium) and 3′ end labeled using a digoxigenin oligonucleotide tailing kit (Roche Diagnostics, Meylan, France).
Hybridization was carried out in a wet chamber at room temperature for 15 h in RPN 3310 hybridization buffer (Amersham, Buckinghamshire, United Kingdom) containing 50% deionized formamide and 100 pmol of labeled oligonucleotide per ml. After hybridization, the slides were washed for 5 min at 4°C in 4× SSC, 15 min plus 45 min at room temperature in 2× SSC and 15 min plus 45 min at room temperature in 1× SSC. After 1 h of incubation with HRP-conjugated sheep antidigoxigenin antibodies (1/500), cytochemical detection was carried out using the Tyramide Signal Amplification-Direct system (NEN Life Science Products-France SA, Paris, France). Slides were mounted with Mowiol mounting medium and observed as described above.
RESULTS
Specific glycoproteins are differently expressed on M cells and dome enterocytes.
We used MAb 58 and MAb 214, which recognize M cells and dome enterocytes, respectively (Fig. 1; see reference 16), and compared their labeling in the different rabbit GALTs with that of other antibodies to rabbit intestinal mucin-related epitopes. This was performed on domes isolated by microdissection, which gave a large surface view of FAE and highlighted differences between adherent mucus and apical membrane-specific labeling (Fig. 2a and c). The amount of adherent mucus varied according to the FAE location. It was extensively present in the sacculus rotundus, less was present in the appendix, and it was almost absent in cecal and Peyer's patches. The fact that sacculus rotundus and appendix domes were buried in surrounding mucosa rich in goblet cells might explain the importance of adherent mucus in these tissues.
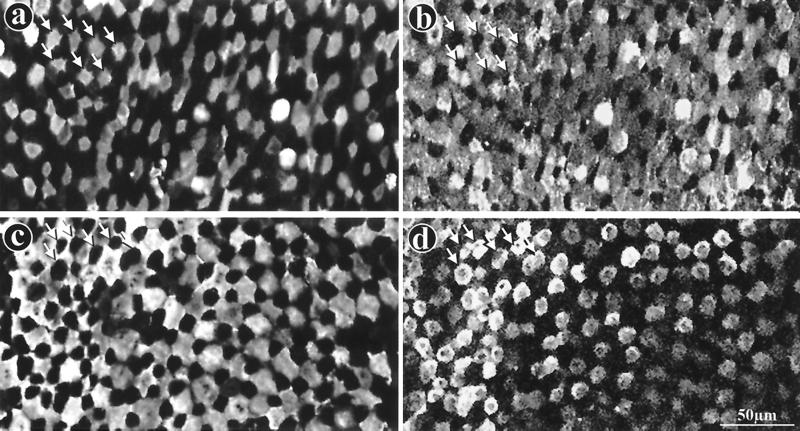
FIG. 1.
Complementary labeling of M cells and enterocytes by MAb 58 and MAb 214 in rabbit appendix and Peyer's patch FAE. Whole domes from a rabbit appendix (a, b) or a Peyer's patch (c, d) were fixed, double labeled with dichlorotriazinyl aminofluorescein (DTAF)-coupled MAb 58 (a, c) and with MAb 214 and secondary RITC-coupled antibody (b, d), and viewed by confocal microscopy, as described in Materials and Methods. Panels a and b and panels c and d, respectively, are surface views of the same two fields. Note complementarity between M-cell (a, c) and enterocyte (b, d) apical surface staining in both tissues (see arrows pointing to M cells [a and b] and to enterocytes [c and d]). M cells appear to be surrounded by enterocytes in the appendix (a, b) whereas they appear to encompass enterocytes in Peyer's patches (c, d).
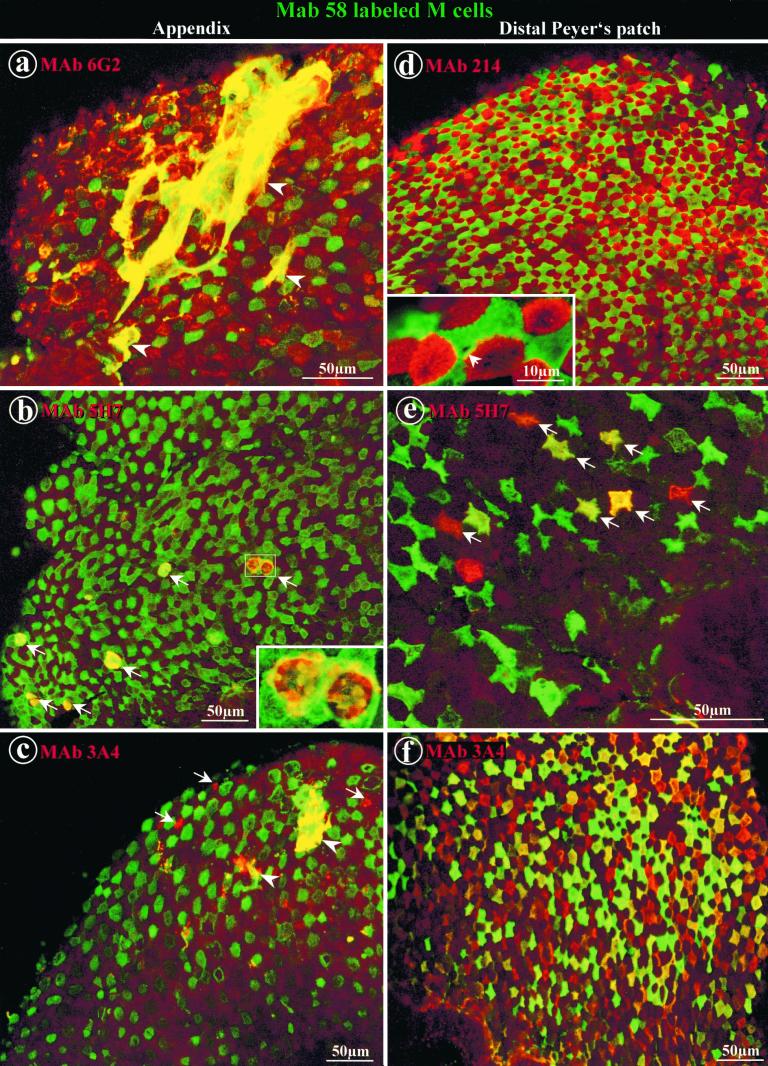
FIG. 2.
Double labeling of whole domes of rabbit appendix and distal Peyer's patches with MAb 58 and other antibodies to mucin-related epitopes. Direct labeling with DTAF-coupled MAb 58 (green) in the appendix (a through c) and in distal Peyer's patches (d through f) was concentrated on the apex of M cells. (a) Indirect labeling of the appendix with MAb 6G2-RITC (red) was located on the apices of dome enterocytes. Filamentous networks of adherent mucus extending over several adjacent cells appeared yellow in color due to colocalization of MAb 58 and MAb 6G2 (arrowheads). (b) Indirect labeling of appendix tissue with MAb 5H7-RITC (red) was observed on a few round-shaped goblet cells at the base of domes (arrows), where it partially colocalized with MAb 58. Note the higher-magnification inset showing the double-labeled secretory granules of the two delineated adjacent goblet cells. (c) Indirect labeling of appendix with MAb 3A4-RITC (red) was present on rare dome enterocytes (arrows) and on adherent mucus (arrowheads). (d) Labeling of a distal Peyer's patch with MAb 214-RITC (red) together with MAb 58 (green) displays a typical mosaic pattern. At higher magnification (inset), packed microvilli are clearly recognizable. A circular area of one M cell appeared not to be labeled by MAb 58 (arrow). (e) Indirect labeling with MAb 5H7-RITC (red) was present on less than 10% of M cells, which are recognizable by their star shape. Colocalization with MAb 58 on these M cells appeared in various colors from green to red (arrows). (f) Indirect labeling with MAb 3A4-RITC (red) was observed on M cells which appeared in various shades of red, yellow, and green due to different concentrations of the two carbohydrate epitopes recognized by MAb 58 and MAb 3A4.
(i) Appendix.
In rabbit appendix tissue, MAb 58 labeled M cells in 80% of the 20 rabbits tested (Fig. 1a and 2a through c). MAb 214 labeled all dome enterocytes of the FAE (Fig. 1b) but not those of the surrounding mushroom-like mucosa, except on the flat apical luminal surface (not shown). MAb 6G2, which is specific to a peptide epitope of a rabbit new intestinal mucin (24), gave the same labeling pattern as MAb 214 on all of the lymphoid tissues tested (Fig. 2a; Table 1). MAb 5H7, which is specific to a small intestine carbohydrate epitope of a rabbit homologue of human Muc2 (24), did not label M cells or dome enterocytes (Fig. 2b). MAb 3A4, which is specific to a carbohydrate epitope present on the filamentous brush border glycocalyx (FBBG) of jejunum enterocytes (19, 20), labeled rare dome enterocytes but not M cells (Fig. 2c). All MAbs colocalized quite well but not totally with MAb 58 in goblet cells of the dome base (see inset in Fig. 2b) and on secreted mucus. The latter displayed a typical filamentous network covering several adjacent cells and did not adhere preferentially to M cells (Fig. 2a and c).
TABLE 1.
Summary of immunofluorescence labeling of enterocytes and M cells in rabbit FAE by the mucin-related MAbs used in this study
| MAb | Epitope (reference) | Antigen(s) (reference)b | Cell type(s) labeled by immunofluorescencea in:
|
||||
|---|---|---|---|---|---|---|---|
| Appendix | Cecal patch | Sacculus rotundus | Distal Peyer's patch | Proximal Peyer's patch | |||
| 58 | Carbohydrate (16) | Muc2 and Mgc (this study) | M | M/E | M | M/E∗ | |
| 214 | Conformational peptide (16) | Mucin (this study) | E | E | E | E | E |
| 6G2 | Peptide (24) | Mucin (24) | E | E | E | E | E |
| 5H7 | Carbohydrate (24) | Muc2 and Mgc (24 and this study) | [M] | [M] | |||
| 3A4 | Carbohydrate (20) | FBBG (20), Muc2 and Mgc (this study) | [E] | M∗ | E∗ | ||
E, enterocytes; M, M cells. Brackets indicate rare labeling, and asterisks indicate instances in which goblet cells and mucus were not stained.
Mgc, M cell glycoconjugate.
(ii) Peyer's patches.
In 80% of the 20 rabbits examined, MAb 58 labeling of M cells was restricted to the most distal Peyer's patches (Table 1). In these Peyer's patches, 20 to 100% of the M cells were labeled, but none of the enterocytes (Fig. 1c and 2d through f) were. In the remaining 20% of rabbits, MAb 58 was not selective for M cells because it also recognized dome enterocytes. There was no correlation between rabbits negative for M-cell labeling in appendix tissue and those negative in Peyer's patches.
MAb 58 labeling was observed on microvilli of M cells with occasional negative circular areas, representing possible migration of lymphoid cells in the lumen (Fig. 2d, inset), as already described (27). MAb 214 (Fig. 1d and 2d) and MAb 6G2 recognized microvilli of all dome enterocytes but not of enterocytes of adjacent villi (data not shown).
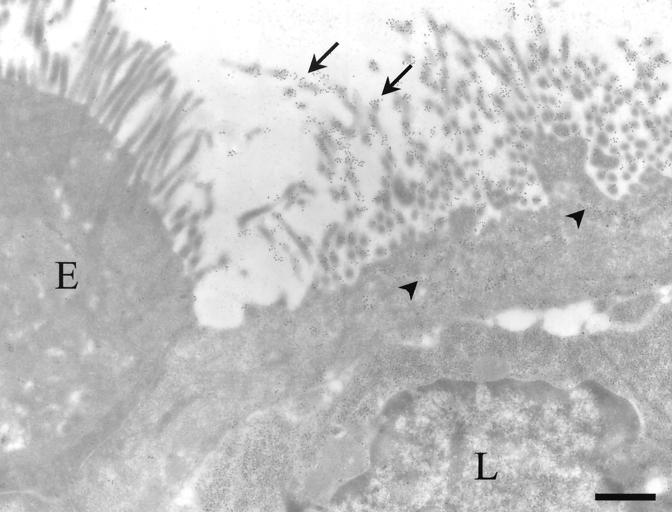
The other anti-oligosaccharide antibodies gave a different labeling pattern compared to that observed in the appendix. In all Peyer's patches, MAb 5H7 labeled about 10% of M cells (Fig. 2e). MAb 3A4 stained most but not all M cells in distal Peyer's patches, similar to MAb 58 (Fig. 2f). Double labeling with MAb 58 showed various intensities of the two corresponding MAbs, indicating that the labeling with each antibody varied according to glycosylation pattern differences between M cells (see the different colors from green to red in Fig. 2f). Electron microscopy indicated that MAb 3A4 was concentrated on the apical microvilli and endocytic vesicles of M cells (Fig. 3), similar to the pattern observed with MAb 58 (16). Furthermore, MAb 3A4 did not recognize FBBG molecules on dome enterocytes, as it does on microvilli from normal jejunum enterocytes (20). However, in proximal Peyer's patches, MAb 3A4 labeled few dome enterocytes (Table 1), a finding also noted in normal jejunum (19, 20). In all Peyer's patches, goblet cell labeling was faint for MAb 3A4, while MAb 58 labeled only a few goblet cells in the most distal Peyer's patches of the ileum.
FIG. 3.
Electron micrograph of an M cell labeled with MAb 3A4. The labeling (10-nm gold) was concentrated on the microvilli (arrows) and on the membranes of apical vesicles from M cells (arrowheads). Enterocytes (E) and lymphocytes in the basolateral pocket (L) were devoid of significant labeling. Bar, 2 μm.
(iii) Cecal patch.
In all rabbits tested, dome enterocytes were regularly and strongly labeled by MAb 214 or MAb 6G2. Unlike appendix and Peyer's patch labeling, all villus enterocytes were also recognized by both MAbs. No specific labeling of either dome enterocytes or M cells was observed with the other MAbs tested (Table 1).
(iv) Sacculus rotundus.
Depending on the rabbits, MAb 58 specificity ranged from strict labeling of M cells to dome enterocyte labeling (Table 1). MAb 214 or MAb 6G2 specifically but weakly stained dome enterocytes. No labeling of either dome enterocytes or M cells was observed with the other MAbs tested (Table 1).
From these data, it is clear that apical membrane from M cells expresses several glycosidic epitopes related to one or more mucins. Since MAb 58 specifically recognized the apex from M cells, secretory granules from goblet cells, and secreted mucus, we hypothesize that on M cells MAb 58 could recognize either a membranous mucin (hereafter called a mucin form of antigen 58) or a membrane glycoprotein (hereafter called a membrane form of antigen 58) which shares glycosidic epitopes with mucins. Therefore, we decided to use mucin and/or glycoprotein isolation methods to characterize the molecules recognized by MAb 58 at the apex of M cells.
Purification of a mucin form of antigen 58 on cesium chloride gradient. (i) Antigen 58 enrichment in carbonate-washed membranes.
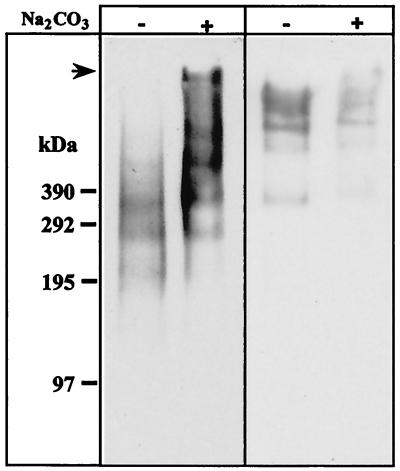
In previous studies, we showed that antigen 58 was recovered in carbonate-washed membranes but that antigen 214 was not recovered (16). The signal detected for MAb 58 by Western blotting decreased with the number of carbonate washes (not shown), consistent with mechanical degradation due to membrane pellet sonication (16). To avoid this degredation, membranes were recovered on a 2 M sucrose cushion. Hence, antigen 58 was significantly enriched by carbonate washing whereas antigen 214 was partially resistant (Fig. 4). SDS-PAGE was performed on continuous 2 to 10% gradient polyacrylamide gels to distinguish bands migrating over 200 kDa.
FIG. 4.
Antigen 58 enrichment and antigen 214 partial solubilization in a modified preparation of carbonate-washed membranes. Immunoblotting with MAb 58 (left panel) and MAb 214 (right panel) of 50 μg of total membranes treated with Na2CO3, or untreated, run on an SDS–2 to 10% PAGE gradient under reducing (for MAb 58) or nonreducing (for MAb 214) conditions, followed by transfer to an Immobilon-P membrane. The top of the gel (arrow) and molecular masses (in kilodaltons) are indicated on the left-hand side of the figure.
(ii) Distribution of antigen 58 following different extraction procedures.
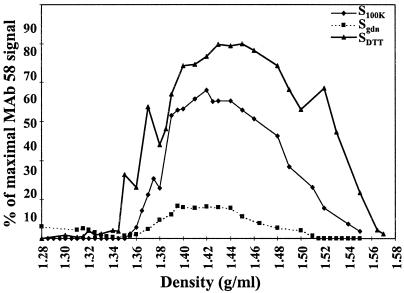
Freshly prepared carbonate-washed membranes were treated successively with 6 M gdn-HCl to release membrane-associated mucins (29), with 0.5% Triton X-100 to release integral membrane proteins, and with 10 mM DTT to release insoluble mucins such as Muc2 (1, 13) that might have sedimented with the membranes. Supernatants of the different extraction steps were submitted to isopycnic density gradient centrifugation in cesium chloride (1). The collected fractions (1.1 ml) were assessed by dot blot for the presence of different antigens, and quantifications were performed. Sixty percent of antigen 58 was found in the DTT supernatant (SDTT), 34% in the 100,000 × g supernatant (S100K), and 6% in the gdn-HCl supernatant (Sgdn) (Fig. 5). Antigen 58 was not detected in carbonate (Scarb) and Triton X-100 supernatants (STX). The percentage of antigen 58 present in Sgdn increased while that present in SDTT decreased upon storage of the carbonate-washed membranes at −20°C (data not shown), suggesting that antigen 58 degradation may be responsible for the release of antigen 58 in Sgdn.
FIG. 5.
Antigen 58 distribution after different extraction procedures and on a CsCl isopycnic density gradient. The initial membrane preparation (100,000 × g pellet) was carbonate washed and submitted successively to 6 M gdn-HCl, 0.5% Triton X-100 and 10 mM DTT, as described in Materials and Methods. The 100,000 × g (S100K, ⧫), gdn-HCl (Sgdn, ■) and DTT (SDTT, ▴) supernatants were subjected to density gradient centrifugation in 6 M CsCl–gdn-HCl. Fractions of 1.1 ml were collected from the top to the bottom of the gradient and analyzed for density, and 2 μl of each fraction was used for a dot blot assay of reactivity with MAb 58. No signal was detected for the Scarb and STX fractions.
(iii) Behavior of other mucin-related antigens following extraction treatments.
In S100K, all mucin-related antigens were present in large amounts (Table 2). In Scarb, antigens 214 and 6G2 were largely present, antigen 5H7 was poorly detected, and antigens 58 and 3A4 were not detected. In Sgdn, antigens 58, 214, and 6G2 were present whereas the others were not. In SDTT, antigens 58, 5H7, and 3A4 were the only molecules detected. None of the antigens tested were detected in STX (Table 2).
TABLE 2.
Mucin-related antigen release after membrane extractiona
| MAb | S100K | Scarb | Sgdn | STx | SDTT |
|---|---|---|---|---|---|
| 58 | ++ | − | + | − | +++ |
| 214 | +++ | ++ | ++ | − | NA |
| 6G2 | +++ | ++ | ++ | − | − |
| 5H7 | +++ | + | − | − | + |
| 3A4 | ++ | − | − | − | +++ |
Signal intensity was evaluated on dot blots with corresponding MAbs: +++, strong; ++, moderate; +, weak; −, null; NA, not applicable.
(iv) Sedimentation patterns on cesium chloride gradient.
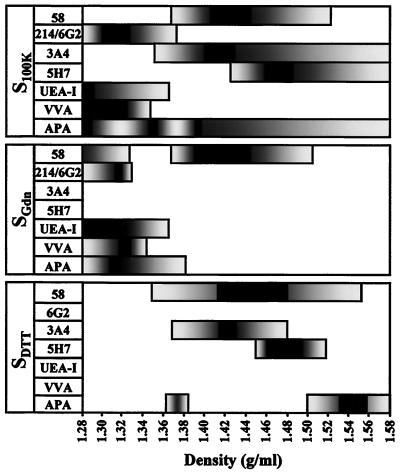
In S100K, Sgdn, and SDTT, a similar broad peak was obtained with antigen 58, with the density (d) ranging from 1.37 to 1.50 g/ml (Fig. 5). In S100K, antigen 3A4 and antigen 5H7 partially cosedimented with antigen 58 (d3A4 = 1.35 to 1.58 and d5H7 = 1.43 to 1.58 g/ml) whereas antigen 214 and antigen 6G2 sedimented in lower-density fractions (d = 1.28 to 1.38 g/ml [Fig. 6]). We also monitored the sedimentation pattern of glycoconjugates with lectins previously used to label rabbit appendix FAE (9). Molecules containing terminal fucose and terminal N-acetylgalactosamine detected by Ulex europaeus agglutinin and Vicia villosa agglutinin, respectively, were only present at the top of the S100K gradient (d = 1.28 to 1.35 g/ml) (Fig. 6). Terminal galactose residues recognized by Abrus precatorius agglutinin were present throughout the S100K gradient (Fig. 6).
FIG. 6.
Distribution of mucin-related epitopes and lectin-detected sugars after CsCl isopycnic density gradient centrifugation. The fractions collected from CsCl isopycnic density gradients of S100K, Sgdn, and SDTT and containing antigen 58 (see Fig. 5) were further analyzed for dot blot reactivity with other mucin-related MAbs and lectins. Reactivities that were >20% (grey) and >90% (black) of the maximal signal obtained with the corresponding MAbs or lectins are indicated as a function of the density.
Antigen 58 was purified from Sgdn since, under these conditions only, other antigens or lectin-detected glycoconjugates did not cosediment (Fig. 6). Protein detection with Coomassie blue gave no staining for Sgdn fractions of >1.38-g/ml densities, while carbohydrate detection with PAS gave a faint pink staining, which corresponded to the antigen 58 peak, and stronger unidentified staining, which corresponded to the bottom of the gradient (data not shown). The fractions containing antigen 58 in Sgdn, ranging from d = 1.38 to 1.48 g/ml, were thus pooled for further biochemical analysis and called Fgdn-58.
In SDTT, a broad peak was obtained with antigen 58 (d = 1.35 to 1.55 g/ml), including those of antigen 3A4 (d = 1.37 to 1.47 g/ml) and antigen 5H7 (d = 1.45 to 1.51 g/ml) (Fig. 6). The antigen 58 peak was thus separated into two pools; one contained fractions ranging from d = 1.38 to 1.44 g/ml, including the major antigen 3A4 peak (hereafter called FDTT-58-3A4), and the second contained fractions from d = 1.45 to 1.51 g/ml, covering the antigen 5H7 peak (called FDTT-58-5H7).
MAb 58, MAb 5H7, and MAb 3A4 recognized different Muc2 carbohydrate epitopes in rabbit appendix.
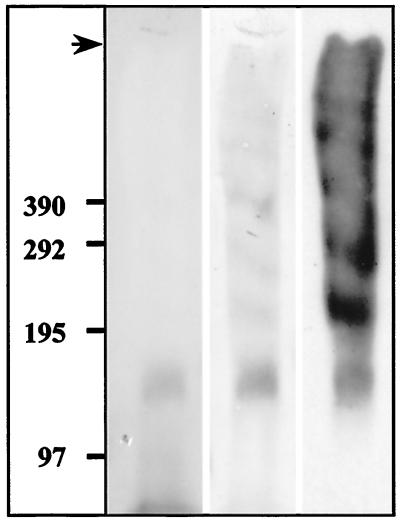
Electrotransferred Fgdn-58 gave faint negative staining with Coomassie blue except for a 140-kDa band (Fig. 7). The staining intensity of this band increased upon storage, suggesting that Fgdn-58 may be a degradation fragment issued from a poorly glycosylated portion of mucin. PAS staining gave several bands from the top of the gel down to the 140-kDa band (Fig. 7). In Western blots, all of these bands were recognized by MAb 58, confirming the purity of the antigen 58 peak (Fig. 7). A 15-amino-acid N-terminal sequence of the 140-kDa band was obtained, consisting of Pro-His-Tyr-Val-Thr-Phe-Asp-Gly-Leu-Tyr-Tyr-Ser-X-Gln-Gly. This sequence was 100% identical to the N-terminal sequence of the link peptide, a proteolytic cleavage fragment of the carboxyl terminus of human Muc2, attached to the rest of the molecule by intramolecular disulfide bonds. This proteolytic cleavage also occurs on purified Muc2 (14) as observed with antigen 58. We concluded that MAb 58 recognized a carbohydrate epitope present on the C-terminal part of Muc2.
FIG. 7.
Biochemical characterization of purified antigen 58 from Sgdn. An aliquot of the Sgdn fraction containing antigen 58 (see Fig. 6) was run on an SDS-2 to 10% PAGE gradient under reducing conditions, transferred to Immobilon-P membranes, stained with Coomassie blue (first lane) and then PAS (second lane), and immunolabeled with MAb 58 (third lane). The top of the gel (arrow) and molecular sizes (in kilodaltons) are indicated on the left-hand side of the figure.
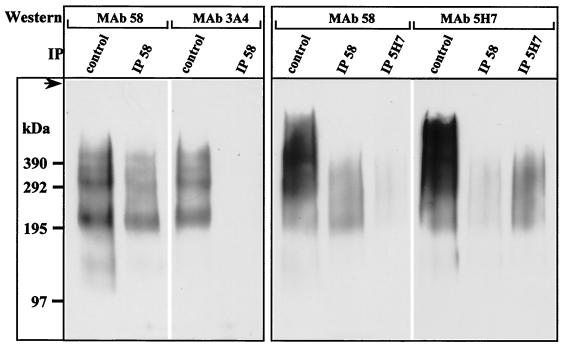
In humans and rats, Muc2 is the only mucin described so far that is solubilized after disulfide bridge reduction by DTT (1, 13). It was thus very likely that FDTT-58-3A4 and FDTT-58-5H7 contained at least two distinct glycoforms of Muc2 obtained from membranes washed with carbonate, gdn-HCl, and Triton X-100 and solubilized only after DTT reduction. This was further confirmed by Western blot analysis of FDTT-58-3A4 and FDTT-58-5H7. MAb 58 and MAb 3A4 produced similar patterns for FDTT-58-3A4, whereas MAb 58 and MAb 5H7 gave similar smears for FDTT-58-5H7 (Fig. 8, control lanes). Since the FDTT-58-5H7 density was higher than that of FDTT-58-3A4, there was presumably greater glycosylation of antigen 5H7 than of antigen 3A4, which could explain why more diffuse bands were issued from FDTT-58-5H7. Immunoprecipitation performed with MAb 58 on FDTT-58-3A4 completely abolished the MAb 3A4 signal (Fig. 8, left panel), indicating that although both MAb 58 and MAb 3A4 recognized Muc2 epitopes, these epitopes were expressed on two different glycoforms. When immunoprecipitation was performed with MAb 58 on FDTT-58-5H7, a decrease in the MAb 5H7 signal was observed relative to the MAb 58 signal (Fig. 8, right panel). Conversely, the MAb 58 signal decreased when immunoprecipitation was performed with MAb 5H7. MAb 58 and MAb 5H7 epitopes were therefore expressed on both the same glycoform and on different glycoforms of Muc2.
FIG. 8.
Immunoprecipitation of SDTT fractions. The SDTT fractions (see Fig. 6) containing either antigens 58 and 3A4 (left panel) or antigens 58 and 5H7 (right panel) were divided into 30-μl aliquots. These aliquots were run on an SDS-2 to 10% PAGE gradient without (control) or after immunoprecipitation (IP) with MAb 58 (IP 58) or MAb 5H7 (IP 5H7) coupled to protein A-Sepharose. After transfer to Immobilon-P membrane, Western blotting was performed with MAb 58, MAb 3A4, or MAb 5H7. The top of the gel (arrow) and molecular sizes (in kilodaltons) are indicated on the left. Immunoprecipitation with MAb 3A4 was not performed since it was an IgM molecule and could not be used the same way.
MAb 214 and MAb 6G2 recognized distinct peptidic epitopes of the same mucin.
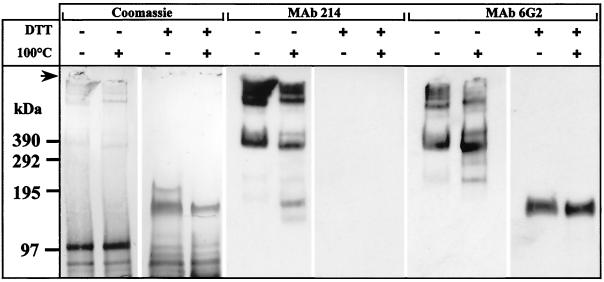
The S100K fractions enriched for antigens 214 and 6G2 (d = 1.28 to 1.36 g/ml [Fig. 6]) were pooled, run on SDS-PAGE, and transferred onto Immobilon-P. Under nonreducing conditions, Coomassie blue staining produced a 400-kDa band and at least three other bands at the top of the gel (Fig. 9). Several bands and smears below 200 kDa were also detected. The 400-kDa and higher bands were all detected by both MAbs 214 and 6G2 (Fig. 9). Lower putative degradation fragments were considerably increased after heating at 100°C before SDS-PAGE and correlated with a decrease in the signal of the high-molecular-weight bands (Fig. 9). Since both MAbs produced identical immunofluorescence staining and identical Western blot patterns after extraction and sedimentation of the antigens on a density gradient, they were presumed to recognize the same mucin. The 400-kDa and higher bands were also detected in carbonate-washed membranes (Fig. 4), suggesting that the same mucin was recognized in S100K and in carbonate-washed membranes.
FIG. 9.
: Biochemical characterization of antigen 214- and 6G2-enriched fractions from S100K. Thirty microliters of the antigen 214- or 6G2-enriched fraction from S100K (see Fig. 6) was treated as indicated above the lanes, run on SDS-2 to 10% gradient PAGE, and transferred to Immobilon-P membrane before Coomassie blue staining and immunolabeling with either MAb 214 or MAb 6G2. The top of the gel (arrow) and molecular sizes (in kilodaltons) are indicated at the left.
When DTT was added to antigen 214- and 6G2- enriched fractions before loading of the gels, the 400-kDa and higher bands disappeared in Coomassie blue, whereas a major 150-kDa band appeared (Fig. 9). As previously described (16), the MAb 214 epitope was destroyed by DTT reduction whereas MAb 6G2 still recognized the 150-kDa band (Fig. 9). An N-terminal amino acid sequence of this 150-kDa band has already been published (24), which corresponds to a novel intestinal mucin whose C terminus is cleaved, similar to the Muc2 link peptide.
Muc2 mRNA and protein core were not detected in rabbit and rat M cells.
To determine whether M cells express Muc2 mRNA, in situ hybridization experiments were performed with an anti-sense oligonucleotide corresponding to the human homologue of the N-terminal sequence of the 140-kDa Muc2 link peptide. Muc2 mRNA was not detected in the FAE of rabbit appendix and distal Peyer's patches (Fig. 10), except at the base of the appendix domes where some goblet cells were labeled (data not shown). Intense staining of goblet cells in adjacent villi served as a positive control for our probe (Fig. 10). We also used a probe corresponding to the N-terminal sequence of the rat link peptide on rat Peyer's patches and the cecal patch; again, no labeling of M cells could be detected, although goblet cells were intensely stained. Finally, we used the MAb WE9 (25), which recognizes the peptidic core of human, rat, and mouse Muc2 (32). We did not detect any signal in the FAE of rat Peyer's and cecal patches, but goblet cells from adjacent villi were labeled. MAb WE9 did not recognize rabbit Muc2. We concluded that the apical markers of M cells were probably not a membranous form of Muc2 but rather one or more membrane glycoproteins expressing the same glycosidic epitopes.
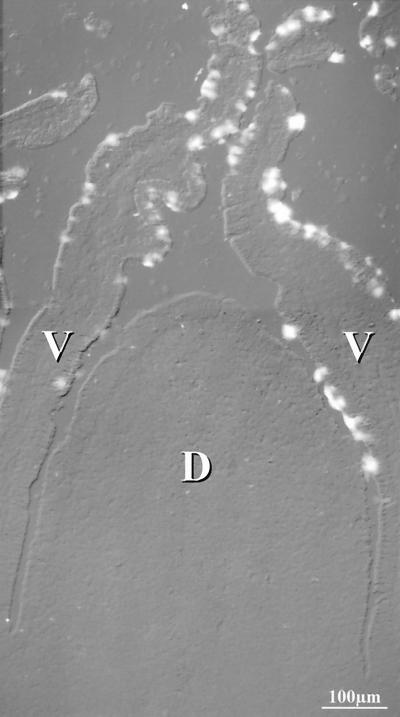
FIG. 10.
In situ hybridization of Muc2 probe to rabbit distal Peyer's patch. Superposition of differential interference contrast and fluorescein-Tyramide signal amplification of the Muc2 probe showed the absence of staining of the dome epithelium (D), whereas goblet cells on the adjacent villi (V) were intensely stained.
Attempts to characterize a membrane form of antigen 58.
To separate membrane glycoproteins from the already characterized soluble Muc2, we solubilized membrane proteins from the crude membrane fraction using Triton X-100. Surprisingly, Muc2 was still detected by Western blot analysis in the Triton fraction although no new band appeared (not shown). Another attempt was made by reducing the membranes with DTT to solubilize Muc2 before pelleting the membrane proteins; Muc2 was still the only species detected in the pellet by MAb 58 (not shown). The heavy contamination of all fractions by Muc2 prevented us from characterizing a membrane glycoprotein recognized by MAb 58 but did not rule out its presence.
DISCUSSION
We used monoclonal antibodies to study the distribution of different epitopes expressed at the apical plasma membrane of FAE in rabbit intestine, including appendix, cecal patch, sacculus rotundus, and Peyer's patches. Three of the antibodies, i.e., MAb 58, MAb 5H7, and MAb 3A4, specifically recognized the apical membrane of M cells although differences were observed according to the tissue concerned (see Table 1). The carbohydrate epitopes detected with these three MAbs were also expressed on the rabbit homologue of the major human-secreted intestinal mucin Muc2 (31), as demonstrated by biochemical studies and by microsequencing (24 and this study). However, we did not detect the presence of Muc2 or its mRNA in rabbit or rat FAE. It thus seemed unlikely that M cells expressed a Muc2 variant at their apical surface, unless its level was too low to be detected in our conditions. Furthermore, this mucin is not known to be membranous. It was also clear that luminal mucus containing Muc2 was always colabeled by several MAbs and did not adhere preferentially to M cells on the FAE since it covered several adjacent cells with no distinction between M cells and enterocytes (see Fig. 2a and c). The most likely hypothesis is that M cells express, on their apical surface, a glycoconjugate(s) that shares carbohydrate epitopes with Muc2. Several attempts to characterize such a glycoconjugate(s) have been unsuccessful. This could be due to the small amount of the M-cell membrane form of antigen 58 and/or to the large quantity of Muc2 which contaminates all fractions, even after DTT reduction or Triton extraction. Muc2 is known to be difficult to solubilize (31). Massive enrichment of M-cell fractions used will be necessary to detect such a molecule.
The fact that M cells express, on their apical surface, three different carbohydrate epitopes also expressed by Muc2 is of considerable interest since several intestinal pathogens such as Campylobacter upsaliensis, Yersinia enterocolitica, Shigella boydii, and Shigella sonnei bind purified intestinal mucins through their carbohydrate moiety (17, 26, 30). Elsewhere, a strain of human pathogenic Escherichia coli has been shown to bind carbohydrates of the link peptide of Muc2 (28), which was identified in our study. Interaction with mucin carbohydrates could explain how most pathogens may be trapped in the mucus gel to be discarded, while a small amount could be sampled by M cells and transcytosed to the mucosal immune system where they induce an immune response. Carbohydrate epitopes expressed on mucins and labeled by antibodies are generally more complex than those recognized by lectins and could thus provide better specificity (25). New antibodies should be used to determine whether other carbohydrate epitopes from Muc2 might also be expressed by M cells, especially in tissues where the three antibodies used in this study did not quantitatively label these cells (e.g., proximal Peyer's patches). Studies should be performed on other species to assess whether the presence of a mucin carbohydrate epitope on M cells is a general phenomenon. The antibodies used in the present study did not cross-react with human, mouse, rat, or hamster intestinal samples.
MAb 58 could be the best tool for M-cell studies, since it had the widest tissue distribution compared to the other two MAbs (see Table 1). MAb 3A4 labeling was restricted to M cells from distal Peyer's patches. In proximal Peyer's patches, MAb 3A4 labeled only dome enterocytes, and MAb 58 indifferently labeled a subpopulation of M cells and/or dome enterocytes. We were thus able to distinguish distal from proximal Peyer's patches, indicating that there are major differences in glycoconjugate expression at the surface of M cells, depending on their localization along the small intestine. Differences in glycoconjugate expression as detected by lectin labeling have been described between M cells of small and large intestines in mouse and rabbit (5, 9, 12) subjects but not within Peyer's patches. As infection studies are generally performed on Peyer's patches, it may be important to report their localization accurately along the intestine because they may not all have the same binding properties. MAb 58 and MAb 3A4 double labeling also indicated glycosylation pattern differences among M cells within a single distal Peyer's patch, as already described for rabbit cecal patches (10) and mouse Peyer's patches (12).
MAb 3A4 was originally raised against FBBG molecules of the rabbit jejunum, where it recognizes a glycosidic structure containing a terminal O-acetylated sialic acid (20). This epitope is mostly absent from jejunum Muc2, since only a few crypt goblet cells were labeled in this part of the intestine (19). However, our electron microscopic study in distal Peyer's patches demonstrated that MAb 3A4 did not label FBBG on FAE enterocytes. Our biochemical studies clearly indicate that the MAb 3A4 carbohydrate epitope was also expressed on Muc2 in appendix tissue. Hence, we assume that the MAb 3A4 does label the FBBG of the jejunum but also glycoconjugates on M cells from distal Peyer's patches and Muc2 in secreted mucus from the cecum, appendix, and colon (see Table 1).
Finally, MAb 214 and MAb 6G2 labeling was widely distributed in the different rabbit GALTs. These antibodies recognized the same newly described mucin molecule (16), an excellent negative marker for M cells present on the apical membrane of dome enterocytes from all GALTs studied. MAb 214 and MAb 6G2 bind peptidic epitopes, which may be the reason for their wide distribution on enterocytes from different GALTs. The MAb 6G2 signal was not detected in SDTT. Antigen 214/6G2 present in the Sgdn, therefore, cannot be a degradation product issued from an insoluble disulfide-linked complex as is the case for Muc2 but behaves as a membrane-associated form of mucin which can be extracted by gdn-HCl, as is the case for human ASGP1 (29). Antigen 214/6G2 may be a soluble mucin secreted by goblet cells essentially found in S100K and a dome enterocyte apical membrane-associated mucin in Sgdn. It was recently shown that, besides the presence of an appropriate bacterial (or viral) receptor, another important criterion for selective binding of pathogens might be free accessibility to epithelial apical membranes, which could depend on the thickness of the membrane glycocalyx (8, 22). We propose that this membrane-associated mucin could act as a protective barrier preventing pathogens from direct contact with the apical membrane of enterocytes. Its absence on the M-cell apical surface could enhance pathogen accessibility, whereas the presence of Muc2 carbohydrates on these cells could supply an environment favorable for initial attachment of the pathogens.
ACKNOWLEDGMENTS
We thank Marian Neutra for helpful discussions; Daniel K. Podolsky and Kathryn Devaney (Massachusetts General Hospital, Boston, Mass.) for the gift of WE9 monoclonal antibody; Susanne Maroux (Faculté des Sciences of Marseille Saint Jérôme, France) for the gift of MAbs 5H7, 6G2, and 3A4; and M. Boutillon (Centre Commun de Séquençage des Protéines, Lyon, France) for performing the microsequencing. We gratefully acknowledge B. Nguyen for excellent technical help; P. Paulet for the photographic work; and C. Astier, J. Sainte-Marie, J. R. Bonami, and G. Devau for kind help in setting up the experiments. Electron microscopy was performed at the Centre Régional d'Imagerie Cellulaire (Montpellier, France).
This work was supported by the Centre National de la Recherche Scientifique (UMR 5539 and Cell Biology project 96033), the Association pour la Recherche sur le Cancer (contract 6844), the Ligue Nationale contre le Cancer. H.L. was a fellow of the Délégation à la Recherche et au Développement and the Association pour la Recherche sur le Cancer.
REFERENCES:
- 1.Carlstedt I, Herrmann A, Karlsson H, Sheehan J, Fransson L A, Hansson G C. Characterization of two different glycosylated domains from the insoluble mucin complex of rat small intestine. J Biol Chem. 1993;268:18771–18781. [PubMed] [Google Scholar]
- 2.Chambraud L, Bernadac A, Gorvel J P, Maroux S. Renewal of goblet cell mucus granules during the cell migration along the crypt-villus axis in rabbit jejunum: an immunolabeling study. Biol Cell. 1989;65:151–162. doi: 10.1111/j.1768-322x.1989.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 3.Clark M A, Hirst B H, Jepson M A. M cell surface β1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer's patch M cells. Infect Immun. 1998;66:1237–1243. doi: 10.1128/iai.66.3.1237-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark M A, Jepson M A, Simmons N L, Booth T A, Hirst B H. Differential expression of lectin-binding sites defines mouse intestinal M-cells. J Histochem Cytochem. 1993;41:1679–1687. doi: 10.1177/41.11.7691933. [DOI] [PubMed] [Google Scholar]
- 5.Clark M A, Jepson M A, Simmons N L, Hirst B H. Differential surface characteristics of M cells from mouse intestinal Peyer's and caecal patches. Histochem J. 1994;26:271–280. [PubMed] [Google Scholar]
- 6.Falkow S, Isberg R R, Portnoy D A. The interaction of bacteria with mammalian cells. Annu Rev Cell Biol. 1992;8:333–363. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- 7.Foster N, Clark M A, Jepson M A, Hirst B H. Ulex europaeus 1 lectin targets microspheres to mouse Peyer's patch M cells in vivo. Vaccine. 1998;16:536–541. doi: 10.1016/s0264-410x(97)00222-3. [DOI] [PubMed] [Google Scholar]
- 8.Frey A, Giannasca K T, Weltzin R, Giannasca P J, Reggio H, Lencer W I, Neutra M R. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med. 1996;184:1045–1059. doi: 10.1084/jem.184.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gebert A, Hach G. Differential binding of lectins to M cells and enterocytes in the rabbit cecum. Gastroenterology. 1993;105:1350–1361. doi: 10.1016/0016-5085(93)90139-4. [DOI] [PubMed] [Google Scholar]
- 10.Gebert A, Posselt W. Glycoconjugate expression defines the origin and differentiation pathway of intestinal M cells. J Histochem Cytochem. 1997;45:1341–1350. doi: 10.1177/002215549704501003. [DOI] [PubMed] [Google Scholar]
- 11.Giannasca P J, Boden J A, Monath T P. Targeted delivery of antigen to hamster nasal lymphoid tissue with M-cell-directed lectins. Infect Immun. 1997;65:4288–4298. doi: 10.1128/iai.65.10.4288-4298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannasca P J, Giannasca K T, Falk P, Gordon J I, Neutra M R. Regional differences in glycoconjugates of intestinal M cells in mice: potential targets for mucosal vaccines. Am J Physiol Gastrointest Liver Physiol. 1994;267:G1108–G1121. doi: 10.1152/ajpgi.1994.267.6.G1108. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann A, Davies J R, Lindell G, Martensson S, Packer N H, Swallow D M, Caristedt I. Studies on the “insoluble” glycoprotein complex from human colon. J Biol Chem. 1999;274:15828–15836. doi: 10.1074/jbc.274.22.15828. [DOI] [PubMed] [Google Scholar]
- 14.Khatri I A, Forstner G G, Forstner J F. Susceptibility of the cysteine-rich N-terminal and C-terminal ends of rat intestinal mucin Muc2 to proteolytic cleavage. Biochem J. 1998;331:323–330. doi: 10.1042/bj3310323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraehenbuhl J P, Neutra M R. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992;72:853–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- 16.Lelouard H, Reggio H, Mangeat P, Neutra M, Montcourrier P. Mucin-related epitopes distinguish M cells and enterocytes in rabbit appendix and Peyer's patches. Infect Immun. 1999;67:357–367. doi: 10.1128/iai.67.1.357-367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantle M, Husar S D. Binding of Yersinia enterocolitica to purified, native small intestinal mucins from rabbits and humans involves interactions with the mucin carbohydrate moiety. Infect Immun. 1994;62:1219–1227. doi: 10.1128/iai.62.4.1219-1227.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marra A, Isberg R R. Invasin-dependent and invasin-independent pathways for translocation of Yersinia pseudotuberculosis across the Peyer's patch intestinal epithelium. Infect Immun. 1997;65:3412–3421. doi: 10.1128/iai.65.8.3412-3421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maury J, Bernadac A, Rigal A, Maroux S. Expression and glycosylation of the filamentous brush border glycocalyx (FBBG) during rabbit enterocyte differentiation along the crypt-villus axis. J Cell Sci. 1995;108:2705–2713. doi: 10.1242/jcs.108.7.2705. [DOI] [PubMed] [Google Scholar]
- 20.Maury J, Nicoletti C, Guzzo-Chambraud L, Maroux S. The filamentous brush border glycocalyx, a mucin-like marker of enterocyte hyper-polarization. Eur J Biochem. 1995;228:323–331. [PubMed] [Google Scholar]
- 21.Neutra M R, Kraehenbuhl J P. Transepithelial transport and mucosal defence I: the role of M cells. Trends Cell Biol. 1992;2:134–138. doi: 10.1016/0962-8924(92)90099-9. [DOI] [PubMed] [Google Scholar]
- 22.Neutra M R, Mantis N J, Frey A, Giannasca P J. The composition and function of M cell apical membranes: implications for microbial pathogenesis. Semin immunol. 1999;11:171–181. doi: 10.1006/smim.1999.0173. [DOI] [PubMed] [Google Scholar]
- 23.Neutra M R, Pringault E, Kraehenbuhl J P. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu Rev Immunol. 1996;14:275–300. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]
- 24.Nicoletti C, Maury J, Massey-Harroche D, Maroux S. Cellular and subcellular localization along the digestive and pulmonary tracts of a rabbit intestinal mucin differing from MUC2 and containing a 150 kDa light chain. Eur J Cell Biol. 1997;73:321–331. [PubMed] [Google Scholar]
- 25.Podolsky D K, Fournier D A, Lynch K E. Development of anti-human colonic mucin monoclonal antibodies: characterization of multiple colonic mucin species. J Clin Investig. 1986;77:1251–1262. doi: 10.1172/JCI112428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajkumar R, Devaraj H, Niranjali S. Binding of Shigella to rat and human intestinal mucin. Mol Cell Biochem. 1998;178:261–268. doi: 10.1023/a:1006844125976. [DOI] [PubMed] [Google Scholar]
- 27.Regoli M, Borghesi C, Bertelli E, Nicoletti C. A morphological study of the lymphocyte traffic in Peyer's patches after an in vivo antigenic stimulation. Anat Rec. 1994;239:47–54. doi: 10.1002/ar.1092390106. [DOI] [PubMed] [Google Scholar]
- 28.Sajjan S U, Forstner J F. Role of the putative “link” glycopeptide of intestinal mucin in binding of piliated Escherichia coli serotype O157:H7 strain CL-49. Infect Immun. 1990;58:868–873. doi: 10.1128/iai.58.4.868-873.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherblom A P, Carraway K L. A complex of two cell surface glycoproteins from ascites mammary adenocarcinoma cells. J Biol Chem. 1980;255:12051–12059. [PubMed] [Google Scholar]
- 30.Sylvester F A, Philpott D, Gold B, Lastovica A, Forstner J F. Adherence to lipids and intestinal mucin by a recently recognized human pathogen, Campylobacter upsaliensis. Infect Immun. 1996;64:4060–4066. doi: 10.1128/iai.64.10.4060-4066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tytgat K M, Buller H A, Opdam F J, Kim Y S, Einerhand A W, Dekker J. Biosynthesis of human colonic mucin: Muc2 is the prominent secretory mucin. Gastroenterology. 1994;107:1352–1363. doi: 10.1016/0016-5085(94)90537-1. [DOI] [PubMed] [Google Scholar]
- 32.van Klinken B J, Einerhand A W, Duits L A, Makkink M K, Tytgat K M, Renes I B, Verburg M, Büller H A, Dekker J. Gastrointestinal expression and partial cDNA cloning of murine Muc2. Am J Physiol Gastrointest Liver Physiol. 1999;276:G115–G124. doi: 10.1152/ajpgi.1999.276.1.G115. [DOI] [PubMed] [Google Scholar]