Abstract
Infectious microbial diseases can easily be transferred from person to person in the air or via high contact surfaces. As a result, researchers must aspire to create materials that can be implemented in surface contact applications to disrupt pathogen growth and transmission. This study examines the antimicrobial properties of polyacrylonitrile (PAN) nanofibers coated with silver nanoparticles (AgNPs) and silver(I,III) oxide. PAN was homogenized with varied weight concentrations of silver nitrate (AgNO3) in N,N-dimethylformamide solution, a common organic solvent that serves as both an electrospinning solvent and as a reducing agent that forms AgNPs. The subsequent colloids were electrospun into nanofibers, which were then characterized via various analysis techniques, including scanning electron microscopy, transmission electron microscopy, energy-dispersive X-ray analysis, dynamic light scattering, and X-ray photoelectron spectroscopy. A total of 10 microbes, including 7 strains of Gram-positive bacteria, 2 strains of Gram-negative bacteria, and Candida albicans, were incubated with cutouts of various PAN-AgNP nanocomposites using disk diffusion methods to test for the nanocomposites’ antimicrobial efficiency. We report that our electrospun PAN-AgNP nanocomposites contain 100% AgO, a rare, mixed oxidation state of silver(I,III) oxide that is a better sterilizing agent than conventional nanosilver. PAN-AgNP nanocomposites also retain a certain degree of antimicrobial longevity; samples stored for approximately 90 days demonstrate a similar antimicrobial activity against Escherichia coli (E. coli) and Lactobacillus crispatus (L. crispatus) when compared to their newly electrospun counterparts. Moreover, our results indicate that PAN-AgNP nanocomposites successfully display antimicrobial activity against various bacteria and fungi strains regardless of their resistance to conventional antibiotics. Our study demonstrates that PAN-AgNP nanocomposites, a novel polymer material with long-term universal antimicrobial stability, can potentially be applied as a universal antimicrobial on surfaces at risk of contracting microbial infections and alleviate issues related to antibiotic overuse and microbial mutability.
1. Introduction
Infectious microbes are easily transmitted in the air or from person to person via high contact surfaces. Most microbial infections are caused by bacteria, while approximately 10% of all microbial infections are induced by fungi.1 Antibiotics and antifungal drugs are conventionally used to control these two types of major infective diseases. However, the overuse of antimicrobial drugs promotes pathogenic microbial mutation and could possibly develop drug resistance.2
In microbiology, bacteria species are often distinguished and categorized into one of two groups (Gram-positive or Gram-negative) via the Gram Stain method. The result of the Gram Stain is analogous to the bacterium’s type of cell wall.3 The differentiation between Gram-positive and Gram-negative bacteria is a conventional method used to choose antibiotics for various infectious diseases.4 Gram-negative bacteria have a unique outer membrane, in which its outer leaflet comprises lipopolysaccharide (LPS), which plays a crucial role as a barrier that prevents the passive diffusion of hydrophobic substances, such as antibiotics and detergent, into the bacterial cell.5 The two-membrane bilayers of Gram-negative bacteria are also separated by the periplasm, a space that helps break down enzymes from toxic substances.6 On the other hand, Gram-positive bacteria do not have an outer membrane; instead, they possess multiple layers of densely packed and ordered peptidoglycan chains that help protect the cell.7
Multiple instances of drug-resistant bacteria have already been found.8 For example, Klebsiella pneumoniae (K. pneumoniae), a Gram-negative bacterium that causes complicated urinary tract infections, produces extended-spectrum β-lactamase (ESBL); ESBL-producing organisms render β-lactam antibiotics ineffective and induce refractory infections.9,10 Another common antibiotic-resistant strain is Staphylococcus aureus (S. aureus), a Gram-positive bacterium. S. aureus infections are usually treated with penicillin; when penicillin fails to treat the infection, methicillin will be used to neutralize the bacteria.11,12 Recent spikes in nosocomial pneumonia morbidity have been attributed to the rapid spread of methicillin-susceptible and methicillin-resistant isolates of S. aureus (MSSA and MRSA), which have proven to resist standard antibiotic therapy.13
Candida albicans (C. albicans) is considered to be a primary species responsible for the majority of all systemic fungal infections.1C. albicans is an opportunistic pathogenic yeast that is a common member of the human gut fungal flora.14,15 Unlike bacteria, the stronger cell wall of C. albicans is mainly composed of complex polymers of glucose (β1,3- and β1,6-glucan), chains of N-acetylglucosamine (chitin), and cell wall mannoproteins.16,17 Due to the complex structure of fungi, eukaryotic yeast cells are more capable of enduring unfavorable conditions created by antimicrobial agents than prokaryotic bacteria species.18
Microorganisms tend to remain on high contact surfaces for extended periods of time if they are not eliminated.19 Hence, antimicrobial agents should be implemented to exterminate harmful pathogens before transmission and infection. However, conventional antibiotics cannot be placed onto high contact surfaces to anticipate microbial transmission.20 Therefore, solid antimicrobial agents such as nanofiber coatings or nanocomposites should be implemented to exterminate microbes the moment they come into contact with patients and carriers.21
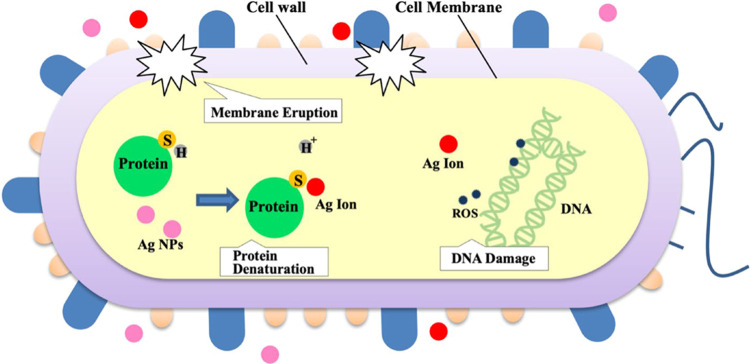
Metals such as silver (Ag) have been utilized in the past as antimicrobial agents.22 Throughout history, silver has been used as a form of wound dressing for burns and bacterial infections.23 When synthesized into silver nanoparticles (AgNPs), silver undergoes significant physiochemical changes, allowing it to obtain improved permeability in pathogens compared to its bulk counterparts due to an increased surface area-to-volume ratio.24,25 As AgNPs come into contact with microbes, they can bind to their cell wall and cell membrane and begin inhibiting respiration.26 For instance, AgNPs can destroy the cell wall of C. albicans by disrupting its β-glucan synthase, as β-glucan is the main constituent within the cell wall of C. albicans.16,27 Likewise, the positively charged silver ions of the nanoparticles rupture the negatively charged peptidoglycan bacteria cell wall via depolarization and release the bacteria’s internal cell contents.26,28 During this process, reactive oxygen species (ROSs) are also created, in turn interrupting DNA replication.26,29 Biochemical processes inside the cell are also disrupted as silver ions interact with sulfur (S) containing biomolecules, replacing their respective H+ groups and altering their molecular structure.26,30 The antimicrobial properties of AgNPs result in the subsequent biochemical degradation and death of the targeted pathogen (Figure 1).
Figure 1.
Schematic diagram demonstrating the antimicrobial mechanism of AgNPs and Ag ions, which induces protein, DNA, and cell membrane damage.
Previous studies have used carbon-based polymers to create nanoparticle-based surface coatings used in antimicrobial nanocomposite engineering applications; this is because organic polymers possess a high surface area-to-volume ratio, are malleable, and have a compact structure.31,32 Among various carbon polymers, polyacrylonitrile (PAN) is optimal for antimicrobial experimentation due to its ability for effective fibril formation via electrospinning.33,34 PAN has stable mechanical and thermal properties, is able to degrade before reaching its melting point, and possesses a high carbon yield, making it an ideal polymer that could be used in solution-based electrospinning formation.35,36 Our recent study on polyacrylonitrile-copper nanoparticle (PAN-CuNP) nanofibers took advantage of these characteristics and successfully synthesized a series of antibacterial copper-based nanocomposites.37
The chemical formation of AgNPs and their synthesis upon PAN can be initiated with N,N-dimethylformamide (DMF). While DMF is a conventional solvent commonly used in organic chemistry applications, it can reduce metal salts in the presence of water during colloidal solution formation (Scheme 1) due to its high oxidation potential (1.9 V vs Standard Hydrogen Electrode).38 Moreover, DMF has a high dielectric constant, is an electrically conductive solvent, and has a low vapor pressure.39 Metal nanoparticle containing carbon polymer nanofibers are usually synthesized with electrospinning techniques; therefore, DMF can also be used as an electrospinning solvent for PAN due to its electrical properties. DMF also improves the crystallinity and decreases the diameter of hydrophilic electrospun fibers, further increasing the surface area-to-volume ratio for electrospun polymer nanocomposites.40 Hence, PAN/DMF/AgNP colloidal solutions could be spun into polyacrylonitrile-silver nanoparticle (PAN-AgNP) filaments via the use of an electrospinner. The high voltage electric field gradient created by the electrospinner spins, solidifies, and coagulates the colloidal solution into solid nanofibers (Figure 2).
Scheme 1. DMF Reduction of Metal Ions in the Presence of Water.
Figure 2.

Schematic diagram of electrospinner creating carbon-based polymer nanofibers.
This paper poses as a significant and impactful extension in the field of surface-based antimicrobials. PAN-AgNP nanocomposites will be tested for their antimicrobial efficiency against a broad range of 10 microbes; previous studies in this subject area have not tested their nanofibers against such a large variety of pathogens. PAN-AgNP nanofibers synthesized from different weight concentrations (wt %) of AgNO3 will be observed and compared for their differences in antimicrobial activity against seven strains of Gram-positive bacteria, including Lactobacillus crispatus (L. crispatus), MSSA, S. aureus, Staphylococcus epidermidis (S. epidermidis), Enterococcus faecalis (E. faecalis), Streptococcus agalactiae (S. agalactiae), Streptococcus pneumoniae (S. pneumoniae), two strains of Gram-negative bacteria, including Escherichia coli (E. coli) and K. pneumoniae, and one strain of fungus, that is, C. albicans. PAN-AgNP nanocomposites will be analyzed and measured for their antimicrobial efficiency in zone of inhibition (ZOI) tests against these microbes.
Moreover, we aim to analyze the characteristics of PAN-AgNP nanofibers and their subsequent NPs after the nanocomposites have been synthesized via chemical reduction and electrospinning procedures. The nanocomposites will also be characterized with scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy-dispersive X-ray (EDX) analysis, dynamic light scattering (DLS), and X-ray photoelectron spectroscopy (XPS). These tests will allow us to expound upon and analyze the properties of our nanocomposites. Using these characterization techniques, we also recognized that our electrospun PAN-AgNP nanofibers contain 100% AgO, a rare, mixed oxidation state of silver(I,III) oxide that is a better sterilizing agent than conventional nanosilver, in turn demonstrating that we have synthesized a novel antimicrobial material.41
2. Experimental Section
2.1. PAN/DMF/AgNP Colloidal Solutions
Before synthesizing PAN-AgNP nanofibers with an electrospinner, different electrospinning solutions were prepared. Four different PAN solutions were created by dissolving 10 wt % of PAN (Merck Co., Ltd., Sigma-Aldrich Company, Neihu, Taipei, Taiwan) in 50 mL of DMF (Merck Co., Ltd.). Different concentrations (5, 10, and 15% wt % w.r.t to weight of PAN) of AgNO3 (Merck Co., Ltd.) were dissolved in three of the solutions, while the fourth solution was left as a pure PAN/DMF control that will not contain any AgNPs. The solutions were stirred in the dark under an aluminum foil covering with a 50 mm × 8 mm magnetic stir bar (Merck Co., Ltd.) at 300 rpm for 24 h. After the chemicals have homogenized, color changes can be observed in the solutions, in turn signifying the formation of AgNPs in PAN/DMF solution.
2.2. Electrospinning
5 mL of PAN/DMF/AgNP solution synthesized from a specific concentration of AgNO3 (0, 5, 10, and 15% wt % w.r.t to weight of PAN) was loaded into a 10 mL single use syringe (Terumo Co., Ltd., Shibuya City, Tokyo, Japan). The syringe was affixed to a syringe pump (Inovenso Co., Ltd., Istanbul, Turkey) and connected to an Inovenso Basic System electrospinner (Inovenso Co., Ltd.) via a single use plastic tube (Terumo Co., Ltd., Shibuya City, Tokyo, Japan) attached to an electrospinning nozzle (Inovenso Co., Ltd.). A 250 × 250 mm2 piece of aluminum foil was attached to the collection platform of the electrospinner, which was locked in a position 100 mm away from the electrospinning nozzle. The negative electrode clip was then attached to the aluminum foil to allow for the creation of an electric field during the electrospinning process. The electrospinner operated at a constant voltage of 30 kV, and the solution injection rate was set at 5 mL/h. The process of electrospinning concludes once the precursor solution has been completely spun into PAN-AgNP nanofibers.
2.3. PAN-AgNP Nanofiber and AgNP Characterization
A SU8220 scanning electron microscope (Hitachi High-Technologies Corporation, Tokyo, Japan) was used to image all nanofibers in this study. EDX analysis was performed using the scanning electron microscope to analyze elemental contents of the nanofibers.
The sizes of nanoparticles in PAN/DMF/AgNP colloidal solution were measured using a Talos F200X G2 transmission electron microscope (Thermo Fisher Scientific, Waltham, MA, USA). 15% PAN/DMF/AgNP solution was coated onto a copper grid at a thickness of 100 nm and analyzed using TEM techniques. EDX analysis was conducted in the TEM to analyze the elemental contents of the nanoparticles in the colloidal solution.
DLS (Beckman Coulter, Inc., Brea, CA, USA) techniques were also used to determine the size distribution of nanoparticles suspended in PAN/DMF/AgNP solution.
A K-Alpha X-ray photoelectron spectrometer (Thermo Fisher Scientific) was used to identify elements that covered the surface of nanofibers and provide information regarding their chemical state.
A Cary 630 Fourier transform infrared spectrophotometer (Agilent Technologies, Santa Clara, California, United States) was utilized to detect different functional groups in PAN-AgNP nanofibers.
A D8 Discover X-ray diffraction (XRD) spectrophotometer (Bruker Corporation, Billerica, Massachusetts, United States) was also used to identify the crystalline structure of PAN-AgNP nanofibers.
2.4. Microbial Culture Preparation and Serial Dilution
Microbial media and growth plates were prepared prior to growing various microbe strains. Sterilized Trypticase Soy Broth (TSB) (Merck Co., Ltd.) and de Man, Rogosa, Sharpe (MRS) (Merck Co., Ltd.) broth were used for all microbe cultures used in this study. Similarly, sterilized Trypticase Soy Agar (TSA) (Merck Co., Ltd.) and MRS agar (Merck Co., Ltd.) were poured and solidified in 100 × 15 mm petri dishes.
Cultures of L. crispatus (Bioresource Collection and Research Center, Hsinchu, Taiwan) were grown in MRS broth; on the other hand, MSSA, S. aureus, S. epidermidis, E. faecalis, S. agalactiae, S. pneumoniae, E. coli, K. pneumoniae, and C. albicans (Bioresource Collection and Research Center) were grown in TSB. All cultures were prepared in a shaking incubator (Thermo Fisher Scientific) set at 37 °C and 200 rpm for 24 h. The cultures were diluted to a 0.5 MacFarland bacterial turbidity standard using a UV–VIS optical density spectrophotometer (Vernier Software & Technology, Beaverton, OR, USA). 50 μL of different microbes was added to their corresponding nutrient plate, and sterilized cell spreaders were used to equally distribute the microbes on the plate.
2.5. ZOI Antimicrobial Tests
A 6.5 mm diameter hole puncher (SDI Corporation, Changhua City, Taiwan) was used to cut out all nanocomposite and control disks. All four types of nanocomposite disk cutouts synthesized from different concentrations of AgNO3 (0, 5, 10, and 15% wt % w.r.t to weight of PAN) as well as bulk silver disks and antimicrobial disks (ampicillin for all microbe strains except C. albicans; ketoconazole for C. albicans) of the same diameter were placed onto corresponding nutrient plates for all 10 microbes used in this study.
Another distinct group of nanocomposites of the same aforementioned concentrations were preserved under room temperature for 90 days. After 90 days of preservation, they were also cut out into disks of diameter 6.5 mm and used in ZOI antimicrobial tests against L. crispatus and E. coli.
The plates were then incubated with the aforementioned disks at 37 °C for 24 h in a non-shaking incubator (Thermo Fisher Scientific). After incubation, the inhibition diameter of the various fiber and control disks were quantified with ImageJ (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA), a prominent Java-based image analysis program used to process ZOI tests.
3. Results
3.1. Elemental Analysis of PAN-AgNP Nanofibers with SEM and EDX Analysis
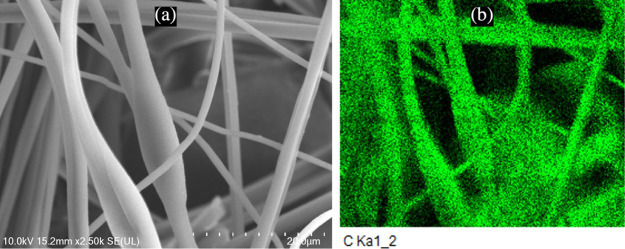
Pure PAN nanofibers were first analyzed in a SEM. As seen in Figure 3a, it is evident that PAN nanofibers create a compact scaffolding structure. Elemental distribution tests also confirm that PAN is a carbon polymer by detecting carbon in EDX analysis (Figure 3b).
Figure 3.
(a) SEM image of pure PAN nanofibers and (b) elemental distribution of carbon in the SEM image of pure PAN nanofibers.
SEM techniques also confirmed the presence of AgNPs on PAN nanofibers. As seen in Figure 4, it can be observed that AgNPs are scattered and distributed atop PAN-AgNP nanofibers. Table 1 and Figure S1 also indicate that higher intensities of silver were found on PAN nanofibers synthesized from higher wt % concentrations of AgNO3.
Figure 4.
(a) SEM image of 5% PAN-AgNP nanofibers, (b) elemental distribution of silver in the SEM image of 5% PAN-AgNP nanofibers, (c) SEM image of 10% PAN-AgNP nanofibers, (d) elemental distribution of silver in the SEM image of 10% PAN-AgNP nanofibers, (e) SEM image of 15% PAN-AgNP nanofibers, and (f) elemental distribution of silver in the SEM image of 15% PAN-AgNP Nanofibers.
Table 1. Average Silver Weight Concentration for PAN-AgNP Nanofibers Synthesized from Different wt % Concentrations of AgNO3.
| sample classification | average elemental weight concentration (%) |
|---|---|
| 5% wt % concentration | 4.12 |
| 10% wt % concentration | 7.85 |
| 15% wt % concentration | 10.08 |
3.2. Characterization of AgNP Size with TEM and DLS
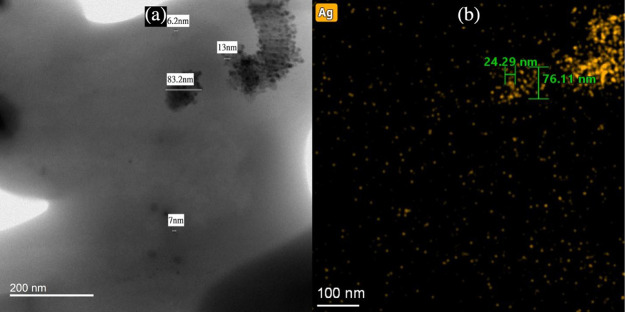
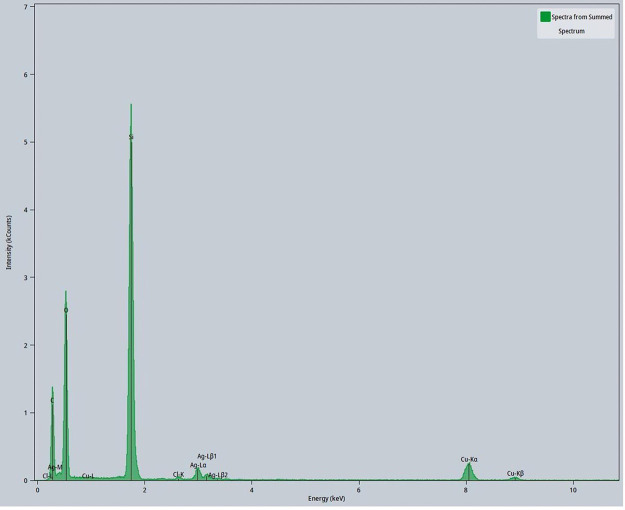
AgNPs were also characterized for their particle size using TEM analysis. Examples of this are found in Figure 5a, which depicts the TEM image of multiple AgNPs. AgNPs were observed to be scattered in PAN/DMF/AgNP colloidal solution and were measured for their size. AgNPs analyzed using TEM techniques had relatively spherical structures that varied in size and shape. The elemental distribution of silver (Figures 5b and S2) and their subsequent EDX spectrum (Figure 6) peaks also demonstrate that the particles imaged with the TEM were indeed AgNPs as high intensities of silver were measured in regions that contained the nanoparticles.
Figure 5.
(a) TEM image of AgNPs of various sizes in 15% PAN/DMF/AgNP colloidal solution and (b) elemental distribution of silver in the TEM image of 15% PAN/DMF/AgNP colloidal solution.
Figure 6.
EDX spectrum of 15% PAN/DMF/AgNP colloidal solution.
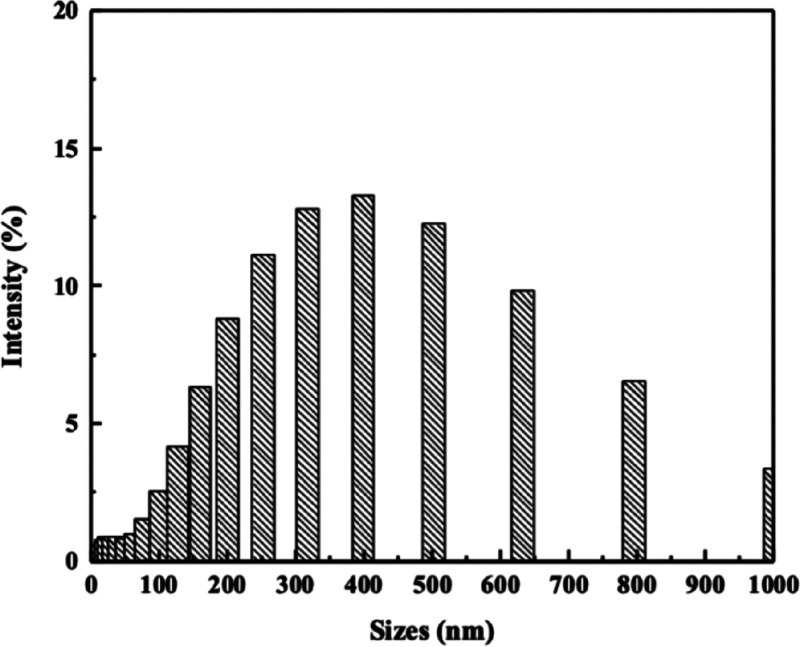
DLS was also used measure the size of AgNPs found in PAN/DMF/AgNP solution. Results from DLS-reinforced AgNP diameter measurements obtained from the TEM (Figure 7). Larger measurements could be clusters of AgNPs being identified together as a single particle.
Figure 7.
Dynamic light scattering spectrum of 15% PAN/DMF/AgNP colloidal solution.
3.3. Analysis of New and Preserved PAN-AgNP Nanocomposites with XPS
XPS analysis was utilized to further identify the chemical state of elements on the surface of PAN-AgNP nanofibers. 100% AgO in its stable form of silver(I, III) oxide was measured on both new (Figure 8a and Table S1) and preserved (Figure 8a and Table S2) 15% PAN-AgNP nanocomposite samples. Supplementary O 1s (Figure 8c and Table S3), C 1s (Figure 8d and Table S4), and N 1s (Figure 8e and Table S5) XPS elemental atomic weight quantifications have also been measured for New 15% PAN-AgNP nanocomposite samples.
Figure 8.
Silver X-ray photoelectron spectrum of (a) new and (b) preserved 15% PAN-AgNP nanofibers and (c) O 1s, (d) C 1s, and (e) N 1s X-ray photoelectron spectrum of new 15% PAN-AgNP nanofibers.
3.4. Antimicrobial Efficiency Comparison between Preserved and New PAN-AgNP Nanocomposites
As seen in Figure S5, preserved PAN-AgNP nanofibers (shown on the left) had a brown-black hue, while new PAN-AgNP nanofibers were white.
E. coli (Gram-negative bacterium) and L. crispatus (Gram-positive bacterium) were used to evaluate the antimicrobial efficiency and longevity of PAN-AgNPs. As BioSafety Level 1 (BSL-1) bacteria, both bacteria are relatively accessible and can serve as preliminary tests for future experiments against other microbes or pathogens.
When comparing Figures 9 and 10, it can be observed that all disks and nanocomposites had a higher antimicrobial efficiency against L. crispatus compared to E. coli for both new and preserved samples. New PAN-AgNP nanocomposites generally had better antimicrobial efficiency against E. coli and L. crispatus when compared to nanocomposites that were preserved for 90 days (Tables 2 and 3); however, preserved samples also retained a certain degree of antimicrobial activity. PAN-AgNP nanocomposites synthesized from higher wt % concentrations of AgNO3 also possess more antimicrobial activity.
Figure 9.
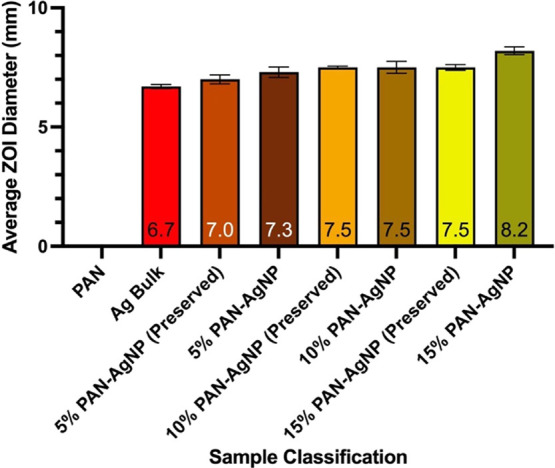
Average antimicrobial efficiency of new and preserved PAN-AgNP nanocomposites synthesized from different wt % of AgNO3 and bulk silver disks against E. coli over three independent trials. Error bars represent standard error.
Figure 10.

Average antimicrobial efficiency of new and preserved PAN-AgNP nanocomposites synthesized from different wt % of AgNO3 and bulk silver disks against L. crispatus over three independent trials. Error bars represent standard error.
Table 2. Antimicrobial Efficiency of New and Preserved PAN-AgNP Nanocomposites Synthesized from Different wt % of AgNO3 and Bulk Silver Disks against E. coli.
| sample classification | new |
preserved |
||
|---|---|---|---|---|
| average ZOI diameter (mm) | standard deviation | average ZOI diameter (mm) | standard deviation | |
| pure PAN nanofiber | 0 | 0 | N/A | N/A |
| Ag bulk | 6.7 | 0.09 | N/A | N/A |
| 5% PAN-AgNP nanofiber | 7.3 | 0.22 | 7.0 | 0.19 |
| 10% PAN-AgNP nanofiber | 7.5 | 0.25 | 7.5 | 0.05 |
| 15% PAN-AgNP nanofiber | 8.2 | 0.16 | 7.5 | 0.12 |
Table 3. Antimicrobial Efficiency of PAN-AgNP Nanocomposites Synthesized from Different wt % of AgNO3 and Bulk Silver Disks against L. crispatus.
| sample classification | new |
preserved |
||
|---|---|---|---|---|
| average ZOI diameter (mm) | standard deviation | average ZOI diameter (mm) | standard deviation | |
| pure PAN nanofiber | 0 | 0 | N/A | N/A |
| Ag bulk | 7.6 | 0.46 | N/A | N/A |
| 5% PAN-AgNP nanofiber | 11.5 | 0.17 | 9.8 | 0.34 |
| 10% PAN-AgNP nanofiber | 12.0 | 0.22 | 10.2 | 0.37 |
| 15% PAN-AgNP nanofiber | 13.4 | 0.05 | 10.4 | 0.42 |
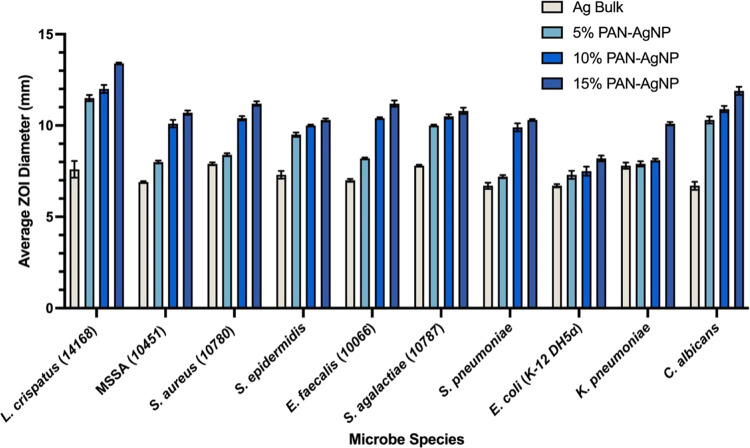
3.5. Antimicrobial Efficiency Tests against Various Microbes
Table 4 demonstrates that pure PAN nanocomposites synthesized from 0% of AgNO3 do not possess any antimicrobial properties against all 10 microbes used in this study.
Table 4. Antimicrobial Efficiency of PAN-AgNP Nanocomposites Synthesized from Different wt % Concentrations of AgNO3 and Bulk Silver and Antimicrobial Disks against Various Microbes.
| microbes |
samples |
||||||
|---|---|---|---|---|---|---|---|
| strains | gram stain | antimicrobial | PAN | Ag bulk | 5% Ag | 10% Ag | 15% Ag |
| AVG (SD) | AVG (SD) | AVG (SD) | AVG (SD) | AVG (SD) | AVG (SD) | ||
| L. crispatus (14168) | + | 37.5 (1.84) | 0 | 7.6 (0.46) | 11.5 (0.17) | 12.0 (0.22) | 13.4 (0.05) |
| MSSA (10451) | + | 0 | 0 | 6.9 (0.05) | 8.0 (0.08) | 10.1 (0.21) | 10.7 (0.12) |
| S. aureus (10780) | + | 6.8 (0.12) | 0 | 7.9 (0.08) | 8.4 (0.08) | 10.4 (0.12) | 11.2 (0.12) |
| S. epidermidis | + | 0 | 0 | 7.3 (0.21) | 9.5 (0.12) | 10.0 (0.05) | 10.3 (0.08) |
| E. faecalis (10066) | + | 0 | 0 | 7.0 (0.08) | 8.2 (0.05) | 10.4 (0.05) | 11.2 (0.17) |
| S. agalactiae (10787) | + | 0 | 0 | 7.8 (0.05) | 10.0 (0.05) | 10.5 (0.12) | 10.8 (0.17) |
| S. pneumoniae | + | 8.3 (0.12) | 0 | 6.7 (0.17) | 7.2 (0.08) | 9.9 (0.22) | 10.3 (0.05) |
| E. coli (K-12 DH5α) | – | 6.8 (0.25) | 0 | 6.7 (0.09) | 7.3 (0.22) | 7.5 (0.25) | 8.2 (0.16) |
| K. pneumoniae | – | 0 | 0 | 7.8 (0.17) | 7.9 (0.14) | 8.1 (0.08) | 10.1 (0.09) |
| C. albicans | N/A | 0 | 0 | 6.7 (0.22) | 10.3 (0.19) | 10.9 (0.17) | 11.9 (0.22) |
All silver containing bulk and nanofiber disk samples demonstrated antimicrobial activity against all 10 microbes in question (Table 4 and Figure 11). PAN-AgNP nanocomposites synthesized from increased wt % concentrations of AgNO3 show higher antimicrobial activity. All PAN-AgNP nanocomposite samples also appear to have better antimicrobial efficiency when compared to their bulk silver foil counterparts.
Figure 11.
Average antimicrobial efficiency of PAN-AgNP nanocomposites and bulk silver disks against various microbes over three independent trials. Error bars represent standard error.
Our data indicate that PAN-AgNP nanocomposites show more antimicrobial activity against Gram-positive bacteria and C. albicans compared to Gram-negative bacteria. This trend in antimicrobial efficiency is not influenced by individual antibiotic resistance; moreover, ampicillin or ketoconazole antimicrobial disk inhibition efficiency does not directly correlate to PAN-AgNP nanocomposite ZOI diameter.
4. Discussion
This study successfully created an alternative antimicrobial nanocomposite by using chemical reduction and electrospinning methodologies. DMF, while conventionally used as an organic solvent in chemistry experiments, plays three crucial roles in our study—it is a prominent electrospinning solvent, a potent metal salt reducing agent, and also acts as a metal nanoparticle protectant.38 Because of its dielectric, low vapor pressure nature, DMF serves as a great electrospinning base as charged DMF colloidal solutions will be able to overcome their own surface tension before evaporating, subsequently decreasing the diameter of the Taylor Cone.39 DMF also prevents the agglomeration of NPs by acting as a protectant in surfactant-free colloidal nanoparticle solution synthesis.42
Therefore, as seen from SEM, TEM, EDX analysis, DLS, and XPS tests, silver and its oxidized NPs have successfully formed in PAN/DMF solution and electrospun PAN nanofibers. EDX analysis spectra from both SEM and TEM characterizations further confirm the presence of AgNPs in PAN/DMF solution and PAN nanofibers. XPS tests revealed that preserved and new AgNP nanofibers both contain silver oxide; both samples were reported to contain 100% AgO, showing that silver has been reduced via DMF and stabilized over 90 days at room temperature on PAN. Antimicrobial ZOI test results show that our method of synthesis via chemical reduction and electrospinning proves to be successful. Our ZOI data for preserved and new PAN-AgNP nanocomposites also indicate that AgNP carbon polymer could retain their antimicrobial efficiency when preserved at room temperature for 90 days.
Data from ZOI tests indicate that all silver nanocomposite disks outperformed their bulk counterparts against all 10 microbes used in this study. Hence, this reveals that once silver has been synthesized into a nanoparticle form, it becomes a potent antimicrobial because of its increased surface area-to-volume ratio. AgNPs can also bind to microbes and disrupt their organic and chemical processes, eventually causing cellular death.43 In this case, pure PAN polymers do not have any antimicrobial properties but serve as a carrier for metal NPs, allowing them to avoid agglomeration and in turn interact with and eliminate more pathogens.44
Conventional antibiotics are prone to mutant resistance because they combat strains or genotypes of bacteria by targeting species-exclusive enzymes and specialized biochemical processes in individual and specific locations within the cell.45,46 On the other hand, AgNPs and its subsequent ions eliminate microbes by simultaneously exerting their antimicrobial properties in multiple areas of the bacterial cell; examples include instigating membrane eruption, creating DNA damage, and denaturing proteins.47−49 AgNPs pose a lower risk of bacterial resistance development as opposed to antibiotics because of these discrepancies.50,51
Our results also indicate that PAN-AgNP nanocomposites possess relatively less antimicrobial activity against Gram-negative bacteria compared to Gram-positive bacteria and C. albicans. Differences in nanocomposite antimicrobial efficiency against Gram-positive bacteria, Gram-negative bacteria, and C. albicans possibly originated from the distinct molecular compositions of their cell walls.52 The presence of an additional LPS-based outer membrane in Gram-negative bacteria possibly limits the antimicrobial efficiency of AgNPs.5
5. Conclusions
Characterization techniques including SEM, TEM, EDX analysis, DLS, and XPS validated the formation of AgNPs on PAN nanofibers.
We report that PAN-AgNP nanocomposites have more antimicrobial efficiency when compared to their bulk counterparts. Our data also indicate that PAN-AgNP nanocomposites synthesized from higher wt % concentrations of AgNO3 demonstrated more antimicrobial efficiency against all 10 microbes compared to samples synthesized from lower concentrations of metal salts.
The creation of polyacrylonitrile silver(I,III) oxide nanoparticle nanocomposites in this study positively impacts the development of new surface-based polymer pharmaceuticals. Conventional antimicrobial drugs like antibiotics are often expensive and are usually stored under specific conditions. Antibiotics can induce mutant resistance in microbes, while microbes treated with AgNPs do not develop mutant resistance. Hence, PAN-AgNP nanofibers can be used as an effective alternative antimicrobial material that is more cost-effective and easier to store in certain industries or fields of study. PAN-AgNP nanocomposites can also be engineered or implemented onto high contact surfaces such as masks, pantyliners, pads, carpets, or surgical equipment. Patients and medical personnel will also no longer have to combat antibiotic failure and microbial mutations with the development universal antimicrobials like PAN-AgNP nanofibers and nanocomposites.
Acknowledgments
Regarding sample characterization and analysis, we extend our gratitude to Wei-Lung Tseng and Guang-Hong Zheng of the National Sun Yat-Sen University for granting access to their DLS equipment and offering advice on methods related to DLS sample analysis. The authors also express their appreciation toward Le Shin Chang of JOYCOM BIO-CHEM Co. Ltd. for providing the L. crispatus bacteria strain used in this study. The authors further thank Sampson Chiang of Materials Analysis Technology Incorporated (MA-tek) for granting access to their SEM, TEM, XPS, FTIR, and XRD and offering advice on methods related to SEM, TEM, XPS, FTIR, and XRD sample analyses. The authors also thank Sean Tsao of Taipei American School for acquiring resources.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c06208.
SEM EDX spectrum of 5% PAN-AgNP nanofiber, 10% PAN-AgNP nanofiber, and 15% PAN-AgNP nanofiber; HAADF-TEM image of PAN/DMF/AgNP colloidal solution, silver, carbon, oxygen, and silicon elemental distribution in TEM image of PAN/DMF/AgNP colloidal solution; Ag 3d XPS atomic weight quantification of new 15% PAN-AgNP nanofiber sample; Ag 3d XPS atomic weight quantification of preserved 15% PAN-AgNP nanofiber sample; O 1s atomic weight quantification of new 15% PAN-AgNP nanofiber sample; C 1s atomic weight quantification of new 15% PAN-AgNP nanofiber sample; N 1s atomic weight quantification of new 15% PAN-AgNP nanofiber sample; FTIR Spectrum of 15% PAN-AgNP nanofiber sample; XRD spectrum of 15% PAN-AgNP nanofiber sample; preserved and new PAN-AgNP nanofibers; and PAN/DMF/AgNO3 mixture prior to homogenization versus homogenized PAN-AgNP colloidal solution (PDF)
Author Contributions
W.B.W.: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, and writing—original draft and editing. C.-Y.P.: project administration, resources, and supervision. E.-Y.H.: project administration, resources, and supervision. B.-J.P.: conceptualization, methodology, and writing—review and editing. J.H.: funding acquisition, project administration, resources, supervision, and writing—review and editing. J.C.C.: conceptualization, methodology, funding acquisition, project administration, resources, supervision, and writing—review and editing.
This research was funded by the Taipei American School. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Mba I. E.; Nweze E. I. The use of nanoparticles as alternative therapeutic agents against Candida infections: an up-to-date overview and future perspectives. World J. Microbiol. Biotechnol. 2020, 36, 163. 10.1007/s11274-020-02940-0. [DOI] [PubMed] [Google Scholar]
- Browne K.; Chakraborty S.; Chen R.; Willcox M. D.; Black D. S.; Walsh W. R.; Kumar N. A new era of antibiotics: The clinical potential of antimicrobial peptides. Int. J. Mol. Sci. 2020, 21, 7047. 10.3390/ijms21197047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi N.; Sapra A.. Gram staining, 2020. [PubMed]
- Barenfanger J.; Graham D. R.; Kolluri L.; Sangwan G.; Lawhorn J.; Drake C. A.; Verhulst S. J.; Peterson R.; Moja L. B.; Ertmoed M. M.; Moja A. B.; Shevlin D. W.; Vautrain R.; Callahan C. D. Decreased mortality associated with prompt Gram staining of blood cultures. Am. J. Clin. Pathol. 2008, 130, 870–876. 10.1309/AJCPVMDQU2ZJDPBL. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Meredith T. C.; Kahne D. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr. Opin. Microbiol. 2013, 16, 779–785. 10.1016/j.mib.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I.; Salama N. R. The gram-negative bacterial periplasm: Size matters. PLoS Biol. 2018, 16, e2004935 10.1371/journal.pbio.2004935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J.; Chang J.; Singh M. Peptidoglycan architecture of Gram-positive bacteria by solid-state NMR. Biochim. Biophys. Acta, Biomembr. 2015, 1848, 350–362. 10.1016/j.bbamem.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K. L.; Morris S. K. Travel and the spread of drug-resistant bacteria. Curr. Infect. Dis. Rep. 2018, 20, 29. 10.1007/s11908-018-0634-9. [DOI] [PubMed] [Google Scholar]
- Padmini N.; Ajilda A. A. K.; Sivakumar N.; Selvakumar G. Extended spectrum β-lactamase producing Escherichia coli and Klebsiella pneumoniae: critical tools for antibiotic resistance pattern. J. Basic Microbiol. 2017, 57, 460–470. 10.1002/jobm.201700008. [DOI] [PubMed] [Google Scholar]
- Shaikh S.; Fatima J.; Shakil S.; Rizvi S. M. D.; Kamal M. A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J. Biol. Sci. 2015, 22, 90–101. 10.1016/j.sjbs.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S. Y.; Davis J. S.; Eichenberger E.; Holland T. L.; Fowler V. G. Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. S.; De Lencastre H.; Garau J.; Kluytmans J.; Malhotra-Kumar S.; Peschel A.; Harbarth S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. 10.1038/nrdp.2018.33. [DOI] [PubMed] [Google Scholar]
- Wang W.-Y.; Hsueh P.-R.; Tsao S.-M.; Group T. S. Genotyping of methicillin-resistant Staphylococcus aureus isolates causing invasive infections using spa typing and their correlation with antimicrobial susceptibility. Int. J. Antimicrob. Agents 2022, 59, 106525 10.1016/j.ijantimicag.2022.106525. [DOI] [PubMed] [Google Scholar]
- Oliveira W. A. D.; Pereira F. D. O.; Luna G. C. D. G. D.; Lima I. O.; Wanderley P. A.; Lima R. B. D.; Lima E. D. O. Antifungal activity of Cymbopogon winterianus Jowitt ex Bor against Candida albicans. Braz. J. Microbiol. 2011, 42, 433–441. 10.1590/S1517-83822011000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. Candida albicans. Curr. Biol. 2012, 22, R620–R622. 10.1016/j.cub.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Teparić R.; Mrša V. Proteins involved in building, maintaining and remodeling of yeast cell walls. Curr. Genet. 2013, 59, 171–185. 10.1007/s00294-013-0403-0. [DOI] [PubMed] [Google Scholar]
- Chaffin W. L. Candida albicans cell wall proteins. Microbiol. Mol. Biol. Rev. 2008, 72, 495–544. 10.1128/MMBR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panáček A.; Kolář M.; Večeřová R.; Prucek R.; Soukupová J.; Kryštof V.; Hamal P.; Zbořil R.; Kvítek L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. 10.1016/j.biomaterials.2009.07.065. [DOI] [PubMed] [Google Scholar]
- Cobrado L.; Silva-Dias A.; Azevedo M.; Rodrigues A. High-touch surfaces: microbial neighbours at hand. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2053–2062. 10.1007/s10096-017-3042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh D. L.; Berry J.; Yarboro S. R.; Dahners L. E. Better prophylaxis against surgical site infection with local as well as systemic antibiotics: an in vivo study. J. Bone Joint Surg. Am. 2009, 91, 1907. 10.2106/JBJS.G.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homaeigohar S.; Boccaccini A. R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. 10.1016/j.actbio.2020.02.022. [DOI] [PubMed] [Google Scholar]
- Zhitnitsky D.; Rose J.; Lewinson O. The highly synergistic, broad spectrum, antibacterial activity of organic acids and transition metals. Sci. Rep. 2017, 7, 44554. 10.1038/srep44554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M.; Yadav A.; Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Hoseinzadeh E.; Makhdoumi P.; Taha P.; Hossini H.; Stelling J.; Amjad Kamal M. A review on nano-antimicrobials: metal nanoparticles, methods and mechanisms. Curr. Drug Metab. 2017, 18, 120–128. 10.2174/1389200217666161201111146. [DOI] [PubMed] [Google Scholar]
- Wang W. B.; Dezieck A.; Peng B.-J. Measuring Size-Dependent Enthalpy Alterations in Dry Milled White Rice via Bomb Calorimetry. J. Food Nutr. Res. 2022, 10, 74–80. 10.12691/jfnr-10-1-10. [DOI] [Google Scholar]
- Chatterjee A. K.; Chakraborty R.; Basu T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101 10.1088/0957-4484/25/13/135101. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J. A.; Caballero S.; Díaz L. A.; Guerrero M. A.; Ruiz J.; Ortiz C. C. High antifungal activity against candida species of monometallic and bimetallic nanoparticles synthesized in nanoreactors. ACS Biomater. Sci. Eng. 2018, 4, 647–653. 10.1021/acsbiomaterials.7b00511. [DOI] [PubMed] [Google Scholar]
- Konieczny J.; Rdzawski Z. Antibacterial properties of copper and its alloys. Arch. Mater. Sci. Eng. 2012, 56, 53–60. [Google Scholar]
- Samoilova N.; Krayukhina M.; Naumkin A.; Anuchina N.; Popov D. Silver nanoparticles doped with silver cations and stabilized with maleic acid copolymers: specific structure and antimicrobial properties. New J. Chem. 2021, 45, 14513–14521. 10.1039/D1NJ02478G. [DOI] [Google Scholar]
- Khodashenas B.; Ghorbani H. R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. 10.1016/j.arabjc.2014.12.014. [DOI] [Google Scholar]
- Lemraski E. G.; Jahangirian H.; Dashti M.; Khajehali E.; Sharafinia S.; Rafiee-Moghaddam R.; Webster T. J. Antimicrobial double-layer wound dressing based on chitosan/polyvinyl alcohol/copper: in vitro and in vivo assessment. Int. J. Nanomed. 2021, 16, 223. 10.2147/IJN.S266692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persano L.; Camposeo A.; Tekmen C.; Pisignano D. Industrial upscaling of electrospinning and applications of polymer nanofibers: a review. Macromol. Mater. Eng. 2013, 298, 504–520. 10.1002/mame.201200290. [DOI] [Google Scholar]
- Xu J.; Feng X.; Chen P.; Gao C. Development of an antibacterial copper (II)-chelated polyacrylonitrile ultrafiltration membrane. J. Membr. Sci. 2012, 413, 62–69. 10.1016/j.memsci.2012.04.004. [DOI] [Google Scholar]
- Yusof N.; Ismail A. Post spinning and pyrolysis processes of polyacrylonitrile (PAN)-based carbon fiber and activated carbon fiber: A review. J. Anal. Appl. Pyrolysis 2012, 93, 1–13. 10.1016/j.jaap.2011.10.001. [DOI] [Google Scholar]
- Bashir Z. A critical review of the stabilisation of polyacrylonitrile. Carbon 1991, 29, 1081–1090. 10.1016/0008-6223(91)90024-D. [DOI] [Google Scholar]
- Rangarajan P.; Yang J.; Bhanu V.; Godshall D.; McGrath J.; Wilkes G.; Baird D. Effect of comonomers on melt processability of polyacrylonitrile. J. Appl. Polym. Sci. 2002, 85, 69–83. 10.1002/app.10655. [DOI] [Google Scholar]
- Wang W. B.; Clapper J. C. Antibacterial Activity of Electrospun Polyacrylonitrile Copper Nanoparticle Nanofibers on Antibiotic Resistant Pathogens and Methicillin Resistant Staphylococcus aureus (MRSA). Nanomaterials 2022, 12, 2139. 10.3390/nano12132139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoriza-Santos I.; Liz-Marzán L. M. N, N-dimethylformamide as a reaction medium for metal nanoparticle synthesis. Adv. Funct. Mater. 2009, 19, 679–688. 10.1002/adfm.200801566. [DOI] [Google Scholar]
- Du L.; Xu H.; Zhang Y.; Zou F. Electrospinning of polycaprolatone nanofibers with DMF additive: The effect of solution proprieties on jet perturbation and fiber morphologies. Fibers Polym. 2016, 17, 751–759. 10.1007/s12221-016-6045-3. [DOI] [Google Scholar]
- Chen J.; Harrison I. Modification of polyacrylonitrile (PAN) carbon fiber precursor via post-spinning plasticization and stretching in dimethyl formamide (DMF). Carbon 2002, 40, 25–45. 10.1016/S0008-6223(01)00050-1. [DOI] [Google Scholar]
- Wang C.; Liu W.; Cao H.; Jia L.; Liu P. Cellulose nanofibers aerogels functionalized with AgO: Preparation, characterization and antibacterial activity. Int. J. Biol. Macromol. 2022, 194, 58–65. 10.1016/j.ijbiomac.2021.11.164. [DOI] [PubMed] [Google Scholar]
- Nagata T.; Obora Y. N, N-dimethylformamide-protected single-sized metal nanoparticles and their use as catalysts for organic transformations. ACS Omega 2020, 5, 98–103. 10.1021/acsomega.9b03828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnihotri S.; Mukherji S.; Mukherji S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: elucidation of the mechanism of bactericidal action of silver. Nanoscale 2013, 5, 7328–7340. 10.1039/c3nr00024a. [DOI] [PubMed] [Google Scholar]
- Vollath D.; Szabo D. Coated nanoparticles: a new way to improved nanocomposites. J. Nanopart. Res. 1999, 1, 235–242. 10.1023/A:1010060701507. [DOI] [Google Scholar]
- Magiorakos A.-P.; Srinivasan A.; Carey R. B.; Carmeli Y.; Falagas M.; Giske C.; Harbarth S.; Hindler J.; Kahlmeter G.; Olsson-Liljequist B.; Paterson D. L.; Rice L. B.; Stelling J.; Struelens M. J.; Vatopoulos A.; Weber J. T.; Monnet D. L. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- Van Duin D.; Paterson D. L. Multidrug-resistant bacteria in the community: trends and lessons learned. Infect. Dis. Clin. 2016, 30, 377–390. 10.1016/j.idc.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava S.; Bera T.; Roy A.; Singh G.; Ramachandrarao P.; Dash D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103 10.1088/0957-4484/18/22/225103. [DOI] [PubMed] [Google Scholar]
- Morones J. R.; Elechiguerra J. L.; Camacho A.; Holt K.; Kouri J. B.; Ramírez J. T.; Yacaman M. J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346. 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- Gogoi S. K.; Gopinath P.; Paul A.; Ramesh A.; Ghosh S. S.; Chattopadhyay A. Green fluorescent protein-expressing escherichia c oli as a model system for investigating the antimicrobial activities of silver nanoparticles. Langmuir 2006, 22, 9322–9328. 10.1021/la060661v. [DOI] [PubMed] [Google Scholar]
- Stabryla L. M.; Johnston K. A.; Diemler N. A.; Cooper V. S.; Millstone J. E.; Haig S.-J.; Gilbertson L. M. Role of bacterial motility in differential resistance mechanisms of silver nanoparticles and silver ions. Nat. Nanotechnol. 2021, 16, 996–1003. 10.1038/s41565-021-00929-w. [DOI] [PubMed] [Google Scholar]
- Naqvi S. Z. H.; Kiran U.; Ali M. I.; Jamal A.; Hameed A.; Ahmed S.; Ali N. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int. J. Nanomed. 2013, 8, 3187–3195. 10.2147/IJN.S49284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Thabaiti S. A.; Khan Z.; Manzoor N. Biosynthesis of silver nanoparticles and its antibacterial and antifungal activities towards Gram-positive, Gram-negative bacterial strains and different species of Candida fungus. Bioprocess Biosyst. Eng. 2015, 38, 1773–1781. 10.1007/s00449-015-1418-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.