Abstract
Estrogen receptor alpha (ERα) is a ligand-responsive transcription factor critical for sex determination and development. Recent reports challenge the canonical view of ERα function by suggesting an activity beyond binding dsDNA at estrogen-responsive promotor elements: association with RNAs in vivo. Whether these interactions are direct or indirect remains unknown, which limits the ability to understand the extent, specificity, and biological role of ERα-RNA binding. Here we demonstrate that an extended DNA-binding domain of ERα directly binds a wide range of RNAs in vitro with structural specificity. ERα binds RNAs that adopt a range of hairpin-derived structures independent of sequence, while interacting poorly with single- and double-stranded RNA. RNA affinities are only 4-fold weaker than consensus dsDNA and significantly tighter than nonconsensus dsDNA sequences. Moreover, RNA binding is competitive with DNA binding. Together, these data show that ERα utilizes an extended DNA-binding domain to achieve a high-affinity/low-specificity mode for interacting with RNA.
An increasing number of transcription factors (TFs), previously thought to solely bind DNA, have been reported to also bind RNA via their DNA-binding domains (DBDs).1 In these unexpected TF–RNA interactions, the RNA acts as scaffolds,2 guides,3 or decoys,4 controlling TF promoter occupancy and transcriptional output.5−9 One TF proposed to associate with RNA is the estrogen receptor alpha (ERα).3,7 ERα is a ligand-responsive TF that mediates the function of estrogen, a steroid hormone that plays myriad roles in reproductive system physiology, including regulating growth and development.10−14 Like most TFs, ERα binds a specific dsDNA estrogen response element (ERE) TGGTCAnnnTGACCA, via its DBD (Figure 1A).
Figure 1.
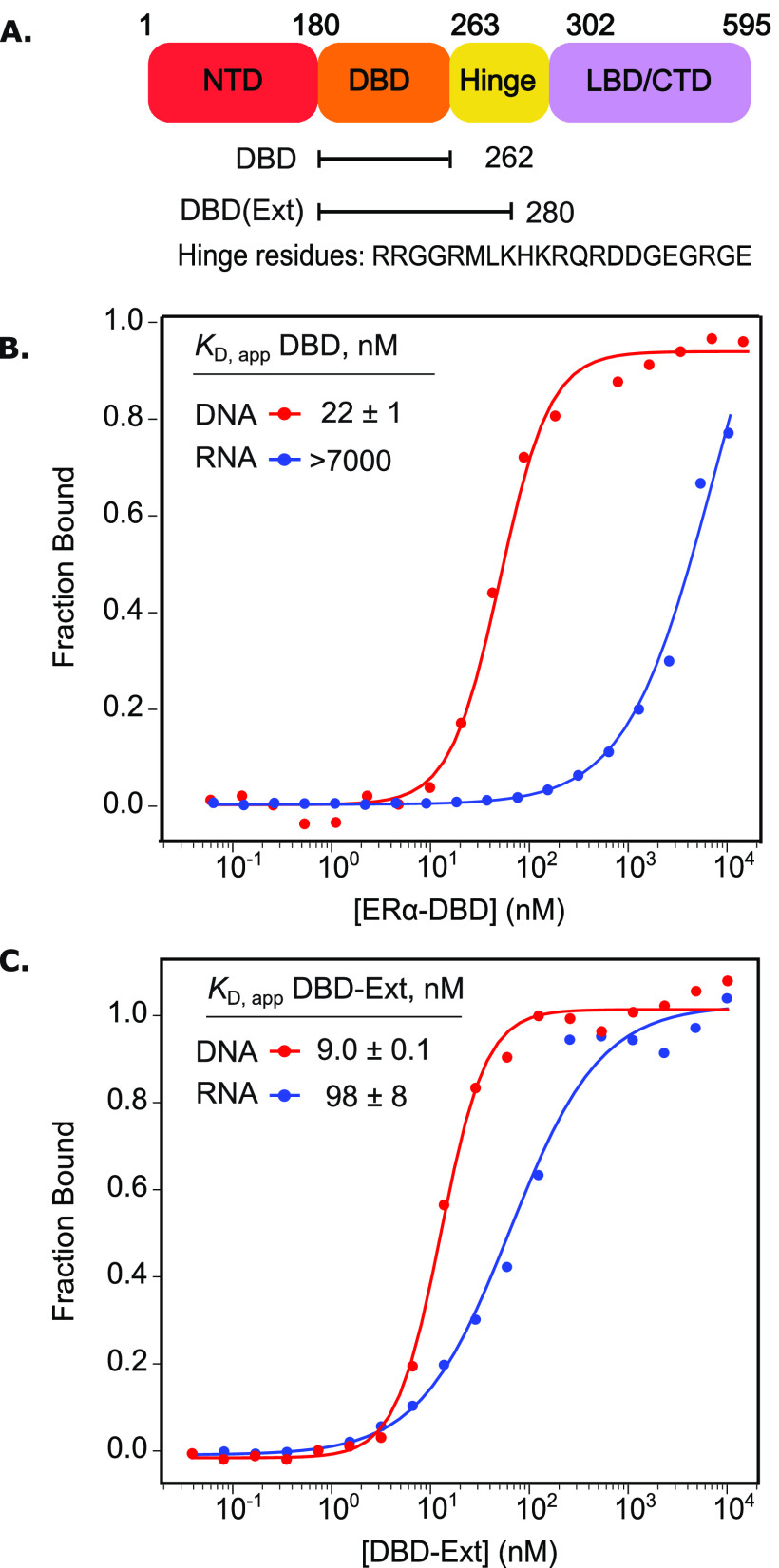
Residues in ERα’s hinge region are required for RNA binding. (A) Domain map of ERα, including the N-terminal domain, DBD, hinge region, and ligand binding/C-terminal domain. (B) and (C) FA-binding curves for ERα-DBD (B) or DBD-Ext (C) bound to duplex DNA (ERE) or an RNA hairpin derived from HOTAIR. N ≥ 3, standard error of the mean reported.
Recent discoveries, however, suggest ERα transcriptional regulation occurs through RNA binding.15−18 Certain enhancer RNAs (eRNAs) facilitate estrogen-induced ERα gene repression, while the lncRNA HOTAIR targets ERα to its promoter to positively regulate transcription via chromatin remodeling.3,7 Recently, RNA-binding activity by ERα in the cytoplasm has been characterized.17 Whether the ERα/RNA interaction is direct or mediated by other proteins remains unknown, as association between ERα and RNA is based on in vivo pull-down experiments. Additionally, systematic deletion of ERα’s functional domains in vivo demonstrated that the region spanning ERα’s DBD is required for association with RNA.7 A survey of the features required for ERα–RNA binding would improve our understanding of transcriptional regulation via TF–RNA interactions.
While in vivo studies suggest that the ERα-DBD interacts with RNA,3 it has previously been shown that it does not bind the lncRNA Gas5 directly,19 motivating us to readdress this issue. To approach this question, we used an in vitro fluorescence anisotropy (FA) assay to measure direct binding with highly purified components and performed binding in a buffered solution containing 100 mM NaCl. As expected, the DBD (AAs 180–262) binds fluorescently labeled DNA ERE with a KD,app of 22 ± 1 nM (Figure 1B).19 Consistent with the prior study,19 we did not observe direct DBD binding to several RNA hairpins derived from the lncRNA HOTAIR (Figure 1B, all RNA sequences are listed in Table S1).
A previous study for a related TF revealed the addition of basic residues from the hinge region, C terminal to the DBD, conferred high affinity RNA binding (Figure 1A).20 Compellingly, this region was independently implicated in cytoplasmic ERα–RNA association in mammalian cells.17 To test if this region assists in direct RNA binding, we purified a second ERα construct with 18 additional residues extending into the hinge region, termed DBD-Ext (AAs 180–280, Figure 1A, Table S2). In striking contrast to the binding of the canonical DBD, we found DBD-Ext robustly binds RNA derived from HOTAIR with a KD,app of 98 ± 8 nM (Figure 1C).
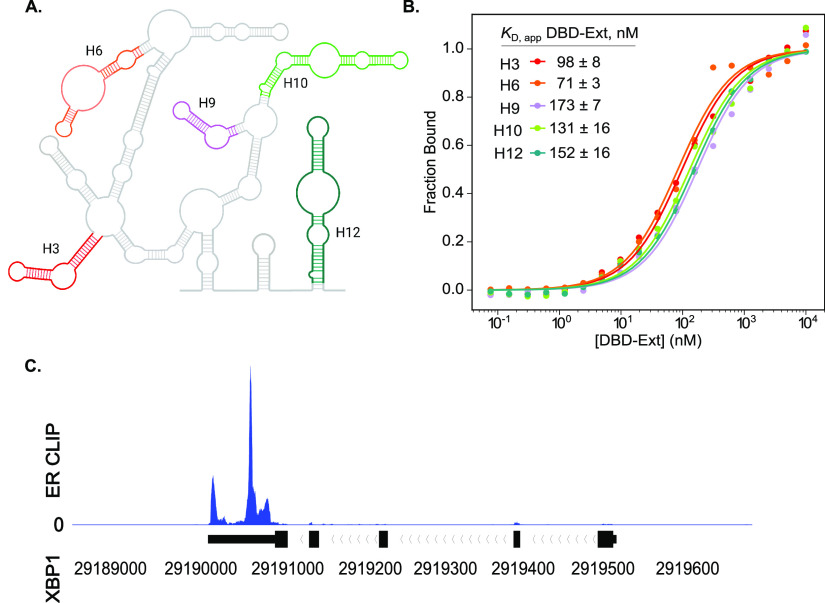
We next investigated the specificity of RNA binding by DBD-Ext. We chose various RNAs found to pull down with ERα, which include lncRNAs, eRNAs, and mRNAs.3,7,17 An RNA hairpin has been shown to be the minimal binding element for the TFs Sox2 and glucocorticoid receptor (GR), and we asked if ERα behaves similarly.20,21 We first tested hairpins derived from the 5′ domain of the highly structured HOTAIR.22 Sfold and UNAfold23,24 secondary structure calculations predicted these fold exclusively as hairpins (Figure 2A, Figure S1). We found that DBD-Ext binds all of the HOTAIR-derived hairpins, which have no sequence relationship or conservation, with similar KD,app (Figure 2B).
Figure 2.
DBD-Ext binds a range of biological RNAs. (A) Schematic of Domain 1 from the secondary structure of HOTAIR.(22) Colored hairpins were prepared separately while maintaining their secondary structures. (B) FA-binding data for various HOTAIR hairpins shown in (A). N ≥ 3, standard error of the mean reported. (C) Integrative Genome Viewer (IGV) example of HITS-CLIP data, showing ERα bound to the mRNA XBP1.
To determine if other biologically defined RNAs bound DBD-Ext, we tested the affinity for eRNAs and mRNAs obtained from publicly available HITS-CLIP data sets.17 We took >200 nucleotide-window sequences that overlapped with the most reads, calculated their secondary structures,23,24 and transcribed segments of RNAs (Figure 2C, Figure S1). These structured RNAs, including XBP1, TFF1, and GREB1, ranging from 24 to 75 nucleotides, bound within the same affinity range as the hairpins from HOTAIR (Figure S3). Therefore, we conclude that DBD-Ext binds a range of structured, biological RNAs in vitro.
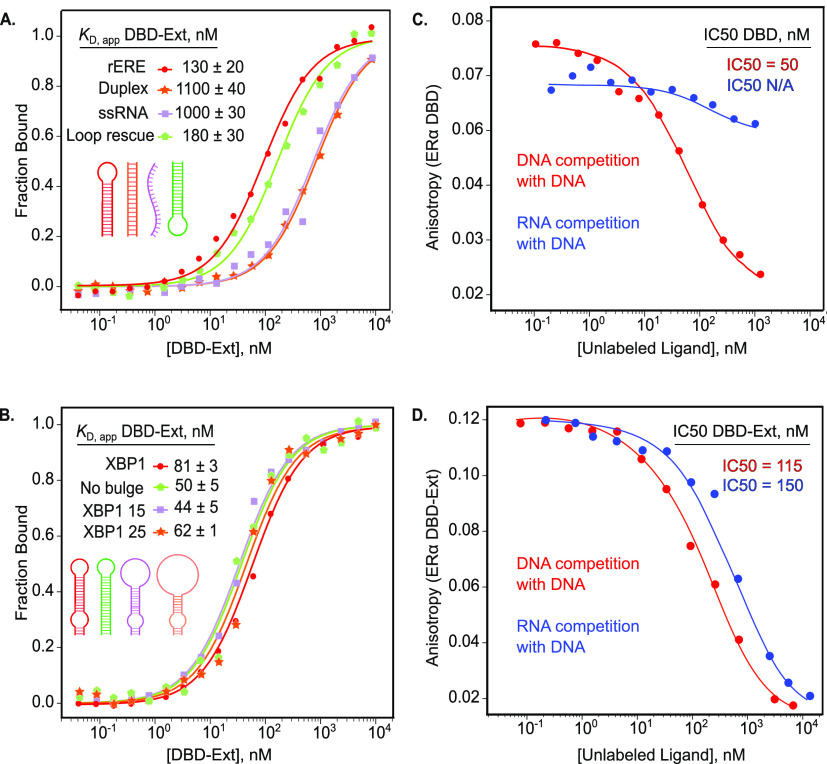
To further determine sequence or structural features of RNA required for ERα binding, we made a hairpin RNA derived from the ERE consensus sequence. This RNA sequence, rERE, contains the two six-nucleotide half sites and a terminal loop. DBD-Ext bound rERE with a KD,app of 130 ± 20 nM (Figure 3A). To test if the sequence, not the hairpin, is required for binding, we made an RNA duplex (duplex rERE) of the same consensus sequence that lacked secondary structure. Binding affinity to the RNA duplex rERE was reduced dramatically to 1.1 μM. Moreover, DBD-Ext bound single-stranded RNA (ssRNA) with the same reduced affinity, demonstrating that DBD-Ext binds poorly to single-stranded and duplexed, unstructured RNA (Figure 3A). To test if binding can be rescued, we created a modified hairpin, adding the terminal loop back to the opposite end of duplex rERE. We found that re-establishing the hairpin at the opposite end of the nonbinding duplex rescues high affinity binding (Figure 3A). Thus, the RNA hairpin structure is sufficient for binding, regardless of sequence or orientation. This lack of sequence specificity for RNA is in stark contrast to ERα’s DNA binding, wherein affinity for dsDNA without the consensus binding site is reduced 40-fold (Figure S2).25,26
Figure 3.
DBD-Ext binds RNA hairpins. (A) Normalized FA binding of DBD-Ext to RNA from the DNA consensus sequence, rERE (red). rERE was made into a standard duplex (orange), the hairpin structure was rescued by the addition of a loop on the opposite end (green). (B) FA-binding curves of DBD-Ext bound to a hairpin from XBP1 (red), no internal bulge (green), and enlarged stem loops (purple and orange). DBD (C) or DBD-Ext (D) bound to labeled DNA-ERE and competed off by titration of unlabeled ERE DNA (red) or XBP1 RNA (blue). N ≥ 3, standard error of the mean reported.
Many biological targets of ERα contain internal bulges that could facilitate binding, such as in the case of SMAD3.5 We therefore asked if other structural features play a role in RNA recognition. We started with the mRNA XBP1 identified in the HITS-CLIP analysis, which contains internal and terminal loops.17 We first eliminated the internal bulge, creating just a hairpin RNA; affinity was unchanged (Figure 3B). To further test how the size of the terminal loop affects binding for the XBP1 hairpin, we created separate constructs, with enlarged 15 and 25 nucleotide terminal loops. For all constructs, the KD,app remained in the range of the parent XBP1 construct (50–81 nM) (Figure 3B). Therefore, neither the internal bulge nor the size or sequence of the terminal loop confers specificity.
To determine if ERα-binding activity of RNA and DNA is mutually exclusive, we performed competition experiments in which unlabeled DNA (red) or XBP1 RNA (blue) was added to prebound protein/DNA complex. Unlabeled DNA competed labeled DNA off both ERα DBD and DBD-Ext, as expected (Figure 3C,D). However, unlabeled RNA effectively competed off labeled DNA bound to DBD-Ext, but not DBD alone (Figure 3C,D), further demonstrating residues extending past the DBD are required for ERα–RNA binding. This suggests that though the binding domains differ for DNA and RNA, they overlap to enough of an extent that binding one precludes interacting with the other.
This study of ERα’s ability to directly bind RNAs advances our understanding of TF–RNA interactions, supporting the interpretation that the observed in vivo associations are the result of direct binding. ERα binds RNA competitively with DNA, augmenting the canonical DBD with an additional region that confers full RNA-binding activity. Importantly, ERα discriminates RNAs on the basis of structure, binding a suite of RNAs including hairpins of eRNAs, mRNAs, and lncRNAs independent of sequence or length. As RNA hairpins are a pervasive element in cellular RNA, our results suggest that ERα interacts extensively with RNA in the cell, consistent with HITS-CLIP data.27−29
A second important finding is that ERα requires residues from the hinge region to interact with RNA (Figure 1).17 Little is known about the function of hinge regions of nuclear hormone receptors (NHRs), as most structures suggest flexible, disordered domains.25,30,31 Notably, this region was found to be necessary for GR–RNA binding as well.20 An alignment of other NHRs shows basic residues are highly conserved within their hinge regions (Figure S4), suggesting this is a general phenomenon for this class of proteins.
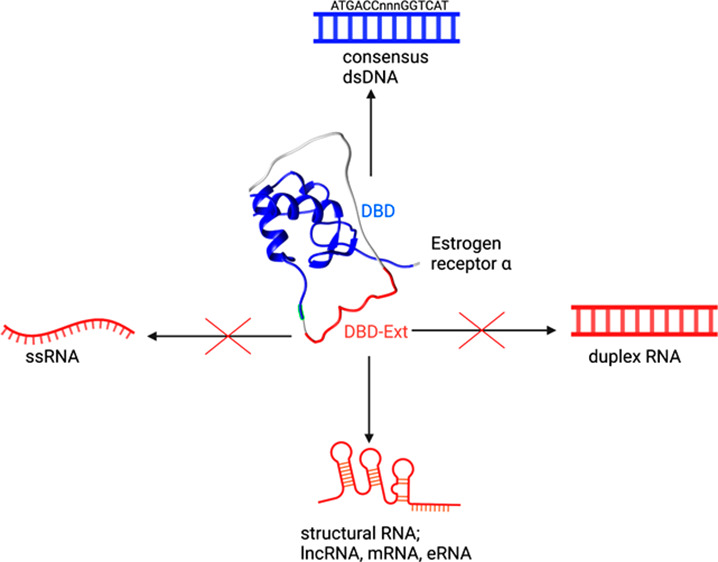
These observations also raise the question as to the purpose of ERα–RNA binding and provide insight into existing models.3,4,7 Given that ERα localizes to chromatin, where a large number of mature, nascent, and highly structured noncoding RNAs are present at intracellular concentrations near or above the KD,app of ERα, RNA binding may significantly impact ERα function.32−35 On one hand, RNA could influence the localization of ERα, with nascent, structural transcripts keeping ERα associated with chromatin during transcription (Figure 4, top). Conversely, ERα bound to a non-ERE promoter (with a lower KD than ERE elements, Figure S2) could be competed off by an RNA with multiple hairpins, acting as a molecular sponge to titrate away ERα and terminate transcription (Figure 4, bottom).
Figure 4.
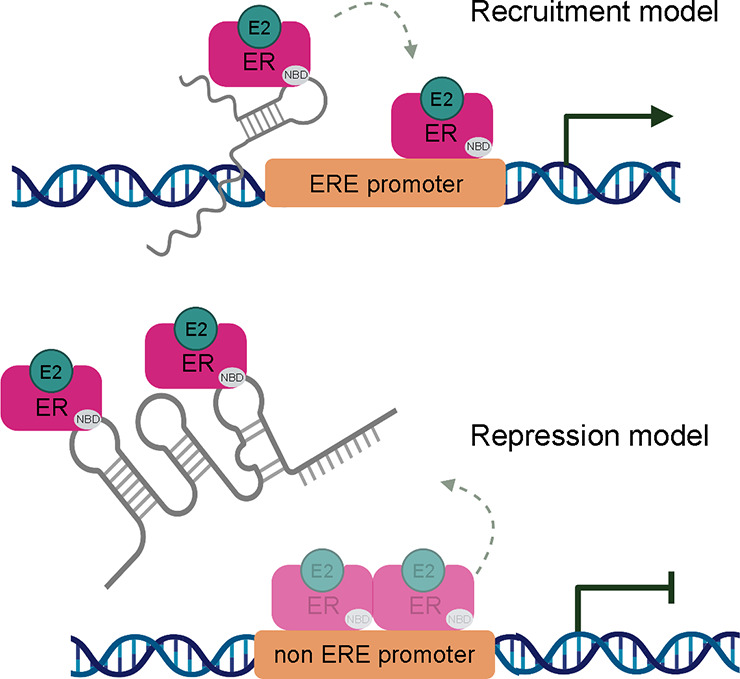
Models of ERα–RNA binding. Top: Recruitment model wherein ERα binds nascently transcribed RNA or other transcripts in cis, guiding it to the ERE promoter. Chromatin occupancy increases and transcription is upregulated. Bottom: Repression model wherein ERα bound to non-ERE promoter can be sequestered away by binding a lncRNA with multiple binding sites, decreasing promoter occupancy, and downregulating transcription. Nucleic acid binding domain (NBD) shown in gray, estrogen bound (E2).
Characterizing this interaction sheds light on the greater theme of RNA structural recognition that has emerged for other TFs such as Sox2, SMAD3, and GR.5,20,21 Our findings suggest this represents an important, and unappreciated, mechanism of transcriptional regulation. Further investigation of these associations in vivo is paramount to understanding the role of ERα–RNA binding in the cell.
Acknowledgments
The authors thank the Shared Instrument Pool (SIP) core facility (RRID SCR_018986), for the use of the Typhoon FLA 9500 and centrifuges.
Glossary
Abbreviations
- DBD
DNA-binding domain
- TF
transcription factor
- ERα
estrogen receptor alpha
- RBD
RNA binding domain
- FA
fluorescence anisotropy
- NHR
nuclear hormone receptor
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.biochem.2c00536.
Materials and methods, figures of folding predictions, binding curves, DBDs, and tables of nucleic acid ligand sequences and protein sequences, (PDF)
Accession Codes
ESR1: P03372
Author Contributions
H.R.S., N.C.L., D.S.W., and R.T.B. designed the research. H.R.S conducted experimental research and wrote the paper with feedback from all authors. D.S.W. and R.T.B. supervised the project.
This work was supported by a grant from the National Institutes of Health to D.S.W. and R.T.B. (R01 GM120347) and a grant from the National Science Foundation to D.S.W. (NSF 1716425).
The authors declare no competing financial interest.
Supplementary Material
References
- Hudson W. H.; Ortlund E. A. The Structure, Function and Evolution of Proteins That Bind DNA and RNA. Nat. Rev. Mol. Cell Biol. 2014, 15 (11), 749–760. 10.1038/nrm3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigova A. A.; Abraham B. J.; Ji X.; Molinie B.; Hannett N. M.; Guo Y. E.; Jangi M.; Giallourakis C. C.; Sharp P. A.; Young R. A. Transcription Factor Trapping by RNA in Gene Regulatory Elements. Science 2015, 350 (6263), 978–981. 10.1126/science.aad3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M.; Lee J. H.; Zhang Z.; De La Rosa R.; Bi M.; Tan Y.; Liao Y.; Hong J.; Du B.; Wu Y.; Scheirer J.; Hong T.; Li W.; Fei T.; Hsieh C.-L.; Liu Z.; Li W.; Rosenfeld M. G.; Xu K. Enhancer RNAs Mediate Estrogen-Induced Decommissioning of Selective Enhancers by Recruiting ERα and Its Cofactor. Cell Rep. 2020, 31 (12), 107803. 10.1016/j.celrep.2020.107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. C.; Chang H. Y. Molecular Mechanisms of Long Noncoding RNAs. Mol. Cell 2011, 43 (6), 904–914. 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey T. H.; Pyle A. M. The SMAD3 Transcription Factor Binds Complex RNA Structures with High Affinity. Nucleic Acids Res. 2017, 45 (20), 11980–11988. 10.1093/nar/gkx846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y.; Lee J. T. YY1 Tethers Xist RNA to the Inactive X Nucleation Center. Cell 2011, 146 (1), 119–133. 10.1016/j.cell.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello A.; Bacci L.; Re A.; Ripoli C.; Pierconti F.; Pinto F.; Masetti R.; Grassi C.; Gaetano C.; Bassi P. F.; Pontecorvi A.; Nanni S.; Farsetti A. MALAT1 and HOTAIR Long Non-Coding RNAs Play Opposite Role in Estrogen-Mediated Transcriptional Regulation in Prostate Cancer Cells. Sci. Rep. 2016, 6 (1), 38414. 10.1038/srep38414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassiday L. A.; Maher L. J. III Having It Both Ways: Transcription Factors That Bind DNA and RNA. Nucleic Acids Res. 2002, 30 (19), 4118–4126. 10.1093/nar/gkf512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y.; Izumi H.; Torigoe T.; Ishiguchi H.; Yoshida T.; Itoh H.; Kohno K. Binding of RNA to P53 Regulates Its Oligomerization and DNA-Binding Activity. Oncogene 2004, 23 (25), 4371–4379. 10.1038/sj.onc.1207583. [DOI] [PubMed] [Google Scholar]
- Björnström L.; Sjöberg M. Mechanisms of Estrogen Receptor Signaling: Convergence of Genomic and Nongenomic Actions on Target Genes. Mol. Endocrinol. 2005, 19 (4), 833–842. 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Alluri P. G.; Speers C.; Chinnaiyan A. M. Estrogen Receptor Mutations and Their Role in Breast Cancer Progression. Breast Cancer Res. 2014, 16 (6), 494. 10.1186/s13058-014-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaşar P.; Ayaz G.; User S. D.; Güpür G.; Muyan M. Molecular Mechanism of Estrogen–Estrogen Receptor Signaling. Reprod. Med. Biol. 2017, 16 (1), 4–20. 10.1002/rmb2.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J. S. Mechanisms of Oestrogen Receptor (ER) Gene Regulation in Breast Cancer. Eur. J. Endocrinol. 2016, 175 (1), R41–R49. 10.1530/EJE-16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourdy P.; Guillaume M.; Fontaine C.; Adlanmerini M.; Montagner A.; Laurell H.; Lenfant F.; Arnal J.-F. Estrogen Receptor Subcellular Localization and Cardiometabolism. Mol. Metab. 2018, 15, 56–69. 10.1016/j.molmet.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge C. M. Estrogen Receptor Interaction with Estrogen Response Elements. Nucleic Acids Res. 2001, 29 (14), 2905–2919. 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniappan M.; Nguyen L.; Grimm S. L.; Xi Y.; Xia Z.; Li W.; Coarfa C. The Genomic Landscape of Estrogen Receptor α Binding Sites in Mouse Mammary Gland. PLoS One 2019, 14 (8), e0220311 10.1371/journal.pone.0220311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.; Huangyang P.; Wang Y.; Xue L.; Devericks E.; Nguyen H. G.; Yu X.; Oses-Prieto J. A.; Burlingame A. L.; Miglani S.; Goodarzi H.; Ruggero D. ERα Is an RNA-Binding Protein Sustaining Tumor Cell Survival and Drug Resistance. Cell 2021, 184 (20), 5215–5229.e17. 10.1016/j.cell.2021.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley K. J.-L.; Maher L. J. Analysis of P53-RNA Interactions in Cultured Human Cells. Biochem. Biophys. Res. Commun. 2007, 363 (2), 381–387. 10.1016/j.bbrc.2007.08.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson W. H.; Pickard M. R.; de Vera I. M. S.; Kuiper E. G.; Mourtada-Maarabouni M.; Conn G. L.; Kojetin D. J.; Williams G. T.; Ortlund E. A. Conserved Sequence-Specific LincRNA-Steroid Receptor Interactions Drive Transcriptional Repression and Direct Cell Fate. Nat. Commun. 2014, 5, 5395. 10.1038/ncomms6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet N. V.; Lammer N. C.; Holmes Z. E.; Batey R. T.; Wuttke D. S. The Glucocorticoid Receptor DNA-Binding Domain Recognizes RNA Hairpin Structures with High Affinity. Nucleic Acids Res. 2019, 47 (15), 8180–8192. 10.1093/nar/gkz486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes Z. E.; Hamilton D. J.; Hwang T.; Parsonnet N. V.; Rinn J. L.; Wuttke D. S.; Batey R. T. The Sox2 Transcription Factor Binds RNA. Nat. Commun. 2020, 11, 1805. 10.1038/s41467-020-15571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somarowthu S.; Legiewicz M.; Chillón I.; Marcia M.; Liu F.; Pyle A. M. HOTAIR Forms an Intricate and Modular Secondary Structure. Mol. Cell 2015, 58 (2), 353–361. 10.1016/j.molcel.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y.; Chan C. Y.; Lawrence C. E. Sfold Web Server for Statistical Folding and Rational Design of Nucleic Acids. Nucleic Acids Res. 2004, 32, W135–W141. 10.1093/nar/gkh449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham N. R.; Zuker M.. 2008. UNAFold. In Bioinformatics: Structure, Function and Applications; Keith J. M., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, pp 3–31, 10.1007/978-1-60327-429-6_1. [DOI] [Google Scholar]
- Schwabe J. W. R.; Chapman L.; Finch J. T.; Rhodes D. The Crystal Structure of the Estrogen Receptor DNA-Binding Domain Bound to DNA: How Receptors Discriminate between Their Response Elements. Cell 1993, 75 (3), 567–578. 10.1016/0092-8674(93)90390-C. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Zakharov M. N.; Khan S. H.; Miki R.; Jang H.; Toraldo G.; Singh R.; Bhasin S.; Jasuja R. The Dynamic Structure of the Estrogen Receptor. J. Amino Acids 2011, 2011, 1–7. 10.4061/2011/812540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng N.-J.; Cieplak P. Free Energy Profile of RNA Hairpins: A Molecular Dynamics Simulation Study. Biophys. J. 2010, 98 (4), 627–636. 10.1016/j.bpj.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters J. The Nature of Preferred Hairpin Structures in 16S-like RRNA Variable Regions. Nucleic Acids Res. 1992, 20 (8), 1843–1850. 10.1093/nar/20.8.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutell R. R. Collection of Small Subunit (16S- and 16S-like) Ribosomal RNA Structures. Nucleic Acids Res. 1994, 22 (17), 3502–3507. 10.1093/nar/22.17.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak M.; Lefebvre P.; Staels B. General Molecular Biology and Architecture of Nuclear Receptors. Curr. Top. Med. Chem. 2012, 12 (6), 486–504. 10.2174/156802612799436641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikum E. R.; Liu X.; Ortlund E. A. The Nuclear Receptor Superfamily: A Structural Perspective. Protein Sci. Publ. Protein Soc. 2018, 27 (11), 1876–1892. 10.1002/pro.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O.; Morton J. G.; Rosbash M.; Richardson M. Three Abundance Classes in HeLa Cell Messenger RNA. Nature 1974, 250 (463), 199–204. 10.1038/250199a0. [DOI] [PubMed] [Google Scholar]
- Werner M. S.; Ruthenburg A. J. Nuclear Fractionation Reveals Thousands of Chromatin-Tethered Noncoding RNAs Adjacent to Active Genes. Cell Rep. 2015, 12 (7), 1089–1098. 10.1016/j.celrep.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A.; Penman S. Small Molecular Weight Monodisperse Nuclear RNA. J. Mol. Biol. 1968, 38 (3), 289–304. 10.1016/0022-2836(68)90387-2. [DOI] [PubMed] [Google Scholar]
- Zieve G.; Penman S. Small RNA Species of the HeLa Cell: Metabolism and Subcellular Localization. Cell 1976, 8 (1), 19–31. 10.1016/0092-8674(76)90181-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.